Abstract
The proximal humerus is a common location for both primary benign and malignant bone tumors and may require sacrificing deltoid muscles, axillary nerve and/or rotator cuff along with proximal humerus resection. Thus, post operatively shoulder movements are restricted. The main goals of reconstruction are to maintain a stable shoulder so that the function of elbow and hand can be optimized. Various reconstruction options are available after proximal humerus resection. We present our experience in using implant-cement spacers as a primary reconstruction option for limb salvage in the primary tumors of proximal humerus. All cases were retrieved from our prospectively maintained surgical database. 142 patients (96 males and 46 females) with a median age of 17.5 years (3–70 years) were operated with implant cement spacer between January 2006 and April 2019. Median follow up was 34 months (1–174 months). Functional outcome of the surgery was assessed in survivors by Musculoskeletal Tumor Society score (MSTS). Implant survival was assessed by Kaplan Meier analysis and competing risk analysis. On last follow up, out of 142 cases, 81 patients had died, 54 are alive and seven were lost to follow up. 18(13%) patients underwent revision surgery for symptomatic proximal migration, implant failure or infection. Four (2.8%) patients underwent forequarter amputation for local recurrence. The five years implant survival (IS) by Kaplan Meier analysis was 79.6% and as per competing risk analysis, the chances of implant revision are 12% and 18% at five and ten years respectively. Mean MSTS score in survivors was 71% (60–80%). Implant cement spacer is a cost-effective alternative for reconstruction of proximal humerus with revision rates and function comparable to other reconstructions in cases where deltoid, axillary nerve and/or rotator cuff are excised.
Keywords: Bone tumor, Reconstruction, Nail cement spacer, Shoulder, Humerus
Abbreviation
- Musculoskeletal tumor society
MSTS
- Implant survival
IS
- Nail cement spacer
NCS
- Plate cement spacer
PCS
- Multi-disciplinary clinics
MDC
- Kuntscher nail
K nail
- Endotracheal tube
ET tube
- Corona virus disease 19
COVID 19
- Osteoarticular allograft
OA
- Allograft-prosthesis combo
APC
- Claviculo pro humero
CPH
- Reverse shoulder arthroplasty
RSA
- Free vascularized fibula graft
FVFG
- F/U
Follow up
1. Introduction
The proximal humerus is a common location for both malignant and benign primary bone lesions.1, 2, 3 With the advances in adjuvant therapy and reconstructive options, limb salvage surgery is the preferred treatment whenever feasible. In most tumors of the proximal humerus needing resection, limb salvage necessitates proximal humerus resection with sacrifice of deltoid muscle, axillary nerve and rotator cuff to attain safe oncological margins.4 The resultant functional morbidity is significant. The functional outcome is directly dependent on the extent of proximal humerus resection and the extent of residual abductor function at shoulder.5 Even with minimal residual shoulder function6 limb salvage gives superior functional and cosmetic results compared to amputation. A stable shoulder with adequate arm length can facilitate good elbow and hand function. Most reconstructive options thus merely function as spacers7 in the absence of useful shoulder function.
Though cement spacers are often used in oncology to salvage infected prostheses there are few reports of them being used as a primary reconstruction option for limb salvage in the upper limb. We present our series of proximal humerus resection reconstructed with an implant cement spacer as an alternative to expensive prosthetic replacement. The purpose of this study is to evaluate the results of this procedure in terms of complications, mechanical durability and functional outcome.
2. Materials and methods
Between January 2006 and April 2019, 238 patients underwent proximal humerus resection for primary bone tumors [142(59.6%) Implant cement spacer, 88(37%) megaprosthesis, 7(3%) reverse shoulder tumor prosthesis and 1(0.4%) claviculo pro humero]. In 142 patients, a cement spacer reinforced with either an intramedullary nail (NCS) or a plate (PCS) was used for reconstruction. Details of clinical history, imaging and treatment records and post treatment surveillance were retrieved using electronic medical records and case record files. Clinical details, radiology and histopathology of all cases were revisited by senior authors for the purpose of this study. Ethical committee approval was obtained before conducting this study.
All the cases were discussed in a multidisciplinary clinic (MDC) for diagnosis, staging and treatment planning. A bi planar plain radiograph along with magnetic resonance imaging (MRI) was done to assess disease extent locally. Patients underwent core needle biopsy for histopathological confirmation. If material from a biopsy done earlier at another institution was available, slides and blocks were reviewed at our institution. All patients were staged as per institutional protocol depending on the histopathology. There were 96(68%) male and 46 (32%) female patients. The median age at the time of surgery was 17.5 years. 127(89.5%) patients had malignant tumors [64 (45%) osteosarcoma, 48 (34%) Ewing sarcoma, 11 (7.7%) chondrosarcoma and 4(2.8%) others] and 15(10.5%) patients had benign aggressive tumors [14 (9.8%) Giant cell tumors and 1 (0.7%) chondroblastoma].
114 patients with malignant tumors (64 osteosarcoma, 48 Ewing sarcoma, 2 others) received pre-operative neo-adjuvant therapy according to the standard institutional guidelines. 4 cases received pre-operative radiotherapy (2 cases of Ewing sarcoma in view of large soft tissue component, 1 case of osteosarcoma received radiotherapy prior to seeking treatment at our institute and 1 case of high-grade sarcoma in view of large soft tissue component). Appropriate adjuvant therapy was administered after surgery in the form of chemotherapy in 114 patients (64 osteosarcoma, 48 Ewing sarcoma, 2 others) and post-operative radiotherapy was given in 19 patients of Ewing sarcoma. Patients with Chondrosarcoma, Giant cell tumor, Malignant peripheral nerve sheath tumor, Synovial sarcoma and Chondroblastoma were treated only surgically. Seventeen patients had distant metastasis at presentation, (7 Ewing sarcoma and 10 osteosarcoma). All patients were treated with curative intent.
Based on detailed pre-operative evaluation by the senior authors, patients in whom proximal humerus resection included sacrifice of deltoid muscle and/or axillary nerve and/or rotator cuff resulting in considerable loss of post-operative shoulder function were selected for reconstruction with NCS/PCS to stabilize the shoulder while maintaining adequate arm length to facilitate good elbow and hand function. When at least 5 cm of bone was available proximal to the olecranon fossa after resection we preferred to reconstruct with NCS [intramedullary Kuntscher nail (K nail) anchored with bone cement]. When <5 cm of bone was available proximal to the olecranon fossa, a plate and screws were used along with bone cement (PCS) to anchor the implant to bone.
Of the 142 patients of NCS/PCS, 108(76%) underwent NCS, 31(22%) underwent PCS and 3 (2%) underwent a combination of plate and nail cement spacer (for better anchorage and stability in cases with extremely short residual stump).
2.1. Surgical technique
Surgery was done in beach chair position under general anesthesia. After tumor resection as per oncologic principles, the length of residual bone was reassessed. In patients with adequate residual bone (>/ = 5 cm from olecranon fossa), medullary canal was gradually reamed and prepared to receive a snugly fitting K nail of appropriate diameter(7). The K nail was inserted into the residual bone and the required extra medullary length reconfirmed. Depending on the size of the medullary canal an endotracheal tube (ET tube) of appropriate outer diameter was attached to a conventional cement injection syringe kit. The distal portion of the nozzle of the cement injection syringe was cut to ensure a snug fit with the ET tube. The ET tube was cut to correspond to the length of the residual intramedullary canal to enable adequate cementing.8 After injecting the cement, the K nail was inserted into the medullary canal (Fig. 1) and the predetermined extra medullary length was reconfirmed. Occasionally, additional cement was applied at the implant bone interface to provide additional stability and distribute stress. When a PCS was the reconstruction modality used (<5 cm from olecranon fossa), a dynamic compression plate or a column specific distal humerus locking plate or a customized plate of appropriate length was fixed with screws to the distal humerus with intramedullary cement insertion to improve screw hold9, 10 As the canal in this part of the humerus is extremely narrow especially in pediatric age groups, we used 5 cc syringes filled with bone cement to inject cement in the canal. The proximal end of the nail or plate was anchored to the lower half of the glenoid, preserved scapula or available length of clavicle (in Tikoff-Lindberg resection) with polypropylene mesh11, 12 (Prolene™-Johnson and Johnson, Ethicon division, Aurangabad, India) (Fig. 1). The mesh was passed through the eye of the nail or the proximal most screw hole of the plate and anchored in place with non-absorbable braided polyester sutures (EthibondR-Johnson and Johnson, Ethicon division, Aurangabad, India). Depending on surgeon preference we added a cement blob (23 cases) at the proximal end of the construct incorporating the mesh to prevent soft tissue injury in case of proximal migration (Fig. 1). The residual muscles including tendon of long head of biceps (if adequate tendon length was available) were sutured to the mesh and to each other across the construct to provide stability. The wound was closed in layers over a negative suction drain.
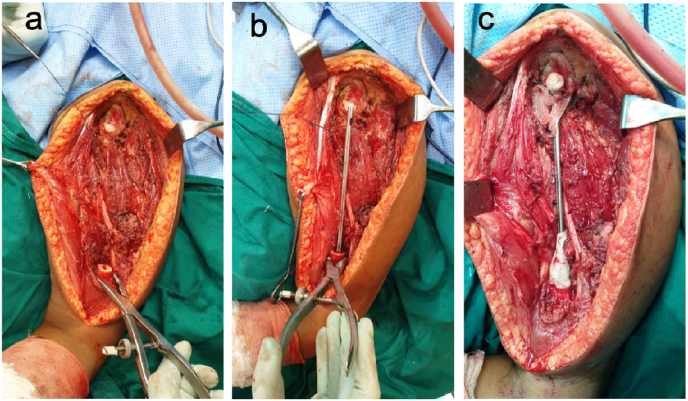
Fig. 1.
a) Tumor bed after tumor resection showing cut end of humerus and exposed glenoid cavity. b) K nail of appropriate diameter and length inserted into the remnant humeral canal with bone cement. c) Proximal end of the nail anchored to the glenoid with Prolene mesh and non-absorbable sutures. Cement cone extended onto the distal part of nail for additional stability. Cement blob added to the proximal end to prevent proximal migration.
The limb was kept in a shoulder immobilizer for six weeks. All patients were allowed immediate gravity eliminated movements of the elbow, forearm, wrist, and hand ensuring complete extension of the elbow to prevent any postoperative stiffness. Graded shoulder movements were commenced after six weeks.
For assessing mechanical durability, the end point of the study was date of implant failure necessitating revision surgery, date of last follow-up for survivors or date of death. To reinforce the validity of mechanical durability, competing risk survivorship estimation was done for implant survival in addition to Kaplan-Meier analysis as there were several patients who succumbed due to various reasons. Functional outcomes were assessed in survivors without implant failure using Musculo Skeletal Tumor Society (MSTS) score13 (functional assessment could not be done for the patients who succumbed before the start of this study).
3. Results
At last follow-up, 81 of 142 cases (57%) patients had died (78-disease, 1-secondary malignancy, 1- chemotherapy related cardiomyopathy, 1- COVID 19 related complications), 54(38%) were alive and 7(5%) were lost to follow-up. Median follow up was 34 months (1–174 months). Median follow up of survivors was 70 months (25th percentile - 45 and 75th percentile - 109 months).
For the sake of analysis, cases of PCS and plate & nail cement spacer were grouped together as PCS. Hence 108(76%) patients were reconstructed with NCS (Fig. 2) and 34(24%) were reconstructed with PCS (Fig. 3).
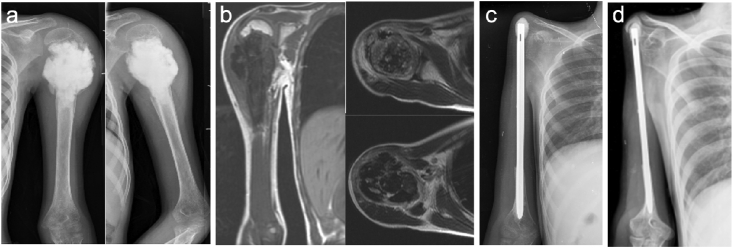
Fig. 2.
29yr lady with non-metastatic Osteosarcoma of left proximal humerus. a) Pre-operative x-ray. b) MRI T1 coronal section showing the medullary extent and T2 axial sections showing extra-medullary extent of the tumor. c) Post-operative x-ray with Nail cement spacer(NCS). d) X-ray at 10 years follow-up.
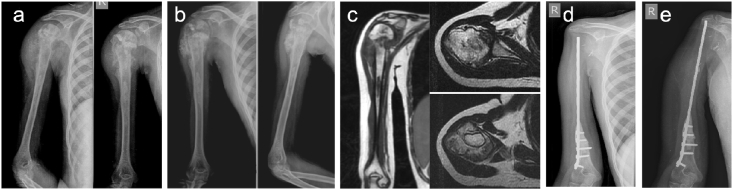
Fig. 3.
12yr girl with non-metastatic Osteosarcoma of right proximal humerus. a) Pre-chemotherapy x-ray. b) Post-chemotherapy x-ray. c) MRI T1 coronal section showing the medullary extent and T2 axial sections showing extra-medullary extent of the tumor. d) Post-operative x-ray with Plate cement spacer (PCS). e) X-ray at 4.5 years follow-up.
Superficial skin necrosis was seen in three patients (Clavien Dindo grade 1),14 these were managed conservatively. One patient of NCS with infection needed a wound lavage (Clavien Dindo grade 3B), while another was managed conservatively (on palliative care for distant metastasis). One patient with NCS with proximal migration (on palliative care for distant metastasis) was managed conservatively. Nine patients with NCS and three patients with PCS who had asymptomatic proximal migration of the implant at the shoulder and one patient with NCS who had inferior subluxation of the implant noted on radiographs were observed with no active intervention (n = 13, 9%).
Two patients had a prominent acromion process, of which one required partial excision while another needed no intervention. Three patients of NCS group and one patient of PCS group underwent forequarter amputation for local recurrence (n = 4, 2.8%).
Eighteen (13%) patients needed revision surgeries for implant related complications. Two patients with PCS and six patients with NCS were revised for symptomatic proximal migration (n = 8, 6%), three patients with PCS and four patients with NCS had implant failure/breakage (n = 7, 5%), three patients with NCS had infection requiring revision of construct (n = 3, 2%).
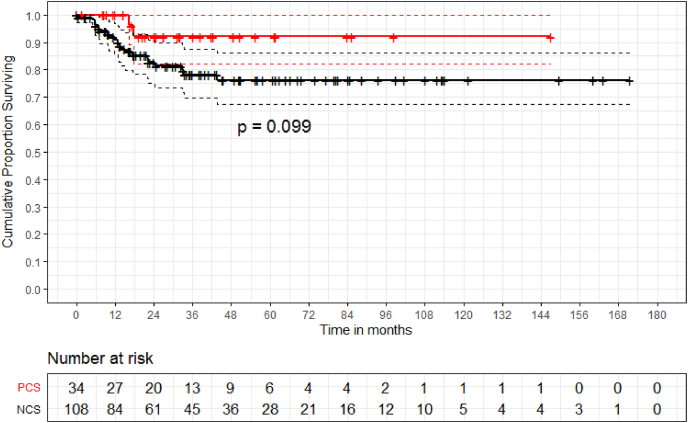
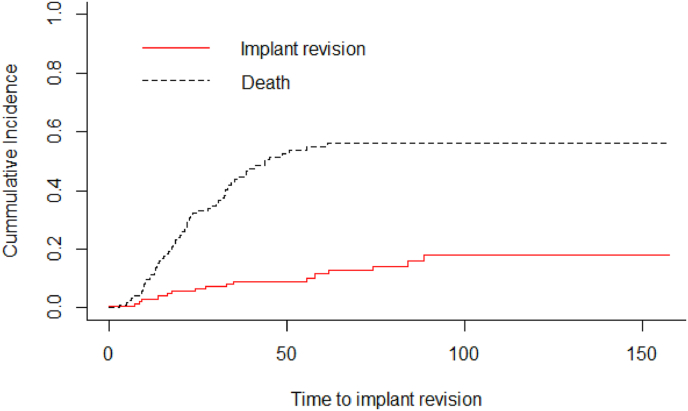
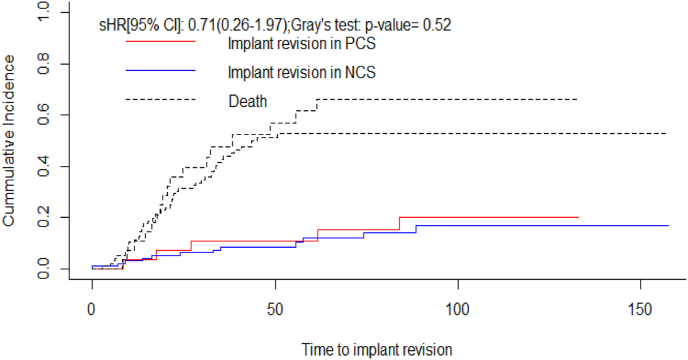
One of the 23 cases (4%) where a cement blob was added at the proximal end of the construct had asymptomatic proximal migration whereas 21 of 119 cases (17%) without cement blob had proximal migration (8 symptomatic proximal migration requiring re-operation, 1 symptomatic proximal migration on palliative care did not undergo re-operation and 12 asymptomatic proximal migration). Five years implant survival (IS) by Kaplan Meier analysis was 79.6% (Fig. 4). In patients with NCS and PCS the implant survival was 76.2% and 91.7% respectively (p-value = 0.249) (Fig. 5). As per competing risk analysis, the chances of implant revision are 12% (NCS -12%, PCS -11%) and 18% (NCS -17%, PCS -20%) at five and ten years respectively (Fig. 6)(Fig. 7).
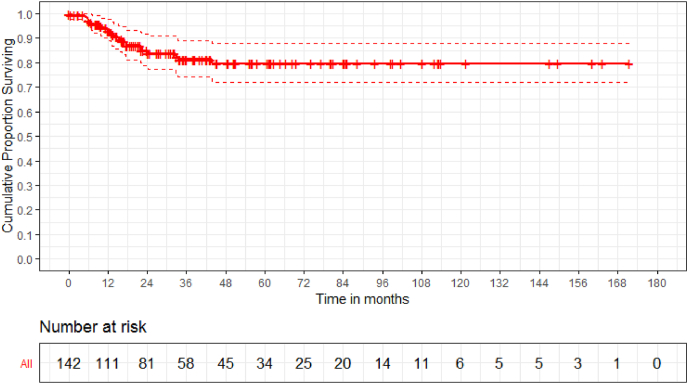
Fig. 4.
The Kaplan-Meier implant survivorship estimate curves with end point taken as date of implant failure necessitating revision surgery, date of death or date of last follow-up for survivors among all 142 patients shows 79.6% implant survival at 5 years.
Fig. 5.
The Kaplan-Meier implant survivorship estimate curves with end point taken as implant failure necessitating revision surgery, date of death or date of last follow-up for survivors shows implant survival of 76.2% and 91.7%(p-value = 0.099) in NCS and PCS group respectively at 5 years.
Fig. 6.
The Gray's test for competing risk analysis, when death was considered as a competing risk to implant revision, the risk of implant revision in the study was 12% and 18% at five and ten years respectively.
Fig. 7.
The Gray's test for competing risk analysis, when death was considered as a competing risk to implant revision, the risk of implant revision in NCS group was 12% and 17% at five and ten years respectively. The risk of implant revision in PCS group was 11% and 20% at five and ten years respectively.
3.1. Functional outcomes
The functional outcome was assessed at the most recent follow up for patients who are alive without implant failure (n = 40/142). The overall mean MSTS score was 71% (60–80%). The mean MSTS score for NCS and PCS was 71% and 72% respectively.
4. Discussion
After resection of proximal humerus tumors, in cases where the deltoid muscle and axillary nerve are retained after attaining safe margins prosthetic replacement (proximal humerus megaprosthesis and reverse shoulder prosthesis) of the proximal humerus remain the reconstructive modality of choice.2, 15 When abductor function is severely compromised owing to the need to sacrifice the deltoid muscle and axillary nerve to attain safe margins, most modalities of reconstruction act merely as spacers to stabilize the shoulder while maintaining the length of the arm to enable good elbow and hand function. In such cases where post-operative shoulder function is unlikely, the cost of prosthesis can often be a limiting factor in resource constrained settings. Biologic reconstruction modalities like free fibula autograft and claviculo pro humero are complex procedures, associated with donor site morbidity and problems with union.16,17 Allografts are not readily available and are associated with fracture, non-union and infection.17
Implant cement spacer (NCS/PCS) offers a simple, inexpensive alternative reconstruction modality in such cases as K-nails and bone fixation plates are readily available. NCS/PCS are versatile and can be used for intra-articular, extra articular or Tikoff-Lindberg resection of the proximal humerus of varying lengths18. The cost of an implant cement spacer is approximately 4500 INR(80 USD) compared to 45,000 INR(600 USD) for a cemented proximal humerus megaprosthesis at our center. This is a technically less demanding procedure with ease of use, reproducibility and does not require any specialized instrumentation. Unlike in free vascularized fibula grafting, no special expertise or skills is required. Unlike biological reconstructions [vascularized or non-vascularized fibula, osteoarticular allografts(OA), allograft-prosthesis combo(APC) or claviculo pro humero(CPH)], NCS/PCS is not dependent on bone healing16,17 which may be delayed in those undergoing post-operative radiation, chemotherapy and patients can be rehabilitated early.
The narrow profile of nails and plates compared to bulky prosthesis facilitates easy wound closure even if excision of soft tissue and skin is required for oncological clearance.
22 patients reconstructed with NCS/PCS had proximal migration of the implant. While proximal migration has been noted with prosthesis too,18 the tip of nail or plate being sharp can result in discomfort and need for surgical intervention in case of proximal migration (8 cases needed repeat surgery for this). Adding a cement blob at the proximal end of the nail/plate incorporating the mesh and ensuring secure anchorage of the mesh in the scapula can decrease proximal migration. Only 4% cases where cement blob was added at the proximal end of the construct had proximal migration whereas 17% cases without cement blob had proximal migration.
Our re-operation rate of 13% is comparable to re-operation rates of 10–47% documented in a systematic review of various methods of reconstruction after proximal humerus resection by Dubina et al. (proximal humerus prosthesis, APC, OA, RSA, CPH, vascularized and non-vascularized fibula etc.)5
Our overall implant survival(IS)of 78.% at five years is comparable with results of a systematic analysis by Teunis et al.6 in which IS at five years were calculated for 616 patients from 29 studies. IS ranged from 38% to 100% in the prosthesis group (341 patients); 33%–100% in the OA group (143 patients), and 33%–100% in APC group (132 patients). At the time of last follow up 57% of our patients had died. While we acknowledge that these spacers being mechanical constructs may eventually fail in long term survivors, they can be easily revised. In early failures we prefer to revise with a similar NCS/PCS. In late failures/long term survivors we prefer a vascularized fibula as a more durable biologic reconstruction as the patient is free of any adjuvant therapy (chemotherapy or radiotherapy) which may impair biologic healing and the possibility of disease recurrence is low.
Our findings are reinforced by the functional outcomes documented in other studies using NCS; Kundu et al. [63.63% (range 50–66.7%)](7) and Rafalla and Adbullah [65% (range 55–70%)](18) (Table 1).
Table 1.
Comparing results of our study with other proximal humerus reconstruction studies published in literature.
| Author | Osteoarticular allograft (OA) | Allograft prosthetic composite (APC) | Megaprosthesis | Free vascularized fibula (FVFG) | Claviculo pro humero (CPH) | Implant cement spacer | F/U in months (range, mean/median) | Complications requiring revision surgery |
|---|---|---|---|---|---|---|---|---|
| Present study | _ | _ | _ | _ | _ | (n = 142) MSTS- 71% (n = 40) 5-year implant survival (IS)- 79.6% |
1–174 months 34 months (median) |
13% (n = 18) 8 symptomatic proximal migration, 7 implant failure, 3 infection |
| Kundu Z S et al.7 | _ | _ | _ | _ | _ | (n = 14) MSTS- 63% |
12–52 months 30 months (mean) |
Nil |
| Rafalla A A et al.17 | _ | _ | (n = 8) MSTS-65% |
_ | _ | (n = 12) MSTS-65% |
12–75 months 26 months (mean) |
? (1 subluxation in megaprosthesis group -? Reoperation) |
| Potter et al.2 | (n = 17) MSTS- 71% 5-year IS- 56% |
(n = 16) MSTS-79% 5-year IS- 91% |
(n = 16) MSTS- 69% 5-year IS- 100% |
_ | _ | _ | 24–214 months 98 months (median) 113 months (mean) |
33% (n = 16) 8(47%) in OA, 4(25%) in APC, 4(25%) in megaprosthesis |
| Van de Sande et al.18 | (n = 13) MSTS-77% 5-year IS- 9% |
(n = 10) MSTS-72% 5-year IS- 60% |
(n = 14) MSTS-77% 5-year IS- 88% |
_ | _ | 9–300 months 120 months (mean) |
32% (n = 12) 8(61%) in OA, 3(30%) in APC, 1(7%) in megaprosthesis |
|
| Teunis et al.6 | (n = 143) MSTS- 50 to 78% (n = 84) 5-year IS- 33 to 100% |
(n = 132) MSTS- 57 to 91% (n = 141) 5-year IS- 33 to 100% |
(n = 341) MSTS- 61 to 77% (n = 141) 5-year IS - 38 to 100% |
_ | _ | _ | 24–204 months | ? (Fractures were more common in OA and APC group) |
| El-Sherbiny M(19) | _ | _ | (n = 13) MSTS- 71% |
(n = 11) MSTS- 73% |
_ | _ | 19–92 months | 9% (n = 1) 1 Bone grafting for nonunion in FVFG (1 stress fracture in FVFG- immobilization) |
| Dubina A et al.5 | (n = 167) MSTS- 74% |
(n = 106) MSTS- 73% |
(n = 761) MSTS- 72% |
(n = 43) MSTS- 73% |
(n = 19) MSTS- 83% |
_ | 14–231 months 71 months (mean) |
10–47% 47% in CPH, 34% in OA, 26% in APC, 10% in megaprosthesis, 14% in FVFG |
| Rödl RW et al.14 | (n = 11) MSTS- 74% 5 year IS- 75% |
_ | (n = 19) MSTS- 79% 5 year IS- 83% |
_ | (n = 15) MSTS- 82% 5 year IS- 79% |
_ | 23–106 months 59 months (mean) |
33% (n = 15) 3(27%) in OA, 2(10%) in megaprosthesis, 10(66%) in CPH |
4.1. Limitations of the study
The limitations of this study are those inherent to any retrospective study. As we have the largest sample size of proximal humerus resections reconstructed with NCS/PCS, published from a single institute which have been treated by the same team we believe the results of our study will be useful.
5. Conclusion
With similar revision rates, implant survival and functional outcome, NCS/PCS offers a simple, cost-effective, and reliable alternative in proximal humerus resection when oncological concerns necessitate sacrifice of proximal humerus motors and/or the axillary nerve.
References
- 1.Unni K.K., Inwards C.Y. sixth ed. vol. 10. 2012. Dahlin's Bone Tumors: General Aspects and Data on 10,165 Cases; p. 165. (Dahlin's Bone Tumors: General Aspects and Data on). Cases, sixth ed. [Google Scholar]
- 2.Potter B.K., Adams S.C., Pitcher J.D., Malinin T.I., Temple H.T. Proximal humerus reconstructions for tumors. Clin Orthop Relat Res. 2009;467(4):1035–1041. doi: 10.1007/s11999-008-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano-Calderón S.A., Chen N. Proximal humerus allograft prosthetic composites: technique, outcomes, and pearls and pitfalls. Curr Rev Musculoskelet Med. 2015;8(4):324–333. doi: 10.1007/s12178-015-9306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourdet C, Audard V, Anract P, Biau D, Saint F. Is it safe to preserve the deltoid when resecting the proximal humerus for a primary malignant bone tumour ? A COMPARATIVE STUDY. :9–11. [DOI] [PubMed]
- 5.Dubina A., Shiu B., Gilotra M., Hasan S.A., Lerman D., Ng V.Y. What is the optimal reconstruction option after the resection of proximal humeral tumors? A systematic review. Open Orthop J. 2017;11(1):203–211. doi: 10.2174/1874325001711010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teunis T., Nota S.P.F.T., Hornicek F.J., Schwab J.H., Lozano-Calderón S.A. Outcome after reconstruction of the proximal humerus for tumor resection: a systematic review. Clin Orthop Relat Res. 2014;472(7):2245–2253. doi: 10.1007/s11999-014-3474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kundu Z.S., Gogna P., Gupta V., Kamboj P., Singla R., Sangwan S.S. Proximal humeral reconstruction using nail cement spacer in primary and metastatic tumours of proximal humerus. Strateg Trauma Limb Reconstr. 2013;8(3):149–154. doi: 10.1007/s11751-013-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gundavda M.K., Nayak P., Agarwal M.G. Novel modification of second generation intramedullary PMMA cementing technique for narrow upper and lower extremity canals. J Clin Orthop Trauma [Internet] 2016;7(1):66–69. doi: 10.1016/j.jcot.2015.08.006. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puri A., Gulia A. An inexpensive reconstruction method after resection in tumors of the proximal humerus with extensive involvement of the diaphysis. Int J Shoulder Surg. 2011;5(2):44–46. doi: 10.4103/0973-6042.83196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S., Gulia A., Kurisunkal V., Parikh M., Gupta S. vol. 25. Wolters Kluwer Medknow Publications; 2019. Principles of management of extremity skeletal metastasis; pp. 580–586. (Indian Journal of Palliative Care). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puri A., Agarwal M. Use of polypropylene mesh to stabilize skeletal reconstructions after resection for bone tumors. J Surg Oncol. 2007 Feb 1;95(2):158–160. doi: 10.1002/jso.20595. [Internet] [cited 2020 Feb 2]. Available from: [DOI] [PubMed] [Google Scholar]
- 12.Tang X., Guo W., Yang R., Tang S., Ji T. Synthetic mesh improves shoulder function after intraarticular resection and prosthetic replacement of proximal humerus. Clin Orthop Relat Res. 2015;473(4):1464–1471. doi: 10.1007/s11999-015-4139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enneking W.F., Dunham W., Gebhardt M.C., Malawar M., Pritchard D.J. Clinical Orthopaedics and Related Research. 1993. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system; pp. 241–246. [PubMed] [Google Scholar]
- 14.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rödl R.W., Gosheger G., Gebert C., Lindner N., Ozaki T., Winkelmann W. Reconstruction of the proximal humerus after wide resection of tumours. J Bone Jt Surg - Ser B. 2002;84(7):1004–1008. doi: 10.1302/0301-620x.84b7.12989. [DOI] [PubMed] [Google Scholar]
- 16.Barbier D., De Billy B., Gicquel P., Bourelle S., Journeau P. Is the clavicula pro humero technique of value for reconstruction after resection of the proximal humerus in children? Clin Orthop Relat Res. 2017;475(10):2550–2561. doi: 10.1007/s11999-017-5438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu T., Zhang Q., Guo X., Zhang X., Li Z., Li X. Treatment and outcome of malignant bone tumors of the proximal humerus: biological versus endoprosthetic reconstruction. BMC Musculoskelet Disord [Internet. 2014;15(1):1–9. doi: 10.1186/1471-2474-15-69. Available from: BMC Musculoskeletal Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafalla A.A., Abdullah E.S.A. Endoprosthetic replacement versus cement spacer in reconstruction of proximal humerus after tumor resection: cost and benefits. J Orthop Surg. 2017;25(2):1–5. doi: 10.1177/2309499017713937. [DOI] [PubMed] [Google Scholar]