Abstract
Considering the value of guinea fowl keets, successful incubation of eggs is particularly desirable in this poultry species. This study evaluated the effect of egg storage duration on egg quality, heat production, hematological parameters during embryonic development and post hatch performance of guinea fowl broilers. A total of 800 hatching eggs of guinea fowl were used for this study. Before incubation, 12 eggs per treatment were used to analyse egg quality. Then, eggs were numbered, weighed, and assigned to 2 treatment groups of 400 eggs each according to storage duration of 5, and 10 d at a temperature of 18°C. The eggs were set for incubation at 37.7°C and 55% relative humidity for 28 d in a forced-draft incubator. To determine heat production as a measure of metabolism, 60 eggs in each replicate were transferred to respiratory cages post hatch two 12 wk old guinea fowl were also used to determine heat production. CO2 and O2 were recorded to calculate heat production at internal pipping, hatch and at 12 wk of age. The hatched keets were reared for 12 wk and data were collected on feed intake, body weight and feed conversion ratio. Blood samples were collected at hatch and at 12 wk of age from 24 guinea fowls per treatment to analyze haematological parameters. The results showed that embryos and guinea fowls at 12 wks of age from eggs stored for 5 d had higher (P ˂ 0.05) heat production and body weights. However, a significant higher (P ˂ 0.05) level of basophile, eosinophils, and lymphocytes was observed in guinea fowls from 10 d storage egg. It was concluded that extended duration of egg storage negatively influenced the metabolic rate of embryos. It also impacted hematological parameters which may suggest influence on immune response during embryonic and post-hatch growth.
Key words: egg storage, egg quality, meat-type guinea fowl, heat production, haematological parameter
INTRODUCTION
Prolonged egg storage has been repeatedly shown to result in substantial extension of incubation duration (Tona et al., 2003), decreased hatchability (Yassin et al., 2008) as well as chick quality (Tona et al., 2003). Moreover, similarly, other studies have confirmed that storage results in decrease in albumen, perivitellin membrane, yolk, blastoderm quality, gas exchange and embryonic metabolism when fertile eggs are stored for long duration (Bakst and Akuffo 1999; Lapão et al., 1999; Fasenko, 2007). The mechanism of the survival of embryos during egg storage is still not completely well understood. Fasenko (1996) showed that turkey embryos of eggs stored for 14 d were more dependent on gluconeogenesis during pipping and hatching than embryos of eggs stored for 4 d.
It is well known that, during the hatching process and subsequent growth, the birds undergo many physiological changes, which considerably alter their thermal balance. Thermal equilibrium is intrinsically related to environmental conditions which broilers are exposed to. For example, it is known that the performance and productivity of broilers depends on air temperature, relative humidity and air speed (Giloh et al., 2012). Thus, in order to have a clear understanding of their homeostasis, it is important to investigate their metabolic heat production (Randall et al., 2008). Birds have greater respiratory volume in comparison with mammals and respiratory evaporation is an important component of energy balance of these subjects (DaSilva and Maia, 2013). Buzala et al. (2015) reported that selection of broiler chickens leads to increased metabolic activities. Moreover, Nascimento et al. (2017) reported the relationship between oxygen consumption and body weight, and heat production. So, heat production increased with increasing body weight. Collin et al. (2003) measured heat production of broiler chicks between the 10 and 14th days of their rearing period and reported physiological change.
Thus, at hatch, several parameters can be measured on 1-day-old chicks that can predict overall performance and the health of chickens. One important parameter is the immune system. Indeed, newly hatched chicks are initially protected against infection by maternal antibodies in the egg yolk (Hamal et al., 2006). A functional immune system must be in place to develop a protective immune response against major pathogens. Korver, 2012 reported that the ability of the birds to resist pathogens efficiently and thus avoid productive loss due to disease or death may be due to cellular and humoral immunocompetence. According to Frandson (1981), lymphocytes are important in forming barriers against local disease conditions and may be involved in antibody formation. Similary, Viertlboeck and Göbel (2008) reported that, T cells play a central role in acquired immunity and in particular in cellular immune responses. They may belong to the T cell lines αβ or γδ expressing the αβ or γδ chains of the T cell receptor. Thus, hematological parameters are the biomarkers to indicate on animal's health status. Thus, nutritional and respiratory deficiencies can be diagnosed by hematological analysis by analyzing for erythrocyte and leukocyte cells. Evidence in the literature shows that organisms are able to respond to stressors by causing increases in hematocrit, reductions in lymphocyte count and changes in the heterophil-to-lymphocyte ratio (Barton et al., 1987; Chamblee et al., 1989; Maxwell et al., 1990).
Very little information exists in the literature on the mechanism used to determine metabolic heat production and cellular immune response of birds and even less on guinea fowl. The hypothesis of this study was that heat production and the state of the immune system of guinea fowls may be related to egg storage duration prior to incubation. The aim of this study is to investigate the effects of duration of guinea fowl hatching egg on heat production, hematological parameters, and post-hatch growth performance of their keets.
MATERIAL AND METHODS
Experimental Design
Due to the absence of Animal Care Committee available at the University of Lomé at the time of this research, the research was conducted under the supervision of the research team leader following the guidelines of the Canadian Council on Animal Care (2009).
A total of 800 hatching eggs of Galor G1543 guinea fowl breeders provided by Incubel n.v. (Hoogstraten, Belgium) were used for this study. They were numbered, weighed and assigned to two treatment groups of 400 eggs each. Each group was assigned to different storage duration of 5 and 10 d at 18°C and relative humidity of 70%. A sample of 12 eggs per storage time was used to measure their quality before incubation. Prior to incubation, the eggs from each treatment group were weighed again and divided into 4 replications of 100 eggs each. The eggs were incubated in the same incubator (Petersime incubator Visio 96, Zulte, Belgium) at a temperature of 37.7°C, relative humidity of 55% and turning once an hour until 23 d. At 23 d of incubation, 60 eggs in each replicate were transferred to respiratory cages. CO2 and O2 were recorded to assess heat production at internal pipping and hatch. At hatch, quality of 1-day-old keet was evaluated and 180 (45 × 4 repetitions) keets per treatment were reared for 12 wk, including starter (0–4 wks), grower (4–8 wks), and finisher (8–11 wks) stages. The replicates were randomly distributed in an open-sided poultry house in order to avoid positional effects. The birds were raised on a deep litter system with a stocking density of 20 birds per m2 and photoperiod of 23 h of light during the first 4 (wk) of age. But, from 5 (wk) of age onward, the stocking density was 10 birds/m2 and light was reduced gradually to natural photoperiod of 16L/8D at the end of wk 6 of age. Feed and water were supplied ad libitum. Body weights and feed intake were recorded weekly. Mortality was recorded according to the treatments during the 12 wk of rearing. At 12 wk of age, 8 guinea fowls (2 birds/replicate) per treatment were selected at random to determine heat production. Also, at 12 wk of age, 24 guinea fowls (6 birds/replicate) per treatment were selected at random and slaughtered to determine carcass yield. Blood samples were collected at hatch and 12 wk of age to determined hematological parameters. Also, intestinal morphometric parameters were recorded according to the treatments.
Determination of Egg Quality Before Storage
The eggs weighed (12 per storage duration) before incubation were broken out to measure albumen Haugh units (HU). For each broken egg, the egg quality measurement system was used to record thick albumen height, by connecting a transmission box and a balance to a personal computer. The balance type KERN 440-43N, with an accuracy of 0.01 g and a maximum weight of 400 g was used. After calibration of the albumen height gauge, the height of albumen was measured (±0.25 mm) with a vertically mounted micrometer with an electronic path. After each measurement, the micrometer was cleaned with distilled water and absorbent paper. The standard software program version 2A calculated albumen HU with egg weight and albumen height (Tona et al., 2002). Proportion of eggshell, albumen, and yolk were calculated according to:
| (1) |
X = was eggshell, albumen, or yolk.
Determination of Heat Production
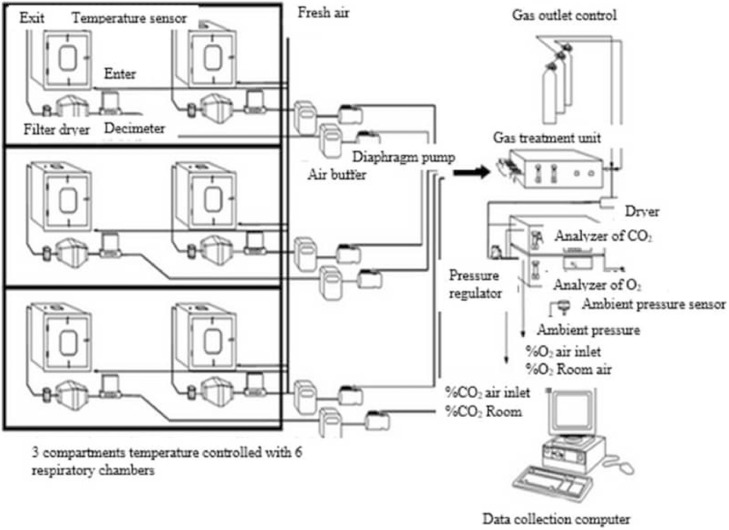
The basic framework of the devise of respiration chambers was constructed with chamber and the control station. The respiration chamber has 3 rooms with isothermal doors. There are 2 cage units positioned on the floor of each room (0.3 m × 0.55 m × 0.5 m). The environmental conditions in the cage were the same as those in the hatcher. These conditions were temperature of 36.7°C and relative humidity of 55%. Each cage can hold 60 eggs or 2 adults’ guinea fowls and hermetic seal during periods of measurement of gaseous exchange. Each cage was configured and connected by piping at the control station (Figure 1). A total of 60 hatching eggs per treatment in each replicate were transferred into the cages after candling on 23 d of incubation and were allowed to remain there until hatching. The amount of oxygen consumed and the amount of CO2 produced was recorded automatically every 10 s during 24 h for internal pipping and hatch. At 12 wk of age, 2 guinea fowls were transferred in each cage unit and birds were provided feed and water in the cage. The air conditioners are positioned above each of the cage and cooling capacity is thermostatically controlled.
Figure 1.
System of respiration chamber used for physiological measurements of heat production by broiler embryo and guinea fowls at 12 wk.
The data collected was used to calculate heat production. The formula corrected of Brouwer (1965) was used to calculate heat production as:
| (2) |
where O2 and CO2 are in dm3 of dry gas /24 h, and H is in kj/kg/ h.
Body Weight, Weight Gain, Feed Intake, FCR, and Carcass Yield
The keets were weighed at hatch and then weekly until 12 of age. Feed intake was recorded daily and was used to calculate average feed intake per week and per bird. Body weights and feed intakes were used to calculate average body weight gains and feed conversion ratios. At 12 wk of age, samples of 24 birds per treatment were slaughtered. Carcass weight was recorded. These weights were used to determine the carcass yields: Carcass yield = 100 × carcass weight/body weight (3).
Hematological Parameters
Before slaughtering, approximately 3 mL of blood was collected from the wing vein of 12 birds (6 per replication) into tubes pretreated with ethylene diamine tetra acetic acid (EDTA). Blood was used to determine hematological parameters such as red blood cells (RBC), white blood cells (WBC), hemoglobin (Hb), hematocrit, lymphocytes, basophils, eosinophils (Mid), granulocytes (Gram), and platelet count (PLT). Hematological parameters were analyzed by using Mindray BC-2800 Analyzer (Mindray, Guangzhou, China), which is a fully automated hematology analyzer. The analyses were performed using 3 reagents (Lyse Mindray BC-2800, Rinse Mindray BC-2800, Diluent Mindray BC-2800), which are directly incorporated in the analyzer and automatically pipetted by the PLC during counting. For each hematological parameter, all the samples were run in the same essay in order to avoid inter essay variability.
Morphometric Intestinal Parameters
At slaughter, the small intestine with the pancreas was removed carefully from the birds and suspended vertically to apply uniform tension for length measurements. For length and weight measurements, the small intestine was demarcated into 3 segments: duodenum (from gizzard to entry of the bile and pancreatic ducts), jejunum (from entry of the ducts to yolk stalk), and ileum (from yolk stalk to ileocecal junction). The pancreas was removed and weighed.
Statistical Analysis
The data collected were analyzed using GraphPad Prism 5 software (GraphPad Software Inc, San Diego, CA). The t test was used to analyze data on heat production and hematological parameters, weight of keet, and absolute weight of organ, feed consumption, guinea fowl live weight, and carcass yield. Data on mortality rates 6 were analyzed using the Chi-square test (χ²). The probability P ˂ 0.05 was considered the significance level. The results are presented as a mean plus or minus the standard error of the mean (M ± ESM).
RESULTS
Quality of Hatching Eggs of Guinea Fowl
A significant increase in yolk weight (P < 0.05) and a significant decrease of albumen weight and Haugh Unit were observed in hatching eggs stored for 10 d compared to 5 d (Table 1). There was, however, no significant effect of storage duration on shell weight and the yolk color (Table 1).
Table 1.
Quality of the hatching egg at 5 d and 10 d storage before incubation according to storage duration.
| Storage time (d) |
||
|---|---|---|
| Parameters | 5 | 10 |
| Egg weight (g) | 51.68 ± 0.69 | 51.16 ± 0.89 |
| Shell weight (g) | 9.38 ± 0.20b | 9.8± 0.23a |
| Yolk weight (g) | 15.22 ± 0.26b | 16.65 ± 0.58a |
| Albumen weight(g) | 27.08 ± 0.44a | 24.71 ± 0.24b |
| Haugh Unit Score | 64.49 ± 1.5a | 57.67 ± 3.46b |
| Roch Scale score | 8.03 ± 0.17 | 7.52 ± 0.17 |
Within row, data sharing different superscripts are significantly different (P < 0.05). Mean ± SEM.
Heat Production at Internal Pipping, Hatching and at 12 Wk of Age
Table 2 shows that heat production increased significantly with the stage of development. Heat production in keets from eggs stored for 5 d and heat production in guinea fowls at 12 wk of age were higher (P ˂ 0.05) than those from eggs stored for 10 d. Similar heat productions were observed during internal pipping in both groups.
Table 2.
Heat production at internal pipping, hatching and slaughter according to different storage duration.
| Storage time (d) |
||
|---|---|---|
| Heat production (kj/kg/h) | 5 | 10 |
| Egg | 274.6± 54.66 | 259.1± 48.25 |
| Keet | 522.5± 16.23a | 361.1±9.4b |
| Guinea fowl (12 wk) | 1079.94 ± 24.64a | 816.74 ± 12.17b |
Within row, data sharing different superscripts are significantly different (p < 0.05). Mean ± SEM.
Effect of Egg Storage Duration on Quality of Keet at Hatch
Shown in Table 3 are 1-day-old keet, yolk sac and organs weights at hatch. The 1-day-old keets were comparable in all treatment. But, the yolk-free weights of keets from eggs stored for 5 d were significantly higher (P ˂ 0.05) compared to eggs stored for 10 d. However, absolute yolk sac, liver, and heart were not affected by storage duration.
Table 3.
Weights of 1-day-old keet, yolk – free body, yolk sac, liver, and heart at hatch according to different storage duration.
| Storage time (d) |
||
|---|---|---|
| Parameters | 5 | 10 |
| Keet (g) | 33.67 ± 0.77 | 33.27 ± 0.75 |
| Yolk-free body (g) | 30.01 ± 0.43a | 28.32 ± 0.68b |
| Yolk sac (g) | 3.66 ± 0.14 | 4.95 ± 1.23 |
| Heart (g) | 0.31 ± 0.01 | 0.31 ± 0.01 |
| Liver (g) | 0.76 ± 0.02 | 0.69 ± 0.02 |
Within row, data sharing different superscripts are significantly different (P < 0.05). Mean ± SEM.
Effect of Storage Duration on Hematological Parameters in Keet at Hatching and Guinea Fowl at 12 Wk of Age
Blood samples from the 2 treatments at hatch (Table 4) and at 12 wk of age (Table 5) revealed that, Mid and Lymphocyte count in guinea fowl from eggs stored for 10 d were higher (P ˂ 0.05) compared to eggs stored for 5 d, both at hatching and at 12 wk of age. On the other hand, PLT of the birds from eggs stored for 10 d was lower (P ˂ 0.05) compared to 5 d at hatching. But, PLT measured at 12 wk of age was higher (P < 0.05) in guinea fowl hatched from eggs stored for 10 d compared to those hatched from eggs stored for 5 d. However, storage had no effect on concentrations of RBC, HCT, HGB, MCV, WBC, Gran.
Table 4.
Hematological parameters values in guinea fowl hatchlings according to egg storage duration.
| Storage time (d) |
||
|---|---|---|
| Parameters | 5 | 10 |
| RBC (× 106/μL) | 1.98 ± 0.05 | 2.09 ± 0.04 |
| HCT (%) | 32.15 ± 1.01 | 35.69 ± 1.3 |
| HGB (g/dL) | 12.35 ± 0.90 | 13.95 ± 0.42 |
| MCV (fL) | 170.8± 3.16 | 170.4± 3.65 |
| WBC (× 103/μL) | 49.86 ± 3.21 | 53.76 ± 4.5 |
| Mid (× 109/L) | 9.96 ± 1.4b | 14.03 ± 0.42a |
| Gran(× 109/L) | 36.64 ± 1.97 | 35.85 ± 3.17 |
| Lymphocyte (× 109/L) | 1.30 ± 0.30 b | 2.46 ± 0.4a |
| PLT (× 103/μL) | 60.69 ± 2.61a | 41.92 ± 3.35b |
Abbreviations: Gran, granulocytes; HCT, hematocrit; HGB, hemoglobin; MCV, mean cell volume; Mid, basophilic and cosinophilic; PLT, platelet count; RBC, red blood cell count; WBC, white blood cell count.
Within row, data sharing different superscripts are significantly different (P < 0.05). Mean ± SEM.
Table 5.
Hematological parameters values of guinea fowl at 12 wk of age according to egg storage duration.
| Storage time (d) |
||
|---|---|---|
| Parameters | 5 | 10 |
| RBC (× 1012/L) | 2.47 ± 0.04 | 2.53 ± 0.08 |
| HCT (%) | 41.37 ± 0.63 | 42.03 ± 1.14 |
| HGB (g/dL) | 14.17 ± 0.22 | 14.31 ± 0.37 |
| MCV (fL) | 167.4± 1.73 | 166.5± 1.71 |
| WBC (× 109/L) | 197.5± 3.15 | 196.0± 3.59 |
| Mid (× 109/L) | 70.45 ± 1.06b | 75.05 ± 1.34a |
| Gran(× 109/L) | 95.73 ± 1.22 | 88.05 ± 2.64 |
| Lymphocyte (× 109/L) | 22.46 ± 1.19b | 32.8± 2.03a |
| PLT (× 103/μL) | 16.79 ± 1.01b | 22.17 ± 2.53a |
Abbreviations: Gran, granulocytes; HCT, hematocrit; HGB, hemoglobin; MCV, mean cell volume; Mid, basophilic and eosinophilic; PLT, platelet count; RBC, red blood cell count; WBC, white blood cell count.
Within row, data sharing different superscripts are significantly different (P < 0.05). Mean ± SEM.
Effect of Hatching Egg Storage on Body Weight, Feed Intake, Average Daily Gain, Feed Conversion Ratio, Mortality Rate During Rearing Period, and Carcass Yield at 12 Wk
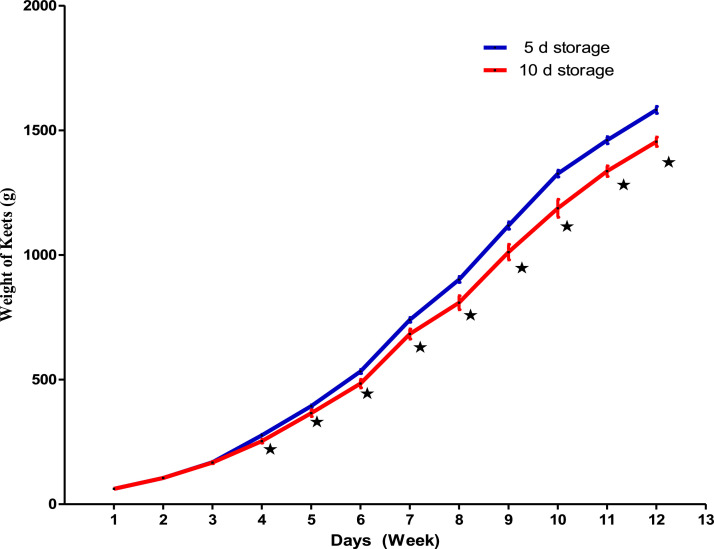
Body weights of the birds increased with age. Between 1 and 2 wk, body weights were not affected by treatments (Figure 2). However, from the 3rd to the 12th wk of age, the weight of guinea fowl from eggs stored for 5 d were higher (P ˂ 0.05) than guinea fowls from eggs stored for 10 d.
Figure 2.
Weight change of guinea fowl during the 12 wk according to the different storage duration * indicates the difference between treatments (P ˂ 0.05).
The feed intake result (Table 6) shows an increase (P ˂ 0.05) in guinea fowl from 10 d storage eggs compared to guinea fowl from 5 d storage. Also, the feed conversion ratio and the mortality were higher in guinea fowls from eggs stored for 10 d (P ˂ 0.05) than those guinea fowls from 5 d. However, the average daily gain was similar in both eggs storage treatment. Moreover, carcass yields were higher (P < 0.05) in guinea fowl from eggs stored for 5 d than those guinea fowls from eggs stored for 10 d.
Table 6.
Feed intake, average daily gain, feed conversion ratio, mortality during rearing, and carcass yield according to storage duration.
| Storage time (d) |
||
|---|---|---|
| Parameters | 5 | 10 |
| Feed intake (g/d/keet) | 64± 10.38b | 81.05 ± 14.6a |
| Daily weight gain (g/d) | 19.74 ±2.46 | 18.08 ±2.24 |
| Feed conversion ratio | 2.58 ±0.31b | 4.11 ±0.96a |
| Mortality (%) | 1.94 ±0.48b | 3.29 ±0.33a |
| Carcass yield (%) | 70.91 ±1.87a | 60.71 ±4.34b |
Within row, data sharing different superscripts are significantly different (P < 0.05).
Effect of Storage Duration on Intestinal Morphometry in Guinea Fowls at 12 Wk of Age
As shown in Table 7, weight and length of the duodenum in guinea fowls from eggs stored for 10 d was significantly higher (P ˂ 0.05) than those hatched from eggs stored for 5 d. On the other hand, the weight/length ratio of the duodenum of the birds from 10 d egg storage was lower compared to 5 d. Storage had no effect on the weights and length of the entire intestine, jejunum and ileum.
Table 7.
Intestinal morphometry.
| Storage time (d) |
|||
|---|---|---|---|
| Parameters | 5 | 10 | |
| Whole intestine | 16.18 ± 1.83 | 18.5± 0.87 | |
| Weight (g/kg of guinea fowl) | Duodenum | 3.14 ± 0.24b | 3.9± 0.19a |
| Jejunum | 6.73 ± 0.43 | 5.45 ± 0.43 | |
| Ileum | 4.82 ± 0.4 | 4.42 ± 0.34 | |
| Whole intestine | 91.98 ± 2.7 | 94.04 ± 3.51 | |
| Length (cm/kg of guinea fowl) | Duodenum | 9.13 ± 0.65b | 13.01 ± 0.64a |
| Jejunum | 29.97 ± 1.3 | 28.33 ± 1.47 | |
| Ileum | 24.70 ± 0.89 | 24.29 ± 1.22 | |
| Whole intestine | 0.17 ± 0.01 | 0.19 ± 0.01 | |
| Weight/Length (g/cm) | Duodenum | 0.34 ± 0.01a | 0.3± 0.01b |
| Jejunum | 0.21 ± 0.01 | 0.19 ± 0.01 | |
| Ileum | 0.2± 0.02 | 0.18 ± 0.01 | |
Within row, data sharing different superscripts are significantly different (P < 0.05). Mean ± SEM.
DISCUSSION
It is well known that embryo development and its viability depends on the quality of the hatching egg. Thus any factor that affects eggs quality will have effect on embryo development. In practical, egg storage is considered as a technical factor that affects egg quality (Dogan and Bayindirli, 1996; Samli et al., 2005). In the current study, the decrease in albumen and the increase in yolk weight could be due to loss of water from albumen, which may have decreased the weight of the albumen thereby altering the yolk-albumen ratio. These results are similar to those of Tona et al. (2004) and Akyurek and Okur (2009) who reported the loss of water from broiler breeder eggs during storage. Although egg weight was similar in both 5 d and 10 d storage duration, this may have been caused by the less loss of water and other gaseous products from the egg during egg storage. Possibly water loss may have been higher in one group especially in eggs stored for 10 d. Haugh unit reduction observed in our result may be caused by the decrease in thick albumen height, because during storage, the ovomucin-lysozyme complex breaks down, and leads to increase the pH of eggs. This result corroborates the findings of Morais et al. (1997) who observed the reduction in the Haugh unit of eggs at 21 d of storage compared to those stored for shorter duration. Based on previous study of Caglayan et al. (2009), Tilki and Saatci (2004) and Redondo (2010) who reported negative effects on albumen weight, yolk weight, albumen height, and Haugh Unit during eggs storage. Thus, in the present findings, an increase in the storage duration had similar deleterious effects on albumen weight and Haugh Unit. According to the USDA standard (2010), eggs are categorized as very good with HU ≥72, good HU 72 ≥60) and bad with HU <60. Therefore, eggs stored for 5 d could be categorized as good quality while those eggs stored for 10 d as poor quality.
In an attempt to gain additional information on the consequence of egg storage, we evaluated the quality of 1 d old keet. At hatch, the absolute weights of 1-day-old keets from both stored eggs were similar. This result was a surprise because egg quality observed previously was different. May be this similarity could be due to the level of yolk resorption in the last phase of incubation. This result has been observed in other studies on chickens and has been associated with a resorption of the remaining yolk by the embryo at the end of incubation (Willemsen et al., 2008). In this regard, it was interesting to observe that the weight of keets that were yolk-free was lower from eggs stored for 10 d. A possible explanation for this result is the lower embryo growth performance caused by the decrease in the total number of embryonic cells (due to both apoptosis and necrosis) as reported in some studies using different chicken egg storage durations (Bloom et al., 1998; Bakst and Akuffo, 1999).
It is well documented that broiler selection for feed efficiency is associated with a higher metabolic rate (Buzala et al., 2015). Thus, it might be argued that the higher heat production found in bird hatched from eggs stored for 5 d as compared to 10 d might be explained by the higher energy used for development. This interpretation is supported by the fact that growth was lower in embryos and birds hatched from eggs stored for 10 d. Singal and Kosin (1969) also suggested that prolonged egg storage may induce irreversible changes in metabolism, which lowers the capacity of cells to maintain vital activities during subsequent incubation. Thus, embryos from eggs stored for long duration were less efficient with energy and protein utilization from the albumen/yolk than embryos in eggs of shorter storage duration. As reported by Lourens et al. (2005), the proteins that were lost, may be used for the energy demanding hatching process because the hepatic glycogen amount on 18 d of incubation was less in prolonged egg storage than in short egg storage. This might have contributed to the lower heat production observed in this group. In the current study, a long storage duration had detrimental effect on both embryo and keet growth. It can be hypothesized, therefore, that keets from eggs stored for 10 d were unable to compensate fully for the negative effects of prolonged egg storage. Thus, the damage caused by prolonged egg storage may be irreversible and may cause embryonic and keet mortality as well as lower growth performance.
Generally, hematological parameters are used in avian species to estimate body condition. Esonu et al. (2001) considered haematological parameters as important indicators of physiological and pathological status in animals. According to our result, the overall erythrocyte parameters revealed little effect of storage duration. However, higher levels of lymphocyte and eosinophilic + basophilic counts were received in the embryos and guinea fowls hatched from egg stored for 10 d. This is suggestive of unpleasant physiological conditions possibly indicating the presence of foreign bodies or antigens in the circulatory system. Indeed, it was reported that when storage period is lengthened, internal quality in eggs decline due to loss of moisture. Evaporation of water and carbon dioxide leads to a decrease of albumen. Indeed, it has been reported that during prolonged storage, the internal quality of eggs decreases due to the degradation of proteins in the albumen thus inducing malnutrition in embryos. As described by Demirel and Kırıkçı (2009), the reduction of the antimicrobial activity of proteins during prolonged storage could have consequences partly on the protection of the embryo against microorganisms and on the other hand on the availability of the nutrients necessary for the embryo to develop properly during incubation and, therefore, may affect embryo development as well as chick growth as reported previously. The hypothesis of a poor feed efficiency was supported in the present study by the observation of a decrease in duodenal weight/length ratio in guinea fowls from eggs stored for 10 d. Indeed, the high feed intake and FCR and low body weight of the long storage time suggest that feed efficiency was negatively effect in birds hatched from eggs stored for longer duration. Thus, this decrease of duodenal weight/length ratio could indicate a poor intestinal absorption which is probably due to the shortening of villi or thickening of the intestinal mucous membrane. According to Dibner (1997) and Uni (1999), nutrient absorption capacity is related to the development of intestinal mucous membranes or intestinal villi and the establishment of active transmembrane transport systems.
In conclusion, extended egg storage duration before incubation had negative consequences on egg quality, embryonic development, keet hatchings, and post-hatch growth of guinea fowls as had previously been reported for chickens.
Acknowledgments
ACKNOWLEDGMENTS
The authors acknowledge the financial support of World Bank grant IDA 5424 through the “Centre d'Excellence Régional sur les Sciences Aviaires (CERSA)” of University of Lomé (Togo) and l'University of Félix Houphouët Boigny.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Akyurek H., Okur A.A. Effect of storage time, temperature and hen age on egg quality in free-range layer hens. J. Anim. Vet. Adv. 2009;8:1953–1958. [Google Scholar]
- Bakst M.R., Akuffo V. Impact of egg storage on embryonic development. Avian Poult. Biol. Rev. 1999;13:125–131. [Google Scholar]
- Barton B., Schreck C.B., Barton L.D. Effects of chronic cortisol administration and daily acute stress on growth. physiological conditions, and stress responses in juvenile rainbow trout. Dis. Aquat. Org. 1987;2:173–185. [Google Scholar]
- Brouwer, E., 1965. Pages 441-443 in Energy Metabolism. Blaxter K.L. ed., Academic Press., London, UK. EAAP-Publication. N°. 11
- Bloom S.E., Muscarella D.E., Lee M.Y., Rachlinski M. Cell death in the avian blastoderm: Resistance to stress induced apoptosis and expression of anti-apoptotic genes. Cell. Death. Differ. 1998;5:529–538. doi: 10.1038/sj.cdd.4400381. [DOI] [PubMed] [Google Scholar]
- Buzala M., Janicki B., Czarnecki R. Consequences of different growth rates in broiler breeder and layer hens on embryogenesis, metabolism and metabolic rate: a review. Poult. Sci. 2015;94:728–733. doi: 10.3382/ps/pev015. [DOI] [PubMed] [Google Scholar]
- Caglayan T., Alasahan S., Kırık K., Gunlu A. Effect of different egg storage periods on some egg quality characteristics and hatchability of partridges (Alectoris graeca) Poult. Sci. 2009;88:1330–1333. doi: 10.3382/ps.2009-00091. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care . Canadian Council on Animal Care; Ottawa, Canada: 2009. CCAC Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing. [Google Scholar]
- Chamblee T.N., Morgan G.W., Schultz C.D. Effect of refeeding shortterm deprivation of feed or water, or both. on selected physiological parameters for broiler chickens. Poult. Sci. 1989;68:1619–1623. doi: 10.3382/ps.0681619. [DOI] [PubMed] [Google Scholar]
- Collin A., Buyse J., Van, As P., Darras V.M., Malheiros R.D., Moraes V.M.B., Reyns G.E., Taouis M., Decuypere E. Cold-induced enhancement of avian uncoupling protein expression, heat production, and triiodothyronine concentrations in broiler chicks. Gen. Comp. Endocrinol. 2003;130:70–77. doi: 10.1016/s0016-6480(02)00571-3. [DOI] [PubMed] [Google Scholar]
- DaSilva R.G., Maia A.S.C. Principles of animal biometeorology. Springer; New York, NY: 2013. [Google Scholar]
- Demirel Ş., Kırıkçı K. Effect of different egg storage times on some egg quality characteristics and hatchability of pheasants (Phasianus colchicus) Poult. Sci. 2009;88:440–444. doi: 10.3382/ps.2008-00131. [DOI] [PubMed] [Google Scholar]
- Dibner J.J. Early development of the digestive tract and the nutritional implications. Poult. Digest. 1997 August 16-19. [Google Scholar]
- Dogan H., Bayindirli L. Mechanism of egg deterioration induced by exposure to high temperatures. Indian J. Anim. Sci. 1996;66:1060–1064. [Google Scholar]
- Esonu B.O., Emenalom O.O., Udedibie A.B.I., Herbert U., Ekpor C.F., Okolie I.C., Iheukwumere F.C. Performance and blood chemistry of weaner pigs fed raw mucuna (velvet bean) Trop. Anim. Health. Prod. 2001;4:49–54. [Google Scholar]
- Fasenko, G. M., 1996. Embryo and poult viability in stored eggs. PhD thesis, Univ. Alberta, Edmonton, Canada.
- Fasenko G.M. Egg storage and the embryo. Poult. Sci. 2007;86:1020–1024. doi: 10.1093/ps/86.5.1020. [DOI] [PubMed] [Google Scholar]
- Frandson R.D. Anatomy and physiology of farm animals. 1st ed. Lea Febiger publishers; Philadelphia: 1981. [Google Scholar]
- Giloh M., Shinder D., Yahav S. Skin surface temperature of broiler chickens is correlated to body core temperature and is indicative of their thermoregulatory status. Poult. Sci. 2012;91:175–188. doi: 10.3382/ps.2011-01497. [DOI] [PubMed] [Google Scholar]
- Hamal K.R., Burgess S.C., Pevzner I.Y., Erf G.F. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poult. Sci. 2006;85:1364–1372. doi: 10.1093/ps/85.8.1364. [DOI] [PubMed] [Google Scholar]
- Korver D. Implications of changing immune function through nutrition in poultry. Anim. Feed Sci. Technol. 2012;173:54–64. [Google Scholar]
- Lapão C., Gama L.T., Soares M.C. Effects of broiler breeder age and length of egg storage on albumen characteristics and hatchability. Poult. Sci. 1999;78:640–645. doi: 10.1093/ps/78.5.640. [DOI] [PubMed] [Google Scholar]
- Lourens A., Brand H., Meijerhof R., Kemp B. Effect of eggshell temperature during incubation on embryo development, hatchability, and post-hatch development. Poult. Sci. 2005;84:914–920. doi: 10.1093/ps/84.6.914. [DOI] [PubMed] [Google Scholar]
- Maxwell M.H., Robertson G.W., Spence S., Corquodale Mc C.C. Comparison of haematological values in restricted- and as libitumfed domestic fowls: white blood cells and thrombocytes. Br. Poult. Sci. 1990;31:399–405. doi: 10.1080/00071669008417270. [DOI] [PubMed] [Google Scholar]
- Morais C.F.A., Campos E.J., Silva T.J.P. Qualidade interna de ovos comercializados em diferentes supermercados na cidade de Uberlândia. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 1997;49:365–373. [Google Scholar]
- Nascimento S.T., Alex S.C.M., Kifle G.G., Nascimento C.C.N. Metabolic heat production and evaporation of poultry. Poult. Sci. 2017;96:2691–2698. doi: 10.3382/ps/pex094. [DOI] [PubMed] [Google Scholar]
- Randall D., Burggren W., French K. 4th rev. ed. Guanabara Koogan; Rio de Janeiro, RJ, Brazil: 2008. Eckert, Fisiologia Animal: Mecanismos e Adaptaçǒes. [Google Scholar]
- Redondo P.G. Effect of long-term storage on the hatchability of red-legged partridge (Alectoris rufa) eggs. Poult. Sci. 2010;89:379–383. doi: 10.3382/ps.2009-00408. [DOI] [PubMed] [Google Scholar]
- Samli H., Agma A., Senkoylu N. Effects of storage time and temperature on egg quality in old laying hens. J. Appl. Poult. Res. 2005;14:548–553. [Google Scholar]
- Singal D.P., Kosin I.L. Induced preincubation aging of the avian egg and subsequent development of the embryo, as revealed by the DNA, RNA, and protein level of its spleen. Proc. Soc. Exper. Biol. Med. 1969;132:871–877. doi: 10.3181/00379727-132-34326. [DOI] [PubMed] [Google Scholar]
- Tilki M., Saatci M. Effects of storage time on external and internal characteristics in partridge (Alectoris graeca) eggs. Revue Med. Vet. 2004;155:561–564. [Google Scholar]
- Tona K., Bamelis F., De Ketelaere B., Bruggeman V., Moreas V.M.B., Buyse J., Onagbesan O., Decuypere E. Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poult. Sci. 2003;82:736–741. doi: 10.1093/ps/82.5.736. [DOI] [PubMed] [Google Scholar]
- Tona K., Bamelis F., DeT Ketelaere B., Bruggeman V., Decuypere E. Effect of inducing molting on albumen quality, hatchability and chick body weight from broiler breeders. Poult. Sci. 2002;81:327–332. doi: 10.1093/ps/81.3.327. [DOI] [PubMed] [Google Scholar]
- Tona K., Onagbesan O., De Ketelaere B., Decuypere E., Bruggeman V. Effects of Age of Broiler Breeders and Egg Storage on Egg Quality, Hatchability, Chick Quality, Chick Weight and Chick Post-Hatch Growth to 42 Days. J. Appl. Poult. Res. 2004;13:10–18. [Google Scholar]
- Uni Z. Functional development of the small intes-tine in domestic birds: cellular and molecular aspects. Poultry and avian Biol. Rev. 1999;10:167–179. [Google Scholar]
- Viertlboeck B., Göbel T.W.F. In: Avian Immunology. Davison F., Kaspers B., Schat K.A., editors. Academic Press; London: 2008. 5-Avian T Cells: Antigen Recognition and Lineages; pp. 91–105. [Google Scholar]
- Willemsen H., Tona K., Bruggeman V., Onagbesan O., Decuypere E. Effects of high CO2 level during early incubation and late incubation in ovo dexamethasone injection on perinatal embryonic parameters and post-hatch growth of broilers. Br. Poult. Sci. 2008;49:222–231. doi: 10.1080/00071660801955654. [DOI] [PubMed] [Google Scholar]
- Yassin H., Velthuis A.G.J., Boerjan M., van Riel J., Huirne R.B.M. Field study on broiler eggs hatchability. Poult. Sci. 2008;87:2408–2417. doi: 10.3382/ps.2007-00515. [DOI] [PubMed] [Google Scholar]
- USDA. 2010. Egg-Grading Manual. Accessed Aug. 2010.