Abstract
Recently, the emerging roles of adipocyte-derived extracellular vesicles (EVs) linking obesity and its comorbidities have been recognized. In obese subjects, adipocytes are having hypertrophic growth and are under stressed. The dysfunction adipocytes dysregulate the assembly of the biological components in the EVs including exosomes. This article critically reviews the current findings on the impact of obesity on the exosomal cargo contents that induce the pathophysiological changes. Besides, this review also summarizes the understanding on how obesity affects the biogenesis of adipocyte-derived exosomes and the exosome secretion. Furthermore, the differences of the exosomal contents in different adipose depots, and the impact of obesity on the exosomes that are derived from the stromal vascular fraction such as the adipose tissue macrophages and adipocyte-derived stem cells will also be discussed. The current development and potential application of exosome-based therapy will be summarized. This review provides crucial information for the design of novel exosome-based therapy for the treatment of obesity and its comorbidities.
Keywords: Adipocyte, Obesity, Extracellular vesicles, Exosomes
Introduction
Obesity is associated with metabolic diseases, such as hypertension, type II diabetes, dyslipidemia, liver disease, as well as heart disease, reproductive disorders, mood disorders, and several forms of cancers. Many of these casual relationships are attributed to the hypertrophic and hyperplastic growth of adipocytes that lead to adipocyte dysfunction. In the past decades, it is well established that the pro-inflammatory state and oxidative stress of the adipocytes deregulate the secretions of adipokines, cytokines, and other factors capable of regulating metabolic homeostasis [1–3]. Dysregulations of these factors underlie many obesity-associated comorbidities.
Recently, the emerging roles of adipocyte-derived extracellular vesicles (EVs) linking obesity and its comorbidities have been recognized. EVs are membrane-bound vesicles including the submicron-size microparticles and the nanometer-size exosomes. These EVs are stably transported in various biofluids, carrying diverse bioactive cargos derived from parent cells [4] and mediating the intracellular communication by transporting and delivering the broad array of biological content such as functional mRNAs, long non-coding RNAs (lncRNAs), microRNAs (miRNAs), DNA fragments, lipids, and proteins to the recipient cells, hence change the cellular biological processes. [5–8].
Studies show that under obese condition, adipocyte-derived EVs or exosomes play a crucial role in mediating the pathogenesis of many diseases. Research starts focusing on the changes of the exosomal contents such as miRNA or proteins that mediate the effects of the dysfunction adipocytes on recipient cells. Since exosome is a cargo of biological contents, a comprehensive evaluation of the effects of the exosomal cargo contents on the recipient cells or roles in disease development can present a holistic picture that reflects the pathophysiological situation. On the contrary, studying the contribution of a single exosomal miRNA or protein to the disease development may provide crucial hints for the development of exosome-based therapy.
Regarding the study of exosomes under obese condition, we are still looking for the answers for many questions. For example, we do not know whether obesity affects the biogenesis of adipocyte-derived exosomes. Exosomes are a cargo of contents; however, whether obesity affects the exosomal cargo packaging process and secretion is largely unknown. Furthermore, whether there is any specific ligand/receptor interaction mediating the adhesion and uptake of adipocyte-derived exosomes by the recipient cells, and whether this process will be affected under obese condition is less studied. Besides, the impact of obesity on exosomal cargo contents in different adipose depots may be different, which deserves investigation as they may exert different impacts on the same recipient cell.
This review aims to summarize the current findings on how obesity affects the contents of the adipocyte-derived exosomes, their biogenesis, and secretion; the pathological impacts of the adipocyte-derived exosomes or EVs under obese condition. The review also summarizes the current development and application of exosomal-based therapy.
Adipocyte-derived extracellular vesicles (EVs)
Obesity is characterized by excess accumulation of adipose tissues. Adipocytes are the main building block of adipose tissues. The non-adipocyte cell types are collectively called stromal vascular fraction that includes preadipocytes, fibroblast, capillary endothelial cells, macrophages, and stem cells.
Connolly et al. use 3T3-L1 as cell model and has revealed that the secretion of EVs or exosomes is increased before adipogenesis [9]. These exosomes have high levels of signaling fatty acids such as arachidonic acid, and adipogenesis markers such as peroxisome proliferator-activated receptor gamma (PPARγ) and preadipocyte factor-1 (PREF1) [9]. Interestingly, the adiponectin level in the secreted exosomes is the highest at day 15 during the course of 3T3-L1 cell differentiation [9]. This study suggests that the exosomal contents are dependent on the differentiation stages of the adipocytes.
Mature adipocytes secret different populations of EVs, namely, the small extracellular vesicles (sEVs) and large extracellular vesicles (lEVs) [10]. IEVs are shed from plasma membrane due to cytoskeleton reorganization and they have negatively charged phosphatidylserine on the outer membrane. lEVs usually range from 50 nm/100 nm to 1 µm in size. sEVs are endosome-derived vesicles formed in multivesicular bodies and released after they fuse with the plasma membrane. sEVs range from 30 to 100 nm in size. [10]. A total of 480 and 168 proteins are identified in lEVs and sEVs, respectively. The distinct peripheral proteins of lEVs are caveolin-1, flotillin-2, and β-actin that are involved in micro-vesicle shedding. lEVs are also characterized by externalized phosphatidylserine. While sEVs are enriched with endosomal sorting complex Alix, tetraspanins CD9, CD63, and CD81, and have high cholesterol level [10]. However, the differences of the physiological or pathological roles between the adipocyte-derived sEVs and lEVs are less defined. Other studies report that adipocyte secret apoptotic bodies (ABs) and microvesicles (MVs). ABs are of size (500 nm–5 µm); they are generated from the disassembly of apoptotic cells. ABs contain a small amount of material from fragmented cells including RNAs, DNA, and lipids from the plasma membranes [11]. MVs are of size 100–500 nm; they are formed by cell membrane budding in response to the increased intracellular calcium levels [11]. However, due to the overlapping size of these EVs, and lack of specific markers for MVs and ABs, it is difficult to differentiate these EVs and characterize their specific functions [12–15]. Profiling of the surface proteins on these different types of adipocyte-derived EVs [16] not only helps to differentiate their identities but also suggests the potential clinical applications of these EVs [17].
Impact of obesity on the exosomal cargo of the adipocyte-derived exosomes
Despite the differentiation stage, obesity or high-fat dietary intervention also affect the exosomal cargo of the adipocyte-derived exosomes. In obese subjects, adipocytes are having hypertrophic growth and they are under stress [18, 19]. The dysfunction adipocytes dysregulate the assembly and sorting of the biological components in the exosomes. Studies have been done with palmitic acid-induced hypertrophic 3T3-L1 adipocyte model. These adipocytes have increased exosomal miR-802-5p content [20]. miR-802-5p downregulates heat shock protein 60 (HSP60) and increases the expression levels of CCAAT/enhancer-binding protein (C/EBP)-homologous protein, and enhances oxidative stress and phosphorylation of c-Jun NH(2)-terminal kinase (JNK) and insulin receptor substrate-1 (IRS1), eventually leading to the development of cardiac insulin resistance [20]. Hypoxia also affects the exosomal contents. As fat pad increases in size, hypoxia occurs within the adipose tissues. Hypoxia increases the adipocyte exosomal proteins that are related to metabolic processes [21]. Study with 3T3-L1 adipocyte models reveals that hypoxic condition increases the exosomal proteins that are related to lipid synthesis such as acetyl-CoA carboxylase, glucose-6-phosphate dehydrogenase, and fatty acid synthase [21]. The expression levels of these proteins are three-to-fourfold higher than those in the normoxic conditions [21]. Indeed, the effects of obesity on the exosomal cargo of the adipocyte-derived exosomes have also been demonstrated in obese patients. Clinical study shows that subcutaneous adipocyte-derived exosomes of the obese patients are enriched in proteins implicated in fatty acid oxidation [22]. While in animal studies, the injection of ob/ob mouse adipose-derived exosomes to control mice induces insulin resistance, promotes differentiation of bone-marrow-derived monocytes into macrophage, and increases pro-inflammatory cytokine secretion [23]. Interestingly, the exosomes secreted from the hypertrophic adipocytes may also have positive feedback on its hypertrophic growth in a paracrine manner. Furthermore, exosomes released from large adipocytes can be transferred to small adipocytes in the adipose tissues to promote lipogenesis and adipocyte hypertrophy [24]. All these studies suggest that obesity has large impact on the exosomal cargo of the adipocyte-derived exosomes, which leads to the pathophysiologic changes.
The impact of obesity on the exosomal cargo of the adipocyte-derived exosomes have been recognized. It is known that the effect of exosomes on recipient cells is hardly attribute to a specific exosomal content, because the recipient cells uptake the cargo without content selective process. Nevertheless, many studies have tried to identify the specific exosomal content that mediates the pathophysiological changes. These findings may be useful for engineering and manipulating the exosomes for disease treatments.
Exosomal long non-coding RNA small nucleolar RNA
Long non-coding RNAs (lncRNAs) are long RNA transcript (> 200 bp) that do not encode proteins. IncRNAs are important regulators of many cellular signaling [25]. Studies have constructed comprehensive non-coding transcriptome for the human adipose tissues, and more than 3000 IncRNA transcripts have been identified [25–27]. These IncRNAs regulate the adipocyte differentiation and function [25].
In the adipose-derived mesenchymal stem cells, studies have identified the IncRNA small nucleolar RNA host gene 9 (lncRNA-SNHG9) that mediates the lncRNA–mRNA interaction networks during adipocyte differentiation [28]. Interestingly, lncRNA-SNHG9 is also found in the adipocyte-derived exosomes of the obese patients and is correlated to the endothelium dysfunction. A study shows that SNHG9 level is reduced in adipocyte-derived exosomes in obese individuals and is further reduced in obese individuals with endothelial dysfunction [29]. Since exosomal SNHG9 alleviates inflammation and apoptosis in endothelial cells [29], its reduction in the exosomes cannot confer the normal functioning of the endothelium in the obese patients.
Exosomal microRNAs (miRNAs)
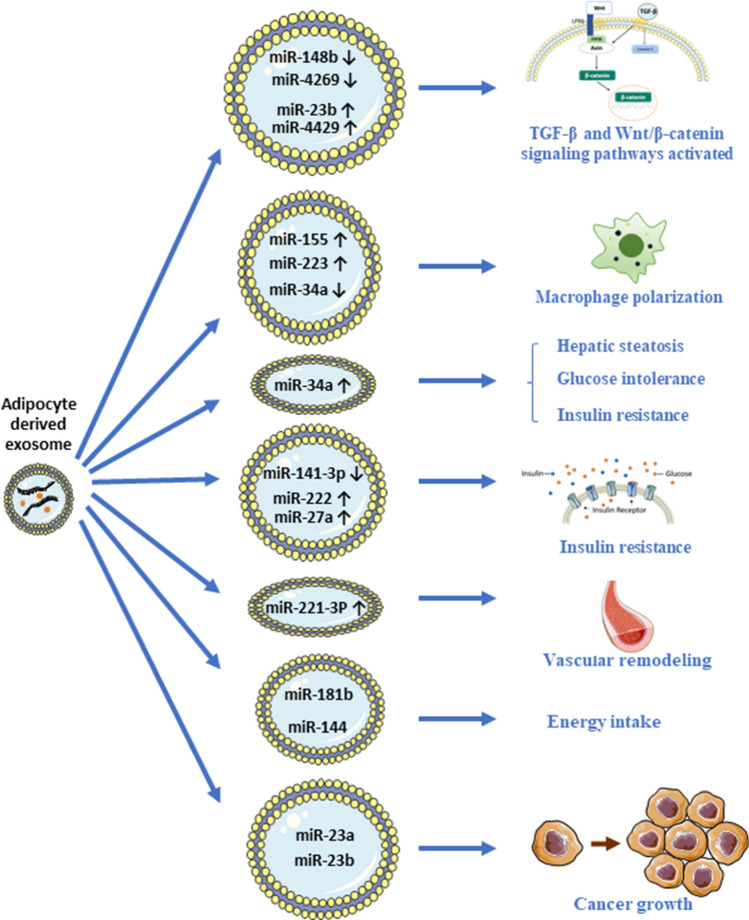
Exosomes may also transport miRNA to regulate the function of the recipient cells. miRNA serves as posttranscriptional regulators of messenger RNA expression and induces changes in the protein products [30, 31]. An elegant study shows that in genetically engineered mice that are deficient for the miRNA-processing enzyme (Dicer) in the adipose tissues (ADicerKO), the circulating miRNA levels are greatly reduced, and the reduction can be reversed after fat depot is transplanted [32], suggesting that adipose tissue is the main source of circulating exosomal miRNA. Under obese condition, dysfunction adipocytes will have dysregulated exosome secretion that attributes to the changes of the circulating miRNA composition [32–37]. Changes in the circulating miRNA composition may underlie the pathological conditions in the obese patients [38]. Indeed, a study shows that the exosomal miRNA profiles of the adipose tissue-derived exosomes isolated from lean and obese individuals are different. For example, exosomal miR-148b (ratio = 0.2 (95% confidence interval = 0.1–0.6)) and miR-4269 (0.3 (0.1, 0.8)) are downregulated, while miR-23b (6.2 (2.2–17.8)) and miR-4429 (3.8 (1.1–13.4)) are upregulated in the obese subjects [19]. The elevation of these miRNAs activates both transforming growth factor-β (TGF-β) and Wnt/β-catenin signaling pathways in the recipient cells and tissues [19]. However, the subsequences after activation of these signaling pathways mediated by the exosomal miRNAs have not been studied.
Macrophages play a crucial role in the development of obesity. During obesity, the number of adipose tissue macrophage (ATM) M1 macrophage increases, which is correlated with adipose tissue inflammation and insulin resistance [39]. On the contrary, M2 macrophages release anti-inflammatory cytokines [39]. Many studies have revealed the effects of the exosomal miRNAs on macrophage polarization. In obese patients, the elevated level of miR-155 in the adipocyte-derived exosomes induces pro-inflammatory M1-macrophage phenotype by activating signal transducer and activator of transcription 1 (STAT1) and repressing STAT6 expression [40]. Another study also shows that the exosomal miR-155 in the mouse visceral adipose tissue-derived exosomes promotes macrophage M1 polarization [41]. The M1 macrophages increase intestine inflammation, which can be reversed by miR-155 inhibitor [41]. Interestingly, other study shows that the elevated level of miR-223 induces M2 macrophage polarization through glycolysis alteration [42]. However, the elevated level of miR-34a in the adipocyte-derived exosomes suppresses M2 macrophage polarization by repressing Krüppel-like factor 4 (KLF4) that leads to obesity-induced adipose inflammation [43]. These data suggest that the effects of the exosomal miRNA on macrophage polarization depend on many factors such as the pathological conditions in the obese subjects.
On the contrary, the prominent effects of the adipocyte-derived exosomal miRNA on insulin sensitivity are consistently reported in different studies. miR-34a contributes to insulin resistance. In adipose tissue-selective miR-34a-KO mice, the ablation of miR-34a induces adipose tissue macrophage polarization that renders the mice resistance to obesity-induced insulin resistance, glucose intolerance, and hepatic steatosis [43]. Interestingly, in obese patients, the increased miR-34a expression in the visceral fat is positively correlated with the parameters of insulin resistance [43]. Besides, exosomes released from obese subject's adipose tissues transferred less miR-141-3p to hepatocytes than exosomes from the lean subject's adipose tissues, hence reduces the glucose uptake and induces insulin resistance in the hepatocytes [44]. In animal studies, it is also found that the elevated level of gonadal adipose tissue-derived exosomal miR222 induces insulin resistance in the liver and skeletal muscle of the obese mice by suppressing the insulin receptor substrate-1 expression [45]. Another study shows that elevated expression of miR-27a in visceral adipose tissue-derived exosomes of high-fat diet-fed mice represses peroxisome proliferator-activated receptor-γ and impairs the insulin signaling after it is taken up by skeletal muscle cells [46]. These studies suggest that not only the exosomal contents, but also the amount of the secreted exosomes will also affect the insulin sensitivity of the recipient organs.
In fact, the adipocyte-derived exosomal miRNA also affects the function of other organs under obese condition. For examples, the perivascular adipose tissue-derived exosomal miR-221-3p mediates vascular remodeling, increases vascular smooth cells (VSMC) proliferation and migration after being taken up by VSMCs in the obese subjects [47]. Interestingly, the exosomes can also go through the blood–brain barrier. A study reports that obese mice secret metastasis associated lung adenocarcinoma transcript 1 (MALAT1)-containing exosomes, which can be internalized by hypothalamic pro-opiomelanocortin (POMC) neurons [48]. MALAT1 is a competitive endogenous RNA (ceRNA) that activates the mTOR-signaling pathway mediated by miR‐181b and miR‐144 in the hypothalamic POMC neurons, and hence increases appetite and weight gain [48]. This is the first few studies demonstrating the adipocyte-derived exosomes can act on hypothalamic neurons to modulate energy intake. Besides, the adipocyte-derived exosomal miRNAs also affect cancer growth. miR-23a and miR-23b in these exosomes confer chemoresistance in hepatocellular cancer cells, and increase the cancer growth and metastasis [49]. The impacts of the miRNAs in the adipocyte-derived exosomes on various tissues and organs under obese condition are summarized in Fig. 1.
Fig. 1.
The impacts of the miRNAs in the adipocyte-derived exosomes on various tissues and organs under obese condition
Exosomal proteins
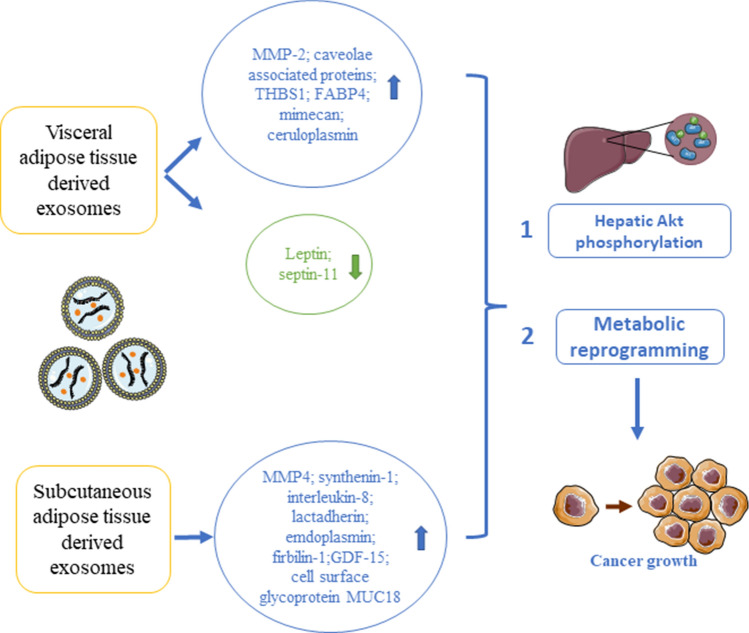
Exosomes also carry proteins. The exosomal proteins constitute an essential part of the human adipose tissue secretome. Hartwig et al. have employed non-targeted proteomics and bioinformatic analysis to reveal the functional roles of the exosomal proteins in the adipocyte-derived exosomes isolated from lean or overweight women. The proteome analysis has identified 897 adipokines in these exosomes (also known as exoadipokines), among which 67 has not yet been described in ExoCarta [50]. These exoadipokines have strong associations with human metabolic diseases including liver lesion (p value 1.04E − 12; 161 proteins), liver fibrosis (p value 8.83E − 10; 14 proteins), and liver cancer (p value 6.63E − 14; 115 proteins), as well as metabolic diseases like diabetes mellitus (p value 2.23E − 20; 60 proteins), glucose metabolism disorders (p value 5.34E − 23; 70 proteins), and metabolic syndrome (p value 2.65E − 08; 13 proteins) [50]. Besides, the protein sequence analysis has revealed that 196 of these exosomal proteins (57%) display secretory signal peptides [50]. Some of the exosomal proteins are linked to signaling pathways such as mTOR-signaling (2.0E − 28), integrin signaling (3.98E − 20), and membrane-mediated processes like endocytosis (5.01E − 18) [50]. Nevertheless, these adipocyte-derived exosomes are isolated from women, whether the exosomal protein profiles will be affected by hormones and are different in different genders, or will they be different under healthy and obese conditions have not been explored. Another interesting study also shows that the adipose tissue-derived EV protein contents are different between obese and lean subjects [54]. In the obese subjects, the protein levels of matrix metalloproteinase-2 (MMP-2), caveolae-associated protein, transforming growth factor-beta-induced protein ig-h3, thrombospondin-1, fatty acid binding protein-4, Mimecan, and ceruloplasmin are elevated in the visceral adipose tissue-derived EVs; while leptin, septin-11 levels are reduced [51]. Interestingly, in the subcutaneous adipose tissue-derived EVs, the protein levels of MMP4, synthenin-1, interleukin-8, lactadherin, emdoplasmin, firbilin-1, growth differentiation factor-15, and cell surface glycoprotein MUC18 are elevated [51]. Although this may not be a comprehensive list showing all the EVs protein changes under obese condition, the analysis suggests that the adipose tissue-derived EVs’ protein contents are different between healthy and obese subjects, and these changes also vary between different adipose depots.
The changes of these adipose tissue-derived exosomal protein contents have implication on insulin resistance in liver and muscle cells in the obese subjects. A study shows that after treating the hepatocytes with adipocyte-derived EVs isolated from overweight or obese patients, Akt phosphorylation in the hepatocytes is increased [52]. The hepatic Akt phosphorylation is negatively correlated to the glucose-6-phosphate expression in both subcutaneous adipose tissue-derived EVs (SAT-EVs) (r = − 0.60, p = 0.01) and omental adipose tissue-derived EVs (OAT-EVs) (r = − 0.74, p = 0.001) [52]. Another study shows that exosomes promote cancer growth. The majority of the adipocyte-derived exosomal proteins is implicated in fatty acid oxidation; these exosomes induce metabolic reprogramming in melanoma cells, and increase the cancer aggressiveness, migration, and invasion [22]. Furthermore, both the number of exosomes secreted and their effects on melanoma cell migration are increased in the obese subjects [22], suggesting that the exosomal proteins play a role in melanoma growth under obese condition. Indeed, the adipocyte-derived exosomes are rich in metabolic enzymes; their roles in lipid metabolism have been recently reviewed [53]. Under obese condition, the different expression profiles of these exosomal metabolic enzymes may also underlie their proatherogenic effects on macrophages [54]. The impacts of the proteins in the adipocyte-derived exosomes on various tissues and organs under obese condition are summarized in Fig. 2.
Fig. 2.
The impacts of the proteins in the adipocyte-derived exosomes on various tissues and organs under obese condition
Exosomal lipids and metabolites
Exosomes also carry lipids and metabolites. In general, most of the lipid enriched in the exosomes are phosphatidylcholine, lysophosphatidylcholine, ether-linked phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylglycerol, phosphatidic acid, ceramide, sphingomyelin, glycosphingolipids, cholesterol, and cholesterol ester [55]. A study shows that the adipocyte-derived exosomes has approximately 25% cholesterol, 25% phosphatidylcholine, 10% sphingomyelin, 10% triglyceride, 6% ceramide, and also some other lipid species such as phosphatidylserine and phosphatidylethanolamine [56]. However, the lipid content in the adipocyte-derived exosomes may be different under obese condition. For example, the fatty acid contents, but not the triglyceride content, are significantly increased in obese murine adipocyte-derived exosomes and is also positively correlated with the body mass index in human subjects [56, 57]. When comparing the lipid content of the adipocyte-derived exosomes with the exosomes derived from other cell types, the adipocyte-derived exosomes contain more sphingomyelin but less phosphatidylserine than the exosomes derived from prostate cancer cells, liver cancer cells, B-lymphocytes, mast cells, dendritic cells, reticulocytes, and platelets ester [55].
The adipocyte-derived exosomes will also mediate the transport of lipids from adipocytes to other cell types such as monocytes and induces the differentiation of bone-marrow-derived monocytes into macrophages [56]. Besides, under obese condition, the enhanced secretion of the exosomes from adipocytes and the elevated levels of the exosomal fatty acids may enhance melanoma fatty acid oxidation by increasing the transport of fatty acids to the cancer cells and hence increases the mitochondrial dynamics and cancer cell migration [57]. In the tumor microenvironment, there is an interaction between adipocytes and cancer cells. With the emerging role of exosomes, it is reasonable to postulate that the adipocyte-derived exosomes can affect the cancer cells at a distance by transporting the lipids to the cancer cells and affect the cancer growth.
Impact of obesity on the biogenesis of adipocyte-derived exosomes
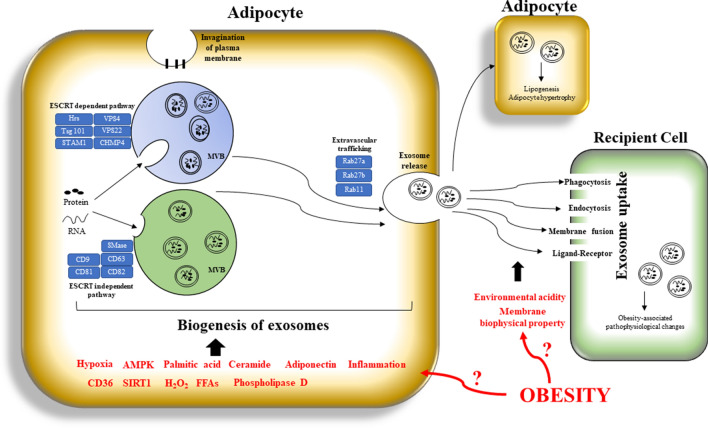
Obesity not only affects the exosomal content, but also the biogenesis of exosomes (Fig. 3). Exosomal production is increased during obesity and is correlated with the onset of obesity-related diseases such as insulin resistance [18, 22, 44]. Interestingly, the increased exosome production in the adipose tissues under obese condition is not observed in other cell types but only in adipocytes [22]. It is believed that adipocytes are under various stress in the hypertrophic growth [18, 19, 58, 59]. The cellular stress may regulate the secretion of exosomes. A study shows that different biological stimuli related to the obesity-associated chronic low-grade inflammation enhance the secretion of exosomes from mature adipocytes [10]. Interestingly, a recent study reveals that a cell’s exosomal release will suppress its own cellular senescence by excreting accumulated harmful cytoplasmic substances [60]. It is reasonable to postulate that the enhanced production of adipocyte-derived exosomes attenuates its own cellular stress that helps to maintain its homeostasis. However, the mechanisms underlying the enhanced production of these exosomes under obese condition are not fully understood.
Fig. 3.
A schematic diagram showing the impact of obesity on the biogenesis, secretion, and uptake of the exosomes derived from mature adipocytes. AMPK, 5′ AMP-activated protein kinase; SIRT1, NAD + -dependent deacetylase Sirtuin 1; H202, hydrogen peroxide; FFAs, free fatty acids; HRs, hepatocyte growth factor-regulated tyrosine kinase substrate; STAM1, signal transducing adaptor molecule
The biogenesis of exosomes involves vesicle budding into discrete endosomes that mature into multivesicular bodies (MVBs). These MVBs release exosomes upon plasma membrane fusion. Alternatively, the vesicles may directly bud off from the plasma membrane or budding at intracellular plasma membrane-connected compartments that results in a delay release [61]. The physicochemical properties determine budding efficiency [11]. Study shows that the budding process is affected by palmitic acid and phospholipase D [62]. Besides, ceramide is an important lipid molecule facilitating membrane curvature of the exosomes that is essential for the vesicular budding. Study shows that the inhibition of the ceramide production impairs exosome biogenesis [63]. In obese subjects, the ceramide species are elevated in the adipose tissues [64], and the enlarged adipocytes are overloaded with palmitic acid [65], both facilitate the budding of MVBs from the adipocytes.
Sorting of the biological contents into the exosomes are processed by the endosomal sorting complex transport (ESCRT)-dependent pathway and also the ESCRT-independent pathway [66]. In the ESCRT-dependent pathway, ESCRT proteins such as Hrs, CHMP4, tumor susceptibility gene 101 (TSG101), signaling transducing adaptor molecule (STAM1), and VPS4 play an important role in the exosome biosynthesis and secretion [67–71]. The ESCRT‐independent pathways regulate the assembly of exosomes in the absence of ESCRTs, which involve different proteins such as the tetraspanins CD9, CD63, CD81, and CD82 [63, 72]. These proteins are usually abundant within the exosomes. However, whether obesity affects the expressions and functions of these proteins for the biogenesis and sorting of biological content in the adipocyte-derived exosomes is less studied.
Exosome secretion is regulated by the Rab proteins. Rab27a and Rab27b are function in MVE docking at the plasma membrane [73]. Silencing two known Rab27 effectors, Slp4 (also known as SYTL4, synaptotagmin-like 4) and Slac2b (also known as EXPH5, exophilin 5), inhibits the exosome secretion [73, 74]. Besides, the effectors of Rab27a and Rab27b such as Slp4, Slac2b, Munc13-4, Rab 35, and Rab 11 also regulate the secretion process [75, 76]. Rab27a has an endogenous role in promoting the maturation of adipocytes from human perivascular adipose progenitor cells [77]. It will be interesting to investigate whether the expression of Rab27a in the mature adipocytes in obese subjects is increased that facilitates the secretion of the exosomes from the adipocytes.
Although the precise mechanism by which obesity regulates the production of adipose tissue-derived exosomes is not clear, AMP-activated protein kinas (AMPK-α1) has been suggested to play a role in this process. In cultured adipocytes and white adipose tissues, the absence of AMPKα1 increases exosome release and exosomal proteins by elevating tumor susceptibility gene 101 (TSG101)-mediated exosome biogenesis. In the palmitic acid-induced hypertrophic adipocyte model, TSG101 facilitates scavenger receptor class B (CD36) sorting into exosomes [78]. Activation of AMPK reduces the adipocyte-mediated exosome release [78]. These findings suggest that the release of adipocyte-derived exosomes is depending on the expression TSG101 and the activity of AMPK. Besides, AMPK also regulates the activity of Sirtuin1 (SIRT1), which is a highly conserved NAD+-dependent protein deacetylase [79]. Interestingly, adipose-specific ablation of Sirt1 in mice (Ad-Sirt1−/− mice) increases exosome production that is due to the defective autophagy activity in Ad-Sirt1−/− mice [80], suggesting a suppressive role of the adipocyte SIRT1 in the exosome production. Secretion of adipocyte-derived exosomes may also be regulated by free fatty acids and hydrogen peroxide in an endocrine manner [24]. A study shows that hydrogen peroxide concentration and catalase activity are increased in visceral fat in overweight and obese subjects when compared to normal-weight subjects (32%, p = 0.038 and 51%, p = 0.043 respectively) [81]. The elevated level of hydrogen peroxide may be a factor that explains the increased secretion of adipocyte-derived exosomes. Besides, adiponectin/T-cadherin system also enhances exosome biogenesis and secretion [82], because mice genetically deficient in either adiponectin or T-cadherin have lower plasma exosome levels [83]. Adiponectin is an adipokine, it has an inverse relationship with adipocyte size [84], and its level is decreased under obese condition [85]. If adiponectin increases the secretion of adipocyte-derived exosomes, the reduced level of adiponectin should reduce the secretion of adipocyte-derived exosomes in obese subjects, which deserves further investigation.
Impact of obesity on the release of adipocyte-derived exosomal content to the recipient cells
The dominant mechanism responsible for the release of exosomal contents to the cytosol of the recipient cells for signaling has not been identified. Exosomes may deliver the cargo to the recipient cell cytosol after they are engulfed via phagocytosis, endocytosis, or membrane fusion [86]. Alternatively, exosomes may depend on direct protein–protein interaction between ligands and cell receptors to elicit intracellular signaling cascades without being internalized [87]. Besides, it has been reported that integrins on exosomes interact with adhesion molecules and matrix proteins on recipient cells that enables active targeting [88]. That is, the ligand–receptor mediates the binding and docking of the exosomes to the target cells [89]. Whether obesity affects the phagocytosis of the exosomes in the recipient cells and the direct protein–protein interaction is largely unknown. Nevertheless, a study shows that uptake of exosomes by melanoma cells depends on the membrane biophysical property such as fluidity and lipid composition that affect the fusion process [90, 91]. Under obese condition, the membrane biophysical property of the recipient cells may be changed and that may affect the up-taking process. Besides, low pH facilitates exosome uptake when compared with a buffered condition [92]. Therefore, the microenvironmental acidity under obese condition may be another factor affecting the exosome uptake by recipient cells (Fig. 3).
Circulating EVs
Obese patients have significantly more circulating EVs than normal control subjects [93]. Interestingly, the elevated levels of EVs can be reversed by low-energy diet [93]. The elevated level of circulating EVs is also observed in obese mouse models [18, 38]. Recently, computational approaches have tried to estimate the tissue or cellular origin of circulating EVs based on RNA-seq data. Interestingly, a study with 101 human plasma samples reports that 99.8% of circulating EVs are generated from hematopoietic cells, and only 0.2% of EVs are derived from tissues [94]. These hematopoietic cells include platelets, erythrocytes, mast cells, neutrophils, eosinophils, and so on [95–97]. However, the abundance of circulating EVs may be affected by preanalytical factors such as blood sample storage time, temperature, and anticoagulants on their secretion [98–100]. The tissues that secret EVs into the circulating include muscle [101], lung [102], liver [103], cardiomyocytes [104], and endothelium [105]. The adipocyte-derived EVs are also found in the human circulation [105]. A study reports that after depleting the circulating EVs secreted by platelet, leukocyte, endothelial cells, and erythrocytes, the EVs remained in the post-depletion samples contain a high level of adipokines and adipocyte proteins [106]. Indeed, many studies also report that EVs positive for adipocyte-specific markers such as adiponectin and resistin are found in mice serum and human plasma [18, 106–108].
However, it would be difficult to differentiate the origin of the circulating EVs, calculate the proportion of the adipocyte-derived EVs in the circulating EVs, and clarify the pathological or physiological roles of each circulating EV subtype. Nevertheless, circulating EVs are potential biomarkers for many diseases, and they also contribute to the development of metabolic diseases and cancers [109, 110]. For example, circulating platelet-derived EVs from patients with type II diabetes have higher levels of CD42 and CD41a when compared to healthy controls [111], suggesting that CD42 and CD41a can serve as biomarkers for type 2 diabetes. Circulating EVs in non-alcoholic fatty liver disease (NAFLD) patients have elevated level of CD14 and V/24/Vb11, which are correlated with alanine aminotransferase and the histological grade of NAFLD [112].
Advance of EVs for clinical purpose
Using exosomes for disease treatment is a feasible strategy, because exosomes are less immunogenic, which is particularly useful for repeat drug dosing [113]. Exosome is also a flexible platform. We can modify the exosomal content with modified exosome-producing cells which have transfected or transduced in culture with the target genes or proteins [114–116]. Alternatively, exosomes can be loaded with therapeutic proteins that can compensate for genetic deficiencies, which has been demonstrated in urea cycle disorders [113]. Another study engineers the exosomes to be avoided being taken up by reticuloendothelial system [113]. Orally delivered exosomes have also been tested; the delivered exosomes can transport the expression vectors to the cells in the gastrointestinal tract [113]. Indeed, many exosome-based therapies are now undergoing clinical trials (Table 1). For example, intravenously delivered exosomes that carry RASG12D siRNA may improve the survival of the pancreatic cancer patients [113]. Regarding the adipose tissue-derived exosomes, they are the potential therapeutic agents for wound healing, and for the treatments of liver damage, metabolic syndrome, and cancers [53].
Table 1.
Ongoing clinical trials for exosome-based therapy
| No | Study title | Study no |
|---|---|---|
| 1 | MSC-Exos Promote Healing of MHs | NCT03437759 |
| 2 | Circulating Exosomes As Potential Prognostic And Predictive Biomarkers In Advanced Gastric Cancer Patients ("EXO-PPP Study") (EXO-PPP) | NCT01779583 |
| 3 | Combined Diagnosis of CT and Exosome in Early Lung Cancer | NCT03542253 |
| 4 |
Effect of Microvesicles and Exosomes Therapy on β-cell Mass in Type I Diabetes Mellitus (T1DM) |
NCT02138331 |
| 5 | iExosomes in Treating Participants With Metastatic Pancreas Cancer With KrasG12D Mutation | NCT03608631 |
| 6 | The Use of Exosomes In Craniofacial Neuralgia | NCT04202783 |
| 7 | Exosomes and Immunotherapy in Non-Hodgkin B-cell Lymphomas (ExoReBLy) | NCT03985696 |
| 8 | Allogenic Mesenchymal Stem Cell Derived Exosome in Patients With Acute Ischemic Stroke | NCT03384433 |
| 9 | Focused Ultrasound and Exosomes to Treat Depression, Anxiety, and Dementias | NCT04202770 |
| 10 | MSC EVs in Dystrophic Epidermolysis Bullosa | NCT04173650 |
| 11 | How Does Prostate Cancer Metastasize? Studying the Role of Secreted Packages (Exosomes) From Fat Tissue in Lean and Obese Patients (EXOPRO) | NCT04167722 |
| 12 | Evaluation of Adipose-Derived Stem Cells Exo.in Treatment of Periodontitis | NCT04270006 |
| 13 | A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia | NCT04276987 |
| 14 | Effect of UMSCs Derived Exosomes on Dry Eye in Patients With cGVHD | NCT04213248 |
| 15 | A Tolerance Clinical Study on Aerosol Inhalation of Mesenchymal Stem Cells Exosomes In Healthy Volunteers | NCT04313647 |
| 16 |
Exosome of Mesenchymal Stem Cells for Multiple Organ Dysfunction Syndrome After Surgical repair of Acute Type A aortic Dissection |
NCT04356300 |
| 17 | A Tolerance Clinical Study on Aerosol Inhalation of Mesenchymal Stem Cells Exosomes In Healthy Volunteers | NCT04313647 |
| 18 | Extracellular Vesicle Infusion Treatment for COVID-19 Associated ARDS (EXITCOVID19) | NCT04493242 |
| 19 | A Clinical Study of Mesenchymal Progenitor Cell Exosomes Nebulizer for the Treatment of Pulmonary Infection | NCT04544215 |
| 20 | The Safety and the Efficacy Evaluation of Allogenic Adipose MSC-Exos in Patients With Alzheimer’s Disease | NCT04388982 |
| 21 | A Clinical Study of Mesenchymal Stem Cell Exosomes Nebulizer for the Treatment of ARDS | NCT04602104 |
| 22 | Safety and Efficiency of Method of Exosome Inhalation in COVID-19 Associated Pneumonia (COVID-19EXO2) | NCT04602442 |
| 23 | The Use of Exosomes for the Treatment of Acute Respiratory Distress Syndrome or Novel Coronavirus Pneumonia Caused by COVID-19 (ARDOXSO) | NCT04798716 |
| 24 | Organicell Flow for Patients with COVID-19 | NCT04384445 |
| 25 | A Prospective Feasibility Study Evaluating Extracellular Vesicles Obtained by Liquid Biopsy for Neoadjuvant Treatment Response Assessment in Rectal Cancer (RECCEV) | NCT04852653 |
| 26 | Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia | NCT04491240 |
| 27 | Function of Circulating Exosomes in Sepsis-induced Immunosuppression | NCT04979767 |
Besides carrying therapeutic agents for disease treatments, exosomes can also serve as biomarkers or diagnostic tools for many diseases including cardiovascular diseases [117], neuronal disease [118], liver diseases [119], kidney diseases [120], lung diseases [121], and cancers of the hepatobiliary system, breast, lung, gastrointestinal tract, skin (melanoma), prostate, and nasopharynx [122–130]. One of the advantages of using exosomes as biomarkers is, exosomes can be found in almost all body fluids, including blood, urine, saliva, breast milk, cerebrospinal fluid, semen, amniotic fluid, and ascites [131]. Exosomes have been suggested to serve as tumor markers for personalized diagnostics and the development of personalized medicine. Many exosomal biomarkers for various cancers have been identified [132]. Obesity is a complicated disease. Although the application of exosomes as biomarkers is a pragmatic approach to develop personalized medicine for treating obesity and its comorbid conditions, the development is still premature. Revealing the exosomal content as biomarkers for the diagnosis of obesity and its comorbidities may help to develop exosome-based personalized medicine.
Perspective
In the further, studies can be done to examine how obesity affects the interaction between the adipocyte-derived exosomes and the target cells and explore more physiological and pathological roles of these adipocyte-derived exosomes. Exosomes are stable in plasma [133, 134]; they transport the exosomal cargo at a distance from the donor tissues to the target tissues [135]. Under obese condition, the exosomal contents of the adipocyte-derived exosomes can be taken up many different cell types, which induce a diverse array of pathological conditions. It is worth noting that the specific ligand/receptor interactions mediate the uptake of exosomes by T cells has been reported. The initial binding/docking of T cells to exosomes is regulated by ICAM-1/LFA-1 [136], a firm adhesion is further facilitated via αL (CD11a), α4 (CD49d), CD44 and ICAM-1 expression on leukocytes, as well as the expressions of tetraspanins CD9 and CD81 on the exosomes [91, 137]. It would be interesting to examine whether a specific ligand/receptor interaction mediates the adhesion and uptake of adipocyte-derived exosomes by the recipient cells, and would these interactions be affected under obese condition. Besides, different adipose depots have different gene expression profiles [138–140]. Therefore, the exosomal contents derived from these adipose depots should be different, and the impacts of these exosomes on the recipient cells should also be different. Indeed, it has been shown that under obese condition, the protein levels of monocyte chemoattractant protein-1 (MCP-1), interleukin-6, and macrophage migration inhibitory factor (MIF) are higher in OAT-EVs when compared to SAT-EVs in the obese subjects [52]. Mimecan and ceruloplasmin are elevated in the visceral adipose tissue-derived EVs; while leptin and septin-11 levels are reduced [51]. Interestingly, in the subcutaneous adipose tissue-derived EVs, the protein levels of MMP4, synthenin-1, interleukin-8, lactadherin, emdoplasmin, firbilin-1, growth differentiation factor-15, and cell surface glycoprotein MUC18 are elevated [51]. However, the differential roles of the exosomal proteins isolated from different adipose depots in obesity-related traits are less studied. Furthermore, the enlarged adipose tissues also contain other cell types but not limited to adipocytes. Indeed, obesity induces local inflammation in the adipose tissues with infiltration of immune cells [141]. Among the adipose-resident immune cells, macrophages are the most abundant cell type that constitutes almost 50% of the total cells of the adipose tissue [142]. The ATM will also secret EVs to the circulation that attribute to the obesity comorbid conditions. Current studies mainly focus on the effect of ATM-derived EVs on insulin sensitivity under obese condition. Study shows that the ATM-derived exosomes of obese mice induce insulin resistance in lean mice, indicating that these exosomes may lead to the development of diabetes under obese condition [143]. The specific exosomal contents that attribute to insulin resistance in liver and muscle are miR-155 that is overexpressed in ATM-derived exosomes in the obese subjects [144]. miR-155 mediates insulin resistance after it is taken up by adipocytes, myotubes, and hepatocytes [144]. Moreover, the adipocyte-derived stem cells (ADSCs) are also present in the adipose tissues. ADSCs mainly play a regenerative role and promote the migration, proliferation, and secretory activity of keratinocytes and fibroblasts [145]. ADSCs not only augment inflammatory processes, but also differentiate into mature adipocytes for building adipose tissues [146]. Interestingly, the ADSC-derived exosomes affect the vascular integrity, in which the exosomal miR-126 regulates the response of endothelial cells to vascular endothelial growth factor. However, a study reports that obesity reduces the ADSC exosomal miR-126 content and hence impairs its pro-angiogenic potential [147, 148], which may underlie the obesity-associated vascular diseases. Besides, ADSC-derived exosomes also mediate the transport of signal transducer and activator of transcription-3 (STAT3) to macrophages, initiates the anti‐inflammatory macrophage polarization, and reduces inflammation [149]. The transfer of STAT3 in the ADSC exosomes also improves the insulin sensitivity and glucose tolerance in high-fat diet-fed mice [149]. Besides, the ADSC exosomes contain other biological contents that reduce hepatic steatosis, induce browning in inguinal white adipose tissue, increase M2 macrophage infiltration into white adipose tissues, promote the proliferation and migration of human osteoarthritic chondrocytes, maintain the chondrocyte matrix by increasing type II collagen synthesis and decreasing matrix metalloproteinase-1 (MMP-1), MMP-3, MMP-13, and ADAM metallopeptidase with thrombospondin type 1 motif 5 (ADAMTS-5) expression [150, 151]. Therefore, the effects of ATM and ADSCs should be taken into consideration when constructing a holistic picture to understand the pathological impact of the exosomes that are derived from the adipose tissues under obese condition.
Exploring the mechanisms underlying the effects of adipocyte-derived or adipose tissue-derived exosomes on obesity and its comorbid conditions may help to develop novel therapeutic strategies. Indeed, in an adipocyte specific Sirt1 knockout mouse model that resulted in obesity, treatment with the exosome production inhibitor, GW4869, significantly reduces body weight and improves insulin sensitivity [80]. Besides, revealing the time point for the changes of the exosomal contents during the course of obesity development may also give us a hint to understand whether the changes are prior to onset of obesity, or they are the outcome of the disease [152].
Conclusion
The impacts of adipocyte-derived exosomes on obesity and its comorbid conditions are emerging. Exosomes are endogenous products that are the ideal tools for therapy. Understanding how obesity affects the biogenesis, cargo packaging, and secretion of the adipocyte-derived exosomes allows us to manipulate the process and reengineer the exosomes. Exosome-based therapy is a novel yet pragmatic approach for the treatment of obesity and its comorbidities.
Author contributions
Conceptualization, writing, and editing: HY Kwan; data curation: MT Chen, KY Xu and B Chen.
Funding
This work was partially supported by HKBU Initiation Grant for Faculty Niche Research Areas (#RC-FNRA-IG/20-21/SCI/03), Research Grant Council of HKSAR HKBU-22103017-ECS, Innovation & Technology Commission #PRP/015/19FX, Free Exploration Basic Research Project in Shenzhen Virtual University Park #2021Szvup131, Natural Science Foundation of Guangdong Province #2021A1515010655 and #2018A0303130122.
Declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontana L, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 3.Fain JN, et al. Transforming growth factor beta1 release by human adipose tissue is enhanced in obesity. Metab Clin Exp. 2005;54:1546–1551. doi: 10.1016/j.metabol.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular vesicles: Novel mediators of cell communication in metabolic disease. Trends Endocrinol Metab. 2017;28(1):3–18. doi: 10.1016/j.tem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013;41:283–287. doi: 10.1042/BST20120192. [DOI] [PubMed] [Google Scholar]
- 6.Choi D, et al. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin Cell Dev Biol. 2017;67:11–22. doi: 10.1016/j.semcdb.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Vicencio JM, et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol. 2015;65:1525–1536. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Pironti G, et al. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation. 2015;131:2120–2130. doi: 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connolly KD, et al. Characterisation of adipocyte-derived extracellular vesicles released pre- and post-adipogenesis. J Extracell Vesicles. 2015;4:29159. doi: 10.3402/jev.v4.29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durcin M, et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J Extracell Vesicles. 2017;6(1):1305677. doi: 10.1080/20013078.2017.1305677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurunathan S, et al. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int J Nanomedicine. 2021;16:1281–1312. doi: 10.2147/IJN.S291956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tauro BJ, et al. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013;12(3):587–598. doi: 10.1074/mcp.M112.021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowal J, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113(8):E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laulagnier K, et al. Characterization of exosome subpopulations from RBL- 2H3 cells using fluorescent lipids. Blood Cells Mol Dis. 2005;35(2):116–121. doi: 10.1016/j.bcmd.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Lai RC, et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828. doi: 10.3402/jev.v5.29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D, et al. Profiling surface proteins on individual exosomes using a proximity barcoding assay. Nat Commun. 2019;10:3854. doi: 10.1038/s41467-019-11486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Q, et al. Clinical applications of exosome membrane proteins. Precis Clin Med. 2020;3(1):54–66. doi: 10.1093/pcmedi/pbaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eguchi A, et al. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J Mol Med. 2016;94(11):1241–1253. doi: 10.1007/s00109-016-1446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrante SC, et al. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res. 2015;77:447–454. doi: 10.1038/pr.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen Z, et al. Hypertrophic adipocyte-derived exosomal miR-802-5p contributes to insulin resistance in cardiac myocytes through targeting HSP60. Obesity. 2020;28:1932–1940. doi: 10.1002/oby.22932. [DOI] [PubMed] [Google Scholar]
- 21.Sano S, et al. Lipid synthesis is promoted by hypoxic in 3T3-L1 cells. Biochem Biophys Res Commun. 2014;445:327–332. doi: 10.1016/j.bbrc.2014.01.183. [DOI] [PubMed] [Google Scholar]
- 22.Lazar I, et al. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: a novel mechanism linking obesity and cancer. Cancer Res. 2016;76(14):4051–4057. doi: 10.1158/0008-5472.CAN-16-0651. [DOI] [PubMed] [Google Scholar]
- 23.Deng Z, et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58:2498–2505. doi: 10.2337/db09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller G, et al. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositolanchored proteins transfer RNA stimulating lipid synthesis. Cell Signal. 2011;23(7):1207–1223. doi: 10.1016/j.cellsig.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Lin JD. Function and mechanism of long noncoding RNAs in adipocyte biology. Diabetes. 2019;68:887–896. doi: 10.2337/dbi18-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, et al. Interrogation of nonconserved human adipose lincRNAs identifies a regulatory role of linc-ADAL in adipocyte metabolism. Sci Transl Med. 2018;10:eaar5987. doi: 10.1126/scitranslmed.aar5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding C, et al. De novo reconstruction of human adipose transcriptome reveals conserved lncRNAs as regulators of brown adipogenesis. Nat Commun. 2018;9:1329. doi: 10.1038/s41467-018-03754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng L, et al. Long non-coding RNA MEG3 inhibits adipogenesis and promotes osteogenesis of human adipose-derived mesenchymal stem cells via miR-140-5p. Mol Cell Biochem. 2017;433(1–2):51–60. doi: 10.1007/s11010-017-3015-z. [DOI] [PubMed] [Google Scholar]
- 29.Song Y, et al. SNHG9, delivered by adipocyte derived exosomes, alleviates inflammation and apoptosis of endothelial cells through suppressing TRADD expression. Eur J Pharmacol. 2020;872:172977. doi: 10.1016/j.ejphar.2020.172977. [DOI] [PubMed] [Google Scholar]
- 30.Takata A, et al. MicroRNAs and liver function. Minerva Gastroenterol Dietol. 2013;59:187. [PubMed] [Google Scholar]
- 31.Pauley KM, Chan EK. MicroRNAs and their emerging roles in immunology. Ann N Y Acad Sci. 2008;1143:226. doi: 10.1196/annals.1443.009. [DOI] [PubMed] [Google Scholar]
- 32.Thomou T. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higuchi C, et al. Identification of circulating miR-101, miR-375 and miR-802 as biomarkers for type 2 diabetes. Metab Clin Exp. 2015;64(4):489–497. doi: 10.1016/j.metabol.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Kong L, et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 2011;48(1):61–69. doi: 10.1007/s00592-010-0226-0. [DOI] [PubMed] [Google Scholar]
- 35.Sun K, et al. Expression and DNA methylation status of microRNA-375 in patients with type 2 diabetes mellitus. Mol Med Rep. 2014;9(3):967–972. doi: 10.3892/mmr.2013.1872. [DOI] [PubMed] [Google Scholar]
- 36.Latreille M, et al. miR-375 gene dosage in pancreatic β-cells: implications for regulation of β-cell mass and biomarker development. J Mol Med. 2015;93(10):1159–1169. doi: 10.1007/s00109-015-1296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karolina DS, et al. Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab. 2012;97(12):E2271–E2276. doi: 10.1210/jc.2012-1996. [DOI] [PubMed] [Google Scholar]
- 38.Hubal EP, et al. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity. 2017;25:102–110. doi: 10.1002/oby.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castoldi A, et al. The macrophage switch in obesity development. Front Immunol. 2016;6:637. doi: 10.3389/fimmu.2015.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, et al. Adipocyte derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J Mol Cell Biol. 2016;8:505–517. doi: 10.1093/jmcb/mjw040. [DOI] [PubMed] [Google Scholar]
- 41.Wei M, et al. Visceral adipose tissue derived exosomes exacerbate colitis severity via pro-inflammatory MiRNAs in high fat diet fed mice. ACS Nano. 2020;14(4):5099–5110. doi: 10.1021/acsnano.0c01860. [DOI] [PubMed] [Google Scholar]
- 42.Dang CP, Leelahavanichkul A. Over-expression of miR-223 induces M2 macrophage through glycolysis alteration and attenuates LPS-induced sepsis mouse model, the cell-based therapy in sepsis. PLoS ONE. 2020;15(7):e0236038. doi: 10.1371/journal.pone.0236038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan Y, et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019;129:834–849. doi: 10.1172/JCI123069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dang Y, et al. Exosomal transfer of obesity adipose tissue for decreased miR-141-3p mediate insulin resistance of hepatocytes. Int J Biol Sci. 2019;15:351–368. doi: 10.7150/ijbs.28522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li D, et al. Gonadal white adipose tissue-derived exosomal MiR-222 promotes obesity-associated insulin resistance. Aging. 2020;12(22):22719–22743. doi: 10.18632/aging.103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu H. Adipocyte-derived exosomal MiR-27a induces insulin resistance in skeletal muscle through repression of PPAR-gamma. Theranostics. 2018;8:2171–2188. doi: 10.7150/thno.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, et al. Perivascular adipose tissue–derived extracellular vesicle miR-221-3p mediates vascular remodeling. FASEB J. 2019;33:12704–12722. doi: 10.1096/fj.201901548R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao J, et al. Adipocyte-derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. Acta Physiol. 2020;228(2):e13339. doi: 10.1111/apha.13339. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, et al. Adipocyte-derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. J Physiol Biochem. 2019;75:391–401. doi: 10.1111/apha.13339. [DOI] [PubMed] [Google Scholar]
- 50.Hartwig S, et al. Exosomal proteins constitute an essential part of the human adipose tissue secretome. BBA Protein Proteomics. 2019;867:140172. doi: 10.1016/j.bbapap.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Camino T, et al. Deciphering adipose tissue extracellular vesicles protein cargo and its role in obesity. Int J Mol Sci. 2020;21:9366. doi: 10.3390/ijms21249366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kranendonk MEG, et al. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity. 2014;22:2216–2223. doi: 10.1002/oby.20847. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, et al. Multifaceted roles of adipose tissue-derived exosomes in physiological and pathological conditions. Front Physiol. 2021;12:9429. doi: 10.3389/fphys.2021.669429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie Z, et al. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J Am Heart Assoc. 2018;7:e007442. doi: 10.1161/JAHA.117.007442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skotland T, et al. An emerging focus on lipids in extracellular vesicles. Adv Drug Deliv Rev. 2020;159:308–321. doi: 10.1016/j.addr.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Flaherty SE, III, et al. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363:989–993. doi: 10.1126/science.aaw2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clement E, et al. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. EMBO J. 2020;39:e102525. doi: 10.15252/embj.2019102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saman S, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sano S, et al. Lipid synthesis is promoted by hypoxic adipocyte derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun. 2014;445:327–333. doi: 10.1016/j.bbrc.2014.01.183. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi A, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 62.Egea-Jimenez AL, Zimmermann P. Phospholipase D and phosphatidic acid in the biogenesis and cargo loading of extracellular vesicles. J Lipid Res. 2018;59(9):1554–1560. doi: 10.1194/jlr.R083964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Talbot CL, Chaurasia B. Ceramides in adipose tissue. Front Endocrinol. 2020;11:407. doi: 10.3389/fendo.2020.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JI, et al. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol. 2015;35(10):686–699. doi: 10.1128/MCB.01321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jadli AS, et al. Inside(sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol Cell Biochem. 2020;4687:77–94. doi: 10.1007/s11010-020-03703-z. [DOI] [PubMed] [Google Scholar]
- 67.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colombo M, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 69.Ghossoub R, et al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:3477. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- 70.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Villarroya-Beltri C, et al. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:3–13. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beach A, et al. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J Ovarian Res. 2014;7:14. doi: 10.1186/1757-2215-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ostrowski M, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 74.Blanc L, Vidal M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases. 2018;9(1–2):95–106. doi: 10.1080/21541248.2016.1264352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerber PP, et al. Rab27a controls HIV-1 assembly by regulating plasma membrane levels of phosphatidylinositol 4,5-bisphosphate. J Cell Biol. 2015;209(3):435–452. doi: 10.1083/jcb.201409082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsu C, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boucher JM, et al. Rab27a regulates human perivascular adipose progenitor cell differentiation. Cardiovasc Drug Ther. 2018;32(5):519–530. doi: 10.1007/s10557-018-6813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan C, et al. A high-fat diet attenuates AMPK α1 in adipocytes to induce exosome shedding and nonalcoholic fatty liver development in vivo. Diabetes. 2021;70(2):577–588. doi: 10.2337/db20-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schug TT, Li X. Sirtuin 1 in lipid metabolism and obesity. Ann Med. 2011;43(3):198–211. doi: 10.3109/07853890.2010.547211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li F, et al. Adiposespecific knockdown of Sirt1 results in obesity and insulin resistance by promoting exosomes release. Cell Cycle. 2019;18:2067–2082. doi: 10.1080/15384101.2019.1638694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akl MG, et al. Perturbed adipose tissue hydrogen peroxide metabolism in centrally obese men: association with insulin resistance. PLoS ONE. 2017;12(5):e0177268. doi: 10.1371/journal.pone.0177268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Obata Y, et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight. 2018;3(8):e99680. doi: 10.1172/jci.insight.99680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kita S, Shimomura I. Stimulation of exosome biogenesis by adiponectin, a circulating factor secreted from adipocytes. J Biochem. 2021;169(2):173–179. doi: 10.1093/jb/mvaa105. [DOI] [PubMed] [Google Scholar]
- 84.Drolet R, et al. Fat depot-specific impact of visceral obesity on adipocyte adiponectin release in women. Obesity. 2009;17(3):424–430. doi: 10.1038/oby.2008.555. [DOI] [PubMed] [Google Scholar]
- 85.Nigro E, et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014:658913. doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hawari FI, et al. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci USA. 2004;101(5):1297–1302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kao CY, Papoutsakis ET. Extracellular vesicles: exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr Opin Biotechnol. 2019;60:89–98. doi: 10.1016/j.copbio.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 90.Parolini I, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morelli AE, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 92.Tian T, et al. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111(2):488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- 93.Pardo F, et al. Extracellular vesicles in obesity and diabetes mellitus. Mol Asp Med. 2018;60:81–91. doi: 10.1016/j.mam.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 94.Li Y, et al. EV-origin: enumerating the tissue-cellular origin of circulating extracellular vesicles using exLR profile. Comput Struct Biotechnol J. 2020;18:2851–2859. doi: 10.1016/j.csbj.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Skokos D, et al. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J Immunol. 2001;166:868–876. doi: 10.4049/jimmunol.166.2.868. [DOI] [PubMed] [Google Scholar]
- 96.Hess C, et al. Ectosomes released by human neutrophils are specialized functional units. J Immunol. 1999;163:4564–4573. [PubMed] [Google Scholar]
- 97.Mazzeo C, et al. Exosome secretion by eosinophils: a possible role in asthma pathogenesis. J Allergy Clin Immunol. 2015;135:1603–1613. doi: 10.1016/j.jaci.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 98.Arraud N, et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12:614–627. doi: 10.1111/jth.12554. [DOI] [PubMed] [Google Scholar]
- 99.Arraud N, et al. Fluorescence triggering: a general strategy for enumerating and phenotyping extracellular vesicles by flow cytometry. Cytom Part A. 2016;89:184–195. doi: 10.1002/cyto.a.22669. [DOI] [PubMed] [Google Scholar]
- 100.Wisgrill L, et al. Peripheral blood microvesicles secretion is influenced by storage time, temperature, and anticoagulants. Cytom Part A. 2016;89:663–672. doi: 10.1002/cyto.a.22892. [DOI] [PubMed] [Google Scholar]
- 101.Guescini M, et al. Muscle releases alpha-sarcoglycan positive extracellular vesicles carrying miRNAs in the bloodstream. PLoS ONE. 2015;10:e0125094. doi: 10.1371/journal.pone.0125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carpi S, et al. Expression analysis of muscle-specific miRNAs in plasma-derived extracellular vesicles from patients with chronic obstructive pulmonary disease. Diagnostics. 2020;10:502. doi: 10.3390/diagnostics10070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Povero D, et al. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS ONE. 2014;9:e113651. doi: 10.1371/journal.pone.0113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu B, et al. The ESCRT-III pathway facilitates cardiomyocyte release of cBIN1-containing microparticles. PLoS Biol. 2017;15:e2002354. doi: 10.1371/journal.pbio.2002354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Combes V, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Connolly KD, et al. Evidence for adipocyte-derived extracellular vesicles in the human circulation. Endocrinology. 2018;159:3259–3267. doi: 10.1210/en.2018-00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ogawa R, et al. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun. 2010;398:723–729. doi: 10.1016/j.bbrc.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 108.Phoonsawat W, et al. Adiponectin is partially associated with exosomes in mouse serum. Biochem Biophys Res Commun. 2014;448:261–266. doi: 10.1016/j.bbrc.2014.04.114. [DOI] [PubMed] [Google Scholar]
- 109.Shah R, et al. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379:958–966. doi: 10.1056/NEJMra1704286. [DOI] [PubMed] [Google Scholar]
- 110.Bhattacharjee R, et al. Exosomal cargo properties, endothelial function and treatment of obesity hypoventilation syndrome: a proof of concept study. J Clin Sleep Med. 2018;14(5):797–807. doi: 10.5664/jcsm.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Müller G. Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. DMSO. 2012;5:247–282. doi: 10.2147/DMSO.S32923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kornek M, et al. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or non-alcoholic steatohepatitis. Gastroenterology. 2012;143:448–458. doi: 10.1053/j.gastro.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cully M. Exosome-based candidates move into the clinic. Nat Rev Drug Discov. 2021;20(1):6–7. doi: 10.1038/d41573-020-00220-y. [DOI] [PubMed] [Google Scholar]
- 114.Rufino-Ramos D, et al. Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J Control Release. 2017;262:47–258. doi: 10.1016/j.jconrel.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 115.Vader P, et al. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–157. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 116.Osorio-Querejeta I, et al. Therapeutic potential of extracellular vesicles for demyelinating diseases; challenges and opportunities. Front Mol Neurosci. 2018;11:434. doi: 10.3389/fnmol.2018.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ye W, et al. Plasma derived exosomes contribute to inflammation via the TLR9-NF-κB pathway in chronic heart failure patients. Mol Immunol. 2017;87:114. doi: 10.1016/j.molimm.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 118.Kanninen KM, et al. Exosomes as new diagnostic tools in CNS diseases. Biochim Biophys Acta. 2016;1862(3):403–410. doi: 10.1016/j.bbadis.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 119.Masyuk AI, Masyuk TV, Larusso NF. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J Hepatol. 2013;59(3):621–625. doi: 10.1016/j.jhep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang W, et al. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol. 2016;311(5):F844–F851. doi: 10.1152/ajprenal.00429.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alipoor SD, et al. Exosomes and exosomal miRNA in respiratory diseases. Mediators Inflamm. 2016;2016:5628404. doi: 10.1155/2016/5628404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ye SB, et al. Tumor derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5:5439–5452. doi: 10.18632/oncotarget.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Silva J, et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer. 2012;51:409–418. doi: 10.1002/gcc.21926. [DOI] [PubMed] [Google Scholar]
- 124.Sandfeld-Paulsen B, et al. Exosomal proteins as diagnostic biomarkers in lung cancer. J Thorac Oncol. 2016;11:1701–1710. doi: 10.1016/j.jtho.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 125.He M, Zeng Y. Microfluidic exosome analysis toward liquid biopsy for cancer. J Lab Autom. 2016;21:599–608. doi: 10.1177/2211068216651035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baran J, et al. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer Immunol Immunother. 2010;59:841–850. doi: 10.1007/s00262-009-0808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arbelaiz A, et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2017;66:1125–1143. doi: 10.1002/hep.29291. [DOI] [PubMed] [Google Scholar]
- 128.Khan S, et al. Early diagnostic value of survivin and its alternative splice variants in breast cancer. BMC Cancer. 2014;14:176. doi: 10.1186/1471-2407-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Logozzi M, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE. 2009;4(4):e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Logozzi M, et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017;403:318–329. doi: 10.1016/j.canlet.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y, et al. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.An T, et al. Exosomes serve as tumour markers for personalized diagnostics owing to their important role in cancer metastasis. J Extracellular Vesicles. 2015;4:27522. doi: 10.3402/jev.v4.27522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang H, et al. Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. Int J Cancer. 2019;144:2501–2515. doi: 10.1002/ijc.31977. [DOI] [PubMed] [Google Scholar]
- 134.Pace KR, Dutt R, Galileo DS. Exosomal L1CAMstimulates glioblastoma cell motility, proliferation, and invasiveness. Int J Mol Sci. 2019;20(16):3982. doi: 10.3390/ijms20163982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McKelvey KJ, et al. Exosomes: mechanism of uptake. J Circ Biomark. 2015;4:7. doi: 10.5772/61186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nolte’t Hoen ENM, et al. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 137.Zech D, et al. Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal. 2014;10:3731. doi: 10.1186/1478-811X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kwok KHM, Lam KSL, Xu A. Heterogeneity of white adipose tissue: molecular basis and clinical implications. Exp Mol Med. 2016;48:e215. doi: 10.1038/emm.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gerhard GS, et al. Gene expression profiling in subcutaneous, visceral and epigastric adipose tissues of patients with extreme obesity. Int J Obesity. 2014;38:371. doi: 10.1038/ijo.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schleinitz D, et al. The genetics of fat distribution. Diabetologia. 2014;57:1276. doi: 10.1007/s00125-014-3214-z. [DOI] [PubMed] [Google Scholar]
- 141.Park YM. Adipose tissue inflammation and metabolic dysfunction: role of exercise. Mo Med. 2014;111(1):65–72. [PMC free article] [PubMed] [Google Scholar]
- 142.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu T, et al. Adipose tissue macrophage derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem Biophys Res Commun. 2019;515:352–358. doi: 10.1016/j.bbrc.2019.05.113. [DOI] [PubMed] [Google Scholar]
- 144.Ying W, et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171(2):372–384. doi: 10.1016/j.cell.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 145.Cai Y, et al. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther. 2020;11(1):312. doi: 10.1186/s13287-020-01831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shukla L, et al. Fat therapeutics: the clinical capacity of adipose-derived stem cells and exosomes for human disease and tissue regeneration. Front Pharmacol. 2020;11:158. doi: 10.3389/fphar.2020.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fish JE, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Togliatto G, et al. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell derived extracellular vesicles (EVs) by impairing miR-126 content: impact on clinical applications. Int J Obes. 2016;40(1):102–111. doi: 10.1038/ijo.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao H, et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67(2):235–247. doi: 10.2337/db17-0356. [DOI] [PubMed] [Google Scholar]
- 150.Woo CH, et al. mall extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J Extracell Vesicles. 2020;9:1735249. doi: 10.1080/20013078.2020.1735249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao H, et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;57(2):235. doi: 10.2337/db17-0356. [DOI] [PubMed] [Google Scholar]
- 152.Zhou Y, Tan C. miRNAs in adipocyte-derived extracellular vesicles: multiple roles in development of obesity-associated disease. Front Mol Biosci. 2020;7:171. doi: 10.3389/fmolb.2020.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]