To the Editor: Previous studies have shown that the BNT162b2 (Pfizer–BioNTech), mRNA-1273 (Moderna), and Ad26.COV2.S (Johnson & Johnson–Janssen) vaccines provide robust protective efficacy against coronavirus disease 2019 (Covid-19). Here, we report comparative kinetics of humoral and cellular immune responses elicited by the two-dose BNT162b2 vaccine (in 31 participants), the two-dose mRNA-1273 vaccine (in 22 participants), and the one-dose Ad26.COV2.S vaccine (in 8 participants). We evaluated antibody and T-cell responses from peak immunity at 2 to 4 weeks after the second immunization in recipients of the messenger RNA (mRNA) vaccines or after the first immunization in recipients of the Ad26.COV2.S vaccine to 8 months (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org).
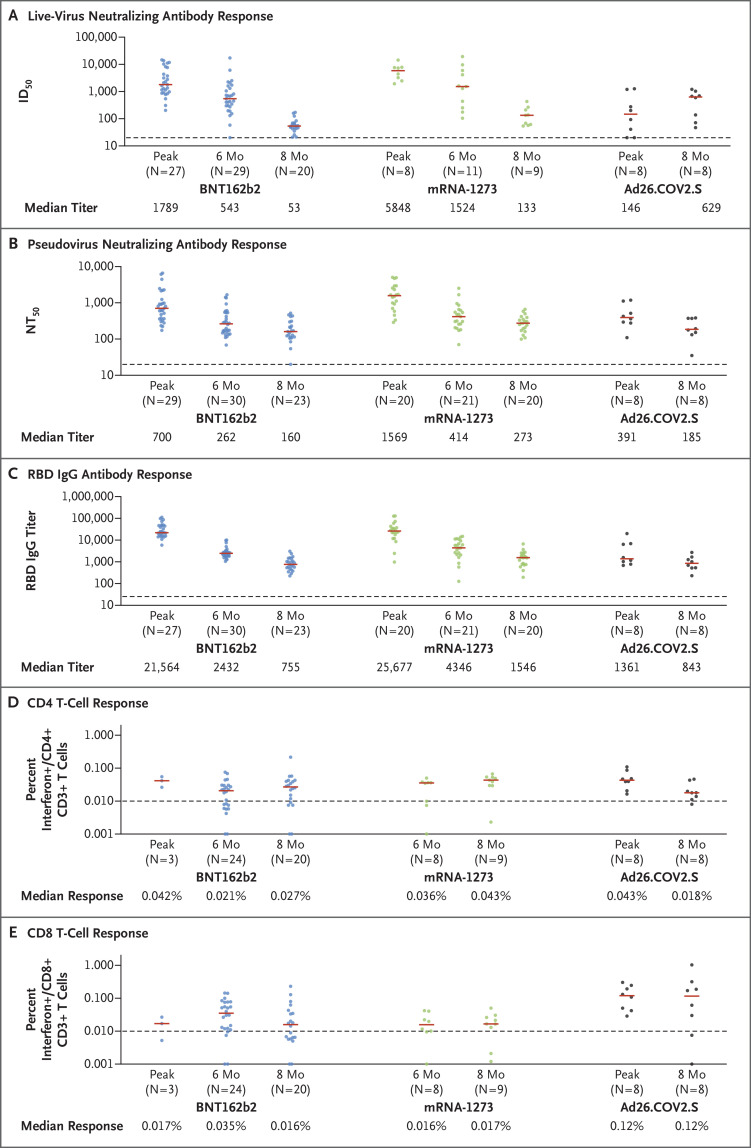
At peak immunity, the BNT162b2 vaccine induced a high median live-virus neutralizing antibody titer (1789), a high median pseudovirus neutralizing antibody titer (700), and a high median binding antibody titer against the receptor-binding domain (RBD) (21,564). However, these titers declined sharply by 6 months after vaccination, as previously reported,1,2 and they declined further by 8 months (Figure 1A through 1C, S1, and S2). By 8 months after BNT162b2 vaccination, the median live-virus neutralizing antibody titer (53), pseudovirus neutralizing antibody titer (160), and RBD-specific binding antibody titer (755) elicited by the vaccine were lower than the peak titers by a factor of 34, 4, and 29, respectively.
Figure 1. Kinetics of Humoral and Cellular Immune Responses Elicited by the BNT162b2, mRNA-1273, and Ad26.COV2.S Vaccines.
Shown are immune responses after vaccination with BNT162b2, mRNA-1273, and Ad26.COV2.S at peak immunity (2 to 4 weeks after the second dose in recipients of the messenger RNA vaccines or 4 weeks after one dose in recipients of the Ad26.COV2.S vaccine) and at 6 months, 8 months, or both after the first dose. Panel A shows the serum 50% inhibitory dilution (ID50) titers in the live-virus neutralizing antibody assay. Red bars indicate medians, dashed lines the limit of detection for each assay, and each dot a single participant. Panel B shows the serum dilution for 50% reduction (NT50) expressed in relative light units in the pseudovirus neutralizing antibody assay. Panel C shows the binding IgG antibody titers against the receptor-binding domain (RBD) in the serum enzyme-linked immunosorbent assay. Intracellular cytokine-staining assays were performed to measure the percentage of interferon-γ production in T cells; Panel D shows this percentage in CD4+ T cells, and Panel E shows this percentage in CD8+ T cells. Flow cytometric gating was performed to identify T cells (which are CD3+) rather than other CD4+- or CD8+-expressing immune cells. All assays were performed with the use of the SARS-CoV-2 WA1/2020 strain. The Ad26.COV2.S vaccine data in Panels B through E were published previously3 and are included here for comparative purposes.
At peak immunity, the mRNA-1273 vaccine also elicited a high median live-virus neutralizing antibody titer (5848), pseudovirus neutralizing antibody titer (1569), and RBD-specific binding antibody titer (25,677). By 8 months after mRNA-1273 vaccination, the median live-virus neutralizing antibody titer was 133, the pseudovirus neutralizing antibody titer was 273, and the median RBD-specific binding antibody titer was 1546; these titers were lower than the peak titers by a factor of 44, 6, and 17, respectively.
The Ad26.COV2.S vaccine induced substantially lower median titers than the mRNA vaccines at peak immunity. At 4 weeks after single-shot Ad26.COV2.S immunization, the median live-virus neutralizing antibody titer was 146, the median pseudovirus neutralizing antibody titer was 391, and the median RBD-specific binding antibody titer was 1361; however, these titers remained relatively stable over 8 months.3 At 8 months, the median live-virus neutralizing antibody titer was 629, the median pseudovirus neutralizing antibody titer was 185, and the median RBD-specific binding antibody titer was 843; these titers were similar to the titers at week 4. With all three vaccines, there were generally stable antibody-dependent cellular phagocytosis and antibody-dependent complement deposition responses (Fig. S3).
Recipients of the BNT162b2 and mRNA-1273 vaccines also had decreases in titers of live-virus neutralizing antibodies, pseudovirus neutralizing antibodies, and RBD- and spike protein (S)–specific binding antibody responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants from peak immunity to 8 months; after Ad26.COV2.S vaccination, however, there were stable or in some cases increasing antibody titers against these variants (Figs. S4 and S5). At 8 months, the median pseudovirus neutralizing antibody titers against the SARS-CoV-2 B.1.617.2 (delta) variant were similar with the BNT162b2 vaccine (67), the mRNA-1273 vaccine (76), and the Ad26.COV2.S vaccine (107).
T-cell responses were assessed by CD4+ and CD8+ intracellular cytokine-staining assays that used pooled S peptides for stimulation (Figure 1D and 1E). At 8 months, the median CD8+ T-cell responses were 0.016% with the BNT162b2 vaccine, 0.017% with the mRNA-1273 vaccine, and 0.12% with the Ad26.COV2.S vaccine. With all three vaccines, T-cell responses showed broad cross-reactivity against SARS-CoV-2 variants (Fig. S6).
These data show differential kinetics of immune responses induced by the mRNA and Ad26.COV2.S vaccines over an 8-month follow-up period. As shown in previous studies,1,2 the BNT162b2 and mRNA-1273 vaccines were characterized by high peak antibody responses that declined sharply by 6 months; these responses declined further by 8 months. Antibody titers in recipients of the mRNA-1273 vaccine were generally higher than those in recipients of the BNT162b2 vaccine. The Ad26.COV2.S vaccine induced lower initial antibody responses, but these responses were relatively stable over the 8-month follow-up period, with minimal-to-no evidence of decline.3 These findings have important implications for waning vaccine immunity, although correlates of protection from SARS-CoV-2 are not yet defined.
Supplementary Appendix
Disclosure Forms
Requests for access to the study data can be submitted to Dr. Barouch at dbarouch@bidmc.harvard.edu.
This letter was published on October 15, 2021, at NEJM.org.
Footnotes
Supported by Janssen Vaccines and Prevention; a grant (CA260476, to Dr. Barouch) from the National Institutes of Health; the Massachusetts Consortium on Pathogen Readiness; the Ragon Institute of MGH, MIT, and Harvard; a grant (to Dr. Barouch) from the Musk Foundation; a grant (HD000849, to Dr. Collier) from the Reproductive Scientist Development Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Burroughs Wellcome Fund; a grant (TR002541, to Dr. Hacker) from the Harvard Clinical and Translational Science Center; and a fellowship from the Hanna H. Gray Fellows Program from the Howard Hughes Medical Institute and an award from the Postdoctoral Enrichment Program of the Burroughs Wellcome Fund (both to Dr. Martinez). The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Pegu A, O’Connell SE, Schmidt SD, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021;373:1372-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falsey AR, Frenck RW Jr, Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. DOI: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch DH, Stephenson KE, Sadoff J, et al. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med 2021;385:951-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.