Abstract
Background
Reports have suggested an association between the development of myocarditis and the receipt of messenger RNA (mRNA) vaccines against coronavirus disease 2019 (Covid-19), but the frequency and severity of myocarditis after vaccination have not been extensively explored.
Methods
We searched the database of Clalit Health Services, the largest health care organization (HCO) in Israel, for diagnoses of myocarditis in patients who had received at least one dose of the BNT162b2 mRNA vaccine (Pfizer–BioNTech). The diagnosis of myocarditis was adjudicated by cardiologists using the case definition used by the Centers for Disease Control and Prevention. We abstracted the presentation, clinical course, and outcome from the patient’s electronic health record. We performed a Kaplan–Meier analysis of the incidence of myocarditis up to 42 days after the first vaccine dose.
Results
Among more than 2.5 million vaccinated HCO members who were 16 years of age or older, 54 cases met the criteria for myocarditis. The estimated incidence per 100,000 persons who had received at least one dose of vaccine was 2.13 cases (95% confidence interval [CI], 1.56 to 2.70). The highest incidence of myocarditis (10.69 cases per 100,000 persons; 95% CI, 6.93 to 14.46) was reported in male patients between the ages of 16 and 29 years. A total of 76% of cases of myocarditis were described as mild and 22% as intermediate; 1 case was associated with cardiogenic shock. After a median follow-up of 83 days after the onset of myocarditis, 1 patient had been readmitted to the hospital, and 1 had died of an unknown cause after discharge. Of 14 patients who had left ventricular dysfunction on echocardiography during admission, 10 still had such dysfunction at the time of hospital discharge. Of these patients, 5 underwent subsequent testing that revealed normal heart function.
Conclusions
Among patients in a large Israeli health care system who had received at least one dose of the BNT162b2 mRNA vaccine, the estimated incidence of myocarditis was 2.13 cases per 100,000 persons; the highest incidence was among male patients between the ages of 16 and 29 years. Most cases of myocarditis were mild or moderate in severity. (Funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.)
In Israel, a nationwide campaign to administer the BNT162b2 messenger RNA (mRNA) vaccine (Pfizer–BioNTech) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started on December 20, 2020. By May 5, 2021, a total of 58.2% of the Israeli population had received at least one dose of vaccine and 54.6% were fully vaccinated.1 On May 27, 2021, the Centers for Disease Control and Prevention (CDC) issued a statement regarding a possible link between vaccination to prevent coronavirus disease 2019 (Covid-19) and myocarditis for both the BNT162b2 vaccine and the mRNA-1273 vaccine (Moderna).2 On June 2, 2021, the Israeli Ministry of Health reported that 148 cases of myocarditis had been identified in Israel between December 2020 and May 2021, around the time of the initiation of the vaccination program. The report noted a possible link between the second vaccine dose and myocarditis among male patients between the ages of 16 and 30 years.3 More recently, researchers in Israel reported that vaccination increased the 42-day risk of myocarditis by a factor of 3.24 (95% confidence interval [CI], 1.55 to 12.44) as compared with the risk among unvaccinated persons, events that were mostly concentrated among young male patients.4
We evaluated the incidence of myocarditis after the receipt of the BNT162b2 mRNA vaccine in a single health care organization (HCO) in Israel and described the clinical course and disease severity from a review of patients’ charts.
Methods
Patients and Oversight
The study was based on the database of Clalit Health Services, the largest HCO in Israel. This health care system provides care for 4.7 million patients (52% of the total population), with membership that is approximately representative of the Israeli population with respect to both socioeconomic status and prevalence of coexisting diseases.5 Outpatient care for patients is provided by the organization, whereas inpatient care is provided by both in-network and out-of-network hospitals. The database that we used in this study has been described previously.6 All data regarding SARS-CoV-2 vaccination, testing, and diagnosis are collected centrally by the Israeli Ministry of Health and are shared daily with health care providers.
The study was approved by the institutional review board at Clalit Health Services. Since the study was based on retrospective data, it was exempt from the provision of patients’ written informed consent. The authors designed the study and collected the data. A subgroup of authors wrote the initial draft of the manuscript. Pfizer–BioNTech had no involvement in the study.
Study Design and Data Collection
In this retrospective cohort study, we evaluated patients who were enrolled in Clalit Health Services and who had been vaccinated during the period from December 20, 2020, through May 24, 2021. We identified suspected cases of myocarditis that had occurred within 42 days after the first dose of the vaccine by searching for codes used in the International Classification of Diseases, Ninth Revision (ICD-9; codes 422, 429.0, 398.0, and 391.2 and their respective subcodes) in electronic health records. A follow-up period of 42 days after the first dose was chosen to allow for approximately 21 days of follow-up after each of the two vaccine doses.
Each suspected case was adjudicated through review of the patient’s electronic health record. A patient was considered to have had myocarditis if the CDC case definition for suspected, probable, or confirmed myocarditis had been met. The case definition had originally been written in 2003 for use in determining the incidence of medical disorders after the administration of the smallpox vaccine7 (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). We then abstracted data from the index hospitalization and from subsequent health records. A description of the identification, adjudication, and abstraction process is provided in the Methods section in the Supplementary Appendix.
Myocarditis was classified as fulminant or nonfulminant, according to the definition used by the American Heart Association.8 Cases of nonfulminant myocarditis were further classified as mild or intermediate, according to published definitions.9 The definitions that were used for the level of severity of myocarditis and left ventricular dysfunction are provided in Table S2.
Statistical Analysis
To estimate the incidence of myocarditis, patients were followed for 42 days after vaccination. In case of death from unrelated causes, termination of membership in the HCO, or the end of the study period (May 24, 2021), the patients’ data were censored. A competing-risks analysis was not performed because death occurred in less than 0.01% of the patients.
We performed Kaplan–Meier analysis to estimate the cumulative incidence of myocarditis and of myocarditis at each severity level at 42 days after the first vaccine dose, stratified according to age and sex. Categorical variables were summarized with the use of counts (percentages), and continuous variables were summarized with the use of means (±SD) if the distribution appeared to be symmetrical or with the use of medians and interquartile ranges if the distribution was not symmetrical. We handled missing data according to the phase of the analysis: for adjudication, a patient for whom data were not available to meet the study criteria was not considered to have had a confirmed case of myocarditis; after adjudication, information for each specific variable is presented only for patients for whom data regarding that variable were available.
Results
Patients
Between December 20, 2020, and May 24, 2021, a total of 2,558,421 Clalit Health Services members received at least one dose of the BNT162b2 mRNA Covid-19 vaccine; of these patients, 2,401,605 (94%) received two doses. Initially, 159 potential cases of myocarditis were identified according to ICD-9 codes during the 42 days after receipt of the first vaccine dose. After adjudication, 54 of these cases were deemed to have met the study criteria for a diagnosis of myocarditis. Of these cases, 41 were classified as mild in severity, 12 as intermediate, and 1 as fulminant.
Of the 105 cases that did not meet the study criteria for a diagnosis of myocarditis, 78 were recodings of previous diagnoses of myocarditis without a new event, 16 did not have sufficient available data to meet the diagnostic criteria, and 7 preceded the first vaccine dose; in 4 cases, a diagnosis of a condition other than myocarditis was determined to be more likely (Fig. S1). Community health records were available for all the patients who had been identified as potentially having had myocarditis. Discharge summaries from the index hospitalization were available for 55 of 81 potential cases (68%) that were not recoding events and for 38 of 54 cases (70%) that met the study criteria.
The characteristics of the patients with myocarditis are provided in Table 1. The median age of the patients was 27 years (interquartile range [IQR], 21 to 35), and 94% were boys and men. Two patients had contracted Covid-19 before they received the vaccine (125 days and 186 days earlier, respectively). Most patients (83%) had no coexisting medical conditions; 13% were receiving treatment for chronic diseases. One patient had mild left ventricular dysfunction before vaccination.
Table 1. Characteristics of the Study Population and Myocarditis Cases at Baseline.*.
| Characteristic | Study Population (N=2,558,421) |
Patients with Myocarditis (N=54) |
|---|---|---|
| Median age (IQR) — yr | 44 (30–63) | 27 (21–35) |
| Sex — no. (%) | ||
| Female | 1,309,988 (51) | 3 (6) |
| Male | 1,248,433 (49) | 51 (94) |
| Coexisting illness — no. (%)† | ||
| Any | 9 (17) | |
| Diabetes mellitus | — | 1 (2) |
| Hypertension | — | 7 (13) |
| Dyslipidemia | — | 5 (9) |
| Coronary artery disease | — | 1 (2) |
| Previous pericarditis | — | 1 (2) |
| Known left ventricular dysfunction | — | 1 (2) |
| Medication use — no. (%)† | ||
| Any | 7 (13) | |
| Aspirin | — | 2 (4) |
| P2Y12 inhibitor | — | 1 (2) |
| Beta-blocker | — | 1 (2) |
| ACE inhibitor or ARB | — | 4 (7) |
| Statin | — | 4 (7) |
| Proton-pump inhibitor | — | 1 (2) |
| Insulin | — | 1 (2) |
| Oral hypoglycemic agent | — | 1 (2) |
ACE denotes angiotensin-converting enzyme, ARB angiotensin-receptor blocker, and IQR interquartile range.
Data regarding coexisting medical conditions and medication use were abstracted from structured fields in notes regarding the patient’s index hospitalization and follow-up. Accordingly, such data were not available for the entire study population. None of the patients had a history of myocarditis.
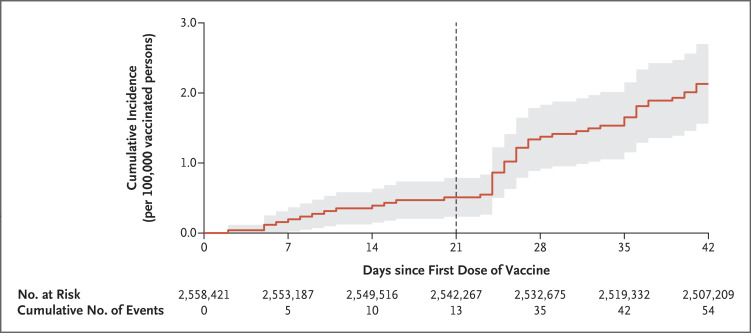
Among the patients with myocarditis, 37 (69%) received the diagnosis after the second vaccine dose, with a median interval of 21 days (IQR, 21 to 22) between doses. A cumulative incidence curve of myocarditis after vaccination is shown in Figure 1. The distribution of the days since vaccination until the occurrence of myocarditis is shown in Figure S2. Both figures show events occurring throughout the postvaccination period and indicate an increase in incidence after the second dose.
Figure 1. Kaplan–Meier Estimates of Myocarditis at 42 Days.
Shown is the cumulative incidence of myocarditis during a 42-day period after the receipt of the first dose of the BNT162b2 messenger RNA coronavirus disease 2019 (Covid-19) vaccine. A diagnosis of myocarditis was made in 54 patients in an overall population of 2,558,421 vaccinated persons enrolled in the largest health care organization in Israel. The vertical line at 21 days shows the median day of administration of the second vaccine dose. The shaded area shows the 95% confidence interval.
Incidence of Myocarditis
The overall estimated incidence of myocarditis within 42 days after the receipt of the first dose per 100,000 vaccinated persons was 2.13 cases (95% confidence interval [CI], 1.56 to 2.70), which included an incidence of 4.12 (95% CI, 2.99 to 5.26) among male patients and 0.23 (95% CI, 0 to 0.49) among female patients (Table 2). Among all the patients between the ages of 16 and 29 years, the incidence per 100,000 persons was 5.49 (95% CI, 3.59 to 7.39); among those who were 30 years of age or older, the incidence was 1.13 (95% CI, 0.66 to 1.60). The highest incidence (10.69 cases per 100,000 persons; 95% CI, 6.93 to 14.46) was observed among male patients between the ages of 16 and 29 years. In the overall population, the incidence per 100,000 persons according to disease severity was 1.62 (95% CI, 1.12 to 2.11) for mild myocarditis, 0.47 (95% CI, 0.21 to 0.74) for intermediate myocarditis, and 0.04 (95% CI, 0 to 0.12) for fulminant myocarditis. Within each disease-severity stratum, the incidence was higher in male patients than in female patients and higher in those between the ages of 16 and 29 than in those who were 30 years of age or older.
Table 2. Incidence of Myocarditis 42 Days after Receipt of the First Vaccine Dose, Stratified According to Age, Sex, and Disease Severity.*.
| Population | Total Study Population |
All Cases of Myocarditis | Fulminant Myocarditis | Intermediate Myocarditis | Mild Myocarditis | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Cases |
Cumulative Incidence (95% CI) |
No. of Cases |
Cumulative Incidence (95% CI) |
No. of Cases |
Cumulative Incidence (95% CI) |
No. of Cases |
Cumulative Incidence (95% CI) |
||
| no. | no./100,000 persons |
no./100,000 persons |
no./100,000 persons |
no./100,000 persons |
|||||
| All vaccinated patients | 2,558,421 | 54 | 2.13 (1.56–2.70) |
1 | 0.04 (0–0.12) |
12 | 0.47 (0.21–0.74) |
41 | 1.62 (1.12–2.11) |
| Male sex | 1,248,433 | 51 | 4.12 (2.99–5.26) |
1 | 0.08 (0–0.24) |
11 | 0.89 (0.36–1.42) |
39 | 3.15 (2.16–4.14) |
| Female sex | 1,309,988 | 3 | 0.23 (0–0.49) |
0 | 0 | 1 | 0.08 (0–0.23) |
2 | 0.15 (0–0.37) |
| Sex and age group | |||||||||
| Either sex, 16–29 yr | 593,648 | 32 | 5.49 (3.59–7.39) |
1 | 0.17 (0–0.50) |
6 | 1.03 (0.21–1.85) |
25 | 4.29 (2.61–5.97) |
| Either sex, ≥30 yr | 1,964,773 | 22 | 1.13 (0.66–1.60) |
0 | 0 | 6 | 0.31 (0.06–0.56) |
16 | 0.82 (0.42–1.22) |
| Male sex, 16–29 yr | 295,288 | 31 | 10.69 (6.93–14.46) |
1 | 0.34 (0–1.01) |
6 | 2.06 (0.41–3.72) |
24 | 8.29 (4.97–11.61) |
| Male sex, ≥30 yr | 953,145 | 20 | 2.11 (1.19–3.04) |
0 | 0 | 5 | 0.53 (0.07–0.99) |
15 | 1.58 (0.78–2.38) |
| Female sex, 16–29 yr | 298,360 | 1 | 0.34 (0–1) |
0 | 0 | 0 | 0 | 1 | 0.34 (0–1) |
| Female sex, ≥30 yr | 1,011,628 | 2 | 0.20 (0–0.48) |
0 | 0 | 1 | 0.10 (0–0.30) |
1 | 0.10 (0–0.29) |
The cumulative incidence per 100,000 persons was estimated with the use of the Kaplan–Meier method.
Clinical and Laboratory Findings
The clinical and laboratory features of myocarditis are shown in Table 3 and Table S3. The presenting symptom was chest pain in 82% of cases. Vital signs on admission were generally normal; 1 patient presented with hemodynamic instability, and none required inotropic or vasopressor support or mechanical circulatory support on presentation. Electrocardiography (ECG) at presentation showed ST-segment elevation in 20 of 38 patients (53%) for whom ECG data were available on admission; the results on ECG were normal in 8 of 38 patients (21%), whereas minor abnormalities (including T-wave changes, atrial fibrillation, and nonsustained ventricular tachycardia) were detected in the rest of the patients. The median peak troponin T level was 680 ng per liter (IQR, 275 to 2075) in 41 patients with available data, and the median creatine kinase level was 487 U per liter (IQR, 230 to 1193) in 28 patients with available data.
Table 3. Presentation, Clinical Course, and Follow-up of 54 Patients with Myocarditis after Vaccination.*.
| Variable | Value |
|---|---|
| Presenting symptoms and signs — no./total no. (%) | |
| Chest pain | 44/54 (81) |
| Palpitations | 1/54 (2) |
| Dyspnea | 3/54 (6) |
| Fever | 5/54 (9) |
| Pericardial effusion | 10/49 (20) |
| Vital signs on admission | |
| Temperature — °C | 37.4±1.0 |
| Blood pressure — mm Hg | |
| Systolic | 122.7±16.8 |
| Diastolic | 72.2±11.0 |
| Heart rate — beats per min | 81.3±17.3 |
| Shock — no./total no. (%) | 1/47 (2) |
| Electrocardiographic findings — no./total no. (%) | |
| Normal | 8/38 (21) |
| ST-segment elevation | |
| Diffuse | 18/38 (47) |
| Nondiffuse | 2/38 (5) |
| T-wave change | 7/38 (18) |
| Atrial fibrillation | 1/38 (3) |
| Nonsustained ventricular tachycardia | 2/38 (5) |
| Laboratory values† | |
| Elevated troponin T — no./total no. (%) | 41/41 (100) |
| Median creatine kinase (IQR) — U/liter | 487 (230–1193) |
| Clinical course during index hospitalization — no./total no. (%) | |
| Need for inotropes or vasopressors | 1/49 (2) |
| Need for mechanical circulatory support | 1/49 (2) |
| Arrhythmias | 1/49 (2) |
Plus–minus values are means ±SD. Data for temperature, blood pressure, and heart rate were available in 37 patients and for the creatine kinase level in 28 patients.
Reference ranges for the laboratory tests are as follows: troponin T, 0 to 14 ng per liter; and creatine kinase, 20 to 180 U per liter. Additional data regarding presentation, clinical course, and follow-up are provided in Table S3.
During hospitalization, cardiogenic shock leading to extracorporeal membrane oxygenation developed in 1 patient. None of the other patients required inotropic or vasopressor support or mechanical ventilation. However, 5% had nonsustained ventricular tachycardia, and 3% had atrial fibrillation. A myocardial biopsy sample obtained from 1 patient showed perivascular infiltration of lymphocytes and eosinophils. The median length of hospital stay was 3 days (IQR, 2 to 4). Overall, 65% of the patients were discharged from the hospital without any ongoing medical treatment.
A patient with preexisting cardiac disease died the day after discharge from an unspecified cause. One patient who had a history of pericarditis and had been admitted to the hospital with myocarditis had three more admissions for recurrent pericarditis, with no further myocardial involvement after the initial episode. Additional clinical descriptions are provided in Table S4.
Echocardiography and Other Cardiac Imaging
Echocardiographic findings were available for 48 of 54 patients (89%) (Table S5). Among these patients, left ventricular function was normal on admission in 71% of the patients. Of the 14 patients (29%) who had any degree of left ventricular dysfunction, 17% had mild dysfunction, 4% mild-to-moderate dysfunction, 4% moderate dysfunction, 2% moderate-to-severe dysfunction, and 2% severe dysfunction. Among the 14 patients with some degree of left ventricular dysfunction at presentation, follow-up echocardiography during the index admission showed normal function in 4 patients and similar dysfunction in the other 10. The mean left ventricular function at discharge was 57.5±6.1%, which was similar to the mean value at presentation. At a median follow-up of 25 days (IQR, 14 to 37) after discharge, echocardiographic follow-up was available for 5 of the 10 patients in whom the last left ventricular assessment before discharge had shown some degree of dysfunction. Of these patients, all had normal left ventricular function; follow-up results on echocardiography were not available for the other 5 patients.
Cardiac magnetic resonance imaging was performed in 15 patients (28%): in 5 patients during the initial admission and in 10 patients at a median of 44 days (IQR, 21 to 70) after discharge. In all cases, left ventricular function was normal, with a mean ejection fraction of 61±6%. Data from quantitative assessment of late gadolinium enhancement were available in 11 patients, with a median value of 5% (IQR, 1 to 15) (Table S6).
Discussion
In this study, we estimated the incidence and clinical course of myocarditis after receipt of the BNT162b2 mRNA Covid-19 vaccine in a large Israeli health provider database. Using published diagnostic criteria, we estimated the incidence of myocarditis to be 2.13 cases per 100,000 vaccinated persons in the 42 days after the first vaccine dose. The highest incidence was observed in male patients between the ages of 16 and 29 years. Most cases were mild or moderate in severity. The diagnosis of myocarditis occurred throughout the postvaccination period, but there appeared to be an increase approximately 3 to 5 days after the second vaccine dose. One patient had cardiogenic shock, and one patient with preexisting cardiac disease died of an unknown cause soon after hospital discharge. Left ventricular dysfunction occurred initially in 29% of the patients with myocarditis who underwent echocardiography; of the patients who underwent additional testing after discharge, the left ventricular function had normalized. The follow-up period in our cohort (median, 83 days) was too short to ascertain the long-term prognosis of patients with myocarditis after vaccination.
Although we cannot directly compare the incidence of myocarditis after vaccination in our study with the incidence in other studies, our data may provide points of reference. On the basis of data from the Vaccine Adverse Events Reporting System, the CDC has estimated that the incidence of myocarditis after any Covid-19 vaccination is 0.48 cases per 100,000 overall and 1.2 cases per 100,000 among vaccine recipients between the ages of 18 and 29 years.10 These estimates are lower than those in our study, possibly due to different methods that were used to identify cases (passive reporting to the CDC vs. electronic health records in our HCO). Other differences between the two estimates include the age of patients included in each study and different time windows for the detection of adverse events. A report from the U.S. military Covid-19 vaccination campaign noted an incidence of 8.2 cases of myocarditis per 100,000 male service members (a total of 23 cases), an estimate that is approximately double the number among men of all ages in our study. Investigators in the military study identified left ventricular dysfunction in 17% of the men (with all cases in the mild or mild-to-moderate range),11 as compared with 29% in our study, a difference that may reflect different age groups, different coexisting conditions, and a shorter follow-up time in the military study.
In the Israeli study by Barda et al.,4 vaccination resulted in 2.7 excess cases of myocarditis per 100,000 vaccinated persons. Although this study used the same HCO database that was used in our study, the incidence estimates between the two studies are not identical. The primary reason for this difference is the different age distributions in the two study populations, coupled with the large heterogeneity of postvaccination myocarditis with respect to age, since the median age was 44 years (IQR, 30 to 63) in our study and 38 years (IQR, 27 to 53) in the study by Barda et al. Another difference between the studies was the method by which cases were ascertained. Barda et al. relied on diagnosis codes to identify cases, whereas we also performed manual adjudication and counted only cases for which sufficient data were available to satisfy the diagnostic criteria of the CDC.
Our study has several limitations. First, a definitive diagnosis of myocarditis is based on the results of endomyocardial biopsy, which was performed in only one patient. The definition of myocarditis that was used in our study does not establish the diagnosis, although it corresponds to the way in which myocarditis is diagnosed in clinical practice. Second, cases could have been missed if the diagnosis had been made in an out-of-network hospital and the reporting to the insurer had been delayed or if the diagnosis had not been entered into the outpatient medical record. In addition, some patients with myocarditis might not have fulfilled the study criteria for the diagnosis because of our lack of access to details regarding the index hospitalization or the lack of recording of specific data in the discharge notes. These issues would presumably lead to underestimating the incidence of myocarditis. Third, data were missing for many of the items related to the clinical course. Fourth, because of the lack of a simultaneously enrolled comparator group, no inferences can be made regarding causality between the vaccine and subsequent development of myocarditis. Finally, the study design did not call for the collection of data regarding the incidence of myocarditis after Covid-19.
In this retrospective cohort study involving persons who were 16 years of age or older in a large Israeli health care system, the estimated incidence of myocarditis in the 42 days after receipt of at least one dose of the BNT162b2 mRNA vaccine was 2.13 cases per 100,000 vaccinated persons and 10.69 cases per 100,000 in male patients between the ages of 16 and 29 years. Most cases of myocarditis were of mild or intermediate severity.
Acknowledgments
We thank Shmuel Banai, M.D., Roy Beigel, M.D., Tal Hasin, M.D., Ariel Roguin, M.D., and Ofra Maimon, M.D., for their assistance with the study.
Supplementary Appendix
Disclosure Forms
This article was published on October 6, 2021, at NEJM.org.
Footnotes
Supported by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Israel Ministry of Health. National Covid-19 data dashboard (https://datadashboard.health.gov.il/COVID-19/general). (In Hebrew).
- 2.Centers for Disease Control and Prevention. Myocarditis and pericarditis following mRNA COVID-19 vaccination. 2021. (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html).
- 3.Surveillance of myocarditis (inflammation of the heart muscle) cases between December 2020 and May 2021 (including). Press release of the Israeli Ministry of Health, June 2021 (https://www.gov.il/en/departments/news/01062021-03).
- 4.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2021;385:1078-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen R, Rabin H. Membership in the health funds. 2017. (https://www.btl.gov.il/Publications/survey/Documents/seker289/seker_289.pdf). (In Hebrew).
- 6.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Update: cardiac-related events during the civilian smallpox vaccination program — United States, 2003. MMWR Morb Mortal Wkly Rep 2003;52:492-496. [PubMed] [Google Scholar]
- 8.Kociol RD, Cooper LT, Fang JC, et al. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation 2020;141(6):e69-e92. [DOI] [PubMed] [Google Scholar]
- 9.Sinagra G, Anzini M, Pereira NL, et al. Myocarditis in clinical practice. Mayo Clin Proc 2016;91:1256-1266. [DOI] [PubMed] [Google Scholar]
- 10.Wallace M, Oliver S. COVID-19 mRNA vaccines in adolescents and young adults: benefit-risk discussion. Presented at the Advisory Committee on Immunization Practices Meeting, Atlanta, June 23–25, 2021. (https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/05-COVID-Wallace-508.pdf). [Google Scholar]
- 11.Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol 2021. June 29 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.