Abstract
Background
Approximately 5.1 million Israelis had been fully immunized against coronavirus disease 2019 (Covid-19) after receiving two doses of the BNT162b2 messenger RNA vaccine (Pfizer–BioNTech) by May 31, 2021. After early reports of myocarditis during adverse events monitoring, the Israeli Ministry of Health initiated active surveillance.
Methods
We retrospectively reviewed data obtained from December 20, 2020, to May 31, 2021, regarding all cases of myocarditis and categorized the information using the Brighton Collaboration definition. We analyzed the occurrence of myocarditis by computing the risk difference for the comparison of the incidence after the first and second vaccine doses (21 days apart); by calculating the standardized incidence ratio of the observed-to-expected incidence within 21 days after the first dose and 30 days after the second dose, independent of certainty of diagnosis; and by calculating the rate ratio 30 days after the second dose as compared with unvaccinated persons.
Results
Among 304 persons with symptoms of myocarditis, 21 had received an alternative diagnosis. Of the remaining 283 cases, 142 occurred after receipt of the BNT162b2 vaccine; of these cases, 136 diagnoses were definitive or probable. The clinical presentation was judged to be mild in 129 recipients (95%); one fulminant case was fatal. The overall risk difference between the first and second doses was 1.76 per 100,000 persons (95% confidence interval [CI], 1.33 to 2.19), with the largest difference among male recipients between the ages of 16 and 19 years (difference, 13.73 per 100,000 persons; 95% CI, 8.11 to 19.46). As compared with the expected incidence based on historical data, the standardized incidence ratio was 5.34 (95% CI, 4.48 to 6.40) and was highest after the second dose in male recipients between the ages of 16 and 19 years (13.60; 95% CI, 9.30 to 19.20). The rate ratio 30 days after the second vaccine dose in fully vaccinated recipients, as compared with unvaccinated persons, was 2.35 (95% CI, 1.10 to 5.02); the rate ratio was again highest in male recipients between the ages of 16 and 19 years (8.96; 95% CI, 4.50 to 17.83), with a ratio of 1 in 6637.
Conclusions
The incidence of myocarditis, although low, increased after the receipt of the BNT162b2 vaccine, particularly after the second dose among young male recipients. The clinical presentation of myocarditis after vaccination was usually mild.
After the emergency use authorization of the BNT162b2 messenger RNA (mRNA) vaccine (Pfizer–BioNTech) against coronavirus disease 2019 (Covid-19) by the Food and Drug Administration,1 authorization was also granted for use in Israel. On December 20, 2020, a national vaccination campaign was initiated that was based on a two-dose regimen spaced 21 days apart.2 The campaign initially targeted health care workers and persons who were 60 years of age or older, and later the vaccine was offered to all persons who were at least 16 years of age. By May 31, 2021, approximately 5.12 million Israeli residents had received two vaccine doses.
At the beginning of the vaccination campaign, a program of passive surveillance was initiated for the monitoring of adverse events within 21 days after the first dose of vaccine and within 30 days after the second dose. Health care providers reported these data to the Ministry of Health, as required by Israeli law. After receipt of reports of myocarditis, the Ministry of Health subsequently initiated active surveillance beginning in February 2021 by requesting that all hospitals report cases of myocarditis, including cases that had been diagnosed since December 2020, with or without pericardial effusion and regardless of vaccination status. Since persons with suspected myocarditis are almost always hospitalized in Israel, such surveillance data should approximate all cases of myocarditis during the period of active surveillance.
The aims of the current study were to present the clinical and epidemiologic characteristics and follow-up findings of cases of myocarditis that were diagnosed in temporal proximity to vaccination and to examine a possible causal relationship between the vaccine and myocarditis.
Methods
Data Source and Case Definition
We retrospectively reviewed data regarding presumptive cases of myocarditis, including clinical and laboratory data and discharge summaries, from medical records obtained from the Ministry of Health database. The focus of the study was the 6 months from December 2020 through May 2021, which included periods of both active and passive surveillance. We used the codes for myocarditis (422.0-9x and 429.0x) of the International Classification of Diseases, 9th Revision (ICD-9), for screening. Records were reviewed by one of four board-certified cardiologists, with advice from a board-certified rheumatologist for verification of the diagnosis of myocarditis. All the reviewers were aware of the vaccination status of the patients.
The diagnostic criteria for myocarditis and degree of certainty of diagnosis were adapted from the case definition and classification of the Brighton Collaboration (Pandemic Emergency Response Process).3 Cases were classified as definitive, probable, possible, having insufficient data, or having an alternative diagnosis. Cases of pericarditis with myocarditis were included among these cases, although pericarditis alone was not included in case counts. We also compared the classification according to the Brighton Collaboration with classifications of myocarditis issued by the Centers for Disease Control and Prevention (CDC) for adverse events after smallpox vaccination.4-6 Additional details regarding the two classification systems are provided in the Methods section and Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Since the study was conducted as part of ongoing clinical surveillance for side effects related to the BNT162b2 vaccine as required by national guidelines, it received a waiver for review by an institutional review board. Pfizer–BioNTech had no role in the collection or analysis of the data or in the reporting of the data in this study.
Statistical Analysis
We used descriptive frequencies, percentages, means, and standard deviations to characterize cases of myocarditis according to age, sex, time elapsed since vaccination, length of hospital stay, and clinical outcome. Incidence curves were examined for the occurrence of new cases of myocarditis during the first 21 days after the first dose of vaccine and 30 days after the second dose, since passive surveillance had usually been terminated at that point. The data were analyzed separately for males and females and according to age group (16 to 19 years, 20 to 24 years, 25 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years or older). To assess the incidence of myocarditis among vaccine recipients, we calculated risk differences, observed-to-expected ratios, and rate ratios between vaccinated and unvaccinated persons.
To calculate the risk difference, we determined the risk of myocarditis per 100,000 persons after the first and second doses of vaccine according to age group and sex. This analysis included only the probable or definite myocarditis cases. In the calculation of the risk differences between the second and first doses, we used the cumulative incidence for a follow-up period of 21 days for both vaccine doses; we computed 95% confidence intervals for the risk difference using the Jeffreys–Perks method. The percentage of the myocarditis risk that could be attributed to the second dose was calculated by dividing the risk difference between the two vaccine doses by the risk after the second dose and expressing the quotient as a percentage.
We compared the observed incidence of myocarditis with the expected incidence using data obtained during the period from 2017 through 2019 in the pre–Covid-19 pandemic era by calculating standardized incidence ratios (after adjustment for age and sex) for all reported cases of myocarditis. We performed this analysis in all myocarditis cases that had occurred in temporal proximity to the vaccination without accounting for the adjudicated category of certainty, because historical cases of myocarditis had not been adjudicated by a team of clinical experts. We calculated approximate 95% confidence intervals for the true standardized incidence ratio by applying the Wilson and Hilferty approximation for chi-square percentiles.7 In addition, to determine whether the standardized incidence ratios could have been overestimated owing to the overreporting of myocarditis cases because of a higher index of clinical suspicion during the surveillance period, we performed a sensitivity analysis in which we determined the minimal number of observed cases that would be needed to produce a significant difference in the standardized incidence ratios for male recipients after the second vaccine dose. This subgroup was chosen post hoc according to the apparent increase in risk observed in male teenagers and young adults.
We compared the incidence of myocarditis among recipients 30 days after the second vaccine dose with the incidence among unvaccinated persons starting on January 11, 2021 (when second vaccine doses were first administered in Israel) up to May 31, 2021, with data reported according to age group and sex. We computed the rate ratio between vaccinated and unvaccinated persons and 95% confidence intervals for each stratum and for the overall study population after adjustment for age and sex using a negative binomial regression model. This analysis included only definite or probable myocarditis cases (Fig. S1).
Since we had no prespecified plan for adjustment of the width of confidence intervals for multiple comparisons in any of these approaches, no definite conclusions can be drawn from these data. We also assessed our findings according to the Bradford Hill causality criteria.
Results
Cases of Myocarditis
Among 9,289,765 Israeli residents who were included during the surveillance period, 5,442,696 received a first vaccine dose and 5,125,635 received two doses (Table 1 and Fig. S2). A total of 304 cases of myocarditis (as defined by the ICD-9 codes for myocarditis) were reported to the Ministry of Health (Table 2). These cases were diagnosed in 196 persons who had received two doses of the vaccine: 151 persons within 21 days after the first dose and 30 days after the second dose and 45 persons in the period after 21 days and 30 days, respectively. (Persons in whom myocarditis developed 22 days or more after the first dose of vaccine or more than 30 days after the second dose were considered to have myocarditis that was not in temporal proximity to the vaccine.) After a detailed review of the case histories, we ruled out 21 cases because of reasonable alternative diagnoses. Thus, the diagnosis of myocarditis was affirmed for 283 cases. These cases included 142 among vaccinated persons within 21 days after the first dose and 30 days after the second dose, 40 among vaccinated persons not in proximity to vaccination, and 101 among unvaccinated persons. Among the unvaccinated persons, 29 cases of myocarditis were diagnosed in those with confirmed Covid-19 and 72 in those without a confirmed diagnosis.
Table 1. Reported Myocarditis Cases, According to Timing of First or Second Vaccine Dose.*.
| Timing | First Vaccine Dose | Second Vaccine Dose | Both Doses | ||||
|---|---|---|---|---|---|---|---|
| No. of Vaccinations |
Myocarditis Cases |
Males/ Females |
No. of Vaccinations |
Myocarditis Cases |
Males/ Females |
Myocarditis Cases |
|
| Six-month study period | 5,442,696 | 19 | 17/2 | 5,125,635 | 117 | 101/16 | 136 |
| December 2020 | 987,013 | 0 | 0/0 | 0 | 0 | 0/0 | 0 |
| January 2021 | 2,109,854 | 4 | 3/1 | 1,844,896 | 13 | 12/1 | 17 |
| February 2021 | 1,613,909 | 6 | 5/1 | 1,546,184 | 47 | 41/6 | 53 |
| March 2021 | 528,069 | 7 | 7/0 | 1,397,609 | 44 | 38/6 | 51 |
| April 2021 | 152,765 | 1 | 1/0 | 253,701 | 13 | 10/3 | 14 |
| May 2021 | 51,086 | 1 | 1/0 | 83,245 | 0 | 0 | 1 |
Data are from medical records, including clinical and laboratory data and discharge summaries, from the Ministry of Health database from December 2020 through May 2021, according to the codes for myocarditis used in the International Classification of Diseases, 9th Revision. Cases of myocarditis were reported within 21 days after the first dose of vaccine and 30 days after the second dose. All cases were clinically reviewed, and only definite or probable cases are shown.
Table 2. Classification of Myocarditis Cases Reported to the Ministry of Health.*.
| Timing of Myocarditis Diagnosis | Brighton Collaboration Classification of Myocarditis | |||||
|---|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | All Levels | |
| number of cases | ||||||
| All cases | 118 | 153 | 3 | 9 | 21 | 304 |
| Vaccinated persons | ||||||
| ≤21 days after first dose and 30 days after second dose | 55 | 81 | 1 | 5 | 9 | 151 |
| >21 days after first dose and 30 days after second dose | 15 | 23 | 0 | 2 | 5 | 45 |
| Unvaccinated persons | 48 | 49 | 2 | 2 | 7 | 108 |
In the Brighton Collaboration classification system for the diagnosis of myocarditis, level 1 indicates definite, level 2 probable, level 3 possible, level 4 insufficient data, and level 5 ruled out. Included are data for persons who had a delayed second dose of vaccine and who received a diagnosis of myocarditis 22 days or longer after the first dose and those in whom myocarditis developed more than 30 days after the second dose, so the diagnosis was not considered to have been made in temporal proximity to vaccination.
Of the 142 persons in whom myocarditis developed within 21 days after the first dose of vaccine or within 30 days after the second dose, 136 received a diagnosis of definite or probable myocarditis, 1 received a diagnosis of possible myocarditis, and 5 had insufficient data. Classification of cases according to the definition of myocarditis used by the CDC 4-6 is provided in Table S1.
Endomyocardial biopsy samples that were obtained from 2 persons showed foci of endointerstitial edema and neutrophils, along with mononuclear-cell infiltrates (monocytes or macrophages and lymphocytes) with no giant cells. No other patients underwent endomyocardial biopsy. The clinical features of myocarditis after vaccination are provided in Table S3.
In the 136 cases of definite or probable myocarditis, the clinical presentation in 129 was generally mild, with resolution of myocarditis in most cases, as judged by clinical symptoms and inflammatory markers and troponin elevation, electrocardiographic and echocardiographic normalization, and a relatively short length of hospital stay. However, one person with fulminant myocarditis died. The ejection fraction was normal or mildly reduced in most persons and severely reduced in 4 persons. Magnetic resonance imaging that was performed in 48 persons showed findings that were consistent with myocarditis on the basis of at least one positive T2-based sequence and one positive T1-based sequence (including T2-weighted images, T1 and T2 parametric mapping, and late gadolinium enhancement). Follow-up data regarding the status of cases after hospital discharge and consistent measures of cardiac function were not available.
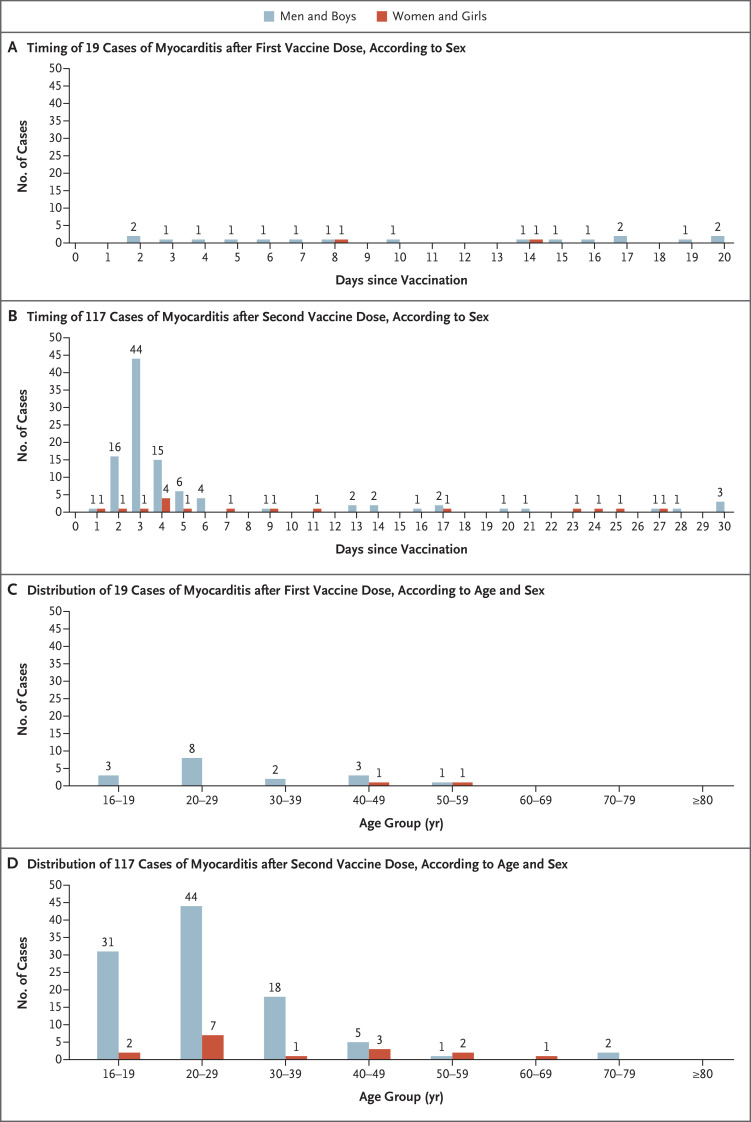
The peak number of cases with proximity to vaccination occurred in February and March 2021; the associations with vaccination status, age, and sex are provided in Table 1 and Figure 1. Of 136 persons with definite or probable myocarditis, 19 presented after the first dose of vaccine and 117 after the second dose. In the 21 days after the first dose, 19 persons with myocarditis were hospitalized, and hospital admission dates were approximately equally distributed over time. A total of 95 of 117 persons (81%) who presented after the second dose were hospitalized within 7 days after vaccination. Among 95 persons for whom data regarding age and sex were available, 86 (91%) were male and 72 (76%) were under the age of 30 years.
Figure 1. Timing and Distribution of Myocarditis after Receipt of the BNT162b2 Vaccine.
Shown is the timing of the diagnosis of myocarditis among recipients of the first dose of vaccine (Panel A) and the second dose (Panel B), according to sex, and the distribution of cases among recipients according to both age and sex after the first dose (Panel C) and after the second dose (Panel D). Cases of myocarditis were reported within 21 days after the first dose and within 30 days after the second dose.
Comparison of Risks According to First or Second Dose
A comparison of risks over equal time periods of 21 days after the first and second doses according to age and sex is provided in Table 3. Cases were clustered during the first few days after the second dose of vaccine, according to visual inspection of the data (Figure 1B and 1D). The overall risk difference between the first and second doses was 1.76 per 100,000 persons (95% confidence interval [CI], 1.33 to 2.19); the overall risk difference was 3.19 (95% CI, 2.37 to 4.02) among male recipients and 0.39 (95% CI, 0.10 to 0.68) among female recipients. The highest difference was observed among male recipients between the ages of 16 and 19 years: 13.73 per 100,000 persons (95% CI, 8.11 to 19.46); in this age group, the percent attributable risk to the second dose was 91%. The difference in the risk among female recipients between the first and second doses in the same age group was 1.00 per 100,000 persons (95% CI, −0.63 to 2.72). Repeating these analyses with a shorter follow-up of 7 days owing to the presence of a cluster that was noted after the second vaccine dose disclosed similar differences in male recipients between the ages of 16 and 19 years (risk difference, 13.62 per 100,000 persons; 95% CI, 8.31 to 19.03). These findings pointed to the first week after the second vaccine dose as the main risk window.
Table 3. Risk of Myocarditis within 21 Days after the First or Second Dose of Vaccine, According to Age and Sex.*.
| Age and Sex | First Dose | Second Dose | Risk Difference (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| Recipients | Cases | Risk per 100,000 Persons | Recipients | Cases | Risk per 100,000 Persons | ||
| number | number | no./100,000 persons | |||||
| Male recipients | |||||||
| All ages | 2,668,894 | 17 | 0.64 | 2,507,210 | 96 | 3.83 | 3.19 (2.37 to 4.02) |
| 16–19 yr | 224,518 | 3 | 1.34 | 199,115 | 30 | 15.07 | 13.73 (8.11 to 19.46) |
| 20–24 yr | 261,741 | 5 | 1.91 | 239,396 | 26 | 10.86 | 8.95 (4.42 to 13.55) |
| 25–29 yr | 246,638 | 3 | 1.22 | 228,988 | 16 | 6.99 | 5.77 (2.02 to 9.58) |
| 30–39 yr | 491,126 | 2 | 0.41 | 461,044 | 17 | 3.69 | 3.28 (1.41 to 5.18) |
| 40–49 yr | 458,268 | 3 | 0.65 | 433,069 | 5 | 1.15 | 0.50 (−0.82 to 1.84) |
| ≥50 yr | 986,603 | 1 | 0.10 | 945,598 | 2 | 0.21 | 0.11 (−0.29 to 0.52) |
| Female recipients | |||||||
| All ages | 2,773,802 | 2 | 0.07 | 2,618,425 | 12 | 0.46 | 0.39 (0.10 to 0.68) |
| 16–19 yr | 219,460 | 0 | 0 | 199,706 | 2 | 1.00 | 1.00 (−0.63 to 2.72) |
| 20–24 yr | 250,556 | 0 | 0 | 231,960 | 5 | 2.16 | 2.16 (0.13 to 4.24) |
| 25–29 yr | 235,575 | 0 | 0 | 219,113 | 0 | 0 | 0 (−0.83 to 0.89) |
| 30–39 yr | 481,045 | 0 | 0 | 451,791 | 1 | 0.22 | 0.22 (−0.37 to 0.84) |
| 40–49 yr | 472,083 | 1 | 0.21 | 444,916 | 2 | 0.45 | 0.24 (−0.61 to 1.11) |
| ≥50 yr | 1,115,083 | 1 | 0.09 | 1,070,939 | 2 | 0.19 | 0.10 (−0.26 to 0.46) |
Among vaccine recipients of all ages and both sexes, the overall difference in the incidence of myocarditis after the second dose as compared with the incidence after the first dose was 1.76 (95% confidence interval [CI], 1.33 to 2.19). The widths of the confidence intervals have not been adjusted for multiple testing.
Observed versus Expected Incidence
Table 4 shows the standardized incidence ratios for myocarditis according to vaccine dose, age group, and sex, as projected from the incidence during the prepandemic period from 2017 through 2019. Myocarditis after the second dose of vaccine had a standardized incidence ratio of 5.34 (95% CI, 4.48 to 6.40), which was driven mostly by the diagnosis of myocarditis in younger male recipients. Among boys and men, the standardized incidence ratio was 13.60 (95% CI, 9.30 to 19.20) for those 16 to 19 years of age, 8.53 (95% CI, 5.57 to 12.50) for those 20 to 24 years, 6.96 (95% CI, 4.25 to 10.75) for those 25 to 29 years, and 2.90 (95% CI, 1.98 to 4.09) for those 30 years of age or older. These substantially increased findings were not observed after the first dose. A sensitivity analysis showed that for male recipients between the ages of 16 and 24 years who had received a second vaccine dose, the observed standardized incidence ratios would have required overreporting of myocarditis by a factor of 4 to 5 on the assumption that the true incidence would not have differed from the expected incidence (Table S4).
Table 4. Standardized Incidence Ratios for 151 Cases of Myocarditis, According to Vaccine Dose, Age, and Sex.
| Age and Sex | First Dose | Second Dose | ||||
|---|---|---|---|---|---|---|
| Observed Cases |
Expected Cases per 2017–2019 Reference* | Standardized Incidence Ratio (95% CI) |
Observed Cases |
Expected Cases per 2017–2019 Reference* | Standardized Incidence Ratio (95% CI) |
|
| number | number | |||||
| All recipients † | 25 | 17.55 | 1.42 (0.92–2.10) |
126 | 23.43 | 5.34 (4.48–6.40) |
| 16–19 yr | ||||||
| Male | 3 | 1.86 | 1.62 (0.32–4.72) |
32 | 2.35 | 13.60 (9.30–19.20) |
| Female | 0 | 0.23 | 0 | 2 | 0.30 | 6.74 (0.76–24.35) |
| 20–24 yr | ||||||
| Male | 5 | 2.33 | 2.14 (0.69–5.00) |
26 | 3.05 | 8.53 (5.57–12.50) |
| Female | 1 | 0.42 | 2.37 (0.03–13.20) |
6 | 0.56 | 10.76 (3.93–23.43) |
| 25–29 yr | ||||||
| Male | 3 | 2.17 | 1.39 (0.28–4.05) |
20 | 2.87 | 6.96 (4.25–10.75) |
| Female | 0 | 0.30 | 0 | 1 | 0.39 | 2.54 (0.03–14.14) |
| ≥30 yr | ||||||
| Male | 10 | 8.13 | 1.23 (0.59–2.26) |
32 | 11.04 | 2.90 (1.98–4.09) |
| Female | 3 | 2.11 | 1.42 (0.29–4.15) |
7 | 2.87 | 2.44 (0.98–4.09) |
Reference data regarding the background incidence of myocarditis were extracted from the Israel National Hospital Discharge Database for the years 2017 through 2019.
Data are listed for the 151 vaccine recipients in whom myocarditis was diagnosed at any level of certainty within 21 days after the first dose and 30 days after the second dose; data for all vaccine recipients have been weighted according to age and sex.
Rate Ratio between Vaccinated and Unvaccinated Persons
Within 30 days after receipt of the second vaccine dose in the general population, the rate ratio for the comparison of the incidence of myocarditis between vaccinated and unvaccinated persons was 2.35 (95% CI, 1.10 to 5.02) according to the Brighton Collaboration classification of definite and probable cases and after adjustment for age and sex. This result was driven mainly by the findings for males in younger age groups, with a rate ratio of 8.96 (95% CI, 4.50 to 17.83) for those between the ages of 16 and 19 years, 6.13 (95% CI, 3.16 to 11.88) for those 20 to 24 years, and 3.58 (95% CI, 1.82 to 7.01) for those 25 to 29 years (Table 5). When follow-up was restricted to 7 days after the second vaccine dose, the analysis results for male recipients between the ages of 16 and 19 years were even stronger than the findings within 30 days (rate ratio, 31.90; 95% CI, 15.88 to 64.08). Concordance of our findings with the Bradford Hill causality criteria is shown in Table S5.
Table 5. Rate Ratios for a Diagnosis of Myocarditis within 30 Days after the Second Dose of Vaccine, as Compared with Unvaccinated Persons (January 11 to May 31, 2021).
| Age and Sex | Vaccinated Group | Unvaccinated Group | Rate Ratio (95% CI) |
||
|---|---|---|---|---|---|
| Person-Days of Follow-up | Cases | Person-Days of Follow-up | Cases | ||
| number | |||||
| All recipients * | 149,786,065 | 117 | 296,377,727 | 98 | 2.35 (1.10–5.02) |
| 16–19 yr | |||||
| Male | 6,018,541 | 31 | 19,135,706 | 11 | 8.96 (4.50–17.83) |
| Female | 6,033,192 | 2 | 17,768,696 | 2 | 2.95 (0.42–20.91) |
| 20–24 yr | |||||
| Male | 7,088,335 | 27 | 20,926,320 | 13 | 6.13 (3.16–11.88) |
| Female | 6,889,399 | 5 | 20,832,407 | 2 | 7.56 (1.47–38.96) |
| 25–29 yr | |||||
| Male | 6,590,263 | 18 | 20,944,595 | 16 | 3.58 (1.82–7.01) |
| Female | 6,417,564 | 1 | 20,943,920 | 0 | 0 |
| ≥30 yr | |||||
| Male | 53,577,403 | 26 | 82,419,957 | 40 | 1.00 (0.61–1.64) |
| Female | 57,171,368 | 7 | 93,406,126 | 14 | 0.82 (0.33–2.02) |
Data for all vaccine recipients have been weighted according to age and sex.
Discussion
During a nationwide vaccination campaign conducted from December 2020 through May 2021 involving more than 5 million residents, the Israeli Ministry of Health recorded 136 cases of definite or probable myocarditis that had occurred in temporal proximity to the receipt of two doses of the BNT162b2 mRNA vaccine — a risk that was more than twice that among unvaccinated persons. This association was highest in young male recipients within the first week after the second dose. In our study, definite or probable cases of myocarditis among persons between the ages of 16 and 19 years within 21 days after the second vaccine dose occurred in approximately 1 of 6637 male recipients and in 1 of 99,853 female recipients.
In most cases, symptoms of myocarditis developed within a few days after the second dose of vaccine. The incidence of myocarditis declined as the number of newly vaccinated persons decreased over time. This finding was suggestive of a possible causal relationship between two doses of the vaccine and the risk of myocarditis. Overall, we estimated that definite or probable cases of myocarditis occurred in the overall Israeli population at a rate of approximately 1 per 26,000 males and 1 per 218,000 females after the second vaccine dose, with the highest risk again among young male recipients. This result may explain why a phase 3 trial of the vaccine, which included only 15,000 male and female recipients,8 showed no cases of myocarditis. The mechanism of vaccine-induced myocarditis is not known but may be related to the active component of the vaccine, the mRNA sequence that codes for the spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), or to the immune response that follows vaccination.
Although selection bias in this study is possible, we consider it unlikely, since we used data from the entire nation. A major limitation of the study is that the calculation of rate ratios was based on individual patient data in the vaccinated group as compared with aggregated data in the unvaccinated group. In addition, the diagnosis of myocarditis was not validated by myocardial biopsy, and acquisition bias could be present, because clinical assessors were aware of vaccination status. Misclassification may have taken place during surveillance, which could have resulted in the underdiagnosis of myocarditis among young patients with chest pain or discomfort who were not referred for evaluation for myocarditis because of a low level of suspicion, despite notifications by the Ministry of Health to health care providers. There was also a possibility of overdiagnosis of cases of myocarditis owing to increased public and medical awareness of this possible side effect of vaccination. However, our sensitivity analysis did not support the occurrence of overreporting as an explanation for our findings. Our calculations of risk difference and rate ratios were confined to cases that had met strict criteria for definite or probable myocarditis, which would tend to reduce ascertainment bias. Another limitation may be the use of the Israel National Hospital Discharge Database for the years 2017 through 2019 as a reference for the background incidence of myocarditis in the analyses of standardized incidence ratios. Those years were different from the period between 2020 and 2021 with respect to viral circulation — including influenza outbreaks in 2017, 2018, and 2019 but not in 2020 and 2021 and Covid-19 morbidity in 2020 and 2021 but not in 2017 through 2019 — and to the lack of systematic reporting of myocarditis during the earlier period. However, hospitalization rates for myocarditis during the period from 2017 through 2019 were similar to those in 2020, and the databases used for these denominators are representative of the unvaccinated population. We were unable to adjust for potential confounders other than age and sex.
Finally, the rates of myocarditis in our study can be compared with those in the Clalit Health Services database in the study by Witberg et al.,9 as now reported in the Journal. That study showed a somewhat lower incidence of myocarditis, possibly because of the different methods that were used. In our study, each vaccination date was recorded to ensure accurate follow-up of 21 days after the first dose and 30 days after the second dose, whereas Witberg et al. followed vaccinees for 42 days after the first dose. The study design may have led to an underestimation of myocarditis cases owing to a shorter follow-up for the second dose. In our study, the rate of myocarditis in the general unvaccinated population was 1 per 10,857 and can be compared with findings indicating that myocarditis was more common after SARS-CoV-2 infection than after vaccination, as reported previously by Barda et al.10
On the basis of data from an Israeli national database, the incidence of myocarditis after two doses of the BNT162b2 mRNA vaccine was low but higher than the incidence among unvaccinated persons and among historical controls. The risk of myocarditis was driven primarily by the increased incidence after the second dose of vaccine and in young male recipients.
Acknowledgments
We thank the following members of the safety committee: Alex Batler, Bella Elran, Michael Askenazi, Rina Mintz, and Yaron Niv; staff members at the medical centers that helped to collect the data; Aharona Freedman of the Israel Center for Disease Control for her contribution to computations of rate ratios; Orna Cohen, Rivka Rich, and Michal Ashkenazy of the Division of Epidemiology and Bela Elran of the Public Health Services at the Ministry of Health for their contributions to data collection; and the residents of Internal Medicine B of the Hadassah Medical Center who made us aware of the association between vaccination and myocarditis.
Supplementary Appendix
Disclosure Forms
This article was published on October 6, 2021, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Emergency use authorization: Pfizer-BioNTech COVID-19 vaccine. Silver Spring, MD: Food and Drug Administration, 2021. (https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine). [Google Scholar]
- 2.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brighton Collaboration. Myocarditis/pericarditis case definition. The Task Force for Global Health, July 16, 2021. (https://brightoncollaboration.us/myocarditis-case-definition-update/).
- 4.Casey C, Vellozzi C, Mootrey GT, et al. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep 2006;55:1-16. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program — United States, 2003. MMWR Morb Mortal Wkly Rep 2003;52:492-496. [PubMed] [Google Scholar]
- 6.Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol 2018;273:183-186. [DOI] [PubMed] [Google Scholar]
- 7.Sahai H, Khurshid A. Statistics in epidemiology: methods, techniques, and applications. Boca Raton, FL: CRC Press, 1996. [Google Scholar]
- 8.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. DOI: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2021;385:1078-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.