Key Points
Question
Is the combination of cetuximab plus avelumab effective as a rechallenge strategy in patients with RAS wild-type (WT) metastatic colorectal cancer?
Findings
This phase 2, single-arm clinical trial provides evidence of clinical activity of combining cetuximab plus avelumab in 77 patients with RAS WT metastatic colorectal cancer (mCRC) who benefited from first-line anti–epidermal growth factor receptor-containing therapy when retreated with cetuximab plus avelumab in third or further lines of therapy as a rechallenge strategy. Median overall survival (mOS) was 11.6 months and reached 17.3 months in patients with baseline RAS/BRAF WT circulating tumor DNA (ctDNA).
Meaning
The magnitude of overall survival benefit obtained with this treatment accompanied with a mild overall toxic effects profile provides a potential new therapeutic option for RAS WT mCRC in the rechallenge setting; the trial also identified that plasma RAS/BRAF WT ctDNA analysis might be used to select patients with mCRC who may benefit from the treatment.
This phase 2 clinical trial examines the effect of cetuximab plus avelumab as a rechallenge strategy in patients with RAS wild-type metastatic colorectal cancer.
Abstract
Importance
Rechallenge therapy with anti–epidermal growth factor receptor (EGFR) drugs has been suggested in patients with chemo-refractory RAS wild-type (WT) metastatic colorectal cancer (mCRC) after initial response to anti–EGFR-based first-line treatment. The association of treatment with cetuximab plus avelumab with overall survival (OS) may be worthy of investigation in this setting.
Objective
To assess the efficacy and safety of cetuximab rechallenge therapy plus avelumab.
Design, Setting, and Participants
This single-arm, multicenter phase 2 trial enrolled patients from August 2018 to February 2020. Eligible patients with RAS WT mCRC had a complete or partial response to first-line chemotherapy plus anti-EGFR drugs, developed acquired resistance, and failed second-line therapy. Baseline circulating tumor DNA (ctDNA) for KRAS, NRAS, BRAF, and EGFR-S492R mutation analysis was done.
Interventions
Patients received avelumab (10 mg/kg every 2 weeks) and cetuximab (400 mg/m2 and, subsequently, 250 mg/m2 weekly) until disease progression or unacceptable toxic effects.
Main Outcomes and Measures
The primary end point was OS. Secondary end points were progression-free survival (PFS), overall response rate (ORR), and safety.
Results
Seventy-seven patients were enrolled (42 men, 35 women; median age, 63 years); 71 had microsatellite stable tumors (MSS), 3 microsatellite instability-high tumors (MSI-H), 3 unknown. The study met the primary end point, with median OS (mOS) of 11.6 months (95% CI, 8.4-14.8 months). Median PFS (mPFS) was 3.6 months (95% CI, 3.2-4.1 months). Common grade-3 adverse events were cutaneous eruption, 11 (14%), and diarrhea, 3 (4%). For 67 of 77 (87%) patients, baseline analysis of plasma circulating tumor DNA (ctDNA) for KRAS, NRAS, BRAF, and EGFR-S492R variations was feasible. Forty-eight patients had WT disease, whereas 19 had mutations. Patients with RAS/BRAF WT ctDNA had mOS of 17.3 months (95% CI, 12.5-22.0 months) compared with 10.4 months (95% CI, 7.2-13.6 months) in patients with mutated ctDNA (hazard ratio [HR], 0.49; 95% CI, 0.27-0.90; P = .02). The mPFS was 4.1 months (95% CI, 2.9-5.2 months) in RAS/BRAF WT patients compared with 3.0 months (95% CI, 2.6-3.5 months) in patients with mutated ctDNA (HR, 0.42; 95% CI, 0.23-0.75; P = .004).
Conclusions and Relevance
The findings of this single-arm phase 2 trial suggest that cetuximab plus avelumab is an active, well tolerated rechallenge therapy in RAS WT mCRC. Plasma ctDNA analysis before treatment may allow selection of patients who could benefit.
Trial Registration
ClinicalTrials.gov Identifier: NCT04561336
Introduction
Anti–epidermal growth factor receptor (EGFR) monoclonal antibodies (mAbs) in combination with FOLFIRI or FOLFOX are valid options for treatment of RAS wild-type (WT) metastatic colorectal cancer (mCRC).1,2,3 It has been suggested that there is potential benefit of retreatment with anti-EGFR mAbs of patients with mCRC who previously responded to first-line therapy.4 The rationale is the assumption that most RAS WT cancer cells during treatment with cetuximab or panitumumab are killed. However, during anti-EGFR mAbs treatment, a genetic selection of RAS mutant cancer cells occurs. These anti–EGFR-resistant clones are responsible for disease progression.5 Analysis of circulating tumor DNA (ctDNA) in the plasma of patients with mCRC has demonstrated that, after progression, a treatment break from anti-EGFR drugs results in RAS mutant cancer cell decay, whereas RAS WT cancer clones increase, thus potentially restoring therapeutic sensitivity to cetuximab or panitumumab.6,7,8
Immune checkpoint inhibitors (ICIs), such as anti-programmed death 1 (PD-1) or anti–programmed death ligand 1 (PD-L1) mAbs, are effective only in patients with microsatellite instability-high (MSI-H) mCRC.9 The immune system may play a fundamental role in modulating response to mAbs therapies in cancer.10 Antibody-dependent cell cytotoxicity (ADCC) is enhanced by IgG1 mAbs, such as cetuximab, and may activate innate and adaptive immune responses. Among ICIs, the anti–PD-L1 IgG1 mAb avelumab has ADCC properties. Cetuximab treatment activates functional cross-talks between natural killer (NK) and dendritic cells; enhances NK cell-mediated ADCC; promotes opsonization of cancer cells by dendritic cells; increases major histocompatibility complex (MHC) class II molecule expression and recruitment of T cells in the tumor microenvironment. These effects may increase cetuximab-induced cancer cell death.11,12,13
Inhibition of PD-L1 with avelumab in combination with the anti-EGFR mAb cetuximab could be a strategy for potentiating antitumor activity.14 To evaluate this hypothesis, we have conducted a prospective, single arm, multicenter phase 2 study of cetuximab plus avelumab as rechallenge treatment in patients with RAS WT mCRC.
Methods
Study Design and Participants
This is a nonprofit, academic, multicenter, open-label, phase 2 trial, involving 8 Italian centers in the GOIM (Gruppo-Oncologico-Italia-Meridionale) network. We enrolled adult patients with RAS (NRAS and KRAS, exon 2-3-4) WT, histologically confirmed metastatic colorectal adenocarcinoma. Patients should have obtained a major response (complete or partial response) during first-line chemotherapy plus an anti-EGFR drug (panitumumab or cetuximab) and should have progressed. Patients should have received at least a second-line therapy after first-line treatment failure. An interval of more than 4 months from last dose of the anti-EGFR drug administered in the first-line treatment was mandatory for enrollment. Additional inclusion/exclusion criteria are detailed in the Trial Protocol available in Supplement 1.
The study (clinical trial registration: NCT04561336) was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization and Good Clinical Practice Guidelines. Ethic Committees of participating centers approved study procedures. All patients provided written informed consent.
Cetuximab at 400 mg/m2 (loading dose) and, subsequently, at 250 mg/m2 weekly, and avelumab intravenously at 10 mg/kg, once every 2 weeks, were given. Details are in the Trial Protocol in Supplement 1. Patients were treated until progression, unacceptable toxic effects, or refusal.
Response Assessment and Biomarkers Analysis
Safety was graded using National Cancer Institute-Common Toxicity Criteria (NCI-CTC) for adverse events (AEs), version 4.03. Tumor assessments for defining partial response (PR), complete response (CR), stable disease (SD), or progressive disease (PD) were performed according to RECIST criteria, version 1.1. Tumor measurements were done at baseline, and every 8 weeks for 40 weeks and every 12 weeks thereafter.
Plasma samples from 67 of 77 (87%) patients, collected at treatment baseline, were suitable to perform ctDNA analysis for KRAS, NRAS, BRAF, and EGFR extracellular domain S492R mutations. Samples were analyzed at Oncologia Medica, Università degli Studi della Campania “L. Vanvitelli” (Naples, Italy), by using the automated Idylla qPCR-based platform.15 More details in Supplement 1.
Statistical Analysis
The primary end point was overall survival (OS). Secondary end points were progression-free survival (PFS), overall response rate (ORR) ([CR] + [PR]) and toxic effects. Primary and secondary end points were also evaluated according to RAS and BRAF mutations determined by testing ctDNA in patient plasma at baseline.
The study aimed to demonstrate a median OS (mOS) of 11 months for cetuximab plus avelumab as compared with historical mOS of 8.0 months with standard third-line treatments,16,17 which corresponds to an improvement of 37.5% in mOS. It was estimated that we would have to enroll 66 patients with one-sided 5% level test. Considering a potential risk of drop-out of patients in 15% of cases, 77 patients were enrolled. The Kaplan-Meier method was used to estimate PFS and OS. Statistical analyses were performed using the SPSS package (version 23, IBM).
Results
Between August 2018 and February 2020, 83 patients were screened and 77 patients were treated (eFigure 1 in Supplement 2). Patient characteristics are reported in eTable 1 in Supplement 2. Patients were treated in first-line with chemotherapy plus an anti-EGFR drug, either cetuximab 38 (49%) or panitumumab 39 (51%). During first-line treatment, 73 (95%) patients achieved PR, whereas 4 (5%) achieved CR; 52 (68%) patients received 2 previous regimens of therapy, whereas 25 (32%) patients had progressed to 3 or more lines of treatment. Seventy-one (92%) patients had microsatellite stable (MSS) cancer; 3 (4%) patients had MSI-H cancer; microsatellite status was unknown in 3 (4%) patients. At the time of data cutoff (December 31, 2020), median duration of follow-up was 19.5 months (interquartile range [IQR], 12.8-22.8 months). Seventy-three (95%) patients progressed, 56 (73%) patients died, whereas 4 (5%) patients were receiving treatment with cetuximab plus avelumab.
Median OS was 11.6 months (95% CI, 8.4-14.8 months) (Figure 1A). Median PFS (mPFS) was 3.6 months (95% CI, 3.2-4.1 months) (Figure 1B). One patient had CR, 5 patients had PR (ORR, 7.8%,); 44 patients (57%) had SD, of these 28 (36%) patients had SD longer than 4 months. Disease control rate (DCR) was 65% (Table).
Figure 1. Kaplan-Meier Estimates.
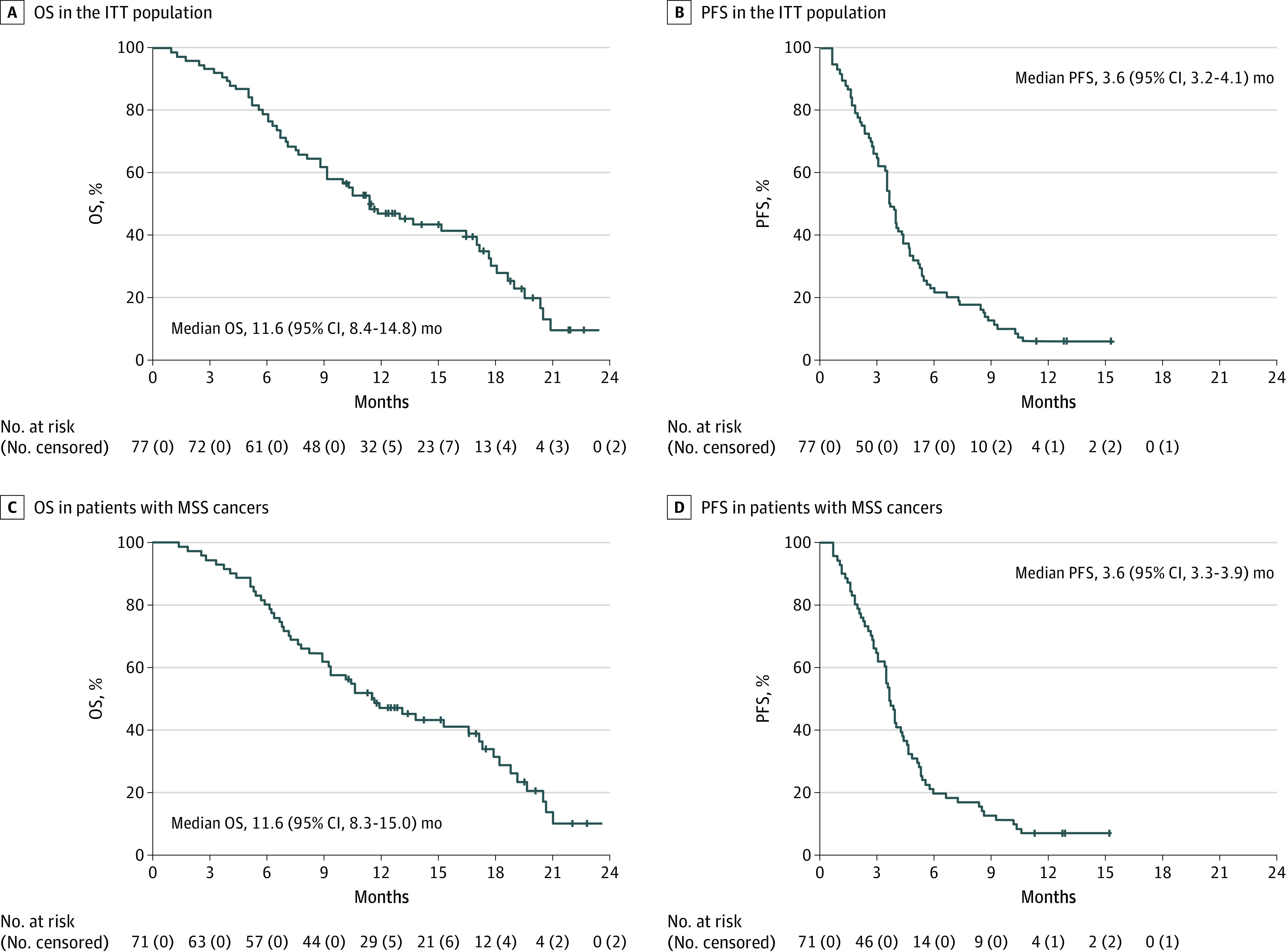
ITT indicates intention-to-treat; MSS, microsatellite stable tumors; OS, overall survival; PFS, progression-free survival. A, Overall survival in the ITT population. B, Progression-free survival in the ITT population. C, Overall survival in patients with microsatellite stable tumors. D, Progression-free survival in patients with microsatellite stable tumors.
Table. Activity and Efficacy in the Intention-to-Treat Population and in Patients With Plasma Available for ctDNA at Baseline.
| Variable | No. | No. (%) [95% CI] | Median (95% CI), mo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR | PR | ORR | SD | SD >4 mo | PD | DCR | mPFS | mOS | ||
| ITT | 77 | 1 (1.3) [0-7] | 5 (6.5) [2-14] | 6 (7.8) [2.9-16.2] | 44 (57.1) [45-68] | 28 (36.4) [25.7-48.1] | 27 (35) [24-47] | 50 (65) [53-75] | 3.6 (3.2-4.1) | 11.6 (8.4-14.8) |
| ITT MSS | 71 | 1 (1.4) [0-7.6] | 5 (7) [2.3-15.7] | 6 (8.5) [3.2-17.5] | 40 (56.3) [44-68.1] | 24 (33.8) [23-46] | 25 (35.2) [24.2-47.5] | 46 (64.8) [52.2-75.8] | 3.6 (3.3-3.9) | 11.6 (8.3-15.0) |
| Basal ctDNA cohort | 67 | 1 (1.5) [0-8] | 4 (6.0) [1.7-14.6] | 5 (7.5) [2.5-16.6] | 39 (58.0) [45.5-70.2] | 25 (37.3) [25.8-50] | 23 (34.3) [23.2-46.9] | 44 (65.7) [53.1-76.8] | 3.9 (3.3-4.5) | 13.8 (7.7-19.9) |
| RAS/BRAF WT | 48 | 1 (2.1) [0.1-11.1] | 3 (6.2) [1.3-17.2] | 4 (8.3) [2.3-20] | 31 (64.6) [49.5-77.8] | 21 (43.8) [29.5-58.8] | 13 (27.1) [15.3-41.8] | 35 (72.9) [58.2-84.7] | 4.1 (2.9-5.2) | 17.3 (12.5-22) |
| RAS or BRAF mutant | 19 | 0 (0) [0-17.6] | 1 (5.3) [0.1-26] | 1 (5.3) [0.1-26] | 8 (42.1) [20.3-66.5] | 4 (21.1) [6.1-45.6] | 10 (52.6) [28.9-75.6] | 9 (47.4) [24.4-71.1] | 3.0 (2.6-3.5] | 10.4 (7.2-13.6) |
| MSS RAS/BRAF WT | 44 | 1 (2.3) [0.1-12] | 3 (6.8) [1.4-18.7] | 4 (9.1) [2.5-21.7] | 28 (63.6) [47.8-77.6] | 18 (40.9) [26.3-56.8] | 12 (27.3) [15-42.8] | 32 (72.7) [57.2-85] | 3.9 (2.8-5) | 17.3 (11.2-23.4) |
Abbreviations: CR, complete response; ctDNA, circulating tumor DNA; DCR, disease control rate; ITT, intention to treat; mOS, median overall survival; mPFS, median progression free survival; MSS, microsatellite stable; ORR, overall response rate; PD, progression disease; PR, partial response; SD, stable disease; WT, wild type.
A post-hoc planned analysis assessed the efficacy of cetuximab plus avelumab in patients according to ctDNA detection of KRAS, NRAS, BRAF, and EGFR-extracellular domain S492R mutations by using plasma samples collected at treatment baseline. Samples from 67 patients were analyzed using Idylla qPCR-based platform (eTable 2 in Supplement 2). Forty-eight patients had KRAS/NRAS/BRAF WT ctDNA, whereas RAS and/or BRAF mutations were found in plasma of 19 patients. No EGFR-extracellular domain S492R mutation was found (eTable 3 in Supplement 2). Patients with RAS/BRAF WT ctDNA had mOS of 17.3 months (95% CI, 12.5-22.0 months) compared with 10.4 months (95% CI, 7.2-13.6 months) in patients with mutated ctDNA (HR, 0.49; 95% CI, 0.27-0.90; P = .02). Median PFS was 4.1 months (95% CI, 2.9-5.2 months) in patients with RAS/BRAF WT ctDNA compared with 3.0 months (95% CI, 2.6-3.5 months) in patients with mutations (HR, 0.42; 95% CI, 0.23-0.75; P = .004) (Figure 2, A and B). Three PR and 1 CR were observed in patients with RAS/BRAF WT ctDNA with DCR of 73% (Table). Overall, PFS ranged from 6 to 15 months in 20 of 48 (41%) patients with RAS/BRAF WT ctDNA compared with PFS less than 6 months in all the 19 patients with mutated ctDNA. Individual patient data for PFS and OS are reported in eFigure 2 in Supplement 2.
Figure 2. Kaplan-Meier Estimates.

A, Overall survival in patients with plasma circulating tumor DNA RAS/BRAF mutational status. B, Progression-free survival in patients with plasma circulating tumor DNA RAS/BRAF mutational status. C, Overall survival in patients with microsatellite stable tumors and plasma ctDNA RAS/BRAF mutational status. D, Progression-free survival in patients with microsatellite stable tumors and plasma ctDNA RAS/BRAF mutational status.
In 71 patients with MSS cancer, mOS was 11.6 months (95% CI, 8.3-15.0 months) and mPFS was 3.6 months (95% CI, 3.3-3.9 months) with ORR of 8.5% (Table and Figure 1, C and D). In 44 patients with MSS cancer and RAS/BRAF WT ctDNA, mOS was 17.3 months (95% CI, 11.2-23.4 months), mPFS was 3.9 months (95% CI, 2.8-5.0 months) compared with 10.4 months (95% CI, 7.2-13.6 months) mOS and to 3.0 months (95% CI, 2.6-3.5 months) mPFS in 19 patients with MSS cancer and mutated ctDNA (OS HR, 0.50; 95% CI, 0.27-0.93; P = .03; PFS HR, 0.42; 95% CI, 0.23-0.77; P = .005) (Table and Figure 2, C and D).
Analysis of ctDNA was performed at progression in 30 of 44 (68%) patients (4 patients were receiving treatment) with RAS/BRAF WT ctDNA at baseline. RAS/BRAF WT ctDNA was confirmed in 20 of 30 (66%) patients, whereas KRAS, NRAS, BRAF, or EGFR-extracellular domain S492R mutations were found in 6, 0, 1, and 3 patients, respectively. Overall, EGFR-S492R was the only mutation found in 1 patient, whereas it was found together with KRAS mutation in 2 patients (eTable 4 in Supplement 2).
A total of 744 cycles (range, 1-30) were administered. Patients received a median of 8 cycles (IQR, 5-12). Most common grade 3 toxic effects were cutaneous eruption (11 [14%] patients) and diarrhea (3 [4%] patients). There were no treatment-related grade 4 or 5 AEs (eTable 5 in Supplement 2). Treatment delay for AEs occurred in 28 (36%) patients and it was related to cetuximab in 23 (30%) patients. Cetuximab dose was reduced in 7 patients for skin rash or diarrhea (eTable 6 in Supplement 2). No treatment discontinuation was reported. Forty-nine of 73 (67%) patients received a further line of treatment after progression (eTable 7 in Supplement 2).
Discussion
The present study shows that rechallenge therapy with cetuximab plus avelumab is well tolerated and feasible in patients with RAS WT mCRC. The primary end point was achieved with mOS of 11.6 months. Disease control was obtained in 65% of patients with mPFS of 3.6 months.
Analysis of plasma ctDNA is a noninvasive tool able to identify the onset of anti-EGFR cancer cell resistance mechanisms.18,19 A significant difference in mOS was observed in patients with RAS/BRAF WT ctDNA at baseline compared with patients with mutated ctDNA (17.3 vs 10.4 months). Also PFS was significantly better. Of note, mPFS was 4.1 months and PFS was longer than 6 (range, 6-15) months in 20 of 48 (41%) patients with RAS/BRAF WT ctDNA; whereas mPFS was 3.0 months in patients with mutated ctDNA and none of them reached PFS of 6 months.
To our knowledge, 3 single-arm prospective studies of cetuximab plus irinotecan have been published. Santini and colleagues4 reported ORR of 53.8% and mPFS of 6.6 months in 39 mCRC patients retreated with irinotecan plus cetuximab. However, the percentage of patients who received cetuximab as true rechallenge treatment rather than as reintroduction without having developed resistance to this drug was not defined. A phase 2 study (CRICKET) reported ORR of 21% and DCR of 54% in 28 mCRC patients treated with irinotecan plus cetuximab in a well-defined third-line rechallenge strategy. Baseline testing for plasma ctDNA was done in 25 patients, of which 13 had RAS WT and 12 RAS mutated ctDNA. In these 13 patients mOS and mPFS were 12.5 and 4 months, respectively; whereas mPFS and mOS in the 12 patients with RAS mutated ctDNA were 1.9 and 5.2 months, respectively.8 Another study20 evaluated cetuximab plus irinotecan treatment in 34 patients with KRAS exon 2 WT cancer at initial disease assessment, as third-line rechallenge therapy. Median PFS and mOS were 2.4 and 8.2 months, respectively. In these studies the therapeutic role of irinotecan in addition to cetuximab was not assessed. Irinotecan may contribute to clinical activity since it has been demonstrated that cetuximab could partially restore irinotecan sensitivity in mCRC patients.21
Immunotherapy has limited activity in mCRC with only evidence of ICIs efficacy in MSI-H or mismatch-repair deficient (dMMR) mCRC.22,23,24,25,26 The IMblaze370 trial, a multicenter phase 3 randomized clinical trial,26 evaluated chemo-refractory patients with MSS mCRC. In testing the efficacy of atezolizumab, an anti–PD-L1 mAb, alone, or in combination with cobimetinib, a selective MEK-1 inhibitor, compared with standard of care regorafenib, the findings failed to demonstrate a survival advantage. In the present study, similar clinical activity was observed in the ITT population (77 patients) as well as in 71 patients with MSS cancer. Moreover, similar differential clinical benefit was reported in the 48 patients with RAS/BRAF WT ctDNA and in those 44 of 48 patients with RAS/BRAF WT ctDNA and MSS cancer compared with patients with mutated ctDNA.
These results may be explained on the basis of the structure of cetuximab and avelumab. They are IgG1 isotypes with ADCC-inducing properties. We have recently provided evidence that cetuximab plus avelumab selectively induces ADCC in human non–small cell lung cancer (NSCLC) cell lines in vitro and has antitumor activity, paralleled by activation of NK cell-driven ADCC, in patients with chemorefractory NSCLC cancer in a proof of concept, single-arm study.12 Translational and functional studies are needed to understand the potential additive and/or synergistic effects of these 2 IgG1 mAbs in patients with mCRC.
Cetuximab plus avelumab was well tolerated, with low incidence of grade 3 cutaneous eruption and diarrhea. This is worth of note because standard-of-care treatments, such as regorafenib or trifluridine-tipiracil, have a limited magnitude of clinical benefit in terms of OS and PFS in chemorefractory mCRC, but with a higher incidence of drug-induced AEs. Moreover, the present study reports that, as a balance between clinical activity and safety of cetuximab plus avelumab, 49 patients (67%) patients received a subsequent line of therapy after progression.
Limitations
The single-arm nature of this study represents the main limitation. A randomized clinical trial is needed to validate the results. We are currently organizing a randomized phase 2 clinical trial to compare cetuximab alone or in combination with avelumab as rechallenge therapy in patients with plasma RAS/BRAF WT ctDNA and MSS cancer.
Conclusions
Cetuximab rechallenge treatment plus avelumab may represent a novel active and safe therapy for a selected subgroup of patients with mCRC. Baseline evaluation of RAS and BRAF mutations in plasma ctDNA may select patients that could benefit from treatment.
Trial Protocol
eFigure 1. Study Consort Diagram
eFigure 2. Swimmer Plot for Overall Survival (A) and Progression Free Survival (B) in Patients With Plasma ctDNA Mutational Status
eTable 1. Baseline Characteristics
eTable 2. Baseline Characteristics of 67 ctDNA RAS/BRAF/EGFRS492R Wild Type and Mutant Patients
eTable 3. Analysis of Patients With Baseline Mutated ctDNA
eTable 4. ctDNA Analysis at Progression of Patient With Baseline Wild Type ctDNA
eTable 5. Treatment-Emerged Adverse Events
eTable 6. Dose Delays and Reductions
eTable 7. Post CAVE mCRC Treatments
Data Sharing Statement
References
- 1.Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692-700. doi: 10.1200/JCO.2014.59.4812 [DOI] [PubMed] [Google Scholar]
- 2.Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023-1034. doi: 10.1056/NEJMoa1305275 [DOI] [PubMed] [Google Scholar]
- 3.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065-1075. doi: 10.1016/S1470-2045(14)70330-4 [DOI] [PubMed] [Google Scholar]
- 4.Santini D, Vincenzi B, Addeo R, et al. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann Oncol. 2012;23(9):2313-2318. doi: 10.1093/annonc/mdr623 [DOI] [PubMed] [Google Scholar]
- 5.Mauri G, Pizzutilo EG, Amatu A, et al. Retreatment with anti-EGFR monoclonal antibodies in metastatic colorectal cancer: Systematic review of different strategies. Cancer Treat Rev. 2019;73:41-53. doi: 10.1016/j.ctrv.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 6.Siravegna G, Bardelli A. Failure is not final: ctDNA-guided rechallenge therapy in colorectal cancer. Ann Oncol. 2019;30(10):1671. doi: 10.1093/annonc/mdz212 [DOI] [PubMed] [Google Scholar]
- 7.Parseghian CM, Loree JM, Morris VK, et al. Anti-EGFR-resistant clones decay exponentially after progression: implications for anti-EGFR re-challenge. Ann Oncol. 2019;30(2):243-249. doi: 10.1093/annonc/mdy509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremolini C, Antoniotti C, Lonardi S, et al. Activity and safety of cetuximab plus modified FOLFOXIRI followed by maintenance with cetuximab or bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: a randomized phase 2 clinical trial. JAMA Oncol. 2018;4(4):529-536. doi: 10.1001/jamaoncol.2017.5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotwals P, Cameron S, Cipolletta D, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17(5):286-301. doi: 10.1038/nrc.2017.17 [DOI] [PubMed] [Google Scholar]
- 11.Collins JM, Gulley JL. Product review: avelumab, an anti-PD-L1 antibody. Hum Vaccin Immunother. 2019;15(4):891-908. doi: 10.1080/21645515.2018.1551671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fasano M, Della Corte CM, Di Liello R, et al. Induction of natural killer antibody-dependent cell cytotoxicity and of clinical activity of cetuximab plus avelumab in non-small cell lung cancer. ESMO Open. 2020;5(5):e000753. doi: 10.1136/esmoopen-2020-000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue Y, Hazama S, Suzuki N, et al. Cetuximab strongly enhances immune cell infiltration into liver metastatic sites in colorectal cancer. Cancer Sci. 2017;108(3):455-460. doi: 10.1111/cas.13162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein A, Binder M, Al-Batran S, et al. Avelumab and cetuximab in combination with FOLFOX in patients with previously untreated metastatic colorectal cancer (MCRC): results of the safety run-in phase of the phase II AVETUX trial (AIO-KRK-0216). J Clin Oncol. 2018;36(15_Suppl):3561-3561. [Google Scholar]
- 15.Vitiello PP, De Falco V, Giunta EF, et al. Clinical practice use of liquid biopsy to identify RAS/BRAF mutations in patients with metastatic colorectal cancer (mCRC): a single institution experience. Cancers (Basel). 2019;11(10):1504-1509. doi: 10.3390/cancers11101504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grothey A, Van Cutsem E, Sobrero A, et al. ; CORRECT Study Group . Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303-312. doi: 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 17.Mayer RJ, Van Cutsem E, Falcone A, et al. ; RECOURSE Study Group . Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909-1919. doi: 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 18.Martinelli E, Ciardiello D, Martini G, et al. Implementing anti-epidermal growth factor receptor (EGFR) therapy in metastatic colorectal cancer: challenges and future perspectives. Ann Oncol. 2020;31(1):30-40. doi: 10.1016/j.annonc.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 19.Martini G, Ciardiello D, Vitiello PP, et al. Resistance to anti-epidermal growth factor receptor in metastatic colorectal cancer: What does still need to be addressed? Cancer Treat Rev. 2020;86:102023. doi: 10.1016/j.ctrv.2020.102023 [DOI] [PubMed] [Google Scholar]
- 20.Masuishi T, Tsuji A, Kotaka M, et al. Phase 2 study of irinotecan plus cetuximab rechallenge as third-line treatment in KRAS wild-type metastatic colorectal cancer: JACCRO CC-08. Br J Cancer. 2020;123(10):1490-1495. doi: 10.1038/s41416-020-01042-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337-345. doi: 10.1056/NEJMoa033025 [DOI] [PubMed] [Google Scholar]
- 22.Ciardiello D, Vitiello PP, Cardone C, et al. Immunotherapy of colorectal cancer: challenges for therapeutic efficacy. Cancer Treat Rev. 2019;76:22-32. doi: 10.1016/j.ctrv.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 23.Andre Thierry, Shiu Kai-Keen, Kim Tae Wonet al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: the phase 3 KEYNOTE-177 Study. Journal Clin Oncol 2020; 38(18_Suppl). doi: 10.1200/JCO.2020.38.18_suppl.LBA4 [DOI] [Google Scholar]
- 24.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182-1191. doi: 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773-779. doi: 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 26.Eng C, Kim TW, Bendell J, et al. ; IMblaze370 Investigators . Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20(6):849-861. doi: 10.1016/S1470-2045(19)30027-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Study Consort Diagram
eFigure 2. Swimmer Plot for Overall Survival (A) and Progression Free Survival (B) in Patients With Plasma ctDNA Mutational Status
eTable 1. Baseline Characteristics
eTable 2. Baseline Characteristics of 67 ctDNA RAS/BRAF/EGFRS492R Wild Type and Mutant Patients
eTable 3. Analysis of Patients With Baseline Mutated ctDNA
eTable 4. ctDNA Analysis at Progression of Patient With Baseline Wild Type ctDNA
eTable 5. Treatment-Emerged Adverse Events
eTable 6. Dose Delays and Reductions
eTable 7. Post CAVE mCRC Treatments
Data Sharing Statement


