Abstract
Stent failure remains one of the greatest challenges for interventional cardiologists. Despite the evolution to superior second- and third-generation drug-eluting stent designs, increasing use of intracoronary imaging and the adoption of more potent antiplatelet regimens, registries continue to demonstrate a prevalence of stent failure or target lesion revascularisation of 15–20%. Predisposition to stent failure is consistent across both chronic total occlusion (CTO) and non-CTO populations and includes patient-, lesion- and procedure-related factors. However, histological and pathophysiological properties specific to CTOs, alongside complex strategies to treat these lesions, may potentially render percutaneous coronary interventions in this cohort more vulnerable to failure. Prevention requires recognition and mitigation of the precipitants of stent failure, optimisation of interventional techniques, including image-guided precision percutaneous coronary intervention, and aggressive modification of a patient’s cardiovascular risk factors. Management of stent failure in the CTO population is technically challenging and itself begets recurrence. We aim to provide a comprehensive review of factors influencing stent failure in the CTO population and strategies to attenuate these.
Keywords: Stent failure, in-stent restenosis, stent thrombosis, chronic total occlusion, stenting strategy
Stent failure (SF) is a widely used term enveloping the aetiologies of in-stent restenosis (ISR), stent thrombosis (ST) and stent fracture (StF). The mechanisms of each vary: ISR is most commonly secondary to neointimal hyperplasia or neoatherosclerosis; ST is often precipitated by malapposition and incomplete stent strut coverage; and StF, although much rarer, is caused most frequently by vessel motion at hinge points. In addition to these factors, a number of patient comorbidities, as well as coronary lesion characteristics, procedural strategies and stent design factors, increase the risk of SF. Despite the evolution of stent platforms and increasing adoption of precision percutaneous coronary intervention (PCI) facilitated by adjunctive calcium modification and the use of intracoronary imaging, SF poses an ongoing challenge to coronary revascularisation. Although the shift from bare-metal stents (BMS) to drug-eluting stents (DES) saw a decrement in overall ISR rates, ISR remains the primary culprit for failure of contemporary PCI.[1]
Target lesion revascularisation (TLR) rates of between 3% and 20% are consistently reported across randomised controlled trials (RCTs) and large registries, regardless of the coronary vessel treated, lesion complexity or the use of image-guided stent optimisation.[2–5] In both the EXCEL and NOBLE trials, with >70% uptake of intravascular ultrasound (IVUS) for left mainstem PCI and the use of DES, TLR rates of approximately 12% were reported.[6,7] Similar trends have been seen in trials of coronary artery bypass grafting (CABG) versus PCI incorporating complex multivessel coronary disease and diabetic cohorts, proven to be at increased SF risk, with TLR rates of 19% in the SYNTAX trial at the 5-year follow-up, 5.7% in the BEST trial and 12.6% in the FREEDOM trial.[8–10]
ISR and TLR are also common phenomena following PCI for chronic total occlusion (CTO), a challenging subset of coronary disease, the treatment of which frequently induces greater vessel trauma. In the ACE-CTO study of 100 patients following implantation of second-generation everolimus-eluting stents (EES), the 12-month TLR rate was 37%.[11] However, significantly lower TLR and target vessel revascularisation (TVR) rates have been shown in both RCTs and registries. For example, an evaluation of long-term clinical outcomes following CTO PCI in three tertiary centres found TLR and ST rates of 17.2% and 1.7%, respectively, with use of DES.[12] More recently, the CONSISTENT CTO study found TVR rates of 4.8% and 17% in non-diabetic and diabetic cohorts, respectively.[13] Although earlier data, such as those from the J-Cypher 5-year outcomes study, demonstrated higher rates of TLR in CTO PCI versus unselected non-CTO PCI, more recent data directly comparing outcomes of CTO PCI versus complex non-CTO PCI found equipoise in target vessel failure (TVF) at the 3-year follow-up.[14,15] This was despite higher cardiovascular comorbidity, a lower degree of procedural success and higher complication rates in the CTO treated cohort.
The aim of this review is to provide a structured and comprehensive summary of the primary factors influencing and predicting SF (Figure 1), primarily ISR, in the CTO population, alongside strategies to mitigate these.
Figure 1: Classification of Factors Affecting Stent Failure in Percutaneous Coronary Intervention for Chronic Total Occlusion.
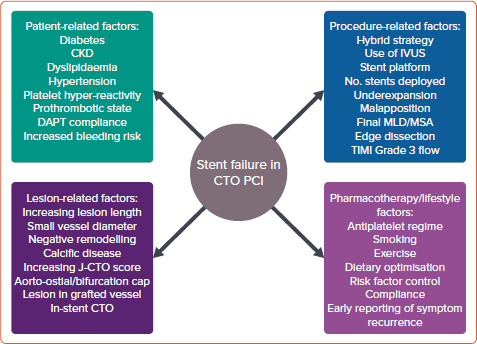
CKD = chronic kidney disease; CTO = chronic total occlusion; DAPT = dual antiplatelet therapy; IVUS = intravascular ultrasound; MLD = minimum lumen diameter; MSA = minimum stent area; PCI = percutaneous coronary intervention; TIMI = thrombolysis in MI.
Patient-related Factors
Numerous comorbidities and clinical entities increase the absolute risk of SF secondary to ISR and ST. In this review, we focus on diabetes, chronic kidney disease (CKD) and dyslipidaemia.
Diabetes
Diabetes is prevalent in approximately 40% of patients undergoing CTO PCI and is an independent predictor of ISR.[16] Intimal hyperplasia is promoted by vessel trauma during stenting, and the effects of hyperinsulinaemia on smooth muscle cells, leading to luminal loss, reduced minimal luminal area and subsequent TLR/TVR.[17] A 980-patient single-centre observational study comparing outcomes in diabetics with HbA1c <7% versus >7% showed increased major adverse cardiovascular events (MACE), driven primarily by repeat revascularisation, in those with poorer glycaemic control (TLR and non-TLR).[18] These findings are echoed in other large studies and a meta-analysis demonstrating trends towards increased ISR and TLR in diabetic cohorts with a higher HbA1c or glucose level at index PCI, with increasing severity of disease predicting increased TLR in a stepwise manner.[19,20]
Furthermore, one large study showed TVR rates to be significantly increased in diabetic patients with poor glycaemic control compared with non-diabetic patients, whereas in diabetic subjects with good glycaemic control the outcomes were equivalent to those in a non-diabetic matched population, reinforcing the importance of vigilant glycaemic management.[21] Similar findings were observed in the CONSISTENT CTO study, in which TVR rates were significantly higher at both 12 months (15.9% versus 4.8%) and 2 years (27.3% versus 7.8%) in diabetic versus non-diabetic cohorts undergoing CTO PCI.[13]
Chronic Kidney Disease
Almost 30% of patients undergoing CTO PCI have Stage III CKD, defined as an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2, either as an end-organ manifestation of diabetes or hypertension or secondary to alternative renal or systemic pathology.[22] CKD is associated with accelerated and aggressive atherosclerotic coronary disease, increased MACE (including post-coronary revascularisation) and higher SF rates. Mechanisms linked to conventional coronary risk factors include activation of the renin–angiotensin–aldosterone system in hypertensive patients, increased insulin-like growth factor in diabetic patients and malignant patterns of dyslipidaemia, including upregulation of LDL receptor expression and increased triglyceridaemia. Furthermore, higher levels of novel coronary risk factors, including homocysteine and certain forms of apolipoprotein, have been consistently found in CKD patients alongside increased oxidative stress and reductions in endothelial nitric oxide levels (crucial for vascular integrity).[23]
Trials in coronary intervention frequently exclude CKD patients, 80% excluding end-stage renal disease and 60% excluding any CKD, due to the challenges of revascularisation in these patients resulting from increased coronary calcification and the need to minimise contrast load.[24] In a pooled analysis of over 12,000 patients in the Korean Multi-Center DES registry, TLF was significantly higher in the CKD versus preserved renal function cohort, with a trend towards increased ST at 30 days.[25] In the Japanese Multicentre Prospective Registry outcomes analysis of 4,749 patients, an eGFR <30 ml/min/1.73 m2 and haemodialysis dependence were associated with reduced procedural success (primarily due to failure of retrograde wire cross), higher in-stent occlusion rates (p<0.001), increased lesion calcification, proximal tortuosity and occlusion length >20 mm, and subsequently higher J-CTO scores.[26] Further, dialysis dependence was a predictor of 12-month major adverse cardiovascular and cerebrovascular events driven by death, TVR and CABG.[26] An eGFR <40 ml/min/1.73 m2, alongside diabetes, left ventricular (LV) ejection fraction <45%, volume-deplete hypotension, increasing age and anaemia, predicts an increased risk of contrast-induced nephropathy (CIN).[27] Irreversible deterioration in eGFR secondary to CIN, leading to progressive CKD, is associated with increased longer-term MACE, mediated through accelerated atherosclerosis, vascular calcification and effects on LV remodelling.[27]
European Society of Cardiology recommendations advocate renal optimisation through transient withholding of nephrotoxic medications, intravenous pre- and post-hydration, limited contrast use (lowest of either <350 ml or <4 ml/kg) and serum creatinine monitoring post procedure.[28] LV end-diastolic pressure-guided fluid replacement in the POSEIDON study and the use of the RenalGuard (RenalGuard Solutions) system in the REMEDIAL II study both demonstrated a reduced risk of CIN compared with matched control cohorts.[29,30] These strategies should be appropriately adopted to optimise both short- and longer-term renal outcomes, preventing irreversible progression of CKD, which itself begets SF. Further, with the advent of precision IVUS-guided PCI, the ability to safely perform complex intervention with minimal or zero contrast use has been demonstrated and is being incorporated into clinical practice.[31]
Dyslipidaemia
Dyslipidaemia is highly prevalent in the CTO population, at over 70% in the European Registry of CTO, and frequently coexists with diabetes and CKD.[32] LDL, the target of statin therapy in preventive cardiovascular pharmacotherapeutics, has long been known as a risk factor for coronary artery disease.[33] More recently, VLDL was found to be an independent predictor of ISR in people with diabetes after DES implantation.[33] This was hypothesised to be mediated via the pathophysiological effects of apolipoproteins (Apo) B and C.[34,35] However, HDL and ApoA1, the so-called ‘good cholesterol’, have been linked in vitro to improved stent biocompatibility through inhibition of smooth muscle cell proliferation, the suppression of inflammation and the prevention of neointimal hyperplasia.[36]
Furthermore, ApoA1 promotes re-endothelialisation following vessel trauma and stent implantation via generation of nitric oxide and facilitation of endothelial repair.[36] This process is fundamental to stent strut coverage and hence prevention of ST. The REVEAL trial was the first to show an association between anacetrapib (a cholesterol ester transfer protein inhibitor)-driven increases in HDL and ApoA1 levels and a reduction in atherosclerotic vascular events, including cardiac death, MI and coronary revascularisation.[37] Hence, rigorous management of cholesterol components, through lowering of LDL and VLDL while increasing HDL, appears important in halting aggressive atherosclerotic processes and optimising physiological stent biocompatibility.
Lesion-related Factors
Lesion length, location, composition and complexity are predictors of SF. Increasing CTO length predicts escalation through the hybrid algorithm and subsequent procedural success. In a retrospective analysis by Tian et al., 5-year outcomes demonstrated CTO length >15 mm to be a predictor of TLR, whereas Ahn et al. found CTO length >30 mm was associated with higher repeat PCI driven by TVR at 2 years.[38,39] In the diabetic cohort of the OPEN-CTO study, approximately 60% had occlusion length >20 mm, whereas in the dissection and re-entry technique (DART) cohort of the CONSISTENT-CTO study, a mean (± SD) lesion length of 32 ± 22 mm was associated with an overall 2-year TVR of 14.9%, reiterating the potential for ISR and, further, development of downstream in-stent CTO.[13,40]
CTO location at the aorto-ostium or bifurcation increases procedural difficulty and carries a higher risk of SF. Aorto-ostial lesions are independent predictors of quantitative coronary angiography-based longitudinal stent deformation (LSD), and carry a higher chance of geographical miss, potentially leaving behind a nidus for TVR/TLR.[41] The presence of a major bifurcation (within the proximal cap, occluded segment or distal cap) has been reported in 26–47% of CTO lesions and is associated with increased procedural complexity and longer-term MACE (primarily driven by reduced side-branch Thrombolysis in Myocardial Infarction [TIMI] flow and periprocedural MI).[42] The strongest predictor of technical success where there is within-CTO side-branch involvement, is luminal side branch wiring at baseline, mitigating side branch loss, although this is not always feasible.[42]
Lesion composition is dependent on CTO duration. Younger lesions display features of organised thrombus and necrotic core. Older lesions contain greater quantities of fibrous tissue and calcium, with a higher prevalence of negative remodelling.[43] Heavily calcified lesions, particularly during the use of a subintimal (SI) strategy when aggressive modification carries an increased risk of perforation, are vulnerable to stent underexpansion or restriction, leading to an increased risk of both ISR and ST.[43] Longer-duration CTO lesions without calcium often demonstrate significant negative remodelling, increasing the risk of vessel rupture with 1: 1 stent sizing and leading to smaller minimum stent area (MSA), another independent predictor of ISR.[43] Pathological considerations for stent sizing are shown in Figure 2.
Figure 2: Considerations and Longer-term Effects for Stent Sizing.
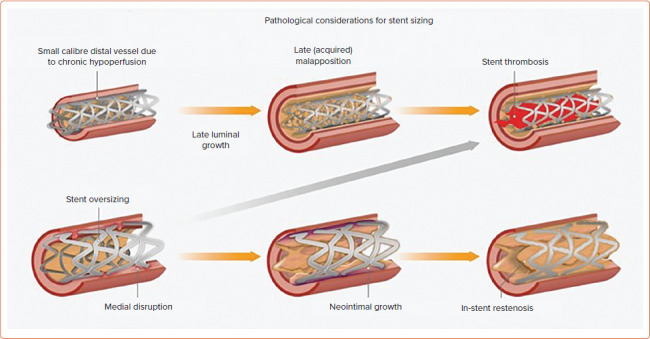
Source: Spratt et al.[43] Reproduced with permission from Optima Education.
Post-CABG, CTO PCI adds an additional dimension of complexity to CTO recanalisation. Up to 40% of venous bypass grafts and 15% of internal mammary artery grafts occlude within 10 years.[44] A study of native vessel patency after CABG demonstrated at least one new CTO in 43.6%.[45] Pre-CABG native vessel stenosis >90% and Canadian Cardiovascular Society Class IV angina at baseline were independent predictors of subsequent postoperative CTO formation.[45] Post-bypass CTO lesions exhibit increased calcification, negative remodelling and blunt proximal cap (Figure 3), rendering wire escalation strategies less successful (38%) than in non-CABG CTO (57%) and requiring early adoption of retrograde or DART strategies.[46,47] Although TVR and MACE were higher in the post-CABG cohort, reassuringly 85% of patients remained event free at 1 year despite the complexity of their disease and procedural technique, alongside increased comorbidity.[47]
Figure 3: Accelerated Development of Proximal Native Vessel Disease Post-Coronary Artery Bypass Grafting on CT Coronary Angiography.
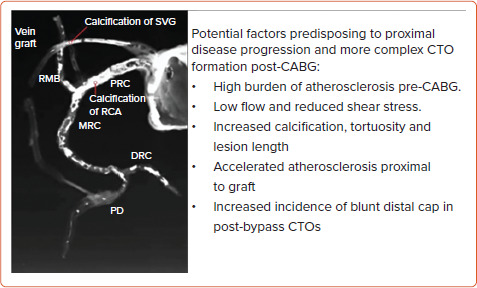
CABG = coronary artery bypass grafting; CTO = chronic total occlusion; DRC = distal right coronary artery; MRC = middle right coronary artery; PD = posterior descending artery; PRC = proximal right coronary artery; RCA = right coronary artery; RMB = right marginal branch; SVG = saphenous vein graft. Source: Spratt et al.[43] Reproduced with permission from Optima Education.
The in-stent (IS) CTO cohort poses further treatment complexity. Specific challenges include luminal wiring of previously under-expanded stents, with a higher likelihood of SI entry at the proximal cap (Figure 4) and increased calcification in these often longer-duration occlusions. Initial experiences of IS-CTO revascularisation reported success rates of 71%, whereas operator up-skilling and the use of novel devices have led to significant improvements, with an 86% success rate reported in both the PROGRESS-CTO registry and another large study by Azzalini et al.[48,49] This is comparable to that of de novo CTO PCI, recognised to be around 90% when performed in high-volume centres by experienced CTO operators.[13,48] Nevertheless, IS-CTO PCI carries a predilection to increased TLR and is independently associated with both increased MACE and repeat revascularisation.[50]
Figure 4: Challenge of Luminal Wiring With In-stent Chronic Total Occlusion.
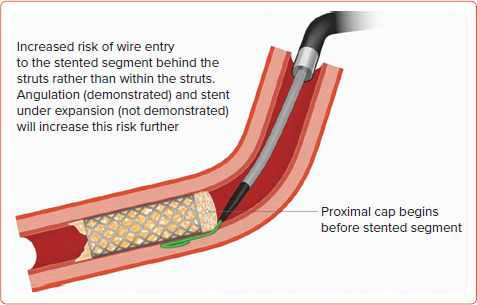
Source: Spratt et al.[43] Reproduced with permission from Optima Education.
The prediction of CTO lesion complexity and procedural success through dedicated scoring systems is well established. Of these systems, the most commonly used J-CTO score has more recently been associated with trends in predicting 5-year TLR.[51] In post-CABG CTO cohorts, the RECHARGE score (which includes a weighting for CABG) was deemed superior to the J-CTO score as an independent predictor of longer-term adverse outcomes, including TVF.[52]
Procedure-related Factors
Chronic Total Occlusion Recanalisation Strategy
Results of studies of the hybrid algorithm components (antegrade wire escalation, antegrade dissection and re-entry, retrograde wire escalation and retrograde dissection and re-entry) are mixed with regard to strategy-associated long-term TLR/TVR. An analysis of predictors of re-occlusion after DES supported CTO PCI in 802 successfully revascularised patients from the Florence CTO Registry, reporting a re-occlusion rate of 7.5%.[53] The use of an SI tracking and re-entry (STAR) technique was associated with the highest re-occlusion rate (57%), compared with 5.1% in the non-STAR cohort.[53] This was echoed in the J-PROCTOR 2 Study, where increased TVR was noted in a retrograde SI tracking cohort, and in a small meta-analysis of five studies where DART was associated with increased longer-term adverse events.[54,55] However, the DART strategy itself is important, with use of newer device-based (CrossBoss and Stingray, Boston Scientific) approaches demonstrating significantly lower rates of MACE compared with older wire-based (STAR, limited antegrade subintimal tracking and controlled antegrade and retrograde tracking and dissection) techniques (8.9% versus 22.1%, respectively).[56] Further, in regression analyses, wire-based DART and stent length were independent predictors of MACE.[56,57] The use of DART in an earlier study by Rinfret et al. had a minimal impact on longer-term outcomes after CTO PCI and, most recently, SI stenting (48.1% of the cohort) in the CONSISTENT-CTO study neither adversely affected intravascular healing at 12 months nor was associated with higher TVR at 2 years.[13,58] Differences between study outcomes, particularly those seen in CONSISTENT-CTO, may be explained by the higher rates (91%) of TIMI grade 3-defined procedural success and the adoption of pre-stenting IVUS (90.5% of cases).[13] The results of the LOTUS ADR/RDR study, assessing long-term outcomes, including binary restenosis at the 13-month angiographic follow-up, 2-year TVR and validation of a dedicated restenosis score (R-Score) of related risk factors, are awaited.[59]
Intravascular Ultrasound
The use of IVUS in all PCIs has increased over the past decade. Global uptake and operator beliefs around the benefits of IVUS are highly variable by region.[60] Although the British Cardiovascular Interventional Society’s national audit data show overall uptake at approximately 12%, a large study by Mentias et al. of more than 1 million Medicare patients in the US demonstrated an increase in IVUS use of only 3.9% (from 3% to 6.9%) between 2009 and 2017.[61,62] However, another multicentre US registry reported a 38% frequency of IVUS.[63] Hence, data are varied, perhaps (in the US) influenced to some extent by reimbursement. In contrast, in Japan, the country with the highest use of IVUS, registry data report >80% uptake.[64] IVUS serves numerous roles, including: resolution of proximal cap ambiguity and guidance of cap penetration; facilitation of antegrade and retrograde DART techniques and prevention of dissection into the aorta; vessel and stent sizing; identification of appropriate landing zones, negative remodelling and calcium; and optimisation of stent expansion and apposition. IVUS also appropriately identifies nodular calcium and the extent to which the SI channel is in the adventitial space. This allows prediction of the risk of perforation with over-zealous post-dilatation, guiding the operator to accept a degree of eccentric stent expansion when appropriate and reasonable. The use of IVUS is associated with improved long-term outcomes in CTO PCI. Although the AVIO trial of angiography versus IVUS-guided PCI of complex lesions showed no significant difference in 2-year MACE, TLR or TVR, subgroup analysis revealed improved minimum lumen diameter (MLD) in the IVUS arm.[65] However, CTO-specific analysis of IVUS versus angiography in the Korean-CTO registry found reductions in MACE, ST and TLR in longer (>30 mm) lesions in the AIR-CTO Study showed significantly lower rates of late lumen loss and ‘in-true-lumen’ stent restenosis and the CTO-IVUS Trial demonstrated reduction in MACE at 12 months.[66–68] Hence, the use of IVUS, both up-front and for stent optimisation, can be deemed crucial in the prevention of longer-term SF and should be routinely used, regardless of CTO lesion complexity or recanalisation strategy.
Importantly, the demonstrated benefits of IVUS are reliant upon accurate image acquisition, interpretation and management of findings. RCTs of IVUS are prescriptive, protocolised to optimise results and often involve operators with greatest expertise, producing optimal outcomes. This is not reflective of real-world practice. Further, the importance of a final IVUS can be exemplified using rates of longitudinal stent deformation (LSD). In registry data, mean rates of approximately 1.2% are reported.[41] However, the EXCEL substudy of stent deformation observed a 6.5% prevalence of LSD.[69] This reiterates the importance of a final imaging run to systematically detect and correct anomalies predicting downstream SF such as LSD, tissue prolapse and edge dissection, in addition to ensuring good stent apposition and expansion.
Stenting Strategy
Stenting strategy, determined by stent platform, diameter, total stent length and post-deployment optimisation, is a key determinant of downstream SF. Evolution from BMS to DES witnessed significant reductions in ISR. This was mediated through reduced neointimal hyperplasia owing to DES bioactivity and reduced ST due to non-endothelialised stent struts, due, in part, to suppression of local inflammatory responses. A meta-analysis of 26,000 patients across 20 studies again supports the superiority of DES, with Picollo et al. demonstrating ST rates at 1- and 5-year follow-up after DES and BMS implantation of 0.6%, 1.1%, 8.4% and 13.4%, respectively, and corresponding TLR rates of 4% and 8.8%, 8.4% and 13.4%, respectively (p<0.001 for all).[70] CTO-specific analysis of DES platforms in the FLORENCE-CTO Registry demonstrated superiority of EES over other DES, with significantly lower re-occlusion rates (3% versus 10.1%).[53] However, other studies have found no significant differences between use of first- and second-generation DES, including Moreno et al., who demonstrated equipoise for restenosis between EES and sirolimus-eluting stent (SES) platforms following CTO PCI.[71–73]
Stent design, including strut thickness, radial strength and longitudinal integrity, affects the risk of LSD, StF and stent usability. Thinner strut designs that allow increased deliverability, trackability and vessel conformability are particularly advantageous in CTOs with tortuous and calcific anatomy or where coronary reconstruction with a ‘metal jacket’ of overlapping stents is required. Early studies of BMS, comparing thick (140 µm) and thin (50 µm) stent struts, found significant differences in both angiographic restenosis and TVR in favour of the thin strut design.[74,75] This is supported by a recent network meta-analysis of 69 randomised controlled trials including over 80,000 patients, which demonstrated reductions in ST and MI with the use of ultrathin versus thick strut DES.[76] The switch from stainless steel to cobalt chromium alloy in these thinner strut platforms allowed preservation of radial strength. However, clinical experience and data show a trade-off in longitudinal strength, rendering thinner strut designs more vulnerable to LSD.[77]
Although infrequent, with rates of 1.2% reported in the literature, LSD precipitates ST, TLF and MACE.[41] Mechanisms relating to LSD are multifactorial, including, in addition to strut thickness, the number of connectors and their orientation. In bench testing, Ormiston et al. demonstrated that stent platforms with two connectors between hoops had reduced longitudinal strength on exposure to external forces compared with designs with six connectors, concluding that fewer connectors directly compromises longitudinal integrity.[78] For example, the Promus Premier platform (Boston Scientific), a newer iteration of the two-connector Promus Element platform, saw the addition of extra connectors at the proximal stent end while maintaining a two-connector design through the main stent body, mitigating the occurrence of LSD while maintaining deliverability.[79] This is an important consideration, particularly in the undertaking of aorto-ostial and bifurcation PCI.
StF, as a precipitant of SF, has been reported to occur at rates of 1–3% and is isolated to DES.[80] Kuramitsu et al. studied StF of EES, finding increased MACE driven by ST and TLR (25.6% versus 2.3% in the non-StF group; p<0.001).[80] Ostial stent location and lesions with hinge motion, tortuosity or increased calcium (prevalent in native and post-CABG CTOs) were independent predictors of StF.[81] Platforms with increased radial strength serve to reduce the risk of StF in such lesions. In contrast to LSD, an increase in connectors confers greater risk of StF due to rigidity and reduced conformability to vessel anatomy and motion.[79] An additional consideration in CTO PCI is the risk of stent undersizing due to negative remodelling, subintimal haematoma or poorer flow. Choosing a platform that can be appropriately overexpanded, particularly in long lesions or where there is distal and proximal vessel size mismatch, is important. This feature of the stent is primarily based on crown and connector design. The data demonstrate that with increasing overexpansion, crown straightening occurs.[82] Although this can increase radial strength, it reduces flexibility and conformity, predisposing to StF. In addition, increased stent cell opening diameter can lead to intrastrut plaque prolapse and impair uniform drug elution, again risking SF.[82] Balancing these factors when making stent choices during CTO PCI is key in longer-term procedural outcomes, and on-going development of the optimal stent platform continues as newer iterations manifest.
Stent length, akin to lesion length, alongside the number of stents deployed, predicts ISR. Ahn et al. studied outcomes after DES in long (>30 mm) versus short (<30 mm) CTO lesions.[83] Although no significant differences in binary restenosis, late lumen loss or MLD were seen at the 6-month angiographic follow-up, higher repeat PCI driven by TVR was noted at 2 years.[83] In the Korean CTO registry, lesion length >20 mm (p<0.01) and the use of at least three DES (p<0.001) were associated with MACE, TVR and TLR at a median follow-up of 22 months.[84] Stent diameter and subsequent MSA are also strong predictors of SF. The combined TAXUS IV, V, VI and ATLAS Workhorse Trials’ IVUS substudy analysis of 1,580 patients demonstrated post-IVUS MSA >5.7 mm[2] predicted 9-month angiographic stent patency.[85] In smaller vessels, postintervention optical coherence tomography-assessed MSA <3.5 mm[2] was found to be a predictor of 9-month ISR and TLR following PCI with a 2.5-mm diameter EES.[86] IVUS analysis of MSA across different stent platforms (zotarolimus-eluting stent, EES and SES), revealed similar optimal MSA cut-off values for predicting ISR (5.2 mm2 and 5.4 mm2), with a smaller MSA predictive of angiographic ISR in first- and second-generation DES.[87] In a CTO-specific study, Kang et al. found that the MLD and stent expansion ratio were independent predictors of ISR.[88] These findings support the importance of image-guided PCI in CTO lesions to: allow accurate estimates of vessel sizing (and therefore stent diameter choice), particularly in the presence of vessel dissection or haematoma; rationalise maximal stent length; identify and appropriately modify calcium to facilitate stent expansion; and optimise stent expansion through post-dilation to achieve greatest MLD, MSA and stent expansion ratio in prevention of downstream ISR and ST.
Pharmacological Factors
Increased vessel trauma, the length of the stented segment and post-revascularisation vessel remodelling, alongside the often highly comorbid nature of this patient group, render the CTO PCI cohort at high risk for SF. RCT evidence over the past decade has led to paradigm shifts in dual antiplatelet regimes, with ticagrelor the preferred addition to aspirin for acute coronary syndrome presentations.[89] In PCI for stable angina, Clopidogrel has remained the second agent of choice in addition to aspirin. Few studies specifically assess optimal platelet reactivity/responsivity or dual antiplatelet therapy choice after CTO PCI. The first analysis of platelet reactivity in a CTO cohort emerged from the FLORENCE CTO registry of more than 1,000 patients who underwent platelet function testing by light transmission aggregometry.[90] The high platelet reactivity cohort had significantly increased cardiac mortality at 3 years compared with an optimal platelet reactivity cohort (p<0.001). Further, when those with high platelet reactivity were identified and clopidogrel (standard of care) switched to either prasugrel or ticagrelor, the survival rates seen were similar to those in the optimal platelet reactivity group.[90] The TIGER trial, although small, demonstrated that ticagrelor pretreatment (compared with clopidogrel) improved downstream coronary vascular flow following CTO recanalisation, with the longer-term potential to improve ischaemia and reduce TLR.[91] This is supported by data identifying TIMI flow grade as an independent predictor of TVF.[92] Although larger RCTs to identify the optimal dual antiplatelet therapy strategy following CTO PCI are required and current guidelines offer no consensus, decisions will be at operator discretion and should factor in comprehensive assessment of patient-specific ischaemic and bleeding risk, alongside the complexity of CTO recanalisation, the extent of vessel stenting and final TIMI flow grade.
Conclusion
SF in both non-CTO and CTO PCI is multifactorial, involving patient comorbidities, cardiovascular risk factor control, lesion complexity, stenting strategy and, finally, antiplatelet and adjunctive medical therapy. As the prevalence of ischaemic risk factors continues to rise, preventive and therapeutic measures to mitigate these are paramount in the reduction of longer-term MACE and repeat revascularisation. Advances in interventional techniques, the development of novel devices and operator upskilling have led to the undertaking of PCI in increasingly complex and high-risk patient subsets. Specific to CTO PCI, increased calcification, the challenges of lesion preparation and stent optimisation in the SI space, where perforation risk is higher, and the potential for stent undersizing at the index procedure is greater, due to negative remodelling and haematoma increase the risk of SF. Therefore, it is crucial to optimise outcomes using a multipronged approach via aggressive medical therapy, lifestyle modification and meticulous image-guided precision PCI to reduce SF in these challenging patient cohorts.
References
- 1.Bonaa K, Mannsverk J, Wiseth R et al. Drug eluting or bare metal stents for coronary artery disease. N Engl J Med. 2016;375:1242–52. doi: 10.1056/NEJMoa1607991. [DOI] [PubMed] [Google Scholar]
- 2.Stolker JM, Cohen DJ, Kennedy KF et al. Repeat revascularisation after contemporary percutaneous coronary intervention; an evaluation of staged, target lesion and other unplanned revascularization procedures during the first year. Circ Cardiovasc Interv. 2012;5:772–82. doi: 10.1161/CIRCINTERVENTIONS.111.967802. [DOI] [PubMed] [Google Scholar]
- 3.Kandzari DE, Leon MB, Meredith I et al. Final 5-year outcomes from the Endeavor zotarolimus-eluting stent clinical trial program: comparison of safety and efficacy with first-generation drug-eluting and bare-metal stents. JACC Cardiovasc Interv. 2013;6:504–12. doi: 10.1016/j.jcin.2012.12.125. [DOI] [PubMed] [Google Scholar]
- 4.Sarno G, Lagerqvist B, Fröbert O et al. Lower risk of stent thrombosis and restenosis with unrestricted use of ‘new-generation’ drug-eluting stents: a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J. 2012;33:606–13. doi: 10.1093/eurheartj/ehr479. [DOI] [PubMed] [Google Scholar]
- 5.Navarese EP, Kowalewski M, Kandzari D et al. First-generation versus second-generation drug-eluting stents in current clinical practise: updated evidence from a comprehensive meta-analysis of randomised clinical trials comparing 31 379 patients. Open Heart. 2014;1:e000064. doi: 10.1136/openhrt-2014-000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone GW, Kappetein AP, Sabik JF et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381:1820–30. doi: 10.1056/NEJMoa1909406. [DOI] [PubMed] [Google Scholar]
- 7.Makikallio T, Holm N, Lindsey M et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective randomised, open label, non-inferiority trial. Lancet. 2016;388:2743–52. doi: 10.1016/S0140-6736(16)32052-9. [DOI] [PubMed] [Google Scholar]
- 8.Mohr FW, Morice MC, Kappetein AP et al. Coronary artery by-pass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–38. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 9.Park SJ, Ahn JM, Kim YH et al. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. 2015;372:1204–12. doi: 10.1056/NEJMoa1415447. [DOI] [PubMed] [Google Scholar]
- 10.Farkouh ME, Domanski M, Sleeper LA et al. Strategies for multivessel revascularisation in patients with diabetes. N Engl J Med. 2012;367:2375–84. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 11.Kotsia A, Navara R, Michael TT et al. The AngiographiC Evaluation of the Everolimus Eluting Stent in Chronic Total Occlusion (ACE-CTO) study. J Invasive Cardiol. 2015;27:393–400. [PubMed] [Google Scholar]
- 12.Mehran R, Claessen BE, Godino C et al. Long-term outcome of percutaneous coronary intervention for chronic total occlusions. JACC Cardiovasc Interv. 2011;4:952–61. doi: 10.1016/j.jcin.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Walsh SJ, Hanratty CG, McEntegart M et al. Intravascular healing is not affected by contemporary approaches in CTO PCI: the CONSISTENT CTO study. JACC Cardiovasc Interv. 2020;13:1448–57. doi: 10.1016/j.jcin.2020.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Kato M, Kimura T, Morimoto T et al. Comparison of five-year outcome of sirolimus-eluting stent implantation for chronic total occlusions versus for non-chronic total occlusion (from the j-Cypher registry). Am J Cardiol. 2012;110:1282–9. doi: 10.1016/j.amjcard.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 15.Azzalini L, Carlino M, Bellini B et al. Long-term outcomes of chronic total occlusion recanalization versus percutaneous coronary intervention for complex non-occlusive coronary artery disease. Am J Cardiol. 2020;125:182–8. doi: 10.1016/j.amjcard.2019.10.034. [DOI] [PubMed] [Google Scholar]
- 16.Salisbury AC, Sapontis J, Grantham JA et al. Outcomes of chronic total occlusion percutaneous coronary intervention in patients with diabetes: insights from the OPEN CTO registry. JACC Cardiovasc Interv. 2017;10:2174–81. doi: 10.1016/j.jcin.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 17.Pfefile B, Dischuneit H. Effect of insulin on growth factors of cultured human arterial smooth muscle cells. Diabetologia. 1981;20:155–8. doi: 10.1007/BF00262020. [DOI] [PubMed] [Google Scholar]
- 18.Hwang JK, Lee SH, Song YB et al. Glycaemic control status after percutaneous coronary intervention and long-term clinical outcomes in patients with type 2 diabetes mellitus. Circ Cardiovasc Interv. 2017;10:e004157. doi: 10.1161/CIRCINTERVENTIONS.117.005616. [DOI] [PubMed] [Google Scholar]
- 19.Orbach A, Halon DA, Rubinshtein R et al. Impact of diabetes and early revascularisation on the need for late and repeat procedures. Cardiovasc Diabetol. 2018;17:25. doi: 10.1186/s12933-018-0669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuo X, Zhang C, Feng J et al. In-hospital short-term and long-term adverse clinical outcomes observed in patients with type 2 diabetes mellitus vs non-diabetes mellitus following percutaneous coronary intervention. Medicine (Baltimore) 2019;98:e14669. doi: 10.1097/MD.0000000000014669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassaian SE, Goodarzynejad H, Boroumand MA et al. Glycosylated hemoglobin (HbA1c) levels and clinical outcomes in diabetic patients following coronary artery stenting. Cardiovasc Diabetol. 2012;11:82. doi: 10.1186/1475-2840-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladwiniec A, Allgar V, Thackray S et al. Medical therapy, percutaneous coronary intervention and prognosis in patients with chronic total occlusions. Heart. 2015;101:1907–14. doi: 10.1136/heartjnl-2015-308181. [DOI] [PubMed] [Google Scholar]
- 23.Yerkey MW, Kernis SJ, Franklin BA et al. Renal dysfunction and acceleration of coronary disease. Heart. 2004;90:961–6. doi: 10.1136/hrt.2003.015503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int. 2006;70:2021–30. doi: 10.1038/sj.ki.5001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JM, Kang J, Lee E et al. Chronic kidney disease in the second-generation drug eluting stent era: pooled analysis of the Korean Multicenter Drug-Eluting Stent registry. JACC Cardiovasc Interv. 2016;9:2097–109. doi: 10.1016/j.jcin.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 26.Nakachi T, Kohsaka S, Yamane M et al. Impact of hemodialysis on procedural outcomes of percutaneous coronary interventions in chronic total occlusion: insights from the Japanese Multi-Center registry. J Am Heart Assoc. 2017;6:e006431. doi: 10.1161/JAHA.117.006431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rear R, Bell RM, Hausenloy DJ. Contrast-induced nephropathy following angiography and cardiac interventions. Heart. 2016;102:638–48. doi: 10.1136/heartjnl-2014-306962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Windecker S, Kolh P, Alfonso F et al. 2014 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2014;35:2541–619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 29.Brar SS, Aharonian V, Mansukhani P et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383:1814–23. doi: 10.1016/S0140-6736(14)60689-9. [DOI] [PubMed] [Google Scholar]
- 30.Briguori C, Visconti G, Focaccio A et al. Renal insufficiency after contrast media administration trial II (REMEDIAL II): RenalGuard system in high-risk patients for contrast-induced acute kidney injury. Circulation. 2011;124:1260–9. doi: 10.1161/CIRCULATIONAHA.111.030759. [DOI] [PubMed] [Google Scholar]
- 31.Ali ZA, Galougahi KK, Nazif T et al. Imaging- and physiology-guided percutaneous coronary intervention without contrast administration in advanced renal failure: a feasibility, safety, and outcome study. Eur Heart J. 2016;37:3090–5. doi: 10.1093/eurheartj/ehw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konstantinidis NK, Werner GS, Deftereos S et al. Temporal trends in chronic total occlusion interventions in Europe: 17626 procedures from the European Registry of Chronic Total Occlusion. Circ Cardiovasc Interv. 2018;11:e006229. doi: 10.1161/CIRCINTERVENTIONS.117.006229. [DOI] [PubMed] [Google Scholar]
- 33.Kannel WB, Castelli WP, Gordon T et al. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham Study. Ann Intern Med. 1971;74:1–12. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- 34.Qin Z, Zheng FW, Zheng C et al. Elevated levels of very low-density lipoprotein cholesterol independently associated with in-stent restenosis in diabetic patients after drug-eluting stent implantation. Chin Med J (Engl) 2017;130:2326–32. doi: 10.4103/0366-6999.213575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin Z, Zhou K, Li YP et al. Remnant lipoproteins play an important role of in-stent restenosis in type 2 diabetes undergoing percutaneous coronary intervention: a single-centre observational cohort study. Cardiovasc Diabetol. 2019;18:11. doi: 10.1186/s12933-019-0819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanags LZ, Wong NKP, Nicholls SJ, Bursill CA. High-density lipoproteins and apolipoprotein A-I improve stent biocompatibility. Arterioscler Thromb Vasc Biol. 2018;38:1691–701. doi: 10.1161/atvbaha.118.310788. [DOI] [PubMed] [Google Scholar]
- 37.Bowman L, Hopewell JC, Chen F et al. Effects of anecatrepib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217–27. doi: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 38.Tian T, Guan C, Gao L et al. Prognostic significance of occlusion length in recanalized chronic total occlusion lesion: a retrospective cohort study with 5-year follow-up. BMJ Open. 2020;10:e038302. doi: 10.1136/bmjopen-2020-038302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn J, Rha SW, Choi B et al. Impact of chronic total occlusion lesion length on six-month angiographic and 2-year clinical outcomes. PLoS One. 2018;13:e0198571. doi: 10.1371/journal.pone.0198571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salisbury AC, Sapontis J, Grantham JA et al. Outcomes of chronic total occlusion percutaneous coronary intervention in patients with diabetes: insights from the OPEN CTO registry. JACC Cardiovasc Interv. 2017;10:2174–81. doi: 10.1016/j.jcin.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 41.Rhee TM, Park KW, Lee JM et al. Predictors and long-term clinical outcome of longitudinal stent deformation: insights from pooled analysis of Korean multicenter drug-eluting stent cohort. Circ Cardiovasc Interv. 2017;10:e005518. doi: 10.1161/CIRCINTERVENTIONS.117.005518. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen-Trong PJ, Rangan BV, Karatasakis A et al. Predictors and outcomes of side-branch occlusion in coronary chronic total occlusion interventions. J Invasive Cardiol. 2016;28:168–73. [PubMed] [Google Scholar]
- 43.Spratt JC, Hanratty CG, Walsh SJ, Wilson SJ. A Guide to Mastering Antegrade CTO PCI. Newcastle Upon Tyne: Optima Education, 2019
- 44.Goldman S, Zadina K, Moritz T et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–56. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 45.Pereg D, Fefer P, Samuel M et al. Native coronary artery patency after coronary artery bypass grafting. JACC Cardiovasc Interv. 2014;7:761–7. doi: 10.1016/j.jcin.2014.01.164. [DOI] [PubMed] [Google Scholar]
- 46.Sakakura K, Nakano M, Otsuka F et al. Comparison of pathology of chronic total occlusion with and without coronary artery bypass graft. Eur Heart J. 2014;35:1683–93. doi: 10.1093/eurheartj/eht422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dautov R, Nguyen CM, Altisent O et al. Recanalisation of CTO in patients with previous coronary artery bypass surgery and consideration of retrograde access via saphenous vein graft. Circ Cardiovasc Interv. 2016;9:e003515. doi: 10.1161/CIRCINTERVENTIONS.115.003515. [DOI] [PubMed] [Google Scholar]
- 48.Tajti P, Karmpaliotis D, Alaswad K et al. The hybrid approach to chronic total occlusion percutaneous coronary intervention: update from the PROGRESS CTO registry. JACC Cardiovasc Interv. 2018;11:1325–35. doi: 10.1016/j.jcin.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 49.Azzalini L, Dautav R, Ojeda S et al. Procedural and long-term outcomes of percutaneous coronary intervention for in-stent chronic total occlusion. JACC Cardiovasc Interv. 2017;10:892–902. doi: 10.1016/j.jcin.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 50.Lee SH, Cho JY, Kim JS et al. A comparison of procedural success rate and long-term clinical outcomes between in-stent restenosis chronic total occlusion and de novo chronic total occlusion using multicenter registry data. Clin Res Cardiol. 2020;109:628–37. doi: 10.1007/s00392-019-01550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abe M, Morimoto T, Morino Y et al. Association between J-CTO score and long-term target lesion revascularization rate after successful chronic total coronary occlusion angioplasty (from the J-CTO Registry). Catheter Cardiovasc Interv. 2019;93:1025–32. doi: 10.1002/ccd.28104. [DOI] [PubMed] [Google Scholar]
- 52.Azzalini L, Ojeda S, Karatasakis A et al. Long-term outcomes of percutaneous coronary intervention for chronic total occlusion in patients who have undergone coronary artery bypass grafting vs those who have not. Can J Cardiol. 2018;34:310–8. doi: 10.1016/j.cjca.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Valenti R, Vergara R, Migliorini A et al. Predictors of re-occlusion after successful drug-eluting stent-supported percutaneous coronary intervention of chronic total occlusion. J Am Coll Cardiol. 2013;61:545–50. doi: 10.1016/j.jacc.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 54.Hasegawa K, Tsuchikane E, Okamura A et al. Incidence and impact on midterm outcome of intimal versus subintimal tracking with both antegrade and retrograde approaches in patients with successful recanalisation of chronic total occlusions: J-PROCTOR 2 study. EuroIntervention. 2017;12:e1868–73. doi: 10.4244/EIJ-D-16-00557. [DOI] [PubMed] [Google Scholar]
- 55.Karatasakis A, Danek BA, Karacsonyi J et al. Mid-term outcomes of chronic total occlusion percutaneous coronary intervention with subadventitial vs. intraplaque crossing: a systematic review and meta-analysis. Int J Cardiol. 2018;253:29–34. doi: 10.1016/j.ijcard.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 56.Azzalini L, Dautov R, Brilakis ES et al. Impact of crossing strategy on midterm outcomes following percutaneous revascularisation of coronary chronic total occlusions. EuroIntervention. 2017;13:978–85. doi: 10.4244/EIJ-D-16-01010. [DOI] [PubMed] [Google Scholar]
- 57.Azzalini L, Dautov R, Brilakis ES et al. Procedural and longer-term outcomes of wire- versus device-based antegrade dissection and re-entry techniques for the percutaneous revascularization of coronary chronic total occlusions. Int J Cardiol. 2017;231:78–83. doi: 10.1016/j.ijcard.2016.11.273. [DOI] [PubMed] [Google Scholar]
- 58.Rinfret S, Ribeiro HB, Nguyen CM et al. Dissection and re-entry techniques and longer-term outcomes following successful percutaneous coronary intervention of chronic total occlusion. Am J Cardiol. 2014;114:1354–60. doi: 10.1016/j.amjcard.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 59.Long-term outcomes of successful chronic total occlusion percutaneous coronary interventions using the antegrade and retrograde dissection and re-entry approach (LOTUS ADR/RDR). Protocol. 2018. https://clinicaltrials.gov/ProvidedDocs/38/NCT03769038/Prot_SAP_000.pdf (accessed 22 June 2021) [DOI] [PubMed]
- 60.Kaskinas KC, Nakamura M, Raber L et al. Current use of intracoronary imaging in interventional practice – results of a European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) clinical practice survey. EuroIntervention. 2018;14:e475–84. doi: 10.4244/EIJY18M03_01. [DOI] [PubMed] [Google Scholar]
- 61.Ludman PF. UK National Audit. Percutaneous Coronary Intervention 1st April 2019 to 31st March 2020. BCIS. http://bcis.org.uk/wp-content/uploads/2021/01/BCIS-Audit-2018-19-data-ALL-4-5-2020-for-web.pdf (accessed 7 June 2021)
- 62.Mentias A, Sarrazin MV, Saad M et al. Long-term outcomes of coronary stenting with and without use of intravascular ultrasound. JACC Cardiovasc Interv. 2020;13:1880–90. doi: 10.1016/j.jcin.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karacsonyi J, Alaswad K, Jaffer FA et al. Use of intravascular imaging during chronic total occlusion percutaneous coronary intervention: insights from a contemporary multicenter registry. J Am Heart Assoc. 2016;5:e003890. doi: 10.1161/JAHA.116.003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuno T, Numasawa Y, Sawano M et al. Real-world use of intravascular ultrasound in Japan: a report from contemporary multicenter PCI registry. Heart Vessels. 2019;34:1728–39. doi: 10.1007/s00380-019-01427-9. [DOI] [PubMed] [Google Scholar]
- 65.Cheiffo A, Latib A, Caussin C et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J. 2013;165:65–72. doi: 10.1016/j.ahj.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Hong SJ, Kim BK, Shin DH et al. Usefulness of intravascular ultrasound guidance in percutaneous coronary intervention with second-generation drug-eluting stents for chronic total occlusions (from the Multicenter Korean-Chronic Total Occlusion Registry). Am J Cardiol. 2014;114:534–40. doi: 10.1016/j.amjcard.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Tian NL, Gami SK, Ye F et al. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study. EuroIntervention. 2015;10:1409–17. doi: 10.4244/EIJV10I12A245. [DOI] [PubMed] [Google Scholar]
- 68.Kim BK, Shin DH, Hong MK et al. Clinical Impact of intravascular ultrasound-guided chronic total occlusion intervention with zotarolimus-eluting versus biolimus-eluting stent implantation. Circ Cardiovasc Interv. 2015;8:e002592. doi: 10.1161/CIRCINTERVENTIONS.115.002592. [DOI] [PubMed] [Google Scholar]
- 69.Maehara A. Frequency and impact of acute stent deformation after PCI of LM artery disease: EXCEL IVUS sub-study. Presented at: XIV European Bifurcation Club, Brussels, 13 October 2018. https://bifurc.eu/wp-content/uploads/2019/05/Frequency-ad-impact-of-acute-stent- deformation-after-PCI-of-LM-artery.pdf (accessed 22 June 2021)
- 70.Picollo R, Bonaa KH, Efthimiou O et al. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 2019;393:2503–10. doi: 10.1016/s0140-6736(19)30474-x. [DOI] [PubMed] [Google Scholar]
- 71.Moreno R, Garcia E, Teles R et al. Randomized comparison of sirolimus-eluting and everolimus-eluting coronary stents in the treatment of total coronary occlusions: results from the chronic coronary occlusion treated by everolimus-eluting stent randomized trial. Circ Cardiovasc Interv. 2013;6:21–8. doi: 10.1161/CIRCINTERVENTIONS.112.000076. [DOI] [PubMed] [Google Scholar]
- 72.Ahn JH, Yang JH, Yu CW et al. First-generation versus second-generation drug-eluting stents in coronary chronic total occlusions: two-year results of a multicenter registry. PLoS One. 2016;11:e0157549. doi: 10.1371/journal.pone.0157549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho MS, Lee PH, Lee SW et al. Comparison of second- and first-generation drug eluting stent for percutaneous coronary chronic total occlusion intervention. Int J Cardiol. 2016;206:7–11. doi: 10.1016/j.ijcard.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 74.Kastrati A, Mehilli J, Dirschinger J et al. Intra-coronary Stenting and Angiographic Results: Strut Thickness Effect on Restenosis Outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816–21. doi: 10.1161/01.CIR.103.23.2816. [DOI] [PubMed] [Google Scholar]
- 75.Pache J, Kastrati A, Mehilli J et al. Intracoronary Stenting and Angiographic Results: Strut Thickness Effect on Restenosis Outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41:1283–8. doi: 10.1016/s0735-1097(03)00119-0. [DOI] [PubMed] [Google Scholar]
- 76.Iantorno M, Lipinski MJ, Garcia-Garcia HM et al. Meta-analysis of the impact of strut-thickness on outcomes in patients with drug eluting stents in a coronary artery. Am J Cardiol. 2018;122:1652–60. doi: 10.1016/j.amjcard.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 77.Williams PD, Mamas MA, Morgan KP et al. Longitudinal stent deformation: a retrospective analysis of frequency and mechanisms. EuroIntervention. 2012;8:267–74. doi: 10.4244/EIJV8I2A41. [DOI] [PubMed] [Google Scholar]
- 78.Ormiston JA, Webber B, Webster MWI. Stent longitudinal integrity: bench insights into a clinical problem. JACC Cardiovasc Interv. 2011;4:1310–7. doi: 10.1016/j.jcin.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 79.Watson T, Webster MWI, Ormiston JA et al. Long and short of optimal stent design. Open Heart. 2017;4:e000680. doi: 10.1136/openhrt-2017-000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuramitsu S, Iwabuchi M, Haraguchi T et al. Incidence and clinical impact of stent fracture after everolimus-eluting stent implantation. Circ Cardiovasc Interv. 2012;5:663–71. doi: 10.1161/CIRCINTERVENTIONS.112.969238. [DOI] [PubMed] [Google Scholar]
- 81.Popma JJ, Tiroch K, Almonacid A et al. A qualitative and quantitative angiographic analysis of stent fracture late following sirolimus-eluting stent implantation. Am J Cardiol. 2009;1:923–9. doi: 10.1016/j.amjcard.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 82.Ng J, Foin N, Ang HY et al. Over-expansion capacity and stent design model: an update with contemporary DES platforms. Int J Cardiol. 2016;221:171–9. doi: 10.1016/j.ijcard.2016.06.097. [DOI] [PubMed] [Google Scholar]
- 83.Ahn J, Rha SW, Choi B et al. Impact of chronic total occlusion lesion length on six-month angiographic and 2-year clinical outcomes. PLoS One. 2018;13:e0198571. doi: 10.1371/journal.pone.0198571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim GS, Kim BK, Shin DH et al. Predictors of poor clinical outcomes after successful chronic total occlusion intervention with drug-eluting stents. Coron Artery Dis. 2017;28:381–6. doi: 10.1097/MCA.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 85.Doi H, Maehara A, Mintz GS et al. An Integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Interv. 2009;2:1269–75. doi: 10.1016/j.jcin.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 86.Matsuo Y, Kubo T, Aoki H et al. Optimal threshold of postintervention minimum stent area to predict in-stent restenosis in small coronary arteries: an optical coherence tomography analysis. Catheter Cardiovasc Interv. 2016;87:e9–14. doi: 10.1002/ccd.26143. [DOI] [PubMed] [Google Scholar]
- 87.Song HG, Kang SJ, Ahn JM et al. Intravascular ultrasound assessment of optimal stent area to prevent in-stent restenosis after zotarolimus-, everolimus-, and sirolimus-eluting stent implantation. Catheter Cardiovasc Interv. 2014;83:873–8. doi: 10.1002/ccd.24560. [DOI] [PubMed] [Google Scholar]
- 88.Kang J, Cho YS, Kijm SW et al. Intravascular ultrasound and angiographic predictors of in-stent restenosis of chronic total occlusion lesions. PLoS One. 2015;10:e0140421. doi: 10.1371/journal.pone.0140421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neumann F-J, Sousa-Uva M, Ahlsson A et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 90.de Gregorio MG, Marcucci R, Migliorini A et al. Clinical implications of “tailored” antiplatelet therapy in patients with chronic total occlusion. J Am Heart Assoc. 2020;9:e014676. doi: 10.1161/JAHA.119.014676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brugaletta S, Gomez-Lara J, Caballero J et al. Ticagrelor vs. clopidogrel for recovery of vascular function immediately after successful chronic coronary total occlusi on recanalization: a randomized clinical trial. Am Heart J. 2018;204:205–9. doi: 10.1016/j.ahj.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 92.Geyer M, Wild J, Hirschmann M et al. Predictors for target vessel failure after recanalization of chronic total occlusions in patients undergoing surveillance coronary angiography. J Clin Med. 2020;9:178. doi: 10.3390/jcm9010178. [DOI] [PMC free article] [PubMed] [Google Scholar]


