Abstract
Background:
Recommended testing for both infants with Zika-associated birth defects (i.e., microcephaly and selected brain or eye anomalies) and infants without birth defects whose mothers had laboratory evidence of possible Zika virus (ZIKV) infection during pregnancy includes nucleic acid amplification testing (NAAT) and immunoglobulin M (IgM) testing within days after birth. Brain and eye defects highly specific for congenital ZIKV infection have been described; sporadic reports have documented negative ZIKV testing in such infants.
Methods:
Infants from the U.S. Zika Pregnancy and Infant Registry and Zika Birth Defects Surveillance with Zika-associated birth defects and maternal and infant laboratory testing for ZIKV and two congenital infections (i.e., cytomegalovirus [CMV] and toxoplasmosis) were reviewed for phenotype and laboratory results. Infants with at least one defect considered highly specific for congenital ZIKV infection were designated as having congenital Zika syndrome (CZS) clinical phenotype for this study.
Results:
Of 325 liveborn infants with Zika-associated birth defects and laboratory evidence of maternal ZIKV infection, 33 (10%) had CZS clinical phenotype; 172 (53%) had ZIKV IgM testing with negative or no ZIKV NAAT. ZIKV IgM was negative in the remaining 121 infants, and for 90%, testing for CMV and toxoplasmosis was missing/incomplete. Among 11 infants testing negative for ZIKV IgM, CMV, and toxoplasmosis, 2 infants had CZS clinical phenotype.
Conclusions:
These data add support to previous reports of negative ZIKV IgM testing in infants with clear maternal and phenotypic evidence of congenital ZIKV infection. Follow-up care consistent with the diagnosis is recommended regardless of infant ZIKV test results.
Keywords: congenital anomalies, Zika virus, Zika-associated birth defects, ZIKV testing
1 |. INTRODUCTION
Zika virus (ZIKV) infection during pregnancy can cause serious birth defects in the developing fetus, primarily affecting the central and peripheral nervous systems (de Araújo et al., 2018; Honein et al., 2017; Krow-Lucal et al., 2018; Rasmussen, Jamieson, Honein, & Petersen, 2016; Reynolds et al., 2017). Zika-associated birth defects include structural anomalies of the brain and eye, including microcephaly (Moore et al., 2017; Rice et al., 2018). A distinctive clinical phenotype, which has been termed congenital Zika syndrome (CZS), has been described with features that are highly specific to congenital ZIKV infection, including severe microcephaly with partially collapsed skull and thin cerebral cortices with subcortical calcifications; macular scarring and focal pigmentary retinal mottling; congenital contractures; and marked early hypertonia and symptoms of extrapyramidal involvement (Moore et al., 2017). Due to the serious birth defects associated with ZIKV infection during pregnancy, it is important that infants exposed to ZIKV in utero receive the recommended testing and clinical evaluation to facilitate early intervention and developmental assistance. A standard evaluation is recommended for all infants born to mothers with possible ZIKV exposure during pregnancy (Adebanjo et al., 2017). It is suggested that providers consider ZIKV laboratory testing for infants based on clinical findings of Zika-associated birth defects or, in the absence of birth defects, maternal laboratory evidence of possible ZIKV infection during pregnancy (Adebanjo et al., 2017). Infants with findings consistent with CZS are highly encouraged to receive the recommended follow-up care regardless of maternal test results.
The time between infection and testing can make the diagnosis of ZIKV challenging in mothers and infants. Most ZIKV infections are asymptomatic, and among women with symptoms, the presentation might be mild. Testing is not recommended for asymptomatic pregnant women with recent possible exposure, but without ongoing exposure (Oduyebo et al., 2017). In 2016, interim guidance for pregnant women residing in an area with active ZIKV transmission, with or without clinical illness consistent with ZIKV disease, included testing women in the first and second trimesters of pregnancy (Peterson et al., 2016); therefore, some women without clinical evidence of ZIKV infection during pregnancy were routinely tested for ZIKV infection. Although there are a few reports of prolonged detection of ZIKV ribonucleic acid (RNA) by nucleic acid amplification testing (NAAT), detection of ZIKV RNA is most accurate within 14 days of infection for pregnant women; therefore, a negative ZIKV NAAT does not rule out infection (Bingham et al., 2016; Oduyebo et al., 2017; Rabe et al., 2016).
Current recommendations include infant ZIKV NAAT (serum and urine) and immunoglobulin M (IgM) testing in serum within a few days after birth (Adebanjo et al., 2017). Plaque reduction neutralization test (PRNT) can be used to confirm congenital ZIKV infection in children at age ≥18 months with negative ZIKV IgM and NAAT at birth (Adebanjo et al., 2017). During the ZIKV pandemic, jurisdictions with endemic dengue, such as Puerto Rico, did not conduct PRNTs on maternal or infant specimens as a confirmatory test for positive ZIKV IgM results due to cross-reactivity with dengue virus (Sharp et al., 2019). Depending on the timing of maternal infection and the fetal immunologic response to the infection, NAAT and IgM antibody testing of the infant at the time of delivery might not be positive (Adebanjo et al., 2017). Infants born with birth defects consistent with CZS clinical phenotype but without infant positive laboratory testing for ZIKV have been previously reported (de Araújo et al., 2018; de Melo Espindola et al., 2021; Melo et al., 2016; Pomar et al., 2018; Rodo et al., 2018). The infants were born to mothers who tested positive for ZIKV by NAAT and/or serology (IgM) during pregnancy; the infants tested negative for ZIKV RNA and IgM at birth. The sensitivity and positive predictive value of laboratory testing for diagnosing infants with CZS clinical phenotype are not currently known (Adebanjo et al., 2017).
Using data from the two U.S. surveillance cohorts, we sought to determine if there were infants similar to those previously reported who had clear clinical evidence of the CZS clinical phenotype but negative postnatal testing for ZIKV, and if so, how prevalent this might be in our cohorts. The two surveillance systems collect maternal and infant ZIKV testing, reported infant physical anomalies, and maternal infectious disease testing during pregnancy, if performed. Because some features of the CZS clinical phenotype are similar to those seen with other congenital infections, we excluded any infant with evidence of congenital cytomegalovirus (CMV) or toxoplasmosis infections or cases in which coinfections could not be ruled out, mother or infant. These findings can be used to evaluate the health risks of infants born to women with ZIKV infection during pregnancy.
2 |. METHODS
We reviewed cases of infants with Zika-associated birth defects (i.e., microcephaly and brain or eye abnormalities at birth) from two U.S.-based surveillance systems launched during the 2016 ZIKV response. First, the U.S. Zika Pregnancy and Infant Registry (USZPIR) collected data on infants born to mothers with laboratory evidence of confirmed or possible ZIKV infection during pregnancy in the U.S. states and territories from December 2015 to March 2018 (Reynolds et al., 2017). Evidence of ZIKV infection during pregnancy was defined as a positive ZIKV NAAT or serologic evidence in a maternal, placental, fetal, or infant specimen. Data were abstracted from medical records from birth through 3 years of age, including growth parameters, physical examination findings, imaging studies, developmental screenings, and other evaluations at multiple time points. Infants from approximately 7,500 completed pregnancies are monitored in this system. Second, the Zika Birth Defects Surveillance (ZBDS) is a population-based surveillance system that collected data on 3,359 infants and fetuses who had a birth defect potentially related to ZIKV infection, regardless of ZIKV laboratory findings, in 22 U. S. states and territories from January 2016 to June 2017 (Olson et al., 2019; Smoots et al., 2020). Infants captured in both surveillance systems were matched to increase data completeness.
Clinical phenotype and laboratory testing results for infants with Zika-associated birth defects were reviewed to determine which infants met the following inclusion criteria: (a) born to women with positive ZIKV testing during pregnancy or from a delivery sample, (b) negative infant ZIKV IgM testing performed in the first 14 days of life to rule out postnatal infection and ZIKV NAAT negative or NAAT not performed, and (c) negative CMV and toxoplasmosis testing in either the mother or infant. Two clinicians reviewed cases for the CZS clinical phenotype. The last criterion required the presence of at least one birth defect considered to be a highly specific component of the pattern of birth defects associated with congenital ZIKV infection (subcortical/cortical calcifications, arthrogryposis, macular scarring and pigmentary anomaly, or cranial anomalies consistent with fetal brain disruption sequence [collapsed skull, overlapping sutures, prominent occiput, and scalp rugae]) (Galang et al., 2020). Among case infants meeting the inclusion criteria, we describe maternal history of timing and symptoms of ZIKV infection; maternal and infant ZIKV testing; CMV, toxoplasmosis, and other testing (i.e., rubella, herpes simplex, and HIV) when available; infants’ physical examination findings and imaging studies; and other relevant information. Sensitivity (SE) and positive predictive value (PPV) were calculated using CZS clinical phenotype as the gold standard for all infants with Zika IgM testing, as well as for infants with negative CMV and toxoplasmosis testing.
3 |. RESULTS
A total of 325 liveborn infants were identified with Zika-associated birth defects and laboratory evidence of possible or confirmed ZIKV infection during pregnancy (Figure 1). Of the 325, 15 infants had negative or no maternal ZIKV testing, and 106 had no postnatal ZIKV testing. Among those with positive maternal ZIKV testing and postnatal ZIKA testing in the infant (n = 204), 172 had Zika IgM testing with negative NAAT or NAAT not performed. One hundred and twenty-one infants had negative ZIKV IgM and 51 had positive or equivocal ZIKV IgM. Among infants with positive or equivocal ZIKV IgM results, 24/51 (47%) had negative testing for congenital CMV and toxoplasmosis reported. Among the ZIKV IgM negative infants, most lacked sufficient information on CMV and toxoplasmosis testing, but 11/121 (9%) had negative testing for CMV and toxoplasmosis.
FIGURE 1.
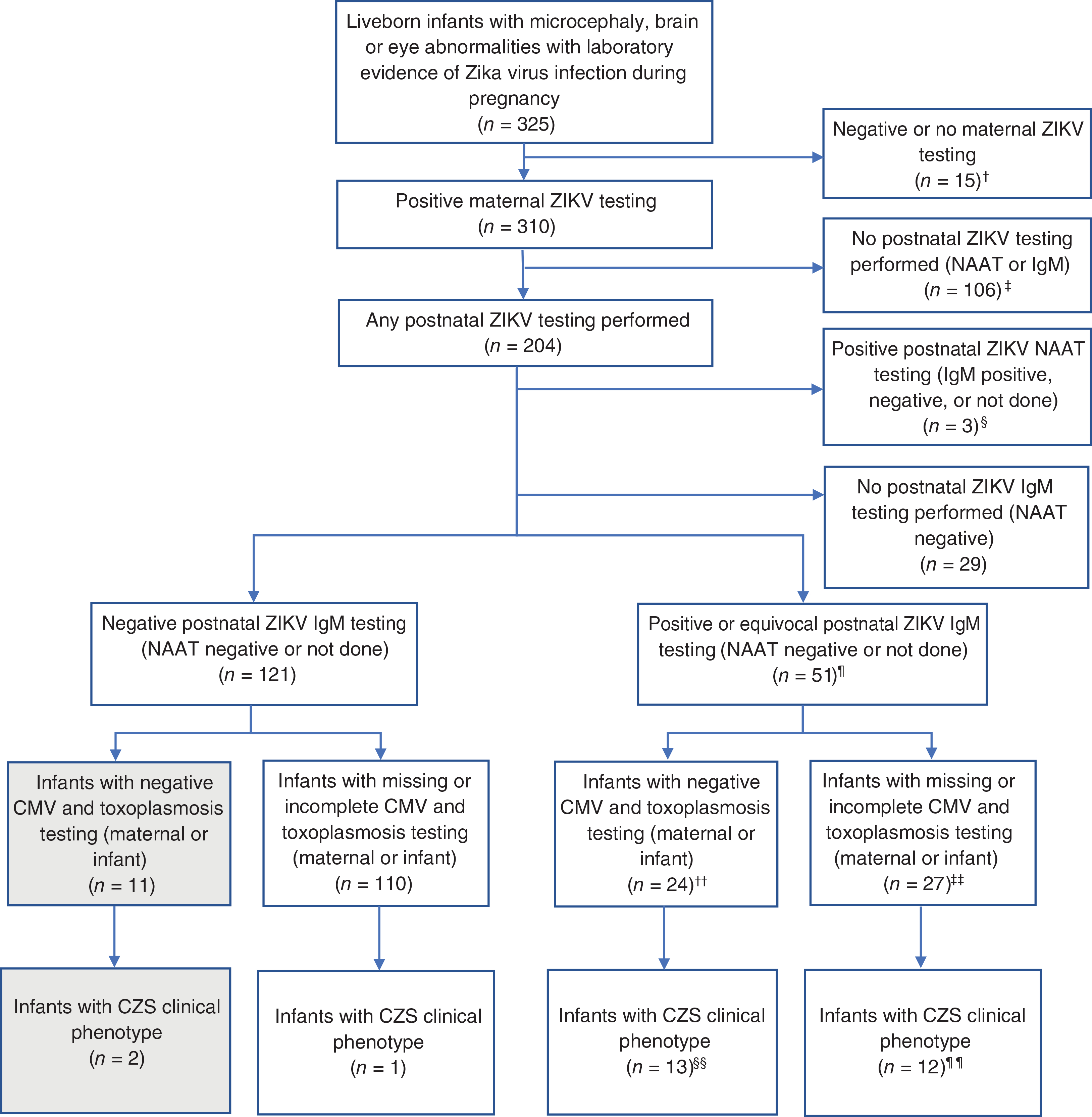
Flowchart of cases with negative ZIKV IgM testing born to mothers with confirmed ZIKV infection during pregnancy and the CZS clinical phenotype from the U.S. Zika Pregnancy and Infant Registry and Zika Birth Defects Surveillance. †One infant excluded had the CZS clinical phenotype; ‡2 infants excluded had the CZS clinical phenotype; §2 infants excluded had the CZS clinical phenotype; ¶48 IgM+, 3 IgM equivocal; ††23 IgM+, 1 IgM equivocal; ‡‡25 IgM+, 2 IgM equivocal; §§12 IgM+, 1 IgM equivocal; ¶¶11 IgM+, 1 IgM equivocal. CMV = cytomegalovirus; CZS = congenital Zika syndrome; IgM = immunoglobulin M; NAAT = nucleic acid amplification testing; ZIKV = Zika virus
A review of Zika-associated birth defect cases for the CZS clinical phenotype identified 33/325 (10%) infants with at least one birth defect considered to be highly specific for congenital ZIKV infection meeting our definition for the CZS clinical phenotype. Two of these 33 infants (6%) were among the 11 with negative IgM testing for ZIKV and negative CMV and toxoplasmosis testing (see Section 4 and Table 1).
TABLE 1.
Sensitivity and positive predictive value for Zika immunoglobulin M testing compared to congenital Zika syndrome (CZS) clinical phenotypes
| CZS clinical phenotype (+) | CZS clinical phenotype (−) | Total | Sensitivity | PPV | |
|---|---|---|---|---|---|
| Cases with complete negative CMV or toxoplasmosis testing | |||||
| IgM+ or equivocal | 13 | 11 | 24 | ||
| IgM− | 2 | 9 | 11 | ||
| Total | 15a | 20 | 35 | 87% | 54% |
| All cases, regardless of CMV or toxoplasmosis testing | |||||
| IgM+ or equivocal | 25 | 26 | 51 | ||
| IgM− | 3 | 118 | 121 | ||
| Total | 28b | 144 | 172 | 89% | 49% |
Abbreviations: CMV = cytomegalovirus; IgM = immunoglobulin M.
15/33 infants with at least one birth defect considered highly specific for congenital ZIKV infection also met testing criteria. Eighteen infants were excluded due to lack of maternal ZIKV testing, no infant postnatal ZIKV testing performed, positive postnatal ZIKV NAAT or did not have complete or negative CMV or toxoplasmosis testing.
28/33 infants with at least one birth defect considered highly specific for congenital ZIKV infection also met testing criteria. Five infants were excluded due to lack of maternal ZIKV testing, no infant postnatal ZIKV testing performed or positive postnatal ZIKV NAAT.
For all infants with ZIKV IgM testing and negative NAAT or NAAT not performed, the SE and PPV of ZIKV IgM assay for detecting CZS phenotype was 89% (25/28) and 49% (25/51), respectively, while for the infants with negative toxoplasmosis and CMV testing, the SE was 87% (13/15) and the PPV was 54% (13/24) (Table 1). Of the 121 infants with negative ZIKV IgM testing, 4 received PRNT testing at or around 18 months of age; 3 were positive and 1 was negative.
4 |. CASE DESCRIPTIONS
Case 1 presented with microcephaly (−4.9 SD), weighed less than the 10th percentile at birth, and had cranial anomalies consistent with fetal brain disruption sequence (Figure 2). Brain ultrasound showed intracranial calcifications (bilateral cerebral peripheral), cerebral/cortical atrophy, and ventriculomegaly and the infant had arthrogryposis. Ophthalmologic examination showed chorioretinal pigmentary changes and optic nerve hypoplasia. At 2.5 months of age, the infant had worsening microcephaly with a head circumference of −7.9 SD, and the infant had developmental delay with hypertonia and spasticity. Vision loss was documented on ophthalmologic examination at 5 months.
FIGURE 2.
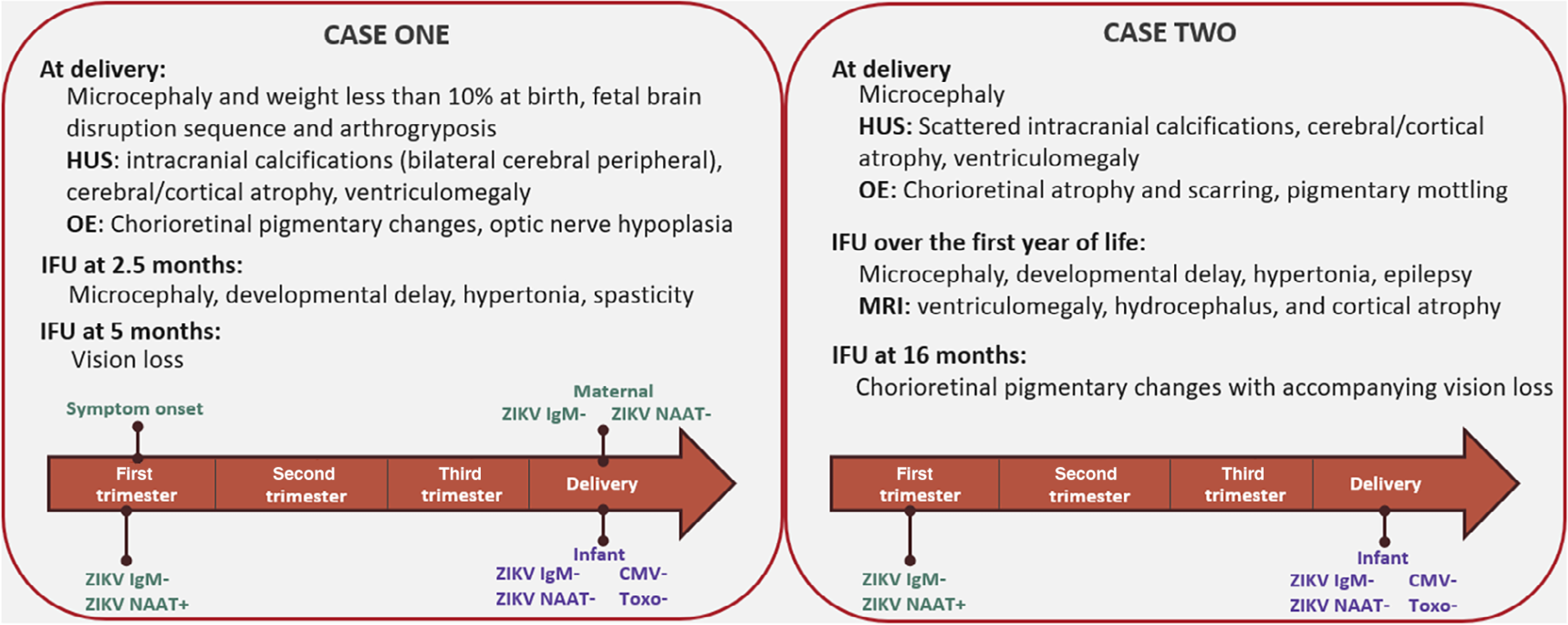
Case descriptions and test results for two infants with birth defects consistent with the congenital Zika syndrome clinical phenotype and negative infant ZIKV IgM. CMV = cytomegalovirus; HUS = head ultrasound; IFU = infant follow-up; IgM = immunoglobulin M; NAAT = nucleic acid amplification testing; OE = ophthalmologic exam; Toxo = toxoplasmosis; ZIKV = Zika virus
Mother had reported symptoms clinically compatible with ZIKV disease in the first trimester. ZIKV NAAT by PCR in serum was positive and ZIKV IgM was negative in the first trimester. Repeat maternal testing at delivery was ZIKV NAAT and ZIKV IgM negative. Infant samples taken at delivery were negative for ZIKV IgM and ZIKV NAAT in serum. Infant ZIKV NAAT in CSF and urine were negative. Mother tested positive for chlamydia (testing type unknown) in the first trimester. She was rubella immune and tested negative for the following: syphilis RPR, HIV, gonorrhea, and varicella in the first trimester. CMV, toxoplasmosis, and herpes IgM testing in the infant at the day of birth was negative, and infant was rubella nonimmune. No PRNT was performed at 18 months of age.
Case 2 presented with microcephaly at birth (−3.5 SD) and brain ultrasound showed scattered intracranial calcifications, cerebral/cortical atrophy, and ventriculomegaly (Figure 2). On ophthalmologic examination, chorioretinal atrophy and scarring, and pigmentary mottling were seen. Infant follow-up over the first year of life demonstrated continued microcephaly on multiple time points, with a head circumference measurement of −2.6 SD at 18 months of age, developmental delay, hypertonia, and epilepsy. Head MRI at 11 months of life showed ventriculomegaly/hydrocephalus and cortical atrophy. Chorioretinal pigmentary changes were confirmed at age 16 months with accompanying vision loss.
Mother did not have symptoms clinically compatible with ZIKV infection during pregnancy. Routine maternal testing in the first trimester was ZIKV NAAT by PCR positive and ZIKV IgM negative. Infant samples taken at delivery were negative for ZIKV IgM in serum and NAAT negative for ZIKV in urine and cord blood. Maternal and infant toxoplasmosis and CMV testing at delivery were negative. Mother tested negative for HIV and syphilis venereal disease research laboratory (VDRL) on the day of delivery. Infant was rubella nonimmune on the day of birth. No PRNT was performed at 18 months of age.
5 |. DISCUSSION
Although there have been previous reports of infants with a CZS clinical phenotype but without supporting laboratory evidence of ZIKV infection and other similar congenital infections ruled out, information about these infants has been limited (de Araújo et al., 2018; de Melo Espindola et al., 2021; Melo et al., 2016; Pomar et al., 2018; Rodo et al., 2018). The data in this report support previous observations of a CZS clinical phenotype with negative ZIKV IgM, and the two cases in this report provide additional information on the CZS clinical phenotype including neurodevelopment as well as testing for ZIKV and other congenital infections. The mother of Case 1 had symptoms of ZIKV infection in the first trimester and the mother of Case 2 was asymptomatic; routine testing revealed both mothers were ZIKV NAAT positive in the first trimester, confirming infection. Infant testing of these cases was not supportive of the clinical diagnosis, likely due to clearance of ZIKV RNA as well as ZIKV IgM antibodies from infant serum, though issues related to test characteristics and performance could have contributed. Neither infant had PRNTs performed at 18 months of age. The infants presented with characteristic clinical findings of CZS and each had at least one highly specific finding, including cranial morphology, consistent with fetal brain disruption sequence (Russell, Weaver, Bull, & Weinbaum, 1984), and arthrogryposis in Case 1 and macular and chorioretinal pigmentary changes and scarring in Case 2. Neurologic and long-term developmental sequelae were found at follow up including continued microcephaly, spasticity, developmental delay, vision loss, and epilepsy.
Overall, SE of ZIKV IgM testing was greater than 85% for detecting infection among infants with the CZS clinical phenotype, but PPV was around 50%. Of those with CZS clinical phenotype and negative CMV and toxoplasmosis testing, 13% (2/15) were IgM negative. For those infants with negative IgM testing, two babies had the CZS clinical phenotype; one additional infant also had the CZS clinical phenotype but CMV and toxoplasmosis congenital infection could not be ruled out. The two infants described above had Zika-associated birth defects consistent with congenital ZIKV infection, but the diagnosis could have been missed due to negative infant ZIKV IgM and ZIKV NAAT at birth. These findings, coupled with cases from prior reports provide support that both laboratory testing, including for other causes, and phenotype characteristics that are consistent with Zika infection during pregnancy are important components to the diagnosis of congenital Zika infection.
There were several limitations of this study. First, these findings are based on surveillance data collected during the 2016 ZIKV outbreak, and a comprehensive evaluation might not have been conducted or reported to CDC. Follow-up data were inconsistently reported as infants may have moved, did not attend all recommended appointments, or were otherwise lost to follow-up. For infants with lack of imaging studies and/or follow-up evaluations a clinical phenotype consistent with CZS might not have been identified leading to misclassification of some infants. Second, ZIKV testing guidelines and availability and characteristics of testing changed during the ZIKV outbreak, and there might have been inconsistency in reporting of data across jurisdictions. In some jurisdictions, a PRNT was recommended for infants at 18 months who tested negative by ZIKV IgM at birth. Third, although the component birth defects in the CZS clinical phenotype have been identified (Moore et al., 2017), the full spectrum of effects of congenital ZIKV infection is still being elucidated—in particular the one that includes a less severe phenotype. For the purposes of identifying cases for the current report, we considered only the most severe CZS clinical phenotype to increase the likelihood of a ZIKV etiology; therefore, it is possible that without confirmatory testing we might have missed infants with a less severe phenotype. Finally, some of the examination findings in infants born to mothers with ZIKV exposure during pregnancy overlap with other congenital infections, most notably CMV and toxoplasmosis. In our surveillance systems which are based on medical record abstraction, CMV or toxoplasmosis testing was missing from the abstracted records for the majority of cases or testing did not consistently indicate whether the specimen was tested for IgM or immunoglobulin G (IgG) antibodies; therefore, we could not rule out other congenital infections for these cases.
In summary, due to challenges with laboratory testing in infants potentially exposed in utero to ZIKV some infants born with maternal evidence of ZIKV infection during pregnancy might have negative infant testing. Infants without laboratory evidence of other congenital infections but with physical findings consistent with the CZS clinical phenotype, including severe microcephaly, subcortical calcifications, macular scarring and focal pigmentary retinal mottling, congenital contractures, and marked early hypertonia, may be considered to have the CZS clinical phenotype. Infants and children with CZS clinical phenotype should be evaluated for the need to receive referrals for a head ultrasound, comprehensive ophthalmologic exam, and automated auditory brainstem response, additional consideration should be given for evaluation by specialty providers to include infectious disease, developmental, and pediatric neurology and additional consultations with other specialists based on the infant’s clinical findings (Adebanjo et al., 2017).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
DATA AVAILABILITY STATEMENT
Data sharing not available.
REFERENCES
- Adebanjo T, Godfred-Cato S, Viens L, Fischer M, Staples JE, Kuhnert-Tallman W, ... Moore CA (2017). Update: Interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection – United States, October 2017. MMWR Morbidity and Mortality Weekly Report, 66(41), 1089–1099. 10.15585/mmwr.mm6641a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham AM, Cone M, Mock V, Heberlein-Larson L, Stanek D, Blackmore C, & Likos A (2016). Comparison of test results for Zika virus RNA in urine, serum, and saliva specimens from persons with travel-associated Zika virus disease – Florida, 2016. MMWR Morbidity and Mortality Weekly Report, 65(18), 475–478. 10.15585/mmwr.mm6518e2 [DOI] [PubMed] [Google Scholar]
- de Araújo TVB, Ximenes RAA, Miranda-Filho DB, Souza WV, Montarroyos UR, de Melo APL, ... Rodrigues LC (2018). Association between microcephaly, Zika virus infection, and other risk factors in Brazil: Final report of a case-control study. Lancet Infectious Diseases, 18(3), 328–336. 10.1016/s1473-3099(17)30727-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo Espindola O, Jaenish T, NielsenSaines K, Carvalhaes de Oliveira RV, Pastorino B, Vasconcelos Z, ... Brasil P (2021). Zika virus neutralizing antibody kinetics in antenatally exposed infants. Journal of Infectious Diseases, 1–9. 10.1093/infdis/jiab054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galang RR, Avila GA, Valencia D, Daza M, Tong VT, Bermúdez AJ, ... Ospina ML (2020). Etiology of microcephaly and central nervous system defects during the Zika epidemic in Colombia. Journal of Pediatrics, 222, 112–119.e113. 10.1016/j.jpeds.2020.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, ... US Zika Pregnancy Registry Collaboration. (2017). Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. Journal of the American Medical Association, 317(1), 59–68. 10.1001/jama.2016.19006 [DOI] [PubMed] [Google Scholar]
- Krow-Lucal ER, de Andrade MR, Cananea JNA, Moore CA, Leite PL, Biggerstaff BJ, ... Paraíba Microcephaly Work Group. (2018). Association and birth prevalence of microcephaly attributable to Zika virus infection among infants in Paraiba, Brazil, in 2015–16: A case-control study. Lancet Child & Adolescent Health, 2(3), 205–213. 10.1016/S2352-4642(18)30020-8 [DOI] [PubMed] [Google Scholar]
- Melo AS, Aguiar RS, Amorim MM, Arruda MB, Melo FO, Ribeiro ST, ... Tanuri A (2016). Congenital Zika virus infection: Beyond neonatal microcephaly. JAMA Neurology, 73(12), 1407–1416. 10.1001/jamaneurol.2016.3720 [DOI] [PubMed] [Google Scholar]
- Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, ... Rasmussen SA (2017). Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatrics, 171(3), 288–295. 10.1001/jamapediatrics.2016.3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduyebo T, Polen KD, Walke HT, Reagan-Steiner S, Lathrop E, Rabe IB, ... Meaney-Delman D (2017). Update: Interim guidance for health care providers caring for pregnant women with possible Zika virus exposure – United States (including U.S. territories), July 2017. MMWR Morbidity and Mortality Weekly Report, 66(29), 781–793. 10.15585/mmwr.mm6629e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SM, Delaney A, Jones AM, Carr CP, Liberman RF, Forestieri NE, ... Cragan JD (2019). Updated baseline prevalence of birth defects potentially related to Zika virus infection. Birth Defects Research, 111(13), 938–940. 10.1002/bdr2.1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EE, Polen KN, Meaney-Delman D, Ellington SR, Oduyebo T, Cohn A, ... Rasmussen SA (2016). Update: Interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure – United States, 2016. MMWR Morbidity and Mortality Weekly Report, 65 (12), 315–322. 10.15585/mmwr.mm6512e2 [DOI] [PubMed] [Google Scholar]
- Pomar L, Vouga M, Lambert V, Pomar C, Hcini N, Jolivet A, ... Baud D (2018). Maternal-fetal transmission and adverse perinatal outcomes in pregnant women infected with Zika virus: Prospective cohort study in French Guiana. BMJ, 363, k4431. 10.1136/bmj.k4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe IB, Staples JE, Villanueva J, Hummel KB, Johnson JA, Rose L, ... Powers AM (2016). Interim guidance for interpretation of Zika virus antibody test results. MMWR Morbidity and Mortality Weekly Report, 65(21), 543–546. 10.15585/mmwr.mm6521e1 [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Jamieson DJ, Honein MA, & Petersen LR (2016). Zika virus and birth defects—reviewing the evidence for causality. New England Journal of Medicine, 374(20), 1981–1987. 10.1056/NEJMsr1604338 [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Jones AM, Petersen EE, Lee EH, Rice ME, Bingham A, ... US Zika Pregnancy Registry Collaboration. (2017). Vital signs: Update on Zika virus-associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure – U.S. Zika Pregnancy Registry, 2016. MMWR Morbidity and Mortality Weekly Report, 66(13), 366–373. 10.15585/mmwr.mm6613e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Galang RR, Roth NM, Ellington SR, Moore CA, Valencia-Prado M, ... Honein MA (2018). Vital signs: Zika-associated birth defects and neurodevelopmental abnormalities possibly associated with congenital Zika virus infection – U.S. territories and freely associated states, 2018. MMWR Morbidity and Mortality Weekly Report, 67(31), 858–867. 10.15585/mmwr.mm6731e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodo C, Suy A, Sulleiro E, Soriano-Arandes A, Anton A, Garcia-Ruiz I, ... Carreras E (2018). In utero negativization of Zika virus in a foetus with serious central nervous system abnormalities. Clinical Microbiology and Infection, 24(5), 549. e1–549.e3. 10.1016/j.cmi.2017.09.022 [DOI] [PubMed] [Google Scholar]
- Russell LJ, Weaver DD, Bull MJ, & Weinbaum M (1984). In utero brain destruction resulting in collapse of the fetal skull, microcephaly, scalp rugae, and neurologic impairment: The fetal brain disruption sequence. American Journal of Medical Genetics, 17(2), 509–521. 10.1002/ajmg.1320170213 [DOI] [PubMed] [Google Scholar]
- Sharp TM, Fischer M, Munoz-Jordan JL, Paz-Bailey G, Staples JE, Gregory CJ, & Waterman SH (2019). Dengue and Zika virus diagnostic testing for patients with a clinically compatible illness and risk for infection with both viruses. MMWR Recommendations and Reports, 68(1), 1–10. 10.15585/mmwr.rr6801a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoots AN, Olson SM, Cragan J, Delaney A, Roth NM, Godfred-Cato S, ... Honein MA (2020). Population-based surveillance for birth defects potentially related to Zika virus infection – 22 states and territories, January 2016-June 2017. MMWR Morbidity and Mortality Weekly Report, 69(3), 67–71. 10.15585/mmwr.mm6903a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not available.


