Abstract
BACKGROUND & AIMS:
Gastrointestinal (GI) motility is regulated by serotonin (5-hydroxytryptamine, 5-HT), which is primarily produced by enterochromaffin (EC) cells in the GI tract. However, the precise roles of EC cell-derived 5-HT in regulating gastric motility remain a major point of conjecture. Using a novel transgenic mouse line, we investigated the distribution of EC cells and the pathophysiological roles of 5-HT deficiency in gastric motility in mice and humans.
METHODS:
We developed an inducible, EC cell-specific Tph1CreERT2/+ mouse, which was used to generate a reporter mouse line, Tph1-tdTom, and an EC cell-depleted line, Tph1-DTA. We examined EC cell distribution, morphology, and subpopulations in reporter mice. GI motility was measured in vivo and ex vivo in EC cell-depleted mice. Additionally, we evaluated 5-HT content in biopsy and plasma specimens from patients with idiopathic gastroparesis (IG).
RESULTS:
Tph1-tdTom mice revealed EC cells were heterogeneously distributed throughout the GI tract with the greatest abundance in the antrum and proximal colon. Two subpopulations of EC cells were identified in the gut: self-renewal cells located at the base of the crypt and mature cells observed in the villi. Tph1-DTA mice displayed delayed gastric emptying, total GI transit, and colonic transit. These gut motility alterations were reversed by exogenous provision of 5-HT. Patients with IG had a significant reduction of antral EC cell numbers and 5-HT content, which negatively correlated with gastric emptying rate.
CONCLUSIONS:
The Tph1CreERT2/+ mouse provides a powerful tool to study the functional roles of EC cells in the GI tract. Our findings suggest a new pathophysiological mechanism of 5-HT deficiency in IG.
Keywords: Enterochromaffin cells, Serotonin, Idiopathic Gastroparesis, Slow Transit Constipation, Gastrointestinal Motility
Introduction
Enterochromaffin (EC) cells are specialized enteroendocrine cells in the gastrointestinal (GI) tract that produce the vast majority (90% to 95%) of serotonin (5-hydroxytryptamine, 5-HT) in the body.1 The synthesis of 5-HT in EC cells is catalyzed by the rate-limited enzyme, tryptophan hydroxylase 1 (TPH1),2 with TPH2 being the neuron-specific TPH isoform.3 The precise physiological roles of EC cell-derived 5-HT are yet to be fully elucidated. It is known that 5-HT potently affects a variety of physiological processes, such as GI motility, secretion, mechanosensation, and pathologies including visceral hypersensitivity and nausea.4, 5 EC cells coordinate bilateral communication through crosstalk between dietary, microbial, and inflammatory factors to which their apical surface is exposed, and to enteric neurons with afferent endings close to their basolateral surface.1, 4, 6-8 Alterations to EC cell populations and/or 5-HT signaling can result in GI dysmotility and secretomotor abnormalities in the gut.9
Idiopathic gastroparesis (IG) is a common form of gastroparesis characterized by delayed gastric emptying without an identifiable origin.10 IG manifests as various GI symptoms, including anorexia, early satiety, bloating, nausea, vomiting, and abdominal pain11 and is often comorbid with other functional GI disorders such as slow transit constipation (STC) and functional dyspepsia.12 Normal control of gastric motility requires a complex coordinated interplay of the sympathetic, parasympathetic, and enteric nervous system, which necessitates a variety of other cell types in the GI tract.13 Functional defects in different cell types, for instance, smooth muscle cells, interstitial cells of Cajal (ICCs), immune cells, and enteric neurons have the potential to hinder gastric motility and are known pathogenesis of gastroparesis.10, 14 However, none of the proposed pathophysiology has impacted the management of gastroparesis to date. Unfortunately, a large group of patients with gastroparesis shows no defects in the aforementioned cell types. Thus, this yet-to-be-identified cause and disconnect give the impetus for exploring other pathological mechanisms of IG, particularly ones that could be actionable with treatment, such as with pharmacological agents.
The functional role of EC cell-derived 5-HT in regulating GI motility has been debated for decades.15, 16 The current competing paradigms are perplexing and controversial as evidenced by global, congenital Tph1−/− murine models.17 An inducible animal model is warranted because of the following reasons: 1) Li and colleagues indicated that Tph1-derived 5-HT is trivial for normal GI motility as gastric emptying, total GI transit, and even colonic motility did not differ between Tph1−/− and wild type mice.18 In contrast, Heredia and colleagues later showed the important role of mucosal 5-HT in colonic propulsion and peristaltic reflexes in the same Tph1−/− model.19 2) 5-HT functions as a growth factor in the early postnatal development in enteric nervous system.18 Congenital Tph1−/− mice exhibited hypertrophic and elongated colons.19 3) EC cells are regenerated from intestinal epithelial stem cells in the cryptic base every 3-5 days.20 Studies on EC cell-derived 5-HT in congenital knockout animal models may have been hampered by the nature of EC cells being such a rapidly regenerating and dynamic cell type. Thus, an inducible animal model that circumvents such developmental and physiological alterations is needed to elucidate the unresolved questions, particularly the precise role of EC cell-derived 5-HT in regulating gut motility.
To interrogate the role of EC cells and their 5-HT in gut motility, we generated a tamoxifen-inducible Tph1CreERT2/+ mouse, a Tph1-tdTom reporter line, and an EC cell depleting Tph1-DTA line. Lineage tracing in the reporter line demonstrated two separate types of EC cells that we termed self-renewal and mature cells. Depletion of EC cells in the Tph1-DTA mice enabled us to evaluate the contribution of EC cell-derived 5-HT on functional GI motility, both in vivo and ex vivo. We found that loss of EC cells and their 5-HT resulted in delayed gastric emptying and slowed colonic motility, while intragastric administration of 5-HT rescued the impaired gut motility in the mice. We also confirmed a reduction of 5-HT content and EC cell numbers in antrum mucosal biopsy specimens from patients with IG compared with healthy controls. The new inducible and reversible Tph1CreERT2/+ mouse line is a powerful tool for studying the functional roles of EC cells in GI physiology and better understanding the link between 5-HT deficiency and the pathophysiology of IG.
Materials and Methods
Note: The full Materials and Methods section is included in the supplementary material.
Generation of Tph1CreERT2/+, Tph1CreERT2+/;Rosa26tdTom/+ (Tph1-tdTom), and Tph1CreERT2/+;Rosa26DTA/+ (Tph1-DTA) Mice
A target vector was constructed with CreERT2 recombined into the last exon of Tph1 (BAC clone) and injected into the embryonic stem cells. Tph1CreERT2/+ mouse was crossed with a Rosa20dTom/tdTom mouse or a Rosa26PDTA/DTA mouse to generate the Tph1-tdTom and Tph1-DTA mice. All procedures that include animal subjects were approved by the Institutional Animal Care and Use Committee (IACUC) at University of Rochester and University of Nevada, Reno (UNR).
Tamoxifen Administration
Tph1-tdTom, Tph1-DTA, C57, Rosa26DTA/+, Tph1CreERT2/+ mice were given tamoxifen (Sigma-Aldrich; 1.0 mg/20g body weight) solubilized in sunflower oil (Sigma-Aldrich) intraperitoneally for 5 consecutive days at 8-16 weeks of age. The analysis was compiled from age- and gender-matched mice from multiple litters.
Functional GI motility Procedures
Gastric emptying test (GET) was performed using GastroSense 750 and an IVIS Lumina III system.21, 22 Fluorescence images were analyzed using Living Image software. Total GI transit time (TGITT) was assessed as the time taken from intragastric gavage of Evans blue solution until the first observation of the blue fecal pellet.22 Colonic transit time (CTT) was measured through the bead expulsion test.22 For 5-HT exogenous provision, GastroSense 750 or Evans blue solution was mixed with 5-HT (serotonin hydrochloride) for GET or TGITT. For CTT and water pellet content measurement, mice were given an intragastric gavage of 5-HT 20 minutes prior to bead placement or fecal pellet collection.
Colonic migrating motor complex (CMMC) Muscle Contractility Recordings
The force generated during each CMMC contraction was recorded on ex vivo colon reparations using independent isometric recording transducers connected via fine suture and micro-hooks.16 Each force transducer was connected to two custom made preamplifiers and data acquired using a PowerLab.
Human Specimens
All human idiopathic gastroparesis and control biopsy/plasma samples and clinical data were received from Stanford University. All human subjects provided informed consent, and all study procedures were approved by the Stanford University and UNR Institutional Review Boards.
Statistics
All data are presented as mean ± SEM. The inferential statistical significance of differences between sample means was evaluated using two-tailed unpaired t-tests. The grouped samples were evaluated using one-way or two-way ANOVA tests using GraphPad Prism.
Results
Generation of an Inducible Tph1CreERT2/+ Knockin Mouse
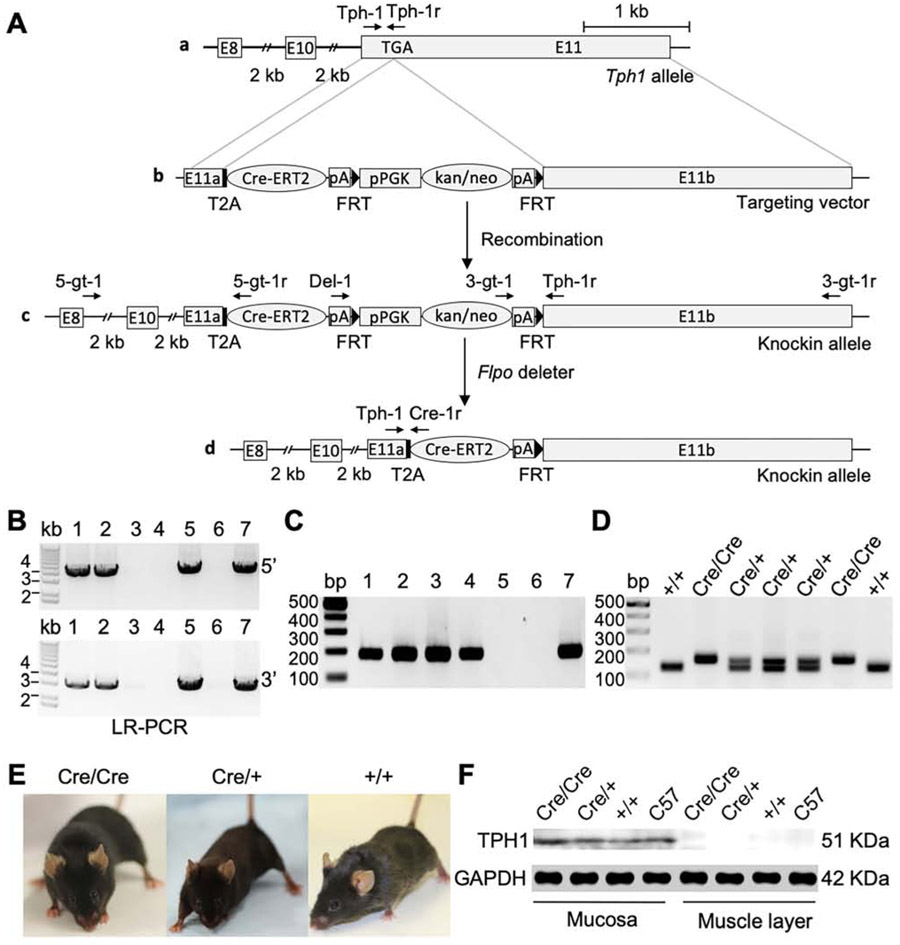
To elucidate the functional roles of EC cells in the GI tract, we generated the Tph1CreERT2/+ mouse. The Cre recombinase was fused to a modified estrogen receptor (CreERT2) and inserted to the end of the open reading frame of the Tph1 gene at exon 11 with a self-cleaving peptide T2A (Figure 1Aa and b). The recombinant transcript with T2A (Tph1-T2A-CreERT2) allows the peptide to be cleaved into two proteins, TPH1 and CreERT2. The targeting vector was introduced into embryonic stem (ES) cells and targeted ES cell clones were screened using 5’ and 3’ external primers (Figure 1Ac; Supplementary Table 1). Four homologous recombination targeted ES cell clones were confirmed and two of them were used to generate a heterozygous Tph1CreERT2-Kan/Neo/+ mouse (Figure 1B). Given kan/neo cassette can include a cryptic splice acceptor and donor that interfere with the expression of their neighboring genes, the kan/neo cassette was removed by crossing the heterozygous mouse with the Flpo deleter Rosa26Flpo/+ mouse (Figure 1Ad). This removal was confirmed by genotyping PCR (Figure 1C). The heterozygous Tph1CreERT2/+ mice were used to develop homozygous Tph1CreERT2/CreERT2 mice. Genotypes were confirmed in wild type, heterozygote, and homozygote, who are viable and fertile (Figure 1D and E). Western blot analysis showed that the TPH1 protein was expressed at normal levels in the heterozygous and homozygous mice (Figure 1F), confirming CreERT2 insertion does not disturb TPH1 expression.
Figure 1.
Generation of Tph1CreERT2/+ mouse. (A) Schematic illustration of the targeted knockin strategy. (a) A Tph1 allele showing exons 8, 10, and 11. A stop codon TGA is shown in exon 11. (b) A targeting vector including the CreERT2 containing a T2A sequence at the end of 5’ coding sequence, followed by pPGK-GB2-driven kan/neo selection cassette flanked by FRT recognition sites, was used to replace the exon 11. (c) A targeted knockin allele after homologous recombination in the mouse embryonic stem (ES) cells. (d) A knockin allele after removal of the kan/neo selection cassette by with a Flpo mouse. (B) Long-range (LR) PCR performed with 5’ primers (5-gt-1/5-gt-1r: 3.8 kb) and 3’ primers (3-gt-1/3-gt-1r: 2.9 kb) in ES cells to identify targeted colonies. (C) Genotyping PCR performed with primers (Del-1 and Tph-1r: 190 bp) confirmed the deletion of kan/neo selection cassette. (D) Genotyping PCR performed with primers (Tph-1, Tph-1r, and Cre-1r) to find Cre/Cre (156 bp), Cre/+ (156 bp/125 bp), and +/+ (125 bp). (E) Gross images of Cre/Cre, Cre/+, and +/+ mice. (F) Expression of TPH1 analyzed by Western blot in the colonic mucosa and smooth muscle tissue of Cre/Cre, Cre/+, and +/+ mice. E, exon; T2A, 2A peptide derived from insect Thosea asigna virus; CreERT2, Cre recombinase fused to a mutant estrogen ligand-binding domain; pA, polyadenylation; pPGK-GB2, combined phosphoglycerate kinase and prokaryotic promoter; kan/neo, kanamycin/neomycin; FRT, flippase recognition target; Flpo, flippase deleter.
EC cells Are Regionally Distributed Throughout the GI Tract
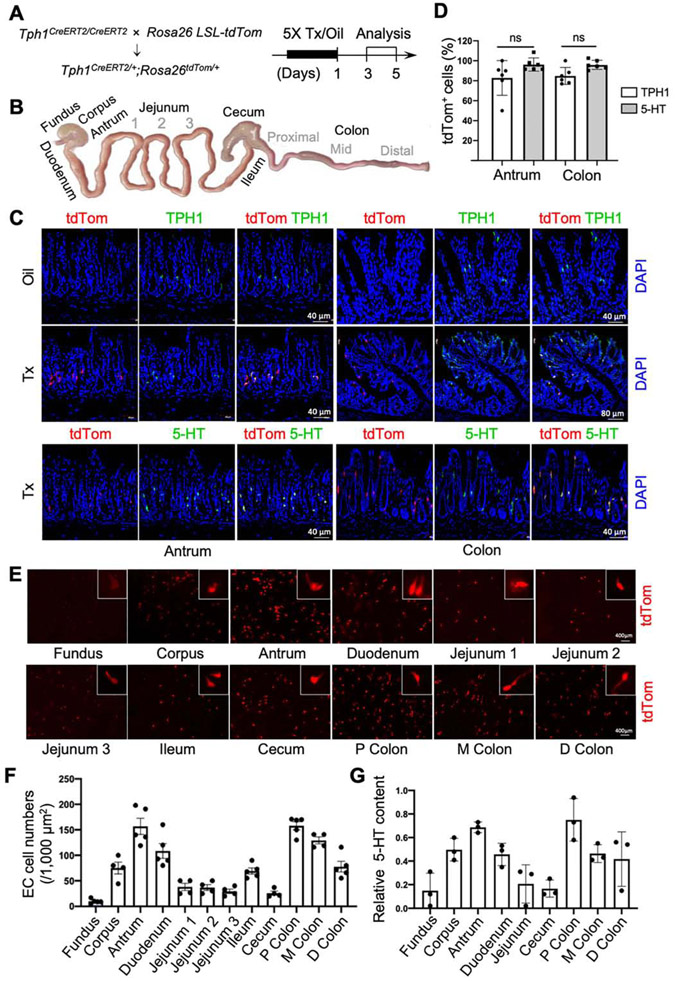
To estimate the specificity of Cre activity in EC cells, we generated reporter Tph1-tdTom mice. The removal of the STOP cassette by CreERT2 upon tamoxifen (Tx) injection allows the expression of tdTom in EC cells. tdTom+ cells were analyzed in 12 different regions of the GI tract from 3 to 5 days post-Tx or sunflower oil (oil) treatment (Figure 2A and B). tdTom+ cells were detected in Tph1-tdTom mice with Tx treatment, but not in oil treatment (Figure 2C). The tdTom+ cells in the antrum, duodenum, and colon mucosa colocalized with antibodies targeting TPH1 and 5-HT (Figure 2C; Supplementary Figure 1A), but not with tryptase, a marker for 5-HT-producing mast cells (Supplementary Figure 1B).23 Most (> 80%) tdTom+ cells in antrum and colon were TPH1 and 5-HT positive (Figure 2D). Taken together, these data confirmed that tdTom marked EC cells specifically.
Figure 2.
EC cells are regionally distributed throughout the GI tract. (A) Schematic diagrams of the reporter line Tph1CreERT2/+;Rosa26tdTom/+ (Tph1-tdTom) and tamoxifen (Tx)/sunflower oil (oil) induction plan. (B) Schematic anatomy of 12 regions in the GI tract analyzed in this study. (C) tdTom+ cells colocalized with TPH1 or 5-HT antibody staining in antrum and colon at day 3 post-Tx treatment. DAPI staining shows cell nuclei. (D) The percentage of EC cells colocalized with TPH1 and 5-HT was quantified in antrum and colon (n=6). (E) Distribution and morphology of representative EC cells in each region. (F) Quantification of EC cells in each region (mean ± SEM, n=5). (G) Mucosal 5-HT content measured by ELISA (mean ± SEM, n=3). P, proximal; M, middle; D, distal. Error bars indicate SEM, unpaired t-test.
We next examined the morphology and distribution of EC cells in 12 regions (Figure 2B) of the GI tract of Tph1-tdTom mice. EC cells were detected in all 12 regions, but cell morphology and distribution were heterogeneous. We observed two common types of EC cell morphology: “wineglass” and “axon-like”,24, 25 the latter of which were most frequently found in the proximal colon (Figure 2E). This regional distribution of EC cells was quantified by tdTom+ cell densities and mucosal 5-HT levels in all regions (Figure 2F and G). The antrum and proximal colon contained more EC cells and 5-HT than any other regions along the GI tract. Collectively, the data confirmed Tph1CreERT2/+ mouse is a reliable model to investigate regional EC cell features in the gut.
Lineage Tracing of Mature and Self-renewal EC Cells in Tph1-tdTom Mice
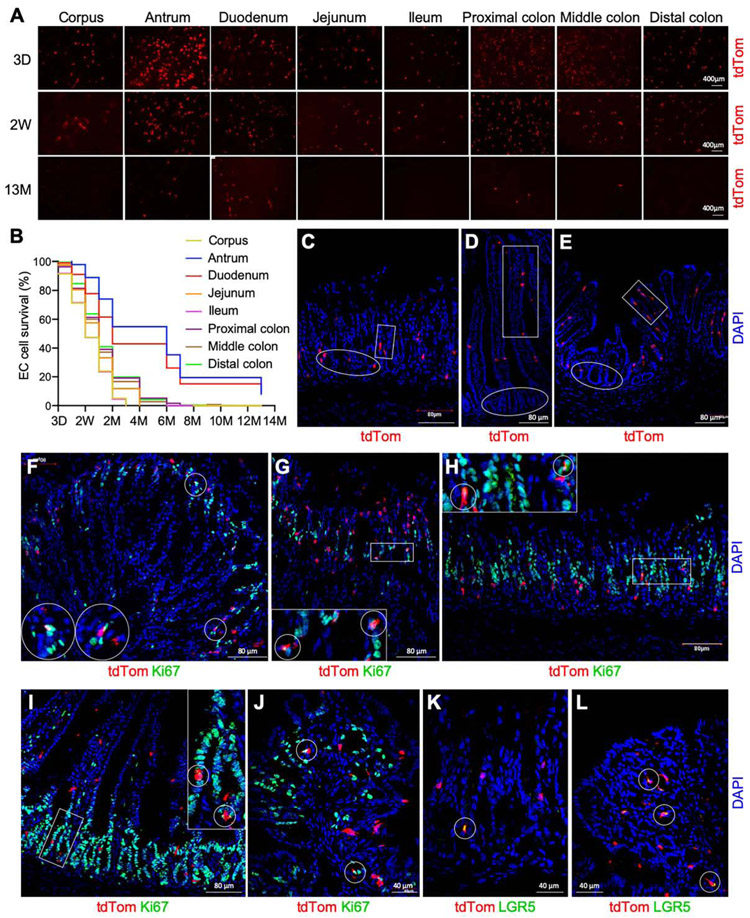
The intestine is a dynamic organ with stem cell division generating epithelial cells that are thought to mature and undergo apoptosis in 3-5 days.26 Although EC cell alterations and 5-HT signaling have been investigated in several gut inflammatory conditions,27 the turnover rate of EC cells in each segment of the GI tract is largely unknown. To explore the regenerative dynamics of EC cells, we monitored and traced EC cells in 8 gut regions from the corpus to the distal colon in the Tph1-tdTom mice. The majority (> 80%) of EC cells in these regions were detectable after 2 weeks post-Tx treatment (Figure 3A). A substantial number of the EC cells were still observed over several months and even over one-year post-Tx treatment (Figure 3A and B). The corpus and ileal EC cells were still discernible 3 months post-Tx treatment, while the EC cells were imperceptible in the jejunum, proximal, middle, and distal colon after 4 to 6 months (Figure 3B; Supplementary Figure 2A). The antral and duodenal EC cells survived till 13 months post-Tx treatment (Figure 3B; Supplementary Figure 2A). Intestinal epithelial cells, including EC cells, differentiate out of the stem cell zone at the base of crypts and migrate apically to the villus as they mature.28, 29 EC cells were found in both the stem cell zone and maturing region in the antrum (pit and gland, Figure 3C), duodenum (villus and crypt, Figure 3D), and colon (villus and crypt, Figure 3E). In the stem cell zone, approximately 0.1% of EC cells in the corpus, antrum, duodenum, and colon were positive for Ki67, at 3 days post-Tx treatment (Figure 3F-J; Supplementary Figure 2F), indicating that these EC cells were actively dividing. Approximately 0.3% of EC cells in the same regions remained Ki67+ at 1 month post-Tx treatment (Supplementary Figure 2B-D and F). Moreover, several EC cells expressed the intestinal stem cell marker Lgr5 at 3 days post-Tx treatment (Figure 3K and L). Lgr5+ EC cells remained 1 month post-Tx treatment (Supplementary Figure 2E). These observations imply that mature EC cells are mostly located in the apical surface and maintained by proliferative self-renewal EC cells, which are often found in the stem cell zone along the length of the GI tract in a stable fashion over a long period of time.
Figure 3.
Lineage tracing of mature and self-renewal EC cells in Tph1-tdTom mice. (A) EC cells in 8 regions of the GI tract at 3 days (D), 2 weeks (W), and 13 months (M) post-Tx treatment. (B) Survival percentage of EC cells from 3 days to 13 months post-Tx treatment (n=7). (C-E) EC cells located in antrum (C), duodenum (D), and colon (E). Boxes indicate cells in the mature zone, and circles indicate cells in the stem cell zone. (F-J) tdTom+ Ki67+ cells in corpus (F and G), antrum (H), duodenum (I), and colon (J) at 3 days post-Tx treatment. Circles and boxes show representative tdTom+ cells colocalized with Ki67. (K, L) tdTom+ Lgr5+ cells in antrum (K) and colon (L). Circles show representative tdTom+ cells colocalized with Lgr5. DAPI staining shows cell nuclei.
Depletion of EC Cells Impairs GI Motility In Vivo
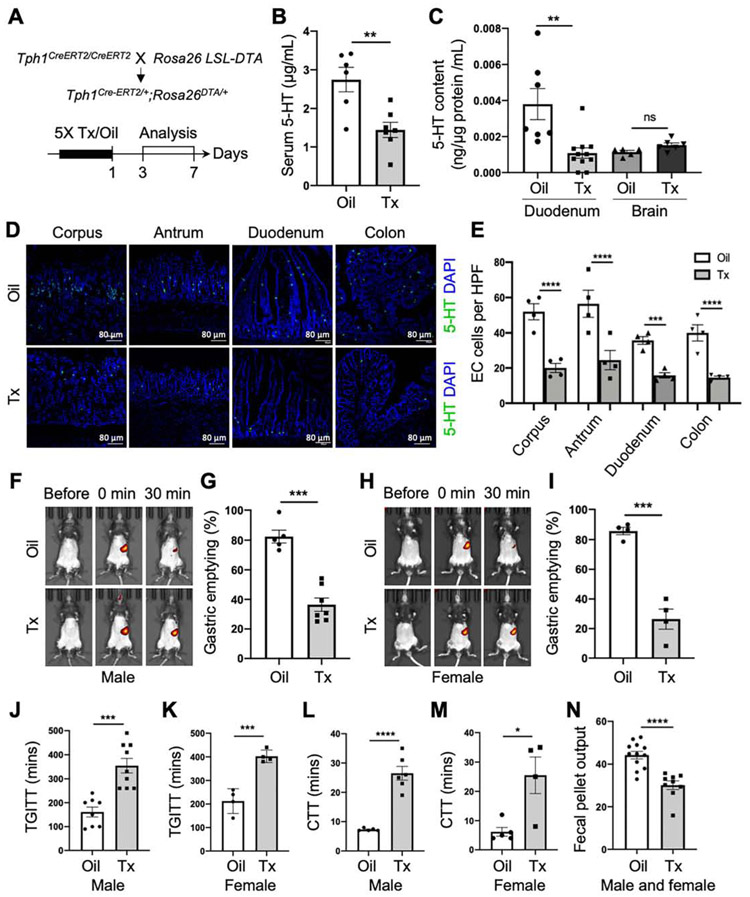
To explore the functional roles of EC cells and 5-HT in GI motility, we generated the Tph1-DTA mice with a conditional expression of diphtheria toxin A (DTA). The removal of the STOP cassette (flanked by LoxP) by CreERT2 upon Tx administration induces DTA activation specifically in Cre-expressing cells. DTA expression results in the inhibition of protein synthesis, leads to apoptotic death of the target cell,30 and thus facilitates EC cell depletion. Both male and female Tph1-DTA mice were administrated with Tx or oil, and EC cell loss was analyzed in the GI tract at 3 to 7 days post-Tx or oil treatment (Figure 4A). 5-HT levels were significantly decreased in the serum and duodenal mucosa of EC cell-depleted mice compared to control mice (Figure 4B). However, 5-HT levels remained unchanged in the brain of EC cell-depleted mice, suggesting that the depletion of EC cells does not affect TPH2-derived 5-HT in neurons (Figure 4C). 60-70% of EC cells were depleted in the corpus, antrum, duodenum, and colon mucosal tissue in EC cell-depleted mice (Figure 4D and E). A significant reduction of TPH1 protein was confirmed in the colonic mucosa of EC cell-depleted mice (Supplementary Figure 3A and B). These results confirm that conditional depletion of EC cells is sufficient to achieve a substantial reduction of mucosal restricted-5-HT in Tph1-DTA mice upon Tx treatment.
Figure 4.
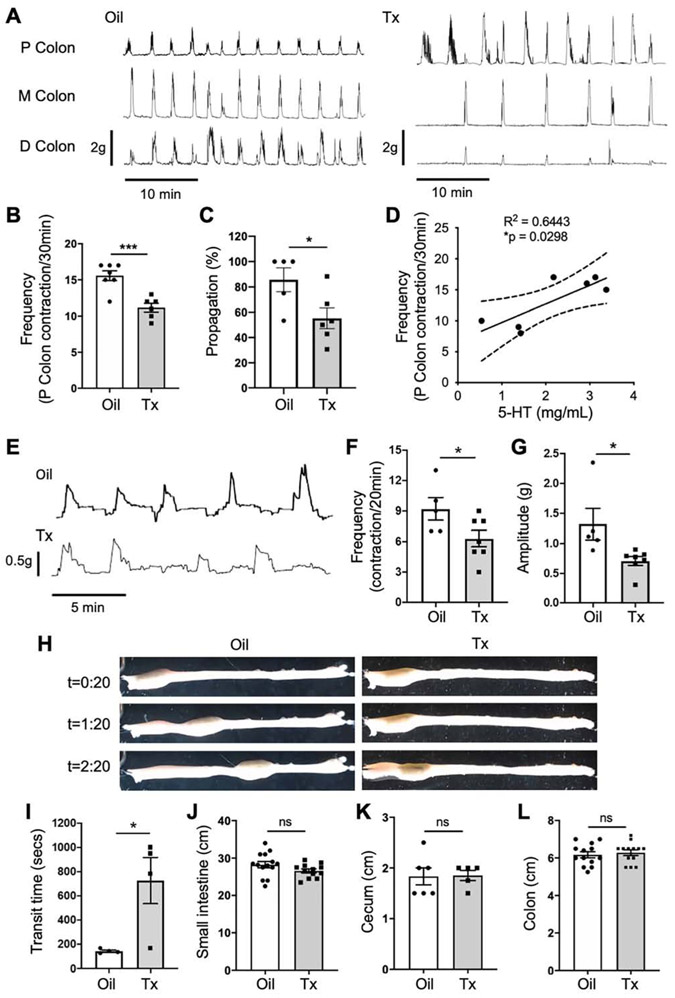
Depletion of EC Cells leads to delayed gastric emptying and slow transit constipation (STC). (A) Schematic diagrams of the EC cell depletion line Tph1CreERT2/+;Rosa26DTA/+ (Tph1-DTA) and Tx induction plan. (B) Serum 5-HT levels measured in the mice with oil or Tx treatment by ELISA (n=6-7). (C) 5-HT content in the duodenum mucosa and brain tissue estimated by ELISA (n=5-11). (D) EC cells stained with 5-HT antibody at day 3 post-Tx treatment. DAPI staining shows cell nuclei. (E) Numbers of EC cells per high-power field (HPF) in the corpus, antrum, duodenum, and colon at day 3 post-Tx treatment in the mice with oil or Tx treatment (Two-way ANOVA, n=4). (F-I) Gastric emptying test (GET) in the males and females (n=4-7). Fluorescent images before and after an intragastric gavage of GastroSense 750 (F and H) and percentage of gastric emptying quantified at 30 mins (G and I). (J, K) Total GI transit time (TGITT) in the males and female mice (n=4-9). (L, M) Colonic transit time (CTT) in the males and female mice (n=4-6). (N) Fecal pellet output (pellet number multiplied by weight) in the mice (n=9-12). Error bars indicate SEM, unpaired t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Given the suggested functional roles of EC cells and 5-HT in gut motility, we hypothesized that depletion of EC cells could impair GI motility in the mouse. We performed GI motility tests in vivo and ex vivo with EC cell-depleted and control mice. The percentage of liquid gastric emptying at 30 minutes was delayed in male and female Tph1-DTA mice 3 days post-Tx treatment as compared to control mice (Figure 4F-I). We also performed GET using solid food and observed a similar pattern of gastric emptying in EC cell-depleted mice (Supplementary Figure 3C-F). TGITT and CTT were significantly delayed EC cell-depleted mice (Figure 4J-M). Furthermore, fecal pellet indices (number, output, water content, and size) supported for STC phenotype exhibited in EC cell-depleted mice (Figure 4N; Supplementary Figure 3G-K). Given the peripheral actions of 5-HT on multiple organs to regulate metabolic homeostasis,29, 31 we noticed the body weight and fasting blood glucose remained the same in EC cell-depleted and control mice (Supplementary Figure 3L-O). To rule out the possible effects of Tx on gut motility, we demonstrated the current dose of Tx did not alter GI motility in C57, Rosa26DTA/+, and Tph1CreERT2/+ mice as compared to no treatment baselines (Supplementary Figure 4A-E).
Due to the rapid turnover rate of mucosal epithelium, we examined how rapidly the GI motility would return to normal after EC cell depletion (Supplementary Figure 5A). TGITT was recovered to normal levels at 14 days post-Tx treatment (Supplementary Figure 5B). Fecal pellet number and output, gastric emptying, and CTT were recovered at 21 days post-Tx treatment (Supplementary Figure 5C-G). This data suggests EC cell depletion lasts for approximately 2 weeks. Taken together, the conditional depletion of EC cells in Tph1-DTA mice results in a notable reduction of 5-HT in the GI tract, leading to markedly delayed gastric emptying and slowed colonic motility.
Depletion of EC Cells Impairs Colonic Motility Ex Vivo
We further investigated the functions of EC cell-derived 5-HT in gut motility by analyzing colonic migrating motor complexes (CMMCs) in EC cell-depleted and control mice. CMMCs were noticeably abnormal in Tph1-DTA mice at 3 days post-Tx compared to the control mice of both sexes (Figure 5A). The frequency of proximal colon contractions and the percentage of the CMMCs fully propagating down the colon length were significantly lower in the EC cell-depleted colon than in controls (Figure 5B and C). To evaluate the possible effect of Tx on CMMCs, the frequency and propagation of proximal colon contractions were compared between Tph1-DTA mice with Oil treatment, C57 and Rosa26DTA/+ mice with Tx treatment. No significant difference in frequency or propagation was observed between these groups (Supplementary Figure 4F and G). CMMC frequency and serum 5-HT levels were positively (R2 = 0.644, P<0.05) correlated (Figure 5D). To demonstrate 5-HT depletion is the causal effect of abnormal neurogenic function in Tph1-DTA mice, we flushed exogenous 5-HT (0.1 μM) to the colon in preparation bath and the frequency of proximal colon contractions returned to a normal range (Supplementary Figure 6A). When a fecal pellet was fixed in place, the amplitude and frequency of colonic contractions were also reduced in the EC cell-depleted colon compared to the controls (Figure 5E-G). The transit time of freely moving pellets was markedly delayed in EC cell-depleted colon (Figure 5H and I). Lastly, we confirmed there were no notable morphological changes in the gut of EC cell-depleted mice, as the length of the small intestine, colon, and cecum remained unchanged (Figure 5J-L) in contrast to the prolonged colon in congenital Tph1−/− mice.19 Collectively, this data demonstrates that conditional EC cell depletion impairs colonic peristalsis and neurogenic motor patterns in Tph1-DTA mice.
Figure 5.
Depletion of EC cells impairs colonic motility ex vivo. (A) Representative mechanical traces from ex vivo colon preparations measured simultaneously at three sites. Traces show colonic migrating motor complexes (CMMCs) in the colon of Tph1-DTA mice with oil or Tx treatment. (B) Frequency of initiated CMMCs from the proximal colon, over a 30-minute baseline period (n=6-7). (C) Percentage of CMMC propagation along the colon from mice with oil or Tx treatment (n=5-6). (D) A nonlinear regression analysis between CMMC frequency and serum 5-HT (n=7). (E-G) Representative mechanical traces, contraction frequency (F) and amplitude (G), against an inserted fixed pellet in ex vivo colon in mice with oil or Tx treatment (n=5-7). (H) Gross images showing the movement of a fixed pellet over time (minutes) after being inserted into the proximal colon. (I) Transit time of a fixed pellet moving from proximal colon to distal colon (n=4). (J-L) Length of small intestine (J), cecum (K), and colon (L) in mice with oil or Tx treatment (n=5-14). P, proximal; M, middle; D, distal. Error bars indicate SEM, unpaired t-test. *p < 0.05, ***p < 0.001.
Exogenous Administration of 5-HT Reverses Delayed Gastric Emptying and STC
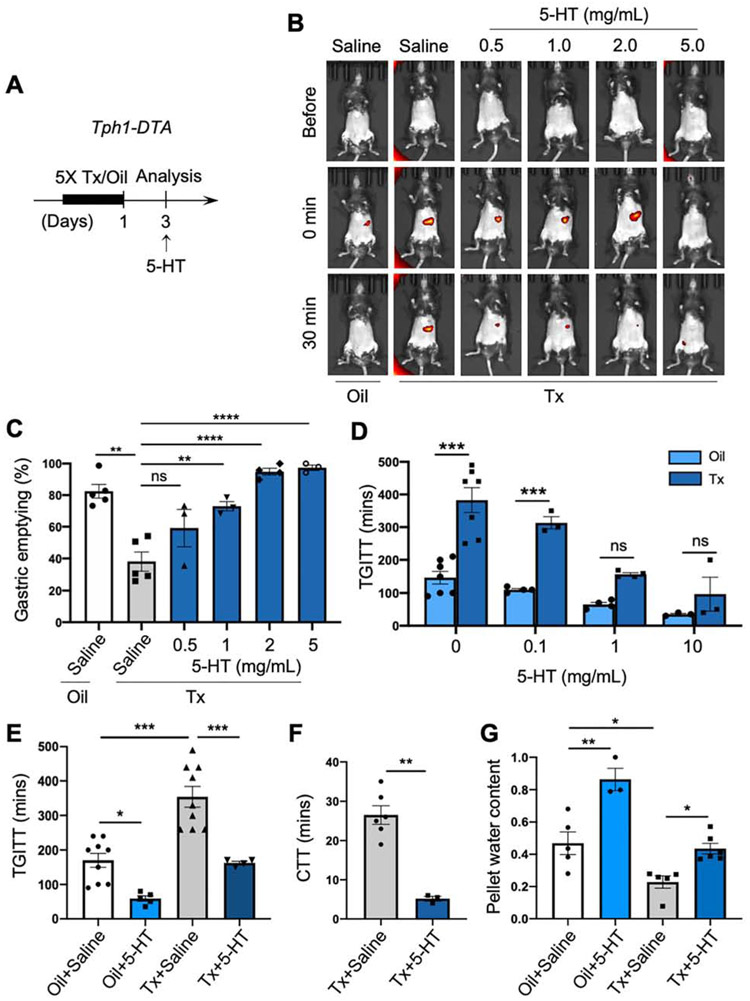
To test whether the provision of 5-HT could rescue the GI dysmotility in EC cell-depleted mice, we provided 5-HT administration and dietary interventions to Tph1-DTA mice post-Tx treatment (Figure 6A). The delayed gastric emptying was reversed in a dose-dependent manner by intragastric administration of 5-HT (Figure 6B and C). At 2 mg/mL, 5-HT normalized gastric emptying in EC cell-depleted mice. At 5 mg/mL, nearly 100% gastric emptying occurred, and GastroSense 750 was detected in the pelvis at 30 minutes indicating accelerated motility (Figure 6B). Similarly, TGITT was improved in a dose-dependent manner by intragastric administration of 5-HT in EC cell-depleted mice (Figure 6D), and normalized at the 1 mg/mL dose (Figure 6E). CTT and fecal pellet water content were significantly improved by intragastric administration of 5-HT in EC cell-depleted mice (Figure 6F and G; Supplementary Figure 6B and C). Further, intragastric 5-HT accelerated TGITT and increased fecal pellet water content in oil-treated control mice as well as TGITT in C57 mice (Figure 6D, E and G; Supplementary Figure 6C and D). Therapeutic strategies rely on dietary intervention represent the first line of treatment for gastroparesis.32 In 5-HT biosynthesis, L-Tryptophan (Trp) is processed into hydroxytryptophan (5-HTP) by TPH1 and is subsequently modified into 5-HT by aromatic L-amino acid decarboxylase (AADC) in EC cells (Supplementary Figure 7A). Due to EC cell depletion, the absence of TPH1 could abolish the biosynthesis of 5-HTP. As the precursor of 5-HT, supplementary diet 5-HTP could be converted to 5-HT by AADC present in neighboring cells. We examined if Trp and/or 5-HTP dietary supplements could rescue impaired motility in EC-depleted mice as a potential treatment option in patients with delayed gut transit, like IG or STC. We switched the chow diet to supplemented diets (Supplementary Table 2) post-Tx/oil treatment (Supplementary Figure 7B). 5-HTP dietary supplements improved fecal pellet output and TGITT in EC cell-depleted mice (Supplementary Figure 7C and D). These results support the dietary 5-HTP might improve GI motility in EC cell-depleted mice.
Figure 6.
Exogenous 5-HT administration rescued delayed gastric emptying and STC. (A) Schematic diagram of Tx induction and exogenous 5-HT administration in Tph1-DTA mice. (B, C) GET after intragastric gavage of 5-HT (GastroSense 750 mixed with 5-HT at 0.5, 1, 2, and 5 mg/mL) or saline in the EC cell-depleted and control mice. Fluorescent images before and after an intragastric gavage (B) and percentage of gastric emptying quantified at 30 mins (C, One-way ANOVA, n=3-5). (D) TGITT after intragastric gavage of 5-HT (Evans blue mixed with 5-HT at 0.1, 1, and 10 mg/mL, Two-way ANOVA, n=3-7). (E) TGITT after intragastric gavage of 5-HT (1 mg/mL mixed with Evans blue) or saline (One-way ANOVA, n=4-9). (F) CTT after an intragastric gavage of 5-HT (1 mg/mL) or saline (unpaired t-test, n=3-6). (G) Normalized fecal pellet water content after an intragastric gavage of 5-HT (10 mg/mL) or saline (One-way ANOVA, n=3-6). Error bars indicate SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Gastric 5-HT Deficiency Is a Potential Pathophysiology of IG
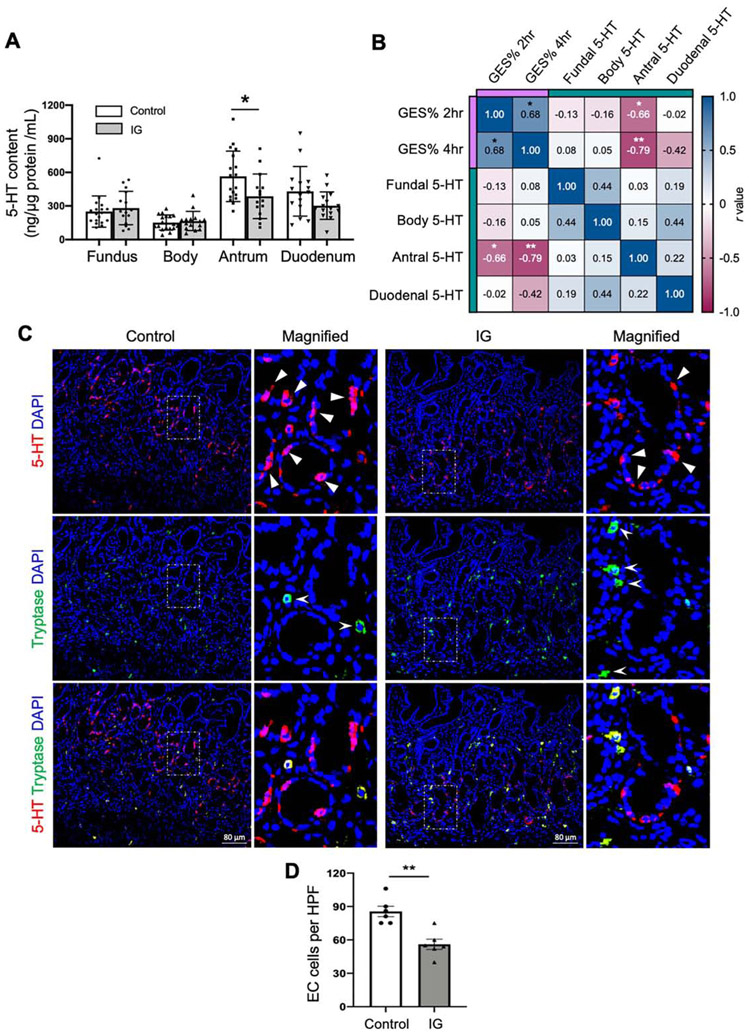
To further explore the translational aspect of 5-HT deficiency in human gut motility disorders, we measured 5-HT content in gastric and duodenal mucosal biopsy specimens and/or blood plasma from patients with IG and healthy controls (Supplementary Table 3). 5-HT levels in healthy controls were differentially presented in the fundus, body, antrum, and duodenum (Figure 7A). Antrum contained the highest 5-HT levels, followed by duodenum in healthy controls. Antral 5-HT levels were significantly reduced in IG as compared to controls (Figure 7A). Plasma 5-HT levels in IG were not decreased as compared to healthy controls (Supplementary Figure 8A). Though the consistency and accuracy of plasma 5-HT is often questionable given its fluctuation under physiological conditions and the variations during platelet-poor plasma collection.33 To test if 5-HT deficiency is associated with gastric emptying, we performed correlation analysis between gastric emptying scintigraphy (GES) % at 2 and 4 hours with 5-HT levels in the IG subjects. We found strong negative correlations between antral 5-HT and GES % at 2 and 4 hours (r=−0.66, P<0.05; r=−0.79, P<0.01) (Figure 7B; Supplementary Table 5). We also evaluated the correlations between 5-HT levels and demographic variables such as age, BMI, and the gastroparesis cardinal symptom index-daily diary scores (Supplementary Figure 8B; Supplementary Table 5). Furthermore, we observed a significant reduction of EC cell numbers in antral mucosal biopsy specimens in IG as compared to healthy controls (Figure 7C and D). These findings imply that antral 5-HT deficiency by reduced EC cells may play a role in delayed gastric emptying in humans.
Figure 7.
Reduced 5-HT in antrum is strongly associated with delayed gastric emptying in human patients with IG. (A) 5-HT content in fundus, body, antrum, and duodenum mucosal biopsy specimens from patients with IG (n=15) along with healthy control samples (n=18) via ELISA assay (One-way ANOVA). (B) Correlation matrix showing the Spearman’s rank correlation among gastric emptying scintigraphy (GES) %, 5-HT in the fundus, body, antrum, duodenum in patients with IG (n=11). (C) 5-HT and tryptase antibodies staining in antral biopsies from healthy controls and patients with IG. Triangles indicate EC cells (5-HT+Tryptase−), arrow heads indicate mast cells (Tryptase+). (D) Quantification of EC cells (5-HT+Tryptase−) counts per high-power field (HPF) (unpaired t-test, n=6). Error bars indicate SEM, *p < 0.05, **p < 0.01.
Discussion
In this study, our inducible EC cell-specific Tph1CreERT2/+ mouse circumvented the limitations of previous congenital knockout animal models and elucidated the functional roles of EC cell-derived 5-HT in gut motility. Conditional loss of EC cells in Tph1-DTA mice resulted in impaired gastric emptying and colonic transit, which were rescued by intragastric administration of 5-HT. Similar to what we found in murine models, 5-HT levels were highest in antral mucosal biopsies from control subjects. Antral mucosa from patients with IG showed a significant reduction of EC cell numbers and 5-HT levels, which strongly correlated with delayed gastric emptying.
Tph1-tdTom reporter mouse revealed the mature EC cells are located in the apical surface and the self-renewal EC cells are in the basolateral surface. This observation is supported by a recent single-cell differentiation mapping of mouse enteroendocrine cells, which showed two EC subpopulations and they were not derived from independent parallel lineages, but result from subsequent stages in EC-cell maturation.34 Our study clarifies the locations of these two subpopulations, with the mature cells primarily located in the apical surface and the self-renewal cells that may serve as precursor cells to maintain EC cell populations in the basolateral surface. Further investigation is warranted to explore whether long-lasting EC cells are truly long-lived or are products of self-renewal or transduced Lgr5+ cells.
In EC cell-depleted mice, both the apical and basolateral EC cells are ablated. We believe that not only apical 5-HT production and secretion are impaired, but the bidirectional crosstalk between basolateral EC cells and afferent nerves is inhibited as well. Basolateral EC cells may function as a “communication circuit” by exploiting its neuropod, a prominent long extension “synapse” structure in enteroendocrine cells with adjacent mucosal epithelium or afferent nerve terminals in the submucosal and myenteric plexus.35 Upon EC cell depletion, the cancelation of electrical signals that transmit from basolateral EC cells onto nearby intrinsic and extrinsic sensory afferent nerve terminals may inhibit the ability to directly transduce information from the gut lumen to the enteric and central nervous system.36, 37 EC cell-depleted mice allow us to dissect the function of EC cell-derived 5-HT as a driver of peristalsis between mucosal and enteric neuronal 5-HT. The impaired gastric and colonic motility was reversed via 5-HT in Tph1-DTA mice. 5-HT deficiency is the primary cause of GI dysmotility in the EC cell-depleted mice. Our data support the concept that endogenous 5-HT released from the mucosa is important to maintain but not essential for generating of major neurogenic motor patterns.15, 38
Enteroendocrine cells in the mouse are remarkably similar to those in humans, making it likely that insights learned from the mouse may contribute to both our understanding and treatment of a variety of human disorders.39 EC cells are also known to secrete secretin, Peptide YY (PYY), and cholecystokinin (CCK), even though 5-HT is the primary hormone produced in these cells.40 EC cell-depleted mice abrogate not only 5-HT production as well as other hormones and peptides secreted from these cells. While secretin, PYY, and CCK are important mediators of gastric and intestinal motility,41 they are primarily synthesized and released from other enteroendocrine cells.42 EC cells are not only heterogeneous with respect to the chemical messengers they secrete, but also in the patterns of expression of important sensory proteins such as nutrient receptors and transporters in the mouse duodenum and colon.43 The Tph1-tdTom reporter mouse line enables us to understand and interrogate EC cell behavior at single-cell resolution across each GI segment and uncover important biological and clinical implications in different diseases. Furthermore, Tph1-DTA mice may advantage the therapeutic target testing for GI motility disorders due to the reversible EC cell depletion. EC cells were naturally regenerated, and gastric and colonic dysmotility returned to normal at 3 weeks post-Tx injection. The conditional EC cell depletion can be repeated in the same mice, which is an advantage using this model. On the other hand, the gastric emptying was estimated via liquid and solid food, technically a semi-solid paste, which is designated reflecting more on the solid gastric emptying. Further investigations exploiting high-resolution of spatiotemporal gastric mapping or [13C]-octanoic acid breath test would be ideal gastric emptying measurements for murine studies.44, 45
The current pathogenesis of gastroparesis involves the cellular abnormalities in the enteric neurons, ICCs, platelet-derived growth factor alpha (PDGFRα)+ cells, smooth muscle cells, and immune cells.8, 14 Yet, dysfunction in nNOS is the only current IG animal model.46 Depletion of EC cells in our mice resulted in phenotypes that resemble those often seen in human IG and STC. Clinically, there is a significant overlap between gastroparesis and functional GI motility disorders.47, 48 A recent study showed that patients with gastroparesis had a higher prevalence of STC than those with normal gastric emptying.12 Reduced fecal output and water content, delayed colonic transit time, and impaired colonic peristalsis and neurogenic motor patterns are the key parameters of the constipation phenotype in clinical practice and murine models.49, 50 EC cell-depleted mice exhibited these parameters discernibly and larger size fecal pellets, which is congruous with the earlier observation in the Tph1−/− mice19 and is probably a consequence of the slower propulsion when mice strain to defecate. Medications, especially 5-HT receptor modulators that promote gastric emptying, have become a cornerstone of gastroparesis management.51 Prucalopride, a 5-HT4 agonist, is approved for the treatment of IG in some countries and also showed improvement for chronic constipation.52 These clinical observations and applications imply that 5-HT deficiency is a potential factor in the pathogenesis of delayed gastric emptying, and gut dysmotility in general. Yet, the co-occurrence of gastroparesis and STC also raise a cause-effect question of these two conditions. These findings are supported by our mouse models, suggesting a principal role of EC cells in the pathophysiology of IG. There is an overwhelming demand to elucidate effective treatment regimens for IG, targeting the underlying pathophysiology instead of regimens aimed at suppressing the associated symptoms.
We report for the first time that antral mucosal 5-HT levels and EC cell numbers are decreased in patients with IG and significantly correlate with gastric emptying. A larger sample size is warranted to sufficiently powered to detect a precise correlation between 5-HT content and gastric emptying and/or cardinal symptoms. Regional abnormalities in motility patterns of the fundus, body, antrum, and pylorus could result in delayed gastric emptying.53 Motility studies have demonstrated that antral hypomotility is a consistent finding in gastroparesis.54 On the other hand, the coordinated interplay between the fundus, body and antrum are crucial for normal gastric emptying. The fundus serves mainly as a reservoir to receive the food bolus, and the antrum crushes, sieves solids and pumps chyme into the duodenum. Additionally, a fundo-antral reflex is believed to increase antral contractions in response to fundal distention and may serve in mixing and peristalsis.55 In delayed emptying, the coordination of pressures between the antrum, pylorus, and duodenum are diminished.56 Furthermore, 5-HT induces fundus relaxation and initiates phasic contractions in the antrum.57 Thus, our data imply that antral 5-HT deficiency has unique effects that might lead to impaired gastric emptying. Given the depletion occurs in the entire GI tract in the mouse model, an antrum-specific EC cell loss or 5-HT inhibition strategy warrants future investigations.
Here, we report a potential mechanistic link between 5-HT deficiency and delayed gastric emptying, illuminating a pathogenic mechanism that may underlie gastroparesis more generally. Furthermore, our study suggests that examining gastric biopsy for 5-HT content may be a potential complementary step for the diagnosis, prognosis, and treatment of IG. The new inducible EC cell mouse model can facilitate future studies of 5-HT-related gut motility, functional GI disorders, but also the peripheral effects of 5-HT in other diseases.
Supplementary Material
BACKGROUND AND CONTEXT
The precise roles of enterochromaffin (EC) cell-derived serotonin in gut motility remains a conjecture. The pathophysiological functions of serotonin in gastric motility is explored in mice and humans.
NEW FINDINGS
Tph1CreERT2/+ mouse revealed the mature EC cells located in apical surface and the self-renewal EC cells in basolateral and uncovered a potential mechanistic link between serotonin deficiency and idiopathic gastroparesis (IG).
LIMITATIONS
The direct evidence to determine whether self-renewal EC cells are long-lived needs further investigations. A large cohort study is warranted to elucidate a precise mechanism of 5-HT deficiency in IG.
IMPACT
This study’s novel approach and findings have intrinsic translational importance in IG. Examining gastric biopsy for serotonin content may be a potential complementary step for diagnosis and treatment of IG.
Acknowledgements
We would like to thank Lin Gan, Ph.D. for their services in generating the Tph1CreERT2 mouse and Benjamin J Weigler, D.V.M. and Walt Mandeville, D.V.M. for their animal services.
Grant support:
Research was supported by NIH grants (DK094886 and DK103055 to S. Ro, and P01 DK41315 to K. Sanders and S. Ro).
Abbreviations used in this paper:
- AADC
aromatic L-amino acid decarboxylase
- CMMC
colonic migrating motor complexes
- CTT
colonic transit time
- DTA
diphtheria toxin A
- EC
enterochromaffin
- GET
gastric emptying test
- GES
gastric emptying scintigraphy
- GI
gastrointestinal
- IG
idiopathic gastroparesis
- Oil
sunflower oil
- STC
slow transit constipation
- TGITT
total GI transit time
- TPH
tryptophan hydroxylase
- Trp
L-tryptophan
- Tx
tamoxifen
- 5-HT
5-hydroxytryptamine
- 5-HTP
5-hydroxytryptophan
Footnotes
Conflict of interest statement (for all authors)
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplemental material includes supplementary methods, eight figures and five tables can be found with this article at Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2021.02.060
References
- 1.Linan-Rico A, Ochoa-Cortes F, Beyder A, et al. Mechanosensory Signaling in Enterochromaffin Cells and 5-HT Release: Potential Implications for Gut Inflammation. Front Neurosci 2016;10:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol 2003;66:1673–80. [DOI] [PubMed] [Google Scholar]
- 3.Costa M, Brookes SJ, Steele PA, et al. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience 1996;75:949–67. [DOI] [PubMed] [Google Scholar]
- 4.Bellono NW, Bayrer JR, Leitch DB, et al. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017;170:185–198 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 2013;10:473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin AM, Yabut JM, Choo JM, et al. The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc Natl Acad Sci U S A 2019;116:19802–19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh R, Zogg H, Wei L, et al. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J Neurogastroenterol Motil 2021;27:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowell MD, Shetzline MA, Moses PL, et al. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr Opin Investig Drugs 2004;5:55–60. [PubMed] [Google Scholar]
- 10.Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015;44:1–7. [DOI] [PubMed] [Google Scholar]
- 11.Yu DH, Ramsey FV, Norton WF, et al. The Burdens, Concerns, and Quality of Life of Patients with Gastroparesis. Digestive Diseases and Sciences 2017;62:879–893. [DOI] [PubMed] [Google Scholar]
- 12.Zikos TA, Kamal AN, Neshatian L, et al. High Prevalence of Slow Transit Constipation in Patients With Gastroparesis. Journal of Neurogastroenterology and Motility 2019;25:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grover M, Farrugia G, Lurken MS, et al. Cellular Changes in Diabetic and Idiopathic Gastroparesis. Gastroenterology 2011;140:1575–U296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grover M, Farrugia G, Stanghellini V. Gastroparesis: a turning point in understanding and treatment. Gut 2019;68:2238–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating DJ, Spencer NJ. What is the role of endogenous gut serotonin in the control of gastrointestinal motility? Pharmacological Research 2019;140:50–55. [DOI] [PubMed] [Google Scholar]
- 16.Keating DJ, Spencer NJ. Release of 5-Hydroxytryptamine From the Mucosa Is Not Required for the Generation or Propagation of Colonic Migrating Motor Complexes. Gastroenterology 2010;138:659–U319. [DOI] [PubMed] [Google Scholar]
- 17.Spencer NJ, Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol 2020;17:338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Chalazonitis A, Huang YY, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci 2011;31:8998–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heredia DJ, Gershon MD, Koh SD, et al. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol 2013;591:5939–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majumdar AP. Regulation of gastrointestinal mucosal growth during aging. J Physiol Pharmacol 2003;54 Suppl 4:143–54. [PubMed] [Google Scholar]
- 21.McCann CJ, Cooper JE, Natarajan D, et al. Transplantation of enteric nervous system stem cells rescues nitric oxide synthase deficient mouse colon. Nat Commun 2017;8:15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh R, Ha SE, Wei L, et al. MiR-10b-5p Rescues Diabetes and Gastrointestinal Dysmotility. Gastroenterology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kushnir-Sukhov NM, Brown JM, Wu Y, et al. Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol 2007;119:498–9. [DOI] [PubMed] [Google Scholar]
- 24.Bertrand RL, Senadheera S, Tanoto A, et al. Serotonin availability in rat colon is reduced during a Western diet model of obesity. American Journal of Physiology-Gastrointestinal and Liver Physiology 2012;303:G424–G434. [DOI] [PubMed] [Google Scholar]
- 25.Gustafsson BI, Bakke I, Tommeras K, et al. A new method for visualization of gut mucosal cells, describing the enterochromaffin cell in the rat gastrointestinal tract. Scand J Gastroenterol 2006;41:390–5. [DOI] [PubMed] [Google Scholar]
- 26.Tan DWM, Barker N. Intestinal Stem Cells and Their Defining Niche. Stem Cells in Development and Disease 2014;107:77–107. [DOI] [PubMed] [Google Scholar]
- 27.Coates MD, Tekin I, Vrana KE, et al. Review article: the many potential roles of intestinal serotonin (5-hydroxytryptamine, 5-HT) signalling in inflammatory bowel disease. Aliment Pharmacol Ther 2017;46:569–580. [DOI] [PubMed] [Google Scholar]
- 28.Krndija D, El Marjou F, Guirao B, et al. Active cell migration is critical for steady-state epithelial turnover in the gut. Science 2019;365:705–710. [DOI] [PubMed] [Google Scholar]
- 29.de Bruine AP, Dinjens WN, Zijlema JH, et al. Renewal of enterochromaffin cells in the rat caecum. Anat Rec 1992;233:75–82. [DOI] [PubMed] [Google Scholar]
- 30.Voehringer D, Liang HE, Locksley RM. Homeostasis and effector function of lymphopenia-induced "memory-like" T cells in constitutively T cell-depleted mice. J Immunol 2008;180:4742–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin AM, Young RL, Leong L, et al. The Diverse Metabolic Roles of Peripheral Serotonin. Endocrinology 2017;158:1049–1063. [DOI] [PubMed] [Google Scholar]
- 32.Camilleri M, Chedid V, Ford AC, et al. Gastroparesis. Nat Rev Dis Primers 2018;4:41. [DOI] [PubMed] [Google Scholar]
- 33.Bearcroft CP, Perrett D, Farthing MJ. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut 1998;42:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gehart H, van Es JH, Hamer K, et al. Identification of Enteroendocrine Regulators by Real-Time Single-Cell Differentiation Mapping. Cell 2019;176:1158–1173 e16. [DOI] [PubMed] [Google Scholar]
- 35.Kaelberer MM, Rupprecht LE, Liu WW, et al. Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction. Annu Rev Neurosci 2020;43:337–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liddle RA. Neuropods. Cellular and Molecular Gastroenterology and Hepatology 2019;7:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Needham BD, Kaddurah-Daouk R, Mazmanian SK. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat Rev Neurosci 2020;21:717–731. [DOI] [PubMed] [Google Scholar]
- 38.Spencer NJ, Sia TC, Brookes SJ, et al. CrossTalk opposing view: 5-HT is not necessary for peristalsis. Journal of Physiology-London 2015;593:3229–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: Development and differentiation of gut endocrine cells. Endocrinology 2004;145:2639–2644. [DOI] [PubMed] [Google Scholar]
- 40.Martins P, Fakhry J, de Oliveira EC, et al. Analysis of enteroendocrine cell populations in the human colon. Cell and Tissue Research 2017;367:161–168. [DOI] [PubMed] [Google Scholar]
- 41.Camilleri M Gastrointestinal hormones and regulation of gastric emptying. Curr Opin Endocrinol Diabetes Obes 2019;26:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diwakarla S, Fothergill LJ, Fakhry J, et al. Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol Motil 2017;29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin AM, Lumsden AL, Young RL, et al. The nutrient-sensing repertoires of mouse enterochromaffin cells differ between duodenum and colon. Neurogastroenterology and Motility 2017;29. [DOI] [PubMed] [Google Scholar]
- 44.Creedon CT, Verhulst PJ, Choi KM, et al. Assessment of gastric emptying in non-obese diabetic mice using a [13C]-octanoic acid breath test. J Vis Exp 2013:e50301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Grady G, Angeli TR, Du P, et al. Abnormal initiation and conduction of slow-wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology 2012;143:589–598 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sivarao DV, Mashimo H, Goyal RK. Pyloric sphincter dysfunction in nNOS−/− and W/Wv mutant mice: animal models of gastroparesis and duodenogastric reflux. Gastroenterology 2008;135:1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghoshal UC, Singh R. Frequency and risk factors of functional gastro-intestinal disorders in a rural Indian population. Journal of Gastroenterology and Hepatology 2017;32:378–387. [DOI] [PubMed] [Google Scholar]
- 48.Kim BJ, Kuo B. Gastroparesis and Functional Dyspepsia: A Blurring Distinction of Pathophysiology and Treatment. J Neurogastroenterol Motil 2019;25:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spencer NJ, Dinning PG, Brookes SJ, et al. Insights into the mechanisms underlying colonic motor patterns. J Physiol 2016;594:4099–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology 2016. [DOI] [PubMed] [Google Scholar]
- 51.Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of Gastroparesis. American Journal of Gastroenterology 2013;108:18–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carbone F, Van den Houte K, Clevers E, et al. Prucalopride in Gastroparesis: A Randomized Placebo-Controlled Crossover Study. Am J Gastroenterol 2019;114:1265–1274. [DOI] [PubMed] [Google Scholar]
- 53.Goyal RK, Guo Y, Mashimo H. Advances in the physiology of gastric emptying. Neurogastroenterol Motil 2019;31:e13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kerlin P Postprandial antral hypomotility in patients with idiopathic nausea and vomiting. Gut 1989;30:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao SS, Kumar A, Harris B, et al. Investigation of fundo-antral reflex in human beings. World J Gastroenterol 2005;11:6676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rayner CK, Horowitz M. New management approaches for gastroparesis. Nat Clin Pract Gastroenterol Hepatol 2005;2:454–62; quiz 493. [DOI] [PubMed] [Google Scholar]
- 57.Moen H, Ertresvaag K, Gerner T. Motor responses to serotonin in isolated guinea pig fundus and antrum. Scand J Gastroenterol 1983;18:145–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.