Key Points
Question
How well did participants assigned to ranibizumab injections for proliferative diabetic retinopathy in a randomized clinical trial comply with their scheduled visits for treatment and monitoring?
Findings
In this post hoc analysis of a randomized clinical trial, 94 of 170 participants (55.3%) had at least 1 instance within 5 years when they were late for their next scheduled appointment by 8 or more weeks, and 50 (29.4%) ultimately dropped out.
Meaning
The likelihood of lapses in care after initial treatment should be considered when choosing treatment for proliferative diabetic retinopathy.
This post hoc analysis of a randomized clinical trial evaluates completion of scheduled examinations among participants assigned to intravitreous injections of ranibizumab for proliferative diabetic retinopathy in a multicenter clinical trial.
Abstract
Importance
The follow-up schedule for individuals with eyes treated with anti–vascular endothelial growth factor agents for proliferative diabetic retinopathy (PDR) requires that patients return frequently for monitoring and repeated treatment. The likelihood that a patient will comply should be a consideration in choosing a treatment approach.
Objective
To describe completion of scheduled examinations among participants assigned to intravitreous injections of ranibizumab for PDR in a multicenter randomized clinical trial.
Design, Setting, and Participants
This post hoc analysis evaluates data from a randomized clinical trial conducted at 55 US sites among 305 adults with proliferative diabetic retinopathy enrolled between February and December 2012. Both eyes were enrolled for 89 participants (1 eye to each study group), with a total of 394 study eyes. The final 2-year visit was completed in January 2015. Data were analyzed from April 2019 to July 2021.
Interventions
Ranibizumab injections for PDR or macular edema.
Main Outcomes and Measures
A long lapse in care of 8 or more weeks past a scheduled examination, dropout from follow-up, visual acuity at 5 years.
Results
Among 170 participants, the median age was 51 years, and 44.7% were female. Through 5 years of follow-up, 94 of 170 participants (55.3%) had 1 or more long lapse in care. Median time to the first long lapse was 210 weeks, and 69 of 94 participants (73.4%) returned for examination after the first long lapse. Fifty of 170 participants (29.4%) dropped out of follow-up by 5 years. Among the 120 participants who completed the 5-year examination, median change from baseline in visual acuity was −2 letters for participants who had 1 or more long lapse compared with +5 letters for those without a long lapse (P = .02). After multivariable adjustment, the odds ratio (95% CI) for baseline associations with 1 or more long lapse was 1.21 (1.03-1.43) for each 5-letter decrement in visual acuity score, 2.19 (1.09-4.38) for neovascularization of the disc and elsewhere, and 3.48 (1.38-8.78) for no prior laser treatment for diabetic macular edema.
Conclusions and Relevance
Over 5 years, approximately half of the participants assigned to ranibizumab for PDR had a long lapse in care despite substantial effort by the DRCR Retina Network to facilitate timely completion of examinations. The likelihood of a long lapse in care during long-term follow-up needs to be considered when choosing treatment for PDR.
Trial Registration
ClinicalTrials.gov Identifier: NCT01489189
Introduction
In 1976, results from the Diabetic Retinopathy Study1 showed that panretinal photocoagulation (PRP) was an effective treatment for proliferative diabetic retinopathy (PDR). To our knowledge, no other treatments for PDR were proven effective until results from the DRCR Retina Network Protocol S2 demonstrated that treatment with intravitreous ranibizumab provided visual acuity (VA) outcomes for 2 years following treatment initiation that were noninferior compared with PRP.2 These results were maintained through 5 years.3 Eyes treated with ranibizumab had less visual field loss, lower incidence of vision-impairing diabetic macular edema (DME), fewer vitrectomies, and fewer retinal detachments through 2 years.3,4 Additionally, results from a clinical trial conducted in the UK demonstrated that treatment with aflibercept provided a better 1-year mean VA score (4-letter difference) compared with treatment with PRP.5
However, the frequency of treatment and monitoring visits is greater for eyes treated with anti–vascular endothelial growth factor (VEGF) agents than for those treated with PRP. Initial PRP treatment is performed in 1 or 2 sessions and about 50% of eyes receive supplemental PRP to treat persistent or worsening PDR. Treatment with anti-VEGF agents typically involves 4 to 6 consecutive monthly loading doses followed by frequent examinations to detect worsening retinopathy that may require additional injections. Eyes assigned to ranibizumab in Protocol S had a mean (SD) of 7 (2) injections for PDR in year 1 followed by 3 (3) injections during each following year and had a median (IQR) of 43 (33-52) visits over the 5-year follow-up period. In contrast, eyes assigned to PRP had a median (IQR) of 21 (17-25) visits and approximately 50% did not require a supplemental PRP session.2,3
Because of the greater need for monitoring and repeat treatment with anti-VEGF therapy, the likelihood that a patient will comply with the follow-up schedule is an important consideration in choosing between treatment approaches.4,6,7 In reports describing patients receiving treatment for PDR in clinical practice settings with either PRP or anti-VEGF agents for up to 5 years, the percentage of patients with lapses in care lasting more than 6 months was 72% and 61% in 2 studies.8,9 Another study reported lapses in care of 12 months or more among 25% of patients with PDR.10 In other reports, a high proportion of patients returning after an extended lapse in care had irreversible VA loss and/or vision-threatening disease progression.11,12 These findings amplify concerns about choosing anti-VEGF treatment to manage PDR. Thus, identifying characteristics of participants likely to comply with the relatively intensive follow-up schedule required for anti-VEGF treatment of PDR is important.
We describe the frequency and pattern of lapses in follow-up among participants assigned to ranibizumab in Protocol S, identify baseline factors associated with lapses in follow-up, and describe complications and VA among participants who returned after a lapse.
Methods
Eligibility for Enrollment and Treatment Assignment
Between February and December 2012, 305 adults with PDR from 55 clinical sites in the US enrolled in Protocol S.2,3 Both eyes were enrolled for 89 participants. A VA letter score of 24 or higher (approximate Snellen equivalent of 20/320) and no prior PRP were required in study eyes. Eyes were randomly assigned to ranibizumab injections of 0.5 mg (n = 191) or PRP (n = 203). Participants with 2 study eyes were randomly assigned one eye to each group. The study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board associated with each clinical site. Study participants provided written informed consent. Participant-reported race and ethnicity were collected based on fixed categories per the National Institutes of Health policy13 and consistent with US Food and Drug Administration guidelines.14 Participants were given $50 at baseline and each completed annual protocol visit $25 per completed nonannual protocol visits, and as needed to cover travel and other visit-related expenses. The final 2-year visit was completed in January 2015. Data were analyzed from April 2019 to July 2021.
Treatment of Participants Assigned to Ranibizumab
Eyes assigned to ranibizumab received injections every 4 weeks through week 24. Injections were deferred at weeks 16 and 20 if neovascularization (NV) had resolved. At 24 weeks, injections continued every 4 weeks if NV had improved or worsened but could be deferred if the investigator judged all NV to be resolved or if NV was stable after 2 consecutive injections. A series of injections resumed if NV worsened later. If an eye met protocol-specified failure or futility criteria based on the investigator’s clinical evaluation, PRP was permitted. Vitrectomy for vitreous hemorrhage was prohibited for at least 8 weeks after onset, while vitrectomy for retinal detachment was at investigator discretion. A series of ranibizumab injections was required at baseline for eyes with DME and vision impairment. Thereafter, injections for DME were at investigator discretion.
Follow-up With Participants Assigned to Ranibizumab
Follow-up visits were scheduled every 4 weeks during year 1 and every 4, 8, 16, or 20 weeks thereafter depending on individual treatment course. Following a change in protocol, 5 of 191 participants (2.6%) opted to return for only annual visits after the 3-year visit. Participants received payment for individual visit completion, financial assistance for protocol-required study eye treatments, and the support of clinical coordinators who made intensive and sustained efforts to facilitate completion of visits. Investigators, coordinators, and the DRCR Retina Network Coordinating Center attempted to locate participants who were overdue for visits using telephone calls, certified letters, and a third-party search service to identify new contact information.15
Measures of Compliance With Follow-up: Short and Long Lapses in Care
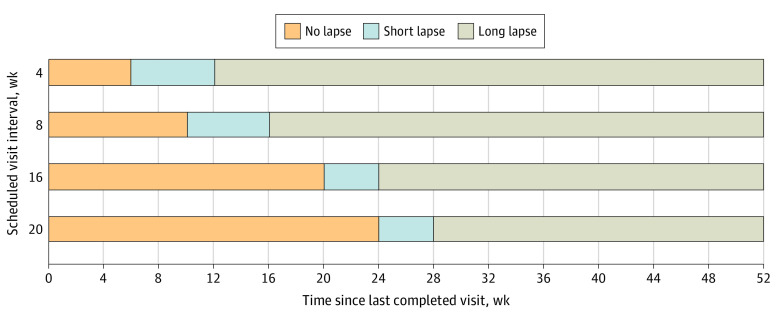
For this post hoc analysis, lapse in care was defined as more than 2 weeks past the target date for a visit that was scheduled either 4 or 8 weeks after a completed visit or more than 4 weeks past the target date for a visit scheduled 16 or more weeks after a completed visit. A short lapse in care occurred when the participant had a lapse in care and returned within 8 weeks from the target date for the next visit. A long lapse in care occurred if the participant did not return by 8 weeks past the target date for the next visit (Figure 1). The length of a lapse in care was calculated from the date of the last completed visit to the date of return. If the participant never returned, the 5-year anniversary of enrollment was used as the end point for the lapse. Any participant who did not complete the 5-year visit was considered to have dropped out.
Figure 1. Lapse in Care by Scheduled Interval From Last Study Visit and Number of Weeks Since the Last Completed Visit.
The 20-week schedule only applied to the visits immediately before an annual visit (ie, 84 weeks, 136 weeks, 188 weeks, and 240 weeks).
Data Analyses
Participants were classified as having no lapses in care, short lapses only, or 1 or more long lapse. Participants who died during the follow-up period were excluded. Kaplan-Meier curves were plotted for time to the first episode of any lapse in care, to the first episode of a long lapse in care, and to dropping out. A modified Functional Comorbidity Index score (range 0 to 16), which was an unweighted sum of 16 concurrent comorbid conditions excluding diabetes and visual impairment from the original version, was derived from participant self-reported preexisting conditions based on MedDRA classification.16,17,18 A backward stepwise variable selection approach was used in logistic regression models of a long lapse with entry selection criterion of P ≤ .10 and stay criterion of P ≤ .05. The C statistic was used to assess the discriminatory performance of the final multivariable model. Kruskal-Wallis test and Fisher exact test were used to compare outcomes between participant groups. P values were 2-sided and were not adjusted for multiple outcomes analyzed. Analyses used SAS version 9.4 (SAS Institute).
Results
Among the 191 participants with an eye assigned to ranibizumab, 21 died during the follow-up period. Among the remaining 170 participants, the median (IQR) age at baseline was 51 (44-59) years, and 76 participants (44.7%) were female. Participant self-reported race and ethnicity were as follows: 35 were Black or African American (20.6%), 42 Hispanic (24.7%), 88 non-Hispanic White (51.8%), and 5 other (2.9%). The median hemoglobin A1c was 8.6% (to convert to proportion of total hemoglobin, multiply by 0.01), 124 participants (72.9%) self-reported type 2 diabetes, and 61 (36.3%) had high-risk PDR (level 71 or higher) (eTable 1 in Supplement 1).
Visit Compliance Through 5 Years
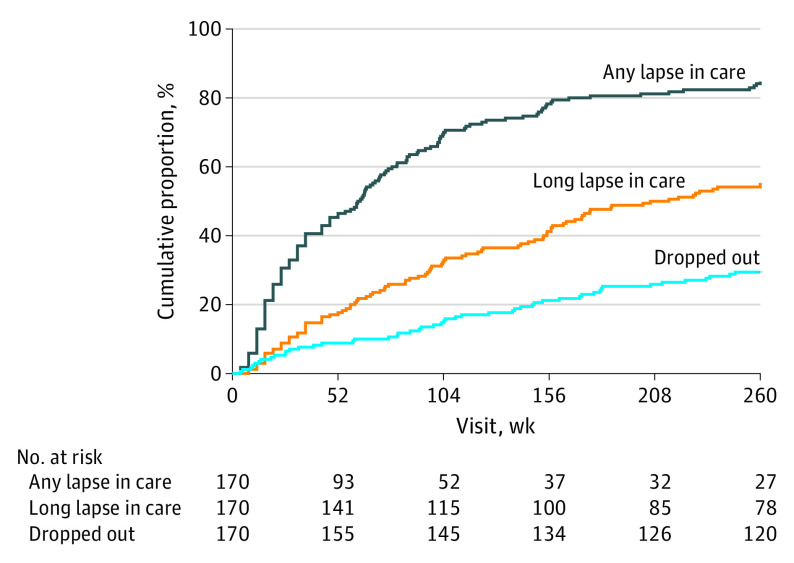
Over 5 years, 26 of 170 participants (15.3%) had no lapses in care (Table 1). For the remaining participants, the median number of episodes of any lapse in care was 3 (range, 0-16) and 63 participants (37.1%) had 4 or more lapses in care. Among the 144 participants with at least 1 lapse in care, the first lapse resulted in dropout from the study for 14 (9.7%), while 130 (90.3%) returned for follow-up. The 5 participants who opted for annual visits only after 3 years each met the criteria for a long lapse because of dropout (after 3 years) or missing scheduled visits before 3 years. Fifty participants (29.4%) had short lapses only and 94 (55.3%) had 1 or more long lapse in care. In the group with 1 or more long lapse, 65 participants (69.1%) had a short lapse preceding the first long lapse, and the first long lapse coincided with dropout from the study in 25 (26.6%). Among 69 participants who returned after the first long lapse, 25 (36.2%) dropped out later. By 52 weeks, 79 (46.5%) had any lapse in care, 30 (17.6%) had a long lapse, and 15 (8.8%) had dropped out; by 104 weeks the corresponding numbers were 119 (70.0%), 55 (32.4%), and 26 (15.3%) (Kaplan-Meier estimates). The median time to the first lapse in care was 62 weeks (95% CI, 44-73) and to the first long lapse was 210 weeks (95% CI, 157-undefined) (Figure 2).
Table 1. Patterns of Lapses in Care Among Participants With an Eye Assigned to Ranibizumab.
| Patterns of lapses in care | No. (%) | Dropped out by 5 y, No. (%) |
|---|---|---|
| Total No. of participants (excluding deaths) | 170 (100) | 50 (29.4) |
| Category of lapses in care | ||
| No lapses | 26 (15.3) | 0 |
| Short lapses only | 50 (29.4) | 0 |
| ≥1 Long lapse | 94 (55.3) | 50 (53.2) |
| No. of episodes of lapse in care | ||
| 0 | 26 (15.3) | 0 |
| 1 | 32 (18.8) | 14 (43.8) |
| 2 | 21 (12.4) | 5 (23.8) |
| 3 | 28 (16.5) | 11 (39.3) |
| 4-6 | 34 (20.0) | 13 (38.2) |
| ≥7 | 29 (17.1) | 7 (24.1) |
| No. of episodes of long lapse in care | ||
| 0 | 76 (44.7) | 0 |
| 1 | 48 (28.2) | 25 (52.1) |
| 2 | 23 (13.5) | 11 (47.8) |
| ≥3 | 23 (13.5) | 14 (60.9) |
Figure 2. Time to Lapse in Care and Dropping Out Among Participants Assigned to Ranibizumab.
Time to first lapse in care was the target date for the next scheduled visit that triggered the lapse in care. Time to dropping out was the last completed visit date. Participants who completed the study without a lapse in care were censored at 260 weeks.
Baseline Factors Associated With Long Lapses in Care
The proportion of participants with a long lapse was higher for non-White individuals (including American Indian/Alaskan Native, Asian, Black, Hispanic, more than 1 race, and unknown or not reported) and in those with worse baseline VA letter score (less than 79 [approximate Snellen equivalent of worse than 20/25]) in the study eye or in the fellow eye, neovascularization of the disc (NVD) and elsewhere (NVE) both present, high-risk PDR, no prior treatment for DME, and no prior laser treatment for DME (Table 2). After applying the backward variable selection approach, the final multivariable model contained 3 baseline factors. Each line (5-letter) worse in study eye VA was associated with an odds ratio (95% CI) for a long lapse in care of 1.21 (1.03-1.43) (eTable 2 in Supplement 1). Having both NVD and NVE in the study eye was associated with an odds ratio (95% CI) of 2.19 (1.09-4.38) and not having had prior laser in the study eye for DME was associated with an odds ratio (95% CI) of 3.48 (1.38-8.78). The C statistic for the model, which is equal to the area under the receiver operating characteristic curve, was 0.68.
Table 2. Long Lapse in Care by Selected Baseline Factors Among Participants Treated With Ranibizumab.
| Baseline factor | No. | Long lapse, No. (%) | P valuea |
|---|---|---|---|
| All participants | 170 | 94 (55.3) | NA |
| Participant factors | |||
| Sex | .35 | ||
| Female | 76 | 39 (51.3) | |
| Male | 94 | 55 (58.5) | |
| Age, y | .88 | ||
| <50 | 75 | 39 (52.0) | |
| ≥50 | 95 | 55 (57.9) | |
| No. of study eyes enrolled | .14 | ||
| 1 | 89 | 54 (60.7) | |
| 2 | 81 | 40 (49.4) | |
| Race and ethnicityb | .003 | ||
| White | 88 | 39 (44.3) | |
| Non-Whitec | 82 | 55 (67.1) | |
| Diabetesd | .24 | ||
| Type 1 | 40 | 19 (47.5) | |
| Type 2 | 124 | 72 (58.1) | |
| Duration of diabetes, y | .24 | ||
| <20 | 96 | 59 (61.5) | |
| ≥20 | 74 | 35 (47.3) | |
| Hemoglobin A1c, %e | .45 | ||
| <9.0 | 97 | 47 (48.5) | |
| ≥9.0 | 67 | 42 (62.7) | |
| Functional Comorbidity Index | .63 | ||
| 0 | 86 | 47 (54.7) | |
| ≥1 | 84 | 47 (56.0) | |
| Fellow eye visual acuity letter score (approximate Snellen equivalent)f | .01 | ||
| ≥79 (20/25 or Better) | 72 | 33 (45.8) | |
| <79 (20/32 or Worse) | 98 | 61 (62.2) | |
| Study eye factors | |||
| Visual acuity letter score (approximate Snellen equivalent)f | .02 | ||
| ≥79 (20/25 or Better) | 83 | 38 (45.8) | |
| <79 (20/32 or Worse) | 87 | 56 (64.4) | |
| OCT central subfield thickness (Stratus equivalent), μmg | .79 | ||
| <250 | 115 | 62 (53.9) | |
| ≥250 | 53 | 31 (58.5) | |
| Presence of DMEg | .46 | ||
| No | 123 | 66 (53.7) | |
| Yes | 45 | 27 (60.0) | |
| Neovascularization on clinical examinationh | .01 | ||
| NVD or NVE only | 108 | 51 (47.2) | |
| NVD and NVE | 57 | 39 (68.4) | |
| Lens status on clinical examination | NA | ||
| Phakic | 152 | 84 (55.3) | |
| Posterior chamber intraocular lens | 18 | 10 (55.6) | |
| Diabetic retinopathy severity (ETDRS level)i | .01 | ||
| ≤65 (Moderate PDR or better) | 107 | 52 (48.6) | |
| ≥71 (High-risk PDR or worse) | 61 | 40 (65.6) | |
| Prior treatment for DME | .03 | ||
| No | 130 | 78 (60.0) | |
| Yes | 40 | 16 (40.0) | |
| Prior focal/grid laser treatment for DME | .009 | ||
| No | 142 | 85 (59.9) | |
| Yes | 28 | 9 (32.1) | |
| Prior anti-VEGF treatment for DME | NA | ||
| No | 150 | 83 (55.3) | |
| Yes | 20 | 11 (55.0) |
Abbreviations: DME, diabetic macular edema; ETDRS, Early Treatment Diabetic Retinopathy Study; NA, not applicable; NVD, neovascularization of the disc; NVE, neovascularization elsewhere; OCT, optical coherence tomography; PDR, proliferative diabetic retinopathy; VEGF, vascular endothelial growth factor.
P values were generated from univariable logistic regression models for having long lapse in care, which were only performed for the baseline factors with more than 20 eyes in each stratum. Baseline numerical factors were included as a continuous variable in the model and were dichotomized for presentation.
Participant-reported race and ethnicity were collected based on fixed categories per the National Institutes of Health policy13 and consistent with US Food and Drug Administration guidelines.14
Includes American Indian/Alaskan Native, Asian, Black, Hispanic, more than 1 race, and unknown or not reported.
Three participants in each group had uncertain type.
Unavailable for 5 participants with a long lapse and 1 without a long lapse. To convert to proportion of total hemoglobin, multiply by 0.01.
Visual acuity letter scores indicate best-corrected visual acuity in the study eye following protocol-defined refraction. Visual acuity was measured using electronic ETDRS visual acuity testing; higher letter scores indicate better vision.
Unavailable for 1 participant in each group.
Unavailable for 4 participants with a long lapse and 1 without a long lapse.
Determined by central reading center on 7 standard stereo field fundus photographs; unavailable for 2 participants in the group with a long lapse.
Outcomes by Visit Compliance
The baseline VA, 5-year VA, and change in VA for the 120 participants who completed the 5-year visit are summarized in Table 3. The median (IQR) change from baseline at the 5-year visit was +4 (−1 to +9) letters in the group having no lapses in care, +5 (+1 to +10) letters for the group with short lapses only, and −2 (−6 to +11) letters in the group with 1 or more long lapse (Table 3; P = .05). The corresponding percentages for a letter loss of 15 or more from baseline to the 5-year visit were 3.8% (1 of 26), 2.0% (1 of 50), and 13.6% (6 of 44) (P = .07). The median (IQR) change in VA letter score from the last completed visit to the return from a short lapse in care was 0 (−4 to +3) and was −1 (−6 to +2) after returning from a long lapse (P = .003). There was no apparent association between the timing of dropout from the study and baseline VA or VA at the last completed visit (eTable 3 in Supplement 1).
Table 3. Outcomes by Lapses in Care Among Participants Completing the 5-Year Visit.
| Outcome | Lapses in care | P valuea | ||
|---|---|---|---|---|
| None | Short only | ≥1 Long | ||
| No. of participants | 26 | 50 | 44 | NA |
| Visual acuity letter scoreb | ||||
| Baseline | NA | |||
| Mean (SD) | 78.8 (12.5) | 78.1 (9.1) | 75.7 (14.0) | |
| Snellen equivalent, mean | 20/32 | 20/32 | 20/32 | |
| Median (IQR) | 80 (76 to 87) | 80 (75 to 85) | 78 (72 to 85) | |
| Snellen equivalent | 20/25 (20/32 to 20/20) | 20/25 (20/32 to 20/20) | 20/32 (20/40 to 20/20) | |
| 5 y | NA | |||
| Mean (SD) | 83.3 (12.8) | 83.0 (14.6) | 71.4 (26.6) | |
| Snellen equivalent, mean | 20/25 | 20/25 | 20/40 | |
| Median (IQR) | 86 (80 to 91) | 85 (80 to 91) | 82 (73 to 87) | |
| Snellen equivalent | 20/20 (20/25 to 20/16) | 20/20 (20/25 to 20/16) | 20/25 (20/40 to 20/20) | |
| Change at 5 y | ||||
| Mean (SD) | +4.6 (11.4) | +4.9 (15.9) | −4.3 (25.1) | NA |
| Median (IQR) | +4 (−1 to +9) | +5 (+1 to +10) | −2 (−6 to +11) | .05c |
| ≥15-Letter loss from baseline at 5 y, No. (%) | 1 (3.8) | 1 (2.0) | 6 (13.6) | .07 |
| PDR-related adverse events during follow-up, No. (%) | ||||
| NVG or NVA | 0 | 2 (4.0) | 5 (11.4) | .12 |
| Tractional retinal detachment | 0 | 4 (8.0) | 3 (6.8) | .47 |
| Vitreous hemorrhage | 8 (30.8) | 26 (52.0) | 29 (65.9) | .02 |
| Vitrectomy | 1 (3.8) | 8 (16.0) | 8 (18.2) | .23 |
Abbreviations: NA, not applicable; NVA, neovascularization of the angle; NVG, neovascular glaucoma; PDR, proliferative diabetic retinopathy.
Kruskal-Wallis test was used to compare change in visual acuity letter score between the 3 groups. Fisher exact test was conducted to compare proportions of each binary outcome between the 3 groups.
Visual acuity letter scores indicate best-corrected visual acuity in the study eye following protocol-defined refraction. Visual acuity was measured using electronic Early Treatment for Diabetic Retinopathy Study (ETDRS) visual acuity testing; higher letter scores indicate better vision. A 5-letter difference in visual acuity letter score is equivalent to 1 Snellen line.
P = .02 from Wilcoxon rank sum test comparing change in visual acuity letter score from baseline between participants with a long lapse and those without a long lapse (median change = +5).
The proportions of eyes with PDR-associated adverse morphologic outcomes during follow-up are displayed in Table 3. The proportions of eyes with vitreous hemorrhage differed among the following groups: no lapse in care (8 of 26 [30.8%]), short lapses only (26 of 50 [52.0%]), and 1 or more long lapse (29 of 44 [65.9%]) (P = .02). Vitreous hemorrhage preceded or occurred remotely from a lapse in care in 22 of 26 participants (84.6%) in the group having short lapses only and 20 of 29 (69.0%) in the group having a long lapse. No instances of neovascular glaucoma (NVG) or neovascularization of the angle (NVA) were detected at the return visit (within 2 weeks) for the group having short lapses only, while NVG or NVA were detected in 2 of 5 eyes (40.0%) returning from a long lapse. All 7 eyes with tractional retinal detachments developed this either before or outside of a lapse in care. Among eyes with long lapses, the proportion of adverse events occurring before the first long lapse was 2 of 5 with NVG or NVA (40.0%), 3 of 3 with tractional retinal detachment (100%), and 11 of 29 with vitreous hemorrhage (37.9%).
Discussion
In this post hoc analysis of a clinical trial, approximately half of the participants assigned to ranibizumab for treatment of PDR who survived through 5 years of follow-up had 1 or more long lapse in care. Approximately 30% of participants dropped out by 5 years and only 15% completed all examinations without any lapse.
DRCR Retina Network clinical center staff for Protocol S enrolled only participants who they believed would be likely to complete the initial 3-year follow-up schedule. Even within the confines of a multicenter clinical trial that provided additional resources to facilitate timely examinations, participants assigned to ranibizumab frequently had lapses in care that could jeopardize outcomes.
Several baseline factors were associated with having a long lapse in care (Table 2), but after adjustment for other factors, only 3 were associated: worse VA, presence of both NVD and NVE on clinical examination, and no prior history of laser treatment for DME. However, the combined ability of these factors to predict a long lapse in care was low, with an area under the receiver operating characteristic curve of 0.68.19
Within 1 year of initiating ranibizumab treatment for PDR, nearly half of the participants (47%) had a lapse in care, including 18% with a long lapse (Figure 2). The cumulative probability of a lapse increased through the 5-year follow-up period, implying that a past record of timely visits did not ensure timely future visits. Nonetheless, 90% of participants returned after their first lapse. The return of a patient following a lapse provided an opportunity for the ophthalmologist to discuss with the patient the outcomes of poor follow-up and options for modifying treatment to include PRP.
Among participants who completed the 5-year examination, those with a long lapse in care had a median (IQR) VA change of −2 (−6 to +11) letters compared with +5 (1 to +10) letters for those without a long lapse. Furthermore, 13.6% of those with a long lapse lost 15 or more letters from baseline to 5 years compared with 2.6% of those without a long lapse. In a previous report on Protocol S,16 the only baseline factor identified as associated with less VA gain at 2 years for eyes assigned to ranibizumab was better baseline VA. Therefore, it is unlikely that the worse baseline VA in eyes with long lapses was responsible for the worse 5-year change from baseline. Many of the instances of NVG, NVA, tractional retinal detachment, and vitreous hemorrhage in eyes with long lapses were detected at times other than a return from a lapse. Thus, while eyes with long lapses may have had worse VA and anatomic outcomes, it is unclear whether this could be attributable to long lapses or to other characteristics (eg, ocular, medical, or social).
The definition of a long lapse in care used in this investigation (an intervisit interval of more than 6 months for individuals asked to return at 16-week intervals) is similar to definitions used in recent studies of visit compliance after initiation of treatment for PDR.8,9,20,21 However, we also considered an interval of more than 3 months a long lapse when the participant was scheduled for more frequent visits while receiving ranibizumab injections for active PDR or DME. Recent studies monitored PDR participants for periods of time ranging between 6 months and 3 to 5 years, and the percentages of PDR participants that were noncompliant varied markedly: 16% in a university center in Egypt (excluding patients who dropped out),20 29% in a university environment in which Austria provided universal health coverage,21 72% in a large retina practice in greater Houston, Texas,8 and 61% in a university center in Boston, Massachusetts.9 Therefore, it is difficult to compare results with Protocol S. Similarly, comparing the risk factors for poor compliance identified in previous studies with those in Protocol S is difficult because different sets of potential risk factors were used. However, most studies have evaluated age, sex, and baseline visual acuity. Age and visual acuity have not shown consistent association with visit adherence. Older age has been associated with worse compliance and9,10,21 better compliance,20 as well as having no significant association.8 Worse baseline VA has been associated with worse compliance20 and better compliance,10 as well as having no significant association.9,21 Sex has not been identified as a risk factor.
Strengths and Limitations
Our study of compliance with follow-up examinations has strengths and weaknesses. The data from Protocol S allowed description over 5 years of the timing of follow-up examinations that were prospectively determined and standardized. The timing of lapses in care and of dropout were available in the data set, as was the percentage of patients returning for examination. However, it remains unknown whether the 29% of participants who dropped out resumed care elsewhere, and the VA and anatomical outcomes of these eyes also remains unknown. The ability of baseline factors to predict who will have a long lapse was low. As the efforts to facilitate follow-up and the financial incentives offered in Protocol S may be unique, the follow-up experience reported herein is likely better than that achieved in most clinical practice settings.
Conclusions
Approximately 15% of participants treated with ranibizumab for PDR in a multicenter clinical trial completed 5 years of follow-up without a lapse in care, and an additional 30% had only short lapses in care that were not associated with worse vision outcomes. Among the approximately 50% of participants who had a long lapse in care, the proportion of individuals with a loss of VA of 15 or more letters was higher than in participants without a long lapse (14% vs 3%); however, it is unclear whether these worse outcomes resulted from missed visits or were related to patient and/or eye characteristics that were more prevalent among participants who miss visits. Although participants with worse VA, with both NVE and NVD, and without prior laser treatment for DME at baseline were more likely to have a long lapse in care, these factors were not highly predictive of who would later have a long lapse. Most (90%) participants in this study returned for at least 1 visit after their first lapse in care. This finding suggests that clinicians may have opportunities to counsel returning patients about the importance of timely follow-up to optimize their outcomes.
eTable 1. Baseline factors of participants and study eye alive through 5 years with eyes assigned to ranibizumab
eTable 2. Multivariable analysis of baseline factors associated with a long lapse in care
eTable 3. Duration of follow-up at time of dropout and visual acuity at baseline and the last completed visit
The DRCR Retina Network members
References
- 1.The Diabetic Retinopathy Study Research Group . Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81(4):383-396. doi: 10.1016/0002-9394(76)90292-0 [DOI] [PubMed] [Google Scholar]
- 2.Gross JG, Glassman AR, Jampol LM, et al. ; Writing Committee for the Diabetic Retinopathy Clinical Research Network . Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146. doi: 10.1001/jama.2015.15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross JG, Glassman AR, Liu D, et al. ; Diabetic Retinopathy Clinical Research Network . Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-1148. doi: 10.1001/jamaophthalmol.2018.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun JK, Glassman AR, Beaulieu WT, et al. ; Diabetic Retinopathy Clinical Research Network . Rationale and application of the Protocol S anti-vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology. 2019;126(1):87-95. doi: 10.1016/j.ophtha.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivaprasad S, Prevost AT, Vasconcelos JC, et al. ; CLARITY Study Group . Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193-2203. doi: 10.1016/S0140-6736(17)31193-5 [DOI] [PubMed] [Google Scholar]
- 6.Ting DSW, Wong TY. Proliferative diabetic retinopathy: laser or eye injection? Lancet. 2017;389(10085):2165-2166. doi: 10.1016/S0140-6736(17)31194-7 [DOI] [PubMed] [Google Scholar]
- 7.Olsen TW. Anti-VEGF pharmacotherapy as an alternative to panretinal laser photocoagulation for proliferative diabetic retinopathy. JAMA. 2015;314(20):2135-2136. doi: 10.1001/jama.2015.15409 [DOI] [PubMed] [Google Scholar]
- 8.Suresh R, Yu HJ, Thoveson A, et al. Loss to follow-up among patients with proliferative diabetic retinopathy in clinical practice. Am J Ophthalmol. 2020;215:66-71. doi: 10.1016/j.ajo.2020.03.011 [DOI] [PubMed] [Google Scholar]
- 9.Green M, Tien T, Ness S. Predictors of lost to follow-up in patients being treated for proliferative diabetic retinopathy. Am J Ophthalmol. 2020;216:18-27. doi: 10.1016/j.ajo.2020.03.023 [DOI] [PubMed] [Google Scholar]
- 10.Obeid A, Gao X, Ali FS, et al. Loss to follow-up in patients with proliferative diabetic retinopathy after panretinal photocoagulation or intravitreal anti-VEGF injections. Ophthalmology. 2018;125(9):1386-1392. doi: 10.1016/j.ophtha.2018.02.034 [DOI] [PubMed] [Google Scholar]
- 11.Wubben TJ, Johnson MW; Anti-VEGF Treatment Interruption Study Group . Anti-vascular endothelial growth factor therapy for diabetic retinopathy: consequences of inadvertent treatment interruptions. Am J Ophthalmol. 2019;204:13-18. doi: 10.1016/j.ajo.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 12.Obeid A, Su D, Patel SN, et al. Outcomes of eyes lost to follow-up with proliferative diabetic retinopathy that received panretinal photocoagulation versus intravitreal anti-vascular endothelial growth factor. Ophthalmology. 2019;126(3):407-413. doi: 10.1016/j.ophtha.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 13.National Institutes of Health . NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. Accessed 15 September 2021. https://grants.nih.gov/grants/funding/women_min/guidelines.htm
- 14.US Food and Drug Administration . Collection of race and ethnicity data in clinical trials. Accessed 15 September 2021. https://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM126396.pdf?source=govdelivery&utm_medium=email&utm_source=govdelivery
- 15.Glassman AR, Beaulieu WT, Stockdale CR, et al. Effect of telephone calls from a centralized coordinating center on participant retention in a randomized clinical trial. Clin Trials. 2020;17(2):195-201. doi: 10.1177/1740774519894229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bressler SB, Beaulieu WT, Glassman AR, et al. ; Diabetic Retinopathy Clinical Research Network . Panretinal photocoagulation versus ranibizumab for proliferative diabetic retinopathy: factors associated with vision and edema outcomes. Ophthalmology. 2018;125(11):1776-1783. doi: 10.1016/j.ophtha.2018.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58(6):595-602. doi: 10.1016/j.jclinepi.2004.10.018 [DOI] [PubMed] [Google Scholar]
- 18.Putrik P, Ramiro S, Lie E, et al. Deriving common comorbidity indices from the MedDRA classification and exploring their performance on key outcomes in patients with rheumatoid arthritis. Rheumatology (Oxford). 2018;57(3):548-554. doi: 10.1093/rheumatology/kex440 [DOI] [PubMed] [Google Scholar]
- 19.Royston P, Altman DG. Visualizing and assessing discrimination in the logistic regression model. Stat Med. 2010;29(24):2508-2520. doi: 10.1002/sim.3994 [DOI] [PubMed] [Google Scholar]
- 20.Abdelmotaal H, Ibrahim W, Sharaf M, Abdelazeem K. Causes and clinical impact of loss to follow-up in patients with proliferative diabetic retinopathy. J Ophthalmol. 2020;2020:7691724. doi: 10.1155/2020/7691724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angermann R, Rauchegger T, Nowosielski Y, et al. Treatment compliance and adherence among patients with diabetic retinopathy and age-related macular degeneration treated by anti-vascular endothelial growth factor under universal health coverage. Graefes Arch Clin Exp Ophthalmol. 2019;257(10):2119-2125. doi: 10.1007/s00417-019-04414-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline factors of participants and study eye alive through 5 years with eyes assigned to ranibizumab
eTable 2. Multivariable analysis of baseline factors associated with a long lapse in care
eTable 3. Duration of follow-up at time of dropout and visual acuity at baseline and the last completed visit
The DRCR Retina Network members