Key Points
Question
Is the 2021 US Preventive Services Task Force (USPSTF) recommendation on lung cancer screening cost-effective?
Findings
In this economic evaluation of the cost-effectiveness of the 2021 USPSTF recommendation on lung cancer screening, a comparative modeling analysis found that relative to the 2013 USPSTF recommendation, the 2021 recommendation was cost-effective despite the expansion of screening eligibility to include younger smokers and those who had at least 20 pack-years of smoking exposure. However, alternative screening strategies that maintained a minimum cumulative smoking exposure of 20 pack-years but expanded screening eligibility to former smokers who had not smoked for more than 15 years were estimated to be more cost-effective than the 2021 USPSTF recommendation.
Meaning
This study found that the 2021 USPSTF recommendation for lung cancer screening was cost-effective relative to the 2013 USPSTF recommendation but that expanding screening eligibility to include former smokers who have not smoked for more than 15 years might further improve the cost-effectiveness of the screening program and warrants further evaluation.
Abstract
Importance
The US Preventive Services Task Force (USPSTF) issued its 2021 recommendation on lung cancer screening, which lowered the starting age for screening from 55 to 50 years and the minimum cumulative smoking exposure from 30 to 20 pack-years relative to its 2013 recommendation. Although costs are expected to increase because of the expanded screening eligibility criteria, it is unknown whether the new guidelines for lung cancer screening are cost-effective.
Objective
To evaluate the cost-effectiveness of the 2021 USPSTF recommendation for lung cancer screening compared with the 2013 recommendation and to explore the cost-effectiveness of 6 alternative screening strategies that maintained a minimum cumulative smoking exposure of 20 pack-years and an ending age for screening of 80 years but varied the starting ages for screening (50 or 55 years) and the number of years since smoking cessation (≤15, ≤20, or ≤25).
Design, Setting, and Participants
A comparative cost-effectiveness analysis using 4 independently developed microsimulation models that shared common inputs to assess the population-level health benefits and costs of the 2021 recommended screening strategy and 6 alternative screening strategies compared with the 2013 recommended screening strategy. The models simulated a 1960 US birth cohort. Simulated individuals entered the study at age 45 years and were followed up until death or age 90 years, corresponding to a study period from January 1, 2005, to December 31, 2050.
Exposures
Low-dose computed tomography in lung cancer screening programs with a minimum cumulative smoking exposure of 20 pack-years.
Main Outcomes and Measures
Incremental cost-effectiveness ratio (ICER) per quality-adjusted life-year (QALY) of the 2021 vs 2013 USPSTF lung cancer screening recommendations as well as 6 alternative screening strategies vs the 2013 USPSTF screening strategy. Strategies with a mean ICER lower than $100 000 per QALY were deemed cost-effective.
Results
The 2021 USPSTF recommendation was estimated to be cost-effective compared with the 2013 recommendation, with a mean ICER of $72 564 (range across 4 models, $59 493-$85 837) per QALY gained. The 2021 recommendation was not cost-effective compared with 6 alternative strategies that used the 20 pack-year criterion. Strategies associated with the most cost-effectiveness included those that expanded screening eligibility to include a greater number of former smokers who had not smoked for a longer duration (ie, ≤20 years and ≤25 years since smoking cessation vs ≤15 years since smoking cessation). In particular, the strategy that screened former smokers who quit within the past 25 years and began screening at age 55 years was associated with screening coverage closest to that of the 2021 USPSTF recommendation yet yielded greater cost-effectiveness, with a mean ICER of $66 533 (range across 4 models, $55 693-$80 539).
Conclusions and Relevance
This economic evaluation found that the 2021 USPSTF recommendation for lung cancer screening was cost-effective; however, alternative screening strategies that maintained a minimum cumulative smoking exposure of 20 pack-years but included individuals who quit smoking within the past 25 years may be more cost-effective and warrant further evaluation.
This economic evaluation assessed the cost-effectiveness of the 2021 US Preventive Services Task Force recommendation for lung cancer screening compared with the 2013 recommendation and alternative screening strategies.
Introduction
In March 2021, the US Preventive Services Task Force (USPSTF) updated its recommendation on lung cancer screening.1,2 Compared with the 2013 USPSTF criteria,3 the new recommendation lowered the starting age for screening from 55 to 50 years and the minimum cumulative smoking exposure from 30 to 20 pack-years, but maintained the annual screening frequency, the stopping age for screening at 80 years, and the time since smoking cessation at 15 years.
The USPSTF periodically updates its recommendations on preventive services to reflect up-to-date evidence on the effectiveness of available interventions and technological advancements. A comparative decision analysis framework was developed by the Cancer Intervention and Surveillance Modeling Network (CISNET) Lung Working Group to inform the USPSTF’s recommendation by estimating the potential health benefits and harms of several lung cancer screening strategies.4,5 The CISNET Lung Working Group estimated that approximately 23% of the US population born in 1960 will be eligible for lung cancer screening based on the 2021 USPSTF recommendation, which is an increase from the approximately 14% of individuals who were eligible under the 2013 USPSTF recommended screening strategy. As a result, the number of low-dose computed tomographic (LDCT) examinations was estimated to increase by 85% (from approximately 227 000 to 419 000 examinations over the lifetime of 100 000 individuals). Whether the projected increase in health benefits accrued from using the 2021 screening recommendation will be sufficient to offset the expected additional costs remains uncertain because costs were not considered in the decision analysis.6
The decision analysis identified 6 consensus-efficient strategies, including the 2021 USPSTF strategy; each strategy maintained the 20 pack-year minimum cumulative smoking exposure eligibility criterion but varied the starting age of screening (50 or 55 years) and the number of years since smoking cessation (≤15, ≤20, or ≤25). However, it is unknown which of these 20 pack-year strategies would be optimal from a cost-effectiveness perspective.
A previous analysis compared the cost-effectiveness of the 2013 USPSTF eligibility criteria with those of the National Lung Screening Trial7 and the Centers for Medicare & Medicaid Services, which start screening at age 55 years and have a 30 pack-year minimum eligibility criterion but differ in their ending age for screening.8 This analysis estimated that all 3 programs were cost-effective at a willingness-to-pay threshold of $100 000 per quality-adjusted life-year (QALY).8 However, it did not evaluate the cost-effectiveness of strategies using the 20 pack-year minimum eligibility criterion or strategies that start screening at age 50 years.
In this economic evaluation, we assessed the cost-effectiveness of the 2021 USPSTF recommendation to examine whether the expected increase in the costs associated with expanded screening eligibility was justified by the expected health benefits. As part of this analysis, we assessed the relative impacts of reducing the minimum pack-years to 20 compared with changing the starting age of screening to 50 years. Moreover, we evaluated the relative cost-effectiveness of the 6 alternative 20 pack-year screening strategies identified by the CISNET decision analysis to identify the strategies that were cost-effective.5
Methods
A comparative modeling approach involving 4 independently developed and validated microsimulation models from the CISNET Lung Working Group was used to assess the cost-effectiveness of the following screening strategies, all of which were based on annual screening frequency: (1) 2021 recommended strategy: age 50 years at screening start, age 80 years at screening end, minimum cumulative smoking exposure of 20 pack-years, and maximum of 15 years since smoking cessation (abbreviated as 50-80-20-15); (2) alternative strategy 1: age 50 years at screening start, age 80 years at screening end, minimum cumulative smoking exposure of 30 pack-years, and maximum of 15 years since smoking cessation (abbreviated as 50-80-30-15); (3) alternative strategy 2: age 55 years at screening start, age 80 years at screening end, minimum cumulative smoking exposure of 20 pack-years, and maximum of 15 years since smoking cessation (abbreviated as 55-80-20-15); (4) alternative strategy 3: age 55 years at screening start, age 80 years at screening end, minimum cumulative smoking exposure of 20 pack-years, and maximum of 20 years since smoking cessation (abbreviated as 55-80-20-20); (5) alternative strategy 4: age 55 years at screening start, age 80 years at screening end, minimum cumulative smoking exposures of 20 pack-years, and maximum of 25 years since smoking cessation (abbreviated as 55-80-20-25); (6) alternative strategy 5: age 50 years at screening start, age 80 years at screening end, minimum cumulative smoking exposure of 20 pack-years, and maximum of 20 years since smoking cessation (abbreviated as 50-80-20-20); and (7) alternative strategy 6: age 50 years at screening start, age 80 years at screening end, minimum cumulative smoking exposure of 20 pack-years, and maximum of 25 years since smoking cessation (abbreviated as 50-80-20-25). These strategies were compared with the 2013 USPSTF recommended strategy (age 55 years at screening start, age 80 years at screening end, minimum cumulative smoking exposure of 30 pack-years, and maximum of 15 years since smoking cessation [abbreviated as 55-80-30-15]). The models used in this analysis were the same as those used to inform the 2013 and 2021 USPSTF recommendations on lung cancer screening.4,5,9
The cost-effectiveness of screening strategies was evaluated from the perspective of the US health care sector, assuming 100% adherence to screening, as in previous studies.5,8,9 Lung cancer–associated events for 1 million men and women were separately simulated using smoking patterns of a 1960 US birth cohort because this cohort was representative of the US population targeted by lung cancer screening. Simulated individuals entered our study at age 45 years and were followed up until death or age 90 years, whichever occurred first, corresponding to a study period from January 1, 2005, to December 31, 2050.
Model Description
The 4 microsimulation models of the CISNET Lung Working Group were independently developed to evaluate screening and differed in their mathematical formulations of lung cancer development. These models included the Microsimulation Screening Analysis–Lung Model from Erasmus University Medical Center,10 the Lung Cancer Policy Model from Massachusetts General Hospital,11 the Lung Cancer Outcomes Simulation from Stanford University,12 and the University of Michigan model.13 The models were calibrated to lung cancer incidence and mortality data from the National Lung Screening Trial7 and the prostate, lung, colorectal, and ovarian cancer screening study.14
Despite the differences in underlying modeling assumptions, the models share a similar overall structure. First, dose-response functions use individual smoking histories to model lung carcinogenesis, and a natural history component is used to simulate disease progression in the absence of interventions. Then the same individual is evaluated in the presence of the intervention of interest, and the 2 life histories are compared to assess the impact of the intervention. The models have been updated to reflect the current practice and management of screening-detected pulmonary nodules.5,15,16 An overview of the models and their assumptions is provided in eTable 1 in the Supplement and in the literature.5,9,14,17
Smoking Histories
Smoking histories representative of the 1960 US birth cohort were obtained through the CISNET smoking history generator.18,19,20 The smoking history generator provides standardized age-specific, sex-specific, and birth cohort–specific smoking histories that include ages at smoking initiation and cessation, smoking frequency, and age at death from causes other than lung cancer. The 4 models use individual-level smoking histories to simulate lung cancer–associated events over the lifetime of individuals, including age at diagnosis, histological features and stage of disease, disease progression, and survival time from diagnosis.
Health Utility and Cost Inputs
The life-years of simulated individuals were adjusted for quality of life using published health utilities associated with aging, lung cancer stage at diagnosis, and terminal care (eTable 2 in the Supplement).21,22,23 Costs associated with screening and diagnostic procedures were obtained from the Centers for Medicare & Medicaid Services 2020 reimbursement rates24 based on their corresponding Current Procedural Terminology25 codes (eTable 3 in the Supplement). Downstream treatment costs associated with specific phases of lung cancer treatment were obtained from a published analysis of data from the Surveillance, Epidemiology, and End Results–Medicare database26 and converted to 2020 US dollars using a 3% annual inflation rate (eTable 4 in the Supplement). Health utilities and costs were standardized and shared across the 4 CISNET models.
Each model estimated the sex-specific health benefits and costs of screening and derived overall population outcomes by aggregating the sex-specific results. Future health and cost outcomes were equally discounted using an annual rate of 3%. A $100 000 per QALY willingness-to-pay threshold was used to define cost-effective strategies.27
Outcome Measures
Primary outcome measures included the incremental cost-effectiveness ratios (ICERs) of the different screening strategies compared with 2013 USPSTF strategy, with ICER calculated as the cost of the i-th strategy minus the cost of the 2013 USPSTF strategy divided by the difference of the QALY of the i-th strategy minus the QALY of the 2013 USPSTF strategy (in which i-th denotes one of the strategies included in our analysis). The costs and QALYs for each strategy were estimated by calculating the arithmetic mean of the results derived from each of the 4 CISNET models. A cost-effectiveness efficiency frontier (in which line segments connect strategies that yield the highest health benefit at a given level of cost) was derived, and ICERs were calculated for each strategy compared with the strategy preceding it on the efficiency frontier. A weakly dominated strategy was defined as a strategy dominated by a linear combination of 2 other strategies, and a strongly dominated strategy was defined as a strategy for which another strategy existed that yielded better health benefit at lower cost.
Secondary outcome measures included reduction in lung cancer mortality, unadjusted life-years gained from screening, ICERs based on unadjusted life-years, number of LDCT screenings, and overdiagnosis rates. Results were presented per 100 000 individuals alive at age 45 years, unless otherwise specified. Individuals were from the general population; thus, individuals who were not eligible for screening were included in the analyses.
Sensitivity Analyses
Univariate sensitivity analyses were conducted to assess the sensitivity of the results to changes in the value of important model input parameters, and efficiency frontiers associated with each set of inputs were identified. We varied the costs of LDCT, downstream treatment by phase of care (initial, continuous, and terminal), and health utilities associated with cancer stages and histological subtypes by 25% higher and lower than their base-case values (eTable 2 and eTable 3 in the Supplement). We also evaluated the cost-effectiveness of screening strategies in which individuals with short life expectancy (<5 years from the time of LDCT screening) were deemed ineligible for screening. The geometric (vs arithmetic) means of model results were also examined. A 2-fold increase in the treatment cost of de novo advanced non–small cell lung cancer was evaluated to measure the impact of using more expensive immunotherapies.
Probabilistic sensitivity analyses were conducted to assess the robustness of our findings to simultaneous changes in the values of input parameters using 100 000 iterations.28 At each iteration, the value of all cost parameters and lung cancer health utilities were concurrently varied by independently sampling their values from the corresponding uniform distribution defined by the boundaries of 25% lower and higher than their base-case values (0.75 multiplied by base-case value and 1.25 multiplied by base-case value, respectively). The probability that each screening strategy was cost-effective at a given willingness-to-pay threshold was calculated by counting the number of times per 100 000 iterations that the ICER was below the specified threshold.
Statistical Analysis
All simulations were performed using R software, version 3.0 (R Foundation for Statistical Computing). Data aggregation and analysis was conducted using Microsoft Excel, version 1902 (Microsoft Corp).
Results
The 2021 USPSTF recommended screening strategy (50-80-20-15) was cost-effective, with an estimated mean ICER of $72 564 (range across 4 models, $59 493 to $85 837) per QALY gained compared with the 2013 USPSTF recommended strategy (55-80-30-15) (Table 1; eTables 5-8 and eFigure 1 in the Supplement). Similar results were obtained when the geometric mean was used to consolidate model-specific results (eg, the estimated geometric mean ICER per QALY gained for 2021 recommended strategy was $73 600 [range across 4 models, $59 493-$85 837] vs the 2013 recommended strategy) (eTable 9 in the Supplement). Lung cancer–specific reductions in mortality, number of screening-detected lung cancer cases, overdiagnosis rates, and the required number of LDCT screenings for each strategy are shown in Table 2.
Table 1. Additional Health Benefits and Costs of 20 Pack-Year Strategies Based on 2021 USPSTF Recommendation vs Strategies Based on 2013 USPSTF Recommendationa.
| Lung cancer screening strategyb | Additional costs incurred, mean (range), $ millionc | Additional QALYs accrued, mean (range) | ICER per QALY gained vs 2013 recommended strategy, mean (range), $c,d | ICER per QALY gained vs preceding strategy on efficiency frontier, mean, $c,d |
|---|---|---|---|---|
| 2013 USPSTF recommended strategy: age 55 y at screening start; age 80 y at screening end; ≥30 pack-years; ≤15 y since smoking cessation (55-80-30-15) | 1 [Reference] | 1 [Reference] | NA | NA |
| Alternative strategy 1: age 50 y at screening start; age 80 y at screening end; ≥30 pack-years; ≤15 y since smoking cessation (50-80-30-15) | 16.61 (7.76-19.93) | 233 (95-328) | 71 205 (58 567-81 788) | 72 561 (Weakly dominated)e |
| Alternative strategy 2: age 55 y at screening start, age 80 y at screening end, ≥20 pack-years, ≤15 y since smoking cessation (55-80-20-15) | 23.16 (22.04-24.73) | 363 (289-470) | 63 719 (52 619-77 229) | 63 719 |
| Alternative strategy 3: age 55 y at screening start, age 80 y at screening end, ≥20 pack-years, ≤20 y since smoking cessation (55-80-20-20) | 31.35 (29.54-33.06) | 482 (379-612) | 65 012 (54 046-77 930) | 68 967 |
| Alternative strategy 4: age 55 y at screening start; age 80 y at screening end; ≥20 pack-years; ≤25 y since smoking cessation (55-80-20-25) | 38.20 (35.79-39.98) | 574 (444-718) | 66 533 (55 693-80 539) | 74 502 |
| 2021 USPSTF recommended strategy: age 50 y at screening start; age 80 y at screening end; ≥20 pack-years; ≤15 y since smoking cessation (50-80-20-15) | 49.59 (47.16-51.12) | 683 (549-859) | 72 564 (59 493-85 837) | 104 273 (Weakly dominated)e |
| Alternative strategy 5: age 50 y at screening start; age 80 y at screening end, ≥20 pack-years; ≤20 y since smoking cessation (50-80-20-20) | 59.07 (56.35-61.15) | 806 (656-1001) | 73 262 (60 500-85 956) | 89 908 (Weakly dominated)e |
| Alternative strategy 6: age 50 y at screening start; age 80 y at screening end; ≥20 pack-years; ≤25 y since smoking cessation (50-80-20-25) | 66.40 (63.57-69.35) | 898 (736-1103) | 73 952 (61 379-86 354) | 87 115 |
Abbreviations: CISNET, Cancer Intervention and Surveillance Modeling Network; ICER, incremental cost-effectiveness ratio; NA, not applicable; QALY, quality-adjusted life-year; USPSTF, US Preventive Services Task Force.
Per 100 000 individuals (both ever eligible and never eligible) alive at age 45 years with no previous cancer diagnosis. All health outcomes and costs were discounted at a 3% annual rate.
All strategies were based on annual screening frequency.
2020 Dollars.
ICERs based on mean QALY and mean cost values across the 4 CISNET models.
A weakly dominated strategy was defined as a strategy dominated by a linear combination of 2 other strategies, and a strongly dominated strategy was defined as a strategy for which another strategy existed that yielded better health benefit at lower cost.
Table 2. Clinical Outcomes and Use of Low-Dose Computed Tomography by Lung Cancer Screening Strategya.
| Screening strategyb | Persons ever screened, % | No. | Overdiagnosis, % | No. | Reduction in lung cancer mortality, % | |||
|---|---|---|---|---|---|---|---|---|
| LDCT screenings | Total lung cancer cases | Screening-detected lung cancer cases | Total lung cancer deaths | Lung cancer deaths averted | ||||
| 2013 USPSTF recommended strategy: age 55 y at screening start; age 80 y at screening end; ≥30 pack-years; ≤15 y since smoking cessation (55-80-30-15) | 14 | 228 348 | 4507 | 985 | 6.1 | 3624 | 364 | 9.1 |
| Alternative strategy 1: age 50 y at screening start; age 80 y at screening end; ≥30 pack-years; ≤15 y since smoking cessation (50-80-30-15) | 16 | 282 194 | 4508 | 1031 | 6.1 | 3611 | 377 | 9.5 |
| Alternative strategy 2: age 55 y at screening start, age 80 y at screening end, ≥20 pack-years, ≤15 y since smoking cessation (55-80-20-15) | 21 | 331 326 | 4521 | 1191 | 6.2 | 3553 | 435 | 10.9 |
| Alternative strategy 3: age 55 y at screening start, age 80 y at screening end, ≥20 pack-years, ≤20 y since smoking cessation (55-80-20-20) | 22 | 370 985 | 4526 | 1269 | 6.2 | 3524 | 464 | 11.6 |
| Alternative strategy 4: age 55 y at screening start; age 80 y at screening end; ≥20 pack-years; ≤25 y since smoking cessation (55-80-20-25) | 23 | 406 086 | 4530 | 1331 | 6.2 | 3501 | 487 | 12.2 |
| 2021 USPSTF recommended strategy: age 50 y at screening start; age 80 y at screening end; ≥20 pack-years; ≤15 y since smoking cessation (50-80-20-15) | 23 | 420 553 | 4522 | 1254 | 6.0 | 3521 | 467 | 11.7 |
| Alternative strategy 5: age 50 y at screening start; age 80 y at screening end, ≥20 pack-years; ≤20 y since smoking cessation (50-80-20-20) | 23 | 465 121 | 4527 | 1333 | 6.0 | 3492 | 496 | 12.4 |
| Alternative strategy 6: age 50 y at screening start; age 80 y at screening end; ≥20 pack-years; ≤25 y since smoking cessation (50-80-20-25) | 24 | 502 220 | 4531 | 1394 | 6.0 | 3469 | 519 | 13.0 |
Abbreviations: CISNET, Cancer Intervention and Surveillance Modeling Network; LDCT, low-dose computed tomography; USPSTF, US Preventive Services Task Force.
Based on the mean values across the 4 CISNET models for a cohort of 100 000 persons (both ever eligible and never eligible) alive at age 45 years with no previous cancer diagnosis.
All strategies were based on annual screening frequency.
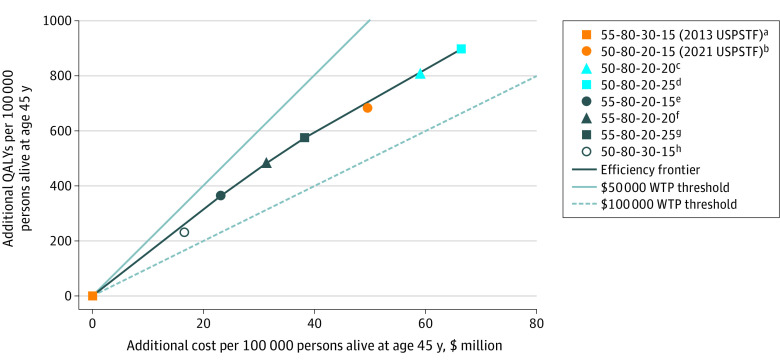
When all 6 screening strategies that used the 20 pack-year criterion (ie, the 2021 recommended strategy plus alternative strategies 2-6) were analyzed together with the USPSTF 2013 recommended strategy, the 2021 USPSTF strategy was not on the cost-effectiveness efficiency frontier (Figure 1; eFigure 2 in the Supplement). The efficiency frontier (based on mean results across the 4 CISNET models) consisted of 4 strategies: alternative strategy 2 (55-80-20-15), alternative strategy 3 (55-80-20-20), alternative strategy 4 (55-80-20-25), and alternative strategy 6 (50-80-20-25). Three of those strategies began screening at age 55 years (alternative strategies 2-4) vs 50 years, and 3 of those strategies were expanded to include former smokers who quit smoking within 20 years (alternative strategy 3) and 25 years (alternative strategies 4 and 6) vs 15 years. Notably, alternative strategy 1 (50-80-30-15) was not on the cost-effectiveness efficiency frontier (mean ICER per QALY gained, $71 205 [range across 4 models, $58 567-$81 788] vs 2013 recommended strategy), whereas alternative strategy 2 (55-80-20-15) was on the efficiency frontier (mean ICER per QALY gained, $63 719 [range across 4 models, $52 619-$77 930] vs 2013 recommended strategy); this finding suggested that the cost-effectiveness of the 2021 USPSTF program was largely associated with lowering the minimum pack-years to 20 rather than lowering the start of screening to age 50 years.
Figure 1. Excess Health Benefits and Costs of the 2021 vs 2013 US Preventive Services Task Force (USPSTF) Strategies for Lung Cancer Screening.
Mean values across the 4 Cancer Intervention and Surveillance Modeling Network (CISNET) models. The cost-effectiveness efficiency frontier comprised lung cancer screening strategies with a minimum cumulative smoking exposure of 20 pack-years. The additional cost accrued and quality-adjusted life-years (QALYs) gained for a given screening strategy vs the 2013 USPSTF screening strategy were normalized per 100 000 persons (both ever eligible and never eligible for screening) who were alive at age 45 years. The cost-effectiveness efficiency frontier was formed by line segments connecting strategies that were not dominated by any other strategy (ie, the most effective screening strategies in terms of QALYs gained at any given cost level). A weakly dominated strategy was defined as a strategy dominated by a linear combination of 2 other strategies, and a strongly dominated strategy was defined as a strategy for which another strategy existed that yielded better health benefit at lower cost. The slope of the line segment connecting 2 strategies represents the number of QALYs gained per unit of cost; the inverse of the slope corresponds to the incremental cost-effectiveness ratio (ICER) of the 2 strategies, which represents the amount of additional cost incurred to obtain 1 additional QALY using the new vs old strategy. The labels of the screening strategies represent the following (left to right, separated by hyphens): starting age of screening (in years), ending age of screening (in years), minimum pack-years of smoking, and maximum number of years since cessation of smoking. All strategies included annual screening frequency. All health outcomes and costs were discounted at a 3% annual rate. WTP indicates willingness-to-pay.
aThe origin of all line segments (strategy 55-80-30-15) corresponds to the 2013 USPSTF strategy, which yielded 5239 total QALYs and cost $319 192 071 per 100 000 persons alive and cancer-free at age 45 years.
bWeakly dominated. Mean ICER per QALY gained (across 4 CISNET models) vs preceding strategy on efficiency frontier: $104 273.
cWeakly dominated. Mean ICER per QALY gained (across 4 CISNET models) vs preceding strategy on efficiency frontier: $89 908.
dMean ICER per QALY gained (across 4 CISNET models) vs preceding strategy on efficiency frontier: $87 115.
eMean ICER per QALY gained (across 4 CISNET models) vs preceding strategy on efficiency frontier: $63 719.
fMean ICER per QALY gained (across 4 CISNET models) vs preceding strategy on efficiency frontier: $68 967.
gMean ICER per QALY gained (across 4 CISNET models) vs preceding strategy on efficiency frontier: $74 502.
hWeakly dominated. Mean ICER per QALY gained (across 4 CISNET models) vs preceding strategy on efficiency frontier: $72 561.
The 2 strategies on the efficiency frontier with the greatest health benefits were those in which screening eligibility was expanded to include former smokers who quit within 25 years and started screening at ages 55 and 50 years (alternative strategy 4 [55-80-20-25]: mean additional QALYs accrued, 574 [range across 4 models, 444-718] and alternative strategy 6 [50-80-20-25]: mean additional QALYs accrued, 898 [range across 4 models, 736-1103]). Notably, alternative strategy 4 (55-80-20-25) had a more favorable mean ICER per QALY gained ($66 533 [range across 4 models, $55 693-$80 539]) than the 2021 recommended strategy ($72 564 [range across 4 models, $59 493-$85 837]) and provided screening coverage to a proportion of the US population identical to that of the 2021 recommended strategy (23.0% for both strategies) (Table 2). Of the 4 strategies on the efficiency frontier (ie, alternative strategies 2, 3, 4, and 6), alternative strategy 6 (50-80-20-25) was the only strategy that started screening at age 50 years. Screening eligibility levels as a function of age for each screening strategy by smoking status are shown in eFigure 3 in the Supplement.
The 2021 USPSTF recommendation was associated with cost-effectiveness compared with the 2013 USPSTF recommendation for both men and women, but the 2021 recommendation was not on the sex-specific cost-effectiveness efficiency frontiers when considering all strategies (eTable 10 and eTable 11 in the Supplement). In general, screening was more cost-effective for women vs men. The 2021 USPSTF recommendation was not on the efficiency frontier when the health benefit was quantified according to lung cancer deaths averted (eFigure 4 in the Supplement). When unadjusted life-years were used to estimate the cost-effectiveness of the strategies, the respective ICERs were lower, but the efficiency frontier and general patterns were similar to those observed using QALYs (eFigure 5 and eFigure 6 in the Supplement). Treatment was the main factor associated with cost, accounting for 73% to 84% of the total cost associated with screening programs (eFigure 7 in the Supplement).
Sensitivity Analysis
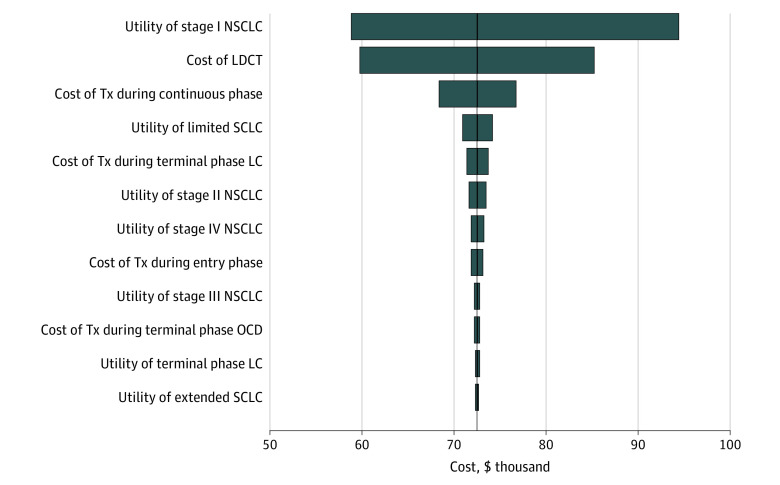
The cost-effectiveness of the 2021 USPSTF strategy compared with the 2013 USPSTF strategy was robust to changes in the value of important input parameters (Figure 2). Univariate sensitivity analyses revealed that the cost-effectiveness efficiency frontier remained identical to the base-case frontier in all scenarios (eTables 12-23 in the Supplement). The ICERs of screening strategies were most sensitive to changes in health utilities for stage I non–small cell lung cancer followed by cost of LDCT screening.
Figure 2. Univariate Sensitivity Analyses of the 2021 vs 2013 US Preventive Services Task Force Screening Strategies for Lung Cancer.
The tornado diagram illustrates the change in the incremental cost-effectiveness ratio (ICER), which was defined as the cost of the 2021 US Preventive Services Task Force (USPSTF) strategy minus the cost of the 2013 USPSTF strategy divided by the difference of the quality-adjusted life-year of the 2021 USPSTF strategy minus the quality-adjusted life-year of the 2013 USPSTF strategy when important input parameters were varied for both strategies (1 strategy at a time) by 25% higher or lower than their base-case values (shown in eTable 2 and eTable 3 in the Supplement). The vertical axis (solid dark line) shows the estimated ICER for the base-case analysis. The baseline ICER was $72 564. The left column of the tornado diagram shows the input parameters in descending order of their impact for the results (eg, the ICER of the 2021 USPSTF strategy vs the 2013 USPSTF strategy was most sensitive to changes in the health utility of stage I non–small cell lung cancer [NSCLC] and the least sensitive to changes in the health utility of extended small cell lung cancer [SCLC]). LC indicates lung cancer; LDCT, low-dose computed tomography; OCD, other causes of death; and Tx, treatment.
When screening was restricted to individuals with at least 5 years of life expectancy, the cost-effectiveness efficiency frontier comprised strategies that provided screening coverage to former smokers who had quit smoking within 20 and 25 years (eTable 24 in the Supplement). When we conducted a within-strategy comparison, which compared each strategy with vs without the life expectancy restriction (eTable 25 in the Supplement), the 2021 USPSTF recommendation without the life expectancy restriction cost $6.95 million more per 100 000 individuals and yielded 56 more QALYs vs the same strategy with the life expectancy restriction. This result corresponded to an ICER of $123 327, suggesting that screening eligible individuals without considering their life expectancy may not be cost-effective. When assuming a 2-fold increase from base-case value for the treatment cost during the initial and continuous disease phases of advanced non–small cell lung cancer (to account for the high cost of modern immunotherapies), the efficiency frontier remained unchanged, despite estimating lower ICERs than the base-case analysis (eTable 26 in the Supplement).
The probabilistic sensitivity analysis revealed that the cost-effectiveness of the 4 strategies on the base-case efficiency frontier was robust to simultaneous changes in important input parameters (eFigure 8 in the Supplement). The 2021 USPSTF recommendation compared with the strategy preceding it on the efficiency frontier (ie, alternative strategy 4 [55-80-20-25]) had a 24% likelihood of being cost-effective when the willingness-to-pay threshold was $100 000.
Discussion
This economic evaluation assessed the cost-effectiveness of the 2021 USPSTF recommendation on lung cancer screening using a comparative modeling approach. When directly compared with the 2013 USPSTF recommendation, the 2021 USPSTF recommendation was cost-effective based on a willingness-to-pay threshold of $100 000 per QALY gained. We found that the cost-effectiveness of the 2021 USPSTF recommendation was largely associated with lowering the minimum cumulative smoking exposure to 20 pack-years rather than lowering the starting age for screening to 50 years. The main findings of this study were robust to changes in the values of input parameters and modeling assumptions and were most sensitive to changes in health utilities for early-stage lung cancer and the cost of LDCT screening.
It is notable that when we included the 5 alternative 20 pack-year strategies in our analysis, the 2021 USPSTF recommendation was not on the efficiency frontier; the frontier consisted of strategies that expanded screening eligibility by including former smokers who had not smoked for more than 15 years. Alternative strategy 4 (55-80-20-25) was estimated to provide screening coverage to a portion of the US population that was similar to that of the 2021 USPSTF recommendation,4,5 yet it was associated with greater cost-effectiveness, improvements in lung cancer–specific mortality, and reductions in the number of LDCT screenings but increases in overdiagnosis. Providing screening coverage to former smokers who have not smoked for more than 15 years has been supported by data from previous studies.29,30,31
The USPSTF decision to lower the starting age for screening to 50 years and the minimum cumulative smoking exposure to 20 pack-years was made in part to address racial disparities in screening eligibility that were associated with the 2013 USPSTF recommendation.2,32,33,34 According to the CISNET decision analysis, the 2021 USPSTF strategy (50-80-20-15) and the 2 strategies on the efficiency frontier that included former smokers who quit smoking within 25 years (ie, alternative strategy 4 [55-80-20-25] and alternative strategy 6 [50-80-20-25]) would reduce racial and sex disparities in screening eligibility.4,5 Whether expanding screening to former smokers who had not smoked for more than 15 years could further reduce racial disparities in practice is unknown and warrants further evaluation.31,35
The findings of this study were notable given that the decision analysis that informed the 2021 USPSTF recommendation did not consider costs. Although previous cost-effectiveness analyses reported that existing lung cancer screening programs requiring at least 30 pack-years of smoking history were cost-effective, the cost-effectiveness of 20 pack-year strategies was not examined.8,36 The ICERs derived from the present analysis were within the range of those published in similar studies.37,38,39,40 Our sex-specific analyses were consistent with previous studies reporting more favorable results for women compared with men.15,36 Downstream treatment costs were the main factor associated with the cost of a lung cancer screening program.8 Although clinicians may, in practice, consider life expectancy when making decisions about lung cancer screening for patients, our findings suggest that formally including life expectancy in the eligibility criteria of a screening program may further improve its cost-effectiveness.
Limitations
This study has limitations. Despite low screening uptake (estimated 5%-17% of eligible individuals),41,42,43,44,45,46 we assumed 100% uptake and adherence, thereby potentially overestimating the health benefits and costs associated with lung cancer screening. Incorporating adherence into modeling is challenging given its potential heterogeneity by age, sex, race and ethnicity, socioeconomic status, smoking status, and access to care.47,48,49,50 Moreover, we did not consider potential issues regarding availability of resources to satisfy the expected increase in screening demand based on the 2021 USPSTF recommendation.51 Our current models are unable to produce race-specific and ethnicity-specific screening outcomes; thus, caution is warranted when generalizing the present results to specific racial and ethnic groups. The models in this study do not consider the increase in risk of developing other cancers (eg, breast cancer52) associated with exposure to screening radiation or the benefits of smoking cessation interventions at the time of lung cancer screening, which have been reported to improve the cost-effectiveness of the overall screening program.23,53,54,55,56 Although a 2014 study found differential cost-effectiveness of lung cancer screening by smoking status,36 we were unable to conduct a subgroup analysis by smoking status because we followed up individuals over their entire life span, and smoking status changes over time. In addition, we did not consider the health benefits and costs associated with modern lung cancer immunotherapies. Incorporating these new treatments will likely improve the cost-effectiveness of screening programs because, as screening programs expand, more lung cancers will be diagnosed at earlier stages when alternative, equally beneficial, and less costly treatment options are available.
Conclusions
This economic evaluation found that the 2021 USPSTF recommendation for lung cancer screening was cost-effective when directly compared with the 2013 USPSTF recommendation. However, the cost-effectiveness of lung cancer screening may be improved if screening coverage is expanded to include former smokers with 20 pack-year smoking histories who have not smoked for more than 15 years; this expansion of screening eligibility warrants further consideration.
eTable 1. Characteristics of the CISNET Models in the Comparative Cost-effectiveness Analysis
eTable 2. Health Utilities Used to Adjust Life-years for Quality of Life Based on Health State of Individuals
eTable 3. Cost of Screening and Diagnostic Procedures
eTable 4. Cost of Downstream Treatment Interventions by Phase of Care, Stage, and Histological Subtype at Specific Ages Used for Base-Case Analysis in 2020 US Dollars
eTable 5. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Erasmus Model
eTable 6. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Massachusetts General Hospital–Harvard Medical School Model
eTable 7. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the University of Michigan Model
eTable 8. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Stanford University Model
eTable 9. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Geometric Mean Across the 4 CISNET Models
eTable 10. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models for US Men Born in 1960
eTable 11. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models for US Women Born in 1960
eTable 12. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Cost of LDCT Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 13. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Cost of Treatment Administered During the First 6 Months From Diagnosis (Entry Phase of Care) Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 14. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Cost of Treatment Administered During the Continuous Phase of Care Was Varied by 25% Higher and Lower Than Its Base-Case Value
eTable 15. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Cost of Treatment Administered During the Last 6 Months of Life From the Time at Lung Cancer Death (Terminal LC phase) by 25% Higher or Lower Than Its Base-Case Value
eTable 16. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Cost of Treatment Administered During the Last 6 Months of Life From the Time at Death From Causes Other Than Lung Cancer (Terminal OCM phase) Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 17. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With Stage I Non–Small Cell Lung Cancer Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 18. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With Stage II Non–Small Cell Lung Cancer Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 19. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With Stage III Non–Small Cell Lung Cancer Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 20. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With Stage IV Non–Small Cell Lung Cancer Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 21. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With Limited-Stage Small Cell Lung Cancer Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 22. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With Extended-Stage Small Cell Lung Cancer Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 23. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With the Last 6 months of Life From Any Cause of Death Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 24. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When Screening Was Restricted to Individuals With Life Expectancy of at Least 5 Years From the Time of Each Screening
eTable 25. Additional Health Benefits Gained and Costs Incurred From Screening Strategies When Screening Was Not Restricted to Individuals With Life Expectancy of at Least 5 Years Relative to the Same Strategy When Screening Was Restricted to Individuals With Life Expectancy of at Least 5 Years From the Time of Screening and Their Corresponding Incremental Cost-effectiveness Ratios
eTable 26. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Cost of Treatment for Advanced Non–Small Cell Lung Cancer Was Doubled
eFigure 1. Model-Specific Incremental Cost and QALYs Relative to the 2013 US Preventive Services Task Force Recommendation on Lung Cancer Screening
eFigure 2. Total Health Benefits (Measured Using QALY) and Costs Associated With Screening Strategies Based on the Mean Values Across the 4 CISNET Models and Their Relative Position to the No Screening Strategy
eFigure 3. Number of Screening-Eligible Individuals per 100 000 Individuals Alive and Cancer-Free at Age 45 Years (Based on Mean Across Models) by Age and Smoking Status Using 20 Pack-Year Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening
eFigure 4. Number of LDCT Screenings vs Number of Lung Cancer Deaths Averted by Screening Strategies Based on Mean Values Across the 4 CISNET Models and Their Resulting Efficiency Frontiers
eFigure 5. Excess Health Benefit Accrued (Measured in Life-Years) and Cost Incurred Relative to the 2013 USPSTF Screening Strategy and the Resulting Cost-effectiveness Efficiency Frontier (Bolded Strategies) Composed of the Lung Cancer Screening Strategies Based on Mean Values Across the 4 CISNET Models
eFigure 6. Total Health Benefits (Measured Using Unadjusted Life-Years) and Costs Associated With Screening Strategies Based on Mean Values Across the 4 CISNET Models and Their Relative Position to the No Screening Strategy
eFigure 7. Total Cost per 100 000 Individuals Alive at Age 45 Years From the General Population Associated With the Screening Programs
eFigure 8. Probabilistic Sensitivity Analysis for Evaluating the Cost-effectiveness of the 7 Strategies Considered in the Base-Case Analysis by Simultaneously Changing the Input Parameter Values
eReferences
References
- 1.U.S. Preventive Services Task Force . Final recommendation statement. lung cancer: screening. U.S. Preventive Services Task Force. March 9, 2021. Accessed March 9, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
- 2.Krist AH, Davidson KW, Mangione CM, et al. ; US Preventive Services Task Force . Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi: 10.1001/jama.2021.1117 [DOI] [PubMed] [Google Scholar]
- 3.Moyer VA; U.S. Preventive Services Task Force . Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi: 10.7326/M13-2771 [DOI] [PubMed] [Google Scholar]
- 4.Meza R, Jeon J, Toumazis I, et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: modeling study for the US Preventive Services Task Force. JAMA. 2021;325(10):988-997. doi: 10.1001/jama.2021.1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meza R, Jeon J, Toumazis I, et al. Evaluation of the Benefits and Harms of Lung Cancer Screening With Low-Dose Computed Tomography: A Collaborative Modeling Study for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality; 2021. Evidence syntheses 198tr. Report 20-05266-EF-2. March 2021. Accessed March 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK568586/ [PubMed]
- 6.U.S. Preventive Services Task Force. USPSTF and cost considerations. U.S. Preventive Services Task Force; 2017. Updated April 2021. Accessed February 14, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/task-force-resources/uspstf-and-cost-considerations
- 7.Aberle DR, Adams AM, Berg CD, et al; National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Criss SD, Cao P, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171(11):796-804. doi: 10.7326/M19-0322 [DOI] [PubMed] [Google Scholar]
- 9.de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(5):311-320. doi: 10.7326/M13-2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ten Haaf K, van Rosmalen J, de Koning HJ. Lung cancer detectability by test, histology, stage, and gender: estimates from the NLST and the PLCO trials. Cancer Epidemiol Biomarkers Prev. 2015;24(1):154-161. doi: 10.1158/1055-9965.EPI-14-0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon PM, Kong CY, Johnson BE, et al. Chapter 9: the MGH-HMS lung cancer policy model: tobacco control versus screening. Risk Anal. 2012;32(suppl 1):S117-S124. doi: 10.1111/j.1539-6924.2011.01652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin RS, Plevritis SK. Comparing the benefits of screening for breast cancer and lung cancer using a novel natural history model. Cancer Causes Control. 2012;23(1):175-185. doi: 10.1007/s10552-011-9866-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazelton WD, Jeon J, Meza R, Moolgavkar SH. Chapter 8: the FHCRC lung cancer model. Risk Anal. 2012;32(suppl 1):S99-S116. doi: 10.1111/j.1539-6924.2011.01681.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMahon PM, Meza R, Plevritis SK, et al. Comparing benefits from many possible computed tomography lung cancer screening programs: extrapolating from the National Lung Screening Trial using comparative modeling. PLoS One. 2014;9(6):e99978. doi: 10.1371/journal.pone.0099978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toumazis I, Tsai EB, Erdogan SA, et al. Cost-effectiveness analysis of lung cancer screening accounting for the effect of indeterminate findings. J Natl Cancer Inst Cancer Spectr. 2019;3(3):pkz035. doi: 10.1093/jncics/pkz035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lung CT Screening Reporting and Data System (Lung-RADS). American College of Radiology; 2014. Accessed October 10, 2020. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads
- 17.Meza R, ten Haaf K, Kong CY, et al. Comparative analysis of 5 lung cancer natural history and screening models that reproduce outcomes of the NLST and PLCO trials. Cancer. 2014;120(11):1713-1724. doi: 10.1002/cncr.28623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon J, Holford TR, Levy DT, et al. Smoking and lung cancer mortality in the United States from 2015 to 2065: a comparative modeling approach. Ann Intern Med. 2018;169(10):684-693. doi: 10.7326/M18-1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holford TR, Meza R, Warner KE, et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964-2012. JAMA. 2014;311(2):164-171. doi: 10.1001/jama.2013.285112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holford TR, Levy DT, McKay LA, et al. Patterns of birth cohort–specific smoking histories, 1965-2009. Am J Prev Med. 2014;46(2):e31-e37. doi: 10.1016/j.amepre.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26(4):391-400. doi: 10.1177/0272989X06290497 [DOI] [PubMed] [Google Scholar]
- 22.Tramontano AC, Schrag DL, Malin JK, et al. Catalog and comparison of societal preferences (utilities) for lung cancer health states: results from the Cancer Care Outcomes Research and Surveillance (CanCORS) study. Med Decis Making. 2015;35(3):371-387. doi: 10.1177/0272989X15570364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol. 2011;6(11):1841-1848. doi: 10.1097/JTO.0b013e31822e59b3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Medicare & Medicaid Services. Physician fee schedule look-up tool. Centers for Medicare & Medicaid Services; 2020. Accessed November 16, 2020. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PFSlookup
- 25.American Medical Association. Practice management: CPT. American Medical Association; 2020. Accessed November 16, 2020. https://www.ama-assn.org/practice-management/cpt
- 26.Sheehan DF, Criss SD, Chen Y, et al. Lung cancer costs by treatment strategy and phase of care among patients enrolled in Medicare. Cancer Med. 2019;8(1):94-103. doi: 10.1002/cam4.1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-797. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 28.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253-1258. doi: 10.1001/jama.1996.03540150055031 [DOI] [PubMed] [Google Scholar]
- 29.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA. 2016;315(21):2300-2311. doi: 10.1001/jama.2016.6255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tindle HA, Stevenson Duncan M, Greevy RA, et al. Lifetime smoking history and risk of lung cancer: results from the Framingham Heart Study. J Natl Cancer Inst. 2018;110(11):1201-1207. doi: 10.1093/jnci/djy041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasquinelli MM, Tammemagi MC, Kovitz KL, et al. Risk prediction model versus United States Preventive Services Task Force lung cancer screening eligibility criteria: reducing race disparities. J Thorac Oncol. 2020;15(11):1738-1747. doi: 10.1016/j.jtho.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 32.Han SS, Chow E, ten Haaf K, et al. Disparities of national lung cancer screening guidelines in the US population. J Natl Cancer Inst. 2020;112(11):1136-1142. doi: 10.1093/jnci/djaa013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivera MP, Katki HA, Tanner NT, et al. Addressing disparities in lung cancer screening eligibility and healthcare access: an official American Thoracic Society statement. Am J Respir Crit Care Med. 2020;202(7):e95-e112. doi: 10.1164/rccm.202008-3053ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF lung cancer screening guidelines among African American adult smokers. JAMA Oncol. 2019;5(9):1318-1324. doi: 10.1001/jamaoncol.2019.1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinsky PF, Lau YK, Doubeni CA. Potential disparities by sex and race or ethnicity in lung cancer screening eligibility rates. Chest. 2021;160(1):341-350. doi: 10.1016/j.chest.2021.01.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Black WC, Gareen IF, Soneji SS, et al. ; National Lung Screening Trial Research Team . Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med. 2014;371(19):1793-1802. doi: 10.1056/NEJMoa1312547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lew JB, Feletto E, Wade S, et al. Benefits, harms and cost-effectiveness of cancer screening in Australia: an overview of modelling estimates. Public Health Res Pract. 2019;29(2):29121913. doi: 10.17061/phrp2921913 [DOI] [PubMed] [Google Scholar]
- 38.Goffin JR, Flanagan WM, Miller AB, et al. Biennial lung cancer screening in Canada with smoking cessation—outcomes and cost-effectiveness. Lung Cancer. 2016;101:98-103. doi: 10.1016/j.lungcan.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 39.Schmaus KD, Gould MK, Silvestri GA, Dinh T. Cost-effectiveness of CT screening for lung cancer by risk and age. Am J Respir Crit Care Med. 2014;189:A6607. [Google Scholar]
- 40.ten Haaf K, Tammemagi MC, Bondy SJ, et al. Performance and cost-effectiveness of computed tomography lung cancer screening scenarios in a population-based setting: a microsimulation modeling analysis in Ontario, Canada. PLoS Med. 2017;14(2):e1002225. doi: 10.1371/journal.pmed.1002225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Lung Association. State of Lung Cancer 2020 Report. American Lung Association; 2020. Accessed December 2, 2020. https://www.lung.org/getmedia/381ca407-a4e9-4069-b24b-195811f29a00/solc-2020-report-final.pdf
- 42.Richards TB, Soman A, Thomas CC, et al. Screening for lung cancer—10 states, 2017. MMWR Morb Mortal Wkly Rep. 2020;69(8):201-206. doi: 10.15585/mmwr.mm6908a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zahnd WE, Eberth JM. Lung cancer screening utilization: a Behavioral Risk Factor Surveillance System analysis. Am J Prev Med. 2019;57(2):250-255. doi: 10.1016/j.amepre.2019.03.015 [DOI] [PubMed] [Google Scholar]
- 44.Kee D, Wisnivesky J, Kale MS. Lung cancer screening uptake: analysis of BRFSS 2018. J Gen Intern Med. 2021;36(9):2897-2899. doi: 10.1007/s11606-020-06236-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narayan AK, Gupta Y, Little BP, Shepard JO, Flores EJ. Lung cancer screening eligibility and use with low-dose computed tomography: results from the 2018 Behavioral Risk Factor Surveillance System cross-sectional survey. Cancer. 2021;127(5):748-756. doi: 10.1002/cncr.33322 [DOI] [PubMed] [Google Scholar]
- 46.Fedewa SA, Kazerooni EA, Studts JL, et al. State variation in low-dose computed tomography scanning for lung cancer screening in the United States. J Natl Cancer Inst. 2021;113(8):1044-1052. doi: 10.1093/jnci/djaa170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanner NT, Brasher PB, Wojciechowski B, et al. Screening adherence in the Veterans Administration Lung Cancer Screening Demonstration Project. Chest. 2020;158(4):1742-1752. doi: 10.1016/j.chest.2020.04.063 [DOI] [PubMed] [Google Scholar]
- 48.Wildstein KA, Faustini Y, Yip R, Henschke CI, Ostroff JS. Longitudinal predictors of adherence to annual follow-up in a lung cancer screening programme. J Med Screen. 2011;18(3):154-159. doi: 10.1258/jms.2011.010127 [DOI] [PubMed] [Google Scholar]
- 49.Spalluto L, Lewis J, Sandler K, Massion P, Dittus R, Roumie C. P3.11-23 Adherence to annual low-dose CT lung cancer screening at a large academic institution. J Thorac Oncol. 2018;13(10):S967-S968. doi: 10.1016/j.jtho.2018.08.1819 [DOI] [Google Scholar]
- 50.Brasher P, Tanner N, Yeager D, Silvestri G. Adherence to annual lung cancer screening within the Veterans Health Administration Lung Cancer Screening Demonstration Project. Chest. 2018;154(4)(suppl):636A-637A. doi: 10.1016/j.chest.2018.08.576 [DOI] [PubMed] [Google Scholar]
- 51.Blom EF, ten Haaf K, Arenberg DA, de Koning HJ. Treatment capacity required for full-scale implementation of lung cancer screening in the United States. Cancer. 2019;125(12):2039-2048. doi: 10.1002/cncr.32026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berrington de Gonzalez A, Kim KP, Berg CD. Low-dose lung computed tomography screening before age 55: estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. J Med Screen. 2008;15(3):153-158. doi: 10.1258/jms.2008.008052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goffin JR, Flanagan WM, Miller AB, et al. Cost-effectiveness of lung cancer screening in Canada. JAMA Oncol. 2015;1(6):807-813. doi: 10.1001/jamaoncol.2015.2472 [DOI] [PubMed] [Google Scholar]
- 54.Bethune R, Wu L, Goodridge D, et al. The clinical benefit and cost-effectiveness of adding a smoking cessation program to a simulated lung cancer screening program in Saskatchewan, Canada. Am J Respir Crit Care Med. 2017;195:AS179. [Google Scholar]
- 55.Cao P, Jeon J, Levy DT, et al. Potential impact of cessation interventions at the point of lung cancer screening on lung cancer and overall mortality in the United States. J Thorac Oncol. 2020;15(7):1160-1169. doi: 10.1016/j.jtho.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cadham CJ, Cao P, Jayasekera J, et al. ; CISNET-SCALE Collaboration . Cost-effectiveness of smoking cessation interventions in the lung cancer screening setting: a simulation study. J Natl Cancer Inst. 2021;113(8):1065-1073. doi: 10.1093/jnci/djab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of the CISNET Models in the Comparative Cost-effectiveness Analysis
eTable 2. Health Utilities Used to Adjust Life-years for Quality of Life Based on Health State of Individuals
eTable 3. Cost of Screening and Diagnostic Procedures
eTable 4. Cost of Downstream Treatment Interventions by Phase of Care, Stage, and Histological Subtype at Specific Ages Used for Base-Case Analysis in 2020 US Dollars
eTable 5. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Erasmus Model
eTable 6. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Massachusetts General Hospital–Harvard Medical School Model
eTable 7. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the University of Michigan Model
eTable 8. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Stanford University Model
eTable 9. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Geometric Mean Across the 4 CISNET Models
eTable 10. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models for US Men Born in 1960
eTable 11. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models for US Women Born in 1960
eTable 12. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Cost of LDCT Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 13. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Cost of Treatment Administered During the First 6 Months From Diagnosis (Entry Phase of Care) Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 14. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Cost of Treatment Administered During the Continuous Phase of Care Was Varied by 25% Higher and Lower Than Its Base-Case Value
eTable 15. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Cost of Treatment Administered During the Last 6 Months of Life From the Time at Lung Cancer Death (Terminal LC phase) by 25% Higher or Lower Than Its Base-Case Value
eTable 16. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Cost of Treatment Administered During the Last 6 Months of Life From the Time at Death From Causes Other Than Lung Cancer (Terminal OCM phase) Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 17. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With Stage I Non–Small Cell Lung Cancer Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 18. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With Stage II Non–Small Cell Lung Cancer Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 19. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With Stage III Non–Small Cell Lung Cancer Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 20. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With Stage IV Non–Small Cell Lung Cancer Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 21. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With Limited-Stage Small Cell Lung Cancer Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 22. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With Extended-Stage Small Cell Lung Cancer Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 23. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Health Utility Associated With the Last 6 months of Life From Any Cause of Death Was Varied by 25% Higher or Lower Than Its Base-Case Value
eTable 24. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When Screening Was Restricted to Individuals With Life Expectancy of at Least 5 Years From the Time of Each Screening
eTable 25. Additional Health Benefits Gained and Costs Incurred From Screening Strategies When Screening Was Not Restricted to Individuals With Life Expectancy of at Least 5 Years Relative to the Same Strategy When Screening Was Restricted to Individuals With Life Expectancy of at Least 5 Years From the Time of Screening and Their Corresponding Incremental Cost-effectiveness Ratios
eTable 26. Additional Health Benefits Gained and Costs Incurred From Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening per 100 000 Individuals Alive at Age 45 Years With No Previous Cancer Diagnosis and Their Respective Incremental Cost-effectiveness Ratios Based on the Mean Values Across the 4 CISNET Models When the Cost of Treatment for Advanced Non–Small Cell Lung Cancer Was Doubled
eFigure 1. Model-Specific Incremental Cost and QALYs Relative to the 2013 US Preventive Services Task Force Recommendation on Lung Cancer Screening
eFigure 2. Total Health Benefits (Measured Using QALY) and Costs Associated With Screening Strategies Based on the Mean Values Across the 4 CISNET Models and Their Relative Position to the No Screening Strategy
eFigure 3. Number of Screening-Eligible Individuals per 100 000 Individuals Alive and Cancer-Free at Age 45 Years (Based on Mean Across Models) by Age and Smoking Status Using 20 Pack-Year Screening Strategies Relative to the 2013 USPSTF Recommendation on Lung Cancer Screening
eFigure 4. Number of LDCT Screenings vs Number of Lung Cancer Deaths Averted by Screening Strategies Based on Mean Values Across the 4 CISNET Models and Their Resulting Efficiency Frontiers
eFigure 5. Excess Health Benefit Accrued (Measured in Life-Years) and Cost Incurred Relative to the 2013 USPSTF Screening Strategy and the Resulting Cost-effectiveness Efficiency Frontier (Bolded Strategies) Composed of the Lung Cancer Screening Strategies Based on Mean Values Across the 4 CISNET Models
eFigure 6. Total Health Benefits (Measured Using Unadjusted Life-Years) and Costs Associated With Screening Strategies Based on Mean Values Across the 4 CISNET Models and Their Relative Position to the No Screening Strategy
eFigure 7. Total Cost per 100 000 Individuals Alive at Age 45 Years From the General Population Associated With the Screening Programs
eFigure 8. Probabilistic Sensitivity Analysis for Evaluating the Cost-effectiveness of the 7 Strategies Considered in the Base-Case Analysis by Simultaneously Changing the Input Parameter Values
eReferences