Key Points
Question
What is the effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support at 28 days in patients with COVID-19 and severe hypoxemia?
Findings
In this randomized trial that included 1000 patients with COVID-19 and severe hypoxemia, treatment with 12 mg/d of dexamethasone resulted in 22.0 days alive without life support at 28 days compared with 20.5 days in those receiving 6 mg/d of dexamethasone. This difference was not statistically significant.
Meaning
Compared with 6 mg of dexamethasone, 12 mg of dexamethasone did not statistically significantly reduce the number of days alive without life support at 28 days.
Abstract
Importance
A daily dose with 6 mg of dexamethasone is recommended for up to 10 days in patients with severe and critical COVID-19, but a higher dose may benefit those with more severe disease.
Objective
To assess the effects of 12 mg/d vs 6 mg/d of dexamethasone in patients with COVID-19 and severe hypoxemia.
Design, Setting, and Participants
A multicenter, randomized clinical trial was conducted between August 2020 and May 2021 at 26 hospitals in Europe and India and included 1000 adults with confirmed COVID-19 requiring at least 10 L/min of oxygen or mechanical ventilation. End of 90-day follow-up was on August 19, 2021.
Interventions
Patients were randomized 1:1 to 12 mg/d of intravenous dexamethasone (n = 503) or 6 mg/d of intravenous dexamethasone (n = 497) for up to 10 days.
Main Outcomes and Measures
The primary outcome was the number of days alive without life support (invasive mechanical ventilation, circulatory support, or kidney replacement therapy) at 28 days and was adjusted for stratification variables. Of the 8 prespecified secondary outcomes, 5 are included in this analysis (the number of days alive without life support at 90 days, the number of days alive out of the hospital at 90 days, mortality at 28 days and at 90 days, and ≥1 serious adverse reactions at 28 days).
Results
Of the 1000 randomized patients, 982 were included (median age, 65 [IQR, 55-73] years; 305 [31%] women) and primary outcome data were available for 971 (491 in the 12 mg of dexamethasone group and 480 in the 6 mg of dexamethasone group). The median number of days alive without life support was 22.0 days (IQR, 6.0-28.0 days) in the 12 mg of dexamethasone group and 20.5 days (IQR, 4.0-28.0 days) in the 6 mg of dexamethasone group (adjusted mean difference, 1.3 days [95% CI, 0-2.6 days]; P = .07). Mortality at 28 days was 27.1% in the 12 mg of dexamethasone group vs 32.3% in the 6 mg of dexamethasone group (adjusted relative risk, 0.86 [99% CI, 0.68-1.08]). Mortality at 90 days was 32.0% in the 12 mg of dexamethasone group vs 37.7% in the 6 mg of dexamethasone group (adjusted relative risk, 0.87 [99% CI, 0.70-1.07]). Serious adverse reactions, including septic shock and invasive fungal infections, occurred in 11.3% in the 12 mg of dexamethasone group vs 13.4% in the 6 mg of dexamethasone group (adjusted relative risk, 0.83 [99% CI, 0.54-1.29]).
Conclusions and Relevance
Among patients with COVID-19 and severe hypoxemia, 12 mg/d of dexamethasone compared with 6 mg/d of dexamethasone did not result in statistically significantly more days alive without life support at 28 days. However, the trial may have been underpowered to identify a significant difference.
Trial Registration
ClinicalTrials.gov Identifier: NCT04509973 and ctri.nic.in Identifier: CTRI/2020/10/028731
This multicenter randomized clinical trial compares the effects of 12 mg/d vs 6 mg/d of dexamethasone in patients with COVID-19 and severe hypoxemia.
Introduction
Patients with critical COVID-19 are characterized by severe pulmonary inflammation and hypoxemia, which often leads to use of high-flow oxygen, mechanical ventilation and, in case of further disease progression, circulatory support and kidney replacement therapy.1
Dexamethasone is recommended by the World Health Organization2 for patients with severe and critical COVID-19 based on a prospective meta-analysis3 of 7 randomized trials reporting reduced short-term mortality with the use of systemic glucocorticoids. The largest of these trials, the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial,4 demonstrated a mortality benefit with 6 mg/d of dexamethasone for up to 10 days. Among the remaining 6 trials in the meta-analysis,3 most evaluated daily doses of glucocorticoids that were higher than 6 mg of dexamethasone (median dose in dexamethasone equivalents, 12 mg [range, 6-16 mg]).5,6,7,8 Higher doses of dexamethasone also have been reported as beneficial in a randomized trial including patients without COVID-19 who had acute respiratory distress syndrome.9 Pharmacodynamic studies suggest dose-dependent activation of the corticosteroid receptor with increasing doses up to 60 mg of prednisone (equivalent to 12 mg of dexamethasone).10
These findings suggest the possibility that higher doses of dexamethasone than the recommended dose of 6 mg/d may benefit patients with COVID-19 who have more severe disease. However, there are concerns about adverse reactions with the use of higher doses of glucocorticoids,11 particularly reports of severe fungal infections, such as mucormycosis, in patients with COVID-19 treated with glucocorticoids.12,13
The COVID STEROID 2 trial was conducted to evaluate the efficacy and safety of a higher dose of dexamethasone in hospitalized adults with COVID-19 and severe hypoxemia. The hypothesis was that a higher daily dose of dexamethasone (12 mg) compared with the currently recommended daily dose (6 mg) would increase the number of days alive without life support at 28 days in these patients.
Methods
Trial Design and Oversight
This trial was an investigator-initiated, international, parallel-group, stratified, blinded randomized clinical trial. The trial protocol was approved by the Danish Medicines Agency, the ethics committee of the Capital Region of Denmark, and institutionally at each trial site. Before enrollment was completed, the trial protocol and statistical analysis plan were published14 and also appear in Supplement 1. The trial was overseen by the Collaboration for Research in Intensive Care and the George Institute for Global Health. A data and safety monitoring committee oversaw the safety of the trial participants and conducted 1 planned interim analysis.
Informed consent was obtained from the patients or their legal surrogates according to national regulations. At many institutions, enrollment was allowed as an emergency procedure (ie, assent was provided by a physician who was not involved in the trial and consent was later obtained from the patient or a relative to continue participation). If consent was withdrawn or not granted, permission was sought from the patient or a relative to continue the collection and use of data.
Trial Sites and Patients
Patients underwent screening and randomization between August 27, 2020, and May 20, 2021, at 26 hospitals (11 in Denmark, 12 in India, 2 in Sweden, and 1 in Switzerland). At 2 of the Danish hospitals, there were multiple sites at intensive care units and departments of infectious diseases and pulmonary medicine so the total number of trial sites was 31.
Eligible patients were aged 18 years or older, hospitalized with confirmed SARS-CoV-2 infection, and required (1) supplementary oxygen at a flow rate of at least 10 L/min (independent of delivery system), (2) noninvasive ventilation or continuous positive airway pressure for hypoxemia, or (3) invasive mechanical ventilation. We excluded patients who (1) were treated with systemic glucocorticoids in doses higher than 6 mg of dexamethasone equivalents for indications other than COVID-19 or had been treated with systemic glucocorticoids for COVID-19 for 5 days or longer, (2) had invasive fungal infection or active tuberculosis, (3) had known hypersensitivity to dexamethasone, and (4) were pregnant. The full details regarding the inclusion and exclusion criteria appear in the eMethods in Supplement 2.
Randomization
Randomization was performed using a centralized, computer-generated allocation sequence stratified by trial site, by age of younger than 70 years, and by whether the patient required invasive mechanical ventilation at the time of screening. Eligible patients were randomly allocated in a 1:1 ratio to 12 mg/d of dexamethasone or 6 mg/d of dexamethasone using varying permuted block sizes of 6 or 8 (Figure 1). Treatment assignments were concealed from patients, clinicians, investigators, trial statisticians, the data and safety monitoring committee, and the management committee when it wrote the first version of the abstract (eMethods in Supplement 2).
Figure 1. Screening, Randomization, and Follow-up of Patients in the COVID STEROID 2 Trial.
aThere were 33 patients who met more than 1 criterion.
bProvided data at baseline and for the analysis of serious adverse reactions.
cRequired exclusion by the Swiss ethics committee.
Interventions
A daily dose with 12 mg of dexamethasone (as 14.4 mg of dexamethasone phosphate) or 6 mg of dexamethasone (as 7.2 mg of dexamethasone phosphate) was suspended in sodium chloride 0.9% (eFigure 1 in Supplement 2) and administered as a masked bolus injection (total volume of 5 mL) intravenously once daily for up to 10 days from randomization. The use of betamethasone was allowed at sites where dexamethasone was not available (1 hospital in Sweden) because the drugs are diastereomers and are likely equipotent.15
A team of unblinded trial staff, who were not involved in the care of trial patients or in the entry of outcome data or the statistical analysis, prepared the masked trial medication from the medication available at local hospital pharmacies (the brand names appear in eFigure 1 in Supplement 2). The staff was instructed not to reveal the treatment allocation unless the participant was subject to emergency unblinding (occurred in 1 patient who was randomized to 6 mg of dexamethasone).
If the patient had been treated with dexamethasone for COVID-19 prior to enrollment, the intervention period was shortened so that no patients received dexamethasone for more than 10 days per the trial protocol. All other interventions were at the discretion of the clinicians; however, we recommended against the use of other immunosuppressive agents for COVID-19. Starting on January 9, 2021, the use of tocilizumab was allowed after the publication of results from the IL-6 receptor antagonists domain of the Randomized, Embedded, Multifactorial, Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial.16
Data Collection and Monitoring
The trial investigators or staff reported any serious adverse events to the coordinating centers and entered the baseline characteristics, process variables, and outcome data from the patient files into web-based case report forms for days 1 to 14, 28-day follow-up, and 90-day follow-up. When available, regional and national registries were used for follow-up and patients or their surrogates were contacted directly if additional data were needed. Trial data were monitored at the sites (including consent and source data verification) by independent monitors according to a prespecified monitoring plan and centrally by staff from the coordinating centers.
Outcomes
The primary outcome was the number of days alive without life support (invasive mechanical ventilation, circulatory support, or kidney replacement therapy) at 28 days after randomization. All outcome definitions appear in the eMethods in Supplement 2).
The secondary outcomes were the number of days alive without life support at 90 days, the number of days alive out of the hospital at 90 days, mortality at 28 days and at 90 days, and the number of patients with 1 or more serious adverse reactions at 28 days (ie, new episodes of septic shock, invasive fungal infection, clinically important gastrointestinal bleeding, or anaphylactic reaction to dexamethasone). Three additional secondary outcomes, including health-related quality of life measured using the 5-dimension, 5-level European Quality of Life questionnaire and the European Quality of Life visual analog scale, will be assessed at 180 days after randomization (Supplement 1).
Sample Size Calculation
We estimated that 1000 patients were required for the trial to have 85% power to show a relative reduction of 15% in 28-day mortality, which is within the range observed in other critical care trials,17 combined with a reduction of 10% in the time requiring life support at a 2-sided α level of 5%, assuming that 30% of patients would die and 10% of patients would still require life support at 28 days in the control group (6 mg of dexamethasone).1
Statistical Analysis
The statistician on the data and safety monitoring committee conducted the interim analysis after the first 500 patients had been followed up for 28 days. The α threshold for the interim analysis was .005 and for the final analysis was .049 per O’Brien-Fleming boundary points.18 Therefore, P < .049 was considered statistically significant and 95.08% CIs (rounded to 95%) were used for the primary outcome analysis. For the secondary mortality outcomes, a hierarchical testing procedure was specified. If the primary outcome was statistically significantly different, the α threshold was reused for 28-day mortality. If 28-day mortality also differed significantly, the α threshold was reused for 90-day mortality; otherwise, an α threshold of .01 was used. For the other secondary outcomes, P < .01 was considered statistically significant and adjusted 99% CIs were used because of multiple comparisons.
The statistical analyses were performed according to the statistical analysis plan with some modifications (a 2-step procedure was used to analyze all binary outcomes due to convergence problems and post hoc sensitivity analyses were added; the details appear in Supplement 1).14 Patients were analyzed according to their randomization group unless they withdrew consent for the use of any data. In the per-protocol population, patients with 1 or more major protocol violations were excluded (eMethods in Supplement 2).
In the primary outcome analysis, the number of days alive without life support within the 28-day period was analyzed using the Kryger Jensen and Lange test19 and was adjusted for stratification variables. The Kryger Jensen and Lange test increases power when used for data sets with zero values (ie, many patients who were expected to have 0 days alive without life support). The results are presented as adjusted means and medians with 95% CIs. The secondary analysis of the primary outcome was adjusted for the stratification variables and additional predefined risk factors at baseline (history of ischemic heart disease or heart failure, diabetes, chronic obstructive pulmonary disease, use of immunosuppressive therapy within prior 3 months, use of circulatory support, and use of kidney replacement therapy) and was performed in the per-protocol population and in prespecified subgroups (including test of interaction using the Wald test). The prespecified subgroups were enrollment geographic region (Europe vs India), age (<70 years vs ≥70 years), chronic use of systemic glucocorticoids vs no use at baseline, presence vs absence of limitations in care, required vs did not require invasive mechanical ventilation, prior use vs no prior use of IL-6 receptor antagonists, and prior use of dexamethasone for up to 2 days vs use for 3 to 4 days prior to randomization. The protocol was changed on January 9, 2021, to include the subgroup analyses by enrollment geographic region and by use of IL-6 receptor antagonists and to exclude septic shock (to reduce the overall number of subgroup analyses).14
For the secondary outcomes, the Kryger Jensen and Lange test19 was used and the logistic regression adjusted for the stratification variables and g-computation or for the generalized linear models with log links and binomial error distributions. Unadjusted Fisher exact testing also was performed.
Logical imputations were made for missing primary outcome data for 2 patients. One patient was declared by a physician on day 25 as being well enough to board an airplane; however, this patient was lost to follow-up and assumed to be alive without life support from days 25 to 28. The relatives of another patient (who died on day 46) reported that the patient had been treated with kidney replacement therapy after hospital discharge, therefore, this patient was assumed to have been receiving kidney replacement therapy from hospital discharge to day 28 (eMethods in Supplement 2).
Best-worst and worst-best imputations were made for 11 patients (who withdrew consent during the 28-day data collection period) without data on the use of life support or vital status from the date of withdrawal. These 11 patients were included in the analysis of serious adverse reactions without imputation of missing data. An additional 2 patients withdrew consent before 90 days (1 was lost to follow-up and 1 had missing life support data at day 90). The 90-day outcomes were analyzed without any imputation. These analyses were performed using R versions 3.6.3 and 4.1.0 (R Foundation for Statistical Computing).
The primary analyses of days alive without life support at 28 days and at 90 days were supplemented post hoc with bootstrapped-adjusted mean differences (50 000 samples) because the observed distribution of these outcomes was markedly skewed (41.4% of all participants were alive without life support at 28 days). In another post hoc analysis decided before database lock, we analyzed the primary outcome by assigning dead participants the worst possible outcome (ie, 0 days alive without life support) as was done in previous trials.20 During the manuscript review process, we added a post hoc analysis of the primary outcome using a linear mixed-effects model with random effects for site and fixed effects for other stratification variables. An additional post hoc analysis for time to death compared the 2 groups using unadjusted Cox regression.
Results
Participants
Between August 27, 2020, and May 20, 2021, 1414 patients were screened and 1000 were randomized (503 were randomized to receive 12 mg/d of dexamethasone and 497 were randomized to receive 6 mg/d of dexamethasone; Figure 1). Of the 1000 patients, 18 did not provide consent to allow the use of any data, therefore, 982 were included in the full analysis data set (median age, 65 [IQR, 55-73] years; 305 [31%] women). Another 8 patients were erroneously randomized and were included in the analyses (eTables 1A-1B and eFigures 2-3 in Supplement 2). Data for the primary outcome were obtained for 971 patients (491 in the 12-mg group and 480 in the 6-mg group). Patient characteristics at baseline were largely similar in the 2 groups; however, the prevalence of coexisting diabetes differed (Table 1). The end of 90-day follow-up was on August 19, 2021.
Table 1. Baseline Characteristics of the Patientsa.
| Characteristic | 12 mg of dexamethasone (n = 497) | 6 mg of dexamethasone (n = 485) |
|---|---|---|
| Country of enrollment, No. (%) | ||
| Denmark | 251 (51) | 234 (48) |
| India | 182 (37) | 187 (39) |
| Sweden | 40 (8) | 39 (8) |
| Switzerland | 24 (5) | 25 (5) |
| Age, median (IQR), y | 65 (56-74) | 64 (54-72) |
| Sex, No. (%) | ||
| Male | 346 (70) | 331 (68) |
| Female | 151 (30) | 154 (32) |
| Weight, median (IQR), kg | 80 (68-96) | 80 (68-95) |
| Coexisting conditions, No. (%)b | ||
| Diabetes | 135 (27) | 163 (34) |
| Ischemic heart disease or heart failure | 67 (14) | 69 (14) |
| Chronic obstructive pulmonary disease | 57 (12) | 56 (12) |
| Immunosuppressive therapy within 3 mo prior to randomization | 40 (8) | 43 (9) |
| Chronic use of systemic glucocorticoids | 13 (3) | 16 (3) |
| Limitations in the use of life support or CPR at randomization, No. (%) | 30 (6) | 25 (5) |
| Time from onset of symptoms to hospitalization, median (IQR), d | (n = 465) 7 (4-9) |
(n = 467) 7 (4-10) |
| Time from hospitalization to randomization, median (IQR), d | 2 (1-3) | 2 (1-3) |
| Place of enrollment, No. (%) | ||
| Intensive care unit | 389 (78) | 393 (81) |
| Hospital ward | 66 (13) | 54 (11) |
| Emergency department | 22 (4) | 21 (4) |
| Intermediate care unit | 20 (4) | 17 (4) |
| Type of oxygen supplementation | ||
| Nasal cannula or open mask, No. (%) | 272 (55) | 258 (53) |
| Flow rate, median (IQR), L/min | 22 (15-40) | 24 (15-40) |
| Noninvasive ventilation or continuous positive airway pressure, No. (%) | 118 (24) | 128 (26) |
| Fio2, median (IQR), % | (n = 114) 58 (50-78) |
(n = 120) 60 (50-71) |
| Duration before randomization, median (IQR), d | 1 (0-1) | 1 (0-1) |
| Invasive mechanical ventilation, No. (%) | 107 (22) | 99 (20) |
| Fio2, median (IQR), % | (n = 106) 60 (45-70) |
(n = 99) 60 (45-85) |
| Duration before randomization, median (IQR), d | 1 (0-1) | 1 (0-1) |
| Level, median (IQR)c | ||
| Pao2, mm Hg | (n = 469) 72 (62-87) |
(n = 462) 71 (61-83) |
| Sao2, % | (n = 492) 94 (91-96) |
(n = 476) 94 (91-96) |
| Lactate concentration, median (IQR), mmol/Ld | (n = 440) 1.6 (1.1-2.3) |
(n = 436) 1.7 (1.2-2.3) |
| Therapies in use at randomizatione | ||
| Dexamethasone, median (IQR), d | 1 (1-2) | 1 (1-3) |
| Antiviral agents | 312 (63) | 318 (66) |
| Remdesivir | 307 (62) | 310 (64) |
| Convalescent plasma | 11 (2) | 17 (4) |
| Other | 9 (2) | 6 (1) |
| Systemic antibacterial agents | 312 (63) | 318 (66) |
| Vasopressors or inotropes | 81 (16) | 68 (14) |
| Anti-inflammatory agents | 58 (12) | 57 (12) |
| IL-6 receptor antagonists | 52 (11) | 47 (10) |
| Janus kinase inhibitors | 8 (2) | 7 (1) |
| Other | 9 (2) | 10 (2) |
| Kidney replacement therapy | 11 (2) | 14 (3) |
Abbreviations: CPR, cardiopulmonary resuscitation; Fio2, fraction of inspired oxygen; Sao2, arterial oxygen saturation.
SI conversion factor: To convert lactate to mg/dL, divide by 0.111.
The definitions of the baseline characteristics appear in the eMethods in Supplement 2.
These were considered as potential effect modifiers and the data were collected from chart review.
Level while receiving oxygen supplementation.
Normal level is less than 2.0 mmol/L.
Expressed as No. (%) unless otherwise indicated.
Trial and Concomitant Interventions
Both groups had received dexamethasone for a median of 1 day before enrollment. The use of respiratory, circulatory, and kidney support and the use of other anti-inflammatory, antiviral, and antibacterial agents was similar between groups at baseline (Table 1).
The assigned trial intervention was received per protocol by 461 of 497 patients (92.7%) in the 12 mg of dexamethasone group and by 446 of 485 (91.9%) in the 6 mg of dexamethasone group (eTable 2 in Supplement 2). The duration of the intervention was similar in the 2 groups (median, 7 days [IQR, 5.0-9.0 days] in the 12-mg group and 7 days [IQR, 6.0-9.0 days] in the 6-mg group; eTable 2 in Supplement 2). During the intervention period, 10 of 497 patients (2.0%) in the 12-mg group and 9 of 485 (1.9%) in the 6-mg group received open-label glucocorticoids (eTable 2 in Supplement 2). Nine (1.8%) patients in the 12-mg group and 11 (2.3%) in the 6-mg group were discharged from the hospital against medical advice within 28 days (eTable 3 in Supplement 2).
Primary Outcome
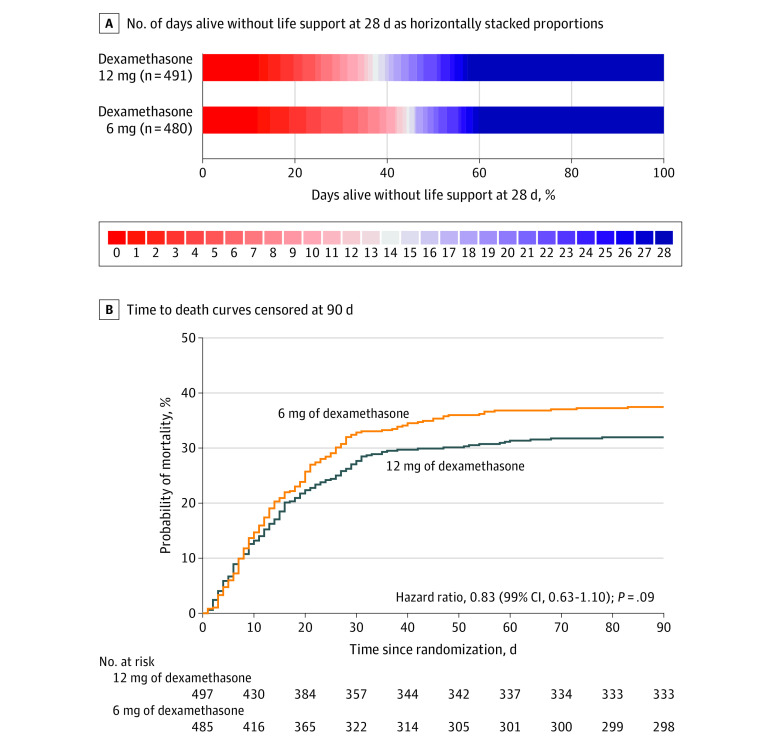
At 28 days after randomization, the median number of days alive without life support was 22.0 days (IQR, 6.0-28.0 days) in the 12 mg of dexamethasone group and 20.5 days (IQR, 4.0-28.0 days) in the 6 mg of dexamethasone group (adjusted mean difference, 1.3 days [95% CI, 0-2.6 days], P = .07; Figure 2 and Table 2). The results were similar in the preplanned (Table 2 and eTables 4-5 in Supplement 2) and in the post hoc sensitivity analyses (Table 2; eFigure 4 and eTables 6-7 in Supplement 2). In the predefined subgroup analysis, no statistically significant heterogeneity was found for the effect of the trial intervention on the primary outcome (Figure 3). The single components of the composite primary outcome were similar between groups (Table 2 and eTable 8 in Supplement 2). The percentages of patients with 28 days alive without life support were 42.6% in the 12-mg group and 40.2% in the 6-mg group.
Figure 2. Distributions of the Primary Outcome and Time to Death Curves to Day 90.
A, Life support was defined as invasive mechanical ventilation, circulatory support, or kidney replacement therapy. There were missing data in 11 patients for the primary outcome. Red represents the worse outcomes and blue represents better outcomes. B, There were 14 patients who were not followed up for the full 90 days (7 patients in each intervention group) and who were included until the last day they were known to be alive. The median follow-up time was 90 days (IQR, 24-90 days) in the 12 mg of dexamethasone group and 90 days (IQR, 20-90 days) in the 6 mg of dexamethasone group. The time to death was compared post hoc using unadjusted Cox regression.
Table 2. Primary and Secondary Outcomes.
| Outcomea | 12 mg of dexamethasone (n = 491) |
6 mg of dexamethasone (n = 480) |
Adjusted mean difference (95% CI)b | Adjusted relative risk (99% CI)b | P value |
|---|---|---|---|---|---|
| Primary outcome | |||||
| No. of days alive without life support at 28 d, median (IQR)c | 22.0 (6.0 to 28.0) | 20.5 (4.0 to 28.0) | 1.3 (0 to 2.6) | .07d | |
| Single components of the composite primary outcomeb | |||||
| No. of days alive without invasive mechanical ventilation at 28 d, median (IQR) | 23.0 (7.0 to 28.0) | 22.0 (5.0 to 28.0) | |||
| No. of days alive without circulatory support at 28 d, median (IQR) | 26.0 (13.0 to 28.0) | 25.0 (9.0 to 28.0) | |||
| No. of days alive without kidney replacement therapy at 28 d, median (IQR) | 28.0 (18.0 to 28.0) | 28.0 (13.8 to 28.0) | |||
| Secondary analysis of the primary outcome | |||||
| No. of days alive without life support at 28 de | 1.2 (−0.1 to 2.4) | .06 | |||
| Unadjusted analysis | 1.3 (−0.1 to 2.7) | .07 | |||
| Secondary outcomes | |||||
| No. of days alive without life support at 90 d, median (IQR) | (n = 489) 84.0 (9.3 to 90.0) |
(n = 478) 80.0 (6.0 to 90.0) |
4.4 (−1.6 to 10.4) | .15f | |
| No. of days alive out of the hospital at 90 d, median (IQR) | (n = 490) 61.5 (0 to 78.0) |
(n = 478) 48.0 (0 to 76.0) |
4.1 (−1.3 to 9.5) | .09 | |
| Mortality | |||||
| At 28 d, No. (%) | 133 (27.1) | 155 (32.3) | −4.5 (−11.5 to 2.3)g | 0.86 (0.68 to 1.08) | .10h |
| At 90 d, No./total (%) | 157/490 (32.0) | 180/478 (37.7) | −4.9 (−12.1 to 2.4)g | 0.87 (0.70 to 1.07) | .09i |
| ≥1 serious adverse reactions, No./total (%)j | 56/497 (11.3) | 65/485 (13.4) | −2.2 (−7.3 to 3.1)g | 0.83 (0.54 to 1.29) | .27k |
| New episodes of septic shock, No. (%) | 42 (8.5) | 50 (10.3) | |||
| Invasive fungal infection, No. (%) | 15 (3.0) | 21 (4.3) | |||
| Clinically important gastrointestinal bleeding, No. (%) | 9 (1.8) | 5 (1.0) | |||
| Anaphylactic reaction to dexamethasone, No. | 0 | 0 | |||
Outcome definitions appear in the eMethods in Supplement 2.
Adjusted for the stratification variables of site, age younger than 70 years, and use of invasive mechanical ventilation unless otherwise indicated. The median differences for the outcomes of days alive without life support and days out of the hospital and the analyses of the single components of the composite outcomes appear in eTable 8 in Supplement 2.
Life support defined as invasive mechanical ventilation, circulatory support, or kidney replacement therapy.
A post hoc analysis of the bootstrapped-adjusted mean difference was performed because the data were markedly skewed (a high proportion [41.4%] of the 28-day counts; P = .05).
Additionally adjusted for the baseline risk factors of history of ischemic heart disease or heart failure, diabetes, chronic obstructive pulmonary disease, use of immunosuppressive therapy within the prior 3 months, use of circulatory support, and use of kidney replacement therapy.
A post hoc analysis of the bootstrapped-adjusted mean difference was performed because the data were markedly skewed (P = .06).
Data are expressed as risk difference (99% CI).
Fisher exact test yielded P = .08.
Fisher exact test yielded P = .07.
Predefined outcome. All serious adverse reactions and serious adverse events appear in eTable 10 in Supplement 2.
Fisher exact test yielded P = .33.
Figure 3. Median Days Alive Without Life Support and the Adjusted Mean Differences in the 7 Predefined Subgroups.

aAdjusted for the stratification variables of site, age younger than 70 years, and use of invasive mechanical ventilation unless otherwise indicated. The median differences for the outcomes of days alive without life support and days out of the hospital and the analyses of the single components of the composite outcomes appear in eTable 8 in Supplement 2.
bDefined as life support or cardiopulmonary resuscitation at randomization.
Secondary Outcomes
Days Alive Without Life Support and Days Alive Out of the Hospital at 90 Days
At 90 days, the median number of days alive without life support was 84.0 days (IQR, 9.3 to 90.0 days) in the 12 mg of dexamethasone group and 80.0 days (IQR, 6.0 to 90.0 days) in the 6 mg of dexamethasone group (adjusted mean difference, 4.4 days [99% CI, −1.6 to 10.4 days]; Table 2 and eFigure 5 in Supplement 2). At 90 days, the median number of days alive and out of the hospital was 61.5 days (IQR, 0 to 78.0 days) in the 12-mg group and 48.0 days (IQR, 0 to 76.0 days) in the 6-mg group (adjusted mean difference, 4.1 days [99% CI, −1.3 to 9.5 days]; Table 2 and eFigure 6 in Supplement 2).
28-Day and 90-Day Mortality
At 28 days, a total of 133 of 491 patients (27.1%) had died in the 12 mg of dexamethasone group and 155 of 480 patients (32.3%) had died in the 6 mg of dexamethasone group (adjusted relative risk, 0.86 [99% CI, 0.68-1.08]; Figure 2, Table 2, and eTable 9 in Supplement 2). At 90 days, 157 of 490 patients (32.0%) had died in the 12-mg group and 180 of 478 patients (37.7%) had died in the 6-mg group (adjusted relative risk, 0.87 [99% CI, 0.70-1.07]; Figure 2, Table 2, and eTable 9 in Supplement 2).
Serious Adverse Reactions and Events
At 28 days, 56 of 497 patients in the 12 mg of dexamethasone group (11.3%) had 1 or more serious adverse reactions compared with 65 of 485 patients (13.4%) in the 6 mg of dexamethasone group (adjusted relative risk, 0.83 [99% CI, 0.54-1.29]; Table 2 and eTable 9 in Supplement 2). The components of the composite adverse reaction outcome appear in Table 2 and eTable 8 in Supplement 2; none had an anaphylactic reaction to dexamethasone.
The total number of patients with 1 or more serious adverse reactions or serious adverse events was 102 (20.5%) in the 12-mg group and 123 (25.4%) in the 6-mg group (eTable 10 in Supplement 2). Extracorporeal membrane oxygenation was used in 3 patients (0.6%) in the 12-mg group and in 14 patients (2.9%) in the 6-mg group (eTable 11 in Supplement 2).
Discussion
In this international, blinded, randomized clinical trial including adults with COVID-19 and severe hypoxemia, treatment with 12 mg/d of dexamethasone compared with 6 mg/d of dexamethasone did not result in significantly more days alive without life support at 28 days. None of the analyzed secondary outcomes were statistically significant and the subgroup analyses did not support heterogeneity for the intervention effect. The number of patients with serious adverse reactions (ie, septic shock, invasive fungal infection, and clinically important gastrointestinal bleeding) appeared similar between the groups.
Other trials have assessed treatments providing anti-inflammatory effects in addition to that of 6 mg of dexamethasone in patients with COVID-19.16,21 Most patients also received dexamethasone as part of usual care in the IL-6 receptor antagonists domains of the REMAP-CAP16 and RECOVERY21 trials. Both trials showed improvements in organ support–free days, short-term mortality, or both; the absolute short-term mortality benefit with tocilizumab was 8 percentage points in the REMAP-CAP trial and 4 percentage points in the RECOVERY trial.16,21 In the current trial, the adjusted point estimate for 28-day mortality was 4.5 percentage points lower in the 12 mg of dexamethasone group than in the 6 mg of dexamethasone group, but this was not statistically significantly different. These differences may be due to differences in anti-inflammatory modulation, patient populations, outcome definitions, statistical frameworks (REMAP-CAP used bayesian statistics), or sample sizes and event rates. Additional analyses of the current trial (outcomes at 180 days and a bayesian analysis of outcomes at 28 days and at 90 days),14,22 and a planned prospective meta-analysis of the trials assessing high-dose vs standard-dose dexamethasone in patients with COVID-19 and hypoxemia23 may provide additional insights.
The strengths of this trial include the pragmatic protocol, its relatively large sample size, inclusion of most of the eligible patients, allocation concealment and blinding, the high percentage of follow-up at 28 days, and the variety of hospitals and countries involved. Patients were enrolled in both Europe (62%) and India (38%), reflecting different patient characteristics and risk factors, practice patterns, and health care systems. Septic shock and invasive fungal infections were prespecified secondary safety outcomes and therefore accurately captured. The results were consistent in multiple sensitivity analyses, as well as in analyses of the per-protocol population and in the prespecified subgroups. Together, these characteristics increase the internal and external validity of our results.
Limitations
This trial had several limitations. First, the null result may reflect limited power to detect statistically significant differences for the primary outcome as well other outcomes and in the subgroup analyses.
Second, some baseline variables such as ethnicity were not collected, and some characteristics such as prevalence of diabetes differed between the groups. However, a predefined secondary analysis adjusting for diabetes and other important risk factors supported the primary result.
Third, the intervention period was only 6 days in some patients per protocol because the trial design allowed up to 4 days of dexamethasone use before enrollment, which may have reduced any effect of the intervention.
Fourth, the distribution of the primary and secondary outcome data was not normal. To mitigate this, a newly developed statistical test that accounts for data sets with many zero values was used, and post hoc bootstrapping was used to test the results further.19
Fifth, the sample size estimation for the primary outcome was based on expected relative differences of 15% in 28-day mortality and of 10% in time requiring life support; these differences may have been too large.
Sixth, changes in the treatment of COVID-19 during the trial (such as increased use of IL-6 receptor antagonists) may have influenced the results.
Conclusions
Among patients with COVID-19 and severe hypoxemia, 12 mg/d of dexamethasone compared with 6 mg/d of dexamethasone did not result in statistically significantly more days alive without life support at 28 days. However, the trial may have been underpowered to identify a significant difference.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
Trial protocol and statistical analysis plan
eMethods
eFigure 1. Masking of Trial Medication
eFigure 2. Number of Enrolments per Site
eFigure 3. Number of Enrolments per Month
eFigure 4. Distributions of the Primary Outcome in the Post Hoc Analyses of the Primary Outcome Assigning Patients Who Had Died at Day 28 the Worst Possible Outcome (i.e., 0 Days Alive Without Life Support)
eFigure 5. Distributions of the Number of Days Alive Without Life Support at 90 days in the Two Intervention Groups
eFigure 6. Distributions of the Number of Days Alive and Out of Hospital at 90 days in the Two Intervention Groups
eTables 1a and 1b. Randomizations in Error and Wrongly Entered Stratification Variables
eTable 2. Trial Medication Administration and Protocol Violations
eTable 3. Discharge Against Medical Advice within 28 Days of Randomization
eTable 4. Results of the Analysis of the Primary Outcome in the Per Protocol Population
eTable 5. Best-Worst/Worst-Best Case Analyses of the Primary Outcome
eTable 6. Post Hoc Analyses of the Primary Outcome Assigning Patients Who had Died at Day 28 the Worst Possible Outcome (i.e., 0 Days Alive Without Life Support)
eTable 7. Post Hoc Analysis of the Primary Outcome Assessing Potential Clustering Effect Among Sites
eTable 8. Analyses of the Single Components of Composite Outcomes
eTable 9. Results of the Unadjusted Analyses of Secondary Outcomes
eTable 10. All Serious Adverse Reactions and Serious Adverse Events
eTable 11. Use of Extracorporeal Membrane Oxygenation within 28 Days of Randomization
eReferences
Nonauthor collaborators
Data sharing statement
References
- 1.Haase N, Plovsing R, Christensen S, et al. Characteristics, interventions, and longer term outcomes of COVID-19 ICU patients in Denmark: a nationwide, observational study. Acta Anaesthesiol Scand. 2021;65(1):68-75. doi: 10.1111/aas.13701 [DOI] [PubMed] [Google Scholar]
- 2.Rochwerg B, Agarwal A, Siemieniuk RA, et al. A living WHO guideline on drugs for COVID-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379 [DOI] [PubMed] [Google Scholar]
- 3.Sterne JAC, Murthy S, Diaz JV, et al. ; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330-1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC, Derde L, Al-Beidh F, et al. ; Writing Committee for the REMAP-CAP Investigators . Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317-1329. doi: 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dequin PF, Heming N, Meziani F, et al. ; CAPE COVID Trial Group and the CRICS-TriGGERSep Network . Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1298-1306. doi: 10.1001/jama.2020.16761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomazini BM, Maia IS, Cavalcanti AB, et al. ; COALITION COVID-19 Brazil III Investigators . Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307-1316. doi: 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munch MW, Meyhoff TS, Helleberg M, et al. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia: the COVID STEROID randomised, placebo-controlled trial. Acta Anaesthesiol Scand. Published online June 17, 2021. doi: 10.1111/aas.13941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villar J, Ferrando C, Martínez D, et al. ; Dexamethasone in ARDS Network . Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267-276. doi: 10.1016/S2213-2600(19)30417-5 [DOI] [PubMed] [Google Scholar]
- 10.Fleishaker DL, Mukherjee A, Whaley FS, Daniel S, Zeiher BG. Safety and pharmacodynamic dose response of short-term prednisone in healthy adult subjects: a dose ranging, randomized, placebo-controlled, crossover study. BMC Musculoskelet Disord. 2016;17:293. doi: 10.1186/s12891-016-1135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams DM. Clinical pharmacology of corticosteroids. Respir Care. 2018;63(6):655-670. doi: 10.4187/respcare.06314 [DOI] [PubMed] [Google Scholar]
- 12.Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(4):102146. doi: 10.1016/j.dsx.2021.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raut A, Huy NT. Rising incidence of mucormycosis in patients with COVID-19: another challenge for India amidst the second wave? Lancet Respir Med. 2021;9(8):e77. doi: 10.1016/S2213-2600(21)00265-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munch MW, Granholm A, Myatra SN, et al. Higher vs lower doses of dexamethasone in patients with COVID-19 and severe hypoxia (COVID STEROID 2) trial: protocol and statistical analysis plan. Acta Anaesthesiol Scand. 2021;65(6):834-845. doi: 10.1111/aas.13795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute of Diabetes and Digestive and Kidney Diseases . LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [Google Scholar]
- 16.Gordon AC, Mouncey PR, Al-Beidh F, et al. ; REMAP-CAP Investigators . Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491-1502. doi: 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landoni G, Comis M, Conte M, et al. Mortality in multicenter critical care trials: an analysis of interventions with a significant effect. Crit Care Med. 2015;43(8):1559-1568. doi: 10.1097/CCM.0000000000000974 [DOI] [PubMed] [Google Scholar]
- 18.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549-556. doi: 10.2307/2530245 [DOI] [PubMed] [Google Scholar]
- 19.Kryger Jensen A, Lange T. A novel high-power test for continuous outcomes truncated by death. arXiv. 2019;1910.12267. [Google Scholar]
- 20.Schoenfeld DA, Bernard GR; ARDS Network . Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772-1777. doi: 10.1097/00003246-200208000-00016 [DOI] [PubMed] [Google Scholar]
- 21.RECOVERY Collaborative Group . Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi: 10.1016/S0140-6736(21)00676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granholm A, Munch MW, Myatra SN, et al. Higher vs lower doses of dexamethasone in patients with COVID-19 and severe hypoxia (COVID STEROID 2) trial: protocol for a secondary bayesian analysis. Acta Anaesthesiol Scand. 2021;65(5):702-710. doi: 10.1111/aas.13793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granholm A. Higher vs standard doses of dexamethasone in patients with COVID-19 and hypoxia: a prospective meta-analysis. Published May 31, 2021. Accessed October 8, 2021. https://osf.io/fr5sv
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eMethods
eFigure 1. Masking of Trial Medication
eFigure 2. Number of Enrolments per Site
eFigure 3. Number of Enrolments per Month
eFigure 4. Distributions of the Primary Outcome in the Post Hoc Analyses of the Primary Outcome Assigning Patients Who Had Died at Day 28 the Worst Possible Outcome (i.e., 0 Days Alive Without Life Support)
eFigure 5. Distributions of the Number of Days Alive Without Life Support at 90 days in the Two Intervention Groups
eFigure 6. Distributions of the Number of Days Alive and Out of Hospital at 90 days in the Two Intervention Groups
eTables 1a and 1b. Randomizations in Error and Wrongly Entered Stratification Variables
eTable 2. Trial Medication Administration and Protocol Violations
eTable 3. Discharge Against Medical Advice within 28 Days of Randomization
eTable 4. Results of the Analysis of the Primary Outcome in the Per Protocol Population
eTable 5. Best-Worst/Worst-Best Case Analyses of the Primary Outcome
eTable 6. Post Hoc Analyses of the Primary Outcome Assigning Patients Who had Died at Day 28 the Worst Possible Outcome (i.e., 0 Days Alive Without Life Support)
eTable 7. Post Hoc Analysis of the Primary Outcome Assessing Potential Clustering Effect Among Sites
eTable 8. Analyses of the Single Components of Composite Outcomes
eTable 9. Results of the Unadjusted Analyses of Secondary Outcomes
eTable 10. All Serious Adverse Reactions and Serious Adverse Events
eTable 11. Use of Extracorporeal Membrane Oxygenation within 28 Days of Randomization
eReferences
Nonauthor collaborators
Data sharing statement