This cohort study assesses the clinical activity and toxic effects of cabozantinib to treat brain metastases in patients with metastatic renal cell carcinoma.
Key Points
Question
What is the clinical activity and safety of cabozantinib for treatment of brain metastases in patients with renal cell carcinoma?
Findings
In this cohort study of 88 patients with renal cell carcinoma and brain metastases treated in 15 institutions across 4 countries, the intracranial response rate was 55% in the cohort of patients with progressing brain metastases without concomitant brain-directed local therapy and 47% in the cohort of patients with stable or progressing brain metastases concomitantly treated by brain-directed local therapy. No unexpected toxic effects or neurological adverse events were reported.
Meaning
These findings show considerable intracranial activity and an acceptable safety profile of cabozantinib in patients with renal cell carcinoma and brain metastases.
Abstract
Importance
Patients with brain metastases from renal cell carcinoma (RCC) have been underrepresented in clinical trials, and effective systemic therapy is lacking. Cabozantinib shows robust clinical activity in metastatic RCC, but its effect on brain metastases remains unclear.
Objective
To assess the clinical activity and toxic effects of cabozantinib to treat brain metastases in patients with metastatic RCC.
Design, Setting, and Participants
This retrospective cohort study included patients with metastatic RCC and brain metastases treated in 15 international institutions (US, Belgium, France, and Spain) between January 2014 and October 2020. Cohort A comprised patients with progressing brain metastases without concomitant brain-directed local therapy, and cohort B comprised patients with stable or progressing brain metastases concomitantly treated by brain-directed local therapy.
Exposures
Receipt of cabozantinib monotherapy at any line of treatment.
Main Outcomes and Measures
Intracranial radiological response rate by modified Response Evaluation Criteria in Solid Tumors, version 1.1, and toxic effects of cabozantinib.
Results
Of the 88 patients with brain metastases from RCC included in the study, 33 (38%) were in cohort A and 55 (62%) were in cohort B; the majority of patients were men (n = 69; 78%), and the median age at cabozantinib initiation was 61 years (range, 34-81 years). Median follow-up was 17 months (range, 2-74 months). The intracranial response rate was 55% (95% CI, 36%-73%) and 47% (95% CI, 33%-61%) in cohorts A and B, respectively. In cohort A, the extracranial response rate was 48% (95% CI, 31%-66%), median time to treatment failure was 8.9 months (95% CI, 5.9-12.3 months), and median overall survival was 15 months (95% CI, 9.0-30.0 months). In cohort B, the extracranial response rate was 38% (95% CI, 25%-52%), time to treatment failure was 9.7 months (95% CI, 6.0-13.2 months), and median overall survival was 16 months (95% CI, 12.0-21.9 months). Cabozantinib was well tolerated, with no unexpected toxic effects or neurological adverse events reported. No treatment-related deaths were observed.
Conclusions and Relevance
In this cohort study, cabozantinib showed considerable intracranial activity and an acceptable safety profile in patients with RCC and brain metastases. Support of prospective studies evaluating the efficacy of cabozantinib for brain metastases in patients with RCC is critical.
Introduction
Brain metastases occur in approximately 10% to 15% of patients with metastatic renal cell carcinoma (RCC)1,2,3,4 and are associated with considerable morbidity and mortality.5,6,7 Local therapies targeting brain metastases, such as stereotactic radiosurgery, whole-brain radiotherapy, and surgery, remain the gold standard of care for these patients.8,9 Pivotal clinical trials of targeted therapies have generally excluded patients with RCC and brain metastases owing to their poor performance status and the need for immediate local therapy. Most data regarding efficacy of these agents against brain metastases have been derived from retrospective studies, expanded access programs, and prospective single-arm studies. In a phase 2 trial of 16 patients with RCC who had untreated brain metastases, treatment with the vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI) sunitinib did not elicit any intracranial responses.10
Cabozantinib targets multiple tyrosine kinase receptors such as VEGFR2, MET, AXL, RET, and KIT, which are important mediators of tumor cell survival, metastasis, and tumor angiogenesis.11 Cabozantinib monotherapy is an established agent in advanced RCC based on the results of 2 prospective randomized clinical trials.12,13,14,15 Neither study allowed inclusion of patients with progressive brain metastases. The outcomes of cabozantinib in patients with RCC and brain metastases have been characterized in 3 single case reports and 1 small retrospective study of 12 patients, suggesting the potential for cabozantinib to cross the blood-brain barrier and treat brain metastases.16,17,18,19 Given the sparse literature and high clinical need, we sought to assess the activity and safety of cabozantinib in patients with brain metastases from RCC, leveraging an international multicenter collaboration.
Methods
Study Design and Patients
We conducted a multicenter, international, retrospective cohort study, including consecutive patients with brain metastases from RCC who were treated with cabozantinib between January 2014 and October 2020 at academic centers with dedicated RCC and neuro-oncology programs. Data were collected from patients treated in the US (9 centers [Dana Farber-Cancer Institute, Huntsman Cancer Institute, The Ohio State University Comprehensive Cancer Center, Duke Cancer Center, Beth Israel Deaconess Medical Center, University of Texas Southwestern Medical Center, University of Colorado Cancer Center, Holden Comprehensive Cancer Center, and Moores Cancer Center]), Belgium (3 centers [Jules Bordet Institute, Institut Roi Albert II, and Leuven Cancer Institute]), France (2 centers [Institut de cancérologie de Strasbourg Europe and Gustave Roussy]), and Spain (1 center [University Hospital 12 de Octubre]). This study received institutional review board approval from the Dana-Farber Cancer Institute and each participating center per their institutional guidelines. Only deidentified data were shared with the coordinating institution. Patients were required to have a histologically proven RCC metastatic to the brain before the initiation of cabozantinib and at least 1 follow-up imaging evaluation of the brain. No other restrictive inclusion criteria were applied. To specifically assess the antitumor activity of cabozantinib on progressing brain metastases, patients were classified in 2 cohorts. Cohort A included patients with progressing brain metastases at cabozantinib initiation and without concomitant brain-directed local therapy (defined as stereotactic radiosurgery, whole-brain radiotherapy, and surgery) within the past 2 months. Radiological intracranial progressive disease at baseline was defined using investigator-assessed modified Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1), principles.20 Cohort B included patients with stable brain metastases at cabozantinib initiation or patients with progressing brain metastases concomitantly treated by brain-directed local therapy. Concomitant brain-directed local therapy was defined as any brain-directed local therapy within 2 months prior to cabozantinib initiation. Thus, patients included in cohort A could have had prior brain-directed local therapy if it occurred longer than 2 months before cabozantinib initiation and if they had evidence of brain metastases progression in between.
Procedure and Radiologic Measurements
Demographic, clinical, pathologic, radiographic, laboratory, treatment, and outcomes data were extracted by investigators from electronic medical records at each institution. Standardized data collection templates were used to minimize interobserver variation. Radiological responses were assessed locally and categorized using modified RECIST v1.1 that allowed evaluation of target lesions 5 mm or larger for intracranial responses and RECIST v1.1 for extracranial responses.20,21 The RECIST v1.1 evaluation by a radiologist was preferred, but if not available, the site investigator performed the measurements. For patients at the Dana-Farber Cancer Institute, radiological responses were reassessed using modified RECIST v1.1 by a dedicated radiologist (K.M.K.) and using Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) criteria to evaluate concordance rate with investigator assessment.22 Clinical and radiological evaluations were assessed regularly according to the standard of care of each participating center. Treatment tolerability was assessed by each investigator using Common Terminology Criteria for Adverse Events (version 4.0 or higher). Toxic effects leading to dose reductions, dose delays, and reasons for cabozantinib discontinuation were collected.
Outcomes
Intracranial and extracranial objective response rates (ORRs) were defined as the sum of complete responses (CRs) and partial responses (PRs) as the best radiological response. Time to treatment failure (TTF) was defined as time (months) from cabozantinib initiation to treatment discontinuation for any reason, including intracranial or extracranial progressive disease, toxic effect, patient preference, or death. Time to brain progression was calculated as time from cabozantinib initiation to the brain progression, whereas off treatment owing to other reason without brain progression was considered as a competing risk. Overall survival (OS) was assessed from cabozantinib initiation and based on death from any cause. Patients were followed up from initiation of cabozantinib until death or December 31, 2020, whichever occurred first.
Statistical Analysis
No formal sample size or power calculations were done a priori owing to the retrospective nature of the study. Descriptive statistics are presented for baseline characteristics at initial diagnosis and cabozantinib initiation separately, with respect to the 2 cohorts at baseline. Intracranial and extracranial response rates are presented with exact binomial 95% CIs. Distributions of TTF and OS have been estimated using the Kaplan-Meier method. Competing risk models estimated cumulative incidences of brain progression at 6 and 12 months. Analyses were completed in SAS, version 9.4 (SAS Institute). All statistical tests were 2-sided.
Results
Baseline Patient Characteristics
A total of 88 patients treated with cabozantinib for advanced RCC with brain metastases across 15 institutions were included. Of these patients, 33 (38%) had radiological evidence of intracranial progression without concomitant brain-directed local therapy at cabozantinib initiation and were included in cohort A, whereas 55 patients (62%) were included in cohort B. In cohort A, median interval from prior brain-directed therapy to cabozantinib initiation was 5 months. Among patients included in cohort B, 35 of 55 (64%) had radiological evidence of intracranial progression with concomitant treatment of brain-directed local therapy (26 undergoing stereotactic radiosurgery, 7 whole-brain radiotherapy, and 5 neurosurgery [patients could have had more than 1 type of therapy]), and 20 of 55 (36%) had stable intracranial disease at cabozantinib initiation.
Overall, the majority of patients were men (n = 69 [78%]) and had a performance status of 0 or 1 (n = 64 [73%]) (Table 1). Median age at cabozantinib initiation was 61 years (range, 34-81 years). International Metastatic RCC Database Consortium risk groups at cabozantinib initiation included favorable (n = 11 [13%]), intermediate (n = 44 [50%]), and poor (n = 32 [36%]). Cabozantinib was mainly administered as second-line treatment (n = 24 [27%]) or third line or beyond (n = 60 [68%]). Previous treatments mainly were VEGFR TKIs (n = 68 [77%]) and single-agent anti–programmed cell death 1 (PD-1) therapy (n = 48 [55%]) (eTable 1 in the Supplement). The median follow-up time after cabozantinib initiation was 17 months (range, 2-74 months). Median time from diagnosis of metastatic RCC to diagnosis of brain metastases was 18 months (range, 0-159 months). At the time of the analysis, 72 (82%) patients had discontinued cabozantinib, and 16 (18%) patients remained on therapy. Reasons for treatment discontinuation included disease progression (n = 50 [57%]), toxic effects (n = 10 [11%]), patient choice (n = 3 [4%]), or other reasons (n = 9 [10%]).
Table 1. Clinicodemographic and Brain Metastases Characteristics at Cabozantinib Initiation.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Cohort A (n = 33)a | Cohort B (n = 55)b | Overall (N = 88) | |
| Age at cabozantinib initiation, median (range), y | 64 (34-81) | 59 (41-80) | 61 (34-81) |
| Gender | |||
| Male | 26 (79) | 43 (78) | 69 (78) |
| Female | 7 (21) | 12 (22) | 19 (22) |
| Histology | |||
| ccRCC | 31 (94) | 52 (95) | 83 (94) |
| nccRCC | 2 (6) | 3 (5) | 5 (6) |
| Papillary | 1 (3) | 1 (2) | 2 (2) |
| Chromophobe | 0 | 1 (2) | 1 (1) |
| Mixed | 1 (3) | 1 (2) | 2 (2) |
| ECOG Performance Status | |||
| 0 | 9 (27) | 9 (16) | 18 (21) |
| 1 | 15 (46) | 31 (56) | 46 (52) |
| 2-3 | 9 (27) | 14 (26) | 23 (26) |
| Unknown | 0 | 1 (2) | 1 (1) |
| IMDC risk groupc | |||
| Favorable | 7 (21) | 4 (7) | 11 (13) |
| Intermediate | 14 (43) | 30 (55) | 44 (50) |
| Poor | 12 (36) | 20 (36) | 32 (36) |
| Unknown | 0 | 1 (2) | 1 (1) |
| No. of prior systemic therapies | |||
| 0 | 1 (3) | 3 (5) | 4 (5) |
| 1 | 8 (24) | 16 (29) | 24 (27) |
| ≥2 | 24 (73) | 36 (66) | 60 (68) |
| Sites of extracranial metastasesd | |||
| Lymph node | 24 (73) | 38 (69) | 62 (70) |
| Lung | 29 (88) | 51 (93) | 80 (91) |
| Bone | 20 (61) | 28 (51) | 48 (55) |
| Liver | 13 (39) | 15 (27) | 28 (32) |
| Other | 14 (42) | 26 (47) | 40 (46) |
| No. of brain metastases | |||
| 1 | 8 (24) | 12 (22) | 20 (23) |
| 2 | 7 (21) | 18 (33) | 25 (28) |
| 3 | 4 (12) | 9 (16) | 13 (15) |
| ≥4 | 14 (43) | 16 (29) | 30 (34) |
| Size of largest brain lesion, median (range), mm | 8 (3-32) | 14 (2-66) | 12 (2-66) |
| Prior brain-directed therapye | |||
| Neurosurgery | 5 (15) | 15 (27) | 20 (23) |
| WBRT | 8 (24) | 11 (20) | 19 (22) |
| Stereotactic radiosurgery | 18 (55) | 42 (76) | 60 (68) |
Abbreviations: ccRCC, clear cell renal cell carcinoma; ECOG, Eastern Cooperative Oncology Group; IMDC, International Metastatic RCC Database Consortium; nccRCC, non–clear cell renal cell carcinoma; WBRT, whole-brain radiotherapy.
Evidence of radiological intracranial progressive disease at baseline without concomitant brain-directed local therapy.
Patients with stable brain metastases at cabozantinib initiation or patients with progressing brain metastases concomitantly treated by brain-directed local therapy. Concomitant brain-directed local therapy was defined as any brain-directed local therapy within 2 months prior to cabozantinib initiation.
Risk groups at the beginning of cabozantinib treatment.
Patients could have more than 1 metastatic site.
Patients could have had more than 1 type of therapy.
Of the 33 patients in cohort A, intracranial disease consisted of a single lesion in 8 (24%) patients (Table 1). The median number of lesions was 3 (range, 1-27), and the median size of the largest brain lesion was 8 mm (range, 3-32 mm). In cohort B, the median size of largest brain lesion was 14 mm (range, 2-66 mm), and the median number of lesions was 2 (range, 1-10).
Intracranial Activity
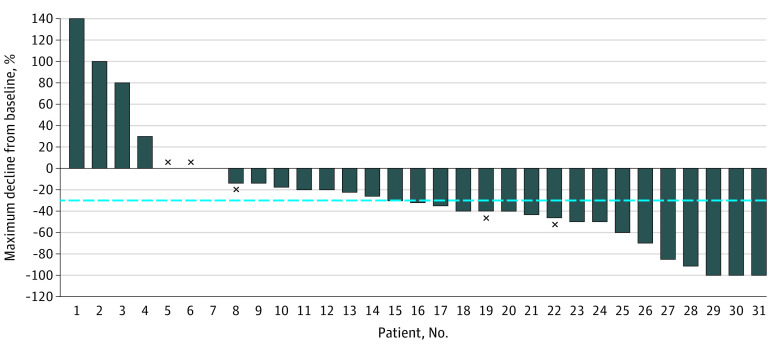
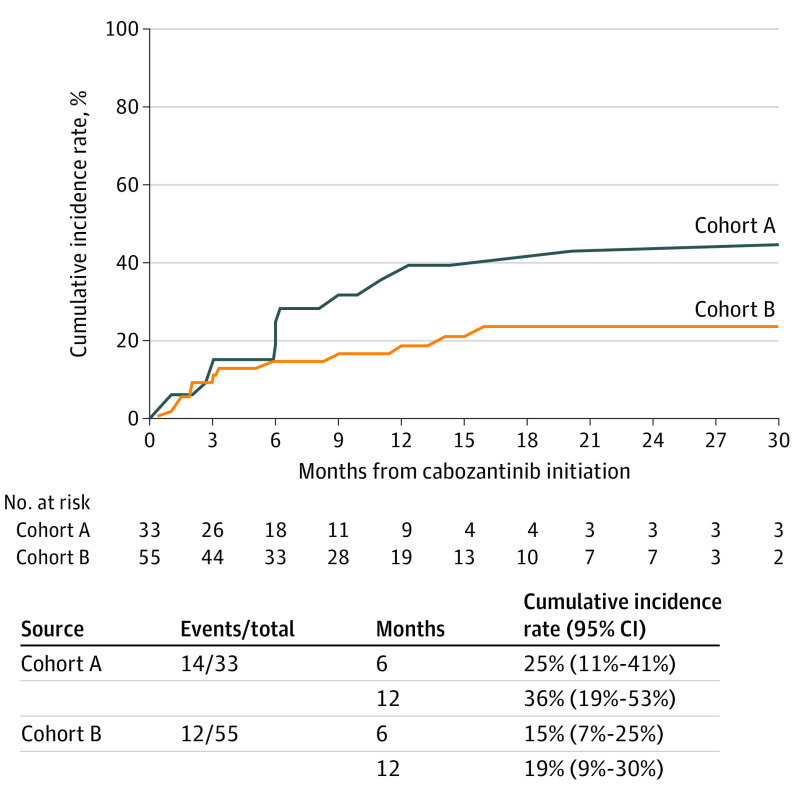
In cohort A, the objective intracranial response rate was 55% (95% CI, 36%-73%), including 3 CRs and 14 PRs (Table 2 and eFigure in the Supplement). Stable disease was the best response for 10 patients, whereas 4 patients did not experience any clinical benefit and experienced primary progressive intracranial disease. Two patients were not evaluable to assess intracranial treatment response owing to lesions smaller than 5 mm. The 3 patients who experienced an intracranial CR with cabozantinib had clear cell RCC: 2 patients had solitary brain lesions (16 mm and 7 mm, respectively), and 1 patient had 2 brain lesions. All received cabozantinib as third-line treatment or later and had received both prior immune checkpoint inhibitor (ICI) and anti-VEGF(R) therapy. Two patients had received prior stereotactic radiosurgery to the intracranial lesion but had documented subsequent progression prior to cabozantinib initiation. Among the 3 patients with CR, 2 experienced PR as best extracranial response. One of these 3 patients experienced brain progression on cabozantinib but only after approximately 3 years of treatment. Figure 1 depicts the best percentage of intracranial tumor decline from baseline in cohort A. Intracranial progression at 6 months was 25% (95% CI, 11%-41%) (Figure 2). In cohort A, subgroup analysis highlighted numerically higher intracranial responses in favorable or intermediate International Metastatic RCC Database Consortium risk groups (eTable 2 in the Supplement).
Table 2. Antitumor Activity of Cabozantinib.
| Measurement | Cohort A (n = 33)a | Cohort B (n = 55)b |
|---|---|---|
| Best intracranial response, No. (%)c | ||
| Complete response | 3 (10) | 1 (2) |
| Partial response | 14 (45) | 24 (45) |
| Stable disease | 10 (32) | 22 (42) |
| Progressive disease | 4 (13) | 6 (11) |
| Response rate, % (95% CI) | ||
| Intracranial | 55 (36-73) | 47 (33-61) |
| Extracranial | 48 (31-66) | 38 (25-52) |
| Outcome, % (95% CI), mo | ||
| TTF | 8.9 (5.9-12.3) | 9.7 (6.0-13.2) |
| OS | 15.0 (9.0-30.0) | 16.0 (12.0-21.9) |
Abbreviations: OS, overall survival; TTF, time to treatment failure.
Evidence of radiological intracranial progressive disease at baseline (prior brain-directed local therapy was allowed if radiological confirmation of intracranial progression was demonstrated before starting cabozantinib).
Patients with stable brain metastases at cabozantinib initiation or patients with progressing brain metastases concomitantly treated by brain-directed local therapy. Concomitant brain-directed local therapy was defined as any brain-directed local therapy within 2 months prior to cabozantinib initiation.
A total of 84 patients were included (31 in cohort A and 53 in cohort B) for intracranial response analysis, excluding 4 patients whose intracranial treatment response was not evaluable owing to lesion size less than 5 mm.
Figure 1. Waterfall Plot of the Best Percentage of Intracranial Tumor Decline From Baseline in Cohort A.
× Indicates that the patient was still on therapy at study completion.
Figure 2. Cumulative Incidence of Brain Progression From Competing Risk Model by Cohort.
In cohort B, the objective intracranial response rate was 47% (95% CI, 33%-61%), including 1 CR and 24 PRs. Primary progressive intracranial disease was reported as best response in 6 patients, of whom all had received prior brain-directed local therapy. Two patients were not evaluable for intracranial response assessment owing to lesion size smaller than 5 mm. Patients treated with concomitant radiation demonstrated an ORR of 54% (19 of 35 patients). Intracranial progression at 6 months in cohort B was 15% (95% CI, 7%-25%) (Figure 2).
A group of clinical interest is patients with leptomeningeal disease. In this small subset (n = 9), cabozantinib intracranial (parenchyma and leptomeningeal disease) and extracranial response rate was 56% and 44%, respectively, and median OS was 12 months.
For patients at the Dana-Farber Cancer Institute (n = 10), concordance rate for intracranial activity between radiologist and site investigator was 90%. In addition, the concordance between RANO-BM criteria and modified RECIST v1.1 evaluation was 90%.
Extracranial Activity and Overall Survival Outcomes
In cohort A, the extracranial response rate was 48% (95% CI, 31%-66%) with no CRs and 16 PRs. Of the 17 patients with intracranial response, 11 patients had extracranial response, and 6 had extracranial stable disease. Median TTF was 8.9 months (95% CI, 5.9-12.3 months) (Figure 3). Median OS was 15 months (95% CI, 9.0-30.0 months), and 21 patients died (Table 2 and Figure 3).
Figure 3. Time to Treatment Failure (TTF) and Overall Survival (OS) in the Study Population.
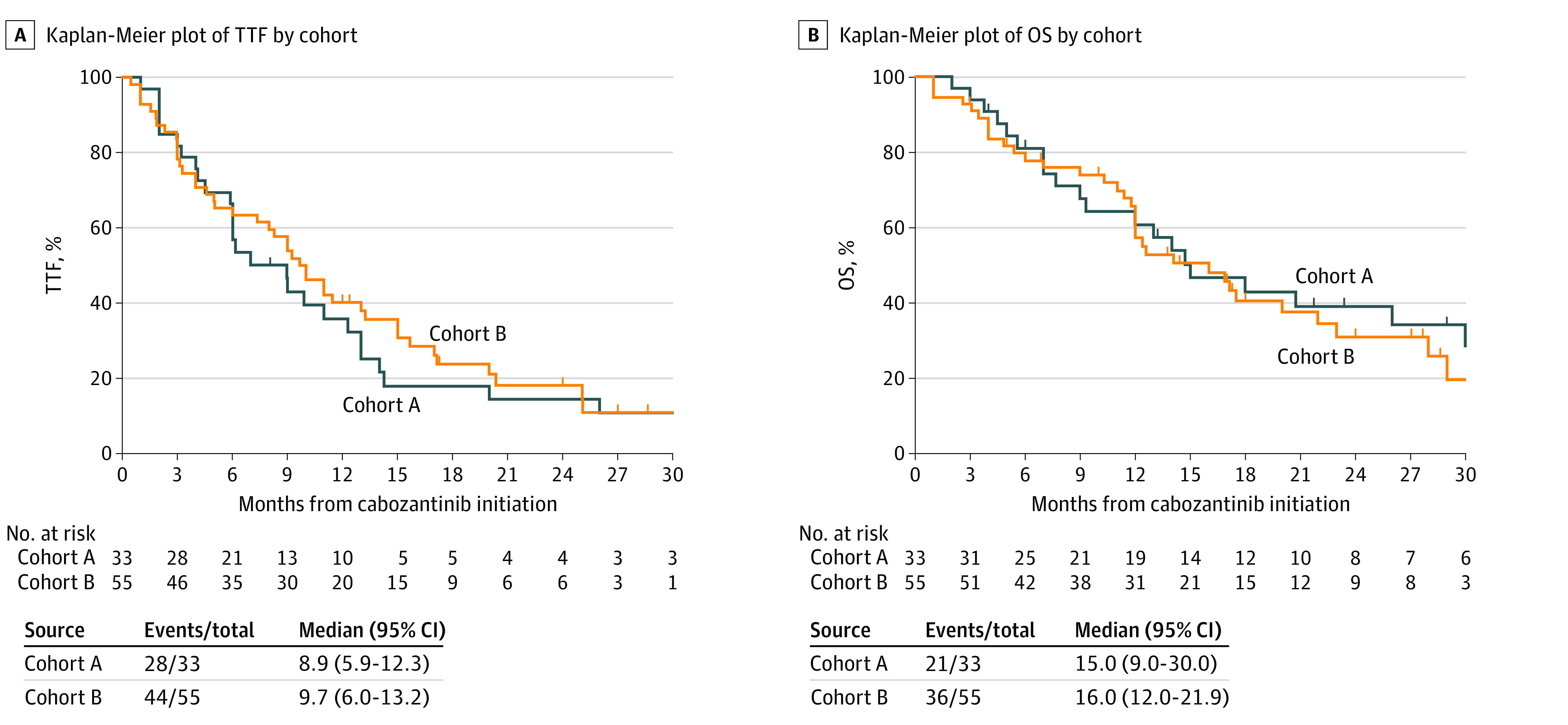
In cohort B, the extracranial response rate was 38% (95% CI, 25%-52%). Of the 25 patients with intracranial response, 14 patients experienced extracranial responses, 8 had extracranial stable disease, and 3 had extracranial progressive disease as the best response. Median TTF was 9.7 months (95% CI, 6.0-13.2 months) (Figure 3). Median OS was 16.0 months (95% CI, 12.0-21.9 months) with 36 deaths (Table 2 and Figure 3). In both cohorts, there were 12 patients (14%) who had intracranial progression and died within 3 months.
Safety Analysis
The most common treatment-related adverse events of any grade recorded in more than 30% of patients were fatigue (n = 68 [77%]), diarrhea (n = 40 [46%]), palmar-plantar erythrodysesthesia (n = 28 [32%]), and nausea (n = 27 [31%]) (eTable 3 in the Supplement). Grade 3 or 4 treatment-related adverse events were reported in 15 patients, the most frequent being fatigue (n = 6 [7%]) and mucositis (n = 4 [5%]). No deaths related to cabozantinib were observed. Forty-eight patients required dose reductions, with 10 permanent discontinuations owing to toxic effects. No neurological toxic effects (eg, seizure, brain hemorrhage, stroke) were reported in the 2 cohorts, including patients who underwent concomitant brain-directed local therapy and cabozantinib.
Discussion
Leveraging the collective experience of 15 institutions, we characterized the intracranial and extracranial activity and safety profile of cabozantinib in patients with brain metastases from RCC in the largest cohort reported to our knowledge. Cabozantinib demonstrated high intracranial activity with a considerable response rate (55%) in heavily pretreated patients who had progressing intracranial disease without concomitant brain-directed local therapy. Across the 2 cohorts of patients with brain metastases from RCC, cabozantinib demonstrated a safety profile consistent with previous pivotal studies12,13 in a healthier population of patients. Importantly, no related neurologic adverse events were recorded during cabozantinib treatment.
Cabozantinib is an established effective agent for advanced RCC based on the results of 2 randomized trials.12,13 Neither study included patients with progressing intracranial disease, while very few patients with stable, adequately treated brain metastases were enrolled. While limited in size and controls, the published literature includes only 3 case reports suggesting antitumor activity and acceptable tolerability of cabozantinib in patients with RCC and brain metastases.17,18,19 One study retrospectively analyzed 12 patients with brain metastases who received cabozantinib; however, presence or absence of radiological intracranial progression at time of cabozantinib initiation was not reported. Of these patients, only 4 with no prior brain-directed local therapy were evaluable for intracranial response and 2 had PR observed.16 In contrast, sunitinib, a standard-of-care TKI therapy for RCC, has not shown substantial efficacy against brain metastases. In a phase 2 trial of sunitinib in 16 patients with RCC and untreated brain metastases, no intracranial responses were reported and only 5 patients had a disease stabilization as best intracranial response, with a short progression-free survival of 2.3 months and a dismal median OS of 6.3 months.10 To our knowledge, there is no data on intracranial efficacy with use of other TKIs such as axitinib, pazopanib, or sorafenib in this subgroup of patients. Based on pivotal trials showing considerable improvements in ORR and OS, ICIs against PD-1 ligand 1 and CTLA-4 are now broadly used in advanced RCC. In the present study, 55% and 23% of the patients received single-agent anti–PD-1 and dual ICI, respectively. Prior immunotherapy may have influenced the observed response rate.23 Recently, a phase 2 study evaluated the intracranial activity of the anti–PD-1 nivolumab in patients with brain metastases from RCC and reported limited intracranial activity with immunotherapy.20 Only 4 of 39 (12%) patients with previously untreated brain metastases experienced intracranial response, all with brain metastases smaller than 10 mm. Recently, cabozantinib in combination with nivolumab has been approved for RCC based on CheckMate 9ER.24 The antitumor activity and safety of the antiangiogenic-ICI combinations require further evaluation in dedicated trials of patients with brain metastases.
Cabozantinib’s observed activity against brain metastases could be related to its inhibition of c-MET. In gliomas, an elevated expression of MET is well characterized and occurs in upward of 35% of primary tumors and 75% of recurrent glioblastomas.25 There can be substantial MET expression in brain metastases in patients with RCC.25 Analysis of whole-brain lysates of nontumor-bearing mice showed 20% of peak plasma levels, suggesting the ability of cabozantinib to cross the blood-brain barrier.26 Cabozantinib is not known to be a substrate of P-glycoprotein, which would inhibit brain penetration.27
Limitations
Limitations of this study include the retrospective nature of the analyses and the lack of central radiological review on all cases, which might have affected tumor response assessment. Nevertheless, in patients at the Dana-Farber Cancer Institute, the concordance rate between radiologist and site investigator was more than 90%. Use of VEGF inhibitors such as cabozantinib may decrease enhancement but not size of the lesion on computed tomography.28 Therefore, we assessed intracranial progression using the modified RECIST v1.1, which is based on the size of the lesion and not its enhancement. In addition, the very low rate of intracranial progression in cohort A at the 6-month time point can be considered a good indicator of cabozantinib activity. We did not apply the RANO-BM criteria to the entire population, although they are increasingly being used and recommended for assessment of brain metastases, especially when evaluating response to VEGF inhibitors.22 In patients at the Dana-Farber Cancer Institute, the concordance between RANO-BM criteria and modified RECIST v1.1 evaluation was 90%. There was missing information regarding concomitant use of systemic corticosteroids with cabozantinib, which could influence the neurological and radiological assessment. We considered time to brain progression and TTF rather than progression-free survival as the primary end points to reflect real-world clinical practice.
Conclusions
Results of this cohort study showed that as patients with RCC live longer given an increasing number of effective treatment options, as well as improved clinician awareness as to the incidence, brain metastases from RCC is an important problem in clinical practice, and patients with brain metastases are underrepresented in clinical trials.29 In the absence of consensus guidelines and prospective data, this international retrospective experience provides evidence that cabozantinib generally can be administered safely and is active in this relatively large population with poor prognosis, as reflected in the poor OS despite cabozantinib intracranial activity. Further investigations are needed to confirm the present findings and to extend evaluation to more symptomatic or aggressive cases not included in this study. Support of studies such as the ongoing French phase 2 CABRAMET study,30 which is evaluating prospectively the efficacy of cabozantinib on brain metastases in patients with RCC, is critical.
eTable 1. Prior systemic therapies to cabozantinib
eTable 2. Antitumor activity of cabozantinib according to baseline characteristics
eTable 3. Overall incidence of adverse events considered related to cabozantinib
eFigure. Gadolinium enhanced brain magnetic resonance imaging of one patient in cohort A (no history of brain radiotherapy). Left cerebellum metastasis measured 7 mm at baseline, 4 mm at 12 week follow-up representing partial response
References
- 1.Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23(4):973-980. doi: 10.1093/annonc/mdr362 [DOI] [PubMed] [Google Scholar]
- 2.Wyler L, Napoli CU, Ingold B, et al. Brain metastasis in renal cancer patients: metastatic pattern, tumour-associated macrophages and chemokine/chemoreceptor expression. Br J Cancer. 2014;110(3):686-694. doi: 10.1038/bjc.2013.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun M, De Velasco G, Brastianos PK, et al. The development of brain metastases in patients with renal cell carcinoma: epidemiologic trends, survival, and clinical risk factors using a population-based cohort. Eur Urol Focus. 2019;5(3):474-481. doi: 10.1016/j.euf.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 4.Suarez-Sarmiento A Jr, Nguyen KA, Syed JS, et al. Brain metastasis from renal-cell carcinoma: an institutional study. Clin Genitourin Cancer. 2019;17(6):e1163-e1170. doi: 10.1016/j.clgc.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 5.Shuch B, La Rochelle JC, Klatte T, et al. Brain metastasis from renal cell carcinoma: presentation, recurrence, and survival. Cancer. 2008;113(7):1641-1648. doi: 10.1002/cncr.23769 [DOI] [PubMed] [Google Scholar]
- 6.Guida A, Albiges L, Derosa L, et al. Prognosis of brain metastasis (BM) in metastatic renal cell carcinoma (mRCC): experience from Gustave Roussy (IGR). J Clin Oncol. 2016;34(suppl 15):4561. doi: 10.1200/JCO.2016.34.15_suppl.4561 [DOI] [Google Scholar]
- 7.Sperduto PW, Deegan BJ, Li J, et al. Estimating survival for renal cell carcinoma patients with brain metastases: an update of the Renal Graded Prognostic Assessment tool. Neuro Oncol. 2018;20(12):1652-1660. doi: 10.1093/neuonc/noy099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patil CG, Pricola K, Sarmiento JM, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst Rev. 2017;9:CD006121. doi: 10.1002/14651858.CD006121.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer E, Pasquier D, Bernadou G, et al. Stereotactic radiation therapy in the strategy of treatment of metastatic renal cell carcinoma: a study of the Getug group. Eur J Cancer. 2018;98:38-47. doi: 10.1016/j.ejca.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 10.Chevreau C, Ravaud A, Escudier B, et al. ; French Group on Renal Cancer . A phase II trial of sunitinib in patients with renal cell cancer and untreated brain metastases. Clin Genitourin Cancer. 2014;12(1):50-54. doi: 10.1016/j.clgc.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 11.Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298-2308. doi: 10.1158/1535-7163.MCT-11-0264 [DOI] [PubMed] [Google Scholar]
- 12.Choueiri TK, Escudier B, Powles T, et al. ; METEOR investigators . Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917-927. doi: 10.1016/S1470-2045(16)30107-3 [DOI] [PubMed] [Google Scholar]
- 13.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35(6):591-597. doi: 10.1200/JCO.2016.70.7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pal SK, Tangen C, Thompson IM Jr, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet. 2021;397(10275):695-703. doi: 10.1016/S0140-6736(21)00152-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez Chanzá N, Xie W, Asim Bilen M, et al. Cabozantinib in advanced non-clear-cell renal cell carcinoma: a multicentre, retrospective, cohort study. Lancet Oncol. 2019;20(4):581-590. doi: 10.1016/S1470-2045(18)30907-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peverelli G, Raimondi A, Ratta R, et al. Cabozantinib in renal cell carcinoma with brain metastases: safety and efficacy in a real-world population. Clin Genitourin Cancer. 2019;17(4):291-298. doi: 10.1016/j.clgc.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 17.Uche A, Sila C, Tanoura T, et al. Brain complete response to cabozantinib prior to radiation therapy in metastatic renal cell carcinoma. Case Rep Urol. 2019;2019:6769017. doi: 10.1155/2019/6769017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciccarese C, Iacovelli R, Mosillo C, Tortora G. Exceptional response to cabozantinib of rapidly evolving brain metastases of renal cell carcinoma: a case report and review of the literature. Clin Genitourin Cancer. 2018;16(5):e1069-e1071. doi: 10.1016/j.clgc.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 19.Négrier S, Moriceau G, Attignon V, et al. Activity of cabozantinib in radioresistant brain metastases from renal cell carcinoma: two case reports. J Med Case Rep. 2018;12(1):351. doi: 10.1186/s13256-018-1875-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flippot R, Dalban C, Laguerre B, et al. Safety and efficacy of nivolumab in brain metastases from renal cell carcinoma: results of the GETUG-AFU 26 NIVOREN multicenter phase II study. J Clin Oncol. 2019;37(23):2008-2016. doi: 10.1200/JCO.18.02218 [DOI] [PubMed] [Google Scholar]
- 21.Qian JM, Mahajan A, Yu JB, et al. Comparing available criteria for measuring brain metastasis response to immunotherapy. J Neurooncol. 2017;132(3):479-485. doi: 10.1007/s11060-017-2398-8 [DOI] [PubMed] [Google Scholar]
- 22.Lin NU, Lee EQ, Aoyama H, et al. ; Response Assessment in Neuro-Oncology (RANO) group . Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270-e278. doi: 10.1016/S1470-2045(15)70057-4 [DOI] [PubMed] [Google Scholar]
- 23.McGregor BA, Lalani AA, Xie W, et al. Activity of cabozantinib after immune checkpoint blockade in metastatic clear-cell renal cell carcinoma. Eur J Cancer. 2020;135:203-210. doi: 10.1016/j.ejca.2020.05.009 [DOI] [PubMed] [Google Scholar]
- 24.Choueiri TK, Powles T, Burotto M, et al. ; CheckMate 9ER Investigators . Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi: 10.1056/NEJMoa2026982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derosa L, Le Teuff G, Khordahi M, et al. Inter and intra-tumor heterogeneity of PD-L1 and MET expression in metastatic renal cell carcinoma (mRCC). J Clin Oncol. 2017;35(suppl 15):4569. doi: 10.1200/JCO.2017.35.15_suppl.4569 [DOI] [Google Scholar]
- 26.Zhang Y, Guessous F, Kofman A, Schiff D, Abounader R. XL-184, a MET, VEGFR-2 and RET kinase inhibitor for the treatment of thyroid cancer, glioblastoma multiforme and NSCLC. IDrugs. 2010;13(2):112-121. [PMC free article] [PubMed] [Google Scholar]
- 27.Heffron TP. Small molecule kinase inhibitors for the treatment of brain cancer. J Med Chem. 2016;59(22):10030-10066. doi: 10.1021/acs.jmedchem.6b00618 [DOI] [PubMed] [Google Scholar]
- 28.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963-1972. doi: 10.1200/JCO.2009.26.3541 [DOI] [PubMed] [Google Scholar]
- 29.Kotecha RR, Flippot R, Nortman T, et al. Prognosis of incidental brain metastases in patients with advanced renal cell carcinoma. J Natl Compr Canc Netw. 2021;19(4):432-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evaluation of cabozantinib in metastatic renal cell carcinoma (mRCC) with brain metastases (CABRAMET). ClinicalTrials.gov identifier: NCT03967522. Updated February 8, 2021. Accessed September 21, 2021. https://clinicaltrials.gov/ct2/show/NCT03967522?term=NCT03967522&draw=2&rank=1
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Prior systemic therapies to cabozantinib
eTable 2. Antitumor activity of cabozantinib according to baseline characteristics
eTable 3. Overall incidence of adverse events considered related to cabozantinib
eFigure. Gadolinium enhanced brain magnetic resonance imaging of one patient in cohort A (no history of brain radiotherapy). Left cerebellum metastasis measured 7 mm at baseline, 4 mm at 12 week follow-up representing partial response