Abstract
BACKGROUND & AIMS:
Constipation is commonly associated with diabetes. Serotonin (5-HT), produced predominantly by enterochromaffin (EC) cells via tryptophan hydroxylase 1 (TPH1), is a key modulator of gastrointestinal (GI) motility. However, the role of serotonergic signaling in constipation associated with diabetes is unknown.
METHODS:
We generated EC cell reporter Tph1-tdTom, EC cell depleted Tph1-DTA, combined Tph1-tdTom-DTA, and interstitial cell of Cajal (ICC)-specific Kit-GCaMP6 mice. Male mice and surgically ovariectomized female mice were fed a high-fat high-sucrose diet to induce diabetes. The effect of serotonergic signaling on GI motility was studied by examining 5-HT receptor expression in the colon and in vivo GI transit, colonic migrating motor complexes (CMMCs), and calcium imaging in mice treated with either a 5-HT2B receptor (HTR2B) antagonist or agonist.
RESULTS:
Colonic transit was delayed in males with diabetes, although colonic Tph1+ cell density and 5-HT levels were increased. Colonic transit was not further reduced in diabetic mice by EC cell depletion. The HTR2B protein, predominantly expressed by colonic ICCs, was markedly decreased in the colonic muscles of males and ovariectomized females with diabetes. Ca2+ activity in colonic ICCs was decreased in diabetic males. Treatment with an HTR2B antagonist impaired CMMCs and colonic motility in healthy males, while treatment with an HTR2B agonist improved CMMCs and colonic motility in males with diabetes. Colonic transit in ovariectomized females with diabetes was also improved significantly by the HTR2B agonist.
CONCLUSION:
Impaired colonic motility in mice with diabetes was improved by enhancing HTR2B signaling. The HTR2B agonist may provide therapeutic benefits for constipation associated with diabetes.
Keywords: Diabetes, Constipation, Serotonin, 5-HT2B, ICCs
Type 2 diabetes mellitus (T2DM) is an increasingly prevalent medical condition affecting approximately 27 million adults in the US.1 Moreover, it is estimated that up to 7 million diabetic individuals remain undiagnosed and that nearly 90 million US adults are prediabetic.1 As the number of individuals affected by T2DM continues to grow, understanding the pathophysiology of the disease process and related symptoms remains essential to enable exploration of novel options to improve the medical management of patients’ conditions. In addition to the severe complications of vascular compromise, nephropathy, neuropathy, infection, and blindness,2 an estimated 50% of T2DM patients suffer from some type of gastrointestinal (GI) motility disorder, ranging from esophageal dysmotility to gastroparesis and constipation.1,2 The relationship between T2DM and GI dysfunction, especially constipation, has been widely observed;3 however this relationship is complex and not well understood.
Serotonin (5-hydroxytryptamine, 5-HT) is a monoamine that acts as a hormone and neurotransmitter.4,5 The largest amount of 5-HT in the body (~95%) is synthesized in enterochromaffin (EC) cells within the GI mucosal layer via the rate-limiting enzyme tryptophan hydroxylase 1 (TPH1).4,5 On a much smaller scale, 5-HT is synthesized in the central nervous system (CNS) and enteric nervous system (ENS) via another isoform of this rate-limiting enzyme, TPH2.5,6 The role of 5-HT in regulating GI motility has been extensively examined but is still under debate.7,8,9,10
5-HT is believed to regulate GI motility through its receptors in enteric neurons and GI smooth muscle cells. There are seven families (5-HT1 to 5-HT7) and multiple subtypes of 5-HT receptors.11 Six of the seven families are G-protein-coupled receptors while the 5-HT3 receptor is a ligand-gated ion channel.11 5-HT induces inhibitory (via 5-HT1A) or excitatory (via 5-HT3 and 5-HT4) neurotransmission.12,13 5-HT also affects smooth muscle contraction (via 5-HT2A) or relaxation (via 5-HT4 and 5-HT7).13 However, the role of 5-HT and its receptors on the function of interstitial cells of Cajal (ICCs) is not well studied.
ICCs are pacemaker cells that generate slow waves (continuous rhythmic electrical oscillations) in the GI tract.14 Ca2+ transients in ICCs initiate active propagation of slow waves via spontaneous transient depolarizations (STDs) that travel to neighboring ICCs or smooth muscle cells through gap junctions to coordinate smooth muscle contraction.14,15 Thus, impaired functioning of ICCs is associated with GI dysmotility symptoms, such as constipation and gastroparesis.16,17 5-HT induces the proliferation of ICCs through the 5-HT2B receptor (HTR2B).18,19 However, it is unknown how T2DM impacts HTR2B signaling and therefore influences Ca2+ transients in ICCs.
In this study, we used multiple mouse lines: the EC cell reporter Tph1-tdTom, EC cell depleted Tph1-DTA, combined Tph1-tdTom-DTA, and ICC-specific Kit-GCaMP6 mice. The mice were fed a high-fat high-sucrose diet (HFSHD) to induce diabetes and changes in serotonergic signaling were examined. We found that colonic motility is improved by the activation of HTR2B signaling in mice with diabetes.
Methods
Note: The full Methods section is included in the Supplementary Material.
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Nevada, Reno.
In Vivo Functional GI Motility Tests
To measure total GI transit time, overnight fasted mice were orally gavaged with 0.1 mL of Evans blue semi-solid solution and monitored every 10 minutes until the first blue fecal pellet appeared.20 To measure colonic transit time, a 3 mm glass bead was inserted into the colon (3 cm from the anus) of overnight fasted mice.20 To measure effects of agonist or antagonist treatment, overnight fasted and isolated mice were treated with either the HTR2B agonist (BW723C86, Sigma, 1 μg/40 g body weight) or HTR2B antagonist (LY266097, Tocris, 5 μg/40 g body weight), which were diluted with saline and delivered to the mice via IP injection. The glass bead was inserted, and the colonic transit time was measured on the same day before and 15 minutes after IP injection. To compare the effects of long-term agonist treatment to saline treatment on colonic transit time, saline or the HTR2B agonist diluted with saline (BW 723C86, Sigma, 0.5 μg/40 g body weight) was delivered to the mice via IP injection daily for seven days. Colonic transit time was measured on the eighth day.
Tension Recording of Colonic Migrating Motor Complexes (CMMCs)
Colonic muscle contractions were measured as previously described.10 On-going tension was recorded with Acqknowledge 3.2.6 (Biopac Systems). 0.05 μM of HTR2B agonist (BW723C86, Tocris) and 0.05 μM HTR2B antagonist (LY 266097, Tocris) were used for drug treatments. Each drug was dissolved in Krebs buffer and continuously perfused into colonic tissue using a peristaltic pump (model MINIPULS 3; GILSON Inc). Acqknowledge software was used for the frequency and amplitude measurements of the colonic motor complex (CMC) for a 30-minute period and the percent propagation was measured as [(the frequency of distal colon CMC for 30 minutes/the frequency of proximal colon CMC for 30 minutes) X 100].
Data Analysis
Data are expressed as mean ± standard error of the mean (SEM). GraphPad Prism, version 8.0 (GraphPad Software) was used for data visualization.
Results
Colonic 5-HT is Increased in Male Mice with Diabetes
The phenotypic characteristics of mice fed a ND and HFHSD are shown in Figure 1. The body weight of male and female C57BL/6J mice fed a HFHSD for four months was significantly greater than the body weight of male and female mice fed a ND (Figure 1A). However, only the males fed the HFHSD became hyperglycemic (≥170 mg/dL fasting blood glucose level), while females fed the HFHSD did not (Figure 1B). This was further confirmed by glucose intolerance and insulin resistance in HFHSD-fed male mice when compared to ND-fed male mice (Figure 1C and D). Additionally, female mice did not develop glucose intolerance or insulin resistance after being fed a HFHSD. These data indicate that a HFHSD induced obesity in both male and female mice, but only induced diabetes in the male mice.
Figure 1.
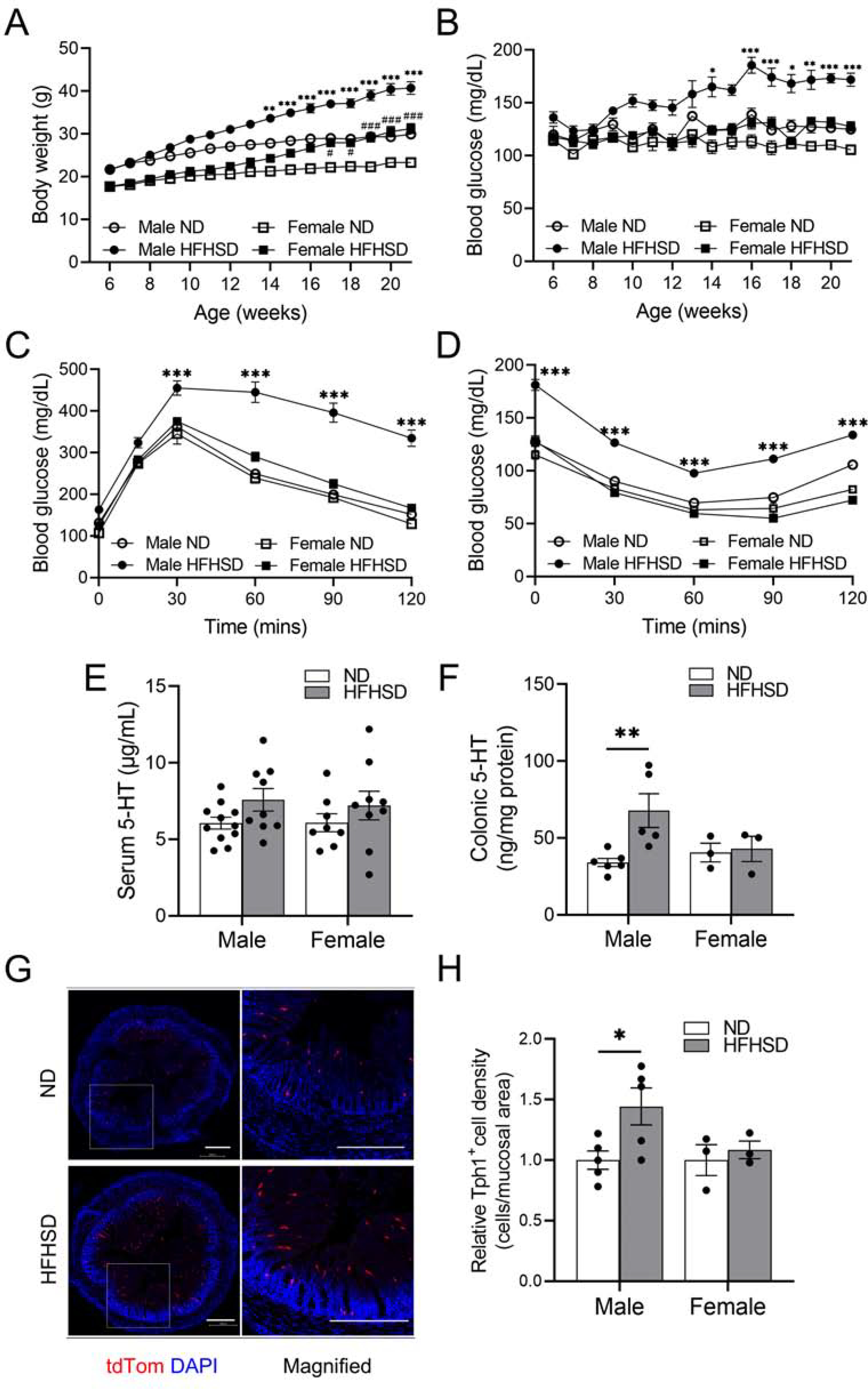
Colonic 5-HT is increased in male mice with diabetes. (A) Body weight and (B) fasting blood glucose levels of male and female C57BL/6J mice fed a ND or HFHSD measured for four months (n = 6 to 13 mice). (C) Glucose tolerance tests and (D) insulin tolerance tests of male and female C57BL/6J mice fed a ND or HFHSD (n = 7 to 10 mice). (E) Serum 5-HT (n = 8 to 11 mice) and (F) colonic tissue 5-HT levels (n = 3 to 6 mice) of male and female C57BL/6J mice fed a ND or HFHSD. (G) Representative colonic tissue histology from tamoxifen injected Tph1-tdTom mice (scale bars = 300 μm). (H) Colonic Tph1+ cell density of male and female Tph1-tdTom mice fed a ND or HFHSD (n = 3 to 5 mice). (A, B, C, D) 3-way ANOVA, Tukey’s multiple comparisons test, and (E, F, H) 2-way ANOVA, Sidak’s multiple comparisons test were used. *P < 0.05, **P < 0.01, ***P < 0.001 vs ND male mice; #P < 0.05, ###P <0.001 vs ND female mice.
To investigate if 5-HT levels are changed in the HFHSD-fed mice, we measured 5-HT levels in the sera and GI mucosal tissues using ELISA. We observed that although there was no statistically significant difference in serum and duodenal 5-HT levels between ND- and HFHSD-fed male and female mice (Figure 1E; Supplementary Figure 1A), HFHSD-induced diabetic male mice had significantly increased 5-HT levels in the colon compared to ND-fed male mice (Figure 1F). To determine whether this increase reflected an increased number of 5-HT-secreting EC cells, we measured the density of these cells by using the tamoxifen-inducible Tph1-tdTom mice that we previously generated.56 Upon tamoxifen injection, EC cells in the small intestine and colon expressed tdTom robustly (Supplementary Figure 1B). Similar to C57BL/6J male mice, Tph1-tdTom (C57BL/6 background strain) male mice fed a HFHSD for four months had significantly increased body weight and developed diabetes as defined by impaired glucose tolerance and reduced insulin sensitivity compared to ND-fed male mice. Tph1-tdTom female mice fed a HFHSD for four months gained weight modestly and did not become diabetic (data not shown). Tph1-tdTom male, but not female, mice with HFHSD-induced diabetes had increased Tph1+ cell density in the colon (Figure 1G and H). These data suggest that the elevated levels of colonic 5-HT result from an increased density of Tph1+ cells in the HFHSD-induced diabetic male mice.
Ex Vivo and In Vivo Colonic Motility Are Impaired in Diabetic Male Mice
In order to investigate the effects of a HFHSD on GI motility, we measured GI motility in both ex vivo preparations as well as in in vivo models (Figure 2). In ex vivo preparations of isolated colon, we analyzed colonic migrating motor complexes (CMMCs), which are rhythmic propagating contractions thought to underlie peristalsis in the colon of many mammals.21,22 In HFHSD-induced diabetic male mice, the mean contractile frequency was reduced in the distal colon (Figure 2A and B). Moreover, the percentage of CMMCs that propagated to more distal colonic segments was lower in HFHSD-induced diabetic male mice than in ND-fed male mice (Figure 2C). However, the contraction amplitude in the proximal colon of HFHSD-fed male mice was higher than that of ND-fed male mice (Figure 2D). The use of CMMCs, which are neurogenic in nature, to examine the effects of manipulations to cells other than neuronal cells may have limitations, as many of the spatiotemporal features of CMMCs are largely dictated by the ENS. To overcome these limitations and study GI motility without GI damage, we examined motility in vivo by measuring total GI transit and colonic transit times in mice fed a ND or HFHSD. We observed that HFHSD-induced diabetic male mice had significantly delayed total GI and colonic transit times compared to ND-fed male mice (Figure 2E and F). In contrast, we did not observe significant differences in GI motility between ND- and HFHSD-fed female mice in either ex vivo preparations (Supplementary Figure 2A–D) or in vivo models (Figure 2E and F). Taken together, these studies suggest that HFHSD-induced diabetic male mice exhibit impaired GI motility.
Figure 2.
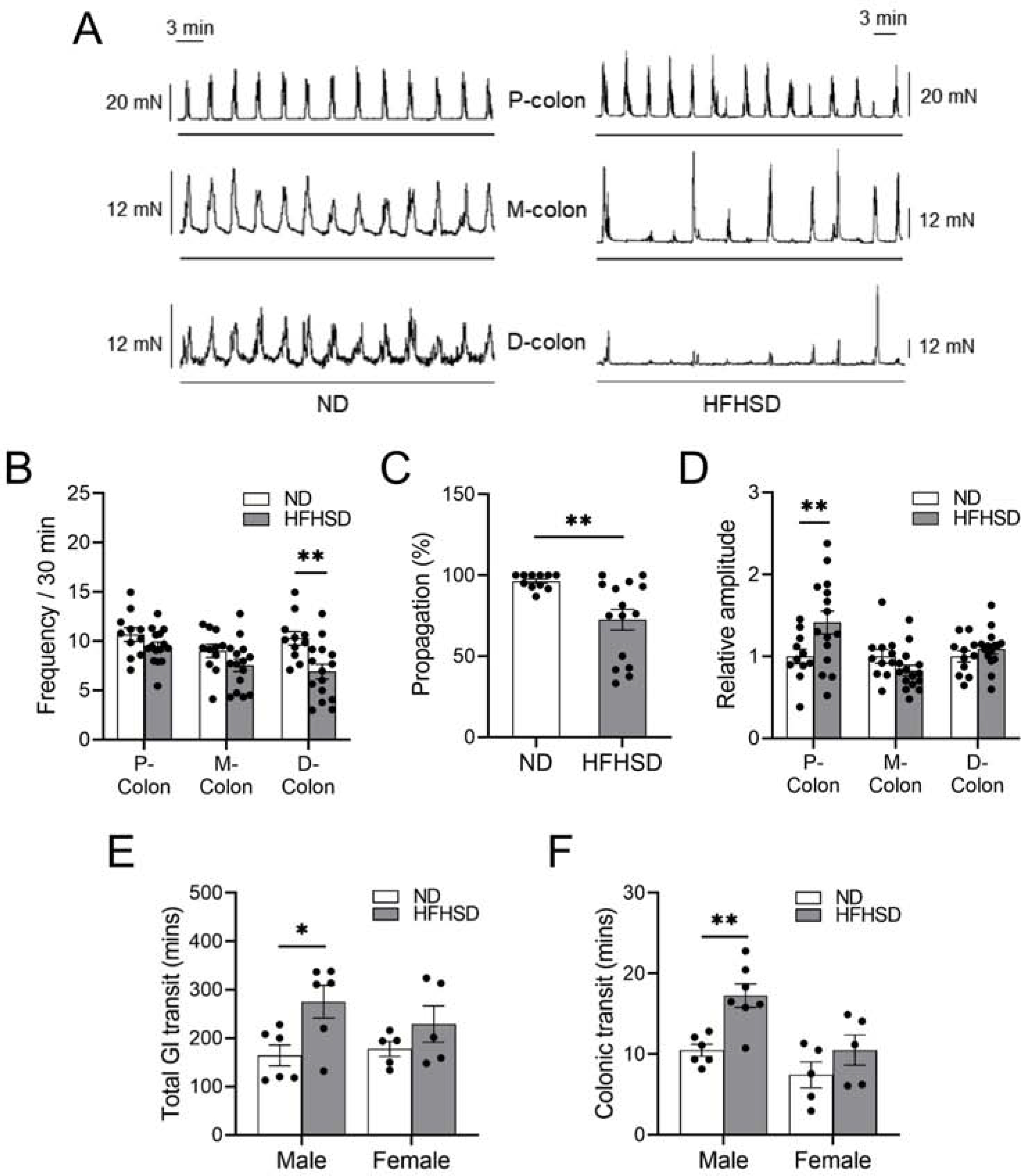
Ex vivo and in vivo colonic motility are impaired in diabetic mice. (A) Representative CMMCs and (B) colonic motor complex (CMC) frequencies in each colonic region (P- proximal, M- mid, D- distal), (C) percent propagation and (D) comparison of the relative amplitudes in each colonic region between ND- and HFHSD-fed C57BL/6J male mice (n = 11 to 15 mice). (E) Total GI transit time (n = 5 to 6 mice), (F) colonic transit time (n = 5 to 7 mice) of male and female C57BL/6J mice fed a ND or HFHSD. (B, D, E, F) 2-way ANOVA, Sidak’s multiple comparisons test, and (C) Student’s unpaired t-test were used. *P < 0.05, **P < 0.01
Depletion of EC Cell-Derived 5-HT Alters GI Motility in Healthy Mice, but not in Diabetic Mice
To examine whether mucosal 5-HT plays a critical role in GI motility, we used tamoxifen-inducible Tph1-DTA mice.56 Following tamoxifen injection, Tph1+ EC cells robustly expressed diphtheria toxin A (DTA), leading to death of EC cells by apoptosis.56 We found a significant reduction in serum 5-HT levels in Tph1-DTA mice fed a ND and a HFHSD on day 3 and 10 following five consecutive days of tamoxifen injections. Serum samples taken 15 and 20 days after the last tamoxifen injection showed gradual recovery of 5-HT levels (Figure 3A and B; Supplementary Figure 3A). Similar to the reduction in serum 5-HT levels, tamoxifen injected Tph1-DTA mice displayed decreased colonic 5-HT levels 3 days after the last tamoxifen injection (Supplementary Figure 3B). We also confirmed that there was a significant reduction of Tph1+ cell density in tamoxifen injected Tph1-tdTom-DTA mice 3 days after the last tamoxifen injection compared to tamoxifen injected Tph1-tdTom mice (Figure 3C and D).
Figure 3.
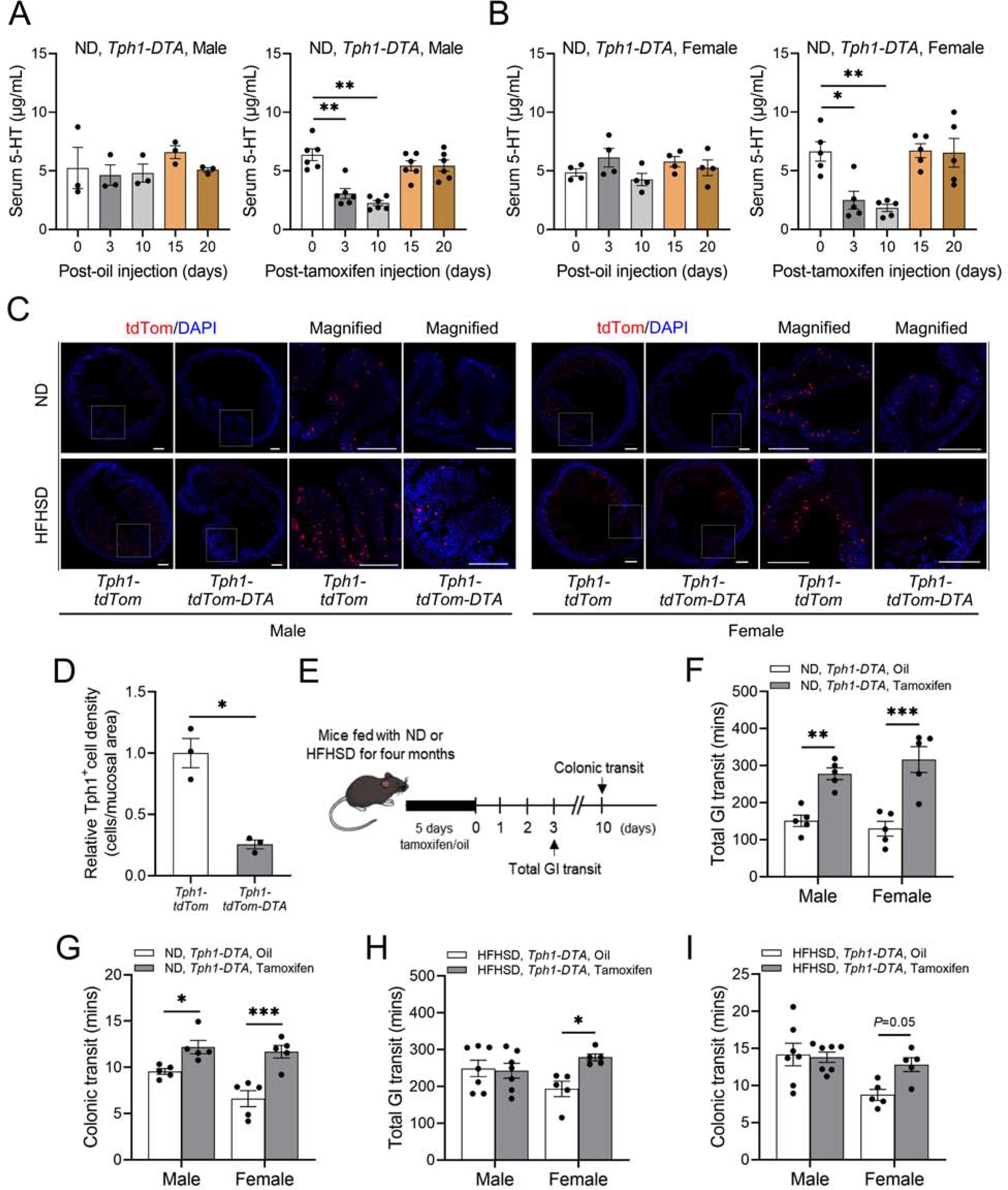
Depletion of EC cell-derived 5-HT decreases GI motility in healthy mice, but not in diabetic mice. (A, B) Serum 5-HT levels in oil injected (n = 3 to 4 mice) and tamoxifen injected Tph1-DTA mice fed a ND (n = 5 to 6 mice). (C) Representative colonic tdTom+ cells (red) in Tph1-tdTom-DTA mice fed a ND or a HFHSD (Scale bars = 300 μm). (D) Relative Tph1+ cell density comparison between ND-fed Tph1-tdTom and Tph1-tdTom-DTA male mice (n = 3 mice). (E) Experimental design of the tamoxifen or oil injections and GI functional tests in Tph1-DTA mice. (F) Total GI transit time and (G) colonic transit time of Tph1-DTA (oil or tamoxifen injected) mice fed a ND (n = 5 mice). (H) Total GI transit time and (I) colonic transit time of Tph1-DTA (oil or tamoxifen injected) mice fed a HFHSD (n = 5 to 7 mice). (A, B) 1-way ANOVA, Dunnett’s multiple comparisons test, (D) Student’s unpaired t-test, and (F, G, H, I) 2-way ANOVA, Sidak’s multiple comparisons test were used. *P < 0.05, **P < 0.01, ***P < 0.001
Next, we measured GI motility in vivo in Tph1-DTA mice fed a ND or a HFHSD. Total GI transit was measured 3 days after the last tamoxifen or oil injection, and colonic transit was measured 10 days after the last tamoxifen or oil injection (Figure 3E). Tamoxifen injected Tph1-DTA male and female mice fed a ND showed a significant delay of total GI transit and colonic transit times compared to Tph1-DTA mice that were fed a ND and injected with oil (Figure 3F and G). Interestingly, Tph1-DTA male mice fed a HFHSD and injected with tamoxifen showed no significant alterations in GI motility compared to Tph1-DTA male mice fed a HFHSD and injected with oil. In contrast, Tph1-DTA females fed a HFHSD and injected with tamoxifen had decreased motility in comparison to Tph1-DTA females fed a HFHSD and injected with oil (Figure 3H and I). These observations demonstrate that reduction in mucosal 5-HT decreased GI motility in non-diabetic mice, but this procedure did not further decrease motility in diabetic mice, which already exhibit impaired motility compared to normal mice. Instead, these studies, together with the elevated levels of 5-HT observed in diabetic male mice described above, suggest that GI motility in diabetic male mice may be reduced by an alternative serotonergic signaling mechanism.
Protein Expression of HTR2B is Significantly Decreased in Diabetic Mice
Our findings that the levels of 5-HT are elevated, yet GI motility is reduced, in male diabetic mice suggest that alterations of 5-HT levels per se do not affect motility in these mice. These results may indicate that 5-HT levels fail to regulate motility in any model or context. However, our finding that depletion of EC cells and the concomitant reduction of 5-HT levels lead to decreased motility in ND-fed mice argues against this idea. Alternatively, diabetic mice may fail to exhibit enhanced GI motility in the face of elevated 5-HT levels because their responsivity to 5-HT is impaired. In order to address this issue, we examined 5-HT receptors expressed in the colon of mice. ICCs, smooth muscle cells (SMCs), and enteric neurons play a central role in the regulation of intestinal motility.16,17 We previously reported a GI smooth muscle transcriptome browser to provide transcript expression profiles of SMCs, ICCs, and PDGFRα cells in the jejunum and colon.23 The transcriptome browser data showed high levels of Htr2b and Htr3a gene expression in colonic muscles (Figure 4A). Within colonic ICCs, Htr2b was the most highly expressed 5-HT receptor (Figure 4B). On the other hand, it was verified through the transcriptome browser that the gene expression of Htr2b was very low in colonic mucosa (Supplementary Figure 4A).
Figure 4.
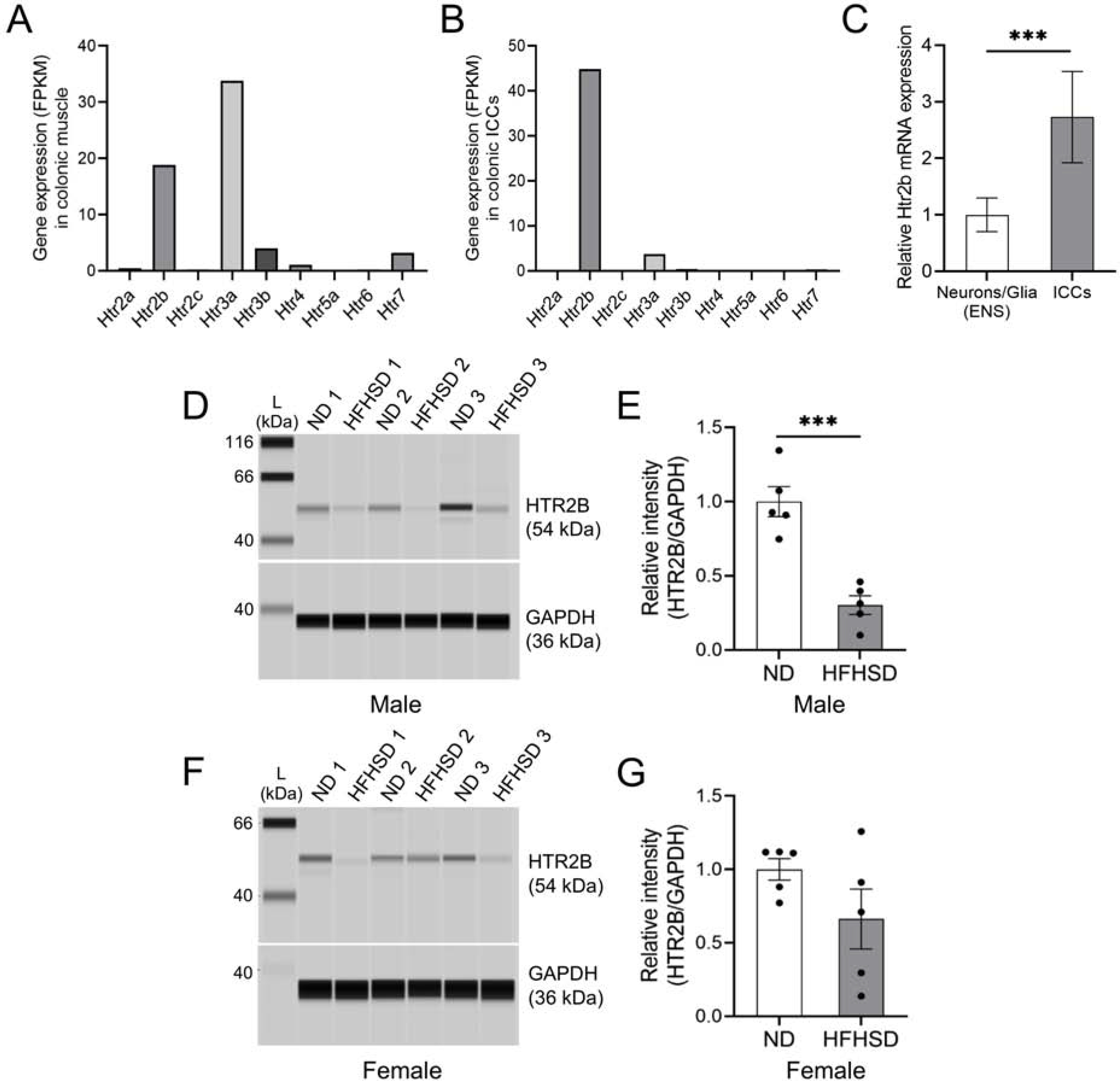
Protein expression of HTR2B is significantly decreased in diabetic mice. (A) mRNA expression levels of 5-HT receptor genes in the colonic muscles and (B) ICCs. (C) Comparison of Htr2b mRNA expression in sorted colonic cells from Sox10-tdTom and Kit-tdTom mice (n = 4 to 5 mice). (D) Comparison and (E) quantification of HTR2B protein in the colonic muscles in both ND- and HFHSD-fed male groups (n = 5 mice). (F) Comparison and (G) quantification of HTR2B protein in the colonic muscles in both ND- and HFHSD-fed female groups (n = 5 mice). (C, E, G) Student’s unpaired t-test was used. ***P < 0.001
To compare the Htr2b gene expression levels between ICCs and other cells in the ENS, we sorted fluorescent cells from the colon of KitCreERT2/+;Rosa26tdTom/+ (Kit-tdTom) mice, which drives expression of tdTom in ICCs, and Sox10Cre/+;Rosa26tdTom/+ (Sox10-tdTom) mice, which drives expression of tdTom in enteric neurons and glia. These mouse lines have been widely used for the study of ICCs and enteric neurons and glia within the ENS.20,24,25 We then examined Htr2b gene expression in each of these sorted cells by RT-qPCR. Frist, we confirmed that the expression of the Kit gene was low in cells in the ENS and that Sox10 expression was low in the Kit+ sorted cells (Supplementary Figure 4B and C). Second, we found that expression of the Htr2b gene was much higher in Kit+ cells than in neuronal and glial cells of the ENS (Figure 4C). These data demonstrate that the Htr2b gene is significantly more expressed in ICCs than enteric neurons and glia.
Because of the role of ICCs in motility, and the robust and selective expression of Htr2b expression within these cells, we decided to examine the expression levels of this receptor in diabetic mice as a potential mediator of impaired 5-HT responsivity. We measured HTR2B protein levels in ND- and HFHSD-fed male (Figure 4D and E) and female mice (Figure 4F and G). Protein assays showed that HTR2B levels were significantly decreased in colonic muscles, in which ICCs reside, of diabetic male mice (Figure 4E). HTR2B levels were also decreased in female mice, but the reduction was not statistically significant (Figure 4G). These results suggest that the reduction of HTR2B expression, presumably within ICCs, may underlie the failure of elevated 5-HT levels to maintain normal motility in diabetic male mice.
The Frequency of Ca2+ Transients in ICCs of Diabetic Mice is Reduced and Can Be Increased by Activation of HTR2B Signaling
To directly measure the functional activity of ICCs in the GI tract, we generated a Kit-GCaMP6 mouse (Figure 5A–D; Supplementary Figure 5A). GCaMP6f is a genetically encoded Ca2+ sensor used to image Ca2+ dynamics.26 In order to measure the activity of ICCs in diabetic mice, Kit-GcaMP6 male mice were fed a HFHSD for four months. These mice had increased body weight, glucose intolerance, and insulin resistance compared to ND-fed Kit-GCaMP6 male mice (Supplementary Figure 5B–D). ND-fed mice displayed a Ca2+ transient frequency of 0.71 ± 0.03 /sec, whereas the Ca2+ transient frequency was significantly reduced to 0.46 ± 0.04 /sec in diabetic Kit-GcaMP6 mice (Figure 5E; Supplementary Video 1 and 2). Moreover, we found that pharmacological modulation of HTR2B receptor activity affects the frequency of Ca2+ transients in ICCs: in ND-fed Kit-GCaMP6 male mice, the HTR2B antagonist (LY266097) decreased the Ca2+ transient frequency (Figure 5F; Supplementary Video 3), whereas in HFHSD-fed Kit-GCaMP6 male mice, the HTR2B agonist (BW723C86) increased the Ca2+ transient frequency (Figure 5G; Supplementary Video 4). Taken together, the data in Figures 4 and 5 show that the reduction of HTR2B expression in HFHSD-induced diabetic mice is accompanied by a decrease of Ca2+ signaling in colonic ICCs. This impairment of ICC activity can be improved by treatment with an HTR2B agonist.
Figure 5.
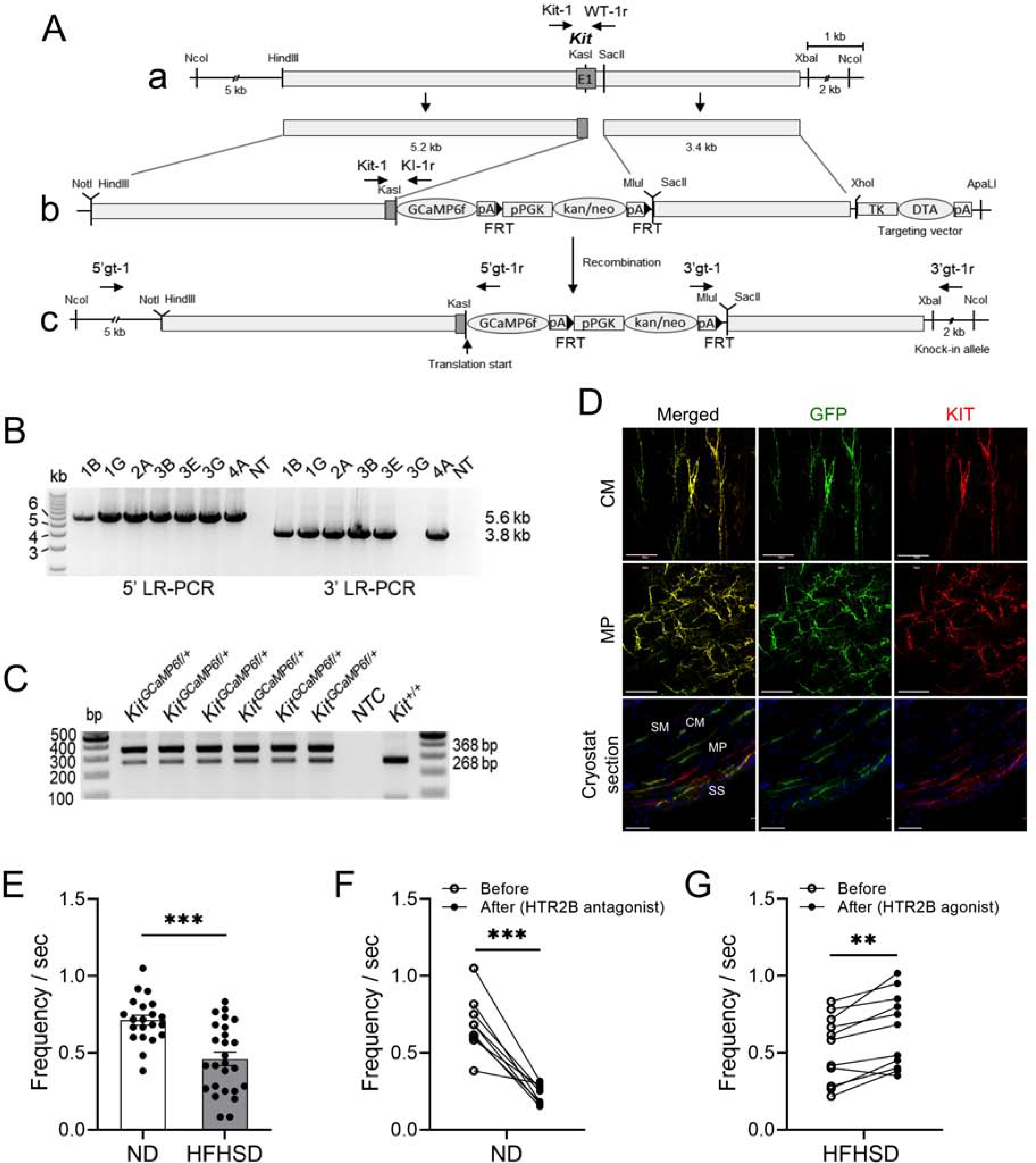
Generation of the Kit-GCaMP6 mouse line to elucidate the activity of ICCs in diabetic mice. (A) Schematic genomic map of the Kit-GCaMP6 mouse line. (B) Long-range (LR) PCR performed with 5’ primers (5’gt-1/5’gt-1r: 5.6 kb) and 3’ primers (3’gt-1/3’gt-1r: 3.8 kb), indicated in c, in ES cells to identify targeted colonies. (C) Genotyping PCR performed with primers (Kit-1, WT-1r, and KI-1r: 368 bp and 268 bp) in the pups of Kit-GCaMP6 mice. (D) Kit-GCaMP6 wholemount immunohistochemistry within the circular (CM), myenteric (MP) layers of the colon and distribution of ICCs in cryostat cross sections in the colonic muscles (SM: submucosa, CM: circular muscle, MP: myenteric plexus and SS: subserosa) from Kit-GCaMP6 mice (Scale bars = 50 μm). (E) Comparison of Ca2+ transient frequency between ND-fed and HFHSD-fed Kit-GCaMP6 mice, (F) Ca2+ transient frequency in ND-fed Kit-GCaMP6 mice before and after LY266097 (50 μM) treatment and (G) Ca2+ transient frequency in HFHSD-fed Kit-GCaMP6 mice before and after BW723C86 (20 μM) treatment (each dot represents a region of ICCs of interest, n= 3 to 4 male mice). (E) Student’s unpaired t-test, and (F, G) paired t-test were used. **P < 0.01, ***P < 0.001
The HTR2B Antagonist Impairs CMMCs and Colonic Motility in ND-Fed Mice and the HTR2B Agonist Improves CMMCs and Colonic Motility in HFHSD-Induced Diabetic Mice
To determine whether the acute perturbation of HTR2B signaling in ND-fed male mice affects motility, CMMCs in the intact colon from these mice were assessed before and after bath application of LY266097 (Figure 6A–D). LY266097 treatment decreased CMMC frequencies in all regions of the colon of ND-fed male mice (Figure 6B). LY266097 treatment selectively reduced the amplitude of CMMCs in the proximal colon (Figure 6D) and exerted no change in CMMC propagation (Figure 6C). In contrast, bath application of BW723C86 failed to affect the frequency, propagation and amplitude of CMMCs in ND-fed mice (Supplementary Figure 6A–D).
Figure 6.
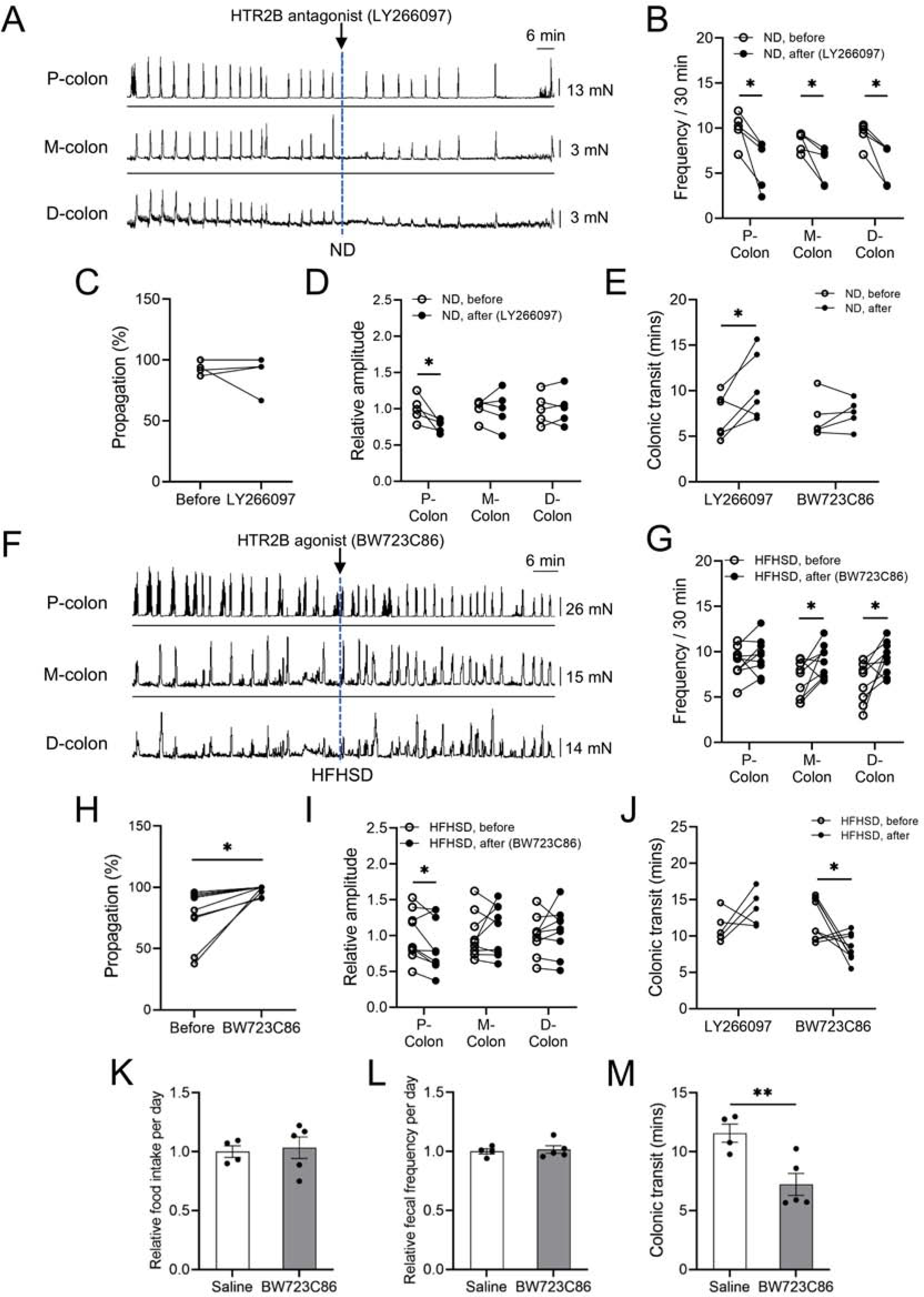
Treatment with the HTR2B antagonist impairs CMMCs and colonic motility in ND-fed mice, while treatment with the HTR2B agonist improves CMMCs and colonic motility in HFHSD-induced diabetic mice. (A) Representative CMMCs; LY266097 was added as indicated by the dotted line. (B) CMC frequencies in each region of the colon, (C) percent propagation and (D) relative amplitude in each colonic region before and after LY266097 treatment in ND-fed C57BL/6J male mice (n = 5 mice). (E) Colonic transit time of ND-fed C57BL/6J male mice injected with LY266097 (5 μg/40 g body weight) or BW723C86 (1 μg/40 g body weight) (n = 5 to 6 mice). (F) Representative CMMCs; BW723C86 was added as indicated by the dotted line. (G) CMC frequencies in each region of the colon, (H) percent propagation (I) relative amplitude in each colonic region from the HFHSD-fed C57BL/6J male mice before and after BW723C86 treatment (n = 9 mice). (J) Colonic transit time from the HFHSD-fed C57BL/6J male mice injected with LY266097 (5 μg/40 g body weight) or BW723C86 (1 μg/40 g body weight) (n = 5 to 9 mice). (K) Comparison of relative food intake per day and (L) relative fecal frequency per day between the two groups during a 7-day period of injections with BW723C86 (0.5 μg/40 g body weight) or saline in the HFHSD-fed mice. (M) Comparison of in vivo colonic transit time 7 days after injection of BW723C86 (0.5 μg/40 g body weight) or saline. (B, C, D, E, G, H, I, J) paired t-test, and (K, L, M) Student’s unpaired t-test were used. *P < 0.05, **P < 0.01
Next, to investigate whether the manipulation of HTR2B signaling in HFHSD-fed male mice, which exhibit elevated 5-HT levels and reduced HTR2B expression, CMMCs in the intact colon from these mice were assessed before and after bath application of BW723C86 (Figure 6F–I). This compound increased CMMC frequencies in the middle and distal colon (Figure 6G) and increased CMMC propagation (Figure 6H). We also noted a 14% decrease in amplitude of CMMCs in the proximal colon (Figure 6I). Conversely, LY266097 treatment of colonic tissue from HFHSD-induced diabetic male mice led to decreased CMMC frequencies in all regions of the colon and a 20.7% decrease in the proximal colonic CMMC amplitude (Supplementary Figure 6E–H).
To determine how LY266097 and BW723C86 treatment affects colonic motility in vivo in ND-fed and HFHSD-fed mice, we delivered LY266097 or BW723C86 to each group of mice through IP injection. Following one injection of LY266097, ND-fed mice, but not HFHSD-fed mice, had significantly increased colonic transit time (Figure 6E and J). Conversely, after just one injection of BW723C86 HFHSD-fed mice, but not ND-fed mice, had significantly decreased colonic transit time (Figure 6E and J). To examine the possible undesirable effects of BW723C86, such as impaired appetite or enhanced defecation, we performed daily injections of the HTR2B agonist for one week in diabetic mice. There were no differences in daily food intake and fecal pellet frequency in HFHSD-fed male mice receiving one week of HTR2B agonist or vehicle (saline) IP injections (Figure 6K and L). However, in vivo colonic transit time was significantly decreased in diabetic mice injected with the HTR2B agonist for one week (Figure 6M), similar to results after one injection. These results suggest that impaired colonic transit in HFHSD-induced diabetic mice can be improved by activating HTR2B signaling.
Ovariectomized and HFHSD-Induced Diabetic Female Mice Develop Constipation, which is Restored by HTR2B Agonist Treatment
As previously observed, a HFHSD did not induce diabetes in female mice (Figure 1B–D). Female sex hormones protect against the development of diabetes in high-fat diet fed female mice.27,28,29 Therefore, we ovariectomized (OVX) 6 week-old female mice to rule out the possible protective effect of female sex hormones and to attempt to induce diabetes in these mice. Following OVX or sham surgery, female mice were fed a HFHSD for 4 months prior to measuring serum estrogen levels and performing glucose and insulin tolerance tests, HTR2B protein assays, and in vivo colonic transit tests (Figure 7A). Estrogen levels were significantly reduced in OVX female mice compared to control (sham surgery) female mice (Figure 7B). After feeding mice a HFHSD for 16 weeks following OVX or sham surgery, the body weight of OVX mice (37.9 ± 0.6 g) was significantly heavier than sham mice (30.2 ± 1.4 g) (Figure 7C). Moreover, OVX mice exhibited glucose intolerance and insulin resistance compared to sham mice, confirming OVX females were diabetic (Figure 7D and E). Of note, OVX mice with diabetes also had delayed colonic transit when compared to sham mice (Figure 7F). Additionally, OVX mice with diabetes displayed significantly decreased HTR2B protein expression in colonic muscles in comparison to sham mice (Figure 7G and H). The delayed colonic transit time and decreased HTR2B expression in colonic muscles are similar to the phenotypes we observed in HFHSD-induced diabetic male mice (Figure 2 and Figure 4). Following a single injection with the HTR2B agonist BW723C86, diabetic OVX mice displayed significantly improved colonic transit time (Figure 7I). These results suggest that without the protective effects of estrogen, female mice develop diabetes similar to male mice fed a HFHSD, and that treatment with an HTR2B agonist may improve colonic motility in postmenopausal females with constipation caused by diabetes.
Figure 7.
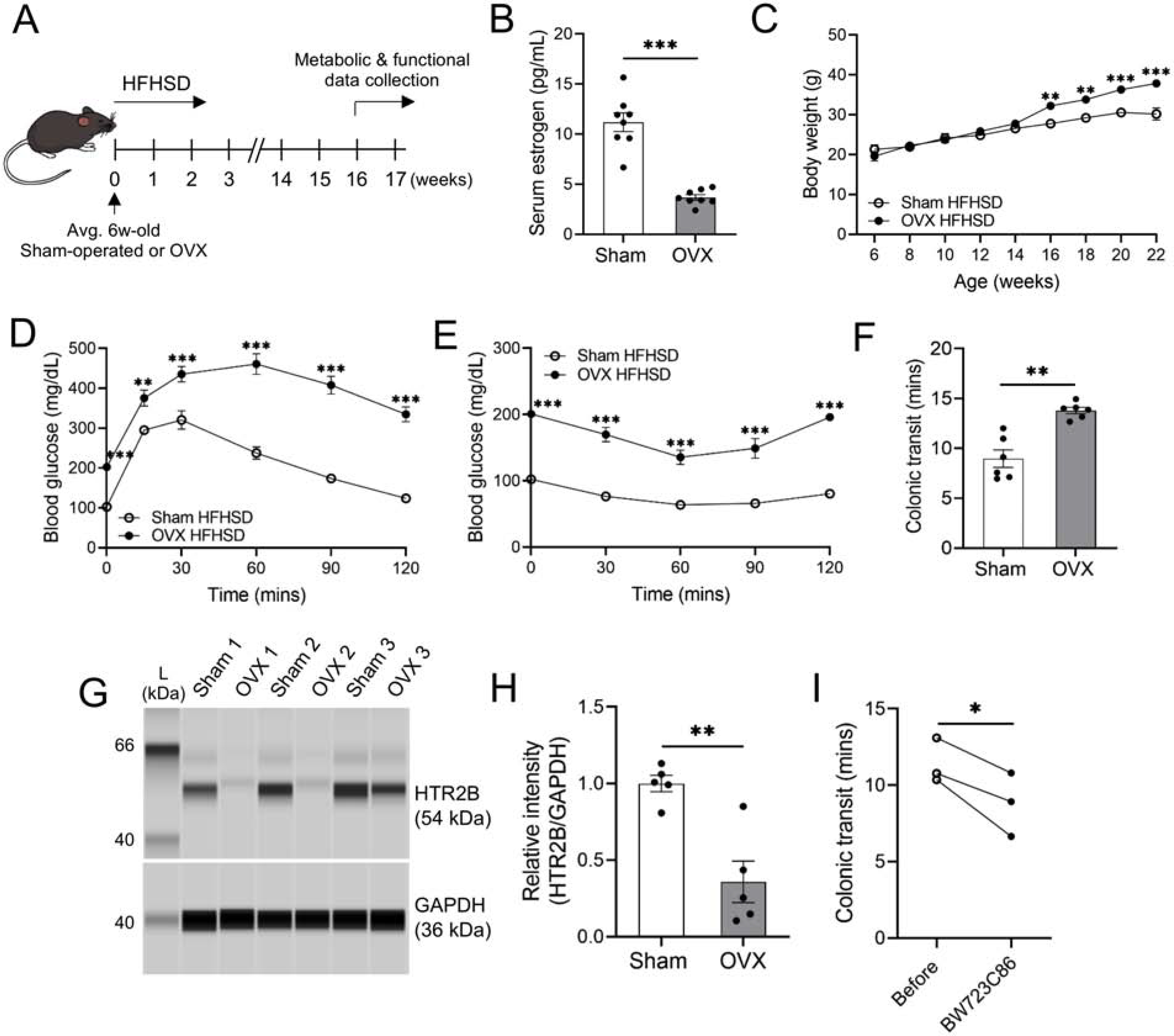
Ovariectomized and HFHSD-induced diabetic female mice develop constipation, which is restored by HTR2B agonist treatment. (A) Representative image of the sham or OVX experiments. (B) Comparison of serum estrogen levels between sham and OVX female mice fed a HFHSD (n = 8 mice). (C) Body weight (n = 5 mice), (D) glucose tolerance test (n = 6 mice) and (E) insulin tolerance test (n = 6 mice) in sham and OVX female mice fed a HFHSD. (F) Colonic transit time in sham and OVX female mice fed a HFHSD (n = 6 mice). (G) Expression of the HTR2B protein in the colonic muscles between the sham and OVX female mice fed a HFHSD. (H) Quantification of HTR2B (n = 5 mice). (I) Colonic transit time of HFHSD-fed OVX mice injected with BW723C86 (1 μg/40 g body weight) (n = 3 mice). (B, F, H) Student’s unpaired t-test, (C, D, E) 2-way ANOVA, Sidak’s multiple comparisons test, and (I) paired t-test were used. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
In this study, we showed that colonic motility was reduced in both male and female diabetic mice through the reduction of serotonergic HTR2B signaling. Moreover, colonic motility was impaired by the HTR2B antagonist LY266097 in healthy male mice and improved by the HTR2B agonist BW723C86 in diabetic male mice as well as diabetic ovariectomized female mice. These data therefore suggest that HTR2B may represent a therapeutic target for improving constipation seen in many diabetic patients.
Multiple cross-sectional studies observing patients with T2DM have documented the notable prevalence of GI dysmotility.30,31 The prevalence of constipation among patients with non-insulin dependent diabetes is reported between 15 to 30%, and is observed more frequently in patients with poor glycemic control and in those who were diagnosed more than 10 years ago.31 Despite the high prevalence, few options exist in a physician’s repertoire to treat constipation in patients with diabetes.3,32 Some treatments that do exist leverage serotonergic signaling, as serotonergic mechanisms have been shown to play an important role in regulating GI motility.10,33 Current pharmaceuticals developed to treat functional bowel disorders target subfamily receptors 5-HT1, 5-HT3, and 5-HT4; however, treatment options are limited and many patients fail to respond to the available agents.34 The physiological complexities of constipation coupled with the limited treatment options necessitate further elucidation of the physiological underpinnings of and potential treatment targets for constipation seen in patients with diabetes.
Despite the fact that most 5-HT in the body is produced by EC cells, the role of EC cell-derived 5-HT on GI motility is still unclear and being investigated. Contrasting results have been reported; some studies showing that 5-HT is not necessary for peristalsis8,9 and other studies indicating that EC cell-derived 5-HT plays a critical role.7,10 Moreover, even in experiments using the same congenital Tph1−/− mouse model, different groups have come to different conclusions with regard to the role of EC cell-derived 5-HT on GI motility.35,10 In our attempt to clarify the relationship between EC cell-derived 5-HT and GI motility using the inducible EC cell depleted Tph1-DTA mice that we generated previously,56 we found that ND-fed male and female mice developed delayed total GI transit and colonic transit when EC cells were conditionally depleted. This result is consistent with the view that EC cell-derived 5-HT is required for normal GI motility, although we cannot rule out the possibility that other EC cell-derived factors may contribute to the control of peristalsis. Interestingly, HFHSD-fed male mice did not exhibit a further delay in total GI transit and colonic transit time when EC cells were conditionally depleted (Figure 3). This observation led us to hypothesize that the cause of GI dysmotility in HFHSD-induced diabetic mice is dysregulation in serotonergic signaling pathways, the identification of which could provide a therapeutic approach to treat constipation in diabetic patients.
To identify the serotonergic signaling changes that affect colonic motility in diabetes, we focused on the 5-HT receptor protein expression in colonic muscles. From transcriptome data obtained from primary jejunal and colonic ICCs, we identified Htr2b as the most highly expressed serotonin receptor in ICCs.23 HTR2B signaling is important for ICC survival and proliferation.18,19 We found that the HTR2B protein was reduced significantly in the colonic muscles of HFHSD-induced diabetic male mice and diabetic ovariectomized female mice. Speculation as to the cause of this change in phenotype can be made based on previous studies: 1) increased colonic 5-HT in HFHSD-fed male mice might be attributable to intestinal inflammation commonly reported in mice fed a high-fat diet.36 In addition, males are more susceptible to intestinal inflammation than females under some conditions.37,38 Moreover, EC cell-derived 5-HT can affect the onset of intestinal inflammation39 and the number of EC cells increases in intestinal inflammation.40 Therefore, the high-fat diet, intestinal inflammation, and EC cell-derived 5-HT appear interconnected, an idea supported by the observation of increased gut 5-HT levels in mice fed a high-fat diet.41 Elevated 5-HT levels can lead to changes in the expression of 5-HT receptors;42,43 thus, decreased HTR2B in diabetic mice may be a phenotype linked to the increase in 5-HT; 2) microRNA (miRNA)-27a (miR-27a) may play a role in the reduction of HTR2B in ICCs of the colonic muscles in diabetic mice. We have published a comparison of miRNA expression in ICCs from diabetic and healthy mice using miRNA-sequencing. Based on these data, miR-27a expression is reduced in the ICCs of diabetic mice.20 Moreover, previous studies have shown that miR-27a directly targets runt related transcription factor 1 (RUNX1),44,45 which represses Htr2b gene transcription in uveal melanoma cells.46,47 The exact pathways regulating Htr2b gene expression in ICCs have yet to be unraveled and the mechanism of Htr2b gene regulation by miRNAs needs to be further studied. This signaling could explain the reduction of the number of colonic ICCs in diabetic mice.48,20
We demonstrated that pharmacological activation of HTR2B significantly increased the frequency of Ca2+ transients in ICCs from HFHSD-fed mice. On the other hand, pharmacological inactivation of HTR2B significantly impaired the generation of Ca2+ transients in ICCs from ND-fed mice. HTR2B is a Gαq/11-coupled serotonin receptor, and the activation of HTR2B through the binding of 5-HT initiates the generation of inositol triphosphate and release of Ca2+ from intracellular stores.49,50 Thus, effects due to modulation of HTR2B on Ca2+ transients in colonic ICCs may occur through the inositol triphosphate regulation of Ca2+ release. It might be suggested that changes in Ca2+ transients in ICC activity mediated by HTR2B signaling could be due to effects in enteric neurons and/or SMCs. However, we showed that Htr2b mRNA is dominantly expressed in ICCs rather than in cells in the ENS using Kit-tdTom and Sox10-tdTom mice (Figure 4). This does not mean that HTR2B is not expressed in cells other than ICCs, and it remains possible that the effects on Ca2+ transients in ICCs, modulated by HTR2B, could result from the activation of responses in additional cell types. Further studies are needed to determine whether HTR2B signaling affects cells other than ICCs in colonic muscles. Data from the current study suggest that HTR2B signaling regulates Ca2+ transients in ICCs, an important process initiating the generation and active propagation of slow waves and regulating smooth muscle contraction.14,51 Thus, the reduction of HTR2B expression in HFHSD-induced diabetic mice potentially interferes with the regulatory processes by which EC cell-derived 5-HT impacts GI motility. However, despite the reduction in its levels, the pharmacological activation of HTR2B with a specific agonist improves motility in diabetic mice both ex vivo and in vivo, suggesting that even these lower levels of HTR2B can still be stimulated.
Although EC cell-derived 5-HT and HTR2B on ICCs have independently been shown to be important regulators of colonic motility, the direct interplay between these two is unclear. Since 5-HT is produced in TPH1-expressing EC cells,5 TPH2-expressing enteric neurons,52 mast cells,53 and stored and carried in platelets,54 the precise functional regulation of ICCs via EC cell-derived 5-HT remains unclear. A previous study revealed that TPH2-expressing enteric neurons maintain close proximity with a subset of ICCs in the colon;55 however, whether all ICCs are subject to modulation by TPH2-expressing enteric neurons is elusive.
In summary, this study describes a new role for serotonergic signaling in the regulation of colonic motility in diabetes. Loss or inhibition of HTR2B signaling, which is observed in diabetic mice, decreases colonic motility, while activation of the receptor restores colonic motility in diabetic mice. Restoration of HTR2B function may provide therapeutic benefits that can improve constipation in diabetic patients.
Supplementary Material
BACKGROUND AND CONTEXT
Constipation is overrepresented in patients with diabetes. Currently available medicine for constipation is often not effective for diabetic patients. Identification of new therapeutic options based on the disease pathogenesis is essential.
NEW FINDINGS
Expression of the 5-HT2B receptor (HTR2B) was significantly decreased in colonic muscles in diabetic mice with constipation. Activation of HTR2B signaling via the HTR2B agonist increased the frequency of Ca2+ transients in ICCs, which improved colonic migrating motor complexes (CMMCs) and colonic motility in diabetic mice with constipation.
LIMITATIONS
We demonstrated the role of 5-HT-HTR2B signaling in the colonic ICCs of mice. Human studies are warranted to elucidate the translational potential of this study.
IMPACT
The HTR2B agonist may be beneficial in the treatment of constipation manifested with diabetes.
Acknowledgements
We would like to thank Benjamin J Weigler, D.V.M. and Walt Mandeville, D.V.M. for their excellent animal services provided to the mice, as well as Sandra M. Poudrier for her outstanding research support.
Grant support:
Research was supported by NIDDK (DK091725, DK094886, DK103055 to S. Ro, and P01 DK41315 to K. Sanders and S. Ro).
Abbreviations used in this paper:
- 5-HT
serotonin
- HTR2B
5-HT2B receptor
- ND
normal diet
- HFHSD
high-fat high-sucrose diet
- ICC
interstitial cell of Cajal
- GI
gastrointestinal
- Oil
sunflower oil
- EC
enterochromaffin
- DTA
diphtheria toxin A
- CMMCs
colonic migrating motor complexes
- TPH
tryptophan hydroxylase
- OVX
ovariectomized
- IP
intraperitoneal, T2DM, type 2 diabetes mellitus
- CNS
central nervous system
- ENS
enteric nervous system
Footnotes
Conflict of interest statement (for all authors)
None.
Supplementary Material
Supplementary material includes supplementary methods, six figures, two tables, and four videos.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Report NDS. National Diabetes Statistics Report, 2020. Natl Diabetes Stat Rep 2020. [Google Scholar]
- 2.Kumar V, Abbas AK, Aster JC. Robbins Basic Pathology, Tenth Edition. 2018. [Google Scholar]
- 3.Chandrasekharan B, Anitha M, Blatt R, et al. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil 2011;23:131–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med 2009;60:355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Merahbi R, Löffler M, Mayer A, et al. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett 2015;589:1728–34. [DOI] [PubMed] [Google Scholar]
- 6.Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol 2009;587:567–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith TK, Gershon MD. CrossTalk proposal: 5-HT is necessary for peristalsis. J Physiol 2015;593:3225–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer NJ, Sia TC, Brookes SJ, et al. CrossTalk opposing view: 5-HT is not necessary for peristalsis. J Physiol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keating DJ, Spencer NJ. Release of 5-Hydroxytryptamine From the Mucosa Is Not Required for the Generation or Propagation of Colonic Migrating Motor Complexes. Gastroenterology 2010;138:659–670.e2. [DOI] [PubMed] [Google Scholar]
- 10.Heredia DJ, Gershon MD, Koh SD, et al. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol 2013;591:5939–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 1999;38:1083–1152. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich C, Kilbinger H. 5-HT1A receptor-mediated inhibition of acetylcholine release from guinea pig myenteric plexus: Potential mechanisms. Neuropharmacology 1996;35:483–488. [DOI] [PubMed] [Google Scholar]
- 13.Ponti F De. Pharmacology of serotonin: what a clinician should know. Gut 2004;53:1520–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders KM, Ward SM, Koh SD. Interstitial Cells: Regulators of Smooth Muscle Function. Physiol Rev 2014;94:859–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cousins HM, Edwards FR, Hickey H, et al. Electrical Coupling between the Myenteric Interstitial Cells of Cajal and Adjacent Muscle Layers in the Guinea-Pig Gastric Antrum. J Physiol 2003;550:829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He CL, Burgart L, Wang L, et al. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology 2000;118:14–21. [DOI] [PubMed] [Google Scholar]
- 17.Zarate N Severe idiopathic gastroparesis due to neuronal and interstitial cells of Cajal degeneration: pathological findings and management. Gut 2003;52:966–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.tharayil v. s., Wouters m. m., Stanich j. e., et al. Lack of serotonin 5-HT 2B receptor alters proliferation and network volume of interstitial cells of Cajal in vivo. Neurogastroenterol Motil 2010;22:462–e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wouters MM, Gibbons SJ, Roeder JL, et al. Exogenous Serotonin Regulates Proliferation of Interstitial Cells of Cajal in Mouse Jejunum Through 5-HT2B Receptors. Gastroenterology 2007;133:897–906. [DOI] [PubMed] [Google Scholar]
- 20.Singh R, Ha SE, Wei L, et al. MiR-10b-5p Rescues Diabetes and Gastrointestinal Dysmotility. Gastroenterology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spencer NJ, Kyloh M, Wattchow DA, et al. Characterization of motor patterns in isolated human colon: are there differences in patients with slow-transit constipation? Am J Physiol Liver Physiol 2012;302:G34–G43. [DOI] [PubMed] [Google Scholar]
- 22.Dickson EJ, Heredia DJ, McCann CJ, et al. The mechanisms underlying the generation of the colonic migrating motor complex in both wild-type and nNOS knockout mice. Am J Physiol Liver Physiol 2010;298:G222–G232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breland A, Ha SE, Jorgensen BG, et al. Smooth Muscle Transcriptome Browser: offering genome-wide references and expression profiles of transcripts expressed in intestinal SMC, ICC, and PDGFRα+ cells. Sci Rep 2019;9:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drokhlyansky E, Smillie CS, Wittenberghe N Van, et al. The Human and Mouse Enteric Nervous System at Single-Cell Resolution. Cell 2020;182:1606–1622.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debbache J, Parfejevs V, Sommer L. Cre-driver lines used for genetic fate mapping of neural crest cells in the mouse: An overview. genesis 2018;56:e23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen T-W, Wardill TJ, Sun Y, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013;499:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettersson US, Waldén TB, Carlsson P-O, et al. Female Mice are Protected against High-Fat Diet Induced Metabolic Syndrome and Increase the Regulatory T Cell Population in Adipose Tissue Maedler K, ed. PLoS One 2012;7:e46057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu B, Allard C, Alvarez-Mercado AI, et al. Estrogens Promote Misfolded Proinsulin Degradation to Protect Insulin Production and Delay Diabetes. Cell Rep 2018;24:181–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel H, Mirhashemi F, Liehl B, et al. Estrogen Deficiency Aggravates Insulin Resistance and Induces β-Cell Loss and Diabetes in Female New Zealand Obese Mice. Horm Metab Res 2013;45:430–435. [DOI] [PubMed] [Google Scholar]
- 30.Fujishiro M, Kushiyama A, Yamazaki H, et al. Gastrointestinal symptom prevalence depends on disease duration and gastrointestinal region in type 2 diabetes mellitus. World J Gastroenterol 2017;23:6694–6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh J-H, Choi M-G, Kang M-I, et al. The Prevalence of Gastrointestinal Symptoms in Patients with Non-Insulin Dependent Diabetes Mellitus. Korean J Intern Med 2009;24:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FELDMAN M. Disorders of Gastrointestinal Motility Associated with Diabetes Mellitus. Ann Intern Med 1983;98:378. [DOI] [PubMed] [Google Scholar]
- 33.Israelyan N, Colle A Del, Li Z, et al. Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression. Gastroenterology 2019;157:507–521.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beattie DT, Smith JAM. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedebergs Arch Pharmacol 2008;377:181–203. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Chalazonitis A, Huang Y -y., et al. Essential Roles of Enteric Neuronal Serotonin in Gastrointestinal Motility and the Development/Survival of Enteric Dopaminergic Neurons. J Neurosci 2011;31:8998–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan Y, Zeng L, Zheng C, et al. Inflammatory Links Between High Fat Diets and Diseases. Front Immunol 2018;9:2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bábíčková J, Tóthová Ľ, Lengyelová E, et al. Sex Differences in Experimentally Induced Colitis in Mice: a Role for Estrogens. Inflammation 2015;38:1996–2006. [DOI] [PubMed] [Google Scholar]
- 38.Homma H, Hoy E, Xu D-Z, et al. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Liver Physiol 2005;288:G466–G472. [DOI] [PubMed] [Google Scholar]
- 39.Li N, Ghia J-E, Wang H, et al. Serotonin Activates Dendritic Cell Function in the Context of Gut Inflammation. Am J Pathol 2011;178:662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Utsumi D, Matsumoto K, Amagase K, et al. 5-HT 3 receptors promote colonic inflammation via activation of substance P/neurokinin-1 receptors in dextran sulphate sodium-induced murine colitis. Br J Pharmacol 2016;173:1835–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi W, Namkung J, Hwang I, et al. Serotonin signals through a gut-liver axis to regulate hepatic steatosis. Nat Commun 2018;9:4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poul E Le, Boni C, Hanoun N, et al. Differential adaptation of brain 5-HT1A and 5-HT1B receptors and 5-HT transporter in rats treated chronically with fluoxetine. Neuropharmacology 2000;39:110–122. [DOI] [PubMed] [Google Scholar]
- 43.Dale E, Pehrson AL, Jeyarajah T, et al. Effects of serotonin in the hippocampus: how SSRIs and multimodal antidepressants might regulate pyramidal cell function. CNS Spectr 2016;21:143–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng Y, Bai H, Hu H. rs11671784 G/A variation in miR-27a decreases chemo-sensitivity of bladder cancer by decreasing miR-27a and increasing the target RUNX-1 expression. Biochem Biophys Res Commun 2015;458:321–327. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Xu M, Ding L, et al. MiR-27a: A Novel Biomarker and Potential Therapeutic Target in Tumors. J Cancer 2019;10:2836–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benhassine M, Guérin S. Transcription of the Human 5-Hydroxytryptamine Receptor 2B (HTR2B) Gene Is under the Regulatory Influence of the Transcription Factors NFI and RUNX1 in Human Uveal Melanoma. Int J Mol Sci 2018;19:3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le-Bel G, Benhassine M, Landreville S, et al. Analysis of the proteasome activity and the turnover of the serotonin receptor 2B (HTR2B) in human uveal melanoma. Exp Eye Res 2019;184:72–77. [DOI] [PubMed] [Google Scholar]
- 48.Ro S, Park C, Jin J, et al. A Model to Study the Phenotypic Changes of Interstitial Cells of Cajal in Gastrointestinal Diseases. Gastroenterology 2010;138:1068–1078.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murray KC, Stephens MJ, Ballou EW, et al. Motoneuron Excitability and Muscle Spasms Are Regulated by 5-HT 2B and 5-HT 2C Receptor Activity. J Neurophysiol 2011;105:731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCorvy JD, Roth BL. Structure and function of serotonin G protein-coupled receptors. Pharmacol Ther 2015;150:129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanders KM, Koh SD, Ward SM. INTERSTITIAL CELLS OF CAJAL AS PACEMAKERS IN THE GASTROINTESTINAL TRACT. Annu Rev Physiol 2006;68:307–343. [DOI] [PubMed] [Google Scholar]
- 52.Young HM, Furness JB. Ultrastructural examination of the targets of serotoninn immunoreactive descending interneurons in the guinea pig small intestine. J Comp Neurol 1995;356:101–114. [DOI] [PubMed] [Google Scholar]
- 53.ENERBÄCK L. Serotonin in Human Mast Cells. Nature 1963;197:610–611. [Google Scholar]
- 54.Vanhoutte PM, Cohen RA. The elusory role of serotonin in vascular function and disease. Biochem Pharmacol 1983;32:3671–3674. [DOI] [PubMed] [Google Scholar]
- 55.Okamoto T, Barton MJ, Hennig GW, et al. Extensive projections of myenteric serotonergic neurons suggest they comprise the central processing unit in the colon. Neurogastroenterol Motil 2014;26:556–570. [DOI] [PubMed] [Google Scholar]
- 56.Wei L, Singh R, Ha S, et al. Serotonin Deficiency is Associated with Delayed Gastric Emptying. Gastroenterology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


