Abstract
Acute respiratory distress syndrome (ARDS) is a significant cause of morbidity and mortality in the intensive care unit (ICU) and is characterized by lung epithelial and endothelial cell injury, with increased permeability of the alveolar-capillary membrane, leading to pulmonary edema, severe hypoxia, and difficulty with ventilation. The most common cause of ARDS is sepsis, and currently, treatment of ARDS and sepsis has consisted mostly of supportive care because targeted therapies have largely been unsuccessful. The molecular mechanisms behind ARDS remain elusive. Recently, a number of microRNAs (miRNAs) identified through high-throughput screening studies in ARDS patients and preclinical animal models have suggested a role for miRNA in the pathophysiology of ARDS. miRNAs are small noncoding RNAs ranging from 18 to 24 nucleotides that regulate gene expression via inhibition of the target mRNA translation or by targeting complementary mRNA for early degradation. Unsurprisingly, some miRNAs that are differentially expressed in ARDS overlap with those important in sepsis. In addition, circulatory miRNA may be useful as biomarkers or as targets for pharmacologic therapy. This can be revolutionary in a syndrome that has neither a measurable indicator of the disease nor a targeted therapy. While there are currently no miRNA-based therapies targeted for ARDS, therapies targeting miRNA have reached phase II clinical trials for the treatment of a wide range of diseases. Further studies may yield a unique miRNA profile pattern that serves as a biomarker or as targets for miRNA-based pharmacologic therapy. In this review, we discuss miRNAs that have been found to play a role in ARDS and sepsis, the potential mechanism of how particular miRNAs may contribute to the pathophysiology of ARDS, and strategies for pharmacologically targeting miRNA as therapy.
Despite recent medical advances, the treatment of sepsis and acute respiratory distress syndrome (ARDS) remains a challenge. As the molecular mechanisms of ARDS remain elusive, mortality remains unacceptably high.1 ARDS is characterized by lung epithelial and endothelial cell injury, causing increased permeability of the alveolar-capillary membrane leading to acute pulmonary edema of noncardiac origin.2–4 Clinically, severe hypoxia, difficulty with gas exchange, and impairment of lung mechanics are observed.2–4 Significant risk factors for developing ARDS include sepsis, shock, pneumonia, gastric aspiration, drowning, trauma, pancreatitis, and blood transfusions.5,6 Worldwide, the incidence of ARDS ranges from 10.1 to 193.4 per 100,000 person-years.7,8 Currently, the treatment of ARDS consists mostly of supportive care with low-tidal volume ventilation, prone positioning, conservative fluid management, and neuromuscular blockade for ventilator dyssynchrony. Targeted therapies to modulate the severity of disease, such as antioxidants,9–12 prostaglandin E1,13,14 neutrophil elastase inhibitors,15 activated protein C,16 statins,17,18 and keratinocyte growth factor,19 have not demonstrated clear benefit. More recently, randomized controlled trials by the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) Prevention and Early Treatment of ALI (PETAL) network did not demonstrate a reduction in mortality with the use of early continuous neuromuscular blockade (Reevaluation of Systemic Early Neuromuscular Blockade [ROSE] study)20 or with the use of vitamin D (Vitamin D to Improve Outcomes by Leveraging Early Treatment [VIOLET] study).21 Similar to ARDS, therapies targeted at moderating inflammation in sepsis have not been shown to be beneficial. These therapies include recombinant activated protein C,22 Toll-like receptor (TLR) antagonists,23 antitumor necrosis factor monoclonal antibody,24–26 tumor necrosis factor receptor antagonists,26,27 interleukin-1 (IL-1) receptor antagonists,28 recombinant human tissue factor pathway inhibitor,29,30 bradykinin antagonist,31 and nitric oxide inhibitors.32–37 Therefore, there is an urgent need to find effective targeted therapy for these patients.
Recently, there has been increasing attention to the role microRNAs (miRNAs) may play in ARDS and sepsis. miRNAs are small, noncoding RNAs ranging from 18 to 24 nucleotides that regulate gene expression via inhibition of target mRNA translation or by targeting complementary mRNA for early degradation.38 A number of therapies targeting miRNAs have been developed or are being developed for various inflammatory disease states.39–44 Promising results from clinical and preclinical studies demonstrate that miRNAs may play roles in the pathophysiology of ARDS and sepsis. Therefore, miRNA mimics or antagomirs (synthetic miRNA inhibitors with sequences complementary to a specific miRNA) may be attractive candidates as targeted therapy. In this review, we discuss miRNAs that have been found to play a role in ARDS and sepsis, the potential mechanism of how particular miRNAs may contribute to ARDS, and strategies for pharmacologically targeting miRNA as therapy.
THE ROLE OF miRNA IN ARDS AND SEPSIS: miRNA AS BIOMARKERS
Extracellular miRNAs exist in biological fluids as passively leaked apoptotic bodies from injured or necrotic cells or as actively secreted and regulated microparticles and exosomes. Among different types of biofluids, miRNAs are found most abundantly in bronchoalveolar lavage fluid (BALF) and blood plasma, with relative expression of miRNAs being highly correlated between different types of biofluids.45 The major source of circulating miRNA in plasma is blood cells,46 and unsurprisingly, many circulating miRNAs that have been suggested to play a role in sepsis and ARDS are highly expressed in blood cells. Recently, circulating miRNAs have been suggested to mediate cell-to-cell communication. Though the exact mechanism remains to be elucidated, experimental evidence of active uptake of miRNA-containing vesicles or exosomes into receptor cells exists.47
miRNA as Biomarkers for ARDS
ARDS is a disease that currently does not have a standardized laboratory diagnosis.48 The clinical diagnosis is made using the Berlin criteria (Figure 1).49 Underdiagnosis of this syndrome is common due to issues with the reliability of these criteria, including interreader variability in the interpretation of chest radiographs.2 Thus, a biomarker or biomarker panel for ARDS may help identify patients to initiate life-saving treatment sooner. A number of circulating miRNAs have been found to be differentially expressed between ARDS patients and controls. Indeed, ARDS patients are reported to have elevated blood miR-155, miR-223, miR-146, and miR-27 levels compared with healthy patients or critically ill patients without ARDS across multiple studies. Other miRNAs have also been found to be differentially expressed in ARDS (Supplemental Digital Content, Table 1, http://links.lww.com/AA/D172), but only miRNAs with ≥3 references supporting its involvement in ARDS as a biomarker or concurrent experimental evidence for a plausible mechanism of action are discussed in detail. The use of miRNA as circulating biomarkers is attractive because the acquisition of a sample does not require invasive sampling, such as biopsy.
Figure 1.
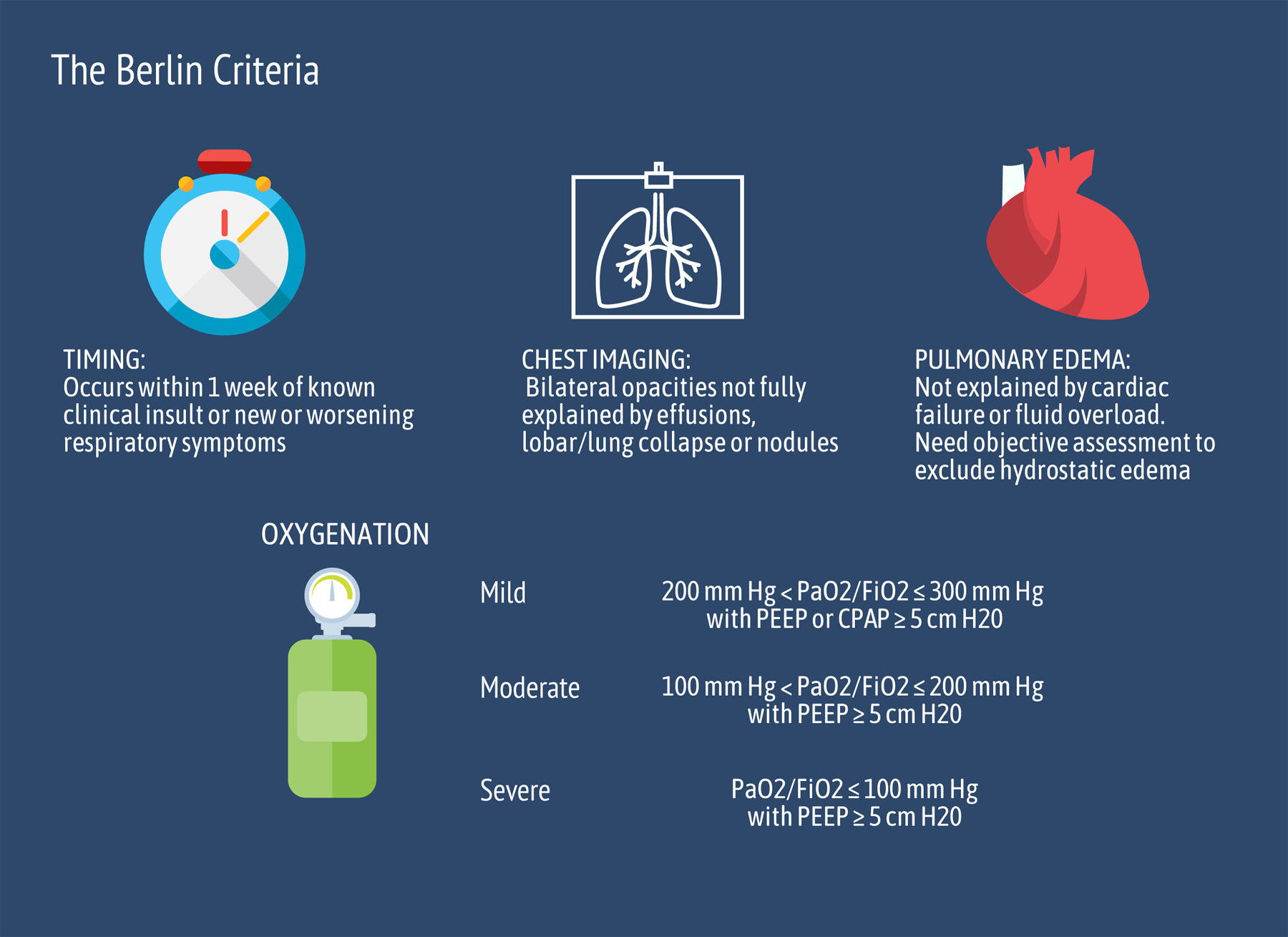
Berlin criteria for the diagnosis of ARDS. These criteria were decided on by a panel of experts from the American Thoracic Society, the Society of Critical Care Medicine, and the European Society of Intensive Care Medicine in 2012 and were a modification from the previously widely used American-European Consensus Conference criteria. The Berlin criteria stipulate that the maximum period between risk factor exposure and development of ARDS be within 7 d, in addition to the requirement for chest imaging and exclusion of hydrostatic pulmonary edema. The Berlin criteria also further classified severity of illness based on oxygen and a minimum level of support with PEEP or CPAP. ARDS indicates acute respiratory distress syndrome; CPAP, continuous positive airway pressure; Fio2, fraction of inspired oxygen; Pao2, partial pressure of oxygen; PEEP, positive end-expiratory pressure.
miR-155.
miR-155 plays a major role in immune regulation50 and is highly expressed in lymphoid cells and moderately expressed in the lung in humans.51 miR-155 was consistently increased in BALF, whole blood, plasma, and serum of ARDS patients compared with healthy patients or other critically ill patients without ARDS across 5 studies.52–56 These findings correlate with data obtained from experimental animal models of ARDS.52–54,57,58 Its upregulation in a number of easily obtainable biofluids also makes miR-155 an attractive candidate biomarker for the diagnosis of ARDS.
miR-223.
miR-223 is involved in myeloid differentiation and function.59 This miRNA is highly expressed in the myeloid cell line and not well-expressed by lung epithelium.60 miR-223 expression was increased in leukocytes and plasma of patients with ARDS compared with healthy controls61 and ventilated non-ARDS patients.62 As the exudative and inflammatory phase of ARDS progressed, miR-223 levels were increased in the leukocytes of blood samples drawn on day 7 of ICU admission compared with those drawn on day 3 in patients diagnosed with ARDS.61 An miRNA profiling study performed in a rat lipopolysaccharides (LPS)-induced model of ARDS also found similarly increased expression of miR-223 in the lung.63 Thus, plasma levels of miR-223 may be useful for diagnosing ARDS, but further clinical validation studies are needed to establish cutoff values for delineating different phases of ARDS.
miR-146.
miR-146 is an important regulator of immune function55,64 and is expressed highly in leukocytes, monocytes, and endothelial cells and moderately low levels in alveolar and bronchial epithelial cells.60 In plasma and serum from ARDS patients, miR-146 expression was increased compared with healthy controls or otherwise critically ill patients without ARDS.55,56 The findings from these human biomarker studies correspond with the observed increase of miR-146 expression in experimental mouse models of ARDS,65,66 making this miRNA an attractive biomarker for ARDS also. However, relative to miR-155 and miR-223, there are fewer human studies corroborating its importance in ARDS.
miR-27.
miR-27 is highly expressed in epithelial cells, while miR-27b is highly expressed in hepatocytes and smooth muscle cells and moderately expressed in alveolar and bronchial epithelial cells.60 This family of miRNAs plays a role in lipid metabolism and the inflammatory response.67,68 Both miR-27a and miR-27b expression were increased in serum, plasma, and leukocytes of patients with ARDS compared with non-ARDS or healthy controls.56,61,69 Expression of miR-27a was also noted to be increased in survivors of ARDS compared to nonsurvivors, and miR-27b was differentially expressed between patients with pulmonary causes of ARDS compared with extrapulmonary causes of ARDS.69 In patients already diagnosed with ARDS, expression of miR-27b was increased in leukocytes on day 7 of diagnosis compared to day 3.61 Thus, miR-27a and 27b may be useful as biomarkers of ARDS.
miRNA as Biomarkers for Sepsis
A number of miRNAs have been found to be differentially expressed between sepsis patients compared with healthy controls and thus may be useful as biomarkers of sepsis (Supplemental Digital Content, Table 2, http://links.lww.com/AA/D172). Traditionally, protein biomarkers such as C-reactive protein, procalcitonin, angiopoietins,70 and serum lactate71–73 have been used as biomarkers for sepsis. However, because miRNAs inhibit the translation of target mRNA into proteins, earlier detection of pathology may be identified with changes in miRNA expression. miRNA may also help distinguish sepsis from other noninfectious causes for systemic inflammatory response syndrome (SIRS). Furthermore, biomarkers in sepsis that are able to distinguish the hyperinflammatory phase from the immunosuppressive phase of sepsis may also help develop specific therapies for targeted treatment.74
miR-223.
Conflicting results are reported on the expression of miR-223 in sepsis. Elevated miR-223 levels have been shown in serum75 and whole blood76,77 of patients with sepsis when compared with healthy controls. Among septic patients, miR-223 was decreased in nonsurvivors compared to survivors.78 miR-223 levels were also decreased in patients with SIRS compared with healthy controls.79 However, other studies found that there is no change in plasma miR-22380 between patients with sepsis and healthy controls or between sepsis patients compared to those with other critical illnesses.81 The blood samples in studies that showed no difference between patients with sepsis and those without sepsis were potentially collected earlier, at ICU admission81 or in the emergency department,80 than in other studies where samples were collected within 24 hours of ICU admission. It is possible that the ostensible lack of differential expression was due to relatively small sample sizes and insufficient time elapsed for differential expression to become apparent.
miR-146.
In clinical studies of sepsis, miR-146 levels in blood were differentially expressed in sepsis patients compared to healthy controls. Two studies reported miR-146 downregulation in serum and plasma of septic patients relative to patients with SIRS and healthy controls.79,82 Similarly, downregulation of miR-146 was also found in plasma exosomes from septic patients relative to healthy controls.83 However, a study comparing sepsis patients, septic shock patients, and healthy controls found no difference in expression levels of miR-146 in serum or plasma between either group.80 These discrepant findings may be attributable to differences in defining sepsis between studies. Overall, circulating miR-146 levels appear to be downregulated in sepsis.
Biomarker Panels for ARDS and Sepsis
An miRNA panel demonstrating increased expression of miR-155, miR-223, miR-146, miR-27a, and miR-27b and decreased expression of miR-150 would likely favor the diagnosis of ARDS. Most biomarker studies involving humans did not specify the cause of ARDS. However, one study found that miR-221 and miR-27b plasma levels were significantly lower in patients with extrapulmonary causes of ARDS when compared with patients with pulmonary causes of ARDS.84 Further miRNA profiling studies are needed to determine the expression profile of ARDS patients during the immunosuppressive phase, because the majority of the studies published thus far are from samples obtained early, during the acute hyperinflammatory phase.
For distinguishing ARDS from sepsis, increased expression of miR-122,77,78,83,85 miR-223,75–77 and miR-48379,85 and decreased expression of miR-26a,77,83,84 miR-146,79,83,86 miR-150,76,77,87–89 and miR-34276,87 would favor a diagnosis of sepsis. While there is a fair amount of overlap in the miRNAs that are differentially expressed between diseased and healthy patients for both ARDS and sepsis, miR-146 appears to be the differentiating miRNA between those 2 diseases. In patients with sepsis, miR-146a was usually significantly downregulated,79,83,86 whereas miR-146a expression was increased in ARDS55,56 when compared with healthy controls. Indeed, in experimental models of ARDS and sepsis, miR-146 seems to exert dual effects in different immune cells: having anti-inflammatory effects in T cells,90 while promoting proinflammatory functions in macrophages.91 Increased expression of miR-125b has also been suggested to distinguish sepsis with ARDS from sepsis alone.92
In distinguishing sepsis from SIRS, one study84 found that plasma levels of miR-191, miR-192, miR-23a, miR-26a, miR-30a, and miR-30d were decreased in severe sepsis compared to severe SIRS. Interestingly, miR-146a and miR-223 were both downregulated in patients with SIRS compared to healthy controls.79 Finally, increased expression of miR-122, miR-193, and miR-483 and decreased expression of miR-15a, miR-16, miR-223, and miR-150 were associated with nonsurvival in patients with sepsis.78 Many of these differentially expressed miRNAs have targets that are involved in major inflammatory signaling pathways such as nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), signal transducer and activator of transcription (STAT), protein kinase B (AKT), and TLR. A number of these miRNA also have targets involved with stress-activated c-Jun N-terminal kinase (JNK) signaling pathway, which promotes apoptosis and will be discussed in further detail in the next section.
THE FUNCTIONAL ROLE OF miRNA IN ARDS
miRNA as Pharmacologic Targets in ARDS
miRNAs are involved in the regulation of many biological processes and therefore are attractive candidates as novel pharmacologic targets. Animal models of ARDS have provided insight into the mechanistic role of miRNAs in ARDS pathophysiology.93 Because multiple triggers lead to the common pathway of ARDS in humans, multiple animal models of ARDS have been developed to reflect the various clinical features seen in ARDS.94 Examples of these include intratracheal instillation of acid, bacteria, or saline to simulate aspiration, pneumonia, or drowning, respectively.94 Other models mimic the effects of sepsis with the intraperitoneal injection of LPS or by cecal ligation and puncture (CLP).94 Indeed, across these various models, miRNAs have been demonstrated to play both protective and detrimental roles in ARDS pathophysiology, from regulating the release of inflammatory cytokines66,95–100 to enhancing cell survival and recovery from injury.54,101–103 A summary of these functions is detailed in Figure 2.
Figure 2.
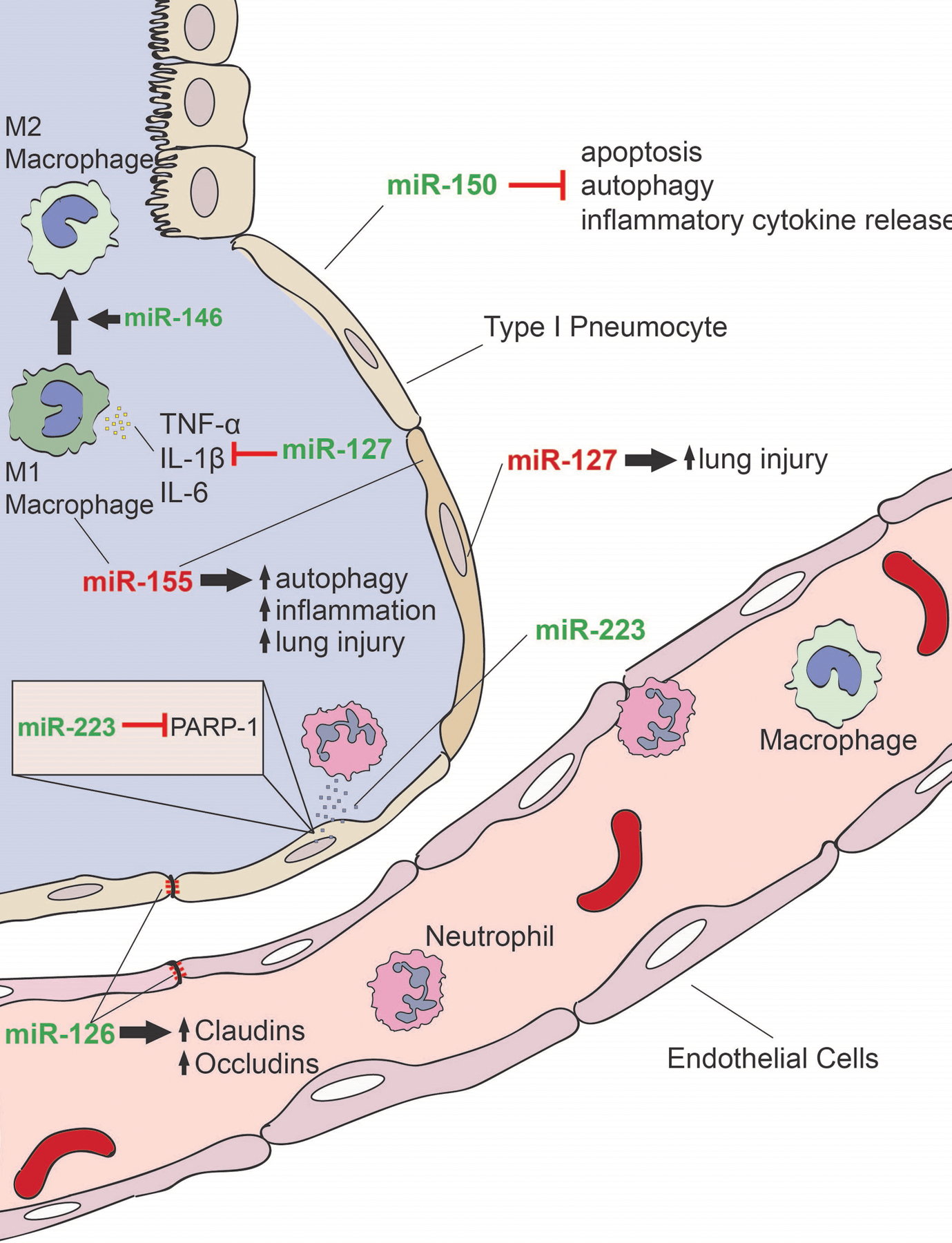
The role of miRNA in ARDS. A number of miRNAs have been demonstrated to play a role in the pathophysiology of ARDS. miRNA with predominately proinflammatory functions are denoted in red, while miRNA with predominately anti-inflammatory functions is denoted in green. miR-150 decreases cell apoptosis, autophagy, and release of inflammatory cytokines. miR-146 promotes the M2 phenotype in macrophages, promoting wound healing and tissue repair. miR-127 decreases production of TNF-α, IL-1β, and IL-6 decreasing severity of lung injury in vivo but has also been associated with lung injury with overexpression. miR-155 increased autophagy bodies in alveolar macrophages and lung tissue, promoting inflammation and injury. Shuttling of miR-223 from neutrophils to alveolar epithelial cells was protective against VILI, possibly mediated via repression of PARP-1 in epithelial cells. miR-126 promotes expression of claudins and occludins to improve epithelial and endothelial integrity. Therapeutic targeting of these miRNAs could potentially serve as novel treatment approaches for ARDS. ARDS indicates acute respiratory distress syndrome; IL, interleukin; miRNA, microRNA; PARP, poly(ADP-Ribose) polymerase 1; TNF-α, tumor necrosis factor α; VILI, ventilator-induced lung injury.
miR-126.
miR-126 is reported to be increased in lungs and BALF from mice that have received intratracheal LPS compared with controls.104,105 miR-126 has also been found to be abundant in exosomes originating from human endothelial progenitor cells.106 Intratracheal administration of these exosomes reduced the severity of lung injury in a murine intratracheal LPS lung injury model.105 Here, miR-126 is reported to confer its protective actions by increasing expression of tight junction proteins, claudins, and occludins, thereby maintaining lung alveolar epithelial barrier integrity.105 It is hypothesized that miR-126 acts via its targets phosphoinositide-3-kinase regulatory subunit 2 (PIK3R2), high-mobility group protein 1 (HMGB1), and vascular endothelial growth factor (VEGF) α105 and by increasing Ras-related C3 botulinum toxin substrate 1 (Rac1) and AKT signaling. Collectively, reduced expression of these proteins resulted in improved epithelial and endothelial cell barrier integrity.105 Taken altogether, therapeutic overexpression of miR-126 could potentially improve pulmonary barrier function in ARDS.
miR-146.
Another miRNA found to confer protective characteristics in experimental models of ARDS is miR-146. Macrophages play seemingly contrasting roles in ARDS by releasing inflammatory cytokines that recruit neutrophils leading to more tissue damage in the classically activated phenotype (M1) and by producing anti-inflammatory cytokines such as IL-10 and promoting wound healing activity in the alternatively activated phenotype (M2).101 miR-146 has been shown to promote the M2 phenotype in macrophages.65 Overexpression of miR-146 was associated with decreased inflammation and attenuated lung injury in response to acid aspiration and intratracheal LPS ARDS models.65,66 These protective effects may be mediated by a reduction in proinflammatory proteins, tumor necrosis factor receptor–associated factor-6 (TRAF6) and IL-1 receptor–associated kinase 1 (IRAK1), which are proposed targets of miR-146.66 Therapeutic overexpression of miR-146 in ARDS patients may, therefore, inhibit the exaggerated inflammatory response characteristic of ARDS.
miR-150.
miR-150 has been demonstrated to be protective in the context of ARDS. Li et al95 showed that miR-150 regulates apoptosis, inflammation, cytokine production, and metabolism by targeting AKT serine/threonine kinase 3 (AKT3), which in turn inhibits JNK signaling in A549 alveolar basal epithelial cells. miR-150 regulation of the immune response to infection via NF-κB signaling may also protect against LPS-induced injury in vitro.95 Furthermore, miR-150 targets the proinflammatory growth factor angiopoietin-2 (Ang2), leading to reannealing of adherens junctions and promoting healing from vascular injury in in vitro model and murine intraperitoneal LPS or CLP models of ARDS.102 Upregulation of miR-150 may be therapeutic by limiting processes contributing to cellular injury seen in ARDS.
miR-223.
miR-223 is increased in various ARDS models including acute lung injury induced by mitochondrial damage-associated molecular patterns, intratracheal LPS, intratracheal Klebsiella, and ventilator-induced lung injury.59,96,97,103,107 In neutrophils, miR-223 has been shown to target nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain containing 3 (NLRP3),96,97 an important intracellular sensor that activates the NLRP3 inflammasome in response to infection, damage-associated molecular patterns, and environmental irritants.108 Negative regulation of poly(ADP-ribose) polymerase 1 (PARP-1), an enzyme involved in single-strand DNA break repair, may contribute to the observed anti-inflammatory effects of miR-223 because miR-223 deficiency has been associated with more severe lung injury in response to ventilator-induced lung injury, while experimental overexpression of miR-223 is associated with an attenuated inflammatory response.103 Other proposed targets of miR-223 include the Ras homolog family member B (RhoB),98 a regulator of the proinflammatory cytokines IL-1β, IL-6, and tumor necrosis factor α (TNF-α) in the context of hypoxia.99 Therapeutic upregulation of miR-223 could promote DNA repair and limit damage caused by exaggerated inflammatory responses.
miR-127.
In contrast, 2 miRNAs have also been identified as detrimental to ARDS survival. miR-127 was shown to be increased in the lung and BALF of ventilator-induced lung injury and intratracheal LPS models of ARDS in several studies.109–111 Upregulation of miR-127 using mimics was associated with enhanced pulmonary inflammation110 and injury, while downregulation of miR-127 resulted in attenuated injury and decreased activation of NF-κB.110,111 The targets through which miR-127 has been proposed to act include B-cell lymphoma 6 protein (Bcl-6),111 a transcriptional regulator of a number of immune cells, and dual specificity phosphatase 1 (Dusp1), a phosphatase that promotes JNK signaling pathway, which contributes to inflammatory responses.110 However, a study using a bleomycin-induced lung injury model and an IgG immune complex model of ARDS in mice demonstrated a decrease in miR-127 expression in the lungs.100 In this study, lentiviral-induced overexpression of miR-127 was found to decrease production of TNF-α, IL-1β, and IL-6 by macrophages in vitro and intranasal administration of miR-127 in mice attenuated IgG immune complex-induced lung injury in vivo.100 Given these mixed results, pharmacologic downregulation of miR-127 could attenuate severity of lung injury in certain subtypes of ARDS, while therapeutic upregulation would be advantageous in others.
miR-155.
miR-155 has also been shown to promote inflammation in ARDS. Increased expression of miR-155 has been demonstrated in a number of ARDS models, including CLP models52 and intratracheal LPS administration.53 Overexpression of miR-155 was associated with increased microtubule-associated protein 1A/1B-light chain 3 (LC3) II/I expression, an autophagosome-associated protein, and higher number of autophagy bodies in LPS-treated rat alveolar macrophages and in the lung tissue of mice subjected to a CLP model of septic lung injury.52 Additionally, injection of serum exosomes enriched in miR-155 from mice subjected to acute lung injury elicited lung inflammation in previously healthy mice.58 Furthermore, knockdown of miR-155 partially reversed LPS-induced lung injury in mice.53 A number of studies have indicated that these effects of miR-155 are mediated through suppressors of cytokine signaling-1 (Socs-1),53,57,58 a negative regulator of inflammatory cytokines interferon (IFN) α/β/γ, IL-12/23, IL-4/13, and the IL-2 family cytokines.112 Other reported targets of miR-155 include TGF-β activated kinase 1 (MAP3K7) binding protein 2 (TAB 2),52 which is required for the IL-1–induced activation of NF-κB, the proinflammatory MAPK8/JNK,113 and the Wnt signaling pathway,54 which is involved in cell fate specification, proliferation, and migration. Therapeutic downregulation of miR-155 may suppress the expression of inflammatory cytokines to limit lung injury in ARDS.
Overall, the differentially expressed circulating miRNAs in ARDS patients appear to regulate the release of inflammatory cytokines and maintenance of cell barrier integrity, as shown in animal models of ARDS.
miRNA as Pharmacologic Targets in Sepsis
The role of some miRNAs has been elucidated through animal models of sepsis (Figure 3). Similar to experimental models of ARDS, a number of murine models have been used to simulate different aspects of sepsis, that is, endotoxemia by LPS administration, host response to pathogens using exogenous bacterial infection, and loss of endogenous protective barrier in CLP models of sepsis.114 Although translational limitations of these models to human pathology exist, understanding the molecular mechanisms of miRNA expression and the changes that result from varying miRNA expression can lead to a better understanding of the pathophysiology underlying sepsis and help develop new miRNA-based sepsis therapies.
Figure 3.
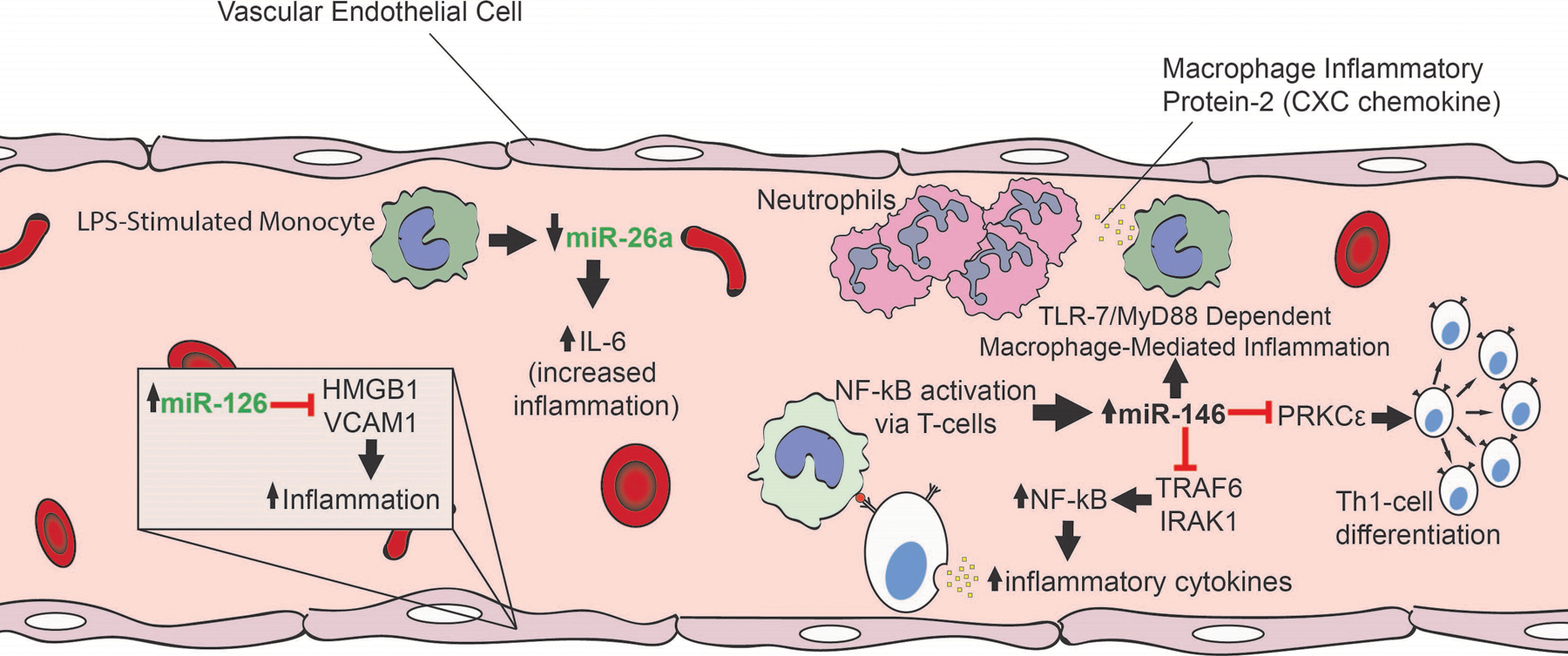
The mechanistic role of miRNA in sepsis. A number of miRNAs have been demonstrated to play a role in the pathophysiology of sepsis. miRNA with predominately anti-inflammatory functions is denoted in green. miR-126 suppresses LPS-induced expression of proinflammatory mediators, HMGB1, and VCAM1. miR-26a was downregulated in LPS-stimulated monocytes, leading to an increase in IL-6 expression. miR-146 had both pro- and anti-inflammatory functions. Activation of NF-κB via T-cell receptors resulted in increased expression of miR-146a, which then represses NF-κB activators TRAF6 and IRAK1, in a negative feedback loop, dampening the inflammatory response. miR-146a also targets PRKCε, which phosphorylates STAT4, promoting Th1-cell differentiation processes in human CD4(+) T lymphocytes. miR-146 may also promote inflammation through TLR7−/− and MyD88 mediated macrophage activation resulting in increased expression of MIP-2 and IL-6. Therapeutic targeting of these miRNAs could potentially serve as novel treatment approaches for sepsis. CXC indicates Cysteine-X-cysteine motif; HMGB1, high-mobility group box 1; IL, interleukin; IRAK1, interleukin-1 receptor–associated kinase 1; LPS, lipopolysaccharides; MIP-2, macrophage inflammatory protein-2; miRNA, microRNA; MyD88, myeloid differentiation primary response 88; NF-κB, nuclear factor κ-lightchain-enhancer of activated B cells; PRKCε, protein kinase C ε; STAT4, signal transducer and activator of transcription 4; Th1, T helper type 1; TLR7, Toll-like receptor 7; TRAF6, tumor necrosis factor receptor–associated factor-6; VCAM1, vascular cell adhesion molecule 1.
miR-126.
In models of sepsis, miR-126 is a protective miRNA that was upregulated in extracellular vesicles in the blood of septic mice compared to healthy controls.91,106 Zhou et al106 found miR-126 to be enriched in exosomes of endothelial progenitor cells from human cord blood and that injection of these exosomes attenuated cytokine and chemokine production in a murine CLP sepsis model. In human vascular endothelial cells, miR-126 was also shown to suppress LPS-induced expression of HMGB1 and vascular cell adhesion molecule 1 (VCAM1), both important mediators of inflammation.106 Conversely, miR-126 inhibition in human vascular endothelial cells led to increased expression of HMGB1 and VCAM1 after LPS stimulation.115,116 As a result, therapeutic overexpression of miR-126 may be beneficial in sepsis by limiting proinflammatory effects of HMGB1 and VCAM1.
miR-146.
miRNA-146 has been shown to be anti-inflammatory and to regulate T-cell function in mice and humans. In miR-146–deficient mice, increased expression of Stat1, resulted in IFN-γ–mediated autoimmunity and expression of miR-146 in regulatory T cells (Treg), prevented uncontrolled inflammation.117 miR-146 also acts as a regulator of T-cell responses through its role in the negative feedback control of T-cell receptor–induced NF-κB activity. Activation of NF-κB via T-cell receptors resulted in increased expression of miR-146a, which then repressed NF-κB activators TRAF6 and IRAK1.90 In T cells from septic patients, levels of miR-146 have been shown to be decreased, resulting in increased protein kinase C ε (PRKCε) expression and T helper type 1 (Th1)–cell differentiation, leading to excessive inflammation characteristic of early sepsis.118 Altogether, these results indicate that miR-146 may play a role in the pathophysiology of sepsis, by regulating T-cell function.
In sepsis models, miR-146 may also promote inflammation through macrophage activation. Xu et al91 found that wild-type bone marrow–derived macrophages treated with miR-146–enriched extracellular vesicles derived from septic mice had increased expression of macrophage inflammatory protein-2 (MIP-2) and IL-6, whereas bone marrow–derived macrophages from TLR7−/− and myeloid differentiation primary response 88 (MyD88)−/− mice treated with miR-146 enriched extracellular vesicles did not induce this effect. This suggests that the effects of miR-146 are mediated by TLR signaling pathways because TLR7 and MyD88 are critical players in this proinflammatory pathway. Additionally, peritoneal injection of miR-146 enriched extracellular vesicles from the plasma of septic mice into healthy wild-type mice resulted in a marked increase in peritoneal neutrophils.91 Taken as a whole, therapeutic downregulation of miR-146 may have a role in limiting the exaggerated inflammatory responses seen in sepsis.
THERAPEUTIC TARGETING OF miRNAs: STRATEGIES AND CHALLENGES
As of the writing of this review, there are not yet miRNA-based therapeutics in development for the treatment of ARDS. However, clinical trials have been initiated to evaluate the use of miRNA-based therapies in other diseases. There are several strategies for increasing and decreasing miRNA levels (Figure 4). miRNA can be increased by delivery of miRNA mimic or an expression vector (usually a plasmid or virus) (Figure 5A). Conversely, apparent levels of miRNA can be inhibited by anti-miRNA oligonucleotides (AMOs), miRNA sponges, or small molecular inhibitors of specific miRNA (SMIRs) (Figure 5B).
Figure 4.
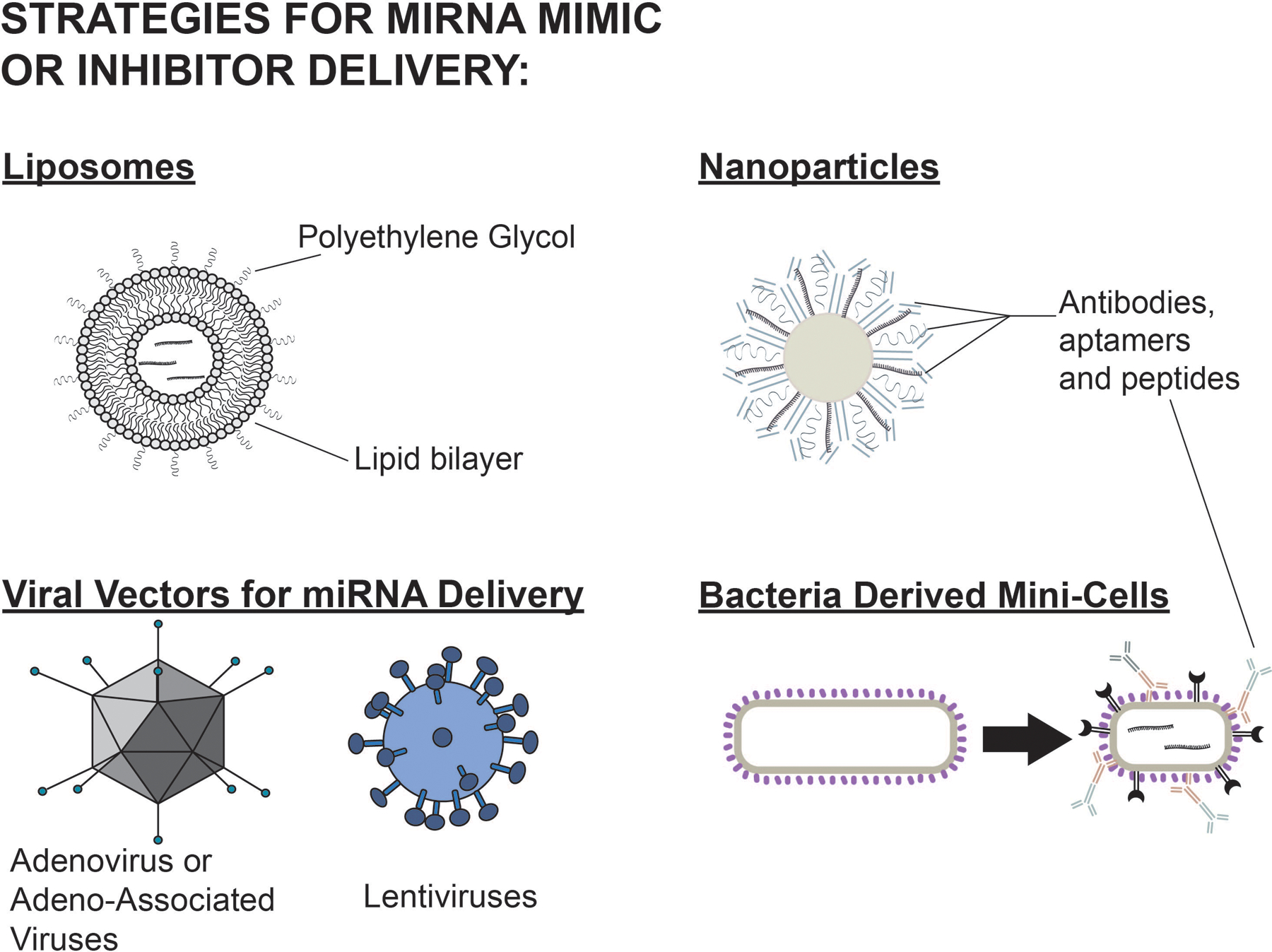
Delivery strategies for miRNA mimics and inhibitors. Examples of these include liposomes, nanoparticles, bacterial minicells, and viral vectors. Modifications to liposomes such as PEG help to evade macrophages and decrease clearance. Antibodies, aptamers, and peptides can increase targeting capabilities. Liposomes and nanoparticles have a low risk of immunogenicity or genomic integration but have lower transfection efficiency. Viral vectors have a higher transfection efficiency but a higher risk of immunogenicity and genomic integration of vector components. miRNA indicates microRNA; PEG, polyethylene glycol.
Figure 5.
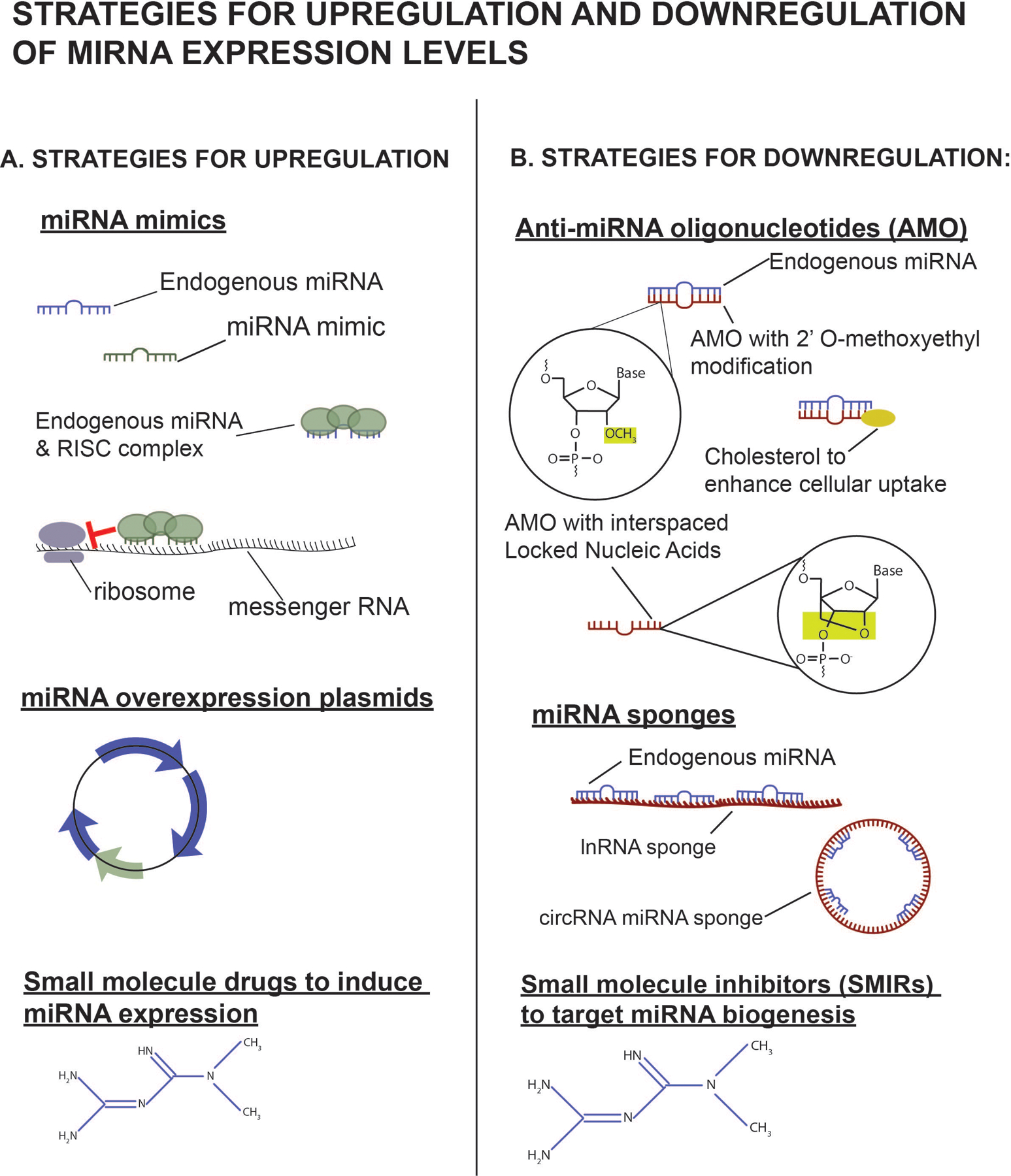
Strategies to upregulate and downregulate miRNA expression levels. A, Strategies for upregulation. In the cytoplasm, miRNA mimics can interact with RISCs and inhibit miRNA translation or target mRNA for early degradation. Overexpression plasmids can be packaged into viral vectors to increase expression levels of desired miRNAs in targeted cells. Small molecules can also be used to induce miRNA expression. B, Strategies for downregulation. AMOs bind endogenous miRNA, preventing interaction with target mRNA. Chemical modifications to AMOs can increase miRNA binding affinity, cellular uptake, and enhance resistance to degradation. lnRNA and circular RNA can act as decoys and bind miRNA. Small molecules inhibitors can also target miRNA at any point during biogenesis. AMO indicates anti-miRNA oligonucleotide; lnRNA, long noncoding RNA; miRNA, microRNA; RISC, RNA-induced silencing complex.
Therapeutic Inhibition of miRNAs
Miravirsen, which was in phase II clinical trials as of 2017 for the treatment of hepatitis C (National Clinical Trial NCT01200420, NCT01727934, NCT01872936), is a prime example of the use of an AMO. miR-122 is a liver-specific miRNA previously identified as an important factor for efficient hepatitis C virus replication.119 Therefore, decreased levels of miR-122 would be desirable to inhibit viral replication. Miravirsen is a complimentary AMO sequence that binds miR-122, thus inhibiting disease progression.120 AMOs are not simply oligonucleotide sequences that are complementary to their target miRNA. Various chemical modifications are made to these sequences, such as the addition of 2′ O-methoxyethyl and the use of interspaced locked nucleic acid (LNA) ribonucleotides, to make the AMOs more resistant to degradation by nucleases and enhance binding affinity. Likewise, the addition of N,N-diethyl-4-(4-nitronaphthalen-1-ylazo)-phenylamine (ZEN) to the 3′ end of the AMO sequence also increases binding affinity to the miRNA and inhibits exonuclease degradation, whereas cholesterol enhances cellular uptake of the AMO. Lima et al121 have reviewed the advantages and disadvantages of these chemical modifications to AMOs.
Another strategy for downregulating miRNA levels is the use of miRNA sponges. These are circular or linear noncoding RNA with high-affinity binding sites for target endogenous miRNAs. These sequences act as a decoy by “sponging” up endogenous miRNA and the accompanying multiprotein RNA-induced silencing complex (RISC) that normally targets mRNA for degradation, allowing the target mRNA sequences of those miRNA to be translated. A single miRNA sponge may be engineered to bind multiple of a single miRNA species or multiple different miRNAs and can be cloned into lentiviral vectors for cellular delivery.122,123 The advantage of using this approach is that multiple miRNA species may be targeted using a single vector. miRNA sponges have been used in in vitro124,125 and in vivo animal models126,127; however, none are being studied for use in clinical trials as of yet. Interestingly, this is not an exclusively artificial means for downregulating miRNA activity because there are naturally occurring, endogenous long noncoding RNA that may regulate miRNA activity in the same fashion.128–130
Another approach for downregulating miRNA levels is the use of SMIRs. The first of such molecules to be identified was a diazobenzene that inhibited miR-21, an oncogenic miRNA.131 Bioinformatic methods are being used to enhance the screening process for identifying small compound-miRNA interactions, in hopes of developing a marketable drug.132 However, the development of this area has been slow compared to the development of AMOs. Because small molecule inhibitors are not oligonucleotides, 1 advantage is that they do not experience as many concerns about stability from nuclease degradation, difficulties with cellular uptake, low oral bioavailability, or other pharmacokinetics issues.133 Another advantage is that SMIRs can target the miRNA at any stage in its generation, from primary or precursor miRNA in the nucleus to miRNA duplex in the cytoplasm to the mature miRNA sequence.134 These strategies for inhibiting miRNA function may be useful for disease processes for which ≥1 miRNAs are found to be upregulated.
Therapeutic Overexpression of miRNA
There are 2 main strategies for increasing the effects of a miRNA (Figure 5A). One method is to deliver a mimic and the other is to overexpress the miRNA using a viral vector.135 Overexpression has been mainly been contained in preclinical trials, but the first miRNA mimic to reach phase 1 clinical trials was MRX34 (miRNA Therapeutics, NCT01829971), a liposomal, intravenously delivered miR-34a mimic developed to treat advanced solid tumors.136 Unfortunately, the trial was ended early due to immune-related adverse events. The development of miRNA mimics for clinical use has been hindered by difficulty with adequate delivery to the desired target cells or tissue.137 Lentiviruses, adenoviruses, and adeno-associated viruses have been used to target cells to produce the desired mature miRNA, but concerns exist regarding genomic integration of these vectors leading to cancer or provoking an excessive immune response.138 Another delivery strategy is the use of liposomes, such as those used for MRX34. Recently, the use of bacterial-derived minicells (TargomiRs) coated with antibodies for receptors of cancer cells was tested in phase 1 clinical trials (NCT02369198).137 These TargomiRs were tested for the delivery of miR-16 mimics for the treatment of malignant pleural mesothelioma.139 Liposomal, nanoparticle, and antibody-based delivery strategies could ostensibly also be used for targeted downregulation of miRNA with AMOs. In addition, small molecule drugs may also be effective in increasing expression of miRNA. ABX464, currently undergoing phase 2 clinical trials (NCT03760003, NCT04023396, NCT03905109, NCT03368118), is an example of one such drug. ABX464 increases the expression of the anti-inflammatory miRNA, miR-124, and may be useful in the treatment of inflammatory bowel diseases.140 All of these strategies may be useful for increasing apparent levels of target miRNA known to have decreased expression in ARDS and sepsis, such as miR-150 and miR-223, for the purposes of treatment.
Remaining Challenges
Off-target effects remain a concern for miRNA as therapeutics. While the probability of off-target effects can be predicted using bioinformatics approaches, the magnitude of the effect may not be fully apparent until in vivo studies are conducted.141 Cell and tissue targeting of the miRNA therapeutic may also play a key role in minimizing undesirable side effects, as well as administration of the lowest effective dose.142 Because the pathology of ARDS is primarily concentrated in the lung, targeted administration via inhalation may also help avoid systemic toxicity. While the pleiotropic nature of miRNAs makes off-target effects unquestionably concerning, similar nonspecific responses have also occurred with traditional therapeutics targeting single protein-coding genes.143
Because the testing of miRNA as biomarkers is not yet commercially available as a diagnostic test, it is difficult to comment on the cost of the test relative to other modalities. Ostensibly, miRNA could detect pathology earlier in the disease state due to its position in the DNA to protein pathway. However, it remains to be seen whether this difference would be clinically relevant. The sensitivity and specificity of miRNAs as diagnostic tests would be determined during the validation process in study populations larger than the individual studies mentioned in this review. Thus, future studies will need to demonstrate how miRNAs compare to other biomarkers/interventions regarding efficacy and cost/benefit analyses.
CONCLUDING REMARKS
MiRNAs have been demonstrated to mediate the inflammatory response to injury and infection, as seen in sepsis and ARDS. The future use of miRNAs as biomarkers for ARDS and sepsis appears to be promising. A unique miRNA biomarker panel may offer an opportunity for earlier detection, channeling resources appropriately for patients who are likely to become more severely ill as predicted by their miRNA expression profile. The ability to classify whether a patient is in the hyperinflammatory phase versus the immunosuppressive phase of the course of illness based on miRNA expression profile may also help guide treatment selection. Furthermore, miRNA-based therapies may offer another opportunity to provide targeted therapy for ARDS and sepsis, where current care is mostly supportive. Overexpression of anti-inflammatory miRNA may help alleviate some of the organ damage that occurs in the hyperinflammatory phase of sepsis and ARDS, while administration of an antagomir for miRNA that is found to promote inflammation, apoptosis, or increase cell barrier permeability could lead to a favorable outcome. Some challenges remain in the development of these therapies. The potential systemic off-target effects of delivering an miRNA mimic or antagonist are still unknown. Additionally, development of technology for targeted delivery of miRNA mimics or antagonists is still ongoing. Some strategies include the use of double-stranded miRNA oligonucleotides to be taken up by endogenous miRNA processing enzymes, miRNA expression vectors, antibody-coated nanoparticles, or liposomes to deliver the miRNA into targeted cells.144 As more research is completed on the mechanistic pathways of ARDS and its interrelationship with sepsis is elucidated, the importance of the role of miRNA these diseases remains strong.
Supplementary Material
Funding:
M.E. received grants from R01-HL129051 and R01-HL147586; and H.E. received grants from R01-DK097075, R01-HL098294, POI-HL114457, R01-DK082509, R01-HL109233, R01-DK109574, R01-HL119837, and R01-HL133900. X.Y. received grants from the American Thoracic Society Unrestricted Grant, American Heart Association Career Development Award (19CDA34660279), American Lung Association Catalyst Award (CA-622265), the Center for Clinical and Translational Sciences, McGovern Medical School Pilot Award (1UL1TR003167–01), and Parker B. Francis Fellowship.
GLOSSARY
- AKT
protein kinase B
- AKT3
AKT serine/threonine kinase 3
- AMO
anti-miRNA oligonucleotides
- Ang2
angiopoietin-2
- ARDS
acute respiratory distress syndrome
- BALF
bronchoalveolar lavage fluid
- Bcl-6
B-cell lymphoma 6 protein
- CLP
cecal ligation and puncture
- CPAP
continuous positive airway pressure
- CXC
Cysteine-X-cysteine motif
- Dusp1
dual specificity phosphatase 1
- Fio2
fraction of inspired oxygen
- HMBG1
high-mobility group protein 1
- ICU
intensive care unit
- IFN
interferon
- IgG
immunoglobulin G
- IL
interleukin
- IRAK1
interleukin-1 receptor–associated kinase 1
- JNK
c-Jun N-terminal kinase
- LC3
1A/1B-light chain 3
- LNA
locked nucleic acid
- LPS
lipopolysaccharides
- MAPK8
mitogen-activated protein kinase 8
- MIP-2
macrophage inflammatory protein-2
- miRNAs
microRNAs
- MyD88
myeloid differentiation primary response 88
- NCT
National Clinical Trial
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
- NHLBI
National Heart, Lung, and Blood Institute
- NIH
National Institutes of Health
- NLRP3
nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain containing 3
- Pao2
partial pressure of oxygen
- PARP-1
poly(ADP-ribose) polymerase 1
- PEEP
positive end-expiratory pressure
- PETAL
Prevention and Early Treatment of ALI
- PIK3R2
phosphoinositide-3-kinase regulatory subunit 2
- PRKCε
protein kinase C ε
- Rac1
Ras-related C3 botulinum toxin substrate 1
- RhoB
Ras homolog family member B
- RISC
RNA-induced silencing complex
- ROSE
Reevaluation of Systemic Early Neuromuscular Blockade
- SIRS
systemic inflammatory response syndrome
- SMIRs
small molecular inhibitors of specific miRNA
- SOCS-1
suppressors of cytokine signaling-1
- STAT
signal transducer and activator of transcription
- TAB 2
TGF-β activated kinase 1 (MAP3K7) binding protein 2
- Th1
T helper type 1
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor α
- TRAF6
tumor necrosis factor receptor–associated factor-6
- Treg
regulatory T cells
- VCAM1
vascular cell adhesion molecule 1
- VEGF
vascular endothelial growth factor
- VILI
ventilator-induced lung injury
- VIOLET
Vitamin D to Improve Outcomes by Leveraging Early Treatment
- ZEN
N,N-diethyl-4-(4-nitronaphthalen-1-ylazo)-phenylamine
Footnotes
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
DISCLOSURES
Name: Lisa K. Lee, MD, MSCR.
Contribution: This author helped draft and revise the manuscript.
Name: Lejla Medzikovic, PhD.
Contribution: This author helped draft and revise the manuscript.
Name: Mansoureh Eghbali, PhD.
Contribution: This author helped draft and revise the manuscript.
Name: Holger K. Eltzschig, MD, PhD.
Contribution: This author helped draft and revise the manuscript.
Name: Xiaoyi Yuan, PhD.
Contribution: This author helped draft and revise the manuscript.
This manuscript was handled by: Alexander Zarbock, MD.
REFERENCES
- 1.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. [DOI] [PubMed] [Google Scholar]
- 2.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710. [DOI] [PubMed] [Google Scholar]
- 3.Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primer. 2019;5:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. [DOI] [PubMed] [Google Scholar]
- 5.Saguil A, Fargo M. Acute respiratory distress syndrome: diagnosis and management. Am Fam Physician. 2012;85:352–358. [PubMed] [Google Scholar]
- 6.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 7.Rezoagli E, Fumagalli R, Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Ann Transl Med. 2017;5:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eworuke E, Major JM, Gilbert McClain LI. National incidence rates for acute respiratory distress syndrome (ARDS) and ARDS cause-specific factors in the United States (2006–2014). J Crit Care. 2018;47:192–197. [DOI] [PubMed] [Google Scholar]
- 9.Heyland D, Muscedere J, Wischmeyer PE, et al. ; Canadian Critical Care Trials Group. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368:1489–1497. [DOI] [PubMed] [Google Scholar]
- 10.Bernard GR, Wheeler AP, Arons MM, et al. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The antioxidant in ARDS study group. Chest. 1997;112:164–172. [DOI] [PubMed] [Google Scholar]
- 11.Jepsen S, Herlevsen P, Knudsen P, Bud MI, Klausen NO. Antioxidant treatment with N-acetylcysteine during adult respiratory distress syndrome: a prospective, randomized, placebo-controlled study. Crit Care Med. 1992;20:918–923. [DOI] [PubMed] [Google Scholar]
- 12.The ARDS Clinical Trials Network, National Heart, Lung, and Blood Institute, National Institutes of Health. Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30:1–6. [DOI] [PubMed] [Google Scholar]
- 13.Abraham E, Baughman R, Fletcher E, et al. Liposomal prostaglandin E1 (TLC C-53) in acute respiratory distress syndrome: a controlled, randomized, double-blind, multicenter clinical trial. TLC C-53 ARDS Study Group. Crit Care Med. 1999;27:1478–1485. [DOI] [PubMed] [Google Scholar]
- 14.Bone RC, Slotman G, Maunder R, et al. Randomized double-blind, multicenter study of prostaglandin E1 in patients with the adult respiratory distress syndrome. Prostaglandin E1 Study Group. Chest. 1989;96:114–119. [DOI] [PubMed] [Google Scholar]
- 15.Zeiher BG, Artigas A, Vincent JL, et al. ; STRIVE Study Group. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med. 2004;32:1695–1702. [DOI] [PubMed] [Google Scholar]
- 16.Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178:618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truwit JD, Bernard GR, Steingrub J, et al. ; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370:2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAuley DF, Laffey JG, O’Kane CM, et al. ; HARP-2 Investigators; Irish Critical Care Trials Group. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371:1695–1703. [DOI] [PubMed] [Google Scholar]
- 19.McAuley DF, Cross LM, Hamid U, et al. Keratinocyte growth factor for the treatment of the acute respiratory distress syndrome (KARE): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Respir Med. 2017;5:484–491. [DOI] [PubMed] [Google Scholar]
- 20.Moss M, Huang DT, Brower RJ, et al. ; National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginde AA, Brower RG, Caterino JM, et al. ; National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Early high-dose Vitamin D3 for critically Ill, Vitamin D-deficient patients. N Engl J Med. 2019;381:2529–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martí-Carvajal AJ, Solà I, Gluud C, Lathyris D, Cardona AF. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012;12:CD004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice TW, Wheeler AP, Bernard GR, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010;38:1685–1694. [DOI] [PubMed] [Google Scholar]
- 24.Abraham E, Anzueto A, Gutierrez G, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- 25.Abraham E, Wunderink R, Silverman H, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha Mab Sepsis Study Group. JAMA. 1995;273:934–941. [PubMed] [Google Scholar]
- 26.Cohen J, Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med. 1996;24:1431–1440. [DOI] [PubMed] [Google Scholar]
- 27.Abraham E, Laterre PF, Garbino J, et al. Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit Care Med. 2001;29:503–510. [DOI] [PubMed] [Google Scholar]
- 28.Opal SM, Fisher CJ Jr, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25:1115–1124. [DOI] [PubMed] [Google Scholar]
- 29.Abraham E, Reinhart K, Opal S, et al. ; OPTIMIST Trial Study Group. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–247. [DOI] [PubMed] [Google Scholar]
- 30.Abraham E, Reinhart K, Svoboda P, et al. Assessment of the safety of recombinant tissue factor pathway inhibitor in patients with severe sepsis: a multicenter, randomized, placebo-controlled, single-blind, dose escalation study. Crit Care Med. 2001;29:2081–2089. [DOI] [PubMed] [Google Scholar]
- 31.Fein AM, Bernard GR, Criner GJ, et al. Treatment of severe systemic inflammatory response syndrome and sepsis with a novel bradykinin antagonist, deltibant (CP-0127). Results of a randomized, double-blind, placebo-controlled trial. CP-0127 SIRS and Sepsis Study Group. JAMA. 1997;277:482–487. [PubMed] [Google Scholar]
- 32.Petros A, Lamb G, Leone A, Moncada S, Bennett D, Vallance P. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc Res. 1994;28:34–39. [DOI] [PubMed] [Google Scholar]
- 33.Avontuur JA, Boomsma F, van den Meiracker AH, de Jong FH, Bruining HA. Endothelin-1 and blood pressure after inhibition of nitric oxide synthesis in human septic shock. Circulation. 1999;99:271–275. [DOI] [PubMed] [Google Scholar]
- 34.Grover R, Zaccardelli D, Colice G, Guntupalli K, Watson D, Vincent JL. An open-label dose escalation study of the nitric oxide synthase inhibitor, N(G)-methyl-L-arginine hydrochloride (546C88), in patients with septic shock. Glaxo Wellcome International Septic Shock Study Group. Crit Care Med. 1999;27:913–922. [DOI] [PubMed] [Google Scholar]
- 35.Schoonover LL, Stewart AS, Clifton GD. Hemodynamic and cardiovascular effects of nitric oxide modulation in the therapy of septic shock. Pharmacotherapy. 2000;20:1184–1197. [DOI] [PubMed] [Google Scholar]
- 36.Vincent JL, Privalle CT, Singer M, et al. Multicenter, randomized, placebo-controlled phase III study of pyridoxalated hemoglobin polyoxyethylene in distributive shock (PHOENIX). Crit Care Med. 2015;43:57–64. [DOI] [PubMed] [Google Scholar]
- 37.López A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. [DOI] [PubMed] [Google Scholar]
- 40.Gallant-Behm CL, Piper J, Dickinson BA, Dalby CM, Pestano LA, Jackson AL. A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Repair Regen. 2018;26:311–323. [DOI] [PubMed] [Google Scholar]
- 41.Rayner KJ, Sheedy FJ, Esau CC, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rotllan N, Ramírez CM, Aryal B, Esau CC, FernándezHernando C. Therapeutic silencing of microRNA-33 inhibits the progression of atherosclerosis in Ldlr−/− mice–brief report. Arterioscler Thromb Vasc Biol. 2013;33:1973–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallant-Behm CL, Piper J, Lynch JM, et al. A MicroRNA-29 Mimic (Remlarsen) Represses extracellular matrix expression and fibroplasia in the skin. J Invest Dermatol. 2019;139:1073–1081. [DOI] [PubMed] [Google Scholar]
- 44.Seto AG, Beatty X, Lynch JM, et al. Cobomarsen, an oligonucleotide inhibitor of miR-155, co-ordinately regulates multiple survival pathways to reduce cellular proliferation and survival in cutaneous T-cell lymphoma. Br J Haematol. 2018;183:428–444. [DOI] [PubMed] [Google Scholar]
- 45.Godoy PM, Bhakta NR, Barczak AJ, et al. Large differences in small RNA composition between human biofluids. Cell Rep. 2018;25:1346–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pritchard CC, Kroh E, Wood B, et al. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res (Phila). 2012;5:492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui M, Wang H, Yao X, et al. Circulating MicroRNAs in cancer: potential and challenge. Front Genet. 2019;10:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pham T, Rubenfeld GD. Fifty years of Research in ARDS. The epidemiology of acute respiratory distress syndrome. A 50th birthday review. Am J Respir Crit Care Med. 2017;195:860–870. [DOI] [PubMed] [Google Scholar]
- 49.Marco Ranieri V, Rubenfeld GD, Taylor Thompson B, et al. ; ARDS Definition Task Force. Acute respiratory distress syndrome: the berlin definition. JAMA. 2012;307:2526–2533. [DOI] [PubMed] [Google Scholar]
- 50.Mashima R Physiological roles of miR-155. Immunology. 2015;145:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludwig N, Leidinger P, Becker K, et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016;44:3865–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu F, Nie C, Zhao N, et al. MiR-155 alleviates septic Lung injury by inducing autophagy via inhibition of transforming growth factor-β-activated binding protein 2. Shock. 2017;48:61–68. [DOI] [PubMed] [Google Scholar]
- 53.Dong G, Liu S, Liu J, et al. Upregulation of miR-155 contributes to the suppression of inflammatory responses by targeting Socs1 in LPS-induced acute lung injury. Int J Clin Exp Pathol. 2016;9:7010–7019. [Google Scholar]
- 54.Jiang J, Song Z, Zhang L. miR-155–5p promotes progression of acute respiratory distress syndrome by inhibiting differentiation of bone marrow mesenchymal stem cells to Alveolar type II epithelial cells. Med Sci Monit. 2018;24:4330–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han Y, Li Y, Jiang Y. The prognostic value of plasma MicroRNA-155 and MicroRNA-146a level in severe sepsis and sepsis-induced acute Lung injury patients. Clin Lab. 2016;62:2355–2360. [DOI] [PubMed] [Google Scholar]
- 56.Wu X, Wu C, Gu W, Ji H, Zhu L. Serum exosomal MicroRNAs predict acute respiratory distress syndrome events in patients with severe community-acquired pneumonia. Biomed Res Int. 2019;34:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Yuan Z, Syed M, Panchal D, et al. TREM-1-accentuated lung injury via miR-155 is inhibited by LP17 nanomedicine. Am J Physiol Lung Cell Mol Physiol. 2016;310:L426–L438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang K, Yang J, Guo S, Zhao G, Wu H, Deng G. Peripheral circulating exosome-mediated delivery of miR-155 as a novel mechanism for acute lung inflammation. Mol Ther. 2019;27:1758–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan X, Berg N, Lee JW, et al. MicroRNA miR-223 as regulator of innate immunity. J Leukoc Biol. 2018;104:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Rie D, Abugessaisa I, Alam T, et al. ; The FANTOM Consortium. An integrated expression atlas of miRNAs and their promoters in human and mouse. Nat Biotechnol. 2017;35:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narute P, Seam N, Tropea M, et al. Temporal changes in microrna expression in blood leukocytes from patients with the acute respiratory distress syndrome. Shock. 2017;47:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evankovich J, Lear T, Baldwin C, et al. Toll-like receptor 8 stability is regulated by Ring Finger 216 in response to circulating MicroRNAs. Am J Respir Cell Mol Biol. 2020;62:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park J, Jeong S, Park K, Yang K, Shin S. Expression profile of microRNAs following bone marrow-derived mesenchymal stem cell treatment in lipopolysaccharide-induced acute lung injury. Exp Ther Med. 2018;15:5495–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Testa U, Pelosi E, Castelli G, Labbaye C. miR-146 and miR-155: two key modulators of immune response and tumor development. Non-Coding RNA. 2017;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vergadi E, Vaporidi K, Theodorakis EE, et al. Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR-146a induction in mice. J Immunol. 2014;192:394–406. [DOI] [PubMed] [Google Scholar]
- 66.Zeng Z, Gong H, Li Y, et al. Upregulation of miR-146a contributes to the suppression of inflammatory responses in LPS-induced acute lung injury. Exp Lung Res. 2013;39:275–282. [DOI] [PubMed] [Google Scholar]
- 67.Xie W, Li L, Zhang M, et al. MicroRNA-27 prevents atherosclerosis by suppressing lipoprotein lipase-induced lipid accumulation and inflammatory response in apolipoprotein E knockout mice. PloS One. 2016;11:e0157085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z, Ruan Z, Mao Y, et al. miR-27a is up regulated and promotes inflammatory response in sepsis. Cell Immunol. 2014;290:190–195. [DOI] [PubMed] [Google Scholar]
- 69.Zheng Y, Liu S, Sun Q, et al. Plasma microRNAs levels are different between pulmonary and extrapulmonary ARDS patients: a clinical observational study. Ann Intensive Care. 2018;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biron BM, Ayala A, Lomas-Neira JL. Biomarkers for sepsis: what is and what might be? Biomark Insights. 2015;10(suppl 4):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–1677. [DOI] [PubMed] [Google Scholar]
- 72.Bryant Nguyen H, Loomba M, Yang JJ. Early lactate clearance is associated with biomarkers of inflammation, coagulation, apoptosis, organ dysfunction and mortality in severe sepsis and septic shock. J Inflamm (Lond). 2010;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Standage SW, Wong HR. Biomarkers for pediatric sepsis and septic shock. Expert Rev Anti Infect Ther. 2011;9:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Faix JD. Biomarkers of sepsis. Crit Rev Clin Lab Sci. 2013;50:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang HJ, Zhang PJ, Chen WJ, Feng D, Jia YH, Xie LX. Four serum microRNAs identified as diagnostic biomarkers of sepsis. J Trauma Acute Care Surg. 2012;73:850–854. [DOI] [PubMed] [Google Scholar]
- 76.Möhnle P, Hirschberger S, Hinske LC, et al. MicroRNAs 143 and 150 in whole blood enable detection of T-cell immunoparalysis in sepsis. Mol Med. 2018;24:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reithmair M, Buschmann D, Märte M, et al. Cellular and extracellular miRNAs are blood-compartmentspecific diagnostic targets in sepsis. J Cell Mol Med. 2017;21:2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie L. Serum MicroRNA signatures identified by Solexa sequencing predict sepsis patients’ mortality: a prospective observational study. PloS ONE. 2012;7:e38885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang JF, Yu ML, Yu G, et al. Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem Biophys Res Commun. 2010;394:184–188. [DOI] [PubMed] [Google Scholar]
- 80.Puskarich MA, Nandi U, Shapiro NI, Trzeciak S, Kline JA, Jones AE. Detection of microRNAs in patients with sepsis. J Acute Dis. 2015;4:101–106. [Google Scholar]
- 81.Benz F, Tacke F, Luedde M, et al. Circulating microRNA-223 serum levels do not predict sepsis or survival in patients with critical illness. Dis Markers. 2015;2015:384208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang L, Wang HC, Chen C, et al. Differential expression of plasma miR-146a in sepsis patients compared with non-sepsis-SIRS patients. Exp Ther Med. 2013;5:1101–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Real JM, Ferreira LRP, Esteves GH, et al. Exosomes from patients with septic shock convey miRNAs related to inflammation and cell cycle regulation: new signaling pathways in sepsis? Crit Care. 2018;22:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caserta S, Kern F, Cohen J, Drage S, Newbury SF, Llewelyn MJ. Circulating plasma microRNAs can differentiate human sepsis and systemic inflammatory response syndrome (SIRS). Sci Rep. 2016;6:28006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang HJ, Deng J, Wang JY, et al. Serum miR-122 levels are related to coagulation disorders in sepsis patients. Clin Chem Lab Med. 2014;52:927–933. [DOI] [PubMed] [Google Scholar]
- 86.Vasilescu C, Dragomir M, Tanase M, et al. Circulating miRNAs in sepsis-A network under attack: an in-silico prediction of the potential existence of miRNA sponges in sepsis. PloS One. 2017;12:e0183334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vasilescu C, Rossi S, Shimizu M, et al. MicroRNA fingerprints identify miR-150 as a plasma prognostic marker in patients with sepsis. PloS One. 2009;4:e7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roderburg C, Luedde M, Vargas Cardenas D, et al. Circulating microRNA-150 serum levels predict survival in patients with critical illness and sepsis. PloS One. 2013;8:e54612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.How CK, Hou SK, Shih HC, et al. Expression profile of MicroRNAs in gram-negative bacterial sepsis. Shock. 2015;43:121–127. [DOI] [PubMed] [Google Scholar]
- 90.Yang L, Boldin MP, Yu Y, et al. miR-146a controls the resolution of T cell responses in mice. J Exp Med. 2012;209:1655–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu J, Feng Y, Jeyaram A, Jay SM, Zou L, Chao W. Circulating plasma extracellular vesicles from septic mice induce inflammation via microRNA- and TLR7-dependent mechanisms. J Immunol. 2018;201:3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li S, Zhao D, Cui J, Wang L, Ma X, Li Y. Correlation of microRNA-125a/b with acute respiratory distress syndrome risk and prognosis in sepsis patients. J Clin Lab Anal. 2020;34:e23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neudecker V, Brodsky KS, Kreth S, Ginde AA, Eltzschig HK. Emerging roles for microRNAs in perioperative medicine. Anesthesiology. 2016;124:489–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li P, Yao Y, Ma Y, Chen Y. MiR-150 attenuates LPS-induced acute lung injury via targeting AKT3. Int Immunopharmacol. 2019;75:105794. [DOI] [PubMed] [Google Scholar]
- 96.Feng Z, Qi S, Zhang Y, et al. Ly6G+ neutrophil-derived miR-223 inhibits the NLRP3 inflammasome in mitochondrial DAMP-induced acute lung injury. Cell Death Dis. 2017;8:e3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang D, Lee H, Wang X, et al. A potential role of microvesicle-containing miR-223/142 in lung inflammation. Thorax. 2019;74:865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan Y, Lu K, Ye T, Zhang Z. MicroRNA-223 attenuates LPS-induced inflammation in an acute lung injury model via the NLRP3 inflammasome and TLR4/NF-κB signaling pathway via RHOB. Int J Mol Med. 2019;43:1467–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang G, Su J, Zhang M, et al. RhoB regulates the function of macrophages in the hypoxia-induced inflammatory response. Cell Mol Immunol. 2017;14:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie T, Liang J, Liu N, et al. MicroRNA-127 inhibits lung inflammation by targeting IgG Fcγ receptor I. J Immunol. 2012;188:2437–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rajput C, Tauseef M, Farazuddin M, et al. MicroRNA-150 suppression of angiopoetin-2 generation and signaling is crucial for resolving vascular injury. Arterioscler Thromb Vasc Biol. 2016;36:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Neudecker V, Brodsky KS, Clambey ET, et al. Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice. Sci Transl Med. 2017;9:eaah5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guo C, Goodwin A, Buie JNJ, et al. A stromal cell-derived factor 1α analogue improves endothelial cell function in lipopolysaccharide-induced acute respiratory distress syndrome. Mol Med. 2016;22:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou Y, Li P, Goodwin AJ, et al. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit Care. 2019;23:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou Y, Li P, Goodwin AJ, et al. Exosomes from endothelial progenitor cells improve the outcome of a Murine model of sepsis. Mol Ther. 2018;26:1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neudecker V, Yuan X, Bowser JL, Eltzschig HK. MicroRNAs in mucosal inflammation. J Mol Med Berl Ger. 2017;95:935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang C, Xiao X, Chintagari NR, Breshears M, Wang Y, Liu L. MicroRNA and mRNA expression profiling in rat acute respiratory distress syndrome. BMC Med Genomics. 2014;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ying H, Kang Y, Zhang H, et al. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J Immunol. 2015;194:1239–1251. [DOI] [PubMed] [Google Scholar]
- 111.Li Q, Ge YL, Li M, et al. miR-127 contributes to ventilator-induced lung injury. Mol Med Rep. 2017;16:4119–4126. [DOI] [PubMed] [Google Scholar]
- 112.Liau NPD, Laktyushin A, Lucet IS, et al. The molecular basis of JAK/STAT inhibition by SOCS1. Nat Commun. 2018;9:1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takaesu G, Kishida S, Hiyama A, et al. TAB 2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell. 2000;5:649–658. [DOI] [PubMed] [Google Scholar]
- 114.Buras JA, Holzmann B, Sitkovsky M. Animal Models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. [DOI] [PubMed] [Google Scholar]
- 115.Magna M, Pisetsky DS. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol Med Camb Mass. 2014;20:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal. 2011;15:1607–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu LF, Boldin MP, Chaudhry A, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142:914–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Möhnle P, Schütz SV, van der Heide V, et al. MicroRNA-146a controls Th1-cell differentiation of human CD4 + T lymphocytes by targeting PRKCε: molecular immunology. Eur J Immunol. 2015;45:260–272. [DOI] [PubMed] [Google Scholar]
- 119.Schult P, Roth H, Adams RL, et al. microRNA-122 amplifies hepatitis C virus translation by shaping the structure of the internal ribosomal entry site. Nat Commun. 2018;9:2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gebert LFR, Rebhan MAE, Crivelli SEM, Denzler R, Stoffel M, Hall J. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 2014;42:609–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lima JF, Cerqueira L, Figueiredo C, Oliveira C, Azevedo NF. Anti-miRNA oligonucleotides: a comprehensive guide for design. RNA Biol. 2018;15:338–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barta T, Peskova L, Hampl A. miRNAsong: a web-based tool for generation and testing of miRNA sponge constructs in silico. Sci Rep. 2016;6:36625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang J, Liu L, Xu T, et al. miRspongeR: an R/Bioconductor package for the identification and analysis of miRNA sponge interaction networks and modules. BMC Bioinformatics. 2019;20:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu X, Abraham JM, Cheng Y, et al. Synthetic circular RNA functions as a miR-21 sponge to suppress gastric Carcinoma cell proliferation. Mol Ther Nucleic Acids. 2018;13:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hollensen AK, Andersen S, Hjorth K, et al. Enhanced tailored MicroRNA sponge activity of RNA Pol II-transcribed TuD Hairpins relative to ectopically expressed ciRS7-derived circRNAs. Mol Ther Nucleic Acids. 2018;13:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kent OA, Steenbergen C, Das S. In vivo nanovector delivery of a heart-specific microRNA-sponge. J Vis Exp JoVE. 2018;136:57845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bofill-De Ros X, Santos M, Vila-Casadesús M, et al. Genome-wide miR-155 and miR-802 target gene identification in the hippocampus of Ts65Dn Down syndrome mouse model by miRNA sponges. BMC Genomics. 2015;16:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cesana M, Cacchiarelli D, Legnini I, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tay Y, Kats L, Salmena L, et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell. 2011;147:344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17:272–283. [DOI] [PubMed] [Google Scholar]
- 131.Gumireddy K, Young DD, Xiong X, Hogenesch JB, Huang Q, Deiters A. Small molecule inhibitors of microRNA miR-21 function. Angew Chem Int Ed Engl. 2008;47:7482–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qu J, Chen X, Sun YZ, et al. In silico prediction of small molecule-miRNA associations based on the HeteSim algorithm. Mol Ther Nucleic Acids. 2019;14:274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Connelly CM, Deiters A. Identification of inhibitors of microRNA function from small molecule screens. In: Arenz C, ed. MiRNA Maturation: Methods and Protocols. Methods in Molecular Biology. Totowa, NJ: Humana Press; 2014:147–156. [DOI] [PubMed] [Google Scholar]
- 134.Jayaraj GG, Nahar S, Maiti S. Nonconventional chemical inhibitors of microRNA: therapeutic scope. Chem Commun (Camb). 2015;51:820–831. [DOI] [PubMed] [Google Scholar]
- 135.Zöllner H, Hahn SA, Maghnouj A. Lentiviral overexpression of miRNAs. Methods Mol Biol. 2014;1095:177–190. [DOI] [PubMed] [Google Scholar]
- 136.Beg MS, Brenner AJ, Sachdev J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. 2017;35:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reid G, Kao SC, Pavlakis N, et al. Clinical development of TargomiRs, a miRNA mimic-based treatment for patients with recurrent thoracic cancer. Epigenomics. 2016;8:1079–1085. [DOI] [PubMed] [Google Scholar]
- 138.Kortylewski M, Nechaev S. How to train your dragon: targeted delivery of microRNA to cancer cells in vivo. Mol Ther. 2014;22:1070–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Zandwijk N, Pavlakis N, Kao SC, et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017;18:1386–1396. [DOI] [PubMed] [Google Scholar]
- 140.Vautrin A, Manchon L, Garcel A, et al. Both anti-inflammatory and antiviral properties of novel drug candidate ABX464 are mediated by modulation of RNA splicing. Sci Rep. 2019;9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ishida M, Selaru FM. miRNA-Based therapeutic strategies. Curr Anesthesiol Rep. 2013;1:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bartoszewski R, Sikorski AF. Editorial focus: understanding off-target effects as the key to successful RNAi therapy. Cell Mol Biol Lett. 2019;24:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hanna J, Hossain GS, Kocerha J. The potential for microRNA therapeutics and clinical research. Front Genet. 2019;10:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maltby S, Plank M, Tay HL, Collison A, Foster PS. Targeting MicroRNA function in respiratory diseases: mini-review. Front Physiol. 2016;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


