Abstract
Aim: To explore the predictive values of different small vessel disease (SVD) scores on functional recoveries and the clinical cerebrovascular events in mild intracerebral hemorrhage (ICH).
Methods: In this study, we enrolled conscious and mild ICH patients without surgery and further divided them into the cerebral amyloid angiopathy (CAA)-ICH group and hypertension (HTN)-ICH group. The severity of individual SVD markers, including lacunes, cerebral microbleeds (CMBs), enlarged perivascular spaces (EPVS), white matter hyperintensity (WMH), and cortical superficial siderosis (cSS), was evaluated. The original SVD score, modified SVD score, refined SVD score, and CAA-SVD score and the total number of SVD markers were further calculated. Functional recoveries were evaluated using the modified Rankin scale. Recurrences of stroke were defined as readmission to the hospital with a definite diagnosis of stroke.
Results: A total of 163 ICH patients (60 CAA-ICH and 103 HTN-ICH) were included in the study. The CAA-SVD score (OR=3.429; 95% confidence interval (CI)=1.518–7.748) had the best predictive effect on functional dependence in the CAA-ICH group, among which cSS severities probably played a vital role (OR=4.665; 95% CI=1.388–15.679). The total number of SVD markers [hazard ratio (HR)=3.765; 95% CI=1.467–9.663] can better identify stroke recurrences in CAA-ICH. In HTN-ICH, while the total number of SVD markers (HR=2.136; 95% CI=1.218–3.745) also demonstrated association with recurrent stroke, this effect seems to be related with the influence of lacunes (HR=5.064; 95% CI=1.697–15.116).
Conclusions: The CAA-SVD score and the total number of SVD markers might identify mild CAA-ICH patients with poor prognosis. However, it would be better to focus on lacunes rather than on the overall burden of SVD to predict recurrent strokes in HTN-ICH.
Keywords: Different Small Vessel Disease Scores, CAA-related ICH, HTN-related ICH, Functional recoveries, Clinical cerebrovascular events
Introduction
Cerebral small vessel disease (CSVD) is a disorder of the penetrating cerebral small vessels, such as arterioles, capillaries, and venules 1) . Lacunas, cerebral microbleeds (CMBs), enlarged perivascular spaces (EPVS), white matter hyperintensities (WMH), and cortical superficial siderosis (cSS) are all imaging markers of CSVD 2 - 6) . Previous studies have demonstrated the clinical significance of individual and separated CSVD markers on cognitive impairments, functional recoveries, and stroke recurrences in patients with stroke 7 - 9) . However, different CSVD markers could be simultaneously presented in one patient, and recent studies have attempted to convey more information by conducting different semi-quantitative assessments of the cumulative burden.
The concept of the original SVD score 10 , 11) and the modified SVD score 12) , evaluated by lacunas, CMBs, EPVS, and WMHs, was first introduced in ischemic stroke patients. Moreover, it has been pointed out that the original SVD score significantly influenced the functional recoveries of ICH patients 13) . Other scholars believed that the total number of the four SVD markers previously mentioned could also predict the adverse functional outcome of ICH 14) . Besides, to accurately calculate the total SVD burden in cerebral amyloid angiopathy (CAA) patients, the cerebral amyloid angiopathy-small vessel disease (CAA-SVD) score evaluating lobar CMBs, centrum semiovale (CSO)-EPVS, WMH, and cSS was further proposed to demonstrate the underlying pathological severity of CAA 15) . Subsequently, researchers found that the CAA-SVD score could be adapted to predict the risk of ICH recurrence in patients with CAA 16) . In this study, for the first time, we put those five individual markers together and summarized the effect of different SVD scores on the prognosis of ICH patients with different etiologies. Furthermore, as deep CMBs and hypertensive ICH may be closely related 17) , we proposed the refined SVD score in the HTN-ICH group by placing more emphasis on deep CMBs, especially severe deep CMBs.
In our years of clinical practice, we have found that clinicians usually focus more on comatose ICH patients requiring surgical treatments due to the high rates of mortality and disability. However, for mild and conscious ICH patients supported with conservative medication, neurologists may be less proactive in the follow-up due to the relatively good prognosis. Thus, we enrolled mild spontaneous ICH patients without surgical treatment and further divided them into the CAA-ICH or HTN-ICH groups. We would like to explore the following questions: (1) among mild ICH patients with different etiologies, whether individualized and calculated burden of SVD markers could predict functional dependence and stroke recurrence, and (2) in different semi-quantitative methods of the SVD burden, whether there is a difference in the predictive ability of prognosis. The analysis might help neurologists identify mild ICH patients with poor outcomes and take effective preventive measures in advance.
Methods
Patients
This study retrospectively analyzed prospectively collected data from an observational study of conscious and spontaneous ICH patients without surgery. Patients were recruited from August 2012 to October 2019 in the neurology wards in seven independent general hospitals with stroke units accredited by the Chinese Stroke Association. These seven hospitals were Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Ruijin North Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Pudong District Gongli Hospital, Jiaxing First Municipal Hospital, Zhongshan Hospital Qingpu Branch affiliated to Fudan University, Minhang Hospital affiliated to Fudan University, and Haiyan County People’s Hospital.
According to the modified Boston criteria, the inclusion criteria for CAA-ICH patients were as follows: 1) age ≥ 55 years; 2) multiple hemorrhages restricted to the lobar, cortical, or corticosubcortical regions (cerebellar hemorrhage allowed) or single lobar, cortical, or corticosubcortical hemorrhage and focal or disseminated superficial siderosis; and 3) the absence of other causes of hemorrhage 18 , 19) . The inclusion criteria for the HTN-ICH patients were as follows: 1) ≥ 1 year of hypertension history; 2) hemorrhages (ICH and CMBs) restricted to the deep regions with or without deep microbleeds but no lobar microbleeds 20) . The exclusion criteria were as follows: 1) ICH secondary to brain tumors, trauma, or hematological diseases; 2) mixed hemorrhage (ICH and CMBs) in the lobar and deep regions; 3) deep hematomas without hypertension; 4) lobar, cortical, or subcortical hematomas with age <55 years; 5) examination with computed tomography (CT) scan over 3 days or magnetic resonance imaging (MRI) over 7 days following onset; 6) without completed or qualified clinical and imaging data; 7) lost to follow-up; and 8) pregnancy or refusal to participate in the study.
This study was approved by the ethics committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Written informed consent for data collection and clinical outcomes was obtained from the patient or a legally authorized representative in all cases.
Imaging Analysis
All patients were examined via cranial CT within 3 days and MRI within 7 days following ICH onset. A GE Signa HDxT 3.0 T superconducting MRI system was utilized. All patients were first examined with axial T1WI, T2WI, and then diffusion-weighted imaging and susceptibility-weighted imaging (SWI). In this multicenter study, different hospitals used the same imaging parameters. All imaging data were randomly read and recorded by two senior radiologists under double-blind conditions. The inter-rater reliability of neuroimaging variable was tested using the kappa statistic.
Neuroimaging markers for CSVD were evaluated according to the STandards for Reporting Vascular changes on nEuroimaging (STRIVE criteria) 21) . Lacunes were defined as rounded or ovoid lesions of cerebrospinal fluid signal, that is, hyperintensities on a T2-weighted sequence with corresponding hypointensities with a hyperintense rim on fluid-attenuated inversion recovery (FLAIR), ranging from 3 to 20 mm in diameter in bilateral hemispheres 22) . CMBs were defined as round or oval foci with a low signal or signal loss on SWI, generally with diameters ranging from 2 to 5 mm and a maximum of 10 mm. CMB severity was classified as mild ( n =1), moderate ( n =2–4), or severe ( n ≥ 5) 23) . EPVS were defined as <3 mm punctate or linear hyperintensities on a T2-weighted sequence with corresponding hypointensities on a T1/FLAIR sequence. EPVS were rated in the regions of the centrum semiovale (CSO) and basal ganglia (BG). EPVS severity was classified as mild (≤ 10 EPVS), moderate (11–20 EPVS), or severe (≥ 20 EPVS) 24) . WMHs were identified as white matter hyperintensities on the FLAIR sequence. WMHs in the deep (DWMH) and periventricular (PVWMH) regions were graded from 0 to 3 using the semi-quantitative Fazekas scale. WMH severity was classified as mild (Fazekas score=1–2), moderate (Fazekas score=3–4), or severe (Fazekas score=5–6) 24) . cSS was defined as curvilinear signal loss on gradient-recalled echo following the cortical gyral surface, and cSS severity was classified as focal (≤ 3 sulci) or disseminated (>3 sulci) 27) . In this study, only one patient had acute convexity subarachnoid hemorrhage (cSAH), lacking statistical value. Thus, these data were not reflected in the paper.
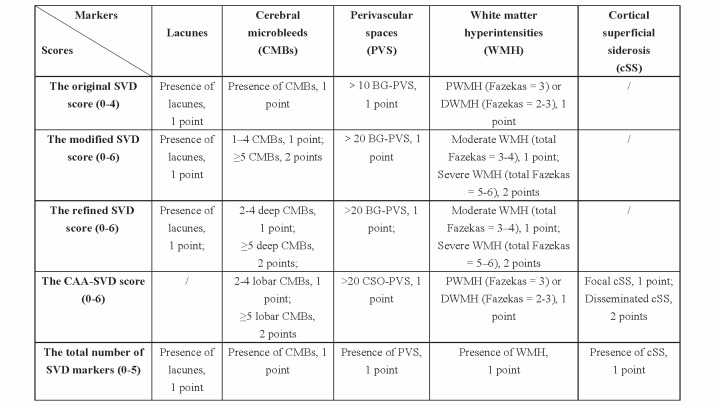
The original SVD score (0–4) 10) was assessed as follows: presence of lacunes, 1 point; presence of CMBs, 1 point; >10 BG-EPVS, 1 point; and periventricular WMH (Fazekas=3) or deep WMH (Fazekas=2–3), 1 point. The modified SVD score (0–6) 12) was assessed as follows: presence of lacunes, 1 point; 1–4 CMBs, 1 point; ≥ 5 CMBs, 2 points; >20 BG-EPVS, 1 point; moderate WMH (total Fazekas=3–4), 1 point; and severe WMH (total Fazekas=5–6), 2 points. The CAA-SVD score (0–6) 28) was assessed as follows: 2–4 lobar CMBs, 1 point; ≥ 5 lobar CMBs, 2 points; >20 CSO-EPVS, 1 point; confluent deep WMHs (Fazekas score=2 or 3) or irregular periventricular WMHs extending into the deep white matter (Fazekas score=3): 1 point focal; cSS, 1 point; and disseminated cSS, 2 points. Since patients with deep CMBs were excluded from the CAA-ICH group and those with lobar CMBs were excluded from the HTN-ICH group, we proposed the refined SVD score in HTN-ICH patients by placing more emphasis on deep CMBs, especially severe deep CMBs: presence of lacunes, 1 point; 2–4 deep CMBs, 1 point, ≥ 5 deep CMBs, 2 points; >20 BG-EPVS, 1 point; moderate WMH (total Fazekas=3–4), 1 point; and severe WMH (total Fazekas=5–6), 2 points. The total number of SVD markers was assessed as follows: 1 point for each of the presence of lacunes, CMBs, EPVS, WMHs, and cSS ( Fig.1 ) .
Fig.1.
Features and categories of different small vessel disease scores
Prognoses
Within 1 year after the onset, patients were followed up via telephone every 3 months by trained professional neurologists. We used two prognostic end points: functional dependence and stroke recurrence. Functional dependence was measured 3 months after the onset and was defined as modified Rankin scale (mRS) >2 29) . Recurrent stroke was defined as a sudden new neurologic deficit that suits the definition of ischemic stroke or ICH. Patients with suspected recurrent stroke received repeat neuroimaging in the form of cranial CT or MRI to support the diagnosis.
Statistical Methods
We consulted professional statisticians to conduct the statistical analysis. The SPSS 22.0 software (SPSS Inc., Chicago, IL, USA) and the GraphPad Prism 8.0 software (GraphPad, San Diego, CA, USA) were utilized. ICH patients were divided into the CAA-ICH group and HTN-ICH group. The baseline clinical data and imaging data of these two groups were then compared. Continuous variables were analyzed using the Kolmogorov–Smirnov normal test. The data following a normal distribution were expressed as mean±standard deviation, and a t test was employed. For non-normal continuous variables, data were expressed as medians (interquartile ranges), and the Mann–Whitney U test was employed. Categorical variables were expressed as frequencies or percentages and analyzed using either the χ 2 test or Fisher’s exact test.
Univariable logistic regression was employed with functional dependence as dependent variable and different SVD scores as independent variables. Backward stepwise multiple logistic regression analysis was then conducted. Age, sex, and baseline clinical characteristics associated with functional dependence in the univariable analysis ( P <0.1) were further used as covariates. Univariable Cox regression was employed to analyze the relationship between stroke recurrence and different SVD scores. Backward stepwise multiple Cox regression analysis was conducted, with age, sex, and vascular risk factors fully adjusted.
Data Availability Statement
Patient-related data will be shared upon request by the corresponding author or any qualified investigator.
Results
Study Population
A total of 163 patients (60 with CAA-ICH and 103 with HTN-ICH) were finally included in the study ( Fig.2 ) . Three patients died during the 1-year follow-up: two died of recurrent stroke, whereas the other one died of heart disease. Compared with HTN-ICH patients, CAA-ICH patients were more likely to be older and have larger hematoma volumes but milder clinical symptoms upon admission, as assessed by the National Institutes of Health Stroke Scale (NIHSS) score. In addition, CAA-ICH patients ( P <0.05 for all) were found to have more SVD markers (cSS, severe CSO-EPVS, and severe WMH) and higher CAA-SVD score. Baseline clinical characteristics and radiological outcomes are presented in Table 1 .
Fig.2.
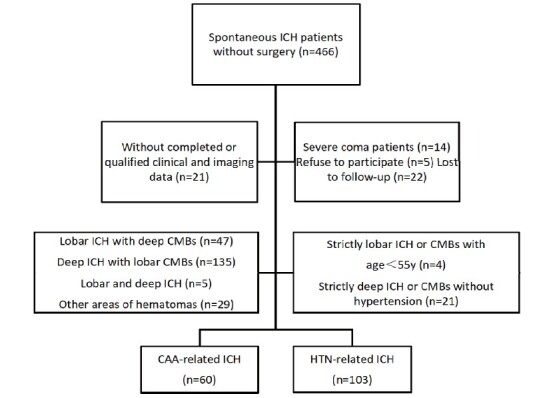
Study flowchart
Table 1. Baseline Characteristics of the Study Population.
| Total Cohort ( N = 163) | CAA-related ICH ( n = 60) | HTN-related ICH ( n = 103) | P value | |
|---|---|---|---|---|
| Baseline clinical characteristics | ||||
| Age, means±SD | 63.3±13.6 | 71.0±9.9 | 58.8±11.8 | <0.001 |
| Sex, male (%) | 100 (61.3) | 32 (53.2) | 68 (66.0) | 0.109 |
| Hypertension, n (%) | 132 (80.9) | 29 (48.3) | 103 (100) | <0.001 |
| Diabetes mellitus, n (%) | 27 (16.6) | 10 (16.7) | 17 (16.5) | 0.542 |
| Dyslipidemia, n (%) | 38 (23.3) | 15 (25.0) | 23 (22.3) | 0.698 |
| Atrial fibrillation, n (%) | 6 (3.9) | 4 (6.7) | 2 (1.9) | 0.194 |
| Previous stroke, n (%) | 23 (14.1) | 13 (21.7) | 10 (9.7) | 0.034 |
| Antiplatelet use , n (%) | 27 (16.6) | 12 (20.0) | 15 (14.6) | 0.300 |
| Statin use, n (%) | 15 (9.2) | 7 (11.7) | 8 (7.8) | 0.209 |
| Admission NIHSS, median (IQR) | 4 (2-6) | 2 (1-5) | 4 (2-7) | 0.001 |
| Admission GCS, median (IQR) | 15 (15-15) | 15 (15-15) | 15 (15-15) | 0.319 |
| Hematoma volume, means±SD | 9.4±10.4 | 12.4±13.2 | 7.6±7.9 | 0.004 |
| Imaging characteristics | ||||
| Lacunes, n (%) | 26 (16.0) | 7 (11.7) | 19 (18.4) | 0.254 |
| CMBs, n (%) | 65 (39.9) | 22 (36.2) | 43 (41.7) | 0.523 |
| 1 | 28 (17.2) | 13 (21.7) | 15 (14.6) | 0.246 |
| 2~4 | 28 (17.2) | 7 (11.7) | 21 (20.4) | 0.155 |
| ≥ 5 | 9 (5.5) | 2 (3.3) | 7 (6.8) | 0.488 |
| lobar | 16 (9.8) | 16 (26.7) | 0 (0) | <0.001 |
| deep | 39 (23.9) | 0 (0) | 39 (37.9) | <0.001 |
| > 20 CSO-EPVS, n (%) | 34 (20.8) | 23 (38.3) | 11 (10.7) | <0.001 |
| > 20 BG-EPVS, n (%) | 18 (11.0) | 5 (8.3) | 13 (12.6) | 0.400 |
| WMHs, n (%) | 122 (74.8) | 46 (76.7) | 76 (73.8) | 0.683 |
| 1-2 | 37 (22.7) | 8 (13.3) | 29 (28.2) | 0.029 |
| 3-4 | 48 (29.4) | 18 (30.0) | 30 (29.1) | 0.906 |
| 5-6 | 37 (22.7) | 20 (33.3) | 17 (16.5) | 0.013 |
| cSS, n (%) | 11 (6.7) | 9 (15.0) | 2 (1.9) | 0.002 |
| Disseminated cSS | 7 (4.3) | 6 (10.0) | 1 (1.0) | 0.006 |
| Focal cSS | 4 (2.5) | 3 (5.0) | 1 (1.0) | 0.109 |
| The original SVD score, median (IQR) | 1 (0-2) | 1 (1-2) | 1 (0-2) | 0.570 |
| The modified SVD score, median (IQR) | 1 (1-3) | 1 (1-3) | 1 (0-2) | 0.567 |
| The refined SVD score, median (IQR) | 1 (0-2) | 1 (0-2) | 1 (0-2) | 0.822 |
| The CAA-SVD score, median (IQR) | 1 (0-1) | 1 (0-2) | 0 (0-1) | <0.001 |
| Total number of SVD markers, median (IQR) | 1 (1-2) | 1 (1-2) | 1 (1-2) | 0.779 |
| Recurrent stroke | 21 (12.9) | 8 (13.3) | 13 (12.6) | 0.975 |
Abbreviation: HTN = Hypertension; CAA = Cerebral amyloid angiopathy; ICH = Intracerebral Hemorrhage; NIHSS = National Institutes of Health Stroke Scale; GCS = Glasgow Coma Scale; cSS = Cortical Superficial Siderosis; CMBs = Cerebral Microbleeds; WMHs = White Matter Hyperintensities; EPVS = Enlarged Perivascular Spaces; CSO = Centrum Semiovale ; BG = Basal Ganglia; SVD = Small Vessel Disease; Results are expressed as numbers (column %), means (SD) or medians (interquartile range) as appropriate.
Univariate Analysis of Factors Associated with Functional Dependence
In the CAA-ICH group, 11 patients (18.3%) were found to be functionally dependent 3 months after onset. Univariable analyses revealed that cSS (OR=4.322; 95% CI=1.624–11.503; P =0.003) and the CAA-SVD score (OR=3.299; 95% CI=1.656–6.572; P =0.001) demonstrated stronger associations with functional dependence compared with other individual and total burden of SVD markers.
In the HTN-ICH group, 19 patients (18.4%) were found to exhibit functional dependence. The modified SVD score (OR=1.764; 95% CI=1.151–2.704; P =0.009) and the refined SVD score (OR=1.893; 95% CI=1.202–2.980; P =0.006) both demonstrated correlations with functional dependence in the univariable analyses ( Table 2 ) .
Table 2. Univariate analysis of factors associated with functional dependence * .
| CAA-related ICH ( n = 60) | HTN-related ICH ( n = 103) | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Baseline clinical characteristics | ||||
| Age | 1.082 (1.007-1.162) | 0.031 | 1.031 (0.987-1.076) | 0.176 |
| Male | 0.942 (0.253-3.501) | 0.929 | 0.875 (0.301-2.544) | 0.807 |
| Hypertension | 0.737 (0.198-2.740) | 0.649 | / | / |
| Diabetes mellitus | 1.111 (0.201-6.143) | 0.904 | 0.541 (0.113-2.596) | 0.443 |
| Dyslipidemia | 1.025 (0.365-6.348) | 0.563 | 1.126 (0.438-7.462) | 0.413 |
| Previous stroke | 0.768 (0.144-4.089) | 0.757 | 1.118 (0.218-5.740) | 0.894 |
| Antiplatelet use | 1.061 (0.222-5.078) | 0.941 | 1.023 (0.136-3.046) | 0.867 |
| Statin use | 0.702 (0.114-4.338) | 0.703 | 0.921 (0.256-5.471) | 0.643 |
| Admission NIHSS | 1.167 (0.984-1.384) | 0.076 | 1.384 (1.187-1.614) | <0.001 |
| Admission GCS | 1.825 (0.303-10.979) | 0.511 | 0.426 (0.209-0.869) | 0.019 |
| Hematoma volume | 1.052 (1.000-1.108) | 0.051 | 1.057 (0.998-1.120) | 0.059 |
| Imaging markers of CSVD | ||||
| Lacunes | 2.057 (0.330-12.809) | 0.439 | 1.370 (0.425-4.419) | 0.598 |
| CMBs | 1.126 (0.855-1.482) | 0.399 | 1.190 (973-1.4554) | 0.090 |
| EPVS | 2.763 (0.712-10.725) | 0.142 | 1.818 (0.651-5.075) | 0.254 |
| WMH | 1.458 (1.007-2.111) | 0.046 | 1.215 (0.955-1.545) | 0.113 |
| cSS | 4.322 (1.624-11.503) | 0.003 | 2.822 (0.697-11.429) | 0.146 |
| The original SVD score | 2.361 (1.033-5.398) | 0.042 | 1.378 (0.854-2.223) | 0.189 |
| The modified SVD score | 2.249 (1.203-4.208) | 0.011 | 1.764 (1.151-2.704) | 0.009 |
| The refined SVD score | / | / | 1.893 (1.202-2.980) | 0.006 |
| The CAA-SVD score | 3.299 (1.656-6.572) | 0.001 | / | / |
| Total number of SVD markers | 3.728 (1.502-9.252) | 0.005 | 1.424 (0.800-2.538) | 0.230 |
Abbreviation: HTN = Hypertension; CAA = Cerebral amyloid angiopathy; ICH = Intracerebral Hemorrhage; NIHSS = National Institutes of Health Stroke Scale; GCS = Glasgow Coma Scale; cSS = Cortical Superficial Siderosis; CMBs = Cerebral Microbleeds; WMHs = White Matter Hyperintensities; EPVSs = Enlarged Perivascular Spaces; CSO = Centrum Semiovale ; BG = Basal Ganglia; SVD = Small Vessel Disease.
* Functional dependence was measured 3 months after onset and defined as the modified Rankin scale (mRS) > 2
Univariate Analysis of Factors Associated with Stroke Recurrence
In the CAA-ICH group, eight patients (13.3%) have suffered recurrent stroke within 1 year of ICH onset, including three cases of ischemic stroke and five cases of hemorrhagic stroke. The total number of SVD markers (OR=3.839; 95% CI=1.505–9.794; P =0.005) demonstrated the highest hazard ratio (HR) in univariable analyses among different SVD scores.
In the HTN-ICH group, 13 patients (12.6%) had suffered recurrent stroke, including 10 cases of ischemic stroke and three cases of hemorrhagic stroke. Univariable analyses revealed that stroke recurrence was associated with the original SVD score, modified SVD score, refined SVD score, and total number of SVD markers. However, those correlations may be significantly related to the influence of lacunes (OR=6.132; 95% CI=2.054–18.311; P =0.001) ( Table 3 ) .
Table 3. Univariate analysis of factors associated with stroke recurrences.
| CAA-related ICH ( n = 60) | HTN-related ICH ( n = 103) | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Baseline clinical characteristics | ||||
| Age | 0.964 (0.893-1.041) | 0.352 | 1.032 (0.985-1.081) | 0.186 |
| Male | 2.073 (0.495-8.677) | 0.318 | 1.226 (0.401-3.748) | 0.721 |
| Hypertension | 0.577 (0.138-2.413) | 0.451 | / | / |
| Diabetes mellitus | 1.861 (0.375-9.227) | 0.447 | 0.632 (0.174-2.298) | 0.486 |
| Dyslipidemia | 1.223 (0.142-7.537) | 0.637 | 0.728 (0.331-1.601) | 0.429 |
| Previous stroke | 0.494 (0.061-4.016) | 0.510 | 1.700 (0.377-7.678) | 0.490 |
| Antiplatelet use | 0.756 (0.222-4.078) | 0.529 | 0.576 (0.067-5.387) | 0.689 |
| Statin use | 1.275 (0.653-4.338) | 0.264 | 0.835 (0.321-3.965) | 0.189 |
| Admission NIHSS | 1.116 (0.960-1.297) | 0.153 | 1.124 (0.986-1.280) | 0.081 |
| Admission GCS | 0.749 (0.223-2.519) | 0.641 | 0.728 (0.331-1.601) | 0.429 |
| Hematoma volume | 1.033 (1.001-1.066) | 0.045 | 1.012 (0.950-1.079) | 0.710 |
| Imaging markers of CVSD | ||||
| Lacunes | 2.120 (0.428-10.510) | 0.357 | 6.132 (2.054-18.311) | 0.001 |
| CMBs | 1.108 (0.892-1.376) | 0.354 | 0.797 (0.258-2.455) | 0.692 |
| EPVS | 0.771 (0.184-3.227) | 0.722 | 1.957 (0.658-5.825) | 0.228 |
| WMH | 1.376 (0.936-2.024) | 0.105 | 1.251 (0.965-1.622) | 0.091 |
| cSS | 1.979 (0.941-4.160) | 0.072 | 2.176 (0.747-6.343) | 0.154 |
| The original SVD score | 2.449 (1.076-5.575) | 0.033 | 1.911 (1.193-3.061) | 0.007 |
| The modified SVD score | 1.904 (1.051-3.448) | 0.034 | 1.586 (1.047-2.403) | 0.030 |
| The refined SVD score | / | / | 1.589 (1.007-2.507) | 0.047 |
| The CAA-SVD score | 1.894 (1.216-2.950) | 0.005 | / | / |
| Total number of SVD markers | 3.839 (1.505-9.794) | 0.005 | 2.136 (1.218-3.745) | 0.008 |
Abbreviation: HTN = Hypertension; CAA = Cerebral Amyloid Angiopathy; ICH = Intracerebral Hemorrhage; NIHSS = National Institutes of Health Stroke Scale; GCS = Glasgow Coma Scale; cSS = Cortical Superficial Siderosis; CMBs = Cerebral Microbleeds; WMHs = White Matter Hyperintensities; EPVSs = Enlarged Perivascular Spaces; CSO = Centrum Semiovale ; BG = Basal Ganglia; SVD = Small Vessel Disease.
Multiple Logistic Regression Analysis Between Functional Dependence and Different SVD Scores
After conducting univariate analyses to determine the factors associated with poor functional outcomes, age, sex, and variables with a P <0.10 in Table 2 were used in the multivariate logistic regression as covariates. The multivariate analyses revealed that the CAA-SVD score (OR=3.429; 95% CI=1.518–7.748; P =0.003) was probably better to predict functional dependence in the CAA-ICH group ( Table 4 ) . Conversely, in the HTN-ICH group, the admission NIHSS (OR=1.556; 95%CI=1.262-1.918; P <0.001) may have a stronger effect on functional dependence compared with individuals and total burden of CSVD markers ( Table 5 ) .
Table 4. Multivariable adjusted models exploring associations of individual SVD markers and different SVD scores and functional dependence * in CAA-related ICH patients .
| OR (95% CI) | P value | |
|---|---|---|
| Model 1: individual SVD markers | ||
| Age | 1.098 (0.983-1.226) | 0.097 |
| Admission NIHSS | 1.211 (0.967-1.516) | 0.095 |
| Hematoma volume | 0.985 (0.903-1.075) | 0.735 |
| Lacunes | 0.349 (0.023-5.400) | 0.451 |
| Lobar CMBs | 2.486 (0.360-17.156) | 0.355 |
| Severe CSO-EPVS | 4.778 (0.568-400.125) | 0.150 |
| WMH | 2.881 (0.234-35.407) | 0.408 |
| cSS | 4.665 (1.388-15.679) | 0.013 |
| Model 2: different total SVD scores | ||
| Age | 1.120 (1.014-1.237) | 0.026 |
| Admission NIHSS | 1.249 (0.979-1.594) | 0.074 |
| Hematoma volume | 1.023 (0.935-1.120) | 0.617 |
| The original SVD score | 0.129 (0.010-1.640) | 0.114 |
| The modified SVD score | 3.816 (0.321-45.355) | 0.289 |
| The refined SVD score | 0.802 (0.095-6.764) | 0.839 |
| The CAA-SVD score | 3.429 (1.518-7.748) | 0.003 |
| Total number of SVD markers | 1.410 (0.180-11.030) | 0.744 |
Abbreviation: SVD = Small Vessel Disease; CAA = Cerebral amyloid angiopathy; NIHSS = National Institutes of Health Stroke Scale; cSS = Cortical Superficial Siderosis; CMBs = Cerebral Microbleeds; WMH = White Matter Hyperintensities; EPVS = Enlarged Perivascular Spaces; CSO = Centrum Semiovale.
* Functional dependence was measured 3 months after onset and defined as the modified Rankin scale (mRS) >2
Table 5. Multivariable adjusted models exploring associations of individual SVD markers and different SVD scores and functional dependence * in HTN-related ICH patients .
| OR (95% CI) | P value | |
|---|---|---|
| Model 1: individual SVD markers | ||
| Age | 1.095 (1.015-1.181) | 0.019 |
| Admission NIHSS | 1.679 (1.316-2.142) | <0.001 |
| Admission GCS | 0.596 (0.150-2.374) | 0.463 |
| Hematoma volume | 0.977 (0.873-1.093) | 0.685 |
| Lacunes | 0.233 (0.025-1.969) | 0.177 |
| Deep CMBs | 3.195 (0.920-11.101) | 0.068 |
| Severe BG-EPVS | 0.634 (0.050-8.026) | 0.725 |
| WMH | 1.493 (0.514-4.336) | 0.461 |
| cSS | 3.672 (0.478-28.229) | 0.211 |
| Model 2: different total SVD scores | ||
| Age | 1.066 (0.998-1.138) | 0.057 |
| Admission NIHSS | 1.556 (1.262-1.918) | <0.001 |
| Admission GCS | 0.941 (0.277-3.194) | 0.922 |
| Hematoma volume | 0.950 (0.856-1.055) | 0.337 |
| The original SVD score | 0.459 (0.102-2.058) | 0.309 |
| The modified SVD score | 1.514 (0.913-2.512) | 0.108 |
| The refined SVD score | 1.185 (0.184-7.624) | 0.858 |
| The CAA-SVD score | 1.439 (0.277-7.475) | 0.665 |
| Total number of SVD markers | 0.724 (0.213-2.460) | 0.605 |
Abbreviation: SVD = Small Vessel Disease; HTN = Hypertension; NIHSS = National Institutes of Health Stroke Scale; GCS = Glasgow Coma Scale; cSS = Cortical Superficial Siderosis; CMBs = Cerebral Microbleeds; WMH = White Matter Hyperintensities; EPVS = Enlarged Perivascular Spaces; BG = Basal Ganglia.
* Functional dependence was measured 3 months after onset and defined as the modified Rankin scale (mRS) >2
Multiple Cox Regression Analysis Between Stroke Recurrence and Different SVD Scores
The multivariate analyses revealed that the total number of SVD markers (HR=3.765; 95% CI=1.467–9.663; P =0.006) might had the best predictive value for stroke recurrence in the CAA-ICH group ( Table 6 ) . In the HTN-ICH group, while the total number of SVD markers (HR=2.136; 95% CI=1.218–3.745; P =0.008) also demonstrated association with recurrent stroke, this effect seems to be related with the influence of lacunes (HR=5.064; 95% CI=1.697–15.116; P =0.004) ( Table 7 ) .
Table 6. Multivariable adjusted models exploring associations of individual SVD markers and different total SVD scores and stroke recurrences in CAA-related ICH patients.
| HR (95% CI) | P value | |
|---|---|---|
| Model 1: individual SVD markers | ||
| Age | 0.948 (0.868-1.036) | 0.241 |
| Hematoma volume | 1.022 (0.983-1.062) | 0.266 |
| Lacunes | 2.211 (0.257-19.055) | 0.470 |
| Lobar CMBs | 1.013 (0.177-5.804) | 0.988 |
| Severe CSO-EPVS | 1.596 (0.348-7.328) | 0.548 |
| WMH | 1.376 (0.936-2.024) | 0.105 |
| cSS | 1.953 (0.929-4.107) | 0.078 |
| Model 2: different total SVD scores | ||
| Age | 0.945 (0.874-1.020) | 0.148 |
| Hematoma volume | 1.020 (0.977-1.065) | 0.370 |
| The original SVD score | 1.107 (0.094-12.998) | 0.935 |
| The modified SVD score | 0.549 (0.049-6.218) | 0.629 |
| The refined SVD score | 3.468 (0.447-26.881) | 0.234 |
| The CAA-SVD score | 1.304 (0.548-3.101) | 0.549 |
| Total number of SVD markers | 3.765 (1.467-9.663) | 0.006 |
Abbreviation: SVD = Small Vessel Disease; CAA = Cerebral amyloid angiopathy; cSS = Cortical Superficial Siderosis; CMBs = Cerebral Microbleeds; WMH = White Matter Hyperintensities; EPVS = Enlarged Perivascular Spaces; CSO = Centrum Semiovale.
Table 7. Multivariable adjusted models exploring associations of individual SVD markers and different total SVD scores and stroke recurrences in HTN-related ICH patients.
| HR (95% CI) | P value | |
|---|---|---|
| Model 1: individual SVD markers | ||
| Age | 1.032 (0.985-1.081) | 0.186 |
| Admission NIHSS | 1.108 (0.954-1.288) | 0.179 |
| Lacunes | 5.064 (1.697-15.116) | 0.004 |
| Deep CMBs | 0.572 (0.150-2.174) | 0.412 |
| Severe BG-EPVS | 0.275 (0.020-3.857) | 0.338 |
| WMH | 1.330 (0.606-2.916) | 0.477 |
| cSS | 3.135 (0.799-12.309) | 0.101 |
| Model 2: different total SVD scores | ||
| Age | 1.035 (0.980-1.093) | 0.212 |
| Admission NIHSS | 1.111 (0.968-1.276) | 0.135 |
| The original SVD score | 1.833 (0.702-4.784) | 0.216 |
| The modified SVD score | 0.575 (0.119-2.792) | 0.493 |
| The refined SVD score | 1.056 (0.264-4.232) | 0.939 |
| The CAA-SVD score | 1.582 (0.614-4.074) | 0.342 |
| Total number of SVD markers | 2.136 (1.218-3.745) | 0.008 |
Abbreviation: SVD = Small Vessel Disease; HTN = Hypertension; NIHSS = National Institutes of Health Stroke Scale; cSS = Cortical Superficial Siderosis; CMBs = Cerebral Microbleeds; WMH = White Matter Hyperintensities; EPVS = Enlarged Perivascular Spaces; BG = Basal Ganglia.
Discussion
In this study, we attempted to determine whether different SVD scores have varying predictive effects on the prognosis of mild ICH patients. The results are summarized as follows: (1) The CAA-SVD score may be the best to predict poor functional outcome in CAA-ICH patients. (2) None of the SVD scores demonstrated association with functional dependence in the HTN-ICH group, which was somewhat unexpected. (3) Moreover, the total number of SVD markers was probably better than the CAA-ICH score to identify mild CAA-ICH patients with a high risk of recurrent stroke. (4) In the HTN-ICH group, while the total number of SVD markers was found to be significant for the prediction of recurrent stroke, this effect appeared to be related with the influence of lacunes.
For the first time, we summarized the effect of different SVD scores on the prognosis of mild ICH patients without surgical treatment. Clinicians may be more concerned with the recoveries of severe ICH patients requiring surgery. Thus, our research may help neurologists identify mild ICH patients with poor prognosis and adopt effective preventive measures in advance.
In this study, we found that the CAA-SVD score may be better to predict poor functional recoveries in mild CAA-ICH patients, whereas the total number of SVD markers may be more accurate to identify stroke recurrence in the same cohort. The CAA-SVD score was proposed by the introduction of cSS, which is an emerging SVD marker. There was a hypothesis holding that cSS could reflect repeated hemorrhage in the subarachnoid space from the superficial cortical vessels. Thus, cSS may be a marker of small-vessel fragility 30 , 31) . Previous study has indicated that cSS is a stronger risk factor for ICH recurrence in CAA patients compared with the total CAA-SVD score 16) . We failed to obtain a similar conclusion. However, our study indicated that the total number of SVD markers, rather than the specific disease phenotype, could better identify mild CAA-ICH patients with a high risk of stroke recurrence. This result may suggest the potential of cSS combined with other SVD markers to predict recurrent stroke in mild ICH patients. Furthermore, we innovatively found that the CAA-SVD score was also associated with functional dependence. These findings added to our understanding of the CAA neuroimaging profiles and their relationship with the clinical outcomes. More cases and longer follow-up durations would be required for further verifications.
The limited association between the calculative burden of SVD markers and worse functional outcome in HTN-ICH patients was somewhat unexpected. Prior literature demonstrated that deep CMBs were related to HTN-ICH 32) . Therefore, our expectation was that the refined SVD score would have a more profound effect. This result suggests that when the analysis was restricted to the mild HTN-ICH subgroup, the influence of SVD markers on functional dependence was limited. Thus, it would be better to pay more attention to age and admission NIHSS score to predict the recoveries of neurological function. Our study also suggested that lacunes and the specific imaging characteristics, rather than the overall severity of SVD burden, were responsible for the high risk of stroke recurrence in HTN-ICH patients. The pathophysiological mechanisms of lacunes were related to atherosclerosis and microenvironment of arterioles less than 100 µm. Moreover, further changes in the blood–brain barrier and cerebral blood flow might be the cause of recurrent stroke 33) . Previous study has also reported that lacunes increased the risk of stroke recurrence in non-CAA-ICH patients, which is in agreement with our results 34) .
Our study has important clinical implications, which enable clinicians to better identify secondary prevention measures, especially in conscious and spontaneous ICH patients without surgical treatment, who might be easily ignored by doctors. Another strength of our work is the systematic evaluation and summary of different SVD scores by trained raters using validated scales. Our study also has some limitations. First, due to the rigidity of the inclusion criteria, the sample size in our study was small, and the findings need to be confirmed in future larger studies. Second, patients who could not be definitively diagnosed with CAA-ICH or HTN-ICH were excluded, which limited the strength of some of our results. Third, we did not assess the cognitive function during follow-up. Finally, our study was limited by the small number of some clinical outcomes, with only 8 patients had recurrent hemorrhagic strokes and 13 patients had recurrent ischemic strokes, rendering further analysis difficult. In future study, we would like to include more cases to separately explore the prediction value of different SVD scores on recurrent cerebral hemorrhage and recurrent cerebral infarction. The results may help clinicians to better determine the use of medications, such as anti-platelet drugs and statins.
In conclusion, the CAA-SVD score and the total number of SVD markers might help doctors identify high-risk patients with poor prognosis in the mild CAA-ICH group. It would be better for neurologists to focus on lacunes rather than the overall burden of SVD markers to identify patients who might have recurrent strokes in the mild HTN-ICH group.
Funding Information
This work was sponsored by National Natural Science Foundation of China (81771281) and (81471177).
Compliance with Ethical Standards Conflicts of Interest
The authors declare no conflicts of interest.
Ethical Approval
This study was approved by the ethics committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Written informed consent to collect data and clinical outcomes was obtained from the patient or a legally authorized representative in all cases.
References
- 1).Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol, 2010; 9: 689-701 [DOI] [PubMed] [Google Scholar]
- 2).Shaaban CE, Jorgensen DR, Gianaros PJ, Mettenburg J, Rosano C. Cerebrovascular disease: Neuroimaging of cerebral small vessel disease. Prog Mol Biol Transl Sci, 2019; 165: 225-255 [DOI] [PubMed] [Google Scholar]
- 3).Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol, 2013; 12: 483-497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Roongpiboonsopit D, Charidimou A, William CM, Lauer A, Falcone GJ, Martinez-Ramirez S, Biffi A, Ayres A, Vashkevich A, Awosika OO, Rosand J, Gurol ME, Silverman SB, Greenberg SM, Viswanathan A. Cortical superficial siderosis predicts early recurrent lobar hemorrhage. Neurology, 2016; 87: 1863-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Xu M, Cheng Y, Song Q, Yuan R, Zhang S, Hao Z, Liu M. Total Burden of Cerebral Small Vessel Disease in Recurrent ICH versus First-ever ICH. Aging Dis, 2019; 10: 570-577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Xiong L, Boulouis G, Charidimou A, Roongpiboonsopit D, Jessel MJ, Pasi M, Reijmer YD, Fotiadis P, Ayres A, Merrill E, Schwab K, Blacker D, Gurol ME, Greenberg SM, Viswanathan A. Dementia incidence and predictors in cerebral amyloid angiopathy patients without intracerebral hemorrhage. J Cereb Blood Flow Metab, 2017; 38: 241-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Wang YW, Zhang GM. New Silent Cerebral Infarction in Patients with Acute Non-Cerebral Amyloid Angiopathy Intracerebral Hemorrhage as a Predictor of Recurrent Cerebrovascular Events. Med Sci Monit, 2019; 25: 418-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Caprio FZ, Maas MB, Rosenberg NF, Kosteva AR, Bernstein RA, Alberts MJ, Prabhakaran S, Naidech AM. Leukoaraiosis on Magnetic Resonance Imaging Correlates With Worse Outcomes After Spontaneous Intracerebral Hemorrhage. Stroke, 2013; 44: 642-646 [DOI] [PubMed] [Google Scholar]
- 9).Charidimou A, Boulouis G, Roongpiboonsopit D, Auriel E, Pasi M, Haley K, van Etten ES, Martinez-Ramirez S, Ayres A, Vashkevich A, Schwab KM, Goldstein JN, Rosand J, Viswanathan A, Greenberg SM, Gurol ME. Cortical superficial siderosis multifocality in cerebral amyloid angiopathy. Neurology, 2017; 89: 2128-2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology, 2014; 83: 1228-1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Huijts M, Duits A, van Oostenbrugge RJ, Kroon AA, de Leeuw PW, Staals J. Accumulation of MRI Markers of Cerebral Small Vessel Disease is Associated with Decreased Cognitive Function. A Study in First-Ever Lacunar Stroke and Hypertensive Patients. Front Aging Neurosci, 2013; 5: 72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Lau KK, Li L, Schulz U, Simoni M, Chan KH, Ho SL, Cheung RTF, Küker W, Mak HKF, Rothwell PM. Total small vessel disease score and risk of recurrent stroke: Validation in 2 large cohorts. Neurology, 2017; 88: 2260-2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Lioutas V, Wu B, Norton C, Helenius J, Modak J, Selim M. Cerebral small vessel disease burden and functional and radiographic outcomes in intracerebral hemorrhage. J Neurol, 2018; 265: 2803-2814 [DOI] [PubMed] [Google Scholar]
- 14).Kimura Y, Miwa K, Takasugi J, Oyama N, Todo K, Sakaguchi M, Mochizuki H, Sasaki T. Total small vessel disease score and functional outcomes following acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis, 2020; 29: 105001 [DOI] [PubMed] [Google Scholar]
- 15).Charidimou A, Martinez-Ramirez S, Reijmer YD, Oliveira-Filho J, Lauer A, Roongpiboonsopit D, Frosch M, Vashkevich A, Ayres A, Rosand J, Gurol ME, Greenberg SM, Viswanathan A. Total Magnetic Resonance Imaging Burden of Small Vessel Disease in Cerebral Amyloid Angiopathy: An Imaging-Pathologic Study of Concept Validation. JAMA Neurol, 2016; 73: 994-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Boulouis G, Charidimou A, Pasi M, Roongpiboonsopit D, Xiong L, Auriel E, van Etten ES, Martinez-Ramirez S, Ayres A, Vashkevich A, Schwab KM, Rosand J, Goldstein JN, Gurol ME, Greenberg SM, Viswanathan A. Hemorrhage recurrence risk factors in cerebral amyloid angiopathy: Comparative analysis of the overall small vessel disease severity score versus individual neuroimaging markers. J Neurol Sci, 2017; 380: 64-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM; Microbleed Study Group. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol, 2009; 8: 165-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, van Buchem MA, Bruckmann H, Greenberg SM. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology, 2010; 74: 1346-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Charidimou A, Frosch MP, Al-Shahi Salman R, Baron JC, Cordonnier C, Hernandez-Guillamon M, Linn J, Raposo N, Rodrigues M, Romero JR, Schneider JA, Schreiber S, Smith EE, van Buchem MA, Viswanathan A, Wollenweber FA, Werring DJ, Greenberg SM; International CAA Association. Advancing diagnostic criteria for sporadic cerebral amyloid angiopathy: Study protocol for a multicenter MRI-pathology validation of Boston criteria v2.0. Int J Stroke, 2019; 14: 956-971 [DOI] [PubMed] [Google Scholar]
- 20).Pasi M, Charidimou A, Boulouis G, Auriel E, Ayres A, Schwab KM, Goldstein JN, Rosand J, Viswanathan A, Pantoni L, Greenberg SM, Gurol ME. Mixed-location cerebral hemorrhage/microbleeds. Neurology, 2018; 90: e119-e126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Wardlaw, J M Smith, E E Biessels, G J Cordonnier, C Fazekas, F Frayne, R Lindley, R I O’Brien, J T Barkhof, F Benavente, O R Black, S E Brayne, C Breteler, M Chabriat, H Decarli, C de Leeuw, F E Doubal, F Duering, M Fox, N C Greenberg, S Hachinski, V Kilimann, I Mok, V Oostenbrugge, Rv Pantoni, L Speck, O Stephan, B C Teipel, S Viswanathan, A Werring, D Chen, C Smith, C van Buchem, M Norrving, B Gorelick, P B Dichgans, M. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol, 2013; 12: 822-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, Knottnerus IL, Staals J. Ambulatory blood pressure in patients with lacunar stroke: association with total MRI burden of cerebral small vessel disease. Stroke, 2013; 44: 2995-2999 [DOI] [PubMed] [Google Scholar]
- 23).Xu M, Zhang S, Liu J, Luo H, Wu S, Cheng Y, Liu M. Kidney Dysfunction is Associated with a High Burden of Cerebral Small Vessel Disease in Primary Intracerebral Hemorrhage. Curr Neurovasc Res, 2018; 15: 39-46 [DOI] [PubMed] [Google Scholar]
- 24).Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K, Ayres A, Schwab KM, Martinez-Ramirez S, Goldstein JN, Rosand J, Viswanathan A, Greenberg SM, Gurol ME. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology, 2017; 88: 1157-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Patti J, Helenius J, Puri AS, Henninger N. White Matter Hyperintensity-Adjusted Critical Infarct Thresholds to Predict a Favorable 90-Day Outcome. Stroke, 2016; 47: 2526-2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol, 1987; 149: 351-356 [DOI] [PubMed] [Google Scholar]
- 27).Charidimou A, Jäger RH, Fox Z, Peeters A, Vandermeeren Y, Laloux P, Baron JC, Werring DJ. Prevalence and mechanisms of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology, 2013; 81: 626-632 [DOI] [PubMed] [Google Scholar]
- 28).Charidimou A, Martinez-Ramirez S, Reijmer YD, Oliveira-Filho J, Lauer A, Roongpiboonsopit D, Frosch M, Vashkevich A, Ayres A, Rosand J, Gurol ME, Greenberg SM, Viswanathan A. Total Magnetic Resonance Imaging Burden of Small Vessel Disease in Cerebral Amyloid Angiopathy. JAMA Neurol, 2016; 73: 994-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Delcourt C, Sato S, Zhang S, Sandset EC, Zheng D, Chen X, Hackett ML, Arima H, Hata J, Heeley E, Al-Shahi Salman R, Robinson T, Davies L, Lavados PM, Lindley RI, Stapf C, Chalmers J, Anderson CS; INTERACT2 Investigators. Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology, 2017; 88: 1408-1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Charidimou A, Boulouis G, Xiong L, Jessel MJ, Roongpiboonsopit D, Ayres A, Schwab KM, Rosand J, Gurol ME, Greenberg SM, Viswanathan A. Cortical superficial siderosis and first-ever cerebral hemorrhage in cerebral amyloid angiopathy. Neurology, 2017; 88: 1607-1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Charidimou A, Boulouis G, Xiong L, Pasi M, Roongpiboonsopit D, Ayres A, Schwab KM, Rosand J, Gurol ME, Viswanathan A, Greenberg SM. Cortical Superficial Siderosis Evolution. Stroke, 2019; 50: 954-962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Xu M, Cheng Y, Song Q, Yuan R, Zhang S, Hao Z, Liu M. Total Burden of Cerebral Small Vessel Disease in Recurrent ICH versus First-ever ICH. Aging Dis, 2019; 10: 570-577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Kang DW, Han MK, Kim HJ, Yun SC, Jeon SB, Bae HJ, Kwon SU, Kim JS. New ischemic lesions coexisting with acute intracerebral hemorrhage. Neurology, 2012; 79: 848-855 [DOI] [PubMed] [Google Scholar]
- 34).Wang Y, Zhang G. New Silent Cerebral Infarction in Patients with Acute Non-Cerebral Amyloid Angiopathy Intracerebral Hemorrhage as a Predictor of Recurrent Cerebrovascular Events. Med Sci Monit, 2019; 25: 418-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Patient-related data will be shared upon request by the corresponding author or any qualified investigator.