Abstract
Primary chylomicronemia (PCM) is a rare and intractable disease characterized by marked accumulation of chylomicrons in plasma. The levels of plasma triglycerides (TGs) typically range from 1,000 - 15,000 mg/dL or higher.
PCM is caused by defects in the lipoprotein lipase (LPL) pathway due to genetic mutations, autoantibodies, or unidentified causes. The monogenic type is typically inherited as an autosomal recessive trait with loss-of-function mutations in LPL pathway genes ( LPL , LMF1 , GPIHBP1 , APOC2 , and APOA5 ). Secondary/environmental factors (diabetes, alcohol intake, pregnancy, etc.) often exacerbate hypertriglyceridemia (HTG).
The signs, symptoms, and complications of chylomicronemia include eruptive xanthomas, lipemia retinalis, hepatosplenomegaly, and acute pancreatitis with onset as early as in infancy. Acute pancreatitis can be fatal and recurrent episodes of abdominal pain may lead to dietary fat intolerance and failure to thrive.
The main goal of treatment is to prevent acute pancreatitis by reducing plasma TG levels to at least less than 500-1,000 mg/dL. However, current TG-lowering medications are generally ineffective for PCM. The only other treatment options are modulation of secondary/environmental factors. Most patients need strict dietary fat restriction, which is often difficult to maintain and likely affects their quality of life.
Timely diagnosis is critical for the best prognosis with currently available management, but PCM is often misdiagnosed and undertreated. The aim of this review is firstly to summarize the pathogenesis, signs, symptoms, diagnosis, and management of PCM, and secondly to propose simple diagnostic criteria that can be readily translated into general clinical practice to improve the diagnostic rate of PCM. In fact, these criteria are currently used to define eligibility to receive social support from the Japanese government for PCM as a rare and intractable disease.
Nevertheless, further research to unravel the molecular pathogenesis and develop effective therapeutic modalities is warranted. Nationwide registry research on PCM is currently ongoing in Japan with the aim of better understanding the disease burden as well as the unmet needs of this life-threatening disease with poor therapeutic options.
Keywords: Chylomicronemia, Triglyceride, Pancreatitis, Diagnostic criteria, Treatment guide
1. Definition of Chylomicronemia
Chylomicrons (CMs) are intestine-derived lipoproteins that transport dietary fat to peripheral tissues 1) . CMs are usually quickly cleared from plasma after an overnight fast. The hallmark of chylomicronemia is persistent elevation of CMs in the fasting state (>12h). Plasma triglyceride (TG) levels in chylomicronemia typically range from 1,000 to 15,000 mg/dL or higher 2) . As CMs start to accumulate in plasma when TG levels increase to more than 500 - 1,000 mg/dL 1) , it is practical to screen and suspect chylomicronemia if plasma TGs >1,000 mg/dL 2 , 3) .
2. Metabolism of Chylomicrons
CMs are produced by the intestine after a meal and secreted into the circulation 1) . Their synthesis requires apoB-48 protein, which is transcribed from the edited mRNA of APOB , and microsomal triglyceride transfer protein (MTTP), which incorporates dietary TGs into CMs. More than 90% of lipids in CMs are TGs (derived from dietary fatty acids and TGs), which are hydrolyzed by lipoprotein lipase (LPL) on the endothelial cell surface of peripheral tissues (muscle, heart, adipose tissue, etc.) to liberate free fatty acids (FFAs). FFAs are used as an energy source in peripheral tissues, or stored as TGs in adipose tissues, or re-esterified and secreted as very low-density lipoprotein (VLDL)-TG by the liver. CMs are converted to CM remnants after TG hydrolysis by LPL and then cleared from plasma through endocytosis mainly by the liver.
3. Molecular Basis of Chylomicronemia
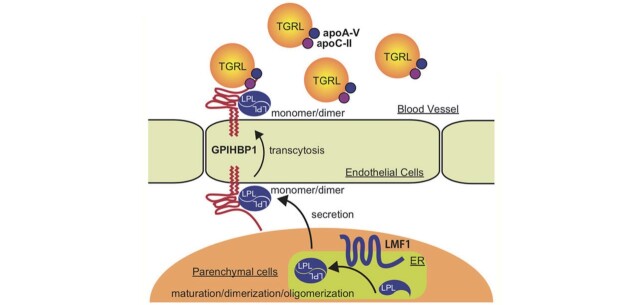
Plasma levels of CMs are affected by primary factors as well as secondary factors 2 , 4 , 5) . Primary factors consist of defects in the proteins that metabolize CMs, such as LPL and its related proteins (apolipoprotein(apo)C-II, apoA-V, glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 (GPIHBP1), lipase maturation factor 1 (LMF1)) ( Fig.1 ) . Secondary factors include conditions that impair CM metabolism, such as poor control of diabetes, excessive alcohol intake, and pregnancy. In primary chylomicronemia (PCM), chylomicronemia usually persists even after management of secondary factors. PCM is caused by defects in LPL pathway genes ( LPL, APOC2, APOA5, GPIHBP1, LMF1 ) due to genetic mutations or presence of autoantibodies against LPL pathway proteins (GPIHBP1, LPL, and apoC-II) 6 - 9) .
Fig.1. Molecular basis of primary chylomicronemia.
Lipoprotein lipase (LPL) hydrolyzes triglycerides (TGs) in TG-rich lipoproteins (TGRLs), such as very low-density lipoproteins (VLDLs) and chylomicrons (CMs) to liberate free fatty acids (FFAs), which are utilized by peripheral tissues (e.g., muscle, heart, and adipose tissues). Activity of LPL is regulated by a quaternary structure (monomer/dimer/oligomer) as well as by multiple LPL-pathway proteins 210-212) . LMF1 is required for the synthesis of LPL in parenchymal cells of these peripheral tissues. GPIHBP1 is a transmembrane protein that tethers LPL on the endothelial cell surface to provide a platform for TG hydrolysis. GPIHBP1 captures LPL in the subendothelial (interstitial) space of peripheral tissues, transports LPL from the subendothelial surface to the luminal surface of endothelial cells by transcytosis, and anchors LPL on the luminal surface facing the bloodstream to facilitate lipolysis. For the hydrolytic activity of LPL, two apolipoproteins, apoC-II and apoA-V, are required. ApoC-II is necessary for the enzymatic activity of LPL. ApoA-V primarily enhances the interaction between TGRL and LPL by forming TGRL-apoA-V-GPIHBP1-LPL complex via its dual binding affinity to TGRL and GPIHBP1. Defects in LPL pathway proteins (LPL, LMF1, GPIHBP1, apoC-II, apoA-V) due to genetic mutations or autoantibodies cause primary chylomicronemia (PCM). LPL activity can be measured after releasing LPL from the luminal surface into the circulation by i.v. injection with heparin.
Abbreviations: apoA-V, apolipoprotein A-V; apoC-II, apolipoprotein C-II; GPIHBP-1, glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1; LMF1, lipase maturation factor 1.
4. Complications of Chylomicronemia
Chylomicronemia is a risk factor for acute pancreatitis 10) . Although the underlying mechanisms are not fully understood, the widely accepted hypothesis is that an excess of FFAs derived from lipolysis of chylomicron-TG by pancreatic lipase damages pancreatic acinar cells or endothelial cells of pancreatic capillaries, leading to activation of inflammatory processes 11) . In addition, high levels of plasma CM may increase the viscosity of pancreatic microcirculation and impair pancreatic blood flow, further exacerbating inflammation. Genetic predisposition may also contribute to the pathogenesis 12) .
Based on this hypothesis, it is generally believed that chylomicronemia per se induces pancreatitis and, therefore, reducing plasma CM levels should lower the risk of pancreatitis 10) . The widely accepted treatment goal is to reduce plasma TG levels to at least less than 1,000 mg/dL 2 , 4 , 5 , 12 - 14) .
5. Primary Factors of Chylomicronemia
Five genes ( LPL , LMF1 , GPIHBP1 , APOC2 , APOA5 ) have been identified as the causative genes of monogenic chylomicronemia ( Fig.1 ) 4 , 5) . All of them are required for the normal function of LPL. Homozygous, compound heterozygous, or double heterozygous loss-of-function mutations of these LPL pathway genes usually cause monogenic chylomicronemia with an autosomal recessive mode of inheritance 15) . Heterozygous mutations or pathological variants in HTG-susceptible genes, including those for hepatic lipase (HL) and apolipoprotein E, may also predispose to chylomicronemia 3 , 5 , 16 - 18) .
Loss-of-function mutations in LPL-pathway genes have been identified in only less than 30-40% of patients suspected of monogenic chylomicronemia 19 , 20) . In some cases, autoantibodies against LPL pathway proteins can cause PCM. Additional genetic factors or unknown causes may also underlie PCM.
• LPL (LPL deficiency, OMIM: 238600): LPL is the most common causative gene of monogenic chylomicronemia. More than 220 pathogenic variants in LPL have been described (missense, nonsense, splicing variants, insertion/deletion of nucleotide(s), deletion/duplication/insertion/rearrangement of exon(s)) 2 , 4 , 15 , 20 - 22) . A list of mutations in LPL has been comprehensively summarized in exon-intron diagrams by Rabacchi C et al. and Rodrigues R et al. 20 , 22) . LPL activity is usually tested in post-heparin plasma taken 10-15 min after iv. heparin injection (10-60 IU/kg body weight) 4 , 23) .
• LMF1 (LMF1 deficiency, OMIM: 246650): LMF1 encodes LMF1, a transmembrane chaperone protein of parenchymal cells (muscle, adipose tissues, etc.). LMF1 is localized to the endoplasmic reticulum and is required for proper synthesis and secretion of LPL and HL. In addition to nonsense mutations that were originally reported, several other mutations, including missense loss-of-function mutations, have been identified in LMF1 24 - 29) .
• GPIHBP1 (GPIHBP1 deficiency, OMIM: 615947): GPIHBP1 encodes GPIHBP1, a transmembrane protein that tethers LPL on the endothelial cell surface to provide a platform for TG hydrolysis 30 , 31) . GPIHBP1 captures LPL in the subendothelial (interstitial) space of peripheral tissues (skeletal muscles, heart, adipose tissues, etc.), transports LPL from the subendothelial surface to the luminal surface of endothelial cells by transcytosis, and anchors LPL on the luminal surface facing the bloodstream to facilitate lipolysis. So far, at least 23 pathogenic mutations in GPIHBP1 have been reported in severe HTG patients, including those causing total GPIHBP1 deficiency 21 , 32 - 36) . Mutations in GPIHBP1 are comprehensively summarized in the exon-intron diagram by Rabacchi et al. 35) . GPIHBP1 deficiency may be differentiated from LPL deficiency in that LPL activity or protein in post-heparin plasma is not totally lacking in GPIHBP1 deficiency 37) .
• APOC2 (apoC-II deficiency, OMIM: 207750): APOC2 encodes apoC-II, a co-factor required for LPL activity. APOC2 is the second most common causative gene of monogenic chylomicronemia. At least 24 mutations have been reported worldwide 2 , 4 , 5 , 38 - 41) , and are comprehensively summarized in the exon-intron diagram by Wolska et al. 41) . In some cases, plasma levels of apoC-II have been severely decreased but are detected with apparently normal electrophoretic patterns (so-called hypoapoC-II) 42 - 45) . In apoC-II deficiency, symptoms often develop at an older age (13-60 years) than in LPL deficiency 1 , 4) . As apoC-II deficient patients tend to be subjected to strict fat restriction at an older age, they may have poorer dietary adherence and more frequent episodes of acute pancreatitis in adulthood than LPL deficient patients, often accompanied by high VLDL levels 2) .
• APOA5 (apoA-V deficiency, OMIM: 144650): APOA5 encodes apoA-V, a co-factor of LPL that enhances interaction between LPL and TG-rich lipoproteins (TGRLs) 46 , 47) . ApoA-V has dual binding affinity for both TGRL and GPIHBP1, thereby forming the TGRL-apoA-V-GPIHBP1-LPL complex to facilitate lipolysis 31 , 48 , 49) . So far, at least 21 mutations in APOA5 have been reported, which are comprehensively summarized in exon-intron diagrams by Albers et al. 50 - 53) . In most cases, severe HTG develops later in life due to the combinatorial effects of primary (genetic) and secondary factors (aging, diabetes, pregnancy, HIV therapy, etc.) 53) . The underlying molecular mechanisms of apoA-V deficient HTG in terms of gene-environmental interactions has only begun to be elucidated 54 , 55) .
• Autoantibodies against LPL pathway proteins : Autoimmune chylomicronemia due to autoantibodies against LPL pathway proteins (GPIHBP1, LPL, apoC-II) has been reported 6 - 9) . Autoimmune chylomicronemia may be complicated by other autoimmune diseases and be ameliorated by steroid or immune-suppressive therapy.
6. Secondary Factors of Chylomicronemia
The following factors have been reported to induce chylomicronemia 3 - 5 , 13 , 35 , 56 - 61) . Among them, excess alcohol consumption and diabetes mellitus are the two most common factors associated with severe HTG 18 , 62 , 63) .
Pregnancy is another important factor that can induce chylomicronemia. Hormonal changes during pregnancy result in hyperlipidemia even in healthy subjects, as a physiological response to ensure nutrient supply to the fetus 64 - 66) . Plasma TG levels progressively increase in healthy subjects by 2- to 4-fold 67) , and markedly increase in subjects with PCM 68 - 74) . Pregnancy increases TGRL (VLDL) production and decreases TGRL clearance 75 - 78) .
• Lifestyle-related factors : Dietary factors such as excessive alcohol intake, high-fat diet, high-carbohydrate diet rich in fructose and other simple sugars. Less exercise and excessive intake of total calories, particularly in subjects who are overweight, have metabolic syndrome, or diabetes mellitus.
• Pathophysiological conditions : Pregnancy, obesity, metabolic syndrome, anorexia nervosa, glucose intolerance, diabetes mellitus with insulin resistance or insulin deficiency, endocrine diseases such as hypothyroidism, acromegaly, Cushing’s syndrome, Nelson’s syndrome, glycogen storage diseases, amyloidosis, renal disease (nephrotic syndrome, proteinuria, uremia, glomerulonephritis, etc.), liver disease, autoimmune disorders, lipodystrophies, Weber-Christian disease, multiple myeloma, paraproteinemia, and lymphoproliferative disorders, etc.
• Medications : Glucocorticoids, oral estrogens (contraceptives, postmenopausal replacement therapies), clomiphene, tamoxifen, exogenous testosterone; retinoids such as isotretinoin, bexarotene; immunosuppressants and anticancer drugs such as sirolimus, cyclosporine A, tacrolimus, capecitabine, cyclophosphamide, asparaginase; antihypertensives such as thiazides, loop diuretics, non-selective β-blockers; bile acid-sequestrants; antiviral drugs such as entecavir, ritonavir and other antiretroviral protease inhibitors; second-generation antipsychotics (atypical antipsychotics), such as dozapine, clozapine, olanzapine, risperidone, quetiapine; antidepressants such as mirtazapine, venlafaxine; selective serotonin reuptake inhibitors (sertraline, etc.); anticonvulsants, such as valproate; anesthetic drugs, such as propofol.
7. Classification of Chylomicronemia
Chylomicronemia was formerly classified as type 1 and 5 hyperlipoproteinemia (HLP) in the Fredrickson classification (WHO classification) 5 , 79) , where increased plasma lipoproteins are CM for type 1 and CM+VLDL for type 5 HLP. However, differentiation of type 1 and type 5 HLP is often difficult because of considerable overlaps in phenotypes and genetic backgrounds 3 , 5 , 17) .
Differentiation of monogenic from polygenic chylomicronemia is also difficult 80) . Even when monogenic chylomicronemia is suspected, causative mutations have been identified in less than 30-40% 19 , 20) .
For practical reasons, we prefer to use the term PCM, which is defined as a condition with persistent elevation of TG >1,000 mg/dL even after management of secondary factors. The term familial chylomicronemia syndrome (FCS) is not used here, as monogenic chylomicronemia is typically autosomal recessive, and most cases are sporadic, without a family history. The term “familial” may confuse both patients and doctors, and may lead to underdiagnosis 3 , 81) .
8. Prevalence of Chylomicronemia
The prevalence of severe HTG (TG >10 mmol/L (885 mg/dL)) is estimated at ~1 in 600 in North America 5) . In a population of 440,240 subjects in the US, severe HTG (886-2,000 mg/dL) and very severe HTG (>2,000 mg/dL) were observed in 0.15% and 0.014%, respectively 36) .
The monogenic type of chylomicronemia is very rare and its frequency is estimated at 1 to 2 per million in the general population 1 , 4 , 5) . The most common causative gene is LPL , followed by other LPL pathway genes ( APOC2 , GPIHBP1 , APOA5 , and LMF1 ) 5 , 15 , 53) .
Severe HTG is more often due to polygenic causes 3 , 82) . Dron et al. reported that the etiology of severe HTG (TG >10 mmol/L (885 mg/dL)) is monogenic in 1.1%, polygenic in 46%, and genetically unidentified in the rest of cases 83) . Another study identified monogenic causes in 0.96% of patients with very severe HTG (TG >20 mmol/L (1770 mg/dL)) recorded at least once during regular medical care 22) .
9. Prevalence of HTG-Induced Acute Pancreatitis
Regardless of the underlying etiology of HTG, the risk of acute pancreatitis increases as TG levels increase, particularly when they exceed 1,000 - 2,000 mg/dL 84) .
HTG is the third leading cause (5-38%) of acute pancreatitis after alcohol and gallstones 1 , 12 , 85 - 87) . HTG is the leading cause (25~50%) of acute pancreatitis during pregnancy 12 , 74 , 88) , which is most often seen in the third trimester (19%, 26%, 53% in the 1 st , 2 nd , and 3 rd trimester, and 2% in postpartum period) 89) . The frequency of HTG-induced acute pancreatitis in pregnancy is estimated as 1 in 1000-12,000 pregnancies 14 , 73 , 74) .
Risk of acute pancreatitis increases by ~4% for every 100 mg/dL increase in plasma TG 56 , 84 , 87 , 90 , 91) . According to the 2010 Endocrine Society guidelines, HTG can be classified into mild HTG (150-199 mg/dL), moderate HTG (200-999 mg/dL), severe HTG (sHTG, 1000-1999 mg/dL), or very severe HTG (vsHTG, >2000 mg/dL). The prevalence of pancreatitis in sHTG and vsHTG is about 10% and 20%, respectively 5 , 13 , 14 , 87 , 92) .
Clinically, it remains difficult to predict if a HTG patient will develop acute pancreatitis or not. Some patients do not develop acute pancreatitis even at TG levels higher than 30,000 mg/dL 1 , 2) . Conversely, patients can develop acute pancreatitis at TG levels of 5-10 mmol/L (442-885 mg/dL) 93) . The underlying etiology of monogenic chylomicronemia seems to confer a potent risk of acute pancreatitis 53 , 94 - 96) . Compared to normolipidemic individuals, the risk of acute pancreatitis increased by 16-fold, 56-fold, and 361-fold in HTG (5-9 mmol/L (442-796 mg/dL)) without LPL deficiency, HTG (>9 mmol/L (796 mg/dL)) without LPL deficiency, and HTG (>9 mmol/L (796 mg/dL)) with LPL deficiency (genetically-confirmed), respectively 94) . Greater cumulative exposure of HTG to the pancreas due to genetic causes may impose higher pancreatitis risk 53 , 94 - 97) (See sections 12 and 16(B) for other suggested risk factors of acute pancreatitis among HTG subjects).
10. Signs, Symptoms, and Complications of Chylomicronemia
Depending on the severity of the mutation, signs, symptoms, and complications of chylomicronemia may manifest as early as in infancy, in childhood, or later in life 4 , 95) .
• Abdominal pain, fat intolerance, failure to thrive : Abdominal pain affects ~60% of monogenic chylomicronemia patients and can be mild to incapacitating 4 , 95 , 96) . Recurrent episodes of abdominal pain may lead to dietary fat intolerance and failure to thrive. Body weight may be lower because of restricted food intake.
• Pancreatitis : Acute pancreatitis can be severe, recurrent, and life-threatening 1 , 4 , 95) . Acute pancreatitis may lead to chronic pancreatitis and diabetes. Irrespective of its etiology, severe HTG should be carefully monitored for the possible complications of pancreatitis. Compared to polygenic chylomicronemia, monogenic chylomicronemia is associated with a higher risk of acute pancreatitis and tends to manifest severe phenotypes 86 , 94 , 95) . According to a survey of lipidologists, ~67% of monogenic patients were hospitalized for acute pancreatitis vs. ~14% of polygenic patients 95 , 96) . It should be noted that high levels of plasma TG may interfere with assays of plasma pancreatic enzymes (lipase, amylase), resulting in falsely low levels 2 , 98 - 100) . HTG-induced acute pancreatitis should not be misdiagnosed due to apparently low serum levels of pancreatic enzymes.
• Lipemic plasma : Lipemic plasma (milky-looking plasma) is characterized by a creamy CM layer floating above the bottom layer, after leaving serum standing overnight in a refrigerator (the “refrigerator” test) 1) . In type 5 HLP, the bottom layer contains VLDLs and has a lactescent appearance. In type 1 HLP, the bottom layer is clear without apparent VLDL accumulation. Type 1 HLP is more frequently observed than type 5 in monogenic chylomicronemia 19) . (See reference by Yuan G et al. for a photograph of lipemic plasma 56) .)
• Eruptive xanthomas : Eruptive xanthomas affect 10-50% of the monogenic type 95 , 96) . They appear when plasma TG levels increase to more than 2,000 mg/dL as yellow papules on the skin of the trunk, buttocks, and extremities (extensor surfaces of the arms and knees) as a result of TG uptake by macrophages (see references by Nayak KR et al. and Yuan G et al. for photographs) 2 , 4 , 56 , 101) . Along with reduction in plasma TG levels, they gradually disappear over several weeks to a few months 2) .
• Lipemia retinalis : Lipemia retinalis affects ~40% of the monogenic type 95 , 96) and is characterized by retinal blood vessels with a milky appearance (a pale pink color) on fundoscopy (see references by Kumar J et al. and Yuan G et al. for photographs) 56 , 102) . Vision is not impaired. It is usually visible when plasma TG levels increase to more than 4,000 mg/dL 2) .
• Hepatomegaly and splenomegaly : Hepatomegaly and splenomegaly affect 10-50% of the monogenic type 95 , 96) as a result of TG uptake by macrophages and other cell types in these tissues 1 , 4) . Both conditions are reversible and rapidly improve within a week, along with reduction in plasma TG levels 2) .
• Other symptoms (fatigue, dyspnea, and neurological symptoms) : Other clinical symptoms include fatigue, dyspnea, and neurological symptoms 1) such as memory impairment (transient memory loss), cognitive impairment, mild dementia, cloudy thought, brain fog, neurosis, irritability, anxiety, and depression 4 , 95 , 103 - 107) . Neurological symptoms affect ~8% of the monogenic type 95) . Although little is known about the underlying mechanisms of these symptoms, large surveillance studies among chylomicronemia patients have shown that they dominantly and adversely affect patients’ quality of life and increase the burden of the disease 104 - 107) .
11. Diagnosis of Chylomicronemia
Undertreatment and underdiagnosis of chylomicronemia are one of the major risks for acute pancreatitis 84 , 87) . In order to achieve early diagnosis and treatment for chylomicronemia, simple diagnostic criteria that can be readily translated into general practice are required.
Based on TG levels that lead to suspicion of chylomicronemia (TG >1,000 mg/dL), clinical manifestations and the available data on diagnostic tests, here we propose diagnostic criteria for PCM ( Table 1 ) .
Table 1. Diagnostic criteria for primary chylomicronemia.
A1 and A2 with exclusion of differential diagnosis in E A1) Plasma TG level ≧1,000 mg/dL (after fasting for 12 hours or longer) A2) Presence of chylomicrons in serum (from appearance of supernatant cream layer after allowing serum to stand for 24 hours or longer at 4℃, ultracentrifugation, or electrophoresis (agarose gel, polyacrylamide gel, or HPLC))
B1) Recurrent episodes of abdominal pain and/or acute pancreatitis B2) Eruptive xanthomas B3) Lipemia retinalis B4) Hepatomegaly and/or splenomegaly B5) Dyspnea B6) Neurological symptoms (cognitive impairment, memory impairment, depression, etc.)
C1) LPL activity and/or protein in post heparin plasma, adipose tissue, or macrophages is absent or markedly decreased (<10% of normal subjects) C2) Plasma apoC-II is absent or markedly decreased (<10% of normal subjects) C3) Plasma apoA-V is absent or markedly decreased (<10% of normal subjects) C4) Autoantibodies against LPL, heparin, apoC-II, or GPIHBP1.
Identification of causative mutation(s) in LPL , APOC2 , GPIHBP1 , LMF1 , or APOA5
Type 3 hyperlipidemia, familial combined hyperlipidemia (FCHL), and secondary hyperlipidemia due to following: excess alcohol intake, nephrotic syndrome, anorexia nervosa, pregnancy, diabetes mellitus, lipodystrophies, Weber-Christian disease, hypothyroidism, acromegaly, Cushing’s syndrome, Nelson’s syndrome, multiple myeloma, systemic lupus erythematosis, malignant lymphoma, sarcoidosis, etc.; medications such as estrogens, steroids, diuretics, β-blockers, antipsychotics including selective serotonin reuptake inhibitors (SSRIs), retinoids such as isotretinoin, antiretroviral protease inhibitors, immunosuppressants, etc. <Diagnosis> Definite: Entry criterion (A) associated with at least one item from C or D Probable: Entry criterion (A) associated with at least one item from B1-B4 Possible: Entry criterion (A) with or without item(s) from B5-B6 |
It should be noted that PCM is genetically confirmed in less than 30-40% of patients suspected of monogenic chylomicronemia 19 , 20) . Therefore, we have set three categories (definite, probable, and possible) so that PCM patients will not be missed even without a genetic diagnosis.
To achieve timely diagnosis and improve the diagnostic rate of PCM, screening of plasma TG levels in the following settings will be helpful. Clinicians across multiple disciplines, such as primary care physicians, gastroenterologists, gynecologists, and other doctors who have occasionally encountered severe HTG patients, should consult lipidologists concerning further diagnostic tests for chylomicronemia.
• Health checkup or opportunistic blood test : All patients who have TG levels of more than 1,000 mg/dL in universal lipid screening 108) or a routine clinic visit should be suspected of chylomicronemia 96 , 109) .
• Acute abdomen (including pancreatitis) : Those who have acute abdomen or are suspected of pancreatitis should have their plasma TG levels measured 96) . Plasma TG should be measured as early as possible after the onset of abdominal pain, as TG levels rapidly decrease within 24-48 hours of onset 110) .
• Pregnancy : Many cases of monogenic chylomicronemia have been discovered in the third trimester of pregnancy 111 - 118) . HTG-induced acute pancreatitis in pregnancy can be lethal to both mother and fetus 119) . Gestational HTG may also increase the risk of hyperviscosity syndrome 120) , pre-eclampsia 121) , fetal macrosomia, and fetal pancreatitis-related complications (in-utero fetal death, preterm labor, and prematurity) 66) . Pregnant women who are suspected of pancreatitis should be tested for plasma TG. Pregnant women at high-risk for HTG-induced acute pancreatitis may benefit from plasma TG screening and monitoring on a weekly basis 122) . Such patients include: those with HTG or pancreatitis prior to or during pregnancy; high predisposition for HTG-induced acute pancreatitis due to diabetes mellitus, obesity, hypertension, hypothyroidism, renal disease, liver disease, family history of HTG, alcohol consumption, and medications that cause HTG; HTG with abdominal pain or other symptoms typical to chylomicronemia 84 , 87 , 122) (See sections 12 and 16(B) for suggested risk factors of acute pancreatitis among HTG patients).
• Family members of chylomicronemia patients : Evaluation of plasma TG levels in family members is beneficial for early diagnosis and management of possible complications 96) .
12. Features for Suspecting Monogenic Chylomicronemia
Clinical features that lead to suspicion of monogenic chylomicronemia have been suggested by previous studies and in expert opinions, but further validation in various cohorts is required 2 - 5 , 15 , 18 , 53 , 80 , 96 , 109 , 123 , 124) . These features may be useful not only for indicating the likelihood of monogenic chylomicronemia but also for predicting a higher risk of pancreatitis 53 , 86 , 94 - 96) :
|
On the other hand, the polygenic type of chylomicronemia is more frequently associated with secondary/environmental factors, such as high-alcohol intake, diabetes mellitus, hypertension, and obesity 2 , 18 , 96) .
13. Treatment of Chylomicronemia
The treatment goal of chylomicronemia is to lower plasma TG levels enough to reduce the risk of pancreatitis. Data from large healthcare databases suggest that sustained HTG (>500 mg/dL) increases the risk of pancreatitis (hazard ratio 1.79 [CI 95%: 1.10-1.28]) 125) and lowering TG from >500 mg/dL to less than 200 mg/dL can reduce the incidence of acute pancreatitis from 1.1 to 0.4 per 100 person-year (adjusted OR 0.45 [CI 95%: 0.34-0.60]) 126) . Due to the rareness of the disease, there have been no randomized control trials (RCTs) to determine treatment TG targets for prevention of pancreatitis. Mainly based on clinical experience, the opinion of experts is to recommend maintaining plasma TG levels below 500-1,000 mg/dL to prevent pancreatitis 2 , 4 , 5 , 12 - 14) .
A) Control of Secondary Factors
Comorbid conditions that aggravate chylomicronemia should be thoroughly evaluated in order to rule them out. If any are present, they should be managed adequately. Bodyweight reduction, reduced calorie intake, and increased energy expenditure through regular physical exercise may help reduce plasma TG levels, particularly in overweight subjects. Regular physical exercise may also help in the non-obese 93 , 127) . Bodyweight should be carefully controlled as rebound weight gain might elicit pancreatitis 13) .
B) Dietary Therapy
Strict dietary control is currently the primary treatment modality for chylomicronemia, although it is often insufficient and difficult to maintain in the long term 128 , 129) . Children and adolescents should be carefully monitored to ensure proper growth and development. Adjustment of social life might be a challenge throughout life 104 - 107) .
• Fat restriction : The mainstay of dietary treatment is a low-fat diet. Restriction of total dietary fat to <15-20 g per day (<10-15 % of total energy intake) is usually required to reduce plasma CMs and prevent pancreatitis 128 , 129) . Under fat restriction, adequate intake of essential fatty acids (EFA; 2-4% of daily calories) and fat-soluble vitamins (A, D, E, and K) should be ensured to avoid deficiency. Signs and symptoms typical of EFA deficiency include: inadequate growth in pediatric patients, dry or dull hair, dry or scaly skin, skin lesions, particularly raised bumps on the skin, soft and brittle nails, and impaired wound healing 129) . Food sources of EFAs include soybeans, tofu, flaxseeds, walnuts, and chia seeds for alpha-linolenic acid (ALA) and whole grains for linoleic acid (LA).
• Medium-chain triglycerides (MCTs) : In a very-low-fat diet, MCTs containing fatty acids of ≦10 carbon atoms in length may be used to provide sufficient calories in meals or infant formula 4 , 36 , 128 , 129) . MCTs are absorbed directly into the circulation via the CM-independent pathway. MCTs may help reduce plasma TG levels 130) . In order to avoid possible adverse effects (diarrhea, abdominal pain, etc.), MCTs should be introduced slowly. The safety of long-term MCTs is not established and patients should be carefully monitored for possible complications such as hepatotoxicity. MCTs should not be confused with coconut oil, which contains lauric acid (C12) and other long-chain fatty acids 129) .
• Carbohydrate restriction : Restriction of carbohydrates, particularly fructose and other simple and refined carbohydrates, is advisable for patients with increased VLDL levels such as those with diabetes mellitus, metabolic syndrome, and obesity. As both CMs and VLDLs are substrates for LPL, reduced production of VLDLs due to carbohydrate restriction enhances the catabolism of CMs by LPL 131) . In cases where carbohydrate intake needs to be adequate, such as in pregnancy-associated HTG, carbohydrate iv may be a better therapeutic choice, as oral carbohydrate intake may produce a greater rise in plasma TG than carbohydrate iv 122 , 132) .
• Alcohol restriction : Alcohol intake should be restricted 4 , 128 , 129) .
C) Lipid-Lowering Medications
Current lipid-lowering medications (fibrates, n-3 polyunsaturated fatty acids (PUFAs), niacin, etc.) generally have little to no TG-lowering effects in patients with PCM, as they lower plasma TGs mainly by enhancing the LPL pathway and reducing VLDL levels. Treatment with n-3 PUFA or fish oil may be useful for lowering TG and preventing pancreatitis as suggested in patients with APOA5 mutations 21) . However, the effect of n-3 PUFAs needs to be monitored carefully, as their effectiveness has only been suggested by small studies without controls 3 , 5) . Fish oil supplements may increase the production of chylomicrons and are contraindicated according to an expert opinion 4 ) . In patients with autoantibodies against LPL pathway proteins (GPIHBP1, LPL, apoC-II), immune-suppressive agents may ameliorate HTG as well as the comorbid autoimmune diseases 7) .
14. Treatment of HTG-Induced Acute Pancreatitis
The clinical course of HTG-induced acute pancreatitis may be more severe than acute pancreatitis due to other causes in terms of complications and mortality rates 12 , 14 , 133 , 134) , but more controlled studies are required to produce firm evidence. Meta analysis of acute pancreatitis is difficult due to the heterogeneity of scoring systems for its severity 86) .
A) Standard Care for Acute Pancreatitis
Treatment of HTG-induced acute pancreatitis is based on standard care, including cessation of oral food intake, admission to hospital, intravenous hydration, hypocaloric parenteral nutrition avoiding excess calories and glucose infusions, pain management, prophylactic antibiotics, and protease inhibitors 12 , 130 , 135) . Any precipitating factors should be treated appropriately (e.g., insulin treatment for diabetes). Patients should be carefully monitored for development of pancreatic complications (necrosis, abscesses, etc.).
B) Specific Therapy for HTG-Induced Acute Pancreatitis
When patients can tolerate, oral TG-lowering medications (fibrates, n-3 PUFAs, niacin, etc.) may be administered 12 , 130 , 135) . MCTs may help reduce plasma TG levels as well as the risk of pancreatitis 130) . With a few exceptions, current TG-lowering medications are not based on the etiology of chylomicronemia. In apoC-II deficiency, infusion of normal human plasma containing apoC-II can greatly reduce plasma TG levels, and plasmapheresis has been suggested as a treatment of choice for pancreatitis due to apoC-II deficiency 4 , 58) .
C) Management of Chronic Pancreatitis
As acute pancreatitis can lead to chronic complications, patients with a history of it are better monitored for complications such as chronic pancreatitis, pancreatic pseudocysts, pancreatic insufficiency, steatorrhea, and insulin-dependent diabetes mellitus 4 , 86) . Although chronic complications are not invariably associated with HTG-induced acute pancreatitis 136) , they are not uncommon despite modern medical care 95) .
D) Other Therapeutic Options for HTG-Induced Acute Pancreatitis
• Insulin (should be individualized) : Insulin therapy is advised in patients with diabetes mellitus. Insulin stimulates LPL activity, thereby reducing plasma TG levels. Administration of insulin or insulin plus glucose may be considered in non-diabetic patients in the case of severe HTG-induced acute pancreatitis 14 , 137 - 139) . Detailed protocols for insulin/glucose administration have been summarized elsewhere 137 , 139) .
• Heparin (not usually recommended) : Heparin infusion has been used as a therapeutic option but is not usually recommended as a monotherapy in treatment guides by experts 12 , 14) . Heparin transiently increases plasma LPL levels by releasing LPL from the endothelial cell surface, which temporarily reduces plasma TG levels. However, heparin can also deplete LPL, causing a rebound increase in plasma TGs 140) . Heparin may increase the risk of pancreatic hemorrhage when pancreatic necrosis is present 66) .
• Heparin plus insulin (should be individualized) : Combination of heparin and insulin may be a therapeutic option for severe HTG-induced acute pancreatitis 14) . Although heparin infusion alone is usually not advised, a recent study has suggested that combination of heparin and insulin may be effective 14) . Evaluation in RCTs is awaited.
• Apheresis for HTG-induced acute pancreatitis (should be individualized) : In the acute setting, apheresis (lipoprotein apheresis (LA), plasmapheresis, or plasma exchange (PEX), etc.) 141 , 142) can rapidly reduce plasma TG levels (40-80%) by directly removing TGRLs, as reported in case reports, case series, and multi-center studies 12 , 14 , 36 , 57) . However, it is not proven whether rapid TG reduction by apheresis leads to better clinical outcomes than other therapeutic modalities in terms of pancreatic complications and mortality 59) . Plasmapheresis is costly, has only a transient TG- lowering effect (usually for a day), and may have adverse reactions (e.g., allergic reactions, anaphylactic shock, infusion-related infections, thromboses, etc.) 14) . A recent systematic review and case-control studies indicated that while plasmapheresis decreased plasma TG, it did not conclusively affect the morbidity or mortality of acute pancreatitis 14 , 86 ). A recent RCT, the first one in HTG-induced acute pancreatitis, has demonstrated that although plasma apheresis lowers plasma TG more efficiently than insulin plus heparin, it is costly and does not lead to better clinical outcomes 143) . In the guideline of the American Society for Apheresis (ASFA), plasmapheresis is a category III indication with Grade 2C recommendation (“optimum role of apheresis therapy is not established. Decision making should be individualized”; “Weak recommendation, low-quality or very low-quality evidence due to observational studies or case series”) 144) , and generally not recommended by experts in treatment guides for chylomicronemia 4 , 5) . Plasmapheresis may be a therapeutic option for: 1) severe HTG-induced acute pancreatitis with persistent TG elevation past the first 48-72h with no other therapeutic choice 57 , 59 , 86 , 120) ; 2) HTG-induced acute pancreatitis in pregnancy or postpartum with no other therapeutic choice 14 , 36 , 74 , 120 , 145 - 149) ; or 3) severe HTG-induced acute pancreatitis with high levels of serum lipase, hypocalcemia, lactic acidosis, worsening inflammation or organ dysfunction 149 , 150) . However, such advice is experience-based, not evidence-based.
• Prophylactic apheresis to prevent HTG-induced acute pancreatitis (should be individualized) : Prophylactic apheresis may be a therapeutic choice for preventing severe recurrent HTG-induced acute pancreatitis but evidence for it is limited to several case reports of HTG-induced acute pancreatitis 12 , 14 , 57 , 151 - 154) and gestational HTG-induced acute pancreatitis 155 , 156) .
15. Treatment of HTG and HTG-induced Acute Pancreatitis in Pregnancy
There are currently no formal guidelines for gestational HTG and HTG-induced acute pancreatitis due to the rarity of these conditions and insufficient evidence. The treatment approach for gestational HTG and HTG-induced acute pancreatitis is well summarized by Wong et al. 66) .
• Dietary therapy (restriction of dietary fat, MCTs, n-3 PUFA) : There have been reports of successful management of HTG and prevention of HTG-induced acute pancreatitis during pregnancy through early intervention with a low-fat or very-low fat diet, MCTs, and n-3 fatty acids 4 , 66 , 113 , 122 , 149 , 157 - 162) . For pregnant women at high-risk of pregnancy-associated pancreatitis, extreme fat restriction to <2 g/day may be required during the 2 nd and 3 rd trimesters for successful delivery 1 , 4 , 122 , 159) . Topical application of sunflower oil or corn oil in pregnancy with an extra-low-fat diet may help prevent EFA deficiency 122 , 129 , 159) . In pregnancy-induced HTG, carbohydrate may need to be restricted, but an adequate amount should be taken. Carbohydrate iv may be a better therapeutic choice during carbohydrate restriction, as carbohydrate per os may produce a greater rise in plasma TG than carbohydrate iv 122 , 132) . The risk of MCTs to the fetus is thought to be low 66) . The safety of maternal n-3 fatty acid supplementation (DHA 2.2 g and EPA 1.1 g/day) for the mother and the fetus has been confirmed through a RCT 66) .
• TG-lowering medications (some are contraindicated in Japan) : There have been several reports on the use of niacin, or fibrates (gemfibrozil and fenofibrate) for pregnancy-associated HTG 66 , 120 , 122 , 159 , 163 - 166) . However, the safety of niacin or fibrate use during pregnancy has not been established, and the use of fibrates during pregnancy is contraindicated in Japan.
• Admission to hospital : For gestational HTG, admission to hospital may be advised in the following cases: suspected pancreatitis, persistent abdominal pain, steep increase in plasma TG in the 3 rd trimester, or TG >40 mmol/L (3540 mg/dL) 66 , 122 , 159) . Gestational acute pancreatitis is managed through standard care, which has been extensively reviewed by Papadakis EP et al. 73) .
• Other therapeutic options : When uncontrollable, further management may include: insulin, insulin pus glucose, insulin plus heparin, or plasmapheresis 36 , 66 , 119 , 120 , 137 - 139 , 149 , 156 , 161) . In a treatment guide for gestational HTG, the use of insulin is recommended only for hyperglycemic pregnant women, and the use of heparin is not recommended due to the paucity of clinical evidence 66) .
16. Unanswered Questions
A) Molecular Basis and New Therapeutic Modalities
Unraveling the molecular basis of chylomicronemia may lead to the development of new therapeutic modalities 167) .
Emerging therapeutic targets for chylomicronemia include: a microsomal triglyceride transfer protein (MTTP) inhibitor (lomitapide) 168 - 170) ; an APOB antisense oligonucleotide (ASO) inhibitor (mipomersen) 171 - 173) ; APOC3 ASO inhibitors, e.g., volanesorsen 174) , which has been approved by the EMA for genetically confirmed chylomicronemia at high-risk for pancreatitis 93) ; diacylglycerol O-acyltransferase 1 (DGAT1) inhibitors (AZD7687, LCQ908 (Pradigastat)) 175 - 177) ; angiopoietin-like protein 3 (ANGPTL3) inhibitors, e.g., ANGPTL3 ASOs (IONIS-ANGPTL3-L Rx ) 178) and ANGPTL3 antibody (evinacumab) 179 - 182) .
For these new therapies, potential adverse effects need to be carefully evaluated, including fatty liver associated diseases for lomitapide and mipomersen 168 - 170 , 172) as a consequence of their inhibition of VLDL secretion; thrombocytopenia for volanesorsen 174) ; gastrointestinal adverse effects (diarrhea, nausea, etc.) for DGAT inhibitors 175 - 177) , consistent with the fact that homozygous loss-of-function mutations of DGAT1 cause a congenital diarrheal disorder (OMIM: 615863) 183) .
Other agents under development include CAT-2003, a niacin-eicosapentaenoic acid conjugate that blocks sterol regulatory element-binding protein (SREBP). Inhibition of SREBP-1c has ameliorated environment-induced severe HTG in mouse models of hyperlipidemia, including apoA-V deficient mice, by blocking secretion of large-sized VLDL particles 54 , 55) .
Orlistat, an inhibitor of intestinal lipase, may help reduce TG levels in PCM 21 , 184 , 185) but may have adverse effects such as oily stools and fat-soluble vitamin insufficiency 128) .
Treatment that targets a specific genetic cause of PCM is not available. A gene therapy for LPL (alipogene tiparvovec) was approved by the EMA in 2012, but is costly and has been withdrawn from the market 94 , 186) .
C) Genotype-Phenotype Relationship of PCM
The genetic etiology of chylomicronemia may influence the risk of complications such as pancreatitis and atherosclerotic diseases 86 , 94 - 96) . The benefit, risk, and cost-effectiveness of genetic testing for chylomicronemia should be carefully evaluated 15 , 109 , 201 - 203) . Some expert reviews, which include the Consensus Panel report of the European Atherosclerosis Society, do not recommend routine genetic testing for severe HTG 3 , 204) .
D) Underdiagnosis and Undertreatment
Underdiagnosis of chylomicronemia is one of the major risks for pancreatitis 84 , 87) . A web-based patient survey reported that patients with chylomicronemia typically visit 5 physicians (range, 1-30) on average before receiving a final diagnosis of chylomicronemia 106 , 107) . Owing to the variety of symptoms and complications, patients with chylomicronemia may visit not only lipidologists and endocrinologists but also other diverse specialists, such as primary care physicians, pediatricians, obstetricians, emergency physicians, gastroenterologists, pancreatologists, and psychologists. Simple diagnostic criteria as well as cooperation among different medical specialists will be necessary to achieve timely diagnosis and treatment.
E) Unmet Needs and Burden of Disease
Due to the rarity of the disease, the clinical experience of each doctor is limited. Large registry studies 205 , 206) as well as patient-oriented observational studies 104 - 107) from the patient’s perspective are useful for understanding the unmet needs and burden of the disease from the physical, psychological, social, and financial viewpoints 104 - 107) .
A recent web-based patient survey revealed physical, emotional, and cognitive symptoms that are relevant to patient’s quality of life but have not been recognized by physicians 207 , 208) , including abdominal pain (41%), fatigue (23%), feeling sad/down/blue/depressed (18%), difficulty in concentrating (16%), impaired judgment (11%), brain fog (8%), forgetfulness (8%), and recent memory loss (5%) 106 , 107) . This survey also revealed actual handicaps felt at school, in society, and work, and family-related issues 106 , 107) .
Self-monitoring of plasma TG may be an unmet need that could help patients with the long-term management of the disease. It may enable patients to individualize their low-fat diets, hopefully leading to fewer episodes of acute pancreatitis 209) .
F) Support for Patients
The mainstay of the current treatment for chylomicronemia is dietary interventions. Supporting information and materials for patients on diets will help develop recipes and menu plans that would be more enjoyable and sustainable. Information and support for patients can be found at FCS Foundation (www.livingwithfcs.org; www.facebook.com/livingwithfcs), FCS Focus (fcsfocus.com), LPLD Alliance (UK) (www.lpldalliance.org), the National Organization for Rare Disorders (NORD) (https://rarediseases.org), and the Japan Intractable Diseases Information Center (https://www.nanbyou.or.jp/entry/4883). Supportive care from other healthcare professionals, such as medical social workers and mental health professionals, will be necessary to reduce the burden of the disease as well as to improve the quality of life of patients with chylomicronemia 105 - 107) .
Acknowledgments and Notice of Grant Support
This work has been supported by Health, Labour and Welfare Sciences Research Grant for Research on Rare and Intractable Diseases (H30-nanji-ippan-003).
Conflicts of Interest
Atsushi Nohara has nothing to disclose. Hayato Tada has nothing to disclose. Masatsune Ogura has received honoraria from Amgen Inc., Astellas Pharma Inc. Sachiko Okazaki has received scholarship grants from Minophagen Pharmaceutical Co., Ltd., Kowa Company, Ltd. Koh Ono has nothing to disclose. Hitoshi Shimano has nothing to disclose. Hiroyuki Daida has received honoraria from Amgen Inc., Daiichi-Sankyo Co., Ltd., Kowa Co., Ltd., and MSD K.K., Novartis Pharma K.K., Bayer Yakuhin, Ltd. and received clinical research funding from Canon Medical Systems Corporation, Philips Japan, Ltd., Toho Holdings Co., Ltd., Asahi Kasei Corporation, and Inter Reha Co., Ltd. HD has also received scholarship grants from Nippon Boehringer Ingelheim Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sanofi K.K., MSD K.K., Daiichi-Sankyo Co., Ltd., Pfizer Co., Ltd., Mitsubishi Tanabe Pharma Corp., Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Teijin Pharma, Ltd., Shionogi & Co., Ltd., Actelion Pharmaceuticals, Ltd., Actelion Ltd., Kowa Co., Ltd., Bayer Yakuhin, Ltd. HD has also courses endowed by companies, including Philips Japan, Ltd., ResMed, Fukuda Denshi Co., Ltd., and Paramount Bed Co., Ltd. Kazushige Dobashi has nothing to disclose. Toshio Hayashi has nothing to disclose. Mika Hori has nothing to disclose. Kota Matsuki has nothing to disclose. Tetsuo Minamino has nothing to disclose. Shinji Yokoyama has nothing to disclose. Mariko Harada-Shiba has received stock holdings or options from Liid Pharma, honoraria from Amgen Inc., Astellas Pharma Inc., Sanofi, and scholarship grants from Aegerion Pharmaceuticals, Inc., Recordati Rare Diseases Japan, and Kaneka Corporation. Katsunori Ikewaki has nothing to disclose. Yasushi Ishigaki has nothing to disclose. Shun Ishibashi has received honoraria from Kowa Co., Ltd., and a scholarship grant from Ono Pharmaceutical Co., Ltd. Kyoko Inagaki has nothing to disclose. Hirotoshi Ohmura has nothing to disclose. Hiroaki Okazaki has received scholarship grants from Minophagen Pharmaceutical Co., Ltd., Kowa Company, Ltd. Masa-aki Kawashiri has nothing to disclose. Masayuki Kuroda has nothing to disclose. Masahiro Koseki has received clinical research funding from Kowa Company, Ltd., Rohto Pharmaceutical Co., Ltd. Takanari Gotoda has nothing to disclose. Shingo Koyama has nothing to disclose. Yoshiki Sekijima has nothing to disclose. Manabu Takahashi has nothing to disclose. Yasuo Takeuchi has nothing to disclose. Misa Takegami has nothing to disclose. Kazuhisa Tsukamoto has received honoraria from Bayer Yakuhin, Ltd., MSD Ltd., Takeda Pharmaceutical Company Ltd., and scholarship grants from Mitsubishi Tanabe Pharma Corporation., Bayer Yakuhin, Ltd., Sanofi K.K. Atsuko Nakatsuka has nothing to disclose. Kimitoshi Nakamura has nothing to disclose. Satoshi Hirayama has nothing to disclose. Hideaki Bujo has nothing to disclose. Daisaku Masuda has received clinical research funding from MSD K.K., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Kowa Co., Ltd. Takashi Miida has nothing to disclose. Yoshihiro Miyamoto has nothing to disclose. Takeyoshi Murano has nothing to disclose. Takashi Yamaguchi has nothing to disclose. Shizuya Yamashita has received honoraria from Kowa Company, Ltd., MSD K.K. Masashi Yamamoto has nothing to disclose. Koutaro Yokote has received honoraria from Kowa Company, Ltd., MSD K.K., Astellas Pharma Inc., Mitsubishi Tanabe Pharma Corp., Amgen K.K., Takeda Pharmaceutical Company Limited, Sanofi K.K., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Daiichi-Sankyo Co., Ltd., Novartis Pharma K.K., Sumitomo Dainippon Pharma Co., Ltd., Kyowa Kirin Co., Ltd., Pfizer Japan Inc., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., Taisho Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., and received clinical research funding from Taisho Pharmaceutical Co., Ltd. KY has also received scholarship grants from Mitsubishi Tanabe Pharma Corp., Takeda Pharmaceutical Co., Ltd., MSD K.K., Pfizer Japan Inc., Novo Nordisk Pharma Ltd., Taisho Pharmaceutical Co., Ltd., Kao Corporation, Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Daiichi-Sankyo Co., Ltd., Teijin Pharma, Ltd., Shionogi Co., Ltd., Bayer Yakuhin, Ltd. Jun Wada has nothing to disclose.
References
- 1).Brunzell JD, Deeb SS. Familial lipoprotein lipase deficiency, apoC-II deficiency, and hepatic lipase deficiency. The metabolic and molecular bases of inherited disease, 2001; 2: 2789-2816 [Google Scholar]
- 2).Gotoda T, Shirai K, Ohta T, Kobayashi J, Yokoyama S, Oikawa S, Bujo H, Ishibashi S, Arai H, Yamashita S, Harada-Shiba M, Eto M, Hayashi T, Sone H, Suzuki H, Yamada N, Research Committee for Primary Hyperlipidemia, Research on Measures against Intractable Diseases by the Ministry of Health, Labour and Welfare in Japan. Diagnosis and management of type I and type V hyperlipoproteinemia. J Atheroscler Thromb, 2012; 19: 1-12 [DOI] [PubMed] [Google Scholar]
- 3).Hegele RA, Ginsberg HN, Chapman MJ, Nordestgaard BG, Kuivenhoven JA, Averna M, Borén J, Bruckert E, Catapano AL, Descamps OS, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Raal FJ, Ray KK, Santos RD, Stalenhoef AFH, Stroes E, Taskinen M-RR, Tybjærg-Hansen A, Watts GF, Wiklund O, Panel EASC. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol, 2014; 2: 655-666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Burnett JR, Hooper AJ, Hegele RA. Familial Lipoprotein Lipase Deficiency [Internet]. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Stephens K, Amemiya A, editors. GeneReviews®. Seattle (WA): University of Washington, Seattle; 1993 [cited 2019 Oct 28]. Available from: http: //www.ncbi.nlm.nih.gov/books/NBK1308/ [PubMed] [Google Scholar]
- 5).Brahm AJ, Hegele RA. Chylomicronaemia--current diagnosis and future therapies. Nat Rev Endocrinol, 2015; 11: 352-362 [DOI] [PubMed] [Google Scholar]
- 6).Kihara S, Matsuzawa Y, Kubo M, Nozaki S, Funahashi T, Yamashita S, Sho N, Tarui S. Autoimmune hyperchylomicronemia. N Engl J Med, 1989; 320: 1255-1259 [DOI] [PubMed] [Google Scholar]
- 7).Beigneux AP, Miyashita K, Ploug M, Blom DJ, Ai M, Linton MF, Khovidhunkit W, Dufour R, Garg A, McMahon MA, Pullinger CR, Sandoval NP, Hu X, Allan CM, Larsson M, Machida T, Murakami M, Reue K, Tontonoz P, Goldberg IJ, Moulin P, Charrière S, Fong LG, Nakajima K, Young SG. Autoantibodies against GPIHBP1 as a Cause of Hypertriglyceridemia. N Engl J Med, 2017; 376: 1647-1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Inoue M, Kawamura M, Yamamoto H, Takahashi T, Kihara S. Juvenile-onset systemic lupus erythematosus with severe hypertriglyceridemia induced by anti-apolipoprotein C-II antibody. Pediatr Int, 2019; 61: 201-203 [DOI] [PubMed] [Google Scholar]
- 9).Miyashita K, Lutz J, Hudgins LC, Toib D, Ashraf AP, Song W, Murakami M, Nakajima K, Ploug M, Fong LG, Young SG, Beigneux AP. Chylomicronemia from GPIHBP1 autoantibodies. J Lipid Res, 2020; 61: 1365-1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Havel RJ. Pathogenesis, differentiation and management of hypertriglyceridemia. Adv Intern Med, 1969; 15: 117-154 [PubMed] [Google Scholar]
- 11).Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, Durgampudi C, Karlsson JM, Lee K, Bae KT, Furlan A, Behari J, Liu S, McHale T, Nichols L, Papachristou GI, Yadav D, Singh VP. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med, 2011; 3: 107ra110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Valdivielso P, Ramírez-Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med, 2014; 25: 689-694 [DOI] [PubMed] [Google Scholar]
- 13).Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, Stalenhoef AFH, Endocrine society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab, 2012; 97: 2969-2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Rawla P, Sunkara T, Thandra KC, Gaduputi V. Hypertriglyceridemia-induced pancreatitis: updated review of current treatment and preventive strategies. Clin J Gastroenterol, 2018; 11: 441-448 [DOI] [PubMed] [Google Scholar]
- 15).Hegele RA, Berberich AJ, Ban MR, Wang J, Digenio A, Alexander VJ, D’Erasmo L, Arca M, Jones A, Bruckert E, Stroes ES, Bergeron J, Civeira F, Witztum JL, Gaudet D. Clinical and biochemical features of different molecular etiologies of familial chylomicronemia. Journal of Clinical Lipidology, 2018; 12: 920-927.e4 [DOI] [PubMed] [Google Scholar]
- 16).Lewis GF, Xiao C, Hegele RA. Hypertriglyceridemia in the Genomic Era: A New Paradigm. Endocrine Reviews, 2015; 36: 131-147 [DOI] [PubMed] [Google Scholar]
- 17).Brown WV, Goldberg IJ, Young SG. JCL Roundtable: Hypertriglyceridemia due to defects in lipoprotein lipase function. J Clin Lipidol, 2015; 9: 274-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Murase T, Okubo M, Ebara T, Mori Y. Severe hypertriglyceridemia in Japan: Differences in causes and therapeutic responses. J Clin Lipidol, 2017; 11: 1383-1392 [DOI] [PubMed] [Google Scholar]
- 19).Surendran RP, Visser ME, Heemelaar S, Wang J, Peter J, Defesche JC, Kuivenhoven JA, Hosseini M, Péterfy M, Kastelein JJP, Johansen CT, Hegele RA, Stroes ESG, Dallinga-Thie GM. Mutations in LPL, APOC2, APOA5, GPIHBP1 and LMF1 in patients with severe hypertriglyceridaemia. J Intern Med, 2012; 272: 185-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Rabacchi C, Pisciotta L, Cefalù AB, Noto D, Fresa R, Tarugi P, Averna M, Bertolini S, Calandra S. Spectrum of mutations of the LPL gene identified in Italy in patients with severe hypertriglyceridemia. Atherosclerosis, 2015; 241: 79-86 [DOI] [PubMed] [Google Scholar]
- 21).Chokshi N, Blumenschein SD, Ahmad Z, Garg A. Genotype-phenotype relationships in patients with type I hyperlipoproteinemia. J Clin Lipidol, 2014; 8: 287-295 [DOI] [PubMed] [Google Scholar]
- 22).Rodrigues R, Artieda M, Tejedor D, Martínez A, Konstantinova P, Petry H, Meyer C, Corzo D, Sundgreen C, Klor HU, Gouni-Berthold I, Westphal S, Steinhagen-Thiessen E, Julius U, Winkler K, Stroes E, Vogt A, Hardt P, Prophet H, Otte B, Nordestgaard BG, Deeb SS, Brunzell JD. Pathogenic classification of LPL gene variants reported to be associated with LPL deficiency. J Clin Lipidol, 2016; 10: 394-409 [DOI] [PubMed] [Google Scholar]
- 23).Nilsson-Ehle P, Schotz MC. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res, 1976; 17: 536-541 [PubMed] [Google Scholar]
- 24).Péterfy M, Ben-Zeev O, Mao HZ, Weissglas-Volkov D, Aouizerat BE, Pullinger CR, Frost PH, Kane JP, Malloy MJ, Reue K, Pajukanta P, Doolittle MH. Mutations in LMF1 cause combined lipase deficiency and severe hypertriglyceridemia. Nat Genet, 2007; 39: 1483-1487 [DOI] [PubMed] [Google Scholar]
- 25).Cefalù AB, Noto D, Arpi ML, Yin F, Spina R, Hilden H, Barbagallo CM, Carroccio A, Tarugi P, Squatrito S, Vigneri R, Taskinen M-R, Péterfy M, Averna MR. Novel LMF1 nonsense mutation in a patient with severe hypertriglyceridemia. J Clin Endocrinol Metab, 2009; 94: 4584-4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Péterfy M, Bedoya C, Giacobbe C, Pagano C, Gentile M, Rubba P, Fortunato G, Di Taranto MD. Characterization of two novel pathogenic variants at compound heterozygous status in lipase maturation factor 1 gene causing severe hypertriglyceridemia. J Clin Lipidol, 2018; 12: 1253-1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Serveaux Dancer M, Di Filippo M, Marmontel O, Valéro R, Piombo Rivarola MDC, Peretti N, Caussy C, Krempf M, Vergès B, Mahl M, Marçais C, Moulin P, Charrière S. New rare genetic variants of LMF1 gene identified in severe hypertriglyceridemia. J Clin Lipidol, 2018; 12: 1244-1252 [DOI] [PubMed] [Google Scholar]
- 28).Liu Y, Xu J, Tao W, Yu R, Zhang X. A Compound Heterozygous Mutation of Lipase Maturation Factor 1 is Responsible for Hypertriglyceridemia of a Patient. J Atheroscler Thromb, 2019; 26: 136-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Chen W-W, Yang Q, Li X-Y, Shi X-L, Pu N, Lu G-T, Tong Z-H, Chen J-M, Li W-Q. Identification of a novel and heterozygous LMF1 nonsense mutation in an acute pancreatitis patient with severe hypertriglyceridemia, severe obesity and heavy smoking. Lipids Health Dis, 2019; 18: 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Ioka RX, Kang M-J, Kamiyama S, Kim D-H, Magoori K, Kamataki A, Ito Y, Takei YA, Sasaki M, Suzuki T, Sasano H, Takahashi S, Sakai J, Fujino T, Yamamoto TT. Expression cloning and characterization of a novel glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein, GPI-HBP1. J Biol Chem, 2003; 278: 7344-7349 [DOI] [PubMed] [Google Scholar]
- 31).Beigneux AP, Davies BSJ, Gin P, Weinstein MM, Farber E, Qiao X, Peale F, Bunting S, Walzem RL, Wong JS, Blaner WS, Ding Z-M, Melford K, Wongsiriroj N, Shu X, de Sauvage F, Ryan RO, Fong LG, Bensadoun A, Young SG. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab, 2007; 5: 279-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Rios JJ, Shastry S, Jasso J, Hauser N, Garg A, Bensadoun A, Cohen JC, Hobbs HH. Deletion of GPIHBP1 causing severe chylomicronemia. J Inherit Metab Dis, 2012; 35: 531-540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Adeyo O, Goulbourne CN, Bensadoun A, Beigneux AP, Fong LG, Young SG. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 and the intravascular processing of triglyceride-rich lipoproteins. J Intern Med, 2012; 272: 528-540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Patni N, Brothers J, Xing C, Garg A. Type 1 hyperlipoproteinemia in a child with large homozygous deletion encompassing GPIHBP1. J Clin Lipidol, 2016; 10: 1035-1039.e2 [DOI] [PubMed] [Google Scholar]
- 35).Rabacchi C, D’Addato S, Palmisano S, Lucchi T, Bertolini S, Calandra S, Tarugi P. Clinical and genetic features of 3 patients with familial chylomicronemia due to mutations in GPIHBP1 gene. J Clin Lipidol, 2016; 10: 915-921.e4 [DOI] [PubMed] [Google Scholar]
- 36).Chyzhyk V, Kozmic S, Brown AS, Hudgins LC, Starc TJ, Davila AD, Blevins TC, Diffenderfer MR, He L, Geller AS, Rush C, Hegele RA, Schaefer EJ. Extreme hypertriglyceridemia: Genetic diversity, pancreatitis, pregnancy, and prevalence. J Clin Lipidol, 2019; 13: 89-99 [DOI] [PubMed] [Google Scholar]
- 37).Franssen R, Young SG, Peelman F, Hertecant J, Sierts JA, Schimmel AWM, Bensadoun A, Kastelein JJP, Fong LG, Dallinga-Thie GM, Beigneux AP. Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects. Circ Cardiovasc Genet, 2010; 3: 169-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Breckenridge WC, Little JA, Steiner G, Chow A, Poapst M. Hypertriglyceridemia associated with deficiency of apolipoprotein C-II. N Engl J Med, 1978; 298: 1265-1273 [DOI] [PubMed] [Google Scholar]
- 39).Yamamura T, Sudo H, Ishikawa K, Yamamoto A. Familial type I hyperlipoproteinemia caused by apolipoprotein C-II deficiency. Atherosclerosis, 1979; 34: 53-65 [DOI] [PubMed] [Google Scholar]
- 40).Okubo M, Toromanovic A, Ebara T, Murase T. Apolipoprotein C-II Tuzla: a novel large deletion in APOC2 caused by Alu-Alu homologous recombination in an infant with apolipoprotein C-II deficiency. Clin Chim Acta, 2015; 438: 148-153 [DOI] [PubMed] [Google Scholar]
- 41).Wolska A, Dunbar RL, Freeman LA, Ueda M, Amar MJ, Sviridov DO, Remaley AT. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis, 2017; 267: 49-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Fojo SS, Beisiegel U, Beil U, Higuchi K, Bojanovski M, Gregg RE, Greten H, Brewer HB. Donor splice site mutation in the apolipoprotein (Apo) C-II gene (Apo C-IIHamburg) of a patient with Apo C-II deficiency. J Clin Invest, 1988; 82: 1489-1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Streicher R, Geisel J, Weisshaar C, Avci H, Oette K, Müller-Wieland D, Krone W. A single nucleotide substitution in the promoter region of the apolipoprotein C-II gene identified in individuals with chylomicronemia. J Lipid Res, 1996; 37: 2599-2607 [PubMed] [Google Scholar]
- 44).Okubo M, Hasegawa Y, Aoyama Y, Murase T. A G+1 to C mutation in a donor splice site of intron 2 in the apolipoprotein (apo) C-II gene in a patient with apo C-II deficiency. A possible interaction between apo C-II deficiency and apo E4 in a severely hypertriglyceridemic patient. Atherosclerosis, 1997; 130: 153-160 [DOI] [PubMed] [Google Scholar]
- 45).Takase S, Osuga J, Fujita H, Hara K, Sekiya M, Igarashi M, Takanashi M, Takeuchi Y, Izumida Y, Ohta K, Kumagai M, Nishi M, Kubota M, Masuda Y, Taira Y, Okazaki S, Iizuka Y, Yahagi N, Ohashi K, Yoshida H, Yanai H, Tada N, Gotoda T, Ishibashi S, Kadowaki T, Okazaki H. Apolipoprotein C-II deficiency with no rare variant in the APOC2 gene. J Atheroscler Thromb, 2013; 20: 481-493 [DOI] [PubMed] [Google Scholar]
- 46).Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science, 2001; 294: 169-173 [DOI] [PubMed] [Google Scholar]
- 47).van der Vliet HN, Sammels MG, Leegwater AC, Levels JH, Reitsma PH, Boers W, Chamuleau RA. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J Biol Chem, 2001; 276: 44512-44520 [DOI] [PubMed] [Google Scholar]
- 48).Merkel M, Loeffler B, Kluger M, Fabig N, Geppert G, Pennacchio LA, Laatsch A, Heeren J. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem, 2005; 280: 21553-21560 [DOI] [PubMed] [Google Scholar]
- 49).Merkel M, Heeren J. Give me A5 for lipoprotein hydrolysis! J Clin Invest, 2005; 115: 2694-2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Marçais C, Verges B, Charrière S, Pruneta V, Merlin M, Billon S, Perrot L, Drai J, Sassolas A, Pennacchio LA, Fruchart-Najib J, Fruchart J-C, Durlach V, Moulin P. Apoa5 Q139X truncation predisposes to late-onset hyperchylomicronemia due to lipoprotein lipase impairment. J Clin Invest, 2005; 115: 2862-2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Priore Oliva C, Pisciotta L, Li Volti G, Sambataro MP, Cantafora A, Bellocchio A, Catapano A, Tarugi P, Bertolini S, Calandra S. Inherited apolipoprotein A-V deficiency in severe hypertriglyceridemia. Arterioscler Thromb Vasc Biol, 2005; 25: 411-417 [DOI] [PubMed] [Google Scholar]
- 52).Albers K, Schlein C, Wenner K, Lohse P, Bartelt A, Heeren J, Santer R, Merkel M. Homozygosity for a partial deletion of apoprotein A-V signal peptide results in intracellular missorting of the protein and chylomicronemia in a breast-fed infant. Atherosclerosis, 2014; 233: 97-103 [DOI] [PubMed] [Google Scholar]
- 53).D’Erasmo L, Di Costanzo A, Cassandra F, Minicocci I, Polito L, Montali A, Ceci F, Arca M. Spectrum of Mutations and Long-Term Clinical Outcomes in Genetic Chylomicronemia Syndromes. Arterioscler Thromb Vasc Biol, 2019; 39: 2531-2541 [DOI] [PubMed] [Google Scholar]
- 54).Okazaki H, Goldstein JL, Brown MS, Liang G. LXR-SREBP-1c-phospholipid transfer protein axis controls very low density lipoprotein (VLDL) particle size. J Biol Chem, 2010; 285: 6801-6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Takanashi M, Kimura T, Li C, Tanaka M, Matsuhashi A, Yoshida H, Noda A, Xu P, Takase S, Okazaki S, Iizuka Y, Kumagai H, Ikeda Y, Gotoda T, Takahashi M, Yagyu H, Ishibashi S, Yamauchi T, Kadowaki T, Liang G, Okazaki H. Critical Role of SREBP-1c Large-VLDL Pathway in Environment-Induced Hypertriglyceridemia of Apo AV Deficiency. Arterioscler Thromb Vasc Biol, 2019; 39: 373-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ, 2007; 176: 1113-1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Stefanutti C, Di Giacomo S, Vivenzio A, Labbadia G, Mazza F, D’Alessandri G, Russi G, De Silvestro G, Marson P. Therapeutic plasma exchange in patients with severe hypertriglyceridemia: a multicenter study. Artif Organs, 2009; 33: 1096-1102 [DOI] [PubMed] [Google Scholar]
- 58).Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S, American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Nursing, Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation, 2011; 123: 2292-2333 [DOI] [PubMed] [Google Scholar]
- 59).Click B, Ketchum AM, Turner R, Whitcomb DC, Papachristou GI, Yadav D. The role of apheresis in hypertriglyceridemia-induced acute pancreatitis: A systematic review. Pancreatology, 2015; 15: 313-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Inayat F, Zafar F, Riaz I, Younus F, Baig AS, Imran Z. Hypertriglyceridemic Pancreatitis: Is Insulin Monotherapy A Feasible Therapeutic Option? Cureus, 2018; 10: e3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, Jacobson TA, Engler MB, Miller M, Robinson JG, Blum CB, Rodriguez-Leyva D, de Ferranti SD, Welty FK, American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation, 2019; 140: e673-e691 [DOI] [PubMed] [Google Scholar]
- 62).Greenberg BH. Primary Type V Hyperlipoproteinemia: A Descriptive Study in 32 Families. Ann Intern Med, 1977; 87: 526-534 [DOI] [PubMed] [Google Scholar]
- 63).Tada H, Kawashiri M-A, Nakahashi T, Yagi K, Chujo D, Ohbatake A, Mori Y, Mori S, Kometani M, Fujii H, Nohara A, Inazu A, Mabuchi H, Yamagishi M, Hayashi K. Clinical characteristics of Japanese patients with severe hypertriglyceridemia. J Clin Lipidol, 2015; 9: 519-524 [DOI] [PubMed] [Google Scholar]
- 64).Basaran A. Pregnancy-induced hyperlipoproteinemia: review of the literature. Reprod Sci, 2009; 16: 431-437 [DOI] [PubMed] [Google Scholar]
- 65).Ghio A, Bertolotto A, Resi V, Volpe L, Di Cianni G. Triglyceride metabolism in pregnancy. Adv Clin Chem, 2011; 55: 133-153 [DOI] [PubMed] [Google Scholar]
- 66).Wong B, Ooi TC, Keely E. Severe gestational hypertriglyceridemia: A practical approach for clinicians. Obstet Med, 2015; 8: 158-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Amundsen AL, Khoury J, Iversen PO, Bergei C, Ose L, Tonstad S, Retterstøl K. Marked changes in plasma lipids and lipoproteins during pregnancy in women with familial hypercholesterolemia. Atherosclerosis, 2006; 189: 451-457 [DOI] [PubMed] [Google Scholar]
- 68).Warth MR, Arky RA, Knopp RH. Lipid metabolism in pregnancy. II. Altered lipid composition in intermediage, very low, low and high-density lipoprotein fractions. J Clin Endocrinol Metab, 1975; 41: 649-655 [DOI] [PubMed] [Google Scholar]
- 69).Potter JM, Nestel PJ. The hyperlipidemia of pregnancy in normal and complicated pregnancies. Am J Obstet Gynecol, 1979; 133: 165-170 [DOI] [PubMed] [Google Scholar]
- 70).Salameh WA, Mastrogiannis DS. Maternal hyperlipidemia in pregnancy. Clin Obstet Gynecol, 1994; 37: 66-77 [DOI] [PubMed] [Google Scholar]
- 71).Alvarez JJ, Montelongo A, Iglesias A, Lasunción MA, Herrera E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J Lipid Res, 1996; 37: 299-308 [PubMed] [Google Scholar]
- 72).Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine, 2002; 19: 43-55 [DOI] [PubMed] [Google Scholar]
- 73).Papadakis EP, Sarigianni M, Mikhailidis DP, Mamopoulos A, Karagiannis V. Acute pancreatitis in pregnancy: an overview. Eur J Obstet Gynecol Reprod Biol, 2011; 159: 261-266 [DOI] [PubMed] [Google Scholar]
- 74).Russi G. Severe dyslipidemia in pregnancy: The role of therapeutic apheresis. Transfus Apher Sci, 2015; 53: 283-287 [DOI] [PubMed] [Google Scholar]
- 75).Kekki M, Nikkilä EA. Plasma triglyceride turnover during use of oral contraceptives. Metab Clin Exp, 1971; 20: 878-889 [DOI] [PubMed] [Google Scholar]
- 76).Schaefer EJ, Foster DM, Zech LA, Lindgren FT, Brewer HB, Levy RI. The Effects of Estrogen Administration on Plasma Lipoprotein Metabolism in Premenopausal Females. The Journal of Clinical Endocrinology & Metabolism, 1983; 57: 262-267 [DOI] [PubMed] [Google Scholar]
- 77).Desoye G, Schweditsch MO, Pfeiffer KP, Zechner R, Kostner GM. Correlation of hormones with lipid and lipoprotein levels during normal pregnancy and postpartum. J Clin Endocrinol Metab, 1987; 64: 704-712 [DOI] [PubMed] [Google Scholar]
- 78).Lamon-Fava S, Diffenderfer MR, Barrett PHR, Wan WY, Postfai B, Nartsupha C, Dolnikowski GG, Schaefer EJ. Differential Effects of Estrogen and Progestin on Apolipoprotein B100 and B48 Kinetics in Postmenopausal Women. Lipids, 2018; 53: 167-175 [DOI] [PubMed] [Google Scholar]
- 79).Fredrickson DS, Lees RS. A SYSTEM FOR PHENOTYPING HYPERLIPOPROTEINEMIA. Circulation, 1965; 31: 321-327 [DOI] [PubMed] [Google Scholar]
- 80).Moulin P, Dufour R, Averna M, Arca M, Cefalù AB, Noto D, D’Erasmo L, Di Costanzo A, Marçais C, Alvarez-Sala Walther LA, Banach M, Borén J, Cramb R, Gouni-Berthold I, Hughes E, Johnson C, Pintó X, Reiner Ž, van Lennep JR, Soran H, Stefanutti C, Stroes E, Bruckert E. Identification and diagnosis of patients with familial chylomicronaemia syndrome (FCS): Expert panel recommendations and proposal of an “FCS score.” Atherosclerosis, 2018; 275: 265-272 [DOI] [PubMed] [Google Scholar]
- 81).Brown WV, Goldberg I, Duell B, Gaudet D. Roundtable discussion: Familial chylomicronemia syndrome: Diagnosis and management. J Clin Lipidol, 2018; 12: 254-263 [DOI] [PubMed] [Google Scholar]
- 82).Brown WV, Gaudet D, Goldberg I, Hegele R. Roundtable on etiology of familial chylomicronemia syndrome. J Clin Lipidol, 2018; 12: 5-11 [DOI] [PubMed] [Google Scholar]
- 83).Dron JS, Wang J, Cao H, McIntyre AD, Iacocca MA, Menard JR, Movsesyan I, Malloy MJ, Pullinger CR, Kane JP, Hegele RA. Severe hypertriglyceridemia is primarily polygenic. J Clin Lipidol, 2019; 13: 80-88 [DOI] [PubMed] [Google Scholar]
- 84).Toth PP, Grabner M, Ramey N, Higuchi K. Clinical and economic outcomes in a real-world population of patients with elevated triglyceride levels. Atherosclerosis, 2014; 237: 790-797 [DOI] [PubMed] [Google Scholar]
- 85).Sekimoto M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, Hirota M, Kimura Y, Takeda K, Isaji S, Koizumi M, Otsuki M, Matsuno S, JPN. JPN Guidelines for the management of acute pancreatitis: epidemiology, etiology, natural history, and outcome predictors in acute pancreatitis. J Hepatobiliary Pancreat Surg, 2006; 13: 10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Carr RA, Rejowski BJ, Cote GA, Pitt HA, Zyromski NJ. Systematic review of hypertriglyceridemia-induced acute pancreatitis: A more virulent etiology? Pancreatology, 2016; 16: 469-476 [DOI] [PubMed] [Google Scholar]
- 87).Rashid N, Sharma PP, Scott RD, Lin KJ, Toth PP. Severe hypertriglyceridemia and factors associated with acute pancreatitis in an integrated health care system. J Clin Lipidol, 2016; 10: 880-890 [DOI] [PubMed] [Google Scholar]
- 88).Geng Y, Li W, Sun L, Tong Z, Li N, Li J. Severe acute pancreatitis during pregnancy: eleven years experience from a surgical intensive care unit. Dig Dis Sci, 2011; 56: 3672-3677 [DOI] [PubMed] [Google Scholar]
- 89).Ramin KD, Ramin SM, Richey SD, Cunningham FG. Acute pancreatitis in pregnancy. Am J Obstet Gynecol, 1995; 173: 187-191 [DOI] [PubMed] [Google Scholar]
- 90).Murphy MJ, Sheng X, MacDonald TM, Wei L. Hypertriglyceridemia and acute pancreatitis. JAMA Intern Med, 2013; 173: 162-164 [DOI] [PubMed] [Google Scholar]
- 91).Pedersen SB, Langsted A, Nordestgaard BG. Nonfasting Mild-to-Moderate Hypertriglyceridemia and Risk of Acute Pancreatitis. JAMA Intern Med, 2016; 176: 1834-1842 [DOI] [PubMed] [Google Scholar]
- 92).Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol, 2014; 48: 195-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93).Authors/Task Force Members, ESC Committee for Practice Guidelines (CPG), ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis, 2019; 290: 140-205 [DOI] [PubMed] [Google Scholar]
- 94).Gaudet D, de Wal J, Tremblay K, Déry S, van Deventer S, Freidig A, Brisson D, Méthot J. Review of the clinical development of alipogene tiparvovec gene therapy for lipoprotein lipase deficiency. Atheroscler Suppl, 2010; 11: 55-60 [DOI] [PubMed] [Google Scholar]
- 95).Gaudet D, Blom D, Bruckert E, Stroes E, Kastelein J, John K, Malloy M, Moulin P, Retterstøļl K, Hughes S, Tsimikas S, Witztum J. Acute Pancreatitis is Highly Prevalent and Complications can be Fatal in Patients with Familial Chylomicronemia: Results From a Survey of Lipidologist. Journal of Clinical Lipidology, 2016; 10: 680-681 [Google Scholar]
- 96).Paquette M, Bernard S, Hegele RA, Baass A. Chylomicronemia: Differences between familial chylomicronemia syndrome and multifactorial chylomicronemia. Atherosclerosis, 2019; 283: 137-142 [DOI] [PubMed] [Google Scholar]
- 97).Zafrir B, Jubran A, Hijazi R, Shapira C. Clinical features and outcomes of severe, very severe, and extreme hypertriglyceridemia in a regional health service. Journal of Clinical Lipidology, 2018; 12: 928-936 [DOI] [PubMed] [Google Scholar]
- 98).Fallat RW, Vester JW, Glueck CJ. Suppression of amylase activity by hypertriglyceridemia. JAMA, 1973; 225: 1331-1334 [PubMed] [Google Scholar]
- 99).Melnick S, Nazir S, Gish D, Aryal MR. Hypertriglyceridemic pancreatitis associated with confounding laboratory abnormalities. J Community Hosp Intern Med Perspect, 2016; 6: 31808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100).Singh A, Shrestha M, Anand C. Acute pancreatitis with normal amylase and lipase--an ED dilemma. Am J Emerg Med, 2016; 34: 940.e5-7 [DOI] [PubMed] [Google Scholar]
- 101).Nayak KR, Daly RG. Images in clinical medicine. Eruptive xanthomas associated with hypertriglyceridemia and new-onset diabetes mellitus. N Engl J Med, 2004; 350: 1235 [DOI] [PubMed] [Google Scholar]
- 102).Kumar J, Wierzbicki AS. Images in clinical medicine. Lipemia retinalis. N Engl J Med, 2005; 353: 823 [DOI] [PubMed] [Google Scholar]
- 103).Stefanutti C, Labbadia G, Morozzi C. Severe hypertriglyceridemia-related acute pancreatitis. Ther Apher Dial, 2013; 17: 130-137 [DOI] [PubMed] [Google Scholar]
- 104).Ahmad Z, Halter R, Stevenson M. Building a better understanding of the burden of disease in familial chylomicronemia syndrome. Expert Rev Clin Pharmacol, 2017; 10: 1-3 [DOI] [PubMed] [Google Scholar]
- 105).Gelrud A, Williams KR, Hsieh A, Gwosdow AR, Gilstrap A, Brown A. The burden of familial chylomicronemia syndrome from the patients’ perspective. Expert Rev Cardiovasc Ther, 2017; 15: 879-887 [DOI] [PubMed] [Google Scholar]
- 106).Davidson M, Stevenson M, Hsieh A, Ahmad Z, Crowson C, Witztum JL. The burden of familial chylomicronemia syndrome: interim results from the IN-FOCUS study. Expert Rev Cardiovasc Ther, 2017; 15: 415-423 [DOI] [PubMed] [Google Scholar]
- 107).Davidson M, Stevenson M, Hsieh A, Ahmad Z, Roeters van Lennep J, Crowson C, Witztum JL. The burden of familial chylomicronemia syndrome: Results from the global IN-FOCUS study. J Clin Lipidol, 2018; 12: 898-907.e2 [DOI] [PubMed] [Google Scholar]
- 108).Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics, 2011; 128 Suppl 5: S213-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109).Buonuomo PS, Rabacchi C, Macchiaiolo M, Trenti C, Fasano T, Tarugi P, Bartuli A, Bertolini S, Calandra S. Incidental finding of severe hypertriglyceridemia in children. Role of multiple rare variants in genes affecting plasma triglyceride. Journal of Clinical Lipidology, 2017; 11: 1329-1337.e3 [DOI] [PubMed] [Google Scholar]
- 110).Dominguez-Muñoz JE, Malfertheiner P, Ditschuneit HH, Blanco-Chavez J, Uhl W, Büchler M, Ditschuneit H. Hyperlipidemia in acute pancreatitis. Relationship with etiology, onset, and severity of the disease. Int J Pancreatol, 1991; 10: 261-267 [PubMed] [Google Scholar]
- 111).Watts GF, Morton K, Jackson P, Lewis B. Management of patients with severe hypertriglyceridaemia during pregnancy: report of two cases with familial lipoprotein lipase deficiency. Br J Obstet Gynaecol, 1992; 99: 163-166 [DOI] [PubMed] [Google Scholar]
- 112).Ma Y, Liu MS, Ginzinger D, Frohlich J, Brunzell JD, Hayden MR. Gene-environment interaction in the conversion of a mild-to-severe phenotype in a patient homozygous for a Ser172-->Cys mutation in the lipoprotein lipase gene. J Clin Invest, 1993; 91: 1953-1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113).Glueck CJ, Streicher P, Wang P, Sprecher D, Falko JM. Treatment of severe familial hypertriglyceridemia during pregnancy with very-low-fat diet and n-3 fatty acids. Nutrition, 1996; 12: 202-205 [DOI] [PubMed] [Google Scholar]
- 114).Saravanan P, Blumenthal S, Anderson C, Stein R, Berkelhammer C. Plasma exchange for dramatic gestational hyperlipidemic pancreatitis. J Clin Gastroenterol, 1996; 22: 295-298 [DOI] [PubMed] [Google Scholar]
- 115).Suga S, Tamasawa N, Kinpara I, Murakami H, Kasai N, Onuma T, Ikeda Y, Takagi A, Suda T. Identification of homozygous lipoprotein lipase gene mutation in a woman with recurrent aggravation of hypertriglyceridaemia induced by pregnancy. J Intern Med, 1998; 243: 317-321 [DOI] [PubMed] [Google Scholar]
- 116).McGladdery SH, Frohlich JJ. Lipoprotein lipase and apoE polymorphisms: relationship to hypertriglyceridemia during pregnancy. J Lipid Res, 2001; 42: 1905-1912 [PubMed] [Google Scholar]
- 117).Bartha I, Dinya T, Seres I, Paragh G, Ross C, Hayden MR, Biró S, Vargha G. Acute hypertriglyceridemic pancreatitis during pregnancy due to homozygous lipoprotein lipase gene mutation. Clin Chim Acta, 2009; 400: 137-138 [DOI] [PubMed] [Google Scholar]
- 118).Xie S-L, Chen T-Z, Huang X-L, Chen C, Jin R, Huang Z-M, Zhou M-T. Genetic Variants Associated with Gestational Hypertriglyceridemia and Pancreatitis. PLoS ONE, 2015; 10: e0129488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119).Gürsoy A, Kulaksizoglu M, Sahin M, Ertugrul DT, Ozer F, Tutuncu NB, Demirag NG. Severe hypertriglyceridemia-induced pancreatitis during pregnancy. J Natl Med Assoc, 2006; 98: 655-657 [PMC free article] [PubMed] [Google Scholar]
- 120).Goldberg AS, Hegele RA. Severe hypertriglyceridemia in pregnancy. J Clin Endocrinol Metab, 2012; 97: 2589-2596 [DOI] [PubMed] [Google Scholar]
- 121).Gallos ID, Sivakumar K, Kilby MD, Coomarasamy A, Thangaratinam S, Vatish M. Pre-eclampsia is associated with, and preceded by, hypertriglyceridaemia: a meta-analysis. BJOG, 2013; 120: 1321-1332 [DOI] [PubMed] [Google Scholar]
- 122).Al-Shali K, Wang J, Fellows F, Huff MW, Wolfe BM, Hegele RA. Successful pregnancy outcome in a patient with severe chylomicronemia due to compound heterozygosity for mutant lipoprotein lipase. Clin Biochem, 2002; 35: 125-130 [DOI] [PubMed] [Google Scholar]
- 123).Stroes E, Moulin P, Parhofer KG, Rebours V, Löhr J-M, Averna M. Diagnostic algorithm for familial chylomicronemia syndrome. Atheroscler Suppl, 2017; 23: 1-7 [DOI] [PubMed] [Google Scholar]
- 124).Tremblay K, Gaudet D, Khoury E, Brisson D. Dissection of Clinical and Gene Expression Signatures of Familial versus Multifactorial Chylomicronemia. J Endocr Soc, 2020; 4: bvaa056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125).Christian JB, Arondekar B, Buysman EK, Johnson SL, Seeger JD, Jacobson TA. Clinical and economic benefits observed when follow-up triglyceride levels are less than 500 mg/dL in patients with severe hypertriglyceridemia. J Clin Lipidol, 2012; 6: 450-461 [DOI] [PubMed] [Google Scholar]
- 126).Christian JB, Arondekar B, Buysman EK, Jacobson TA, Snipes RG, Horwitz RI. Determining triglyceride reductions needed for clinical impact in severe hypertriglyceridemia. Am J Med, 2014; 127: 36-44.e1 [DOI] [PubMed] [Google Scholar]
- 127).Huffman KM, Hawk VH, Henes ST, Ocampo CI, Orenduff MC, Slentz CA, Johnson JL, Houmard JA, Samsa GP, Kraus WE, Bales CW. Exercise effects on lipids in persons with varying dietary patterns-does diet matter if they exercise? Responses in Studies of a Targeted Risk Reduction Intervention through Defined Exercise I. Am Heart J, 2012; 164: 117-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128).Williams L, Wilson DP. Editorial commentary: Dietary management of familial chylomicronemia syndrome. J Clin Lipidol, 2016; 10: 462-465 [DOI] [PubMed] [Google Scholar]
- 129).Williams L, Rhodes KS, Karmally W, Welstead LA, Alexander L, Sutton L, patients and families living with FCS. Familial chylomicronemia syndrome: Bringing to life dietary recommendations throughout the life span. J Clin Lipidol, 2018; 12: 908-919 [DOI] [PubMed] [Google Scholar]
- 130).Ewald N, Hardt PD, Kloer H-U. Severe hypertriglyceridemia and pancreatitis: presentation and management. Curr Opin Lipidol, 2009; 20: 497-504 [DOI] [PubMed] [Google Scholar]
- 131).Brunzell JD, Hazzard WR, Porte D, Bierman EL. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J Clin Invest, 1973; 52: 1578-1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132).DenBesten L, Reyna RH, Connor WE, Stegink LD. The different effects on the serum lipids and fecal steroids of high carbohydrate diets given orally or intravenously. J Clin Invest, 1973; 52: 1384-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133).Deng L-H, Xue P, Xia Q, Yang X-N, Wan M-H. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol, 2008; 14: 4558-4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134).Nawaz H, Koutroumpakis E, Easler J, Slivka A, Whitcomb DC, Singh VP, Yadav D, Papachristou GI. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol, 2015; 110: 1497-1503 [DOI] [PubMed] [Google Scholar]
- 135).Tsuang W, Navaneethan U, Ruiz L, Palascak JB, Gelrud A. Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol, 2009; 104: 984-991 [DOI] [PubMed] [Google Scholar]
- 136).Kawashiri M, Higashikata T, Mizuno M, Takata M, Katsuda S, Miwa K, Nozue T, Nohara A, Inazu A, Kobayashi J, Koizumi J, Mabuchi H. Long-term course of lipoprotein lipase (LPL) deficiency due to homozygous LPL(Arita) in a patient with recurrent pancreatitis, retained glucose tolerance, and atherosclerosis. J Clin Endocrinol Metab, 2005; 90: 6541-6544 [DOI] [PubMed] [Google Scholar]
- 137).Mikhail N, Trivedi K, Page C, Wali S, Cope D. Treatment of severe hypertriglyceridemia in nondiabetic patients with insulin. Am J Emerg Med, 2005; 23: 415-417 [DOI] [PubMed] [Google Scholar]
- 138).Khan R, Jehangir W, Regeti K, Yousif A. Hypertriglyceridemia-Induced Pancreatitis: Choice of Treatment. Gastroenterology Res, 2015; 8: 234-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139).Inayat F, Zafar F, Baig AS, Chaudhry NA, Aslam A, Khan ZH, Iqbal MJ. Hypertriglyceridemic Pancreatitis Treated with Insulin Therapy: A Comparative Review of 34 Cases. Cureus, 2018; 10: e3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140).Weintraub M, Rassin T, Eisenberg S, Ringel Y, Grosskopf I, Iaina A, Charach G, Liron M, Rubinstein A. Continuous intravenous heparin administration in humans causes a decrease in serum lipolytic activity and accumulation of chylomicrons in circulation. J Lipid Res, 1994; 35: 229-238 [PubMed] [Google Scholar]
- 141).Schuff-Werner P, Fenger S, Kohlschein P. Role of lipid apheresis in changing times. Clin Res Cardiol Suppl, 2012; 7: 7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142).Stefanutti C, Julius U. Treatment of primary hypertriglyceridemia states--General approach and the role of extracorporeal methods. Atheroscler Suppl, 2015; 18: 85-94 [DOI] [PubMed] [Google Scholar]
- 143).He W-H, Yu M, Zhu Y, Xia L, Liu P, Zeng H, Zhu Y, Lv N-H. Emergent Triglyceride-lowering Therapy With Early High-volume Hemofiltration Against Low-Molecular-Weight Heparin Combined With Insulin in Hypertriglyceridemic Pancreatitis: A Prospective Randomized Controlled Trial. J Clin Gastroenterol, 2016; 50: 772-778 [DOI] [PubMed] [Google Scholar]
- 144).Schwartz J, Winters JL, Padmanabhan A, Balogun RA, Delaney M, Linenberger ML, Szczepiorkowski ZM, Williams ME, Wu Y, Shaz BH. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher, 2013; 28: 145-284 [DOI] [PubMed] [Google Scholar]
- 145).Thompson GR. Lipoprotein apheresis. Curr Opin Lipidol, 2010; 21: 487-491 [DOI] [PubMed] [Google Scholar]
- 146).Ewald N, Kloer H-U. Treatment options for severe hypertriglyceridemia (SHTG): the role of apheresis. Clin Res Cardiol Suppl, 2012; 7: 31-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147).Basar R, Uzum AK, Canbaz B, Dogansen SC, Kalayoglu-Besisik S, Altay-Dadin S, Aral F, Ozbey NC. Therapeutic apheresis for severe hypertriglyceridemia in pregnancy. Arch Gynecol Obstet, 2013; 287: 839-843 [DOI] [PubMed] [Google Scholar]
- 148).Safi F, Toumeh A, Abuissa Qadan MA, Karaz R, AlAkdar B, Assaly R. Management of familial hypertriglyceridemia-induced pancreatitis during pregnancy with therapeutic plasma exchange: a case report and review of literature. Am J Ther, 2014; 21: e134-136 [DOI] [PubMed] [Google Scholar]
- 149).Gupta N, Ahmed S, Shaffer L, Cavens P, Blankstein J. Severe hypertriglyceridemia induced pancreatitis in pregnancy. Case Rep Obstet Gynecol, 2014; 2014: 485493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150).Newman CB, Blaha MJ, Boord JB, Cariou B, Chait A, Fein HG, Ginsberg HN, Goldberg IJ, Murad MH, Subramanian S, Tannock LR. Lipid Management in Patients with Endocrine Disorders: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism, 2020; 105: 3613-3682 [DOI] [PubMed] [Google Scholar]
- 151).Piolot A, Nadler F, Cavallero E, Coquard JL, Jacotot B. Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: value of regular plasmapheresis. Pancreas, 1996; 13: 96-99 [DOI] [PubMed] [Google Scholar]
- 152).Hovland A, Hardersen R, Mollnes TE, Lappegård KT. Selective whole blood lipoprotein apheresis to prevent pancreatitis in drug refractory hypertriglyceridemia. JOP, 2010; 11: 467-469 [PubMed] [Google Scholar]
- 153).Schaap-Fogler M, Schurr D, Schaap T, Leitersdorf E, Rund D. Long-term plasma exchange for severe refractory hypertriglyceridemia: a decade of experience demonstrates safety and efficacy. J Clin Apher, 2009; 24: 254-258 [DOI] [PubMed] [Google Scholar]
- 154).Saleh MA, Mansoor E, Cooper GS. Case of familial hyperlipoproteinemia type III hypertriglyceridemia induced acute pancreatitis: Role for outpatient apheresis maintenance therapy. World J Gastroenterol, 2017; 23: 7332-7336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155).Niro J, Sapin V, Constantin J-M, Cotte B, Lebel A, Roszyk L, R Eglizot null, Tauveron I, Jacquetin B, Lémery D, Gallot D. [Management of gestational hypertriglyceridemia by plasmapheresis]. Gynecol Obstet Fertil, 2007; 35: 1133-1135 [DOI] [PubMed] [Google Scholar]
- 156).Sivakumaran P, Tabak SW, Gregory K, Pepkowitz SH, Klapper EB. Management of familial hypertriglyceridemia during pregnancy with plasma exchange. J Clin Apher, 2009; 24: 42-46 [DOI] [PubMed] [Google Scholar]
- 157).Mizushima T, Ochi K, Matsumura N, Ichimura M, Ishibashi T, Tsuboi K, Harada H. Prevention of hyperlipidemic acute pancreatitis during pregnancy with medium-chain triglyceride nutritional support. Int J Pancreatol, 1998; 23: 187-192 [DOI] [PubMed] [Google Scholar]
- 158).Athyros VG, Giouleme OI, Nikolaidis NL, Vasiliadis TV, Bouloukos VI, Kontopoulos AG, Eugenidis NP. Long-term follow-up of patients with acute hypertriglyceridemia-induced pancreatitis. J Clin Gastroenterol, 2002; 34: 472-475 [DOI] [PubMed] [Google Scholar]
- 159).Tsai EC, Brown JA, Veldee MY, Anderson GJ, Chait A, Brunzell JD. Potential of essential fatty acid deficiency with extremely low fat diet in lipoprotein lipase deficiency during pregnancy: A case report. BMC Pregnancy Childbirth, 2004; 4: 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160).Greenberg JA, Bell SJ, Ausdal WV. Omega-3 Fatty Acid supplementation during pregnancy. Rev Obstet Gynecol, 2008; 1: 162-169 [PMC free article] [PubMed] [Google Scholar]
- 161).Nelson-Piercy C, Crook MA. Severe hypertriglyceridemia complicating pregnancy, management by dietary intervention and omega-3 fatty acid supplementation. Nutrition, 2009; 25: 1098-1099 [DOI] [PubMed] [Google Scholar]
- 162).Takaishi K, Miyoshi J, Matsumura T, Honda R, Ohba T, Katabuchi H. Hypertriglyceridemic acute pancreatitis during pregnancy: prevention with diet therapy and omega-3 fatty acids in the following pregnancy. Nutrition, 2009; 25: 1094-1097 [DOI] [PubMed] [Google Scholar]
- 163).Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract, 1999; 5: 33-36 [DOI] [PubMed] [Google Scholar]
- 164).Abu Musa AA, Usta IM, Rechdan JB, Nassar AH. Recurrent hypertriglyceridemia-induced pancreatitis in pregnancy: a management dilemma. Pancreas, 2006; 32: 227-228 [DOI] [PubMed] [Google Scholar]
- 165).Hill AJ, Pacheco LD, Saade G, Hankins GDV. Familial hypertriglyceridemia in pregnancy. Int J Gynaecol Obstet, 2014; 125: 80-81 [DOI] [PubMed] [Google Scholar]
- 166).Lim R, Rodger SJ, Hawkins TL-A. Presentation and management of acute hypertriglyceridemic pancreatitis in pregnancy: A case report. Obstet Med, 2015; 8: 200-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167).Hegele RA, Tsimikas S. Lipid-Lowering Agents. Circ Res, 2019; 124: 386-404 [DOI] [PubMed] [Google Scholar]
- 168).Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, Averna MR, Sirtori CR, Shah PK, Gaudet D, Stefanutti C, Vigna GB, Du Plessis AME, Propert KJ, Sasiela WJ, Bloedon LT, Rader DJ, Phase 3 HoFH Lomitapide Study investigators. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet, 2013; 381: 40-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169).Sacks FM, Stanesa M, Hegele RA. Severe hypertriglyceridemia with pancreatitis: thirteen years’ treatment with lomitapide. JAMA Intern Med, 2014; 174: 443-447 [DOI] [PubMed] [Google Scholar]
- 170).Blom DJ, Averna MR, Meagher EA, du Toit Theron H, Sirtori CR, Hegele RA, Shah PK, Gaudet D, Stefanutti C, Vigna GB, Larrey D, Bloedon LT, Foulds P, Rader DJ, Cuchel M. Long-Term Efficacy and Safety of the Microsomal Triglyceride Transfer Protein Inhibitor Lomitapide in Patients With Homozygous Familial Hypercholesterolemia. Circulation, 2017; 136: 332-335 [DOI] [PubMed] [Google Scholar]
- 171).Raal FJ, Santos RD, Blom DJ, Marais AD, Charng M-J, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, Tribble DL, Flaim JD, Crooke ST. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet, 2010; 375: 998-1006 [DOI] [PubMed] [Google Scholar]
- 172).Panta R, Dahal K, Kunwar S. Efficacy and safety of mipomersen in treatment of dyslipidemia: a meta-analysis of randomized controlled trials. J Clin Lipidol, 2015; 9: 217-225 [DOI] [PubMed] [Google Scholar]
- 173).Gryn SE, Hegele RA. Novel therapeutics in hypertriglyceridemia. Curr Opin Lipidol, 2015; 26: 484-491 [DOI] [PubMed] [Google Scholar]
- 174).Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, Yang Q, Hughes SG, Geary RS, Arca M, Stroes ESG, Bergeron J, Soran H, Civeira F, Hemphill L, Tsimikas S, Blom DJ, O’Dea L, Bruckert E. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N Engl J Med, 2019; 381: 531-542 [DOI] [PubMed] [Google Scholar]
- 175).Denison H, Nilsson C, Löfgren L, Himmelmann A, Mårtensson G, Knutsson M, Al-Shurbaji A, Tornqvist H, Eriksson JW. Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes Obes Metab, 2014; 16: 334-343 [DOI] [PubMed] [Google Scholar]
- 176).Meyers CD, Tremblay K, Amer A, Chen J, Jiang L, Gaudet D. Effect of the DGAT1 inhibitor pradigastat on triglyceride and apoB48 levels in patients with familial chylomicronemia syndrome. Lipids Health Dis, 2015; 14: 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177).Meyers CD, Amer A, Majumdar T, Chen J. Pharmacokinetics, pharmacodynamics, safety, and tolerability of pradigastat, a novel diacylglycerol acyltransferase 1 inhibitor in overweight or obese, but otherwise healthy human subjects. J Clin Pharmacol, 2015; 55: 1031-1041 [DOI] [PubMed] [Google Scholar]
- 178).Graham MJ, Lee RG, Brandt TA, Tai L-J, Fu W, Peralta R, Yu R, Hurh E, Paz E, McEvoy BW, Baker BF, Pham NC, Digenio A, Hughes SG, Geary RS, Witztum JL, Crooke RM, Tsimikas S. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N Engl J Med, 2017; 377: 222-232 [DOI] [PubMed] [Google Scholar]
- 179).Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, McCarthy S, Van Hout CV, Bruse S, Dansky HM, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Habegger L, Lopez A, Penn J, Zhao A, Shao W, Stahl N, Murphy AJ, Hamon S, Bouzelmat A, Zhang R, Shumel B, Pordy R, Gipe D, Herman GA, Sheu WHH, Lee I-T, Liang K-W, Guo X, Rotter JI, Chen Y-DI, Kraus WE, Shah SH, Damrauer S, Small A, Rader DJ, Wulff AB, Nordestgaard BG, Tybjærg-Hansen A, van den Hoek AM, Princen HMG, Ledbetter DH, Carey DJ, Overton JD, Reid JG, Sasiela WJ, Banerjee P, Shuldiner AR, Borecki IB, Teslovich TM, Yancopoulos GD, Mellis SJ, Gromada J, Baras A. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N Engl J Med, 2017; 377: 211-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180).Gaudet D, Gipe DA, Pordy R, Ahmad Z, Cuchel M, Shah PK, Chyu K-Y, Sasiela WJ, Chan K-C, Brisson D, Khoury E, Banerjee P, Gusarova V, Gromada J, Stahl N, Yancopoulos GD, Hovingh GK. ANGPTL3 Inhibition in Homozygous Familial Hypercholesterolemia. N Engl J Med, 2017; 377: 296-297 [DOI] [PubMed] [Google Scholar]
- 181).Ahmad Z, Banerjee P, Hamon S, Chan K-C, Bouzelmat A, Sasiela WJ, Pordy R, Mellis S, Dansky H, Gipe DA, Dunbar RL. Inhibition of Angiopoietin-Like Protein 3 With a Monoclonal Antibody Reduces Triglycerides in Hypertriglyceridemia. Circulation, 2019; 140: 470-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182).Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P, Ali S, Banerjee P, Chan K-C, Gipe DA, Khilla N, Pordy R, Weinreich DM, Yancopoulos GD, Zhang Y, Gaudet D, ELIPSE HoFH Investigators. Evinacumab for Homozygous Familial Hypercholesterolemia. N Engl J Med, 2020; 383: 711-720 [DOI] [PubMed] [Google Scholar]
- 183).Haas JT, Winter HS, Lim E, Kirby A, Blumenstiel B, DeFelice M, Gabriel S, Jalas C, Branski D, Grueter CA, Toporovski MS, Walther TC, Daly MJ, Farese RV. DGAT1 mutation is linked to a congenital diarrheal disorder. J Clin Invest, 2012; 122: 4680-4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184).Nakou ES, Filippatos TD, Agouridis AP, Kostara C, Bairaktari ET, Elisaf MS. The effects of ezetimibe and/or orlistat on triglyceride-rich lipoprotein metabolism in obese hypercholesterolemic patients. Lipids, 2010; 45: 445-450 [DOI] [PubMed] [Google Scholar]
- 185).Blackett P, Tryggestad J, Krishnan S, Li S, Xu W, Alaupovic P, Quiroga C, Copeland K. Lipoprotein abnormalities in compound heterozygous lipoprotein lipase deficiency after treatment with a low-fat diet and orlistat. J Clin Lipidol, 2013; 7: 132-139 [DOI] [PubMed] [Google Scholar]
- 186).Gaudet D, Méthot J, Déry S, Brisson D, Essiembre C, Tremblay G, Tremblay K, de Wal J, Twisk J, van den Bulk N, Sier-Ferreira V, van Deventer S. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther, 2013; 20: 361-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187).Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med, 1998; 339: 653-658 [DOI] [PubMed] [Google Scholar]
- 188).Schneider A, Barmada MM, Slivka A, Martin JA, Whitcomb DC. Clinical characterization of patients with idiopathic chronic pancreatitis and SPINK1 Mutations. Scand J Gastroenterol, 2004; 39: 903-904 [DOI] [PubMed] [Google Scholar]
- 189).Chen J-M, Férec C. Chronic pancreatitis: genetics and pathogenesis. Annu Rev Genomics Hum Genet, 2009; 10: 63-87 [DOI] [PubMed] [Google Scholar]
- 190).Whitcomb DC. Genetic aspects of pancreatitis. Annu Rev Med, 2010; 61: 413-424 [DOI] [PubMed] [Google Scholar]
- 191).Tremblay K, Dubois-Bouchard C, Brisson D, Gaudet D. Association of CTRC and SPINK1 gene variants with recurrent hospitalizations for pancreatitis or acute abdominal pain in lipoprotein lipase deficiency. Front Genet, 2014; 5: 90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192).Benlian P, De Gennes JL, Foubert L, Zhang H, Gagné SE, Hayden M. Premature atherosclerosis in patients with familial chylomicronemia caused by mutations in the lipoprotein lipase gene. N Engl J Med, 1996; 335: 848-854 [DOI] [PubMed] [Google Scholar]
- 193).Ebara T, Okubo M, Horinishi A, Adachi M, Murase T, Hirano T. No evidence of accelerated atherosclerosis in a 66-yr-old chylomicronemia patient homozygous for the nonsense mutation (Tyr61-->stop) in the lipoprotein lipase gene. Atherosclerosis, 2001; 159: 375-379 [DOI] [PubMed] [Google Scholar]
- 194).Maki KC, Guyton JR, Orringer CE, Hamilton-Craig I, Alexander DD, Davidson MH. Triglyceride-lowering therapies reduce cardiovascular disease event risk in subjects with hypertriglyceridemia. J Clin Lipidol, 2016; 10: 905-914 [DOI] [PubMed] [Google Scholar]
- 195).Shah NP, Cho L, Ahmed HM. Familial Chylomicronemia Syndrome: Clinical Characteristics and Long-Term Cardiovascular Outcomes. J Am Coll Cardiol, 2018; 72: 1177-1179 [DOI] [PubMed] [Google Scholar]
- 196).Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, Tardif J-C, Ballantyne CM, REDUCE-IT Investigators. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med, 2019; 380: 11-22 [DOI] [PubMed] [Google Scholar]
- 197).Musunuru K, Kathiresan S. Genetics of Common, Complex Coronary Artery Disease. Cell, 2019; 177: 132-145 [DOI] [PubMed] [Google Scholar]
- 198).Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration, Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, Di Angelantonio E, Boekholdt SM, Ouwehand W, Watkins H, Samani NJ, Saleheen D, Lawlor D, Reilly MP, Hingorani AD, Talmud PJ, Danesh J. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet, 2010; 375: 1634-1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199).Do R, Stitziel NO, Won H-H, Jørgensen AB, Duga S, Angelica Merlini P, Kiezun A, Farrall M, Goel A, Zuk O, Guella I, Asselta R, Lange LA, Peloso GM, Auer PL, NHLBI Exome Sequencing Project, Girelli D, Martinelli N, Farlow DN, DePristo MA, Roberts R, Stewart AFR, Saleheen D, Danesh J, Epstein SE, Sivapalaratnam S, Hovingh GK, Kastelein JJ, Samani NJ, Schunkert H, Erdmann J, Shah SH, Kraus WE, Davies R, Nikpay M, Johansen CT, Wang J, Hegele RA, Hechter E, Marz W, Kleber ME, Huang J, Johnson AD, Li M, Burke GL, Gross M, Liu Y, Assimes TL, Heiss G, Lange EM, Folsom AR, Taylor HA, Olivieri O, Hamsten A, Clarke R, Reilly DF, Yin W, Rivas MA, Donnelly P, Rossouw JE, Psaty BM, Herrington DM, Wilson JG, Rich SS, Bamshad MJ, Tracy RP, Cupples LA, Rader DJ, Reilly MP, Spertus JA, Cresci S, Hartiala J, Tang WHW, Hazen SL, Allayee H, Reiner AP, Carlson CS, Kooperberg C, Jackson RD, Boerwinkle E, Lander ES, Schwartz SM, Siscovick DS, McPherson R, Tybjaerg-Hansen A, Abecasis GR, Watkins H, Nickerson DA, Ardissino D, Sunyaev SR, O’Donnell CJ, Altshuler D, Gabriel S, Kathiresan S. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature, 2015; 518: 102-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200).Tremblay K, Méthot J, Brisson D, Gaudet D. Etiology and risk of lactescent plasma and severe hypertriglyceridemia. J Clin Lipidol, 2011; 5: 37-44 [DOI] [PubMed] [Google Scholar]
- 201).Fu J, Kwok S, Sinai L, Abdel-Razek O, Babula J, Chen D, Farago E, Fernandopulle N, Leith S, Loyzer M, Lu C, Malkani N, Morris N, Schmidt M, Stringer R, Whitehead H, Ban MR, Dubé JB, McIntyre A, Johansen CT, Cao H, Wang J, Hegele RA. Western Database of Lipid Variants (WDLV): a catalogue of genetic variants in monogenic dyslipidemias. Can J Cardiol, 2013; 29: 934-939 [DOI] [PubMed] [Google Scholar]
- 202).Berberich AJ, Hegele RA. The role of genetic testing in dyslipidaemia. Pathology, 2019; 51: 184-192 [DOI] [PubMed] [Google Scholar]
- 203).Ng DM, Burnett JR, Bell DA, Hegele RA, Hooper AJ. Update on the diagnosis, treatment and management of rare genetic lipid disorders. Pathology, 2019; 51: 193-201 [DOI] [PubMed] [Google Scholar]
- 204).Garg A, Garg V, Hegele RA, Lewis GF. Practical definitions of severe versus familial hypercholesterolaemia and hypertriglyceridaemia for adult clinical practice. Lancet Diabetes Endocrinol, 2019; 7: 880-886 [DOI] [PubMed] [Google Scholar]
- 205).Steinhagen-Thiessen E, Stroes E, Soran H, Johnson C, Moulin P, Iotti G, Zibellini M, Ossenkoppele B, Dippel M, Averna MR, GENIALL Investigators. The role of registries in rare genetic lipid disorders: Review and introduction of the first global registry in lipoprotein lipase deficiency. Atherosclerosis, 2017; 262: 146-153 [DOI] [PubMed] [Google Scholar]
- 206).Ng DM, Hooper AJ, Bellgard MI, Burnett JR. The role of patient registries for rare genetic lipid disorders. Curr Opin Lipidol, 2018; 29: 156-162 [DOI] [PubMed] [Google Scholar]
- 207).Huang C-Q, Dong B-R, Wu H-M, Zhang Y-L, Wu J-H, Lu Z-C, Flaherty JH. Association of cognitive impairment with serum lipid/lipoprotein among Chinese nonagenarians and centenarians. Dement Geriatr Cogn Disord, 2009; 27: 111-116 [DOI] [PubMed] [Google Scholar]
- 208).Morley JE, Banks WA. Lipids and cognition. J Alzheimers Dis, 2010; 20: 737-747 [DOI] [PubMed] [Google Scholar]
- 209).Hauenschild A, Ewald N, Schnell-Kretschmer H, Porsch-Oezcueruemez M, Kloer H-U, Hardt PD. Successful long-term treatment of severe hypertriglyceridemia by feedback control with lipid self-monitoring. Ann Nutr Metab, 2008; 52: 215-220 [DOI] [PubMed] [Google Scholar]
- 210).Lookene A, Zhang L, Hultin M, Olivecrona G. Rapid subunit exchange in dimeric lipoprotein lipase and properties of the inactive monomer. J Biol Chem, 2004; 279: 49964-49972 [DOI] [PubMed] [Google Scholar]
- 211).Beigneux AP, Allan CM, Sandoval NP, Cho GW, Heizer PJ, Jung RS, Stanhope KL, Havel PJ, Birrane G, Meiyappan M, Gill JE, Murakami M, Miyashita K, Nakajima K, Ploug M, Fong LG, Young SG. Lipoprotein lipase is active as a monomer. Proc Natl Acad Sci USA, 2019; 116: 6319-6328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212).Gunn KH, Roberts BS, Wang F, Strauss JD, Borgnia MJ, Egelman EH, Neher SB. The structure of helical lipoprotein lipase reveals an unexpected twist in lipase storage. Proc Natl Acad Sci USA, 2020; 117: 10254-10264 [DOI] [PMC free article] [PubMed] [Google Scholar]