Abstract
Aim: We established a method to evaluate the lipid concentrations, size and particle numbers (PNs) of lipoprotein subclasses by gel permeation chromatography (GP-HPLC). Nuclear magnetic resonance (NMR) is widely used to analyze these parameters of lipoprotein subclasses, but differences of the two methods are unknown. Current study compared the PNs of each lipoprotein subclass measured by GP-HPLC and NMR, and assessed the effect of a selective PPARα modulator, pemafibrate.
Methods: Lipoprotein profiles of 212 patients with dyslipidemia who participated in the phase 2 clinical trial of a selective PPARα modulator, pemafibrate, were analyzed by two methods, GP-HPLC and NMR, which were performed with LipoSEARCH (Skylight Biotech) and LipoProfile 3 (LabCorp), respectively. GP-HPLC evaluated the PNs of 18 subclasses, consisting of CM, VLDL1-5, LDL1-6, and HDL1-6. NMR evaluated the PNs of 9 subclasses, consisting of large VLDL & CM, medium VLDL, small VLDL, IDL, large LDL, small LDL, large HDL, medium HDL and small HDL.
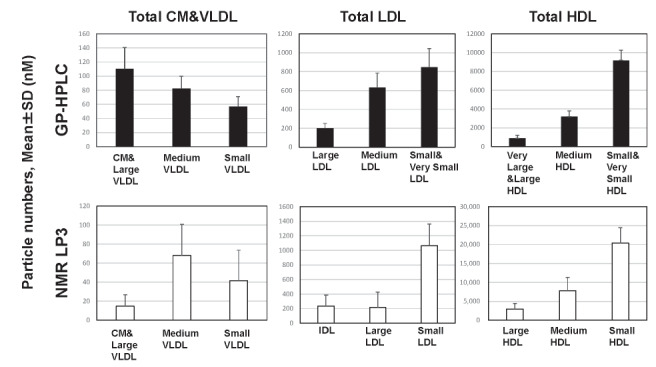
Results: Three major classes, total CM&VLDL, total LDL and total HDL were obtained by grouping of corresponding subclasses in both methods and PNs of these classes analyzed by GP-HPLC were correlated positively with those by NMR. The correlation coefficients in total CM&VLDL, total LDL and total HDL between GP-HPLC and NMR was 0.658, 0.863 and 0.798 (all p <0.0001), respectively. The PNs of total CM&VLDL, total LDL and total HDL analyzed by GP-HPLC was 249.5±51.7nM, 1,679±359 nM and 13,273±1,564 nM, respectively, while those by NMR was 124.6±41.8 nM, 1,514±386 nM and 31,161±4,839 nM, respectively. A marked difference in the PNs between the two methods was demonstrated especially in total HDL.
The number of apolipoprotein (Apo) B molecule per one ApoB-containing lipoprotein particle, total CM&VLDL plus total LDL, was 1.10±0.05 by GP-HPLC, while 1.32±0.18 by NMR. The number of ApoA-I per one HDL particle was 3.40±0.17 by GP-HPLC, but only 1.46±0.15 by NMR, much less than reported previously.
From the phase 2 clinical trial, randomizing 212 patients to pemafibrate 0.025-0.2 mg BID, fenofibrate 100 mg QD, or placebo groups, pemafibrate reduced the PNs of CM, large VLDL1-VLDL3 and medium VLDL4, but not small VLDL5 by GP-HPLC. It significantly decreased the PNs of smaller LDL and larger HDL particles, but increased those of larger LDL and smaller HDL particles. In contrast, NMR showed marked variations in the effect of pemafibrate on lipoprotein PNs, and no significant size-dependent changes.
Conclusions: GP-HPLC evaluates the lipoprotein PNs more accurately than NMR and can be used for assessing the effects of lipid-lowering drugs on lipoprotein subclasses.
Keywords: Lipoprotein analysis, GP-HPLC, NMR, Dyslipidemia, Selective PPARα modulator
Introduction
Epidemiological studies have demonstrated that serum low-density lipoprotein (LDL)-cholesterol (LDL-C) levels are positively, while high-density lipoprotein (HDL)-cholesterol (HDL-C) levels are negatively correlated with atherosclerotic cardiovascular disease (ASCVD) 1) . LDL-C lowering drugs, such as statins, were shown to be effective for primary and secondary prevention of ASCVD 2 - 3) . By contrast, HDL-targeted drugs such as niacin 4) and cholesteryl ester transfer protein (CETP) inhibitors 5 - 8) have failed to reduce cardiovascular (CV) events, although they increased HDL-C. Developments of certain HDL-targed drugs were terminated because of drug accumulation in adipose tissues 9 - 10) . Furthermore, mendelian randomization analyses showed that genetic mechanisms that raise HDL-C levels do not lower risk of myocardial infarction 11) , suggesting that serum HDL-C levels are not causally associated with ASCVD. The use of HDL-C levels might be limited to assessing the CV risks and responses to therapies. The LDL-C or HDL-C represents only total cholesterol contents in LDL or HDL, respectively. Although they are largely proportional to the actual particle numbers (PNs) of LDL or HDL, more accurate methods to measure PNs are warranted.
There are several methods to assess lipoproteins. The sequential or density gradient ultracentrifugation of lipoproteins has been a standard for quantitative analysis of lipoproteins for many years 12 - 14) . Agarose or polyacrylamide gel (PAG) disc electrophoresis has also been used to qualitatively analyze the abnormalities of lipoproteins in dyslipidemic patients. Most widely used method in recent years is nuclear magnetic resonance spectroscopy (NMR) 15 - 24) . In NMR method for lipoproteins, lipid methyl groups emit resonances that are unique to the chemical environments of lipoprotein particles, and NMR makes it possible not only to analyze the lipid concentrations and size of lipoprotein subclasses, but also to quantify the PNs of lipoprotein subclasses with different size 25) .
Similar information of the characteristics of lipoproteins can be obtained by gel permeation-high performance liquid chromatography (GP-HPLC), which was developed by Okazaki and Hara 26 - 27) . Another HPLC method using anion-exchange column (AEX-HPLC) is currently available for clinical use in Japan 28 - 31) . The GP-HPLC method has been used widely to comprehensively analyze the lipoprotein abnormalities in patients with a variety of genetic disorders 32 - 35) as well as those in animals 36 - 39) . This system can directly determine total cholesterol (Cho), free cholesterol (FC), triglycerides (TG), and phospholipids (PL) concentrations in each lipoprotein subclass, respectively, according to lipoprotein particle size. Thus, the lipid compositions and size of lipoprotein subclasses can be evaluated. Furthermore, we have recently developed an analytical method to evaluate the PNs of lipoprotein subclasses by GP-HPLC, using a mathematical simulation model called as “spherical particle model” 40) . Thus far, 13 studies using this new method have been published 41 - 53) . However, only two of them directly compared the results of GP-HPLC with those of NMR method 42 - 43) . In particular, it remains unknown how different the lipid concentrations, size and PNs of each lipoprotein calculated by this GP-HPLC system are from those by NMR method.
Furthermore, this GP-HPLC analysis was applied to elucidate the changes of size, lipid composition and PNs of lipoproteins after the administration of pemafibrate (K-877), a selective PPARα modulator (SPPARMα) with extremely high PPARα agonist activity as well as selectivity. We already reported the efficacy and safety of pemafibrate compared with placebo and fenofibrate in dyslipidemic patients with high TG and low HDL-C levels. Pemafibrate markedly improved TG and HDL-C levels 54 - 65) . In the first phase 2 clinical trial 54) , we further assessed the efficacy of pemafibrate on the PNs of lipoprotein subclasses by GP-HPLC in comparison with NMR LipoProfile3 (LP3) (LabCorp, USA). NMR-LP3 was the system of NMR of LabCorp, which modified the previous analysis system of LipoProfile 2 by LipoScience (USA). The NMR sample data were originally provided by LP2 and were subsequently recalculated by LP3 algorithm, which is the major system of NMR lipoprotein analysis worldwide. From the methodological principles, NMR and GP-HPLC were previously considered preferable for the determination of PNs/size and lipid levels, respectively. In the current study, we aimed to compare the lipid concentrations, size and PNs of each lipoprotein subclass calculated by GP-HPLC and NMR to assess their validity.
Materials and Methods
Study Participants
We enrolled 212 dyslipidemic patients with high TG and low HDL-C levels, who were undertaken the randomized, double blind, active- and placebo-controlled phase 2 trial of pemafibrate, where the study protocol and patient data are summarized in the previous report 54) . In brief, these patients included men and postmenopausal women aged 20 to 74 years who had a history of documented dyslipidemia and plasma TG of 200 mg/dL or higher as well as HDL-C less than 50 mg/dL in men or 55 mg/dL in women. In this double blind, placebo-controlled, parallel-group 12-week phase 2 clinical trial, which randomized 224 patients to pemafibrate 0.025, 0.05, 0.1, 0.2 mg BID, fenofibrate 100 mg QD, or placebo (1:1:1:1:1:1) groups, 212 dyslipidemic patients with data of GP-HPLC and NMR were selected. LP3 was selected, because that LP3 has been widely used as a second-generation NMR analysis process.
The study protocol and amendment were approved by the independent ethic committee or institutional review board before the commencement of study. The study was conducted in accordance with the principle of the Declaration of Helsinki, and under the guidelines of Good Clinical Practice and the International Conference on Harmonization. All study participants provided written informed consent prior to involvement. This trial was registered with JAPIC Clinical Trials Information, number Japic CTI-101331 54) . Venous blood was drawn after overnight fasting for 10 h at the baseline of this trial. Serum was separated by a low-speed centrifugation (3,000 rpm, 15 min) and aliquots were frozen at −80℃ until use. The study took place between November 22, 2010 and July 7, 2011 as described 54) and sample storage time at −80℃ was less than 7 months untill split samples were measured by GP-HPLC and NMR-LP2. Furthermore, the study protocol of comparison of GP-HPLC and NMR methods in the Japic CTI-101331 Study was approved by the Ethical Committee of Osaka University Hospital.
Evaluation of Plasma Lipids, Lipoproteins and Apolipoproteins
Lipoprotein levels were measured by direct enzymatic methods. Apolipoprotein levels were measured by immunoturbidity methods. Other laboratory parameters were analyzed by standardized laboratory methods. All measurements were performed by LSI Medience Corporation (Tokyo, Japan) or its affiliates as described previously 41 , 54) . Serum concentrations of LDL-C and HDL-C were measured by homogeneous assays, using Determiner L HDL-C and LDL-C kits from Kyowa Medex Co., Ltd. (currently Hitachi Chemical Diagnostics Systems Co.,Ltd.), Japan.
Analysis of Plasma Lipoproteins by GP-HPLC
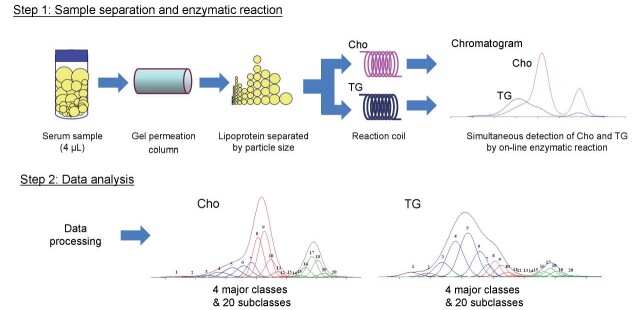
The assessment for the size and lipid concentrations of serum lipoproteins by GP-HPLC was performed at Skylight Biotech Inc. (Akita, Japan) as described previously 66 - 67) . Furthermore, the PNs of each lipoprotein subclass were evaluated by GP-HPLC as described previously 40 - 41 , 68) . Serum samples were obtained and aliquots were frozen at −80℃ for both GP-HPLC and NMR analysis. For GP-HPLC analysis, the fresh-frozen serum samples were sent to Akita, Japan, for analysis. In brief, lipoproteins in serum aliquots (4 µl) after thawing were separated with tandemly connected Skylight PakLP1-AA gel permeation columns (Skylight Biotech Inc., Akita, Japan, 300 mm×4.6 mm I.D.). The lipoproteins were separated into 20 subclasses based upon Gaussian approximation, as shown in Step 2 of Supplementary Fig.1 , and the lipid concentration, size and PNs of lipoproteins were analyzed. The particle size of each lipoprotein was determined by the retention time of each peak observed on a chromatogram using a linear calibration curve 69) . The cholesterol and TG levels of 20 subclasses were defined by component peak analyses on the basis of lipoprotein particle size with the Gaussian curve fitting technique. The following definitions of lipoprotein particle sizes are shown in Table 1 .
Supplementary Fig.1. Methods of GP-HPLC (LipoSEARCH).
Individual curves of 20 subclasses by Gaussian curve fitting method 40, 67-68) are shown in color as follows : CM1 and CM2 (peak number 1 and 2), brown; VLDL1−VLDL5 (peak number 3-7) , blue; LDL1−LDL6 (peak number 8-13), red; HDL1−HDL7 (peak number 14-20), green.
Table 1.
| GP-HPLC | NMR LipoProfile 3 (LP3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Major class | Particle number (nM) | Major subclass | Subclass | Particle diameter (nm) | Particle number (nM) | Major class | Particle number (nM) | Subclass | Particle diameter (nm) | Particle number (nM) |
| Total CM & VLDL | 249.5±51.7 | Chylomicron | CM | 82.4 | 4.2±3.3 | Total CM & VLDL | 124.6±41.8 | Large VLDL & Chylomicron | > 60.0 | 14.8±11.9 |
| Large VLDL | VLDL1 | 64.0 | 9.8±4.4 | |||||||
| VLDL2 | 53.6 | 29.4±10.1 | ||||||||
| VLDL3 | 44.5 | 66.9±15.4 | ||||||||
| Medium VLDL | VLDL4 | 36.8 | 82.2±17.7 | Medium VLDL | 42.0-60.0 | 68.1±32.8 | ||||
| Small VLDL | VLDL5 | 31.3 | 57.0±14.1 | Small VLDL | 29.0-42.0 | 41.6±32.0 | ||||
| Total LDL | 1,679±359 | Large LDL | LDL1 | 28.6 | 201.0±51.0 | Total LDL | 1,514±386 | IDL | 23.0-29.0 | 235.0±150.0 |
| Medium LDL | LDL2 | 25.5 | 631.0±154.0 | Large LDL | 20.5-23.0 | 215.0±211.0 | ||||
| Small LDL | LDL3 | 23.0 | 516.4±124.0 | Small LDL | 18.0-20.5 | 1,064±297 | ||||
| Very Small LDL | LDL4 | 20.7 | 200.3±52.4 | |||||||
| LDL5 | 18.6 | 60.1±20.6 | ||||||||
| LDL6 | 16.7 | 70.2±15.8 | ||||||||
| Total HDL | 13,273±1,564 | Very Large HDL | HDL1 | 15.0 | 59.7±16.4 | Total HDL | 31,161±4,839 | Large HDL | 9.4-14.0 | 2,963±1,483 |
| HDL2 | 13.5 | 135.8±28.2 | ||||||||
| Large HDL | HDL3 | 12.1 | 706.0±286.5 | |||||||
| Medium HDL | HDL4 | 10.9 | 3,190±594 | Medium HDL | 8.2-9.4 | 7,796±3,550 | ||||
| Small HDL | HDL5 | 9.8 | 5,584±844 | Small HDL | 7.3-8.2 | 20,405±4,067 | ||||
| Very Small HDL | HDL6 | 8.8 | 3,597±586 | |||||||
Particle numbers (nM) are shown as mean±SD.
HDL7 was excluded from the analysis because of inaccuracy due to its discoidal shape.
Chylomicrons (CM) subclasses: CM1 and CM2, >90 nm and 75 nm in diameter, respectively.
VLDL subclasses: large VLDL (VLDL1, VLDL2 and VLDL3, 64.0, 53.6 and 44.5 nm in diameter, respectively), medium VLDL (VLDL4, 36.8 nm in diameter), and small VLDL (VLDL5, 31.3 nm in diameter), respectively.
LDL subclasses: large LDL (LDL1, 28.6 nm in diameter), medium LDL (LDL2, 25.5 nm in diameter), small LDL (LDL3, 23.0 nm in diameter) and very small LDL (LDL4, LDL5 and LDL6, 20.7, 18.6 and 16.7 nm in diameter), respectively.
HDL subclasses: very large HDL (HDL1 and HDL2, 15.0 and 13.5 nm in diameter, respectively), large HDL (HDL3, 12.1 nm in diameter), medium HDL (HDL4, 10.9 nm in diameter), small HDL (HDL5, 9.8 nm in diameter) and very small HDL (HDL6 and HDL7, 8.8 and 7.6 nm in diameter), respectively.
The PNs of the lipoprotein subclasses were calculated using a “spherical particle model” 40) . We focused on the sum volume of cholesteryl ester (CE) and TG within the core of lipoprotein. We demonstrated the ratio of FC to (CE+TG) in a certain subclass is almost constant, except HDL7 fraction, which includes discoidal pre-beta 1 HDL. The PNs of lipoproteins were calculated, using the formula as described previously 40) . Two subclasses of CMs were mixed into one, so the PNs of 19 subclasses were calculated. There is a possibility that the HDL7 subclass is the smallest discoidal HDL and it may not fit the spherical core model. So, HDL7 was excluded from this analysis. Supplementary Fig.1 shows the schematic principle of GP-HPLC method from two chromatograms directly detecting cholesterol and TG concentrations as well as the PNs calculation of lipoprotein subclasses for representative individual sample.
Analysis of Plasma Lipoproteins by NMR
The fresh-frozen samples on dry ice were sent to the United States for NMR analysis. NMR spectra of thawed serum were originally obtained at the LabCorp (LP2) and the digitized data were subsequently analyzed for lipoprotein particle concentration and size reanalyzed by LP3 system of LipoScience using LP-3 spectral deconvolution algorithm 70 - 71) . The following subclass categories were investigated: large VLDL and CM (>60 nm), medium VLDL (42–60 nm), small VLDL (29–42 nm), IDL (23–29 nm), large LDL (20.5–23 nm), small LDL (18–20.5 nm), large HDL (9.4–14 nm), medium HDL (8.2–9.4 nm), and small HDL (7.3–8.2 nm), respectively as shown in Table 1 .
Comparison of the Effects of a Selective PPARα modulator (SPPARMα) on Plasma Lipoproteins between GP-HPLC and NMR
To further investigate the differences between GP-HPLC and NMR, we utilized these methods for detailed analysis of the effect of lipid-lowering drugs on the lipoprotein subclasses. We compared the effect of a SPPARMα, pemafibrate (K-877), developed by Kowa Company, Ltd. (Tokyo, Japan), on the PNs of lipoproteins between GP-HPLC and NMR analysis. For comparison of GP-HPLC and NMR analyses of lipoproteins in dyslipidemic patients, the data of 212 dyslipidemic patients before the administration of pemafibrate, who were assigned to pemafibrate 0.025, 0.05, 0.1, 0.2 mg BID, fenofibrate 100 mg QD, or placebo (1:1:1:1:1:1) groups, were combined and analyzed ( Supplementary Fig.2 ) .
Supplementary Fig.2.
Study protocol of pemafibrate (K-877)
Statistical Analysis
Data were expressed as the mean±SD. The differences of the mean values were analyzed by Student t -test. Correlations between various variables were presented as the Spearman correlation coefficient ( r -value) with a P -value <0.05 considered to be statistically different. One sample t -test was used to assess the difference from the baseline to the endpoint (week 12).
Results
1. Comparison between GP-HPLC and NMR in Particle Number and Size of Lipoproteins and Their Subclasses
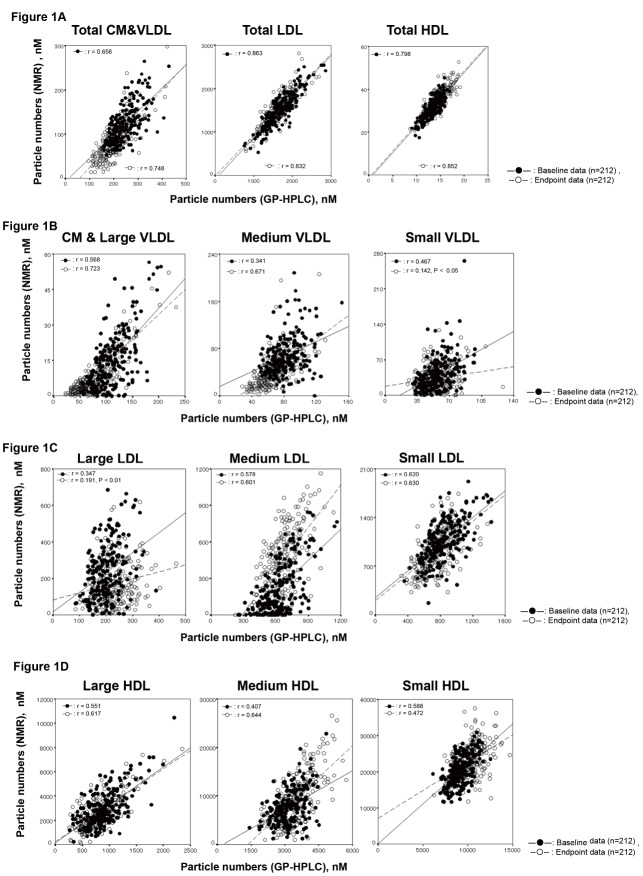
The PNs of lipoproteins of 212 samples were evaluated by GP-HPLC and NMR analysis, both of which separated lipoproteins according to their particle size. Correlations between GP-HPLC and NMR analysis of PNs of major classes and subclasses of lipoproteins are shown in Fig.1 . Fig.1-A compares the PNs of major lipoprotein classes. In GP-HPLC analysis, CM and subclasses of VLDL1-5, LDL1-6 and HDL1-6 were grouped and compared as total CM & VLDL, total LDL and total HDL, respectively. In NMR analysis, 1) total CM & VLDL (CMs and large VLDL, medium VLDL and small VLDL), 2) total LDL (IDL, large LDL and small LDL), and 3) total HDL (large HDL, medium HDL and small HDL) were grouped and compared as total CM & VLDL, total LDL and total HDL, respectively ( Table 1 ) . The correlation coefficients in total CM&VLDL, total LDL and total HDL at the baseline ( n =212) between GP-HPLC and NMR were 0.658, 0.863 and 0.798 (all P <0.0001), respectively. The correlation coefficients in total CM&VLDL, total LDL and total HDL at the endpoint ( n =212) between GP-HPLC and NMR were 0.748, 0.832 and 0.852 (all P <0.0001), respectively. These three major lipoprotein classes in dyslipidemic patients analyzed by GP-HPLC correlated positively with those by NMR in both baseline and endpoint data.
Fig.1. Comparison of PNs in major lipoprotein classes and their subclasses between GP-HPLC and NMR (closed circle: baseline data n =212, open circle: endpoint data n =212) .
The horizontal axis is the data by GP-HPLC and vertical axis shows those by NMR, respectively. The r values were calculated by Spearman correlation. All p values are p <0.0001 except for small VLDL and large LDL at the endpoint.
1-A: Correlation of PNs in major lipoprotein classes between GP-HPLC and NMR.
1-B: Correlation of PNs in total CM & VLDL subclasses between GP-HPLC and NMR. In GP-HPLC analysis, CM and VLDL1-VLDL3 subclasses were grouped and compared as CM & large VLDL.
1-C: Correlation of PNs in LDL subclasses between GP-HPLC and NMR. In GP-HPLC analysis, LDL1 was described as large LDL, LDL2 was described as medium LDL and LDL3–LDL6 were grouped and described as small LDL. IDL and large LDL by NMR were described as large LDL and medium LDL, respectively, in order to compare three LDL subclasses by NMR.
1-D: Correlation of PNs in HDL subclasses between GP-HPLC and NMR. In subclasses of GP-HPLC analysis, HDL1–HDL3 and HDL5–HDL6 were grouped, described and compared as large HDL and small HDL, respectively.
As shown in Table 1 , the PNs of these major classes, total CM&VLDL, total LDL and total HDL analyzed by GP-HPLC were 249.5±51.7 nM, 1,679±359 nM and 13,273±1,564 nM, respectively, while those by NMR were 124.6±41.8 nM, 1,514±386 nM and 31,161±4,839 nM, respectively. The PNs of total HDL measured by NMR were 2-fold higher than those by GP-HPLC. In contrast, the PNs of CM&VLDL measured by GP-HPLC were 2-fold higher than those by NMR.
The PNs of each lipoprotein subclass were further analyzed. Fig.1-B compares the PNs of CM & VLDL subclasses. In GP-HPLC analysis, CM and VLDL1–VLDL3 subclasses were grouped as CM & large VLDL and compared with NMR. The correlation coefficients of CM & Large VLDL, medium VLDL and small VLDL at the baseline ( n =212) between GP-HPLC and NMR were 0.568, 0.341 and 0.467 (all P <0.0001), respectively. The correlation coefficients of CM & Large VLDL, medium VLDL and small VLDL at the endpoint ( n =212) between GP-HPLC and NMR were 0.723 ( P <0.0001), 0.671 ( P <0.0001) and 0.142 ( P <0.05), respectively. These correlations between GP-HPLC and NMR were not so good, and marked variations especially small VLDL were demonstrated. The PNs of CM & large VLDL were calculated much lower in NMR compared with GP-HPLC.
Fig.1-C compares the PNs of each LDL subclass between GP-HPLC and NMR. In GP-HPLC analysis, LDL1 was described as large LDL, LDL2 as medium LDL, and LDL3 as small LDL, while LDL4–LDL6 were grouped and described as very small LDL. In NMR analysis, IDL and large LDL were described as large LDL and medium LDL, respectively. In order to compare three LDL subclasses of NMR with GP-HPLC, small LDL and very small LDL in GP-HPLC were combined and compared with small LDL in NMR. The correlation coefficients in large LDL, medium LDL and small LDL at the baseline between GP-HPLC and NMR were 0.347, 0.578 and 0.620 (all P <0.0001), respectively. The correlation coefficients in large LDL, medium LDL and small LDL at the endpoint between GP-HPLC and NMR were 0.191 ( P <0.01), 0.601 ( P <0.0001) and 0.630 ( P <0.0001), respectively. The absolute PNs of medium LDL by GP-HPLC analysis showed markedly higher value compared to those by NMR. The absolute PNs of large LDL were much less than those of medium LDL and small LDL in both GP-HPLC and NMR analysis.
Fig.1-D compares the PNs of each HDL subclass (large HDL, medium HDL and small HDL) between GP-HPLC and NMR. In subclasses of GP-HPLC analysis, HDL1–HDL3 and HDL5–HDL6 were grouped and described as large HDL and small HDL, respectively. HDL4 was named medium HDL in GP-HPLC analysis. The correlation coefficients in large HDL, medium HDL and small HDL at the baseline between GP-HPLC and NMR were 0.551, 0.407 and 0.588 (all P <0.0001), respectively. The correlations were generally good for large HDL and small HDL, but not so good for medium HDL. The correlation coefficients in large HDL, medium HDL and small HDL at the endpoint between GP-HPLC and NMR were 0.617, 0.644 and 0.472 (all P <0.0001), respectively. The correlations were generally good for large HDL and medium HDL, but not so good for small HDL. The absolute PNs of each HDL subclass, large, medium and small HDL, by NMR was about 2~3-fold of that by GP-HPLC, respectively.
Correlations among major classes and subclasses by GP-HPLC and NMR analysis are shown in Supplementary Table 1 . The average PNs of 18 subclasses consisting of CM, VLDL1–VLDL5, LDL1–LDL6, and HDL1–HDL6 by GP-HPLC and 9 subclasses consisting of large VLDL & CM, medium VLDL, small VLDL, IDL, large LDL, small LDL, large HDL, medium HDL and small HDL by NMR are shown in Table 1 .
Supplementary Table 1. Correlations of PNs among total CM & VLDL and their subclasses measured by GP-HPLC and NMR (LP-3).
| GP-HPLC | NMR LipoProfile 3 (LP-3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Total CM & VLDL | CM & Large VLDL | Medium VLDL | Small VLDL | |||||
| r | P | r | P | r | P | r | P | |
| Total CM & VLDL | 0.658 | <0.0001 | 0.313 | <0.0001 | 0.502 | <0.0001 | 0.226 | 0.0009 |
| CM & Large VLDL | 0.560 | <0.0001 | 0.568 | <0.0001 | 0.576 | <0.0001 | -0.096 | 0.1623 |
| Medium VLDL | 0.617 | <0.0001 | -0.046 | 0.5041 | 0.341 | <0.0001 | 0.468 | <0.0001 |
| Small VLDL | 0.430 | <0.0001 | -0.163 | 0.0177 | 0.170 | 0.0130 | 0.467 | <0.0001 |
| GP-HPLC | NMR LipoProfile 3 (LP-3) | |||||||
| Total LDL | IDL | Medium LDL | Small LDL | |||||
| r | P | r | P | r | P | r | P | |
| Total LDL | 0.863 | <0.0001 | 0.329 | <0.0001 | 0.516 | <0.0001 | 0.615 | <0.0001 |
| Large LDL | 0.687 | <0.0001 | 0.347 | <0.0001 | 0.593 | <0.0001 | 0.284 | <0.0001 |
| Medium LDL | 0.868 | <0.0001 | 0.314 | <0.0001 | 0.578 | <0.0001 | 0.520 | <0.0001 |
| Small LDL | 0.750 | <0.0001 | 0.257 | 0.0002 | 0.291 | <0.0001 | 0.620 | <0.0001 |
| GP-HPLC | NMR LipoProfile 3 (LP-3) | |||||||
| Total HDL | Large HDL | Medium HDL | Small HDL | |||||
| r | P | r | P | r | P | r | P | |
| Total HDL | 0.798 | <0.0001 | 0.390 | <0.0001 | 0.324 | <0.0001 | 0.532 | <0.0001 |
| Large HDL | 0.312 | <0.0001 | 0.551 | <0.0001 | 0.202 | 0.0032 | -0.044 | 0.5194 |
| Medium HDL | 0.747 | <0.0001 | 0.477 | <0.0001 | 0.407 | <0.0001 | 0.329 | <0.0001 |
| Small HDL | 0.657 | <0.0001 | 0.110 | 0.1091 | 0.166 | 0.0157 | 0.588 | <0.0001 |
Baseline data ( n = 212)
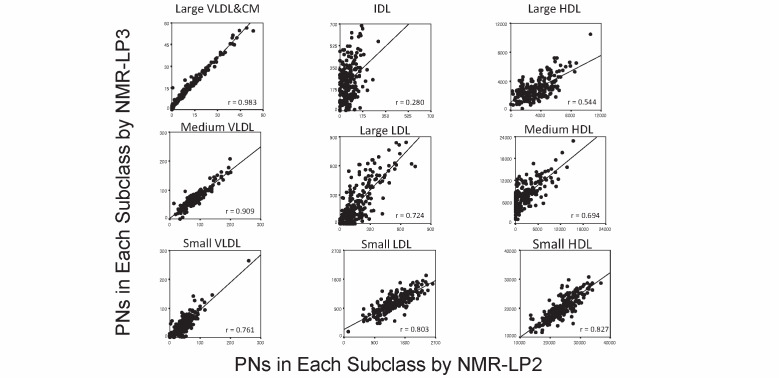
The NMR algorithm LP3 was used in the current study to compare the PNs with GP-HPLC, but we also compared the PNs in each subclass between NMR algorithm LP2 and LP3. As shown in Supplementary Fig.3 , the PNs of large VLDL & CM, medium VLDL, small VLDL, small LDL and small HDL by NMR algorithm LP2 were well correlated with those by NMR algorithm LP3. However, these correlations were not so good in IDL, large LDL, large HDL and medium HDL. Especially in IDL and medium HDL subclass, NMR algorithm LP2 showed many zero values. However, the number of these zero values was decreased in NMR algorithm LP3.
Supplementary Fig.3. Comparison of the PNs in each subclass between NMR-LP2 and LP3 (baseline data n =212) .
The horizontal axis is the data by NMR-LP2 and vertical axis shows those by NMR-LP3, respectively. The r values were calculated by Spearman correlation and those in the parenthesis by Spearman correlation. All p values were p <0.0001.
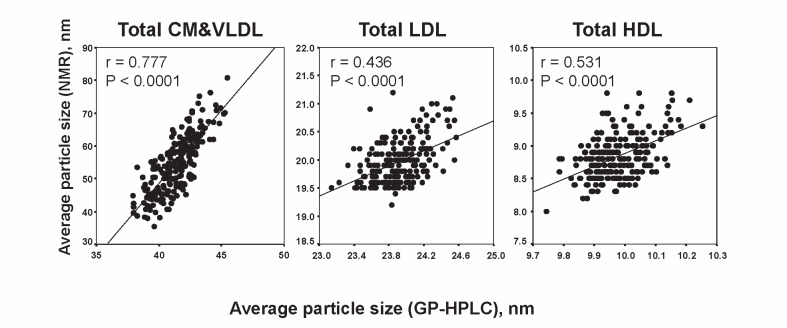
The correlations of average particle sizes of total CM & VLDL, total LDL and total HDL between GP-HPLC and NMR are shown in Fig.2 , where the horizontal axis exhibits the data by GP-HPLC and the vertical axis shows those by NMR, respectively. The correlation coefficients between GP-HPLC and NMR in each major class were 0.777, 0.436 and 0.531 (all P <0.0001), respectively. A good correlation between GP-HPLC and NMR was observed in total CM & VLDL, while a moderate correlations were observed in total LDL and total HDL. The average particle size of total CM & VLDL, total LDL and total HDL analyzed by GP-HPLC was 41.37±1.55 nm, 23.91±6.27 nm and 9.97±0.09 nm, respectively, while that by NMR was 54.60±8.84 nm, 19.97±0.39 nm and 8.83±0.34 nm, respectively.
Fig.2.
Correlations of average particle sizes of total CM&VLDL, total LDL and total HDL between GP-HPLC and NMR (baseline data n =212)
2. Comparison of the Distribution of Lipoprotein Subclasses between GP-HPLC and NMR
The distribution of each lipoprotein subclass was compared between GP-HPLC and NMR. As shown in Fig.3 , the distribution of total CM & VLDL subclasses was markedly different between GP-HPLC and NMR. CM & large VLDL were dominant in GP-HPLC analysis (44%), while medium VLDL was the major subclass of lipoproteins in NMR analysis (55%). Furthermore, the PNs of small LDL were markedly dominant (71%) within total LDL by NMR analysis, whereas it was 50% by GP-HPLC analysis. The PNs of medium LDL was 38% by GP-HPLC analysis, whereas it was very low (14%) by NMR analysis. The distribution of HDL subclasses was comparable between GP-HPLC and NMR analysis.
Fig.3.
Comparison of lipoprotein particle distribution by size between GP-HPLC and NMR (baseline data n =212)
3. Comparison of Lipid Concentrations in Lipoproteins between GP-HPLC and NMR
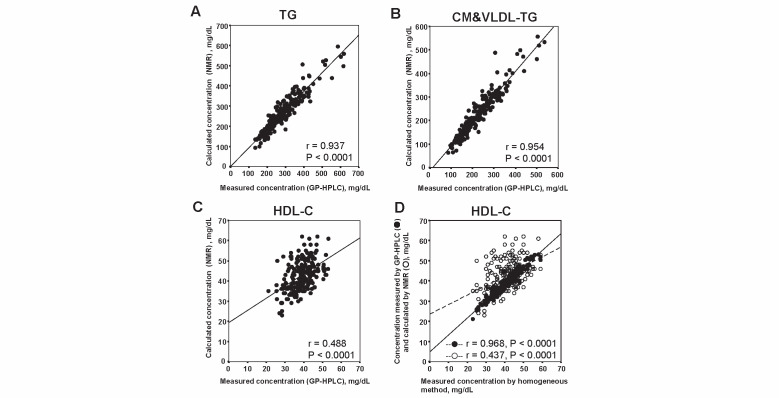
GP-HPLC can measure the cholesterol and TG concentrations in total serum, major classes of lipoproteins and their subclasses. In contrast, NMR can evaluate the concentrations of only total TG, CM&VLDL-TG and HDL-C. Therefore, we could only focus on the difference between GP-HPLC and NMR in the concentrations of only total TG, CM&VLDL-TG and HDL-C. As shown in Fig.4 , the correlation coefficients between GP-HPLC and NMR in total TG ( Fig.4-A ) , CM&VLDL-TG ( Fig.4-B ) and HDL-C ( Fig.4-C ) were 0.937, 0.954 and 0.488 (all P <0.0001), respectively. Very good correlations between GP-HPLC and NMR were observed in total TG and CM&VLDL-TG, whereas a less good correlation between GP-HPLC and NMR was demonstrated in HDL-C. The HDL-C concentration by GP-HPLC was highly correlated with that by homogeneous HDL-C assay (r=0.968, P <0.0001), however the HDL-C concentration determined by NMR was not so well correlated with that assay (r=0.437, P <0.0001) ( Fig.4-D ) .
Fig.4.
Correlations of lipid concentrations in lipoproteins between GP-HPLC and NMR (A: TG, B: CM&VLDL-TG, C: HDL-C) and correlations of HDL-C (D) measured by homogeneous method and HDL-C measured by GP-HPLC (closed circle) and calculated by NMR(open circle), respectively(baseline data n =212)
4. Correlations of Serum Apolipoprotein (Apo)B and ApoA-1 Levels to the Particle Numbers of ApoB-containing Lipoproteins and HDL
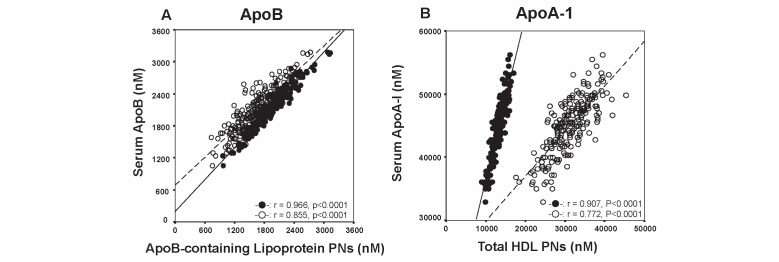
We next evaluated the correlations of serum ApoB and ApoA-1 levels to the PNs of ApoB-containing lipoproteins and HDL and compared these correlations between GP-HPLC and NMR. The horizontal axis in Fig.5-A depicts the PNs of ApoB-containing lipoproteins, while the vertical axis shows serum ApoB concentration in nM, where we used the value of 550,000 as a molecular weight of ApoB. The correlations of serum ApoB molecules and the PNs of ApoB-containing lipoproteins were evaluated. The correlation coefficient was very good in GP-HPLC analysis (r=0.966, P <0.0001), however it was slightly reduced in NMR analysis (r=0.855, P <0.0001). The slope of the regression line indicates the number of ApoB molecule per one particle of ApoB-containing lipoprotein. The slope was approximately 1.0 in GP-HPLC analysis, however it was definitely larger than 1.0 in NMR analysis. The slope data by GP-HPLC analysis but not NMR confirmed the previous finding that one ApoB-containing lipoprotein contains one ApoB molecule.
Fig.5.
Correlations of serum ApoB (A) and ApoA-1 (B) levels in nM to the PNs of ApoB-containing lipoproteins and HDL(baseline data n =212) for GP-HPLC (closed circle) and NMR-LP3 (open circle)
In contrast, a marked difference in the PNs of ApoA-1-containing HDL was observed between GP-HPLC and NMR. Fig.5-B demonstrates the correlations of serum ApoA-1 molecules and the PNs of ApoA-1-containing HDL, where we used the value of 23,800 as a molecular weight of ApoA-I. The correlation coefficient between serum ApoA-I molecules and the PNs of HDL was very good in GP-HPLC analysis (r=0.907, P <0.0001), however it was reduced in NMR analysis (r=0.772, P <0.0001). Furthermore, the slope of the regression line indicates the number of ApoA-I molecule per one particle of HDL, which appeared much higher in GP-HPLC analysis than in NMR analysis.
Table 2 compares the estimated PNs of ApoB and ApoA-1 molecules in ApoB- and ApoA-1-containing lipoproteins, respectively. The number of ApoB in one ApoB-containing lipoprotein was 1.10±0.05 (95% CI: 1.09~1.11) in GP-HPLC, whereas it was 1.32±0.18 (95% CI: 1.29~1.34) in NMR analysis. The number of ApoB in one ApoB-containing lipoprotein in NMR analysis was much higher than expected. The number of ApoA-1 molecule in one particle of ApoA-1-containing HDL was 3.40±0.17 in GP-HPLC analysis, but it was calculated to be 1.46±0.15 in NMR analysis.
Table 2. Comparison of the Calculated Numbers of ApoB and ApoA-1 Molecule in One Lipoprotein Particle.
| GP-HPLC | NMR (LP3) | |||
|---|---|---|---|---|
| Mean (SD) | [95% CI] | Mean (SD) | [95% CI] | |
|
Number of ApoB Molecule in One ApoB-containing Lipoprotein Particle Number of ApoA-1 Molecule in One HDL Particle |
1.10 (0.05) 3.40 (0.17) |
1.09 – 1.11 3.38 – 3.42 |
1.32 (0.18) 1.46 (0.15) |
1.29 – 1.34 1.44 – 1.48 |
5. Effects of Pemafibrate on PNs of Each Lipoprotein Subclass Determined by GP-HPLC and NMR
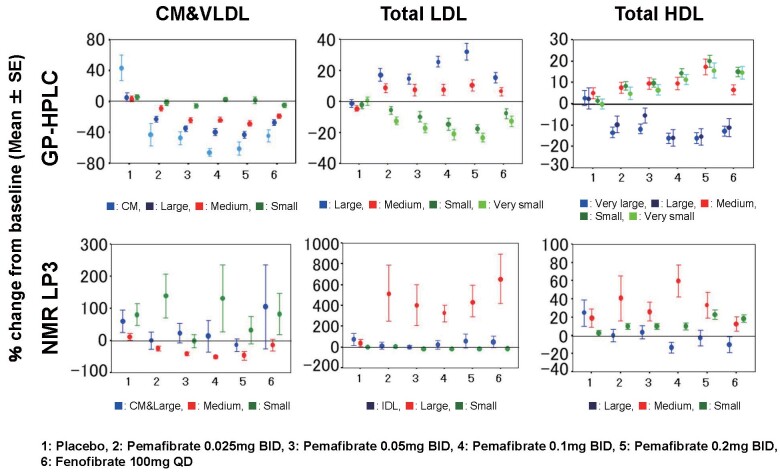
We compared the effect of a SPPARMα, pemafibrate (K-877) on the PNs of lipoproteins between GP-HPLC and NMR analysis ( Fig.6 ) . The details of the percent changes from baseline of other parameters are summarized in Supplementary Table 2-1 (3 major lipoprotein classes), Supplementary Table 2-2 (lipoprotein subclasses) and Supplementary Table 2-3 (average size of major lipoprotein classes, total TG, CM&VLDL-TG and HDL-C). Consistent and dose-dependent reductions in the PNs of CM, large VLDL and medium VLDL, but not small VLDL were observed after pemafibrate treatment in GP-HPLC analysis.
Fig.6. Comparison of percent changes of PNs by GP-HPLC and NMR (LP3) for lipoprotein subclasses after treatment with pemafibrate and fenofibrate.
The vertical axis compares the percent changes from the baseline of PNs of lipoprotein subclasses by GP-HPLC (upper panel) and NMR (lower panel) in each treatment group; 1: placebo, 2: Pemafibrate 0.025 mg BID, 3: Pemafibrate 0.05 mg BID, 4: Pemafibrate 0.1 mg BID, 5: Pemafibrate 0.2 mg BID, 6: Fenofibrate 100 mg QD, respectively. Each lipoprotein subclass is shown in color, and the data represents the mean±SE.
Supplementary Table 2-1. Percent change from baseline of PNs in 3 major classes of lipoproteins evaluated by GP-HPLC and NMR.
| Placebo | Pemafibrate | Fenofibrate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.025 mg BID | 0.05 mg BID | 0.1 mg BID | 0.2 mg BID | 100 mg QD | |||||||||
| Mean(SD) | P value | Mean(SD) | P value | Mean(SD) | P value | Mean(SD) | P value | Mean(SD) | P value | Mean(SD) | P value | ||
| GP-HPLC | |||||||||||||
| CM & VLDLnM | Week 0 | 248.4(49) | 240.5(46) | 248.4(55) | 241.0(57) | 261.3(57) | 256.8(47) | ||||||
| nM | Week 12 | 256.7(70) | 202.0(40) | 182.5(48) | 175.7(45) | 181.2(48) | 202.1(46) | ||||||
| % Change | 3.7(22) | 0.3116 | -14.9(15) | 0.0000 | -25.6(16) | 0.0000 | -25.8(17) | 0.0000 | -29.2(19) | 0.0000 | -20.6(15) | 0.0000 | |
| total LDL | Week 0 | 1657(322) | 1654(315) | 1589(346) | 1678(415) | 1817(330) | 1704(390) | ||||||
| nM | Week 12 | 1604(329) | 1646(259) | 1511(330) | 1580(360) | 1730(292) | 1658(339) | ||||||
| % Change | -2.9(11) | 0.1145 | 0.9(13) | 0.6952 | -2.3(17) | 0.4039 | -3.5(19) | 0.2722 | -3.7(15) | 0.1456 | -1.0(16) | 0.7095 | |
| total HDL | Week 0 | 13288(1580) | 13343(1705) | 13339(1385) | 13474(1766) | 13229(1441) | 12967(1554) | ||||||
| nM | Week 12 | 13417(1557) | 14070(2109) | 14294(1937) | 14779(2317) | 15176(2152) | 14294(2127) | ||||||
| % Change | 1.4(9) | 0.3637 | 5.7(12) | 0.0092 | 7.4(12) | 0.0006 | 9.8(11) | 0.0000 | 15.3(16) | 0.0000 | 10.4(11) | 0.0000 | |
| NMR | |||||||||||||
| CM&VLDL | Week 0 | 119.8(41) | 122.2(45) | 122.7(38) | 116.9(37) | 130.4(51) | 135.5(38) | ||||||
| nM | Week 12 | 123.9(56) | 94.5(38) | 71.8(29) | 64.7(27) | 57.7(30) | 93.2(39) | ||||||
| % Change | 5.9(38) | 0.3659 | -10.8(26) | 0.0001 | -39.7(22) | 0.0000 | -40.8(28) | 0.0000 | -44.2(46) | 0.0000 | -30.1(27) | 0.0000 | |
| total LDL | Week 0 | 1494(376) | 1497(431) | 1428(411) | 1520(377) | 1650(337) | 1498(373) | ||||||
| nM | Week 12 | 1431(354) | 1522(347) | 1388(372) | 1481(347) | 1646(380) | 1534(296) | ||||||
| % Change | -2.7(18) | 0.3800 | 6.5(28) | 0.1946 | 0.6(25) | 0.8907 | 0.7(26) | 0.8816 | 3.7(33) | 0.5123 | 6.5(26) | 0.1387 | |
| total HDL | Week 0 | 31103(5270) | 31264(4932) | 31354(5005) | 31722(5181) | 31074(4235) | 30447(4585) | ||||||
| nM | Week 12 | 32291(5592) | 33727(5838) | 34405(6180) | 35694(6450) | 37317(6023) | 33847(5807) | ||||||
| % Change | 4.7(13) | 0.0425 | 8.4(14) | 0.0018 | 10.1(14) | 0.0001 | 12.9(13) | 0.0000 | 18.7(20) | 0.0000 | 11.7(15) | 0.0000 | |
Supplementary Table 2-2. Percent change from baseline of PNs in subclasses of lipoproteins evaluated by GP-HPLC and NMR.
| Placebo | Pemafibrate | Fenofibrate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.025 mg BID | 0.05 mg BID | 0.1 mg BID | 0.2 mg BID | 100 mg QD | |||||||||
| Mean(SD) | P value | Mean(SD) | P value | Mean(SD) | P value | Mean(SD) | P value | Mean(SD) | P value | Mean(SD) | P value | ||
| GP-HPLC | |||||||||||||
| CM | Week 0 | 4.9(4.2) | 4.5(3.5) | 4.1(3.3) | 4.2(3.1) | 3.3(3.3) | 3.9(2.4) | ||||||
| nM | Week 12 | 5.2(3.6) | 1.6(1.4) | 1.7(2.0) | 1.3(1.6) | 0.8(1.1) | 1.6(1.2) | ||||||
| % Change | 43.2(99.7) | 0.0150 | -41.6(83.7) | 0.0075 | -47.8(49.5) | 0.0000 | -66.2(31.4) | 0.0000 | -61.3(52.2) | 0.0000 | -44.5(46.9) | 0.0000 | |
| Large VLDL | Week 0 | 109.8(29.3) | 101.3(25.6) | 105.9(27.4) | 104.2(30.6) | 106.0(28.2) | 109.2(29.1) | ||||||
| nM | Week 12 | 113.5(41.5) | 77.4(24.9) | 68.0(28.0) | 60.8(25.5) | 56.3(21.3) | 77.4(26.6) | ||||||
| % Change | 5.1(31.6) | 0.3426 | -22.6(20.5) | 0.0000 | -35.2(21.9) | 0.0000 | -39.8(25.7) | 0.0000 | -43.5(27.1) | 0.0000 | -27.5(22.9) | 0.0000 | |
| Medium | Week 0 | 79.6(16.1) | 79.0(16.4) | 81.2(19.1) | 79.6(19.1) | 88.4(19.9) | 85.3(13.8) | ||||||
| VLDL | Week 12 | 81.8(21.9) | 69.3(13.1) | 59.7(14.8) | 59.5(16.4) | 60.7(16.8) | 68.1(14.7) | ||||||
| nM | % Change | 3.0(21.2) | 0.4126 | -9.6(22.5) | 0.0196 | -24.8(18.0) | 0.0000 | -24.2(18.0) | 0.0000 | -29.4(21.7) | 0.0000 | -19.5(15.9) | 0.0000 |
| Small VLDL | Week 0 | 54.1(11.9) | 55.8(13.8) | 57.1(17.3) | 53.0(13.3) | 63.6(15.1) | 58.4(10.6) | ||||||
| nM | Week 12 | 56.3(12.1) | 53.7(12.9) | 53.1(16.4) | 54.1(15.2) | 63.4(18.6) | 55.0(11.7) | ||||||
| % Change | 5.6(18.6) | 0.0827 | -1.7(20.4) | 0.6261 | -5.8(16.8) | 0.0444 | 2.5(16.6) | 0.3710 | 1.9(27.7) | 0.6941 | -4.9(17.9) | 0.1077 | |
| Large LDL | Week 0 | 192.8(43.7) | 196.0(54.4) | 195.5(58.8) | 192.7(45.1) | 227.4(55.4) | 204.2(41.0) | ||||||
| nM | Week 12 | 188.4(43.6) | 222.3(52.9) | 219.9(62.1) | 238.3(54.1) | 288.9(65.2) | 231.7(47.0) | ||||||
| % Change | -1.3(15.2) | 0.6041 | 16.3(24.0) | 0.0004 | 14.4(19.5) | 0.0001 | 25.5(20.4) | 0.0000 | 31.5(32.5) | 0.0000 | 15.2(21.0) | 0.0001 | |
| Medium LDL | Week 0 | 619.0(144.1) | 612.9(148.7) | 592.8(156.4) | 628.4(169.8) | 688.7(133.5) | 642.7(163.2) | ||||||
| nM | Week 12 | 588.3(145.7) | 654.2(128.7) | 618.9(138.9) | 654.8(146.3) | 735.2(128.3) | 672.7(159.6) | ||||||
| % Change | -4.6(11.0) | 0.0195 | 9.0(16.8) | 0.0042 | 7.4(22.9) | 0.0557 | 7.5(21.4) | 0.0435 | 8.6(19.2) | 0.0125 | 6.3(15.7) | 0.0209 | |
| Small LDL | Week 0 | 514.1(113.7) | 510.5(97.7) | 476.0(107.7) | 523.7(155.0) | 552.5(113.8) | 523.3(140.1) | ||||||
| nM | Week 12 | 499.5(116.8) | 478.1(90.3) | 422.8(113.6) | 430.8(132.3) | 445.0(97.1) | 469.3(116.8) | ||||||
| % Change | -2.2(13.2) | 0.3210 | -5.2(14.2) | 0.0416 | -9.8(20.7) | 0.0066 | -14.7(23.7) | 0.0007 | -18.2(16.3) | 0.0000 | -7.8(20.0) | 0.0243 | |
| Very Small | Week 0 | 331.4(75.1) | 334.1(66.9) | 304.2(68.7) | 333.4(94.8) | 348.4(79.8) | 334.0(91.1) | ||||||
| LDL | Week 12 | 327.5(77.2) | 291.3(61.6) | 249.0(68.6) | 255.9(84.6) | 260.8(58.6) | 284.3(76.5) | ||||||
| nM | % Change | 0.2(16.7) | 0.9440 | -12.0(13.2) | 0.0000 | -17.3(17.5) | 0.0000 | -21.2(20.9) | 0.0000 | -23.3(17.3) | 0.0000 | -12.7(20.5) | 0.0007 |
| Very Large | Week 0 | 189.5(33.3) | 204.5(45.0) | 194.7(41.6) | 193.9(40.0) | 195.0(35.4) | 196.1(35.0) | ||||||
| HDL | Week 12 | 191.9(41.9) | 173.6(31.8) | 170.2(43.1) | 159.3(30.0) | 160.9(26.0) | 168.4(28.1) | ||||||
| nM | % Change | 2.6(21.1) | 0.4707 | -13.5(14.0) | 0.0000 | -11.9(13.7) | 0.0000 | -16.3(14.4) | 0.0000 | -16.1(15.0) | 0.0000 | -12.8(14.1) | 0.0000 |
| Large HDL | Week 0 | 615.7(208.7) | 791.5(378.5) | 741.1(295.8) | 684.6(328.3) | 705.7(238.4) | 701.0(227.7) | ||||||
| nM | Week 12 | 623.5(257.7) | 711.4(362.0) | 703.8(346.1) | 562.5(293.2) | 581.2(215.5) | 598.4(231.3) | ||||||
| % Change | 2.4(29.6) | 0.6416 | -9.8(21.9) | 0.0152 | -5.7(21.1) | 0.1099 | -16.1(22.9) | 0.0002 | -15.6(22.5) | 0.0003 | -11.2(25.0) | 0.0108 | |
| Medium HDL | Week 0 | 3031(571.3) | 3224(606.8) | 3282(597.7) | 3251(682.4) | 3170(561.5) | 3175(545.6) | ||||||
| nM | Week 12 | 3137(558.1) | 3474(833.0) | 3574(766.0) | 3529(812.4) | 3691(842.9) | 3390(771.2) | ||||||
| % Change | 5.0(15.4) | 0.0650 | 7.5(14.6) | 0.0060 | 9.5(16.2) | 0.0011 | 9.5(17.5) | 0.0026 | 17.3(21.5) | 0.0000 | 6.6(14.3) | 0.0087 | |
| Small HDL | Week 0 | 5720(903.2) | 5492(770.8) | 5597(763.4) | 5726(1027) | 5552(700.3) | 5414(866.0) | ||||||
| nM | Week 12 | 5775(910.0) | 5933(930.5) | 6116(896.8) | 6545(1319) | 6632(1109) | 6214(1201) | ||||||
| % Change | 1.5(10.1) | 0.4008 | 8.3(11.9) | 0.0003 | 9.7(11.3) | 0.0000 | 14.4(12.1) | 0.0000 | 19.9(16.8) | 0.0000 | 14.9(13.7) | 0.0000 | |
| Very Small | Week 0 | 3732(595.5) | 3631(586.5) | 3524(573.1) | 3617(572.2) | 3607(520.3) | 3481(663.5) | ||||||
| HDL | Week 12 | 3690(596.4) | 3779(698.3) | 3730(702.0) | 3984(620.6) | 4111(642.0) | 3923(621.1) | ||||||
| nM | % Change | -0.3(13.9) | 0.9135 | 4.7(16.5) | 0.1084 | 6.4(14.4) | 0.0104 | 11.2(14.3) | 0.0000 | 15.5(20.7) | 0.0001 | 14.5(17.6) | 0.0000 |
| NMR | |||||||||||||
| CM & Large | Week 0 | 18.0(13.8) | 14.2(9.9) | 14.6(12.3) | 15.6(12.5) | 11.8(9.7) | 14.5(12.5) | ||||||
| VLDL | Week 12 | 18.2(13.8) | 7.1(6.3) | 8.5(8.6) | 6.8(6.4) | 5.2(4.8) | 7.6(6.0) | ||||||
| nM | % Change | 59.3(204.7) | 0.0957 | -0.7(152.1) | 0.9806 | 23.5(181.7) | 0.4425 | 13.7(294.2) | 0.7851 | -14.3(115.8) | 0.4709 | 105.1(768.2) | 0.4238 |
| Medium | Week 0 | 67.8(29.8) | 70.0(37.7) | 65.5(26.7) | 64.6(26.8) | 69.4(38.8) | 71.9(36.9) | ||||||
| VLDL | Week 12 | 68.8(41.8) | 47.7(22.9) | 36.0(21.5) | 28.8(15.4) | 23.1(14.1) | 46.4(26.0) | ||||||
| nM | % Change | 10.4(64.7) | 0.3473 | -24.6(42.1) | 0.0021 | -41.7(31.8) | 0.0000 | -51.6(27.1) | 0.0000 | -47.3(82.5) | 0.0021 | -14.9(106.5) | 0.4053 |
| Small VLDL | Week 0 | 34.0(25.8) | 38.0(30.1) | 42.6(32.6) | 36.7(26.7) | 49.2(44.1) | 49.0(28.3) | ||||||
| nM | Week 12 | 37.0(27.2) | 39.7(25.9) | 27.3(18.8) | 29.1(18.7) | 29.3(22.1) | 39.2(23.9) | ||||||
| % Change | 81.0(197.9) | 0.0230 | 138.4(392.4) | 0.0512 | -2.5(125.9) | 0.9044 | 131.6(624.7) | 0.2146 | 32.0(250.5) | 0.4554 | 82.8(379.5) | 0.2053 | |
| IDL | Week 0 | 236.0(158.0) | 245.2(141.4) | 217.4(122.5) | 204.1(154.8) | 263.5(173.4) | 244.3(151.5) | ||||||
| nM | Week 12 | 196.5(141.9) | 172.5(106.4) | 166.8(99.6) | 131.4(96.8) | 158.9(88.0) | 192.4(153.6) | ||||||
| % Change | 76.4(349.6) | 0.2049 | 15.2(203.6) | 0.6700 | 2.5(102.5) | 0.8812 | 26.1(264.1) | 0.5569 | 62.5(420.4) | 0.3849 | 53.3(340.9) | 0.3613 | |
| Large LDL | Week 0 | 179.6(185.7) | 208.4(210.9) | 218.7(201.7) | 229.1(226.9) | 274.2(241.5) | 182.3(196.5) | ||||||
| nM | Week 12 | 162.1(178.5) | 398.9(248.0) | 414.7(253.9) | 492.9(251.7) | 653.9(257.9) | 469.0(222.9) | ||||||
| % Change | 39.1(194.5) | 0.2882 | 522.2(1535.3) | 0.0636 | 404.2(1096.2) | 0.0489 | 324.5(450.1) | 0.0003 | 444.4(847.2) | 0.0066 | 659.5(1353.0) | 0.0097 | |
| Small LDL | Week 0 | 1078(274.9) | 1043(354.0) | 992.1(268.5) | 1087(298.0) | 1112(293.2) | 1071(298.6) | ||||||
| nM | Week 12 | 1072(277.6) | 950.9(309.4) | 806.1(325.6) | 856.6(282.3) | 833.1(295.3) | 872.8(216.5) | ||||||
| % Change | 2.6(26.1) | 0.5549 | 7.3(79.0) | 0.6005 | -15.2(35.6) | 0.0134 | -16.9(30.9) | 0.0024 | -15.2(50.7) | 0.0851 | -13.3(30.2) | 0.0124 | |
| Large HDL | Week 0 | 2546(1193) | 3558(2065) | 3051(1495) | 2917(1473) | 2791(1380) | 2944(1053) | ||||||
| nM | Week 12 | 2743(1301) | 3276(1682) | 2922(1466) | 2281(971.0) | 2409(1141) | 2397(1100) | ||||||
| % Change | 24.9(84.6) | 0.0904 | 0.3(40.9) | 0.9626 | 3.7(45.3) | 0.6202 | -13.1(36.6) | 0.0386 | -2.5(49.2) | 0.7678 | -9.8(52.2) | 0.2654 | |
| Medium | Week 0 | 6954(2549) | 7273(3251) | 8427(4578) | 7333(4020) | 8511(2904) | 8214(3435) | ||||||
| HDL | Week 12 | 7717(3510) | 7964(4657) | 9768(5556) | 10142(5855) | 10706(5105) | 9011(5104) | ||||||
| nM | % Change | 19.2(59.6) | 0.0644 | 41.1(142.7) | 0.1079 | 26.3(62.9) | 0.0155 | 60.4(105.6) | 0.0015 | 33.5(83.2) | 0.0231 | 12.8(48.1) | 0.1193 |
| Small HDL | Week 0 | 21606(4293) | 20439(4013) | 19873(3422) | 21464(4543) | 19789(3729) | 19292(4043) | ||||||
| nM | Week 12 | 21840(4020) | 22482(5688) | 21730(4653) | 23275(5079) | 24203(4852) | 22439(4477) | ||||||
| % Change | 2.9(17.5) | 0.3414 | 10.3(18.5) | 0.0030 | 10.3(20.1) | 0.0037 | 10.6(21.5) | 0.0057 | 23.2(30.2) | 0.0001 | 18.9(24.3) | 0.0000 | |
Supplementary Table 2-3. Percent change from baseline of particle size, total TG, CM&VLDL-TG and HDL-C in major classes of lipoproteins evaluated by GP-HPLC and NMR.
| Placebo | Pemafibrate | Fenofibrate | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.025 mg BID | 0.05 mg BID | 0.1 mg BID | 0.2 mg BID | 100 mg QD | |||||||||
| Mean(SD) | P value | Mean(SD) | P value | Mean(SD) | P value | Mean(SD) | P value | Mean(SD) | P value | Mean(SD) | P value | ||
| GP-HPLC | |||||||||||||
| CM & VLDL | Week 0 | 41.8(1.6) | 41.5(1.8) | 41.4(1.6) | 41.5(1.5) | 40.7(1.5) | 41.3(1.3) | ||||||
| Average Size | Week 12 | 41.7(1.7) | 40.0(1.6) | 39.7(2.0) | 39.1(1.9) | 38.4(1.3) | 39.9(1.4) | ||||||
| nm | % Change | -0.1(4.1) | 0.8856 | -3.5(3.6) | 0.0000 | -4.0(3.7) | 0.0000 | -5.7(3.7) | 0.0000 | -5.7(4.0) | 0.0000 | -3.3(4.0) | 0.0000 |
| total LDL | Week 0 | 23.9(0.3) | 23.9(0.3) | 23.9(0.3) | 23.9(0.3) | 24.0(0.3) | 23.9(0.2) | ||||||
| Average Size | Week 12 | 23.8(0.3) | 24.1(0.3) | 24.3(0.3) | 24.3(0.3) | 24.5(0.3) | 24.2(0.3) | ||||||
| nm | % Change | -0.1(1.0) | 0.4872 | 1.1(0.8) | 0.0000 | 1.4(1.0) | 0.0000 | 1.9(1.1) | 0.0000 | 2.1(1.4) | 0.0000 | 1.2(1.2) | 0.0000 |
| total HDL | Week 0 | 9.7(0.1) | 9.8(0.1) | 9.8(0.1) | 9.7(0.1) | 9.7(0.1) | 9.8(0.1) | ||||||
| Average Size | Week 12 | 9.7(0.1) | 9.7(0.1) | 9.7(0.1) | 9.7(0.1) | 9.7(0.1) | 9.7(0.1) | ||||||
| nm | % Change | 0.3(1.1) | 0.1781 | -0.5(0.8) | 0.0018 | -0.4(0.9) | 0.0059 | -0.8(0.9) | 0.0000 | -0.6(0.8) | 0.0000 | -0.8(1.1) | 0.0000 |
| total TG | Week 0 | 302.6(104.6) | 287.8(86.8) | 287.8(95.1) | 285.8(105.9) | 270.4(83.3) | 291.6(83.8) | ||||||
| mg/dL | Week 12 | 322.3(126.3) | 201.1(64.1) | 184.6(81.6) | 166.6(71.5) | 151.9(56.3) | 199.9(67.3) | ||||||
| % Change | 10.8(38.2) | 0.1038 | -27.8(22.4) | 0.0000 | -34.3(22.2) | 0.0000 | -39.0(24.0) | 0.0000 | -40.4(23.9) | 0.0000 | -28.2(24.8) | 0.0000 | |
| CM & VLDL- | Week 0 | 244.1(96.4) | 224.5(78.3) | 226.6(82.5) | 225.6(93.2) | 211.4(72.8) | 230.4(75.9) | ||||||
| TG | Week 12 | 259.6(112.7) | 149.2(58.3) | 136.8(74.2) | 118.3(62.1) | 101.7(49.9) | 149.1(59.8) | ||||||
| mg/dL | % Change | 12.3(42.8) | 0.0972 | -30.9(26.1) | 0.0000 | -38.5(24.9) | 0.0000 | -44.9(26.3) | 0.0000 | -47.8(28.8) | 0.0000 | -31.6(27.3) | 0.0000 |
| HDL-C | Week 0 | 38.5(5.9) | 39.0(6.2) | 39.0(5.7) | 39.3(6.4) | 40.0(5.9) | 38.2(6.3) | ||||||
| mg/dL | Week 12 | 38.0(6.2) | 43.3(8.4) | 45.0(8.7) | 45.4(8.0) | 47.7(8.8) | 43.6(8.0) | ||||||
| % Change | -0.9(11.2) | 0.6497 | 11.1(12.6) | 0.0000 | 15.2(13.9) | 0.0000 | 16.2(14.2) | 0.0000 | 20.3(21.5) | 0.0000 | 14.3(13.0) | 0.0000 | |
| NMR | |||||||||||||
| CM & VLDL | Week 0 | 56.9(9.1) | 55.0(8.8) | 54.6(9.8) | 55.8(8.2) | 52.2(8.4) | 53.2(8.5) | ||||||
| Average Size | Week 12 | 57.5(9.9) | 50.4(7.1) | 53.3(9.6) | 51.4(7.7) | 51.3(7.7) | 51.1(6.5) | ||||||
| nm | % Change | 2.0(16.1) | 0.4673 | -6.8(16.7) | 0.0263 | -0.9(16.4) | 0.7383(15.6) | -6.5 | 0.0165 | -1.1(18.3) | 0.7298 | -1.9(14.8) | 0.4536 |
| total LDL | Week 0 | 19.9(0.3) | 20.0(0.4) | 20.0(0.3) | 20.0(0.4) | 20.1(0.4) | 19.9(0.4) | ||||||
| Average Size | Week 12 | 19.8(0.3) | 20.4(0.6) | 20.6(0.6) | 20.7(0.6) | 21.1(0.5) | 20.6(0.5) | ||||||
| nm | % Change | -0.2(1.8) | 0.5353 | 2.1(2.4) | 0.0000 | 3.1(2.5) | 0.0000 | 3.7(2.4) | 0.0000 | 4.7(4.0) | 0.0000 | 3.6(3.0) | 0.0000 |
| total HDL | Week 0 | 8.7(0.3) | 8.9(0.4) | 8.8(0.4) | 8.8(0.4) | 8.8(0.3) | 8.9(0.3) | ||||||
| Average Size | Week 12 | 8.8(0.3) | 8.7(0.4) | 8.6(0.3) | 8.5(0.3) | 8.5(0.3) | 8.5(0.2) | ||||||
| nm | % Change | 1.1(4.2) | 0.1465 | -2.2(3.4) | 0.0010 | -2.0(3.9) | 0.0027 | -2.9(4.1) | 0.0001 | -3.2(4.4) | 0.0001 | -4.4(3.3) | 0.0000 |
| total TG | Week 0 | 302.6(104.6) | 287.8(86.8) | 287.8(95.1) | 285.8(105.9) | 270.4(83.3) | 291.6(83.8) | ||||||
| mg/dL | Week 12 | 322.3(126.3) | 201.1(64.1) | 184.6(81.6) | 166.6(71.5) | 151.9(56.3) | 199.9(67.3) | ||||||
| % Change | 9.4(41.9) | 0.1951 | -28.7(24.9) | 0.0000 | -34.0(23.3) | 0.0000 | -42.1(22.1) | 0.0000 | -38.8(32.4) | 0.0000 | -27.1(29.4) | 0.0000 | |
| CM & VLDL- | Week 0 | 244.1(96.4) | 224.5(78.3) | 226.6(82.5) | 225.6(93.2) | 211.4(72.8) | 230.4(75.9) | ||||||
| TG | Week 12 | 259.6(112.7) | 149.2(58.3) | 136.8(74.2) | 118.3(62.1) | 101.7(49.9) | 149.1(59.8) | ||||||
| mg/dL | % Change | 13.2(49.3) | 0.1232 | -33.8(29.6) | 0.0000 | -40.7(27.0) | 0.0000 | -50.0(25.7) | 0.0000 | -47.2(42.2) | 0.0000 | -32.5(32.7) | 0.0000 |
| HDL-C | Week 0 | 38.5(5.9) | 39.0(6.2) | 39.0(5.7) | 39.3(6.4) | 40.0(5.9) | 38.2(6.3) | ||||||
| mg/dL | Week 12 | 38.0(6.2) | 43.3(8.4) | 45.0(8.7) | 45.4(8.0) | 47.7(8.8) | 43.6(8.0) | ||||||
| % Change | 3.1(14.3) | 0.2096 | -0.2(15.0) | 0.9489 | 4.8(15.4) | 0.0682 | 3.1(14.3) | 0.2065 | 3.6(18.5) | 0.2559 | 2.2(15.2) | 0.3926 | |
Regarding LDL, GP-HPLC showed consistent and dose-dependent reductions in the PNs of small LDL and very small LDL after pemafibrate treatment. Furthermore, GP-HPLC showed consistent and dose-dependent increases in the PNs of large LDL and medium LDL after pemafibrate treatment. Regarding HDL, pemafibrate showed consistent and dose-dependent changes in reductions of very large HDL and large HDL and increases in medium, small and very small HDL in GP-HPLC analysis.
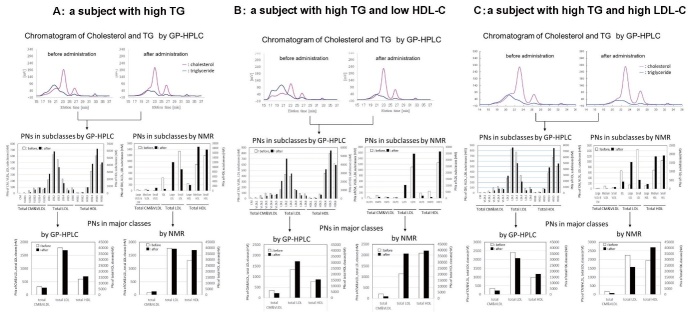
Supplementary Fig.4 shows the representative GP-HPLC chromatograms directly monitoring cholesterol and TG as well as the PNs of each subclass and 3 major classes before and after pemafibrate administration, which are the data of 3 types of subjects, namely, (A) a high TG, (B) a high TG with low HDL-C and (C) a high TG with high LDL-C. The quantification of cholesterol and TG concentration detected by online enzymatic reactions are presented as chromatograms, separating 20 subclasses by Gaussian fitting technique as shown in Supplementary Fig.1 . The PNs of each lipoprotein subclass was directly calculated with cholesterol and TG concentration, and the details of calculation of lipoprotein PNs from cholesterol and TG concentration of 20 subclasses by GP-HPLC can be referred to the reference 40) and 68). The algorithm of Gaussian fitting technique for GP-HPLC and the detailed data of “spherical particle model” can be referred to the patent 68) . The distribution and changes of PNs in 18 lipoprotein subclasses and 3 major classes before and after administration are shown in column graphs. These GP-HPLC chromatograms obtained by simultaneous detection of cholesterol and TG may provide much more qualitative and quantitative information on the whole lipoprotein profiles for each patient.
Supplementary Fig. 4. Representative GP-HPLC chromatograms directly monitoring cholesterol (pink line) and TG (blue line) as well as the PNs of each subclass and 3 major classes by GP-HPLC and NMR-LP3 before (open column) and after (closed column) pemafibrate administration.
4-A: A 44-year-old male subject with high TG (415 mg/dL). Pemafibrate 0.1mg BID was administered.
4-B: A 36-year-old male subject with high TG (650 mg/dL) and low HDL-C (30 mg/dL). Pemafibrate 0.1 mg BID was administered.
4-C: A 49-year-old male subject with high TG (345 mg/dL) and high LDL-C (210 mg/dL). Pemafibrate 0.2 mg BID was administered.
Discussion
The current study has, for the first time, compared the PNs of each lipoprotein subclass measured by GP-HPLC and NMR, and also assessed the effect of a selective PPARα modulator, pemafibrate in dyslipidemic patients. The PNs of three major lipoprotein classes, total CM&VLDL, total LDL and total HDL analyzed by GP-HPLC correlated positively with those by NMR. However, there were marked variations in the PNs of lipoprotein subclasses between the two methods. Although there have been several methods to measure lipoproteins in serum or plasma 28 , 40 - 41) , only GP-HPLC and NMR method can evaluate whole lipoprotein subclasses from CM to HDL according to their particle size and also determine the PNs of major lipoprotein classes as the assembly of subclasses in serum or plasma without any pretreatment. Both methods are considered to be appropriate to quantitatively and qualitatively evaluate many samples quickly, and therefore they have especially been applied to clinical studies.
Firstly, GP-HPLC can separate serum lipoproteins in gel permeation columns according to the difference in particle size and has continuously been applied to detect and measure cholesterol and TG directly by an enzymatic reaction as presented in Supplementary Fig.1 . Accordingly, the data with GP-HPLC method, TC, LDL-C and HDL-C showed a good correlation and correspondence with CDC (Center for Disease Control and Prevention) reference methods 34 , 69) . In the current study, the correlations of serum ApoB molecules, ApoA-1 molecules and HDL-C with the PNs of ApoB-containing lipoproteins, ApoA-1-containing lipoproteins and HDL-C concentration by GP-HPLC showed a good correlation. In contrast to GP-HPLC, NMR signals in NMR method basically depend on particle diffusion rates according to lipoprotein size 25) . The NMR analysis of each lipoprotein subclass is performed using a whole 1 H-NMR signal intensity from methyl groups contained in the hydrocarbon chains; 2 methyl groups in free cholesterol (FC), 3 in TG, 3 in CE, and 2 in phospholipids, respectively 25 , 40) . Therefore, the direct measurements for each lipid component can not be done from 1 H-NMR signal information. Therefore, the NMR method may be suitable for the determination of lipoprotein PNs and the evaluation of lipoprotein particle size. In contrast, the GP-HPLC method is more suited for the evaluation of lipoprotein lipid concentrations than the NMR method.
Secondly, NMR method requires a certain volume (up to 200 µL) of serum samples because of low sensitivity 25) , while GP-HPLC method requires only few microliter of serum due to it’s high sensitivity. Furthermore, the high reproducibility of GP-HPLC method may not require to measure the samples in duplicate, while the low reproducibility of NMR method might require duplicate or triplicate measurements 18 , 19) . Otvos et al. reported that methyl signals corresponding to CMs are increased after a meal compared to fasting state in the same individual 15) . In GP-HPLC, CMs can be detected at void volume of gel-permeation columns in hypertriglyceridemic subjects as shown in Supplementary Fig.4 , subject B. Therefore, the very good correlation for CM & Large VLDL-TG values between the GP-HPLC and NMR as shown in Fig.4 can be clearly explained. In contrast, methyl signals of NMR corresponding to HDL are buried in the right end region of observed peak although signals corrsponding to CM are clearly shown in the Figure 6 of Reference 15) . These data suggest one of the possible reasons for a big difference in the PNs of total HDL between GP-HPLC and NMR. The relation between measurement temperature and methyl signal strength from 15 to 45°C 15) also shows a marked dependence of methyl signal strength on temperature, implying the low reproducibility of NMR method.
In the current study, the NMR algorithm LP3 was used to compare with GP-HPLC. However, we have compared the PNs in each subclass between NMR algorithm LP2 and LP3 as demonstrated in Supplementary Fig.3 . In IDL and medium HDL subclass, NMR algorithm LP2 showed many zero values, which are frequently reported, for example, by Soedamah-Muthu et al. 18) , however it has been improved in NMR algorithm LP3. We also evaluated the correlations between HDL-C level with homogeneous HDL-C assay and HDL-C level determined by NMR in subjects ( n =212) at the baseline. The mean±SD of serum HDL-C determined by homogeneous HDL-C assay was 40.8±6.9 mg/dL, while those of HDL-C by GP-HPLC was 39.0±6.9 mg/dL. The correlation of HDL-C leves determined by homogeneous HDL-C assay and GP-HPLC was very good (r=0.968, p <0.0001). In contrast, the mean±SD of serum HDL-C level by NMR-LP3 was 42.9±7.4 mg/dL and the correlation of serum HDL-C level between homogeneous HDL-C assay and by NMR-LP3 was not so good (r=0.437, p <0.0001), as shown in Fig.4-D .
Furthermore, the number of ApoB molecule in one particle of ApoB-containing lipoprotein was 1.10 and 1.32 in GP-HPLC and NMR, respectively. However, the number of ApoA-1 molecule in one particle of ApoA-1-containing HDL was 1.46 by NMR-LP3 and 1.56 by NMR-LP2, respectively (data not shown), while it was 3.40 in GP-HPLC analysis. In the large-scale study by NMR 21 - 22) , the number of ApoB molecule and ApoA-I moleclues in one lipoproetein particle are reported to be 1.35-1.34 and 1.50-1.51, respectively. The current literature shows that the number of ApoA-1 molecules in one HDL particle was estimated to be approximately 2~5 72 - 74) . In our current GP-HPLC analysis, the number of apoA molecules per one HDL particle and that of apoB molecules per one apoB-containing lipoprotein particle was 1.10 and 3.40, respectivey, ( Fig.5 and Table 2 ) , indicating the validity of GP-HPLC method for evaluation of the PNs using a mathematical simulation model called as “spherical particle model” 40 , 68) .
In NMR analysis, the PNs of small LDL % in total LDL were markedly dominant (~71%), whereas it was ~50% in GP-HPLC analysis. GP-HPLC analysis showed that the PNs of small LDL was calculated to be 41% of total LDL for control subjects 41) and 40.2% of total LDL for YUKAWA study subjects 44) . These values were in a good accordance with the results of the current study, suggesting the reliability of GP-HPLC method. On the other hand, the PNs of small LDL % in total LDL were reported to be from 42.9% to 68.8% by NMR (LP2 and LP3) 21 - 24) .
We have shown the benefits of GP-HPLC method in comparison with NMR especially in assessing the efficacy of antilipidemic drugs on the PNs, sizes and concentrations of lipoproteins before and after therapy. In the current study, we analyzed the serum samples of dyslipidemic subjects treated with a SPPARMα, pemafibrate, and compared the data between GP-HPLC and NMR. GP-HPLC showed a dose-dependent and consistent change in the PNs, sizes and concentrations of lipoproteins after drug administration. GP-HPLC could demonstrate more detailed changes than NMR in the size, concentration, composition and PNs of major lipoproteins as well as their subclasses after administration of pemafibrate. GP-HPLC may be useful for analysis of lipoprotein profiles as well as a quantitative and qualitative evaluation of atherogenic lipoproteins such as small dense LDL and VLDL remnants, leading to comprehensive assessment of dyslipidemia risk. As we have reported recently 44 , 48) , GP-HPLC may be applicable and suitable for the analysis of subtle changes in lipoprotein subclasses after treatment with lipid-modifying drugs. It also seems valuable for its application to mega studies involving multiple laboratories if a certain calibrator is used for GP-HPLC 41 , 75) .
The GP-HPLC using gel permeation column inherently has an insufficient capacity of lipoprotein separation. In ultracentrifugation analysis, major lipoproteins are separated using Gaussian curve fitting method 76) . In order to resolve this problem, we used the Gaussian curve fitting method as presented in Step 2: Data analysis of Supplementary Fig.1 . All of 20 lipoprotein subclasses can not be confirmed as physical and real peaks. HDL subclasses of HDL3−HDL7 have been confirmed as physical and real peaks by rechromatography technique 77) . VLDL4, LDL2 and HDL4 are the major component peaks of VLDL, LDL and HDL in healthy men, respectively, while VLDL3, LDL3, and HDL5 are those of VLDL, LDL and HDL in patients with familial lipoprotein lipase (LPL) deficiency. VLDL5 and HDL2 are those of patients with familial type III hyperlipidemia associated with apoE2/2 and CETP deficiency 40 , 67 - 68 , 78) . Since LDL4−LDL6 and HDL1, minor components between LDL and HDL, are virtual and unreal peaks, they are frequently analyzed by grouping as major subclasses, very small LDL and very large HDL, as presented in Table 1 .
In conclusion, we have compared the lipoproteins and their subclasses analyzed by GP-HPLC and NMR. The PNs of major lipoprotein classes of total CM&VLDL, total LDL and total HDL in dyslipidemic patients analyzed by GP-HPLC correlated positively with those by NMR. However, the PNs of lipoprotein subclasses differed between two methods. The PNs of HDL by NMR were about 2-fold higher than that by GP-HPLC. The number of ApoA-I in one HDL particle was calculated to be 3.40 by GP-HPLC, while it was 1.46 by NMR, much less than that reported in the literature. We have shown a clear evidence that GP-HPLC can evaluate the PNs, size and lipid concentrations of major lipoproteins and their subclasses more accurately than NMR. GP-HPLC might be used especially when assessing the detailed effects of lipid-lowering drugs on lipoprotein subclasses.
Limitations of the Study
The current study has several limitations. Firstly, we used the study samples of a phase 2 clinical trial of pemafibrate and the subjects were limited to those with dyslipidemia. Secondly, we have not performed similar analyses for comparison between GP-HPLC and NMR in different clinical studies using other drugs for dyslipidemia, although we have already shown a consistent and detailed changes of lipoprotein subclasses in studies administering PCSK9 inhibitor evolocumab 44) and omega-3 polyunsaturated fatty acids 48) .
Funding
The original phase 2 study of pemafibrate was funded by Kowa Company, Ltd. The study sponsor had a role in its study design, data collection, analysis, and interpretation. However, the comparison of GP-HPLC and NMR in lipoprotein subfractions after pemafibrate administration was performed by M.O., T.O., D.M., and S.Y. independently from Kowa Company, Ltd.
Conflict of Interests
S.Y. reports personal fees from Kowa during the conduct of the study; personal fees from Kowa, MSD, Amgen Astellas BioPharma, and Sanofi; and grants from MSD, Bayer Yakuhin, Nippon Boehringer Ingelheim, Takeda Pharmaceutical, Ono Pharmaceutical, Astellas Pharma, Mitsubishi Tanabe Pharma, Kyowa Medex, and Rohto Pharmaceutical outside of the submitted work. S.Y. and M.O. report advisory fee from Skylight Biotech. T.O. has no conflict of interest. D.M. reports personal fees from Kowa during the conduct of the study; personal fees from Kowa, MSD, Amgen Astellas BioPharma, and Sanofi; and grants from MSD, Bayer Yakuhin, Nippon Boehringer Ingelheim, Takeda Pharmaceutical, Astellas Pharma, Mitsubishi Tanabe Pharma, Kyowa Medex, and Rohto Pharmaceutical outside of the submitted work. H.A. reports personal fees from Kowa during the conduct of the study; personal fees from Daiichi Sankyo, Astellas Pharma, Sanofi, Kowa, MSD, Amgen Astellas BioPharma, and Abbott Japan; and grants from Daiichi Sankyo outside of the submitted work. K.Y. reports personal fees from Kowa during the conduct of the study; personal fees from Astellas Pharma, Amgen Astellas BioPharma, AstraZeneca, Bayer, MSD, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho, Kissei Pharmaceutical, Kyowa Hakko Kirin, Kaken Pharmaceutical, Kowa, Kowa Pharmaceutical, Sanofi, Shionogi, Daiichi Sankyo, Taisho Toyama Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Eli Lilly Japan, Nippon Boehringer Ingelheim, Novartis Pharma, Novo Nordisk Pharma, Pfizer Japan, Mochida Pharmaceutical, and Teijin Pharma; and grants from Astellas Pharma, MSD, Ono Pharmaceutical, Kowa Pharmaceutical, Kyowa Hakko Kirin, Shionogi, Daiichi Sankyo, Taisho Toyama Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Teijin Pharma, Eli Lilly Japan, Nippon Boehringer Ingelheim, Pfizer Japan, Novo Nordisk Pharma, and Mochida Pharmaceutical outside of the submitted work. E.A. reports personal fees from Kowa during the conduct of the study; personal fees from Astellas Pharma, MSD, Sanofi, Ono Pharmaceutical, and Novo Nordisk Pharma; and grants from Astellas Pharma, Ono Pharmaceutical, Nippon Boehringer Ingelheim, Taisho Toyama Pharmaceutical, Daiichi Sankyo, MSD, Sanofi, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Novartis Pharma, Novo Nordisk Pharma, and Eli Lilly Japan outside of the submitted work. S.I. reports personal fees from Kowa during the conduct of the study; personal fees from MSD, Sanofi, Amgen Astellas BioPharma, Kowa Pharmaceutical, and Kowa; and grants from Eli Lilly Japan, Sanofi, Astellas Pharma, Nippon Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Daiichi Sankyo, Takeda Pharmaceutical, Ono Pharmaceutical, Shionogi, Teijin Pharma, and Kowa Pharmaceutical outside of the submitted work.
References
- 1).Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W, Atherosclerosis Risk in Communities Study Group. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation, 2001; 104: 1108-1113 [DOI] [PubMed] [Google Scholar]
- 2).Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RGJ, de Craen AJM, Knopp RH, Nakamura H, Ridker P, van Domburg R, Deckers JW. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ, 2009; 338: b2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet (London, England), 2010; 376: 1670-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med, 2011; 365: 2255-2267 [DOI] [PubMed] [Google Scholar]
- 5).Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B; ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med, 2007; 357: 2109-2122 [DOI] [PubMed] [Google Scholar]
- 6).Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, Ruzyllo W, Bachinsky WB, Lasala GP, Tuzcu EM; ILLUSTRATE Investigators. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med, 2007; 356: 1304-1316 [DOI] [PubMed] [Google Scholar]
- 7).Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS; dal-OUTCOMES Investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med, 2012; 367: 2089-2099 [DOI] [PubMed] [Google Scholar]
- 8).Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Jose Nicolau C, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE, ACCELERATE Investigators. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med, 2017; 376: 1933-1942 [Google Scholar]
- 9).HPS3/TIMI55-REVEAL Collaborative Group, Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med, 2017; 377: 1217-1227 [DOI] [PubMed] [Google Scholar]
- 10).Krishna R, Gheyas F, Liu Y, Hagen DR, Walker B, Chawla A, Cote J, Blaustein RO, Gutstein DE. Chronic administration of anacetrapib is associated with accumulation in adipose and slow elimination. Clin Pharmacol Ther, 2017; 102: 832-840 [DOI] [PubMed] [Google Scholar]
- 11).Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart AFR, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, De Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PIW, Klungel OH, Anke-Hilse Maitland-van der Zee, Peters BJM, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VHM, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WMM, Boer JMA, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, Mokhtari NEEI, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O’Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet, 2012; 380: 572-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Lindgren FT, Elliott HA, Gofman JW. The ultracentrifugal characterization and isolation of human blood lipids and lipoproteins, with applications to the study of atherosclerosis. J. Phys. Colloid Chem, 1951; 55: 80-93 [DOI] [PubMed] [Google Scholar]
- 13).Gofman JW, Lindgren FT, Elliott H. Ultracentrifugal studies of lipoproteins of human serum. J Biol Chem, 1949; 179: 973-979 [PubMed] [Google Scholar]
- 14).Lindgren FT, Freeman NK, Ewing AM, Jensen LC. Serum lipoprotein distribution, flotation rates and protein analysis. J Am Oil Chem Soc, 1966; 43: 281-285 [DOI] [PubMed] [Google Scholar]
- 15).Otvos JD, Jeyarajah EJ, Bennett DW. Quantification of plasma lipoproteins by proton nuclear magnetic resonance spectroscopy. Clin Chem, 1991; 37: 377-386 [PubMed] [Google Scholar]
- 16).Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem, 1992; 38: 1632-1638 [PubMed] [Google Scholar]
- 17).AACC Lipoproteins and Vascular Diseases Division Working Group on Best Practices; Cole TG, Contois JH, Csako G, McConnell JP, Remaley AT, Devaraj S, Hoefner DM, Mallory T, Sethi AA, Warnick GR. Association of apolipoprotein B and nuclear magnetic resonance spectroscopy-derived LDL particle number with outcomes in 25 clinical studies: assessment by the AACC Lipoprotein and Vascular Diseases Division Working Group on Best Practices. Clin Chem, 2013; 59: 752-770 [DOI] [PubMed] [Google Scholar]
- 18).Soedamah-Muthu SS, Colhoun HM, Thomason MJ, Betteridge DJ, Durrington PN, Hitman GA, Fuller JH, Julier K, Mackness MI, Neil HAW, CARDS Investigators. The effect of atorvastatin on serum lipids, lipoproteins and NMR spectroscopy defined lipoprotein subclasses in type 2 diabetic patients with ischaemic heart disease. Atherosclerosis, 2003; 167: 243-255 [DOI] [PubMed] [Google Scholar]
- 19).Liao Y, Kwon S, Shaughnessy S, Wallace P, Amy Hutto A, Jenkins AJ, Klein RL, Garvey WT. Critical evaluation of adult treatment panel III criteria in identifying insulin resistance with dyslipidemia. Diabetes Care, 2004; 27: 978-983 [DOI] [PubMed] [Google Scholar]
- 20).Hopkins PN, Pottala JV, Nanjee MN. A comparative study of four independent methods to measure LDL particle concentration. Atherosclerosis, 2015; 243: 99-106 [DOI] [PubMed] [Google Scholar]
- 21).Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation, 2009; 119: 931-939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Chasman DI, Paré G, Mora S, Hopewell JC, Peloso G, Clarke R, Cupples LA, Hamsten A, Kathiresan S, Mälarstig A, Ordovas JM, Ripatti S, Parker AN, Miletich JP, Ridker PM. Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet, 2009; 5: e1000730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Kuller LH, Grandits G, Cohen JD, Neaton JD, Prineas R, Multiple Risk Factor Intervention Trial Research Group. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis, 2007; 195: 122-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Duprez DA, Otvos J, Tracy RP, Feingold KR, Greenland P, Gross MD, Lima JAC, Mackey RH, Neaton JD, Sanchez OA, Jacobs DR. High-density lipoprotein subclasses and noncardiovascular, noncancer chronic inflammatory-related events versus cardiovascular events: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc, 2015; 4: e002295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Mallol R, Rodriguez MA, Brezmes J, Masana L, Correig X. Human serum/plasma lipoprotein analysis by NMR: application to the study of diabetic dyslipidemia. Review Prog Nucl Magn Reson Spectrosc, 2013; 70: 1-24 [DOI] [PubMed] [Google Scholar]
- 26).Hara I, Okazaki M, Ohno Y. Rapid analysis of cholesterol of high density lipoprotein and low density lipoproteins in human serum by high performance liquid chromatogrphy. J Biochem (Tokyo), 1980; 87: 1863-1865 [DOI] [PubMed] [Google Scholar]
- 27).Hara I, Okazaki M. High performance liquid chromatography of serum lipoproteins. In: Methods in Enzymology (Albers J.J., Segrest J.P. eds.); 1986: Vol. 129, Academic Press, San Diego, pp. 57-78 [DOI] [PubMed] [Google Scholar]
- 28).Hirowatari Y, Yoshida H. Innovatively established analysis method for lipoprotein profiles based on high-performance anion-exchange liquid chromatography. J Atheroscler Thromb, 2019; 26: 1027-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Hirowatari Y, Yoshida H, Kurosawa H, Doumitu K-I, Tada N. Measurement of cholesterol of major serum lipoprotein classes by anion-exchange HPLC with perchlorate ion-containing eluent. J Lipid Res, 2003; 44: 1404-1412 [DOI] [PubMed] [Google Scholar]
- 30).Manita D, Hirowatari Y, Yoshida H. A rapid anion-exchange chromatography for measurement of cholesterol concentrations in five lipoprotein classes and estimation of lipoprotein profiles in male volunteers without overt diseases. Ann Clin Biochem, 2015; 52(Pt 6): 638-646 [DOI] [PubMed] [Google Scholar]
- 31).Manita D, Yoshida H, Koyama I, Nakamura M, Hirowatari Y. Verification of low-density lipoprotein cholesterol levels measured by anion-exchange high performance liquid chromatography in comparison with beta quantification reference measurement procedure. J Appl Lab Med, 2020 Nov 4; jfaa144. doi: 10.1093/jalm/jfaa144. Online ahead of print [DOI] [PubMed] [Google Scholar]
- 32).Okazaki M, Hara I, Tanaka A, Kodama T, Yokoyama S. Decreased serum HDL3 cholesterol levels in cirrhosis of the liver. N Engl J Med, 1981; 304: 1608 [DOI] [PubMed] [Google Scholar]
- 33).Matsuzawa Y, Sho N, Kameda K, Kubo M, Hirobe K, Tarui S, Yamamoto A, Okazaki M, Hara I. Characterization of lipoprotein abnormalities in type III hyperlipoproteinemia associated with apoE3 deficiency (E2/2 phenotype). J Jpn Atheroscler Soc, 1984; 6: 1243-1248 [Google Scholar]
- 34).Okazaki M, Usui S, Nakamura M, Yamashita S. Evaluation of an HPLC method for LDL-cholesterol determination in patients with various lipoprotein abnormalities in comparison with beta-quantification. Clin Chim Acta, 2008; 395: 62-67 [DOI] [PubMed] [Google Scholar]
- 35).Yamashita S, Matsuzawa Y, Okazaki M, Kako H, Yasugi T, Akioka H, Hirano K, Tarui S. Small polydisperse low density lipoproteins in familial hyperalphalipoproteinemia with complete deficiency of cholesteryl ester transfer activity. Atherosclerosis, 1988; 70: 7-12 [DOI] [PubMed] [Google Scholar]
- 36).Papazyan R, Liu X, Liu J, Dong B, Plummer EM, Lewis RD 2nd, Roth JD, Young MA. FXR activation by obeticholic acid or non-steroidal agonists induces a human-like lipoprotein cholesterol change in mice with humanized chimeric liver. J Lipid Res, 2018; 59: 982-993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Ishiguro K, Kurata R, Shimada Y, Sameshima Y, Kume T. Effects of a sweetpotato protein digest on lipid metabolism in mice administered a high-fat diet. Heliyon, 2016; 2: e00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Shimba Y, Togawa H, Senoo N, Ikeda M, Miyoshi N, Morita A, Miura S. Skeletal muscle-specific PGC1α overexpression suppresses atherosclerosis in apolipoprotein E-knockout mice. Sci Rep, 2019; 9: 4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Amano A, Kondo Y, Noda Y, Ohta M, Kawanishi N, Machida S, Mitsuhashi K, Senmaru T, Fukui M, Takaoka O, Mori T, Kitawaki J, Ono M, Saibara T, Obayashi H, Ishigami A. Abnormal lipid/lipoprotein metabolism and high plasma testosterone levels in male but not female aromatase-knockout mice. Arch Biochem Biophys, 2017; 622: 47-58 [DOI] [PubMed] [Google Scholar]
- 40).Okazaki M, Yamashita S. Recent advances in analytical methods on lipoprotein subclasses: Calculation of particle numbers from lipid levels by gel permeation HPLC using “spherical particle model”. J Oleo Sci, 2016; 65: 265-282 [DOI] [PubMed] [Google Scholar]
- 41).Okada T, Ohama T, Okazaki M, Kanno K, Matsuda H, Sairyo M, Zhu Y, Saga A, Kobayashi T, Masuda D, Koseki M, Nishida M, Sakata Y, Yamashita S. Particle number analysis of lipoprotein subclasses by gel permeation HPLC in patients with cholesteryl ester transfer protein deficiency. PLoS One, 2018; 13: e0190875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Silbernagel G, Pagel P, Pfahlert V, Genser B, Scharnagl H, Kleber ME, Delgado G, Ohrui H, Ritsch A, Grammer TB, Koenig W, März W. High-density lipoprotein subclasses, coronary artery disease, and cardiovascular mortality. Clin Chem, 2017; 63: 1886-1896 [DOI] [PubMed] [Google Scholar]
- 43).Ide K, Koshizaka M, Tokuyama H, Tokuyama T, Ishikawa T, Maezawa Y, Takemoto M, Yokote K. N-3 polyunsaturated fatty acids improve lipoprotein particle size and concentration in Japanese patients with type 2 diabetes and hypertriglyceridemia: A pilot study. Lipids Health Dis, 2018; 17: 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Masuda D, Kiyosue A, Hirayama A, Shimauchi J, López JAG, Miyawaki K, Yamashita S. Evolocumab effects on lipoproteins, measured by high-performance liquid chromatography. J Atheroscler Thromb, 2020; 27: 1183-1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Kinoshita C, Nagano T, Seki N, Tomita Y, Sugita T, Aida Y, Itagaki M, Satoh K, Sutoh S, Abe H, Tsubota A, Aizawa Y. Hepatitis C virus G1b infection decreases the number of small low-density lipoprotein particles. World J Gastroenterol, 2016; 22: 6716-6725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Kanda E, Ai M, Okazaki M, Yoshida M, Maeda Y. Association of high-density lipoprotein subclasses with chronic kidney disease progression, atherosclerosis, and Klotho. PLoS One, 2016; 11: e0166459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Kamijo Y, Ishii H, Yamamoto T, Kobayashi K, Asano H, Miake S, Kanda E, Urata H, Yoshida M. Potential impact on lipoprotein subfractions in type 2 diabetes. Clin Med Insights Endocrinol Diabetes, 2019; 12: 1179551419866811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Masuda D, Miyata Y, Matsui S, Yamashita S. Omega-3 fatty acid ethyl esters improve low-density lipoprotein subclasses without increasing low-density lipoprotein-cholesterol levels: A phase 4, randomized study. Atherosclerosis, 2020; 292: 163-170 [DOI] [PubMed] [Google Scholar]
- 49).Araki R, Ushino R, Fujie K, Ueyama Y, Suzuki H, Nakata Y, Hashimoto K. Effect of partially-abraded brown rice consumption on body weight and the indicators of glucose and lipid metaboism in pre-diabetoc adults: A randomized controlled troal. Clin Nutr ESPEN, 2017; 19: 9-15 [Google Scholar]
- 50).Usui N, Iwata K, Miyachi T, Takagai S, Wakusawa K, Nara T, Tsuchiya KJ, Matsumoto K, Kurita D, Kameno Y, Wakuda T, Takebayashi K, Iwata Y, Fujioka T , Hirai T, Toyoshima M, Ohnishi T, Toyota T, Maekawa M, Yoshikawa T, Maekawa M, Nakamura K, Tsujii M, Sugiyama T, Mori N, Matsuzaki H. VLDL-specific increases of fatty acids in autism spectrum disorder correlate with social interaction. EBioMedicine, 2020; 58: 102917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Beaufrère H, Gardhouse S, Ammersbach M. Lipoprotein characterization in Quaker parrots (Myiopsitta monachus) using gel-permeation high-performance liquid chromatography. Vet Clin Pathol, 2020; 49: 417-427 [DOI] [PubMed] [Google Scholar]
- 52).Tani S, Yagi T, Matsuo R, Kawauchi K, Atsumi W, Matsumoto N, Okumura Y. Administration of eicosapentaenoic acid may alter lipoprotein particle heterogeneity in statin-treated patients with stable coronary artery disease: A pilot 6-month randomized study. J Cardiol, 2020; 76: 487-498 [DOI] [PubMed] [Google Scholar]
- 53).Iwaki A, Moriwaki K, Sobajima T, Taniguchi M, Yoshimura S-I, Kunii M, Kanda S, Kamada Y, Miyoshi E, Harada A. Loss of Rab6a in the small intestine causes lipid accumulation and epithelial cell death from lactation. FASEB J, 2020; 34: 9450-9465 [DOI] [PubMed] [Google Scholar]
- 54).Ishibashi S, Yamashita S, Arai H, Araki E, Yokote K, Suganami H, Fruchart JC, Kodama T. Effects of K-877, a novel selective PPARα modulator (SPPARMα), in dyslipidaemic patients: A randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis, 2016; 249: 36-43 [DOI] [PubMed] [Google Scholar]
- 55).Yamashita S, Masuda D, Matsuzawa Y. Pemafibrate, a new selective PPARα modulator: drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr Atheroscler Rep, 2020; 22: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Yamashita S, Arai H, Yokote K, Araki E, Matsushita M, Nojima T, Suganami H, Ishibashi S. Efficacy and safety of pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα): pooled analysis of phase 2 and 3 studies in dyslipidemic patients with or without statin combination. Int J Mol Sci, 2019; 20: 5537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Araki E, Yamashita S, Arai H, Yokote K, Satoh J, Inoguchi T, Nakamura J, Maegawa H, Yoshioka N, Tanizawa Y, Watada H, Suganami H, Ishibashi S. Efficacy and safety of pemafibrate in people with type 2 diabetes and elevated triglyceride levels: 52-week data from the PROVIDE study. Diabetes Obes Metab, 2019; 21: 1737-1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Yamashita S, Masuda D, Matsuzawa Y. Clinical applications of a novel selective PPARα modulator, pemafibrate, in dyslipidemia and metabolic diseases. J Atheroscler Thromb, 2019; 26: 389-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Yokote K, Yamashita S, Arai H, Araki E, Suganami H, Ishibashi S. Long-term efficacy and safety of pemafibrate, a novel selective peroxisome proliferator-activated receptor-α modulator (SPPARMα), in dyslipidemic patients with renal impairment. Int J Mol Sci, 2019; 20: 706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Yamashita S, Arai H, Yokote K, Araki E, Suganami H, Ishibashi S; K-877 Study Group. Effects of pemafibrate (K-877) on cholesterol efflux capacity and postprandial hyperlipidemia in patients with atherogenic dyslipidemia. J Clin Lipidol, 2018; 12: 1267-1279.e4 [DOI] [PubMed] [Google Scholar]
- 61).Matsuba I, Matsuba R, Ishibashi S, Yamashita S, Arai H, Yokote K, Suganami H, Araki E. Effects of a novel selective peroxisome proliferator-activated receptor-α modulator, pemafibrate, on hepatic and peripheral glucose uptake in patients with hypertriglyceridemia and insulin resistance. J Diabetes Investig, 2018; 9: 1323-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Arai H, Yamashita S, Yokote K, Araki E, Suganami H, Ishibashi S; K-877 Study Group. Efficacy and safety of pemafibrate versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: a multicenter, placebo-controlled, double-blind, randomized trial. J Atheroscler Thromb, 2018; 25: 521-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Araki E, Yamashita S, Arai H, Yokote K, Satoh J, Inoguchi T, Nakamura J, Maegawa H, Yoshioka N, Tanizawa Y, Watada H, Suganami H, Ishibashi S. Effects of pemafibrate, a novel selective PPARα modulator, on lipid and glucose metabolism in patients with type 2 diabetes and hypertriglyceridemia: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care, 2018; 41: 538-546 [DOI] [PubMed] [Google Scholar]
- 64).Arai H, Yamashita S, Yokote K, Araki E, Suganami H, Ishibashi S; K-877 Study Group. Efficacy and safety of K-877, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), in combination with statin treatment: Two randomised, double-blind, placebo-controlled clinical trials in patients with dyslipidaemia. Atherosclerosis, 2017; 261: 144-152 [DOI] [PubMed] [Google Scholar]
- 65).Ishibashi S, Arai H, Yokote K, Araki E, Suganami H, Yamashita S; K-877 Study Group. Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor α modulator, in patients with dyslipidemia: Results from a 24-week, randomized, double blind, active-controlled, phase 3 trial. J Clin Lipidol, 2018; 12: 173-184 [DOI] [PubMed] [Google Scholar]
- 66).Usui S, Hara Y, Hosaki S, Okazaki M. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J Lipid Res, 2002; 43: 805-814 [PubMed] [Google Scholar]
- 67).Okazaki M, Usui S, Ishigami M, Sakai N, Nakamura T, Matsuzawa Y, Yamashita S. Identification of unique lipoprotein subclasses for visceral obesity by component analysis of cholesterol profile in high-performance liquid chromatography. Arterioscler Thromb Vasc Biol, 2005; 25: 578-584 [DOI] [PubMed] [Google Scholar]
- 68).Okazaki M. United States Patent, Patent No. US 8,268,626, US 10,048,283, Japan Patent, Patent No.JP 3899041, JP4729580, JP 4786765, JP6442482, Canada Patent, Patent No.CA2588693, EU Patent, Patent No.EP 1825251, 3128328, China Patent, Patent No.CN ZL200580047081.X [Google Scholar]
- 69).Usui S, Nakamura M, Jitsukata K, Nara M, Hosaki S, Okazaki M. Assessment of between-instrument variations in a HPLC method for serum lipoproteins and its traceability to reference methods for total cholesterol and HDL-cholesterol. Clin Chem, 2000; 46: 63-72 [PubMed] [Google Scholar]
- 70).Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med, 2006; 26: 847-870 [DOI] [PubMed] [Google Scholar]
- 71).Williams PT, Zhao XQ, Marcovina SM, Otvos JD, Brown BG, Krauss RM. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis, 2014; 233: 713-720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Huang R, Silva RAGD, Jerome WG, Kontush A, Chapman MJ, Curtiss LK, Hodges TJ, Davidson WS. Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat Struct Mol Biol, 2011; 18: 416-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Colvin PL, Moriguchi E, Barrett PH, Parks JS, Rudel LL. Small HDL particles containing two apoA-I molecules are precursors in vivo to medium and large HDL particles containing three and four apoA-I molecules in nonhuman primates. J Lipid Res, 1999; 40: 1782-1792 [PubMed] [Google Scholar]
- 74).Segrest JP, Cheung MC, Jones MK. Volumetric determination of apolipoprotein stoichiometry of circulating HDL subspecies. J Lipid Res, 2013; 54: 2733-2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Furusyo N, Ai M, Okazaki M, Ikezaki H, Ihara T, Hayashi T, Hiramine S, Ura K, Kohzuma T, Schaefer EJ, Hayashi J. Serum cholesterol and triglyceride reference ranges of twenty lipoprotein subclasses for healthy Japanese men and women. Atherosclerosis, 2013; 231: 238-245 [DOI] [PubMed] [Google Scholar]
- 76).Cone JT, Segrest JP, Chung BH, Ragland JB, Sabesin SM, Glasscock A. Computerized rapid high resolution quantitative analysis of plasma lipoproteins based upon single vertical spin centrifugation. J Lipid Res, 1982; 23: 923-935 [PubMed] [Google Scholar]
- 77).Okazaki M, Hagiwara N, Hara I. Heterogeneity of human serum high density lipoproteins on high performance liquid chromatography. J Biochem, 1982; 92: 517-524 [DOI] [PubMed] [Google Scholar]
- 78).Yamashita S, Hui DY, Wetterau JR, Sprecher DL, Harmony JA, Sakai N, Matsuzawa Y, Tarui S. Characterization of plasma lipoproteins in patients heterozygous for human plasma cholesteryl ester transfer protein (CETP) deficiency: plasma CETP regulates high-density lipoprotein concentration and composition. Metabolism, 1991; 40: 756-763 [DOI] [PubMed] [Google Scholar]