Abstract
AV7909 is a next-generation anthrax vaccine under development for post-exposure prophylaxis (PEP) following suspected or confirmed Bacillus anthracis exposure, when administered in conjunction with the recommended antibacterial regimen. AV7909 consists of the FDA approved BioThrax® vaccine (Anthrax Vaccine Adsorbed; AVA) and an immunostimulatory Toll-like receptor 9 (TLR9) agonist oligodeoxynucleotide (ODN) adjuvant, CPG 7909. The purpose of this study was to evaluate the potential systemic and local toxicity of AV7909 when administered via repeat intramuscular (IM) injection to the right thigh muscle (biceps femoris) to male and female Sprague Dawley rats. The vaccine was administered on Days 1, 15 and 29 and the animals were assessed for treatment-related effects followed by a two-week recovery period to evaluate the persistence or reversibility of any toxic effects. The AV7909 vaccine produced no apparent systemic toxicity based on evaluation of clinical observations, body weights, body temperature, clinical pathology, and anatomic pathology. Necrosis and inflammation were observed at the injection sites as well as in regional lymph nodes and adjacent tissues and were consistent with immune stimulation. Antibodies against B. anthracis protective antigen (PA) were detected in rats treated with the AV7909 vaccine, confirming relevance of this animal model for the assessment of systemic toxicity of AV7909. In contrast, sera of rats that received saline or soluble CPG 7909 alone were negative for anti-PA antibodies. Overall, three IM immunizations of Sprague Dawley rats with AV7909 were well tolerated, did not induce mortality or any systemic adverse effects, and did not result in any delayed toxicity.
Keywords: anthrax, vaccine, animal, toxicity, rats, CPG 7909, AV7909
1. INTRODUCTION
Anthrax is a zoonotic infectious disease, caused by Bacillus anthracis, which is highly lethal and poses a major biological threat. The novel AV7909 vaccine, currently under development for post-exposure prophylaxis of anthrax, consists of the FDA-approved BioThrax® (Anthrax Vaccine Adsorbed; AVA) Alhydrogel®-adjuvanted drug substance and an immunostimulatory Toll-like receptor 9 (TLR9) agonist, CPG 7909, an oligodeoxynucleotide (ODN).
Preclinical studies have been conducted with CPG 7909-adjuvanted vaccine candidates against diseases caused by Plasmodium, Pseudomonas, and Mycobacterium species1,2,3. These studies showed induction of an innate cellular and humoral immune response, consistent with TLR9 activation, characterized by the production of pro-inflammatory cytokines which in turn may enhance the vaccine efficacy4. Similarly, CPG 7909 enhanced immunogenicity and efficacy of anthrax vaccines in mice, guinea pigs, and nonhuman primates (NHP)5,6,7,8. In clinical studies, the AV7909 candidate vaccine produced an increased specific anti-protective antigen (PA) immune response, compared to the approved BioThrax6,9,10. Importantly, safety and tolerability of AV7909 in clinical studies were not different from AVA10,11,12.
Preclinical vaccine evaluation in relevant animal models includes all aspects of testing, product characterization, and proof of concept/immunogenicity studies. In particular, safety testing in animals is an essential prerequisite to moving a candidate vaccine to the clinic13. Repeat-dose toxicity studies in animals can help establish the maximum tolerated dose in an animal model and aid in selecting dose levels in the subsequent clinical studies. If no major safety signals are revealed, a single repeat-dose toxicity study in one relevant species may be sufficient14.
The purpose of this preclinical toxicology study was to evaluate potential local and systemic toxicity of a novel anthrax vaccine AV7909 in a rat model following three intramuscular (IM) administrations on Days 1, 15 and 29 followed by a two-week recovery period.
2. MATERIALS AND METHODS
2.1. Test and Control Articles
Two liquid formulations of AV7909 lots containing different amounts of CPG 7909, were manufactured at Emergent BioSolutions. AV7909 Lot 1 contained 0.5 mL AVA and 0.5 mg CPG 7909 per dose. AV7909 Lot 2 contained 0.5 mL AVA and 0.25 mg CPG 7909 per dose. Both test articles were manufactured by Emergent BioSolutions (Lansing, MI). The control article 0.9% sodium chloride was obtained from Hospira, Inc. (Rocky Mount, NC). The CPG 7909 adjuvant control was purchased from Girindus America (Cincinnati, OH) as dry powder and was reconstituted on each day of dosing to provide 0.5 mg in 0.5 mL of saline.
2.2. Experimental animals
A total of 160 (80 male and 80 female) Sprague Dawley rats (Crl: CD®), 11 weeks of age, were obtained from Charles River Laboratories (Portage, MI). Male (weight range 254.4 to 340.1 g) and female (weight range 178.5 to 228.4 g) rats were randomized into four groups. All rats had ad libitum access to certified feed (Certified Global Harlan Teklad Laboratory Diet 2018C) and water, except when fasted prior to the scheduled blood collection and necropsy. There were no contaminants present in the feed, water or bedding at levels that could have an impact on the study.
The study was conducted in accordance with the provisions of the USDA Animal Welfare Act, the PHS Policy on Humane Care and Use of Laboratory Animals, and the US Interagency Research Animal Committee Principles for the Utilization and Care of Research Animals. The protocols were approved by Institutional Animal Care and Use Committee (IACUC). Minimum number of animals were used for the study to achieve biological significance based on the study design in accordance with industry standard15.
The rat model was selected as previous studies in rats have shown strong immune response to PA16 and CPG 7909 ODN17. This model is also widely used in toxicology studies.
2.3. Experimental design
Animals were randomly assigned to four experimental groups as shown in Table 1. The test and control articles were administered via IM injection on Days 1, 15, and 29 (N + 1, where N = the intended number of immunizations in humans). The animals from the main groups were sacrificed on Day 31 (two days after the last immunization), while the animals from the recovery groups were sacrificed on Day 43 (14 days after the last immunization). The route of administration (IM) is the same as that intended in humans.
Table 1:
Treatment group assignment
| Group | Treatment | Immunization Schedule (Days) | Number of Animals per Group | |||
|---|---|---|---|---|---|---|
| Main (Day 31) | Recovery (Day 43) | |||||
| Males | Females | Males | Females | |||
| 1 | 0.9% Saline (control) | 0, 15, 29 | 10 | 10 | 10 | 10 |
| 2 | 0.5 mg CPG 7909 | 0, 15, 29 | 10 | 10 | 10 | 10 |
| 3 | AV7909 Lot 1 | 0, 15, 29 | 10 | 10 | 10 | 10 |
| 4 | AV7909 Lot 2 | 0, 15, 29 | 10 | 10 | 10 | 10 |
2.4. Dose Administration and Injection Site Observations
A dose of 0.5 mL constituting the full human dose was administered on the right thigh (biceps femoris) of each animal on each scheduled vaccination day. The hair around the injection site was trimmed before administration as needed to perform visual observations. The vaccine dose was not adjusted for body weight and all animals received the full human dose to evaluate any potential local toxicity of the vaccine candidate containing the CPG 7909 adjuvant13. The dose volume justification was reviewed and approved by IACUC. Injection sites were observed for erythema/eschar and/or edema and graded according to the modified method of Draize18. Dermal Draize observations were performed at the injection site prior to dosing, 4 ± 0.5 hours post dose, and 24 ± 2 hours post dose using the Draize scoring scale as described in Table 2.
Table 2.
Dermal Draize Observations
| Score | Grade | Edema | Erythema |
|---|---|---|---|
| 0 | None | No swelling | Normal color |
| 1 | Minimal | Slight swelling; indistinct border | Light pink; indistinct |
| 2 | Mild | Defined swelling; distinct border | Bright pink/pale red; distinct |
| 3 | Moderate | Defined swelling; raised border (≤ 1 mm) | Bright red; distinct |
| 4 | Severe | Pronounced swelling; raised border (>1 mm) | Dark red; pronounced |
2.5. Clinical Observations, Body Temperature and Body Weights
Cage-side observations were performed at least twice a day and included observation for mortality, moribundity, general health and signs of toxicity. Full clinical observations were performed weekly, one day prior to termination and on the day of termination. These observations included evaluation of skin and fur characteristics, the injection site, eye and mucous membranes, respiratory, circulatory, autonomic and central nervous systems, somatomotor and behavior patterns. The time of onset, location, dimensions, appearance, and progression of any grossly visible or palpable tumors were also observed. Ophthalmologic observations were conducted using indirect ophthalmoscopy and slit lamp biomicroscopy (as needed) following administration of 1% Tropicamide® mydriatic solution. Individual body weights were recorded for all animals, on a weekly basis and were also performed one day prior and on the day of termination. Body temperature was recorded for all animals prior to dosing, 24 ± 2 hours post dose and 48 ± 2 hours post dose.
2.6. Clinical Pathology
Clinical pathology tests including clinical chemistry, hematology, blood coagulation, and urinalysis were performed as essential and standard parts of preclinical safety assessment of human pharmaceuticals. Prior to sample collection animals were fasted overnight (with water available). The animals were anesthetized via CO2/O2 inhalation and blood was collected via puncture of the abdominal aorta followed by humane euthanasia. The core tests included chemistry, hematology, blood coagulation standard parameters described in literature and relevant guidance documents19,20.
2.7. Necropsy
The main study animals were sacrificed on Day 31 and recovery animals were sacrificed on Day 43. On each sacrifice day 10 animals/sex were euthanized via carbon dioxide inhalation followed by exsanguination. Animals were necropsied immediately after death. Necropsy included examination of the external surface of the body and all orifices; the cranial, injection sites, thoracic, abdominal and pelvic cavities, and their contents; and the collection of tissues. The organs were weighed as soon as possible after dissection at scheduled necropsies. Paired organs were weighed together, with the exception of iliac lymph nodes as noted below. Adrenal glands, brain, heart, kidneys, iliac lymph nodes (left and right separately), liver, lung (with bronchi), ovaries (with oviducts), spleen, testes (with epididymides), thymus, and uterus (with cervix) were all weighed. Tissues were preserved in 10% neutral buffered formalin (NBF) except eyes and testes that were first preserved in modified Davidson’s fixative and then transferred to NBF. Two bone marrow smears were prepared from the left femur of all animals. Slides were air dried, fixed in methanol, and stored at room temperature until disposal at the end of the study.
2.8. Histopathology
All protocol-required tissues were embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined by a board-certified pathologist. For evaluation of local and systemic toxicity, samples were collected from the standard list of tissues, as recommended by regulatory guidance documents14,21.
2.9. Immunogenicity Assessment
Blood samples were collected into serum separator tubes prior to necropsy on Days 31 (main) and 43 (recovery) via puncture of the abdominal aorta. Blood was allowed to clot at room temperature for at least 30 minutes and serum isolated by centrifugation. The serum samples were tested by enzyme-linked immunosorbent assay (ELISA) for antibodies against B. anthracis PA to confirm the animals’ exposure to the vaccine. Anti-PA antibody levels in rat sera were determined using an assay based on the original anti-PA immunoglobulin subtype G (IgG) ELISA method developed at the CDC22, using purified recombinant PA (rPA) as the solid-phase immobilized antigen and an enzyme-conjugated anti-gamma chain secondary antibody as the reporter system. The quantitative antibody levels were calculated using linear regression against a standard curve, and the mean anti-PA IgG concentration was reported in μg/mL.
Anthrax toxin-neutralizing antibody levels in rat serum samples were measured using anthrax lethal toxin (LT) neutralization assay (TNA) as described previously23. The assay is designed to measure and quantify the functional ability of serum to neutralize B. anthracis LT activity using a cell-based cytotoxicity method. Serum-mediated neutralization of anthrax LT manifests as a suppression of cytotoxicity and hence preservation of cell viability.
2.10. Statistical Analysis
All appropriate quantitative in-life data were analyzed for test article effects by a parametric or nonparametric analysis of variance (ANOVA) using SigmaStat™ Statistical Software version 1.0. For all data, normality was determined by the Kolmogorov-Smirnov test, homogeneity of variances was determined by Levene’s test and one-way ANOVA test. Nonparametric Kruskal-Wallis ANOVA was used to transform data rank-wise if the normality or equal variance tests failed. For parametric data, if the ANOVA indicated statistical significance among experimental groups then the Dunnett’s t-test was used to delineate which groups (if any) differed from the control. For non-parametric data, if the ANOVA indicated statistical significance among experimental groups then the Dunn’s test was used to delineate which groups (if any) differed from the control. All statistical tests were performed at the 0.05 level of significance (p < 0.05), after accounting for multiple comparisons.
3. RESULTS
3.1. Clinical Observations
No treatment-related adverse effects or mortality were noted during cage-side or clinical observations following administration of AV7909. However, some low-occurrence observations not related to the treatment and common among laboratory rats were noted. These observations included three animals with alopecia, one animal with abrasion away from the injection site, one animal with mucoid feces, and one animal with hunched posture on Day 1 and with rough hair coat from Days 8 to 43.
Based on the results of the Draize scoring, none of the main nor recovery group animals exhibited any signs of edema or erythema throughout the course of the study (data not shown).
The normal body temperature for rats is approximately 38–39°C and individual animals did not exceed the upper limit of this range, except female animals in Groups 1 (39.50°C) and 2 (39.62°C) at 48 hours following the third immunization, which exhibited temperature above 39°C and was considered incidental and not treatment-related.
AV7909-treatment did not cause any ocular lesions in rats. However, one male rat in Group 4 showed hemorrhage in the fundus of the left eye on Day 31. The spontaneous lesions in the retina, including retinal hemorrhage, can be observed during normal ocular development and in naïve adult rats24,25. Therefore, due to the low incidence and based on review of the concurrent control data, the finding was considered incidental and not related to the test article administration.
3.2. Food Consumption and Body Weight
Overall, there were no significant differences in the mean total food consumption in the treated groups (Groups 2–4) relative to the control group (Group 1) within the main and recovery animals. All animals that were vaccinated with AV7909 test article showed gradual increase in weight over the course of the study, indicating normal gain in body weight and that the vaccine did not have any effect on food consumption. There were no significant differences in the mean total absolute body weight change for the Days 1–29 or Days 1–42 intervals Figure 1.
Figure 1. Body Weight Change.
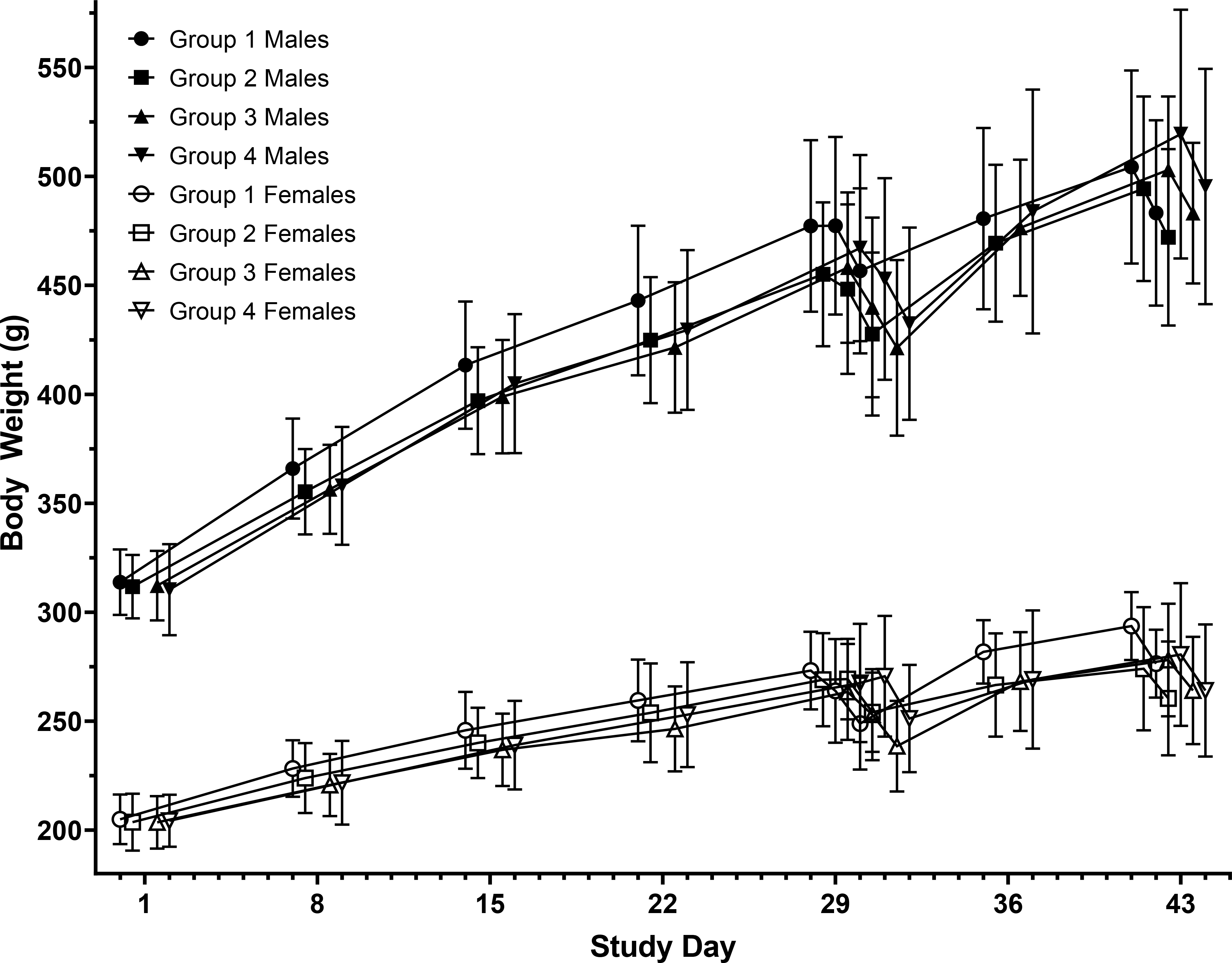
Body weight change is presented as mean and standard deviation. A steady increase in body weight over the course of the study in both male and female rats that were administered with the AV7909 test article was noted except on Days 29 and 43 where there was a slight decrease in body weights as the rats were fasting.
Group 1 – 0 mg (control article) Group 2 – 0.5 mg CPG 7909
Group 3 – 0.5 mL AV7909 Lot 1 (0.5 mL AVA and 0.5 mg CPG 7909)
Group 4 – 0.5 mL AV7909 Lot 2 (0.5 mL AVA and 0.25 mg CPG 7909)
3.3. Clinical Pathology
3.3.1. Hematology
Animals in Groups 3 and 4 that were treated with AV7909 test articles experienced a decrease in hemoglobin (HGB) and hematocrit (HCT) levels on Day 31 as seen in Table 3 below. The test article effects on mean HGB and HCT values were minimal in extent. All mean values were within the normal range for Sprague Dawley rats and were considered not adverse. The absolute neutrophil (ABNEUT) and absolute monocyte (ABMONO) counts in Groups 3 and 4 increased on Day 31. Although mild in extent, these effects exceeded the historical reference range in rats. The effects were attributed to vaccination-related microscopic inflammation and necrosis at the injection sites and considered not adverse. In animals exposed to CPG 7909 (Groups 2, 3 and 4), the absolute basophil (ABBAS) levels increased which was attributed to the immunostimulatory effect of the adjuvant.
Table 3:
Hematology results
| Group | Treatment | Gender | HGB (g/dL) | HCT (%) | ABNEUT (K/μL) | ABBAS (K/μL) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 31 | Day 43 | Day 31 | Day 43 | Day 31 | Day 43 | Day 31 | Day 43 | |||
| 1 | 0.9% Saline (control) | M | 16.06 | 15.85 | 48.29 | 47.09 | 2.127 | 2.156 | 0.070 | 0.031 |
| 2 | 0.5 mg CPG 7909 | M | 16.00 | 16.68 | 49.05 | 49.54 | 1.527 | 2.221 | 0.199* | 0.039 |
| 3 | AV7909 Lot 1 | M | 15.13* | 16.13 | 46.23 | 47.48 | 4.307* | 2.539 | 0.160* | 0.042 |
| 4 | AV7909 Lot 2 | M | 15.09* | 15.61 | 46.36 | 46.67 | 4.573* | 2.550 | 0.184* | 0.041 |
| 1 | 0.9% Saline (control) | F | 15.61 | 16.04 | 46.19 | 46.28 | 1.313 | 1.660 | 0.059 | 0.027 |
| 2 | 0.5 mg CPG 7909 | F | 15.49 | 15.63 | 46.09 | 45.19 | 1.100 | 1.379 | 0.153* | 0.030 |
| 3 | AV7909 Lot 1 | F | 14.31* | 15.83 | 42.63* | 46.31 | 4.069* | 1.912 | 0.156* | 0.038 |
| 4 | AV7909 Lot 2 | F | 14.37* | 15.25 | 43.09* | 44.29 | 3.667* | 1.918 | 0.130* | 0.034 |
M—male; F—female; HCT – Hematocrit; HGB – Hemoglobin; ABNEUT – Absolute neutrophil number; ABBAS - Absolute basophil number
— Significantly different from the control value, p < 0.05
This adjuvant effect was deemed secondary to immune stimulation and considered not adverse. There were no test article or adjuvant-related effects on hematology parameters on Day 43 as summarized in Table 3.
3.3.2. Clinical Chemistry
Clinical chemistry data (Table 4) were consistent with immune response associated with AV7909 vaccine and were not adverse. A slight increase (~13%) in serum globulin (GLOB) values was noted in the AV7909-treated groups (Groups 3 and 4) compared to Group 1 on Days 31 and 43. The serum albumin (ALB) levels decreased in animals exposed to CPG 7909 in Groups 2, 3 and 4 that also showed a decrease in the albumin/globulin (A/G) ratios on Day 31. No changes in C-reactive protein levels were found on Days 31 or 43, except in Group 3 female rats that received AV7909 with the highest CPG 7909 dose. These changes were considered non-adverse and consistent with immunostimulatory effect of CPG 7909 with no gender-based differences in responses.
Table 4:
Key clinical chemistry results
| Group | Treatment | Gender | GLOB | A/G ratio | ALB | CRP (mg/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 31 | Day 43 | Day 31 | Day 43 | Day 31 | Day 43 | Day 31 | Day 43 | |||
| 1 | 0.9% Saline (control) | M | 2.83 | 2.76 | 1.22 | 1.18 | 3.46 | 3.28 | 1.22 | 1.47 |
| 2 | 0.5 mg CPG 7909 | M | 2.87 | 2.83 | 1.15 | 1.20 | 3.27 | 3.38 | 1.24 | 1.26 |
| 3 | AV7909 Lot 1 | M | 3.20 | 3.15* | 0.93 | 1.09 | 2.95 | 3.42 | 1.52 | 1.16 |
| 4 | AV7909 Lot 2 | M | 3.20 | 3.17* | 0.93 | 1.07 | 3.00 | 3.37 | 1.69 | 0.92* |
| 1 | 0.9% Saline (control) | F | 2.84 | 2.94 | 1.37 | 1.32 | 3.83 | 3.86 | 1.56 | 1.17 |
| 2 | 0.5 mg CPG 7909 | F | 2.93 | 2.86 | 1.24 | 1.35 | 3.62 | 3.80 | 1.71 | 1.04 |
| 3 | AV7909 Lot 1 | F | 3.36 | 3.18* | 0.94 | 1.21 | 3.20 | 3.79 | 2.04* | 1.35 |
| 4 | AV7909 Lot 2 | F | 3.36 | 3.19* | 0.98 | 1.20 | 3.30 | 3.80 | 1.58 | 0.98 |
M—male; F—female; GLOB – Globulin; ALB – Albumin
— Significantly different from the control value, p < 0.05
3.3.3. Coagulation
The prothrombin time (PT) values increased in animals exposed to AV7909 (Groups 3 and 4) on Days 31 and 43 (Table 5). A minimal (less than 10%) increase in PT values was observed and considered not adverse in the absence of clinical or microscopic evidence of hemorrhage. Treatment with CPG 7909 also led to a slight increase in the plasma fibrinogen (FIB) values in Groups 2, 3 and 4. This finding was consistent with the immune stimulation associated with administration of adjuvant or vaccine and thus considered non-adverse.
Table 5:
Coagulation results
| Group | Treatment | Gender | PT (sec) | FIB (mg/dL) | ||
|---|---|---|---|---|---|---|
| Day 31 | Day 43 | Day 31 | Day 43 | |||
| 1 | 0.9% Saline (control) | M | 15.77 | 15.55 | 377.1 | 367.0 |
| 2 | 0.5 mg CPG 7909 | M | 16.55* | 15.91 | 528.4* | 366.3 |
| 3 | AV7909 Lot 1 | M | 16.97* | 16.05 | 858.7* | 356.3 |
| 4 | AV7909 Lot 2 | M | 17.22* | 16.45* | 798.9* | 409.2 |
| 1 | 0.9% Saline (control) | F | 15.30 | 15.30 | 284.7 | 279.9 |
| 2 | 0.5 mg CPG 7909 | F | 15.21 | 15.57 | 378.4* | 253.0 |
| 3 | AV7909 Lot 1 | F | 16.44* | 15.77* | 749.6* | 248.1 |
| 4 | AV7909 Lot 2 | F | 16.66* | 16.10* | 605.2* | 255.7 |
M - male; F - female; PT – prothrombin time; FIB – Fibrinogen
- Significantly different from the control value, p < 0.05
3.4. Necropsy and Histopathology
3.4.1. Organ Weights
The organ weight changes are summarized in Table 6. Animals sacrificed at main necropsy had treatment-related increases in weights of the right iliac lymph node and spleen. Increased absolute and relative (to body and brain weight) right iliac lymph node weight occurred in animals in Groups 2, 3, and 4 when compared to the control group (Group 1). The increased weight correlated with clinical pathology findings of increased GLOB values in Groups 3 and 4 and microscopically with minimal to moderate lymphoid hyperplasia. Spleen weight relative to body and brain weight was increased in Group 3 males. Absolute and relative spleen weights were increased in Group 3 and 4 females. These findings are consistent with immune stimulation and are non-adverse.
Table 6:
Organ weights
| Group | Treatment | Gender | Spleen/body weight (Absolute weight (g)) | Right iliac lymph node/body weight (Absolute weight (g)) | ||
|---|---|---|---|---|---|---|
| Day 31 | Day 43 | Day 31 | Day 43 | |||
| 1 | 0.9% Saline (control) | M | 0.20185 | 0.18538 | 0.00512 | 0.00497 |
| 2 | 0.5 mg CPG 7909 | M | 0.21713 | 0.18386 | 0.02247* | 0.00919 |
| 3 | AV7909 Lot 1 | M | 0.25762* | 0.20618 | 0.04104* | 0.01423* |
| 4 | AV7909 Lot 2 | M | 0.22858 | 0.22321* | 0.01851 | 0.01922* |
| 1 | 0.9% Saline (control) | F | 0.24144 | 0.20484 | 0.00507 | 0.00407 |
| 2 | 0.5 mg CPG 7909 | F | 0.24560 | 0.21416 | 0.03892* | 0.00749 |
| 3 | AV7909 Lot 1 | F | 0.31254* | 0.22574 | 0.06455* | 0.02210* |
| 4 | AV7909 Lot 2 | F | 0.29673* | 0.24408* | 0.02507* | 0.02528* |
M—male; F—female
— Significantly different from the control value, p < 0.05
Animals sacrificed at recovery necropsy also had treatment-related weight increases for right iliac lymph nodes and spleen. Absolute and relative right iliac lymph node weights were increased in animals in Groups 3 and 4, correlating with increased GLOB values and microscopically with lymphoid hyperplasia. This finding was consistent with immune stimulation and was non-adverse. Absolute spleen weight was increased in Group 4 males, and spleen weight relative to body weight was increased in Group 4 males and females. This change correlated microscopically with increased splenic hematopoiesis in Group 4 males and was non-adverse.
3.4.2. Macroscopic Findings
In animals sacrificed at main necropsy, test article-related changes occurred at the injection site in Groups 3 and 4 (9/20 animals), and in the inguinal lymph node in one Group 4 female. Masses at the injection site were intramuscular, tan, white, or yellow, firm, semi-firm, or soft (cut surface), often multilobular, and contained caseous material. These macroscopic changes correlated with microscopic necrosis and inflammation of the muscle and surrounding fascia, occasionally extending into the subcutis. Treatment-related enlargement of the right iliac lymph node occurred in Groups 2 (7/20 animals), 3 (16/20 animals), and 4 (9/20 animals), and one Group 3 female showed enlargement of the right inguinal lymph node. These lymph node findings correlated microscopically with lymphoid hyperplasia.
In animals sacrificed at the recovery necropsy, test article-related injection site masses were observed in Groups 3 (11/20 animals) and 4 (12/20 animals), and in a single animal in the adjuvant-treated Group 2 (1/20 animals) and the saline control Group 1 (1/20 animals). The morphology of these masses was similar to those observed in the main necropsy animals. The macroscopic masses observed in Group 1 and Group 2 animals were not present microscopically, while in Group 3 and 4 the masses correlated microscopically with necrosis and inflammation of the muscle and surrounding fascia, occasionally extending into the subcutis. Two Group 3 animals had test article-related masses in abdominal skeletal muscle, which were tan, firm, and contained caseous material. These masses correlated microscopically with necrosis and inflammation of the muscle and surrounding fascia. The lesions likely were an extension of changes at the adjacent injection site. Treatment-related enlargement of the right iliac lymph node also occurred in Groups 2 (3/20 animals), 3 (7/20 animals), and 4 (10/20 animals), and correlated microscopically with minimal to mild lymphoid hyperplasia.
3.4.3. Microscopic Findings
In animals sacrificed at the main necropsy, test article-related adverse changes occurred at the injection site in Groups 3 (19 / 20 animals) and 4 (14 / 20 animals). Mild to marked necrosis occurred within muscle at the injection site for Groups 3 and 4 (primarily Group 3), accompanied by mild to moderate chronic, chronic-active, or granulomatous inflammation which often extended to the surrounding fascia and occasionally into the subcutis. Necrosis and/or inflammation were occasionally observed in sections of skeletal muscle taken from areas adjacent to the injection site. These inflammatory changes correlated with alterations in peripheral leukocyte counts. Minimal to mild subacute inflammation at the injection site occurred in Groups 2, 3, and 4 (primarily Groups 2 and 4) without injection site necrosis. These inflammatory changes correlated with increase in peripheral leukocyte counts and were treatment-related that were considered adverse. Treatment-related minimal to moderate lymphoid hyperplasia occurred in iliac lymph nodes (primarily in the right node, ipsilateral to the injection site) in Groups 2, 3, and 4, and in the inguinal lymph node in one Group 3 animal. This change was consistent with immune stimulation, correlated with increased serum globulin values for Groups 3 and 4, and was non-adverse. Test article-related changes were also observed in the lymph nodes. Scattered accumulations of macrophages occurred in the iliac lymph nodes of animals in animals in Groups 3 and 4. These findings were non-adverse. One Group 4 animal had test article-related necrosis and chronic inflammation in an inguinal lymph node, a change that was adverse. Four Group 4 females had test article-related mild increases in macrophage numbers in the spleen, possibly correlating with an increased spleen weight. This finding was associated with a normal clearance mechanism and was non-adverse.
In animals sacrificed at the recovery necropsy, test article-related adverse changes occurred at the injection site of animals in Groups 3 (12 / 20 animals) and 4 (14 / 20 animals). These changes correlated with masses observed macroscopically and consisted of mild to marked necrosis accompanied by minimal to moderate chronic, or mild to moderate granulomatous, inflammation. Inflammation was occasionally observed in routine sections of skeletal muscle taken from areas adjacent to the injection site. In two Group 3 animals, areas of necrosis and inflammation in the abdominal skeletal muscle were similar microscopically to changes at the injection site and correlated with macroscopic masses. These lesions likely were an extension of changes at the adjacent injection site. Treatment-related minimal to mild chronic inflammation also occurred at injection sites in the absence of injection site necrosis in Groups 2, 3, and 4. This finding represented a normal progression from the subacute inflammation present on Day 31 observed at injection sites that lacked necrotic changes. These inflammatory changes were considered to be treatment-related and adverse. Treatment-related minimal to mild lymphoid hyperplasia also occurred in the right iliac lymph nodes (ipsilateral to the injection site) in Groups 2, 3, and 4. This change was consistent with immune stimulation, correlated with increased serum globulin values for Groups 3 and 4, and was considered non-adverse. Test article-related changes were also observed in the iliac lymph nodes. Scattered accumulations of foamy macrophages occurred in the iliac lymph nodes of animals in Groups 3 and 4. This finding was non-adverse. Two Group 4 females had test article-related minimal to mild increases in macrophage numbers in the spleen. This finding was related to a normal clearance mechanism, and non-adverse. Minimal to mild increased splenic hematopoiesis was observed in Group 3 and 4 males, likely a compensatory response to the decreased HGB and HCT levels noted on Day 31 and correlating with the increased spleen weight for Group 4 males. This change was test article-related but non-adverse.
3.5. Immunogenicity
Immunogenicity of the vaccine was evaluated using the anti-PA IgG ELISA and toxin neutralization assay (TNA) as described below. Both assays demonstrated a robust immune response generated in rats by AV7909 vaccination, thus confirming the specific biological effect expected from the vaccine and immunostimulatory effect of the CPG 7909 adjuvant.
3.5.1. Anti-PA IgG ELISA
Serum samples from Days 31 and 43 were analyzed using anti-PA ELISA. As expected, the serum samples from the control group and CPG 7909 adjuvant (Group 1 and 2) did not have quantifiable levels of PA-specific IgG (below the limit of quantitation (LOQ) of 0.8 μg/mL). In contrast, animals in Groups 3 and 4 generated significant antibody levels with specificity to PA, indicative of a robust immune response to AV7909. The results are shown in Figure 2.
Figure 2. Anti-PA IgG levels as measured by ELISA.
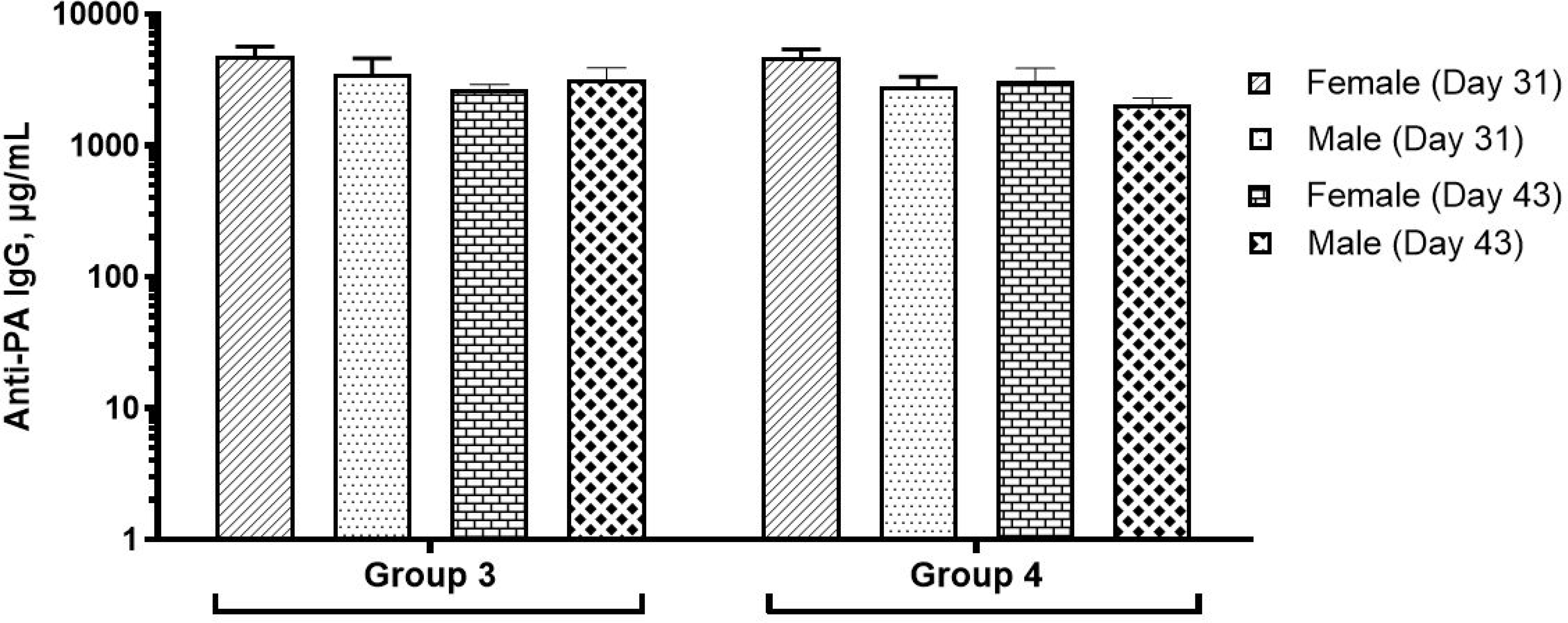
Anti-PA IgG levels in rats are reported as mean and standard error for the group of animals that were administered with the AV7909 vaccine. No detectable levels of anti-PA IgG were observed in control group animals or the adjuvant-alone group and hence not included in the figure
Group 3 – 0.5 mL AV7909 Lot 1 (0.5 mL AVA and 0.5 mg CPG 7909)
Group 4 – 0.5 mL AV7909 Lot 2 (0.5 mL AVA and 0.25 mg CPG 7909)
3.5.2. TNA
In addition to the high anti-PA antibody levels, AV7909 induced a strong functional immune response in both groups of vaccinated animals (Groups 3 and 4), as measured by the toxin-neutralizing antibody levels (Figure 3). Consistent with the ELISA data, the serum effective dilution that neutralizes 50% of the cytotoxic effect of the anthrax lethal toxin (ED50) values in the control group and the group that was administered with CPG 7909 alone were not quantifiable (data not shown).
Figure 3. Toxin-neutralizing antibody (ED50) levels as measured by TNA.
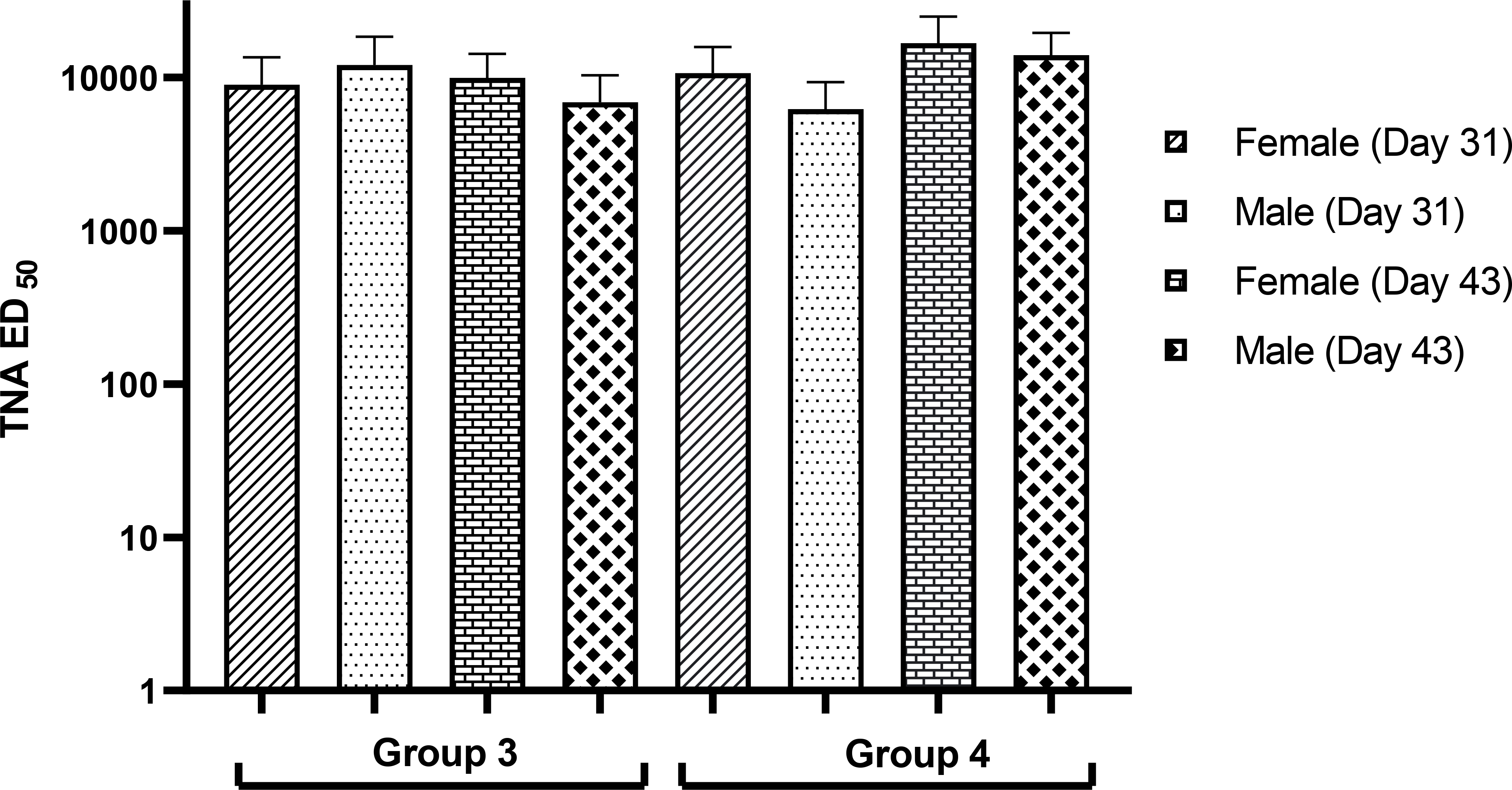
TNA ED50 (the dilution that neutralizes 50% of the cytotoxic effect of the anthrax lethal toxin) levels presented as mean and standard deviation. ED50 values were obtained from animals that were immunized with three doses of AV7909 vaccine. In the AV7909-vaccinated group, median ED50 values were in the order of 10000 that suggests strong immune response generated by the vaccine. The toxin neutralizing antibodies were not detectable (below limit of quantitation) in the control and CPG 7909 alone groups and not shown in the figure.
Group 3 – 0.5 mL AV7909 Lot 1 (0.5 mL AVA and 0.5 mg CPG 7909)
Group 4 – 0.5 mL AV7909 Lot 2 (0.5 mL AVA and 0.25 mg CPG 7909)
4. DISCUSSION
Identifying the appropriate dosage of a vaccine plays a vital role in balancing its ability to be effective within safety limits. Assessing systemic toxicity and local reactogenicity of a vaccine in animals can help predict these effects in human subjects and define the appropriate safe dose levels. A repeat-dose toxicity study in animals, in which the number of vaccinations exceed that intended in the clinic, is, therefore, an important tool for evaluating the safety of the vaccine and can help identify potential safety signals that should be closely monitored in the clinic.
In this repeat-dose toxicity study, the maximum dose levels that could be safely tolerated in adult rats was determined to be 0.5 mL of AVA with 0.5 mg of CPG 7909. The study demonstrated that the AV7909 vaccine was safe and, well tolerated in adult rats. AV7909 was able to induce a robust immune response without causing any significant effects on target organs or any systemic immediate or delayed adverse effects. Importantly, a strong antibody response, including the functional TNA response, which is, known to be predictive of vaccine efficacy10, confirmed the appropriateness of using the rat model to evaluate preclinical safety of the AV7909 vaccine.
Although local reactogenicity associated with immune stimulation was noted, these observations are consistent with the findings in other toxicology studies with AV79 0 926,27,28. Indeed, such effects are observed frequently, especially with adjuvanted vaccines. Furthermore, the 14-day recovery period, which is in line with current regulatory guidance for preclinical safety evaluation of vaccines, may not be sufficiently long to observe complete resolution of the local reactions29. Similar local reactogenicity observations were reported for other CpG ODN-adjuvanted vaccines, including those against hepatitis B30,31, influenza32, malaria33,34,35, pneumococcus36,37, cytomegalovirus, and tetanus38. Of note, in the recent repeat-dose toxicity study in juvenile rats27, AV7909 was also well tolerated with no severe adverse effects or morbidity and induced a strong neutralizing antibody response. Taken together, these data indicate a favorable safety profile for CPG 7909 when used as a part of the vaccine adjuvant system.
A comprehensive review of repeat-dose toxicity studies in rat or rabbit models with a variety of vaccines administered IM reported acute and/or chronic inflammation at the injection sites, often accompanied by lymphoid hyperplasia and/or increased weight in draining lymph nodes and the spleen, as well as necrosis of muscle fibers within the injection sites. These findings were interpreted as an expected reaction associated with vaccine- and/or adjuvant-induced immune response, and the investigational vaccines were deemed to be well tolerated39. The author concluded that a recovery period of 2 to 4 weeks was sufficient to show evidence of reversibility of injection site effects and that the changes can be interpreted as related to an expected reaction to vaccination, associated with generation of an immune response augmented by the presence of an adjuvant. Myofiber necrosis at the injection site, with or without mineralization, was also observed in a rabbit repeat-dose toxicity study of two different formulations of a malaria vaccine candidate, one of which, selected based on a more favorable immunogenicity profile, subsequently progressed to Phase 2 and 3 clinical studies40. Necrosis of muscle fibers at the injection site, following IM administration, was also reported in repeat-dose toxicity studies with other vaccines, including inactivated enterovirus vaccine candidate41 and nucleic acid-based HIV vaccine candidates42, both of which were found to be safe and well tolerated, and advanced to Phase 1 clinical studies.
Local reactions such as erythema, edema, inflammation, and pain have been described following CpG ODN administration in humans30,43,44. However, CpG ODNs were generally well tolerated, with little and transient side effects. In most cases, local inflammatory lesions resolved in less than 24 h45. The inflammatory effects observed in this study are also consistent with those commonly seen with Alhydrogel-adjuvanted vaccines46.
Overall, the results of this study demonstrated that repeated IM administration of AV7909 to Sprague Dawley rats did not induce any systemic toxicity. The study, therefore, supports the use of the AV7909 vaccine in humans when administered as two IM injections.
5. ACKNOWLEDGMENTS
The authors would like to thank the technical staff at Inotiv for the animal immunization, toxicological assessments, necropsy, and histopathology; Andrea Harris for the anti-PA ELISA testing; and Katalin Baranji for the htpTNA testing.
8. FUNDING
This project was funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Grant No. 5 U01 AI078169–02.
Footnotes
DECLARATION OF CONFLICT(S) OF INTERESTS
No conflict(s) of interests were declared by the author(s) with respect to the research, authorship, and/or publication of this manuscript.
9 REFERENCES
- 1.Mullen GE, Aebig JA, Dobrescu G, Rausch K, Lambert L, Long CA, Miles AP, Saul A. Enhanced antibody production in mice to the malaria antigen AMA1 by CPG 7909 requires physical association of CpG and antigen. Vaccine. 2007;25(29):5343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian F, Rausch KM, Muratova O, Zhou H, Song G, Diouf A, Lambert L, Narum DL, Wu Y, Saul A, Miller LH, Long CA, Mullen GE. Addition of CpG ODN to recombinant Pseudomonas aeruginosa ExoProtein A conjugates of AMA1 and Pfs25 greatly increases the number of responders. Vaccine. 2008;26(20):2521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu S, Chen H, Ma J, Chen Q, Deng H, Gong F, Huang H, Shi C. CpG7909 adjuvant enhanced immunogenicity efficacy in mice immunized with ESAT6-Ag85A fusion protein, but does not confer significant protection against Mycobacterium tuberculosis infection. J Appl Microbiol 2013;115(5):1203–11. [DOI] [PubMed] [Google Scholar]
- 4.Scheiermann J and Klinman DM Clinical evaluation of CpG Oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine. 2014; 32(48), 6377–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu M, Hine PM, James Jackson W, Giri L, Nabors GS. Increased potency of BioThrax anthrax vaccine with the addition of the C-class CpG oligonucleotide adjuvant CPG 10109. Vaccine. 2007;25(3):526–34. [DOI] [PubMed] [Google Scholar]
- 6.Savransky V, Shearer JD, Gainey MR, Sanford DC, Sivko GS, Stark GV, Li N, Ionin B, Lacy MJ, Skiadopoulos MH. Correlation between anthrax lethal toxin neutralizing antibody levels and survival in guinea pigs and nonhuman primates vaccinated with the AV7909 anthrax vaccine candidate. Vaccine. 2017; 35(37):4952–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smiley AM, Sanford DC, Triplett CA, Callahan D, Frolov V, Look J, Ruiz C, Reece JJ, Miles A, Ruiz E, Ionin B, Shearer JD, Savransky V., Comparative immunogenicity and efficacy of thermostable (lyophilized) and liquid formulation of anthrax vaccine candidate AV7909. Vaccine, 2019; 37(43):6356–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shearer JD, Henning L, Sanford DC, Li Na, Skiadopoulous MH, Reece JJ, Ionin B, Savransky V. Efficacy of the AV7909 anthrax vaccine candidate in guinea pigs and nonhuman primates following two immunizations two weeks apart. Vaccine. 2021; 39(1):1–5. [DOI] [PubMed] [Google Scholar]
- 9.Klinman DM. CPG Oligonucleotides Accelerate and Boost the Immune Response Elicited by AVA, The Licensed Anthrax Vaccine. Expert Review of Vaccines. 2006; 5(3):365–9. [DOI] [PubMed] [Google Scholar]
- 10.Rynkiewicz D, Rathkopf M, Sim I, Waytes AT, Hopkins RJ, Giri L, DeMuria D, Ransom J, Quinn J, Nabors GS, Nielsen CJ. Marked enhancement of the immune response to BioThrax® (Anthrax Vaccine Adsorbed) by the TLR9 agonist CPG 7909 in healthy volunteers. Vaccine. 2011. August 26;29(37):6313–20. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins RJ, Daczkowski NF, Kaptur PE, Muse D, Sheldon E, LaForce C, Sari S, Rudge TL, Bernton E. Randomized, double-blind, placebo-controlled, safety and immunogenicity study of 4 formulations of Anthrax Vaccine Adsorbed plus CPG 7909 (AV7909) in healthy adult volunteers. Vaccine. 2013;31(30):3051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkins RJ, Kalsi G, Montalvo-Lugo VM, Sharma M, Wu Y, Muse DD, Sheldon EA, Hampel FC, Lemiale L. Randomized, double-blind, active-controlled study evaluating the safety and immunogenicity of three vaccination schedules and two dose levels of AV7909 vaccine for anthrax post-exposure prophylaxis in healthy adults. Vaccine. 2016. April 19;34(18):2096–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Guidelines on Nonclinical Evaluation of Vaccines, Annex 1. WHO Technical Report Series, No. 927, 2005 [Google Scholar]
- 14.Guidelines on the nonclinical evaluation of vaccine adjuvants and adjuvanted vaccines, Annex 2 WHO Technical Report Series, No. 987, 2014 [Google Scholar]
- 15.Regulatory Toxicology, 2nd Edition. Editor Taylor Shayne C. Gad. and Francis Publisher, 2001 [Google Scholar]
- 16.Ivins BE, Ezzell JW Jr, Jemski J, Hedlund KW, Ristroph JD, Leppla SH. Immunization studies with attenuated strains of Bacillus anthracis. Infect Immun 1986; 52:454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullen GE, Giersing BK, Ajose-Popoola O, Davis HL, Kothe C, Zhou H, Aebig J, Dobrescu G, Saul A, Long CA. Enhancement of functional antibody responses to AMA1-C1/Alhydrogel, a Plasmodium falciparum malaria vaccine, with CpG oligodeoxynucleotide. Vaccine. 2006;24(14):2497–505. [DOI] [PubMed] [Google Scholar]
- 18.Draize JH 1959. Appraisal of the Safety of Chemicals in Foods, Drugs and Cosmetics. Editorial Committee of The Association of Food and Drug Officials of the United States, Bureau of Food and Drugs, Austin, TX [Google Scholar]
- 19.International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH), 2008 [DOI] [PubMed]
- 20.Weingand K, Brown G, Hall R, et al. , Harmonization of animal clinical pathology testing in toxicity and safety studies. The Joint Scientific Committee for International Harmonization of Clinical Pathology Testing, Journal of the Society of Toxicology. 1996;29(2):198–201 [PubMed] [Google Scholar]
- 21.Bregman CL, Adler RR, Morton DG, Regan KS, Yano BL; Society of Toxicologic Pathology. Recommended tissue list for histopathologic examination in repeat-dose toxicity and carcinogenicity studies: a proposal of the Society of Toxicologic Pathology (STP). Toxicol Pathol 2003;31(2):252–3. [DOI] [PubMed] [Google Scholar]
- 22.Quinn CP, Semenova VA, Elie CM, Romero-Steiner S, Greene C, Li H, Stamey K, Steward-Clark E, Schmidt DS, Mothershed E, Pruckler J, Schwartz S, Benson RF, Helsel LO, Holder PF, Johnson SE, Kellum M, Messmer T, Thacker WL, Besser L, Plikaytis BD, Taylor TH Jr, Freeman AE, Wallace KJ, Dull P, Sejvar J, Bruce E, Moreno R, Schuchat A, Lingappa JR, Martin SK, Walls J, Bronsdon M, Carlone GM, Bajani-Ari M, Ashford DA, Stephens DS, Perkins BA. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg Infect Dis 2002;8(10):1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Soroka SD, Taylor TH Jr, Stamey KL, Stinson KW, Freeman AE, Abramson DR, Desai R, Cronin LX, Oxford JW, Caba J, Pleatman C, Pathak S, Schmidt DS, Semenova VA, Martin SK, Wilkins PP, Quinn CP. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J Immunol Methods. 2008;333(1–2):89–106. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki K, Koga H, Inoue K, Suzuki K, Suzuki H. Spontaneous intraocular hemorrhage in rats during postnatal ocular development. Comp Med 2014;64(1):34–43. [PMC free article] [PubMed] [Google Scholar]
- 25.Heywood R. Some clinical observations on the eyes of Sprague-Dawley rats. Lab Anim 1973;7(1):19–27. [DOI] [PubMed] [Google Scholar]
- 26.Savransky V, Lacy M, Ionin B, Skiadopoulos MH, Shearer J. Repeat-Dose Toxicity Study of a Lyophilized Recombinant Protective Antigen-Based Anthrax Vaccine Adjuvanted With CpG 7909. Int J Toxicol 2019;38(3):163–172. [DOI] [PubMed] [Google Scholar]
- 27.Zmarowski A, Ballin JD, Sharits J, Carrico K, Novak J, Shearer J, Blauth B, Ionin B, Reece J, Savransky V. Repeat Dose Toxicity Study of the AV7909 Anthrax Vaccine Candidate in Juvenile Rats. Int J Toxicol. 2020. July 21:1091581820941412. doi: 10.1177/1091581820941412. [DOI] [PubMed] [Google Scholar]
- 28.Mylchreest E, Smiley A., Bailin J et al. Developmental and reproductive safety evaluation of AV7909 anthrax vaccine candidate in rats. 2021;113(1):32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernau M, Kremer-Rücker PV, Kreuzer LS, Schwanitz S, Cussler K, Hoffmann A, Scholz AM. Magnetic resonance imaging to detect local tissue reactions after vaccination in sheep in vivo. Vet Rec Open. 2017;4(1):e000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper CL, Davis HL, Morris ML, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. 2004; 24(6):693–701 [DOI] [PubMed] [Google Scholar]
- 31.Cooper CL, Davis HL, Angel JB, et al. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005; 19(14):1473–1479. [DOI] [PubMed] [Google Scholar]
- 32.Cooper CL, Davis HL, Morris ML, et al. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine. 2004; 22(23–24):3136–3143. [DOI] [PubMed] [Google Scholar]
- 33.Duncan CJ, Sheehy SH, Ewer KJ, et al. Impact on malaria parasite multiplication rates in infected volunteers of the protein-inadjuvant vaccine AMA1-C1/Alhydrogel ϸ CPG 7909. PLoS One. 2011;6(7):e22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullen GE, Ellis RD, Miura K, et al. Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS One. 2008;3(8):e2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traore B, Koné Y, Doumbo S, Doumtabé D, Traoré A, Crompton PD, Mircetic M, Huang CY, Kayentao K, Dicko A, Sagara I, Ellis RD, Miura K, Guindo A, Miller LH, Doumbo OK, Pierce SK. The TLR9 agonist CpG fails to enhance the acquisition of Plasmodium falciparum-specific memory B cells in semi-immune adults in Mali. Vaccine. 2009;27(52):7299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Offersen R, Melchjorsen J, Paludan SR, Ostergaard L, Tolstrup M, Sogaard OS. TLR9-adjuvanted pneumococcal conjugate vaccine induces antibody-independent memory responses in HIV infected adults. Hum Vaccin Immunother. 2012;8(8):1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sogaard OS, Lohse N, Harboe ZB, et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin Infect Dis 2010;51(1):42–50. [DOI] [PubMed] [Google Scholar]
- 38.La Rosa C, Longmate J, Lacey SF, et al. Clinical evaluation of safety and immunogenicity of PADRE–cytomegalovirus (CMV) and tetanus–CMV fusion peptide vaccines with or without PF03512676 adjuvant. J Infect Dis 2012;205(8):1294–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baldrick P Dose site reactions and related findings after vaccine administration in safety studies. J Appl Toxicol 2016;36(8):980–90. [DOI] [PubMed] [Google Scholar]
- 40.Segal L, Morelle D, Blee M, Moore E, Damsten M, Liu KC, Destexhe E, Garçon N. Local tolerance and systemic toxicity of single and repeated intramuscular administrations of two different formulations of the RTS,S malaria candidate vaccine in rabbits. Regul Toxicol Pharmacol 2015;71(2):269–78. [DOI] [PubMed] [Google Scholar]
- 41.Zhou XB, Lu JJ, Jiang YS, Huo Y, Wang JF, Zhou KF, Li B. A safety study of inactivated Enterovirus 71 vaccine. Hum Vaccin Immunother 2013;9(7):1430–7. [DOI] [PubMed] [Google Scholar]
- 42.Ondondo B, Brennan C, Nicosia A, Crome SJ, Hanke T. Absence of systemic toxicity changes following intramuscular administration of novel pSG2.HIVconsv DNA, ChAdV63.HIVconsv and MVA.HIVconsv vaccines to BALB/c mice. Vaccine. 2013;31(47):5594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic THl-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother 2004;27(6):460–71. [DOI] [PubMed] [Google Scholar]
- 44.Vicari AP, Luu R, Zhang N, Patel S, Makinen SR, Hanson DC, Weeratna RD, Krieg AM. Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol Immunother 2009;58(4):615–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tosi I, Bureau F, Farnir F, Denoix JM, Lekeux P, Art T. Effects of a P-class CpG-ODN administered by intramuscular injection on plasma cytokines and on white blood cells of healthy horses. Vet Immunol Immunopathol 2018;201:57–61. [DOI] [PubMed] [Google Scholar]
- 46.Watkinson A, Soliakov A, Ganesan A, Hirst K, Lebutt C, Fleetwood K, Fusco PC, Fuerst TR, Lakey JH. Increasing the potency of an alhydrogel-formulated anthrax vaccine by minimizing antigen-adjuvant interactions. Clin Vaccine Immunol 2013;20(11):1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]


