Abstract
Background
COVID-19 continues to pose a significant healthcare challenge throughout the world. Comorbidities including diabetes and hypertension are associated with a significantly higher mortality risk. However, the effect of cirrhosis on COVID-19 outcomes has yet to be systematically assessed.
Objectives
To assess the reported clinical outcomes of patients with cirrhosis who develop COVID-19 infection.
Design/Method
PubMed and EMBASE databases were searched for studies included up to 3 February 2021. All English language primary research articles that reported clinical outcomes in patients with cirrhosis and COVID-19 were included. The study was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The risk of bias was assessed using the Quality In Prognostic Score (QUIPS) risk-of-bias assessment instrument for prognostic factor studies template. Meta-analysis was performed using Cochrane RevMan V.5.4 software using a random effects model.
Results
63 studies were identified reporting clinical outcomes in patients with cirrhosis and concomitant COVID-19. Meta-analysis of cohort studies which report a non-cirrhotic comparator yielded a pooled mortality OR of 2.48 (95% CI: 2.02 to 3.04). Analysis of a subgroup of studies reporting OR for mortality in hospitalised patients adjusted for significant confounders found a pooled adjusted OR 1.81 (CI: 1.36 to 2.42).
Conclusion
Cirrhosis is associated with an increased risk of all-cause mortality in COVID-19 infection compared to non-cirrhotic patients. Patients with cirrhosis should be considered for targeted public health interventions to prevent COVID-19 infection, such as shielding and prioritisation of vaccination.
Keywords: cirrhosis, COVID-19, chronic liver disease
Background
COVID-19 first came to global attention in December 2019, when the Wuhan Municipal Health Commission in China reported cases of a novel ‘viral pneumonia’. Since then, the virus has spread with alarming rapidity across the globe, leading to the WHO declaring a global pandemic on 11 March 2020.1 As of 14 March 2021, the WHO reports 119 million global cumulative confirmed cases of COVID-19, with 2.6 million attributed deaths.2
Observational studies have identified several risk factors associated with COVID-19 mortality. A meta-analysis including 38 906 patients showed the summary relative risk of death was 1.8 (95% CI: 1.6 to 2.0) for hypertension, 1.5 (95% CI: 1.4 to 1.7) for diabetes and 1.6 (95% CI: 1.9 to 3.8) for chronic liver disease (CLD).3 Another meta-analysis including 51 225 patients reported pooled OR of 1.09 for obesity (95% CI: 0.84 to 1.41), 2.98 for cardiovascular disease (95% CI: 2.51 to 3.53), 2.61 for hypertension (95% CI: 2.19 to 3.17), 2.12 for diabetes (95% CI: 1.79 to 2.52) and 1.80 for CLD (95% CI: 1.35 to 2.39).4
Many studies have examined the impact of CLD on the prognosis of COVID-19; however, CLD encompasses a heterogeneous group of patients with a variety of aetiologies as well as a spectrum of severity of liver fibrosis and dysfunction. Aetiologies, such as non-alcoholic fatty liver disease (NAFLD), have a high coprevalence with obesity and diabetes, two other conditions associated with increased mortality in COVID-19.5 Cirrhosis represents the end stage of CLD. Development of infections in patients with cirrhosis is a well-established poor prognostic factor. Meta-analysis of studies examining the clinical outcome of patients with cirrhosis and any infection reported a mortality of 38%.6 Factors proposed to contribute to this include cirrhosis-associated immune dysfunction as well as altered gut microbiome.7 8
Understanding the impact of concomitant cirrhosis in patients with COVID-19 is clinically important for several reasons. From a clinical perspective, it would inform decision-making on day-to-day treatment decisions, escalation and resuscitation status as well as on how to direct resources effectively. From a public health perspective, it would help shape healthcare policy-making regarding targeting of interventions such as vaccination prioritisation and shielding. This is particularly important in resource limited settings. On a lesser note, the pandemic has resulted in a drastic reduction in hepatology outpatient face-to-face consultations. The risk of contracting COVID-19 while in hospital for routine bloods or surveillance imaging should be balanced appropriately against the risks of delaying access to these services.
To address this need, we performed a systematic literature review and meta-analysis to examine all primary studies reporting mortality of COVID-19 in patients with established cirrhosis.
Methods
A systematic search of PubMed and EMBASE databases was performed for papers available on 3 February 2021. The study was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Search terms “cirrhosis”, “chronic liver disease” and “liver disease” were combined with terms “COVID-19”, “coronavirus”, “SARS-CoV-2” and “ncov-19” in all possible permutations. After duplicates were excluded, all titles and abstracts were screened independently by two authors (PM and CH) for relevance and consideration of further review. Full texts were assessed by both authors (PM and CH) for consideration of inclusion. ML’s review was performed in instances of disagreement in author inclusion. Eligible studies included any English language primary research study reporting adult patients with cirrhosis with concomitant acute SARS-CoV-2 infection and reported any clinical outcome including mortality, hospitalisation or mechanical ventilation. No exclusion criteria were applied regarding the definition of cirrhosis within the paper, and all manuscripts which reported patients being cirrhotic were considered. Review articles and systematic reviews were excluded. Reported cases in patients who had undergone liver transplantation were also excluded. A prepublished protocol was not created.
Data were extracted using a defined spreadsheet and included study design, inclusion criteria, definition of cirrhosis, definition of COVID-19, length of follow-up, reported mortality, adjusted mortality, hospitalisation rate, intubation/ventilation rate, cirrhotic decompensation, reporting of cirrhosis aetiology and reporting of cirrhosis severity including Child–Turcotte–Pugh Score, the Model of End-Stage Liver Disease (MELD) Score or compensation/decompensation status. Decompensation of cirrhosis included reported new or worsening hepatic encephalopathy, ascites, jaundice, coagulopathy, spontaneous bacterial peritonitis or variceal bleeding.
Studies that reported cirrhosis mortality alongside a non-cirrhotic comparator group were considered for meta-analysis assessment of all-cause mortality. These papers were assessed independently by two authors for risk of bias using the Quality In Prognostic Score (QUIPS) risk-of-bias assessment instrument for prognostic factor studies template.9 Studies were assessed for consideration of risk of bias under six domains including study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding and statistical analysis and reporting. Studies were scored as low, medium or high risk of bias within each domain. Disagreement between authors was resolved by consensus. Within the ‘study-confounding’ domain, we assessed for the reporting and adjustment for known prognostic factors including age, gender, diabetes, obesity, cardiovascular disease, hypertension and lung disease.3 We accepted statistical adjustment through cohort matching as well as multivariate regression analysis. We stipulated inclusion of a minimum of 10 patients with cirrhosis for a study to be deemed low risk of bias within the ‘prognostic factor’ domain. This is concordant with the previous systematic review and meta-analysis of clinical outcome in patients with cirrhosis and infections.6 The primary outcome examined was all-cause mortality.
Meta-analysis to assess the pooled OR for mortality was conducted using RevMan V.5.4.10 In cases where papers reported from the same cohort, only the paper reporting the largest cirrhotic cohort was included to prevent multiple reporting of the same patient cases. In cases where published abstracts and full articles of the same study were identified in our search, results from the complete paper were included. Crude OR was calculated from absolute values of total patients and patient deaths reported. RevMan calculator was used to derive absolute values from available data where it was not reported. Adjusted OR was input as reported. Interstudy heterogeneity was reported using the τ2, χ2 and I2 statistical tests. A random effects model was used to perform meta-analysis, given the inherent variability of observational studies.
Results
Search and study characteristics
After removal of duplicates, 891 study titles and abstracts were reviewed. Three hundred and sixty-four studies progressed to full article review (figure 1). Sixty-three studies were included in the final cohort (table 1). Ten studies were published in conference abstracts, with the remaining fifty-three papers comprising full articles and letters. Three studies were published as both full articles and abstracts. Country of origin included 22 studies from Europe, 14 studies from Asia, 19 studies from North American, 3 studies from South America, 1 study from Africa, 1 study from the Middle East and 3 international studies incorporating patients from different continents.
Figure 1.

Flow diagram of study selection process.
Table 1.
Characteristics of accepted studies
| Studies | Country | Number of cirrhotic patients | Aetiology | Child–Turotte–Pugh (A/B/C) |
Hospitalised | Decompensation of cirrhosis during admission | Mechanical ventilation | All-cause mortality (%) |
| Published abstracts | ||||||||
| Neppala et al17 | USA | 1 | ARLD (1/1) | NR | 1/1 | 1/1 | 1/1 | 1/1 (100%) |
| Rozenshteyn et al18 | USA | 1 | ARLD (1/1) | NR | 1/1 | 1/1 | NR | NR |
| Garrido et al19 | Portugal | 3 | NR | NR | 3/3 | NR | NR | 2/3 (66.7%) |
| Joshi et al20 | USA | 3 | ARLD (1/3) NASH (1/3) HCV (1/3) |
B (1/3) C (2/3) |
3/3 | 3/3 | 1/3 | 1/3 (33.3%) |
| Mangia et al21 | Italy | 10 | Viral (3/10) Other (7/10) |
NR | 10/10 | NR | NR | 7/10 (70%) |
| Mandour et al22 | UK | 10 | NR | NR | 10/10 | NR | NR | 3/10 (30%) |
| Suresh et al23 | USA | 21 | NR | NR | 21/21 | NR | NR | 10/21 (47.6%) |
| Mendizabal et al24 | Latin America | 24 | NR | NR | 24/24 | NR | NR | 6/24 (25%) |
| Satapathy et al25 | USA | 84 | NR | NR | 84/84 | NR | NR | NR |
| Choudhury et al26 | Asia | 121 | NR | NR | NR | NR | NR | 29/121 (24%) |
| Case reports | ||||||||
| Airoldi et al27 | Italy | 1 | HCV (1/1) | B (1/1) | 1/1 | 1/1 | 0/1 | 0/1 (0%) |
| Artru et al28 | Switzerland | 1 | ARLD/NASH (1/1) | C (1/1) | 1/1 | 0/1 | 0/1 | 0/1 (0%) |
| Culver et al29 | France | 1 | ARLD (1/1) | B (1/1) | 1/1 | 1/1 | 1/1 | NR |
| El Kassas et al30 | Egypt | 1 | HCV (1/1) | NR | 1/1 | 1/1 | 1/1 | 0/1 (0%) |
| Gerstein et al31 | USA | 1 | ARLD (1/1) | NR | 1/1 | 1/1 | 0/1 | 0/1 (0%) |
| Glynn et al32 | Ireland | 1 | ARLD (1/1) | B (1/1) | 1/1 | 0/1 | 0/1 | 0/1 (0%) |
| Grosse et al33 | Germany | 1 | NASH (1/1) | NR | 1/1 | 1/1 | 0/1 | 0/1 (0%) |
| Umair et al34 | Qatar | 1 | Cryptogenic (1/1) | B (1/1) | 1/1 | 1/1 | 1/1 | 1/1 (100%) |
| Kreivenaite et al35 | Lithuania | 1 | HCV (1/1) | B (1/1) | 1/1 | 0/1 | 0/1 | 0/1 (0%) |
| Mangiameli et al36 | France | 1 | RHF (1/1) | NR | 1/1 | 0/1 | 0/1 | 0/1 (0%) |
| Martini et al37 | Italy | 1 | AILD (1/1) | NR | 1/1 | 1/1 | 0/1 | 0/1 (0%) |
| Passarelli et al38 | Brazil | 1 | NR | NR | 1/1 | 1/1 | 1/1 | 1/1 (100%) |
| Qiu et al39 | China | 1 | ARLD (1/1) | NR | 1/1 | 1/1 | 0/1 | 0/1 (0%) |
| Rhee et al40 | USA | 1 | NASH (1/1) | NR | 1/1 | 1/1 | 1/1 | 1/1 (100%) |
| Zelman et al41 | USA | 1 | ARLD (1/1) | NR | 1/1 | 1/1 | 0/1 | 0/1 (0%) |
| Case series | ||||||||
| Rela et al42 | India | 2 | NASH (1/2) Crypotgenic (1/2) |
C (2/2) | 2/2 | 2/2 | 2/2 | 2/2 (100%) |
| Eisa et al43 | USA | 2 | ARLD (2/2) | NR | 2/2 | 2/2 | 1/2 | 2/2 (100%) |
| Kapuria et al44 | USA | 3 | ARLD (3/3) | C (3/3) | 3/3 | 3/3 | 3/3 | 3/3 (100%) |
| Qi et al45 | China | 3 | HBV (1/3), ARLD (1/3) Schistosomiasis (1/3) |
B (1/3) C (2/3) |
3/3 | 3/3 | 1/3 | 2/3 (66.7%) |
| Kulkarni et al46 | India | 9 | ARLD (5/9), AILD (2/9), cryptogenic (1/9), NASH (1/9) |
NR | NR | 7/9 | 4/9 | 4/9 (44.4%) |
| Liu et al47 | China | 17 | HBV (12/17), HCV (2/17) Other (3/17) |
A (15/17), B (1/17) C (1/17) |
NR | NR | 2/17 | 3/17 (17.6%) |
| Shalimar et al48 | India | 22 | ARLD (8/22) Cryptogenic (6/22) Viral (4/22) AILD (2/22) Other (2/22) |
A (8/22) B (8/22) C (6/22) |
22/22 | NR | N/A | 3/22 (13.6%) |
| Kumar et al49 | India | 57 | ARLD (25/57), NASH (13/57), cryptogenic (9/57) Viral (7/57), AILD (3/57) |
A (11/57), B (20/57) C (26/57) |
38/57 | 29 - 38/57 | 8/57 | 8/57 (14%) |
| Single-centre cohort | ||||||||
| Di Giorgio et al11 | Italy | 1 | AILD (1/1) | NR | 1/1 | 1/1 | 0/1 | 0/1 (0%) |
| Rigamonti et al12 | Italy | 1 | AILD (1/1) | NR | 1/1 | NR | 0/1 | 0/1 (0%) |
| Kroemer et al50 | USA | 3 | ARLD (2/3) AILD (1/3) |
NR | 3/3 | NR | 1/3 | 1/3 (33.3%) |
| Forlano et al51 | UK | 6 | NAFLD (6/6) | A (3/6) B (2/6) C (1/6) |
6/6 | NR | NR | 3/6 (50%) |
| Guerra Veloz et al52 | Spain | 7 | HCV (4/7) Other (3/7) |
A (5/7) B (2/7) |
7/7 | NR | NR | 3/7 (42.9%) |
| Shalimar et al53 | India | 26 | ARLD (9/26), NAFLD (2/26) HBV (3/26), HCV (2/26), AILD (4/26), cryptogenic (6/26) |
A (1/26) | NR | 18/26 | 1/26 | 11/26 (42.3%) |
| Torres-Macho et al54 | Spain | 31 | NR | NR | 31/31 | NR | NR | 9/31 (29%) |
| Multicentre cohort | ||||||||
| Li et al55 | China | 2 | HBV (2/2) | NR | 2/2 | 0/2 | 0/2 | 0/2 (0%) |
| Ji et al56 | China | 3 | NR | NR | 3/3 | 0/3 | NR | 1/3 (33.3%) |
| Gerussi et al57 | Italy | 4 | AILD (4/4) | A (3/4) B (1/4) |
3/4 | NR | NR | 1/4 (25%) |
| Marjot et al58 | UK | 6† | NR | NR | NR | NR | NR | 6/6 (100%) |
| Hashemi et al59 | USA | 9 | ARLD (3/9) NAFLD (1/9) HCV (4/9) HBV (1/9) |
NR | 2/2 | NR | NR | 5/9 (55.6%) |
| Mangia et al60 | Italy | 10 | Metabolic (7/10) HCV (3/10) |
NR | 10/10 | NR | NR | 7/10 (70%) |
| Lee et al61 | South Korea | 14 | HBV (5/14) ARLD (5/14) HCV (2/14) AILD (1/14) Cryptogenic (1/14) |
A (9/14) B (5/14) |
14/14 | 0/14 | 3/14 | 4/14 (28.6%) |
| Nathwani et al62 | UK | 21 | ARLD (10/27) Other (17/27) |
A (8/21) B/C (13/21) |
21/21 | 6/21 | 1/27 | 8/21 (38.1%) |
| Qi et al63 | China | 21 | HBV (9/21) HCV (2/21) ARLD (2/21) Schistosomiasis (1/21) AILD (1/21) Other (6/21) |
A (16/21) B (3/21) C (2/21) |
21/21 | NR | 3/21 | 5/21 (23.8%) |
| Bajaj et al64 | USA | 37 | HCV (9/37) ARLD (9/37) NASH (9/37) HCV+ARLD (4/37) Others (6/37) |
NR | 37/37 | NR | 14/37 | 11/37* (29.7%) |
| Iavarone et al65 | Italy | 50 | HCV (14/50) HBV (5/50) ARLD (12/50) NAFLD (3/50) Other/Multiple (16/50) |
A (26/50) B (18/50) C (6/50) |
48/50 | 12/50 | 2/50 | 17/50 (34%) |
| Singh et al66 | USA | 50 | NR | NR | NR | NR | NR | 10/50 (20%) |
| Berenguer et al67 | Spain | 54 | NR | NR | 54/54 | NR | NR | 26/54 (48.1%) |
| Mendizabal et al68 | Latin America | 55 | NR | NR | 55/55 | NR | NR | 21/55 (38.2%) |
| Frager et al69 | USA | 83 | NR | NR | 83/83 | NR | 22/83 | 30/83 (36.1%) |
| Butt et al13 | USA | 93 | HCV (79/93) Other (14/93) |
NR | 23/79 | NR | NR | 7/93 (7.5%) |
| Gottlieb et al70 | USA | 207 | NR | NR | 100/207 | NR | NR | NR |
| Kim et al71 | USA | 227 | NR | NR | NR | NR | NR | 57/227 (25.1%) |
| Ioannou et al72 | USA | 305 | HCV-related (144/305) Other (161/305) |
NR | 163/305 | NR | 40/305 | 52/305 (17%) |
| Multinational registry | ||||||||
| Sarin et al73 | Asia | 43 | Metabolic (14/43) Viral (26/43) ARLD (2/43) Other (1/43) |
NR | 43/43 | 14/43 | NR | 7/43 (16.3%) |
| Moon et al74 | International | 103 | NR | A (46/103) B (30/103) C (27/103) |
98/103 | 39/103 | 18/103 | 41/103 (39.8%) |
| Marjot et al75 | International | 386 | NAFLD (102/386) ARLD (158/386) HBV (37/386) HCV (72/386) |
A (171/386) B (124/386) C (91/386) |
345/386 | 179/386 | 71/386 | 123/386 (31.9%) |
| Marjot et al76 | International | 509 | NR | A (231/509) B 163/509) C (115/509) |
NR | NR | NR | 161/509 (31.6%) |
**Death/hospice.
†Cirrhotic patients from UK multicentre comparator cohort.
AILD, autoimmune liver disease; ARLD, alcohol-related liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus; MAFLD, metabolic associated fatty liver disease; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NR, not reported for cirrhotic patients; RFH, right heart failure.
Study design was varied and included 17 case reports, 9 case series, 10 single-centre cohort studies, 22 multicentre cohort studies and 5 registry studies. The majority of cohort studies had a retrospective design, with only seven studies reporting a prospective or ambispective data collection. Two studies included a prospective telephone-based survey of their autoimmune liver disease patient cohort to screen for COVID-19 symptoms.11 12 COVID-19 was defined by reverse transcription PCR testing/laboratory confirmed SARS-CoV-2 infection or in accordance with WHO criteria in 60/63 studies. Cirrhosis definition was varied, with the majority of studies presenting patients as having a premorbid diagnosis of cirrhosis. Nine studies stipulated additional histological, clinical, endoscopic or imaging features of cirrhosis. Three studies incorporated non-invasive serological screening tools, with one study using Fibrosis-4 Index as its primary determinator of cirrhosis.13 Follow-up was defined as reaching a clinical endpoint such as death, discharge or liver transplant in 29 studies. Thirteen studies employed minimum follow-up period or a censoring date. Twenty studies did not provide a clear follow-up period.
Twenty-seven studies reported further decompensation of cirrhosis associated with COVID-19 infection. Twenty-five studies reported patients receiving intubation and mechanical ventilation.
Risk-of-bias assessment
Overall, 10/63 papers were found to be at low risk of bias across all domains. Common areas for potential bias included low number of cirrhotic patients, lack of confounder reporting and lack of adjustment for confounders.
Meta-analysis of cohort studies
All studies that reported all-cause mortality in a cohort of 10 or more patients with cirrhosis and COVID-19 alongside a non-cirrhotic COVID-19 comparator group were incorporated into the meta-analysis. Overall, 26 studies included 10 or more cirrhotic patients with COVID-19. Two published abstracts were excluded as they were already included as published full articles. Five studies were excluded as they did not report a non-cirrhotic COVID-19 comparator or did not report a mortality outcome. One abstract was excluded as it contained insufficient information to extract an OR. Two studies were excluded as they reported from the same registry as a larger third study which was included.
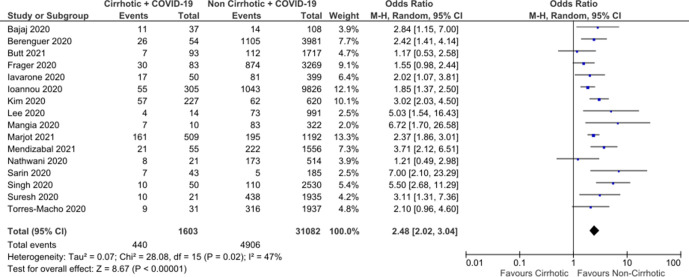
In total, 16 studies were included in the meta-analysis, producing a total of 1603 cirrhotic patients with COVID-19 compared with 31 082 non-cirrhotic patients with COVID-19 (figure 2). In the majority of studies (14/16), this included all other patients with COVID-19 including a proportion of patients with CLD without cirrhosis. Overall, 2/16 studies only reported patients with CLD without cirrhosis to provide a comparator group. A funnel plot showed a degree of publication bias towards studies reporting greater associated risk; however, this was within the smaller studies (figure 3). Using a random-effect model, a pooled crude OR for all-cause mortality for patients with cirrhosis was calculated as 2.48 (95% CI: 2.02 to 3.04). Moderate interstudy heterogeneity was found. Sensitivity analysis removing studies which only had a CLD comparator showed minimal change to the associated mortality OR to 2.64 (95% CI: 2.08 to 3.36).
Figure 2.
Meta-analysis of crude mortality OR comparing cirrhotic patients with COVID-19 with non-cirrhotic patients with COVID-19.
Figure 3.
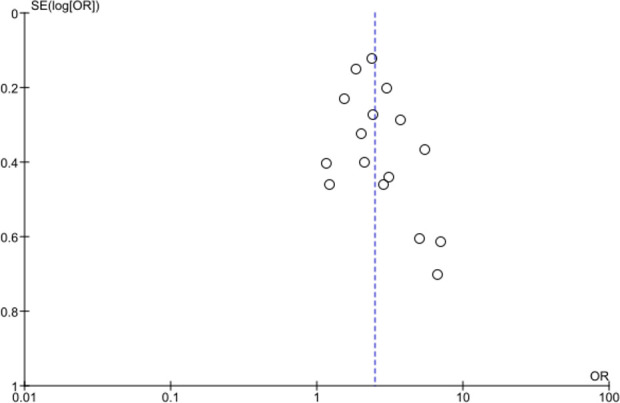
Funnel plot of studies included in the meta-analysis of crude mortality OR.
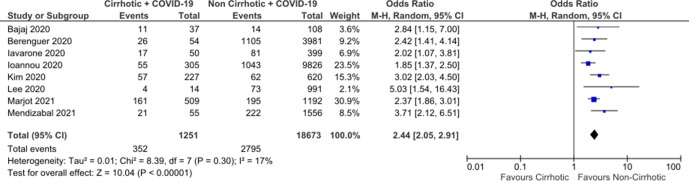
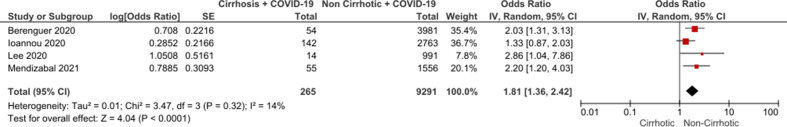
Inclusion of only eligible studies with a low risk of bias yielded an OR 2.44 (95% CI: 2.05 to 2.91) without significant interstudy variability (figure 4). Five low risk-of-bias studies reported adjusted ORs for mortality between all cirrhotic patients and non-cirrhotic comparators. One study adjusted based on cohort matching for age and gender alone. Four studies reported adjusted ORs based on multivariate regression analysis incorporating age, gender as well as significant comorbidities including diabetes and cardiovascular disease in hospitalised patients. Pooled analysis of these four studies produced an adjusted OR of 1.81 (CI: 1.36 to 2.42) (figure 5). Two eligible low-risk studies reported adjusted ORs for mortality by disease severity, suggesting worsening mortality with more advanced cirrhosis (table 2).
Figure 4.
Meta-analysis of studies at lower risk of bias.
Figure 5.
Meta-analysis of adjusted mortality OR in studies comparing cirrhotic inpatients with COVID-19 and non-cirrhotic inpatients with COVID-19.
Table 2.
Studies reporting adjusted ORs for severity subgroups of cirrhosis compared with non-cirrhotic chronic liver disease comparator
Discussion
To the best of our knowledge, this study is the first to systematically examine and analyse the literature to describe the clinical outcome of patients with cirrhosis who have concomitant COVID-19. Pooled crude OR for mortality of 2.48 (95% CI: 2.02 to 3.04) is comparable to other established significant prognostic factors such as diabetes, hypertension and cardiovascular disease.4 This additional mortality risk persisted on analysis of adjusted ORs in hospitalised cirrhotic patients, suggesting cirrhosis poses an additional risk independent of its association with other comorbidities, such as diabetes and cardiovascular disease in patients with NAFLD. Mortality risk is potentially higher in patients with more advanced cirrhosis. Further studies with subgroup outcome reporting based on severity of cirrhosis are required to fully evaluate this; however, to assess this appropriately large patient numbers will be required, likely only achievable by large multinational or registry-based studies.
This study provides evidence to support targeted interventions aimed at protecting patients with cirrhosis from COVID-19, such as prioritisation for vaccination, shielding and limitation of hospital attendance with support from telemedical interventions where appropriate. Healthcare professionals should be aware of the associated heightened COVID-19 mortality in patients with cirrhosis and the potential risk of associated cirrhotic decompensation. However, the associated mortality risk in cirrhosis is not out-keeping with other common comorbidities such as cardiovascular disease or diabetes. Therefore, all cirrhotic patients should still be considered for mechanical ventilation or escalation to intensive care unit on an individual basis.
Following the date of censoring, further studies have been published which may have been suitable for inclusion and it is important to consider these. Ge et al have reported data from the N3C Consortium in the USA which uses electronic healthcare record data to identify patients who underwent SARS-CoV-2 testing or had related symptoms.14 In total, 8941 patients with cirrhosis and COVID-19 were identified. When compared with SARS-COV-2 patients with non-cirrhotic CLD, they report an adjusted 30-day mortality HR of 3.31.14 This risk is higher than adjusted risks for hospitalised patients identified in our systematic review, likely due to the high proportion of non-hospitalised patients in this study and the difference in risk of hospitalisation between groups (CLD 22.9% vs cirrhosis 50.1%).14 Observational studies within our meta-analysis include predominantly patients who were hospitalised or presented to hospitals. This is likely due to changes in the availability and ease of access to SARS-CoV-2 testing in the community over time as the response to the pandemic has progressed.
Mallet et al have reported the outcomes of hospitalised COVID-19 from the French National Hospital Discharge database including 3207 patients with concomitant cirrhosis.15 Comparing cirrhotic patients to all non-cirrhotic patients produced a mortality OR of 1.73 (1.59–1.88) which is in line with our findings. Adjusted OR for 30-day mortality in compensated cirrhosis (0.71; 0.63–0.80) and decompensated cirrhosis (2.21; 1.94–2.51) were provided, highlighting the importance of delineating cirrhosis severity when prognosticating outcome.15 Mendizabal et al have published an update from their prospective study on hospitalised patients with COVID-19, which was already included in this systematic review.16 This update provided an adjusted OR 3.1 (1.9–4.8) for patients with cirrhosis.16 This represents an increase in reported mortality compared with their prior publication. However, they also report an increase in overall mortality for both cirrhotic patients (46.9% from 38.2%) and all non-cirrhotic patients (19.5% from 14.3%). As the pandemic progresses, regional variations in SARS-CoV-2 variant predominance, pressure on healthcare resources, public health policy and access and uptake of vaccination are likely to become more significant when predicting patient outcomes than in the first phase of the global pandemic with potentially increasing heterogeneity in reported outcomes.
The study has several limitations including the heterogeneity of study design and characteristics, the heterogeneity of the comparator group and the relatively small sample size with 34 out of 63 studies reporting fewer than 10 patients with cirrhosis. Although steps were taken to prevent multiple reporting of patient cases during meta-analysis, it is possible that cases reported are also included in registry-based studies and may be reported concurrently.
Conclusion
Systematic review and meta-analysis of observational studies of reporting COVID-19 in patients with cirrhosis supports an increased mortality rate compared with non-cirrhotic patients. Mortality is likely higher in those with more advanced cirrhosis. Patients with cirrhosis should be considered for targeted measures to prevent COVID-19, such as prioritisation of vaccination and shielding.
Footnotes
Contributors: PM—authorship of manuscript, data collection, quality assessment and data analyses. CH—data collection, quality assessment and review of manuscript. ML—data collection, quality assessment and review of manuscript. All authors take reponsibility for the accuracy and reporting of the data presented as guarantors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The datasets generated and/or analysed during the current study are not publicly available due to data protection reasons.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.WHO . Listings of WHO’s response to COVID-19, 2021. Available: https://www.who.int/news/item/29-06-2020-covidtimeline
- 2.WHO . COVID-19 Weekly epidemiological update, 2021. Available: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20210316_weekly_epi_update_31.pdf?sfvrsn=c94717c2_14&download=true
- 3.Dorjee K, Kim H, Bonomo E, et al. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One 2020;15:e0243191. 10.1371/journal.pone.0243191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesas AE, Cavero-Redondo I, Álvarez-Bueno C, et al. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS One 2020;15:e0241742. 10.1371/journal.pone.0241742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Ayada I, Zhang X. Estimating global prevalence of metabolic dysfunction-associated fatty liver disease in overweight or obese adults. Clin Gastroenterol Hepatol 2021:00208–1. 10.1016/j.cgh.2021.02.030 [DOI] [PubMed] [Google Scholar]
- 6.Arvaniti V, D'Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010;139:1246–56. 10.1053/j.gastro.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 7.Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014;61:1385–96. 10.1016/j.jhep.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 8.Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014;60:940–7. 10.1016/j.jhep.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–37. 10.7326/0003-4819-144-6-200603210-00010 [DOI] [PubMed] [Google Scholar]
- 10.Collaboration TC . Review Manager (RevMan) [Computer program] Version 5.4, 2020. [Google Scholar]
- 11.Di Giorgio A, Nicastro E, Speziani C, et al. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol 2020;73:702–5. 10.1016/j.jhep.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigamonti C, Cittone MG, De Benedittis C, et al. Rates of symptomatic SARS-CoV-2 infection in patients with autoimmune liver diseases in northern Italy: a telemedicine study. Clin Gastroenterol Hepatol 2020;18:2369–71. 10.1016/j.cgh.2020.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butt AA, Yan P, Chotani RA, et al. Mortality is not increased in SARS-CoV-2 infected persons with hepatitis C virus infection. Liver Int 2021;41:1824–31. 10.1111/liv.14804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge J, Pletcher MJ, Lai JC. Outcomes of SARS-CoV-2 infection in patients with chronic liver disease and cirrhosis: a national COVID cohort collaborative study. Gastroenterology 2021;73. 10.1053/j.gastro.2021.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallet V, Beeker N, Bouam S, et al. Prognosis of French COVID-19 patients with chronic liver disease: a national retrospective cohort study for 2020. J Hepatol 2021;75:848–55. 10.1016/j.jhep.2021.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendizabal M, Ridruejo E, Piñero F, et al. Comparison of different prognostic scores for patients with cirrhosis hospitalized with SARS-CoV-2 infection. Ann Hepatol 2021;25:100350. 10.1016/j.aohep.2021.100350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neppala S, Caravella J, Camero A, et al. S2113 COVID-19-Associated Coagulopathy and GI Bleed in a Cirrhotic Patient. Am J Gastroenterol 2020;115:S1113–4. 10.14309/01.ajg.0000710500.70986.6b [DOI] [Google Scholar]
- 18.Rozenshteyn F, Shah M, Adeyemo O, et al. S2437 Budd-Chiari Syndrome Secondary to SARS-CoV-2 Infection. Am J Gastroenterol 2020;115:S1292. 10.14309/01.ajg.0000711796.23564.34 [DOI] [Google Scholar]
- 19.Garrido M, Guedes T, Falcão D. Impact of abnormal liver tests and chronic liver disease in hospitalized COVID-19 patients’ outcomes-a still growing understanding. Hepatology 2020;72:278A–9. [Google Scholar]
- 20.Joshi TV, Bilalis MV, Brown J, et al. S2456 Clinical Outcomes of Acute-on-Chronic Liver Failure in Patients With SARS-CoV-2 Infection. Am J Gastroenterol 2020;115:S1300–1. 10.14309/01.ajg.0000711872.40515.f4 [DOI] [Google Scholar]
- 21.Mangia A, Verucchi G, Ciancio A. Are HCV antibodies positive cirrhotic patients at lower risk of death as compared to cirrhotic of different etiologies when infected by COVID-19? Hepatology 2020;72:259A. [Google Scholar]
- 22.Mandour MO, Rafique KK, Koh JM. Characteristics of SARS-COV2 and liver cirrhosis-a single-centre experience in the United Kingdom. Hepatology 2020;72:261A–2. [Google Scholar]
- 23.Suresh S, Siddiqui MB, Ghanimeh MA. Clinical outcomes in hospitalized COVID-19 patients with chronic liver disease and cirrhosis. Hepatology 2020;72:263A. [Google Scholar]
- 24.Mendizabal M, Risruejo E, Pinero F. Abnormal liver function tests on admission are associated with increased mortality in hospitalized patients with COVID-19: preliminary results from a large Latin American cohort. Hepatology 2020;72:79A–80. [Google Scholar]
- 25.Satapathy SK, Roth NC, Kvasnovsky C. Acute-On-Chronic liver failure related to COVID-19 infection is associated with increased in-hospital mortality. Hepatology 2020;72:80A–1. [Google Scholar]
- 26.Choudhury AK, Zheng M, Lau G. Apcolis score predicts outcome in patients of cirrhosis with SARS-COV-2 infection-data from ongoing apasl covid liver injury spectrum (apcolis-i) study. Hepatology 2020;72:76A–7. [Google Scholar]
- 27.Airoldi A, Perricone G, De Nicola S, et al. COVID-19-related thrombotic microangiopathy in a cirrhotic patient. Dig Liver Dis 2020;52:946. 10.1016/j.dld.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Artru F, Alberio L, Moradpour D, et al. Acute immune thrombocytopaenic purpura in a patient with COVID-19 and decompensated cirrhosis. BMJ Case Rep 2020;13:e236815. 10.1136/bcr-2020-236815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culver A, Arbelot C, Bechis C, et al. First description of SARS-CoV-2 in ascites. IDCases 2020;21:13015. 10.1016/j.idcr.2020.e00836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Kassas M, Al Shafie A, Abdel Hameed AS, et al. Emergency endoscopic variceal band ligation in a COVID-19 patient presented with hematemesis while on mechanical ventilation. Dig Endosc 2020;32:812–5. 10.1111/den.13694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerstein S, Khatri A, Roth N, et al. Coronavirus disease 2019 and extra-pulmonary tuberculosis co-infection - A case report and review of literature. J Clin Tuberc Other Mycobact Dis 2021;22:100213. 10.1016/j.jctube.2021.100213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glynn E, Ryan J. Mild COVID-19 despite end stage liver disease. Irish Medical Journal 2020;113:1–2.32298555 [Google Scholar]
- 33.Große K, Kramer M, Trautwein C, et al. SARS-CoV-2 as an extrahepatic precipitator of acute-on-chronic liver failure. Liver Int 2020;40:1792–3. 10.1111/liv.14540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan MU, Mushtaq K, Alkaabi SR. Acute-On-Chronic liver failure: possibly the main culprit of increased mortality in COVID-19 patients with liver disease. Gastroenterology 2021;160:1894–5. 10.1053/j.gastro.2020.06.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreivenaite E, Gedgaudas R, Valantiene I, et al. COVID-19 in a patient with liver cirrhosis. J Gastrointestin Liver Dis 2020;29:263–6. 10.15403/jgld-2440 [DOI] [PubMed] [Google Scholar]
- 36.Mangiameli G, Al Zreibi C, Caudron J, et al. Unexpected evolution of COVID-19 in a heart transplant patient with multimorbidity recently submitted to thoracic surgery. Minerva Chir 2020;75:467–8. 10.23736/S0026-4733.20.08367-4 [DOI] [PubMed] [Google Scholar]
- 37.Martini S, Patrono D, Pittaluga F, et al. Urgent liver transplantation soon after recovery from COVID‐19 in a patient with decompensated liver cirrhosis. Hepatol Commun 2021;5:144–5. 10.1002/hep4.1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Passarelli VC, Perosa AH, de Souza Luna LK, et al. Detected SARS-CoV-2 in ascitic fluid followed by Cryptococcemia: a case report. SN Compr Clin Med 2020;2:2414–8. 10.1007/s42399-020-00574-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu H, Wander P, Bernstein D, et al. Acute on chronic liver failure from novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Liver Int 2020;40:1590–3. 10.1111/liv.14506 [DOI] [PubMed] [Google Scholar]
- 40.Rhee Y, Chan EL, Eswaran SL, et al. Fatal COVID‐19 in a patient with End‐Stage liver disease Wait‐Listed for liver transplantation: an Evidence‐Based review of COVID‐19 screening modalities prior to transplant. Clin Liver Dis 2020;15:246–50. 10.1002/cld.990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zelman S, Holzwanger E, Malik R, et al. Alcoholic hepatitis and COVID-19: the question of steroids. ACG Case Rep J 2020;7:e00504. 10.14309/crj.0000000000000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rela M, Patil V, Narasimhan G, et al. COVID-19 in decompensated cirrhosis. Hepatol Int 2020;14:1125–7. 10.1007/s12072-020-10092-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisa M, Kennedy R, Teferra R, et al. SARS-COV-2 infection in patients with Alcohol-Associated hepatitis: metabolic similarities and treatment challenges. Am J Gastroenterol 2021;116:847–8. 10.14309/ajg.0000000000001002 [DOI] [PubMed] [Google Scholar]
- 44.Kapuria D, Upadhyay S, Shekhar R, et al. Alcoholic liver disease and COVID-19 pneumonia: a case series. J Clin Transl Hepatol 2020;8:463–6. 10.14218/JCTH.2020.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi X, Wang J, Li X, et al. Clinical course of COVID-19 in patients with pre-existing decompensated cirrhosis: initial report from China. Hepatol Int 2020;14:478–82. 10.1007/s12072-020-10051-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulkarni AV, Parthasarathy K, Kumar P, et al. Early liver transplantation after COVID-19 infection: the first report. Am J Transplant 2021;21:2279–84. 10.1111/ajt.16509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu F, Long X, Ji G, et al. Clinically significant portal hypertension in cirrhosis patients with COVID-19: clinical characteristics and outcomes. J Infect 2020;81:178–80. 10.1016/j.jinf.2020.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaishnav M, Elhence A, et al. Outcome of conservative therapy in coronavirus disease-2019 patients presenting with gastrointestinal bleeding. J Clin Exp Hepatol 2021;11:327–33. 10.1016/j.jceh.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar P, Sharma M, Sulthana SF, et al. Severe acute respiratory syndrome coronavirus 2-related acute-on-chronic liver failure. J Clin Exp Hepatol 2021;11:404–6. 10.1016/j.jceh.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kroemer A, Khan K, Plassmeyer M, et al. Inflammasome activation and pyroptosis in lymphopenic liver patients with COVID-19. J Hepatol 2020;73:1258–62. 10.1016/j.jhep.2020.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forlano R, Mullish BH, Mukherjee SK, et al. In-Hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One 2020;15:e0240400. 10.1371/journal.pone.0240400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerra Veloz MF, Cordero Ruiz P, Ríos-Villegas MJ, et al. Liver manifestations in COVID-19 and the influence of pre-existing liver disease in the course of the infection. Rev Esp Enferm Dig 2021;113:103–9. 10.17235/reed.2020.7627/2020 [DOI] [PubMed] [Google Scholar]
- 53.Elhence A, Vaishnav M, Kumar R. Poor outcomes in patients with cirrhosis and Corona Virus Disease-19. Indian J Gastroenterol 2020;39:285–91. 10.1007/s12664-020-01074-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torres-Macho J, Ryan P, Valencia J, et al. The PANDEMYC score. An easily applicable and interpretable model for predicting mortality associated with COVID-19. J Clin Med 2020;9:3066. 10.3390/jcm9103066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, Li C, Wang J, et al. A case series of COVID-19 patients with chronic hepatitis B virus infection. J Med Virol 2020;92:2785–91. 10.1002/jmv.26201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji D, Zhang D, Yang T, et al. Effect of COVID-19 on patients with compensated chronic liver diseases. Hepatol Int 2020;14:701–10. 10.1007/s12072-020-10058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerussi A, Rigamonti C, Elia C, et al. Coronavirus disease 2019 (COVID-19) in autoimmune hepatitis: a lesson from immunosuppressed patients. Hepatol Commun 2020;4:1257–62. 10.1002/hep4.1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webb GJ, Marjot T, Cook JA, et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol 2020;5:1008–16. 10.1016/S2468-1253(20)30271-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hashemi N, Viveiros K, Redd WD, et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: a multicentre United States experience. Liver Int 2020;40:2515–21. 10.1111/liv.14583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mangia A, Cenderello G, Verucchi G, et al. Is positivity for hepatitis C virus antibody predictive of lower risk of death in COVID-19 patients with cirrhosis? World J Clin Cases 2020;8:5831–4. 10.12998/wjcc.v8.i22.5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee YR, Kang MK, Song JE, et al. Clinical outcomes of coronavirus disease 2019 in patients with pre-existing liver diseases: a multicenter study in South Korea. Clin Mol Hepatol 2020;26:562–76. 10.3350/cmh.2020.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nathwani R, Mukherjee S, Forlano R, et al. Letter: liver disease and COVID-19-not the perfect storm. Aliment Pharmacol Ther 2020;52:572–4. 10.1111/apt.15872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qi X, Liu Y, Wang J, et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut 2021;70:433–6. 10.1136/gutjnl-2020-321666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bajaj JS, Garcia-Tsao G, Biggins SW, et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut 2021;70:531–6. 10.1136/gutjnl-2020-322118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iavarone M, D'Ambrosio R, Soria A, et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol 2020;73:1063–71. 10.1016/j.jhep.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology 2020;159:768–71. 10.1053/j.gastro.2020.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berenguer J, Ryan P, Rodríguez-Baño J, et al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect 2020;26:1525–36. 10.1016/j.cmi.2020.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mendizabal M, Piñero F, Ridruejo E, et al. Prospective Latin American cohort evaluating outcomes of patients with COVID-19 and abnormal liver tests on admission. Ann Hepatol 2021;21:100298. 10.1016/j.aohep.2020.100298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frager SZ, Szymanski J, Schwartz JM, et al. Hepatic predictors of mortality in severe acute respiratory syndrome coronavirus 2: role of initial aspartate Aminotransferase/Alanine aminotransferase and preexisting cirrhosis. Hepatol Commun 2021;5:1–10. 10.1002/hep4.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gottlieb M, Sansom S, Frankenberger C, et al. Clinical course and factors associated with hospitalization and critical illness among COVID-19 patients in Chicago, Illinois. Acad Emerg Med 2020;27:963–73. 10.1111/acem.14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim D, Adeniji N, Latt N, et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol 2021;19:1469–79. 10.1016/j.cgh.2020.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ioannou GN, Liang PS, Locke E, et al. Cirrhosis and severe acute respiratory syndrome coronavirus 2 infection in US veterans: risk of infection, hospitalization, ventilation, and mortality. Hepatology 2021;74:322–35. 10.1002/hep.31649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarin SK, Choudhury A, Lau GK, et al. Pre-Existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; the APCOLIS study (APASL COVID-19 liver injury spectrum study). Hepatol Int 2020;14:690–700. 10.1007/s12072-020-10072-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moon AM, Webb GJ, Aloman C, et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol 2020;73:705–8. 10.1016/j.jhep.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marjot T, Moon AM, Cook JA, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol 2021;74:567–77. 10.1016/j.jhep.2020.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marjot T, Buescher G, Sebode M, et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol 2021;74:1335–43. 10.1016/j.jhep.2021.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. The datasets generated and/or analysed during the current study are not publicly available due to data protection reasons.