Abstract
Poorly regulated reward seeking is a central feature of substance use disorder. Recent research shows that rewarding drug-related experiences induce synchronous activation of a discrete number of neurons in the nucleus accumbens that are causally linked to reward-related contexts. Here we comprehensively characterize the specific ensemble of neurons built through experience that are linked to seeking behavior. We additionally address the question of whether or not addictive drugs usurp the neuronal networks recruited by natural rewards by evaluating cocaine- and sucrose-associated ensembles within the same mouse. We used FosCreERT2/+/Ai14 transgenic mice to tag cells activated by and potentially encoding cocaine and sucrose seeking. We tagged ~1% of neurons in the core subregion of the accumbens (NAcore) activated during cue-induced seeking for cocaine or sucrose. The majority of tagged cells in the seeking ensembles were D1-MSNs, and specifically activated during seeking, not during extinction or when mice remained in the home cage. To compare different reward-specific ensembles within the same mouse, we used a dual cocaine and sucrose self-administration protocol allowing reward-specific seeking. Using this model, we found ~70% distinction between the cells constituting the cocaine-compared to the sucrose-seeking ensemble. Establishing that cocaine recruits an ensemble of NAcore neurons largely distinct from neurons recruited into an ensemble coding for sucrose seeking suggest a finely tuned specificity of ensembles. The findings allow further exploration of the mechanisms that transform reward-based positive reinforcement into maladaptive drug seeking.
Introduction
Reward-based positive reinforcement is a shared evolutionary survival strategy across species [1]. However, in drug addiction reward seeking becomes maladaptive and endangers survival [2]. Here, we address a fundamental unanswered question in addiction research: whether or not the same network-specific mechanisms underlie seeking of drug and biological/natural rewards. While drug and natural rewards such as sucrose clearly involve overlapping brain nuclei [3], it remains unknown whether an organism uses the same neuronal ensembles to initiate cued drug and sucrose seeking.
An increasingly popular approach to dissect neuronal ensembles is permanently labeling (tagging) neurons that are active during a specific behavioral task based on the expression of immediate early genes such as Fos or Arc [4, 5]. This approach employs transgenic mouse lines and has been used to tag activated neurons during fear conditioning [6], sensory recognition [7], and affective experiences [8]. In the addiction field, Hope and colleagues developed a β–galactosidase/Daun02 strategy to establish a causal link between an ensemble of neurons in the nucleus accumbens (a brain circuitry hub for reward processing) selectively activated by drug-associated context and cocaine-induced behavioral sensitization [9]. Using the same strategy, this finding was extended to ensembles in the prefrontal cortex driving extinction learning in rats previously exposed to drugs of abuse or food self-administration [10–13]. More recently, a Tet-Off gene expression system coupled with multiple combinations of TRE-dependent viruses was used to tag linked ensembles of neurons in the hippocampus and the core subregion of the nucleus accumbens (NAcore) that mediate cocaine conditioned place preference (CPP) [14]. Authors showed the necessity and sufficiency of these ensembles to store and retrieve of the cocaine CPP memory, and in addition established a preferential connection between the neuronal ensembles in both regions. Concurrent with this study, another recent publication demonstrated necessity and enhanced connectivity amongst neuronal ensembles activated by repeated non-contingent cocaine exposure in the corticostriatal pathway [15]. Convergent findings from these and other recent studies using different biomarkers reveal that only ~2–5% of cells encode a putative cocaine ensemble, including deltaFosB immune-labeling [16], Daun02-inactivation of c-Fos expressing neurons [9], and in vivo recordings during reward-taking behavior [17]. Accumbens ensembles associated with non-contingent cocaine reward memories primarily recruit D1-medium spiny neurons (MSNs) [14], although D2-MSNs and parvalbumin+ interneurons were also identified in context-induced cocaine-seeking ensembles [18].
A few studies also used poly-reward procedures to investigate how competing rewards impact goal-directed behaviors. In vivo measurements of neuronal firing in NAc reveal ~20% overlap between neurons responding to self-administration of different types of reward such as cocaine, water, regular chow or sucrose [17, 19–22], while a study using fluorescence in situ hybridization reports that 50% of activated neurons in the infralimbic prefrontal cortex respond to both ethanol and saccharin [23]. Recently, a dual reward training procedure exposing Fos-LacZ rats to cocaine and palatable food pellets showed these rewards activate functionally different ensembles in the ventromedial prefrontal cortex [24].
Taken together, the data to date suggest a hypothesis that coding cocaine-associated behaviors in the nucleus accumbens involves finely tuned ensembles. Here we evaluate not only the tuning of ensembles, but the specificity of ensembles by testing two hypotheses. (1) Specific ensembles underlie cue-induced cocaine seeking. (2) Specific ensembles underlie cue-induced seeking of cocaine and biological rewards. To test the first hypothesis we employed a cocaine intravenous self-administration protocol followed by extinction training and cue-induced cocaine seeking. FosCreERxAi14 mice (c-Fos-TRAP) were trained on this protocol. This transgenic mouse line allows Cre-dependent expression of tdTomato (Tom+) in neurons expressing c-Fos only in the presence of 4-hydroxytamoxifen (4-OHT) [7], resulting in durable labeling of the neurons in the NAcore specifically activated during extinction learning in the self-administration context, and cued cocaine-seeking. We also employed c-Fos-TRAP mice in a new model of alternate cocaine and sucrose self-administration that uniquely allowed us to directly compare the NAcore ensemble associated with seeking an addictive drug (cocaine) versus an ensemble of neurons activated by seeking a biological reward (sucrose).
Materials and methods
c-Fos-TRAP mice
Male and female c-Fos-TRAP mice were obtained from crossing female Ai14 knock-in mice (B6.cCg-Gt(ROSA) 26Sortm14(CAG-tdTomato)Hze/J, stock# 007914) with male Fos-CreERT2 mice (B6.129(Cg)-Fostm1.1(cre/ERT2)Luo/J, Stock# 021882) [7] (The Jackson Laboratory, ME, USA). Fos-CreERT2 Wild Type/Ai14 heterozygous mice were used to validate the dual cocaine and sucrose SA. All groups were composed of both male and female c-Fos-TRAP mice, and all mice were single-housed on a 12:12 reverse light cycle throughout the experiments. Sample size resulted from previous mouse self-administration experience and 10% attrition rate on mouse intravenous drug self-administration, usually due to failed catheter patency. All mice were maintained on mild food restriction (with access to 80% of their daily intake) throughout behavioral testing to increase operant conditioning responses. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Assessment and Accreditation of Laboratory Animal Care.
Single and dual rewards self-administration, extinction training and cue-induced reward-specific seeking
At week 12 and older (20–25 g), all mice included in single cocaine or dual cocaine/sucrose self-administration underwent catheter implantation. All mice were anesthetized with isoflurane (induction 3–5% v/v, maintenance 1–2% v/v). An incision in the neck of the mouse allowed the insertion of an indwelling jugular catheter 10–12 mm into the vein. The catheter was sutured in place, and the entry port head-mounted and secured with dental cement. Single sucrose SA did not require additional surgery.
All groups underwent self-administration training on a FR1 (10 days) reinforcement schedule (2 h per day) in standard mouse modular test chambers (Med Associates, Fairfax, VT) equipped with two nose-pokes (NP). For mice undergoing single cocaine or sucrose self-administration, active NP resulted in delivery of a cocaine infusion (0.5 mg/kg/inf, NIDA, USA) or sucrose pellet (15 mg, Bio-Serv, NJ, USA) paired with a light cue inside of the NP, with the other NP serving as the inactive operand. Dual cocaine/sucrose self-administration mice self-administered both rewards on alternate days (1 type of reward/day), with groups counterbalanced for first day reward delivery. Within a cocaine session, poking of active NP port induced a cocaine infusion (0.5 mg/kg/inf, NIDA, USA) paired with a light cue inside of the NP port. Within a sucrose session, the sucrose reward (15 mg pellet, Bio-Serv, NJ, USA) and paired inside-port light cue were associated with NP of the inactive cocaine port to differentiate of cocaine versus sucrose seeking. Mice failing a brevital catheter patency test the last day of self-administration were discarded (8 out of 72 total mice catheterized for self-administration).
Following successful acquisition of self-administration (a minimum of 10 days of 10 infusions/pellets or more) mice entered extinction training, during which time a NP had no consequences. Cue-induced seeking was evaluated once extinction criterion was achieved, i.e., when the average active NPs during the 3 last days of extinction was equal or less than <30% of the average of the last 3 days of self-administration, which usually occurred after 10–12 days of extinction training. For dual cocaine and sucrose self-administration, the extinction criteria was similar to single reward, and was calculated in function of the average of the last 3 sessions of cocaine for the cocaine port and the last 3 sucrose sessions for the sucrose port.
Reinstated reward seeking was tested for 30 min without reward delivery and in the presence of reward-specific cues, i.e., cocaine/sucrose seeking was induced by presenting the light cue previously paired with cocaine/sucrose. In some mice we conducted a cue competition test that lasted 30 min, during which cocaine and sucrose cues were presented simultaneously and contingently in response to respective port entries.
Immununohistochemistry (IHC)
Mice were deeply anesthetized with a 2/1 combination of ketamine (body weight, Vedco, Missouri, USA) and xylaxine (Akorn, Illinois, USA), perfused with phosphate-buffered saline followed by 3.7% formaldehyde (Fisher Scientific, NH, USA), and brains were post-fixed for 24 h. Brains were sliced in ¼ series of 50 μm sections using a cryostat, allowing the use of alternate slices for multiple IHC experiments. Free-floating sections were rinsed in PBS-Triton (0.25%) and incubated in normal goat serum and primary antibodies (Anti-phospho-cFos, Cell Signaling Technology, #5348, 1:1000, MA, USA; Anti-NeuN, #MAB377, 1:1000, Millipore Sigma, MA, USA) overnight at 4 °C. After multiple PBS-Triton rinses, sections were incubated in Alexa-Fluor conjugated secondary antibodies (Life Technologies, 1:1000).
Ensemble acquisition and quantification
To allow tdTomato expression and tagging of different ensembles, mice were divided into 3 groups: cued-reinstatement (RST), extinction (EXT) test or home cage (HC), (Fig. 1a). Immediately following their respective behavioral test sessions, mice were injected with >70% Z isomer 4-OH-tamoxifen (4-OHT, 50 mg/kg, i.p.) [7] (Millipore Sigma, MA, USA). Mice in the control group with no 4-OHT received an i.p. vehicle injection instead. All mice were injected with saline daily for at least 5 days prior to 4-OHT injection, to habituate the mice and decrease injection-induced c-Fos expression. In this protocol, cells labeled by 4-OHT with tdTomato (Tom+) were active during the initial cued-seeking or extinguished behavior, while c-Fos immunoreactivity labeled cells that were active during the second seeking test.
Fig. 1. Cocaine seeking ensembles in the NAcore.
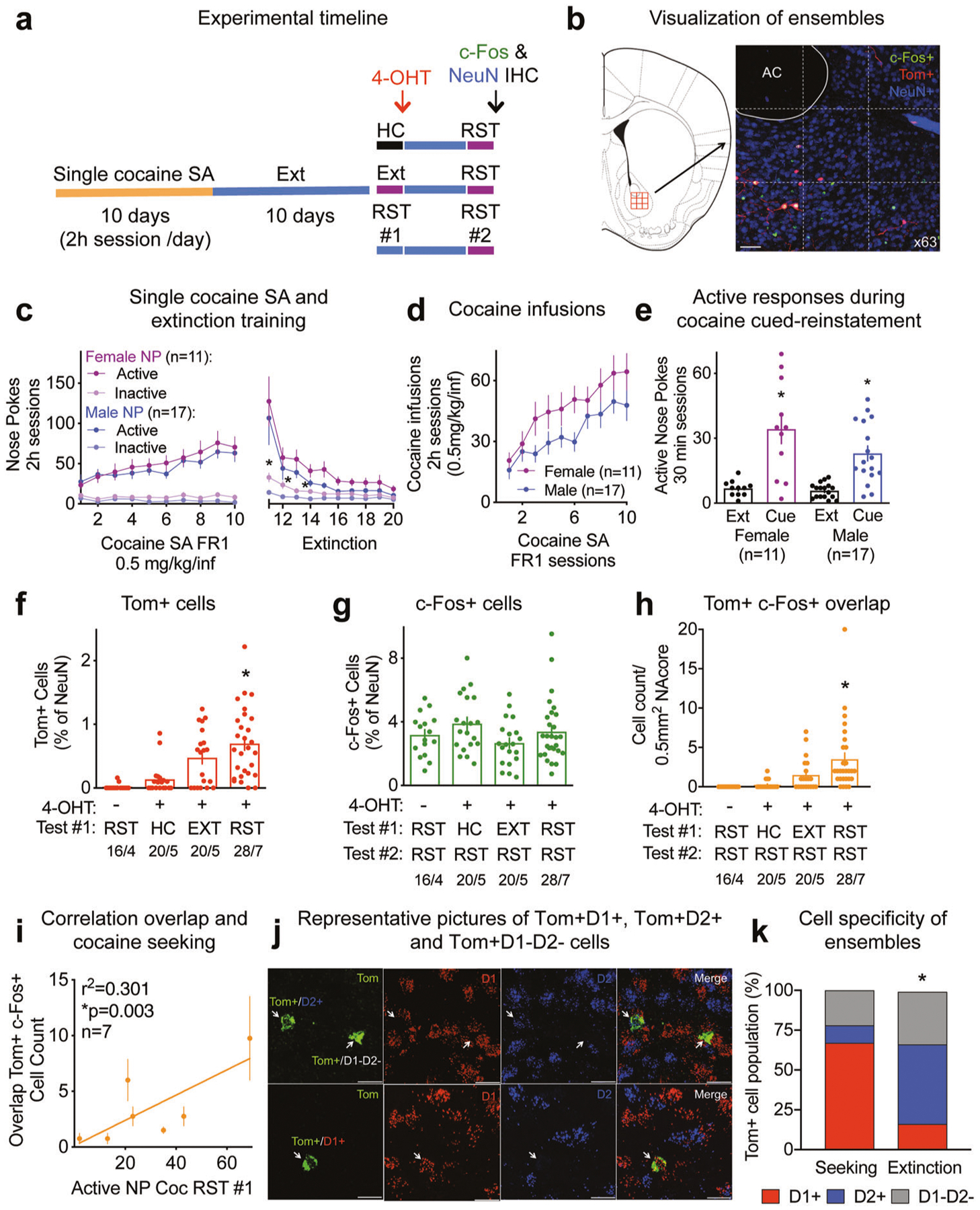
See Supplementary Table ST1 for full statistical analysis. a Experimental timeline. 4-OHT was injected in mice remaining in the home cage (HC), immediately after an extinction (EXT) session or the first cued-seeking reinstatement (RST). b Visualization of ensembles (Tom+ cells) and colocalization with c-Fos and NeuN immune-labeling. Automatic 3 × 3 tiles scanned the tissue to create a 0.5 mm2 mosaic of a portion of the NAcore. The micrograph shows tdTomato filling the soma and dendrites of cells activated during cocaine seeking (Tom+ in red), c-Fos expression in the nucleus (c-Fos+ in green), the overlap between the two in yellow and NeuN as a neuronal marker (NeuN+ in blue). Bar = 50 μm. c Male and female c-Fos-TRAP mice cocaine self-administration (0.5 mg/kg/inf) and extinction responding, *p < 0.05 comparing inactive NP between sexes. d Male and female c-Fos-TRAP mice cocaine intake. e Male and female c-Fos-TRAP mice active nose pokes during cue-induced reinstatement for cocaine, *p < 0.001 for extinction/reinstatement factor. f Percentage of Tom+ cells for No 4-OHT (− 4-OHT), Home cage (HC), EXT and RST groups, *p < 0.05 comparing to HC group. g Percentage of cells expressing c-Fos for − 4-OHT, HC, EXT and RST groups. h Cell count of cells co-expressing Tom and c-Fos in − 4-OHT, HC, EXT and RST groups, *p < 0.05 comparing to HC group. i Correlation between the number of cells co-expressing Tom+ and c-Fos+ and cocaine seeking measured as number of active nose pokes (NP) during reinstatement #1 (RST#1) for the RST/RST group (n = 7), *p = 0.003. j RNAscope representative pictures of Tom+D1+, Tom+D2+ and Tom+D1−D2− cells. Bar = 20 μm. k Proportion of D1+, D2+ and D1−D2− subpopulations in the seeking (n = 116 Tom+ cells, 7 mice) and extinction (n = 49 Tom+ cells, 4 mice) ensembles, *p < 0.0001 comparing seeking ensembles to the cells activated during extinction using Chi2 test as in Chi Square, NOT REFERENCE #2). Single cocaine self-administration behavior of the subset of mice used for RNAscope is included in Figs. 1c–e, S1a, b. Quantifications are presented as number of mosaics/animal, all statistical analysis are performed per animal.
We used super-resolution confocal imaging (DMi8 microscope, Leica, Germany) paired with the LAS X hyugens hyvolution deconvolution software to visualize the specific ensembles and their colocalization with c-Fos; our marker for neuronal activation. To minimize observer bias while quantifying the number of Tom+, c-Fos+ and NeuN+ cells, the anterior commissure was first set into the field of view empirically by an investigator blind to treatment groups. We next automatized the acquisition tiling of the 3 × 3 (512 × 512 pixels) array of images at 63× magnification in red for tdTomato (552 nm), green for c-Fos (488 nm) and far red for NeuN (638 nm). The stitching was also automatized, creating a 0.5 mm 2 9-tile mosaic of a portion of the NAcore, with a composite 1536 × 1536 pixel field (Fig. 1b). Once 3 × 3 tilled arrays were collected and stitched they were manually checked for accuracy and fidelity. Next, semi-automated cell counts were performed for each channel (tdTomato, c-Fos and NeuN) using IMARIS software (Bitplane, MA). During this process Tom+, c-Fos+ or NeuN+ cells were initially marked in an automated fashion based on of a particular nuclei or cell body seed size using the spots function (7 μm for c-Fos and NeuN and 10 μm for tdTomato) and the intensity of the signal in that channel (voxel intensity). All automated counts for each channel were then checked manually, by an investigator that was blind to treatment groups. We acquired up to 4 mosaics per animal. Quantification is expressed as number of mosaics/animal.
RNAscope® in situ hybridization
We used the RNAscope® multiplex fluorescent v2 assay (Advanced Cell Diagnostics, CA) to visualize and amplify target RNA: tdTomato (Probe-tdTomato-C1, Cat #317041), D1-MSN (Probe-Mm-Drd1a-C2, Cat #406491), D2-MSN (Probe-Mm-Drd2-C3, Cat #406501). We followed the detailed protocol provided in the kit, briefly mice brains were fresh frozen in −30 °C isopentane and cut in 16 μm sections at the cryostat. The sliced tissue underwent fixation, dehydratation, and hydrogen peroxide steps as instructed, but the protease step was skipped since it caused excessive tissue damage. After incubating the tissue with the corresponding probes for 2 h at 40 °C, and performing multiple washing steps, the signal was amplified, incubated with fluorophores (#NEL744E001KT TSA Plus Cyanine 3, #NEL741E001KT TSA Plus Fluorescein, #NEL745E001KT TSA Plus Cyanine 5, Perkin Elmer, MA, USA) and each channel was developed with its corresponding HRP signal. Tissue was counterstained with DAPI and results were visualized using the Leica confocal microscope system described above.
Data analysis and statistics
Complete statistical analysis is reported in Supplementary Tables ST1–ST4. Acquisition and quantification were performed by investigators blinded to treatment groups. When two groups were compared, the data were statistically analyzed using a two-tailed paired or unpaired Student’s t test. Differences in cell specificity between seeking ensembles and cells activated during extinction were determined using Chi2. To compare multiple measurements in the same experiment, the data were analyzed using 1- or 2-way ANOVAs. Dunnett’s or Sidak’s post hoc test were applied for multiple comparisons when applicable and p < 0.05 was considered statistically significant. All statistics were performed per animal, and values reported as mean ± SEM. Aside from the mice failing the brevital catheter patency test (8 out of 72), no samples or data were excluded from statistical analysis. Quantification of the overlap between tdTomato (Tom+) and c-Fos (c-Fos+) was calculated in function of the Tom+ cells as (Tom+c-Fos+) × 100/Tom+, and additionally normalized to chance [(Tom+/NeuN+) × (c-Fos+/NeuN+)] × 100 [6, 25]. Statistical tests were conducted using the Prism (Graphpad, La Jolla, CA) software package and all numerical data were analyzed using a D’Agostino-Pearson normality test followed by a Kruskal–Wallis or Mann–Whitney test when one or more groups were found to be not normally distributed.
Results
Cocaine seeking recruited specific ensembles
c-Fos-TRAP mice underwent 10 days of single cocaine self-administration (0.5 mg/kg/infusion) followed by 10–12 days of extinction training (Fig. 1a). To tag the cocaine seeking ensembles or cells activated during extinction, mice were injected with 4-OHT after a first cued-reinstatement (RST#1) or after an extinction session (EXT) (Fig. 1a). Following 3–7 days of additional extinction, all mice were reinstated (RST#2), perfused, and IHC was performed to visualize c-Fos and NeuN expression (Fig. 1b). In this protocol, cells labeled by 4-OHT with tdTomato (Tom+) were active during the initial cued-seeking or extinguished behavior, while c-Fos immunoreactivity labeled cells that were active during the second seeking test. There were no differences between male and female mice regarding active NP discrimination during acquisition of self-administration (Fig. 1c), but male mice showed accelerated extinction learning for the inactive NP (Fig. 1c). Likewise, cocaine intake (Fig. 1d) and cue-induced seeking (Fig. 1e, Supplementary Fig. S1A) were statistically equivalent between sexes; although there was a trend for males to self-administer less cocaine (Fig. 1d, p = 0.073). There was no significant ordering effect between reinstatements in the groups that were tested twice (Supplementary Fig. S1b).
Tomato expression was absent in a group that was injected with vehicle instead of 4-OHT (Fig. 1f), confirming that the tagging of c-Fos expressing cells was 4-OHT dependent. Injection with 4-OHT in mice remaining in the home cage (HC) showed a percentage of Tom+ cells statistically equivalent to the No 4-OHT group (− 4-OHT) (Fig. 1f), in support with previous results showing low c-Fos expression in a familiar environment [26, 27]. Mice injected with 4-OHT after cued-reinstatement (RST) displayed 0.7% of Tom+ neurons (Fig. 1f). This group of neurons, defined as the seeking ensemble, was significantly larger than the groups receiving vehicle (− 4-OHT), remaining in the home cage (HC) or mice that were tagged after an extinction session. The cells activated during extinction, although not significantly different than the control groups but showing a noticeable trend, reached 0.5% of Tom+ neurons, (Fig. 1f). Behavioral responses of the mice used to quantify Tom+ tagging are shown in Supplementary Fig. S1c. Immunoreactivity against c-Fos, measured directly after a 30 min cued-reinstatement session, was similar for all groups (Fig. 1g). Cocaine seeking behavior for the second test is shown in Supplementary Fig. S1d. c-Fos signal did not increase when measured 90 min after the beginning of the session, a time point traditionally used in the literature [28, 29] (Supplementary Fig. S1e). Tom+ and c-Fos+ cells were expressed as a function of the number of cells expressing the neuronal marker NeuN, which was statistically constant between treatment groups (Supplementary Fig. S1f). Reactivation of the cocaine-seeking ensemble, measured as the neurons in which Tom and c-Fos expression overlapped and expressed as cell counts/0.5mm2 of NAcore (Fig. 1h) and percentage of Tom+ cells (Supplementary Fig. S1g), reached 45% in the group undergoing two cue-induced reinstatement tests, a larger percentage than all other groups, including the overlap between the seeking ensemble and the cells activated during extinction, that reached 20% (Fig. 1h, Supplementary Fig. S1g). This remained true when the percentage of overlap of Tom and c-Fos expression was normalized by chance (Supplementary Fig. S1h). Interestingly, the extent of the Tom+/c-Fos+ overlap (as cell counts or percentage of Tom+ cells, Fig. 1i, Supplementary Fig. S1i), positively correlated with cocaine-seeking behavior. The percentage of Tom+ cells was also correlated with cocaine-seeking behavior (Supplementary Fig. S1j). No correlation was observed between c-Fos immunoreactive cells and cocaine-seeking behavior measured during the second cued-reinstatement (Supplementary Fig. S1k).
The cocaine-seeking ensemble recruited mostly D1-MSNs
Many convergent studies indicate that activity in D1-MSNs is recruited to initiate and sustain reward-seeking behaviors [30]. Using RNAscope® in situ hybridization (Fig. 1j), we determined in a subset of mice that 66.7 ± 4.8% of cells recruited in the cocaine-seeking ensemble were D1-MSNs (Fig. 1k). A minority (10.9 ± 2.9%) were D2-MSNs and the remaining 22.4 ± 4.3% were not labeled for either D1 or D2 mRNA. The lack of significant difference with the home cage group prevented us from clearly characterize the cells activated during extinction (Fig. 1f) as the extinction ensemble. However, within the trend of an increase number of activated cells during extinction testing, D2-MSNs constituted the biggest proportion of cells (50.4 ± 9.4%), while D1-MSNs only represented a small minority of cells (16.2 ± 6.4%) (Fig. 1k). A third of the cells activated during extinction testing did not express the D1 or D2 receptor (33.4 ± 8.1%).
Sucrose-seeking and extinction recruited different ensembles
c-Fos-TRAP mice underwent single sucrose self-administration and extinction training using a protocol identical to single cocaine self-administration (Fig. 2a). Male mice emitted more NPs for sucrose pellets than female mice during self-administration acquisition and extinction (Fig. 2b). There was also a significant effect on inactive NP throughout the sessions, but no differences between male and female (Fig. 2b). Despite the sex difference in self-administration and extinction training on active NPs, there were no differences between male and female mice regarding cue-induced seeking, measured as active nose poking (Fig. 2d), and only a trend in sex differences in sucrose intake (Fig. 2c; p = 0.090). There was however a significant difference between sexes for inactive NP during reinstatement (Supplementary Fig. S2a). There was no significant ordering effect between reinstatements in the groups that were tested twice (Supplementary Fig. S1b).
Fig. 2. Sucrose seeking ensembles in the NAcore.
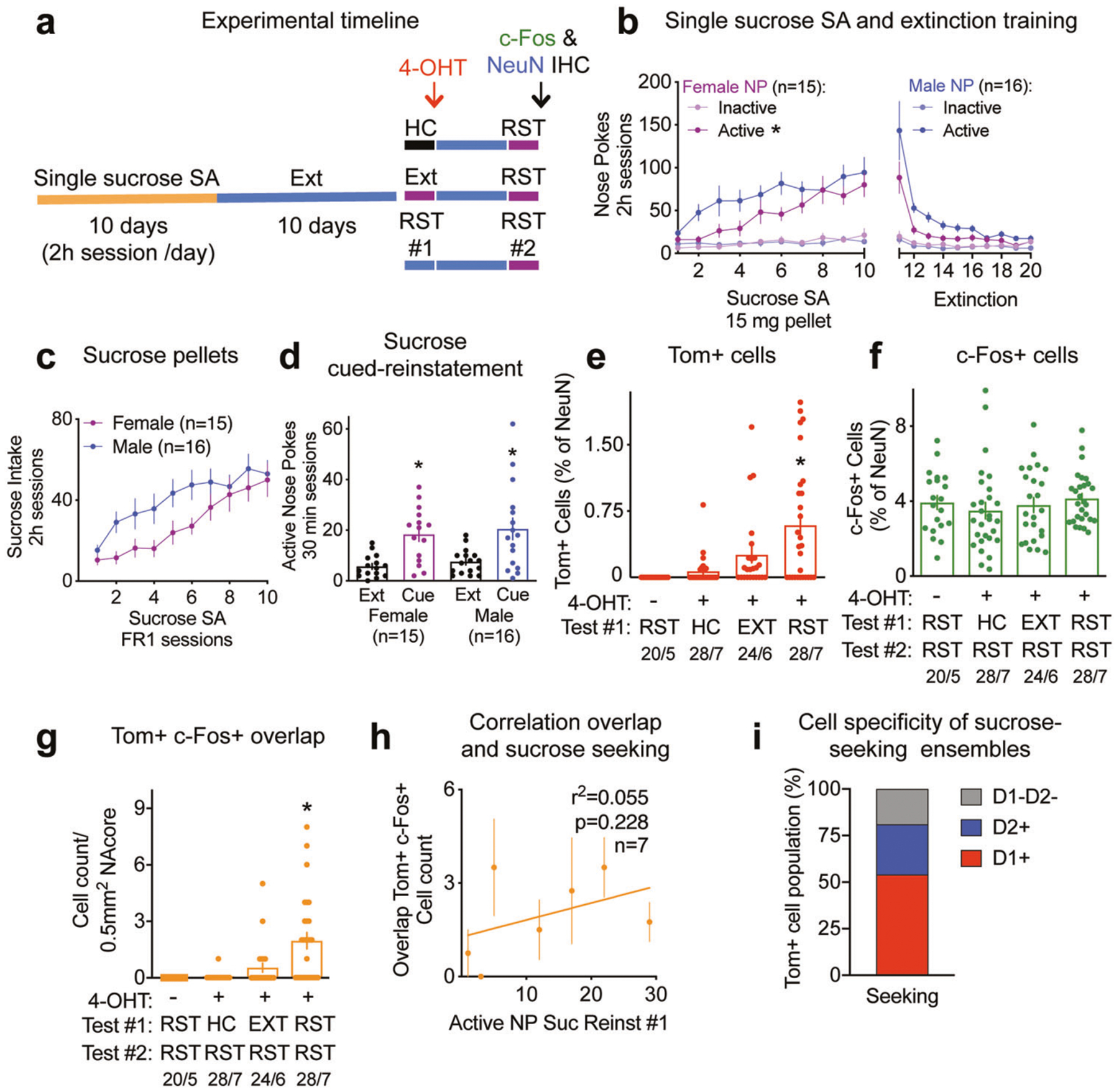
See Supplementary Table ST2 for full statistical analysis. a Experimental timeline. b Male and female c-Fos-TRAP mice sucrose self-administration and extinction training, *p < 0.001 for active nose pokes, sex factor. c Male and female c-Fos-TRAP mice sucrose intake. d Male and female c-Fos-TRAP mice active nose pokes during cue-induced reinstatement for sucrose, *p < 0.001 for extinction/reinstatement factor. e Percentage of Tom+ cells for − 4-OHT, HC, EXT, RST groups, *p < 0.05 comparing to HC group. f Percentage of cells expressing c-Fos for − 4-OHT, HC, EXT, RST groups. g Cell count of cells co-expressing Tom and c-Fos for − 4-OHT, HC, EXT and RST groups, *p < 0.05 comparing to HC group. h Correlation between the number of cells co-expressing Tom+ and c-Fos+ and sucrose seeking measured as number of active nose pokes (NP) during reinstatement #1 (RST#1) for the RST/RST group (n = 7), p = 0.228. i Proportion of D1+, D2+ and D1−D2− subpopulations in the sucrose-seeking ensemble (n = 131 Tom+ cells, 6 mice). Quantifications are presented as number of mosaics/animal, all statistical analysis are performed per animal.
The sucrose-seeking ensemble, measured as percentage of Tom+ cells in the RST+4–0HT group, was significantly larger than all the other groups, including the vehicle group (no 4-OHT), mice receiving 4-OHT after remaining in the home cage (HC) or after an extinction session (EXT) (Fig. 2e). Behavioral responses of the mice used to quantify Tom+ tagging are shown in Supplementary Fig. S2c. Immunoreactivity for c-Fos remained unchanged between all groups (Fig. 2f). Sucrose seeking behavior for the second test is shown in Supplementary Fig. S2d. Tom+ and c-Fos+ cells were expressed in function of the number of cells expressing the neuronal marker NeuN, that was statistically equivalent between groups (Supplementary Fig. S2e). The number of cells co-expressing Tom and c-Fos in the group of mice undergoing two cue-induced reinstatements was significantly higher than the control HC group (Fig. 2g). The overlap of Tom and c-Fos reached 30% in the group undergoing two reinstatements, while no reactivation of the sucrose-seeking ensemble was observed in the −4-OHT and HC groups (Supplementary Fig. S2f). The extinction group showed a trend towards a higher Tom and c-Fos overlap, but this was not significantly different than the control groups (Fig. 2g, Supplementary Fig. S2f). When normalized by chance, only the overlap of the group undergoing two reinstatements was significantly higher than chance (Supplementary Fig. S2g). Although there was no significant correlation between Tom and c-Fos overlap and sucrose seeking (Fig. 2h, Supplementary Fig. S2h), the percentage of Tom+ cells was positively correlated to sucrose seeking during test #1 (Supplementary Fig. S2i). No correlation was observed between c-Fos immunoreactive cells and sucrose seeking behavior measured during the second cued-reinstatement (RST#2) (Supplementary Fig. S2f).
The sucrose-seeking ensemble recruited mostly D1-MSNs
Similar to the cocaine-seeking ensemble (Fig. 1k), the sucrose-seeking ensemble was mostly composed of D1-MSNs (54.08 ± 6.35%), with a smaller proportion of D2-MSNs (27.21 ± 9.38%) and of cells expressing neither (18.71 ± 8.11%) (Fig. 2i). When presented as single rewards, the composition of cocaine- and sucrose-seeking ensembles were thus statistically identical (Chi-square test, Chi2 = 4.91, p = 0.086).
Cue-induced reward-specific seeking after dual cocaine and sucrose self-administration
To compare the sucrose to cocaine seeking ensembles within a mouse, we developed a dual reward protocol where mice underwent cocaine and sucrose self-administration on alternate days, followed by extinction training (Fig. 3a). c-Fos-TRAP mice and WT littermates exhibited significant discrimination between active and inactive NP by cocaine session 2 (Fig. 3b). Mice nose poked more in the cocaine port throughout sucrose sessions, as seen by the saw-tooth appearance of the inactive NP (Fig. 3b), and when comparing nose poke discrimination during stable self-administration, i.e., cocaine and sucrose sessions 6–10 (Fig. 3c). The increased preference for the cocaine port during a sucrose SA session is also revealed in a time course analysis of the 6th sucrose and cocaine session where there was a significant interaction between the ports and time in the cocaine session (Supplementary Fig. S3a) but not in the sucrose session (Supplementary Fig. S3b). Poking was comparable for both ports during extinction training (Fig. 3d). Cocaine and sucrose intake were comparable throughout SA when male and female mice were pooled (Fig. 3e). However, comparing between sexes revealed that while cocaine intake was similar (Fig. 3f), female mice obtained more sucrose pellets than males (Fig. 3g). Importantly, when mice were presented with the cocaine-associated cue, they nose poked significantly more in the port previously associated with cocaine compared to extinction and to the sucrose port (Fig. 3h). Potentiated sucrose seeking was similarly observed when the cue previously associated with sucrose was presented (Fig. 3i). Both males and females maintained a significant preference for the cocaine port when presented with the cocaine-paired cue (Supplementary Fig. S3c). The sucrose-paired cue induced a significant increase in nose poking for the sucrose port, however when divided by sex, there was no significant preference for the sucrose over the cocaine port (Supplementary Fig. S3c). When both cocaine- and sucrose-paired cues were presented simultaneously in a subset of mice, they reinstated to both rewards (i.e., nose poked significantly more than during extinction), but had a preference for the cocaine port (Fig. 3j). This preference became a trend when mice were divided by sex (Supplementary Fig. S3d).
Fig. 3. Dual cocaine and sucrose self-administration and cue-induced reward-specific seeking.
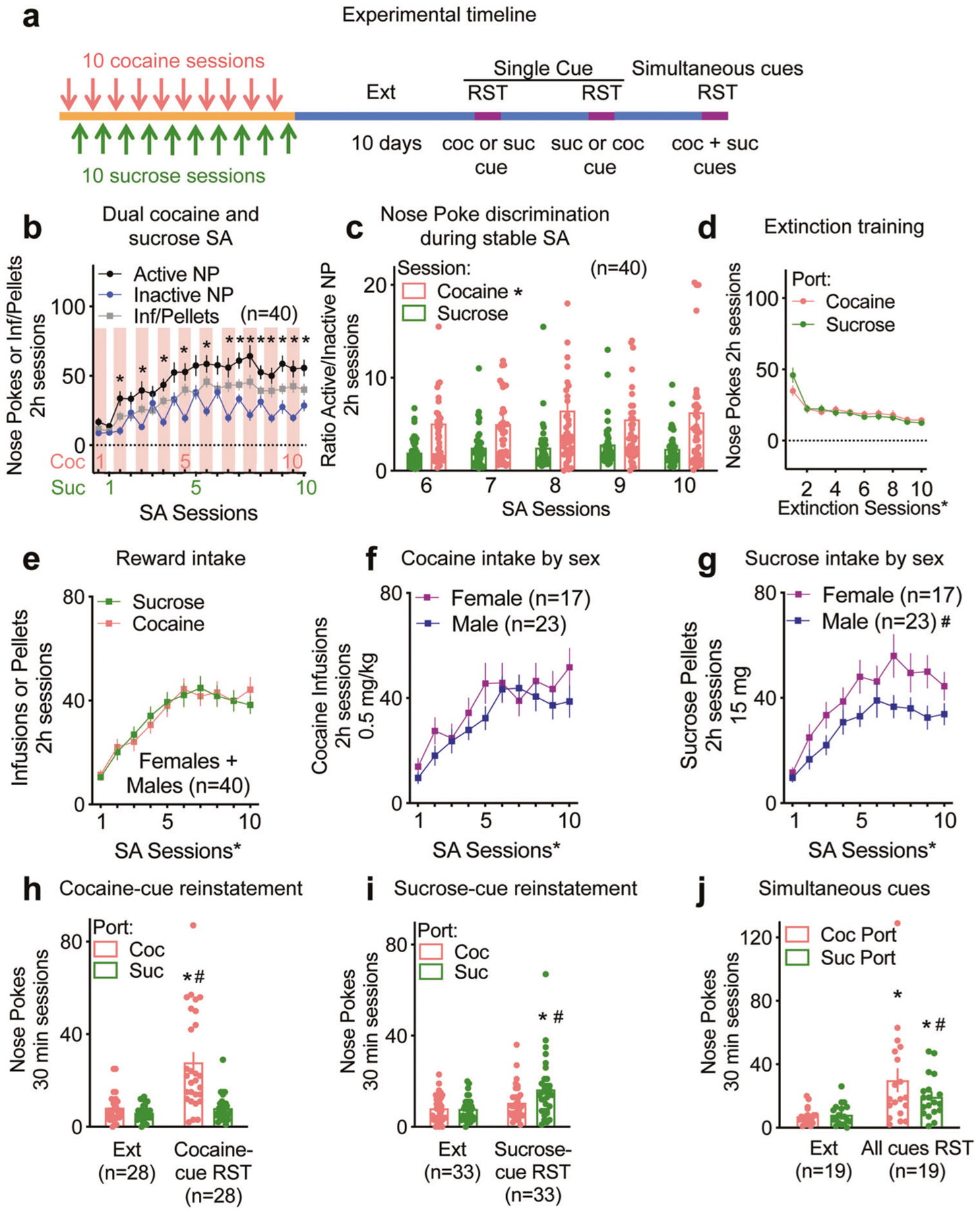
See Supplementary Table ST3 for full statistical analysis. a Experimental timeline of the dual cocaine (coc) and sucrose (suc) self-administration and reinstatements (RST). b Dual cocaine (coc) /sucrose (suc) self-administration, *p < 0.05 comparing active to inactive NP. c Nose Poke discrimination during stable SA (n = 40), *p < 0.05 comparing ports. d Extinction training following dual cocaine and sucrose self-administration, *p < 0.05 comparing extinction sessions. e Cocaine and sucrose intake pooling male and female c-Fos-TRAP mice, *p < 0.05 comparing SA sessions. f Comparison of cocaine intake between male and female c-Fos-TRAP mice, *p < 0.05 comparing SA sessions. g Comparison sucrose intake between male and female c-Fos-TRAP mice, *p < 0.05 comparing SA sessions, #p < 0.05 comparing sexes. h After undergoing dual cocaine and sucrose self-administration and extinction (Ext), a subset of mice were reinstated the cocaine-cue only (cocaine-cue RST), *p < 0.05 comparing extinction to reinstatement, #p < 0.05 comparing cocaine to sucrose port. i After undergoing dual cocaine and sucrose self-administration and extinction (Ext), a subset of mice were reinstated the sucrose-cue only (sucrose-cue RST), *p < 0.05 comparing extinction to reinstatement, #p < 0.05 comparing cocaine to sucrose port. j Simultaneous cue reinstatement in a subset of mice (n = 19), where an NP in any port results in presentation of the cue inside the port. Despite the lack of significance for the port factor, the significant interaction allowed for a Sidak’s multiple comparisons post hoc test that revealed a preference for the cocaine port during all cues reinstatement, *p < 0.05 comparing extinction to reinstatement, #p < 0.05 comparing cocaine to sucrose port.
Cocaine and sucrose seeking ensembles were segregated in the NAcore
After undergoing dual cocaine and sucrose self-administration (Fig. 4a), the 4-OHT tagged cocaine-seeking ensemble was similar in size (~0.9% of NAcore neurons) to the seeking ensembles for cocaine or sucrose self-administered in Figs. 1 and 2 as single rewards (Fig. 4b). However, the sucrose-seeking ensemble was smaller than the cocaine ensemble when mice were exposed to dual self-administration (Fig. 4b). Behavioral responses of the mice used to quantify Tom+ tagging are shown in Supplementary Fig. S4a. Immunoreactive c-Fos expressing neuron number was similar when mice were sacrificed after cocaine- or sucrose-seeking (Fig. 4c). Reward-specific seeking behavior for the second test is shown in Supplementary Fig. S4b. Tom+ and c-Fos+ cells were expressed in function of the number of cells expressing the neuronal marker NeuN, which did not significantly differ between groups (Supplementary Fig. S4c). Notably, the number of cells co-expressing Tom and c-Fos was significantly higher in the group undergoing two cocaine-cue reinstatements than the group exposed first to cocaine-cues followed by sucrose-cues (Fig. 4d). When expressed in percentages of Tom+ neurons, the overlap between Tom+ and c-Fos+ cells reached ~60% when mice sought cocaine twice and decreased to less than 30% when mice were exposed to the two opposing sucrose or cocaine cue (Supplementary Fig. S4d). Similar to cocaine, co-expression of Tom and c-Fos was significantly higher when repeated exposure to sucrose-cues induced seeking repeatedly compared to alternative exposure to sucrose-paied followed by cocaine-paired cues (Fig. 4e). Likewise, the overlap between sucrose-seeking ensembles reached 69%, while the overlap between sucrose- and cocaine-seeking ensembles when the sucrose ensemble was tagged with TdTomato only overlapped by 23% (Supplementary Fig. S4e). When the percentage of overlap between Tom+ and c-Fos+ cells was normalized by chance, the significant decrease between same or different rewards was maintained (Supplementary Fig S4f, g). We found a significant negative correlation between the extent of the cocaine- and sucrose-seeking ensembles overlap expressed as cell counts (Fig. 4f) or percentages of Tom+ cells (Supplementary Fig S4h) and the strength of cocaine seeking, measured as NPs in the cocaine-paired port during cue-induced cocaine reinstatement. A similar trend was observed for sucrose seeking, although the correlation did not reach significance (Fig. 4g, Supplementary Fig S4i).
Fig. 4. Within subject reward-specific ensembles in the NAcore.
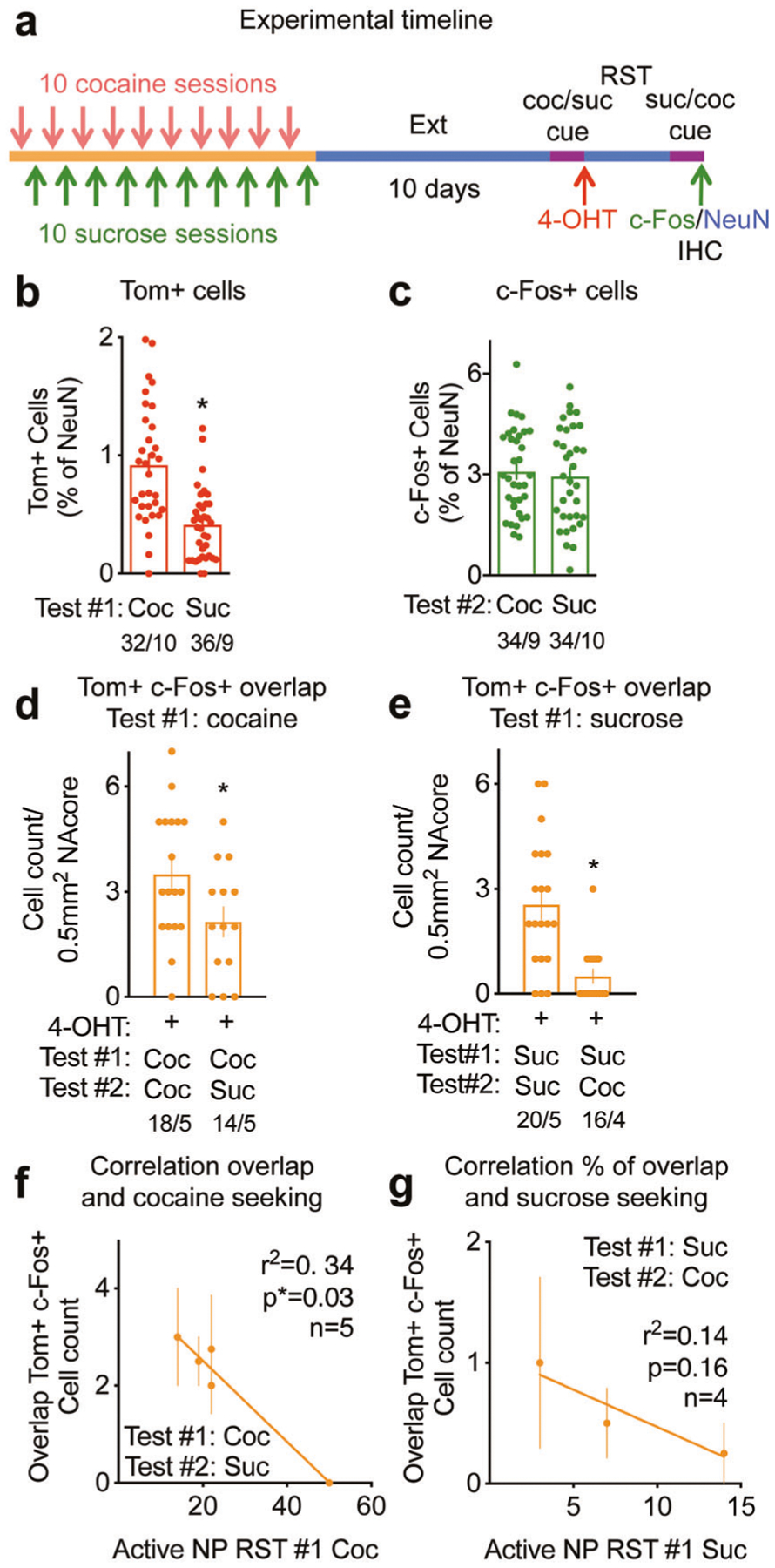
See Supplementary Table ST4 for full statistical analysis. a Experimental timeline of the dual cocaine (coc) and sucrose (suc) self-administration, reinstatements (RST) and subsequent reward-specific ensemble tagging. b Percentage of Tom+ cells after cocaine (coc) or sucrose (suc) seeking during RST#1 (Test#1), *p = 0.005. c Percentage of cells expressing c-Fos after cocaine (coc) or sucrose (suc) seeking during RST#2 (Test#2), p = 0.59. d Cell count of cells expressing Tom and c-Fos in mice that underwent two cue-induced reinstatements for cocaine (Coc/Coc) or a first cue-induced reinstatement for cocaine and a second for sucrose (Coc/Suc), unpaired t test, *p = 0.03. e Cell count of cells expressing Tom and c-Fos in mice that underwent two cue-induced reinstatements for sucrose (Suc/Suc) or a first cue-induced reinstatement for sucrose and a second one for cocaine (Suc/Coc), *p = 0.004. f Correlation between the number of cells co-expressing Tom+ and c-Fos+ and cocaine seeking measured as number of active nose pokes (NP) during reinstatement #1 (RST#1) for the Test #1 Cocaine/Test #2 Sucrose group (n = 5), *p = 0.03. g Correlation between the number of cells co-expressing Tom+ and c-Fos+ and sucrose seeking measured as number of active nose pokes (NP) during reinstatement #1 (RST#1) for the Test #1 Sucrose/Test #2 Cocaine group (n = 4), p = 0.16. Quantifications are presented as number of mosaics/animal, all statistical analysis are performed per animal.
Discussion
Using a semi-automated confocal microscopy acquisition strategy in c-Fos-TRAP mice we identified 0.5–0.9% of neurons in the NAcore that form ensembles in the NAcore and were associated with cocaine or sucrose seeking and extinction. To compare drug- to sucrose-seeking within the same mouse, we developed a model wherein mice self-administered both rewards on alternate days and reward-specific seeking was triggered by distinct reward-specific cues. The data showed that cocaine and sucrose seeking formed distinct ensembles that overlap only partially (up to 30%). Importantly, the percentage of ensemble overlap was negatively correlated to reward seeking responses, implying that overlapping ensemble neurons may be cells that either do not contribute to seeking or may actively blunt seeking behavior.
Seeking ensembles after single reward SA
Using cocaine or sucrose self-administration as single rewards, we found that there was one distinct ensemble that associated with seeking. In mice trained to self-administer only cocaine, a separate extinction ensemble seemed to emerge (Fig. 1f), but its size failed to show statistical significance compared to the home cage control group (p = 0.17). This was also the case in sucrose-trained mice, for which the sucrose-seeking ensemble was significantly larger than the cells activated during extinction (Fig. 2e). The overlap between the cells activated during extinction learning and seeking ensembles likely involves neurons encoding memories of the operant context, while the neurons forming distinct ensembles more likely code for the different behaviors initiated by the extinguished context versus the context when seeking was reinstated by cocaine-paired cues.
We found the cocaine- and sucrose-seeking ensembles in the NAcore recruits mainly D1-MSNs (Figs. 1k, 2i), while D2-MSNs count for half of the composition of the cells activated during extinction testing (Fig. 1k). This result converges with the prevalent argument that activation of the D1 pathway promotes reward seeking, while D2 pathway activation broadly leads to aversion or reduced seeking [31–33]. In addition, we have reported a transient potentiation of NAcore D1-MSNs during cocaine seeking [34] and an increase of D2-/D1-MSNs ratio of AMPA/NMDA associated to extinction learning in the shell subregion of the NAc [35]. When evaluating the types of cells activated during extinction testing (Fig. 1k), the fact that this cells only show a trend towards a difference with cells activated at baseline should be considered (Fig. 1f). While RNAscope data reflecting a majority of D2-MSNs fits with the literature, D2-MSNs could be recruited to have a different role than promoting extinction training. It is indeed likely that the profile of Tom+ activated at baseline (HC) is similar to the D2-MSNs one activated during the extinction session. In support of this hypothesis, a study focused on cell-specific LTD in the NAcore showed that, similar to the dorsal striatum, basal electrophysiological and synaptic properties differ between D1- and D2-MSNs in the NAcore, as the basal frequency of miniature EPSCs is significantly greater in D2-MSNs compared to non-D2-MSNs [36]. Similarly, D2-MSNs Ca2+ transient frequency at baseline is 5 times higher than D1-MSNs [31]. Other studies however, report similar proportions of D1- and D2-MSNs recruited in methamphetamine-seeking ensembles [37, 38]. The discrepancy might result from several factors including region specificity, the cited papers focusing on the dorsal striatum whereas here we narrowed ensemble characterization to the ventral region of the striatum, where the direct and indirect pathways are less clear [39, 40]. Another important distinction between these studies is the presence of extinction learning versus forced or voluntary abstinence inducing progressive increase of drug seeking, or incubation of craving [41], which we do not test for in our procedure. As a whole however, this study (Figs. 1k, 2i) together with others [18, 34, 37, 38] suggest that drug-related ensembles recruit primarily MSNs, but no cell type is exclusively recruited in one type of ensemble. The non-negligible portion of D2-MSNs in the cocaine-seeking ensemble (10.9%) might project to the same glutamatergic, GABAergic or enkephalin-expressing cell populations in the ventral pallidum as D1-MSNs [39, 40, 42] and thus have a similar impact on drug seeking, or it could be part of the 20% overlap of cells between the cocaine-seeking ensemble and the cells activated during extinction reported in Fig. 1h and Supplementary Fig. 1g. Along with MSNs, reward-seeking ensembles likely include other specific cell types commonly found in the NAc such as parvalbumin, somatostatin or cholinergic interneurons [18].
Note that Tom+/c-Fos+ overlap correlates with cocaine seeking but not sucrose seeking behavior. We believe this might reflect the stronger codification of the drug ensemble (cocaine) compared to the one linked to the natural reward (sucrose). In support of this idea, mice undergoing single cocaine self-administration display a higher percentage of Tom+/c-Fos+ compared to mice undergoing single sucrose self-administration, indicating that more Tom+ cells are reactivated during the second cocaine-seeking test than during the sucrose-seeking one. Cocaine-seeking behavior was also found to be more robust than sucrose-seeking (Figs. 1e, 2d), an assessment previously observed in our hands [43].
In addition to the accumbens, other studies using different biomarkers for ensembles find that the prefrontal and orbitofrontal cortices encode context-induced operant responding for heroin during reinstatement and after extended abstinence inducing incubation of craving, respectively [44, 45]. Ensembles mediating extinction of reward seeking have been identified in the prefrontal cortex as distinct from ensembles controlling reward self-administration [10–13]. Although further work needs to be done, the fact that these cortical regions project to NAcore opens the possibility that a circuit ensemble exists where these cortical ensembles are projecting to and stimulating ensembles in the NAcore. This possibility is supported by recent findings showing preferential functional and synaptic connectivity between neuronal ensembles in the CA1 region of the hippocampus and the NAcore, both ensembles coding for cocaine conditioned place preference [14].
Dual cocaine and sucrose self-administration and reward-specific seeking
The cocaine and sucrose self-administration model we developed adds to designs of dual self-administration that have been previously reported [17, 46–50]. A few studies examined the specificity of ensembles after exposure to several rewards. In a series of studies comparing in vivo neuronal firing in the NAc during self-administration of cocaine or different types of natural rewards such as water, regular chow or sucrose [17, 19–22], authors tracked the proportion of neurons responding to one specific reward or to the two different available rewards. They report that between 21–33% of recorded neurons show the same pattern of discharge for sucrose or cocaine rewards at different stages of self-administration, and conclude that most neurons display non-overlapping patterns of activity during responding for sucrose vs. cocaine [17]. In contrast, another study using fluorescence in situ hybridization reports a 50% overlap between neurons activated by both ethanol- and saccharin-paired cues in the infralimbic prefrontal cortex, concluding that ensembles underlying cue-induced seeking of these different rewards largely overlap [23]. Our procedure, in combination with the c-Fos-TRAP mice, similarly allowed within subjects comparisons between the cocaine and sucrose cue-induced seeking ensembles, and characterized ensembles in the NAcore activated by cues in absence of rewards, thereby preventing the latter from directly influencing ensemble-tagging [51].
While cocaine was delivered i.v. and sucrose pellets are ingested, reward intake was similar for both cocaine and sucrose (Fig. 3e). Achieving equivalence in the intensity of active NP operant responding during self-administration acquisition was important because the non-rewarded seeking response is in part regulated by the intensity of the rewarded seeking response [52]. A significant preference for the cocaine-paired port over the sucrose-paired port when both reward-specific cues were available simultaneously (Fig. 3j) corroborates a previous study showing that although mice choose food over cocaine when rewards are available, following extinction training the cue previously associated with cocaine becomes more salient [47]. The relative greater motivational value of cocaine compared to sucrose-associated stimuli is also indicated by the saw-tooth shape of responding on the inactive port (Fig. 3b) produced by a higher level of nose pocking in the cocaine port (i.e., the inactive port during sucrose sessions) throughout sucrose sessions (Fig. 3c and Supplementary Fig. S3a, b).
Notably, the cocaine- and sucrose-seeking ensembles were similar in size in the dual reward model (~1% of NAcore) when mice self-administer the rewards separately. However, while the cocaine-seeking ensemble remained at ~1%, the sucrose-seeking ensemble shrank to ~50% the size of the cocaine ensemble (Fig. 4b). This difference in the seeking ensemble sizes parallels the stronger seeking response induced by cocaine cues compared to sucrose cues (Figs. 3h, i), and corroborates our previous study reporting low cue-induced seeking for sucrose in mice, despite strong self-administration acquisition [43].
Ensemble targeting strategies and impact on reward-specific ensembles
Cell populations tagged with immunohistochemistry (i.e., c-Fos+ cells, ~4%) were larger than those measured by targeted recombination (i.e., Tom+ cells, ~1%), which is similar to what has been reported in the literature [7, 25, 53, 54]. The possibility of the ensemble expanding in neuron number between reinstatements seems unlikely, since c-Fos expression decreases with repeated exposure to the same context or experience [26, 27]. We hypothesize c-Fos dependent Cre-recombination during RST#1 is less efficient than direct c-Fos protein expression during RST#2, since Cre detection requires expressing Cre linked to the estrogen receptor (ER), ER-4-OHT binding, Cre translocation to the nucleus, and subsequent recombination of floxed STOP codon to disinhibition of Tom expression [7]. Additionally, c-Fos protein synthesis requires consistent high levels of Ca2+ influx [4], suggesting that only strong consistent activity will induce expression. Different intensities of immunoreactive c-Fos expression are seen in Fig. 1a, posing the possibility that in cells with low c-Fos expression the process leading to tdTomato expression may not be completed.
Notably, the percentage of Tom+ cells consistently correlated with seeking behavior (Supplementary Fig S1j, S2i), while c-Fos immunoreactive cells that remained equivalent between seeking groups across all experiments (Figs. 1g, 2f, 4c) did not (Supplementary Fig S1k, Fig S2j). This suggests that the TRAP tagging approach might present a greater sensitivity to identify behavior-specific neuronal ensembles relative to c-Fos immunostaining, as proposed in the original study using this technique [7]. To account for this difference, we included counterbalanced exposure to reinstatements to tag different reward-specific ensembles in the dual paradigm and found a similar effect on Tom+/c-Fos+ overlap regardless of reinstatement order (Figs. 4d, e, Supplementary Fig. S4d, e). Interestingly, when mice were exposed to the two rewards, the percentage of overlap within a reward (groups cocaine/cocaine and sucrose/sucrose, Supplementary Fig. S4d, e) was higher than for single rewards. We interpret this result as a more stable allocation of the ensemble cells (Tom+ cells in our study) when discrimination between multiple rewards is needed.
Regarding the overlap between cocaine- and sucrose-seeking ensembles, we found that 30% of the cells activated during cocaine seeking are also activated during sucrose seeking and vice-versa (Figs. 4d, e, Supplementary Fig. S4d, e). This overlap could be due to a presumptive “context ensemble” activated when the mouse is in the box, where everything but the nose pokes is similar between a cocaine and a sucrose-seeking reinstatement test, or to the neurobiological changes induced by cocaine exposure.
Conclusions
To conclude, we developed a novel dual cocaine and sucrose self-administration mouse model that allowed within-subject characterization of reward-specific ensembles. We showed that cocaine-seeking ensembles recruit ~1% of cells in the NAcore, mostly constituted of D1-MSNs. In parallel to seeking ensembles, an extinction learning ensemble recruiting a majority of D2-MSNs seemed to emerge, although its small size prevented it from being statistically distinct from control groups when analyzing the results per mouse. The described seeking ensembles are specific to and correlate with seeking behavior, with very similar results found for sucrose-seeking ensembles. We further established a ~70% distinction between cocaine- and sucrose-seeking ensembles in the NAcore. Similarly, we observed a ~80% distinction between cocaine- or sucrose-seeking ensembles and the cells activated during extinction learning in the extinguished context. These data show that despite many common features between the protocols, notably being in an identical operant context, NAcore ensembles are largely formed in response to more nuanced aspects of the protocol that affect motivation to behave and behavior selection. For example, the ensembles are coding the difference between cocaine and sucrose cues, and the relative motivation of mice to seek reward depending on the motivational valence of the context (i.e., extinction learning versus seeking).
Since constitutive and transient plasticity in the NAcore has been shown to be critical to reward-seeking behaviors [55], it is important for future studies to investigate the electrophysiological properties of the cells recruited by the different reward-specific seeking ensembles. Although it has been established that cells included in these ensembles display robust reward-induced plasticity, some discrepancy remains about ensemble-specific potentiation, via potentiated AMPA/NMDA ratios [14], spine diameter [14] and neuron excitability [56–58], or synaptic scaling via increase of silent synapses and decreased AMPA/NMDA ratios due to prolonged activity [59–61]. Furthermore, in light of reward-memory ensembles emerging throughout the brain [62, 63] and considering the well-described role of the afferent cortico-striatal and efferent striato-pallidal pathways on goal-directed behaviors, dissecting these connections in an ensemble-specific fashion [8, 14, 64] will presumably identify not only preferential inter-regional connectivity between ensembles, but possibly between reward-specific ensembles as well [24]. While further research is needed, if the distinct ensembles are shown to harbor distinct neuronal subtypes it may be possible to use cell-selective targeting approaches to specifically modulate drug reward seeking while leaving natural reward seeking intact.
Supplementary Material
Acknowledgements
The authors thank Drs. Chris Cowan and Makoto Taniguchi for kindly providing the initial cFos-TRAP mice, Jordan Hopkins and Dr. Ahlem Assali for their help optimizing the RNAscope protocol, and the members of the Kalivas lab for helpful comments on the manuscript. This work was supported by NIH DA046522 (A-CB), DA040004 (MDS), DA003906 (PWK), DA12513 (PWK).
Footnotes
Supplementary information The online version of this article (https://doi.org/10.1038/s41380-020-00888-z) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Thorndike EL. Animal intelligence; experimental studies. New York: The Macmillan company; 1911. [Google Scholar]
- 2.American Psychiatric Association., American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th edn. Washington, D.C.: American Psychiatric Association; 2013, xliv, 947 p.pp. [Google Scholar]
- 3.Hadad NA, Knackstedt LA. Addicted to palatable foods: comparing the neurobiology of Bulimia Nervosa to that of drug addiction. Psychopharmacology. 2014;231:1897–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, et al. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14:743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitaker LR, Hope BT. Chasing the addicted engram: identifying functional alterations in Fos-expressing neuronal ensembles that mediate drug-related learned behavior. Learn Mem. 2018;25:455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–3. [DOI] [PubMed] [Google Scholar]
- 7.Guenthner CJ, Miyamichi K, Yang HH, Heller HC, Luo L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron. 2013;78:773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye L, Allen WE, Thompson KR, Tian Q, Hsueh B, Ramakrishnan C, et al. Wiring and molecular features of prefrontal ensembles representing distinct experiences. Cell. 2016;165:1776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren BL, Kane L, Venniro M, Selvam P, Quintana-Feliciano R, Mendoza MP et al. Separate vmPFC ensembles control cocaine self-administration versus extinction in rats. J Neurosci. 2019;39:7394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren BL, Mendoza MP, Cruz FC, Leao RM, Caprioli D, Rubio FJ, et al. Distinct Fos-expressing neuronal ensembles in the ventromedial prefrontal cortex mediate food reward and extinction memories. J Neurosci. 2016;36:6691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laque A, LDN G, Wagner GE, Nedelescu H, Carroll A, Watry D, et al. Anti-relapse neurons in the infralimbic cortex of rats drive relapse-suppression by drug omission cues. Nat Commun. 2019;10:3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suto N, Laque A, De Ness GL, Wagner GE, Watry D, Kerr T et al. Distinct memory engrams in the infralimbic cortex of rats control opposing environmental actions on a learned behavior. Elife. 2016;5:e21920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Y, Zhu H, Liu Z, Chen X, Su X, Ma C et al. A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci. 2019;22:1986–99. [DOI] [PubMed] [Google Scholar]
- 15.Wall NR, Neumann PA, Beier KT, Mokhtari AK, Luo L, Malenka RC. Complementary genetic targeting and monosynaptic input mapping reveal recruitment and refinement of distributed corticostriatal ensembles by cocaine. Neuron. 2019;104:916–30.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattson BJ, Koya E, Simmons DE, Mitchell TB, Berkow A, Crombag HS, et al. Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur J Neurosci. 2008;27:202–12. [DOI] [PubMed] [Google Scholar]
- 17.Cameron CM, Carelli RM. Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. Eur J Neurosci. 2012;35:940–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz FC, Babin KR, Leao RM, Goldart EM, Bossert JM, Shaham Y, et al. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci. 2014;34:7437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carelli RM. The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev. 2002;1:281–96. [DOI] [PubMed] [Google Scholar]
- 20.Carelli RM, Deadwyler SA. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. J Neurosci. 1994;14:7735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carelli RM, Deadwyler SA. Cellular mechanisms underlying reinforcement-related processing in the nucleus accumbens: electrophysiological studies in behaving animals. Pharmacol Biochem Behav. 1997;57:495–504. [DOI] [PubMed] [Google Scholar]
- 22.Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfarr S, Schaaf L, Reinert JK, Paul E, Herrmannsdorfer F, Rossmanith M, et al. Choice for drug or natural reward engages largely overlapping neuronal ensembles in the infralimbic prefrontal cortex. J Neurosci. 2018;38:3507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kane L, Venniro M, Quintana-Feliciano R, Madangopal R, Rubio FJ, Bossert JM et al. Fos-expressing neuronal ensemble in rat ventromedial prefrontal cortex encodes cocaine seeking but not food seeking in rats. Addict Biol. 2020;e12943. 10.1111/adb.12943. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, et al. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat Neurosci. 2019;22:753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manuela M, PP J, Joe H. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33. [DOI] [PubMed] [Google Scholar]
- 27.Struthers WM, DuPriest A, Runyan J. Habituation reduces novelty-induced FOS expression in the striatum and cingulate cortex. Exp Brain Res. 2005;167:136–40. [DOI] [PubMed] [Google Scholar]
- 28.Kovacs KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol. 2008;20:665–72. [DOI] [PubMed] [Google Scholar]
- 29.McReynolds JR, Christianson JP, Blacktop JM, Mantsch JR. What does the Fos say? Using Fos-based approaches to understand the contribution of stress to substance use disorders. Neurobiol Stress. 2018;9:271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bariselli S, Fobbs WC, Creed MC, Kravitz AV. A competitive model for striatal action selection. Brain Res. 2019;1713:70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Pena CJ, et al. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci USA. 2016;113:2726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinsbroek JA, Neuhofer DN, Griffin WC 3rd, Siegel GS, Bobadilla AC, Kupchik YM, et al. Loss of plasticity in the D2-accumbens pallidal pathway promotes cocaine seeking. J Neurosci. 2017;37:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts-Wolfe* D, Bobadilla* AC, Heinsbroek JA, Neuhofer D, Kalivas PW. Drug refraining and seeking potentiate synapses on distinct populations of accumbens medium spiny neurons. J Neurosci. 2018;38:7100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts-Wolfe DJ, Heinsbroek JA, Spencer SM, Bobadilla AC, Smith ACW, Gipson CD et al. Transient synaptic potentiation in nucleus accumbens shell during refraining from cocaine seeking. Addict Biol. 2020;e12943. 10.1111/adb.12943. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Addict Biol. 2020;25:e12759. 10.1111/adb.12759. Epub 2019 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, et al. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci: Off J Soc Neurosci. 2015;35:8232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, et al. Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. J Neurosci: Off J Soc Neurosci. 2017;37:1014–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creed M, Ntamati NR, Chandra R, Lobo MK, Luscher C. Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum. Neuron. 2016;92:214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52. [DOI] [PubMed] [Google Scholar]
- 42.Heinsbroek JA, Bobadilla AC, Dereschewitz E, Assali A, Chalhoub RM, Cowan CW, et al. Opposing regulation of cocaine seeking by glutamate and GABA neurons in the ventral pallidum. Cell Rep. 2020;30:2018–27 e2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bobadilla AC, Garcia-Keller C, Heinsbroek JA, Scofield M, Chareunsouk V, Monforton C et al. Accumbens mechanisms for cued sucrose-seeking. Neuropsychopharmacology. 2017;42:2377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, et al. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, Hope BT. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci. 2012;32:11600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PloS One. 2007;2:e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tunstall BJ, Kearns DN. Cocaine can generate a stronger conditioned reinforcer than food despite being a weaker primary reinforcer. Addict Biol. 2016;21:282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubio FJ, Quintana-Feliciano R, Warren BL, Li X, Witonsky KFR, Soto Del Valle F et al. Prelimbic cortex is a common brain area activated during cue-induced reinstatement of cocaine and heroin seeking in a polydrug self-administration rat model. Eur J Neurosci. 2019;49:165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lynch WJ. Modeling the development of drug addiction in male and female animals. Pharmacol Biochem Behav. 2018;164:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhn BN, Kalivas PW, Bobadilla A-C. Understanding addiction using animal models. Front Behav Neurosci. 2019;13:262. 10.3389/fnbeh.2019.00262. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spencer S, Garcia-Keller C, Roberts-Wolfe D, Heinsbroek JA, Mulvaney M, Sorrell A, et al. Cocaine use reverses striatal plasticity produced during cocaine seeking. Biol Psychiatry. 2017;81:616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–70. [DOI] [PubMed] [Google Scholar]
- 53.Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol. 2013;23:99–106. [DOI] [PubMed] [Google Scholar]
- 54.DeNardo LA, Liu CD, Allen WE, Adams EL, Friedmann D, Dadgar-Kiani E et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Preprint at https://www.biorxiv.org/content/10.1101/295238v1.full. 2018. [DOI] [PMC free article] [PubMed]
- 55.Bobadilla AC, Heinsbroek JA, Gipson CD, Griffin WC, Fowler CD, Kenny PJ, et al. Corticostriatal plasticity, neuronal ensembles, and regulation of drug-seeking behavior. Prog Brain Res. 2017;235:93–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziminski JJ, Sieburg MC, Margetts-Smith G, Crombag HS, Koya E. Regional differences in striatal neuronal ensemble excitability following cocaine and extinction memory retrieval in Fos-GFP mice. Neuropsychopharmacology. 2018;43:718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ziminski JJ, Hessler S, Margetts-Smith G, Sieburg MC, Crombag HS, Koya E. Changes in appetitive associative strength modulates nucleus accumbens, but not orbitofrontal cortex neuronal ensemble excitability. J Neurosci. 2017;37:3160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitaker LR, Warren BL, Venniro M, Harte TC, McPherson KB, Beidel J, et al. Bidirectional modulation of intrinsic excitability in rat prelimbic cortex neuronal ensembles and non-ensembles after operant learning. J Neurosci. 2017;37:8845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koya E, Cruz FC, Ator R, Golden SA, Hoffman AF, Lupica CR, et al. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15: 1556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whitaker LR, Carneiro de Oliveira PE, McPherson KB, Fallon RV, Planeta CS, Bonci A et al. Associative learning drives the formation of silent synapses in neuronal ensembles of the nucleus accumbens. Biol Psychiatry. 2016;80:246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright WJ, Graziane NM, Neumann PA, Hamilton PJ, Cates HM, Fuerst L et al. Silent synapses dictate cocaine memory destabilization and reconsolidation. Nat Neurosci. 2020;23:32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Josselyn SA, Tonegawa S. Memory engrams: recalling the past and imagining the future. Science. 2020;367:eaaw4325. 10.1126/science.aaw4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tonegawa S, Liu X, Ramirez S, Redondo R. Memory engram cells have come of age. Science. 2020;367:eaaw4325. 10.1126/science.aaw4325. [DOI] [PubMed] [Google Scholar]
- 64.Choi JH, Sim SE, Kim JI, Choi DI, Oh J, Ye S, et al. Interregional synaptic maps among engram cells underlie memory formation. Science. 2018;360:430–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


