Abstract
The innate immune system plays key roles in controlling Alzheimer's disease (AD), while secreting cytokines to eliminate pathogens and regulating brain homeostasis. Recent research in the field of AD has shown that the innate immune-sensing ability of pattern recognition receptors on brain-resident macrophages, known as microglia, initiates neuroinflammation, Aβ accumulation, neuronal loss, and memory decline in patients with AD. Advancements in understanding the role of innate immunity in AD have laid a strong foundation to elucidate AD pathology and devise therapeutic strategies for AD in the future. In this review, we highlight the present understanding of innate immune responses, inflammasome activation, inflammatory cell death pathways, and cytokine secretion in AD. We also discuss how the AD pathology influences these biological processes.
Keywords: alzheimer's disease, amyloid-β, tau, innate immunity, inflammasome, NLRP3, MxA, ASC speck, IL-1β, IL-18, caspase-1, pyroptosis, apoptosis, necroptosis, neuroinflammation
Introduction
Alzheimer's disease (AD), the most common dementing illness, is characterized by progressive memory decline and cognitive dysfunction. AD affects more than 44 million people worldwide, with a new patient diagnosed every 4 s. The number of patients with dementia, including AD, is expected to double every 20 years and reach 115 million by 2050, placing huge financial burdens on individuals and society.
The three pathological hallmarks of AD are deposition of extracellular β-amyloid (Aβ), intraneuronal aggregation of neurofibrillary tangles composed of the microtubule-associated protein tau, and neuronal loss called neurodegeneration (Nelson et al., 2012) (Figure 1). In mammalian hosts, Aβ and tau deposits as danger-associated molecular patterns (DAMPs) are recognized and cleared by multiple pattern-recognition receptors (PRRs) including Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), and nucleotide-binding oligomerization domain-like receptor family proteins (NLRs) (de Rivero Vaccari et al., 2014; Heneka et al., 2013; Ising et al., 2019; Tahara et al., 2006; Venegas et al., 2017).
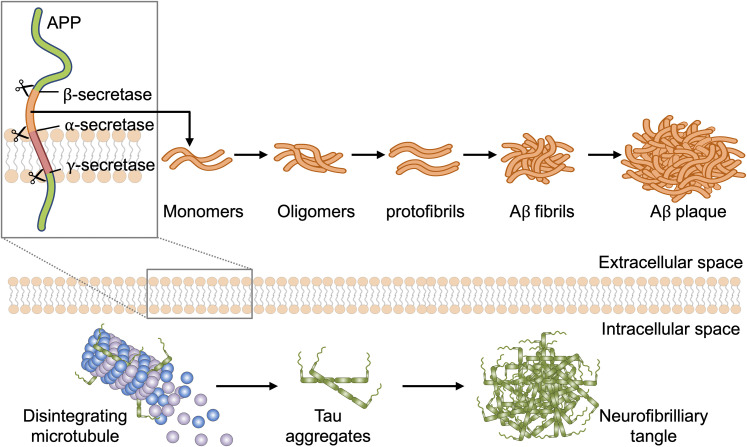
Figure 1.
Aggregation of Aβ and tau. The Aβ precursor APP undergoes processing by proteases α-, β-, and γ-secretase. Aβ monomers can aggregate into Aβ oligomers, protofibrils, fibrils, and ultimately plaques, which are among the hallmarks of AD pathology. Loss of microtubule binding leads to elevated levels of cytosolic tau, thereby increasing the potential for tau–tau interactions. Aggregation of hyperphosphorylated tau protein leads to the assembly of neurofibrillary tangles followed by progressive cytoskeletal changes, disruption of axonal transport, and AD pathology.
In response to DAMPs, some PRRs can assemble large multiprotein complexes known as inflammasomes (Place & Kanneganti, 2018). Upon assembly, the inflammasomes induce membrane pore formation and proinflammatory cytokine processing, leading to a form of inflammatory cell death known as pyroptosis (He et al., 2015; Man et al., 2017; Shi et al., 2015). Innate immune signaling and inflammasome activation are key protective responses against AD (Heneka et al., 2013, 2014; Venegas et al., 2017). However, their activation must be tightly regulated, as excessive activation can lead to neuroinflammation and brain damage. Balancing the host innate immune response has been considered in potential therapeutic approaches for AD, but this balance must be finely tuned to reduce excessive neuroinflammation, while clearing Aβ and tau deposits.
In this review, we discuss innate immune recognition, inflammasome activation, programmed cell death, and cytokine release that occur in response to AD, as well as how innate immune responses regulate the disease to resist development of AD pathology.
Pathogenesis of Alzheimer's Disease
AD is the most common type of dementia and neurodegenerative disease involving neuroinflammation, neuronal loss, and memory decline (Heneka et al., 2015; Nussbaum & Ellis, 2003). Aβ deposition can begin in the brain decades before the onset of clinical symptoms and stimulate tau-mediated AD pathogenesis such as tau hyperphosphorylation, tau aggregation, and triggering neuron-to-neuron spread (Figure 2).
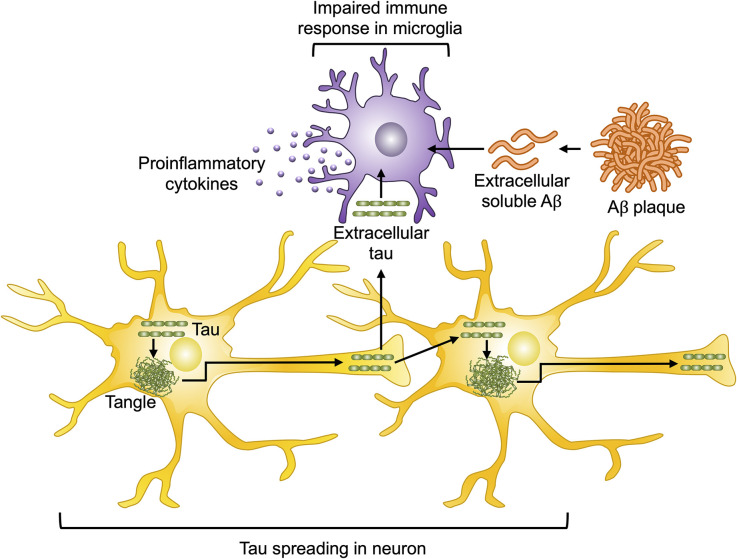
Figure 2.
Aβ and tau pathology in AD. Extracellular Aβ and tau contribute to enhanced bioactivity of microglia, which induces the secretion of proinflammatory cytokines including IL-1β and IL-18. Activated microglia further take up Aβ and tau and ultimately induce impaired immune responses in microglia and sterile inflammation. Several forms of tau contribute to neuron-to-neuron spreading, uptake, and aggregation of tau, thereby leading to tau-induced toxicity.
As Aβ accumulation can induce the formation of neurofibrillary tangles that propagate to produce mild cognitive impairment and dementia, Aβ production has been extensively characterized (Jack et al., 2010). Aβ is a cleavage product of amyloid precursor protein (APP). APP expressed in neurons and glial cells has several physiological roles in mediating cell-to-cell adhesion for neuronal signaling and neurotransmitter release. APP cleavage by proteolytic secretases can generate amyloid peptides, including a peptide of 37–49 amino acids, Aβ, and released into the extracellular space in the brain (Chen et al., 2017). APP processing can follow the following two pathways: amyloidogenic and non-amyloidogenic. The amyloidogenic pathway involves a cleavage event induced by β-secretase such as BACE1, which releases soluble APPβ. The APP C-terminal fragment can then be cleaved by γ-secretases such as presenilin (PS) 1 and 2 at one of the several sites varying from 40 to 44 amino acid residues to generate neurotoxic Aβ peptides. Among Aβs of various lengths, soluble Aβ1−42 is a major component of plaque formation and initiates Aβ aggregation due to its hydrophobic C-terminus, which reduces the flexibility of transformation from α-helical to β-sheet structure. The non-amyloidogenic pathway is mediated by α-secretases such as ADAM 9, 10, and 17, and γ-secretase catalyzes further cleavages to produce non-toxic and possibly beneficial soluble APPα fragments with important neuroprotective functions (Chasseigneaux & Allinquant, 2012; Chen et al., 2017). The Aβ1−42 peptide can induce the production of higher-order assemblies of Aβ, wherein the monomers of Aβ peptide accumulate by misfolding into propagating conformations to freely diffuse through the brain (Chen et al., 2017). Aβ oligomers act as seeds to form insoluble Aβ aggregates know as plaques (Katzmarski et al., 2020). Aβ plaques disrupt cell-to-cell communication, lead to neuronal apoptosis, and activate immune cells such as microglia, thereby triggering inflammation and brain tissue damage (Heneka et al., 2015). Activated microglia further take up Aβ and tau and ultimately induce impaired immune responses in microglia and sterile inflammation (Busche & Hyman, 2020) (Figure 2).
Tau is a type II microtubule-associated protein that is highly expressed in neurons and moderately expressed in oligodendrocytes and astrocytes in the mammalian brain. Under normal condition, tau mainly modulates the stability of microtubules in axons by direct interaction with tubulin. Tau also has synaptic functions regulated by post-translational modifications (PTMs), including phosphorylation and proteolytic cleavage (Wesseling et al., 2020). However, abnormal PTMs induce tau hyperphosphorylation, which causes it to dissociate from microtubules and to enhance tau aggregation (self-assembly) (Alonso et al., 2018). Therefore, hyperphosphorylation of tau substantially impairs the stability of microtubules in nerve cells, which is involved in the pathogenesis of AD. The hyperphosphorylated tau is released by exosomal processes, which can be detected in the cerebrospinal fluid and blood of patients with AD (Fiandaca et al., 2015; Jia et al., 2019), with the amounts detected correlating with cognitive impairment. Although exosomal tau is a potential biomarker for AD, it is necessary to consider how to achieve high sensitivity with diagnostic tools early in the onset of AD. Extracellular misfolded tau released from pathological cells via exosomes can enter naive cells such as neurons and microglia, leading to tau-induced toxicity and convert monomeric physiological tau via endocytosis (Hardy & Selkoe, 2002; He et al., 2018; LaFerla et al., 1995; Selkoe & Hardy, 2016) (Figure 2).
Innate Immune Recognition in Alzheimer's Disease
As resident immune effector cells of the central nervous system, microglia play a crucial role in regulating brain homeostasis and mediating innate immune responses in AD (Clayton et al., 2017; Hanisch & Kettenmann, 2007). Microglia sense a variety of microbial molecules known as pathogen-associated molecular patterns (PAMPs) as well as host-derived DAMPs via PRRs, including TLRs, RLRs, and NLRs (Kigerl et al., 2014) (Table 1). Microglia associated with AD are commonly in an activated state with upregulated expression of PRRs. PRRs drive signal transduction pathways that induce an inflammatory response and secretion of pro-inflammatory cytokines, including type I interferons (IFNs), thereby leading to the microglia-associated clearance of debris via phagocytosis in AD (Heneka et al., 2014; McDonough et al., 2017). Although microglia are integral for the phagocytosis and degradation of Aβ, their chronic activation can provoke impaired neuroinflammatory responses, with the potential to induce Aβ production and neuronal distress (Hansen et al., 2018; Hemonnot et al., 2019; Sarlus & Heneka, 2017).
Table 1.
Effects and Functions of Innate Immune Sensor in AD.
| Sensor | Extracellular Aβ deposits | Intracellular neurofibrillary tangles (pTau) | Inflammatory cytokines | Cognition function | Reference |
|---|---|---|---|---|---|
| TLR2 | Increase | N/A | Increase (TNF-α, IL-1β, IL-8) | Impair | (Liu et al., 2012) |
| Increase | N/A | N/A | Impair | (McDonald et al., 2016) | |
| TLR4 | Decrease | N/A | Increase (IL-1β) | Improve | (Song et al., 2011) |
| N/A | Decrease | Increase (TNF-α, IL-1β, IL-8) | Improve | (Qin et al., 2016) | |
| Increase | N/A | Increase (IL-6, TNF-α) | Impair (AD patient) | (Walter et al., 2007) | |
| Increase | N/A | Increase (TNF-α, IL-1β, IL-6) | Impair | (Balducci et al., 2017) | |
| RIG-I | Increase | N/A | N/A | Impair | (de Rivero Vaccari et al., 2014) |
| NLRP3 | Increase | N/A | Increase (IL-1β) | Impair | (Heneka et al., 2013) |
| N/A | Increase | Increase (IL-1β) | Impair | (Ising et al., 2019) | |
| Increase | Increase | Increase (IL-1β) | N/A | (Stancu et al., 2019) | |
| Increase | N/A | N/A | N/A | (Yin et al., 2018) | |
| Increase | N/A | Increase (TNF-α, IL-1β, IL-6) | Impair | (Lonnemann et al., 2020) | |
| AIM2 | Increase | N/A | No significant (IL-1β) | No significant | (Wu et al., 2017) |
| Extracellular ASC speck | Increase | N/A | Increase (IL-1β) | N/A | (Friker et al., 2020) |
| Increase | N/A | N/A | Impair | (Venegas et al., 2017) |
TLR- and RLR-Mediated Alzheimer's Disease Recognition
TLR- and RLR-mediated signaling leads to the secretion of type I IFNs and proinflammatory cytokines. TLRs are membrane-bound PRRs expressed in the microglia of mice, rats, and humans, and their activation can be beneficial or detrimental to the host (Bsibsi et al., 2002; Olson & Miller, 2004; Zhang et al., 2013). In microglia, the activation of TLR2 together with CD14, a coreceptor of TLR4, induces an immune response associated with fibrillar Aβ phagocytosis (Reed-Geaghan et al., 2009). Moreover, TLR2 and TLR4 activation in microglia enhances the production of Aβ peptides with 42 amino acids (Aβ1−42), which are key pathogenic species in AD (Chen et al., 2006). Some studies have shown that the inhibition of TLR2 activation attenuates glial cell reactivity, leading to reduced Aβ deposits and improved cognitive function in APP/PS1 double transgenic mice (McDonald et al., 2016). Furthermore, Tlr2/4-deficient mice are protected from neurocognitive and behavior impairment after immunization with Aβ1–42 peptide (Vollmar et al., 2010). Tlr2–/– microglia induce proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 and enhance Aβ clearance (Jana et al., 2008; Liu et al., 2012).
APP/PS1 transgenic mice of an AD model deficient in TLR4 exhibit reduced microglial activation, reduced cognitive function, and increased Aβ deposition (Song et al., 2011). In a transgenic mouse model carrying a mutation in tau (P301S), mild and chronic stimulation of TLR4 with lipopolysaccharide reduced the level of cerebral phosphorylated tau proteins and improved memory (Qin et al., 2016). In contrast, the activation of TLR4 reduced the clearance of microglial Aβ1−42 peptides by modulating the activity of the scavenger receptor CD36 (Li et al., 2015). Pronounced TLR4 expression in APP transgenic mice and increased TLR4 expression in the brains of patients with AD are associated with Aβ plaques (Walter et al., 2007). The activation of TLR4 is necessary for glial cell activation, which resulted in memory impairment in a mouse model of AD (Balducci et al., 2017). A recent study suggested that alterations in microglial TLR4 signaling contribute to AD pathogenesis in APP/PS1 double transgenic mice (Go et al., 2016). Collectively, the activation of TLR2 and TLR4 in the initial stages of AD has beneficial effects in Aβ clearance, whereas chronic activation contributes to Aβ aggregation.
RIG-I is a cytosolic PRR that canonically senses 5′-triphosphate double-stranded RNA. Although one study revealed that RIG-I was elevated in the temporal cortex and plasma of patients with mild cognitive impairment (de Rivero Vaccari et al., 2014), the detailed molecular mechanism of how RIG-I is involved in AD pathology remains unclear. Thus, further studies are required to identify the in vivo dominant innate sensor in AD.
Aβ complexed with nucleic acids triggers TLR- and RLR-derived type I IFN responses in microglia, resulting in complement-mediated synapse destruction in AD (Roy et al., 2020). Therefore, IFNs constitute a pivotal element within the neuroinflammatory network of AD and critically contribute to neuropathogenic processes.
NLRP3 Inflammasomes in AD
Innate immune cells in the brain play major roles in cytokine production and inflammatory signaling. Among the proinflammatory cytokines, IL-1β and IL-18 are the key mediators of the inflammatory response; increased levels of these proteins correlate with the severity of AD in patients (Forlenza et al., 2009; King et al., 2018; Ng et al., 2018; Ojala et al., 2009). The release of IL-1β and IL-18 requires proteolytic maturation of pro-IL-1β and pro-IL-18. This process is mediated by inflammasome formation and protease caspase-1 activation (Man et al., 2017). Inflammasome sensors can recognize PAMPs and/or DAMPs produced during pathogen infection and cellular instability, respectively (Kanneganti, 2010). Among the sensors, the most well-characterized sensor is the NLR family pyrin domain-containing 3 (NLRP3), which has been implicated in several diseases such as autoinflammatory diseases, obesity, colitis, and pathogen-mediated diseases including influenza A viruses and corona viruses (Gurung & Kanneganti, 2016; Kanneganti et al., 2006a, 2006b; Karki et al., 2015; Lee et al., 2020; Lee & Ryu, 2021; Stienstra et al., 2011; Thomas et al., 2009; Zaki et al., 2010).
The NLRP3 inflammasome has emerged as a trigger of AD pathogenesis. The mRNA and protein expressions of NLRP3 are upregulated in the monocytes of patients with AD (Saresella et al., 2016). Loss of the NLRP3 inflammasome (NLRP3/caspase-1) in APP/PS1 mice protects against long-term potentiation deficits and attenuates spatial memory deficits and the Aβ burden (Heneka et al., 2013).
Furthermore, exogenous-aggregated tau activates the NLRP3 inflammasome in the microglia (Stancu et al., 2019), and fibrillar Aβ-containing brain homogenates induce tau pathology in an NLRP3-dependent manner (Ising et al., 2019). Consistently, NLRP3 inflammasome inhibitor (MCC950, blocks NLRP3-ASC-induced IL-1β secretion and ASC oligomerization) reduces phosphorylation of tau and accumulation of Aβ in the hippocampus induced by exogenous tau seeding in TauP301S transgenic mice (Stancu et al., 2019). Other NLRP3 inflammasome inhibitors, such as the drug JC-124 and fenamate non-steroidal anti-inflammatory drugs, exert beneficial effects in mouse models of AD (Daniels et al., 2016; Yin et al., 2018). Collectively, these findings suggest that the NLRP3 inflammasome contributes to AD pathology.
MxA Inflammasomes in AD
Besides NLRP3, MxA also acts as an inflammasome sensor in respiratory epithelial cells (Lee et al., 2018, 2019). The Mx genes, which are present in almost all vertebrates, are interferon-regulated genes whose expression is upregulated following IFN production, which leads to an antiviral response, particularly against RNA viruses (Haller et al., 2015). Following influenza A virus infection, MxA recognizes the influenza virus A nucleoprotein, which then interacts with the inflammasome adapter protein and apoptosis-associated speck-like protein containing a CARD (ASC), and leads to caspase 1 activation and IL-1β and IL-18 production (Lee et al., 2018, 2019).
It has been reported that MxA polymorphisms are associated with risk and age-at-onset in AD and accelerate cognitive decline in patients with AD (Ma et al., 2012). Another study showed that MxA is found in senile plaques of the AD brain and the presence of MxA in reactive microglia could contribute to AD pathology (Yamada et al., 1994). Given that IL-1β and IL-18 are associated with the severity of AD in patients (Forlenza et al., 2009; King et al., 2018; Ng et al., 2018; Ojala et al., 2009), it will be interesting to reveal whether MxA inflammasome is required for AD exacerbation.
Extracellular ASC Specks in AD
Inflammasome sensors, such as NLRP3, are intracellular proteins expressed in macrophages and are activated by a wide variety of DAMPs, such as alum, silica, uric acid, and amyloid-β, following their engulfment by macrophages. Activated NLRP3 facilitates ASC oligomerization to form large, intracellular macromolecular aggregates known as ASC specks. It has been reported that ASC specks are released into the extracellular space, where ASC propagates inflammatory responses via prion-like transmission mediated by engulfment in neighboring macrophages (Baroja-Mazo et al., 2014; Franklin et al., 2014). In AD pathology, in the extracellular space, ASC specks bind to Aβ1–42 to accelerate its oligomerization, indicating the cross-seeding activity of ASCs in Aβ aggregation, which boosts its toxicity in microglia (Friker et al., 2020; Venegas et al., 2017) (Figure 3). Recently, it has been reported that the extracellular ASC specks are engulfed by Arf6-deficient macrophages, and IL-1β production is reduced in Arf6−/− macrophages compared with that in wild-type macrophages (Lee et al., 2021a). Although detailed molecular mechanisms of how intracellular ASC specks are released into the extracellular space and the role of these molecules in neuroinflammation remain largely unknown, the extracellular ASC specks may induce Arf6 dependent-secondary inflammasomes in neighboring microglia and AD pathology.
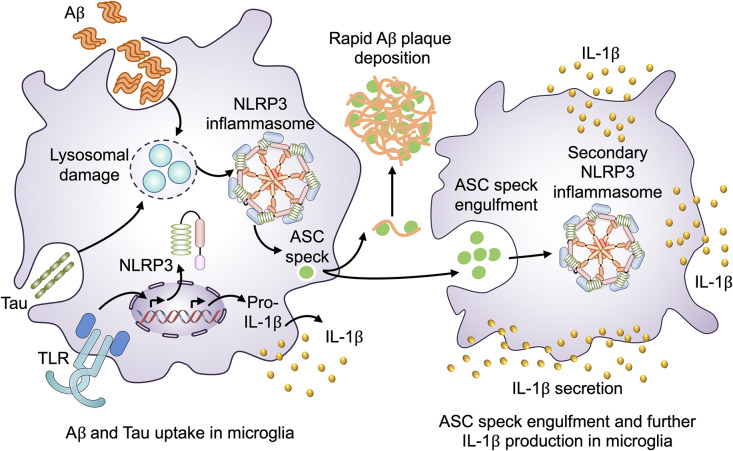
Figure 3.
Extracellular ASC specks in AD. Activation of microglia by a TLR agonist leads to transcriptional induction of genes encoding components of the NLRP3 inflammasome and pro-IL-1β. Upon Aβ and tau uptake, lysosomal damage leads to the assembly of the NLRP3 inflammasome, which eventually induces the release of IL-1β and oligomerization of ASC to form ASC specks. Extracellular ASC specks interact with Aβ aggregates in the extracellular space to promote their deposition as plaques. Extracellular ASC specks can be engulfed by neighboring microglia and contribute to the exacerbation of neuroinflammation via further production of IL-1β.
Programmed Cell Death and Proinflammatory Cytokines in Alzheimer's Disease
Cell death plays an important role in limiting disease progression. However, inflammatory cell death also results in the release of proinflammatory cytokines and cellular contents, including PAMPs and DAMPs, which can induce severe inflammation (Bergsbaken et al., 2009; Pasparakis & Vandenabeele, 2015) (Figure 4). Therefore, cell death is considered a double-edged sword during disease progression.
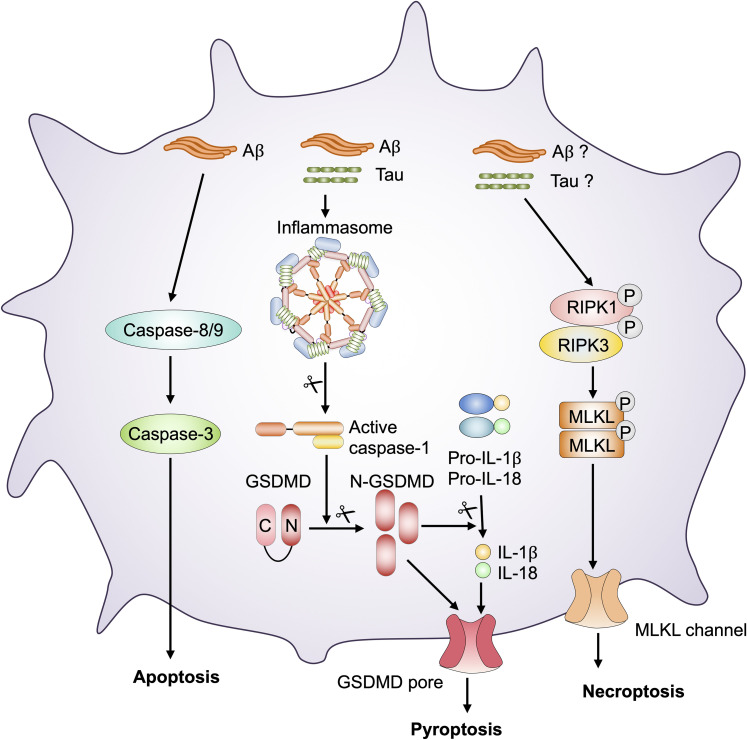
Figure 4.
Programmed cell death pathways in AD pathology. In AD, Aβ and tau deposition act as danger-associated molecular patterns (DAMPs) and stimulate inflammasome assembly, resulting in the activation of caspase-1. Activated caspase-1 cleaves pro–IL-1β, pro–IL-18, and gasdermin D (GSDMD). The N-terminal fragment of GSDMD may then oligomerize within membranes to form membrane pores and execute pyroptosis. Aβ and tau deposition initiates a signaling cascade mediated by caspase-8 and −9 activation. Caspase-8 and −9 induce the activation of caspase-3 to drive apoptosis. AD exacerbation by Aβ and tau deposition initiates necroptosis, a receptor-interacting serine/threonine-protein kinase 1 (RIPK1) and RIPK3 complex-dependent form of inflammatory cell death that depends on the activation of the protein mixed lineage kinase domain-like pseudokinase (MLKL) to form channels in the membrane.
Pyroptosis in Alzheimer's Disease
Pyroptosis is a form of inflammatory cell death mediated by inflammasomes and gasdermin (Shi et al., 2015). Inflammasome assembly leads to the activation of inflammatory caspases [caspase-1 (human and mouse), −4 (human), −5 (human), or −11 (mouse)], which proteolytically cleave and release the N-terminal fragment of gasdermin D (GSDMD) to form pores in the plasma membrane (He et al., 2015; Man et al., 2017; Shi et al., 2015). Caspase-1-dependent GSDMD cleavage leads to the release of IL-1β and IL-18 (Man et al., 2017).
Recombinant Aβ1-42 can induce caspase-1 activation, GSDMD cleavage, and pyroptosis in mouse cortical neurons (Han et al., 2020; Tan et al., 2014). NLRP3 inflammasome and caspase-1 activation, along with IL-1β release, have also been observed when fibrillar Aβ is phagocytosed by microglia (Halle et al., 2008). Furthermore, recombinant tau protein can activate the NLRP3 inflammasome and induce IL-1β release from microglia (Stancu et al., 2019). A recent study revealed that microglia isolated from Tau22 mice, which express human tau mutations involved in frontotemporal dementia, stimulate pyroptosis, as evidenced by NLRP3 inflammasome and caspase-1 activation and IL-1β release (Ising et al., 2019).
Necroptosis in Alzheimer's Disease
Necroptosis is a form of inflammatory cell death mediated by mixed-lineage kinase domain-like pseudokinase (MLKL). MLKL is oligomerized by the phosphorylation of receptor-interacting serine/threonine-protein kinase 1 (RIPK1) and RIPK3; the oligomerized MLKL is translocated to the plasma membrane and forms channels (Dondelinger et al., 2014).
In the temporal gyrus of human patients with AD, RIPK1 and MLKL show robust expression, and necroptosis is associated with reduced brain weight of patients with AD (Caccamo et al., 2017). Furthermore, necroptosis exacerbates cognitive deficits in APP/PS1 transgenic mice (Caccamo et al., 2017). Another study revealed that APP/PS1 mice treated with pharmacological inhibitors of RIPK1 or Ripk1D138N/D138N mice exhibit reductions in Aβ burden, inflammatory cytokine levels, and memory deficits (Ofengeim et al., 2017).
Apoptosis in Alzheimer's Disease
Apoptosis is executed by caspase-3 and −7 following the activation of upstream initiator caspases including caspase-8/10 or −9. Pyroptosis and necroptosis are inflammatory and lytic cell death processes in which cytokines are released to induce inflammation and alert immune cells, whereas apoptosis has been historically considered to be immunologically silent. However, recent studies have suggested that apoptosis is not always immunologically silent, as crosstalk occurs between apoptotic proteins and the molecules executing lytic cell death (Lee et al., 2020; Place et al., 2021). The apoptotic protein caspase-3 has been reported to activate gasdermin E to induce lytic cell death (Rogers et al., 2019). Caspase-8 activates GSDMD under specific conditions (Lee et al., 2020; Place et al., 2021).
In the context of AD, APP is a substrate for caspase-3-mediated cleavage, which contributes to Aβ plaque formation, synaptic loss, and behavioral changes associated with AD (Gervais et al., 1999). Other studies have reported that the activation of caspase-3, -8, and -9 further induces Aβ plaque formation and AD progression (Rohn et al., 2001, 2002; Stadelmann et al., 1999; Su et al., 2001). Collectively, these studies suggest that the apoptotic pathways may also contribute to AD progression.
Recent studies have found extensive crosstalk between programmed cell death pathways, establishing the concept of PANoptosis (pyroptosis, apoptosis, necroptosis) (Gurung et al., 2014; Kuriakose et al., 2016; Lamkanfi et al., 2008; Lee et al., 2020, 2021b; Malireddi et al., 2010, 2021; Place et al., 2021). Although inflammasome activation is primarily associated with gasdermin D (GSDMD)-mediated pyroptosis, there is emerging evidence of the contribution of inflammasome components in driving PANoptosis (Christgen et al., 2020; Gurung et al., 2014; Kesavardhana et al., 2020; Kuriakose et al., 2016; Lee et al., 2020, 2021b; Malireddi et al., 2020, 2021; Place et al., 2021; Zheng et al., 2020).
The AIM2 innate immune sensor, which is activated by double-stranded DNA, has been implicated in AD, that is, Aim2 deficiency reduces Aβ deposition and microglial activation (Wu et al., 2017). Recently, it has been reported that AIM2 regulates the innate immune sensors pyrin and ZBP1 to drive inflammatory signaling and PANoptosis (Lee et al., 2021b). It will be interesting to reveal whether AIM2 PANoptosis exerts beneficial effects in AD.
Inflammatory Cytokines
Microglia-associated PRRs drive signal transduction pathways that induce inflammatory cell death pathways and secretion of pro-inflammatory cytokines, including type I IFNs, thereby leading to the microglia-associated clearance of debris via phagocytosis in AD (Heneka et al., 2014; McDonough et al., 2017).
However, over activation of inflammatory cell death pathways can lead to critical brain damage and severe neuroinflammation (Bergsbaken et al., 2009; Pasparakis & Vandenabeele, 2015). Inflammatory cell death leads to the release of proinflammatory cytokines and chemokines, which may contribute to AD pathology.
Indeed, Aβ-stimulated human monocytes release chemokines such as IL-8, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), and MIP-1β in vitro. Microglia cultured from rapid autopsies of patients with AD and nondemented patients revealed increased expression of IL-8, MCP-1, and MIP-1α after experimental exposure to Aβ (Xia & Hyman, 1999).
Many proinflammatory cytokines have been shown to be produced by neurons or microglia and the levels of IL-1α, IL-1β, IL-6, TNF-α, granulocyte-macrophage colony-stimulating factor, and IFN-α are increased in AD brain tissue (Friker et al., 2020; Ising et al., 2019; Rubio-Perez & Morillas-Ruiz, 2012; Stancu et al., 2019; Venegas et al., 2017). Proinflammatory cytokines such as IL-18, IL-1β, IL-6, and TNF-α are released by astrocytes and microglia in response to Aβ plaques or following tau hyperphosphorylation (Forloni et al., 1997; Wang et al., 2015). Recent studies revealed that the combination of TNF-α and IFN-γ induces inflammatory cell death, tissue damage, and cytokine shock syndromes (Karki & Kanneganti, 2021; Karki et al., 2021). Furthermore, treating with neutralizing antibodies against TNF-α and IFN-γ protected mice during SARS-CoV-2 infection, sepsis, hemophagocytic lymphohistiocytosis, and cytokine shock (Karki & Kanneganti, 2021; Karki et al., 2021). Therefore, it is important to identify the innate immune processes contributing to cytokine release and potential associations between cytokine signaling and inflammatory cell death in AD pathology.
Innate Immune Signals Regulate Adaptive Immunity
The innate immune and adaptive immune systems interact with each other. Although innate immune responses control the progression of AD, adaptive immune responses also contribute to restraining AD pathogenesis. B and T lymphocytes are the main cellular components of adaptive immunity that are responsible for antigen-specific antibody secretion and cell-mediated immunity, respectively. The adaptive immune response plays a key role in the development of adequate control against toxic molecules, such as misfolded tau and Aβ (Anderson et al., 2014; Cantrell, 2015). The triple transgenic (3xTg-AD) mouse model shows tau and Aβ accumulation in the brain increasing with age and changes in their immune system. A recent study has shown that CD4+/CD8+ lymphocyte ratio in the blood of 3xTg-AD mice increased compared with that of WT mice (St-Amour et al., 2014), suggesting a universal deficit in the adaptive immune response; the finding is consistent with abnormal lymphocyte populations in patients with AD (Larbi et al., 2009; Pellicanò et al., 2012; Pirttilä et al., 1992; Saresella et al., 2011; Schindowski et al., 2006; Speciale et al., 2007).
Innate immune sensing by PRRs instructs adaptive immunity (Palm & Medzhitov, 2009). Therefore, innate and adaptive immune systems function cooperatively, and crosstalk between peripheral and central immunity such as cytokine and chemokine signaling likely plays an important albeit understudied role in AD. Recent studies have shown that peripheral neutrophils and T-regulatory cells profoundly affect AD pathogenesis (Baruch et al., 2015; Zenaro et al., 2015). A recent study revealed Aβ plaque formation, neuroinflammation, and microglial activation in 5XFAD mice (Marsh et al., 2016). Additionally, the loss of IgG-producing B cells impairs microglial phagocytosis, thereby inducing Aβ deposition and worsening AD pathology (Marsh et al., 2016).
Adaptive immunity in AD pathology has not been studied using genetic deletion of key PRRs. The use of knockout mice lacking key innate sensors in specific immune cell subsets will reveal the cell type-specific requirements for these sensors in instructing various aspects of adaptive immunity in AD pathology.
Therapies for Alzheimer Disease
Currently, there is no effective therapy for AD except aducanumab; a new therapy for early AD was approved by the FDA in 2021. Aducanumab is a human monoclonal antibody that selectively targets aggregated Aβ (Sevigny et al., 2016). Given the increasing incidence of AD globally, effective treatment strategies for AD are urgently needed. Over the last two decades, several drugs that decrease Aβ production or increase Aβ clearance in the brain have been identified. Although Aβ plaques are the neuropathological hallmark of AD, they are poorly correlated with AD severity and cognitive dysfunction (Arriagada et al., 1992; Giannakopoulos et al., 2003). Patients with AD in whom brain Aβ plaques were cleared by anti-Aβ immunotherapy showed no cognitive benefit (Holmes et al., 2008).
One possible target for intervention is neuroinflammation. Indeed, neuroinflammatory biomarkers shed light on therapeutic target candidates such as soluble TREM2, RAGE, CD38, CD33, and TNF-α (Table 2). Several studies have consistently identified a link between the inflammasome and AD, and microglia play a central role in linking inflammation with neurodegeneration (Friker et al., 2020; Heneka et al., 2013; Salter & Stevens, 2017; Venegas et al., 2017). In contrast, an efficient adaptive immune system may prevent AD pathogenesis by modulating microglial function (Marsh et al., 2016). Further studies are needed to understand the role of the innate immune system and microglia to explore whether immune-based therapies can be designed for AD.
Table 2.
Target and Immunity Mechanism of Therapeutic Agents in Clinical Trials for Alzheimer’s Disease.
| Agent | Target/Mechanism a | Mechanism of action | Clinical trial phase a | Reference |
|---|---|---|---|---|
| Atuzaginstat (COR388) | Inflammation/Infection | Small molecule; bacterial protease inhibitor targets gingipain produced by Porphyromonas gingivalis; reduces neuroinflammation | III | (Haditsch et al., 2020) |
| Azeliragon (TTP488) | Amyloid/ inflammation | Small molecule inhibitor; RAGE antagonist; reduces Aβ transport into the brain; mitigates toxic effects of oligomers and reduces inflammation | III | (Lue et al., 2001) |
| NE3107 (HE30286) | Inflammation | MAPK-1/3 inhibitor; reduces proinflammatory NFκB activation | III | (Ahlem et al., 2009) |
| AL002 (anti-TREM2) | Inflammation | Immunotherapy; monoclonal antibody; targets microglial TREM2 receptors; promotes microglial clearance of Aβ and reduces neurotoxicity | II | (Wang et al., 2020) |
| ALZT-OP1 (cromolyn + ibuprofen) | Inflammation | Small molecule and combination therapy (cromolyn; approved anti-asthma drug, ibuprofen; approved anti-inflammatory drug); reduces aggregation of Aβ and induces neuroprotective microglial activation | II | (Brazier et al., 2017; Zhang et al., 2018) |
| Daratumumab (anti-CD38) | Inflammation/Immunity | Immunotherapy (FDA-approved for the treatment of multiple myeloma); monoclonal antibody; targets CD38 on glia cells; regulates microglial activity | II | (Blacher et al., 2015; Guerreiro et al., 2020) |
| Dasatinib + quercetin | Inflammation/Immunity | Senolytic therapy; tyrosine kinase inhibitor (dasatinib) and flavonoid (quercetin, nutritional supplement); reduces senescent cells and tau aggregation | II | (Zhang et al., 2019) |
| GB301 | Inflammation/Immunity | Cell therapy drug; autologous regulatory T cells; reduces neuroinflammation | II | (Dansokho et al., 2016; Plascencia-Villa and Perry, 2020) |
| Lenalidomide | Inflammation/Immunity | FDA-approved cancer drug; reduces inflammatory cytokines; regulates innate and adaptive immune responses | II | (Decourt et al., 2020) |
| Montelukast (MK0476) | Inflammation | Small molecule; cysteinyl leukotriene type 1 (cysLT-1) receptor antagonist; affects inflammatory processes, neuronal injury, blood-brain-barrier integrity, and Aβ protein accumulation | II | (Lai et al., 2014; Morin et al., 2014) |
| Pepinemab (VX15) | Inflammation | Immunotherapy; monoclonal antibody inhibitor of semaphoring 4D (SEMA4D); reduces inflammatory cytokine release | II | (LaGanke et al., 2017) |
| AL003 | Inflammation | Immunotherapy; monoclonal antibody targeting SIGLEC-3 (CD33); reactivates microglia and immune cells in the brain; improves microglial clearance of toxic proteins | I | (Estus et al., 2019) |
| Edicotinib | Inflammation | Small molecule; CSF-1R antagonist; blocks microglial proliferation and production of cytokines (IL1β and TNFα) | I | (Mancuso et al., 2019) |
| XPro1595 | Inflammation | Protein biologic; soluble TNFα inhibitor; reduces neuroinflammation | I | (MacPherson et al., 2017) |
Target/mechanism and clinical trial phases are based on ClinicalTrials.gov (2021).
Tau-targeted therapies are another active area of investigation. As tau pathology correlates better with cognitive impairments than Aβ lesions, targeting tau is expected to be more effective than Aβ clearance once clinical symptoms are evident (Congdon & Sigurdsson, 2018). Unfortunately, most initial anti-tau therapies based on the inhibition of kinases or tau aggregation or on stabilization of microtubules have been discontinued due to their lack of efficacy and toxicity (Congdon & Sigurdsson, 2018). Currently, most tau-targeted therapies in clinical trials are immunotherapies, which have shown promise in numerous pre-clinical studies (Congdon & Sigurdsson, 2018).
Conclusions
Research in the past two decades have substantially expanded our understanding of innate immune responses in AD pathology. Emerging evidence suggests that aberrant microglial function in innate immune responses and cell death are inextricably linked to AD pathology. Therefore, innate immune responses and cell death must be finely controlled to reduce excessive neuroinflammation during the clearance of deposited Aβ and tau. Nevertheless, the following questions remain unanswered. What is the dominant innate immune sensor-derived signaling pathway that controls neuroinflammation and clearance of Aβ and tau deposition? What types of cell death are involved in neuroinflammation in AD? How can the innate immune response be balanced to reduce excessive neuroinflammation while retaining its support for the adaptive immune response? This association among inflammasome, inflammatory cell death, and neuroinflammation in AD is being actively pursued by numerous studies, and exploring the roles of these factors will help identify new drug targets for combating this devastating neurodegenerative disease.
Acknowledgments
We apologize to our colleagues in the field whose work could not be cited because of space limitations.
Abbreviations
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- ASC
apoptosis-associated speck-like protein containing a CARD
- Aβ
β-amyloid
- DAMP
danger-associated molecular pattern
- GSDMD
N-terminal fragment of gasdermin D
- IFN
interferon
- IL
interleukin
- MCP-1
monocyte chemoattractant protein-1
- MLKL
mixed-lineage kinase domain-like pseudokinase
- NLR
nucleotide-binding oligomerization domain-like receptor family protein
- NLRP3
NLR family pyrin domain-containing 3
- PAMP
pathogen-associated molecular pattern
- PRR
pattern-recognition receptor
- PTM
post-translational modification
- RIG-I
retinoic acid-inducible gene I
- RLR
retinoic acid-inducible gene I-like receptor
- TLR
Toll-like receptor.
Footnotes
Author Contributions: S.L., H.C., and J.R. outlined the manuscript. S.L., H.C., and J.R. wrote the manuscript. S.L., H.C., and J.R. critically revised and approved the final version of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (grant number 19K16665).
ORCID iD: SangJoon Lee https://orcid.org/0000-0001-7500-782X
References
- Ahlem C., Auci D., Mangano K., Reading C., Frincke J., Stickney D., Nicoletti F. (2009). HE3286: A novel synthetic steroid as an oral treatment for autoimmune disease. Annals of the New York Academy of Sciences, 1173, 781–790. 10.1111/j.1749-6632.2009.04798.x [DOI] [PubMed] [Google Scholar]
- Alonso A. D., Cohen L. S., Corbo C., Morozova V., Elidrissi A., Phillips G., Kleiman F. E. (2018). Hyperphosphorylation of tau associates with changes in its function beyond microtubule stability. Frontiers in Cellular Neuroscience, 12, 338. 10.3389/fncel.2018.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. M., Olson K. E., Estes K. A., Flanagan K., Gendelman H. E., Mosley R. L. (2014). Dual destructive and protective roles of adaptive immunity in neurodegenerative disorders. Translational Neurodegeneration, 3(1), 25. 10.1186/2047-9158-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada P. V., Growdon J. H., Hedley-Whyte E. T., Hyman B. T. (1992). Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology, 42(3 Pt 1), 631–639. 10.1212/WNL.42.3.631 [DOI] [PubMed] [Google Scholar]
- Balducci C., Frasca A., Zotti M., La Vitola P., Mhillaj E., Grigoli E., Iacobellis M., Grandi F., Messa M., Colombo L., Molteni M., Trabace L., Rossetti C., Salmona M., Forloni G. (2017). Toll-like receptor 4-dependent glial cell activation mediates the impairment in memory establishment induced by β-amyloid oligomers in an acute mouse model of Alzheimer’s disease. Brain Behavior and Immunity, 60, 188–197. 10.1016/j.bbi.2016.10.012 [DOI] [PubMed] [Google Scholar]
- Baroja-Mazo A., Martín-Sánchez F., Gomez A. I., Martínez C. M., Amores-Iniesta J., Compan V., Barberà-Cremades M., Yagüe J., Ruiz-Ortiz E., Antón J., Buján S., Couillin I., Brough D., Arostegui J. I., Pelegrín P. (2014). The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nature Immunology, 15(8), 738–748. 10.1038/ni.2919 [DOI] [PubMed] [Google Scholar]
- Baruch K., Rosenzweig N., Kertser A., Deczkowska A., Sharif A. M., Spinrad A., Tsitsou-Kampeli A., Sarel A., Cahalon L., Schwartz M. (2015). Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nature Communications, 6, 7967. 10.1038/ncomms8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T., Fink S. L., Cookson B. T. (2009). Pyroptosis: Host cell death and inflammation. Nature Reviews Microbiology, 7(1), 99–109. 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacher E., Dadali T., Bespalko A., Haupenthal V. J., Grimm M. O., Hartmann T., Lund F. E., Stein R., Levy A. (2015). Alzheimer’s disease pathology is attenuated in a CD38-deficient mouse model. Annals of Neurology, 78(1), 88–103. 10.1002/ana.24425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazier D., Perry R., Keane J., Barrett K., Elmaleh D. R. (2017). Pharmacokinetics of cromolyn and ibuprofen in healthy elderly volunteers. Clinical Drug Investigation, 37, 1025–1034. 10.1007/s40261-017-0549-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M., Ravid R., Gveric D., Van Noort J. M. (2002). Broad expression of toll-like receptors in the human central nervous system. Journal of Neuropathology & Experimental Neurology, 61(11), 1013–1021. 10.1093/jnen/61.11.1013 [DOI] [PubMed] [Google Scholar]
- Busche M. A., Hyman B. T. (2020). Synergy between amyloid-β and tau in Alzheimer’s disease. Nature Neuroscience, 23(10), 1183–1193. 10.1038/s41593-020-0687-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A., Branca C., Piras I. S., Ferreira E., Huentelman M. J., Liang W. S., Readhead B., Dudley J. T., Spangenberg E. E., Green K. N., Belfiore R., Winslow W., Oddo S. (2017). Necroptosis activation in Alzheimer’s disease. Nature Neuroscience, 20(9), 1236–1246. 10.1038/nn.4608 [DOI] [PubMed] [Google Scholar]
- Cantrell D. (2015). Signaling in lymphocyte activation. Cold Spring Harbor Perspectives in Biology, 7(6). 10.1101/cshperspect.a018788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasseigneaux S., Allinquant B. (2012). Functions of Aβ, sAPPα and sAPPβ : Similarities and differences. Journal of Neurochemistry, 120(Suppl 1), 99–108. 10.1111/j.1471-4159.2011.07584.x [DOI] [PubMed] [Google Scholar]
- Chen G. F., Xu T. H., Yan Y., Zhou Y. R., Jiang Y., Melcher K., Xu H. E. (2017). Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacologica Sinica, 38(9), 1205–1235. 10.1038/aps.2017.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Iribarren P., Hu J., Chen J., Gong W., Cho E. H., Lockett S., Dunlop N. M., Wang J. M. (2006). Activation of toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. Journal of Biological Chemistry, 281(6), 3651–3659. 10.1074/jbc.M508125200 [DOI] [PubMed] [Google Scholar]
- Christgen S., Zheng M., Kesavardhana S., Karki R., Malireddi R. K. S., Banoth B., Place D. E., Briard B., Sharma B. R., Tuladhar S., Samir P., Burton A., Kanneganti T. D. (2020). Identification of the PANoptosome: A molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis). Frontiers in Cellular and Infection Microbiology, 10, 237. 10.3389/fcimb.2020.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton K. A., Van Enoo A. A., Ikezu T. (2017). Alzheimer’s disease: The role of microglia in brain homeostasis and proteopathy. Frontiers in Neuroscience, 11, 680. 10.3389/fnins.2017.00680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E. E., Sigurdsson E. M. (2018). Tau-targeting therapies for Alzheimer disease. Nature Reviews. Neurology, 14(7), 399–415. 10.1038/s41582-018-0013-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. J., Rivers-Auty J., Schilling T., Spencer N. G., Watremez W., Fasolino V., Booth S. J., White C. S., Baldwin A. G., Freeman S., Wong R., Latta C., Yu S., Jackson J., Fischer N., Koziel V., Pillot T., Bagnall J., Allan S. M., … , Brough D. (2016). Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nature Communications, 7, 12504. 10.1038/ncomms12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansokho C., Ait Ahmed D., Aid S., Toly-Ndour C., Chaigneau T., Calle V., Cagnard N., Holzenberger M., Piaggio E., Aucouturier P., Dorothée G. (2016). Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain, 139(Pt 4), 1237–1251. 10.1093/brain/awv408 [DOI] [PubMed] [Google Scholar]
- Decourt B., Wilson J., Ritter A., Dardis C., Difilippo F. P., Zhuang X., Cordes D., Lee G., Fulkerson N. D., St Rose T., Hartley K., Sabbagh M. N. (2020). MCLENA-1: A phase II clinical trial for the assessment of safety, tolerability, and efficacy of lenalidomide in patients with mild cognitive impairment Due to Alzheimer’s disease. Open Access Journal of Clinical Trials, 12, 1–13. 10.2147/OAJCT.S221914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rivero Vaccari J. P., Brand F. J., 3rd, Sedaghat C., Mash D. C., Dietrich W. D., Keane R. W. (2014). RIG-1 receptor expression in the pathology of Alzheimer’s disease. Journal of Neuroinflammation, 11, 67. 10.1186/1742-2094-11-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondelinger Y., Declercq W., Montessuit S., Roelandt R., Goncalves A., Bruggeman I., Hulpiau P., Weber K., Sehon C. A., Marquis R. W., Bertin J., Gough P. J., Savvides S., Martinou J. C., Bertrand M. J., Vandenabeele P. (2014). MLKL Compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Reports, 7(4), 971–981. 10.1016/j.celrep.2014.04.026 [DOI] [PubMed] [Google Scholar]
- Estus S., Shaw B. C., Devanney N., Katsumata Y., Press E. E., Fardo D. W. (2019). Evaluation of CD33 as a genetic risk factor for Alzheimer’s disease. Acta Neuropathologica, 138(2), 187–199. 10.1007/s00401-019-02000-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiandaca M. S., Kapogiannis D., Mapstone M., Boxer A., Eitan E., Schwartz J. B., Abner E. L., Petersen R. C., Federoff H. J., Miller B. L., Goetzl E. J. (2015). Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s & Dementia, 11(6), 600–607.e601. 10.1016/j.jalz.2014.06.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlenza O. V., Diniz B. S., Talib L. L., Mendonça V. A., Ojopi E. B., Gattaz W. F., Teixeira A. L. (2009). Increased serum IL-1beta level in Alzheimer’s disease and mild cognitive impairment. Dementia and Geriatric Cognitive Disorders, 28(6), 507–512. 10.1159/000255051 [DOI] [PubMed] [Google Scholar]
- Forloni G., Mangiarotti F., Angeretti N., Lucca E., De Simoni M. G. (1997). Beta-amyloid fragment potentiates IL-6 and TNF-alpha secretion by LPS in astrocytes but not in microglia. Cytokine, 9(10), 759–762. 10.1006/cyto.1997.0232 [DOI] [PubMed] [Google Scholar]
- Franklin B. S., Bossaller L., De Nardo D., Ratter J. M., Stutz A., Engels G., Brenker C., Nordhoff M., Mirandola S. R., Al-Amoudi A., Mangan M. S., Zimmer S., Monks B. G., Fricke M., Schmidt R. E., Espevik T., Jones B., Jarnicki A. G., Hansbro P. M., … , Latz E. (2014). The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nature Immunology, 15(8), 727–737. 10.1038/ni.2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friker L. L., Scheiblich H., Hochheiser I. V., Brinkschulte R., Riedel D., Latz E., Geyer M., Heneka M. T. (2020). β-Amyloid clustering around ASC fibrils boosts its toxicity in microglia. Cell Reports, 30(11), 3743–3754.e3746. https://doi.org/10.1016/j.celrep.2020.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais F. G. Xu D. Robertson G. S. Vaillancourt J. P. Zhu Y. Huang J. Leblanc A. Smith D. Rigby M. Shearman M. S. Clarke E. E. Zheng H. Van Der Ploeg L. H. Ruffolo S. C. Thornberry N. A. Xanthoudakis S. Zamboni R. J. Roy S.… Nicholson D. W. (1999). Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell, 97(3), 395–406. 10.1016/S0092-8674(00)80748-5 [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P., Herrmann F. R., Bussière T., Bouras C., Kövari E., Perl D. P., Morrison J. H., Gold G., Hof P. R. (2003). Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology, 60(9), 1495–1500. 10.1212/01.WNL.0000063311.58879.01 [DOI] [PubMed] [Google Scholar]
- Go M., Kou J., Lim J. E., Yang J., Fukuchi K. I. (2016). Microglial response to LPS increases in wild-type mice during aging but diminishes in an Alzheimer’s mouse model: Implication of TLR4 signaling in disease progression. Biochemical and Biophysical Research Communications, 479(2), 331–337. 10.1016/j.bbrc.2016.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro S., Privat A. L., Bressac L., Toulorge D. (2020). CD38 In neurodegeneration and neuroinflammation. Cells, 9(2). 10.3390/cells9020471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P., Anand P. K., Malireddi R. K., Vande Walle L., Van Opdenbosch N., Dillon C. P., Weinlich R., Green D. R., Lamkanfi M., Kanneganti T. D. (2014). FADD And caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. Journal of Immunology, 192(4), 1835–1846. 10.4049/jimmunol.1302839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P., Kanneganti T. D. (2016). Autoinflammatory skin disorders: The inflammasomme in focus. Trends in Molecular Medicine, 22(7), 545–564. 10.1016/j.molmed.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haditsch U., Roth T., Rodriguez L., Hancock S., Cecere T., Nguyen M., Arastu-Kapur S., Broce S., Raha D., Lynch C. C., Holsinger L. J., Dominy S. S., Ermini F. (2020). Alzheimer’s disease-like neurodegeneration in Porphyromonas gingivalis infected neurons with persistent expression of active gingipains. Journal of Alzheimer’s Disease, 75(4), 1361–1376. 10.3233/JAD-200393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., Golenbock D. T. (2008). The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature Immunology, 9(8), 857–865. 10.1038/ni.1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O., Staeheli P., Schwemmle M., Kochs G. (2015). Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends in Microbiology, 23(3), 154–163. 10.1016/j.tim.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Han C., Yang Y., Guan Q., Zhang X., Shen H., Sheng Y., Wang J., Zhou X., Li W., Guo L., Jiao Q. (2020). New mechanism of nerve injury in Alzheimer’s disease: Β-amyloid-induced neuronal pyroptosis. Journal of Cellular and Molecular Medicine, 24(14), 8078–8090. 10.1111/jcmm.15439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch U. K., Kettenmann H. (2007). Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience, 10(11), 1387–1394. 10.1038/nn1997 [DOI] [PubMed] [Google Scholar]
- Hansen D. V., Hanson J. E., Sheng M. (2018). Microglia in Alzheimer’s disease. Journal of Cell Biology, 217(2), 459–472. 10.1083/jcb.201709069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J., Selkoe D. J. (2002). The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science (new York, N Y ), 297(2), 353–356. 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- He W. T., Wan H., Hu L., Chen P., Wang X., Huang Z., Yang Z. H., Zhong C. Q., Han J. (2015). Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Research, 25(12), 1285–1298. 10.1038/cr.2015.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Guo J. L., Mcbride J. D., Narasimhan S., Kim H., Changolkar L., Zhang B., Gathagan R. J., Yue C., Dengler C., Stieber A., Nitla M., Coulter D. A., Abel T., Brunden K. R., Trojanowski J. Q., Lee V. M. (2018). Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nature Medicine, 24(1), 29–38. 10.1038/nm.4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemonnot A. L., Hua J., Ulmann L., Hirbec H. (2019). Microglia in Alzheimer disease: Well-known targets and new opportunities. Frontiers in Aging Neuroscience, 11, 233. 10.3389/fnagi.2019.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., Carson M. J., El Khoury J., Landreth G. E., Brosseron F., Feinstein D. L., Jacobs A. H., Wyss-Coray T., Vitorica J., Ransohoff R. M., Herrup K., Frautschy S. A., Finsen B., Brown G. C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., … , Kummer M. P. (2015). Neuroinflammation in Alzheimer’s disease. The Lancet. Neurology, 14(4), 388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., Kummer M. P., Latz E. (2014). Innate immune activation in neurodegenerative disease. Nature Reviews Immunology, 14(7), 463–477. https://doi.org/10.1038/nri3705 [DOI] [PubMed] [Google Scholar]
- Heneka M. T., Kummer M. P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T. C., Gelpi E., Halle A., Korte M., Latz E., Golenbock D. T. (2013). NLRP3 Is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature, 493(7434), 674–678. 10.1038/nature11729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C., Boche D., Wilkinson D., Yadegarfar G., Hopkins V., Bayer A., Jones R. W., Bullock R., Love S., Neal J. W., Zotova E., Nicoll J. A. (2008). Long-term effects of Abeta42 immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet (London, England), 372(9634), 216–223. 10.1016/S0140-6736(08)61075-2 [DOI] [PubMed] [Google Scholar]
- Ising C., Venegas C., Zhang S., Scheiblich H., Schmidt S. V., Vieira-Saecker A., Schwartz S., Albasset S., Mcmanus R. M., Tejera D., Griep A., Santarelli F., Brosseron F., Opitz S., Stunden J., Merten M., Kayed R., Golenbock D. T., Blum D., … , Heneka M. T. (2019). NLRP3 Inflammasome activation drives tau pathology. Nature, 575(7784), 669–673. 10.1038/s41586-019-1769-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C. R., Jr., Knopman D. S., Jagust W. J., Shaw L. M., Aisen P. S., Weiner M. W., Petersen R. C., Trojanowski J. Q. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet. Neurology, 9(1), 119–128. 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana M., Palencia C. A., Pahan K. (2008). Fibrillar amyloid-beta peptides activate microglia via TLR2: Implications for Alzheimer’s disease. Journal of Immunology, 181(10), 7254–7262. 10.4049/jimmunol.181.10.7254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Qiu Q., Zhang H., Chu L., Du Y., Zhang J., Zhou C., Liang F., Shi S., Wang S., Qin W., Wang Q., Li F., Wang Q., Li Y., Shen L., Wei Y., Jia J. (2019). Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimer’s & Dementia, 15(8), 1071–1080. 10.1016/j.jalz.2019.05.002 [DOI] [PubMed] [Google Scholar]
- Kanneganti T. D. (2010). Central roles of NLRs and inflammasomes in viral infection. Nature Reviews Immunology, 10(10), 688–698. 10.1038/nri2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti T. D., Body-Malapel M., Amer A., Park J. H., Whitfield J., Franchi L., Taraporewala Z. F., Miller D., Patton J. T., Inohara N., Núñez G. (2006a). Critical role for cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. Journal of Biological Chemistry, 281(48), 36560–36568. 10.1074/jbc.M607594200 [DOI] [PubMed] [Google Scholar]
- Kanneganti T. D., Ozören N., Body-Malapel M., Amer A., Park J. H., Franchi L., Whitfield J., Barchet W., Colonna M., Vandenabeele P., Bertin J., Coyle A., Grant E. P., Akira S., Núñez G. (2006b). Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature, 440(7081), 233–236. 10.1038/nature04517 [DOI] [PubMed] [Google Scholar]
- Karki R., Kanneganti T. D. (2021). The ‘cytokine storm’: Molecular mechanisms and therapeutic prospects. Trends in Immunology, 42(8), 681–705. 10.1016/j.it.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R., Man S. M., Malireddi R. K. S., Gurung P., Vogel P., Lamkanfi M., Kanneganti T. D. (2015). Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host & Microbe, 17(3), 357–368. 10.1016/j.chom.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R., Sharma B. R., Tuladhar S., Williams E. P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R. K. S., Schreiner P., Neale G., Vogel P., Webby R., Jonsson C. B., Kanneganti T. D. (2021). Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell, 184(1), 149–168.e117. 10.1016/j.cell.2020.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmarski N., Ziegler-Waldkirch S., Scheffler N., Witt C., Abou-Ajram C., Nuscher B., Prinz M., Haass C., Meyer-Luehmann M. (2020). Aβ oligomers trigger and accelerate Aβ seeding. Brain Pathology, 30(1), 36–45. 10.1111/bpa.12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavardhana S., Malireddi R. K. S., Burton A. R., Porter S. N., Vogel P., Pruett-Miller S. M., Kanneganti T. D. (2020). The Zα2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. Journal of Biological Chemistry, 295(24), 8325–8330. 10.1074/jbc.RA120.013752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl K. A., De Rivero Vaccari J. P., Dietrich W. D., Popovich P. G., Keane R. W. (2014). Pattern recognition receptors and central nervous system repair. Experimental Neurology, 258, 5–16. 10.1016/j.expneurol.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E., O’brien J. T., Donaghy P., Morris C., Barnett N., Olsen K., Martin-Ruiz C., Taylor J. P., Thomas A. J. (2018). Peripheral inflammation in prodromal Alzheimer’s and Lewy body dementias. Journal of Neurology Neurosurgery & Psychiatry, 89(4), 339–345. 10.1136/jnnp-2017-317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriakose T., Man S. M., Malireddi R. K., Karki R., Kesavardhana S., Place D. E., Neale G., Vogel P., Kanneganti T. D. (2016). ZBP1/DAI Is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Science Immunology, 1(2). 10.1126/sciimmunol.aag2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferla F. M., Tinkle B. T., Bieberich C. J., Haudenschild C. C., Jay G. (1995). The Alzheimer’s A beta peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nature Genetics, 9(1), 21–30. 10.1038/ng0195-21 [DOI] [PubMed] [Google Scholar]
- Laganke C., Samkoff L., Edwards K., Jung Henson L., Repovic P., Lynch S., Stone L., Mattson D., Galluzzi A., Fisher T. L., Reilly C., Winter L. A., Leonard J. E., Zauderer M. (2017). Safety/tolerability of the anti-semaphorin 4D antibody VX15/2503 in a randomized phase 1 trial. Neurology(R) Neuroimmunology & Neuroinflammation, 4(4), e367. 10.1212/NXI.0000000000000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J., Mei Z. L., Wang H., Hu M., Long Y., Miao M. X., Li N., Hong H. (2014). Montelukast rescues primary neurons against Aβ1-42-induced toxicity through inhibiting CysLT1R-mediated NF-κB signaling. Neurochemistry International, 75, 26–31. 10.1016/j.neuint.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Lamkanfi M., Kanneganti T. D., Van Damme P., Vanden Berghe T., Vanoverberghe I., Vandekerckhove J., Vandenabeele P., Gevaert K., Núñez G. (2008). Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Molecular and Cellular Proteomics, 7(12), 2350–2363. 10.1074/mcp.M800132-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbi A., Pawelec G., Witkowski J. M., Schipper H. M., Derhovanessian E., Goldeck D., Fulop T. (2009). Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. Journal of Alzheimer’s Disease, 17(1), 91–103. 10.3233/JAD-2009-1015 [DOI] [PubMed] [Google Scholar]
- Lee S., Channappanavar R., Kanneganti T. D. (2020). Coronaviruses: Innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Trends in Immunology, 41(12), 1083–1099. 10.1016/j.it.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Hirohama M., Noguchi M., Nagata K., Kawaguchi A. (2018). Influenza A virus infection triggers pyroptosis and apoptosis of respiratory epithelial cells through the Type I interferon signaling pathway in a mutually exclusive manner. Journal of Virology, 92(14). 10.1128/jvi.00396-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Ishitsuka A., Kuroki T., Lin Y. H., Shibuya A., Hongu T., Funakoshi Y., Kanaho Y., Nagata K., Kawaguchi A. (2021a). Arf6 exacerbates allergic asthma through cell-to-cell transmission of ASC inflammasomes. JCI Insight, 6(16). 10.1172/jci.insight.139190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Ishitsuka A., Noguchi M., Hirohama M., Fujiyasu Y., Petric P. P., Schwemmle M., Staeheli P., Nagata K., Kawaguchi A. (2019). Influenza restriction factor MxA functions as inflammasome sensor in the respiratory epithelium. Science Immunology, 4(40). 10.1126/sciimmunol.aau4643 [DOI] [PubMed] [Google Scholar]
- Lee S., Karki R., Wang Y., Nguyen L. N., Kalathur R. C., Kanneganti T. D. (2021b). AIM2 Forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature, 597(7876), 415–419. 10.1038/s41586-021-03875-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Ryu J.-H. (2021). Influenza viruses: Innate immunity and mRNA vaccines. Frontiers in Immunology, 12(3534). 10.3389/fimmu.2021.710647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Melief E., Postupna N., Montine K. S., Keene C. D., Montine T. J. (2015). Prostaglandin E2 receptor subtype 2 regulation of scavenger receptor CD36 modulates microglial Aβ42 phagocytosis. American Journal of Pathology, 185(1), 230–239. 10.1016/j.ajpath.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Liu Y., Hao W., Wolf L., Kiliaan A. J., Penke B., Rübe C. E., Walter J., Heneka M. T., Hartmann T., Menger M. D., Fassbender K. (2012). TLR2 is a primary receptor for Alzheimer’s amyloid β peptide to trigger neuroinflammatory activation. Journal of Immunology, 188(3), 1098–1107. 10.4049/jimmunol.1101121 [DOI] [PubMed] [Google Scholar]
- Lonnemann N., Hosseini S., Marchetti C., Skouras D. B., Stefanoni D., D’alessandro A., Dinarello C. A., Korte M. (2020). The NLRP3 inflammasome inhibitor OLT1177 rescues cognitive impairment in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America, 117(50), 32145–32154. 10.1073/pnas.2009680117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue L. F., Walker D. G., Brachova L., Beach T. G., Rogers J., Schmidt A. M., Stern D. M., Yan S. D. (2001). Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: Identification of a cellular activation mechanism. Experimental Neurology, 171(1), 29–45. 10.1006/exnr.2001.7732 [DOI] [PubMed] [Google Scholar]
- Ma S. L., Huang W., Tang N. L., Lam L. C. (2012). Mxa polymorphisms are associated with risk and age-at-onset in Alzheimer disease and accelerated cognitive decline in Chinese elders. Rejuvenation Research, 15(5), 516–522. 10.1089/rej.2012.1328 [DOI] [PubMed] [Google Scholar]
- Macpherson K. P., Sompol P., Kannarkat G. T., Chang J., Sniffen L., Wildner M. E., Norris C. M., Tansey M. G. (2017). Peripheral administration of the soluble TNF inhibitor XPro1595 modifies brain immune cell profiles, decreases beta-amyloid plaque load, and rescues impaired long-term potentiation in 5xFAD mice. Neurobiology of Disease, 102, 81–95. 10.1016/j.nbd.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi R. K., Ippagunta S., Lamkanfi M., Kanneganti T. D. (2010). Cutting edge: Proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. Journal of Immunology, 185(6), 3127–3130. 10.4049/jimmunol.1001512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi R. K. S., Gurung P., Kesavardhana S., Samir P., Burton A., Mummareddy H., Vogel P., Pelletier S., Burgula S., Kanneganti T. D. (2020). Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. Journal of Experimental Medicine, 217(3). 10.1084/jem.20191644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi R. K. S., Karki R., Sundaram B., Kancharana B., Lee S., Samir P., Kanneganti T. D. (2021). Inflammatory cell death, PANoptosis, Mediated by cytokines in diverse cancer lineages inhibits tumor growth. Immunohorizons, 5(7), 568–580. 10.4049/immunohorizons.2100059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S. M., Karki R., Kanneganti T. D. (2017). Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunological Reviews, 277(1), 61–75. 10.1111/imr.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso R., Fryatt G., Cleal M., Obst J., Pipi E., Monzón-Sandoval J., Ribe E., Winchester L., Webber C., Nevado A., Jacobs T., Austin N., Theunis C., Grauwen K., Daniela Ruiz E., Mudher A., Vicente-Rodriguez M., Parker C. A., Simmons C., … , Perry V. H. (2019). CSF1R Inhibitor JNJ-40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain, 142(10), 3243–3264. 10.1093/brain/awz241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S. E., Abud E. M., Lakatos A., Karimzadeh A., Yeung S. T., Davtyan H., Fote G. M., Lau L., Weinger J. G., Lane T. E., Inlay M. A., Poon W. W., Blurton-Jones M. (2016). The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proceedings of the National Academy of Sciences of the United States of America, 113(9), E1316–E1325. 10.1073/pnas.1525466113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald C. L., Hennessy E., Rubio-Araiz A., Keogh B., Mccormack W., Mcguirk P., Reilly M., Lynch M. A. (2016). Inhibiting TLR2 activation attenuates amyloid accumulation and glial activation in a mouse model of Alzheimer’s disease. Brain Behavior and Immunity, 58, 191–200. 10.1016/j.bbi.2016.07.143 [DOI] [PubMed] [Google Scholar]
- Mcdonough A., Lee R. V., Weinstein J. R. (2017). Microglial interferon signaling and white matter. Neurochemical Research, 42(9), 2625–2638. 10.1007/s11064-017-2307-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin N., Jourdain V. A., Morissette M., Grégoire L., Di Paolo T. (2014). Long-term treatment with l-DOPA and an mGlu5 receptor antagonist prevents changes in brain basal ganglia dopamine receptors, their associated signaling proteins and neuropeptides in parkinsonian monkeys. Neuropharmacology, 79, 688–706. 10.1016/j.neuropharm.2014.01.014 [DOI] [PubMed] [Google Scholar]
- Nelson P. T., Alafuzoff I., Bigio E. H., Bouras C., Braak H., Cairns N. J., Castellani R. J., Crain B. J., Davies P., Del Tredici K., Duyckaerts C., Frosch M. P., Haroutunian V., Hof P. R., Hulette C. M., Hyman B. T., Iwatsubo T., Jellinger K. A., Jicha G. A., … , Beach T. G. (2012). Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. Journal of Neuropathology & Experimental Neurology, 71(5), 362–381. 10.1097/NEN.0b013e31825018f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng A., Tam W. W., Zhang M. W., Ho C. S., Husain S. F., Mcintyre R. S., Ho R. C. (2018). IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: Systematic review and meta-analysis. Scientific Reports, 8(1), 12050. 10.1038/s41598-018-30487-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum R. L., Ellis C. E. (2003). Alzheimer’s disease and Parkinson’s disease. New England Journal of Medicine, 348(14), 1356–1364. 10.1056/NEJM2003ra020003 [DOI] [PubMed] [Google Scholar]
- Ofengeim D., Mazzitelli S., Ito Y., Dewitt J. P., Mifflin L., Zou C., Das S., Adiconis X., Chen H., Zhu H., Kelliher M. A., Levin J. Z., Yuan J. (2017). RIPK1 Mediates a disease-associated microglial response in Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America, 114(41), E8788–e8797. 10.1073/pnas.1714175114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala J., Alafuzoff I., Herukka S. K., Van Groen T., Tanila H., Pirttilä T. (2009). Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiology of Aging, 30(2), 198–209. 10.1016/j.neurobiolaging.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Olson J. K., Miller S. D. (2004). Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. Journal of Immunology, 173(6), 3916–3924. 10.4049/jimmunol.173.6.3916 [DOI] [PubMed] [Google Scholar]
- Palm N. W., Medzhitov R. (2009). Pattern recognition receptors and control of adaptive immunity. Immunological Reviews, 227(1), 221–233. 10.1111/j.1600-065X.2008.00731.x [DOI] [PubMed] [Google Scholar]
- Pasparakis M., Vandenabeele P. (2015). Necroptosis and its role in inflammation. Nature, 517(7534), 311–320. 10.1038/nature14191 [DOI] [PubMed] [Google Scholar]
- Pellicanò M., Larbi A., Goldeck D., Colonna-Romano G., Buffa S., Bulati M., Rubino G., Iemolo F., Candore G., Caruso C., Derhovanessian E., Pawelec G. (2012). Immune profiling of Alzheimer patients. Journal of Neuroimmunology, 242(1-2), 52–59. 10.1016/j.jneuroim.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Pirttilä T., Mattinen S., Frey H. (1992). The decrease of CD8-positive lymphocytes in Alzheimer’s disease. Journal of the Neurological Sciences, 107(2), 160–165. 10.1016/0022-510X(92)90284-R [DOI] [PubMed] [Google Scholar]
- Place D. E., Kanneganti T. D. (2018). Recent advances in inflammasome biology. Current Opinion in Immunology, 50, 32–38. 10.1016/j.coi.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place D. E., Lee S., Kanneganti T. D. (2021). PANoptosis in microbial infection. Current Opinion in Microbiology, 59, 42–49. 10.1016/j.mib.2020.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plascencia-Villa G., Perry G. (2020). Status and future directions of clinical trials in Alzheimer’s disease. International Review of Neurobiology, 154, 3–50. 10.1016/bs.irn.2020.03.022 [DOI] [PubMed] [Google Scholar]
- Qin Y., Liu Y., Hao W., Decker Y., Tomic I., Menger M. D., Liu C., Fassbender K. (2016). Stimulation of TLR4 attenuates Alzheimer’s disease-related symptoms and pathology in tau-transgenic mice. Journal of Immunology, 197(8), 3281–3292. 10.4049/jimmunol.1600873 [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan E. G., Savage J. C., Hise A. G., Landreth G. E. (2009). CD14 And toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. Journal of Neuroscience, 29(38), 11982–11992. 10.1523/JNEUROSCI.3158-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C., Erkes D. A., Nardone A., Aplin A. E., Fernandes-Alnemri T., Alnemri E. S. (2019). Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nature Communications, 10(1), 1689. 10.1038/s41467-019-09397-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn T. T., Head E., Nesse W. H., Cotman C. W., Cribbs D. H. (2001). Activation of caspase-8 in the Alzheimer’s disease brain. Neurobiology of Disease, 8(6), 1006–1016. 10.1006/nbdi.2001.0449 [DOI] [PubMed] [Google Scholar]
- Rohn T. T., Rissman R. A., Davis M. C., Kim Y. E., Cotman C. W., Head E. (2002). Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiology of Disease, 11(2), 341–354. 10.1006/nbdi.2002.0549 [DOI] [PubMed] [Google Scholar]
- Roy E. R., Wang B., Wan Y. W., Chiu G., Cole A., Yin Z., Propson N. E., Xu Y., Jankowsky J. L., Liu Z., Lee V. M., Trojanowski J. Q., Ginsberg S. D., Butovsky O., Zheng H., Cao W. (2020). Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease. Journal of Clinical Investigation, 130(4), 1912–1930. 10.1172/JCI133737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Perez J. M., Morillas-Ruiz J. M. (2012). A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Thescientificworldjournal, 2012, 756357. 10.1100/2012/756357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter M. W., Stevens B. (2017). Microglia emerge as central players in brain disease. Nature Medicine, 23(9), 1018–1027. 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- Saresella M., Calabrese E., Marventano I., Piancone F., Gatti A., Alberoni M., Nemni R., Clerici M. (2011). Increased activity of Th-17 and Th-9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer’s disease. Brain Behavior and Immunity, 25(3), 539–547. 10.1016/j.bbi.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Saresella M., La Rosa F., Piancone F., Zoppis M., Marventano I., Calabrese E., Rainone V., Nemni R., Mancuso R., Clerici M. (2016). The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Molecular Neurodegeneration, 11, 23. 10.1186/s13024-016-0088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlus H., Heneka M. T. (2017). Microglia in Alzheimer’s disease. Journal of Clinical Investigation, 127(9), 3240–3249. 10.1172/JCI90606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindowski K., Peters J., Gorriz C., Schramm U., Weinandi T., Leutner S., Maurer K., Frölich L., Müller W. E., Eckert A. (2006). Apoptosis of CD4+ T and natural killer cells in Alzheimer’s disease. Pharmacopsychiatry, 39(6), 220–228. 10.1055/s-2006-954591 [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Hardy J. (2016). The amyloid hypothesis of Alzheimer’s disease at 25 years. Embo Molecular Medicine, 8(6), 595–608. 10.15252/emmm.201606210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny J., Chiao P., Bussière T., Weinreb P. H., Williams L., Maier M., Dunstan R., Salloway S., Chen T., Ling Y., O’gorman J., Qian F., Arastu M., Li M., Chollate S., Brennan M. S., Quintero-Monzon O., Scannevin R. H., Arnold H. M., … , Sandrock A. (2016). The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature, 537(7618), 50–56. 10.1038/nature19323 [DOI] [PubMed] [Google Scholar]
- Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature, 526(7575), 660–665. 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- Song M., Jin J., Lim J. E., Kou J., Pattanayak A., Rehman J. A., Kim H. D., Tahara K., Lalonde R., Fukuchi K. (2011). TLR4 Mutation reduces microglial activation, increases Aβ deposits and exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Journal of Neuroinflammation, 8, 92. 10.1186/1742-2094-8-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speciale L., Calabrese E., Saresella M., Tinelli C., Mariani C., Sanvito L., Longhi R., Ferrante P. (2007). Lymphocyte subset patterns and cytokine production in Alzheimer’s disease patients. Neurobiology of Aging, 28(8), 1163–1169. 10.1016/j.neurobiolaging.2006.05.020 [DOI] [PubMed] [Google Scholar]
- Stadelmann C., Deckwerth T. L., Srinivasan A., Bancher C., Brück W., Jellinger K., Lassmann H. (1999). Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease. Evidence for apoptotic cell death. American Journal of Pathology, 155(5), 1459–1466. 10.1016/S0002-9440(10)65460-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Amour I., Paré I., Tremblay C., Coulombe K., Bazin R., Calon F. (2014). IVIg protects the 3xTg-AD mouse model of Alzheimer’s disease from memory deficit and Aβ pathology. Journal of Neuroinflammation, 11, 54. 10.1186/1742-2094-11-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancu I. C., Cremers N., Vanrusselt H., Couturier J., Vanoosthuyse A., Kessels S., Lodder C., Brône B., Huaux F., Octave J. N., Terwel D., Dewachter I. (2019). Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathologica, 137(4), 599–617. 10.1007/s00401-018-01957-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra R., Van Diepen J. A., Tack C. J., Zaki M. H., Van De Veerdonk F. L., Perera D., Neale G. A., Hooiveld G. J., Hijmans A., Vroegrijk I., Van Den Berg S., Romijn J., Rensen P. C., Joosten L. A., Netea M. G., Kanneganti T. D. (2011). Inflammasome is a central player in the induction of obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America, 108(37), 15324–15329. 10.1073/pnas.1100255108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. H., Zhao M., Anderson A. J., Srinivasan A., Cotman C. W. (2001). Activated caspase-3 expression in Alzheimer’s and aged control brain: Correlation with Alzheimer pathology. Brain Research, 898(2), 350–357. 10.1016/S0006-8993(01)02018-2 [DOI] [PubMed] [Google Scholar]
- Tahara K., Kim H. D., Jin J. J., Maxwell J. A., Li L., Fukuchi K. (2006). Role of toll-like receptor signalling in Abeta uptake and clearance. Brain, 129(Pt 11), 3006–3019. 10.1093/brain/awl249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M. S., Tan L., Jiang T., Zhu X. C., Wang H. F., Jia C. D., Yu J. T. (2014). Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death & Disease, 5(8), e1382. 10.1038/cddis.2014.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. G., Dash P., Aldridge J. R., Jr., Ellebedy A. H., Reynolds C., Funk A. J., Martin W. J., Lamkanfi M., Webby R. J., Boyd K. L., Doherty P. C., Kanneganti T. D. (2009). The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity, 30(4), 566–575. 10.1016/j.immuni.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas C., Kumar S., Franklin B. S., Dierkes T., Brinkschulte R., Tejera D., Vieira-Saecker A., Schwartz S., Santarelli F., Kummer M. P., Griep A., Gelpi E., Beilharz M., Riedel D., Golenbock D. T., Geyer M., Walter J., Latz E., Heneka M. T., … (2017). Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature, 552(7685), 355–361. 10.1038/nature25158 [DOI] [PubMed] [Google Scholar]
- Vollmar P., Kullmann J. S., Thilo B., Claussen M. C., Rothhammer V., Jacobi H., Sellner J., Nessler S., Korn T., Hemmer B. (2010). Active immunization with amyloid-beta 1-42 impairs memory performance through TLR2/4-dependent activation of the innate immune system. Journal of Immunology, 185(10), 6338–6347. 10.4049/jimmunol.1001765 [DOI] [PubMed] [Google Scholar]
- Walter S., Letiembre M., Liu Y., Heine H., Penke B., Hao W., Bode B., Manietta N., Walter J., Schulz-Schuffer W., Fassbender K. (2007). Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cellular Physiology and Biochemistry, 20(6), 947–956. 10.1159/000110455 [DOI] [PubMed] [Google Scholar]
- Wang S., Mustafa M., Yuede C. M., Salazar S. V., Kong P., Long H., Ward M., Siddiqui O., Paul R., Gilfillan S., Ibrahim A., Rhinn H., Tassi I., Rosenthal A., Schwabe T., Colonna M. (2020). Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. Journal of Experimental Medicine, 217(9). 10.1084/jem.20200785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. Y., Tan M. S., Yu J. T., Tan L. (2015). Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Annals of Translational Medicine, 3(10), 136. 10.3978/j.issn.2305-5839.2015.03.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling H., Mair W., Kumar M., Schlaffner C. N., Tang S., Beerepoot P., Fatou B., Guise A. J., Cheng L., Takeda S., Muntel J., Rotunno M. S., Dujardin S., Davies P., Kosik K. S., Miller B. L., Berretta S., Hedreen J. C., Grinberg L. T., … , Steen J. A. (2020). Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease. Cell, 183(6), 1699–1713.e1613. 10.1016/j.cell.2020.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. J., Hung Y. F., Liu H. Y., Hsueh Y. P. (2017). Deletion of the inflammasome sensor Aim2 mitigates Aβ deposition and microglial activation but increases inflammatory cytokine expression in an Alzheimer disease mouse model. Neuroimmunomodulation, 24(1), 29–39. 10.1159/000477092 [DOI] [PubMed] [Google Scholar]
- Xia M. Q., Hyman B. T. (1999). Chemokines/chemokine receptors in the central nervous system and Alzheimer’s disease. Journal of Neurovirology, 5(1), 32–41. 10.3109/13550289909029743 [DOI] [PubMed] [Google Scholar]
- Yamada T., Horisberger M. A., Kawaguchi N., Moroo I., Toyoda T. (1994). Immunohistochemistry using antibodies to alpha-interferon and its induced protein, MxA, in Alzheimer’s and Parkinson’s disease brain tissues. Neuroscience Letters, 181(1-2), 61–64. 10.1016/0304-3940(94)90560-6 [DOI] [PubMed] [Google Scholar]
- Yin J., Zhao F., Chojnacki J. E., Fulp J., Klein W. L., Zhang S., Zhu X. (2018). NLRP3 Inflammasome inhibitor ameliorates amyloid pathology in a mouse model of Alzheimer’s disease. Molecular Neurobiology, 55(3), 1977–1987. 10.1007/s12035-017-0467-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki M. H., Boyd K. L., Vogel P., Kastan M. B., Lamkanfi M., Kanneganti T. D. (2010). The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity, 32(3), 379–391. 10.1016/j.immuni.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenaro E., Pietronigro E., Della Bianca V., Piacentino G., Marongiu L., Budui S., Turano E., Rossi B., Angiari S., Dusi S., Montresor A., Carlucci T., Nanì S., Tosadori G., Calciano L., Catalucci D., Berton G., Bonetti B., Constantin G., … (2015). Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nature Medicine, 21(8), 880–886. 10.1038/nm.3913 [DOI] [PubMed] [Google Scholar]
- Zhang C., Griciuc A., Hudry E., Wan Y., Quinti L., Ward J., Forte A. M., Shen X., Ran C., Elmaleh D. R., Tanzi R. E. (2018). Cromolyn reduces levels of the Alzheimer’s disease-associated amyloid β-protein by promoting microglial phagocytosis. Scientific Reports, 8(1), 1144. 10.1038/s41598-018-19641-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Kishimoto Y., Grammatikakis I., Gottimukkala K., Cutler R. G., Zhang S., Abdelmohsen K., Bohr V. A., Misra Sen J., Gorospe M., Mattson M. P. (2019). Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nature Neuroscience, 22(5), 719–728. 10.1038/s41593-019-0372-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. K., Liu J. T., Peng Z. W., Fan H., Yao A. H., Cheng P., Liu L., Ju G., Kuang F. (2013). Different TLR4 expression and microglia/macrophage activation induced by hemorrhage in the rat spinal cord after compressive injury. Journal of Neuroinflammation, 10, 112. 10.1186/1742-2094-10-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Karki R., Vogel P., Kanneganti T. D. (2020). Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell, 181(3), 674–687.e613. 10.1016/j.cell.2020.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]