Abstract
Background:
Previous studies showed increasing number of children with a life-limiting or life-threatening condition who may benefit from input from pediatric palliative care services.
Aim:
To estimate the current prevalence of children with a life-limiting condition and to model future prevalence of this population.
Design:
Observational study using national inpatient hospital data. A population-based approach utilizing ethnic specific population projections was used to estimate future prevalence.
Setting/participants:
All children aged 0–19 years with a life-limiting condition diagnostic code recorded in Hospital Episodes Statistics data in England from 2000/01 to 2017/18.
Results:
Data on 4,543,386 hospital episodes for 359,634 individuals were included. The prevalence of children with a life-limiting condition rose from 26.7 per 10,000 (95%CI 26.5–27.0) in 2001/02 to 66.4 per 10,000 (95% CI: 66.0–66.8) in 2017/18. Using a more restricted definition of a life-limiting condition reduced the prevalence from 66.4 to 61.1 per 10,000 (95%CI 60.7–61.5) in 2017/18. Highest prevalence was in the under 1-year age group at 226.5 per 10,000 and children with a congenital abnormality had the highest prevalence (27.2 per 10,000 (95%CI: 26.9–27.5)).
The prevalence was highest among the most deprived group and in children of Pakistani origin.
Predicted future prevalence of life-limiting conditions ranged from 67.0 (95%CI 67.7–66.3) to 84.22 (95%CI 78.66–90.17) per 10,000 by 2030.
Conclusions:
The prevalence of children with a life-limiting or life-threatening condition in England has risen over the last 17 years and is predicted to increase. Future data collections must include the data required to assess the complex health and social care needs of these children.
Keywords: Life-limiting condition, life-threatening condition, child, pediatrics, palliative care, hospital episode statistics
What is already known?
There is higher prevalence of children with life-limiting and life-threatening conditions in areas of higher deprivation and in ethnic minority groups.
What your paper adds?
The prevalence of children with life-limiting and life-threatening conditions in England is still rising and likely to continue to rise to 2030 in England.
Implications for practice, theory or policy
There are growing numbers of children with life-limiting and life-threatening conditions and this highlights the need for palliative care services to work closely with other pediatric services.
Future data collection should incorporate measures of the complexity of health and social care needs of these child and their families.
Introduction
One key difference between pediatric palliative care and adult palliative care is that the World Health Organization recommendation which states that pediatric palliative care “begins when illness is diagnosed and continues regardless whether a child is treated for the disease or not”. 1 This means that children and their families may require care and support for a prolonged period of time, more than 20 years in some instances. 2
It can be challenging to estimate which children may benefit from palliative care input. Using the number of children who have died can underestimate the ongoing need. 3 In the UK and other countries the terminology life-limiting and life-threatening conditions have been used to describe the population of children who may benefit from input from pediatric palliative care services.4,5 In some other countries, including the US, the term Chronic Complex Conditions is often used to describe a similar population of children. Life-limiting conditions are those for which there is no reasonable hope of cure and from which children or young people will die. 6 Life-threatening conditions are those for which curative treatment may be feasible but can fail, such as cancer. 6 Together life-limiting condition and life-threatening (hereafter life-limiting condition) are a very heterogeneous group with nearly 400 individual diagnoses being classified as life-limiting or life-threatening. 7
Previous studies have shown an increasing number of children living with a life-limiting condition with a reported 40,000 children and young people having an life-limiting condition in England in 2009/108 and 6661 in Scotland in 2015. 9 These studies have also indicated that prevalence of children with a life-limiting condition varies by ethnicity, deprivation status and geographical region.8–10
There has been an increase in the last few decades in the number of pediatric palliative care and hospice services that provide palliative and end of life care for children, but there is little information about the models of care, quality, resource implications and outcomes of children and families who use these services. These services vary in their professional configuration, services provided, funding sources and population served 6 and many have developed locally with heavy reliance on individual clinician and third sector organizations including children’s hospices. 11 As a result, delivery of palliative care for children is “inconsistent and incoherent”. 7 Planning for development of current and future services requires up to date information on the population of children and young people who may benefit from these services. 12
This study aimed to estimate the current prevalence of children with a life-limiting condition in England and to model future prevalence of this population up to 2030 to inform planning of pediatric palliative care services.
Method
Data sources
Individual level pseudonymized patient data was obtained from NHS Digital. 13 Hospital Episode Statistics Admitted Patient Care (HES) were linked to the Office for National Statistics (ONS) mortality data. HES includes information on all admitted care in NHS hospitals in England, with clinical diagnoses and procedures, demographic and geographical information on Government office region of residence (GOR). 14 The ONS data contained the date and cause of death if the child had died.
For denominators, population counts by ethnic group were obtained as mid-year estimates (2001–2017) and projections to 2030 from ETHPOP (http://ethpop.org). 15 This source was necessary rather than ONS subnational estimates because the ETHPOP cohort component model 16 incorporates detailed demographic information by ethnic group in relation to newborns, mortality, and most importantly, both subnational migration and international migration. Any changes in ethnic-specific demographic rates are aligned with ONS projection rate assumption changes. ETHPOP data were available as subnational populations by ethnic group, age, and sex.
Study population
All admitted patient care HES data for children aged 0–19 years who had a life-limiting condition recorded between 1st April 2000 and 31st March 2018 were obtained for this study. Life-limiting conditions were identified using a previously developed diagnostic coding framework using 777 4-digit ICD-10 codes (Supplemental Table 1). 8
Eligibility criteria:
had a diagnosis of one of the life-limiting condition ICD -10 codes in the current year or a previous year (from April 2001)
had a hospital admission in the year of analysis 1
were ⩽19 years old
were resident in England.
The age was assigned as the age at the first hospital episode in a year. Sex was categorized as the most commonly reported sex across all records.
Diagnoses were grouped according to eleven diagnostic groups (neurology, hematology, oncology, metabolic, respiratory, circulatory, gastrointestinal, genitourinary, perinatal, congenital, and other). 8 No attempt was made to prioritize multiple diagnoses for individuals; therefore, a child may have more than one life-limiting diagnosis.
Self-reported ethnicity for each hospital episode was coded according to the 16 2001 Census groups. 17 Eight ethnic groups were made by collapsing these groups:
White (White: British, White: Irish, Other White)
Black (Black or Black British: Black Caribbean, Black or Black British: Black African, Black or Black British: Other Black)
Indian (Asian or Asian British: Indian)
Pakistani (Asian or Asian British: Pakistani)
Bangladeshi (Asian or Asian British: Bangladeshi)
Chinese
Mixed (Mixed: White and Black Caribbean, Mixed: White and Black African, Mixed: White and Asian, Mixed: Other Mixed)
Other Asian.
Individuals were assigned their most commonly recorded ethnicity.
An index of multiple deprivation (IMD2010) score 18 was assigned to each individual based on the 2001 Lower-layer Super Output Area (LSOA) 19 of residence in that year and 10 population weighted categories were created (Category 1 – least deprived) based on these scores.
Analysis
Current prevalence
The prevalence and 95% confidence intervals 20 were calculated per 10,000 population at risk (aged 0–19 years) by dividing the number of individuals with an life-limiting condition by the population estimates for that year according to the ETHPOP dataset. 15 Prevalence was calculated as an overall total per 10,000 and stratified by age group, diagnostic category, ethnicity, and deprivation category.
A further calculation of prevalence using a restricted definition of life-limiting condition was undertaken which excluded the following sets of diagnoses (identified by the study advisory board):
(i) Perinatal disorders were assumed not to be relevant after the first birthday 2
(ii) Oncology cases 5 years after diagnosis, which were assumed to be resolved
(iii) Early stage (1–3) kidney failure.
Modeling of future prevalence
A population based modeling approach, utilizing the ETHPOP data, was used to allow for the prevalence differences by age group, sex, GOR, and ethnicity. 21 This modeling approach automatically adjusts for changing population demographics and does not require separation of incidence, survival, and migration.
HES data from 2004/5 to 2016/17 were the base data for these calculations due to greater missing data for ethnicity prior to 2004/5 and the introduction of a “Payment by Results” system in 2004 which may have affected coding practices. 14
The annual probability of an individual having a life-limiting condition was estimated using the following logistic regression equation:
where P is the probability of an individual having a life-limiting condition and xi are the values of the demographic variables included in the model: sex, age group, ethnic group, and GOR (all categorical). The year terms in the model reflected changes in probability of an individual having a life-limiting condition which were not explained by demographics, that is, increases in survival and/or incidence rates of life-limiting condition over time. Inclusion of a linear year term alone would result in predicted numbers with life-limiting condition being forced to be monotonic with year (i.e. always increasing or always decreasing). Hence a quadratic year term was included.
The regression coefficients were then applied to predict (from ETHPOP data 15 ) numbers of individuals with each unique combination of demographic values in years 2018–2030 expected to have a life-limiting condition (product of the probability of having a life-limiting condition for a unique demographic combination and the number of individuals with that unique demographic combination). These predictions were then summed across the unique demographic combinations to give annual totals of expected individuals with life-limiting condition.
Where NLLC is the annual predicted number of individuals with a life-limiting condition; Pd is the probability of an individual in unique demographic combination group (d) having a LLC and Nd is the number of individuals predicted to be in that unique demographic group.
Three models were developed with differing underlying data:
Model 1: used estimates of numbers of individuals with LLC from 2004/5 to 2016/17
Model 2: as model 1, but using estimates from the restricted definition of LLC
Model 3: as model 1, but predictions used a fixed value of 2017 for year to predict numbers if there was no future change in survival or incidence.
All data manipulation was undertaken using Microsoft SQL server and statistical analysis using STATA version 15 (Stata Corp, Collage Station, TX).
Results
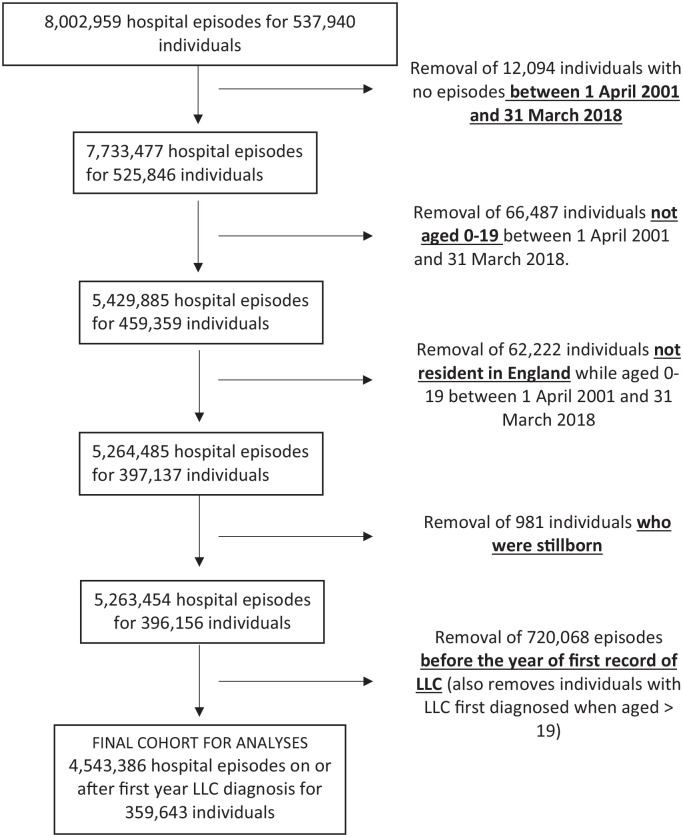
The final analysis dataset contained information on 4,543,386 hospital episodes for 359,634 individuals (Figure 1).
Figure 1.
Flow diagram of inclusion criteria.
There were minimal missing data for sex (n = 2064 (0.2%)) or deprivation (n = 3330 (0.3%)) but more missing data for ethnicity (29,740 (2.8%) overall) notably higher in the earlier years (7% in 2001/02).
Current prevalence
The absolute number and prevalence of children with a life-limiting condition each year rose from 30,535 (26.7 per 10,000 (95% confidence interval [95%CI] 26.5–27.0)) in 2001/02 to 86,625 (66.4 per 10,000 [95% CI: 66.0–66.8]) in 2017/18 (Table 1).
Table 1.
Overall numbers and annual prevalence (per 10,000 population) of children (0–19 years) with life-limiting conditions in England by age group for financial years 2001/02–2017/18.
| Year | Number of patients | Prevalence per 10,000 population |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age 0–19 years | 95% CI | Age <1 year | 95% CI | Age 1–5 years | 95% CI | Age 6–10 years | 95% CI | Age 11–15 years | 95% CI | Age 16–19 years | 95% CI | ||
| 2001/02 | 32,975 | 26.7 | 26.5–27.0 | 130.1 | 127.2–133.1 | 31.3 | 30.7–31.9 | 19.4 | 18.9–19.9 | 18.7 | 18.2–19.2 | 17.6 | 17.1–18.2 |
| 2002/03 | 36,688 | 29.7 | 29.4–30.0 | 131.2 | 128.2–134.2 | 37.3 | 36.6–38.0 | 21.8 | 21.3–22.3 | 20.9 | 20.4–21.4 | 19.4 | 18.8–19.9 |
| 2003/04 | 39,819 | 32.2 | 31.9–32.5 | 134.2 | 131.3–137.2 | 41.7 | 40.9–42.4 | 23.8 | 23.2–24.3 | 22.1 | 21.6–22.6 | 21.5 | 20.9–22.0 |
| 2004/05 | 42,114 | 34.0 | 33.7–34.3 | 134.4 | 131.4–137.3 | 44.9 | 44.1–45.6 | 25.0 | 24.4–25.5 | 23.3 | 22.8–23.8 | 22.9 | 22.3–23.4 |
| 2005/06 | 45,974 | 37.1 | 36.7–37.4 | 141.9 | 138.9–144.9 | 48.4 | 47.6–49.2 | 27.8 | 27.3–28.4 | 25.4 | 24.9–26.0 | 25.5 | 24.9–26.1 |
| 2006/07 | 49,285 | 39.7 | 39.3–40.0 | 158.9 | 155.8–162.0 | 50.5 | 49.7–51.3 | 30.2 | 29.6–30.8 | 26.3 | 25.7–26.9 | 26.8 | 26.1–27.4 |
| 2007/08 | 52,633 | 42.2 | 41.8–42.5 | 158.7 | 155.7–161.8 | 52.9 | 52.0–53.7 | 33.2 | 32.5–33.8 | 28.5 | 27.9–29.1 | 28.7 | 28.0–29.3 |
| 2008/09 | 56,436 | 45.0 | 44.6–45.4 | 177.5 | 174.3–180.6 | 54.3 | 53.5–55.1 | 35.5 | 34.8–36.2 | 29.7 | 29.1–30.3 | 30.0 | 29.4–30.7 |
| 2009/10 | 59,851 | 47.5 | 47.1–47.9 | 187.2 | 183.9–190.4 | 56.2 | 55.4–57.0 | 38.4 | 37.7–39.1 | 31.5 | 30.9–32.1 | 31.7 | 31.0–32.4 |
| 2010/11 | 63,256 | 49.9 | 49.5–50.3 | 189.7 | 186.4–193.0 | 58.9 | 58.1–59.8 | 40.7 | 40.0–41.1 | 33.6 | 33.0–34.3 | 33.4 | 32.8–34.1 |
| 2011/12 | 64,420 | 50.7 | 50.3–51.1 | 174.7 | 171.5–177.8 | 59.9 | 59.1–60.8 | 42.6 | 41.8–43.3 | 35.5 | 34.8–36.2 | 34.6 | 33.8–35.3 |
| 2012/13 | 69,036 | 54.1 | 53.7–54.5 | 188.1 | 184.9–191.3 | 63.2 | 62.3–64.0 | 45.4 | 44.7–46.2 | 38.5 | 37.8–39.2 | 36.0 | 35.3–36.7 |
| 2013/14 | 73,608 | 57.5 | 57.1–57.9 | 212.3 | 208.9–215.7 | 65.7 | 64.8–66.6 | 46.6 | 45.8–47.4 | 41.4 | 40.6–42.1 | 38.5 | 37.8–39.3 |
| 2014/15 | 77,163 | 60.1 | 59.7–60.5 | 221.4 | 217.9–225.0 | 67.9 | 67.1–68.8 | 48.6 | 47.8–49.4 | 44.5 | 43.7–45.2 | 40.8 | 40.0–41.6 |
| 2015/16 | 81,172 | 62.9 | 62.4–63.3 | 231.7 | 228.1–235.3 | 71.9 | 71.0–72.8 | 49.6 | 48.8–50.4 | 46.8 | 46.0–47.5 | 42.9 | 42.1–43.7 |
| 2016/17 | 84,270 | 64.9 | 64.5–65.4 | 235.7 | 232.1–239.3 | 73.1 | 72.1–74.0 | 51.0 | 50.3–51.8 | 49.2 | 48.4–49.9 | 45.9 | 45.1–46.7 |
| 2017/18 | 86,625 | 66.4 | 66.0–66.8 | 226.5 | 223.0–230.1 | 75.1 | 74.2–76.0 | 52.6 | 51.9–53.4 | 50.9 | 50.1–51.7 | 48.6 | 47.8–49.5 |
95% CI: 95% Confidence intervals.
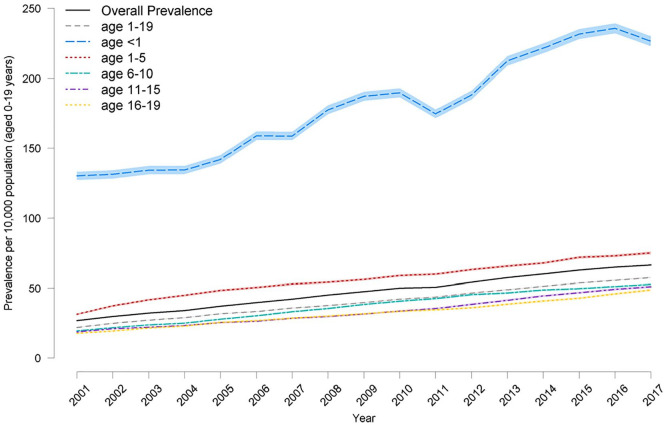
The prevalence was highest in the under 1-year age-group at 226.5 per 10,000 (95% confidence interval [95%CI: 223.0–230.1]) in 2017/2018 (Figure 2). There was a rise in prevalence in all age-groups.
Figure 2.
Prevalence of life-limiting conditions (with 95% confidence intervals in lighter shading) by age 2001/2–2017/18.
The prevalence of life-limiting conditions was significantly higher among boys (72.5 per 10,000 [95%CI 71.8–73.1]) versus girls 60.0 per 10,000 [95%CI 59.4–60.6] (2017/18) although there was no difference in the rise in prevalence between sexes in the study time period.
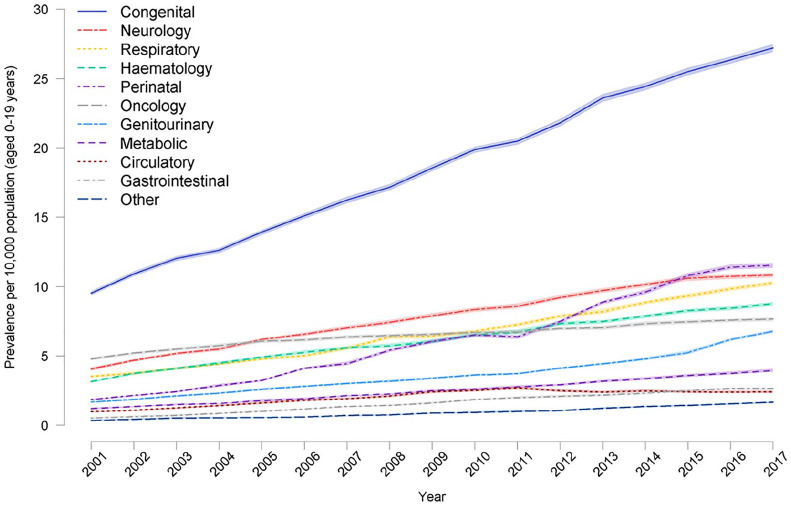
Children with a congenital abnormality had the highest prevalence (2017/18 was 27.2 per 10,000 (95%CI: 26.9–27.5)), with neurological conditions next highest (10.8 per 10,000 [95%CI 10.7–11.0]) (Figure 3). There was an increase in prevalence in all diagnostic groups during 2000/01 to 2017/18.
Figure 3.
Prevalence of life-limiting conditions (with 95% confidence intervals in lighter shading) in children (age 0–19) by diagnostic group for 2001/02–2017/18.
The prevalence of life-limiting condition s was highest in the most deprived group (88.6 per 10,000 [95% CI: 87.0–90.2] (2017/18)) and there was a gradient with deprivation with the lowest prevalence in the least deprived group (48.7 per 10,000 [95% CI: 47.5–49.9] (2017/18)). There was some evidence of a greater difference in prevalence between the least and most deprived groups over time (Supplemental Figure 1).
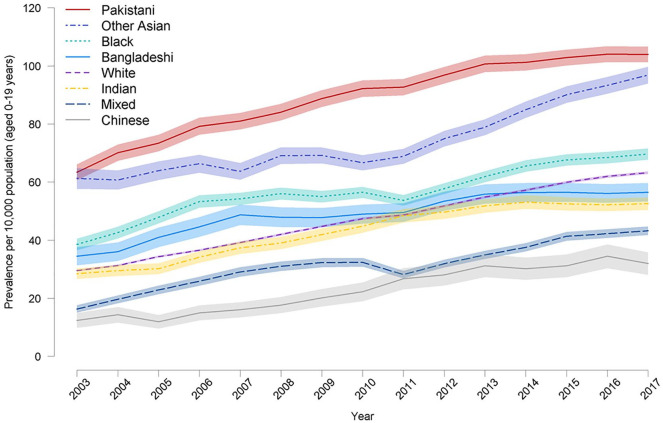
The prevalence of life-limiting condition s rose in all ethnic groups but children of Pakistani origin had the highest prevalence of life-limiting condition (103.9 per 10,000 [95%CI: 101.2–106.6]) (2017/18) children of Chinese origin (32.0 per 10,000 [95% CI: 28.1–35.8] (2017/18)) the lowest (Figure 4). Children from Black and Other Asian groups also had higher prevalence than the White population. Congenital anomalies had the highest prevalence in all ethnic groups but prevalence was higher in the Pakistani and Other Asian groups. The prevalence of neurological, hematological and metabolic diagnoses was also higher in the Pakistani population than the White population (see Supplemental Figures 2–12).
Figure 4.
Prevalence of life-limiting conditions (with 95% confidence intervals in lighter shading) in children (age 0–19) by ethnic group for 2001/02–2017/18.
Using the more restricted definition of a life-limiting condition reduced the overall prevalence from 66.4 to 61.1 per 10,000 (95%CI 60.7–61.5) in 2017/18.
Future prevalence
The predicted future number of children with a life-limiting condition by 2030 ranged from 96,275 (95%CI 95,318–97,242) for the most conservative model (model 3), to 116,770 (95%CI 108,894–125,209) (model 2) to 121,023 (95%CI 113,031–129,573) (model 1).
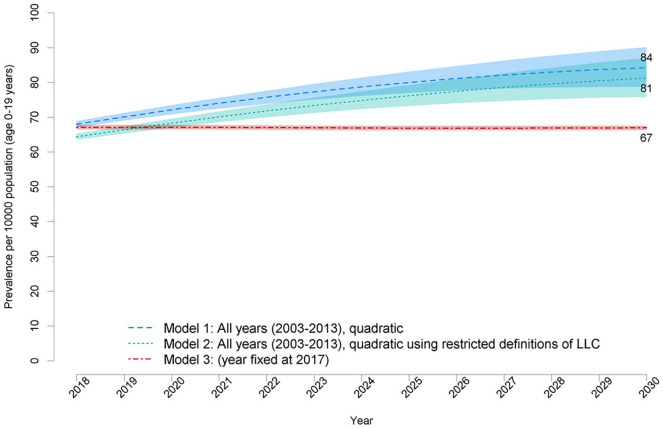
These equate to a prevalence of 67.0 (95%CI 67.7–66.3) per 10,000 (Model 3), 81.26 (95%CI 75.78–87.13) per 10,000 (Model 2) and 84.22 (95%CI 78.66–90.17) per 10,000 (Model 1) (Figure 5, Supplemental Table 2).
Figure 5.
Predicted prevalence (with 95% confidence intervals in lighter shading) of children (age 0–19) with life-limiting conditions s for 2018–2030.
Discussion
There has been a marked rise in prevalence of children with a life-limiting or life-threatening condition in England and this increase is predicted to continue. This highlights the need for pediatric palliative care services to work closely with other pediatric services.
A study using similar datasets from Scotland has shown a less marked rise in prevalence and no increase in this population from 2014/15 to 2016/17. 22 This is despite their being a similar prevalence in 2003/4 in Scotland and England (31.5 per 10,000 population vs 32.2 per 10,000 population respectively). The different demographics of the population, especially the larger ethnic minority populations in England (14.0% in England and Wales compared to 3.5% Scotland) which have higher fertility rates23,24 may partly explain this difference as may the different socio-economic circumstances of ethnic minorities in Scotland. 25 However, there may also be differences in healthcare coding practices due to differences in commissioning of services, mainly the lack of payment by results policies in Scotland.
A previous study, which aimed to estimate the prevalence of children who would require palliative care globally, estimated that 20.1 per 10,000 children would require palliative care in the UK, although this study used a different methodology and focused on diagnoses which cause child death worldwide especially in low and middle income countries. 26
The increase in prevalence is largest in the under 1-year age-group. There is also a marked difference in the prevalence among ethnic groups with higher prevalence for Pakistani, Other Asian and Black ethnic minority groups compared to the White population. Some of the life-limiting diagnoses, including some genetic conditions and congenital disorders23,24 are more common in these ethnic groups. These results highlight the need for services for children to be flexible enough to meet the cultural and religious needs of all children.
Children with congenital anomalies had the highest prevalence in the current study and previous similar studies.8,22,27 A recent Belgian study reported cardiovascular diseases as the most common underlying cause of complex chronic conditions, followed by neurological conditions. 28 This apparent discrepancy is possibly due to congenital cardiac diseases being classified as congenital in the current study but under cardiac conditions in the Belgium study. 28
More children with a life-limiting condition lived in areas of higher deprivation which is important when planning the location and accessibility of those services. Furthermore, there is some evidence of increasing disparities in the proportion of children with a life-limiting condition living in areas of higher deprivation versus lower deprivation over time.
To what extent the increase in prevalence found in this analysis is due to a true increase in incidence or due to an increase in survival, diagnosis or improved coding is difficult to ascertain from these data. While there is evidence of increased recording of life-limiting diagnoses in the under 1-year age-group it is not possible to differentiate between true increase in the number of children being diagnosed (i.e. increase in incidence) and changes in coding practices. Given the number of electronic medical recording systems being introduced in the NHS the latter cannot be discounted. In terms of increased survival, there is evidence of increased survival in some specific conditions for example, very preterm infants 29 and Duchenne Muscular Dystrophy,30,31 increased use of medical technologies such as home ventilation 32 and more aggressive management of complications in these children via care in Intensive Care Units. 33 All these will contribute to an increase in prevalence.
As the predictions of future prevalence are based on previous trends and predicted changes in population it is unsurprising that the estimates of future numbers and prevalence show a steady increase with an estimated prevalence of between 67.0 and 84.2 per 10,000 population. Although it is difficult to predict what the actual future prevalence of children with life-limiting conditions will be, there is nothing in these current data that would suggest that this prevalence is likely to reduce in the next 10 years. This is consistent with other estimates across the age range in the UK of the rise in need for palliative care services in the future. 34
Strengths and limitations
This study used transparent and repeatable methodology over a 17-year period which enabled assessment of change over time.
As a child is only required to have one recording of a life-limiting condition or life-threatening condition to be included in these analyses we may have included individuals whose life-threatening event is no longer life limiting, for example, a life-threatening event around the time of birth or in the neonatal period.
Utilizing data based on diagnoses alone does raise some challenges. The prevalence calculations using the restricted definitions of life-limiting condition made little difference in 2017/18 but did result in a more marked difference by 2030. There is large variation in the severity and prognosis within some of these diagnoses for example, quadriplegic cerebral palsy and this is further compounded by the grouping of some diagnoses within ICD 10, that is, not all diagnoses have their own code. Therefore, we may be capturing come children who may not require palliative care.
Children cared for by pediatric palliative care services are often very complex therefore understanding the complexity of the child’s condition 35 (e.g. use of technology such as home –ventilation) and the child’s needs 35 (e.g. requirement for round-the-clock care) would provide a more accurate estimate of which children would require palliative care services. However, these data are not routinely collected in the English National Health Service. There is an attempt to collect these data for example, community services dataset, 36 but unless explicitly linked to financial payments, these data will be inconsistently collected and the quality of data questionable.
A projection exercise such as this, does by its nature involve substantial assumptions about the similarity of future trends to past trends, and about the future trends in health improvement which may or may not be correct. 37 Alongside any uncertainties in numbers diagnosed, the population projections used as denominators are subject to variation due to variations in future demography. Since small area data are not available in the population projections and the future of area deprivation is not possible to determine, it was not possible to include deprivation in this model.
Conclusions
The prevalence of children with a life-limiting or life-threatening condition in England is predicted to continue to rise. The prevalence is higher in ethnic minority groups which may in part explain this rise.
These data are important for healthcare planners and policymakers as children and young people will require healthcare services which may include pediatric palliative care.
The prevalence is by far the greatest in children under 1 year of age, and this group should be seen as a priority for receiving palliative care as mortality rate is also highest in the under 1 year age-group.
Further research needs to identify the needs and complexity of these children which goes beyond their underlying diagnoses. This can only be resolved by recording of complexity and needs rather than diagnoses alone.
Supplemental Material
Supplemental material, sj-pdf-1-pmj-10.1177_0269216320975308 for Estimating the current and future prevalence of life-limiting conditions in children in England by Lorna K Fraser, Deborah Gibson-Smith, Stuart Jarvis, Paul Norman and Roger C Parslow in Palliative Medicine
Acknowledgments
The authors wish to thank the members of the advisory board of this study who provided clinical input to this study.
Previous research from England only included children in a year if they had a hospital admission for one of the LLC codes.
Assumption is that if they had an ongoing LLC/LTC after age 1 this would be recoded e.g. a baby with severe birth asphyxia would be recoded as having cerebral palsy.
Footnotes
Author contributions: Lorna Fraser, Deborah Gibson-Smith, Stuart Jarvis, Paul Norman and Roger Parslow have all
(i) Made a substantial contribution to the concept or design of the work; or acquisition, analysis or interpretation of data,
(ii) Drafted the article or revised it critically for important intellectual content,
(iii) Approved the version to be published,
(iv) Have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LF receives research grant funding from Martin House Children’s Hospice.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by The True Colours Trust. Stuart Jarvis is funded by a National Institute for Health Research (NIHR) Doctoral Research Fellowship (award DRF-2018-11-ST2-013) for this research project. This publication presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Lorna Fraser is funded by a National Institute for Health Research (NIHR) Career Development Fellowship (award CDF-2018-11-ST2-002) for this research project. This publication presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Research ethics and patient consent: This study has NHS REC approval (16/M/0013). “This study was supported by an advisory board which included patient organizations, but not patients or families. The findings of the study were discussed with members of the Family Advisory Board of the Martin House Research Centre”.
ORCID iDs: Lorna K Fraser  https://orcid.org/0000-0002-1360-4191
https://orcid.org/0000-0002-1360-4191
Stuart Jarvis  https://orcid.org/0000-0001-8447-0306
https://orcid.org/0000-0001-8447-0306
Paul Norman  https://orcid.org/0000-0002-6211-1625
https://orcid.org/0000-0002-6211-1625
Data management and sharing: Individual level data from this study cannot be shared as per NHS Digital data sharing approvals terms and conditions.
Supplemental material: Supplemental material for this article is available online.
References
- 1. World Health Organization. WHO definition of palliative care, http://www.who.int/cancer/palliative/definition/en/ (2011). [DOI] [PubMed]
- 2. Liben S, Papadatou D, Wolfe J. Paediatric palliative care: challenges and emerging ideas. Lancet 2008; 371: 852–864. [DOI] [PubMed] [Google Scholar]
- 3. Jarvis S, Fraser LK. Comparing routine inpatient data and death records as a means of identifying children and young people with life-limiting conditions. Palliat Med 2017; 32: 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldman A. ABC of palliative care: special problems of children. BMJ 1998; 316: 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hain R, Devins M, Hastings R, et al. Paediatric palliative care: development and pilot study of a ‘Directory’ of life-limiting conditions. BMC Palliat Care 2013; 12: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Together for Short Lives. A guide to children’s palliative care. Bristol: Together for Short Lives, 2018. [Google Scholar]
- 7. Noyes J, Edwards RT, Hastings RP, et al. Evidence-based planning and costing palliative care services for children: novel multi-method epidemiological and economic exemplar. BMC Palliat Care 2013; 12: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fraser LK, Miller M, Hain R, et al. Rising national prevalence of life-limiting conditions in children in England. Pediatrics 2012; 129: e923–e929. [DOI] [PubMed] [Google Scholar]
- 9. Fraser LK, Jarvis SW, Moran NE, et al. Children in Scotland requiring palliative care. York: University of York, 2015. [Google Scholar]
- 10. Sidebotham P, Fraser J, Fleming P, et al. Patterns of child death in England and Wales. Lancet 2014; 384: 904–914. [DOI] [PubMed] [Google Scholar]
- 11. Hain R, Heckford E, McCulloch R. Paediatric palliative medicine in the UK: past, present, future. Arch Dis Child 2012; 97: 381–384. [DOI] [PubMed] [Google Scholar]
- 12. Craft A, Killen S. Palliative care service for children and young people in England. London, UK: Crown, 2007. [Google Scholar]
- 13. Health & Social Care Information Centre. A guide to linked mortality data from hospital episode statistics and the office for national statistics. Health & Social Care Information Centre, 2015. [Google Scholar]
- 14. Herbert A, Wijlaars L, Zylbersztejn A, et al. Data resource profile: hospital episode statistics admitted patient care (HES APC). Int J Epidemiol 2017; 46: 1093–1093i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wohland P, Burkitt M, Norman P, et al. EthPOP database, https://www.ethpop.org/ (2016, 2019).
- 16. Rees P, Wohland P, Norman P, et al. A local analysis of ethnic group population trends and projections for the UK. J Popul Res 2011; 28: 149–183. [Google Scholar]
- 17. NOMIS. Census 2011 - Ethnic group by sex by age. Durham, UK: NOMIS, 2013. [Google Scholar]
- 18. Department for Communities and Local Government. Indices of deprivation, http://www.communities.gov.uk/communities/research/indicesdeprivation/deprivation10/ (2011).
- 19. Statistics OfN. Introduction to output areas - the building block of census geography, https://www.ons.gov.uk/census/2001censusandearlier/dataandproducts/outputgeography/outputareas (2020, accessed 11 February 2020).
- 20. Bland JM. Standard error and confidence interval for a proportion. An introduction to medical statistics. 4th ed. Oxford: Oxford University Press, 2015, pp.105–106. [Google Scholar]
- 21. AD MCL. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997; 349(9064): 1498–1504. [DOI] [PubMed] [Google Scholar]
- 22. NHS National Services Scotland. Children in Scotland requiring palliative care (ChiSP) 2. Edinburgh, UK, 2018. https://chas-assets.s3.eu-west-1.amazonaws.com/sites/59dde5b10f7d33796f8cd11b/assets/5f5b87aa0f7d337f7f7636e7/ChiSP3-Report.pdf [Google Scholar]
- 23. Sheridan E, Wright J, Small N, et al. Risk factors for congenital anomaly in a multiethnic birth cohort: an analysis of the Born in Bradford study. Lancet 2013; 382: 1350–1359. [DOI] [PubMed] [Google Scholar]
- 24. Firth C, Petherick E, Oddie SJ. Infant deaths from congenital anomalies: novel use of child death overview panel data. Arch Dis Child 2018; 103: 1027–1032. [DOI] [PubMed] [Google Scholar]
- 25. Walsh D, Buchanan D, Douglas A, et al. Increasingly diverse: the changing ethnic profiles of Scotland and Glasgow and the implications for population health. Appl Spat Anal Policy 2019; 12: 983–1009. [Google Scholar]
- 26. Connor SR, Downing J, Marston J. Estimating the global need for palliative care for children: a cross-sectional analysis. J Pain Symptom Manage 2017; 53: 171–177. [DOI] [PubMed] [Google Scholar]
- 27. Fraser LK, Jarvis SW, Moran N, et al. Children in Scotland requiring palliative care: identifying numbers and needs (The ChiSP Study). York: University of York, 2015. [Google Scholar]
- 28. Friedel M, Gilson A, Bouckenaere D, et al. Access to paediatric palliative care in children and adolescents with complex chronic conditions: a retrospective hospital-based study in Brussels, Belgium. BMJ Paediatr Open 2019; 3: e000547–e000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santhakumaran S, Statnikov Y, Gray D, et al. Survival of very preterm infants admitted to neonatal care in England 2008–2014: time trends and regional variation. Arch Dis Child Fetal Neonatal Ed 2018; 103: F208–F215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eagle M, Baudouin SV, Chandler C, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord 2002; 12: 926–929. [DOI] [PubMed] [Google Scholar]
- 31. Van Ruiten HJA, Marini Bettolo C, Cheetham T, et al. Why are some patients with Duchenne muscular dystrophy dying young: an analysis of causes of death in North East England. Eur J Paediatr Neurol 2016; 20: 904–909. [DOI] [PubMed] [Google Scholar]
- 32. NCEPOD. Balancing the pressures: a review of the quality of care provided to children and young people aged 0-24 years who were receiving long-term ventilation. London: NCEPOD, 2020. [DOI] [PubMed] [Google Scholar]
- 33. Fraser LK, Parslow R. Children with life-limiting conditions in paediatric intensive care units: a national cohort, data linkage study. Arch Dis Child 2018; 103(6): 540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Etkind SN, Bone AE, Gomes B, et al. How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Med 2017; 15: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horridge KA, Harvey C, McGarry K, et al. Quantifying multifaceted needs captured at the point of care. Development of a disabilities terminology set and disabilities complexity scale. Dev Med Child Neurol 2016; 58: 570–580. [DOI] [PubMed] [Google Scholar]
- 36. Horridge KA, Mcgarry K, Williams J, et al. Prospective pilots of routine data capture by paediatricians in clinics and validation of the disabilities complexity scale. Dev Med Child Neurol 2016; 58: 581–588. [DOI] [PubMed] [Google Scholar]
- 37. Buchan JC, Norman P, Shickle D, et al. Failing to plan and planning to fail. Can we predict the future growth of demand on UK Eye Care Services? Eye 2019; 33: 1029–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pmj-10.1177_0269216320975308 for Estimating the current and future prevalence of life-limiting conditions in children in England by Lorna K Fraser, Deborah Gibson-Smith, Stuart Jarvis, Paul Norman and Roger C Parslow in Palliative Medicine