Abstract
Background:
De-escalation of axillary surgery after neoadjuvant chemotherapy (NAC) requires careful patient selection. We sought to determine predictors of nodal pathologic complete response (ypN0) among patients treated on CALGB 40601 or 40603, which tested NAC regimens in HER2+ and triple-negative breast cancer (TNBC), respectively.
Methods:
760 patients with stage II-III HER2+ or TNBC were analyzed. Those who had axillary surgery before NAC (n=122), or who had missing pre-treatment clinical nodal status (cN) (n=58) or ypN status (n=41) were excluded. The proportion of patients with ypN0 disease was estimated for those with and without breast pathologic complete response (pCR) according to pre-treatment nodal status.
Results:
In 539 patients, the overall ypN0 rate was 76.3% (411/539) – 93.2% (245/263) in patients with breast pCR and 60.1% (166/276) with residual breast disease (RD) (P<0.0001). For patients who were cN0 pre-treatment, the ypN0 rate was 88.8% (214/241), 96.3% (104/108) with breast pCR and 82.7% (110/133) with RD. For patients who were cN1, 66.2% (157/237) converted to ypN0, 91.7% (111/121) with breast pCR and 39.7% (46/116) with RD. For patients who were cN2/3, 65.6% (40/61) converted to ypN0, 88.2% (30/34) with breast pCR and 37.0% (10/27) with RD. In multivariable analysis, only pre-treatment clinical nodal status and breast pCR/RD were associated with ypN0 status (both P<0.0001).
Conclusions:
Breast pCR and pre-treatment nodal status are predictive of ypN0 axillary nodal involvement, with < 5% residual nodal disease among cN0 patients who experience breast pCR. These findings support the incorporation of axillary surgery de-escalation strategies into NAC trials.
Keywords: breast cancer, axillary surgery after neoadjuvant chemotherapy, axillary surgery de-escalation, nodal response to neoadjuvant chemotherapy
Introduction
In the treatment of breast cancer patients, there has been a significant increase in the use of neoadjuvant chemotherapy (NAC) and a corresponding decrease in upfront surgery1–3. Patients treated with NAC experience equivalent overall survival and local-regional recurrence rates as those treated with adjuvant chemotherapy4 but NAC has become standard for most cases of stage II-III human epidermal growth factor receptor 2 (HER2)-positive (+) and triple negative breast cancer (TNBC)5, 6 for two reasons. First, NAC allows for an in vivo assessment of response to prognosticate and to identify patients with residual disease as candidates for additional systemic therapy7, 8. Second, NAC can optimize local regional outcomes by converting patients with inoperable breast tumors to operable, or by transforming those deemed candidates for mastectomy-only into candidates for breast conservation9. Moreover, NAC often downstages axillary disease, potentially allowing some patients to have less invasive nodal surgery10, 11.
Downstaging axillary disease with NAC provides an opportunity to de-escalate axillary surgery and reduce the morbidity of treatment. However, this requires accurate prediction of axillary response to NAC. Several large clinical trials tested the feasibility of performing sentinel lymph node biopsy (SLNB) to surgically stage the axilla for cN1 patients who converted to cN0 after NAC11–15. Rates of SLN identification ranged from 80.1 to 92.9% and SLNB false negative rates ranged from 9.6 to 15%, which largely exceeded the trials’ prespecified acceptable failure rates. Similarly, axillary imaging has failed to reliably predict the ypN status of the axilla after NAC, with sensitivity rates reported between 48.5% and 69.8% for ultrasound16–18, 38% and 61% for MRI18, 19, and 63.2% for PET-CT18. Thus, current surgical techniques and imaging modalities lack the sensitivity to accurately predict the ypN0 axilla.
Accurate preoperative identification of the ypN0 axilla after NAC could potentially avoid unnecessary axillary surgery. Two neoadjuvant trials, Cancer and Leukemia Group B (CALGB) 40601 (HER2+)20 and 40603 (TNBC)21, tested NAC regimens with a primary endpoint of pCR in the breast. In this current study, we sought to determine rates of nodal pCR (ypN0) as well as factors associated with ypN0 among patients treated on the trials. We hypothesized that there would be very low rates of residual disease (RD) in the lymph nodes (ypN+) among patients who had a breast pCR, including both clinically node negative and node positive at presentation. The goal of this study is to identify factors that would have the potential to further refine patient selection for axillary surgery de-escalation strategies.
Methods
Data Source
A total of 759 patients were enrolled on the CALGB 40601/40603 trials. CALGB is now part of the Alliance for Clinical Trials in Oncology. CALGB 40601 tested lapatinib, trastuzumab, or both in addition to paclitaxel20. Inclusion criteria for CALGB 40601 were patients over 18 years with stage II-III HER2-postive breast cancer, with tumors at least 1 cm in size. Multicentric and bilateral disease were allowed if at least one of the tumors met inclusion criteria. CALGB 40603 tested the addition of carboplatin and/or bevacizumab to paclitaxel, followed by dose dense doxorubicin and cyclophosphamide with or without bevacizumab21. Inclusion criteria for CALGB 40603 were patients with stage II-III operable, noninflammatory TNBC who were otherwise well. In both trials, estrogen and progesterone receptor expression of < 10% was considered negative, and HER2 was considered negative if the immunohistochemical (IHC) staining was 0–1+, or 2+ with a fluorescence in situ hybridization HER2/CEP17 ratio of <2.0. For the current analysis, patients who underwent axillary surgery prior to NAC (n=122), or those with missing cN status (n=58) or missing ypN status (n=41) were excluded.
Definition of Variables
The rates of breast pCR20, 21, eligibility and rates of breast conservation9, and axillary management22 from the CALGB 40601/40603 trials have been reported previously. Per-protocol imaging assessments and surgical assessments were consistent for both trials. Receptor statuses including HER2 were determined locally. Clinical nodal (cN) status was determined by physical exam; axillary imaging at presentation was strongly encouraged but not required. For patients with clinically palpable lymph nodes, percutaneous sampling was strongly encouraged but not required. The performance of SLNB or ALND before NAC was allowed. Following NAC, ALND was recommended for patients with pretreatment cN+ disease, regardless of clinical nodal response, but definitive axillary surgery was left to the discretion of the treating surgeon22. Breast pCR was defined as no residual invasive disease (ypT0/ypTis). Nodal RD (ypN+) disease was considered ≥ 0.2 mm of disease determined by hematoxylin and eosin staining. Immunohistochemical staining for nodal assessment was used per institutional standards and ypN0(i+) was considered ypN0 disease.
Statistical Analysis
The primary endpoint was the nodal status after NAC (ypN0 versus ypN+) for patients treated on CALGB 40601 or CALGB 40603. Baseline patient and disease characteristics were compared between patients with ypN0 disease and those with ypN+ disease. Chi-square tests (or Fisher’s exact test, when required) were used for categorical variables and the Wilcoxon rank sum test was used for continuous variables. The proportion of patients who had ypN0 disease was estimated with a binomial point estimate and corresponding 95% confidence interval overall and for subgroups defined by baseline cN status, baseline cT category, and post-NAC breast disease status (pCR versus RD). These rates were also determined within each pre-NAC cN cohort (cN0, cN1, cN2–3) by constructed tumor subtype (HER2+/HR+, HER2+/HR-, TNBC). To determine patient selection criteria for consideration of axillary surgery de-escalation, analyses determined the rate of ypN0 disease among patients who had breast pCR, within pre-NAC cN0 and cN+ cohorts by tumor subtype and pre-NAC clinical T category; these analyses were repeated for patients who had breast RD. Multivariable logistic models were used to determine factors associated with ypN0 status in the presence of others. The models included treatment arm as an adjusting variable and included cT, cN, tumor subtype, tumor grade, and in-breast response as variables. Long-term outcomes (overall survival [OS] and local regional recurrence free survival [LRRFS]) were compared among the patient groups defined by ypN status and breast disease status using Cox proportional hazard models. Time variables for these long-term outcomes were calculated by the time from randomization on their original studies. Statistical significance was determined at a 0.05 two-sided alpha level. Analyses were performed in SAS (3.8). National Cancer Institute Central Institutional Review Board (IRB) approval was obtained for the CALGB 40601 and CALGB 40603 trials (ClinicalTrials.gov identifiers are NCT00770809 (CALGB 40601) and NCT00861705 (CALGB 40603)). Each participant signed an IRB-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. All analyses were based on the study database frozen on July 15, 2020.
Results
A total of 539 patients were included in the analysis: 41.0% (221) from CALGB 40601 and 59.0% (318) from CALGB 40603. Patients were evenly allocated to treatment regimens as they were randomly assigned, except to the paclitaxel plus lapatinib arm of CALGB 40601, which closed early20 and thus comprises only 21.7% of patients from that study (8.9% (48/539) of total patients in this study).
Of the 539 patients, 44.7% (241) presented with cN0 disease, 44.0% (237) with cN1, and 11.3% (61) with cN2–3. Overall, 48.8% (263/539) of patients experienced a breast pCR. 76.3% (411/539) of patients were determined to have ypN0 disease. ypN0 rates varied by treatment regimen (Table 1, P=0.013), which is consistent with the parent trials20, 21. Compared to patients with residual nodal disease, ypN0 patients had smaller tumors (mean size of 4.5 cm vs 5.5 cm, P=0.0019), lower clinical T stage (P=0.0016), were more likely to have been cN0 at baseline (P<0.0001), to have been treated with SLNB as definitive axillary surgery (P<0.0001) and to have experienced breast pCR (P<0.0001). Patient age, race, ethnicity, menopausal status, tumor subtype and tumor grade did not significantly differ between ypN0 and ypN+ patients. (Table 1)
Table 1.
Patient Characteristics
| (Row percentages) | ypN0 N=411 (76.3%) |
ypN+ N=128 (23.7%) |
Total N=539 |
P-value |
|---|---|---|---|---|
| Trial | 0.261 | |||
| CALGB 40601 | 163 (73.8%) | 58 (26.2%) | 221 | |
| CALGB 40603 | 248 (78.0%) | 70 (22.0%) | 318 | |
| Treatment arm | 0.0131 | |||
| Paclitaxel | 65 (83.3%) | 13 (16.7%) | 78 | |
| Paclitaxel and Bevacizumab | 52 (66.7%) | 26 (33.3%) | 78 | |
| Paclitaxel and Carboplatin | 65 (79.3%) | 17 (20.7%) | 82 | |
| Paclitaxel, Carboplatin and Bevacizumab | 66 (82.5%) | 14 (17.5%) | 80 | |
| Paclitaxel and Trastuzumab | 66 (74.2%) | 23 (25.8%) | 89 | |
| Paclitaxel, Trastuzumab and Lapatinib | 68 (81.0%) | 16 (19.0%) | 84 | |
| Paclitaxel and Lapatinib | 29 (60.4%) | 19 (39.6%) | 48 | |
| Age at registration (years) | 0.182 | |||
| Mean (SD) | 48.8 (10.3) | 50.5 (10.7) | 49.2 (10.4) | |
| Median | 49.0 | 49.0 | 49.0 | |
| Range | (24.0–79.0) | (26.0–75.0) | (24.0–79.0) | |
| Racial group | 0.931 | |||
| Missing | 15 | 7 | 22 | |
| Asian | 15 (71.4%) | 6 (28.6%) | 21 | |
| Black | 67 (77.0%) | 20 (23.0%) | 87 | |
| Other | 5 (71.4%) | 2 (28.6%) | 7 | |
| White | 309 (76.9%) | 93 (23.1%) | 402 | |
| Ethnicity | 0.491 | |||
| Missing | 34 | 20 | 54 | |
| Hispanic/Latino | 36 (81.8%) | 8 (18.2%) | 44 | |
| Non-Hispanic | 341 (77.3%) | 100 (22.7%) | 441 | |
| Tumor subtype | ||||
| HER2+ | 163 (73.8%) | 58 (26.2%) | 221 | 0.261 |
| TNBC | 248 (78.0%) | 70 (22.0%) | 318 | |
| Clinical stage | <0.00011 | |||
| 2 | 291 (82.0%) | 64 (18.0%) | 355 | |
| 3 | 120 (65.2%) | 64 (34.8%) | 184 | |
| Clinical tumor size (cm) | 0.00192 | |||
| N | 404 | 123 | 527 | |
| Mean (SD) | 4.5 (2.5) | 5.5 (3.1) | 4.8 (2.7) | |
| Median | 4.0 | 5.0 | 4.0 | |
| Range | (0.0–22.0) | (0.0–16.0) | (0.0–22.0) | |
| Clinical T category | 0.00161 | |||
| Missing | 9 | 5 | 14 | |
| 1 | 20 (69.0%) | 9 (31.0%) | 29 | |
| 2 | 265 (82.3%) | 57 (17.7%) | 322 | |
| 3 | 105 (67.3%) | 51 (32.7%) | 156 | |
| 4 | 12 (66.7%) | 6 (33.3%) | 18 | |
| Clinical N category | <0.00011 | |||
| 0 | 214 (88.8%) | 27 (11.2%) | 241 | |
| 1 | 157 (66.2%) | 80 (33.8%) | 237 | |
| 2 | 33 (67.3%) | 16 (32.7%) | 49 | |
| 3 | 7 (58.3%) | 5 (41.7%) | 12 | |
| Tumor grade | 0.401 | |||
| Missing | 21 | 12 | 33 | |
| High | 309 (78.4%) | 85 (21.6%) | 394 | |
| Intermediate | 73 (72.3%) | 28 (27.7%) | 101 | |
| Low | 8 (72.7%) | 3 (27.3%) | 11 | |
| Breast surgery | 0.0481 | |||
| Missing | 0 | 1 | 1 | |
| Lumpectomy | 203 (80.2%) | 50 (19.8%) | 253 | |
| Mastectomy | 208 (73.0%) | 77 (27.0%) | 285 | |
| Axillary Surgery | <0.00011 | |||
| Missing | 70 | 7 | 77 | |
| SLNB | 124 (91.9%) | 11 (8.1%) | 135 | |
| ALND | 217 (66.4%) | 110 (33.6%) | 327 | |
| Breast outcome | <0.00011 | |||
| Breast RD | 166 (60.1%) | 110 (39.9%) | 276 | |
| Breast pCR | 245 (93.2%) | 18 (6.8%) | 263 | |
Chi-Square
Kruskal Wallis
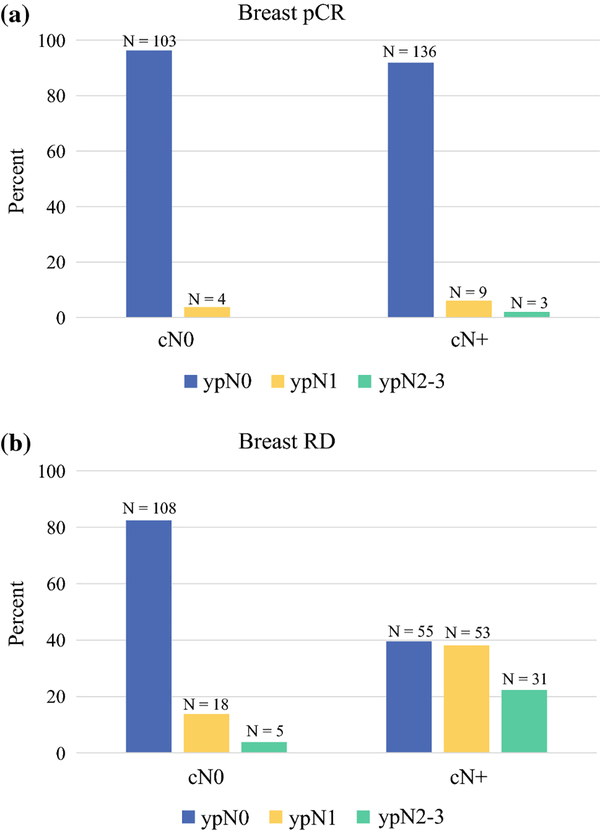
Among cN0 patients at presentation, 88.7% (214/241) were ypN0: the ypN0 rate was 96.3% (104/108) for patients with a breast pCR and 82.7% (110/133) for patients with breast RD. Among cN1 patients at presentation, 66.2% (157/237) were ypN0: the ypN0 rate was 91.7% (111/121) for patients with breast pCR and 39.7% (46/116) for patients with breast RD. Among cN2/3 patients at presentation, 65.6% (40/61) were ypN0; the ypN0 rate was 88.2% (30/34) among those with breast pCR and 37.0% (10/27) among those with breast RD. There were no significant differences in ypN disease rates by tumor subtype, for patients with breast pCR or breast RD. (Table 2)
Table 2.
ypN0 and ypN+ rates among patients who experienced breast pCR or had residual disease (RD), by clinical nodal status, N=539
| Breast pCR (N=263) | ||||
| N (%) | ypN0 | ypN+ | Total | P-value |
| cN0, 108 (41.1%) | 104 (96.3%) | 4 (3.7%) | 108 | 11 |
| HER2+ | 35 (97.2%) | 1 (2.8%) | 36 | |
| TNBC | 69 (95.8%) | 3 (4.2%) | 72 | |
| cN1, 121 (46.0%) | 111 (91.7%) | 10 (8.3%) | 121 | 11 |
| HER2+ | 50 (90.9%) | 5 (9.1%) | 55 | |
| TNBC | 61 (92.4%) | 5 (7.6%) | 66 | |
| cN2/cN3, 34 (12.9%) | 30 (88.2%) | 4 (11.8%) | 34 | 11 |
| HER2+ | 11 (91.7%) | 1 (8.3%) | 12 | |
| TNBC | 19 (86.4%) | 3 (13.6%) | 22 | |
| Breast RD (N=276) | ||||
| N (%) | ypN0 | ypN+ | Total | P-value |
| cN0, 133 (48.2%) | 110 (82.7%) | 23 (17.3%) | 133 | 0.241 |
| HER2+ | 38 (77.6%) | 11 (22.4%) | 49 | |
| TNBC | 72 (85.7%) | 12 (14.3%) | 84 | |
| cN1, 116 (42.0%) | 46 (39.7%) | 70 (60.3%) | 116 | 0.341 |
| HER2+ | 25 (44.6%) | 31 (55.4%) | 56 | |
| TNBC | 21 (35.0%) | 39 (65.0%) | 60 | |
| cN2/cN3, 27 (9.8%) | 10 (37.0%) | 17 (63.0%) | 27 | 0.691 |
| HER2+ | 4 (30.8%) | 9 (69.2%) | 13 | |
| TNBC | 6 (42.9%) | 8 (57.1%) | 14 | |
Fisher Exact p-value
After excluding patients with missing cT category, estimated ypN disease rates (ypN0, ypN1, or ypN2–3) for patients with breast pCR or breast RD are presented in Figure 1. There were no differences in ypN disease rates by cT category in any tumor subtype. (Supplemental Tables 1 and 2) However, among these patients with known cT category and a breast pCR, there was an increasing volume of residual nodal disease based on cN status. The 4 patients who were cN0, experienced a breast pCR, but had residual nodal disease each had only 1 positive lymph node. Of the 9 patients who were cN1, experienced a breast pCR, but had residual nodal disease, 6 patients had 1 positive node, 2 had 2 positive nodes, and 1 had 3 positive nodes. Lastly, the 3 patients who were cN2/3, experienced a breast pCR, but had residual nodal disease had 5, 14, and 15 positive nodes.
Figure 1(a, b).
Figure 1 presents ypN0, ypN1, and ypN2–3 disease rates for patients who presented with cN0 and cN+ disease and experienced a) breast pCR (N = 255) or b) breast RD (N = 270). Patients with missing clinic T category were excluded from this analysis.
In a multivariable analysis, presenting clinical nodal status and breast pathologic response were significantly associated with ypN0 disease. (Table 3) Compared to cN0 patients, patients with cN+ disease at presentation are over 80% less likely to have ypN0 disease, with odds ratios ranging from 0.16–0.18 (P<0.0001). Compared to patients with breast RD, patients with breast pCR were 12 times more likely to have ypN0 disease (OR 12.55, CI 6.73–23.42, P<0.0001). Treatment arm was not significantly associated with nodal pCR on multivariable analysis (P=0.25).
Table 3.
Multivariable analysis to determine factors associated with nodal pCR (ypN0 disease) among patients in trials 40601/40603. (N=493, patients with missing grade[N=32], missing T category [N=13], or missing both grade/T category [N=1] were excluded from the total study population [N=539] for this analysis).
| Events/Total | Odds Ratio (95% CI) | P-value | |
|---|---|---|---|
| Treatment arm | 0.262 | ||
| Paclitaxel | 62/74 | Reference | |
| Paclitaxel and Bevacizumab | 48/65 | 0.38 (0.14, 0.99) | 0.0491 |
| Paclitaxel and Carboplatin | 55/70 | 0.54 (0.20, 1.45) | 0.221 |
| Paclitaxel, Carboplatin and Bevacizumab | 57/68 | 0.59 (0.20, 1.70) | 0.331 |
| Paclitaxel and Trastuzumab | 63/87 | 0.60 (0.24, 1.50) | 0.271 |
| Paclitaxel, Trastuzumab and Lapatinib | 66/83 | 0.74 (0.28, 1.94) | 0.541 |
| Paclitaxel and Lapatinib | 29/46 | 0.30 (0.11, 0.82) | 0.0181 |
| Clinical T category | 0.112 | ||
| 1 | 18/26 | Reference | |
| 2 | 251/306 | 2.42 (0.81, 7.23) | 0.121 |
| 3 | 101/146 | 1.33 (0.44, 4.09) | 0.611 |
| 4 | 10/15 | 1.14 (0.21, 6.37) | 0.881 |
| Clinical N category | <0.00012 | ||
| 0 | 198/223 | Reference | |
| 1 | 146/217 | 0.16 (0.09, 0.30) | <0.00011 |
| 2 | 30/44 | 0.18 (0.07, 0.47) | 0.00041 |
| 3 | 6/9 | 0.17 (0.03, 1.02) | 0.0531 |
| Tumor subtype | 12 | ||
| HER2+ | 158/216 | Reference | |
| TNBC | 222/277 | 1.00 (1.00, 1.00) | 11 |
| Tumor grade | 0.152 | ||
| High | 304/385 | 1.18 (0.24, 5.78) | 0.841 |
| Intermediate | 68/97 | 0.62 (0.12, 3.25) | 0.571 |
| Low | 8/11 | Reference | |
| In-breast response | <0.00012 | ||
| RD | 155/252 | Reference | <0.00011 |
| pCR | 225/241 | 12.55 (6.73, 23.42) |
Covariate Wald p-value
Type 3 Wald p-value
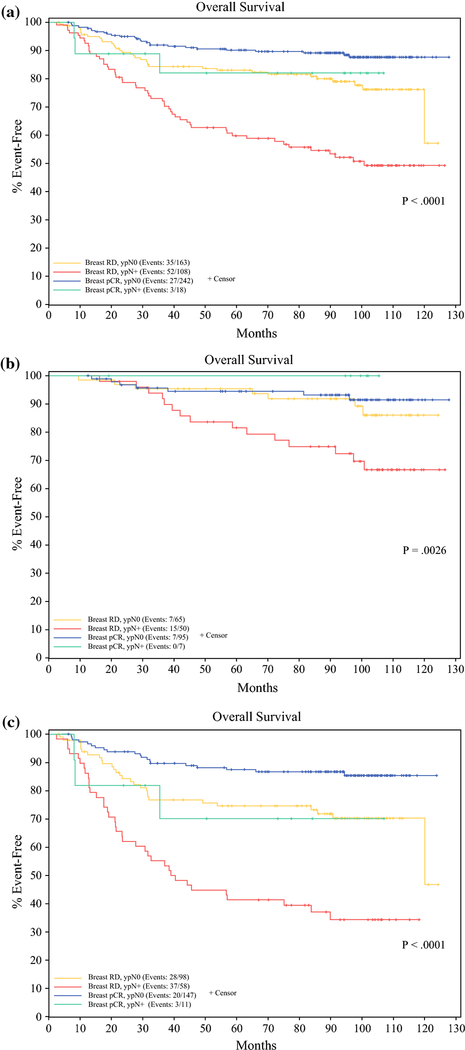
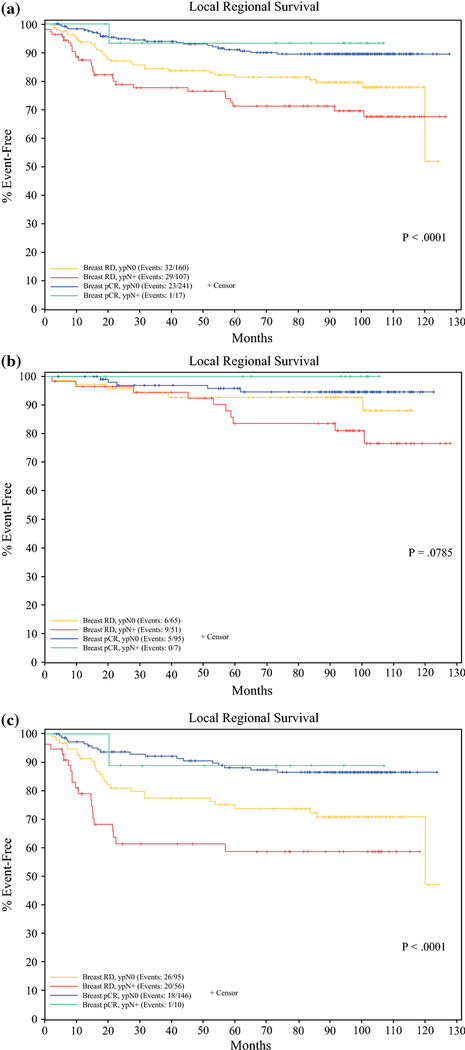
Median follow-up for the combined cohort was 103.0 months. Overall survival was significantly worse among patients with breast RD and ypN+ disease than those with either breast or nodal pCR, and best among patients with both breast pCR/ypN0 disease among all patients (P<0.0001), and among both CALGB 40601 (P=0.0026) and CALGB 40603 patient cohorts (P<0.0001). (Figure 2) Median overall survival at 5 years was 59.9% (95% CI 51.3–69.9%) for all patients with breast RD and ypN+ disease, 83.0% (77.4–89.1%) for all patients with breast RD and ypN0 disease, 82.1% (65.4–100%) for all patients with breast pCR and ypN+ disease, and 90.2% (86.4–94.1%) for all patients with breast pCR and ypN0 disease. This was also true of LRRFS among all patients (P<0.0001) and the CALGB 40603 patient cohort (P<0.0001), but not the CALGB 40601 patient cohort (P=0.0785). (Figure 3) LRRFS at 5 years was 71.2% (62.5–81.2%) for all patients with breast RD and ypN+ disease, 81.4 (75.3–87.9%) for all patients with breast RD and ypN0 disease, 93.3% (81.5–100%) for all patients with breast pCR and ypN+ disease, and 91.0% (87.3–94.9%) for all patients with breast pCR and ypN0 disease. For all patient cohorts defined by breast pathologic response and nodal status LRRFS and OS were superior for HER2+ patients treated on CALGB 40601 compared to TNBC patients treated on CALGB 40603.
Figure 2(a-c).
Overall Survival for patients based on breast RD/breast pCR and nodal RD (ypN+)/nodal pCR (ypN0) for a) all patients included in the analysis b) the 40601 patient cohort, and c) the 40603 patient cohort.
Figure 3(a-c).
Local Regional Recurrence Free Survival for patients based on breast RD/breast pCR and nodal RD (ypN+)/ nodal pCR (ypN0) for a) all patients included in the analysis b) the 40601 patient cohort, and c) the 40603 patient cohort.
Discussion
Over the last three decades one of the major goals of breast cancer surgery research has been to determine which breast cancer patients can safely forego ALND and avoid the associated surgical morbidity and potential for lymphedema. As a result, far fewer ALNDs are being performed in both the upfront surgery23, 24 and NAC populations25, 26. This reduction has been more pronounced in patients choosing upfront surgery, in whom the safe omission of ALND is based on large randomized clinical trial data and predicated on proper patient selection, including negative clinical nodal status (cN0)23 and negative axillary imaging27. In the NAC setting, de-escalation of axillary surgery demands careful patient selection to ensure that this will not compromise long-term oncologic outcomes. Among patients treated with NAC, those with chemotherapy responsive disease, low to moderate axillary burden and a high likelihood of pCR are reasonable candidates to consider for future axillary surgery de-escalation trials28–30. The current study examines two large NAC trials, CALGB 40601 and 40603, to determine rates and variables associated with pathologic nodal disease after NAC among HER2+ and TNBC, two subtypes now conventionally treated with neoadjuvant timing of systemic therapy.
Overall, this study revealed high rates of nodal clearance among patients with HER2+ and TNBC who received NAC, consistent with prior reports31, 32. We demonstrate that 76% of all Stage II-III HER2+ and TNBC patients treated with NAC will have ypN0 disease. Among clinically node negative patients, nearly 90% of patients have ypN0 disease. Among patients with involved axillary nodes up front, two-thirds of cN1–3 patients will have their nodal metastases eradicated after NAC. While these results might suggest that pretreatment clinical nodal status (cN0) might be sufficient to support omission of axillary surgery in these tumor subtypes, the presence of residual nodal disease in about 10% of such patients raises concerns about such an approach.
In this analysis, we found that clinical nodal status and breast response were associated with nodal pCR, independent of phenotype, clinical tumor size, and treatment regimen on multivariable analysis. For example, of cN0 patients who experience a breast pCR only 3.7% have ypN+ disease. These findings are consistent with several prior retrospective analyses demonstrating that breast pCR is highly associated with ypN0 disease (range 97.7–100%)33–38. Thus, if we knew there was a breast pCR we could start to consider the omission of axillary staging, which supports efforts to noninvasively or less invasively identify breast pCR. Several prospective multicenter studies have tested the feasibility of using post-NAC percutaneous tumor biopsy to try to accurately identify patients who experienced a breast pCR. However, these trials have fallen short thus far, reporting false negative rates between 18 and 50%39–42. Despite these disappointing early results, investigators have persisted in trying to improve the prediction of breast pCR and to identify patients less likely to have residual nodal disease after NAC. In a study out of the Netherlands, 303 cT1–3 N0 patients underwent NAC, MRI, and axillary surgical staging. 95.5% of patients with a radiologic complete response by MRI experienced a nodal pCR, including 100% of HER2+ and 98% of TNBC patients43. Whether this is sufficient to justify omission of pathologic examination of the axillary nodes has not been evaluated prospectively. To this end, the Netherlands Cancer Institute is designing a trial called “Avoiding Sentinel Lymph Node Biopsy in Breast Cancer Patients After Neoadjuvant Chemotherapy (ASICS)”44, which plans to enroll 340 patients in a prospective non-inferiority single-arm registration trial to examine the use of imaging response to select patients for omission of axillary surgery. An alternative trial design is being undertaken in the European Breast Cancer Research Association of Surgical Trialists (EUBREAST)-01 clinical trial45. Investigators plan to enroll 267 patients with cT1–3 N0 HER2+ or TNBC to a single arm prospective registration trial to examine the use of breast response as determined by lumpectomy after NAC to select patients for omission of axillary surgery. These are the type of forward thinking clinical trials that could support de-escalation of axillary surgery in NAC patients.
However, in the quest for axillary surgery de-escalation, it is important to exhibit caution for some patient subgroups. Specifically, in the current study cN2/3 patients had higher rates of ypN+ disease even among patients who experienced a breast pCR (12%). Patients with cN2 disease have been understudied thus far. The prior trials of SLNB after NAC included cN2 patients but represented very small samples sizes – only 38 patients in ACOSOG Z107113 and 10 patients in SN FNAC15. A single-institution study of 602 cN+ patients treated with NAC and axillary surgery revealed low regional recurrence rates after SLNB alone for patients who had ypN0 disease at a median follow up of 34 months, but this study only included 19 cN2 and 42 cN3 patients, 6 and 13 of whom, respectively, underwent SLNB46. In a retrospective study of 221 cN2 patients treated with NAC, 40.3% had ypN0 disease and 59.7% had ypN+ disease. Clinical and radiologic response, and HER2+ and TNBC constructed subtypes were associated with ypN0 disease47. The current standard of care is for all cN2/3 patients to undergo ALND independent of their response to NAC. Clearly, more research is needed to identify patients with advanced nodal disease who can be considered for axillary de-escalation strategies after NAC.
The current study does have some limitations. First, axillary imaging and biopsy of abnormal nodes were strongly recommended but not required; documentation of axillary imaging was variable. Thus, clinical nodal status may be inaccurate; for example, some patients who were deemed cN0 by physical exam only may have been classified as cN1 by ultrasound/percutaneous nodal sampling pre-NAC. Furthermore, we cannot compare patients who had physical exam-detected cN1 disease versus those who had imaging detected cN1 disease, which is a current topic of debate. Another limitation is that clinical nodal status was only recorded at baseline; we do not know what the ypN0 rates might have been in cN+ patients who became ycN0, by physical examination or imaging studies, after NAC. The standard of care for management of the axilla in patients receiving NAC has changed substantially since these studies were designed. One hundred twenty-two patients (presumably cN0 by physical exam) were excluded from this analysis because they underwent SLNB or ALND before NAC, approaches which have largely been replaced by nodal sampling after NAC, since the status of the nodes after NAC is felt to have greater prognostic value, especially for determining which patients should receive more intensive post-operative systemic therapy. Interestingly, the HER2+ patients in CALGB 40601 received only taxol and anti-HER2 directed therapy. Conventional therapy at the time would have been polychemotherapy, so nodal conversion may be even higher than we report. Overall though, the incidence of ypN+ disease was low, so it is difficult to draw firm conclusions from this relatively small sample size, specifically regarding further refinement of patient selection by cT category. Lastly, we only examined HER2+ and TNBC subtypes, so these findings cannot be applied to all breast cancer patients, like those with hormone receptor positive disease. Despite these limitations, this study reports low rates of pathologic nodal disease among HER2+ and TNBC patients who were treated with NAC, especially among patients who achieved breast pCR, in the context of two multicenter prospective randomized clinical trials.
Conclusions
Patients with chemotherapy sensitive disease who experience breast pCR may be candidates for axillary surgery de-escalation. Overall, 89% of cN0 patients were indeed ypN0 and 66% of cN1 and cN2/3 patients had eradication of disease in the axilla. Among patients with a breast pCR only 4% of cN0 patients and 8% of cN1 patients had ypN+ disease. However, further studies are needed, including well-designed clinical trials and long-term oncologic outcomes data, before we may be able to recommend omission of axillary staging in select patients receiving NAC. Should such trials be pursued, clinical nodal status and breast pathologic complete response should be considered as selection criteria.
Supplementary Material
Synopsis:
De-escalating axillary surgery after neoadjuvant chemotherapy requires careful patient selection. In HER2+/TNBC breast cancer patients treated on CALGB 40601/40603, pre-treatment nodal status and breast pathologic response correlated with pathologic nodal status. These findings may guide the design of future trials.
Acknowledgements:
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA233178, UG1CA233180, UG1CA233253, UG1CA233337, and UG1CA233373. Also supported in part by funds from Glaxo Smith Kline (40601) and Genentech (40603) and by grants from The Breast Cancer Research Foundation (40601 and 40603). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. https://acknowledgments.alliancefound.org.
Footnotes
Disclosures: WMS discloses honoraria from Lilly Oncology, not pertaining to the topic of this manuscript.
MP - Investor in start-up biotech company, PEEL Therapeutics, Inc. No clinical or commercial products at this time. Stock ownership AstraZeneca (< $3000)
ClinicalTrials.gov Identifiers: NCT00770809 (CALGB 40601) and NCT00861705 (CALGB 40603)
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Al-Hilli Z, Boughey JC, Hoskin TL, Heins CN, Hieken TJ. Increasing Use of Neoadjuvant Treatment for T1 and T2 HER2-Positive Tumors. Ann Surg Oncol. October 2015;22(10):3369–75. [DOI] [PubMed] [Google Scholar]
- 2.Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant Chemotherapy Use in Breast Cancer is Greatest in Excellent Responders: Triple-Negative and HER2+ Subtypes. Ann Surg Oncol. August 2018;25(8):2241–2248. [DOI] [PubMed] [Google Scholar]
- 3.Zeidman M, Schmidt H, Alberty-Oller JJ, et al. Trends in neoadjuvant chemotherapy versus surgery-first in stage I HER2-positive breast cancer patients in the National Cancer DataBase (NCDB). Breast Cancer Res Treat. January 4 2021. [DOI] [PubMed] [Google Scholar]
- 4.Asselain BBW, Bartlett J, Bergh J, Bergsten-Nordstrom E, Bliss J. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. Jan 2018;19(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puig CA, Hoskin TL, Day CN, Habermann EB, Boughey JC. National Trends in the Use of Neoadjuvant Chemotherapy for Hormone Receptor-Negative Breast Cancer: A National Cancer Data Base Study. Ann Surg Oncol. May 2017;24(5):1242–1250. [DOI] [PubMed] [Google Scholar]
- 6.Curigliano G, Burstein HJ, E PW, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. August 1 2017;28(8):1700–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. June 1 2017;376(22):2147–2159. [DOI] [PubMed] [Google Scholar]
- 8.von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. February 14 2019;380(7):617–628. [DOI] [PubMed] [Google Scholar]
- 9.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg. 2015;262(3):434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. August 15 2002;95(4):681–95. [DOI] [PubMed] [Google Scholar]
- 11.Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer:National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. May 1 2006;24(13):2019–27. [DOI] [PubMed] [Google Scholar]
- 12.Classe JM, Bordes V, Campion L, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol February 10 2009;27(5):726–32. [DOI] [PubMed] [Google Scholar]
- 13.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. October 9 2013;310(14):1455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. June 2013;14(7):609–18. [DOI] [PubMed] [Google Scholar]
- 15.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. January 20 2015;33(3):258–64. [DOI] [PubMed] [Google Scholar]
- 16.Boughey JC, Ballman KV, Hunt KK, et al. Axillary Ultrasound After Neoadjuvant Chemotherapy and Its Impact on Sentinel Lymph Node Surgery: Results From the American College of Surgeons Oncology Group Z1071 Trial (Alliance). J Clin Oncol. October 20 2015;33(30):3386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morency D, Dumitra S, Parvez E, et al. Axillary Lymph Node Ultrasound Following Neoadjuvant Chemotherapy in Biopsy-Proven Node-Positive Breast Cancer: Results from the SN FNAC Study. Ann Surg Oncol. December 2019;26(13):4337–4345. [DOI] [PubMed] [Google Scholar]
- 18.Hieken TJ, Boughey JC, Jones KN, Shah SS, Glazebrook KN. Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Ann Surg Oncol. October 2013;20(10):3199–204. [DOI] [PubMed] [Google Scholar]
- 19.Moo TA, Jochelson MS, Zabor EC, et al. Is Clinical Exam of the Axilla Sufficient to Select Node-Positive Patients Who Downstage After NAC for SLNB? A Comparison of the Accuracy of Clinical Exam Versus MRI. Ann Surg Oncol. December 2019;26(13):4238–4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carey LA, Berry DA, Cirrincione CT, et al. Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab With or Without Lapatinib. J Clin Oncol. 2016;34(6):542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ollila DW, Cirrincione CT, Berry DA, et al. Axillary Management of Stage II/III Breast Cancer in Patients Treated with Neoadjuvant Systemic Therapy: Results of CALGB 40601 (HER2-Positive) and CALGB 40603 (Triple-Negative). JACS. 2017;224(4):688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. February 9 2011;305(6):569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrow M, Van Zee KJ, Patil S, et al. Axillary Dissection and Nodal Irradiation Can Be Avoided for Most Node-positive Z0011-eligible Breast Cancers: A Prospective Validation Study of 793 Patients. Ann Surg. September 2017;266(3):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong SM, Weiss A, Mittendorf EA, King TA, Golshan M. Surgical Management of the Axilla in Clinically Node-Positive Patients Receiving Neoadjuvant Chemotherapy: A National Cancer Database Analysis. Ann Surg Oncol. October 2019;26(11):3517–3525. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen TT, Hoskin TL, Day CN, et al. Decreasing Use of Axillary Dissection in Node-Positive Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Ann Surg Oncol. September 2018;25(9):2596–2602. [DOI] [PubMed] [Google Scholar]
- 27.Diepstraten SC, Sever AR, Buckens CF, et al. Value of preoperative ultrasound-guided axillary lymph node biopsy for preventing completion axillary lymph node dissection in breast cancer: a systematic review and meta-analysis. Ann Surg Oncol. January 2014;21(1):51–9. [DOI] [PubMed] [Google Scholar]
- 28.Mamtani A, Barrio AV, King TA, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg Oncol. October 2016;23(11):3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Hilli Z, Hoskin TL, Day CN, Habermann EB, Boughey JC. Impact of Neoadjuvant Chemotherapy on Nodal Disease and Nodal Surgery by Tumor Subtype. Ann Surg Oncol. November 27 2017;27(10):017–6263. [DOI] [PubMed] [Google Scholar]
- 30.Pilewskie M, Zabor EC, Mamtani A, Barrio AV, Stempel M, Morrow M. The Optimal Treatment Plan to Avoid Axillary Lymph Node Dissection in Early-Stage Breast Cancer Patients Differs by Surgical Strategy and Tumor Subtype. Ann Surg Oncol. November 2017;24(12):3527–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. October 2014;260(4):608–14; discussion 614–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fayanju OM, Ren Y, Thomas SM, et al. The Clinical Significance of Breast-only and Node-only Pathologic Complete Response (pCR) After Neoadjuvant Chemotherapy (NACT): A Review of 20,000 Breast Cancer Patients in the National Cancer Data Base (NCDB). Ann Surg. October 2018;268(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tadros AB, Yang WT, Krishnamurthy S, et al. Identification of Patients With Documented Pathologic Complete Response in the Breast After Neoadjuvant Chemotherapy for Omission of Axillary Surgery. JAMA Surg. July 1 2017;152(7):665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barron AU, Hoskin TL, Day CN, Hwang ES, Kuerer HM, Boughey JC. Association of Low Nodal Positivity Rate Among Patients With ERBB2-Positive or Triple-Negative Breast Cancer and Breast Pathologic Complete Response to Neoadjuvant Chemotherapy. JAMA Surg. December 1 2018;153(12):1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samiei S, van Nijnatten TJA, de Munck L, et al. Correlation Between Pathologic Complete Response in the Breast and Absence of Axillary Lymph Node Metastases After Neoadjuvant Systemic Therapy. Ann Surg. March 2020;271(3):574–580. doi: 10.1097/sla.0000000000003126 [DOI] [PubMed] [Google Scholar]
- 36.Shi ZQ, Qiu PF, Liu YB, et al. Neo-adjuvant chemotherapy and axillary de-escalation management for patients with clinically node-negative breast cancer. Breast J. November 2019;25(6):1154–1159. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, Li J, Fan Z, et al. Association of higher axillary pathologic complete response rate with breast pathologic complete response after neoadjuvant chemotherapy. Ann Transl Med. August 2020;8(16):992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolberg HC, Kühn T, Krajewska M, et al. Residual Axillary Burden After Neoadjuvant Chemotherapy (NACT) in Early Breast Cancer in Patients with a priori Clinically Occult Nodal Metastases - a transSENTINA Analysis. Geburtshilfe Frauenheilkd. December 2020;80(12):1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Loevezijn AA, van der Noordaa MEM, van Werkhoven ED, et al. Minimally Invasive Complete Response Assessment of the Breast After Neoadjuvant Systemic Therapy for Early Breast Cancer (MICRA trial): Interim Analysis of a Multicenter Observational Cohort Study. Ann Surg Oncol. December 2 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heil J, Pfob A, Sinn HP, et al. Diagnosing Pathologic Complete Response in the Breast After Neoadjuvant Systemic Treatment of Breast Cancer Patients by Minimal Invasive Biopsy: Oral Presentation at the San Antonio Breast Cancer Symposium on Friday, December 13, 2019, Program Number GS5–03. Ann Surg. July 9 2020. [DOI] [PubMed] [Google Scholar]
- 41.Basik MCR, De Los Santos JF, Umphrey HR, Julian TB, Mamounas EP, White J, Lucas PC, Balanoff C, Tan AR, Weber JJ, Edmonson DA, Brown-Glaberman UA, Diego EJ, Teshome M, Matsen CB, Seaward SA, Wapnir IL, Wager JL, Tjoe JA, Thompson AM, Wolmar N. Primary analysis of NRG-BR005, a phase II trial assessing accuracy of tumor bed biopsies in predicting pathologic complete response (pCR) in patients with clinical/radiologic complete response after neoadjuvant chemotherapy (NCT) to explore the feasibility of breast-conserving treatment without surgery. Paper presented at San Antonio Breast Cancer Symposium; December 2019; San Antonio, TX. https://www.abstractsonline.com/pp8/#!/7946/presentation/2169. [Google Scholar]
- 42.Tasoulis MK, Lee HB, Yang W, et al. Accuracy of Post-Neoadjuvant Chemotherapy Image-Guided Breast Biopsy to Predict Residual Cancer. JAMA Surg. October 7 2020;155(12):e204103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Noordaa MEM, van Duijnhoven FH, Cuijpers FNE, et al. Toward omitting sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with clinically node-negative breast cancer. Br J Surg October 8 2020. [DOI] [PubMed] [Google Scholar]
- 44.Avoiding Sentinel Lymph Node Biopsy in Breast Cancer Patients After Neoadjuvant Chemotherapy (ASICS). ClinicalTrials.gov Identifier: NCT04225858. Accessed January 15, 2021. https://clinicaltrials.gov/ct2/show/NCT04225858
- 45.Reimer T, Glass A, Botteri EA-O, Loibl S, ORCID DGOA-. Avoiding Axillary Sentinel Lymph Node Biopsy after Neoadjuvant Systemic Therapy in Breast Cancer: Rationale for the Prospective, Multicentric EUBREAST-01 Trial. LID; - 10.3390/cancers12123698 [doi] LID - 3698. (2072–6694 (Print)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piltin MA, Hoskin TL, Day CN, Davis J Jr., Boughey JC. Oncologic Outcomes of Sentinel Lymph Node Surgery After Neoadjuvant Chemotherapy for Node-Positive Breast Cancer. Ann Surg Oncol. November 2020;27(12):4795–4801. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Tejedor A, Fernandez-Gonzalez S, Ortega R, et al. Can we avoid axillary lymph node dissection in N2 breast cancer patients with chemo-sensitive tumours such as HER2 and TNBC? Breast Cancer Res Treat. October 17 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.