Abstract
Antivirals already on the market and expertise gained from the SARS and MERS outbreaks are gaining momentum as the most effective way to combat the coronavirus outbreak. SARS-CoV-2 has caused considerable mortality due to respiratory failure, highlighting the immediate need for successful therapies as well as the long-term need for antivirals to combat potential emergent mutants of coronaviruses. There are constant viral mutations are being observed due to which world is experiencing different waves of SARS-CoV-2. If our understanding of the virology and clinical presentation of COVID-19 grows, so does the pool of possible pharmacological targets. In COVID-19, the difficulties of proper analysis of current pre-clinical/clinical data as well as the creation of new evidence concerning drug repurposing will be crucial. The current manuscript aims to evaluate the repurposing of an anti-HIV drug Darunavir Ethanolate in COVID-19 treatment with in silico study and we discuss the therapeutic progress of Darunavir Etanolate, to prevent SARS-CoV-2 replication, which supports its clinical assessment for COVID-19 therapy.
Keywords: Darunavir, Drug repurposing, Molecular docking, COVID-19, SARS-COV-2, Darunavir Etanolate
Graphical abstract
1. Introduction
COVID-19 is a disease that is caused by the novel coronavirus known as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). As of now, many newer strains of the virus have been found in some countries and vaccines are ineffective on newer strains and any specific drug molecule is also not available for such new strain of virus hence, the necessity of drug repurposing and treatment identification is emerging demand of the current situation [1]. Unmet needs for modern antivirals include increased effectiveness, oral bioavailability, usefulness for prophylaxis as well as treatment, and understanding that combined therapy can improve efficacy and avoid drug resistance [[2], [3]]. Clinical trials and high throughput screens of repurposed drugs can show a safe and successful medication that also happens to treat COVID-19; however, drugs found using this strategy would almost certainly need more structural optimization to improve antiviral effectiveness against coronaviruses or reduce side effects [4]. The clinical evidence as a part of clinical trials with Remdesvir and favipiravir suggests that there is a need to find the alternate repurpose drug for COVID-19 management until a new therapeutic agent is approved.
2. SARS-CoV-2 viral lifecycle
SARS-CoV-2, like most other coronaviruses, does indeed have a positive-sense single-stranded genomic RNA that is approximately 30 kb in length, making it one of the biggest known RNA genomes [5]. The coronavirus genome is organised as follows: 5′-leader-UTR-replicase-S (Spike)-E (Envelope)-M (Membrane)-N (Nucleocapsid)-3′ UTR-poly (A) tail, with accessory genes intermingled within coding sequences at the 3′ end of the genome [6]. The S protein (150 kDa), which is used to obtain ER entry by an N-terminal signal chain, is heavily glycosylated by N-linked glycosylation. The homotrimer of the S-encoded virus is the virus's characteristic spike shape [7,8].
Trimeric S glycoprotein is a fusion protein of class I and encourages its adherence to the host receptor [9,10]. These proteins' operation aids in the transport of the viral genome to the replica-transcriptase (RTC) complex, which then inserts the encapsidated genome into viral particles. A fifth structural protein, hemagglutinin-esterase (HE), is present in a subset of beta coronaviruses and aids S-protein-mediated cell entry and viral propagation via the mucosa [11].
As a receptor alphacoronaviruses use APN [12], Angiostensin converter (ACE2) enzymes are used as receptors by SARS-CoV, SARS-CoV-2, and HCoV-NL63 [[13], [14], [15], [16]]. S1 and S2 are the S protein's two regions. The interaction between S1 and its cognate receptor causes the S protein to change/modify [17]. The virus must gain access to the host cell cytosol following receptor binding by endosomal cysteine protease cathespins [18,19], TMPRRS2 (transmembrane protease serine 2) or TMPRSS11D implements S1/S2 cleavage to invoke the S-protein and the viral and cellular membrane fusion. S protein cleavage operates at two locations inside the S2 protein element with the first effective cleavage to distinguish the RBD(receptor binding domain) [20] and fusion domain [20] of the S protein [21].
S2 cleavage exposes a membrane-embedded fusion peptide, which then attaches two heptad repeats to S2 to form an antiparallel six-helix collection, and this package formation allows the viral and cellular membranes to merge, allowing fusion and subsequent release of the viral genome into the cytoplasm. Since the endosome is the primary site for the activation of the toll receptor, which triggers an innate immune response, the endosome is ultimately bypassed by accessing the cell through TMPRSS2 and evading the innate host immune systems [22].
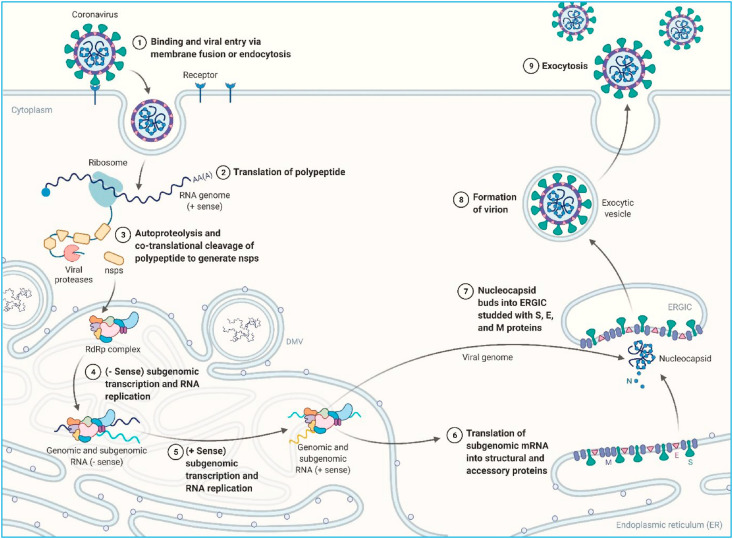
The coronavirus lifecycle continues to translate the replicase gene from the virion genomic RNA after the viral nucleocapsid escapes and becomes uncoated (Fig. 1 ) [6,23,24]. CoV amplification begins with the translation of the virus genome's 5′ proximal ORFs (ORF1a and ORF1b), which results in the synthesis of two significant pp1a (4382 amino acid) and pp1ab (7073 amino acid) replicase polyproteins. In certain instances, the ribosome eliminates/removes/unwind the pseudoknot form and continues translation before rep1a stop codon is encountered.
Fig. 1.
Lifecycle of the SARS-CoV-2 in the host cell.
Following that, multiple nsps are placed in the RTC (replicase transcriptase complex) to initiate the RNA synthesis process and are then in charge of replicating and transcribing the RNA [25]. The replica-polymerase transcribes the entire positive range of genomic RNA as a total negative range template to direct the formation of new genome RNAs and overlap subgenomic negative range templates. Subgenomic RNAs act as messenger RNAs for the structural and accessory genes found downstream of the polyprotein replicase.
Appropriate fold and maturation of viral transmembrane protein (especially S) also depend heavily on ER protein chaperons such as calnexin [26]. Upon translation alteration particulate matter is installed/placed in the intermediate compartment of ER-Golgi (ERGIC) and arranged with the M protein [27,28]. Homotypic M protein interaction provides a scaffold for morphogenesis, whereas M − N and M − S interactions promote the deployment of structural elements on the location [29]. E protein leads also to the assembly of particles by communicating with M and causing membrane curvature [30]. Next, coronavirus particles budded into the ERGIC are eventually transferred in smooth vesicles and exchanged via the secretory path to exocytosis.
3. Immunopathogenesis of SARS-CoV-2
During the entry of replicated viral particles into the cell, its antigen is accessed by the antigen-presenting cells (APC) which is a dominant part of the host's antiviral immunity. In this response, viral peptides are accessed by major histocompatibility complex (MHC) or human leukocyte antigen (HLA) and then further recognized by virus-specific cytotoxic T lymphocytes (CTLs) [31]. Specifically, in the case of SARS-CoV-2, the antigen presentation is mainly obtained by the MHC I molecules [32]. However, MHC II molecules also take part in its presentation [31].
After the viral presentation by APC, the innate immune system of the host instantly gets activated to eliminate the viral particles from the body without injuring the host cells. The innate immune system is responsible for protecting the host cells until the acquired immunity gets developed maybe within 7 or more days after the infection [33]. APC system will further activate the B cells and cytotoxic T cells.
As a part of the humoral response, B cells will develop virus-specific antibodies such as IgM and IgG. The SARS-specific IgM antibodies are almost disappeared after 12 weeks, whereas the IgG antibody can last for a longer period, specifying that the IgG antibody may chiefly play a protective role against SARS-CoV-2 [34]. Due to this mechanism, the reduced level of B cells and increased level of antibodies (IgM and IgG) are found in the infected patients. Hence, the measurement of both antibodies can provide a higher understanding of the diagnosis of acute infection [35]. These antibodies can prevent the re-infection in the same patient but the World Health Organization (WHO) specified that currently there is no proof presenting that recovered patients are completely sheltered from re-infection [36]. Though, SARS-CoV-2 infected rhesus macaques verified that primary infection will produce successful protection from re-infection [37].
CD4+ and CD8+ T cells also play a key role in the pathogenesis of the disease [38]. These cells further produce other cells like dendritic cells, macrophages, neutrophils, and natural killer (NK) cells that are also found to be involved in providing innate immunity. Type I interferons (IFN) which are generally produced by virally infected cells are similarly supposed to be involved in COVID-19 infection [39]. Furthermore, S protein-specific CD4+ T cells were also noticed in infected patients [40]. Some studies have shown that the CD4+ and CD8+ T cell populations are decreased in response to indicating that overall T cell response becomes impaired during the progression of the disease especially in severe cases [35,41,42]. Virus-specific memory CD8+ T cells were revealed to protect infected persons from mortality [41]. However, the role of CD4+ T cells in the control of disease infection is still unclear [35].
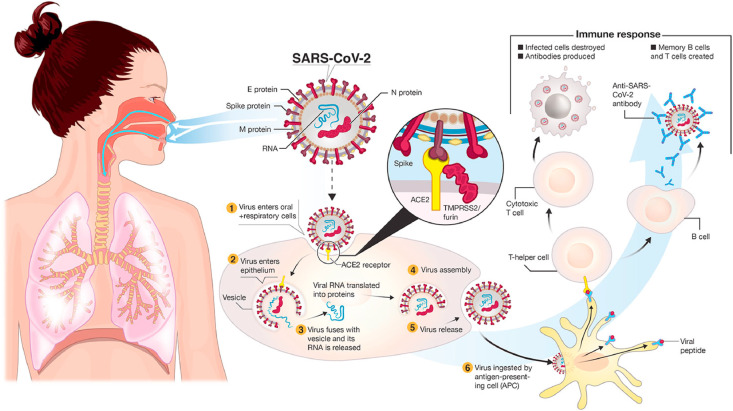
Such innate immune response along with the successively developed acquired immune response is sufficient to get rid of the infection in about 80% of patients who can recover mostly without any antiviral treatments; conversely these responses may not be strong enough to destroy the virus in the leftover infected patients. In such patients, initiation of consequent inflammatory response and recruitment of excess numbers of dendritic cells, T cells, B cells, NK cells, neutrophils, and monocytes/macrophages take place due to continuous viral replication [43]. Such responses can lead to moderate to severe lung damage. The extra cells are assumed to be recruited by several cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-6] and chemokines [CCL2/MCP-1, CCL3/MIP-1α, and CXCL10/IP-10] produced by infected airway epithelial cells and alveolar macrophages [44]. A fatal and uncontrolled anti-inflammatory response can take place due to the release of a large number of pro-inflammatory cytokines (IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF-α, TGFβ, etc.) and chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10, etc.) [45,46]. This event is known as a “cytokine storm” which can induce an intense attack by the immune system to the body which further leads to the ARDS (acute respiratory distress syndrome) followed by multiple organ failure and finally to the death in severe patients. ARDS is found as one of the major responsible reasons for the death in COVID-19 patients [47]. Fig. 2 is labelled for a better understanding of the immunopathogenesis of SARS-CoV-2.
Fig. 2.
Transmission and life-cycle of SARS-CoV-2 causing COVID-19 (Adapted from Ref. [48] under CC BY).
4. Drug repurposing and its approaches for SARS-CoV-2 treatment
Drug repurposing is also known as drug reprofiling, repositioning, or re-tasking. It is the approach to identify the newer uses of previously approved or investigational drugs other than the original indication of that drug. This approach offers several rewards and benefits over developing an exclusively new drug molecule for any disease [49]. It reduces the cost and time of drug development. Additionally, phase I clinical trials can be avoided as human safety data has been already established [50]. Several drug candidates (Table 1 ) and biologics (Table 2 ) have been tested for repurposing to treat COVID-19.
Table 1.
Drug Candidates (small molecules) evaluated for the COVID-19 as a part of Drug Repurposing.
| Class of the Drug | Drug | Function | Clinical Outcome | Reference |
|---|---|---|---|---|
| Antiviral (RNA viruses) | Favipiravir | An antiviral medication was utilized for the treatment of flu and it was additionally endorsed for use in clinical preliminaries as a treatment for nCoV-2019 pneumonia. | It can produce a better therapeutic response in the prevention of disease progression and enhancement of viral clearance along with radiological improvements. | [51,52] |
| Antimalarial, amebicides | Chloroquine phosphate | It is a seasoned enemy of jungle fever/anti-malaria medicate which has indicated a wide scope of antiviral impacts, which incorporates anti-coronavirus | It can efficaciously treat pneumonia in COVID-19 but still, there are several disadvantages so, more RCTs (Randomized controlled trials) are required. | [53,54] |
| Antimalarial | Hydroxy chloroquine sulfate | Hydroxychloroquine is a jungle fever/malarial drug which has indicated viability against coronavirus in lab condition, it was first endorsed in 1995 by FDA under the name of Plaquenil. It has additionally been utilized to treat lupus and joint pain/arthritis. | The drug can significantly improve pneumonia in patients with body temperature normalization and shortening of cough remission time. However, the RCT done by Self et al. doesn't support the use of this drug in COVID-19. | [55,56] |
| Anthelmintics | Ivermectin | An opponent of parasite drugs (anti-parasite) which have proved effective in in-vitro/cell infection against SARS-CoV-2. | Early intervention of the drug can produce faster viral clearance and prevent significant immune involvement. But some RCTs suggest the inefficacy of the drug. | [[57], [58], [59], [60]] |
| Analgesic | Colchicine | It is a more established anti-inflammatory drug and is being concentrated to forestall the complexity of COVID-19 in high hazard patients. | It has been found to reduce the need for oxygen therapy and hospitalization along with clinical improvisation with a reduction in CRP (C- reactive protein) level. It also reduces the hospitalization events and death rate in non-hospitalized patients. | [61,62] |
| HIV protease inhibitor | Darunavir | It is the drug approved for the treatment of HIV and used in the combination with cobicistat to inhibit the viral main protease. It has been assumed that it can also inhibit the protease of the SARS-CoV-2. | In one of the RCT it was found that the drug was well tolerated without any major side effects. Though, it is found effective in in silico studies, it was not found effective in in vitro study. More trials shoul be conducted for the final conclusion about the efficacy. | [63,64] |
| Antiviral | EIDD-2801 (Molnupiravir) | It is an extensive range of oral antiviral that could be utilized as a potential prophylactic or treatment for COVID-19 and different coronaviruses. | Wahl et al. have claimed by their in vivo study that the drug can inhibit viral replication and can be used to prevent or treat the infection. | [65] |
| Antimalarial, antibacterial | Hydroxy chloroquine and azithromycin | COVID-19 patients are treated with a mix of the anti-malaria medication (hydroxychloroquine) and the macrolide antibacterial medication azithromycin, and the patients taking the mix were virologically relieved within six days of treatment. | No stronger evidence of antiviral activity and viral clearance was observed by this drug combination. | [66,67] |
| Protease inhibitor | Camostat mesylate | It is a Protease inhibitor to treat incessant pancreatitis. In vitro analyzes discovered it hinders a mechanism in SARS-CoV-2, which the virus uses to enter human cells. It is assessed that 180 COVID-19 patients aged between 18 and 110 were being enlisted for second phase preliminary studies that will inspect 30 days changes in infection diversity and mortality. | It can decrease the severity of the disease and prevent the viral spread in the lungs by inhibiting the TMPRSS2 and related proteases. | [68,69] |
| Viral fusion inhibitor | Umifenovir | It is an antiviral medication promoted under the name of Arbidol and utilized against flu and as of now being examined for the treatment of COVID-19. | It can potentially improve the clinical and lab status, including oxygen concentration, ICU requirements, hospitalization time, chest CT value, WBC, and ESR. It was found efficacious with supportive therapy in mild to moderate COVID-19 symptomatic patients without any side effects. | [70,71] |
| Antiviral | Remdesivir | Antiviral medication and is under examination in clinical trials in China, the UK, and US. It has exhibited in vitro and in vivo in animal models against the viral pathogens that cause MERS and SARS, which are coronaviruses basically (structurally) like SARS-CoV-2. | It reduces the infection and provides faster recovery to adult patients. However, it does not effective in RCT done by Wang et al. and Spinner et al. Wang et al. have also reported adverse events in 66% of the patients. | [72,73,74] |
| Corticosteroids | Methylprednisolone | It is a glucocorticoid and at present, it is being examined for its wellbeing/safety and adequacy in the treatment of novel coronavirus pneumonia. | It reduces the hospital stay, need for ventilation and improves the clinical status. It also lowers the hyper inflammation status. | [75,76] |
| Antiviral (HIV) | Lopinavir and ritonavir | A mixture of medication to treat HIV and this medication has been examined in blend with influenza medication Also, it has been seen that patient had caused the total to recoup after suffering from acute COVID-19 related pneumonia | These drugs are found to have good pharmacokinetic properties without any adverse events and potential in vivo profile but RCTs did by Horby et al. and Cao et al. do not support the use of these drugs for COVID-19. | [[77], [78], [79], [80]] |
Table 2.
Biologics evaluated for the COVID-19 as a part of Drug Repurposing.
| Class of the Drug | Drug | Function | Clinical Outcome | Reference |
|---|---|---|---|---|
| IL-6 inhibitor | Sarilumab | An interleukin-6 (IL-6) receptor opponent/antagonist utilized against rheumatoid joint pain and is being studied as a potential treatment against intense respiratory misery disorder (ARDS: acute respiratory distress syndrome) in acutely sick patients with COVID-19. | It was found ineffective in phase III RCT and open-label cohort study however, faster recovery was associated with the drug in patients having minor lung consolidation at baseline. | [81,82] |
| Immunosuppressant | Baricitinib | It is a Janus kinase inhibitor showcased under the brand name Oluminat and used to treat rheumatoid joint inflammation and now it is being utilized in the treatment of COVID-19 patients. | It is a safe and promising drug for moderate COVID-19 pneumonia observed in the retrospective multicenter study and it also blocks the viral penetration into the cell but it should be used with cautions. | [83,84] |
| Kinase inhibitor | Ruxolitinib | It is created to treat inflammatory and autoimmune ailments and advertised under the name Jakavi and it is being examined for COVID-19 patients with serious respiratory side effects related to the cytokines storm immune response. | It is found to inhibit the cytokine storm one of the lethal events of the disease and also prevents multiorgan failure and hyperinflammation. Faster improvement in symptoms and CT scan, recovery from lymphopenia without side effects are observed in RCT. | [85,86] |
| IL-6 inhibitor | Tocilizumab (Altizumab) | It is mainly an immunosuppressant drug used to treat rheumatoid arthritis. As it inhibits the interleukins production it is being examined for COVID-19. | It can lower the mortality rate by preventing and decreasing the inflammatory response It was also found to reduce the requirement of mechanical ventilation. However, it was not found to prevent intubation and death in moderately ill patients reported by Stone et al. | [[87], [88], [89]] |
| Antiangiogenic | Bevacizumab | It is a VEGF inhibitor delivers as a treatment for intense respiratory distress condition (ARDS: acute respiratory distress syndrome) in acutely sick patients with COVID-19 pneumonia. | It shows potential clinical efficacy by shortening the oxygen-support duration and improving oxygenation when combined with standard care in severe COVID-19 patients. A drastic survival benefit was also observed in critical patients by this drug. | [90,91] |
| Chemokine receptor blocker | Leronlimab | A CCR5 opponent/antagonist has demonstrated promise against the cytokine storm in acute sick COVID-19 patients. | A high recovery rate along with reduced inflammatory markers and CRP was observed by Yang et al. | [92] |
| Interferons | IFN β-1a/b | Interferons (IFNs) are a family of cytokines that play a vital role to protect against viral infections as a part of the human innate immune system They can be a potential treatment for COVID-19 as per their in vitro and in vivo antiviral properties | Monfared et al. have reported that early administration of the IFN β-1a was found to reduce mortality significantly and increase discharge rate in severe patients but it was not found to change response time in RCT. IFN β-1b was found to reduce the mortality rate and it also shortened the time of recovery along with increased discharge rate | [93,94] |
As of now, remdesivir and favipiravir are used to manage the disease condition but they have several limitations. Remdesivir is found to produce crucial adverse events when it combined with corticosteroids [95]. Also, it cannot provide statistically significant clinical benefits in the RCT (Randomized controlled trial) performed and also 66% of the patients got serious adverse events from the drug [72]. Similarly, several adverse events such as hepatic enzymes elevation, nausea and vomiting and tachycardia have been observed from the use of favipiravir. Severe and lethal events happened more commonly in men and patients above the age of 64 years. Blood and lymphatic disorders, cardiac disorders, hepatobiliary disorders, injury poisoning, and procedural complications were found as a more common ADEs (Adverse drug events) [96]. Hence, repurposing of other drugs is highly desired and patient safety is also the concern. As per the clinical data obtained, the above two antiviral agents are not much effective in managing COVID-19.
5. Darunavir acting through polypeptide packing
Before the formation of virions in case of SARS-CoV-2, the functional proteins are cleaved from the polypeptide chains which have been translated from the viral RNAs. This cleavage is mediated by the viral main proteases enzyme. It is interesting to note here that SARS-CoV-2 protease has 96% similarity with the proteases of SARS-CoV. Several protease inhibitors which are used in the treatment of HIV-1 virus infection are found to be effective against SARS-CoV-2. The inhibition of SARS-CoV-2 protease using HIV-1 protease inhibitors have been validated through some in-silico and in-vitro approaches [97]. Darunavir (originally approved by the FDA in 2006) is a protease inhibitor drug used along with the other HIV protease inhibitors as well as ritonavir to manage the infection of HIV-1 efficaciously. Darunavir is considered for combating the resistance to standard HIV therapy as a second generation protease inhibitor and it is generally used in combination with cobicistat to produce more therapeutic effectiveness.
Darunavir is being studied as a probable treatment for SARS-CoV-2, because of its in vitro results supporting its potency to eradicate this infection. Some clinical trials are on-going and are predicted to conclude soon.
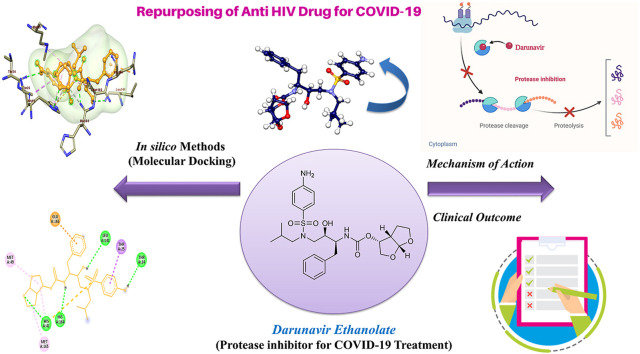
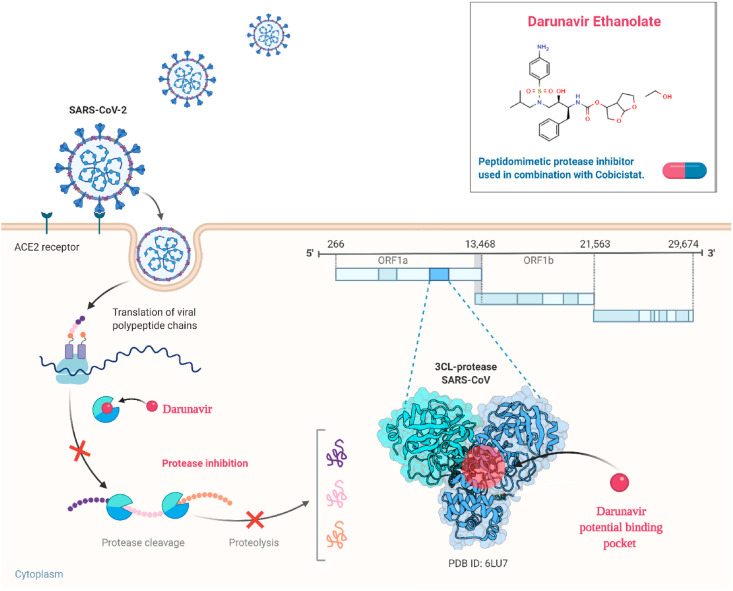
Various protease inhibitors such as saquinavir, amprenavir, indinavir, nelfinavir, ritonavir, and lopinavir are accepted by FDA to utilize in HIV therapy. Some of them are being also considered against the SARS-CoV-2 infection as a part of drug repurposing. As discussed earlier, darunavir is a second-generation non-peptide protease inhibitor having enhanced binding affinity, reduced dissociation rate and more potency than the other protease inhibitors due to its diverse chemical structure. Darunavir was acknowledged as one of the promising hits for inhibition of chymotrypsin-like protease or main protease of SARS-CoV-2 through computational drug design methods (Fig. 3 ).
Fig. 3.
Mechanism of action of Darunavir Ethanolate in eradicating SARS-CoV-2 from the host.
Recently in Shanghai, 30 potential agents against COVID-19 including darunavir with potential antiviral activity against SARS-CoV2 have been revealed using in-silico and an enzyme activity based screening [98]. Excitingly, darunavir has found with the wide safety margin along with the very low therapeutic doses to cause cytotoxic effects. In an in-vitro study, darunavir at 300 μM concentration was found to inhibit replication of SARS-CoV-2 virus by 280 times more than the untreated group [99]. Further, in Italy, darunavir tablet with the dose of 600 mg at every 12 h has been utilized with other anti-viral agents and supportive therapy to clinically manage the patients having COVID-19 with a range of MEWS [100]. It has very quick oral absorption and terminal elimination half-life of 15 h. Approx. 95% of the drug remains plasma protein bound and gets totally metabolized by CYP3A4. Hence, co-administration of small doses of ritonavir (CYP3A4 inhibitor) can further enhance the bioavailability of the darunavir however; combination therapy with other CYP3A4 inhibitors (e.g. statins) with darunavir/ritonavir necessitates the cautions or is even contraindicated sometimes.
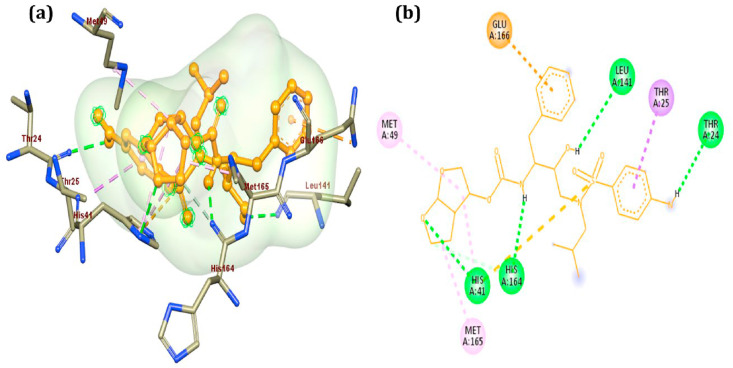
We have also performed the molecular docking of the darunavir ethanolate on the SARS-CoV-2 main protease (PDB ID: 6LU7) using Autodock Vina to ensure the binding affinity of the drug (Fig. 4 ) [101]. We observed the binding affinity −7.8 kcal/mol which is superior to the co-crystallised ligand (−7 kcal/mol). This result provides one more evidence that darunavir ethanolate can be the potential inhibitor of the SARS-CoV-2 protease.
Fig. 4.
Interaction of Darunavir Ethanolate with main protease (6LU7).
6. Preclinical and clinical studies
There is a very less amount of evidence of preclinical and clinical trials of the use of darunavir in COVID-19 hence, more studies should be conducted to evaluate the potential of the drug. Chen et al. have evaluated the darunavir/cobicistat for safety and efficacy in COVID-19 patients with their single-center, randomized, and open-label trial (NCT04252274), and they found that the drugs are well tolerated but they have not observed any significant benefit in clinical improvisation and viral clearance [63]. At the same time, darunavir was not found equally effective during in vitro study when compared with remdesivir as a positive control [64]. An observational, retrospective trial is ongoing on 200 patients in Qatar (NCT04425382) to evaluate the safety and efficacy of Darunavir/Cobicistat vs. Lopinavir/Ritonavir [102]. Mostly the antiviral drugs works well in the combination therapy hence it is essential to evaluate the potential of darunavir ethanolate with different clinical set up and studies which will provide a better holistic picture. The drug is used as boosting agent in the HIV Therapy hence more number of trials shall be designed using different combination of the drug to get the more fruitful results. It is well evident from the current efforts to test the molecule against SARS-CoV-2 that alone it will not be much effective. Johnson & Johnson is testing its antiviral medicines, notably darunavir, in vitro for possible SARS-CoV-2 resistance. As per the Janssen, they have supported three numbers of open labelled randomized clinical trials in china but the data is still not available and published [103]. There was no advantage to darunavir therapy beyond conventional care in hospitalized adult patients (Very limited number) with severe Covid-19. Future trials in severely ill individuals may assist to confirm or rule out the probability of a therapy benefit. There is another trial is going on in chine (ChiCTR2000029541; ICTPR) using a combination of arunavir/cobicistat and thymosin with enrolment of 100 patients and results are awaited. On the other hand, a different trail is established with combination of darunavir/ritonavir and atomised interferon (NCT04291729; ClinicalTrials.gov) with enrolment of 50 patients and results are not public yet. There is a large randomized blinded trial is planned in the spain (NCT04304053; ClinicalTrials.gov) which is ongoing with 3040 patients enrolled and still recruiting to evaluate the darunavir/cobicistat safety and efficacy. This all studies under development suggest that molecule has potential but waiting for the fruitful outcome to be declared in the clinical setup.
7. Adverse events
There are no major adverse event observed to date for the Darunavir Ethanolate but as we have very little amount of data for the safety trials, further clinical trials should be performed to have the actual safety profile of the drug. Mild diarrhea and renal dysfunction were observed in patients who received darunavir/cobicistat in comparison with standard care [63]. The drug should be used cautiously in patients having cardiac comorbidities as increased risk of myocardial infarction in HIV patients who were on the treatment of darunavir. A detail pharmacological profile should further be investigated before regular use of this drug in COVID-19 patients. A recent research linked darunavir usage to an elevated risk of myocardial infarction in HIV patients, concluding that darunavir raises the risk of cardiovascular disease (CVD). As a result, it should be administered with caution in individuals with underlying heart problems [104]. Prior to regular usage of this medicine, further pharmacological characteristics may be examined during COVID usage in a total of 19 patients.
8. Conclusion and future remarks
The enormity of the morbidity and mortality imposed on the global population in less than a year has forced the unavoidable decision that finding and developing successful COVID-19 antiviral drugs is imperative and should be prioritized. There are few vaccines approved but the viral mutations render them ineffective for providing complete protection against SARS-CoV-2. Many medications are presently being repurposed utilising fundamental understanding of viral aetiology and pharmacodynamics, as well as computational methods. In the current context, drug repositioning might be viewed as a potential therapy option for COVID-19. Darunavir is identified as a potential drug to inhibit the SARS-CoV-2 viral protein synthesis by inhibiting one of the vital main proteases enzyme through in silico studies. However, there is too little amount of evidence from preclinical and clinical trials available to ensure its safety and efficacy. It has been also found well tolerated in clinical trials. Hence, more studies should be carried out to evaluate the results obtained from in silico studies. There are ample scope to further evaluate the potential of this candidate under clinical setup globally as the recent clinical evidences reveals many complications with Remdesvir and Favipiravir which are currently prescribed by the medicinal practitioners for COVID-19 management of moderate to severe cases.
Credit author statement
Dr Divyang J Dave and Vivek P Chavda - Conceptualization, Supervision, Writing-Reviewing and Editing; Vivek P Chavda, Normi Gajjar and Nirav Shah - Writing- Original draft and revised manuscript preparation; Vivek P Chavda and Normi Gajjar – Figure Preparation. All authors reviewed the final version of the manuscript and approved their authorship for the work. Fig. 1, Fig. 3 are grafted using Biorender.com.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Vivek P Chavda is thankful to L M College of pharmacy for providing a suitable facility for carrying out the literature search for this work. The authors would like to acknowledge Dr. Lalit Vora (Queen’s University, Belfast, UK) for his support in the figure preparation.
References
- 1.Rubin R. COVID-19 vaccines vs variants - determining how much immunity is enough. JAMA, J. Am. Med. Assoc. 2021;325:1241–1243. doi: 10.1001/jama.2021.3370. [DOI] [PubMed] [Google Scholar]
- 2.Richman D.D. Antiviral drug discovery to address the COVID-19 pandemic. mBio. 2020;11 doi: 10.1128/mBio.02134-20. e02134-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chavda Vivek P., Vora Lalitkumar, Vihol Disha. COVAX-19Ⓡ Vaccine: Completely blocks virus transmission to non-immune individuals. Clin. Complem. Med. Pharmacol. 2021;1(1):100001–100004. doi: 10.1016/j.ccmp.2021.100004. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villamagna A.H., Gore S.J., Lewis J.S., Doggett J.S. The need for antiviral drugs for pandemic coronaviruses from a global Health perspective. Front. Med. 2020;7:998. doi: 10.3389/fmed.2020.596587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses Methods Protoc. 2015;1–23 doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delmas B., Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J. Virol. 1990;64:5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham R.L., Baric R.S. Recombination, reservoirs, and the modular spike: mechanisms of coronavirus cross-species transmission. J. Virol. 2010;84:3134–3146. doi: 10.1128/jvi.01394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White J.M., Delos S.E., Brecher M., Schornberg K. Structures and mechanisms of viral membrane fusion proteins. Crit. Rev. Biochem. Mol. Biol. 2008;43:189–219. doi: 10.1080/10409230802058320.Structures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosch B.J., van der Zee R., de Haan C.A.M., Rottier P.J.M. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/jvi.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelissen L.A., Wierda C.M., van der Meer F.J., Herrewegh A.A., Horzinek M.C., Egberink H.F., et al. Hemagglutinin-esterase, a novel structural protein of torovirus. J. Virol. 1997;71:5277–5286. doi: 10.1128/jvi.71.7.5277-5286.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reguera J., Santiago C., Mudgal G., Ordoño D., Enjuanes L., Casasnovas J.M. Structural bases of coronavirus attachment to host aminopeptidase N and its inhibition by neutralizing antibodies. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann H., Pyrc K., Van Der Hoek L., Geier M., Berkhout B., Pöhlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M., Kleine-Weber H., Schroeder S., Mü M.A., Drosten C., Pö S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor article SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:1–10. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J. Virol. 2020 doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu K., Li W., Peng G., Li F. Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor. Proc. Natl. Acad. Sci. U. S. A. 2009;106:19970–19974. doi: 10.1073/pnas.0908837106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelus B.D., Schickli J.H., Blau D.M., Weiss S.R., Holmes K.V. Conformational changes in the spike glycoprotein of murine coronavirus are induced at 37 C either by soluble murine CEACAM1 receptors or by pH 8. J. Virol. 2003;77:830–840. doi: 10.1128/jvi.77.2.830-840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian Z., Dominguez S.R., Holmes K.V. Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosch B.J., Bartelink W., Rottier P.J.M. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J. Virol. 2008;82:8887–8890. doi: 10.1128/jvi.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y., Zhou Y., Liu S., Kou Z., Li W., Farzan M., et al. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem. Biophys. Res. Commun. 2004;324:773–781. doi: 10.1016/j.bbrc.2004.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirato K., Kanou K., Kawase M., Matsuyama S. Clinical isolates of human coronavirus 229E bypass the endosome for cell entry. J. Virol. 2017;91 doi: 10.1128/jvi.01387-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Host factors in coronavirus replication. Curr. Top. Microbiol. Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim Y., Ng Y., Tam J., Liu D. Human coronaviruses: a review of virus–host interactions. Diseases. 2016;4:26. doi: 10.3390/diseases4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukushi M., Yoshinaka Y., Matsuoka Y., Hatakeyama S., Ishizaka Y., Kirikae T., et al. Monitoring of S Protein maturation in the endoplasmic reticulum by calnexin is important for the infectivity of severe acute respiratory syndrome coronavirus. J. Virol. 2012;86:11745–11753. doi: 10.1128/jvi.01250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klumperman J., Locker J.K., Meijer A., Horzinek M.C., Geuze H.J., Rottier P.J. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J. Virol. 1994;68:6523–6534. doi: 10.1128/jvi.68.10.6523-6534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturman L.S., Holmes K.V. The molecular biology of coronaviruses. Adv. Virus Res. 1983;28:35–112. doi: 10.1016/S0065-3527(08)60721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogue B.G., Machamer C.E. Coronavirus structural proteins and virus assembly. Nidoviruses. 2014:179–200. doi: 10.1128/9781555815790.ch12. [DOI] [Google Scholar]
- 30.Lim K.P., Liu D.X. The missing link in coronavirus assembly. Retention of the avian coronavirus infectious bronchitis virus envelope protein in the pre-Golgi compartments and physical interaction between the envelope and membrane proteins. J. Biol. Chem. 2001;276:17515–17523. doi: 10.1074/jbc.M009731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J., Wu P., Gao F., Qi J., Kawana-Tachikawa A., Xie J., et al. Novel immunodominant peptide presentation strategy: a featured HLA-A∗2402-Restricted cytotoxic T-lymphocyte epitope stabilized by intrachain hydrogen bonds from severe acute respiratory syndrome coronavirus nucleocapsid protein. J. Virol. 2010;84:11849–11857. doi: 10.1128/jvi.01464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Li G., Chen X., Xu A. Profile of specific antibodies to the SARS-associated coronavirus. N. Engl. J. Med. 2003;349:508–509. doi: 10.1056/nejm200307313490520. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu Y. Understanding the immunopathogenesis of COVID-19: its implication for therapeutic strategy. World J Clin Cases. 2020;8:5835–5843. doi: 10.12998/wjcc.v8.i23.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization (WHO) 2020. “Immunity Passports” in the Context of COVID-19. [Google Scholar]
- 37.Deng W., Bao L., Liu J., Xiao C., Liu J., Xue J., et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020;369(80):818–823. doi: 10.1126/science.abc5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rokni M., Ghasemi V., Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: comparison with SARS and MERS. Rev. Med. Virol. 2020;30:1–6. doi: 10.1002/rmv.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pia L. SARS-CoV-2-reactive T cells in patients and healthy donors. Nat. Rev. Immunol. 2020;20:353. doi: 10.1038/s41577-020-0333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Channappanavar R., Fett C., Zhao J., Meyerholz D.K., Perlman S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014;88:11034–11044. doi: 10.1128/jvi.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bender A.E. Food additives. Sci. Prog. 1988;72:549–562. [PubMed] [Google Scholar]
- 43.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 44.Morris G., Bortolasci C.C., Puri B.K., Olive L., Marx W., O'Neil A., et al. The pathophysiology of SARS-CoV-2: a suggested model and therapeutic approach. Life Sci. 2020;258 doi: 10.1016/j.lfs.2020.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cameron M.J., Bermejo-Martin J.F., Danesh A., Muller M.P., Kelvin D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133:13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Funk C.D.L.C., Aa A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front. Pharmacol. 2020;11:937. doi: 10.3389/fphar.2020.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., et al. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 50.Harsha Agarwal S.I. Drug Discov from Technol Networks; 2021. Drug Repurposing: Advantages and Key Approaches. [Google Scholar]
- 51.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020;6:1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coomes E.A., Haghbayan H. Favipiravir, an antiviral for COVID-19? J. Antimicrob. Chemother. 2020;75:2013–2014. doi: 10.1093/jac/dkaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/BST.2020.01047. [DOI] [PubMed] [Google Scholar]
- 54.Tang D., Li J., Zhang R., Kang R., Klionsky D.J. Chloroquine in fighting COVID-19: good, bad, or both? Autophagy. 2020;16:2273–2275. doi: 10.1080/15548627.2020.1796014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. 2020;7 doi: 10.1101/2020.03.22.20040758. [DOI] [Google Scholar]
- 56.Self W.H., Semler M.W., Leither L.M., Casey J.D., Angus D.C., Brower R.G., et al. Effect of hydroxychloroquine on clinical status at 14 Days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA, J. Am. Med. Assoc. 2020;324:2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed S., Karim M.M., Ross A.G., Hossain M.S., Clemens J.D., Sumiya M.K., et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int. J. Infect. Dis. 2021;103:214–216. doi: 10.1016/j.ijid.2020.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pott-Junior H., Bastos Paoliello M.M., Miguel A. de QC., da Cunha A.F., de Melo Freire C.C., Neves F.F., et al. Use of ivermectin in the treatment of Covid-19: a pilot trial. Toxicol Reports. 2021;8:505–510. doi: 10.1016/j.toxrep.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.López-Medina E., López P., Hurtado I.C., Dávalos D.M., Ramirez O., Martínez E., et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA, J. Am. Med. Assoc. 2021;325:1426–1435. doi: 10.1001/jama.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajter J.C., Sherman M.S., Fatteh N., Vogel F., Sacks J., Rajter J.-J. Ivermectin as a potential treatment for mild to moderate COVID-19 – a double blind randomized placebo-controlled trial. MedRxiv. 2020 2020.06.06.20124461. [Google Scholar]
- 61.Lopes M.I.F., Bonjorno L.P., Giannini M.C., Amaral N.B., Benatti M.N., Rezek U.C., et al. Beneficial effects of colchicine for moderate to severe COVID-19: an interim analysis of a randomized, double-blinded, placebo controlled clinical trial. MedRxiv. 2020 doi: 10.1101/2020.08.06.20169573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tardif J., Bouabdallaoui N., Allier P.L.L. Efficacy of colchicine in non-hospitalized patients with COVID-19. MedRxiv. 2021 [Google Scholar]
- 63.Chen J., Xia L., Liu L., Xu Q., Ling Y., Huang D., et al. Antiviral activity and safety of darunavir/Cobicistat for the treatment of COVID-19. Open Forum Infect Dis. 2020;7:1–5. doi: 10.1093/ofid/ofaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Meyer S., Bojkova D., Cinatl J., Van Damme E., Buyck C., Van Loock M., et al. Lack of antiviral activity of darunavir against SARS-CoV-2. Int. J. Infect. Dis. 2020;97:7–10. doi: 10.1016/j.ijid.2020.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wahl A., Gralinski L.E., Johnson C.E., Yao W., Kovarova M., Dinnon K.H., et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451–457. doi: 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Maladies Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lover A.A. Quantifying treatment effects of hydroxychloroquine and azithromycin for COVID-19: a secondary analysis of an open label non-randomized clinical trial. MedRxiv. 2020 doi: 10.1101/2020.03.22.20040949. 0–4. [DOI] [Google Scholar]
- 68.Hofmann-Winkler H., Moerer O., Alt-Epping S., Bräuer A., Büttner B., Müller M., et al. Camostat mesylate may reduce severity of coronavirus disease 2019 sepsis: a first observation. Crit Care Explor. 2020;2 doi: 10.1097/cce.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffmann M., Hofmann-Winkler H., Smith J.C., Krüger N., Arora P., Sørensen L.K., et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine. 2021;65 doi: 10.1016/j.ebiom.2021.103255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nojomi M., Yassin Z., Keyvani H., Makiani M.J., Roham M., Laali A., et al. Effect of Arbidol (Umifenovir) on COVID-19: a randomized controlled trial. BMC Infect. Dis. 2020;20:1–10. doi: 10.1186/s12879-020-05698-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yethindra V., Tagaev T., Uulu M.S., Parihar Y. Efficacy of umifenovir in the treatment of mild and moderate COVID-19 patients. Int. J. Res. Market. 1999;11:113–127. [Google Scholar]
- 72.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of covid-19 — final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spinner C.D., Gottlieb R.L., Criner G.J., Arribas López J.R., Cattelan A.M., Soriano Viladomiu A., et al. Effect of remdesivir vs standard care on clinical status at 11 Days in patients with moderate COVID-19: a randomized clinical trial. JAMA, J. Am. Med. Assoc. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ranjbar K., Moghadami M., Mirahmadizadeh A., Fallahi M.J., Khaloo V., Shahriarirad R., et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect. Dis. 2021;21:1–8. doi: 10.1186/s12879-021-06045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu J., Zheng X., Huang Y., Shan H., Huang J. Successful use of methylprednisolone for treating severe COVID-19. J. Allergy Clin. Immunol. 2020;146:325–327. doi: 10.1016/j.jaci.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gregoire M., Le Turnier P., Gaborit B.J., Veyrac G., Lecomte R., Boutoille D., et al. Lopinavir pharmacokinetics in COVID-19 patients. J. Antimicrob. Chemother. 2020;75:2702–2704. doi: 10.1093/jac/dkaa195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horby P.W., Mafham M., Bell J.L., Linsell L., Staplin N., Emberson J., et al. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S., et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lescure F.-X., Honda H., Fowler R.A., Lazar J.S., Shi G., Wung P., et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9 doi: 10.1016/s2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Della-Torre E., Campochiaro C., Cavalli G., De Luca G., Napolitano A., La Marca S., et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann. Rheum. Dis. 2020;79:1277–1285. doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cantini F., Niccoli L., Nannini C., Matarrese D., Natale ME Di, Lotti P., et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Favalli E.G., Biggioggero M., Maioli G., Caporali R. Baricitinib for COVID-19: a suitable treatment? Lancet Infect. Dis. 2020;20:1012–1013. doi: 10.1016/S1473-3099(20)30262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.La Rosée F., Bremer H.C., Gehrke I., Kehr A., Hochhaus A., Birndt S., et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. 2020;34:1805–1815. doi: 10.1038/s41375-020-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020;146:137–146. doi: 10.1016/j.jaci.2020.05.019. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N. Engl. J. Med. 2021;384:20–30. doi: 10.1056/nejmoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kahan T. 2021. Tocilizumab in COVID-19 : Some Clarity amid Controversy. 1599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., et al. Efficacy of tocilizumab in patients hospitalized with covid-19. N. Engl. J. Med. 2020;383:2333–2344. doi: 10.1056/nejmoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pang J., Xu F., Aondio G., Li Y., Fumagalli A., Lu M., et al. Efficacy and tolerability of bevacizumab in patients with severe Covid-19. Nat. Commun. 2021;12:1–10. doi: 10.1038/s41467-021-21085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Mahtab M., Akbar S.M., Huq A.F., Khan M.S.I., Islam M.A., Mazumder M.A., et al. Extraordinary survival benefits of severe and critical patients with COVID-19 by immune modulators: the outcome of a clinical trial in Bangladesh. Euroasian J. Hepato-Gastroenterol. 2021;10:68–75. doi: 10.5005/jp-journals-10018-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang B., Fulcher J.A., Ahn J., Berro M., Goodman-Meza D., Dhody K., et al. Clinical characteristics and outcomes of coronavirus disease 2019 patients who received compassionate-use leronlimab. Clin. Infect. Dis. 2020;90095:1–8. doi: 10.1093/cid/ciaa1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davoudi-monfared E., Rahmani H., Khalili H., Hajiabdolbaghi M., Salehi M. A randomized clinical trial of the efficacy and safety of interferon -1a in treatment of severe COVID-19. Antimicrob. Agents Chemother. 2020;64:1–14. doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rahmani H., Davoudi-Monfared E., Nourian A., Khalili H., Hajizadeh N., Jalalabadi N.Z., et al. Interferon β-1b in treatment of severe COVID-19: a randomized clinical trial. Int. Immunopharm. 2020;88 doi: 10.1016/j.intimp.2020.106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Young B., Tan T.T., Leo Y.S. The place for remdesivir in COVID-19 treatment. Lancet Infect. Dis. 2021;21:20–21. doi: 10.1016/S1473-3099(20)30911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaur R.J., Charan J., Dutta S., Sharma P., Bhardwaj P., Sharma P., et al. Favipiravir use in COVID-19: analysis of suspected adverse drug events reported in the WHO database. Infect. Drug Resist. 2020;13:4427–4438. doi: 10.2147/IDR.S287934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ortega J.T., Serrano M.L., Pujol F.H., Rangel H.R. Unrevealing sequence and structural features of novel coronavirus using in silico approaches: the main protease as molecular target. EXCLI J. 2020;19:400–409. doi: 10.17179/excli2020-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.simm A. Joint research team of Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Shanghai University of Science and Technology has discovered a batch of old and traditional Chinese medicines that may have therapeutic effects on new pneumonia. Sci Res Trends. 2020 https://www.simm.ac.cn/xwzx/kydt/202001/t20200125_5494417.html [Google Scholar]
- 99.Sharma A., Tiwari S., Deb M.K., Marty J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Colalto C. Volatile molecules for COVID-19: a possible pharmacological strategy? Drug Dev. Res. 2020 doi: 10.1002/ddr.21716. 10.1002/ddr.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trott AJO O., AutoDock Vina. Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hamad Medical Corporation Darunavir/cobicistat vs. Lopinavir/ritonavir in COVID-19 pneumonia in Qatar (DOLCI) https://clinicaltrials.gov/ct2/show/NCT04425382 Clin Identifier NCT04425382 2020.
- 103.Janssen Lack of evidence to support use of darunavir-based treatments for SARS-CoV-2. 2021. https://www.jnj.com/lack-of-evidence-to-support-darunavir-based-hiv-treatments-for-coronavirus
- 104.Triant V.A., Siedner M.J. Darunavir and cardiovascular risk: evaluating the data to inform clinical care. J. Infect. Dis. 2020;221:498–500. doi: 10.1093/infdis/jiz482. [DOI] [PMC free article] [PubMed] [Google Scholar]