Abstract
Objectives:
Recently, Stereotactic Body Radiotherapy (SBRT) has been suggested for managing hepatocellular carcinoma (HCC) curatively. Thus, we conducted this clinical study to evaluate retrospectively the effect of individualized audio-visual (AV) coaching, respiratory modulated SBRT.
Materials and Methods:
Between 2014 and 2018, 29 patients with inoperable Barcelona Clinic Liver Cancer (BCLC) stage 0-B HCC received AV coaching, respiratory-modulated SBRT. We constructed a task-oriented multidisciplinary team to establish a standard operation process of respiratory modulation procedures and developed our AV coaching devices. In the training period, a goodness-of-fit test was applied individually. SBRT was delivered with a total dose of 40–54 Gy in 5–6 fractions individually. Freedom from local progression (FFLP) and overall survival (OS) were estimated using SPSS (version 17, SPSS Inc., Chicago, IL, USA) life tables.
Results:
The patient characteristics were as follows: 32.7 ± 16 mm in maximum tumor diameter (range 11–94); BCLC stage 0: 3.4%, BCLC A: 48.3%, BCLC B: 48.3%; Child-Pugh classification A: 86.2%, Child-Pugh classification B: 13.8%, and a median of 2 prior liver-directed treatments (range 0–7). One-, 2-, and 3-year rates of FFLP of SBRT were 96.6%, 96.6%, and 96.6%, respectively. One-, 2-, and 3-year rates of OS were 81.5%, 72.4%, and 67.2%, respectively. No adverse event (AE) occurred in 41.4% of patients, 48.3% developed grade (G) 1–2 AE, 10.3% had G3 AE and none had G4-5 AE.
Conclusion:
Respiration-modulated SBRT is a promising noninvasive treatment option for patients with inoperable and localized HCC. Our data show that SBRT provides comparable tumor control to historical curative options like surgery and radiofrequency ablation of localized tumors. Thus, we are conducting a further prospective clinical trial with the intent to demarcate the clinical effectiveness of SBRT in a larger population of patients with HCC.
KEYWORDS: Hepatocellular carcinoma, Stereotactic ablative radiotherapy, Stereotactic body radiation therapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third- and second-most prevalent cause of cancer death in the Asia-Pacific region and in Taiwan, respectively [1,2,3]. In recent decades, significant advances have been made in HCC treatment, both curative and noncurative. For curative intent, liver resection, or transplantation can achieve a 5-year overall survival (OS) rate as high as 70%–75% [4,5]. However, fewer than 30% of patients with HCC are suitable for surgery or transplantation. Nonsurgical local treatment options include percutaneous ethanol injection, cryoablation, microwave ablation, and radiofrequency ablation (RFA); emerging treatment modalities such as Stereotactic Body Radiotherapy (SBRT) and hypofractionated proton therapy may serve as a bridge to liver transplantation or as alternatives to liver resection [6].
Recently, SBRT, also called stereotactic ablative radiotherapy (RT), has emerged as a potentially curative treatment with acceptable toxicity and excellent local control (LC) [7,8]. SBRT is an advanced RT technique that delivers an extremely high dose of radiation over a shortened treatment course, requiring only 3–6 fractions, delivered within 2 weeks. Due to its combining image guided RT, effective immobilization, respiratory modulation procedures, modified contouring, unique normal organ at risk (OAR) constraints and extremely strict quality assurance (QA), SBRT delivers precise and highly conformal dose distributions while delivering a much lower dose to the tissues around the tumor. This characteristic spares a large portion of the normal liver and lowers the risk of side effects while achieving higher tumor control than conventional RT.
During SBRT, liver tumor and upper abdominal normal organ motion can significantly impact RT planning and delivery. To achieve high precision, numerous uncertainties involved in computed tomography (CT) simulation, target delineating, planning, image guiding, and finally, delivery of the precise dose must be minimized. Methodologies have been developed to accommodate the potential error at each step of the process. The greatest of these is respiratory motion, which may distort the image up to 20–25 mm in the craniocaudal direction, in addition to causing substantial rotation and liver shape deformation [9,10,11].
To minimize the respiratory motion-related uncertainty, several kinds of respiratory motion management (RMM) procedures have been developed for any motion >5 mm [12,13]. The American Association of Physicists in Medicine Task Group 76 reported 5 management strategies commonly used worldwide: (1) motion-encompassing (free breathing and design target to compensate for full range of motion), (2) respiratory gating (only deliver treatment during select portions of the respiratory cycle), (3) breath-hold (patients freeze and hold their breath with assistance during the treatment), (4) forced shallow breathing with abdominal compression (using a device to limit diaphragm and chest wall expansion and educate patients to breath shallowly), and (5) real-time tumor-tracking (follow target as it moves by fiducial implants or radiation beam) [12]. Accessory devices have also been developed to aid the RMM procedure, such as a system to guide breathing via audio-visual (AV) biofeedback [14,15].
However, as an emerging treatment, evidence is still limited on the efficacy and toxicity of SBRT. Thus, we hypothesized that SBRT can achieve excellent tumor control with acceptable toxicity and conducted this clinical study to apply individualized AV coaching, respiratory-modulated SBRT and evaluate its effectiveness.
MATERIALS AND METHODS
Research ethics and data collection
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the institute (approval number: B10404010). Informed written consent was waived by the IRB because the study was a retrospective data analysis. We retrospectively retrieved and analyzed data from medical records and our prospective departmental database, i.e., our Integrated RT Oncology Information Platform [16,17].
From 2014 to 2018, 29 patients [Table 1] with inoperable Barcelona Clinic Liver Cancer (BCLC) stage 0-B HCC were treated with AV coaching, respiratory-modulated SBRT. Freedom from local progression (FFLP) and OS were defined as the study endpoints.
Table 1.
Patient characteristics
| Demographic characteristics | |
| Number of patients | n=29 |
| Age (years) | 69.6±12.7 (49-92) |
| Clinical characteristics | |
| Tumor size (mm) | 32.7±16.0 (11-94) |
| Number of treated lesions, median | 2 (1-4) |
| Number of prior local liver treatments, median | 2 (0-7) |
| Median number of prior local liver treatments ≥2, n (%) | 18 (62.1) |
| Gender (male), n (%) | 18 (62.1) |
| Clinical T category, n (%) | |
| T1 | 8 (27.6) |
| T2 | 18 (62.1) |
| T3 | 3 (10.3) |
| AJCC stage, n (%) | |
| I | 7 (24.1) |
| II | 20 (69.0) |
| III | 2 (6.9) |
| BCLC stage, n (%) | |
| 0 | 1 (3.4) |
| A | 14 (48.3) |
| B | 14 (48.3) |
| Child-Pugh classification | |
| Score | 5.4±0.8 (5-8) |
| A, n (%) | 25 (86.2) |
| B, n (%) | 4 (13.8) |
| Liver disease, n (%) | |
| Cirrhosis (+) | 28 (96.6) |
| Hepatitis B (+) | 11 (37.9) |
| Hepatitis C (+) | 15 (51.7) |
| Adverse event, n (%) | |
| No AE | 12 (41.4) |
| G1-2 | 14 (48.3) |
| ≥ G3 | 3 (10.3) |
| RT characteristics | |
| RT dose (Gy), median | 50 (40-54) |
| Normal liver volume (liver-GTV, cc) | 1034.2±181.5 (653.0-1361.5) |
| Normal liver mean dose (cGy) | 807.3±293.4 (137.2-1492.3) |
| GTV volume (cc) | 30.5±38.0 (2.4-165.1) |
| RMM methods | |
| Breath-hold | 10 DEBH, 3 DIBH |
| Respiratory gating | 10 |
| Compression | 6 |
| RT field number | |
| Patients number of who used coplanar fields plus noncoplanar fields | 27 |
| Number of coplanar fields plus noncoplanar fields used | |
| Coplanar fields | 1.8±0.5 (1-3) |
| Noncoplanar fields | 1.9±0.7 (0-3) |
| RT beam on time (min) | 2.8±1.1 (1.2-6.3) |
| MU | 2694.5±599.3 (1852.5-4202.8) |
RT: Radiotherapy, AJCC: American Joint Committee on Cancer, BCLC: Barcelona clinic liver cancer, AE: Adverse event, GTV: Gross tumor volume, RMM: Respiratory motion management, DEBH: Deep expiration breath-hold, DIBH: Deep inspiration breath-hold, MU: Monitor units
Stereotactic body radiotherapy treatment
Equipment
Linear accelerator and treatment planning system.
A TrueBeam™ or Trilogy® (Varian Medical Systems, Palo Alto, CA, USA) linear accelerator was used. RMM procedures with a Real-time Position Management™ (RPM) system (Varian Medical System, Palo Alto, CA, USA) and Image-Guided RT (IGRT) were used to overcome positioning uncertainty. The Eclipse™ Treatment Planning System Version 13.6 (Varian Medical System, Palo Alto, CA, USA) was used for treatment planning.
Custom visual aids with verbal coaching.
The vendor RPM system can only show the respiratory wave on the monitor screen in the control room for the therapist and lacks a display screen in the treatment room. Thus, the patient can only be coached by verbal instructions. We this developed a system to involve the patient using AV biofeedback coaching.
AV biofeedback coaching is a technique which specifically aims to guide breathing by showing patients how to adjust their breathing in real-time through display of a patient-specific waveform and in addition to verbal instructions. Our AV biofeedback coaching system hardware device was adapted from that developed by Venkat et al. [14,15], using an optical head-mounted display or 11-inch screen to display the real-time respiratory waveform [Figure 1a and b]. In the treatment room, the custom monitor displays the breathing waveform to help the patient maintain reproducible deep breath-hold or gating wave, accordingly. In the control room, the therapist can see the same waveform to coach the patient verbally. Once the respiratory position exceeds the threshold on the RPM system, the TrueBeam™ or Trilogy® will beam off automatically.
Figure 1.
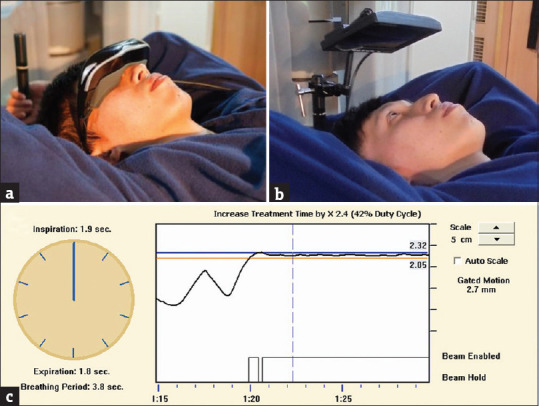
Custom audio-visual coaching accessories to allow patients to see their on-board respiratory wave. (a) An optical head-mounted display was used as a 1st generation device and (b) an 11-inch screen as the 2nd generation device to show the Real-time Position Management™ system's screen directly. (c) Display of the deep inspiration breath-hold respiratory curve on the screen (black line) allows patients to use biofeedback to hold their breath and keep their motion within a 2–3 mm preset threshold for 10–20 s
Respiratory motion management procedures
RMM procedures, including deep inspiration breath-hold (DIBH), deep expiration breath-hold (DEBH), respiratory gating technique, and abdominal compression with four-dimensional CT (4D-CT) encompassing internal target volume (ITV), were used to minimize the inter-fraction and intra-fraction error while the respiratory motion was ≥5 mm [12]. Furthermore, we developed a personalized goodness-of-fit protocol to guide each patient and select the suitable RMM method.
The protocol was as follows: we selected the suitable RMM by testing, in order: (1) DEBH, (2) DIBH, (3) gating with 4D-CT, and (4) abdominal compression with 4D-CT encompassing ITV. Breath-hold depended on the patient's ability to maintain at least 10 s of hold based on the patient's tolerance and endurance.
In general, we arranged a training appointment 1–2 days before CT simulation to first test whether the patient could exhale and then hold the breath for 10–30 s to tolerate DEBH [Figure 1c]. If not, then we tested DIBH. If the patient had difficulty in performing both breath-hold procedures, we then tested the respiratory gating and selected 5 of 10 respiratory cycle phases for treatment. The gating window was typically distributed on end-exhalation, as it is more reproducible. If the respiratory pattern was not regular, we would coach the patient to use shallow breathing and use abdominal compressor and the 4D-CT to get the ITV, which encompasses all the motion during the respiratory cycle. We also used AV biofeedback to allow the patient and therapist to monitor the respiratory curve in real-time and coordinate via simultaneous verbal coaching.
Contour and treatment course
SBRT was contoured on the breath-hold acquired CT or on the reconstructed average CT while using 4D-CT. The treatment was delivered in 5–6 fractions on every other day within 2 weeks, and prescription doses ranged 40–54 Gy (median, 50 Gy in 5 fractions) depending on tumor size, location, and normal tissue constraints. IGRT with cone beam CT before each fraction was mandatory. No expansion was done from Gross Target Volume (GTV) to Clinical Target Volume (CTV). Margins to generate the Plan Target Volume (PTV) from CTV were 5–10 mm according to RMM technique and finding.
Dose constraints, treatment plan and quality assurance
The prescribed isodose encompassed 95% of PTV and 100% of CTV. Non-coplanar volumetric-modulated arc therapy (VMAT) is frequently used to achieve rapid dose fall off outside the PTV. Besides the routine monthly and yearly QA, multileaf collimator QA is also performed monthly. Plan QA was mandatory for all patients and a dry run of gantry, collimator and couch was performed using noncoplanar VMAT with RMM. Critical organ constraints were done according to the Radiation Therapy Oncology Group (RTOG) 1112 protocol [18] and the central hepatobiliary tract limit was <37 cc with V26 and <45 cc with V21 [19].
Statistics and data analysis
FFLP was defined as no progressive or recurrent disease within or at the margin of the SBRT treatment field according to the modified Response Evaluation Criteria In Solid Tumors criteria [20]. We used SPSS (version 17, SPSS Inc., Chicago, IL, USA) to analyze data. Demographic data were analyzed using the Chi-square test and Wilcoxon rank sum test. FFLP and OS were estimated using the Kaplan-Meier method. The log-rank test was applied to assess the curve difference between groups. Multivariate analysis was performed using Cox proportional hazard regression. Hazard ratio with a 95% confidence interval was provided to demarcate the effective size in conjunction with P values. A P < 0.05 was considered to indicate statistical significance.
RESULTS
Patient characteristics
The study included 29 HCC patients. The median age of the patients was 69.6 ± 12.7 (range 49–92) years and 62.1% were male. In all, 96.6% of patients had cirrhosis, 37.9% had Hepatitis B, and 51.7% had Hepatitis C. Most (86.2%) patients were Child-Pugh classification (Child-Pugh) A, and 13.8% were Child-Pugh B.
Most patients were classed at early-to-intermediate BCLC stage: 3.4% were at stage 0, 48.3% were at BCLC stage A and 48.3% were at BCLC stage B. Fully 93.1% of patients were previously treated with invasive locoregional methods with a median 2 (range 0–7) prior liver-directed treatments such as liver resection, RFA, transcatheter arterial chemoembolization (TACE), or TACE with drug-eluting beads. Only in 6.9% of patients had SBRT as the first treatment modality [Table 1].
Radiotherapy treatment parameters
The median number of treated lesions was 2 (range 1–4) and the mean tumor volume was 30.5 ± 38.0 (range 2.4–165.1) cm3. The mean treated tumor size was 32.7 ± 16 mm in maximum diameter (range 11–94 mm); 44.8% of treated tumors were <20 mm and 55.2% were ≥20 mm.
Twenty-seven patients used the 11-inch screen and only 2 patients used video goggles due to the smaller size of the display screen when using the goggles. There were 13 breath-hold (10 DEBH, 3 DIBH), 10 respiratory gating, and 6 abdominal compression with 4D-CT encompassing ITV patients.
The median prescribed dose to the tumor was 50 (range 40–54) Gy. The mean dose to the uninvolved liver (whole liver-GTV) was 8.07 ± 2.93 (range 1.37–14.92) Gy and the mean uninvolved liver volume was 1034.2 ± 181.5 (range 653.0–1361.5) cm3. Coplanar with noncoplanar beams were used in 27 patients and coplanar only in 2 patients. The mean number of coplanar fields was 1.8 ± 0.5 (range 1–3) and the mean number of noncoplanar fields is 1.9 ± 0.7 (range 0–3). The mean RT beam on time was 2.8 ± 1.1 (range 1.2–6.3) minutes and the mean MU delivered was 2694.5 ± 599.3 (range 1852.5–4202.8).
Local control and survival
The mean follow-up time after RT was 21.6 (range 7–46) months. The 1-, 2-, and 3-year FFLP rates of SBRT were 96.6%, 96.6%, and 96.6%, respectively [Figure 2]. The 1-, 2-, and 3-year OS rates were 81.5%, 72.4%, and 67.2%, respectively [Figure 3].
Figure 2.
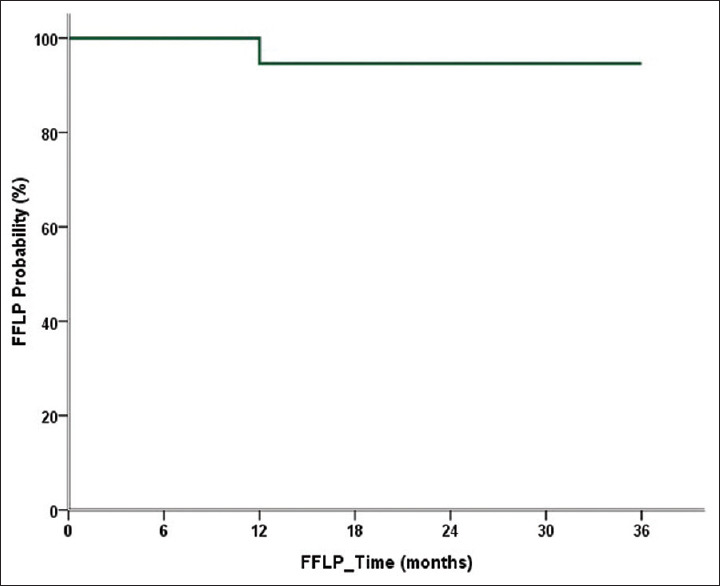
Freedom from local progression of stereotactic body radiotherapy by time. The 1-, 2-, and 3-year rates of Freedom from local progression were all 96.6%
Figure 3.
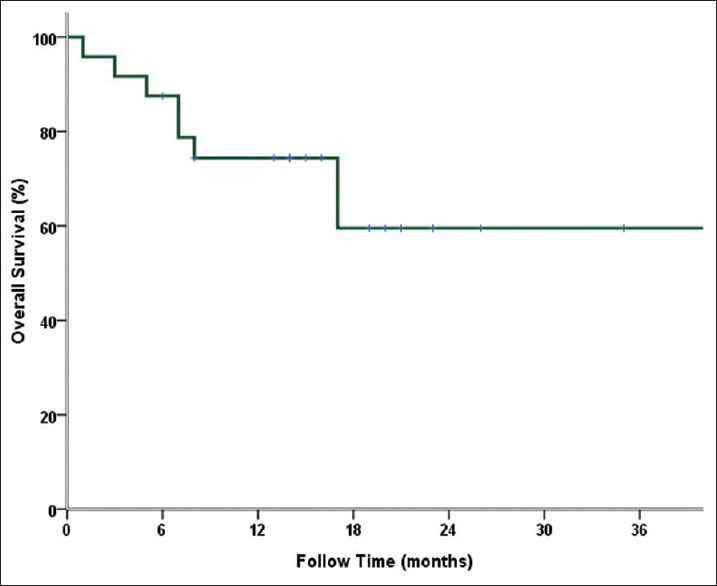
Overall survival of stereotactic body radiotherapy by time. The 1-, 2-, and 3-year OS rates were 81.5%, 72.4%, and 67.2%, respectively
Univariate analysis was performed on the parameters likely to influence treatment efficacy, as shown in Table 2. Univariate analysis showed that tumor size and volume were significant related to 2-year FFLP. The 2-year FFLP rates were 100% in tumors <45 mm and 60% in tumors ≥45 mm (P = 0.001). The 2-year rates of FFLP were 100% in GTV <30 cc and 75% in GTV ≥30 cc (P = 0.02). The 2-year OS of the patients who received <2 other prior local liver treatments was 51.9%; it was 85.1% if the number was ≥2 (P = 0.046). The difference in OS by number of prior local liver treatments might be the result of small sample size. The 2-year OS for those using breath-hold, respiratory gating, and abdominal compression were 100%, 45.7% and 66.7%, respectively (P = 0.14). The 2-year FFLP for those using breath-hold, respiratory gating, and abdominal compression were 43.1%, 28.0%, and 0%, respectively (P = 0.28). The sample size was insufficient to reveal any difference in results by RMM method.
Table 2.
Univariate analysis of outcomes in the study population
| 2 years OS (%) | P | 2 years FFLP (%) | P | |
|---|---|---|---|---|
| Age (years) | ||||
| <65 | 90.9 | 0.16 | 91.7 | 0.78 |
| ≥65 | 59.9 | 94.1 | ||
| Gender | ||||
| Male | 69.9 | 0.47 | 88.9 | 0.26 |
| Female | 77.9 | 100.0 | ||
| Tumor size (mm) | ||||
| <30 | 62.7 | 0.98 | 100.0 | 0.16 |
| ≥30 | 79.4 | 86.7 | ||
| <45 | 70.8 | 0.58 | 100.0 | 0.001 |
| ≥45 | 80.0 | 60.0 | ||
| GTV volume (cc) | ||||
| <30 | 70.8 | 0.31 | 100.0 | 0.02 |
| ≥30 | 56.3 | 75.0 | ||
| RMM methods | ||||
| Breath-hold (n=13) | 100.0 | 0.14 | 84.6 | 0.28 |
| Respiratory gating (n=10) | 45.7 | 100.0 | ||
| Compression (n=6) | 66.7 | 100.0 | ||
| Number of treated lesions | ||||
| <2 | 75.2 | 0.88 | 84.6 | 0.11 |
| ≥2 | 69.9 | 100.0 | ||
| Number of prior local liver treatments | ||||
| <2 | 51.9 | 0.046 | 100.0 | 0.26 |
| ≥2 | 85.1 | 88.9 | ||
| Clinical T category | ||||
| cT1 | 72.9 | 0.94 | 100.0 | 0.17 |
| cT2 | 75.0 | 94.4 | ||
| cT3 | 50.0 | 66.7 | ||
| AJCC stage | ||||
| I | 83.3 | 0.76 | 100.0 | 0.63 |
| II | 72.1 | 90.0 | ||
| III | 50.0 | 100.0 | ||
| BCLC stage | ||||
| 0 | 100.0 | 0.82 | 100.0 | 0.33 |
| A | 65.0 | 100.0 | ||
| B | 77.1 | 85.7 | ||
| Child-Pugh classification | ||||
| A | 81.0 | 0.08 | 100.0 | 0.14 |
| B | 25.0 | 85.7 | ||
| Hepatitis B | ||||
| Negative | 74.1 | 0.98 | 88.9 | 0.26 |
| Positive | 71.6 | 100.0 | ||
| Hepatitis C | ||||
| Negative | 77.1 | 0.76 | 85.7 | 0.14 |
| Positive | 69.3 | 100.0 |
RMM: Respiratory motion management, GTV: Gross tumor volume, AJCC: American Joint Committee on Cancer, BCLC: Barcelona clinic liver cancer, OS: Overall survival, FFLP: Freedom from local progression.
Toxicity
Acute toxicity
Twelve patients (41.4%) had no AEs while 14 patients (48.3%) developed grade 1–2 AE, mostly fatigue, anemia and decreased platelet count, according to the Common Terminology Criteria for Adverse Events v4.03. Three (10.3%) patients developed grade 3 AE including anemia, rising aminotransferase, alanine aminotransferase and total bilirubin and no grade 4–5 AEs occurred. Among the 3 patients who developed grade 3 AEs, two were Child-Pugh B before treatment and only one met the definition of non-classic radiation-induced liver disease.
Chronic toxicity
Three cases (10.3%) of treatment-related grade 2 and 1 case (3.4%) of grade 3 + hepatobiliary toxicity were observed.
DISCUSSION
Conventional RT was once thought to have only a salvage or palliative role in HCC treatment, mainly used in portal vein invasion, inferior vena cava/heart invasion or thrombosis, and distant metastasis [21,22,23,24,25]. Technological improvements across the whole RT workflow process have created state-of-the-art precision RT such as stereotactic radiosurgery or SBRT, which can deliver submillimeter precise doses while also increasing the therapeutic ratio, delivering a higher dose to the tumor while rapidly lowering the unwanted dose to normal tissue surrounding the target.
The advantages of SBRT include that it is both a non-invasive and well-tolerated procedure, not limited by age and only needing a short period of convalescence. Indeed, most SBRT can be completed within only 2 weeks compared to conventional RT, which take 4–6 weeks and produces a greater tumoricidal effect by requiring much larger biologically effective doses. It also produces more endothelial cell vascular damage and may even stimulate the release of tumor antigens, increasing antigen-presenting cells to activate the immune system and augment the systemic effect [26,27].
The ablative effect of SBRT also makes it a potential curative treatment option comparable to surgery and RFA, the current standard. Liver resection and liver transplantation provide the best curative treatments, with LC rates of 80%–100% in the first 2 years [4,21,28,29,30]. RFA also achieves excellent 1-year LC rates of 70%–97.6% for single small HCC ≤3 cm [5,31,32,33]. On the other hand, the 1-year LC of SBRT historically ranges 65%–100% [8,34,35]; this represents a potential third curative option for unresectable tumors or those inaccessible to RFA.
Our SBRT study showed good results, with a high 3-year FFLP rate of 96.6% compared to the historical data, despite a mean tumor size slightly larger than 3 cm. One reason for the satisfactory result may be that well personalized RMM procedures allowed high-precision treatment. Other possible reasons may be the more stringent dose constraints following the latest RTOG protocol and early deployment of more stringent QA procedures than required by current laws and regulations.
RMM is one of the most effective approaches to minimizing the uncertainty of patient motion caused by respiration, because the motion magnitude may be >20 mm [9,10,11]. Three individualized RMM methods were used in our protocol, in this order: (1) breath-hold, including DEBH and DIBH, (2) respiratory gating during specific 4D-CT amplitude or phase, and (3) abdominal compression with 4D-CT encompassing ITV. Although differences were not statistically significant, the 2-year FFLP for these techniques revealed that breath-hold and gating seemed slightly better than the compression modalities in our study.
Of these three RMM methods, compression with motion-encompassing by ITV is the simplest and most passive, and can be considered for all kinds of patients. However, it enlarges the RT field to cover all the possible positions and adds a margin to encompass ITV. Thus, this method typically irradiates a larger volume of the normal liver compared to other strategies [12].
Respiratory gating is used to irradiate the target volume only when it moves into specific respiratory phases or amplitudes within 4D-CT, significantly reducing the ITV margin [12,13]. Jeong et al. used respiratory-gated VMAT to deliver SBRT to HCC patients; the 3-year LC rate was 97.0% [36]. However, because the radiation beam is on only during a selected period (30%-50%) of the respiratory cycle, gating usually takes longer to deliver the same dose than other methods.
Breath holding allows treatment without the need of an ITV and therefore has great potential to reduce the artifacts caused by irregular breathing patterns [37,38]. Almost half of the SBRT patients in our study used breath-hold, which is preferred due to better CT simulation image quality, less uncertainty in image processing, high reproducibility, and also greater efficiency, because the dose is delivered continuously during the breath-hold period [39]. Mast et al. showed that using breath-hold in liver SBRT can reduce the margin >10 mm compared to free breathing [40]. Oliver et al. found that DIBH errors are generally larger than those with DEBH [38]. DEBH also produces better dose distribution than DIBH due to an increase in the abdominal cavity volume when the diaphragm is elevated during deep-exhale, with a slightly lower dose to the liver and other OAR. Thus, the order of RMM selection in our protocol was DEBH followed by DIBH.
To better facilitate patient involvement in the RMM procedure, we developed custom accessory devices for AV biofeedback guidance. The used of audio coaching and visual feedback combined into biofeedback guidance are proven to stabilize respiration better than free breathing or audio coaching alone [14,15].
The present study has several limitations, including a retrospective design, short follow-up duration and relatively small sample size, which may increase the likelihood of type II errors. Despite these limitations, our data confirmed that AV coaching, respiration modulated SBRT demonstrated noninferior tumor control to that of surgery and RFA. To further clarify the benefit, we are conducting a prospectively randomized trial to demarcate the clinical effectiveness of SBRT in managing localized HCC (ClinicalTrials.govIdentifier: NCT02921139).
CONCLUSION
AV Coaching, respiration-modulated SBRT is a promising noninvasive treatment option for patients with inoperable and localized HCC. Further randomized clinical trials should be considered to directly compare it to surgery in a head-to-head manner.
Financial support and sponsorship
Nil.
Conflicts of interest
There is no conflict of interest.
REFERENCES
- 1.Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. Global Burden of Disease Cancer Collaboration. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–27. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of hepatocellular carcinoma in the asia-pacific region. Gut Liver. 2016;10:332–9. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang CJ, Lo WC, Yang YW, You SL, Chen CJ, Lai MS. Incidence and survival of adult cancer patients in Taiwan, 2002-2012. J Formos Med Assoc. 2016;115:1076–88. doi: 10.1016/j.jfma.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: A 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502–9. doi: 10.1097/SLA.0b013e318148c704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int. 2017;11:317–70. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delis SG, Dervenis C. Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J Gastroenterol. 2008;14:3452–60. doi: 10.3748/wjg.14.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajyaguru DJ, Borgert AJ, Smith AL, Thomes RM, Conway PD, Halfdanarson TR, et al. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: Analysis of the national cancer database. J Clin Oncol. 2018;36:600–8. doi: 10.1200/JCO.2017.75.3228. [DOI] [PubMed] [Google Scholar]
- 8.Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34:452–9. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutz CM, Poulsen PR, Fledelius W, Offersen BV, Thomsen MS. Setup error and motion during deep inspiration breath-hold breast radiotherapy measured with continuous portal imaging. Acta Oncol. 2016;55:193–200. doi: 10.3109/0284186X.2015.1045625. [DOI] [PubMed] [Google Scholar]
- 10.Stevens CW, Munden RF, Forster KM, Kelly JF, Liao Z, Starkschall G, et al. Respiratory-driven lung tumor motion is independent of tumor size, tumor location, and pulmonary function. Int J Radiat Oncol Biol Phys. 2001;51:62–8. doi: 10.1016/s0360-3016(01)01621-2. [DOI] [PubMed] [Google Scholar]
- 11.Seppenwoolde Y, Shirato H, Kitamura K, Shimizu S, van Herk M, Lebesque JV, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53:822–34. doi: 10.1016/s0360-3016(02)02803-1. [DOI] [PubMed] [Google Scholar]
- 12.Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys. 2006;33:3874–900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- 13.Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37:4078–101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 14.Pollock S, O’Brien R, Makhija K, Hegi-Johnson F, Ludbrook J, Rezo A, et al. Audiovisual biofeedback breathing guidance for lung cancer patients receiving radiotherapy: A multi-institutional phase II randomised clinical trial. BMC Cancer. 2015;15:526. doi: 10.1186/s12885-015-1483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkat RB, Sawant A, Suh Y, George R, Keall PJ. Development and preliminary evaluation of a prototype audiovisual biofeedback device incorporating a patient-specific guiding waveform. Phys Med Biol. 2008;53:N197–208. doi: 10.1088/0031-9155/53/11/N01. [DOI] [PubMed] [Google Scholar]
- 16.Yeh PH, Hung SK, Lee MS, Chiou WY, Lai CL, Tsai WT, et al. Implementing web-based ping-pong-type e-communication to enhance staff satisfaction, multidisciplinary cooperation, and clinical effectiveness: A SQUIRE-compliant quality-improving study. Medicine (Baltimore) 2016;95:e5236. doi: 10.1097/MD.0000000000005236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin YH, Hung SK, Lee MS, Chiou WY, Lai CL, Shih YT, et al. Enhancing clinical effectiveness of pre-radiotherapy workflow by using multidisciplinary-cooperating e-control and e-alerts: A SQUIRE-compliant quality-improving study. Medicine (Baltimore) 2017;96:e7185. doi: 10.1097/MD.0000000000007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang B, Tan Y, Sun H, Fan J, Tang Z, Ji Y. Higher intratumor than peritumor expression of DUSP6/MKP-3 is associated with recurrence after curative resection of hepatocellular carcinoma. Chin Med J (Engl) 2014;127:1211–7. [PubMed] [Google Scholar]
- 19.Toesca DA, Osmundson EC, Eyben RV, Shaffer JL, Lu P, Koong AC, et al. Central liver toxicity after SBRT: An expanded analysis and predictive nomogram. Radiother Oncol. 2017;122:130–6. doi: 10.1016/j.radonc.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 21.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–74. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 23.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68:723–50. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 24.Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109–13. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
- 25.Korean Liver Cancer A, National Cancer C. 2018 Korean liver cancer association-national cancer center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019;13:227–99. doi: 10.5009/gnl19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macià I, Garau M. Radiobiology of stereotactic body radiation therapy (SBRT) Rep Pract Oncol Radiother. 2017;22:86–95. doi: 10.1016/j.rpor.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88:254–62. doi: 10.1016/j.ijrobp.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: Patterns and prognosis. Liver Transpl. 2004;10:534–40. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 29.de’Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J Gastroenterol. 2015;21:11185–98. doi: 10.3748/wjg.v21.i39.11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sapisochin G, Goldaracena N, Astete S, Laurence JM, Davidson D, Rafael E, et al. Benefit of treating hepatocellular carcinoma recurrence after liver transplantation and analysis of prognostic factors for survival in a large euro-american series. Ann Surg Oncol. 2015;22:2286–94. doi: 10.1245/s10434-014-4273-6. [DOI] [PubMed] [Google Scholar]
- 31.Bae SH, Park HC. Local modalities for inoperable hepatocellular carcinoma: Radiofrequency ablation versus stereotactic body radiotherapy. Ann Transl Med. 2018;6:S3. doi: 10.21037/atm.2018.08.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–6. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata T, Shibata T, Maetani Y, Isoda H, Hiraoka M. Radiofrequency ablation for small hepatocellular carcinoma: Prospective comparison of internally cooled electrode and expandable electrode. Radiology. 2006;238:346–53. doi: 10.1148/radiol.2381041848. [DOI] [PubMed] [Google Scholar]
- 34.Gerum S, Jensen AD, Roeder F. Stereotactic body radiation therapy in patients with hepatocellular carcinoma: A mini-review. World J Gastrointest Oncol. 2019;11:367–76. doi: 10.4251/wjgo.v11.i5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaub SK, Hartvigson PE, Lock MI, Høyer M, Brunner TB, Cardenes HR, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: Current trends and controversies. Technol Cancer Res Treat. 2018;17:1533033818790217. doi: 10.1177/1533033818790217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeong Y, Jung J, Cho B, Kwak J, Jeong C, Kim JH, et al. Stereotactic body radiation therapy using a respiratory-gated volumetric-modulated arc therapy technique for small hepatocellular carcinoma. BMC Cancer. 2018;18:416. doi: 10.1186/s12885-018-4340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Yamamoto T, Pollock S, Berger J, Diehn M, Graves EE, et al. The impact of audiovisual biofeedback on 4D functional and anatomic imaging: Results of a lung cancer pilot study. Radiother Oncol. 2016;120:267–72. doi: 10.1016/j.radonc.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Oliver PA, Yewondwossen M, Summers C, Shaw C, Cwajna S, Syme A. Influence of intra- and interfraction motion on planning target volume margin in liver stereotactic body radiation therapy using breath hold. Adv Radiat Oncol. 2021;6:100610. doi: 10.1016/j.adro.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbas H, Chang B, Chen ZJ. Motion management in gastrointestinal cancers. J Gastrointest Oncol. 2014;5:223–35. doi: 10.3978/j.issn.2078-6891.2014.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mast M, Kouwenhoven E, Roos J, van Geen S, van Egmond J, van Santvoort J, et al. Two years’ experience with inspiration breath-hold in liver SBRT. Tech Innov Patient Support Radiat Oncol. 2018;7:1–5. doi: 10.1016/j.tipsro.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


