Abstract
Indocyanine green (ICG), a US Food and Drug Administration-approved fluorescent compound, has been on the medical stage for more than 60 years. Current uses include hepatic function evaluation before surgical procedure and fundus evaluation. The large safety margin and near-infrared fluorescent optical advantage of the drug have proved useful in several clinical trials of intraoperative systems for tumor removal. Several nanoparticle-sized formulations for thermal ablation and photodynamic therapy have also been evaluated in animal experiments. Studies have attempted to manipulate ICG as a reporter fluorophore with initial success. In this article, we reviewed ICG's histological applications, chemical and physical properties, current clinical applications, ongoing clinical trials, and biomedical studies and prospects. We believe that ICG could be used with novel biotechnological techniques, such as fluorescent endoscopy and photoacoustic equipment, in a range of biomedical fields.
KEYWORDS: Fluorescent dye, Indocyanine green, Near-infrared, Optical Imaging
INTRODUCTION
With the rising cost of new drug development and the growing concerns of the new drug's adverse reactions, interest has been focusing on the medicines that have been in the pharmaceutical markets for decades. Aspirin is one of the most well-known examples. People use aspirin since antiquity for relieving pain and fever. However, its application in cancer treatment and cardiovascular prevention is still ongoing [1,2,3]. Indocyanine green (ICG) is another less-known drug that stands in the medical field for 60 years. The optical fluorescence characteristic of ICG made its first launch in the medical field. As the imaging technique progresses into emerges. ICG along with novel imaging technique has been applied in diagnostic medical imaging, surgical procedure guidance, and new cancer therapy strategy. This review discusses the physical, chemical, and biological characteristics of ICG and its current usage in the clinical field, ongoing trials, and the potential applications proven in animal studies.
HISTORY OF INDOCYANINE GREEN IN BIOMEDICINE
ICG was developed by Kodak during World War II for color imaging purposes. The medical applications of ICG were approved by the United States Food and Drug Administration in 1959. In 1960, Fox reported the characteristics of ICG and the results of use in the Mayo Clinics [4]. ICG was originally, and is still, used to determine liver function. In 1963, Walker applied ICG to determine renal blood flow, owing to its fluorescent characteristics. In 1965, Huffman investigated its applicability in detecting cardiac murmurs [5,6]. ICG has since been applied to evaluate physiological brain perfusion [7]. Several perfusion and angiographic applications have been implemented with the advancement of fluorescence imaging.
BIOCHEMICAL AND PHYSICAL PROPERTIES OF INDOCYANINE GREEN
Chemical and optical properties
ICG is a closed-chain, water-soluble cyanine dye with two benzoyl indole moieties [Figure 1]. The molecular weight of ICG is 774.96. Sodium iodide is added to ICG to increase its solubility, which imparts ICG a dark brown color [Figure 2a]. The ICG solution becomes green when diluted with water [Figure 2b]. The excitation wavelength of ICG is 600–900 nm with a maximum wavelength of 875 nm in the blood, whereas the maximum emission wavelength is 830 nm, and emission wavelength range of 750–950 nm [8,9]. The excitation and emission wavelengths are located in the near-infrared (NIR) range, which has the highest tissue penetration depth. ICG is thus an ideal molecular imaging signal source that could be used in various in vivo animal models [10].
Figure 1.
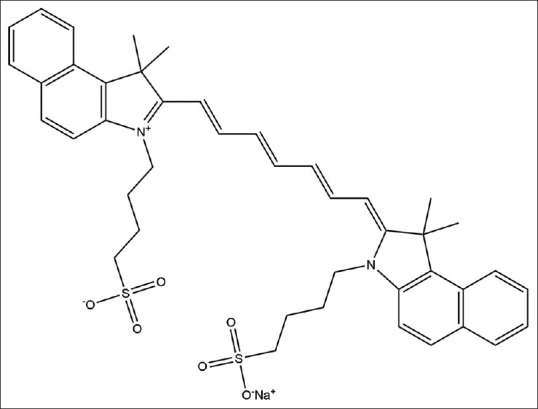
Chemical structure of indocyanine green
Figure 2.
(a) Indocyanine green in the original constitution with the addition of sodium iodide, displaying a dark brown color. (b) Indocyanine green diluted with phosphate-buffered saline (1 mg/mL) displaying a green color
Pharmacokinetics
ICG is not absorbed from the gastrointestinal tract. Intravenous injection of ICG is rapidly conjugated by plasma protein (mostly serum albumin) and eliminated by the liver through secretion into the biliary tree in the unconjugated form [11]. The half-life of ICG is 120–240 s [12]. ICG is a high safety drug with an LD50 of 80 mg/kg according to an animal study (https://www.drugbank.ca/drugs/DB09374).
Cellular uptake of indocyanine green
The uptake of ICG occurs in the hepatocytes. Various transporters carry ICG into the cytoplasm [13,14]. Organic anion transporting polypeptide1B3 (OATP1B3) and Na+-taurocholate co-transporting polypeptide (NTCP) are two of the principal transporters responsible for the metabolism of ICG in hepatocytes [14]. Transfecting various cancer cell lines using OATP1B3 or NTCP further verified these findings [15,16,17]. Although ICG was transported through transporters similar to bilirubin, findings indicated that intestinal bilirubin transporters are not responsible for the transportation of ICG [18].
CLINICAL PRACTICE OF INDOCYANINE GREEN
Retinal evaluation
Fluorescein angiography and ICG angiography are used for retinal evaluation. However, ICG offers a more precise view of choroidal circulation. ICG is a more favorable fluorescent contrast medium than other light-emitting fluorescent dyes because of its infrared fluorescent characteristics. Infrared fluorescent light passes more readily through pigment, turbid fluid, lipid, and hemorrhages, which is common in the optical tract of the eyeball. ICG angiography is favorable for detecting choroidal neovascularization in exudative age-related macular degeneration compared with fluorescein angiography [19,20]. ICG angiography is also an adjunctive tool for differentiating retinal or choroidal tumors [21] and has been applied to aid in diagnosing choroidal inflammatory disorders [22]. Moreover, ICG has been used to detect various pathologic conditions, including vascularized retinal pigment epithelium (RPE) detachment, polypoidal choroidal vasculopathy, traumatic choroidal rupture, diabetic maculopathy, central serous retinopathy, and macular drusen [23]. Although ICG angiography can cause phototoxicity in human RPE cells, which is caused by oxidative damage under continuous ambient light [24], the adverse effects of ICG angiography are very low, and thus ICG is considered a very safe ophthalmic examination [25].
Liver function test
The liver is the largest solid internal organ in the human body. Therefore, major liver surgery remains a high-risk procedure. The hepatic functional reserve of the liver is immediately reduced following injury (e.g., hepatectomy). ICG is an excellent, noninvasive, quantitative, dynamic assessment tool for evaluating liver function among patients in the intensive care unit or during major liver surgery [26]. The plasma disappearance rate (PDR) and fractional retention at 15 min (R15) are the two parameters used to measure ICG clearance during perioperative liver function monitoring [27].
Most patients requiring liver resection surgery have underlying liver disease. Preoperative assessment of liver function is crucial in selecting candidates for safe hepatic resection to prevent postoperative hepatic failure and death after hepatectomy [28]. Makuuchi et al. reported that ICG R15 plays a determining role in hepatic resection outcomes among patients with small hepatocellular carcinomas [29]. Including ICG R15 as an indicator has improved the operative safety of cirrhotic liver resection [30]. Schwarz et al. determined that ICG is a prognostic factor in both major and minor liver surgeries and proposed that the optimal cutoff value to accurately predict liver dysfunction was PDR <19.5%/min and R15 >5.6% for preoperative ICG clearance test, based on a study in a Western population [31]. ICG R15 is now routinely used for preoperative liver function assessments in numerous countries, including Taiwan [32].
ICG also has various valuable intraoperative uses. Intraoperative measurements of the ICG clearance rate are useful to estimate patients’ future liver remnant function during hepatectomy, which helps to determine the preservation of a sufficient amount of liver parenchyma and prevents postoperative liver failure [33]. Furthermore, fluorescence imaging using ICG is promising for intraoperative monitoring during liver surgery [34], and it has been used in liver tumor localization during surgery [35]. ICG fluorescence imaging also helps to identify additional small surface liver tumors during liver resection for cancer [36]. Kobayashi et al. reported five types of ICG fluorescence staining techniques to identify portal vein (PV) territory identification during liver resection, which included transhepatic PV injection and intravenous injection of ICG. These techniques may help to accurately identify the anatomic location during surgery [36]. An increasing number of intraoperative ICG uses during liver surgery are under investigation and require validation in future research studies.
APPLICATIONS UNDER CLINICAL TRIALS
Indocyanine green application in general abdominal surgery
The high tissue penetrance, low tissue absorption, and high visibility of critical but small vasculature using ICG have garnered attention in the surgical field [37]. Over 100 clinical trials registered in the USA are currently verifying the feasibility of ICG-assisted imaging guidance for the surgical removal of tumors and metastatic lymph nodes [38,39,40]. ICG metabolization into the bile duct enables visualization of the bile duct during cholecystectomy. Moreover, bile duct injuries are the most common complication during cholecystectomies. More than 4% of laparoscopic cholecystectomy for acute cholecystitis cause varying degrees of bile duct injury [41]. Consequently, ICG exhibits an operational decisional role by identifying cholecystic ducts during laparoscopic cholecystectomies [42] and can aid in determining the adequate perfusion for colectomy by intravenous injection of ICG [43]. The proximal transection lines of 112 colorectal cancer patients were evaluated. The intravenous injection of ICG successfully demonstrated that the appropriate transection line prevents anastomotic surgery.
Indocyanine green in fluorescence-guided chest surgery
Video-assisted thoracoscopic surgery (VATS) has become the mainstream thoracic surgery. However, identification of vasculature during operation is sometimes difficult because of the limited field of view. Furthermore, observation of the vasculature may be hindered by other tissue or organs. However, the vasculature could be visualized clearly using ICG injection, even when blocked by lung tissue, in which air space contributes to most of the lung volume. Ligation of the segmental artery and injection of ICG into the peripheral systemic vein during VATS segmentectomy facilitates the identification of segmental lines [44]. Furthermore, ICG has been used to determine the perfusion status of the anastomosis during esophagectomy and gastric tube reconstruction [45]. Lymphatic drainage is difficult to visualize in a standard light source; however, with the assistance of fluorescent thoracoscopy, ICG delivered subcutaneously and eventually drained into the thoracic duct can be identified clearly. This new technique could thus facilitate repair of thoracic duct leakage [46].
An increasing number of trivial occult lung cancers have been diagnosed as the use of computed tomography in early lung cancer detection increases. Certain small tumors are difficult to identify through palpation during surgical removal. ICG has been used to visualize these tumors. ICG is delivered intravenously 24 h before the surgical procedure. Notably, some occult tumors that cannot be recognized by other examinations or palpation were detected after ICG delivery. These occult tumors were later demonstrated to be malignant in most cases [47,48]. The authors also reported that the detection depth was 1.3 cm, and the smallest diameter of the nodule was 0.2 cm [47]. No conclusions regarding the mechanism of ICG accumulation in these malignant tumors have been drawn. However, the enhanced permeability and retention effect is believed to be the most likely underlying mechanism [49].
Sentinel lymph node dissection
Sentinel lymph node dissection is the standard procedure for breast cancer surgery. The identification of sentinel lymph nodes is usually performed using blue dye or radioisotope methods. ICG has been proposed for sentinel lymph node dissection, and clinical trials have been conducted [50,51]. A prospective study reported that up to 89% of sentinel lymph nodes were detected. The authors determined that the limiting factor compared with the radioisotope method, which could reach 96% sensitivity, was a body mass index >40. ICG has also been used for melanoma sentinel lymph node biopsy in 14 cases with the administration of both radioisotope and ICG. The sentinel lymph node detection results are comparable [52]. Despite the lower sensitivity of ICG compared with that of the radioisotope method, ICG is less expensive, has real-time imaging capability, and has no severe adverse effect. Therefore, ICG remains an avenue for sentinel lymph node dissection but requires the development of more sensitive fluorescent detection. The sentinel lymph node detection results are comparable [52].
TRANSLATIONAL AND EXPERIMENTAL INVESTIGATIONS OF INDOCYANINE GREEN
Photodynamic therapy
ICG is also a photosensitizer (a compound that can transfer optical energy into reactive oxygen species), which may be used to eradicate tumors. Several in vitro and in vivo studies on the hepatoma cell line Huh-7 have verified the efficacy of ICG at a dose of 5 mg/kg [53]. The ICG was also encapsulated into nanoparticles, such as liposomes and lactosomes. The photodynamic therapy efficacy of the liposomes and lactosomes was verified on gall bladder cancer cell lines and in vivo mouse xenografts [54].
Photothermal therapy
The thermal energy of temperatures above 42.5°C can destroy tissue, enabling tumor ablation [55]. The photothermal effects of ICG have been investigated in several cell lines, including breast cancer 4T1 cells and animal xenograft models [56]. A study treated 4T1 tumor-bearing mice with NIR LASER at an intensity of 1 W/cm2 and ICG at a concentration of 0.1 mg/mL (total quantity of ICG was 200 μL). Intratumoral injections were administered following LASER ablation. Significant tumor necrosis and lymphocytes infiltrate were observed with histochemical observation.
APPLICATIONS IN BIOMEDICAL RESEARCH
Indocyanine green as a reporter probe
ICG is metabolized in the liver where varieties of cell membrane transporters are involved in ICG transportation. These transporters are ideal reporter systems that could be used for in vivo molecular imaging such as transporter–ICG combination. The most widely investigated ICG-related reporter protein is OATP1B3, which can carry both ICG and gadoxetate disodium (Gd-EOB-DTPA), a magnetic resonance imaging contrast agent. Research on the feasibility of ICG-OATP1B3 has been conducted, and the results demonstrated that OATP1B3 has dual imaging capability [17]. NTCP is another protein responsible for transporting ICG into the cytoplasm. The efficacy of the ICG-NTCP combination reporter system has also been demonstrated both in vitro and in vivo [16].
Indocyanine green as an imaging indicator for drug screening
NTCP is the portal for hepatitis B virus (HBV) and hepatitis D virus (HDV) infections into the hepatocyte. Blocking the NTCP with an inhibitor is a prevention strategy for HBV infection. NTCP is a portal for both ICG and HBV into the cytoplasm. High-throughput screening for inhibitors could be performed by identifying the intracellular intensity of ICG, which would indicate the ideal inhibitors for HBV/HDV infection prevention. This concept has been verified in cellular and animal studies [16].
Apical sodium-dependent bile salt transporters (ASBTs), which are responsible for nonalcoholic fatty liver disease, have also been investigated [57]. ICG is transported by ASBTs and could thus be used as an indicator for in vitro drug screening. A conceptual experiment has been established [58].
Photoacoustic imaging using indocyanine green
Photoacoustic imaging is an emerging technology that uses laser targeting on chromophores to emit sound, which is collected for imaging [59]. Endogenous chromophores, such as melanin or ICG, are candidates for photoacoustic imaging. ICG has advantages over other chromophores because of its NIR optical characteristics which are associated with higher tissue depth detection. These conceptual imaging systems have been verified through in vivo lung cancer allograft detection in mice. The efficacy of ICG in tumor vascular leakage photoacoustic imaging has been investigated [60].
CONCLUSION
Because ICG was unveiled in the clinical field, it has displayed superior optical characteristics that have not been surpassed by new technologies. The short half-life of <5 min, high safety dosage, and long-term historical usage of ICG have increased its applicability in numerous new clinical fields, such as photodynamic therapy, photoacoustic imaging, and reporter systems. ICG has the potential to be applied for personalized and precise cancer treatments.
Financial support and sponsorship
This research was funded by the Buddhist Tzu Chi Medical Foundation (TCRD-TPE-106-34).
Conflicts of interest
Dr. Jong-Kai Hsiao, an editorial board member at Tzu Chi Medical Journal, had no role in the peer review process of or decision to publish this article. The other author declared no conflict of interest in writing this paper.
Acknowledgments
We thank I-Jui Hsu for his drawing of the chemical structure of ICG.
REFERENCES
- 1.Yeomans ND. Aspirin: Old drug, new uses and challenges. J Gastroenterol Hepatol. 2011;26:426–31. doi: 10.1111/j.1440-1746.2010.06569.x. [DOI] [PubMed] [Google Scholar]
- 2.FitzGerald GA, Mayo G, Price P, Takahara K. Aspirin in cardiovascular disease; biochemical pharmacology and clinical trials. Prog Clin Biol Res. 1989;301:97–106. [PubMed] [Google Scholar]
- 3.Rothwell PM, Cook NR, Gaziano JM, Price JF, Belch JF, Roncaglioni MC, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: Analysis of individual patient data from randomised trials. Lancet. 2018;392:387–99. doi: 10.1016/S0140-6736(18)31133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox IJ, Wood EH. Indocyanine green: Physical and physiologic properties. Proc Staff Meet Mayo Clin. 1960;35:732–44. [PubMed] [Google Scholar]
- 5.Walker JG, Silva H, Lawson TR, Ryder JA, Shaldon S. Renal blood flow in acute renal failure measured by renal arterial infusion of indocyanine green. Proc Soc Exp Biol Med. 1963;112:932–5. doi: 10.3181/00379727-112-28214. [DOI] [PubMed] [Google Scholar]
- 6.Huffman TA, Goodwin RS, Leighton RF, Ryan JM, Wooley CF. Intracardiac phonocardiography in the differential diagnosis of continuous murmurs. Ann Intern Med. 1965;63:904–5. [Google Scholar]
- 7.Kogure K, Choromokos E. Infrared absorption angiography. J Appl Physiol. 1969;26:154–7. doi: 10.1152/jappl.1969.26.1.154. [DOI] [PubMed] [Google Scholar]
- 8.Benson RC, Kues HA. Fluorescence properties of indocyanine green as related to angiography. Phys Med Biol. 1978;23:159–63. doi: 10.1088/0031-9155/23/1/017. [DOI] [PubMed] [Google Scholar]
- 9.Landsman ML, Kwant G, Mook GA, Zijlstra WG. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J Appl Physiol. 1976;40:575–83. doi: 10.1152/jappl.1976.40.4.575. [DOI] [PubMed] [Google Scholar]
- 10.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–8. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 11.Cherrick GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green: Observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollins B, Noe B, Henderson JM. Fluorometric determination of indocyanine green in plasma. Clin Chem. 1987;33:765–8. [PubMed] [Google Scholar]
- 13.Cusin F, Fernandes Azevedo L, Bonnaventure P, Desmeules J, Daali Y, Pastor CM. Hepatocyte Concentrations of Indocyanine Green Reflect Transfer Rates Across Membrane Transporters. Basic Clin Pharmacol Toxicol. 2017;120:171–8. doi: 10.1111/bcpt.12671. [DOI] [PubMed] [Google Scholar]
- 14.de Graaf W, Häusler S, Heger M, van Ginhoven TM, van Cappellen G, Bennink RJ, et al. Transporters involved in the hepatic uptake of (99m) Tc-mebrofenin and indocyanine green. J Hepatol. 2011;54:738–45. doi: 10.1016/j.jhep.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 15.Wu MR, Huang YY, Hsiao JK. Use of Indocyanine Green (ICG), a Medical Near Infrared Dye, for Enhanced Fluorescent Imaging-Comparison of Organic Anion Transporting Polypeptide 1B3 (OATP1B3) and Sodium-Taurocholate Cotransporting Polypeptide (NTCP) Reporter Genes. Molecules. 2019;24:2295. doi: 10.3390/molecules24122295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu MR, Huang YY, Hsiao JK. Role of sodium taurocholate cotransporting polypeptide as a new reporter and drug-screening platform: Implications for preventing hepatitis B virus infections. Mol Imaging Biol. 2020;22:313–23. doi: 10.1007/s11307-019-01373-y. [DOI] [PubMed] [Google Scholar]
- 17.Wu MR, Liu HM, Lu CW, Shen WH, Lin IJ, Liao LW, et al. Organic anion-transporting polypeptide 1B3 as a dual reporter gene for fluorescence and magnetic resonance imaging. FASEB J. 2018;32:1705–15. doi: 10.1096/fj.201700767R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagawa T, Endo N, Ebisu G, Yamaoka I. Fecal imaging demonstrates that low-methoxyl pectin supplementation normalizes gastro-intestinal transit in mice given a liquid diet. Physiol Rep. 2018;6:e13662. doi: 10.14814/phy2.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonekawa Y, Miller JW, Kim IK. Age-related macular degeneration: Advances in management and diagnosis. J Clin Med. 2015;4:343–59. doi: 10.3390/jcm4020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Lee DE, Park YJ. ICG-enhanced digital angiography and photocoagulation of choroidal neovascularization in age-related macular degeneration. Korean J Ophthalmol. 1995;9:59–65. doi: 10.3341/kjo.1995.9.1.59. [DOI] [PubMed] [Google Scholar]
- 21.Callaway NF, Mruthyunjaya P. Widefield imaging of retinal and choroidal tumors. Int J Retina Vitreous. 2019;5:49. doi: 10.1186/s40942-019-0196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agrawal RV, Biswas J, Gunasekaran D. Indocyanine green angiography in posterior uveitis. Indian J Ophthalmol. 2013;61:148–59. doi: 10.4103/0301-4738.112159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen RB, Hathaway M, Rogers J, Pedro J, Garcia P, Dobre GM, et al. Simultaneous OCT/SLO/ICG imaging. Invest Ophthalmol Vis Sci. 2009;50:851–60. doi: 10.1167/iovs.08-1855. [DOI] [PubMed] [Google Scholar]
- 24.Sato T, Karasawa Y, Ishikawa S, Taguchi M, Muraoka T, Ito M, et al. Potential phototoxicity of indocyanine green in retinal pigment epithelial cells after angiography under ambient illumination. Oxid Med Cell Longev 2018. 2018 doi: 10.1155/2018/6065285. 6065285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hope-Ross M, Yannuzzi LA, Gragoudas ES, Guyer DR, Slakter JS, Sorenson JA, et al. Adverse reactions due to indocyanine green. Ophthalmology. 1994;101:529–33. doi: 10.1016/s0161-6420(94)31303-0. [DOI] [PubMed] [Google Scholar]
- 26.De Gasperi A, Mazza E, Prosperi M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J Hepatol. 2016;8:355–67. doi: 10.4254/wjh.v8.i7.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tralhao JG, Hoti E, Oliveiros B, Botelho MF, Sousa FC. Study of perioperative liver function by dynamic monitoring of ICG-clearance. Hepatogastroenterology. 2012;59:1179–83. doi: 10.5754/hge09726. [DOI] [PubMed] [Google Scholar]
- 28.Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39:107–16. doi: 10.1111/j.1872-034X.2008.00441.x. [DOI] [PubMed] [Google Scholar]
- 29.Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304. doi: 10.1002/ssu.2980090404. [DOI] [PubMed] [Google Scholar]
- 30.Wu CC, Yeh DC, Lin MC, Liu TJ, P’Eng FK. Improving operative safety for cirrhotic liver resection. Br J Surg. 2001;88:210–5. doi: 10.1046/j.1365-2168.2001.01653.x. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz C, Plass I, Fitschek F, Punzengruber A, Mittlböck M, Kampf S, et al. The value of indocyanine green clearance assessment to predict postoperative liver dysfunction in patients undergoing liver resection. Sci Rep. 2019;9:8421. doi: 10.1038/s41598-019-44815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu CC. Progress of liver resection for hepatocellular carcinoma in Taiwan. Jpn J Clin Oncol. 2017;47:375–80. doi: 10.1093/jjco/hyx007. [DOI] [PubMed] [Google Scholar]
- 33.Lau L, Christophi C, Nikfarjam M, Starkey G, Goodwin M, Weinberg L, et al. Assessment of liver remnant using ICG clearance intraoperatively during vascular exclusion: Early experience with the ALIIVE technique. HPB Surg 2015. 2015 doi: 10.1155/2015/757052. 757052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr. 2016;5:322–8. doi: 10.21037/hbsn.2015.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim HJ, Chiow AK, Lee LS, Tan SS, Goh BK, Koh YX, et al. Novel method of intraoperative liver tumour localisation with indocyanine green and near-infrared imaging. Singapore Med J. 2021;62:182–9. doi: 10.11622/smedj.2019137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi Y, Kawaguchi Y, Kobayashi K, Mori K, Arita J, Sakamoto Y, et al. Portal vein territory identification using indocyanine green fluorescence imaging: Technical details and short-term outcomes. J Surg Oncol. 2017;116:921–31. doi: 10.1002/jso.24752. [DOI] [PubMed] [Google Scholar]
- 37.Boni L, David G, Mangano A, Dionigi G, Rausei S, Spampatti S, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc. 2015;29:2046–55. doi: 10.1007/s00464-014-3895-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeLong JC, Chakedis JM, Hosseini A, Kelly KJ, Horgan S, Bouvet M. Indocyanine green (ICG) fluorescence-guided laparoscopic adrenalectomy. J Surg Oncol. 2015;112:650–3. doi: 10.1002/jso.24057. [DOI] [PubMed] [Google Scholar]
- 39.Ohdaira H, Yoshida M, Okada S, Tsutsui N, Kitajima M, Suzuki Y. New method of indocyanine green fluorescence sentinel node mapping for early gastric cancer. Ann Med Surg (Lond) 2017;20:61–5. doi: 10.1016/j.amsu.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baiocchi GL, Diana M, Boni L. Indocyanine green-based fluorescence imaging in visceral and hepatobiliary and pancreatic surgery: State of the art and future directions. World J Gastroenterol. 2018;24:2921–30. doi: 10.3748/wjg.v24.i27.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ambe PC, Jansen S, Macher-Heidrich S, Zirngibl H. Surgical management of empyematous cholecystitis: A register study of over 12,000 cases from a regional quality control database in Germany. Surg Endosc. 2016;30:5319–24. doi: 10.1007/s00464-016-4882-1. [DOI] [PubMed] [Google Scholar]
- 42.Ambe PC, Plambeck J, Fernandez-Jesberg V, Zarras K. The role of indocyanine green fluoroscopy for intraoperative bile duct visualization during laparoscopic cholecystectomy: An observational cohort study in 70 patients. Patient Saf Surg. 2019;13:2. doi: 10.1186/s13037-019-0182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wada T, Kawada K, Takahashi R, Yoshitomi M, Hida K, Hasegawa S, et al. ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc. 2017;31:4184–93. doi: 10.1007/s00464-017-5475-3. [DOI] [PubMed] [Google Scholar]
- 44.Zhou J, Yang F, Jiang G, Wang J. Applications of indocyanine green based near-infrared fluorescence imaging in thoracic surgery. J Thorac Dis. 2016;8:S738–43. doi: 10.21037/jtd.2016.09.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zehetner J, DeMeester SR, Alicuben ET, Oh DS, Lipham JC, Hagen JA, et al. Intraoperative assessment of perfusion of the gastric graft and correlation with anastomotic leaks after esophagectomy. Ann Surg. 2015;262:74–8. doi: 10.1097/SLA.0000000000000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamiya K, Unno N, Konno H. Intraoperative indocyanine green fluorescence lymphography, a novel imaging technique to detect a chyle fistula after an esophagectomy: Report of a case. Surg Today. 2009;39:421–4. doi: 10.1007/s00595-008-3852-1. [DOI] [PubMed] [Google Scholar]
- 47.Okusanya OT, Holt D, Heitjan D, Deshpande C, Venegas O, Jiang J, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg. 2014;98:1223–30. doi: 10.1016/j.athoracsur.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim HK, Quan YH, Choi BH, Park JH, Han KN, Choi Y, et al. Intraoperative pulmonary neoplasm identification using near-infrared fluorescence imaging. Eur J Cardiothorac Surg. 2016;49:1497–502. doi: 10.1093/ejcts/ezv367. [DOI] [PubMed] [Google Scholar]
- 49.Jiang JX, Keating JJ, Jesus EM, Judy RP, Madajewski B, Venegas O, et al. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am J Nucl Med Mol Imaging. 2015;5:390–400. [PMC free article] [PubMed] [Google Scholar]
- 50.Grischke EM, Röhm C, Hahn M, Helms G, Brucker S, Wallwiener D. ICG fluorescence technique for the detection of sentinel lymph nodes in breast cancer: results of a prospective open-label clinical trial. Geburtshilfe Frauenheilkd. 2015;75:935–40. doi: 10.1055/s-0035-1557905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papathemelis T, Jablonski E, Scharl A, Hauzenberger T, Gerken M, Klinkhammer-Schalke M, et al. Sentinel lymph node biopsy in breast cancer patients by means of indocyanine green using the Karl Storz VITOM® fluorescence camera. Biomed Res Int 2018. 2018 doi: 10.1155/2018/6251468. 6251468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGregor A, Pavri SN, Tsay C, Kim S, Narayan D. Use of indocyanine green for sentinel lymph node biopsy: Case series and methods comparison. Plast Reconstr Surg Glob Open. 2017;5:e1566. doi: 10.1097/GOX.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shirata C, Kaneko J, Inagaki Y, Kokudo T, Sato M, Kiritani S, et al. Near-infrared photothermal/photodynamic therapy with indocyanine green induces apoptosis of hepatocellular carcinoma cells through oxidative stress. Sci Rep. 2017;7:13958. doi: 10.1038/s41598-017-14401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hishikawa H, Kaibori M, Tsuda T, Matsui K, Okumura T, Ozeki E, et al. Near-infrared fluorescence imaging and photodynamic therapy with indocyanine green lactosomes has antineoplastic effects for gallbladder cancer. Oncotarget. 2019;10:5622–31. doi: 10.18632/oncotarget.27193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bailey CA, Cowan TM, Liu VG, Lemley EC, Chen WR. Optimization of selective hyperthermia. J Biomed Opt. 2004;9:648–54. doi: 10.1117/1.1689977. [DOI] [PubMed] [Google Scholar]
- 56.Long S, Xu Y, Zhou F, Wang B, Yang Y, Fu Y, et al. Characteristics of temperature changes in photothermal therapy induced by combined application of indocyanine green and laser. Oncol Lett. 2019;17:3952–9. doi: 10.3892/ol.2019.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ge MX, Niu WX, Ren JF, Cai SY, Yu DK, Liu HT, et al. A novel ASBT inhibitor, IMB17-15, repressed nonalcoholic fatty liver disease development in high-fat diet-fed Syrian golden hamsters. Acta Pharmacol Sin. 2019;40:895–907. doi: 10.1038/s41401-018-0195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu MR, Hsiao JK. Apical sodium-dependent bile acid cotransporter, A novel transporter of indocyanine green, and its application in drug screening. Int J Mol Sci. 2020;21:2202. doi: 10.3390/ijms21062202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zackrisson S, van de Ven SMWY, Gambhir SS. Light in and sound out: Emerging translational strategies for photoacoustic imaging. Cancer Res. 2014;74:979–1004. doi: 10.1158/0008-5472.CAN-13-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okumura K, Yoshida K, Yoshioka K, Aki S, Yoneda N, Inoue D, et al. Photoacoustic imaging of tumour vascular permeability with indocyanine green in a mouse model. Eur Radiol Exp. 2018;2:5. doi: 10.1186/s41747-018-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]



