Abstract
Resistance of bacteria, fungi and cancer cells to antibiotics and other drugs is recognized as one of the major problems in current medicine. Therefore, a search for new biologically active compounds able to either kill pathogenic cells or inhibit their growth is mandatory. Hard-to-reach habitats appear to be unexplored sources of microorganisms producing previously unknown antibiotics and other molecules revealing potentially therapeutic properties. Caves belong to such habitats, and Actinobacteria are a predominant group of microorganisms occurring there. This group of bacteria are known for production of many antibiotics and other bioactive compounds. Interestingly, it was demonstrated previously that infection with bacteriophages might enhance production of antibiotics by them. Here, we describe a series of newly isolated strains of Actinobacteria that were found in caves from the Tatra Mountains (Poland). Phage induction tests indicated that some of them may bear active prophages able to produce virions upon treatment with mitomycin C or UV irradiation. Among all the examined bacteria, two newly isolated Streptomyces sp. strains were further characterized to demonstrate their ability to inhibit the growth of pathogenic bacteria (strains of Staphylococcus aureus, Salmonella enterica, Enterococcus sp., Escherichia coli, and Pseudomonas aeruginosa) and fungi (different species and strains from the genus Candida). Moreover, extracts from these Streptomyces strains reduced viability of the breast-cancer cell line T47D. Chemical analyses of these extracts indicated the presence of isomers of dichloranthrabenzoxocinone and 4,10- or 10,12-dichloro-3-O-methylanthrabenzoxocinone, which are putative antimicrobial compounds. Moreover, various previously unknown (unclassified) molecules were also detected using liquid chromatography–mass spectrometry, suggesting that tested Streptomyces strains may synthesize a battery of bioactive compounds with antibacterial, antifungal, and anticancer activities. These results indicate that further studies on the newly isolated Actinobacteria might be a promising approach to develop novel antibacterial, antifungal, and/or anticancer drugs.
Keywords: cave microorganisms, Actinobacteria, antibacterial activity, antifungal activity, anticancer activity
1. Introduction
Resistance of pathogenic bacteria to most, if not all, antibiotics available for medical use is one of the major problems in current medicine. This apparent antimicrobial agent-resistance crisis may lead to serious health problems, with millions of fatal cases of bacterial infections, if no effective actions are conducted to solve this problem [1]. Selection of antibiotic-resistant bacterial strains has been ascribed mainly to the misuse of antibiotics in medicine and animal farming [2]. However, irrespective of the actual cause of appearance of many multiple antimicrobial-resistant bacterial strains, there is an urgent need to find novel ways to treat bacterial infections effectively [3]. It is estimated that total cost of antibiotic resistance is currently as high as USD 55 billion per year worldwide, and this cost may increase up to USD 100 trillion by 2050; this can be accompanied by about 10 million death cases per year caused by infections with antibiotic-resistant microbes [1].
In this light, it is crucial to take intensive actions to prevent the putative scenario described above. The World Health Organization has presented an action plan to solve this problem, which is based on understanding of mechanisms of antibiotic resistance, strengthening the knowledge through extensive research, reducing incidence of infection, optimizing the use of antibiotics, and ensuring investment in countering antimicrobial resistance [1]. Several approaches can be proposed to find novel ways to combat infections caused by pathogenic bacteria. Among them, intensification of vaccination, the use of bacteriophages or products of expression of their genes, the use of herbal products, and searching for novel antibiotics appear to be the most promising options [3].
One may assume that discovery of novel antimicrobial molecules is possible mainly by exploring rarely investigated environments [4]. These include marine waters, glaciers, hot springs, underground lakes, hydrothermal vents, and caves. In fact, such a strategy might be effective; however, technical difficulties related to investigation of hardly accessible habitats may be a serious drawback for attempts to isolate previously unknown microorganisms and compounds produced by them [4]. On the other hand, this can be one of a very few options to solve the antimicrobial-resistance crisis. Therefore, in this work we focused on isolation of microbial strains from a hardly accessible mountain cave and tested if such strains can produce previously unknown compounds revealing strong antimicrobial and/or anticancer activities.
Previous attempts to find antimicrobial compounds produced by microorganisms occurring in caves were relatively rare but indicated a strong potential, despite difficulties in obtaining the biological material and in culturing newly isolated strains. It was found that actinomycetes are predominant microorganisms isolated from caves [5]. When exploring caves, bacteria could be isolated from rock wall [6], cave soil [7], sediment [8], water [8], and moonmilk deposits [9]. Isolation and characterization of microbial strains from caves, including their antibacterial, antifungal, anti-inflammatory, antioxidative, and anticancer activities, and identification of some specific compounds responsible for such biological actions were reviewed recently [4]. These strains and compounds are promising. However, difficulties in cultivation of these microorganisms as well as specificity of detected compounds to some bacterial or fungal species indicate that further studies on a higher number of isolates are reasonable. Interestingly, it was reported that antibiotic production by Streptomyces venezuelae strain ISP5230 can be enhanced under various environmental conditions, such as elevated temperature and ethanol treatment, as well as after infection with bacteriophage SV1 [10]. Moreover, characterization of bacteriophage phiSASD1-endoced endolysin and holin, as potential antibacterial drugs, was reported recently [11]. Therefore, one might suggest that this phage, infecting Streptomyces avermitilis, can be an important source of genes encoding newly identified antibacterial agents, active against various pathogenic strains of Staphylococcus aureus, Sarcina lutea, and Enterococcus faecalis [11]. The studies summarized above encouraged us to search for previously unknown microbes, derived from caves, that might reveal various antibacterial, antifungal, and anticancer properties.
2. Results
2.1. Isolation and Identification of Bacteria from the Szczelina Chochołowska Cave
Using samples of water and moonmilk from the Szczelina Chochołowska cave (Tatra Mountains, Poland; Figure 1), we obtained 24 isolates of bacteria. These isolates were cultured under laboratory conditions, and on the basis of DNA isolation and sequencing of the 16S rRNA gene, they were identified at the genus or species level. All of them belong to Actinobacteria, and to the following genera: Arthrobacter, Frigoribacterium, Microbacterium, Nocardia, Nocardiopsis, Rhodococcus, Streptomyces, and Tomitella (Table 1).
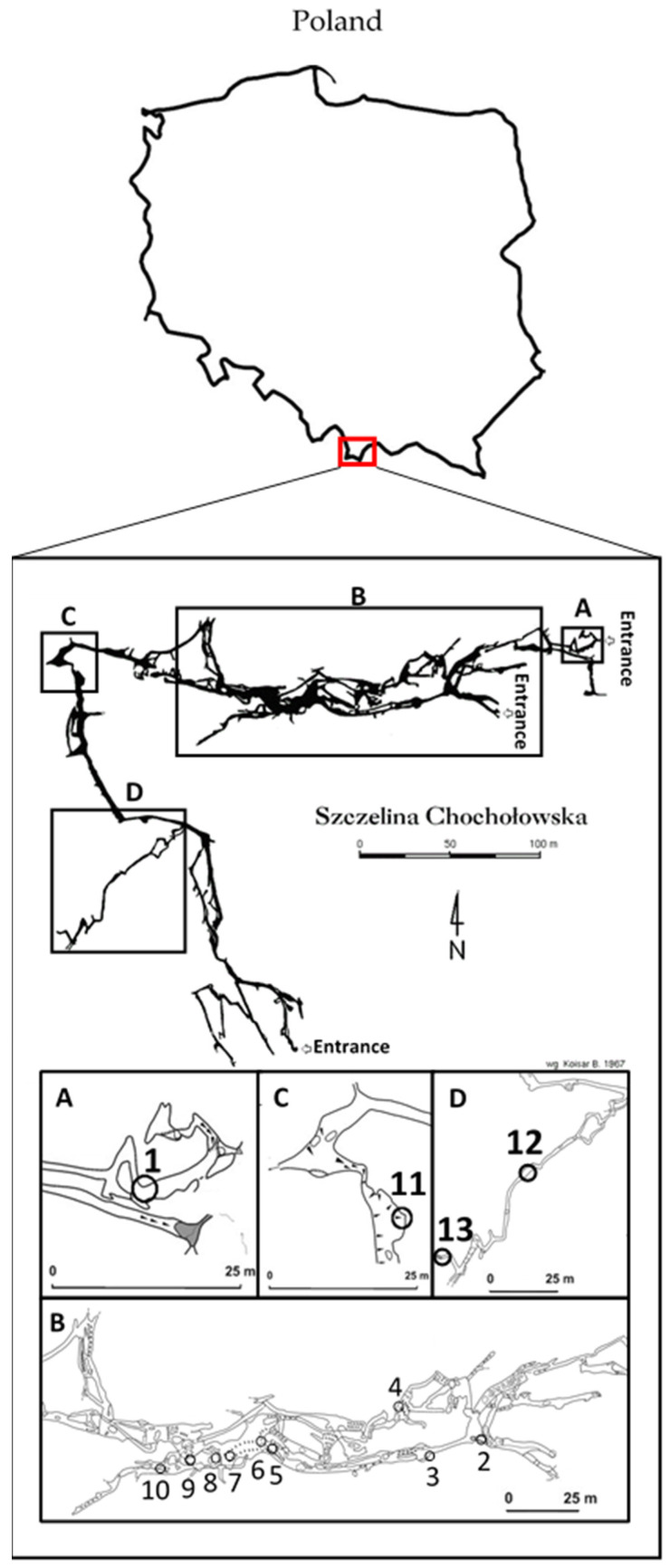
Figure 1.
A map of the Szczelina Chochołowska cave (19°48’43″.140 E 49°14’45″.401 N) (WGS84 coordinates). A map of Poland is shown at the top of the figure with the square indicating the region in which the cave is located. Regions marked as (A–D) are enlarged at the bottom of the figure. Numbers indicate places of collection of samples. Details are described in Section 4.2.
Table 1.
Actinobacteria isolates from the Szczelina Chochołowska cave, with identification of the closest relatives determined on the basis of comparison of 16S rDNA sequences.
| Isolate 1 | Strongest 16S rDNA Sequence Match (BLASTN) | |||
|---|---|---|---|---|
| Organism | Accession No. 2 | Bits | % | |
| JHARAB1_N | Arthrobacter sp. strain VTT E-052904 | EF093123 | 2691 | 99.9 |
| JHARN2 | Rhodococcus sp. strain UFZ-B528 | AF235012 | 2667 | 99.9 |
| JSZCO2 | Microbacterium sp. strain JSZCO2 | KU643207 | 2705 | 100 |
| JSZCZL7 | Nocardia sp. strain JSZCL7 | KU643201 | 2219 | 99.9 |
| M1_4 | Nocardia sp. strain OAct 132 | JX047071 | 2671 | 99.9 |
| M1_7 | Arthrobacter sp. strain 3S-5 | KM434250 | 2670 | 99.9 |
| M1_9 | Tomitella biformata strain AHU 1821 | NR_112905 | 2575 | 98.9 |
| M2_1 | Arthrobacter sp. (uncultured clone) | KJ650689 | 2671 | 100 |
| M2_11 | Frigoribacterium sp. strain FB3 | AM933497 | 2657 | 100 |
| M2_15 | Rhodococcus jialingiae strain djl-6-2 16S | NR_115708 | 2675 | 99.9 |
| M2_4 | Arthrobacter sp. strainRKS6-4 | GQ477171 | 2670 | 99.9 |
| M2_9 | Streptomyces sp. strain MM56 | KU714908 | 2684 | 100 |
| M3_10 | Streptomyces sp. strain MM56 | KU714908 | 2679 | 99.9 |
| M3_8 | Arthrobacter sp. strain 3S-5 | KM434250 | 2647 | 99.7 |
| M3_9 | Arthrobacter sp. strain MNPB6 | FM213396 | 2555 | 98.3 |
| M4_18 | Rhodococcus maanshanensis strain GMC121 | AB741451 | 2465 | 97.6 |
| M4_21 | Arthrobacter sp. strain EM0174 | HM165266 | 2559 | 98.5 |
| M4_24 | Streptomyces sp. strain MM56 | KU714908 | 2679 | 99.9 |
| M4_9 | Nocardiopsis umidischolae strain NBRC 100349 | NR_112746 | 2690 | 100 |
| M5_2 | Nocardia sp. strain OAct 132 | JX047071 | 2644 | 99.6 |
| M5_6 | Nocardia sp. strain OAct 132 | JX047071 | 2633 | 99.4 |
| M5_8 | Streptomyces sp. strain MM56 | KU714908 | 2694 | 100 |
| M5_9 | Streptomyces sp. strain MM56 | KU714908 | 2675 | 99.9 |
| W2_1 | Microbacterium phyllosphaerae IHBB 11136 | KR085857 | 2686 | 100 |
1 GenBank accession numbers for 16S rDNA sequences of the isolates are KU643201.1, KU643207.1, MG758033.1, and MG758033.1. 2 GenBank accession numbers for genomic sequences of the organisms with the strongest 16S rDNA sequence matches to the isolates are provided.
2.2. Prophage Induction and Determination of the Presence of Phage Virions
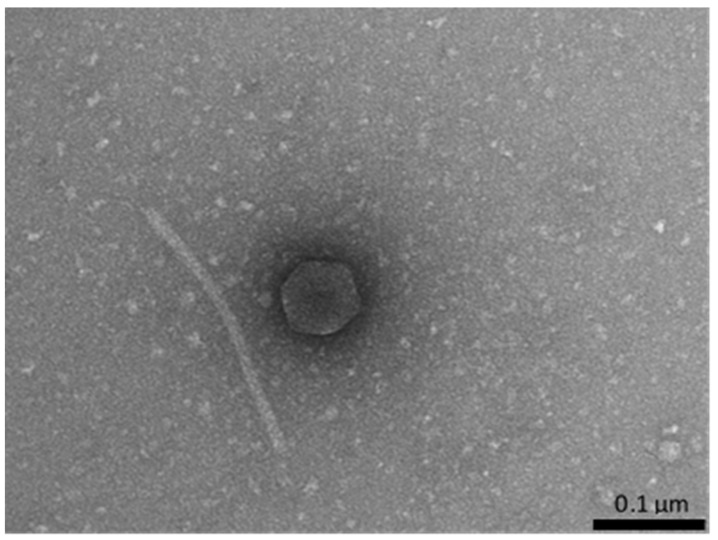
Since bacteriophage presence has been reported previously as a factor enhancing production of antibiotics by Actinobacteria [10], we tested whether the isolated strains contain inducible prophages. Cultures of the isolates were either treated with 0.5 μg/mL mitomycin C or UV-irradiated, and following further cultivation and centrifugation, supernatants were tested for the presence of phage virions using electron microscopy. Bacteriophage virions were found in samples derived from the Nocardia sp. strain JSZCL7 (Figure 2). However, we were not able to propagate the isolated phage under laboratory conditions. The host strain of Nocardia could not be effectively infected with this phage, most probably due to immunity of the lysogen, and we could not find a non-lysogenic strain sensitive to this phage. Moreover, virions appeared very fragile under laboratory conditions and in buffers employed in this study, which could be observed as damaged or disconnected heads and tails (Figure 2). Thus, we failed to obtain single plaques of this phage and to propagate it for further analyses. Nevertheless, the presence of bacteriophages in samples of isolated bacteria may suggest that this group of viruses might be taken into consideration in further studies on bioactive compound-producing Actinobacteria, as putative modulators of syntheses of such substances.
Figure 2.
Electron micrograph of the virion of a bacteriophage isolated after induction of the Nocardia sp. strain JSZCL7 with 0.5 μg/mL mitomycin C. Virions were very unstable under laboratory conditions, which is exemplified by disconnected head and tail of the virion.
2.3. Preliminary Screening for Production of Antibacterial Compounds by the Isolates and Selection of Strains for Further Investigation
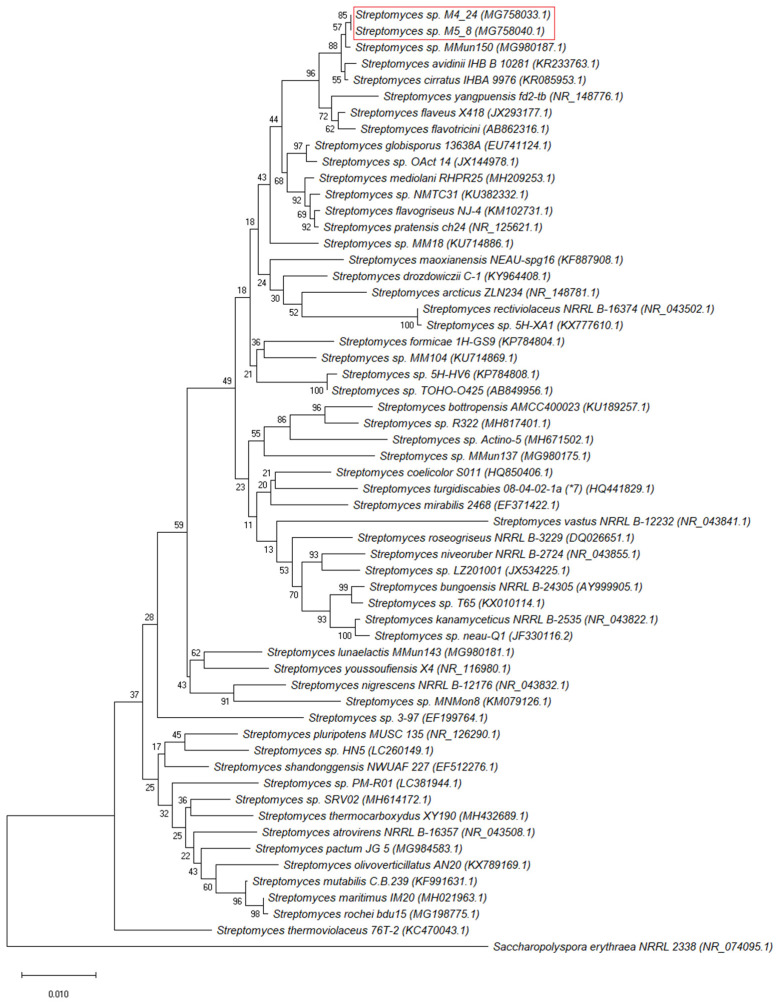
To test abilities of the actinobacterial isolates from the Szczelina Chochołowska cave to produce antimicrobial compounds, we used a battery of pathogenic bacterial and fungal strains (Table 2). The preliminary screening, using the streak-test, indicated that 3 isolates revealed the highest antibacterial and antifungal properties when contacting other microorganisms (estimated as the number of strains in which growth was inhibited). These isolates were M2_9, M4_24, and M5_8, all belonging to Streptomyces sp. (Table 1). Analyses of 16S rDNA sequences revealed that this fragment of the genome is identical in M2_9 and M5_8, thus only one of these strains (M5_8) was investigated further for antimicrobial activities. We also performed phylogenetic analyses, based on 16S rDNA, which indicated that M4_24 and M5_8 are closely related strains (Figure 3).
Table 2.
Bacterial and fungal strains used for determination of antimicrobial activities of isolated Actinobacteria from the Szczelina Chochołowska cave.
| Bacterial or Fungal Strain | Source |
|---|---|
| Staphylococcus aureus MRSA 200 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA ATCC 6538 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 108 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 271 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 203 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 122 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 116 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 115 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 342 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 352 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 44 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 298 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 199 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 343 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 297 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 202 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 124 | Medical University of Gdańsk |
| Staphylococcus aureus MRSA 149 | Medical University of Gdańsk |
| Salmonella enterica Virchow 41 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Enteritidis 64 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Kentucky 1368 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Heidelberg 16 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Cholerasuis 1439 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Typhimurium 12 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Typhimurium 13 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Agona 1408 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Thompson 39 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Gallinarum 74 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Hadar 1784 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Cholerasuis 39 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Infantis 155 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Bovismorbificans 300 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Seftenberg 87 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Newport 50 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Newport 51 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Cholerasuis 37 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Dubin 65 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Saindpaul 435 | National Salmonella Center, Gdańsk, Poland |
| Salmonella enterica Enteritidis 1392 | National Salmonella Center, Gdańsk, Poland |
| Escherichia coli STEC 35 | University of Gdańsk collection |
| Escherichia coli STEC 36 | University of Gdańsk collection |
| Escherichia coli STEC 37 | University of Gdańsk collection |
| Escherichia coli STEC 38 | University of Gdańsk collection |
| Escherichia coli STEC 39 | University of Gdańsk collection |
| Pseudomonas aeruginosa 02113 | University of Gdańsk collection |
| Pseudomonas aeruginosa 02109 | University of Gdańsk collection |
| Pseudomons aeruginosa 02108 | University of Gdańsk collection |
| Pseudomonas aeruginosa RA743 | University of Gdańsk collection |
| Bacillus subtilis 3610 | University of Gdańsk collection |
| Bacillus subtilis wt168 | University of Gdańsk collection |
| Bacillus megaterium | University of Gdańsk collection |
| Bacillus cereus | University of Gdańsk collection |
| Candida parapsilosis D2 | Bruss Laboratories, Gdynia, Poland |
| Candida glabrata D3 | Bruss Laboratories, Gdynia, Poland |
| Candida tropicalis D4 | Bruss Laboratories, Gdynia, Poland |
| Candida dubliniensis D5 | Bruss Laboratories, Gdynia, Poland |
| Candida albicans D6 | Bruss Laboratories, Gdynia, Poland |
| Candida albicans D7 | Medical University of Gdańsk |
| Candida albicans D8 | Medical University of Gdańsk |
| Candida albicans D9 | University Clinical Centre in Gdańsk |
| Candida albicans E1 | University Clinical Centre in Gdańsk |
| Candida guilliermondii E2 | University Clinical Centre in Gdańsk |
| Candida guilliermondii E3 | University Clinical Centre in Gdańsk |
| Candida albicans E4 | University Clinical Centre in Gdańsk |
| Candida albicans E5 | University Clinical Centre in Gdańsk |
| Candida glabrata E6 | University Clinical Centre in Gdańsk |
| Candida glabrata E7 | University Clinical Centre in Gdańsk |
| Candida sp. E8 | University Clinical Centre in Gdańsk |
| Candida sp. E9 | University Clinical Centre in Gdańsk |
Figure 3.
Phylogenetic analysis of Streptomyces M4_24 and M5_8 strains. The alignment and a Neighbor-Joining (NJ) tree, based on Jukes–Cantor Genetic Distance Model, was constructed using the MEGA X software, with Saccharopolyspora erythrea NRRL 2338 as an outgroup. Bootstrap values are shown from 1000 replicates.
2.4. Antibacterial and Antifungal Activities of Streptomyces M4_24 and M5_8 Strains
To assess antibacterial and antifungal activities of Streptomyces M4_24 and M5_8 strains, we performed a streak-test and measured the width of growth inhibition zones of various pathogenic strains of bacteria and fungi after contacting the newly isolated Actinobacteria, as indicated in Supplementary Material Figure S1.
We found that M4_24 and M5_8 isolates revealed antimicrobial activities against most tested strains of bacteria (Table 3) and fungi (Table 4), while M4_24 was generally more effective than M5_8 when considering the number of strains in which growth was halted.
Table 3.
Effects of Streptomyces M4_24 and M5_8 isolates on growth inhibition of bacterial strains. The width of the inhibition zone was measured in the streak-test after 48 h of incubation.
| Bacteruial Strain | Growth Inhibition Zone (mm) 1 | |
|---|---|---|
| M4_24 | M5_8 | |
| S. aureus MRSA 200 | 5.0 ± 1.0 | 5.5 ± 1.5 |
| S. aureus MRSA ATCC 6538 | 7.5 ± 0.3 | 6.5 ± 0.5 |
| S. aureus MRSA 108 | 5.0 ± 2.0 | 6.5 ± 0.5 |
| S. aureus MRSA 271 | 7.5 ± 1.5 | 7.5 ± 0.5 |
| S. aureus MRSA 203 | 6.5 ± 1.5 | 7.0 ± 1.0 |
| S. aureus MRSA 122 | 4.5 ± 0.5 | 4.75 ± 1.25 |
| S. aureus MRSA 116 | 5.0 ± 1.0 | 5.5 ± 0.5 |
| S. aureus MRSA 115 | 6.75 ± 0.75 | 5.75 ± 0.75 |
| S. aureus MRSA 342 | 0.0 | 0.0 |
| S. aureus MRSA 352 | 6.0 ± 1.0 | 5.25 ± 1.75 |
| S. aureus MRSA 44 | 12.0 ± 3.0 | 0.0 |
| S. aureus MRSA 298 | 4.0 ± 0.0 | 0.0 |
| S. aureus MRSA 199 | 8.0 ± 2.0 | 0.0 |
| S. aureus MRSA 343 | 8.0 ± 1.0 | 0.0 |
| S. aureus MRSA 297 | 7.25 ± 1.25 | 6.75 ± 0.25 |
| S. aureus MRSA 202 | 8.25 ± 1.25 | 3.5 ± 0.5 |
| S. aureus MRSA 124 | 3.0 ± 0 | 3.0 ± 0.0 |
| S. aureus MRSA 149 | 6.0 ± 0.0 | 6.0 ± 0.0 |
| S. enterica Virchow 41 | 0.0 | 0.0 |
| S. enterica Enteritidis 64 | 6.0 ± 1.0 | 0.0 |
| S. enterica Kentucky 1368 | 5.5 ± 0.5 | 8.0 ± 1.0 |
| S. enterica Heidelberg 16 | 6.0 ± 1.0 | 5.0 ± 1.0 |
| S. enterica Cholerasuis 1439 | 11.5 ± 0.5 | 0.0 |
| S. enterica Typhimurium 12 | 7.5 ± 1.5 | 0.0 |
| S. enterica Typhimurium 13 | 6.5 ± 0.5 | 0.0 |
| S. enterica Agona 1408 | 0.0 | 0.0 |
| S. enterica Thompson 39 | 0.0 | 0.0 |
| S. enterica Gallinarum 74 | 5.5 ± 0.5 | 0.0 |
| S. enterica Hadar 1784 | 0.0 | 0.0 |
| S. enterica Cholerasuis 39 | 6.5 ± 1.5 | 5.0 ± 2.0 |
| S. enterica Infantis 155 | 6.5 ± 0.5 | 8.0 ± 10 |
| S. enterica Bovismorbificans 300 | 6.25 ± 0.25 | 0.0 |
| S. enterica Seftenberg 87 | 5.0 ± 1.0 | 4.5 ± 0.5 |
| S. enterica Newport 50 | 5.5 ± 0.5 | 5.0 ± 1.0 |
| S. enterica Newport 51 | 5.5 ± 0.5 | 5.0 ± 1.0 |
| S. enterica Cholerasuis 37 | 4.5 ± 0.5 | 6.5 ± 0.5 |
| S. enterica Dubin 65 | 6.5 ± 0.5 | 4.5 ± 0.5 |
| S. enterica Saindpaul 435 | 3.0 ± 1.0 | 0.0 |
| S. enterica Enteritidis 1392 | 9.75 ± 0.25 | 4 ± 0 |
| Enterococcus sp. | 10.5 ± 0.5 | 8.0 ± 1.0 |
| E. coli 35 | 8.5 ± 1.5 | 6.5 ± 0.5 |
| E. coli 36 | 10.5 ± 1.5 | 7.0 ± 1.0 |
| E. coli 37 | 10.0 ± 1.0 | 7.0 ± 0.5 |
| E. coli 38 | 11.0 ± 0.5 | 6.5 ± 0.5 |
| E. coli 39 | 8.5 ± 0.5 | 8.5 ± 0.5 |
| B. subtilis 3610 | 7.0 ± 1.0 | 0.0 |
| B. subtilis wt168 | 8.0 ± 1.0 | 0.0 |
| B. megaterium | 8.0 ± 1.0 | 0.0 |
| B. cereus | 7.0 ± 2.0 | 0.0 |
| P. aeruginosa 02113 | 8.0 ± 2.0 | 6.0 ± 1.0 |
| P. aeruginosa 02109 | 6.5 ± 0.5 | 5.0 ± 0.5 |
| P. aeruginosa 02108 | 10.0 ± 1.0 | 5.0 ± 0.0 |
| P. aeruginosa RA743 | 8.0 ± 1.0 | 7.0 ± 2.0 |
1 Mean values from 3 independent experiments ± SD are demonstrated.
Table 4.
Effects of Streptomyces M4_24 and M5_8 isolates on growth inhibition of fungal strains. The width of the inhibition zone was measured in the streak-test after 48 h of incubation.
| Fungal Strain | Growth Inhibition Zone (mm) 1 | |
|---|---|---|
| M4_24 | M5_8 | |
| Candida parapsilosis D2 | 0.0 | 0.0 |
| Candida glabrata D3 | 10.3 ± 2.1 | 18.7 ± 3.5 |
| Candida tropicalis D4 | 0.0 | 0.0 |
| Candida dubliniensis D5 | 5.7 ± 1.5 | 0.0 |
| Candida albicans D6 | 3.3 ± 1.2 | 0.0 |
| Candida albicans D7 | 3.7 ± 0.6 | 3.0 ± 1.0 |
| Candida albicans D8 | 0.0 | 0.0 |
| Candida albicans D9 | 4.3 ± 1.5 | 2.3 |
| Candida albicans E1 | 3.3 ± 2.3 | 0.0 |
| Candida guilliermondii E2 | 6.0 ± 2.6 | 0.0 |
| Candida guilliermondii E3 | 6.3 ± 0.6 | 0.0 |
| Candida albicans E4 | 3.3 ± 0.6 | 0.0 |
| Candida albicans E5 | 0.0 | 0.0 |
| Candida glabrata E6 | 12.0 ± 4.4 | 5.7 ± 2.5 |
| Candida glabrata E7 | 6.0 ± 2.0 | 2.0 ± 3.5 |
| Candida sp. E8 | 3.7 ± 0.6 | 3.7 ± 1.2 |
| Candida sp. E9 | 3.3 ± 1.2 | 3.3 ± 1.5 |
1 Mean values from 3 independent experiments ± SD are demonstrated.
2.5. Anticancer Activities of Streptomyces M2_9, M4_24 and M5_8 Isolates
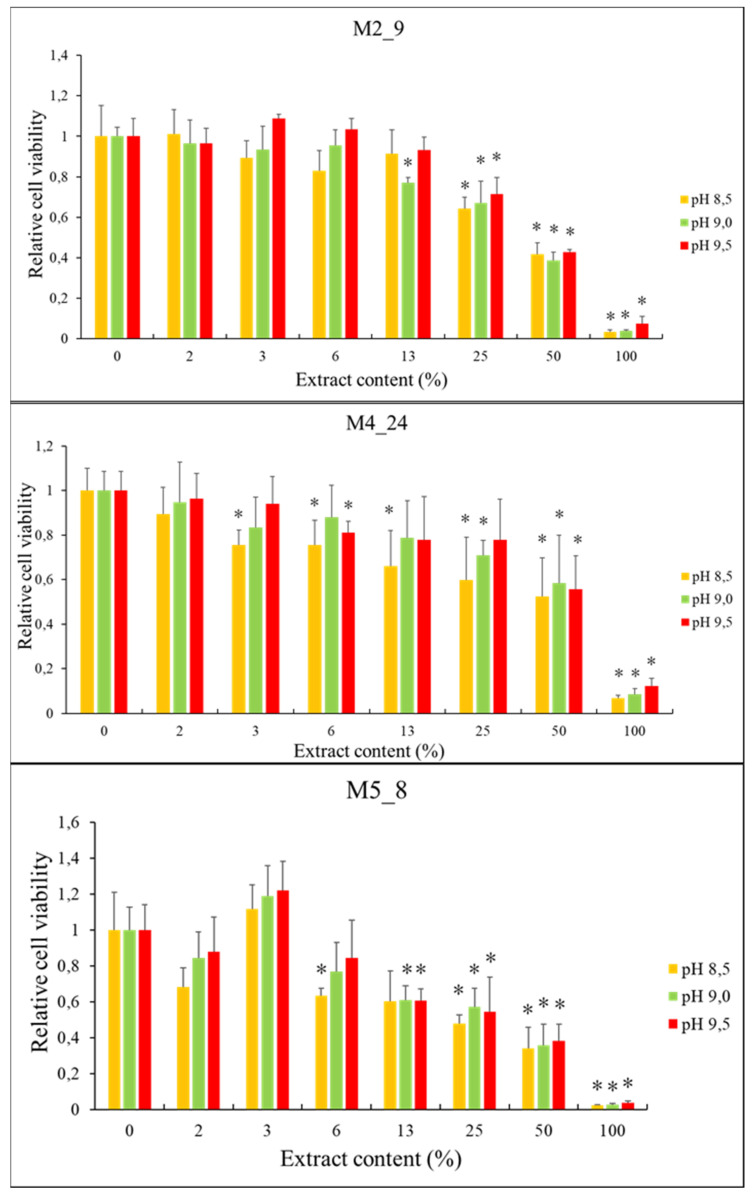
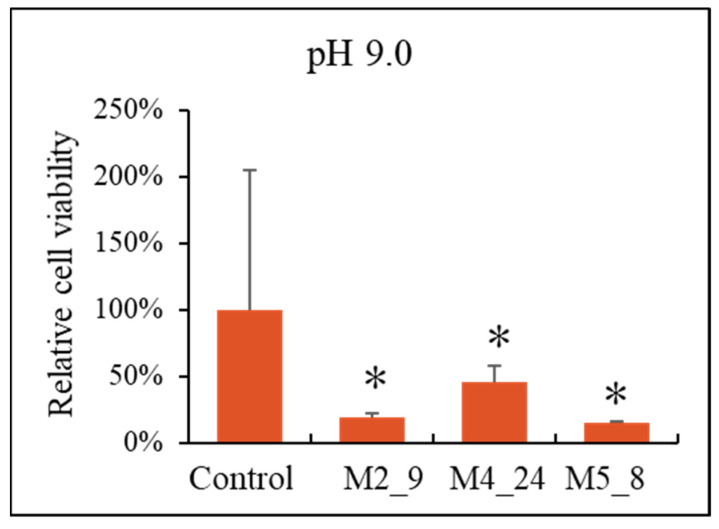
To determine if the Streptomyces isolates produce anticancer compounds, we tested the effects of extracts of cell cultures on the viability of breast-cancer cells (the T47D cell line). To obtain the extracts, Actinobacteria were cultured in liquid media with various pH values. We found that viability of the tested cancer cells decreased significantly with increasing concentrations of the extracts from investigated Actinobacteria, irrespective of the pH value of the medium used for Streptomyces cultivation (Figure 4). In control experiments, we did not observe any significant reduction of viability of non-transformed cells, the HDFa cell line, except conditions of 100% extract (results not shown). Therefore, to test if the observed effects on the breast cancer cells are caused by the compounds present in the extract rather than from dilution of eukaryotic cell-culture medium by the extract, we performed control experiments in which the medium used for cultivation of Actinobacteria was used instead of the extract. When we compared effects of the Actinobacteria-free medium with the analogous medium but derived from Streptomyces cultures, we found that inhibition of viability of T47D cells was specific for the extracts of actinobacterial cultures (Figure 5).
Figure 4.
Effects of extracts from cultures of Streptomyces M2_9, M4_24, and M5_8 isolates on viability of T47D cells (assessed by the MTT test). Mean values from 3 independent experiments ± SD are demonstrated. Asterisks (*) indicate statistically significant differences (p < 0.05) relative to results obtained for samples with no extract (0% extract content).
Figure 5.
Effects of the bacterial culture medium alone (control, the value assumed to be 100%) and extracts from cultures of Streptomyces M4_9, M4_24, and M5_8 isolates on viability of T47D cells (assessed by the MTT test). Mean values from 3 independent experiments ± SD are demonstrated. Asterisks (*) indicate statistically significant differences (p < 0.05) relative to the control.
2.6. Chemical Analyses of Extracts from Cultures of Streptomyces M2_9, M4_24 and M5_8 Isolates
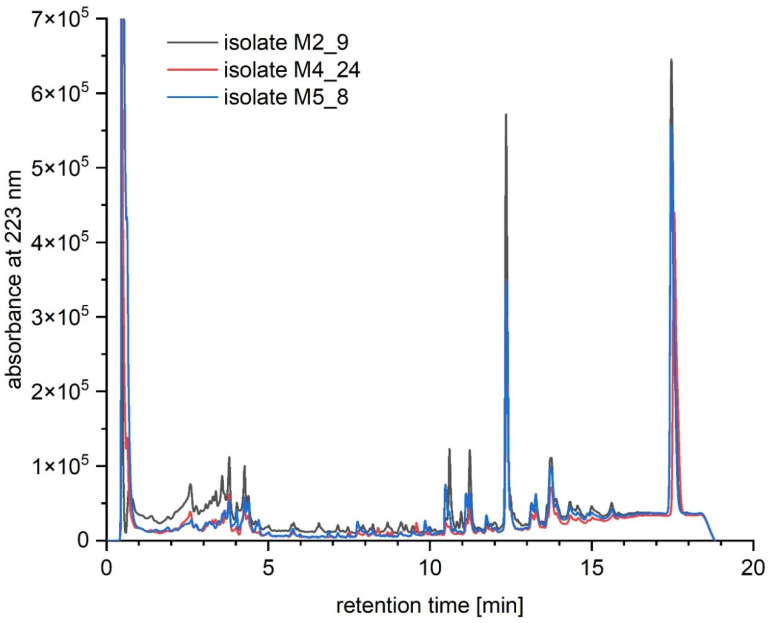
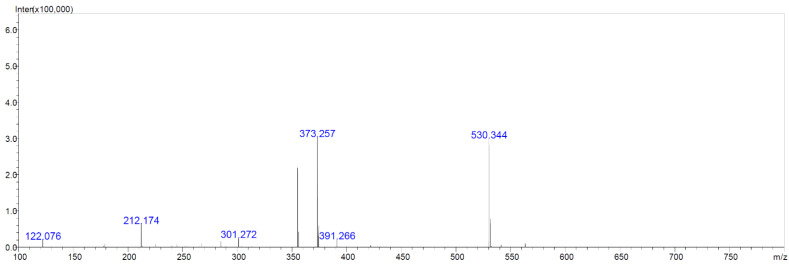
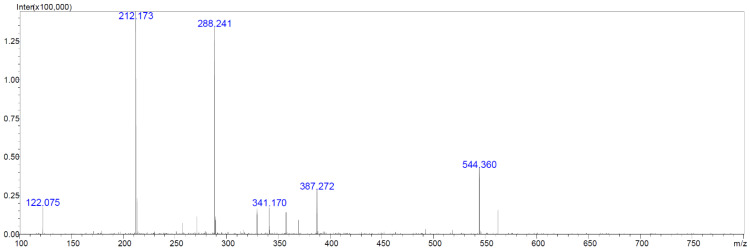
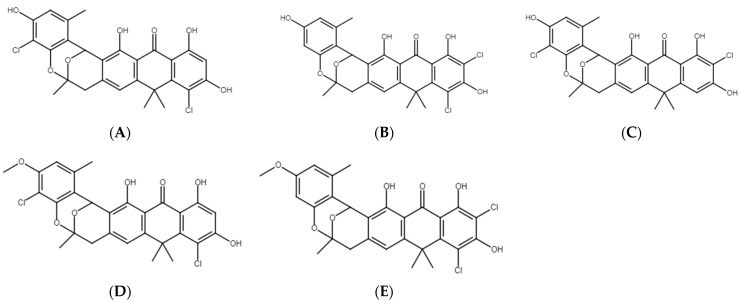
We aimed to test which compounds, potentially responsible for antibacterial, antifungal and anticancer activities of Streptomyces M2_9, M4_24 and M5_8 isolates, are produced by the investigated Actinobacteria. Therefore, we analyzed extracts of cultures of these bacteria. All analyzed crude extracts presented similar chemical profiles (Figure 6). The screening for known bioactive metabolites in the extracts, using the Dictionary of Natural Products, revealed that the analyzed isolates can produce many natural products previously not detected in Streptomyces spp. Only two compounds could be preliminarily predicted, using search parameters described in Section 4.10. These compounds were identified as the isomers of dichloranthrabenzoxocinone (4,10-; 4,12- or 10,12-; m/z 530.344; Figure 7) and 4,10- or 10,12-dichloro-3-O-methylanthrabenzoxocinone (m/z 544.360; Figure 8). Their formulas are presented in Figure 9. They are described to exhibit moderate antibacterial activities [12]. Other metabolites found in the analyzed extracts appear to be novel, which supports the potential of cave Actinobacteria as the producers of previously unknown bioactive substances.
Figure 6.
Overlayed chromatograms obtained by UHPLC of crude extracts of cultures of Streptomyces isolates M2_9 (black), M4_24 (red), M5_8 (blue).
Figure 7.
LC-MS chromatogram of the isomer of dichloroanthrabenzoxocinone (M = 529.366 Da).
Figure 8.
LC-MS chromatogram of the isomer of dichloro-3-O-methylanthrabenzoxocinone (M = 543.393 Da).
Figure 9.
Chemical structures of the preliminarily detected natural products produced by Streptomyces isolates M2_9, M4_24, and M5_8. (A) 4,10-Dichloroanthrabenzoxocinone, (B) 10,12-Dichloroanthrabenzoxocinone, (C) 4,12-Dichloroanthrabenzoxocinone, (D) 4,10-Dichloro-3-O-methylanthrabenzoxocinone, (E) 10,12-Dichloro-3-O-methylanthrabenzoxocinone.
3. Discussion
Since microorganisms occurring in hard-to-reach environments were indicated previously as a potent, though still unexplored, source of bioactive compounds, including antibacterial, antifungal, and anticancer substances [4], in this work we aimed to isolate bacteria living in one of the caves that were not investigated to date for this purpose, and to test their potential in producing compounds with useful properties. We chose the Szczelina Chochołowska cave, located in the Tatra Mountains (Poland), and characterized 24 microbial isolates that were subsequently tested for their antibacterial, antifungal, and anticancer activities. All the isolates belong to Actinobacteria, which is not a surprise as it was demonstrated previously that this group of microorganisms is predominant in caves [5]. Since previous studies indicated that bacteriophages may significantly modulate production of antimicrobial agents by Streptomyces [10], we tested if the isolates are lysogenic for bacteriophages. In fact, we were able to induce a prophage from Nocardia sp. strain JSZCL7 and to demonstrate that phage virions can be formed. However, we were not able to find a host in which this bacteriophage could be propagated. The lysogenic strain could not be infected with the same phage, and it is likely that the isolated bacteriophage may be of narrow host range. Moreover, this bacteriophage appeared very fragile under laboratory conditions, which further caused problems with its characterization. Therefore, although we demonstrated the presence of inducible prophages in Actinobacteria isolates from the Szczelina Chochołowska cave, we could not determine whether these viruses are able to modulate production of bioactive compounds by their host cells.
We found that the Streptomyces isolates revealed antibacterial and antifungal activities against various pathogenic strains, as indicated by the streak-test. The inhibition of growth of most of these strains was evident after a contact with either the M4_24 or M5_8 isolate, though the former one was effective against more bacterial and fungal strains. Extracts from cultures of these isolates could also reduce viability of breast-cancer cells (T47D line). Importantly, chemical analyses of such extracts indicated the presence of 4,10-dichloroanthrabenzoxocinone, 10,12-dichloroanthrabenzoxocinone, 4,12-dichloroanthrabenzoxocinone, 4,10-dichloro-3-O-methylanthrabenzoxocinone, and 10,12-dichloro-3-O-methylanthrabenzoxocinone, compounds that are potential antibiotics. In addition, various unknown compounds were also detected, suggesting that a set of novel bioactive molecules produced by Streptomyces M4_24, M2_9, and M5_8 isolates is even larger.
Our results confirmed that Actinobacteria isolated from caves may be a rich source of potential antibiotics. Examples of previous works in this field include isolation of cervamicin A-D from Streptomyces tendae HKI 0179 [13], isolation of strains inhibiting the growth of various Gram-positive [14,15] and Gram-negative [16] bacteria, and identification of undecylprodigiosin, produced by Streptomyces sp. JS520 [17]. In fact, several reports demonstrated that most of the bacterial strains isolated from caves are able to inhibit growth of other bacteria and/or fungi, though only a few active compounds have been identified, such as pyrrolopyrazines pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl), pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl), and 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester [6,7,8,9,18,19,20,21,22,23,24]. Moreover, anticancer activities of compounds derived from strains of bacteria occurring in caves were also reported previously. These compounds include hypogeamicin A, xiakemycin A, huanglongmycin A, and various unidentified molecules [25,26,27].
It seems that anthrabenzoxocinone compounds might be of special interest among substances isolated from Streptomyces strains. Early studies on Streptomyces violaceusniger, isolated in Japan, led to discovery of a potent anthrabenzoxocinone derivative, named BE-24566B, which might be used against MRSA [28]. Two such compounds were then isolated from Streptomyces sp. (MA6657), and their characteristics indicated that they have significant antimicrobial activities against Gram-negative bacteria, with MIC values ranging from 0.5 to 2.0 μg/mL [12]. Genetic analysis of Streptomyces sp. FJS31-2 indicated that its genome contains a gene cluster that is responsible for production of anthrabenzoxocinones with potential antibacterial activities, previously known BE-24566B and newly identified zunyimycin A [29]. The same Streptomyces strain, when cultured under various laboratory conditions, produced other compounds from this group, named zunyimycins B and C, inhibiting growth of both MRSA and enterococci [30]. These results demonstrated a high potential of Actinobacteria to produce different antimicrobial compounds that may be modified under different environmental conditions. In addition, antibiotic activities of natural anthrabenzoxocinones can be enhanced by biochemical modifications [31]. Furthermore, recent studies highlighted a high biodiversity of natural products, including anthrabenzoxocinone compounds, produced by bacteria isolated from natural habitats [32], and a high genetic potential of Streptomyces spp. in production of a variety of bioactive compounds, also including anthrabenzoxocinones [33]. Our results, presented in this report, corroborate these conclusions.
In summary, cave-derived Actinobacteria reveal various antibacterial, antifungal, and anticancer activities, while among them only relatively few biologically active compounds were identified. This indicates that caves are habitats rich in microorganisms producing as-yet unknown substances that might be potentially used in treatment for various infections and/or cancers. Definitely, further more detailed studies are required in this field, and our work fits to this topic, providing further evidence for effectiveness of Actinobacteria isolated from caves in inhibiting growth of pathogenic bacteria and fungi, and reducing viability of cancer cells. Whether modulation of production of bioactive compounds by bacteriophages (as reported previously [10]) is a specific or a more general phenomenon remains to be elucidated.
4. Materials and Methods
4.1. Bacterial Strains
Newly discovered isolates of bacteria from the Szczelina Chochołowska cave are presented in Table 1. Pathogenic bacteria and fungi used in this work were from various sources that are presented in Table 2, together with characteristics of these strains. All Salmonella enterica serotypes were from National Salmonella Center in Gdańsk (Poland). Staphylococcus aureus strains were from the Department of Medical Microbiology, Medical University of Gdansk (Poland) [34]. Pseudomonas aeruginosa, Bacillus spp. and Shiga-toxin producing Escherichia coli were from the collection of the Department of Molecular Biology, University of Gdańsk (Poland). Fungal strains were from Bruss Laboratories, Gdynia (Poland) and University Medical Center of Medical University of Gdańsk, Gdańsk (Poland).
4.2. Cave Description and Sampling
The Szczelina Chochołowska cave is located in the Tatra National Park (TNP; Poland) in Western Tatra. It is situated orographic left slope of the Valley Chochołowska (19°48’43″.140 E 49°14’45″.401 N) (WGS84 coordinates). Szczelina Chochołowska has 2320 m of cave passages and three entrances (1-E exposition at 1051 m a.s.l; 2-SE at 1072 m a.s.l; 3-NE at 1083 m a.s.l), with 60 m of height difference (Figure 1).
Samples of water and moonmilk were collected from the six parts of cave (Figure 1B–D), according to the permission of the Minister of the Environment (Poland) (DLP-III.286.102.2016.MGr) and TNP director (DBN.505/14/15 RÓŻ no 128, DBN.505/14/16 RÓŻ no 128, DBN.505/14/17 RÓŻ no 128). Sampling sites mainly depended on water flow and the presence of speleothems, such as moonmilk deposits. The first sampling site was located relatively close to the entrance (Figure 1A); samples were taken from the ice formations. Five samples were taken from the selected sites, such as small ponds (Figure 1B, sampling sites 3, 4, 5, 8; Figure 1D, sampling site 13), moonmilk (Figure 1B, sampling sites 4, 7, 10; Figure 1C, sampling site 11; Figure 1D, sampling site 12), and water dripping from speleothems (Figure 1C, sampling sites 2, 7, 6, 9). All samples were collected using sterile tubes and disposable pipettes. Withdrawn samples were transported to the Department of Molecular Biology of University of Gdansk, Gdańsk (Poland) laboratories in a cooler packed with ice, and kept in 4 °C until cultivating.
4.3. Bacterial Growth Conditions
Samples of water and moonmilk (Section 4.2) were refrigerated at 4 °C. Serial dilutions (10, 100, and 1000 times in sterile, distilled water) were prepared and plated using Reasoner’s 2A (R2A) agar medium [35] supplemented with water collected from the Szczelina Chochołowska cave. The plates were incubated at either room temperature or 4 °C for 14 days. Isolated colonies were maintained without access to the light on the R2A agar plates at 4 °C for subsequent studies, and glycerol stocks (30% v/v) were kept in a deep-freezer (at −80 °C) for long-term preservation. Liquid cultures were prepared in the R2A liquid medium [35].
Bacterial strains listed in Table 2, when used alone, were cultured in LB liquid or agar media [36].
4.4. Prophage Induction and Electron Microscopy of Bacteriophage Virions
Liquid cultures in the R2A medium, supplemented with 10 mM MgSO4 and 10 mM CaCl2, were incubated to OD600 ~0.3. Mitomycin C was added to 0.5 μg/mL and the incubation was prolonged for 24 h. Alternatively, 25 mL of the culture was transferred to a Petri dish, and irradiated with UV (at 320 nm wavelength) for 5 s, and incubated as described above. Then, the culture was centrifuged (8000× g, 10 min, room temperature), and the supernatant was treated with DNase I (2 μg/mL) and RNase A (2 μg/mL) for 30 min. Polyethylene glycol was added to final concentration 10%, and the mixture was stirred for 24 h at 4 °C. Following centrifugation (10,000× g, 10 min, 4 °C), the pellet was suspended in TM buffer (10 mM Tris-HCl, 10 mM MgSO4, pH 7.2), and filtered through 0.22 μm microbiological filter. After triple extraction with 0.33 volume of chloroform and centrifugation (8000× g, 5 min, room temperature), the lysate was kept at 4 °C.
Transmission electron microscopic analyses of phage visions were conducted as described previously [37]. Briefly, negatively stained (with uranyl acetate) virions were observed and photographed under a Philips CM 100 electron microscope.
4.5. Streak-Test
Tested isolates of Actinobacteria were streaked perpendicularly on R2A agar plates and left to grow in the dark at room temperature for 48 or 72 h. Pathogenic bacteria and fungi were then streaked diagonally onto the plates with grown isolates and left for 24 h in the dark at room temperature. Growth-inhibition zones were measured after incubation. Each experiment was performed in triplicate.
4.6. Molecular Identification of Isolates and Phylogenetic Analyses
Identification of isolates was based on the molecular analysis of 16S rRNA gene sequences. Whole genome DNA was extracted using an Ultraclean Microbial DNA Isolation Kit (MO BIO, Carlsbad, CA USA) following the manufacturer’s protocol. DNA concentration was determined using Nanodrop ND-1000 Spectrophotometer and agarose gel electrophoresis. Sequences of 16S rRNA genes were PCR-amplified with oligonucleotides 785F/907R and sequenced by Macrogen Inc. (Amsterdam, The Netherlands). The sequences were checked for potential chimeric artifacts using the DECIPHER online tool. The sequences obtained in this study were deposited in GenBank with accession numbers KU643201.1, KU643207.1, MG758033.1, and MG758033.1. Sequences were compared to NCBI GenBank database using BLASTn to identify the closest relatives based on 16S rRNA sequences. The alignment and a Neighbor-Joining (NJ) tree [38], based on the Jukes–Cantor Genetic Distance Model [39], was constructed using the MEGA X software [40], with Saccharopolyspora erythrea NRRL 2338 as an outgroup. Graphical processing was conducted with Inkscape 0.92.4.
4.7. Preparation of Extracts from Cultures of Isolates
Material from colonies of Streptomyces isolates were streaked onto multiple R2A agar plates and left to grow in the dark at room temperature for 5 days. The R2A agar with grown bacteria was then cut into stripes and suspended in 2 L of the liquid R2A medium. The mixture was shaken and incubated in the dark, at room temperature, for 14 days. The mixture was stirred every 2 days in order to enable bacteria and metabolites to diffuse into liquid medium.
4.8. Cancer and Non-Transformed Cell Lines and Cell Cultures
Breast-cancer cell line T47D [41] was purchased from Sigma Aldrich (Darmstadt, Germany), and used for cell-culture experiments. HDFa cell line [42] was used as a control of non-transformed cells. The cells were cultured at 37 °C in a humidified atmosphere with 5% CO2, in the DMEM medium, containing the penicillin–streptomycin mixture, and supplemented with 10% fetal bovine serum, as described previously [42].
4.9. Estimation of Cells’ Viability
Viability of eukaryotic cells was estimated as described previously [43], using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) test. Briefly, 3 × 103 cells were passaged wells of the 96-well plate and incubated overnight. Extracts of Streptomyces cultures were added to indicated final concentrations, and the incubation prolonged for another 24 h at 37 °C. Following addition of 25 µL of the 4 mg/mL MTT solution to each well, and 3-h incubation at 37 °C, 100 µL of DMSO was added to dissolve formazan crystals. Metabolic activity of cells was estimated by measurement of absorbance at 570 nm and 620 nm (using Victor3 microplate reader) and comparison to control samples (untreated cells, i.e., 0% extract). Each experiment was repeated 3 times. Statistical significance of differences between results of experiments with extracts and controls was tested using ANOVA with a Tukey post hoc test. The differences were considered significant when p < 0.05.
4.10. Chemical Analyses
In order to extract secondary metabolites, each liquid culture of Streptomyces isolate was poured into a flask with an equal volume of ethyl acetate and agitated overnight at 100 rpm at room temperature. The organic phase was separated and dried over anhydrous MgSO4, and the solvent was evaporated on a rotatory evaporator.
Crude extracts were analyzed by liquid chromatography UHPLC (Nexera-i, Shimadzu) with a Kinetex-C8 column (2.1 mm × 100 mm, 2.6 µm, 100 Å) using 15 min linear gradient of 5–100% B (B-80% acetonitrile) in 0.1% aqueous trifluoroacetic acid, and liquid chromatography–mass spectrometry, using LC-MS-IT-TOF (Shimadzu, Kyoto, Japan) with a Kromasil-C8 column (1 mm × 250 mm, 5 µm, 90 Å). The mass detection was performed in positive ion mode.
The screening for known compounds was performed using the Dictionary of Natural Products database, version 30.1 (https://dnp.chemnetbase.com/faces/chemical/ChemicalSearch.xhtmlm, accessed on 14 September 2021) with the following search parameters: biological source of natural product and the accurate molecular mass. Compounds were considered to be preliminarily identified when the difference in accurate mass was lower than 0.05.
Acknowledgments
The authors thank Jurand Sobiecki for his assistance at early stages of this project, Beata Furmanek for providing compounds for bacteriological media, and members of the Laboratory of Electron Microscopy of University of Gdansk for an excellent service during bacteriophage analyses.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10101212/s1, Figure S1: Antibacterial and antifungal activities of newly isolated Streptomyces strains M4_24 and M5_8, as revealed by the streak-test. Name of the Streptomyces strain and time of incubation are shown at the bottom of each panel. Names of streaked bacterial and fungal strains (and their origin (collection) are provided in left and right panels.
Author Contributions
Conceptualization, D.L., K.K.-K., Ł.G., P.G., E.W., L.G., K.P., G.W. and A.W.; methodology, W.J., D.L., K.K.-K., P.G., Ł.G., E.W., L.G. and K.P.; investigation, W.J., P.B., D.L., K.K.-K., Ł.G., E.W., W.D., L.G. and K.P.; resources, D.L.; data curation, D.L. and P.G.; writing—original draft preparation, W.J., D.L., K.K.-K., P.G., G.W. and A.W.; writing—review and editing, W.J., K.K.-K., Ł.G., P.G., L.G., K.P., G.W. and A.W.; visualization, W.J., P.B., D.L., K.K.-K., Ł.G., E.W., L.G., and K.P.; supervision, P.G., K.P., G.W. and A.W.; project administration, P.G., K.P., G.W. and A.W.; funding acquisition, G.W. and A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Gdansk (task grant no. 531-D020-D242-21) and the Institute of Biochemistry and Biophysics of Polish Academy of Sciences (task grant no. PN-32).
Data Availability Statement
DNA sequences determined in this study were deposited in GenBank with accession numbers KU643201.1, KU643207.1, MG758033.1, and MG758033.1.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morehead M.S., Scarbrough C. Emergence of Global Antibiotic Resistance. Prim. Care. 2018;45:467–484. doi: 10.1016/j.pop.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Aslam B., Wang W., Arshad M.I., Khurshid M., Muzammil S., Rasool M.H., Nisar M.A., Alvi R.F., Aslam M.A., Qamar M.U., et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018;11:1645–1658. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bragg R.R., Meyburgh C.M., Lee J.-Y., Coetzee M. Potential Treatment Options in a Post-Antibiotic Era. Adv. Exp. Med. Biol. 2018;1052:51–61. doi: 10.1007/978-981-10-7572-8_5. [DOI] [PubMed] [Google Scholar]
- 4.Cyske Z., Jaroszewicz W., Żabińska M., Lorenc P., Sochocka M., Bielańska P., Grabowski Ł., Gaffke L., Pierzynowska K., Węgrzyn G. Unexplored Potential: Biologically Active Compounds Produced by Microorganisms from Hard-to-Reach Environments and Their Applications. Acta Biochim. Pol. 2021 doi: 10.18388/abp.2020_5887. [DOI] [PubMed] [Google Scholar]
- 5.Rangseekaew P., Pathom-aree W. Cave Actinobacteria as Producers of Bioactive Metabolites. Front. Microbiol. 2019;10:387. doi: 10.3389/fmicb.2019.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yücel S., Yamaç M. Selection of Streptomyces Isolates from Turkish Karstic Caves against Antibiotic Resistant Microorganisms. Pak. J. Pharm. Sci. 2010;23:1–6. [PubMed] [Google Scholar]
- 7.Jiang Z., Guo L., Chen C., Liu S., Zhang L., Dai S., He Q., You X., Hu X., Tuo L., et al. Xiakemycin A, a Novel Pyranonaphthoquinone Antibiotic, Produced by the Streptomyces Sp. CC8-201 from the Soil of a Karst Cave. J. Antibiot. 2015;68:771–774. doi: 10.1038/ja.2015.70. [DOI] [PubMed] [Google Scholar]
- 8.Klusaite A., Vickackaite V., Vaitkeviciene B., Karnickaite R., Bukelskis D., Kieraite-Aleksandrova I., Kuisiene N. Characterization of Antimicrobial Activity of Culturable Bacteria Isolated from Krubera-Voronja Cave. Int. J. Speleol. 2016;45:275–287. doi: 10.5038/1827-806X.45.3.1978. [DOI] [Google Scholar]
- 9.Adam D., Maciejewska M., Naômé A., Martinet L., Coppieters W., Karim L., Baurain D., Rigali S. Isolation, Characterization, and Antibacterial Activity of Hard-to-Culture Actinobacteria from Cave Moonmilk Deposits. Antibiotics. 2018;7:28. doi: 10.3390/antibiotics7020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doull J.L., Singh A.K., Hoare M., Ayer S.W. Conditions for the Production of Jadomycin B by Streptomyces Venezuelae ISP5230: Effects of Heat Shock, Ethanol Treatment and Phage Infection. J. Ind. Microbiol. 1994;13:120–125. doi: 10.1007/BF01584109. [DOI] [PubMed] [Google Scholar]
- 11.Lu N., Sun Y., Wang Q., Qiu Y., Chen Z., Wen Y., Wang S., Song Y. Cloning and Characterization of Endolysin and Holin from Streptomyces Avermitilis Bacteriophage PhiSASD1 as Potential Novel Antibiotic Candidates. Int. J. Biol. Macromol. 2020;147:980–989. doi: 10.1016/j.ijbiomac.2019.10.065. [DOI] [PubMed] [Google Scholar]
- 12.Herath K.B., Jayasuriya H., Guan Z., Schulman M., Ruby C., Sharma N., MacNaul K., Menke J.G., Kodali S., Galgoci A., et al. Anthrabenzoxocinones from Streptomyces Sp. as Liver X Receptor Ligands and Antibacterial Agents. J. Nat. Prod. 2005;68:1437–1440. doi: 10.1021/np050176k. [DOI] [PubMed] [Google Scholar]
- 13.Herold K., Gollmick F.A., Groth I., Roth M., Menzel K.-D., Möllmann U., Gräfe U., Hertweck C. Cervimycin A-D: A Polyketide Glycoside Complex from a Cave Bacterium Can Defeat Vancomycin Resistance. Chemistry. 2005;11:5523–5530. doi: 10.1002/chem.200500320. [DOI] [PubMed] [Google Scholar]
- 14.Nakaew N., Pathom-aree W., Lumyong S. First Record of the Isolation, Identification and Biological Activity of a New Strain of Spirillospora Albida from Thai Cave Soil. Actinomycetologica. 2009;23:1–7. doi: 10.3209/saj.SAJ230102. [DOI] [Google Scholar]
- 15.Nakaew N., Pathom-aree W., Lumyong S. Generic Diversity of Rare Actinomycetes from Thai Cave Soils and Their Possible Use as New Bioactive Compounds. Actinomycetologica. 2009;23:21–26. doi: 10.3209/saj.SAJ230201. [DOI] [Google Scholar]
- 16.Rajput Y., Biswas J., Rai V. Potentiality Test in Antimicrobial Activity and Antibiotic Sensitivity of Subterranean Streptomyces Strains Isolated from Kotumsar Cave of India. Int. J. Biol. Chem. 2012;6:53–60. doi: 10.3923/ijbc.2012.53.60. [DOI] [Google Scholar]
- 17.Stankovic N., Radulovic V., Petkovic M., Vuckovic I., Jadranin M., Vasiljevic B., Nikodinovic-Runic J. Streptomyces Sp. JS520 Produces Exceptionally High Quantities of Undecylprodigiosin with Antibacterial, Antioxidative, and UV-Protective Properties. Appl. Microbiol. Biotechnol. 2012;96:1217–1231. doi: 10.1007/s00253-012-4237-3. [DOI] [PubMed] [Google Scholar]
- 18.Tomova I., Lazarkevich I., Tomova A., Kambourova M., Vasileva-Tonkova E. Diversity and Biosynthetic Potential of Culturable Aerobic Heterotrophic Bacteria Isolated from Magura Cave, Bulgaria. IJS. 2013;42:65–76. doi: 10.5038/1827-806X.42.1.8. [DOI] [Google Scholar]
- 19.Cheeptham N., Sadoway T., Rule D., Watson K., Moote P., Soliman L., Azad N., Donkor K., Horne D. Cure from the Cave: Volcanic Cave Actinomycetes and Their Potential in Drug Discovery. Int. J. Speleol. 2013;42:35–47. doi: 10.5038/1827-806X.42.1.5. [DOI] [Google Scholar]
- 20.Riquelme C., Enes Dapkevicius M.d.L., Miller A.Z., Charlop-Powers Z., Brady S., Mason C., Cheeptham N. Biotechnological Potential of Actinobacteria from Canadian and Azorean Volcanic Caves. Appl. Microbiol. Biotechnol. 2017;101:843–857. doi: 10.1007/s00253-016-7932-7. [DOI] [PubMed] [Google Scholar]
- 21.Maciejewska M., Adam D., Martinet L., Naômé A., Całusińska M., Delfosse P., Carnol M., Barton H.A., Hayette M.-P., Smargiasso N., et al. A Phenotypic and Genotypic Analysis of the Antimicrobial Potential of Cultivable Streptomyces Isolated from Cave Moonmilk Deposits. Front. Microbiol. 2016;7:1455. doi: 10.3389/fmicb.2016.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belyagoubi L., Belyagoubi-Benhammou N., Jurado V., Dupont J., Lacoste S., Djebbah F., Ounadjela F., Benaissa S., Habi S., Abdelouahid D., et al. Antimicrobial Activities of Culturable Microorganisms (Actinomycetes and Fungi) Isolated from Chaabe Cave, Algeria. IJS. 2018;47:189–199. doi: 10.5038/1827-806X.47.2.2148. [DOI] [Google Scholar]
- 23.Ambrožič Avguštin J., Petrič P., Pašić L. Screening the Cultivable Cave Microbial Mats for the Production of Antimicrobial Compounds and Antibiotic Resistance. IJS. 2019;48:295–303. doi: 10.5038/1827-806X.48.3.2272. [DOI] [Google Scholar]
- 24.Paun V.I., Lavin P., Chifiriuc M.C., Purcarea C. First Report on Antibiotic Resistance and Antimicrobial Activity of Bacterial Isolates from 13,000-Year Old Cave Ice Core. Sci. Rep. 2021;11:514. doi: 10.1038/s41598-020-79754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakaew N., Sungthong R., Yokota A., Lumyong S. Nonomuraea Monospora Sp. Nov., an Actinomycete Isolated from Cave Soil in Thailand, and Emended Description of the Genus Nonomuraea. Int. J. Syst. Evol. Microbiol. 2012;62:3007–3012. doi: 10.1099/ijs.0.035220-0. [DOI] [PubMed] [Google Scholar]
- 26.Derewacz D.K., McNees C.R., Scalmani G., Covington C.L., Shanmugam G., Marnett L.J., Polavarapu P.L., Bachmann B.O. Structure and Stereochemical Determination of Hypogeamicins from a Cave-Derived Actinomycete. J. Nat. Prod. 2014;77:1759–1763. doi: 10.1021/np400742p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang L., Pu H., Xiang J., Su M., Yan X., Yang D., Zhu X., Shen B., Duan Y., Huang Y. Huanglongmycin A-C, Cytotoxic Polyketides Biosynthesized by a Putative Type II Polyketide Synthase From Streptomyces Sp. CB09001. Front. Chem. 2018;6:254. doi: 10.3389/fchem.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojiri K., Nakajima S., Fuse A., Suzuki H., Suda H. BE-24566B, a New Antibiotic Produced by Streptomyces Violaceusniger. J. Antibiot. 1995;48:1506–1508. doi: 10.7164/antibiotics.48.1506. [DOI] [PubMed] [Google Scholar]
- 29.Lü Y., Yue C., Shao M., Qian S., Liu N., Bao Y., Wang M., Liu M., Li X., Wang Y., et al. Molecular Genetic Characterization of an Anthrabenzoxocinones Gene Cluster in Streptomyces Sp. FJS31-2 for the Biosynthesis of BE-24566B and Zunyimycin Ale. Molecules. 2016;21:711. doi: 10.3390/molecules21060711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lü Y., Shao M., Wang Y., Qian S., Wang M., Wang Y., Li X., Bao Y., Deng C., Yue C., et al. Zunyimycins B and C, New Chloroanthrabenzoxocinones Antibiotics against Methicillin-Resistant Staphylococcus Aureus and Enterococci from Streptomyces Sp. FJS31-2. Molecules. 2017;22:251. doi: 10.3390/molecules22020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mei X., Yan X., Zhang H., Yu M., Shen G., Zhou L., Deng Z., Lei C., Qu X. Expanding the Bioactive Chemical Space of Anthrabenzoxocinones through Engineering the Highly Promiscuous Biosynthetic Modification Steps. ACS Chem. Biol. 2018;13:200–206. doi: 10.1021/acschembio.7b00743. [DOI] [PubMed] [Google Scholar]
- 32.Zhu C., Lew C.I., Neuhaus G.F., Adpressa D.A., Zakharov L.N., Kaweesa E.N., Plitzko B., Loesgen S. Biodiversity, Bioactivity, and Metabolites of High Desert Derived Oregonian Soil Bacteria. Chem. Biodivers. 2021;18:e2100046. doi: 10.1002/cbdv.202100046. [DOI] [PubMed] [Google Scholar]
- 33.Morshed M.T., Lacey E., Vuong D., Lacey A.E., Lean S.S., Moggach S.A., Karuso P., Chooi Y.-H., Booth T.J., Piggott A.M. Chlorinated Metabolites from Streptomyces Sp. Highlight the Role of Biosynthetic Mosaics and Superclusters in the Evolution of Chemical Diversity. Org. Biomol. Chem. 2021;19:6147–6159. doi: 10.1039/D1OB00600B. [DOI] [PubMed] [Google Scholar]
- 34.Łubowska N., Grygorcewicz B., Kosznik-Kwaśnicka K., Zauszkiewicz-Pawlak A., Węgrzyn A., Dołęgowska B., Piechowicz L. Characterization of the Three New Kayviruses and Their Lytic Activity Against Multidrug-Resistant Staphylococcus Aureus. Microorganisms. 2019;7:471. doi: 10.3390/microorganisms7100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Linde K., Lim B.T., Rondeel J.M.M., Antonissen L.P.M.T., de Jong G.M.T. Improved Bacteriological Surveillance of Haemodialysis Fluids: A Comparison between Tryptic Soy Agar and Reasoner’s 2A Media. Nephrol. Dial. Transplant. 1999;14:2433–2437. doi: 10.1093/ndt/14.10.2433. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J., Russell D.W. Molecular Cloning: A Laboratory Manual. 3rd ed. Cold Spring Harbor Laboratory; New York, NY, USA: 2001. [Google Scholar]
- 37.Kosznik-Kwaśnicka K., Ciemińska K., Grabski M., Grabowski Ł., Górniak M., Jurczak-Kurek A., Węgrzyn G., Węgrzyn A. Characteristics of a Series of Three Bacteriophages Infecting Salmonella Enterica Strains. Int. J. Mol. Sci. 2020;21:6152. doi: 10.3390/ijms21176152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saitou N., Nei M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 39.Jukes T.H., Cantor C.R. Evolution of Protein Molecules. In: Munro H.N., editor. Mammalian Protein Metabolism. Academic Press; New York, NY, USA: 1969. pp. 21–132. [Google Scholar]
- 40.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu S., Kim T., Yoo K.H., Kang K. The T47D Cell Line Is an Ideal Experimental Model to Elucidate the Progesterone-Specific Effects of a Luminal A Subtype of Breast Cancer. Biochem. Biophys. Res. Commun. 2017;486:752–758. doi: 10.1016/j.bbrc.2017.03.114. [DOI] [PubMed] [Google Scholar]
- 42.Pierzynowska K., Gaffke L., Hać A., Mantej J., Niedziałek N., Brokowska J., Węgrzyn G. Correction of Huntington’s Disease Phenotype by Genistein-Induced Autophagy in the Cellular Model. Neuro Mol. Med. 2018;20:112–123. doi: 10.1007/s12017-018-8482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar P., Nagarajan A., Uchil P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018;2018 doi: 10.1101/pdb.prot095505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences determined in this study were deposited in GenBank with accession numbers KU643201.1, KU643207.1, MG758033.1, and MG758033.1.