Abstract
We have previously shown that activation of extracellular signal-regulated kinase (Erk) by epidermal growth factor (EGF) treatment was significantly decreased in mouse fibroblast cells expressing a mutant Shp-2 molecule lacking 65 amino acids in the SH2-N domain, Shp-2Δ46-110. To address the molecular mechanism for the positive role of Shp-2 in mediating Erk induction, we evaluated the activation of signaling components upstream of Erk in Shp-2 mutant cells. EGF-stimulated Ras, Raf, and Mek activation was significantly attenuated in Shp-2 mutant cells, suggesting that Shp-2 acts to promote Ras activation or to suppress the down-regulation of activated Ras. Biochemical analyses indicate that upon EGF stimulation, Shp-2 is recruited into a multiprotein complex assembled on the Gab1 docking molecule and that Shp-2 seems to exert its biological function by specifically dephosphorylating an unidentified molecule of 90 kDa in the complex. The mutant Shp-2Δ46-110 molecule failed to participate in the Gab1-organized complex for dephosphorylation of p90, correlating with a defective activation of the Ras-Raf-Mek-Erk cascade in EGF-treated Shp-2 mutant cells. Evidence is also presented that Shp-2 does not appear to modulate the signal relay from EGF receptor to Ras through the Shc, Grb2, and Sos proteins. These results begin to elucidate the mechanism of Shp-2 function downstream of a receptor tyrosine kinase to promote the activation of the Ras-Erk pathway, with potential therapeutic applications in cancer treatment.
The extracellular signal-regulated kinase (Erk) is highly conserved during evolution from lower eukaryotes, such as yeast, to mammals, and it plays an important role in mediating cellular responses to growth factors and cytokines (43). Erk is directly activated by Mek through dual phosphorylation of a threonine and a tyrosine residue (40, 43). Mek, in turn, is activated by Raf, which is believed to be activated by binding to Ras:GTP (8, 30). Each step of the linear signaling cascade in the Ras pathway can be subjected to modulation by other components, either negative or positive, particularly in mammalian cells. This multilevel regulation creates signal convergence or branching and forms a complicated signaling network in high-eukaryote cells about which little is known. Also poorly understood is the mechanism by which the Ras pathway is activated by a receptor protein tyrosine kinase (R-PTK) or a receptor-associated PTK. One proposed mechanism is that an autophosphorylated R-PTK, such as the epidermal growth factor (EGF) receptor (EGF-R), recruits the Grb2-Sos complex to the membrane, thereby obtaining access to and activating Ras (5, 10, 17, 32, 41). However, this mechanism likely represents only one aspect of many that signals to the Ras-Erk pathway.
Shp-2 is a widely expressed protein tyrosine phosphatase (PTP) that has two tandem repeats of Src homology 2 (SH2) domains at the N-terminal portion (13, 34). This phosphatase seems to participate in signaling events proximal to a variety of R-PTKs, although the complete mechanism is not understood. Interestingly, Shp-2 becomes tyrosine phosphorylated in cells treated with a number of growth factors and cytokines, which leads to its interaction with Grb2 (4, 16, 27). Therefore, it has been hypothesized that Shp-2 acts as an adapter protein to recruit the Grb2-Sos complex to the plasma membrane, thereby contributing to Ras activation. However, mutating the putative Grb2 binding sites did not interfere with the Shp-2 function in mediating the activation of Erk (3, 51). On the other hand, several lines of evidence indicate that the phosphatase catalytic activity is required for Shp-2 function in promoting Erk kinase induction (3, 33, 36, 51). The critical issue is to identify the Shp-2 substrate(s) and determine how a dephosphorylation event(s) contributes to the stimulation of the Ras pathway. Molecular and genetic analyses of Corkscrew (Csw), the Drosophila homologue of Shp-2, have led to the identification of the Daughter of Sevenless (Dos) protein as a putative Csw substrate (20, 39). Dos contains a pleckstrin homology (PH) domain at the N terminus and multiple potential tyrosine phosphorylation sites over the C-terminal portion, and it may act as a docking molecule for a variety of SH2-containing proteins. Genetic epistasis experiments suggest that Csw works together with Dos in signal relay from Sevenless R-PTK to Ras1 in the R7 cell development. Dos was preferentially trapped by a catalytically inactive mutant of Csw in its tyrosine-phosphorylated form (20). Interestingly, a human protein, Gab1 (Grb2-associated binder 1), that was cloned through its physical interaction with Grb2 has the same molecular architecture as Drosophila Dos (21). Several lines of evidence implicate Gab1 in mediating signal transduction into the Ras-Erk pathway from receptors for growth factors and cytokines (15, 22, 50). More recently, we and others have reported another molecule, Gab2, that is structurally related to Gab1 and Dos (18, 35, 56). Both Gab1 and Gab2 are associated with Shp-2 in a tyrosine phosphorylation-dependent fashion and seem to participate in multiple signaling pathways. It remains to be determined how Shp-2 works in concert with Gab1 and/or Gab2 in information flow to Ras from R-PTKs.
In previous experiments, we created a targeted mutant Shp-2 allele in mice which expresses a protein with a deletion of 65 amino acids in the N-terminal SH2 (SH2-N) domain, Shp-2Δ46-110 (42). Homozygous mutant (Shp-2−/−) animals die in the uterus at midgestation with a variety of defects in the mesodermal patterning. Using fibroblast cell lines derived from wild-type and mutant littermates, we demonstrated that Shp-2 plays a positive role in EGF-stimulated Erk activation (45). Chimeric animal analysis with Shp-2−/− embryonic stem cells revealed a signal-enhancing effect of Shp-2 for EGF-R in mammalian development, which is substantiated by genetic epistasis data between the Shp-2 mutant allele and a hypomorphic mutant EGF-R allele, waved-2 (37).
In this study, we have further examined EGF stimulation of the Ras pathway in Shp-2 mutant cells. The results suggest a novel mechanism for Shp-2 function by working in concert with Gab1 as well as a Gab1-associated protein, p90, in promoting the activation of Ras-Erk kinase cascade.
MATERIALS AND METHODS
Cell lines and reagents.
Wild-type and mutant (Shp-2Δ46-110) mouse embryonic fibroblast cell lines were described previously (45). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum. Recombinant mouse EGF was purchased from Becton Dickinson Labware. Antibodies against phosphotyrosine (anti-PY; pY99), Shp-2, Mek-1, Raf-1, glutathione S-transferase (GST), Grb2, Sos-1, and EGF-R were from Santa Cruz Biotechnology, Inc. Anti-Ras antibody (Y13-259) was purchased from Oncogene Sciences, and rabbit anti-Gab1 antiserum was raised against a GST fusion protein containing amino acids 116 to 695 of Gab1. The Superose 6 HR10/30 gel filtration column and high- and low-molecular-weight marker kits were from Pharmacia Biotech Inc. GST fusion proteins containing either the SH2-N or SH2-C domain of Shp-2, Erk, or Mek were expressed in Escherichia coli and affinity purified with glutathione-Sepharose 4B beads (Pharmacia Biotech) using the standard protocol (9, 14).
Immunoprecipitation and immunoblotting.
Control or factor-stimulated cell lysates were prepared with cell lysis buffer (14). Specific antibodies were added to the lysates together with protein A/G-Sepharose 4B beads (Pharmacia Biotech). After incubation at 4°C for 1.5 h, the immunocomplex was washed with HNTG buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 0.1% Triton X-100, 10% glycerol, 1 mM Na3VO4, 0.1 mM ZnCl2, 1 mM phenylmethylsulfonylfluoride, 10 μg each of aprotinin and leupeptin per ml) and then resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to a nitrocellulose membrane and blotted with appropriate antibodies, and signals were detected by enhanced chemiluminescence (ECL analysis kit; Amersham Corp.).
Mek and Raf kinase assay.
The coupled in vitro assays for Mek and Raf kinases were performed basically as previously described (44, 48, 52). Briefly, to measure Mek activity, Mek-1 protein was immunoprecipitated from control or EGF-stimulated cell lysates by anti-Mek-1 antibody. The beads were washed with HNTG buffer and kinase assay buffer (KAB; 20 mM HEPES [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol [DTT], 1 mM Na3VO4) followed by incubation with GST-Erk1 (0.04 μg/μl) and 80 μM ATP in 25 μl KAB for 15 min at 30°C. The supernatant (5 μl) was then mixed with 30 μl of reaction buffer containing myelin basic protein (MBP; 0.67 μg/μl; Gibco), 80 μM ATP, and 5 μCi of [γ-32P]ATP in KAB and incubated for an additional 10 min at 30°C. The incorporation of 32P into MBP was determined as previously described (45) to assess the Mek kinase activity. The kinase activity of Raf was measured by a coupled in vitro kinase assay. Immunoprecipitated Raf-1 protein was washed with HNTG buffer and then with reaction buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 75 mM NaCl, 5 mM EGTA, 1 mM DTT, 1 mM Na3VO4) followed by incubation at 30°C for 20 min with 0.8 μM GST-Mek, 0.8 μM GST-Erk1, and 160 μM ATP. A fraction of the first reaction was transferred to a second reaction mix containing MBP (0.4 μg/μl), 50 μM ATP, and 5 μCi of [γ-32P]ATP and incubated for additional 10 min at 30°C. The Raf kinase activity was reported as the phosphorylation extent of MBP.
Ras activity measurement.
The activity of Ras was determined by the ratio between Ras:GTP and Ras:GDP as described previously (2). Serum-starved cells were in vivo labeled with [32P]-phosphorus (ICN Biomedicals, Inc.) in phosphate-free DMEM followed by stimulation with EGF (100 ng/ml) for various times. Ras protein was then immunoprecipitated from cell lysates with anti-Ras antibody (Y13-259) and Gammabind G Sepharose beads (Pharmacia Biotech). After extensive washes with cell lysis buffer, GTP and GDP were eluted by boiling in the elution buffer (2 mM EDTA, 0.2% SDS, 2 mM DTT). The eluted GTP and GDP were resolved by thin-layer chromatography (TLC) on TLC plates (J. T. Baker) developed in 1 M KH2PO4 (pH 3.5). After autoradiography, GTP and GDP spots were scraped off the TLC plate and quantitated by scintillation counting (Beckman).
Gel filtration chromatography.
A Superose 6 HR10/30 gel filtration column was calibrated with molecular markers including thyroglobulin (669 kDa), apoferritin (440 kDa), catalase (232 kDa), aldolase (158 kDa), bovine serum albumin (66 kDa), chymotrypsin (25 kDa), and RNase A (13.7 kDa). A cell lysate containing 0.5 mg of protein was applied at a flow rate of 0.25 ml/min onto the column, which had been previously equilibrated in 50 mM HEPES (pH 7.5)–150 mM NaCl–0.5 mM EDTA–0.1% Triton X-100 and eluted with the same buffer. Proteins in 750-μl fractions were precipitated with 10% trichloroacetic acid. The precipitates were washed once with acetone, resolved in SDS loading buffer, and subjected to immunoblot analysis.
Far-Western blotting.
Direct interaction between Gab1 and Shp-2 SH2 domains was examined by far-Western blot analysis following a published protocol (6). Gab1 protein was immunoprecipitated from control and EGF-stimulated cell lysates. The immunocomplex was then resolved on an SDS-polyacrylamide gel and transferred to nitrocellulose membrane. The membrane was overlaid with 2 μg purified GST or GST fusion proteins per ml and blotted by anti-GST antibody. The signals were detected by ECL reaction after incubation with the appropriate horseradish peroxidase-conjugated secondary antibody.
RESULTS
Pinpointing the role of Shp-2 in the Ras-Erk pathway.
In previous experiments, we compared growth factor-induced Erk activity between wild-type (+/+) and Shp-2 mutant (−/−) fibroblast cells and found that Erk activation was either severely decreased or blocked in −/− cells (45). This observation points to a positive role of Shp-2 in mediating mitogenic stimulation of Erk, consistent with results from other groups with ectopic expression of a catalytically inactive mutant of Shp-2 (33, 36, 51). To pinpoint the position of Shp-2 that acts in the cytoplasmic signaling cascade leading to Erk activation, we examined the activity of signaling components step by step, upward from Erk, under EGF stimulation.
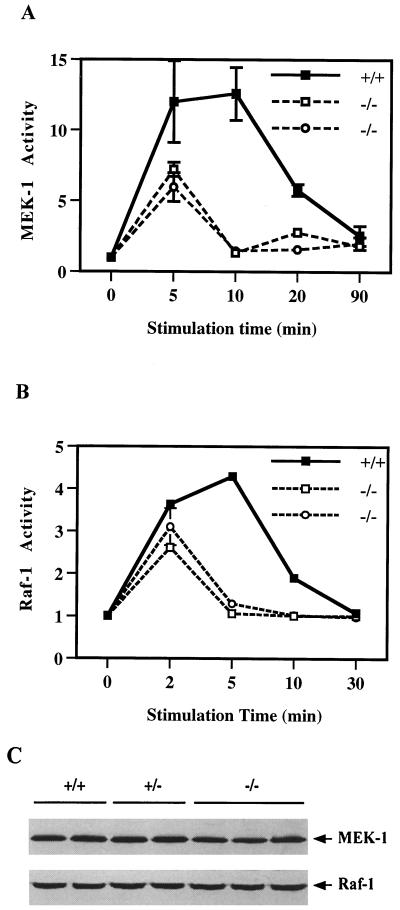
By measuring GST-Erk activity on MBP as a substrate in vitro, we first assessed the activation of Mek, after EGF treatment for 5, 10, 20, and 90 min, in one wild-type and two Shp-2−/− cell lines. As shown in Fig. 1A, EGF induced a transient activation of Mek, with kinetics similar to that for Erk kinase induction, in wild-type cells (45). However, Mek activation by EGF was significantly diminished in Shp-2−/− cells and was remarkably similar to the pattern of reduced Erk induction (45). The Mek activity level did not reach that in wild-type cells and rapidly decreased to the basal level by 10 min of EGF treatment, at which time it had peaked in wild-type cells.
FIG. 1.
Reduced activation of Mek and Raf in Shp-2 mutant cells. Fibroblasts of one wild-type (+/+) and two homozygous Shp-2 mutant (−/−) cell lines were starved in serum-free DMEM medium for 36 h and then stimulated with EGF (100 ng/ml) for indicated times. Mek (A) and Raf (B) kinase activities were measured by a coupled in vitro kinase assay as described in the text. Incorporation of 32P into MBP was determined by scintillation counting to reflect the kinase activities. The protein expression levels of Mek-1 and Raf-1 in two wild-type (+/+), two heterozygous (+/−), and three homozygous mutant (−/−) cell lines were evaluated by immunoblot analysis using specific antibodies against Mek-1 and Raf-1, respectively (C).
We then evaluated the activation of Raf-1, the kinase that activates Mek, using a coupled Mek-Erk kinase assay in vitro. EGF-induced Raf activity was attenuated and decayed much faster in Shp-2−/− cells than in wild-type cells (Fig. 1B). Thus, defective Erk activation in Shp-2 mutant cells is most likely due to a defect in the activation of its upstream kinases, Raf and Mek, in the signaling cascade. To determine whether this result could be explained by decreased expression of these kinases, we performed immunoblot analysis using two wild-type, two Shp-2+/−, and three Shp-2−/− cell lines and found no difference in either Mek-1 or Raf-1 kinase amounts between wild-type and mutant cell lines (Fig. 1C). This result indicates that the difference in EGF-induced kinase activation is not due to an alteration of protein levels but rather is secondary to kinase functional defects.
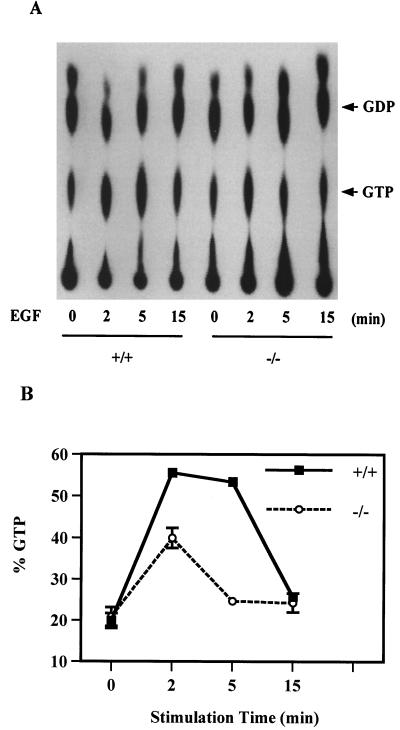
As Raf is apparently activated by binding to active Ras in its GTP-bound form, we next examined Ras activation in wild-type and Shp-2−/− cells, by measuring the ratio of Ras:GTP to Ras:GDP, after EGF stimulation of serum-starved cells for 2, 5, and 15 min (Fig. 2). The relative amount of active Ras:GTP was significantly lower in Shp-2−/− cells than in wild-type cells at 2 min. More strikingly, the active Ras:GTP level had already returned to the basal level by 5 min in Shp-2−/− cells, while a peak level was observed in wild-type cells at this time point. Together, the results in Fig. 1 and 2 suggest that reduced Erk kinase activation in Shp-2−/− cells is due to an incomplete or abortive induction of Ras, Raf, and Mek activities.
FIG. 2.
Decreased Ras activation in Shp-2 mutant cells. Ras protein was immunoprecipitated from 32P-labeled control and EGF-stimulated cell lysates. Following extensive washes with cell lysis buffer, GTP and GDP were eluted from the precipitates and resolved by TLC followed by visualization with autoradiography on X-ray film (A). GTP and GDP spots were removed from the TLC plate and counted in a Beckman scintillation counter. The ratio GTP/(GDP + GTP) was calculated to represent Ras activity (B). Data were averaged from samples of three independent experiments.
Biochemical evidence that the Shp-2Δ46-110 molecule is inert in cells.
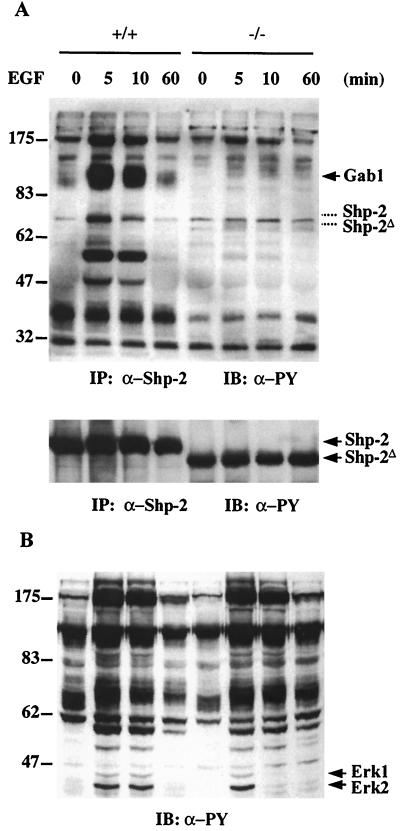
To explore the biochemical basis for Shp-2 function in promoting Ras activation, we searched for differences in protein complex formation between the wild-type and mutant molecules before and after EGF stimulation. Shp-2 was precipitated with a specific antibody raised against the C-terminal tail of Shp-2 that reacts with both wild-type and Shp-2Δ46-110 molecules (38), and the resultant precipitates were immunoblotted with anti-PY antibody. Shp-2 was not significantly tyrosine phosphorylated in response to EGF; however, it became complexed with a number of tyrosine-phosphorylated proteins in wild-type but not mutant cells (Fig. 3A). The overall profiles of EGF-induced protein tyrosine phosphorylation between the wild-type and mutant cells were not significantly different, as detected by immunoblotting of the whole cell lysates with anti-PY antibody (Fig. 3B). This result suggests that the Shp-2Δ46-110 molecule does not exhibit an aberrant phosphatase activity in cells.
FIG. 3.
EGF-induced association of Shp-2 with tyrosine-phosphorylated proteins. (A) Shp-2 protein was immunoprecipitated (IP) from wild-type (+/+) or mutant (−/−) cell lysates, resolved by SDS-PAGE, and immunoblotted (IB) with anti-PY antibody. The same membrane was then stripped and reprobed with an anti-Shp-2 antibody raised against the C-terminal region of Shp-2. The corresponding positions of wild-type and mutant Shp-2 bands were indicated with dashed lines in the upper panel. (B) Equal amounts of whole cell lysates from control or EGF-stimulated cells were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and blotted with an anti-PY antibody.
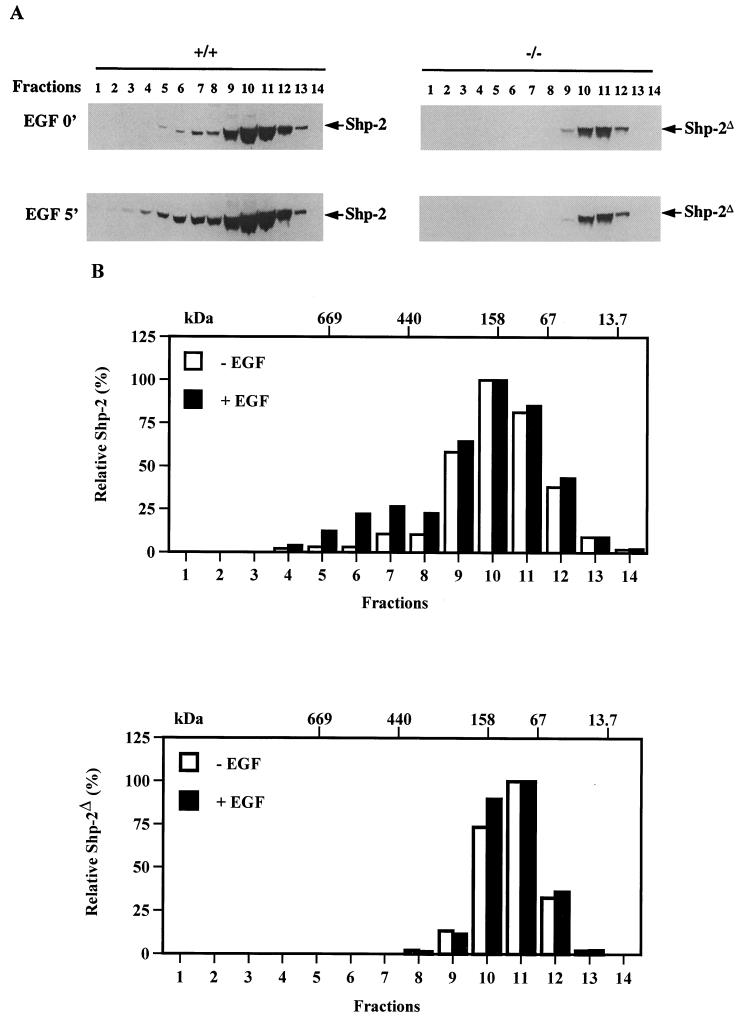
To further investigate whether the Shp-2Δ46-110 protein is able to associate with other cytoplasmic signaling proteins that are not detectable by the anti-PY antibody, we performed Superose gel filtration chromatography. This approach has been successfully used to identify protein complexes involved in cell signaling or cell cycle progression (31). Cell lysates prepared from control and EGF-stimulated fibroblasts were passed through a Superose 6 HR10/30 column, and fractions were collected in a volume of 750 μl. Immunoblot analysis with anti-Shp-2 antibody was done for each fraction to locate the wild-type and mutant Shp-2 proteins. Surprisingly, in unstimulated cells, most of the wild-type and mutant Shp-2 proteins were enriched in fractions with a molecular size of about 150 kDa (Fig. 4). This result suggests that the Shp-2 phosphatase is either a dimer or in a complex with another molecule(s), such as an inhibitor, in resting cells. By comparison, wild-type Shp-2 protein was distributed over a wider range of molecular sizes than Shp-2Δ46-110. Most interestingly, a significant portion of Shp-2 protein shifted to a larger complex of approximately 500 kDa in wild-type cells upon EGF stimulation (Fig. 4). In contrast, no new molecular complex was assembled for the Shp-2Δ46-110 molecule in EGF-stimulated mutant cells (Fig. 4). Thus, the Shp-2Δ46-110 molecule seems to be physiologically inert in cells, since it fails to engage in signaling complexes assembled after growth factor stimulation.
FIG. 4.
Distinct protein complexes of wild-type and mutant Shp-2 in response to EGF stimulation. Control and EGF-treated whole cell lysates were applied on a Superose 6 HR10/30 gel filtration column. Trichloroacetic acid-precipitated proteins from each fraction were separated by SDS-PAGE and immunoblotted with an anti-Shp-2 antibody. Estimated molecular masses were calculated based on the standard curve produced from the molecular weight markers described in the text. (A) Immunoblotting results; (B) densitometry scanning results of the immunoblots shown in panel A.
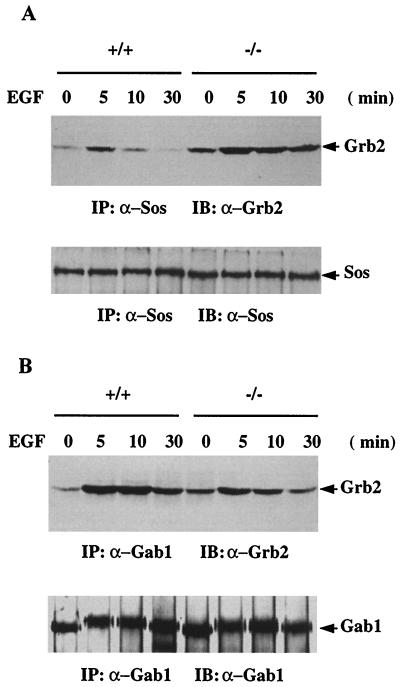
Shp-2 may act through interaction with Gab1.
As shown in Fig. 3A, Shp-2 was complexed with a number of phosphoproteins in EGF-treated wild-type cells. Notably, one protein of approximately 115 kDa was coprecipitated with Shp-2 phosphatase in its highly tyrosine-phosphorylated form in wild-type but not mutant cells. One possible candidate for this protein is Gab1, as Shp-2 was previously reported to associate with Gab1 at 115 kDa (21), and almost 50% of 32P labeling on Gab1 induced by EGF-R kinase was detected on the putative Shp-2 binding site (26). By reblotting the same filter with an anti-Gab1 antibody, we confirmed that it was indeed Gab1 (data not shown).
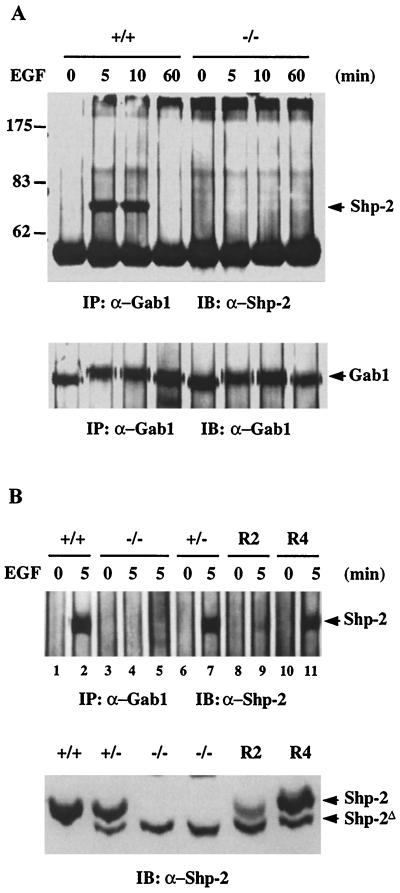
To further investigate the Shp-2–Gab1 interaction, we did a reciprocal coimmunoprecipitation experiment. Gab1 was precipitated from lysates of control and EGF-stimulated cells, and the precipitates were subjected to immunoblot analysis with anti-Shp-2. The results shown in Fig. 5A demonstrate that Shp-2 protein was coprecipitated with Gab1 in EGF-treated wild-type cells. This interaction is obviously transient, since the complex was observed after treatment with EGF for only 5 and 10 min and was dissociated at 60 min. No complex was detected for Gab1 and the Shp-2Δ46-110 molecule at any time point in mutant cells. To confirm this observation, we examined a number of cell lines, including +/+, −/−, +/−, and two −/− cell clones in which a wild-type Shp-2 cDNA was introduced (R2 and R4) (55), before and after EGF treatment for 5 min (Fig. 5B). Interestingly, Gab1 was selectively associated with the wild-type but not the mutant Shp-2 protein, even in the heterozygous (+/−) cells and in the R2 and R4 cells in which both proteins were expressed. As the expression level of the mutant protein was about fourfold lower than that of the wild type, we used fourfold more −/− cell lysate for the immunoprecipitation and still could not detect any mutant Shp-2 coprecipitated with Gab1 (Fig. 5B, lane 5).
FIG. 5.
Coimmunoprecipitation of Gab1 with wild-type but not mutant Shp-2. (A) Anti-Gab1 antiserum (2 μl) was added to 1 ml (1 mg of total protein) of cell lysates from Shp-2+/+ or Shp-2−/− cells stimulated by EGF at the indicated times and immunoprecipitated (IP). The precipitates were immunoblotted (IB) with an anti-Shp-2 antibody that recognizes both wild-type and Shp-2Δ46-110 proteins. The same membrane was then reprobed with an anti-Gab1 antiserum. (B) Gab1 protein was immunoprecipitated from EGF-stimulated wild-type (+/+), heterozygous (+/−), homozygous mutant (−/−), and two rescue cell lines, R2 and R4, in which the wild-type Shp-2 cDNA was reintroduced (55). Each immunoprecipitation was performed with 800 μg of total protein except in lane 5, which had 3 mg of total protein. In the lower panel, equal amounts (40 μg) of cell lysates were directly immunoblotted with the anti-Shp-2 antibody.
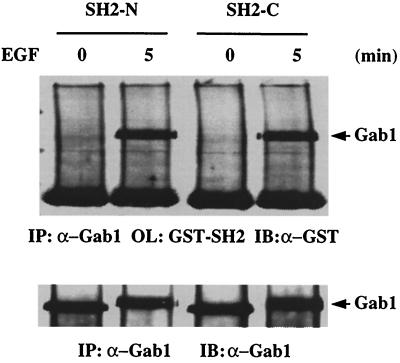
To prove a direct interaction between Gab1 and the Shp-2 SH2 domains, we performed far-Western blot analysis. Immunoprecipitated Gab1 protein was resolved on an SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was blotted with purified GST–SH2-N or GST–SH2-C protein and then probed with anti-GST antibody. Both SH2 domains were able to bind tyrosine-phosphorylated Gab1 in EGF-stimulated cell lysates (Fig. 6). Therefore, although the two SH2 domains of Shp-2 have a similar binding affinities toward Gab1, both of them are required for the interaction to occur in vivo, since the Shp-2Δ46-110 molecule with an intact SH2-C domain is unable to associate with Gab1 in mutant cells.
FIG. 6.
Direct recognition of tyrosine-phosphorylated Gab1 by Shp-2 SH2 domains. Gab1 protein was immunoprecipitated (IP) from control or EGF-stimulated wild-type cell lysates. The immunocomplex was resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and overlaid (OL) with the SH2-N or SH2-C GST fusion protein. Binding of the GST fusion proteins was detected by immunoblotting (IB) the membrane with anti-GST antibody.
Molecular mechanism of Shp-2 function in Ras activation.
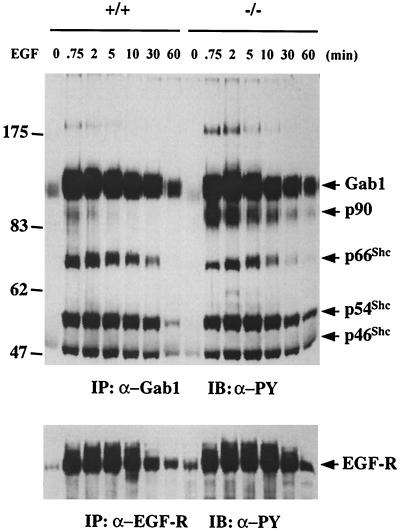
Both Gab1 and Shp-2 have been implicated in activation of the Ras-Erk pathway by growth factors and cytokines, although the mechanism is not understood. The fact that the inability of Shp-2Δ46-110 to participate in a Gab1-centered complex correlates with a defective Ras activation following EGF treatment suggests a physiological significance of the Gab1–Shp-2 partnership in EGF-R signaling. The distinct association of Gab1 with wild-type Shp-2 but not Shp-2Δ46-110 also offered a unique opportunity to determine if Gab1 or other Gab1-associated phosphoproteins are Shp-2 substrates in vivo. Since all of the evidence suggests that promotion of the Ras-Erk pathway by Shp-2 requires its catalytic activity, a dephosphorylation event that occurs in the Gab1 complex in wild-type but not mutant cells may account for the difference of Ras-Erk activation between the two cell types. To address this issue, we compared Gab1 phosphorylation levels in wild-type and mutant cells after EGF stimulation for various times (Fig. 7). Treatment with EGF for only 45 s induced a dramatic increase in Gab1 phosphorylation, and this high level of phosphorylation persisted for more than 30 min. A significant decrease in Gab1 phosphorylation was detected at 60 min, which correlates with the dissociation of the Gab1–Shp-2 complex observed at this time (Fig. 5). There was no obvious difference in the kinetics of EGF-induced Gab1 phosphorylation between wild-type and Shp-2 mutant cells or the tyrosine phosphorylation levels of the three Shc proteins associated with Gab1. However, one prominent difference between these two cell lines was the phosphorylation levels of a Gab1-associated protein at 90 kDa, p90. EGF-induced tyrosine phosphorylation of p90 was much lower and more transient in wild-type cells than in mutant cells. This result identified p90, but not Gab1 or Shc, as a good candidate substrate for the Shp-2 tyrosine phosphatase. A similar pattern of EGF-R tyrosine phosphorylation was observed between the two cell types after EGF treatment (Fig. 7), suggesting that Shp-2 may not have a direct role in modulating the EGF-R activation by EGF. We have previously reported that there is no difference in the expression levels of EGF-R between wild-type and Shp-2 mutant cells (29).
FIG. 7.
Functional interaction between Gab1 and Shp-2. Total cell lysates (1 mg of total proteins) from Shp-2+/+ or Shp-2−/− cells stimulated by 100 ng of EGF per ml for the indicated times were mixed with 2 μl of anti-Gab1 antibody and protein A-Sepharose 4B beads for immunoprecipitation (IP). The precipitates were then immunoblotted (IB) with anti-PY antibody to assess the tyrosine phosphorylation levels of Gab1 as well as Gab1-associated phosphoproteins. In the lower panel, the EGF-R was immunoprecipitated from the same cell lysates and immunoblotted with anti-PY antibody.
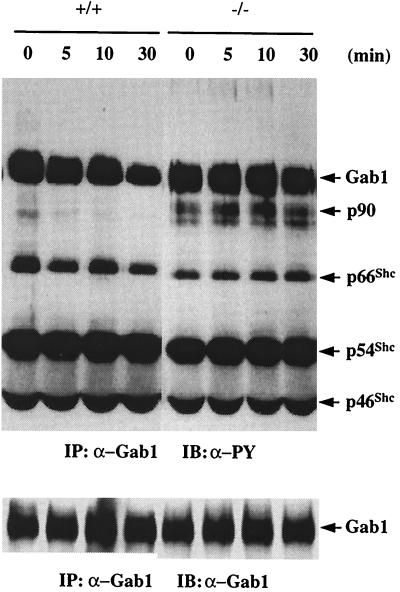
To assess further the effect of Shp-2 dephosphorylation on Gab1 and other phosphoproteins in the complex, we performed an in vitro phosphatase activity assay. Lysates were prepared from EGF-stimulated wild-type and Shp-2 mutant cells. The Gab1 complex was immunoprecipitated, washed with HNTG buffer without phosphatase inhibitor, and then incubated in the phosphatase buffer for various times. The complex was then subjected to immunoblot analysis with anti-PY antibody. Gab1 phosphorylation levels progressively decreased in the complex derived from wild-type cells, which contains Shp-2. More strikingly, Gab1-associated p90 was rapidly decreased in its tyrosine phosphorylation level in the complex originated from wild-type cells (Fig. 8). In contrast, no significant dephosphorylation was observed for Gab1 and p90 in the complex of mutant origin, which is devoid of Shp-2. There was no significant difference in phosphorylation observed in the three Gab1-associated Shc proteins between the two cell types. These results clearly demonstrate a substrate specificity for Shp-2 tyrosine phosphatase in vitro and also suggest that no other tyrosine phosphatase is involved in the Gab1 complex. Gab1 may not be the primary target of Shp-2 phosphatase, and Shc proteins are unlikely the Shp-2 substrates. Upon being recruited into the Gab1 complex, Shp-2 may dephosphorylate p90, an activity which is correlated with the up-regulation of the Ras pathway.
FIG. 8.
Dephosphorylation of Gab1 and Gab1-associated proteins by Shp-2 in vitro. Shp-2+/+ and Shp-2−/− cells were stimulated by 100 ng of EGF per ml for 45 s and lysed in cell lysis buffer; Gab1 was immunoprecipitated (IP) from the cell lysates. The precipitates were washed twice with HNTG buffer without phosphatase inhibitors and, twice with phosphatase buffer (50 mM imidazole [pH 7.5], 10 mM DTT, 5 mM EDTA) and then incubated in 20 μl of phosphatase buffer for 5, 10, and 30 min at 30°C. The reaction was stopped by 5 μl of 5× SDS sample buffer, immunoblotted (IB) by anti-PY antibody, and then reprobed with anti-Gab1 antibody.
Significance of Gab1, Grb2, and Sos interactions in Ras activation.
The aforementioned results raised an interesting point: Shp-2 may not modulate the Ras activation by acting on the Shc proteins that are known to work in concert with Grb2 and Sos. The tyrosine phosphorylation status of the Shc proteins and their association with Gab1 were not altered in Shp-2 mutant cells. The fact that Shp-2 is not significantly phosphorylated on tyrosine in EGF-treated wild-type fibroblast cells exclude the possibility that Shp-2 can act as an adapter to recruit the Grb2-Sos complex. To evaluate further the significance of Grb2-Sos proteins in promoting EGF-stimulated Ras activation, we compared the formation of this complex between wild-type and Shp-2 mutant cells. Sos-1 was immunoprecipitated from cell lysates treated with EGF for 0, 5, 10, or 30 min and immunoblotted with anti-Grb2 (Fig. 9A). The basal level of Grb2-Sos complex in unstimulated cells was higher in mutant than in wild-type cells. Furthermore, EGF treatment induced an enhanced and more prolonged Grb2-Sos association in Shp-2 mutant cells than in wild-type cells. Thus, the amount of Grb2-Sos complex is inversely correlated with the Ras activation. By immunoblotting the anti-Sos immunoprecipitates with anti-PY antibody, we detected very similar profiles of phosphoproteins associated with Sos between wild-type and Shp-2 mutant cells (data not shown). In addition, no difference was observed for plasma membrane relocation of the Sos protein between the two cell types after EGF treatment (data not shown).
FIG. 9.
Alternative binding of Grb2-Gab1 and Grb2-Sos. (A) Sos-1 was immunoprecipitated (IP) from control or EGF-stimulated cell lysates (1 mg of total proteins) and immunoblotted (IB) with an anti-Grb2 antibody. The same membrane was stripped and reprobed by the anti-Sos antibody to determine the amount of Sos protein precipitated from each sample. (B) Immunoprecipitation was performed with an anti-Gab1 antibody, blotted with an anti-Grb2 antibody, and then reprobed with anti-Gab1.
We next examined Grb2-Gab1 interaction by coimmunoprecipitation in the two cell types (Fig. 9B). Consistent with the Sos-Grb2 interaction, the basal level of Grb2-Gab1 complex was slightly higher in mutant than in wild-type cells. However, increased and sustained Grb2-Gab1 complex was detected in wild-type cells compared to mutant cells after EGF stimulation for 5, 10, and 30 min. Thus, there seems to be a competition between Gab1 and Sos for Grb2 binding upon EGF stimulation, which is consistent with previous data (21). Grb2 can bind Gab1 via two mechanisms: through SH3 interaction with the proline-rich motif of Gab1 and by SH2 recognition of a phosphotyrosyl residue. Consistent with a previous result (21), we also failed to detect Sos in the Gab1 complex.
Overall, Ras activity is controlled by the opposing effect of the guanine nucleotide exchange factors, such as Sos, and the Ras GTPase-activating protein (RasGAP). The positive role of Shp-2 in promoting Ras activation could be achieved by suppressing the negative effect of RasGAP. To explore this possibility, we examined the tyrosine phosphorylation of RasGAP and its physical interaction with Shp-2 in EGF-treated cells. We failed to detect a complex consisting of Shp-2 and RasGAP by coimmunoprecipitation, nor did we observe an induced tyrosine phosphorylation of RasGAP under EGF stimulation in either wild-type or Shp-2 mutant cells (data not shown). In previous studies, EGF-induced GAP phosphorylation was observed in cells overexpressing the EGF-R (11, 28). Taken together, our results point to a novel mechanism for the role of Shp-2 in mediating Ras activation by EGF, possibly by modulating the activity of Gab1-associated p90. Shp-2 does not seem to work through the stimulatory Shc, Grb2-Sos pathway or the inhibitory GAP protein.
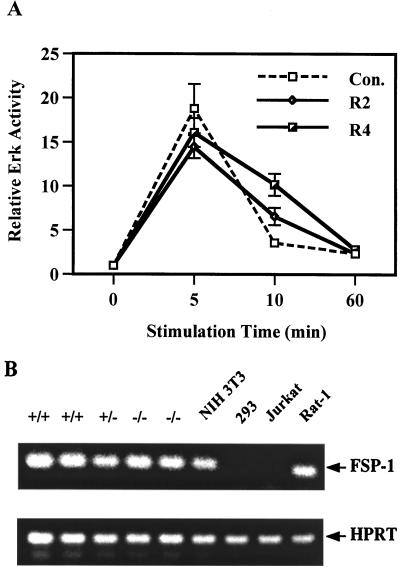
Finally, to prove that the difference of Erk activation between wild-type and mutant cells is due to the lack of a functional Shp-2 protein, we examined the Erk activation by EGF in Shp-2−/− cells reintroduced with wild-type Shp-2 or transfected with the vector only (Fig. 10A). Reintroduction of wild-type Shp-2 was able to partially restore a healthy cellular response to EGF in the Erk induction, and the expression levels of wild-type Shp-2 in R2 and R4 cells (bottom panel in Fig. 5) were proportional to the magnitude of Erk induction.
FIG. 10.
Rescue of defective Erk induction by EGF in Shp-2−/− cells and RT-PCR analysis of the fsp-1 mRNA expression. (A) Vector-transfected control cell line (Con.) and two cell clones expressing different levels of wild-type Shp-2 were stimulated with EGF (100 ng/ml) for indicated times. Erk protein was immunoprecipitated from cell lysates, and the kinase activity was measured by the in vitro kinase assay as described previously (45). (B) Total RNA of different cell lines was purified from 3 × 106 cells by using an RNeasy Mini kit from Qiagen as specified by the manufacturer. Gene-specific primers for mouse fsp-1 (sense primer, 5′-CAG CGA AAG AGG GTG ACA AGT TCA-3′; antisense primer, 5′-ATG TGC GAA GAA GCC AGA GTA AGG-3′) (46, 49) or hypoxanthine phosphoribosyltransferase (HPRT), as a positive control (38), were used to amplify 1 μg of RNA, using a GeneAmp RNA PCR kit (Perkin-Elmer) as instructed by the manufacturer. RT-PCR products were resolved in 1.2% agarose gel and visualized by ethidium bromide staining.
To establish the common fibroblast cell origin of wild-type and mutant cell lines, we performed reverse transcription-PCR (RT-PCR) analysis to examine the expression of mRNA for fibroblast-specific protein 1 (FSP-1) (46, 49). Similar levels of fsp-1 mRNA were detected in Shp-2+/+, Shp-2+/−, and Shp-2−/− cells (Fig. 10B). This mRNA was also detected in NIH 3T3 and Rat1 fibroblast cells but not in 293 epithelial cells or Jurkat lymphoid cells.
DISCUSSION
In this report, we describe several novel observations important for understanding the function of Shp-2 in promoting the Erk pathway. First, biochemical evidence suggests that Shp-2 acts upstream of Ras in the induction of Erk activity by EGF. Second, upon EGF stimulation, Shp-2 is engaged in a multiprotein complex organized by Gab1 and appears to specifically dephosphorylate Gab1-associated p90 and to a minor extent Gab1 itself. Third, the Grb2-Sos complex formation inversely correlates with Ras activation. Finally, we determined that Shp-2Δ46-110 is a loss-of-function molecule in cells, since it fails to participate in the Gab1-centered complex upon growth factor stimulation.
We and others have previously reported that Shp-2 acts to promote growth factor stimulation of Erk kinase activity (33, 36, 45, 51). Following previous observations, we have now extensively examined the critical components upstream of Erk, including Mek, Raf, and Ras. Our results indicate that a diminished stimulation of the conversion of Ras:GDP into Ras:GTP leads to defective activation of the downstream Raf-Mek-Erk kinase cascade by EGF in Shp-2 mutant cells. This is consistent with genetic epistasis data from Drosophila and Caenorhabditis elegans that Shp-2 (Csw, PTP-2) may act upstream of Ras (1, 19).
In exploring the molecular basis for the defective activation of the Ras pathway in Shp-2 mutant cells, we failed to find a positive correlation between the Grb2-Sos complex formation and the activation of Ras. In fact, there was an inverse relationship between the two events. The possibility that Shp-2 acts as an adapter molecule between EGF-R and Grb2-Sos was also ruled out by the fact that Shp-2 is not significantly tyrosine phosphorylated, nor does it associate with Grb2 in EGF-treated fibroblast cells. We have also examined 3T3-L1 preadipocytes and found that Shp-2 was tyrosine phosphorylated by stimulation with platelet-derived growth factor but not insulin-like growth factor I or EGF (data not shown). In previous studies, Shp-2 was found to be phosphorylated on tyrosine in EGF-treated A431 or h1HER cells in which the EGF-R is overexpressed (12, 24). Therefore, Shp-2 is not a substrate for the EGF-R in normal fibroblast cells and is unlikely to act as a docking molecule for the Grb2-Sos complex. Instead, the Shp-2 tyrosine phosphatase must work through a dephosphorylation event in mediating EGF activation of the Ras pathway.
By coimmunoprecipitation, we detected Gab1 as the most abundant phosphoprotein in association with Shp-2. Consistently, Lehr et al. reported recently that phosphorylation of the Shp-2 binding site accounts for almost 50% of the total radioactivity incorporated into Gab1 induced by the EGF-R tyrosine kinase in an in vitro kinase assay (26). Together, these results suggest that Gab1 and Shp-2 are important partners in cytoplasmic signaling. This is consistent with the genetic demonstration of the concerted interaction between Csw and Dos proteins in the control of R7 cell development downstream of the Sevenless tyrosine kinase (20, 39). Now the important issue is how the Shp-2 phosphatase works in the multiprotein complex nested on Gab1. This PH domain-containing protein has been reported to act as a multisubstrate docking molecule to host a multiprotein complex in response to various growth factors and cytokines in a variety of cell types (15, 21–23, 25, 50). Presumably, Shp-2 may act on one or more Gab1 tyrosine phosphorylation sites to modulate its interaction with other SH2-containing proteins; alternatively, it may dephosphorylate a Gab1-associated phosphoprotein(s). Our findings strongly favor the second possibility, since there was no significant difference of the Gab1 phosphorylation levels between wild-type and Shp-2 mutant cells after EGF stimulation. Similar amounts of Shc proteins associated with Gab1 in both the wild-type and mutant cells also suggest Shp-2 does not have a role in dephosphorylating the Shc binding site on Gab1. In addition, Shp-2 apparently does not dephosphorylate the Grb2 binding site to down-regulate Gab1-Grb2 interaction because with the presence of Shp-2 in the Gab1 complex, levels of Gab1-Grb2 association were higher in wild-type cells than in mutant cells. Consistently, mutating the Shp-2 binding site of Gab1 at the C terminus did not change the binding pattern of Gab1 and Grb2 (26).
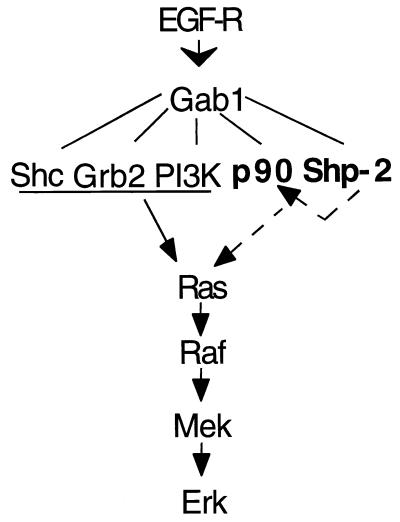
The most interesting part of this report are the data shown in Fig. 7 and 8. We found that with the presence of Shp-2, there was a significant decrease in tyrosine phosphorylation levels of Gab1-associated p90. By incubating the Gab1 complex in a phosphatase buffer in vitro, p90 was rapidly dephosphorylated, while phosphorylation of Gab1 on tyrosine was slowly diminished. Without Shp-2 in the complex, neither of these events was observed. Furthermore, the specificity of Shp-2 on these two proteins was revealed by the finding that tyrosine-phosphorylated Shc proteins were not affected in the two assays. Based on these observations, we propose the following model to explain how Shp-2 operates in contributing signal relay from EGF-R to Ras (Fig. 11). Ligand-activated EGF-R induces phosphorylation of Gab1 on multiple tyrosyl residues, on which a number of proteins bind to form a large signaling complex. Shp-2 binds Gab1 and dephosphorylates p90, which will lead to Ras activation either in a positive stimulatory mechanism or via relief of an inhibitory event. Shp-2 subsequently will dephosphorylate its own binding site on Gab1 and dissociate from this complex. Association of the p90 and Shc proteins with Gab1 has also been detected in B lymphocytes upon surface immunoglobulin engagement and in hematopoietic (UT-7) cells after erythropoietin stimulation (23, 25). Together, these results suggest that Gab1 may be a critical scaffold protein in organizing a signaling complex for Ras activation by growth factors, cytokines, and antigens. Increasing evidence supports the notion that organization of multiprotein complexes is a major biochemical mechanism for the specificity of various cytoplasmic signaling pathways. Similar scaffolding roles of the JIP proteins for components active in the Jnk pathway and the Ksr for members operating in the Erk pathway were reported recently (47, 53, 54).
FIG. 11.
A model of Shp-2 function. It has been known that Shc, Grb2, and phosphoinositol 3-kinase (PI3K) are associated with Gab1 and are involved in mediating Ras activation following EGF treatment. Results from this work allow us to propose a novel mechanism for the role of Shp-2 in promoting the activation of the Ras-Erk pathway by EGF, possibly through dephosphorylation of Gab1-associated p90, which is indicated by arrows with dashed lines.
It can be appreciated that numerous events lead to the activation of the Ras pathway induced by EGF, and Shp-2 manipulates only one aspect of this process. Data from this study show that Shp-2 apparently does not modulate the tyrosine phosphorylation profile and hence the function of Shc proteins. On the other hand, Shp-2 may have other targets that modulate the information flow along the Ras-Erk signaling cascade. Expression of a catalytically inactive mutant of Shp-2 (Csw, PTP-2) interfered with the function of constitutively active Ras or Raf mutants in cell signaling in Drosophila and C. elegans (1, 19). These observations suggest an effect of Shp-2 in signaling downstream of Ras; alternatively, Shp-2 may work on a parallel pathway that makes the Ras signaling permissive. Another study suggests that Shp-2 may regulate the signal relay of the Torso R-PTKs into the Ras pathway by dephosphorylating a phosphotyrosine site on Torso that is engaged in RasGAP binding (7). In this regard, the positive role of Shp-2 in Ras activation may be achieved by removing a negative effector from the R-PTK signal relay. In this and previous studies (12), we did not see a significant phosphatase activity of Shp-2 directly on EGF-R. We also failed to see a direct interaction of Shp-2 with RasGAP or an induced tyrosine phosphorylation of RasGAP in these embryonic fibroblasts stimulated with EGF. Thus, although it is possible that Shp-2 may act to suppress the down-regulation of EGF-stimulated Ras activity, this phosphatase may not directly modulate the RasGAP function.
As previously reported, the targeted mouse mutant Shp-2 allele (Shp-2Δ46-110) expresses a protein with an internal deletion of 65 amino acids in the SH2-N domain (38, 42, 45). One concern has been whether the mutant Shp-2Δ46-110 molecule is a hyperactive enzyme that generates a neomorphic phenotype. Several lines of experimental data have argued against this possibility. For example, the deficient phenotype in Shp-2 mutant cells is similar to that with exogenous expression of a dominant negative mutant of Shp-2 without catalytic activity. Additionally, Shp-2+/− animals appear normal. This report presents convincing biochemical evidence that the Shp-2Δ46-110 protein is biologically inert in cells. In responding to EGF stimulation, Shp-2Δ46-110 fails to bind Gab1 and other cytoplasmic signaling proteins. Another interesting observation reported here is that Shp-2 may exist as a dimer or in a complex in quiescent cells. It will be interesting to determine the physiological significance of the Shp-2 complex, for example, in preventing its function in cell arrest.
In summary, the results in this paper provide a basis for elucidating the molecular mechanism of Shp-2-mediated Erk activation following EGF treatment. The Shp-2-catalyzed reaction in this process may become a new target for pharmaceutical interference of malignant cell growth.
ACKNOWLEDGMENTS
We thank Mark Kaplan, Randy Brutkiewicz, and members of our laboratory for reading the manuscript or helpful comments. This work was supported by grants from National Institutes of Health (R29GM53660 and R01CA78606) and American Cancer Society (RPG-98-273-01-TBE) to G.-S.F., a seed grant for a pilot collaborative project from Indiana University Cancer Center (to G.-S.F. and M.M.), and an operating grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society (to M.P.). Z.Q.S. was supported by a predoctoral fellowship from American Heart Association-Indiana Affiliate, Inc., G.-S.F. had a career development award from American Diabetes Association, and M.P. is a Scientist of the Medical Research Council of Canada.
REFERENCES
- 1.Allard J D, Chang H C, Herbst R, McNeill H, Simon M A. The SH2-containing tyrosine phosphatase corkscrew is required during signaling by sevenless, Ras1 and Raf. Development. 1996;122:1137–1146. doi: 10.1242/dev.122.4.1137. [DOI] [PubMed] [Google Scholar]
- 2.Barnard D, Diaz B, Clawson D, Marshall M. Oncogenes, growth factors and phorbol esters regulate Raf-1 through common mechanisms. Oncogene. 1998;17:1539–1547. doi: 10.1038/sj.onc.1202061. [DOI] [PubMed] [Google Scholar]
- 3.Bennett A M, Hausdorff S F, O'Reilly A M, Freeman R M, Neel B G. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett A M, Tang T L, Sugimoto S, Walsh C T, Neel B G. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc Natl Acad Sci USA. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 6.Carlberg K, Rohrschneider L R. Characterization of a novel tyrosine phosphorylated 100-kDa protein that binds to SHP-2 and phosphatidylinositol 3′-kinase in myeloid cells. J Biol Chem. 1997;272:15943–15950. doi: 10.1074/jbc.272.25.15943. [DOI] [PubMed] [Google Scholar]
- 7.Cleghon V, Feldmann P, Ghiglione C, Copeland T D, Perrimon N, Hughes D A, Morrison D K. Opposing actions of CSW and RasGAP modulate the strength of Torso RTK signaling in the Drosophila terminal pathway. Mol Cell. 1998;2:719–727. doi: 10.1016/s1097-2765(00)80287-7. [DOI] [PubMed] [Google Scholar]
- 8.Davis R J. Transcriptional regulation by MAP kinases. Mol Reprod Dev. 1995;42:459–467. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- 9.Diaz B, Barnard D, Filson A, MacDonald S, King A, Marshall M. Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol Cell Biol. 1997;17:4509–4516. doi: 10.1128/mcb.17.8.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan S E, Weinberg R A. The pathway to signal achievement. Nature. 1993;365:781–783. doi: 10.1038/365781a0. [DOI] [PubMed] [Google Scholar]
- 11.Ellis C, Moran M, McCormick F, Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990;343:377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- 12.Feng G S, Hui C C, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993;259:1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 13.Feng G S, Pawson T. Phosphotyrosine phosphatases with SH2 domains: regulators of signal transduction. Trends Genet. 1994;10:54–58. doi: 10.1016/0168-9525(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 14.Feng G S, Shen R, Heng H H, Tsui L C, Kazlauskas A, Pawson T. Receptor-binding, tyrosine phosphorylation and chromosome localization of the mouse SH2-containing phosphotyrosine phosphatase Syp. Oncogene. 1994;9:1545–1550. [PubMed] [Google Scholar]
- 15.Fixman E D, Holgado-Madruga M, Nguyen L, Kamikura D M, Fournier T M, Wong A J, Park M. Efficient cellular transformation by the Met oncoprotein requires a functional Grb2 binding site and correlates with phosphorylation of the Grb2-associated proteins, Cbl and Gab1. J Biol Chem. 1997;272:20167–20172. doi: 10.1074/jbc.272.32.20167. [DOI] [PubMed] [Google Scholar]
- 16.Fuhrer D K, Feng G S, Yang Y C. Syp associates with gp130 and Janus kinase 2 in response to interleukin-11 in 3T3-L1 mouse preadipocytes. J Biol Chem. 1995;270:24826–24830. doi: 10.1074/jbc.270.42.24826. [DOI] [PubMed] [Google Scholar]
- 17.Gale N W, Kaplan S, Lowenstein E J, Schlessinger J, Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993;363:88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- 18.Gu H, Pratt J C, Burakoff S J, Neel B G. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 19.Gutch M J, Flint A J, Keller J, Tonks N K, Hengartner M O. The Caenorhabditis elegans SH2 domain-containing protein tyrosine phosphatase PTP-2 participates in signal transduction during oogenesis and vulval development. Genes Dev. 1998;12:571–585. doi: 10.1101/gad.12.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbst R, Carroll P M, Allard J D, Schilling J, Raabe T, Simon M A. Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 21.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 22.Holgado-Madruga M, Moscatello D K, Emlet D R, Dieterich R, Wong A J. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci USA. 1997;94:12419–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingham R J, Holgado-Madruga M, Siu C, Wong A J, Gold M R. The Gab1 protein is a docking site for multiple proteins involved in signaling by the B cell antigen receptor. J Biol Chem. 1998;273:30630–30637. doi: 10.1074/jbc.273.46.30630. [DOI] [PubMed] [Google Scholar]
- 24.Lechleider R J, Freeman R, Jr, Neel B G. Tyrosyl phosphorylation and growth factor receptor association of the human corkscrew homologue, SH-PTP2. J Biol Chem. 1993;268:13434–13438. [PubMed] [Google Scholar]
- 25.Lecoq-Lafon C, Verdier F, Fichelson S, Chretien S, Gisselbrecht S, Lacombe C, Mayeux P. Erythropoietin induces the tyrosine phosphorylation of GAB1 and its association with SHC, SHP2, SHIP, and phosphatidylinositol 3-kinase. Blood. 1999;93:2578–2585. [PubMed] [Google Scholar]
- 26.Lehr S, Kotzka J, Herkner A, Klein E, Siethoff C, Knebel B, Noelle V, Bruning J C, Klein H W, Meyer H E, Krone W, Muller-Wieland D. Identification of tyrosine phosphorylation sites in human Gab-1 protein by EGF receptor kinase in vitro. Biochemistry. 1999;38:151–159. doi: 10.1021/bi9818265. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Nishimura R, Kashishian A, Batzer A G, Kim W J, Cooper J A, Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X Q, Pawson T. The epidermal growth factor receptor phosphorylates GTPase-activating protein (GAP) at Tyr-460, adjacent to the GAP SH2 domains. Mol Cell Biol. 1991;11:2511–2516. doi: 10.1128/mcb.11.5.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu X, Qu C K, Shi Z Q, Feng G S. Downregulation of platelet-derived growth factor receptor-β in Shp-2 mutant fibroblast cell lines. Oncogene. 1998;17:441–448. doi: 10.1038/sj.onc.1201988. [DOI] [PubMed] [Google Scholar]
- 30.Marshall M S. Ras target proteins in eukaryotic cells. FASEB J. 1995;9:1311–1318. doi: 10.1096/fasebj.9.13.7557021. [DOI] [PubMed] [Google Scholar]
- 31.Mathias N, Steussy C N, Goebl M G. An essential domain within Cdc34p is required for binding to a complex containing Cdc4p and Cdc53p in Saccharomyces cerevisiae. J Biol Chem. 1998;273:4040–4045. doi: 10.1074/jbc.273.7.4040. [DOI] [PubMed] [Google Scholar]
- 32.McCormick F. Signal transduction. How receptors turn Ras on. Nature. 1993;363:15–16. doi: 10.1038/363015a0. [DOI] [PubMed] [Google Scholar]
- 33.Milarski K L, Saltiel A R. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J Biol Chem. 1994;269:21239–21243. [PubMed] [Google Scholar]
- 34.Neel B G, Tonks N K. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 35.Nishida K, Yoshida Y, Itoh M, Fukada T, Ohtani T, Shirogane T, Atsumi T, Takahashi-Tezuka M, Ishihara K, Hibi M, Hirano T. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood. 1999;93:1809–1816. [PubMed] [Google Scholar]
- 36.Noguchi T, Matozaki T, Horita K, Fujioka Y, Kasuga M. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol Cell Biol. 1994;14:6674–6682. doi: 10.1128/mcb.14.10.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu C K, Yu W M, Azzarelli B, Feng G S. Genetic evidence that shp-2 tyrosine phosphatase is a signal enhancer of the epidermal growth factor receptor in mammals. Proc Natl Acad Sci USA. 1999;96:8528–8533. doi: 10.1073/pnas.96.15.8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qu C K, Shi Z Q, Shen R, Tsai F Y, Orkin S H, Feng G S. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol Cell Biol. 1997;17:5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raabe T, Riesgo-Escovar J, Liu X, Bausenwein B S, Deak P, Maroy P, Hafen E. DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and Ras1 in Drosophila. Cell. 1996;85:911–920. doi: 10.1016/s0092-8674(00)81274-x. [DOI] [PubMed] [Google Scholar]
- 40.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 41.Rozakis-Adcock M, Fernley R, Wade J, Pawson T, Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993;363:83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- 42.Saxton T M, Henkemeyer M, Gasca S, Shen R, Shalaby F, Feng G S, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaeffer H J, Weber M J. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seger R, Seger D, Reszka A A, Munar E S, Eldar-Finkelman H, Dobrowolska G, Jensen A M, Campbell J S, Fischer E H, Krebs E G. Overexpression of mitogen-activated protein kinase kinase (MAPKK) and its mutants in NIH 3T3 cells. Evidence that MAPKK involvement in cellular proliferation is regulated by phosphorylation of serine residues in its kinase subdomains VII and VIII. J Biol Chem. 1994;269:25699–25709. [PubMed] [Google Scholar]
- 45.Shi Z Q, Lu W, Feng G S. The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J Biol Chem. 1998;273:4904–4908. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- 46.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 47.Stewart S, Sundaram M, Zhang Y, Lee J, Han M, Guan K L. Kinase suppressor of Ras forms a multiprotein signaling complex and modulates MEK localization. Mol Cell Biol. 1999;19:5523–5534. doi: 10.1128/mcb.19.8.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stokoe D, Macdonald S G, Cadwallader K, Symons M, Hancock J F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 49.Strutz F, Okada H, Lo C W, Danoff T, Carone R L, Tomaszewski J E, Neilson E G. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi-Tezuka M, Yoshida Y, Fukada T, Ohtani T, Yamanaka Y, Nishida K, Nakajima K, Hibi M, Hirano T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol. 1998;18:4109–4117. doi: 10.1128/mcb.18.7.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang T L, Freeman R, Jr, O'Reilly A M, Neel B G, Sokol S Y. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 52.Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 53.Whitmarsh A J, Cavanagh J, Tournier C, Yasuda J, Davis R J. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- 54.Yasuda J, Whitmarsh A J, Cavanagh J, Sharma M, Davis R J. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol Cell Biol. 1999;19:7245–7254. doi: 10.1128/mcb.19.10.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu D H, Qu C K, Henegariu O, Lu X, Feng G S. Protein tyrosine phosphatase Shp-2 regulates cell spreading, migration and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 56.Zhao C, Yu D H, Shen R, Feng G S. Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1. J Biol Chem. 1999;274:19649–19654. doi: 10.1074/jbc.274.28.19649. [DOI] [PubMed] [Google Scholar]