Abstract
New means to reduce excessive antibiotic use are urgently needed. This study tested dual-light aPDT against Staphylococcus aureus biofilm with different relative ratios of light energy with indocyanine green. We applied single-light aPDT (810 nm aPDT, 405 aBL) or dual-light aPDT (simultaneous 810 nm aPDT and 405 nm aBL), in both cases, together with the ICG photosensitizer with constant energy of 100 or 200 J/cm2. Single-dose light exposures were given after one-day, three-day, or six-day biofilm incubations. A repeated daily dose of identical light energy was applied during biofilm incubations for the three- and six-day biofilms. Using 100 J/cm2 light energy against the one-day biofilm, the dual-light aPDT consisting of more than half of aBL was the most effective. On a three-day maturated biofilm, single-dose exposure to aPDT or dual-light aPDT was more effective than aBL alone. With total light energy of 200 J/cm2, all dual-light treatments were effective. Dual-light aPDT improves the bactericidal effect on Staphylococcus aureus biofilm compared to aPDT or aBL and provides a sustained effect. An increase in the relative ratio of aBL strengthens the antibacterial effect, mainly when the treatment is repeatedly applied. Thus, the light components’ energy ratio is essential with dual-light.
Keywords: biofilm, Staphylococcus aureus, antibacterial photodynamic therapy
1. Introduction
Staphylococcus is a genus of Gram-positive bacteria in the family Staphylococcaceae in the order Bacillales. Despite being part of the harmless skin microbial flora, Staphylococcus aureus is potentially the most dangerous staphylococcal bacteria. Skin infections by S. aureus are common and generally benign, but potentially lethal infections are due once the bacteria enter the bloodstream. [1,2]. Indeed, S. aureus is the leading cause of infectious endocarditis [3,4]. The morbidity and mortality from S. aureus bacteremia have only minor improvement when comparing the reports from the 1940s to 2010s [5,6]. Roughly twenty percent of the general population [7] and 60% of healthcare professionals carry [8] the bacteria.
Local S. aureus infections are commonly treated with peroral antibiotics. However, the use of antibiotics leads to antimicrobial resistance formation, which is one of the most critical threats to modern medicine. S. aureus is notorious for its ability to generate resistance, and the resistant strains are widely spread worldwide [9,10,11]. Multiple challenges relate to the novel antibiotic drug discovery and development [12,13], and diminishing investments for research and development of new antibiotics are reported [14]. The need for new solutions has never been more urgent.
Antimicrobial photodynamic therapy (aPDT) has arisen as an alternative method against microbial infections [15,16,17]. The antimicrobial effect of aPDT is based mainly on the principle that visible light activates an externally applied photosensitizer, resulting in the generation of reactive oxygen species (ROS) that kill bacteria unselectively via an oxidative burst [17]. Antimicrobial blue light (aBL) is suggested to be based on the same principle. However, the photosensitizers in the latter process are inherent molecules within the bacteria itself, such as porphyrins and flavins. [18]. During the last decades, the development of light technology has provided an opportunity for extensive in vitro research, which is now ready to be translated into clinical practice. There is a notable scarcity of papers discussing a combination of antibacterial photodynamic therapy and antibacterial blue light in the literature. Indeed, we have recently published the efficacy of combined aPDT and aBL against streptococcus biofilm, with indocyanine green (ICG) as the photosensitizer. The inherent lack of catalase enzyme in streptococci, which is the bacteria’s main factor in clearing hydrogen peroxide-type reactive oxygen species from the cell, makes streptococcus very vulnerable to aPDT. However, simultaneous application of aBL increased the bactericidal effect significantly [19]. These results encourage the assessment of the treatment method on other clinically important pathogens, as previously discussed, such as Staphylococcus aureus. To the best of our knowledge, our study group is the first to describe the antibacterial effects of dual-light antibacterial therapy combining 405 nm aBL with 810 nm aPDT.
In the present study, we investigated, for the first time, the use of dual-light aPDT against S. aureus. Most papers regarding aPDT are reporting antibacterial effects of a single wavelength. The improvent in efficacy by using dual-light against streptococci might also be valid against staphylococci. Thus, dual-light aPDT could be used instead of antibiotics for superficial skin infections that are often caused by streptococci and staphylococci. Furthermore, this technique might be an accessory tool in the treatment of more severe conditions, such as infective endocarditis, which requires surgical debridement via sternotomy.
2. Materials and Methods
The present study aimed to investigate the effects of aBL (405 nm), aPDT (810 nm combined with a photosensitizer: ICG), and dual-light antibacterial photodynamic therapy (405 and 810 nm combined with a P.S.) on monospecies S. aureus ATCC 25923 incubated for one day before any treatment. This strain is a known biofilm-forming strain [20]. The formed biofilms were divided into subgroups according to the total incubation time and the treatment method. The experiments and the number of assays are shown in Table 1.
Table 1.
Exact details of the test methodology.
| Experiment | Figure | Repeats | Number of Treaments | Radiant Exposure (J/cm2) | Wavelenghts (nm) | Irradiance of 405 nm (mW/cm2) | Irradiance of 810 nm (mW/cm2) | ICG (+/−) | Biofilm Age at the End of Experiment (Days) |
|---|---|---|---|---|---|---|---|---|---|
| 1-day aBL | 1 | 6 | 1 | 100 | 405 | 80 | 0 | − | 1 |
| 1-day aPDT | 1 | 6 | 1 | 100 | 810 | 0 | 100 | + | 1 |
| 1-day single dose 1:3 | 1 | 6 | 1 | 100 | 405 + 810 | 42 | 135 | + | 1 |
| 1-day single dose 1:1 | 1 | 6 | 1 | 100 | 405 + 810 | 73 | 79 | + | 1 |
| 1-day single dose 3:1 | 1 | 6 | 1 | 100 | 405 + 810 | 130 | 38 | + | 1 |
| 1-day control | 1 | 3 | N/A | N/A | N/A | N/A | N/A | − | 1 |
| 1-day aBL | 5 | 6 | 1 | 200 | 405 | 80 | 0 | − | 1 |
| 1-day aPDT | 5 | 6 | 1 | 200 | 810 | 0 | 100 | + | 1 |
| 1-day single dose 1:3 | 5 | 6 | 1 | 200 | 405 + 810 | 42 | 135 | + | 1 |
| 1-day single dose 1:1 | 5 | 6 | 1 | 200 | 405 + 810 | 73 | 79 | + | 1 |
| 1-day single dose 3:1 | 5 | 6 | 1 | 200 | 405 + 810 | 130 | 38 | + | 1 |
| 1-day control | 5 | 6 | N/A | N/A | N/A | N/A | N/A | − | 1 |
| 3-day aBL | 2 | 6 | 1 | 100 | 405 | 80 | 0 | − | 3 |
| 3-day aPDT | 2 | 6 | 1 | 100 | 810 | 0 | 100 | + | 3 |
| 3-day single dose 1:3 | 2 | 6 | 1 | 100 | 405 + 810 | 42 | 135 | + | 3 |
| 3-day single dose 1:1 | 2 | 6 | 1 | 100 | 405 + 810 | 73 | 79 | + | 3 |
| 3-day single dose 3:1 | 2 | 6 | 1 | 100 | 405 + 810 | 130 | 38 | + | 3 |
| 3-day control | 2 | 3 | N/A | N/A | N/A | N/A | N/A | − | 3 |
| 3-day daily dose aBL | 3 | 6 | 3 | 100 | 405 | 80 | 0 | − | 3 |
| 3-day daily dose aPDT | 3 | 6 | 3 | 100 | 810 | 0 | 100 | + | 3 |
| 3-day daily dose 1:3 | 3 | 6 | 3 | 100 | 405 + 810 | 42 | 135 | + | 3 |
| 3-day daily dose 1:1 | 3 | 6 | 3 | 100 | 405 + 810 | 73 | 79 | + | 3 |
| 3-day daily dose 3:1 | 3 | 6 | 3 | 100 | 405 + 810 | 130 | 38 | + | 3 |
| 3-day control | 3 | 3 | N/A | N/A | N/A | N/A | N/A | − | 3 |
| 3-day daily dose aBL | 6 | 6 | 3 | 200 | 405 | 80 | 0 | − | 3 |
| 3-day daily dose aPDT | 6 | 6 | 3 | 200 | 810 | 0 | 100 | + | 3 |
| 3-day daily dose 1:3 | 6 | 6 | 3 | 200 | 405 + 810 | 42 | 135 | + | 3 |
| 3-day daily dose 1:1 | 6 | 6 | 3 | 200 | 405 + 810 | 73 | 79 | + | 3 |
| 3-day daily dose 3:1 | 6 | 6 | 3 | 200 | 405 + 810 | 130 | 38 | + | 3 |
| 3-day control | 6 | 6 | N/A | N/A | N/A | N/A | N/A | − | 3 |
| 6-day aBL | 4 | 6 | 6 | 100 | 405 | 80 | 0 | − | 6 |
| 6-day aPDT | 4 | 6 | 6 | 100 | 810 | 0 | 100 | + | 6 |
| 6-day single dose 1:3 | 4 | 6 | 6 | 100 | 405 + 810 | 42 | 135 | + | 6 |
| 6-day single dose 1:1 | 4 | 6 | 6 | 100 | 405 + 810 | 73 | 79 | + | 6 |
| 6-day single dose 3:1 | 4 | 6 | 6 | 100 | 405 + 810 | 130 | 38 | + | 6 |
| 6-day control | 4 | 3 | N/A | N/A | N/A | N/A | N/A | − | 6 |
ICG, indocyanine green; N/A, not available.
Apart from the species of bacteria studied, the study protocol, details on the biofilm models, and light exposures, in addition to information on colony-forming-unit counting used in the present study, have also been presented accurately elsewhere [19,21].
The methodology in the present study is presented in the following steps. Step 1: The Staphylococcus aureus strain (ATCC 25923) growth in a BHI broth (Bio-Rad 3564014, Bio-Rad Laboratories Inc., Hercules, CA, USA). Step 2: Incubation at +36 degrees C in an air concentration of 5% CO2 for 18 h (NuAire DH autoflow 5500, NuAire Inc., Minneapolis, MN, USA). Step 3: Dilution to the optical density of 0.46, using 0.9% NaCl solution (measured by using a spectrophotometer, Varian Cary 100 Bio UV–VIS, Agilent Technologies, Inc., Santa Clara, CA, USA, and Den 1 McFarland Densitometer, Biosan, Riga, Latvia). Step 4: Biofilm growth in a flat-bottom 96-well plate (Thermo Fisher Scientific Inc., Waltham, MA, USA). Step 5: Placing 100 μL of S. aureus suspension into the well-plates, each primed with 100 μL of BHI broth. (Additional step, Step 5.1: Incubation at a temperature of 36 degrees C and in an air concentration of 5% CO2, in addition to a daily change of the 100 μL BHI broth to a fresh solution.) Step 6: Removal of Growth medium and replacement with indocyanine green solution 250 μg/mL (Verdye, Diagnostic Green, GmBH, Aschheim, Germany). Step 7: Incubation in a dark room for 10 min. Step 8: Washing the biofilm with 0.9% NaCl solution until each well contained a total volume of 200 μL. Step 9: Light exposure, using a custom-made LED light (Lumichip Oy, Espoo, Finland); the time of exposure was calculated by using the known irradiances of the light sources (measured by using a light energy meter, Thorlabs PM 100D and an S121C sensor head, Thorlabs Inc., Newton, NJ, USA, in addition to a spectroradiometer, BTS256, Gigahertz-Optik GmBH, Türkenfeld, Germany). The irradiances of used light, the wavelengths, the number of treatments, and incubation repeats in each biofilm study are presented in Table 1. For example, irradiance times of 1250 and 1000 s were required to reach the desired radiant exposure of 100 J/cm2 for the aBL and aPDT, respectively. Step 10: Changing of the BHI broth and subsequent incubation or biofilm removal for colony-forming-unit counting if the planned study exposure was finished. Step 11: After the light exposure, removal of the entire biofilms into a 1 mL test tube, forming 200 μL of solution. Step 12: Vortexing by using a (Vortex-Genie, Scientific Industries Inc., Bohemia, NY, USA) and serial dilution ranging from 1:1 to 1:100,000, using sterile tipped ART filters (Thermo Fisher Scientific Inc., Waltham, MA, USA). Step 13: Spreading of 100 μL of the resulting biofilm dilution onto a BHI agar plate, using a sterile L-rod. The dilutions were performed according to the observed biofilm mass from the well-plates. Dilution, where the CFU counts were between 30 and 800, were selected for analysis, whereas the results for the CFU 0 analysis were obtained from a solution with a 1:1 dilution factor. Step 14: Incubation for 48 h and photographing of the plates (Leica TCS CARS SP 8X microscope, Leica Microsystems, Wetzlar, Germany) with HC PL APO CS2 20X/0.75 numerical-aperture multi-immersion and HX PL APO CS2 63X/1.2 numerical-aperture water-immersion objectives. Step 15: Staining with a live/dead BacLight bacterial viability kit (Molecular Probes, Invitrogen, Eugene, OR, USA) and incubation in a dark room for 15 min. Step 16: Examination under a confocal scanning laser microscope, with a two-laser system (488 nm argon laser and a 561 nm DPSS laser). The emission windows for the 488 and 561 nm lasers were set at 500–530 nm and 620–640 nm, respectively.
2.1. Outcome Endpoints
The primary outcome endpoints in the present study are the number of viable colony-forming units observed after treatment with aBL, aPDT, or dual-light aPDT and the differences in efficacy between the treatment methods.
2.2. Statistical Analysis
Statistical analysis was performed by using GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA). The continuous variable of colony-forming units was reported as medians. Mann–Whitney U test was used for all univariate analyses. The tests were two-tailed, and a p-value < 0.05 represented statistical significance.
3. Results
3.1. One-Day S. aureus Biofilm Treated Once by 100 J/cm2
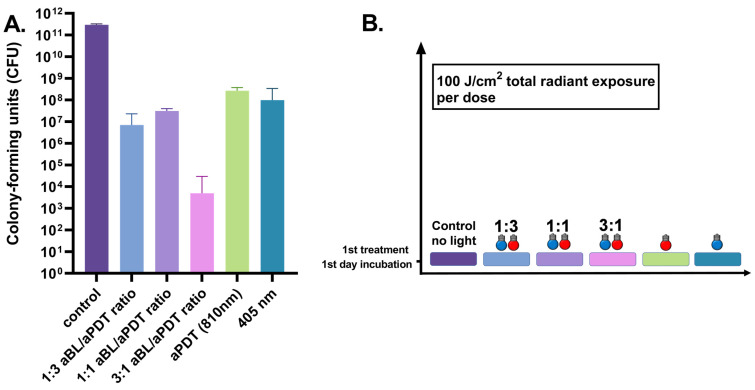
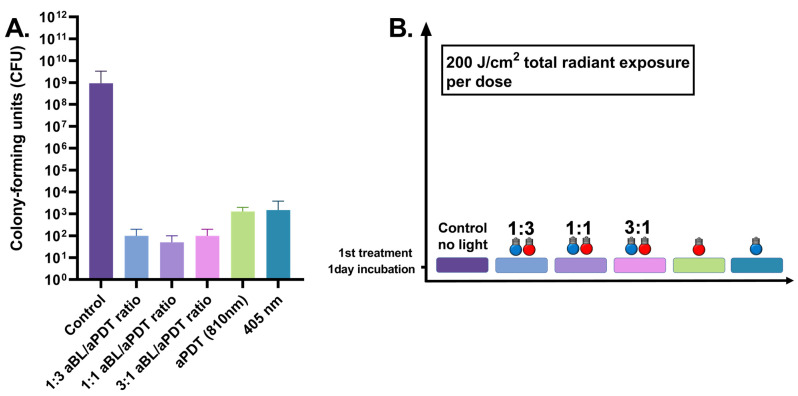
The one-day S. aureus biofilm showed a significant reduction in viability when treated with aBL. A decrease from the median of 1.3 × 109 CFUs (the range being 1.0 × 109–3.3 × 109 CFUs, with three assays) in the control biofilm to a median of 3.1 × 106 CFUs (ranging between 8.0 × 105 and 1.0 × 107 CFUs, with six assays), with p = 0.024. An aPDT treatment with an 810 nm light combined with ICG photosensitizer resulted in the efficacy of a similar scale when compared to aBL. The median number of CFUs was 2.7 × 105 CFUs (the range being 6.0 × 104–3.4 × 106 CFUs, with six assays), with p = 0.024. However, the dual-light combination of one part of aBL and three parts of aPDT, at irradiances of 42 and 135 mW/cm2, respectively, decreased the median of alive bacteria to 3.6 × 104 CFUs (the range being 1.9 × 103–2.4 × 105 CFUs with six assays), with p = 0.024. The bactericidal effect of dual-light with a 1:1 ratio of aBL and aPDT, (with irradiances of 79 and 73 mW/cm2, respectively) decreased the median number of living bacteria to 6.0 × 104 CFUs (the range being 3.0 × 102–2.6 × 104 CFUs with six assays), with p = 0.024. When the amount of aBL was raised to three parts, and aPDT was lowered to one part in the dual-light system with respective irradiances of 130 and 38 mW/cm2, the bacterial viability reduced to 5.0 × 102 (the range being 2.0 × 102–1.6 × 103 CFUs with six assays), with p = 0.024 (Figure 1).
Figure 1.
(A). Effects of different irradiance ratios of aBL and aPDT in the dual-light aPDT system in one-day S. aureus biofilm. One-day S. aureus biofilm, single dose, aBL vs. dual-light 1:3 aBL/aPDT ratio, p = 0.0022; aBL vs. dual-light 1:1 aBL/aPDT ratio, p = 0.015; aBL vs. dual-light 3:1 aBL/aPDT ratio, p = 0.0022; aPDT vs. dual-light 1:3 aBL/aPDT ratio, p = 0.0022; aPDT vs. dual-light 1:1 aBL/aPDT ratio, p = 0.015; aPDT vs. dual-light 3:1 aBL/aPDTratio, p = 0.0022. aBL vs. aPDT, p = 0.046; dual-light aPDT: 1:3 aBL/aPDT ratio vs. 1:1 aBL/aPDT ratio, p = ns; 1:3 aBL/aPDT ratio vs. 3:1 aBL/aPDT ratio, p = ns; 1:1 aBL/aPDT ratio vs. 3:1 aBL/aPDT ratio; p = ns. The y-axis displays the median of CFUs. Six assays were performed for each experiment, and the control biofilm was performed in three assays. The 95% confidence interval (CI) is presented by using t-bars. (B). A schematic representation of the experiment.
3.2. Three-Day S. aureus Biofilm Treated Once by 100 J/cm2
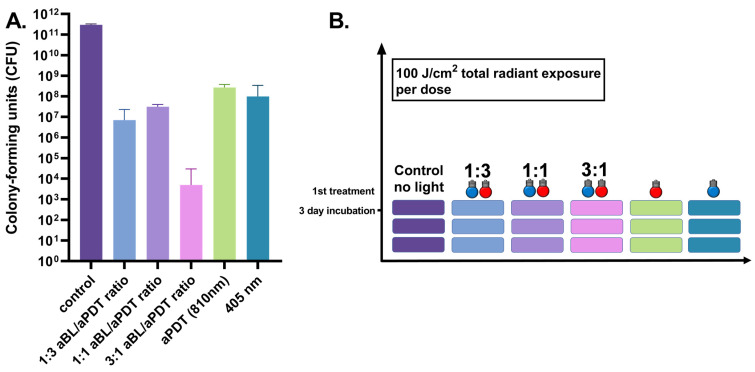
The three-day S. aureus biofilm presented a significantly reduced viability after a single application of aBL, from the control biofilm median of 3.0 × 1011 CFUs (the range being 2.9 × 1011–3.3 × 1012 CFUs, with three assays) to a median of 1.0 × 104 CFUs (the range being 4.9 × 106–3.4 × 108 CFUs, with six assays), with p = 0.024. The use of aPDT showed a slightly lesser degree of antibacterial efficiency than aBL, with an observed median of 2.7 × 108 CFUs (the range being 1.0 × 108–3.8 × 108 CFUs, with six assays), with p = 0.024. An exposure of dual-light in a combination of one part of aBL and three parts of aPDT (at irradiances of 42 and 135 mW/cm2, respectively) decreased the median of live bacteria to 7.1 × 106 CFUs (the range being 3.1–2.3 × 107 CFUs with six assays), with p = 0.024. When the dual-light fractions were administered in a 1:1 ratio (with irradiances of 79 and 73 mW/cm2, respectively), the median of live bacteria decreased to 3.2 × 107 CFUs (the range being 9.4 × 106–4.0 × 107 CFUs with six assays), with p = 0.024. After increasing the amount of aBL to three parts and decreasing aPDT to one part in the dual-light system (with respective irradiances of 130 and 38 mW/cm2), the median number of colony-forming units was 5.0 × 103 (the range being 0.0–7.0 × 103 CFUs with 12 assays), with p = 0.024 (see Figure 2).
Figure 2.
(A). Effects of different irradiance ratios of aBL and aPDT in the dual-light aPDT system in three-day S. aureus biofilm. Three-day S. aureus biofilm, single dose, aBL vs. dual-light 1:3 aBL/aPDT ratio, p = 0.17; aBL vs. dual-light 1:1 aBL/aPDT ratio, p = 1.0; aBL vs. dual-light 3:1 aBL/aPDT ratio, p = 0.0001; aPDT vs. dual-light 1:3 aBL/aPDT ratio, p = 0.0022; aPDT vs. dual-light 1:1 aBL/aPDT ratio, p = 0.0022; aPDT vs. dual-light 3:1 aBL/aPDT ratio, p = 0.0001; aBL vs. aPDT, p = 0.13; dual-light aPDT: 1:3 aBL/aPDT ratio vs. 1:1 aBL/aPDT ratio, p = 0.011; 1:3 aBL/aPDT ratio vs. 3:1 aBL/aPDT ratio, p = 0.0001; 1:1 aBL/aPDT ratio vs. 3:1 aBL/aPDT ratio; p = 0.0001. The y-axis displays the median of CFUs. Six assays were performed for each experiment, and the control biofilm was performed in three assays. The 95% confidence interval (CI) is presented by using t-bars. (B). A schematic representation of the experiment.
3.3. Three-Day S. aureus Biofilm Treated Daily by 100 J/cm2
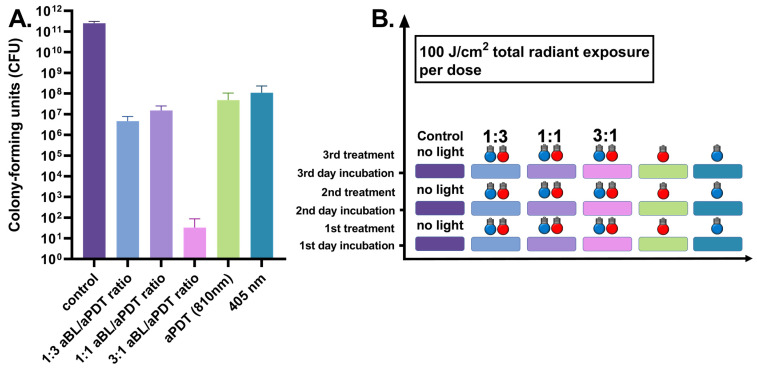
When the three-day S. aureus biofilm was exposed to daily antibacterial therapy, the daily applied aBL significantly reduced the number of bacterial CFUs to a median of 7.5 × 107 (ranging between 1.2 × 107 and 3.5 × 108 CFUs, with six assays) when compared to the control biofilm median of 2.8 × 1011 CFUs (the range being 1.9 × 1011–2.9 × 1011 CFUs, with three assays), with p = 0.024. APDT resulted in a similar antibacterial efficiency, presenting with a median of 3.0 × 107 CFUs (the range being 9.5 × 106–1.6 × 108 CFUs, with six assays), with p = 0.024. The dual-light combination of one part of aBL and three parts of aPDT, at irradiances of 42 mW/cm2 and 135 mW/cm2, respectively, decreased the median of live bacteria to 4.4 × 106 CFUs (a range of 9.0 × 105–1.0 × 107 CFUs with six assays), with p = 0.024. The one-to-one ratio of aBL and 810 nm PDT, with irradiances of 79 and 73 mW/cm2, respectively, decreased the median of live bacteria to 1.6 × 107 CFUs (the range being 3.8 × 106–2.6 × 107 CFUs with six assays), with p = 0.024. When the amount of aBL was raised to three parts, and aPDT was set at one part in the dual-light system with respective irradiances of 130 and 38 mW/cm2, the bacterial viability was reduced to 10 CFUs (ranging between 0 and 140 CFUs with six assays), with p = 0.0012 (see Figure 3).
Figure 3.
(A). Effects of different irradiance ratios of aBL and aPDT in the dual-light aPDT system in three-day S. aureus biofilm, when the antibacterial treatment is given once daily. Three-day S. aureus biofilm, daily dose, aBL vs. dual-light 1:3 aBL/aPDT ratio, p = 0.0022; aBL vs. dual-light 1:1 aBL/aPDT ratio, p = 0.015; aBL vs. dual-light 3:1 aBL/aPDT ratio, p = 0.0022; aPDT vs. dual-light 1:3 aBL/aPDT ratio, p = 0.0043; aPDT vs. dual-light 1:1 aBL/aPDT ratio, p = 0.015; aPDT vs. dual-light 3:1 aBL/aPDT ratio, p = 0.0022; aBL vs. aPDT, p = 0.26; dual-light aPDT: 1:3 aBL/aPDT ratio vs. 1:1 aBL/aPDT ratio, p = 0.03; 1:3 aBL/aPDT ratio vs. 3:1 aBL/aPDT ratio, p = 0.0022; 1:1 aBL/aPDT ratio vs. 3:1 aBL/aPDT ratio; p = 0.0022. The y-axis displays the median of CFUs. Six assays were performed for each experiment, and the control biofilm was performed in three assays. The 95% confidence interval (CI) is presented by using t-bars. (B). A schematic representation of the experiment.
3.4. Six-Day S. aureus Biofilm Treated Daily by 100 J/cm2
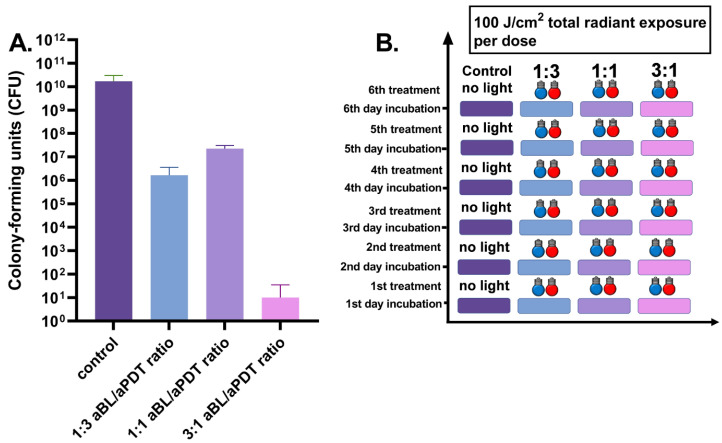
Six-day S. aureus biofilm was exposed to daily applied antibacterial light therapy. The dual-light combination of one part of aBL and three parts of aPDT, at irradiances of 42 and 135 mW/cm2 respectively, decreased the bacterial viability from the control biofilm of 1.0 × 1010 CFUs (the range being 8.0 × 109–3.2 × 1010, with three assays) to a median number of 7.0 × 105 CFUs (ranging between 5.4 × 104 and 4.4 × 106 CFUs with six assays), with p = 0.024. Again, the one-to-one ratio of aBL and 810 nm PDT, with irradiances of 79 and 73 mW/cm2, respectively, decreased the median of live bacteria to 2.2 × 107 CFUs (the range being 1.5 × 107–3.8 × 107 CFUs with six assays), with p = 0.024. When the amount of aBL was set at three parts, and aPDT was reduced to one part of the total amount of light, with irradiances of 130 and 38 mW/cm2, respectively, the number of bacterial colony-forming units reduced to a median of 0 (with a range of 0–61 CFUs with six assays), with p = 0.012 (see Figure 4).
Figure 4.
(A). Effects of different irradiance ratios of aBL and aPDT in the dual-light aPDT system in six-day S. aureus biofilm, when the antibacterial treatment is given once daily. Six-day S. aureus biofilm, daily-dose dual-light aPDT: 1:3 aBL/aPDT ratio vs. 1:1 aBL/aPDT ratio, p = 0.0022; 1:3 aBL/aPDT ratio vs. 3:1 aBL/aPDT ratio, p = 0.0022; 1:1 aBL/aPDT ratio vs. 3:1 aBL/aPDT ratio; p = 0.0022. The y-axis displays the median of CFUs. Six assays were performed for each experiment, and the control biofilm was performed in three assays. The 95% confidence interval (CI) is presented by using t-bars. (B). A schematic representation of the experiment.
3.5. One-Day S. aureus Biofilm Treated Once by 200 J/cm2
The one-day S. aureus biofilm showed a significant reduction in viability when treated with aBL at an energy density of 200 J/cm2. The median number of CFUs decreased from 9.4 × 108 CFUs (with a range of 3.7 × 108–3.3 × 109 CFUs, with six assays) in the control test to 1.5 × 103 CFUs (ranging between 8.0 × 102 and 3.8 × 103 CFUs, with six assays), with p = 0.0022. APDT resulted in a similar scale efficiency as did aBL, decreasing the median number of CFUs to 1.8 × 103 (the range being 6.0 × 102–2.2 × 103 CFUs, with six assays), with p = 0.0022. However, the dual-light combination of one part of aBL and three parts of aPDT, at irradiances of 42 and 135 mW/cm2, respectively, decreased the median of live bacteria to 1.0 × 102 CFUs (ranging between 0.0 and 2.0 × 102 CFUs with six assays), with p = 0.0022. The bactericidal effect of dual-light with a 1:1 ratio of aBL and aPDT (with irradiances of 79 and 73 mW/cm2, respectively) decreased the median number of CFUs to 50 (the range being 0.0–100.0 CFUs with six assays), with p = 0.0022. When the amount of aBL was raised to three parts, and aPDT was set at one part in the dual-light system, with respective irradiances of 130 mW/cm2 and 38 mW/cm2, the bacterial viability was reduced to 100.0 (the range being 0–200.0 CFUs with six assays), with p = 0.0022 (see Figure 5).
Figure 5.
(A). Effects of different irradiance ratios of aBL and aPDT in the dual-light aPDT system in one-day S. aureus biofilm daily at 200 J/cm2. One-day S. aureus biofilm, single dose, aBL vs. dual-light 1:3 aBL/aPDT ratio at 200 J/cm2, p = 0.0022; aBL vs. dual-light 1:1 aBL/aPDT ratio, p = 0.0022; aBL vs. dual-light 3:1 aBL/aPDT ratio, p = 0.0022; aPDT vs. dual-light 1:3 aBL/aPDT ratio, p = 0.0022; aPDT vs. dual-light 1:1 aBL/aPDT ratio, p = 0.0022; aPDT vs. dual-light 3:1 aBL/aPDT ratio, p = 0.0022. aBL vs. aPDT, p = ns; dual-light aPDT: 1:3 aBL/aPDT ratio vs. 1:1 aBL/aPDT ratio, p = ns; 1:3 aBL/aPDT ratio vs. 3:1 aBL/aPDT ratio, p = ns; 1:1 aBL/aPDT ratio vs. 3:1 aBL/aPDT ratio; p = ns. The y-axis displays the median of CFUs. Six assays were performed for each experiment, and the control biofilm was performed in three assays. The 95% confidence interval (CI) is presented by using t-bars; ns = non-significant. (B). A schematic representation of the experiment.
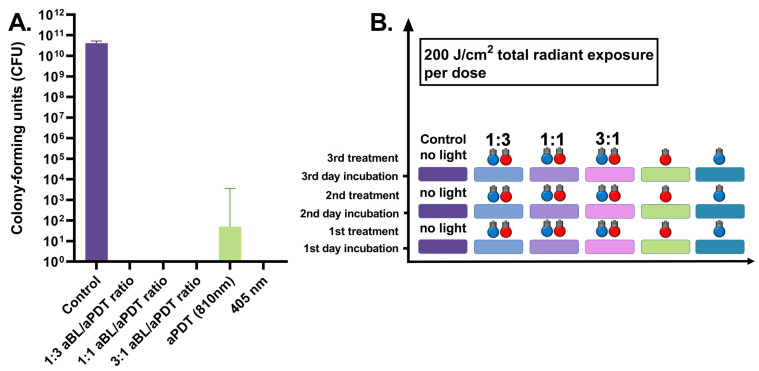
3.6. Three-Day S. aureus Biofilm Treated Daily by 200 J/cm2
When the three-day S. aureus biofilm was exposed to the daily antibacterial therapy, aBL reduced the bacterial viability to almost zero. The median number of CFUs with aBL was 0 (ranging between 0.0 and 210.0 CFUs, with six assays), and the median number of CFUs in the control test was 4.2 × 1010 (the range being 1.6 × 1010–5.2 × 1010 CFUs, with six assays), with p = 0.0022. Similarly, aPDT resulted in a significant reduction in viability as did aBL, presenting with a median of 0.5 CFUs (the range being 0–3.6 × 103 CFUs, with six assays), with p = 0.0022. The dual-light combination of one part of aBL and three parts of aPDT, at irradiances of 42 mW/cm2 and 135 mW/cm2, respectively, decreased the number of living bacteria to 0 CFUs (with a range of 0 CFUs with six assays), with p = 0.0022. The one-to-one ratio of aBL and 810 nm PDT, with irradiances of 79 and 73 mW/cm2, respectively, decreased the median number of CFUs to 0 (the range being 0–1 CFUs with six assays), with p = 0.0022. When the amount of aBL was raised to three parts, and aPDT was set at one part in the dual-light system, with respective irradiances of 130 and 38 mW/cm2, 0 CFUs were found (the range being 0 CFUs with six assays), with p = 0.0022 (see Figure 6).
Figure 6.
(A). Effects of different irradiance ratios of aBL and aPDT in the dual-light aPDT system in three-day S. aureus biofilm, when the antibacterial treatment is given once daily at 200 J/cm2. Three-day S. aureus biofilm, daily dose, aBL vs. dual-light 1:3 aBL/aPDT ratio, p = ns; aBL vs. dual-light 1:1 aBL/aPDT ratio, p = ns; aBL vs. dual-light 3:1 aBL/aPDT ratio, p = ns; aPDT vs. dual-light 1:3 aBL/aPDT ratio, p = ns; aPDT vs. dual-light 1:1 aBL/aPDT ratio, p = ns; aPDT vs. dual-light 3:1 aBL/aPDT ratio, p = ns; aBL vs. aPDT, p = ns; dual-light aPDT: 1:3 aBL/aPDT ratio vs. 1:1 aBL/aPDT ratio, p = ns; 1:3 aBL/aPDT ratio vs. 3:1 aBL/aPDT ratio, p = ns; 1:1 aBL/aPDT ratio vs. 3:1 aBL/aPDT ratio; p = ns. The y-axis displays the median of CFUs. Six assays were performed for each experiment, and the control biofilm was performed in three assays. The 95% confidence interval (CI) is presented by using t-bars; ns = non-significant. (B). A schematic representation of the experiment.
4. Discussion
This study demonstrates the efficacy of dual-light aPDT against S. aureus biofilm when compared to separately applied 810 nm aPDT with ICG photosensitizer or 405 nm aBL. We tested three different combinations of the dual-light to find out the most effective combination against S. aureus. To enable direct comparison between the groups, we kept the radiant exposure constant at 100 J/cm2 and examined the effect of doubling the radiant exposure to 200 J/cm2. The combination of dual-light, where aBL was increased, showed the most effective antibacterial combination against S. aureus. On the one-day biofilm model, the single-dose light exposures at 100 J/cm2 resulted in a decrease of six logarithmic scales in the bacterial count when compared to the control biofilm. The decrease in CFU formation was three logarithmic scales more efficient when compared to aPDT alone and four logarithmic scales more efficient than aBL. Similar results were seen when the three-day biofilm was treated with a single dose of dual-light. When the light exposure was doubled up to 200 J/cm2, the antibacterial efficacy increased expectedly in all the treatment groups, but again, the most effective antibacterial action was seen in the dual-light-treated groups.
We also tested protocols where the S. aureus biofilm was treated daily, simulating clinical treatment scenarios. Investigating repeated treatment effects in biofilm is important. Although aPDT treatment is assumed to be unable to cause resistance [22], the bacteria are capable of adapting to environmental stress [23]. For example, Guffrey et al. (2013) reported a decrease in the inactivation effectiveness of 405 nm blue light against in vitro S. aureus after the fifth bacterial generation was exposed to blue light [24]. However, in the present study, repeated daily treatment with 100 J/cm2 radiant exposure resulted in a persistent antibacterial effect, especially in settings where the relative amount of aBL was higher. When the total daily applied radiant exposure increased to 200 J/cm2, the antibacterial impact increased to the disinfection level in all treatment groups.
Opposite to UV-light bacterial inactivation, visible-light bacterial inactivation has clear advantages in terms of its ease of use and substantially greater safety in a clinical setting. Thus, aPDT has arisen as an alternative therapy to antibiotics in the treatment of bacterial infections. The antimicrobial effect of aPDT is based on the principle that visible light activates an externally applied photosensitizer. This results in the generation of reactive oxygen species (ROS) that kill bacteria unselectively. The use of ICG as a photosensitizer has many benefits, including the low toxicity and the ability to release the absorbed energy as heat through internal conversion, in addition to the fluorescence emission and the ROS producing triplet state. Thus, the depth of penetration might be increased when compared to other photosensitizers. [25]. APDT with ICG has previously shown a significant antibacterial action against S. aureus, studied in the methicillin-resistant strains [26]. ABL is based on the same principle as aPDT, but the photosensitizers in the process are inherent molecules within the bacteria itself, such as porphyrins and flavins [18]. Of the different blue light spectrums, 405 nm aBL has been shown to outperform longer aBL wavelengths in several studies [27,28]. Light coherence has no significant role in the process, and the use of LED light sources would not make a significant difference to laser light sources [29].
A combination of different wavelengths to improve the bactericidal effect against S. aureus has been proposed earlier. In a study by Leanse et al. with a dual-wavelength aBL irradiation approach, the combination of 460 and 405 nm light was more effective against methicillin-resistant S. aureus than separately given irradiations. In this study, the 460 nm light was proposed to inactivate an oxygen quencher staphyloxanthin from the cell surface, which showed a protective action against 405 nm aBL exposure. [30]. Another dual-light approach was conducted by Guffrey et al., wherein the effect of a combination of 405 nm blue light and 880 nm infrared light on S. aureus and Pseudomonas aeruginosa was tested. The bacteria were treated simultaneously with a combination of 405 nm and 880-nm light. The results revealed a significant dose-dependent bactericidal effect, although the S. aureus experiments resulted in statistically significant decreases in bacterial colonies at all dose levels. However, the near-infrared light was applied without a photosensitizer and had only a small effect. [31]. The aforementioned findings were in line with our previous results when we compared the pure NIR light to the combined effect of NIR together with ICG [19]. In the present study, we did not test the antibacterial effect of the sole 810 nm light.
We tested the repeated dosing of aBL and aPDT combination for two reasons. Firstly, to simulate a clinical antibacterial treatment protocol of daily dosing, and secondly, to test the ability of S. aureus to adapt against a repeated dual-light therapy in a short treatment setting. We have recently published data regarding the ability of S. mutans to adapt against both aBL and aPDT, when given separately in a repeated fashion [19]. The adaptation of S. mutans was not seen when aBL and aPDT were combined as a dual-light treatment. In the present study, similarly to our previous findings with S. mutans, we saw the retained antibacterial action abate with S. aureus when aBL or aPDT were used separately. The ability of S. aureus to adapt against the repeated application of 405 nm has also been shown by other groups [30,31]. Although in the recent study, the aPDT showed markedly better antibacterial effect compared to aBL against S. aureus biofilm, the biofilm showed adaptation to repeated exposure. This was seen by increased CFU counts of up to 100-fold when compared to the single-dose aPDT treatment. The response to the repeated adverse environmental stimuli seemed to develop in the early stage, within the first few repeated exposures. In our protocol, the bacteria had one day (approximately 24 h) to build responsive actions. Again, the bacteria were capable of adapting when repeated single wavelength antibacterial light treatment was applied. The dual-light aPDT against S. aureus markedly outperformed both aPDT and aBL in efficacy when compared to the single-use protocol, but most importantly, the synchronized use was able to suppress the ability of the biofilm to adapt to the repeated treatment protocol. This suppression gives great promise to the repeated dual-light treatment protocol in the clinical setting.
Skin and soft-tissue infections are common problems encountered in clinical practice. An increase in the incidence of skin and soft-tissue infections has been observed during previous decades. [32]. Treatment has been significantly complicated by the increasing emergence of multidrug-resistant pathogenic bacteria, especially methicillin-resistant S. aureus [2]. These infections are responsible for a significant percentage of post-surgery infective complications [33,34], patient mortality, and massive healthcare costs in hospitals worldwide [35,36]. Empiric treatment considerations are likely to be changed by the increasing prevalence of antibiotic-resistant bacteria, such as methicillin-resistant S. aureus. Hence, future treatments for local tissue infections could include light as adjunctive therapy in the antibacterial treatment. Our results indicate that the use of a combination of aBL and aPDT indeed provides an increase in the efficacy of bacterial killing, which can be attractive in clinical practice. The combination here presented improves the effect in both single-dose treatments and in repeated protocols. An example where an efficient single treatment would be lucrative is a local antibacterial treatment during surgery, where the local access to the infection site would be limited. Such local antibacterial treatment could be, for example, the disinfection of valve annulus during endocarditis valve replacement surgery or, similarly, during endoprosthesis replacement in orthopedics. On the other hand, the improved and retained antibacterial effect at easily reachable infection sites, such as the local impetigo of superficial wound infection, would be greatly appreciated. This finding already may greatly help to extend the indicated usage of dual-light aPDT. Importantly, it paves the way forward for evaluating dual-light-based aPDT treatments for difficult-to-treat chronic wound infections or burn-wound infections complicated by polymicrobial bacterial biofilms resistant to many treatments now in use.
Limitations
It could be argued that utilizing almost an identical study protocol to investigate the effects of aBL, aPDT, and dual-light aPDT on the viability of S. aureus in the present study, as performed in our previous article reporting on S. mutans [18], would be repetitive. On the contrary, we speculate that the use of similar light irradiances, bacterial culture, and colony-forming-unit counting methods produces valuable comparability. Eventually, in this report, we show the need to increase dual-light dosing against S. aureus, compared to S. mutans. The ability to accurately assess the requirements of successful bacterial eradication is vital during the development of light-based treatments of infections at the time of growing antibiotic resistance.
5. Conclusions
The objective of this proposal was to investigate the utility of a non-antibiotic approach against S. aureus. Our results indicate the effectiveness of a dual-light combination of aBL and aPDT in improving the antibacterial efficacy when compared with aPDT or aBL treatment alone. The dual-light efficacy was sustained in a three-day repetitive use model, and we showed the importance of the amount of aBL for optimal antibacterial efficacy. This study reveals a new effective local antibacterial method against S. aureus, one of the most important pathogens responsible for a significant percentage of common topical skin infections, but also post-surgery infective complications, patient mortality, and massive healthcare costs in hospitals worldwide.
Acknowledgments
Open access funding provided by University of Helsinki.
Author Contributions
Study design, S.N., A.P., J.M., T.S., J.R., E.K., T.T. and T.P.; drafting of the manuscript, S.N., A.P. and T.T; critical review of the manuscript, S.N., P.R., J.M., T.S., J.R., E.K. and T.P.; statistical analysis, T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Instrumentarium Science Foundation. Koite Health Oy provided support in the form of salaries for author S.N. and provided the materials for the study (VAT-2918895-9). The specific roles of the authors are articulated in the Author Contributions section. The funders had no additional role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors of this manuscript have the following competing interests: SN is a paid employee of Koite Health Oy, who provided materials for the study. T.P. is a stock owner and founder of Koite Health Oy and a co-inventor of US20210030874A1, WO2021023915A1, WO2020193870A1, and WO2019234308A1. S.N. is a stock owner and founder of Koite Health Oy and co-inventor of patents WO2020084199A1, US20210030874A1, WO2021023915A1, WO2020193870A1, and WO2019234308A1. J.M. has stock options in Koite Health Oy. J.R. is a stock owner and founder of Koite Health Oy and a co-inventor of patents WO2020084199A1, US20210030874A1, WO2021023915A1, WO2020193870A1, and WO2019234308A1. T.S. is an inventor for US Patents 5652223, 5736341, 5866432, and 6143476 and co-inventor of US Patent 20170023571A1.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ondusko D., Nolt D. Staphylococcus aureus. Pediatr. Rev. 2018;39:287–298. doi: 10.1542/pir.2017-0224. [DOI] [PubMed] [Google Scholar]
- 2.Guo Y., Song G., Sun M., Wang J., Wang Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020;10:107. doi: 10.3389/fcimb.2020.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Federspiel J., Stearns S., Peppercorn A., Chu V., Fowler V., Jr. Increasing U.S. rates of endocarditis with Staphylococcus aureus: 1999–2008. Arch. Intern. Med. 2012;4:363–365. doi: 10.1001/archinternmed.2011.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murdoch D.R., Corey R.G., Hoen B., Miró M., Fowler V.G., Bayer A.S., Karchmer A.W., Olaison L., Pappas P.A., Moreillon P., et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The international collaboration on endocarditis-prospective cohort study. Arch. Intern. Med. 2009;169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kern W. Management of Staphylococcus aureus bacteremia and endocarditis: Progresses and challenges. Curr. Opin. Infect. Dis. 2010;23:346–358. doi: 10.1097/QCO.0b013e32833bcc8a. [DOI] [PubMed] [Google Scholar]
- 6.Skinner D., Keefer C. Significance of bacteremia caused by Staphylococcus aureus A study of one hundred and twenty-two cases and a review of the literature concerned with experimental infection in animals. Arch. Intern. Med. 1941;5:851–875. doi: 10.1001/archinte.1941.00200110003001. [DOI] [Google Scholar]
- 7.den Heijer C.D.J., van Bijnen E.M.E., Paget W.J., Pringle M., Goossens H., Bruggeman C.A., Schellevis F.G., Stobberingh E.E. Prevalence and resistance of commensal Staphylococcus aureus, including methicillin-resistant S. aureus, in nine European countries: A cross-sectional study. Lancet Infect. Dis. 2013;13:409–415. doi: 10.1016/S1473-3099(13)70036-7. [DOI] [PubMed] [Google Scholar]
- 8.Elie-Turenne M.-C., Fernandes H., Mediavilla J.R., Rosenthal M., Mathema B., Singh A., Cohen T.R., Pawar K.A., Shahidi H., Kreiswirth B.N., et al. Prevalence and characteristics of Staphylococcus aureus colonization among healthcare professionals in an urban teaching hospital. Infect. Control Hosp. Epidemiol. 2010;31:574–580. doi: 10.1086/652525. [DOI] [PubMed] [Google Scholar]
- 9.Lee A.S., De Lencastre H., Garau J., Kluytmans J., Malhotra-Kumar S., Peschel A., Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers. 2018;4:18033. doi: 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- 10.Mediavilla J., Chen L., Mathema B., Kreiswirth B. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) Curr. Opin. Microbiol. 2012;15:588–595. doi: 10.1016/j.mib.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Ogawara H. Comparison of antibiotic resistance mechanisms in antibiotic-producing and pathogenic bacteria. Molecules. 2019;24:3430. doi: 10.3390/molecules24193430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plackett B. Why big pharma has abandoned antibiotics. Nature. 2020;586:50–52. doi: 10.1038/d41586-020-02884-3. [DOI] [Google Scholar]
- 13.Mohr K. History of antibiotics research. Curr. Top Microbiol. Immunol. 2016;398:237–272. doi: 10.1007/82_016_499. [DOI] [PubMed] [Google Scholar]
- 14.Årdal C., Balasegaram M., Laxminarayan R., McAdams D., Outterson K., Rex J.H., Sumpradit N. Antibiotic development—Economic, regulatory and societal challenges. Nat. Rev. Microbiol. 2020;18:267–274. doi: 10.1038/s41579-019-0293-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Wang Y., Wang Y., Murray C.K., Hamblin M.R., Hooper D.C., Dai T. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resist. Updat. 2017;33–35:1–22. doi: 10.1016/j.drup.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song H.-H., Lee J.-K., Um H.-S., Chang B.-S., Lee S.-Y., Lee M.-K. Phototoxic effect of blue light on the planktonic and biofilm state of anaerobic periodontal pathogens. J. Periodontal Implant Sci. 2013;43:72–78. doi: 10.5051/jpis.2013.43.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cieplik F., Deng D., Crielaard W., Buchalla W., Hellwig E., Al-Ahmad A., Maisch T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018;5:571–589. doi: 10.1080/1040841X.2018.1467876. [DOI] [PubMed] [Google Scholar]
- 18.Yin R., Dai T., Avci P., Jorge A.E.S., de Melo W., Vecchio D., Huang Y., Gupta A., Hamblin M.R. Light based anti-infectives: Ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr. Opin. Pharmacol. 2013;5:2109–2121. doi: 10.1016/j.coph.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikinmaa S., Alapulli H., Auvinen P., Vaara M., Rantala J., Kankuri E., Sorsa T., Meurman J., Pätilä T. Dual-light photodynamic therapy administered daily provides a sustained antibacterial effect on biofilm and prevents streptococcus mutans adaptation. PLoS ONE. 2020;15:e0232775. doi: 10.1371/journal.pone.0232775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zmantar T., Kouidhi B., Miladi H., Mahdouani K., Bakhrouf A. A microtiter plate assay for Staphylococcus aureus biofilm quantification at various pH levels and hydrogen peroxide supplementation. New Microbiol. 2010;2:137–145. [PubMed] [Google Scholar]
- 21.Nikinmaa S., Patila T. Dual-Light Photodynamic Therapy Administered Daily Provides a Sustained Antibacterial Effect on Biofilm and Prevents Streptococcus mutans Adaptation. 2021. [(accessed on 25 April 2021)]. Available online: https://www.protocols.io/view/dual-light-photodynamic-therapy-administered-daily-bfbcjiiw. [DOI] [PMC free article] [PubMed]
- 22.Tavares A., Carvalho C.M.B., Faustino M.A., Neves M.G.P.M.S., Tomé J.P.C., Tomé A.C., Cavaleiro J.A.S., Cunha Â., Gomes N.C.M., Alves E., et al. Antimicrobial photodynamic therapy: Study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs. 2010;8:91–105. doi: 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onyango L., Alreshidi M. Adaptive metabolism in staphylococci: Survival and persistence in environmental and clinical settings. J. Pathog. 2018;2018:1092632. doi: 10.1155/2018/1092632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guffrey J., Payne W., Jones T., Martin K. Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 nm light from a supraluminous diode array. Photomed. Laser Surg. 2013;31:179–182. doi: 10.1089/pho.2012.3450. [DOI] [PubMed] [Google Scholar]
- 25.Burchard T., Karygianni L., Hellwig E., Follo M., Wrbas T., Wittmer A., Al-Ahmad A. Inactivation of oral biofilms using visible light and water filtered infrared A radiation and indocyanine green. Future Med. Chem. 2019;11:1721–1740. doi: 10.4155/fmc-2018-0522. [DOI] [PubMed] [Google Scholar]
- 26.Wong T.-W., Wu E.-C., Ko W.-C., Lee C.-C., Hor L.-I., Huang I.-H. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus by indocyanine green and near infrared light. Dermatol. Sin. 2018;36:8–15. doi: 10.1016/j.dsi.2017.08.003. [DOI] [Google Scholar]
- 27.Tomb R., White T., Coia J., Anderson J., MacGregor S., Maclean M. Review of the comparative susceptibility of microbial species to photoinactivation using 380–480 nm violet-blue light. Photochem. Photobiol. 2018;3:445–458. doi: 10.1111/php.12883. [DOI] [PubMed] [Google Scholar]
- 28.Hoenes K., Hess M., Vatter P., Spellerberg B., Hessling M. 405 nm and 450 nm photoinactivation of saccharomyces cerevisiae. Eur. J. Microbiol. Immunol. 2018;8:142–148. doi: 10.1556/1886.2018.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masson-Mayers D., Bumah V., Biener G., Raicu V., Enwemeka C. The relative antimicrobial effect of blue 405 nm LED and blue 405 nm laser on methicillin-resistant Staphylococcus aureus in vitro. Lasers Med. Sci. 2015;30:2265–2271. doi: 10.1007/s10103-015-1799-1. [DOI] [PubMed] [Google Scholar]
- 30.Leanse L., Goh X., Cheng J.-X., Hooper D., Dai T. Dual-wavelenght photo-killing of methicillin-resistant Staphylococcus aureus. JCI Insight. 2020;5:e134343. doi: 10.1172/jci.insight.134343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guffrey J., Wilborn J. Effects of combined 405 nm and 880-nm light on Staphylococcus aureus and pseudomonas aeruginosa in vitro. Photomed. Laser Surg. 2006;6:680–683. doi: 10.1089/pho.2006.24.680. [DOI] [PubMed] [Google Scholar]
- 32.Esposito S., Noviello S., Leone S. Epidemiology and microbiology of skin and soft tissue infections. Curr. Opin. Infect. Dis. 2016;2:109–115. doi: 10.1097/QCO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 33.de Carvalho R., Campos C., de Castro Franco L., Rocha A., Ercole F. Incidence and risk factors for surgical site infection in general surgeries. Rev. Lat.-Am. Enfermagem. 2017;25:e2848. doi: 10.1590/1518-8345.1502.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owens C., Stoessel K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008;70:3–10. doi: 10.1016/S0195-6701(08)60017-1. [DOI] [PubMed] [Google Scholar]
- 35.McGarry S., Engemann J., Schmader K., Sexton D., Kaye K. Surgical site infection due to Staphylococcus aureus among elderly patients: Mortality, duration of hospitalization and cost. Infect. Control Hosp. Epidemiol. 2004;6:461–467. doi: 10.1086/502422. [DOI] [PubMed] [Google Scholar]
- 36.Yuasa A., Murata T., Imai K., Yamamoto Y., Fujimoto Y. Treatment procedures and associated medical costs of methicillin-resistant Staphylococcus aureus infection in Japan: A retrospective analysis using a database of Japanese employment-based health insurance. SAGE Open Med. 2019;7:2050312119871181. doi: 10.1177/2050312119871181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.