Abstract
The mammalian Ste20 kinase Nck-interacting kinase (NIK) specifically activates the c-Jun amino-terminal kinase (JNK) mitogen-activated protein kinase module. NIK also binds the SH3 domains of the SH2/SH3 adapter protein Nck. To determine whether Nck functions as an adapter to couple NIK to a receptor tyrosine kinase signaling pathway, we determined whether NIK is activated by Eph receptors (EphR). EphRs constitute the largest family of receptor tyrosine kinases (RTK), and members of this family play important roles in patterning of the nervous and vascular systems. In this report, we show that NIK kinase activity is specifically increased in cells stimulated by two EphRs, EphB1 and EphB2. EphB1 kinase activity and phosphorylation of a juxtamembrane tyrosine (Y594), conserved in all Eph receptors, are both critical for NIK activation by EphB1. Although pY594 in the EphB1R has previously been shown to bind the SH2 domain of Nck, we found that stimulation of EphB1 and EphB2 led predominantly to a complex between NIK/Nck, p62dok, RasGAP, and an unidentified 145-kDa tyrosine-phosphorylated protein. Tyrosine-phosphorylated p62dok most probably binds directly to the SH2 domain of Nck and RasGAP and indirectly to NIK bound to the SH3 domain of Nck. We found that NIK activation is also critical for coupling EphB1R to biological responses that include the activation of integrins and JNK by EphB1. Taken together, these findings support a model in which the recruitment of the Ste20 kinase NIK to phosphotyrosine-containing proteins by Nck is an important proximal step in the signaling cascade downstream of EphRs.
The Eph family of receptor tyrosine kinases (RTKs) is the largest family of RTKs (8, 23, 34). Recent experimental evidence has indicated that members of this family play critical roles in patterning of the embryo. However, Eph receptors (EphRs) are best known for their roles in patterning of the central and peripheral nervous systems and the vascular system (reviewed in references 13, 23 and 34). In the developing nervous system, EphRs function primarily as repulsive cues toward migrating axons and neural crest cells. This activity, coupled with the finding that EphRs and their ligands (ephrins) are expressed in reciprocal compartments in the developing embryo, has led to the suggestion that EphRs function as barriers to migrating axons and cells. In the vascular system, EphRs play a critical role in remodeling of the vascular system (1, 51, 54).
At least 14 different EphRs have been identified (11). These receptors can be divided into two subclasses, EphA and EphB, based on the cell surface ligand with which they interact (12). Ligands that interact with EphA receptors are linked to the cell surface by a glycosylphosphatidylinositol linkage and as a group are referred to as the ephrinA subclass of ligands, whereas ligands that interact with EphB receptors are transmembrane proteins and are referred to as the ephrinB subclass of ligands (11, 12, 23). In comparison to other RTKs, which are usually activated by a single ligand, individual EphRs display various binding affinities for a number of different ephrins, and as a result, a single EphR can be activated to various degrees by a number of different ephrins of the same subclass (23, 34).
Little is known about the intracellular signaling pathways that are responsible for mediating the specific biological roles of members of the Eph family. The mechanism whereby EphRs signal cells is further complicated by the finding that bidirectional signaling occurs between EphB receptors and their ephrinB ligands (4, 22). Thus, specific phenotypes in gene “knockout” studies in mice cannot always be attributed to loss of signaling by an EphR but, rather, may be attributable to the loss of signaling by the corresponding ephrinB ligand, as has been reported for EphB2 knockout mice (19). The finding that EphRs mediate repulsive signals to migrating axons and neural crest cells has led to the idea that members of this family stimulate the reorganization of cytoskeletal elements and/or the adhesive properties of cells; for example, collapse of the axonal growth cone stimulated by ephrins probably can be attributed to Eph-stimulated changes in the cytoskeletal architecture and/or cell adherence (50). Small GTPases of the Rho/Rac family are prime candidates for mediating some of these effects, but a link between EphRs and Rho/Rac family GTPases has not yet been found.
A number of signaling molecules that are recruited to the EphR signaling pathway have now been identified by using the yeast two-hybrid system and by identifying tyrosine-phosphorylated proteins and the proteins with which they associate in Eph-stimulated cells. These studies have led to the identification of several SH2 domain-containing proteins that associate with EphRs including the SH2/SH3 adapter proteins Nck, p85-associated phosphoinositide 3-kinase, SLAP, Grb2, and Grb10, as well as Src family kinases and the Ras GTPase-activating protein (RasGAP) (9, 21, 35, 36, 44–46). In addition, p62dok has recently been shown to be tyrosine phosphorylated in EphB2-stimulated cells, and tyrosine-phosphorylated p62dok in turn binds the SH2 domain of Nck and RasGAP, generating a complex containing these three proteins in EphB2-stimulated cells (21).
As discussed above, one downstream target of Eph receptors is Nck. Nck is a ubiquitously expressed protein composed entirely of a single SH2 and three SH3 domains and thus fits into the adapter class of signaling molecules (29). Nck and related adapter proteins such as Grb2 are thought to regulate signaling pathways downstream of tyrosine kinases by coupling catalytic subunits, bound to their SH3 domains, to phosphotyrosine-containing proteins that interact with their SH2 domains (37, 41, 43). Recent studies with Drosophila have shed light on the probable function of Nck in mammalian cells. The Drosophila homolog of Nck, encoded by dreadlocks (dock), was cloned in a genetic screen searching for proteins that are critical for proper targeting of photoreceptor (R) axons. In dock mutants, R axons show abnormal clumping and crossing over, with some R1 to R6 axons overshooting their target in the lamina and projecting into the medulla in the optic lobe (15). It has been proposed that dock mediates R-cell targeting by functioning as an adapter molecule to couple a signaling molecule bound to its SH3 domain to a receptor tyrosine kinase functioning at the axonal growth cone. EphRs form one class of receptors that may function upstream of dock/Nck at the axonal growth cone. This possibility would be supported by the findings that EphRs regulate the targeting of R axons in both chickens and mammals and that Nck, via its SH2 domain, is recruited to phosphotyrosine-containing proteins in EphB1- and EphB2-stimulated cells (11, 34, 45).
We have previously identified a Ste20-related kinase, Nck interacting kinase (NIK), in mammalian cells that binds the SH3 domains of Nck and is thus a potential downstream effector for the SH3 domains of Nck (47). In addition to binding Nck, NIK is a mitogen-activated protein kinase kinase kinase kinase (MAP4K) that specifically activates the JNK MAPK module (47). Recent genetic studies with Drosophila have demonstrated the roles of NIK as a regulator of the JNK MAPK pathway and as a downstream target of dock and/or a related SH3 domain-containing protein, important for correct targeting of R axons (48; Y.-C. Su, C. Maurel-Zaffran, J. E. Treisman, and E. Y. Skolnick, submitted for publication). We have recently shown that a fly homolog of NIK called misshapen (msn) is present in Drosophila and functions as a MAP4K to stimulate JNK activation and dorsal closure in the Drosophila embryo (48). In addition, msn is required for the correct targeting of photoreceptor axons (Sut et al., submitted). Interestingly, while dock does not act upstream of msn in a pathway leading to JNK activation and dorsal closure, we found that a form of msn that is unable to bind dock is not able to signal the correct targeting R axons. These findings suggest that an interaction between msn, dock, and/or a related SH3 domain containing protein is critical for msn to be activated at the axonal growth cone (Su et al., submitted). Based on these findings, we tested whether NIK is a downstream target of EphRs and whether NIK functions as a downstream effector of Nck in Eph-stimulated cells.
In this report, we show that NIK activity is specifically increased in cells stimulated by two EphRs, EphB1 and EphB2. Coimmunoprecipitation experiments demonstrated that NIK-Nck formed a complex predominantly with p62dok and RasGAP, as well as with an unidentified 145-kDa tyrosine-phosphorylated protein in cells stimulated with EphB1 and EphB2. Tyrosine-phosphorylated p62dok probably binds directly to the SH2 domains of Nck and RasGAP and indirectly to NIK bound to the SH3 domain of Nck. We also demonstrate that NIK couples EphB1 to JNK activation and EphB1-stimulated integrin activation. These findings indicate that the Ste20 kinase NIK is an important downstream target of EphRs.
MATERIALS AND METHODS
Cell lines, cell culture, and ephrinB1 stimulation.
P19, a mouse teratocarcinoma cell line, was cultured in alpha modified Eagle's medium (α-MEM) containing 10% fetal bovine serum (FBS) (45). NG108 cells (mouse neuroblastoma × rat glioma hybrid) stably overexpressing EphB2 (NG108-EphB2) were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS as previously described (21). To stimulate P19 cells, the cells were serum starved for 24 h in Opti-MEM (GIBCO BRL). After treatment with 0.5 mM suramin (Calbiochem) for 3 to 5 h, the cells were stimulated for 20 min at 37°C with 500 ng of ephrin-B1/Fc per ml that was preclustered using anti-human Fc (Jackson ImmunoResearch) (45). NG108-EphB2 cells were stimulated in a similar manner to P19 cells, except that NG108-EphB2 cells were not pretreated with suramin (21) and the starvation period was just 2 h.
Stable P19 cell lines that inducibly express either the wild-type NIK [NIK(WT)] or kinase-inactive NIK [NIK(KD)] were generated using the RevTet-On system (Clontech). Full-length mouse cDNAs of NIK(WT) and NIK(KD) were subcloned into the retroviral vector pRev-TRE. Nonreplicating retroviruses were obtained by transiently transfecting the Phoenix packaging cell line with the regulatory vector pRevTet-On and pRev-TRE–NIK constructs as described previously (16). P19 cells were coinfected either with pRevTet-On/pRev-TRE–NIK(WT) or pRevTet-On/pRev-TRE–NIK(KD), and clones were selected by culturing cells in the presence of 250 μg of hygromicin per ml and 400 μg of Geneticin per ml. Expression of ectopic NIK proteins was induced by culturing in the presence of 1 μg of doxycycline per ml for 48 h.
Antibodies and plasmids.
The anti-NIK antibody is a rabbit polyclonal antibody raised against a glutathione S-transferase (GST) fusion protein corresponding to amino acids 443 to 619 of full-length NIK. Anti-p62dok antibodies were purchased from Santa Cruz, and antibodies against the HA epitope (12CA5) were purchased from Boehringer Mannheim. The antibody 9E10 was used for immunoprecipitation and immunoblotting the Myc-tagged constructs (10). The anti-Nck antibody is a rabbit polyclonal antibody raised against full-length GST-Nck (28). Anti-SH2 domain-containing inositol 5-phosphatase (SHIP2) antibodies were kindly provided by S. Decker (17). HA-tagged and Myc-tagged NIK constructs used for transient-transfection assays were expressed using the vector pRK5 as described previously (47). Kinase inactive NIK contains the substitution of aspartic acid for asparagine in the NIK kinase domain and has been described previously (47). HA-tagged EphB1(Y594F), and HA-tagged EphB1 (K652R) were expressed using the vector pSRα and have been described previously (45).
Kinase assays.
To assay for NIK activity, P19 and NG108-EphB2 cells were stimulated with ephrinB1 as described above. After 15 min, the cells were lysed and NIK was immunoprecipitated using the anti-NIK antibody described above. The immune complex was then washed four times with lysis buffer (47) and three times with kinase buffer (20 mM HEPES [pH 7.4], 10 mM MgCl, 20 mM β-glycerophosphate, 10 mM NaF, 0.2 mM orthovanadate, 1 mM dithiothreitol). After a 20-min incubation at 30°C in kinase buffer containing 10 μCi of [32P]ATP and 5 μg of myelin basic protein, the kinase reaction was terminated by adding an equal volume of 2× sample buffer and the reaction products were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12.5% polyacrylamide).
To assay NIK activity in 293 cells, 293 cells were transfected with the constructs as indicated by using Ca2(PO4)3 (47). At 24 h after transfection, the 293 cells were placed for an additional 24 h in DMEM containing 0.5% FBS and lysed, and NIK activity assays were performed as described above.
JNK activity using GST–c-Jun (containing amino acids 1 to 223 of c-Jun coupled to glutathione-agarose [GST] beads) was determined by incubating 100 μg of lysates from unstimulated or ephrinB1-stimulated P19 cells with 10 μg of GST-cJun for 2 h at 4°C. After the beads were washed three times with lysis buffer and twice with kinase buffer, the kinase reaction was started by incubating beads in 25 μl of kinase buffer containing 20 μM cold ATP and [γ-32P]ATP (15 μCi per sample). After incubation at 30°C for 20 min, the reaction was terminated by boiling the mixtures in sample buffer for 5 min and the reaction products were separated by SDS-PAGE (15% polyacrylamide).
Cell lysis, immunoprecipitation, and immunoblotting.
Cell lysis, immunoprecipitation, and immunoblotting were performed as previously described (43).
Cell attachment assay.
P19 cells were transfected with 6 μg of NIK(WT) or NIK mutants as indicated using Lipofectamine Plus (Life Technologies). At 24 h after transfection, the cells were serum starved in DMEM containing 0.1% bovine serum albumin, and solid-phase attachment assays were performed after 24 h of serum starvation, as described previously (27). Then 48-well plates were precoated with a thin layer of nitrocellulose and incubated overnight at 4°C with phosphate-buffered saline (PBS) containing fibrinogen (1 μg/cm2), either alone or in combination with immunoglobulin G (IgG) control, ephrinB1/Fc, or ephrinB2/Fc (300 ng/cm2). At 2 h before the assay, the wells were washed twice with PBS and blocked for 2 h with 1% bovine serum albumin. The cells were gently trypsinized and replated at a density of 105 cells/well. After 45 to 60 min at 37°C, unattached cells were dislodged by five brisk slaps of the plate on a horizontal surface. The wells were washed with PBS, and adherent cells were fixed in 0.2% glutaraldehyde, stained with 0.5% crystal violet, and quantified by measurement of the optical density at 570 nm. To verify that NIK constructs were expressed at equal levels, half of the transfected cells were lysed after 48 h and immunoblotted with the anti-myc antibody 9E10.
RESULTS
NIK activity is increased in cells stimulated by the EphB1 and EphB2 receptors.
Polyclonal antibodies to NIK were generated by immunizing rabbits with a GST-NIK fusion protein (amino acids 443 to 619) (47). Using immunoprecipitation and immunoblotting, we found that NIK is expressed at its highest levels in cells of neuroepithelial origins, consistent with the idea the NIK functions in the nervous system and is a potential downstream target of EphRs (data not shown). Anti-NIK antibodies, but not preimmune serum, specifically recognize two endogenous proteins that run at molecular masses of 140 and 150 kDa in both P19 and NG108 cells (Fig. 1 and data not shown). The 140-kDa band comigrates with NIK obtained from 293 cells transfected with the NIK cDNA (data not shown). The nature of the 150-kDa band recognized by anti-NIK antibodies is not clear. Our inability to alter the mobility of this band in SDS-polyacrylamide gels by treatment with alkaline phosphatase indicates that this protein does not arise as a result of posttranslational modification by phosphorylation and suggests that it represents an alternatively spliced NIK isoform or a closely related protein (data not shown).
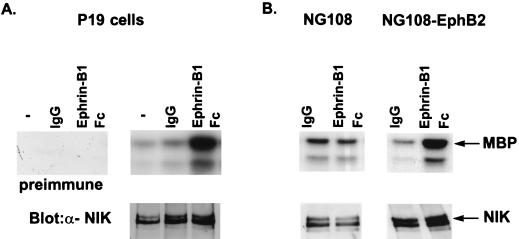
FIG. 1.
Immunoblot and immune complex kinase assay of NIK from P19 and NG108-EphB2 cells stimulated with ephrinB1. Lysates from P19 cells (A) or NG108-EphB2 cells (B) that were unstimulated (−), treated with clustering antibody alone (IgG), or stimulated with preclustered ephrin (Ephrin-B1 Fc) were immunoprecipitated with anti-NIK antibodies. Half the immunoprecipitates were subjected to an in vitro kinase assay using [γ-32P]ATP and myelin basic protein (MBP) as the substrate. Reaction products were separated by SDS-PAGE (12.5% polyacrylamide) and visualized by autoradiography (upper panels). Half of the immunoprecipitate was immunoblotted with the anti-NIK antibodies, to ensure equal levels of NIK expression (lower panels). The anti-NIK antibody (α-NIK) recognizes two isoforms of NIK that run at apparent molecular masses of 140 and 150 kDa, possibly due to alternate splicing of NIK. To control for nonspecific kinase activity coimmunoprecipitating with the anti-NIK antibodies, an immune complex kinase assay was performed on P19 cell lysates using preimmune serum (A). In addition, to demonstrate that the increase in NIK activity in stimulated NG108-EphB2 cells required the EphB2 receptor, NIK activity was assessed in the parental NG108 cell line (B).
To determine whether NIK is a downstream target of EphRs, we tested whether NIK activity is increased in P19 cells stimulated with ephrinB1. P19 is a murine teratocarcinoma cell line that expresses endogenous EphB1 receptors, and stimulation of these cells with ephrinB1 stimulates the activation of EphB1 receptors (45). We found that stimulation of P19 cells with ephrinB1 led to a three- to fivefold increase in NIK activity (Fig. 1A). The increase in NIK activity paralleled the activation of EphB1, in that increased NIK activity was observed only following receptor activation. Increased kinase activity in ephrinB1-stimulated cells was not detected in immunoprecipitates by using preimmune serum, indicating that anti-NIK antibodies do not nonspecifically precipitate a contaminating kinase in stimulated cells. To extend these observations to other Eph family members, we tested whether NIK activity is activated by EphB2 receptors in the neuronal cell line NG108 that ectopically expresses the EphB2 receptor (NG108-EphB2); it has previously been shown that ephrinB1 specifically activates EphB2 in these cells (21). NIK activity is also increased three- to fivefold in NG108-EphB2 cells stimulated with ephrinB1. NIK activity was not induced in parental NG108 cells stimulated with ephrinB1, indicating that the increase in NIK activity is mediated by EphB2 (Fig. 1B). These findings indicate that NIK activity is increased by at least two EphB family members.
Activation of NIK by EphB1 requires both EphB1 kinase activity and Y594 located in the juxtamembrane region of EphB1.
The juxtamembrane region of all EphRs contains a conserved 10-amino-acid motif that includes two tyrosine residues that are targets for phosphorylation by the Eph kinase (9, 23). While the exact function of these tyrosines in Eph signaling remains to be defined, recent evidence indicates that these tyrosines mediate binding to downstream SH2 domain-containing signaling molecules. For EphB1, the first tyrosine residue (pY594) in this motif binds the SH2 domain of Nck and is essential for JNK and integrin activation by EphB1 (27, 45). These findings raise the possibility that direct coupling of NIK to the activated EphB1R by Nck regulates NIK activity.
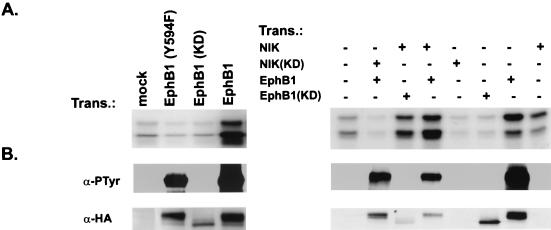
To test whether EphB1 kinase activity and pY594 are required for EphB1 activation of NIK activity, we determined whether endogenous NIK activity is increased in 293 cells transiently transfected with either wild-type or mutant EphB1 receptors containing point mutations that disrupt EphB1 kinase activity [EphB1(K652R)] or pY594 [(EphB1(Y594F)]. 293 cells are suitable for these studies because they do not contain endogenous EphB1 receptors. EphB1 is constitutively active when transfected into 293 cells, presumably because the high level of EphB1 expression in these cells leads to constitutive receptor oligomerization and activation. We found that endogenous NIK activity is also increased three- to fivefold in 293 cells transfected with wild-type EphB1 (Fig. 2). The ability of EphB1 to stimulate an increase in NIK activity required both EphB1 kinase activity and the juxtamembrane tyrosine, since an increase in NIK activity was not seen in cells transfected with either EphB1(K652R), which is kinase dead (KD), or EphB1(Y594F). The increase in NIK kinase activity in cells transfected with wild-type EphB1 is specific for endogenous NIK and is not due to another kinase that coimmunoprecipitates with the anti-NIK antibodies, because the increase in NIK activity was completely blocked when a KD NIK [NIK(KD)] was coexpressed with the wild-type EphB1 receptor (Fig. 2). The overexpression of NIK(KD) would be expected to compete with endogenous NIK for immunoprecipitation with anti-NIK antibodies and thus would be expected to lead to a decrease in immunoprecipitable NIK activity. However, overexpression of NIK(KD) would not be expected to inhibit kinase activity detected in anti-NIK immunoprecipitates if a small amount of a kinase other than NIK (e.g., the activated EphB1 receptor) was responsible for the increase in kinase activity. These results extend our observations with P19 and NG108-EphB2 cells, and indicate that both the EphB1 kinase activity and pY594 are required for EphB1 to activate NIK.
FIG. 2.
NIK activity in 293 cells transfected (Trans.) with wild-type and mutant EphB1 receptors. (A) 293 cells were transfected with expression plasmids containing different cDNAs corresponding to the proteins as indicated. To assess NIK activation, total-protein extracts were immunoprecipitated with anti-NIK antibodies and subjected to an in vitro kinase assay as described in the legend to Fig. 1. (B) Transfected lysates were immunoblotted with antibodies to the HA epitope (12CA5) (α-HA) to control for EphB1 expression (EphB1 was HA epitope tagged). To assess expression of EphB1, the same extracts were precipitated with wheat germ agglutinin and tyrosine-phosphorylated EphB1 was determined by immunoblotting the washed precipitates with antiphosphotyrosine antibodies (α-PTyr).
NIK forms a complex with p62dok, RasGAP, and Nck in P19 and NG108-EphB2 cells stimulated with ephrinB1.
As discussed above, ephrinB1 has previously been shown to stimulate a complex between EphB1 and Nck (45). In contrast to EphB1, the EphB2 receptor has been reported to stimulate tyrosine phosphorylation of p62dok, which in turn binds the SH2 domains of Nck and RasGap (21). p62dok contains an N-terminal pleckstrin homology domain and several tyrosine phosphorylation sites that are contained within consensus SH2 binding motifs (5, 53). p62dok is tyrosine phosphorylated by a number of tyrosine kinases, and tyrosine-phosphorylated p62dok is thought to regulate signaling pathways by functioning as a docking protein for SH2 domain-containing signaling molecules.
To determine whether NIK associates with Nck in P19 and NG108-EphB2 cells, we determined whether NIK and Nck coimmunoprecipitate in either unstimulated or ephrinB1-stimulated cells. We found that Nck coimmunoprecipitates with anti-NIK antibodies in NG108-EphB2 and P19 cells that were either unstimulated or stimulated with ephrinB1 (Fig. 3A). This finding suggests that a preformed NIK-Nck complex is present in cells and is consistent with NIK being a physiological target for the SH3 domains of Nck in vivo. However, we detected only a small amount of Nck that specifically coimmunoprecipitated with anti-NIK antibodies. Thus, the interaction of NIK with Nck in vivo is likely to be of low stoichiometry and/or of low affinity, resulting in a partial disruption of the complex under the immunoprecipitation conditions used. We were unable to detect NIK in anti-Nck immunoprecipitates (Fig. 3A). This may be because our anti-NIK antibodies are less sensitive than our anti-Nck antibodies at immunoblotting. In addition, Nck is present in a large molar excess over NIK in cells (data not shown); thus, most of the Nck present in cells cannot be associated with NIK, making it more difficult to detect NIK in Nck immunoprecipitates.
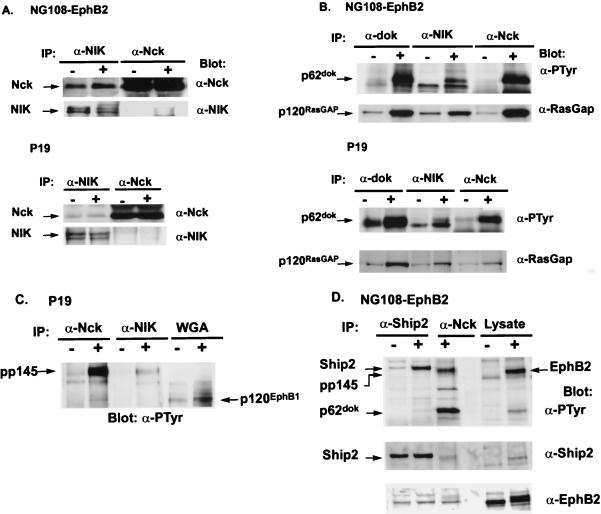
FIG. 3.
Proteins associated with NIK in unstimulated or ephrinB1-stimulated P19 and NG108-EphB2 cells. Cells were treated for 15 to 20 min either with preclustered ephrinB1 (+) or with clustering antibody alone (−). Lysates from P19 or NG108-EphB2 cells were immunoprecipitated (IP) with anti-Nck (α-Nck), anti-NIK (α-NIK), anti-p62dok (α-dok), or anti-SHIP2 antibodies as indicated. (For immunoprecipitation, NIK antibodies were covalently coupled to protein A-Sepharose beads). The washed immunoprecipitates were separated by SDS-PAGE (10% polyacrylamide or as otherwise specified) and, after being transferred to nitrocellulose filters, probed with antibodies as indicated. In panel C, EphB1 was precipitated using wheat germ agglutinin (WGA). In panel D, after the nitrocellulose filter was probed with antiphosphotyrosine (α-PTyr) antibodies, the filter was stripped and reprobed first with anti-SHIP2 antibodies and then with anti-EphB2 antibodies. (A) Antibodies to NIK (α-NIK) coimmunoprecipitate Nck in both unstimulated and ephrinB1-stimulated cells. (B) In stimulated cells, endogenous NIK and Nck coimmunoprecipitate with tyrosine-phosphorylated p62dok and RasGAP which is not tyrosine phosphorylated (data not shown). (C and D) Antibodies to NIK (α-NIK) and Nck (α-Nck) immunoprecipitate an unidentified tyrosine-phosphorylated protein of 145 kDa (pp145), which is not the EphB1 or EphB2 receptor. In panel C, the tyrosine-phosphorylated EphB1 receptor was detected by immunoblotting WGA precipitates with antiphosphotyrosine antibodies as described in the legend to Fig. 2B. The tyrosine-phosphorylated protein at 145 kDa runs at a higher molecular mass than the EphB1 receptor. In stimulated EphB2 cells, pp145, which coimmunoprecipitates with anti-Nck antibodies, is not the EphB2 receptor, since pp145 does not immunoblot with antibodies to EphB2 (D). In panel D, the filter was first probed with antiphosphotyrosine antibodies and, after being stripped, was reprobed with anti-SHIP2 (α-Ship2) and then anti-EphB2 antibodies. The residual bands present in the anti-SHIP2 and the anti-EphB2 blot reflect incomplete stripping of the previously used antibodies. Differences in the concentration of the polyacrylamide gel, 7% (C) versus 10% (D), account for the apparent differences in migration between pp145 and EphB1 compared with pp145 and EphB2. (D) Antibodies to SHIP2 immunoprecipitate tyrosine-phosphorylated SHIP2 from NG108-EphB2 cells stimulated with ephrinB1. pp145 is not SHIP2, since we cannot immunoblot pp145 with antibodies to SHIP2.
To determine whether NIK associates with similar or different proteins in EphB1- and EphB2-stimulated cells, we identified proteins associated with NIK in these cells by using coimmunoprecipitation experiments. In addition to precipitating Nck, anti-NIK antibodies immunoprecipitated a 62-kDa tyrosine-phosphorylated protein from both NG108-EphB2 and P19 cells stimulated with ephrinB1 (Fig. 3B). The tyrosine-phosphorylated 62-kDa protein that coprecipitates with NIK is most probably p62dok, since this band comigrates with tyrosine-phosphorylated p62dok that has been immunoprecipitated with anti-p62dok antibodies (Fig. 3B). In addition, anti-Nck immunoprecipitation detected the same 62-kDa tyrosine-phosphorylated band in these cells stimulated with ephrinB1 (Fig. 3B); a previous study using the same NG108-EphB2 cells has demonstrated that the 62-kDa tyrosine-phosphorylated protein that coimmunoprecipitates with Nck is p62dok (21). We have been unable to confirm by Western blot analysis that the 62-kDa tyrosine-phosphorylated protein that coimmunoprecipitates with NIK and Nck is p62dok due to the unavailability of antibodies that can be used in Western blots of p62dok (21; R. Roth, personal communication). The decreased amount of tyrosine-phosphorylated p62dok detected in anti-NIK immunoprecipitates compared with the amount of p62dok detected in anti-Nck immunoprecipitates is consistent with the idea that NIK associates only indirectly with p62dok via Nck; since only some of the Nck is bound to NIK in cells, more Nck than NIK would be expected to associate with p62dok in ephrin-stimulated cells.
Tyrosine-phosphorylated p62dok associates with the SH2 domains of RasGAP in NG108-EphB2 cells stimulated with ephrinB2 (21). However, RasGAP does not undergo tyrosine phosphorylation in these cells (21). We found that NIK and Nck also form a complex with RasGAP in both NG108-EphB2 and P19 cells stimulated with ephrinB1. While a small amount of RasGAP coimmunoprecipitated with NIK in unstimulated cells, the amount of RasGAP that coimmunoprecipitated with NIK in NG108-EphB2 and P19 cells was increased following stimulation with ephrinB1 (Fig. 3B).
A second tyrosine-phosphorylated protein that coimmunoprecipitated with NIK and Nck in cells stimulated via the EphB1 and EphB2 receptors was present at 145 kDa (pp145) (Fig. 3C and D and data not shown). The identity of this protein is not known. This protein is not EphB1 or EphB2 receptor, phospholipase Cγ, Abl, or NIK, since it does not immunoblot with antibodies to these proteins (Fig. 3C and D and data not shown). Thus, these findings indicate that the predominant proteins with which NIK associates in EphB1- and EphB2-stimulated cells are similar and include Nck, p62dok, RasGAP, and an unidentified 145-kDa tyrosine-phosphorylated protein. It is likely that tyrosine-phosphorylated p62dok is central to this complex and functions to bind the SH2 domains of Nck and RasGAP.
SHIP2 is tyrosine phosphorylated in EphB2-stimulated cells.
In searching for the identity of the 145-kDa protein in EphB1- and EphB2-stimulated cells that coimmunoprecipitates with Nck and NIK, we determined whether pp145 is SHIP2. SHIP2 is a widely expressed 145- to 155-kDa inositol 5-phosphatase that dephosphorylates phosphatidylinositol-3,4,5-trisphosphate [PI(3,4,5)P3] to PI(3,4)P2 (17, 52). In addition, SHIP2 is tyrosine phosphorylated by a number of receptor tyrosine kinases including epidermal growth factor receptor, platelet-derived growth factor receptor, nerve growth factor receptor, and insulin-like growth factor 1 receptor (17). We found that SHIP2 is tyrosine phosphorylated by EphB2. However, SHIP2 is not pp145; while antibodies to SHIP2 immunoblot SHIP2 in anti-SHIP2 immunoprecipitates, they do not immunoblot pp145 (Fig. 3D). In addition, SHIP2 is not present in a complex with Nck, p62dok, or NIK, since neither Nck nor tyrosine-phosphorylated p62dok coimmunoprecipitates with SHIP2 (Fig. 3D and data not shown).
NIK functions to increase EphB1-mediated attachment of P19 cells to fibrinogen.
Integrins can be activated by a number of intracellular signals through a mechanism that is referred to as inside-out signaling. Activation of integrins in this manner results in increased affinity of an integrin toward matrix (25). EphrinB1 stimulation of a number of different cell lines, including endothelial, P19, and 293 cells, stimulates integrin activation as demonstrated by increased adhesiveness of these cells to fibrinogen- or fibronectin-coated plates (27, 46). The increase in cell adhesion observed in these assays is specific because increased cell attachment is not observed when ephrinB1 is added to other control matrices including collagen I or laminin (27, 46). In addition, the EphB1-stimulated increase in attachment of 293 cells to fibronectin requires both EphB1 kinase activity and tyrosine 594, since transfection of Eph mutants lacking kinase activity or tyrosine 594 failed to stimulate integrin-mediated attachment (27). These findings indicate that some of the same signals that mediate NIK activation by EphB1 in 293 cells also mediate EphB1-stimulated integrin activation.
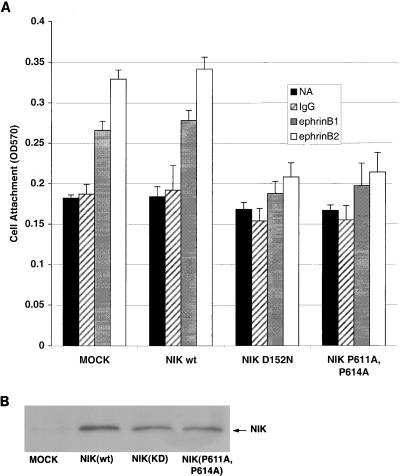
To test whether NIK may be a component of the downstream cytoplasmic signal(s) that couples EphB1 to integrin activation, we determined whether expression of NIK(KD) inhibited EphrinB1-stimulated integrin attachment of P19 cells to fibrinogen. We found that overexpression of NIK(KD) inhibited ephrinB1- and ephrinB2-stimulated attachment of P19 cells to fibrinogen compared with cells transfected with vector control (Fig. 4). In contrast, overexpression of NIK(WT) did not affect EphB1-stimulated attachment of P19 cells (Fig. 4). The finding that NIK(WT) and NIK(KD) have opposite effects in EphB1-stimulated adhesiveness to fibrinogen indicates that the inhibition in attachment of P19 cells by NIK(KD) is not due to a nonspecific effect of NIK overexpression and suggests that activation of NIK is critical for EphB1-stimulated integrin attachment. We excluded the possibility that direct binding of EphR to ephrinB1-Fc accounted for the increase in cell adhesion, because we did not observe an increase in cell attachment when ephrinB1 was added to other control matrices including collagen I or laminin.
FIG. 4.
Solid-phase attachment assay of P19 cells to fibrinogen. (A) P19 cells were transiently transfected with 6 μg of NIK(WT) or mutant NIK(KD), as shown on the x axis. At 48 h after transfection, solid-phase attachment assays (see Materials and Methods) were conducted on fibrinogen-coated plates displaying ephrinB1 or ephrinB2, clustering antibody alone (IgG), or no addition (NA). Adherent cells were stained with crystal violet and quantified by measurement of the optical density at 570 nm (OD570). ∗, P < 0.001; ∗∗, P < 0.01. (B) To confirm the presence of equal levels of NIK expression, lysates were immunoprecipitated with the anti-Myc antibody 9E10 and immunoblotted with the same antibody.
To assess whether binding of NIK to Nck is required for NIK to mediate EphB1-stimulated attachment of P19 cells to these matrices, we tested a NIK mutant in which the proline residues on NIK that mediate binding to the SH3 domains of Nck are mutated to alanine (47). We found that transfection of P19 cells with NIK(P611A, P614A) (47), a mutant which completely inhibits the binding of Nck to NIK in vitro, inhibited ephrinB1- and ephrinB2-stimulated attachment of P19 cells to fibrinogen to a level comparable to that found with NIK(KD). All the NIK constructs were expressed at similar levels in P19 cells, indicating that the differences observed are not due to different levels of expression of the various NIK proteins (Fig. 4B). These findings indicate that NIK plays an important role in mediating one of the signals from EphB1 that stimulates integrin activation and suggests that binding of NIK to Nck is important for NIK to mediate this function.
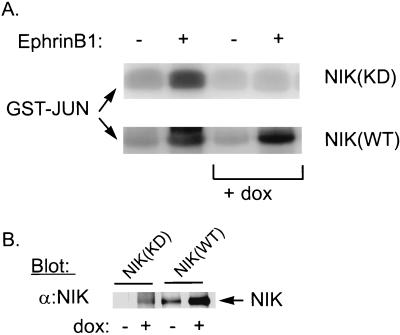
NIK couples EphB1 to JNK activation.
We have previously shown that NIK and msn function as MAP4K to stimulate JNK activation (47, 48). The EphB1 receptor also activates JNK, and activation of JNK by EphB1 is dependent upon tyrosine phosphorylation of Y594 on EphB1 (45). To determine whether NIK mediates JNK activation by EphB1, we used the RevTet-On system to inducibly express NIK(KD) in P19 cells. While JNK activity was increased twofold in P19 cells stimulated with ephrinB1, activation of JNK by ephrinB1 was almost completely inhibited in these cells following the induction of NIK(KD) with doxycycline (Fig. 5A). In contrast, overexpression of NIK(WT) to higher levels than NIK(KD) did not inhibit JNK activation. Thus, these findings suggest that NIK also functions to couple EphB1 to JNK activation. The finding that NIK(KD) but not NIK(WT) blocked EphB1 activation of JNK suggests that NIK(KD) does not nonspecifically inhibit JNK activation by binding and inactivating a downstream MAP3K or by competing the binding of another physiologic target with Nck; under these conditions, inhibition of JNK activation by NIK(KD) would only confirm an essential role for Nck or a downstream MAP3K.
FIG. 5.
EphrinB1-stimulated JNK activation in P19 cells that inducibly express NIK(KD). (A) Stable P19 cell lines that inducibly express HA-tagged NIK(KD) or NIK(WT) were isolated as described in Materials and Methods. To determine whether expression of NIK(KD) or NIK(WT) affects JNK activation by ephrinB1, P19 cell lines growing with (+ dox) or without doxycycline were treated with preclustered ephrin B1 (+) or the clustering IgG alone (−) for 20 min. To assay JNK activity, lysates were incubated with 10 μg of GST-Jun, and after they were washed, an in vitro kinase assay was performed as described previously (43). Reaction products were separated by SDS-PAGE (15% polyacrylamide) and visualized by autoradiography. (B) NIK(KD) and NIK(WT) expression induced by doxycycline was measured by immunoblotting 50 μg of lysates from P19 cells treated for 48 h in the absence (−) or presence (+) of 1 μg of doxycycline (dox) per ml with anti-NIK antibodies (α:NIK).
DISCUSSION
In comparison to other RTKs, little is known about the signaling pathways activated by Eph receptors or the downstream signaling molecules that mediate specific responses to these receptors. In this report, we demonstrate that the Ste20 kinase NIK is specifically activated by two EphB receptors, EphB1 and EphB2. Moreover, NIK activation is required both for EphB1-stimulated integrin attachment to fibrinogen and for activation of JNK. Thus, NIK and the downstream pathways regulated by NIK define a signaling pathway(s) that links EphRs to biological responses.
The mechanism whereby EphRs activate NIK is not yet clear. An attractive hypothesis is that Nck functions as an adapter molecule to couple NIK, bound to its SH3 domains, to tyrosine-phosphorylated proteins in Eph-stimulated cells. A similar model has been proposed and validated for the regulation of Son of Sevenless (Sos) by Grb2 (37, 41). In agreement with this hypothesis, we found that a Nck-NIK complex associates with tyrosine-phosphorylated p62dok in both EphB1- and EphB2-stimulated cells. p62dok localizes to the plasma membrane via its amino-terminal pleckstrin homology domain (32), and thus binding of Nck-NIK to p62dok may be a means of localizing NIK to cellular membranes, where it may be activated. Although a number of different scenarios for NIK activation are possible, it is conceivable that the increased local concentration of NIK induced by the recruitment of NIK by Nck to phosphotyrosine-containing proteins may allow juxtaposed NIK molecules present in the complex to transphosphorylate and activate each other. Such a mechanism would be consistent with the finding that NIK is constitutively active in cells that transiently overexpress high levels of NIK protein, presumably due to constitutive dimerization of NIK induced by high levels of NIK expression (47).
We expected to find that NIK would coimmunoprecipitate with the activated EphB1 receptor because previous studies with the yeast two-hybrid system and with cells have found that Nck binds pY594 in the juxtamembrane region of EphB1 (45). In addition, tyrosine phosphorylation of Y594 on EphB1 is important for EphB1 to stimulate both the activation of the JNK MAPK module and the increase in integrin-mediated attachment to matrix (27, 45). The finding reported here that pY594 is necessary for EphB1 activation of NIK in 293 cells indicates that pY594 also plays an important role in coupling EphRs to NIK activation. However, we have been unable to coimmunoprecipitate the activated EphB1 or activated EphB2Rs with either Nck or NIK in P19 or NG108-EphB2 cells, leading us to favor a model in which interaction of Nck with p62dok may be more relevant for NIK activation in these cells. However, we cannot rule out the possibility that the interaction of Nck-NIK with the activated EphB1R is physiologically relevant to NIK activation and that a lower-affinity interaction and/or a decrease in the stoichiometry of this interaction makes this association undetectable under the conditions used in the coimmunoprecipitation experiments. Alternatively, pY594 may perform other critical functions in EphB1-stimulated cells that are unrelated to binding the SH2 domain of Nck but are critical for EphB1 receptors to signal. For example, the juxtamembrane tyrosines in EphB1 and EphB2 bind the SH2 domains of RasGAP and Src family kinases (9, 21, 55). pY594 may also be required for EphB1 receptors to tyrosine phosphorylate p62dok by either directly facilitating tyrosine phosphorylation of p62dok by EphB1 or indirectly activating an intermediate tyrosine kinase, such as a member of the Src family.
A number of proteins have now been shown to bind the SH3 domains of Nck. These SH3 domains bind, in addition to NIK, the Wiskott-Aldrich syndrome protein, p21-activated protein kinase, Sos, Cbl, and PRK2 (14, 24, 31, 38, 40, 49). While data from several lines of research have suggested that the interaction of Nck with these effector molecules regulates the actin cytoskeleton and/or various MAPK signaling pathways (14, 31, 42, 49), an important question has been which of these molecules are physiological targets for the SH3 domains of Nck in vivo. In this regard, Drosophila p21-activated protein kinase has recently been shown, using genetic methods, to function downstream of dock in the targeting of R axons (20). The findings we report here support a role for NIK as one of the physiological targets for the SH3 domains of Nck in vivo. First, endogenous NIK and Nck coimmunoprecipitate in NG108-EphB2 and P19 cells. Second, the same tyrosine-phosphorylated proteins are immunoprecipitated by both anti-NIK and anti-Nck antibodies in EphB1- and EphB2-stimulated cells. Third, a NIK mutant that fails to interact with Nck inhibited EphB1-stimulated integrin attachment to fibrinogen whereas wild-type NIK had no effect on integrin-mediated attachment. Moreover, the function of NIK as a physiological target of Nck has been confirmed in genetic studies of msn and dock in Drosophila. We found that msn binds the SH3 domains of dock and that amino acids that mediate this binding are required for correct targeting of photoreceptor axons (Su et al., submitted). It should be pointed out that a second molecule related to Nckα (Nckβ; “Ncka” is now used to describe the first Nck molecule cloned) was recently cloned (3, 6). Our studies do not distinguish between these two Nck family members, and thus we cannot determine which Nck family member is more relevant to Eph and NIK signaling.
The above findings suggest that identifying the signaling pathways regulated by NIK in Eph-stimulated cells should better define not only the function of NIK but also the signaling pathways regulated by Nck. In this regard, we found that NIK activation is critical for EphB1 stimulation of integrin activation as manifested by increased adhesiveness of integrins to extracellular matrices. Intracellular signals that activate integrins and increase integrin affinity toward extracellular matrices have been referred to as inside-out signaling (25). Previous studies have demonstrated that EphB1 activation of integrins requires a highly ordered oligomeric EphB1 signaling complex, and, while the capacity of EphB1 to stimulate integrin activation does not correlate with EphB1 tyrosine phosphorylation, EphB1 kinase activity, pY594, and pY929, which mediates binding to the low-molecular-weight phosphotyrosine phosphatase (LMW-PTP), are all required for EphB1-stimulated integrin activation (27, 46). Previous studies have indicated that PI3-kinase, protein kinase C, and the GTP binding proteins R-Ras and Rho can activate signaling pathways that mediate integrin activation, although the mechanism whereby these cytoplasmic signaling pathways activate integrins is still poorly understood (25). The placement of NIK on a pathway to integrin activation is the first demonstration that Ste20 kinases are components of a signaling pathway leading to integrin activation. Thus, the identification of downstream signals regulated by NIK that mediate integrin activation (e.g., JNK) and the mechanism whereby these signals cooperate with other signaling pathways activated by EphB1 (e.g., LMW-PTP) should provide new insights into the mechanisms whereby integrins are regulated by inside-out signals. It will be interesting to determine whether the increased attachment of integrins to matrix induced by EphR play an important biological role in regulating the migration of axons or cells by ephrins, since previous studies have demonstrated an important role for integrin affinity modulation in cell migration (26).
It will be interesting to determine whether NIK plays a role in regulation of the actin cytoskeleton by EphRs, since this function is likely to be critical for the biological function of Ephs. In this regard, Noguchi et al. (32) reported that overexpression of p62dok enhanced cell migration on fibronectin in response to insulin stimulation. This finding, together with the demonstration that p62dok forms a complex with Nck and RasGAP in insulin-stimulated cells, suggests that p62dok functions to couple the insulin receptor to cell migration by serving as a docking protein for Nck and RasGAP p62dok. While a direct role for Nck in mediating this response to insulin stimulation was not tested, it is intriguing to speculate that recruitment of Nck-NIK to p62dok in Eph- and insulin-stimulated cells functions similarly to regulate changes in the cytoskeleton and/or integrin activation. One potential mechanism whereby NIK may regulate the actin cytoskeleton has already been suggested by genetic studies with Drosophila and Caenorhabditis elegans (48). Both the Drosophila homolog of NIK, msn, and the C. elegans homolog, MIG15, regulate signaling pathways that are critical for mediating changes in cell shape in the developing organism (48). In Drosophila, msn functions to mediate changes in cell shape by activating the Drosophila JNK, bsk, which in turn phosphorylates and activates DJUN (33, 48). Activated DJUN in turn mediates changes in cell shape by transcriptionally activating target genes, including dpp. The finding reported here that NIK also couples EphB1 to JNK activation suggests that some of the changes in the actin cytoskeleton induced by EphR may be mediated though a NIK-JNK pathway that is similar to those described in Drosophila and C. elegans. However, some signaling events induced by Eph receptors, such as collapse of the axonal growth cone, occur too quickly to require the transcription of new genes. In this regard, studies with Drosophila have indicated that msn mediates several biological functions that are independent of JNK activation (48). Thus, a full understanding of the biological pathways regulated by NIK will require the identification of these JNK-independent signaling pathways regulated by msn and NIK. Our finding that NIK is activated by EphRs and couples these receptors to two known biological responses, JNK and integrin activation, together with the identification of SHIP2 and pp145 as targets for these receptors, should help to begin to dissect these fascinating but complicated actions of this family of receptors.
ACKNOWLEDGMENTS
We thank S. Decker for the anti-SHIP2 antibodies and N. Gale (Regeneron) for ephrin B1/fc.
This work is supported by National Institutes of Health grants DK49207 (E.Y.S.) and DK47078 (T.O.D.). E.B. is the recipient of a postdoctoral fellowship from Ministerio de Educacion y Cultura, Spain.
REFERENCES
- 1.Adams R H, Wilkinson G A, Weiss C, Diella F, Gale N W, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohme B, VandenBos T, Cerretti D P, Park L S, Holtrich U, Rubsamen-Waigmann H, Strebhardt K. Cell-cell adhesion mediated by binding of membrane-anchored ligandLERK-2 to the EPH-related receptor human embryonal kinase 2 promotes tyrosine kinase activity. J Biol Chem. 1996;217:24747–24752. doi: 10.1074/jbc.271.40.24747. [DOI] [PubMed] [Google Scholar]
- 3.Braverman L E, Quilliam L A. Identification of Grb4/Nckbeta, a src homology 2 and 3 domain-containing adapter protein having similar binding and biological properties to Nck. J Biol Chem. 1999;274:5542–5549. doi: 10.1074/jbc.274.9.5542. [DOI] [PubMed] [Google Scholar]
- 4.Bruckner K, Pasquale E B, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- 5.Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, Clarkson B. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. doi: 10.1016/s0092-8674(00)81840-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, She H, Davis E M, Spicer C M, Kim L, Ren R, Le Beau M M, Li W. Identification of Nck family genes, chromosomal localization, expression, and signaling specificity. J Biol Chem. 1998;273:25171–25178. doi: 10.1074/jbc.273.39.25171. [DOI] [PubMed] [Google Scholar]
- 7.Davis S, Gale N W, Aldrich T H, Maisonpierre P C, Lhotak V, Pawson T, Goldfarb M, Yancopoulos G D. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- 8.Drescher U. The Eph family in the patterning of neural development. Curr Biol. 1997;7:R799–R807. doi: 10.1016/s0960-9822(06)00409-x. [DOI] [PubMed] [Google Scholar]
- 9.Ellis C, Kasmi F, Ganju P, Walls E, Panayotou G, Reith A D. A juxtamembrane autophosphorylation site in the Eph family receptor tyrosine kinase, Sek, mediates high affinity interaction with p59fyn. Oncogene. 1996;12:1727–1736. [PubMed] [Google Scholar]
- 10.Evan G I, Lewis G K, Ramsay G, Bishop J M. Isolation of monoclonal antibodies specific for human c-myc protooncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagan J G, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 12.Gale N W, Holland S J, Valenzuela D M, Flenniken A, Pan L, Ryan T E, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson D G, Pawson T, Davis S, Yancopoulos G D. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 13.Gale N W, Yancopoulos G D. Ephrins and their receptors: a repulsive topic? Cell Tissue Res. 1997;290:227–241. doi: 10.1007/s004410050927. [DOI] [PubMed] [Google Scholar]
- 14.Galisteo M L, Chernoff J, Su Y C, Skolnik E Y, Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- 15.Garrity P A, Rao Y, Salecker I, McGlade J, Pawson T, Zipursky L. Drosophila photoreceptor axon guidance and targeting requires the dreadlocks SH2/SH3 adapter protein. Cell. 1996;85:639–650. doi: 10.1016/s0092-8674(00)81231-3. [DOI] [PubMed] [Google Scholar]
- 16.Grignani F, Kinsella T, Mencarelli A, Valtieri M, Riganelli D, Lanfrancone L, Peschle C, Nolan G P, Pelicci P G. High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/retroviral vector expressing the green fluorescence protein. Cancer Res. 1998;58:14–19. [PubMed] [Google Scholar]
- 17.Habib T, Hejna J A, Moses R E, Decker S J. Growth factors and insulin stimulate tyrosine phosphorylation of the 51C/SHIP2 protein. J Biol Chem. 1998;29:18605–18609. doi: 10.1074/jbc.273.29.18605. [DOI] [PubMed] [Google Scholar]
- 18.Helgason C D, Damen J E, Rosten P, Grewal R, Sorensen P, Chappel S M, Borowski A, Jirik F, Krystal G, Humphries R K. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henkemeyer M, Orioli D, Hendrson J T, Saxton T M, Roder J, Pawson T, Klein R. NUK controls pathfinding of commisural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 20.Hing H, Xiao J, Harden N, Lim L, Zipursky S L. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97:853–863. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 21.Holland S J, Gale N W, Gish G D, Roth R A, Songyang Z, Cantley L C, Henkemeyer M, Yancopoulos G D, Pawson T. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 1997;16:3877–3888. doi: 10.1093/emboj/16.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holland S J, Gale N W, Mbamalu G, Yancopoulos G D, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- 23.Holland S J, Peles E, Pawson T, Schlessinger J. Cell-contact-dependent signalling in axon growth and guidance: Eph receptor tyrosine kinases and receptor protein tyrosine phosphatase beta. Curr Opin Neurobiol. 1998;8:117–127. doi: 10.1016/s0959-4388(98)80015-9. [DOI] [PubMed] [Google Scholar]
- 24.Hu Q, Milfay D, Williams L T. Binding of NCK to SOS and activation of ras-dependent gene expression. Mol Cell Biol. 1995;15:1169–1174. doi: 10.1128/mcb.15.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes P E, Pfaff M. Integrin affinity modulation. Trends Cell Biol. 1998;8:359–364. doi: 10.1016/s0962-8924(98)01339-7. [DOI] [PubMed] [Google Scholar]
- 26.Huttenlocher A, Ginsberg M H, Horwitz A F. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huynh-Do U, Stein E, Lane A A, Liu H, Cerretti D P, Daniel T O. Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through alphavbeta3 and alpha5beta1 integrins. EMBO J. 1999;18:2165–2173. doi: 10.1093/emboj/18.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee C-H, Li W, Nishimura R, Zhou M, Batzer A G, Myers M G, Jr, White M F, Schlessinger J, Skolnik E Y. Nck associates with the SH2 docking protein IRS-1 in insulin stimulated cells. Proc Natl Acad Sci USA. 1993;90:11713–11717. doi: 10.1073/pnas.90.24.11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehman J M, Riethmuller G, Johnson J P. Nck, a melanoma cDNA encoding a cytoplasmic protein consisting of Src homology units SH2 and SH3. Nucleic Acids Res. 1990;18:1048. doi: 10.1093/nar/18.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Sasaki T, Kozieradzki I, Wakeham A, Itie A, Dumont D J, Penninger J M. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 1999;13:786–791. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu W, Katz S, Gupta R, Mayer B J. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr Biol. 1997;7:85–94. doi: 10.1016/s0960-9822(06)00052-2. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi T, Matozaki T, Inagaki K, Tsuda M, Fukunaga K, Kitamura Y, Kitamura T, Shii K, Yamanashi Y, Kasuga M. Tyrosine phosphorylation of p62(Dok) induced by cell adhesion and insulin: possible role in cell migration. EMBO J. 1999;18:1748–1760. doi: 10.1093/emboj/18.7.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noselli S. JNK signaling and morphogenesis in Drosophila. Trends Genet. 1998;14:33–38. doi: 10.1016/S0168-9525(97)01320-6. [DOI] [PubMed] [Google Scholar]
- 34.O'Leary D D, Wilkinson D G. Eph receptors and ephrins in neural development. Curr Opin Neurobiol. 1999;9:65–73. doi: 10.1016/s0959-4388(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 35.Pandey A, Duan H, Dixit V M. Characterization of a novel Src-like adapter protein that associates with the Eck receptor tyrosine kinase. J Biol Chem. 1995;270:19201–19204. doi: 10.1074/jbc.270.33.19201. [DOI] [PubMed] [Google Scholar]
- 36.Pandey A, Lazar D F, Saltiel A R, Dixit V M. Activation of the Eck receptor protein tyrosine kinase stimulates phosphatidylinositol 3-kinase activity. J Biol Chem. 1994;269:30154–30157. [PubMed] [Google Scholar]
- 37.Pawson T. Protein modules and signalling networks. Nature (London) 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 38.Quilliam L A, Lambert Q T, Mickelson-Young L A, Westwick J K, Sparks A B, Kay B K, Jenkins N A, Gilbert D J, Copeland N G, Der C J. Isolation of a NCK-associated kinase, PRK2, an SH3-binding protein and potential effector of Rho protein signaling. J Biol Chem. 1996;271:28772–28776. doi: 10.1074/jbc.271.46.28772. [DOI] [PubMed] [Google Scholar]
- 39.Rameh L E, Cantley L C. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 40.Rivero-Lezcano O M, Marcilla A, Sameshima J H, Robbins K C. Wiskott-Aldrich syndrome protein physically associates with Nck through Src homology 3 domains. Mol Cell Biol. 1995;15:5725–5731. doi: 10.1128/mcb.15.10.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlessinger J. SH2/SH3 signaling molecules. Curr Opin Genet Dev. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 42.Sells M A, Knaus U G, Bagrodia S, Ambrose D M, Bokoch G M, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 43.Skolnik E Y, Lee C H, Batzer A, Vicentini L M, Zhou M, Daley R J, Myers M G, Jr, Backer J M, Ullrich A, White M F, Schlessinger J. Interaction of GRB-2/SEM-5 with insulin receptor substrate-1 (IRS-1) and Shc is implicated in insulin receptor-mediated Ras activation. EMBO J. 1993;12:1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein E, Cerretti D P, Daniel T O. Ligand activation of ELK receptor tyrosine kinase promotes its association with Grb10 and Grb2 in vascular endothelial cells. J Biol Chem. 1996;271:23588–23593. doi: 10.1074/jbc.271.38.23588. [DOI] [PubMed] [Google Scholar]
- 45.Stein E, Huynh-Do U, Lane A A, Cerretti D P, Daniel T O. Nck recruitment to Eph receptor, EphB1/ELK, couples ligand activation to c-Jun kinase. J Biol Chem. 1998;273:1303–1308. doi: 10.1074/jbc.273.3.1303. [DOI] [PubMed] [Google Scholar]
- 46.Stein E, Lane A A, Cerretti D P, Schoecklmann H O, Schroff A D, Van Etten R L, Daniel T O. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 1998;12:667–678. doi: 10.1101/gad.12.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su Y, Han J, Xu S, Cobb M, Skolnik E Y. NIK is a new Ste20-related kinase that binds NCK and MEKK1 and activates the SAPK/JNK cascade via a conserved regulatory domain. EMBO J. 1997;16:1279–1290. doi: 10.1093/emboj/16.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su Y-C, Treisman J, Skolnik E Y. The Drosophila Ste20 related kinase misshapen is required for embryonic dorsal closure and acts via a JNK MAPK on an evolutionary conserved signaling pathway. Genes Dev. 1998;12:2371–2380. doi: 10.1101/gad.12.15.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Symons M, Derry J M, Karkak B, Jiang S, Lemajieu V, McCormick F, Francke U, Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs is implicated in actin polymerization. Cell. 1996;84:723–724. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka E, Sabry J. Making the connection: cytoskeletal rearrangements during growth cone guidance. Cell. 1995;83:171–176. doi: 10.1016/0092-8674(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 51.Wang H U, Chen Z F, Anderson D J. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 52.Wisniewski D, Strife A, Swendeman S, Erdjument-Bromage H, Geromanos S, Kavanaugh W M, Tempst P, Clarkson B. A novel SH2-containing phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase (SHIP2) is constitutively tyrosine phosphorylated and associated with src homologous and collagen gene (SHC) in chronic myelogenous leukemia progenitor cells. Blood. 1999;93:2707–2720. [PubMed] [Google Scholar]
- 53.Yamanashi Y, Baltimore D. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein. Dok Cell. 1997;88:205–211. doi: 10.1016/s0092-8674(00)81841-3. [DOI] [PubMed] [Google Scholar]
- 54.Yancopoulos G D, Klagsbrun M, Folkman J. Vasculogenesis, angiogenesis, and growth factors: ephrins enter the fray at the border. Cell. 1998;93:661–664. doi: 10.1016/s0092-8674(00)81426-9. [DOI] [PubMed] [Google Scholar]
- 55.Zisch A H, Kalo M S, Chong L D, Pasquale E B. Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene. 1998;16:2657–2670. doi: 10.1038/sj.onc.1201823. [DOI] [PubMed] [Google Scholar]