Abstract
Peptostreptococcus micros is a commensal of the oral cavity and the genitourinary tract that rarely causes serious infections. A case of a destructive knee joint infection with rapid progress caused by P. micros is presented. The significance of the microbiological findings was initially not acknowledged, which contributed to a nonsuccessful clinical outcome.
CASE REPORT
An 86-year-old male Caucasian patient (175 cm, 84 kg) developed unspecific pain in the left knee radiating to the hip and was admitted to the Department of Orthopedics. His medical history included minor cerebral vascular insults with no remaining pareses, ulcer, renal calculi, and enlargement of the prostate gland. He had been treated for hypertension and atrial fibrillation for several years. Physical examination revealed no pathological findings, and X ray of the pelvis, left hip, and knee joints showed only a slight chondrocalcinosis in the left knee (Fig. 1A). Furthermore, inspection of the oral cavity revealed that the patient had bridges in both the lower and upper jaw. After 2 days with anti-inflammatory medication, the patient was discharged from the hospital.
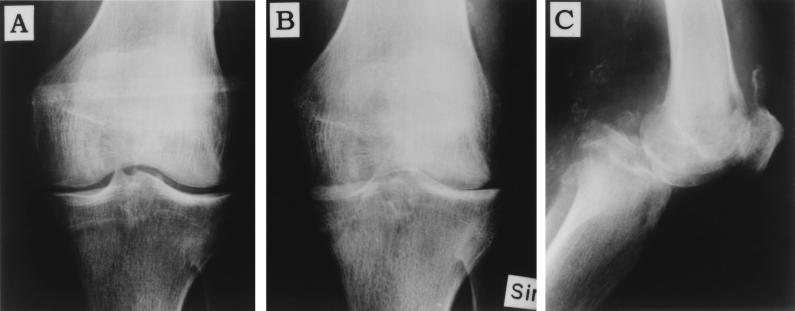
FIG. 1.
Anterio-posterior knee radiograph at first outpatient visit (A) shows a normal distance between the femoral and tibial condyles, indicating a normal cartilage. Eight weeks later (B), the joint line is no longer visible due to destruction of cartilage, attrition of the medical subchondral bone plate of the tibia, and posterior dislocation of the tibia seen in the lateral view (C).
A week later, the patient was again admitted to the local hospital, but this time with acute-onset severe pain in his left knee and hip. The knee joint was aspirated, 25 ml of opaque synovial fluid was recovered, and intravenous cloxacillin therapy was initiated (Table 1). Gram-positive anaerobic micrococci were cultured from the synovial fluid, but no further classification was performed, because the isolate was considered as a contaminant. An additional aspiration was performed the following day. This time, however, no bacteria were isolated. Since the clinical relevance of the bacterial finding was considered vague, it was concluded that the diagnosis most likely was pyrophosphate arthritis. The patient was discharged with anti-inflammatory therapy (diclofenac) and no antibiotics. After 7 weeks from the initial admission, the patient returned to the Department of Orthopedics with the same subjective symptoms. Because the level of inflammatory marker serum C-reactive protein (CRP) was increased compared to the last control value, the patient immediately received antibacterial treatment. Arthroscopy including irrigation of the joint was performed; the synovial fluid was opaque, and culture did not show any bacterial growth. After a week on intravenous cloxacillin therapy, the status of the knee was clinically slightly improved and the patient was discharged (without any additional antibiotics).
TABLE 1.
Time schedule, laboratory findings, and antibacterial therapy
| Time from first admission (wk) | Microorganism isolated | CRP concn (mg/ml) | Sedimentation rate (mm/h) | Plasma leucocyte count (109/liter) | Antibiotic administered (dosage/duration) |
|---|---|---|---|---|---|
| 0 | 113 | 9.6 | |||
| 1a | +/−b | 242 | 7.7 | Cloxacillin (2 g × 3/1 wk) | |
| 2 | 202 | ||||
| 3 | 136 | 6.6 | |||
| 7 | −c | 176 | 8.0 | Cloxacillin (1 g × 3/1 wk) | |
| 8 | 159 | 7.7 | |||
| 10 | +d | 312 | 125 | 13.1 | Penicillin G (1 g × 3/2 wks) |
| 12 | 246 | 85 | 11.5 | ||
| 14 | 28 | 50 | Penicillin V (1 g × 3)e | ||
| 16 | 10 |
Blood glucose/synovial fluid ratio was 1.7.
Gram-positive anaerobic micrococci isolated from the synovial fluid. The second aspiration was performed after initiation of cloxacillin therapy and did not reveal any bacteria.
Aspiration was performed on the third day of cloxacillin treatment.
Bacteria cultured from the synovial fluid and classified as P. micros.
Lifelong therapy with penicillin V was prescribed.
Two weeks later (i.e., 10 weeks after the first hospital visit), he was once again admitted to the local hospital. An additional joint aspiration was performed, and culture of the synovial fluid revealed growth of anaerobic micrococci. The species was classified as Peptostreptococcus micros. As can be seen in Fig. 1B and C, radiographic examination showed almost complete destruction of the cartilage, but also a posterior dislocation of the tibia. Intravenous administration of penicillin G was initiated. Plasma proteins were examined, and an M component classified as immunoglobulin A (IgA) was discovered, suggesting that the patient was suffering from multiple myelomas. However, X-ray examinations did not show any specific skeletal tumor foci. Intravenous antibiotic therapy was given for a prolonged period of time to eradicate the infection, but the function of the knee was permanently impaired, resulting in wheelchair dependence. No reconstructive surgery was performed due to the patient’s age and progressive dementia.
Laboratory tests revealed increased CRP concentrations at several occasions; at the patient’s second admission, the CRP concentration was 242 mg/liter, followed by 176 and 312 mg/liter at the two other hospital visits (Table 1). Increased leukocyte counts (13.1 × 109/liter) were not observed until 10 weeks after the initial contact with the physicians. Analysis of the synovial fluid performed at the second hospital visit showed a decreased blood glucose/synovial fluid ratio of 1.7. Differential counting of synovial leucocytes revealed 92% polymorphonuclear leukocytes and 8% monocytes and lymphocytes. Plasma protein analysis showed an M component (IgA [8 g/liter]), decreased IgG concentration (5.5 g/liter), and hypoalbuminemia (30 g/liter). In addition, the patient had 8% plasma cells in the bone marrow and secreted light κ chain (130 mg/24 h) in the urine, verifying the diagnosis of multiple myelomas.
The bacterial organism was isolated from the synovial fluid after 4 days by using anaerobic flasks with liquid medium (BacT/Alert; Organon Teknika). The isolate was subcultured onto supplemented human blood agar plates containing Columbia II agar, l-cystein, hemin, and vitamin K1 and was found to be obligately anaerobic. The micrococci were classified as P. micros with the RapID ANA II system (>99.9% probability; Innovative Diagnostic Systems). The organism was susceptible to cloxacillin, penicillin, cefuroxime, imipenem, clindamycin, and metronidazole as determined by E-tests (Biodisk). When slides with synovial fluid from the first knee aspiration were Gram stained and reexamined, several gram-positive micrococci were detected scattered among the polymorphonuclear leukocytes.
Discussion.
The gram-positive anaerobic coccus P. micros is one of the most frequently recovered species from the radicular dentin of periodontally diseased teeth (4). In addition, P. micros is occasionally isolated from other oral infections, such as dental periapical abscesses (18) and peritonsillar infections (8). P. micros has been described in several, most commonly mixed, anaerobic infections: e.g., cerebral and pulmonary abscesses, endocarditis, and female genital tract infections (3, 5, 9, 10, 17). We report here a patient with a destructive P. micros infection in a knee joint without any previous history of pathological signs from his joints. The overall infection rate with P. micros has been calculated at 2% of all anaerobic isolates identified at Indiana University Medical Center (1). Despite the fact that monarthritis relatively frequently has a bacterial origin (12), infections with P. micros are rare in joints or other parts of the locomotive organ. In a previous case report in which P. micros and Propionibacterium acnes were isolated from a patient with rheumatoid arthritis, a knee joint prosthesis existed and consequently primed bacterial colonization (14). An apical root abscess was explained as the bacterial focus. Despite the fact that our patient showed poor dental hygiene, an oral origin of P. micros could not be proven. The same fact was also outlined in a paper on a patient with peptostreptococcal vertebral osteomyelitis with no apparent infections at other body sites (11). That patient’s infection resulted in destruction of the intervertebral disk and consequently absence of L4-L5 disk space as revealed by a roentgenogram.
Interestingly, the presence of multiple myelomas with an IgA M component was diagnosed during the course of the P. micros arthritis. The first X ray did not support a local plasma cell tumor (Fig. 1A), but the patient’s newly discovered myeloma may have caused an increased susceptibility to infection. It is a well known fact that patients with multiple myelomas usually display lower IgG concentrations and often suffer from bacterial infections and hence cannot be considered as immunocompetent (13). Here, the peptostreptococcus arthritis was the first presentation of the multiple myelomas; similar cases with Haemophilus influenzae monarthritis as the first clinical finding of multiple myelomas have recently been described (2). The importance of an immunocompetent host with a functional B- and T-cell-mediated immunity has brilliantly been demonstrated with RAG-2 severe combined immunodeficient (SCID) mice (15). One-third of the RAG-2 mice developed endodontic abscesses with P. micros, while no immunocompetent controls had any abscesses, results that indicated a regional dissemination of the infection.
The virulence factors of P. micros have not been fully elucidated. P. micros can be divided into smooth and rough morphotypes (6). When the pathogenicity of the two morphotypes was examined in a mouse model, no difference in abscess-inducing capacity was observed (16). The lesions caused by the rough morphotype were, however, slightly larger than the abscesses induced by the smooth P. micros morphotype. Finally, in a recent investigation, all P. micros strains (n = 12) isolated from amniotic fluid with preterm premature rupture of membranes were demonstrated to display elastolytic activity (7).
Taken together, we have reported an unusual case of P. micros infecting a knee joint in a patient that manifested underlying multiple myelomas. This example of a P. micros infection causing cartilage destruction strongly points out the importance of presenting an early microbiological diagnosis in order to prescribe antibiotics for a prolonged period of time. It is also crucial to classify gram-positive anaerobic micrococci, especially when they are detected without any contaminating skin-derived commensal.
REFERENCES
- 1.Allen S D, Siders J A. . C. Koneman et al. (ed.), Color atlas and textbook of diagnostic microbiology. 4th ed. Philadelphia, Pa: J. B. Lippincott Co.; 1992. Anaerobic bacteria p. 527. [Google Scholar]
- 2.Berthaud V, Milder J, el-Sadr W. Multiple myeloma presenting with Haemophilus influenzae septic arthritis: case report and review of the literature. J Natl Med Assoc. 1993;85:626–628. [PMC free article] [PubMed] [Google Scholar]
- 3.Civen R, Jousimies-Somer H, Marina M, Borenstein L, Shah H, Finegold S M. A retrospective review of cases of anaerobic empyema and update of bacteriology. Clin Infect Dis. 1995;20(Suppl. 2):S224–229. doi: 10.1093/clinids/20.supplement_2.s224. [DOI] [PubMed] [Google Scholar]
- 4.Giuliana G, Ammatuna P, Pizzo G, Capone F, D’Angelo M. Occurrence of invading bacteria in radicular dentin of periodontally diseased teeth: microbiological findings. J Clin Periodontol. 1997;24:478–485. doi: 10.1111/j.1600-051x.1997.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 5.Ingham H R, Selkon J B, Roxby C M. Bacteriological study of otogenic cerebral abscesses: chemotherapeutic role of metronidazole. Br Med J. 1977;ii:991–993. doi: 10.1136/bmj.2.6093.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremer B H A, Magee J T, van Dalen P J, van Steenbergen T J M. Characterization of smooth and rough morphotypes of Peptostreptococcus micros. Int J Syst Bacteriol. 1997;47:363–368. doi: 10.1099/00207713-47-2-363. [DOI] [PubMed] [Google Scholar]
- 7.Mikamo H, Kawazoe K, Sato Y, Tamaya T. Elastase activity of anaerobes isolated from amniotic fluid with preterm premature rupture of membranes. Am J Obstet Gynecol. 1999;180:378–380. doi: 10.1016/s0002-9378(99)70217-6. [DOI] [PubMed] [Google Scholar]
- 8.Mitchelmore I J, Prior A J, Montgomery P Q, Tabaqchali S. Microbiological features and pathogenesis of peritonsillar abscesses. Eur J Clin Microbiol Infect Dis. 1995;14:870–877. doi: 10.1007/BF01691493. [DOI] [PubMed] [Google Scholar]
- 9.Murdoch D A, Mitchelmore I J, Tabaqchali S. Isolation of Peptostreptococcus micros from polymicrobial abscesses. Lancet. 1988;i:594. doi: 10.1016/s0140-6736(88)91393-1. [DOI] [PubMed] [Google Scholar]
- 10.Murdoch D A, Mitchelmore I J, Tabaqchali S. The clinical importance of Gram-positive cocci isolated at St Bartholomew’s Hospital, London, in 1987. J Med Microbiol. 1994;41:36–44. doi: 10.1099/00222615-41-1-36. [DOI] [PubMed] [Google Scholar]
- 11.Papasian C J, McGregor D H, Hodges G R, Kennedy J. Peptostreptococcal vertebral osteomyelitis. J Clin Microbiol. 1986;24:633–635. doi: 10.1128/jcm.24.4.633-635.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sack K. Monarthritis: differential diagnosis. Am J Med. 1997;102(Suppl. 1A):30S–34S. doi: 10.1016/s0002-9343(97)00414-2. [DOI] [PubMed] [Google Scholar]
- 13.Seiden M V, Anderson K C. Multiple myeloma. Curr Opin Oncol. 1994;6:41–49. doi: 10.1097/00001622-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Stoll T, Stucki G, Bruhlmann P, Vogt M, Gschwend N, Michel B A. Infection of a total knee joint prosthesis by Peptostreptococcus micros and Proprionibacterium acnes in an elderly RA patient: implant salvage with longterm antibiotics and needle aspiration/irrigation. Clin Rheumatol. 1996;15:399–402. doi: 10.1007/BF02230366. [DOI] [PubMed] [Google Scholar]
- 15.Teles R, Wang C Y, Stashenko P. Increased susceptibility of RAG-2 SCID mice to dissemination of endodontic infections. Infect Immun. 1997;65:3781–3787. doi: 10.1128/iai.65.9.3781-3787.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dalen P J, van Winkelhoff A J, van Steenbergen T J. Prevalence of Peptostreptococcus micros morphotypes in patients with adult periodontitis. Oral Microbiol Immunol. 1998;13:62–64. doi: 10.1111/j.1399-302x.1998.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 17.Wenisch C, Wiesinger E, Werkgartner T, Makristathis A, Graninger W. Treatment of Peptostreptococcus micros endocarditis teicoplanin. Clin Infect Dis. 1995;21:446–447. doi: 10.1093/clinids/21.2.446. [DOI] [PubMed] [Google Scholar]
- 18.Williams B L, McCann G F, Schoenknecht F D. Bacteriology of dental abscesses of endodontic origin. J Clin Microbiol. 1983;18:770–774. doi: 10.1128/jcm.18.4.770-774.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]