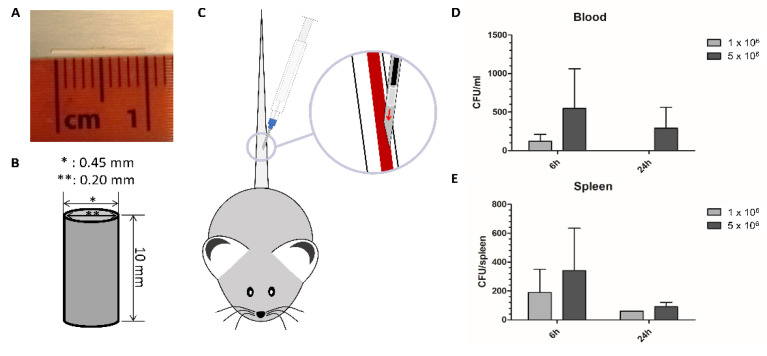
Figure 1.
The implant used for the testing of antimicrobial material properties in the bloodstream is (A) photographically and (B) schematically illustrated (*: uter diameter, **: inner diameter) as well as the implantation procedure (C). Bacterial load of (D) the blood and (E) the spleen are shown by colony forming units (CFU)/mL or per organ for the implants with low (1 × 106) and high (5 × 106) implant contamination concentration.