Abstract
Vitamin D (VD) deficiency is frequently reported in heart transplant (HT) recipients and routinely supplemented. However, the efficacy of VD supplementation on bone mineral density (BMD) and its association with all-cause mortality is underinvestigated. The VD levels and BMD were studied for two years, and the association of VD and BMD with all-cause mortality risk was investigated. Ninety-six HT patients (38.18 ± 12.10 years old; 74% men) were followed up during VD, Ca, and Mg supplementation. Anthropometric measurements, BMD by Dual-energy X-ray absorptiometry (DEXA) scan, VD concentrations, and related biochemical parameters were analyzed before, 1 year, and 2 years after HT. Despite significant improvement of VD3 and 25-hydroxy VD (25OHVD) levels especially in the men, BMD parameters were insignificantly changed. After 2 years, the all-cause mortality rate was 15.6%. High pretransplant levels of 25OHVD failed to improve the survival probability. Cox’s regression showed a 32.7% increased hazard ratio for each unit increase in body mass index (95% CI: 1.015–1.733, p = 0.038), in the VD-deficient group rather than in the VD-sufficient one. In conclusion, VD supplementation improves the biochemical status, especially in VD-deficient HT. However, its impact on the BMD and mortality was not as usually expected. Further investigation of the disturbed VD metabolism in HT is warranted.
Keywords: vitamin D, bone mineral density, supplementation, all-cause mortality, heart transplant
1. Introduction
Vitamin D deficiency (VD-D) is highly prevalent among patients with end-stage organ failure especially heart failure. Further, VD-D is frequently reported in heart transplant (HT) patients [1,2]. Patients eligible for HT are hardly exposed to sunlight due to frequent hospitalization and defective hepatic metabolism for VD due to heart failure-associated hepatic congestion. The VD status is frequently represented by the level of 25 hydroxyvitamin D (25OHVD). The International Osteoporosis Foundation (IOF) reported the definition of the VD-D by having a serum level of 25OHVD less than 25 nmol/L [3]. However, many cutoff points for defining VD-D were also reported, e.g., serum level of 25OHVD < 30 nmol/L [4], and 25OHVD < 50 nmol/L [5]. The 25 nmo/L was suggested as an arbitrary cutoff value especially with a lack of sufficient evidence for the optimal value for non-skeletal effects of VD [6,7], especially in populations with endemic VD-D such as Saudi Arabia. In Saudi Arabia, VD-D (with 25 nmol/L cutoff point) was reported as 44.5% in adults and 49.5% in school children [8]. Heart transplant is still growing in Saudi Arabia, with only 2 cardiac centers available to do HT with a rate of about 30 HT/year [9]. In a recent study, VD-D (reported by 25OHVD < 25 nmol/L) was reported in 10% of the heart transplant patients and 55% of those with orthotopic heart transplants [10]. However, the data about VD status, bone mineral density (BMD), and the impact of VD on survival are deficient.
Osteopenia or osteoporosis may develop in HT patients especially in the first year and with improper preventive management [11]. Glucocorticoids bind osteoprotegerin (OPG); this molecule is necessary for limiting bone resorption. Thus, the decreased amount of OPG reduces BMD in transplantation patients [12]. Notably, it was reported that patients referred to cardiac transplantation generally have low BMD and about 14% of them suffer from osteoporotic vertebral compression fractures [13]. In another report, the prevalence of vertebral fractures was reported as 35% after HT heart transplantation due to increased bone loss [12]. Previous reports from our center showed osteopenia (35%) and osteoporosis (8%) at the lumbar spine of pre-transplant patients, besides significant reduction in pre-transplant BMD compared with that at 1 year after heart transplantation [14]. Nutritional supplementation with VD, Ca, Mg, Zn, and vitamin C are frequently recommended to support BMD in HT patients [2,15]. The International Society for Heart and Lung Transplantation recommended a daily calcium dose of about 1000–1500 mg, and VD 400–1000 IU for HT patients to maintain serum 25OHVD level > 75 nmol/L but with a low level of evidence [16].
The impact of VD and low BMD on all-cause mortality in HT patients is underinvestigated. Some reports showed that a reduced level of 1,25(OH)2VD, measured on the 21st day after HT, was associated with 1-year mortality in HT recipients [17]. This relationship between 1,25(OH)2VD (calcitriol) and mortality, is unclear. Low 1,25(OH)2VD may be because of immunosuppressive medications such as calcineurin inhibitors on the renal enzyme system, or due to bad general health as a consequence following organ transplantation. In another study that used the most common indicator of VD status monitoring (25OHVD), VD supplementation in a dose of 4000 IU did not affect the mortality in patients with end-stage heart failure compared to placebo [18]. Another European randomized controlled trial (NTC01212406) used VD in a high dose of 100,000 IU/month in lung transplantation patients and reported low survival for patients who received VD [19]. Data from another area on the map such as Saudi Arabia are very lacking. Thus, this work aimed to study the changes in VD levels and BMD in VD-supplemented HT patients for two years and to analyze the association of VD levels and BMD with the survival of Saudi HT recipients.
2. Materials and Methods
2.1. Study Design and Setting
A total of ninety-six heart transplant patients at King Faisal Specialist Hospital & Research Centre (KFSH&RC) in Riyadh, Saudi Arabia were investigated and followed up for two years after the procedure had been done. Participants were divided into two groups based on the baseline level of 25OHVD: Group I in which 25OHVD < 25 nmol/L, and Group II with 25OHVD ≥ 25 nmol/L. The Research Ethics Committee (REC) in the College of Applied Medical Sciences, King Saud University reviewed and approved this study protocol under reference number CAMS 93-36/37; date: 01/03/2016. In addition, the REC at KFSH&RC approved it under reference No. 2161051.
2.2. Given Supplementations and Medications
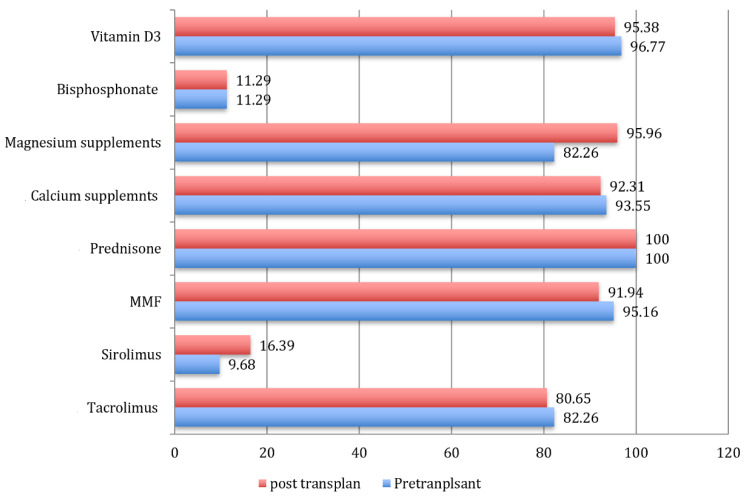
Study participants were on medications as seen in Figure 1. According to the KFSH&RC’s local protocol, a routine dose of vitamin D3 was 10,000 IU (250 μg/day) for patients with VD insufficiency (25OHVD < 50 nmol/L), while those with proven VD-D (by 25OHVD < 25 nmol/L) were given 50,000 IU as a weekly oral dose for 3 months followed by a 10,000 IU maintenance dose. Calcium (Ca) in an oral dose of 1200 mg/day, and magnesium (Mg) 200 mg/day per oral were also routinely given to all patients. Commitment percentages of study participants on other supplements and medications were shown in Figure 1.
Figure 1.
Medications intake by study population in the pre-transplant and post-transplant phases (numbers refer to frequencies of medication use, MMF = mycophenolic acid).
2.3. Anthropometric Parameters
Bodyweight (kg) and height (cm) were used for calculating body mass index (BMI) using the formula; BMI = weight (kg)/height (m)2. Weight was measured to the nearest 0.1 kg by (Scale-Tronix scale, Chicago, IL, USA) while a stadiometer (Seca Co, Hamburg, Germany) was used for height measurement.
2.4. Measurement of VD and Biochemical Parameters
Serum cholecalciferol (VD3), 25OH vitamin D (25OHVD), and intact parathyroid hormone (iPTH) levels (pmol/L) were measured on pretransplant assessment workup appointment, one year, and two years after the transplant had done by using electrochemiluminescence immunoassay, Cobas e411 autoanalyzer (Roche Ltd., Basel, Switzerland). Despite previous reports, the definition of VD-D was suggested in this study to be below 25 nmol/L of the 25OHVD level [20,21,22]. Moreover, levels of Alkaline phosphatase (ALP) (U/L), calcium (mmol/L), phosphorus (mmol/L), magnesium (mmol/L), creatinine (umol/L), urea (mmol/L), potassium (mmol/L), and chloride (mmol/L) were measured by Roche/Hitachi modular Cobas c 701/702 tests.
2.5. Measurement of BMD
The BMD was measured at scheduled appointments for follow-up at pre-transplant, 1 year, and 2 years post-transplant phases and the reports were used for analysis. BMD was assessed by the DEXA scan using a GE medical system Lunar iDEXA (GE Healthcare, Madison, WI, USA). Two sites were selected: lumbar spine (LS) and femoral neck (FN). Due to the relatively young age of our samples, Z-scores (rather than T-scores) were calculated as standard deviations from the mean of the gender- and age-matched controls. Tertials of the BMD results were created as follows: (a) normal BMD: Z-scores above −1; (b) osteopenia: Z-scores between −1 and −2.5 gm/cm2; and (c) osteoporosis: Z-score below −2.5 gm/cm2 [23].
2.6. Sample Size and Satistical Power
The sample size and statistical power were calculated by G*Power software 3.1.9.4 (University of Kiel, Kiel, Germany), considering medium effect size (f = 0.25), alpha error probability at 0.05, power (1-β error probability) = 0.95, number of repeated measurements = 3, and the number of groups = 2. The estimated total sample size was 44 participants (22/group) and the actual power was 0.9557.
2.7. Statistical Analysis
The SPSS tool version 25 (SPSS, IBM, Chicago, IL, USA) was used for processing and analyzing these data. Continuous data were expressed as means ± SD, while dichotomous variables were expressed as percentages and categories. The normal distribution of continuous variables was tested by the Shapiro–Wilk test. For comparison of the three related samples, the Friedman ANOVA test was used with pairwise comparisons. Gender differences and comparisons between groups I and II were done via the Mann–Whitney U test. For survival analysis, Cox’s proportional hazard regression analysis was used with 95% CIs. Results were considered statistically significant at p ≤ 0.05.
3. Results
3.1. Basal Characteristics of Study Participants
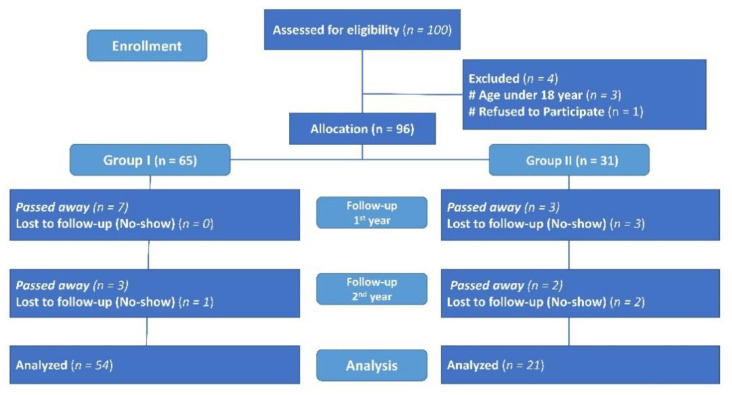
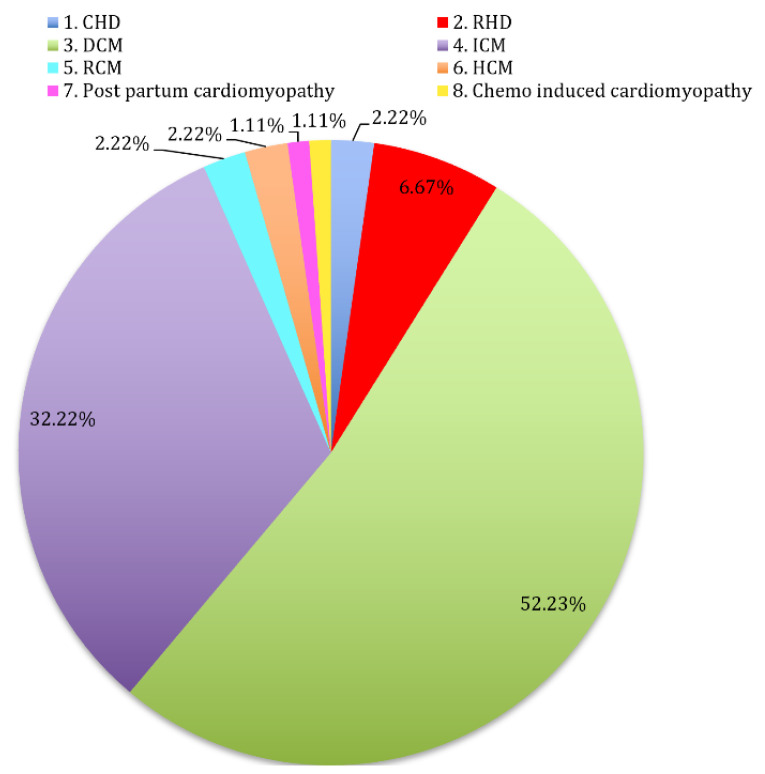
Figure 2 shows participants’ flow throughout the study. As shown in Table 1, pretransplant data of our sample showed younger age, significantly lower BMI, and BMD in the women’s group. Levels of VD3, 25OHVD, PTH, Ca, ALP, Urea, Na, and K were insignificantly different. However, Mg and Cl levels were significantly higher, while creatinine was lower in the women group. Figure 3 shows the cardiovascular events which were diagnosed in our participants. The majority had dilated cardiomyopathy (52.22%), followed by ischemic cardiomyopathy (32.22%), and chemo-induced cardiomyopathy and post-partum cardiomyopathy (1.11%).
Figure 2.
CONSORT diagram showing participants’ flow throughout the study.
Table 1.
Pretransplant characteristics of the study sample.
| Variables | Total (n = 96) |
Men (n = 71) |
Women (n = 25) |
p-Value |
|---|---|---|---|---|
| Age (Years) | 36.17 ± 13.53 | 39.84 ± 12.22 | 32.35 ± 9.31 | 0.031 |
| Height (cm) | 165.33 ± 9.02 | 168.56 ± 7.57 | 156.16 ± 6.02 | <0.001 |
| Weight (kg) | 65.05 ± 17.29 | 69.68 ± 15.87 | 51.90 ± 14.31 | <0.001 |
| BMI (kg/m2) | 23.62 ± 5.37 | 24.50 ± 5.21 | 21.12 ± 5.11 | 0.006 |
| BMD lumbar spine (gm/cm2) | 1.05 ± 0.16 | 1.07 ± 0.16 | 0.95 ± 0.11 | 0.041 |
| Lumbar spine Z-score | −0.31 ± 1.10 | −0.25 ± 1.15 | −0.48 ± 0.96 | 0.380 |
| BMD femoral neck (gm/ cm2) | 0.59 ± 0.49 | 0.67 ± 0.48 | 0.38 ± 0.45 | 0.012 |
| Femoral neck Z-score | −0.10 ± 0.91 | −0.04 ± 0.99 | −0.26 ± 0.60 | 0.293 |
| 25OHVD (nmol/L) | 27.80 ± 23.78 | 27.72 ± 24.63 | 28.04 ± 21.63 | 0.954 |
| Vitamin D3 (nmol/L) | 14.82 ± 14.73 | 15.23 ± 13.63 | 13.68 ± 17.76 | 0.654 |
| Intact parathyroid h. (mmol/L) | 78.15 ± 65.13 | 80.17 ± 61.88 | 72.40 ± 74.67 | 0.611 |
| ALP (U/L) | 96.07 ± 78.76 | 99.97 ± 80.56 | 84.98 ± 73 | 0.416 |
| Calcium (mmol/L) | 2.22 ± 0.18 | 2.20 ± 0.18 | 2.28 ± 0.19 | 0.054 |
| Phosphate (mmol/L) | 1.14 ± 0.34 | 1.15 ± 0.34 | 1.11 ± 0.35 | 0.600 |
| Mg (mmol/L) | 0.95 ± 0.55 | 0.86 ± 0.17 | 1.19 ± 1.02 | 0.011 |
| Creatinine (umol/L) | 91.14 ± 36.80 | 96.01 ± 36.14 | 77.32 ± 35.83 | 0.028 |
| Urea (mmol/L) | 10.14 ± 6.59 | 10.71 ± 5.75 | 8.50 ± 8.45 | 0.149 |
| Sodium (mmol/L) | 136.67 ± 6.43 | 136.04 ± 5.87 | 138.44 ± 7.63 | 0.110 |
| Potassium (mmol/L) | 4.05 ± 0.57 | 4.03 ± 0.56 | 4.10 ± 0.60 | 0.571 |
| Chloride (mmol/L) | 97.62 ± 6.89 | 96.52 ± 6.24 | 100.64 ± 7.80 | 0.010 |
BMI is body mass index; BMD is bone mineral density; 25OHVD is 25 hydroxyvitamin D; ALP is alkaline phosphatase; Mg is magnesium.
Figure 3.
Percentages of main pre-transplant diagnoses among study recipients. (CHD: congenital heart disease, RHD: rheumatoid heart disease, DCM: dilated cardiomyopathy, ICM: ischemic cardiomyopathy, RCM: restrictive cardiomyopathy, HCM: hypertrophic cardiomyopathy).
3.2. Changes in VD and BMD throughout the Study Period
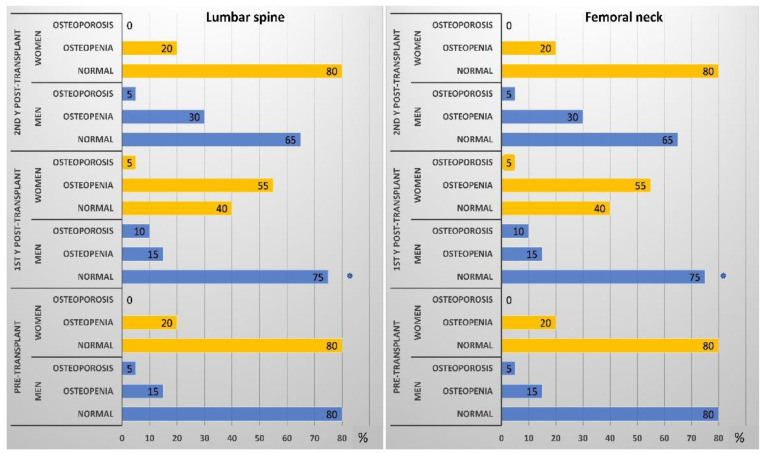
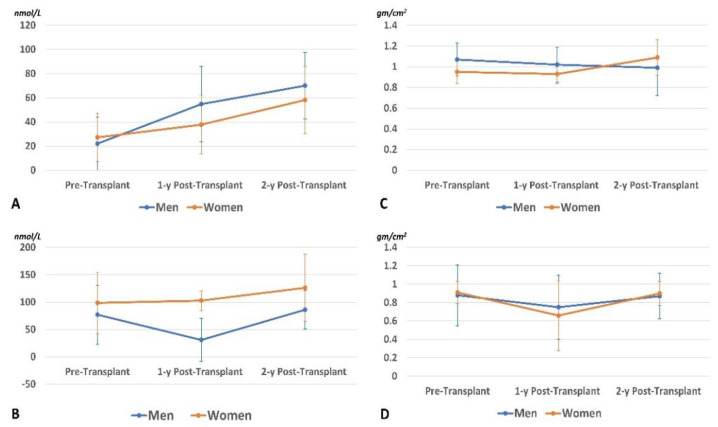
After 2 years, 15 participants passed away (33% women), and 6 dropped out (83% women). The data of remaining participants (n = 75; 20% women) are presented in Table 2 and analyzed by the Friedman ANOVA test with pairwise comparisons. Bodyweight and BMI significantly improved, indicating improvement of nutritional status. Femoral BMD in the men’s group showed a significant reduction after 1 year. After the second year, it returned to a value like that of the pretransplant status. In women, the three measurements were insignificantly different (p trend > 0.05). In the men’s group, levels of 25OHVD and Ca increased progressively throughout the period with a significant reduction of PTH (especially in the first year), and ALP enzyme. However, insignificant changes in their levels were noticed in the women group. The prevalence of VD-D (defined by 25OHVD < 25 nmol/L) is presented in Figure 4. A progressive reduction of the VD_D was noticed, especially in the men’s group, indicating sufficient VD supplementation. Despite supplementation, Mg serum level showed progressive reduction (p < 0.05) in men and women (p = 0.74). Percentages of study participants with osteopenia and osteoporosis at the lumbar spine and femoral neck throughout the study period are presented in Figure 5. Longitudinal changes in the 25OHVD, and PTH, as well as BMD at the lumbar spine and femoral neck, are shown in Figure 6.
Table 2.
Bone mineral density and related biochemical parameters before, 1 year, and 2 years after the heart transplant.
| Variables | Male (n = 60) | p-Value † | Female (n = 15) | p-Value † | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-Transplant Mean ± SD | 1-Y Post-Transplant Mean ± SD | 2-Y Post-Transplant Mean ± SD | Pre-Transplant Mean ± SD | 1-Y Post-Transplant Mean ± SD | 2-Y Post-Transplant Mean ± SD | |||
| Weight (kg) | 70.08 ± 13.24 a,* | 76.95 ± 14.95 b,* | 89.03 ± 12.40 b,* | <0.001 | 52.41 ± 12.56 a | 61.89 ± 17.61 b | 64.35 ± 18.97 b | 0.015 |
| BMI (kg/m2) | 24.75 ± 4.43 a | 26.85 ± 5.37 b,* | 27.21 ± 5.15 b | 0.011 | 21.46 ± 5.20 a | 24.86 ± 6.52 b | 26.27 ± 7.27 b | 0.021 |
| DEXA parameters | ||||||||
| BMD lumbar spine (gm/cm2) | 1.07 ± 0.16 a,* | 1.02 ± 0.17 a,* | 0.99 ± 0.27 a,* | 0.231 | 0.95 ± 0.11 a | 0.93 ± 0.09 a | 1.09 ± 0.17 a | 0.247 |
| Lumbar spine Z-score | −0.28 ± 1.49 a | −0.66 ± 1.39 b,* | −0.50 ± 1.47 a | 0.019 | −0.12 ± 0.99 a | −1.02 ± 0.59 b | −0.80 ± 1.10 a | 0.016 |
| BMD femoral neck (gm/cm2) | 0.88 ± 0.33 a | 0.75 ± 0.35 b,* | 0.87 ± 0.25 a | 0.012 | 0.91 ± 0.12 a | 0.66 ± 0.38 a | 0.90 ± 0.13 a | 0.268 |
| Femoral neck Z-score | −0.09 ± 1.33 a,* | −0.56 ± 1.22 b,* | −0.47 ± 1.18 a,* | 0.106 | −0.10 ± 0.61 a | −0.78 ± 0.75 b | −0.36 ± 0.62 a | 0.007 |
| Biochemical parameters | ||||||||
| 25OHVD (nmol/L) | 22.07 ± 22.02 a | 54.79 ± 31.13 b,* | 70.05 ± 27.58 b,* | <0.001 | 27.20 ± 20.13 a | 37.80 ± 24.36 a | 58.20 ± 27.69 a | 0.143 |
| Vitamin D3 (nmol/L) | 17.07 ± 14.36 a | 40.44 ± 25.43 b,* | 64.00 ± 25.06 c | <0.001 | 17.80 ± 18.83 a | 32.47 ± 19.65 a | 51.21 ± 25.19 a | 0.145 |
| Intact parathyroid h (pmol/L) | 77.40 ± 53.88 a,* | 30.85 ± 39.41 b,* | 86.09 ± 35.36 c | 0.013 | 98.67 ± 55.93 a | 102.80 ± 18.10 a | 126.25 ± 61.1 b | 0.015 |
| ALP (U/L) | 103.70 ± 42.42 a,* | 94.55 ± 42.85 a,* | 81.71 ± 29.10 b | 0.026 | 89.00 ± 40.14 a | 65.00 ± 12.63 a | 63.80 ± 4.55 a | 0.449 |
| Calcium (mmol/L) | 2.18 ± 0.14 a,* | 2.26 ± 0.11 b,* | 2.27 ± 0.9 b,* | 0.024 | 2.25 ± 0.12 a | 2.17 ± 0.04 a | 2.02 ± 0.50 a | 0.449 |
| Phosphate (mmol/L) | 1.10 ± 0.30 a,* | 1.12 ± 0.18 a,* | 1.07 ± 0.16 a | 0.314 | 1.00 ± 0.44 a | 1.09 ± 0.25 a | 1.01 ± 0.34 a | 0.531 |
| Mg (mmol/L) | 0.88 ± 0.12 a,* | 0.72 ± 0.10 b | 0.64 ± 0.18 b | <0.001 | 0.91 ± 0.11 a | 0.71 ± 0.16 a | 0.62 ± 0.05 a | 0.074 |
| Creatinine (umol/L) | 95.85 ± 35.06 a,* | 108.25 ± 52.63 a,* | 95.67 ± 23.88 b | 0.819 | 79.00 ± 23.36 a | 80.40 ± 31.30 a | 79.00 ± 24.22 a | 0.437 |
| Urea (mmol/L) | 10.00 ± 6.33 a,* | 7.30 ± 3.04 b,* | 9.01 ± 10.55 b | 0.030 | 7.60 ± 3.21 a | 7.28 ± 2.82 a | 6.38 ± 1.63 a | 0.819 |
| Sodium (mmol/L) | 135.10 ± 7.40 a,* | 141.3 ± 2.92 a | 140.02 ± 3.44 a | 0.051 | 139.86 ± 9.21 a | 140.43 ± 2.23 a | 145.05 ± 4.34 a | 0.869 |
| Potassium (mmol/L) | 4.07 ± 0.64 a,* | 4.11 ± 0.35 a,* | 4.10 ± 0.15 a | 0.600 | 4.21 ± 0.58 a | 4.31 ± 0.40 a | 4.19 ± 0.39 a | 0.725 |
| Chloride (mmol/L) | 95.45 ± 7.57 a,* | 104.45 ± 3.44 b | 99.33 ± 4.90 a | 0.031 | 104.14 ± 8.21 a | 105.57 ± 2.37 a | 102.90 ± 4.32 a | 0.625 |
†p-values of the three related samples by Friedman’s two-way analysis of variance by ranks. Values with different superscripts (a and b) mean significant vs. pretransplant phase; * Significant versus the related samples in the women group by Mann–Whitney U test; BMI is body mass index; DEXA is dual-energy X-ray absorptiometry; BMD is bone mineral density; 25OHVD is 25 hydroxyvitamin D; ALP is alkaline phosphatase enzyme; Mg is magnesium.
Figure 4.
Prevalence of VD deficiency defined by 25OHVD < 25 nmol/L in men and women’s groups throughout the study period.
Figure 5.
Prevalence of osteopenia and osteoporosis in men and women groups throughout the study period (* means significantly different between men and women by Mann–Whitney U test).
Figure 6.
Longitudinal changes in the 25OHVD level (A), PTH level (B), BMD at the lumbar spine (C), and femoral neck (D).
Table 3 further reports the changes in study parameters in both study groups. Pretransplant body BMI was insignificantly different between group I and group II. However, at the post-transplant assessment points, the BMI was significantly higher in the VD-sufficient group (group II). Besides, longitudinal changes showed a progressive increase of BMI with time, especially in the VD-deficient group. Group I had significantly lower VD3, 25OHVD, and calcium levels. In Group I (VD-D group), significant reductions in BMD parameters were detected after the first year which improved at the second year to be insignificantly different from the pretransplant levels. In Group II, insignificant changes were reported in the three-time points. VD3 level progressively increased with time due to the supplementation, while the 25OHVD level significantly increased in group I rather than group II. Similarly, levels of iPTH, ALP, calcium, and magnesium showed significant changes in group I rather than group II.
Table 3.
Bone mineral density and related biochemical parameters before, 1 year, and 2 years after the heart transplant between study groups.
| Variables | Group I; 25OHVD < 25 nmol/L (n = 54) |
p-Value † | Group II; 25OHVD ≥ 25 nmol/L (n = 21) |
p-Value † | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-Transplant Mean ± SD | 1-y Post-Transplant Mean ± SD | 2-y Post-Transplant Mean ± SD | Pre-Transplant Mean ± SD | 1-y Post-Transplant Mean ± SD | 2-y Post-Transplant Mean ± SD | |||
| Weight (kg) | 64.55 ± 9.22 a | 68.67 ± 12.04 b,* | 70.89 ± 9.67 b,* | 0.035 | 74.57 ± 26.57 a | 83.39 ± 17.79 b | 86.99 ± 19.35 b | 0.057 |
| BMI (kg/m2) | 23.42 ± 3.63 a | 24.92 ± 4.58 b,* | 26.21 ± 5.02 b,* | 0.037 | 27.60 ± 7.51 a | 31.17 ± 4.56 a | 32.67 ± 3.47 a | 0.063 |
| DEXA parameters | ||||||||
| BMD lumbar spine (gm/cm2) | 1.04 ± 0.14 a | 1.01 ± 0.16 a | 0.98 ± 0.26 a | 0.179 | 1.09 ± 0.15 a | 0.83 ± 0.11 a | 1.01 ± 0.16 a | 0.207 |
| Lumbar spine Z-score | −0.19 ± 1.43 a | −0.69 ± 1.28 b | −0.45 ± 1.20 a | 0.002 | −0.40 ± 1.37 a | −0.84 ± 1.33 a | −0.86 ± 1.86 a | 0.368 |
| BMD femoral neck (gm/cm2) | 0.86 ± 0.33 a | 0.71 ± 0.34 b | 0.85 ± 0.25 b | 0.001 | 0.96 ± 0.17 a | 0.79 ± 0.38 a | 0.97 ± 0.13 a | 0.867 |
| Femoral neck Z-score | −0.06 ± 1.09 a | −0.73 ± 0.96 b | −0.49 ± 1.00 a | 0.001 | −0.17 ± 1.58 a | −0.27 ± 1.52 a | −0.34 ± 1.35 a | 0.867 |
| Biochemical parameters | ||||||||
| 25OHVD (nmol/L) | 11.52 ± 6.49 a,* | 48.49 ± 27.11 b | 64.00 ± 22.23 b | <0.001 | 52.86 ± 16.41 a | 58.86 ± 38.52 a | 77.14 ± 38.33 a | 0.368 |
| Vitamin D3 (nmol/L) | 10.97 ± 5.84 a,* | 41.43 ± 24.84 b | 57.67 ± 16.67 b | <0.001 | 33.29 ± 19.35 a | 32.19 ± 22.96 a | 76.14 ± 38.06 b | 0.032 |
| Intact parathyroid h (pmol/L) | 63.50 ± 51.29 a | 23.89 ± 39.54 a | 91.64 ± 43.51 b | 0.002 | 83.86 ± 58.24 a | 37.86 ± 35.92 a | 100.48 ± 45.78 a | 0.368 |
| ALP (U/L) | 91.28 ± 35.60 a | 79.39 ± 26.70 a | 71.01 ± 13.56 a | 0.056 | 125.14 ± 48.61 a | 112.43 ± 59.71 a | 96.43 ± 42.96 a | 0.066 |
| Calcium (mmol/L) | 2.16 ± 0.11 a,* | 2.26 ± 0.99 b | 2.25 ± 0.93 b | 0.016 | 2.27 ± 0.17 a | 2.18 ± 0.12 a | 2.15 ± 0.45 a | 0.565 |
| Phosphate (mmol/L) | 1.14 ± 0.33 a | 1.11 ± 0.22 a | 1.05 ± 0.17 a | 0.454 | 1.10 ± 0.27 a | 1.14 ± 0.25 a | 1.11 ± 0.30 a | 0.867 |
| Mg (mmol/L) | 0.88 ± 0.10 a | 0.72 ± 0.12 b | 0.62 ± 0.17 b | <0.001 | 0.90 ± 0.14 a | 0.70 ± 0.10 a | 0.68 ± 0.11 a | 0.066 |
| Creatinine (umol/L) | 85.94 ± 28.05 a | 105.83 ± 55.69 a | 89.47 ± 25.01 a | 0.486 | 109.29 ± 41.92 a | 94.57 ± 32.35 a | 99.86 ± 22.70 a | 0.156 |
| Urea (mmol/L) | 8.06 ± 4.25 a | 7.31 ± 3.28 b | 5.93 ± 1.86 b | 0.076 | 13.29 ± 7.97 a | 7.26 ± 2.04 a | 15.06 ± 16.70 a | 0.102 |
| Sodium (mmol/L) | 134.50 ± 6.79 a | 141.44 ± 2.79 a | 143.12 ± 4.14 a | 0.251 | 139.71 ± 7.50 a | 140.43 ± 2.70 a | 143.15 ± 3.36 a | 0.867 |
| Potassium (mmol/L) | 4.04 ± 0.54 a | 4.18 ± 0.33 a | 4.07 ± 0.19 a | 0.600 | 4.29 ± 0.75 a | 4.01 ± 0.42 a | 4.09 ± 0.24 a | 0.867 |
| Chloride (mmol/L) | 95.72 ± 7.64 a | 104.83 ± 3.49 b | 98.29 ± 5.91 a | 0.131 | 98.29 ± 5.47 a | 104.14 ± 2.85 a | 102.97 ± 3.34 a | 0.867 |
†p-values of the three related samples by Friedman’s two-way analysis of variance by ranks. Values with different superscripts (a and b) mean significant vs. pretransplant phase; * Significant versus the related samples in Group II by Mann–Whitney U test; BMI is body mass index; DEXA is dual-energy X-ray absorptiometry; BMD is bone mineral density; 25OHVD is 25 hydroxyvitamin D; ALP is alkaline phosphatase enzyme; Mg is magnesium.
3.3. Survival Analysis Based on VD and BMD
Notably, in Group I with VD-D, Cox’s regression analysis showed that for each additional unit of BMI, the hazard increases by about 33% (Table 4). This was not the case in Group II. Besides, in both groups, for each additional unit of 25OHVD or VD3, the hazard ratio (HR) showed insignificant changes (Table 4). Furthermore, the age and BMD parameters had insignificant impacts.
Table 4.
Multivariable Cox proportional hazards regression model based on pretransplant variables.
| Variables | Group I (25OHVD < 25 nmol/L) | Group II (25OHVD ≥ 25 nmol/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |||
| Lower | Upper | Lower | Upper | |||||
| Age | 0.962 | 0.888 | 1.042 | 0.345 | 0.869 | 0.696 | 1.086 | 0.218 |
| BMI | 1.327 | 1.015 | 1.733 | 0.038 | 1.145 | 1.756 | 1.734 | 0.524 |
| Normal BMD at LS | Reference | Reference | ||||||
| Osteopenia at LS | 0.342 | 0.000 | 77,594.19 | 0.865 | 0.008 | 0.000 | - | 0.995 |
| Osteoporosis at LS | 0.000 | 0.000 | - | 0.995 | 0.000 | 0.000 | - | 0.995 |
| Normal BMD at FN | Reference | Reference | ||||||
| Osteopenia at FN | 0.538 | 0.000 | 123,625.63 | 0.922 | 0.004 | 0.000 | - | 0.994 |
| Osteoporosis at FN | 0.000 | 0.000 | - | 0.996 | 0.382 | 0.000 | - | 0.999 |
| VD3 serum level | 0.916 | 0.712 | 1.179 | 0.497 | 0.958 | 0.868 | 1.058 | 0.394 |
| 25OHVD serum level | 0.930 | 0.681 | 1.270 | 0.648 | 0.345 | 0.650 | 1.163 | 0.345 |
HR is hazard ratio; BMI is body mass index; BMD is bone mineral density; LS is lumbar spine; FN id femoral neck; 25OHVD is 25 hydroxyvitamin D; VD3 is vitamin D3.
4. Discussion
This study investigated changes in the VD levels and BMD in HT patients for two years and analyzed the association of VD status (by using an arbitrary cutoff value of 25OHVD, i.e., 25 nmol/L), and BMD with all causes-mortality in vitamin D-deficient and -sufficient groups of HT Saudi recipients. Most of our study participants were on VD supplementation at least by a maintenance dose of 10,000 IU/day. This was successful in the reduction of the percentage of the VD deficiency in both men and women’s groups (Figure 2). Besides, means of the 25OHVD and VD3 serum levels were significantly increased progressively in the men’s group and Group I, while the rise of their levels in the women’s group and group II were insignificant. Indicating that the benefit of VD supplementation is prominent in those with VD-D. Our baseline percentage of the VD-D was much higher than that reported by Stein et al. [24] where severe deficiency (25OHD <25 nmol/L) was found in 16% of heart transplant patients. Moreover, a Slovenian cohort of HT recipients showed 21.3% with severe VD deficiency and 54.7% with mild-to-moderate VD-D. However, these patients were on VD3 supplementation in a dose of 2000 IU/day and Alfacalcidol of 0.5 μg/day [25]. This frequently reported phenomenon is critical, since the VD-D is linked to post-HT bone loss and fracture possibility, sarcopenia, and may aggravate the immunosuppressive action of corticosteroids or calcineurin inhibitors [26]. Besides, it is associated with periodontal disease and gingival inflammation in HT recipients which impair the nutritional intake [27]. The improvement of VD status after HT especially in the VD- and Ca-supplemented men was in line with a previous report by Gilfraguas et al. [28]. Supplementation in addition to relief of hepatic congestion and improvement of general condition with more mobility and sunlight exposure were the causes of VD status improvement [29]. Unfortunately, this was not the case in the women’s group especially in Saudi Arabia where indoor lifestyle and extensive body covering are the traditions.
Bone metabolism and VD status are closely related. Low 25OHVD levels can negatively impact bone turnover biochemical markers. The PTH as an indicator of bone resorption is usually investigated. Baseline measurements of PTH in our sample indicated higher serum levels of iPTH and low normal Ca levels together with low 25OHVD (Table 2 and Table 3). This secondary hyperparathyroidism was improved in the men’s group and the deficient groups after the first year then relapsed later, while in Group II and women, insignificant changes were detected in the first year and a significant increase in iPTH (with insignificant reduction of Ca level) were detected by the second year. This finding was consistent with previous reports [27,29]. The increase in the 25OHVD level leads to normalization of serum calcium and phosphate levels, nevertheless, serum iPTH level remained high, especially in the women’s group and in the second year in the men’s group, indicating a status of persistent hyperparathyroidism in the HT recipients. This persistent secondary hyperparathyroidism occurred in both deficient and sufficient groups in the second year. This finding was consistent with previous reports about HT [30,31], renal transplant adults [32], and up to 50% of children’s kidney transplants [33]. Persistent secondary hyperparathyroidism may then lead to autonomous hyperplasia of parathyroid glands. Besides, perioperative administration of large amounts of citrate during blood transfusion leading to precipitation of calcium resulting in hypocalcemia-induced hyperparathyroidism. This persistent hyperparathyroidism or tertiary hyperparathyroidism is usually reported after successful renal transplantation. PTH levels usually decline significantly within the first 3–6 months after kidney transplantation due to the reduction of the functional mass of parathyroid glands [34]. Persistent hyperparathyroidism despite normalization of renal functions, and overall survival was reported in 25% of kidney transplant recipients 1-year after the procedure. Medical management and even parathyroidectomy may be required in these cases [35,36,37,38,39,40]. Moreover, it may cause serious consequences such as hypercalcemia, organ calcification, hypophosphatemia, and hypercalciuria [34,41].
Despite VD and Ca supplementation, a significant reduction in femoral neck BMD after 1 year was noticed especially in group I, while all remaining measurements were insignificantly different from the pretransplant status, especially in Group II and women. These findings were consistent with previous reports [13,42] about both lung and heart recipients. Compared to pretransplant status, Caffarelli et al. [42] found an increase in the incidence rate of vertebral fractures in the first period post-transplantation (9.6% vs. 25.7%). These vertebral fractures were predicted only by the history of any fracture, while in lung transplant recipients, vertebral fractures were predicted by age, BMD at the femur neck, and history of fracture [43]. The transplantation-associated abnormalities in bone metabolism are generally similar regardless of the transplanted organ, pre-existent low BMD, and previous treatment. Typically, bone loss occurs in the first year after the organ transplant, because of immunosuppressive medications, and the long period of immobilization. Supplementation with VD, Ca, and Mg was not sufficient in the improvement of BMD in the HT population. At least in part, tertiary hyperparathyroidism may be the underlying mechanism. In another hand, Calcitriol (1,25(OH)2VD) supplementation in a dose of 0.5–0.75 µg/day for 12 or 24 months in addition to calcium 600 mg/day in comparison with calcium 600 mg/day produced improvement in the femoral neck (but not at lumbar spine) in the calcitriol groups at 12 months [44]. In another trial, Calcidiol (25OHVD) in a dose of 32,000 IU/week showed a mild improvement of about 4.9% only at the lumbar spine in HT patients [45]
In our study ALP, and urea significantly decreased in the male group rather than the women’s group (Table 2). In the pretransplant phase, congestive hepatopathy and even liver cirrhosis may be evident resulting in impaired hepatic functions such as protein and lipid biosynthesis and decreased ability for detoxification of toxic metabolites. Besides, secretions of hepatic enzymes show an abnormal pattern such as rises in alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) [46]. Post-transplant relief of hepatic congestion greatly correct this abnormal pattern. Moreover, Przybyłowski et al. [10] reported that vitamin D was correlated with kidney functions in heart transplant patients, i.e., improvement of VD status was associated with improvement of renal functions.
Interestingly, Cox’s regression analysis showed that in Group I, for each additional unite of 25OHVD, the hazard for all-cause mortality decreases by 7% (HR = 0.930; 95%CI: 0.681–1.270, p = 0.648), while in Group II, for each additional unite of 25OHVD, the hazard decreases by 65.5% (HR = 0.345; 95%CI: 0.650–1.163, p = 0.345). However, these findings were statistically insignificant (Table 4). The current study finding was in line with Zittermann et al. [18] who reported no effect of 4000 IU/day oral vitamin D supplementation in reduction of mortality in patients with advanced heart failure. After 3 years there was no beneficial latency impact of VD supplementation on all-cause mortality in the same study participants [47]. In a meta-analysis of randomized clinical trials, with >83,000 participants, VD supplementation failed in reducing the risks of major adverse cardiovascular events, stroke, myocardial infarction, cardiovascular disease mortality, or all-cause mortality [48]. In children undergoing hematopoietic stem cell transplant, there was no significant difference in overall survival for those with pretransplant VD deficiency, or sufficiency or optimal level (p = 0.51) [49]. In a renal transplant study, Cox regression analysis showed no significant prediction between 3-month 25OHVD or 3-month 1,25(OH)2VD levels and mortality (HR = 0.97; 95% CI: 0.93–1.02, p = 0.27 for 1 25OHVD unit increase, and for 10 units increase it was 0.86; 95% CI: 0.70–1.06, p = 0.16) [50]. On the other hand, Zittermann et al. [17] found that low postoperative levels of 1,25(OH)2VD were associated with high 1-year mortality in HT recipients. Furthermore, in a large cohort of kidney transplant recipients, survival was better in recipients with sufficient vitamin D which was measured 10 weeks post-transplant [51]. However, we used a different indicator of the VD status (i.e., 25OHVD), and we used the pretransplant level. Another report from patients with chronic heart failure showed about a 14% reduction of all-cause mortality with a 2.7-fold increment in the 25OHVD Level (95% CI: 1–26%; p = 0.04) [52]. Pediatric reports also stated that VD-D was associated with lower survival on a short-term basis after hematopoietic stem cell transplantation in children [53].
This study’s findings indicate a significant increase in the HR of all-cause mortality in the VD-deficient group rather than the VD-sufficient group (HR = 1.327; 95% CI: 1.015–1.733, p = 0.038). Independent of the VD status, a systematic review showed that pretransplant BMI was associated with increased risk of mortality in those with BMI above 30 Kg/m2 (10% increase in HR) and those above 35 kg/m2 (by about 24%) [54]. Moreover, Doumouras et al. [55] added the low BMI to the obesity as independent factors for increased mortality in HT recipients. While Nagendran et al. [56] excluded BMI up to 35 kg/m2 from the factors that worse mortality risk. Our sample’s pretransplant BMI was at the range of normal BMI in Group I, and the overweightedness in Group II. In the general population, obesity may affect the association between VD and cardiac disorders. However, VD supplementation failed to reduce the incidence rate of cardiovascular diseases and mortality [57]. In the HT population, the current study tested the BMI and VD levels in the same Cox’s regression model and resulted in a significant effect of BMI with an insignificant effect of the VD levels. This indicates that the mortality-increasing effect of the BMI is independent of VD.
5. Conclusions
This study tracked the changes of the VD3, 25OHVD, and BMD in VD-supplemented heart transplant recipients for 2 years. Further, it investigated the association of 25OHVD, and BMD with all-cause mortality, based on an arbitrary cutoff value of 25OHVD equal 25 nmo/L. Supplementation with VD3 10,000 IU daily dose, Ca 1200 mg/day, and Mg 200 mg/day were being effective in elevating the serum level of 25OHVD especially in vitamin D deficient HT recipients; however, no significant impact was detected on the preservation of the BMD at measured sites, or on correction of tertiary hyperparathyroidism. Interestingly, the 25OHVD failed to ameliorate the all-cause mortality hazard ratio.
6. Limitations
The main limitation of this study was the lack of a placebo-controlled group. Instead, we used the comparison of VD deficient (Group I) vs. VD sufficient (Group II). The limited number in the women’s group is also a considerable limitation that may affect the obtained results. However, the number of female candidates is usually less than males in many centers all over the world. Another important limitation is the missing of about 21.8% of our participants at the end of the study; either by death (15.6%) or by no-show (6.25%). Missing data are usually common in longitudinal studies. Besides, we did not measure the 1,25(OH)2VD levels and considered the commonly used indicator for VD status which is 25OHVD.
Acknowledgments
All authors thank the Deanship of Scientific Research, King Saud University for funding through the Vice Deanship of Scientific Research Chairs for funding the present study.
Author Contributions
Conceptualization, M.M.A.A. and D.A.A.; methodology, D.A.A., H.M.H., D.A.A.M., M.A. and N.S.; software, M.M.A.A., M.I.H. and D.A.A.; validation, N.S., M.M.A.A. and D.A.A.; formal analysis, D.A.A., H.M.H. and M.M.A.A.; investigation, A.A.A.-K. and D.A.A.; resources, A.A.A.-K., N.S. and M.M.A.A.; data curation, D.A.A.; writing—original draft preparation, M.M.A.A. and M.A.; writing—review and editing, D.A.A.M. and M.M.A.A.; visualization, D.A.A.; supervision, M.M.A.A.; project administration, N.S.; funding acquisition, M.I.H. and A.A.A.-K. All authors have read and agreed to the published version of the manuscript.
Funding
Deanship of Scientific Research, King Saud University for funding through the Vice Deanship of Scientific Research Chairs.
Institutional Review Board Statement
The Research Ethics Committee (REC) in the College of Applied Medical Sciences, King Saud University reviewed and approved this study protocol under reference number CAMS 93-36/37. Also, the REC at KFSH&RC approved it under reference No. 2161051.
Informed Consent Statement
Patient consent was obtained during admission to the cardiac center.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shane E., Mancini D., Aaronson K., Silverberg S.J., Seibel M.J., Addesso V., McMahon D.J. Bone mass, vitamin D deficiency and hyperparathyroidism in congestive heart failure. Am. J. Med. 1997;103:197–207. doi: 10.1016/S0002-9343(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 2.Stein E.M., Shane E. Vitamin D in organ transplantation. Osteoporos. Int. 2011;22:2107–2118. doi: 10.1007/s00198-010-1523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson-Hughes B., Mithal A., Bonjour J.P., Boonen S., Burckhardt P., Fuleihan G.E., Josse R.G., Lips P., Morales-Torres J., Yoshimura N. IOF position statement: Vitamin D recommendation for older adults. Osteoporos. Int. 2010;21:1151–1154. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 4.Veugelers P.J., Pham T., Ekwaru J.P. Optimal vitamin D supplementation doses that minimize the risk for both low and high serum 25-hydroxyvitamin D concentrations in the general population. Nutrients. 2015;7:10189–10208. doi: 10.3390/nu7125527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pludowski P., Karczmarewicz E., Bayer M., Carter G., Chlebna-Sokol D., Czech-Kowalska J., Dębski R., Decsi T., Dobrzańska A., Franek E., et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in central Europe—recommended vitamin D intakes in the general population and groups at risk for vitamin D deficiency. Endokrynol. Pol. 2013;64:319–327. doi: 10.5603/EP.2013.0012. [DOI] [PubMed] [Google Scholar]
- 6.Rosen C.J., Adams J.S., Bikle D.D., Black D.M., Demay M.B., Manson J.E., Murad M.H., Kovacs C.S. The nonskeletal effects of vitamin D: An Endocrine Society scientific statement. Endocr. Rev. 2012;33:456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cianferotti L., Bertoldo F., Bischoff-Ferrari H.A., Bruyere O., Cooper C., Cutolo M., Kanis J.A., Kaufman J.M., Reginster J.Y., Rizzoli R., et al. Vitamin D supplementation in the prevention and management of major chronic diseases not related to mineral homeostasis in adults: Research for evidence and a scientific statement from the European society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO) Endocrine. 2017;56:245–261. doi: 10.1007/s12020-017-1290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaddam I.M., Al-Shaikh A.M., Abaalkhail B.A., Asseri K.S., Al-Saleh Y.M., Al-Qarni A.A., Al-Shuaibi A.M., Tamimi W.G., Mukhtar A.M. Prevalence of vitamin D deficiency and its associated factors in three regions of Saudi Arabia. Saudi Med. J. 2017;38:381–390. doi: 10.15537/smj.2017.4.18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AlHabeeb W., AlAyoubi F., Tash A., AlAhmari L., AlHabib K.F. Attitude of the Saudi community towards heart donation, transplantation, and artificial hearts. Saudi Med. J. 2017;38:742–747. doi: 10.15537/smj.2017.7.18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Przybyłowski P., Wasilewski G., Koc-Żórawska E., Małyszko J. Vitamin D Concentration in Patients after Heart and Kidney Transplantation. Transplant. Proc. 2018;50:2100–2104. doi: 10.1016/j.transproceed.2018.02.171. [DOI] [PubMed] [Google Scholar]
- 11.Löfdahl E., Söderlund S., Rådegran G. Bone mineral density and osteoporosis in heart transplanted patients: A single-center retrospective study at Skåne University Hospital in Lund 1988–2016. Clin. Transplant. 2019;33:13477. doi: 10.1111/ctr.13477. [DOI] [PubMed] [Google Scholar]
- 12.Anastasilakis A., Tsourdi E., Makras P., Polyzos S., Meier C., McCloskey E., Pepe J.Z. Bone disease following solid organ transplantation: A narrative review and recommendations for management from the European Calcified Tissue Society. Bone. 2019;127:401–418. doi: 10.1016/j.bone.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Wang T., O’Sullivan S., Gamble G., Ruygrok P. Bone density in heart or lung transplant recipients—A longitudinal study. Transplant. Proc. 2013;45:2357–2365. doi: 10.1016/j.transproceed.2012.09.117. [DOI] [PubMed] [Google Scholar]
- 14.Selimovic N., Nisar A., Alburaiki J., Khaliel F. Bone density in heart transplanted patients in Kingdom of Saudi Arabia. J. Saudi Heart Assoc. 2015;27:327–328. doi: 10.1016/j.jsha.2015.05.255. [DOI] [Google Scholar]
- 15.Kunutsor S.K., Whitehouse M.R., Blom A.W., Laukkanen J.A. Low serum magnesium levels are associated with increased risk of fractures: A long-term prospective cohort study. Eur. J. Epidemiol. 2017;32:593–603. doi: 10.1007/s10654-017-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel J. Vitamin therapy after heart transplantation. Expert. Rev. Cardiovasc. Ther. 2015;13:1071–1074. doi: 10.1586/14779072.2015.1086268. [DOI] [PubMed] [Google Scholar]
- 17.Zittermann A., Schleithoff S.S., Gotting C., Fuchs U., Kuhn J., Kleesiek K., Tenderich G., Koerfer R. Calcitriol deficiency and 1-yearmortality in cardiac transplant recipients. Transplantation. 2009;87:118–124. doi: 10.1097/TP.0b013e31818c2708. [DOI] [PubMed] [Google Scholar]
- 18.Zittermann A., Ernst J.B., Prokop S., Fuchs U., Dreier J., Kuhn J., Knabbe C., Birschmann I., Schulz U., Berthold H.K., et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): A 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur. Heart J. 2017;38:2279–2286. doi: 10.1093/eurheartj/ehx235. [DOI] [PubMed] [Google Scholar]
- 19.Vos R., Ruttens D., Verleden S.E., Vandermeulen E., Bellon H., Van Herck A., Sacreas A., Heigl T., Schaevers V., Van Raemdonck D.E., et al. High-dose vitamin D after lung transplantation: A randomized trial. J. Heart Lung Transplant. 2017;36:897–905. doi: 10.1016/j.healun.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Sempos C.T., Heijboer A.C., Bikle D.D., Bollerslev J., Bouillon R., Brannon P.M., DeLuca H.F., Jones G., Munns C.F., Bilezikian J.P., et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018;84:2194–2207. doi: 10.1111/bcp.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 22.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G., et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou N.K., Su I.C., Kuo H.L., Chen Y.H., Yang R.S., Wang S.S. Bone mineral density in long-term Chinese heart transplant recipients: A cross-sectional study. Transplant. Proc. 2006;38:2141–2144. doi: 10.1016/j.transproceed.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 24.Stein E.M., Cohen A., Freeby M., Rogers H., Kokolus S., Scott V., Mancini D., Restaino S., Brown R., McMahon D.J., et al. Severe vitamin D deficiency among heart and liver transplant recipients. Clin. Transplant. 2009;23:861–865. doi: 10.1111/j.1399-0012.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakusa M., Vrtovec B., Poglajen G., Janez A., Jensterle M. Endocrine disorders after heart transplantation: National cohort study. BMC Endocr. Disord. 2020;20:54. doi: 10.1186/s12902-020-0533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briffa N.K., Keogh A.M., Sambrook P.N., Eisman J.A. Reduction of immunosuppressant therapy requirement in heart transplantation by calcitriol. Transplantation. 2003;75:2133–2134. doi: 10.1097/01.TP.0000065179.06731.99. [DOI] [PubMed] [Google Scholar]
- 27.Schulze-Späte U., Mizani I., Salaverry K.R., Chang J., Wu C., Jones M., Kennel P.J., Brunjes D.L., Choo T.H., Kato T.S., et al. Periodontitis and bone metabolism in patients with advanced heart failure and after heart transplantation. ESC Heart Fail. 2017;4:169–177. doi: 10.1002/ehf2.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilfraguas L., Guadalix S., Martinez G., Jodar E., Vara J., Gomez-Sanchez M.A., Delgado J., De La Cruz J., Lora D., Hawkins F. Bone loss after heart transplant: Effect of alendronate, etidronate, calcitonin, and calcium plus vitamin D3. Prog. Transplant. 2012;22:237–243. doi: 10.7182/pit2012969. [DOI] [PubMed] [Google Scholar]
- 29.Kerschan-Schindl K., Ruzicka M., Mahr S., Paireder M., Krestan C., Gleiss A., Bieglmayer C., Fialka-Moser V., Pacher R., Grimm M., et al. Unexpected low incidence of vertebral fractures in heart transplant recipients: Analysis of bone turnover. Transpl. Int. 2008;21:255–262. doi: 10.1111/j.1432-2277.2007.00598.x. [DOI] [PubMed] [Google Scholar]
- 30.Shane E., Rivas M., McMahon D.J., Staron R.B., Silverberg S.J., Seibel M.J., Mancini D., Michler R.E., Aaronson K., Addesso V., et al. Bone loss and turnover after cardiac transplantation. J. Clin. Endocrinol. Metabol. 1997;82:1497. doi: 10.1210/jc.82.5.1497. [DOI] [PubMed] [Google Scholar]
- 31.Kerschan-Schindl K., Strametz-Juranek J., Heinze G., Grampp S., Bieglmayer C., Pacher R., Maurer G., Fialka-Moser V., Pietschmann P. Pathogenesis of bone loss in heart transplant candidates and recipients. J. Heart Lung Transplant. 2003;22:843. doi: 10.1016/S1053-2498(02)00806-9. [DOI] [PubMed] [Google Scholar]
- 32.Torregrosa J.V., Fuster D., Pedroso S., Diekmann F., Campistol J.M., Rubí S., Oppenheimer F. Weekly risedronate in kidney transplant patients with osteopenia. Transpl. Int. 2007;20:708. doi: 10.1111/j.1432-2277.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- 33.Vanderstraeten K., De Pauw R., Knops N., Bouts A., Cransberg K., El Amouri A., Raes A., Prytuła A. Body mass index is associated with hyperparathyroidism in pediatric kidney transplant recipients. Pediatric Nephrol. J. Int. Pediatric Nephrol. Assoc. 2021;36:977. doi: 10.1007/s00467-020-04796-w. [DOI] [PubMed] [Google Scholar]
- 34.Moreira C.A., Cochenski Borba V.Z., Kulak J., Jr., Custódio M.R. Osteoporosis after Transplantation. Curr. Osteoporos. Rep. 2012;10:48–55. doi: 10.1007/s11914-011-0083-y. [DOI] [PubMed] [Google Scholar]
- 35.Evenepoel P., Claes K., Kuypers D., Maes B., Bammens B., Vanrenterghem Y. Natural history of parathyroid function and calcium metabolism after kidney transplantation: A single-centre study. Nephrol. Dial. Transplant. 2004;19:1281–1287. doi: 10.1093/ndt/gfh128. [DOI] [PubMed] [Google Scholar]
- 36.Palermo A., Sanesi L., Colaianni G., Tabacco G., Naciu A.M., Cesareo R., Pedone C., Lelli D., Brunetti G., Mori G., et al. A Novel Interplay between Irisin and PTH: From Basic Studies to Clinical Evidence in Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2019;104:3088–3096. doi: 10.1210/jc.2018-02216. [DOI] [PubMed] [Google Scholar]
- 37.Zini M., Attanasio R., Cesareo R., Emmolo I., Frasoldati A., Gianotti L., Guglielmi R., Piovesan A., Procopio M., Scillitani A., et al. AME position statement: Primary hyperparathyroidism in clinical practice. J. Endocrinol. Investig. 2012;35((Suppl. S7)):2–21. [PubMed] [Google Scholar]
- 38.Walker M.D., Cong E., Lee J.A., Kepley A., Zhang C., McMahon D.J., Silverberg S.J. Vitamin D in Primary Hyperparathyroidism: Effects on Clinical, Biochemical, and Densitometric Presentation. J. Clin. Endocrinol. Metab. 2015;100:3443–3451. doi: 10.1210/jc.2015-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silverberg S.J., Clarke B.L., Peacock M., Bandeira F., Boutroy S., Cusano N.E., Dempster D., Lewiecki E.M., Liu J.M., Minisola S., et al. Current issues in the presentation of asymptomatic primary hyperparathyroidism: Proceedings of the Fourth International Workshop. J. Clin. Endocrinol. Metab. 2014;99:3580–3594. doi: 10.1210/jc.2014-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delos Santos R., Rossi A., Coyne D., Maw T.T. Management of Post-transplant Hyperparathyroidism and Bone Disease. Drugs. 2019;79:501–513. doi: 10.1007/s40265-019-01074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sushilkumar S., Guleria S., Crowe D.R., Steenwyk B., Singh S. Progressive Lung Calcification after Orthotopic Heart Transplant. Pediatric Radiol. 2015;45:124–128. doi: 10.1007/s00247-014-3023-z. [DOI] [PubMed] [Google Scholar]
- 42.Caffarelli C., Tomai Pitinca M.D., Alessandri M., Cameli P., Bargagli E., Bennett D., Fossi A., Bernazzali S., Gonnelli S. Timing of Osteoporotic Vertebral Fractures in Lung and Heart Transplantation: A Longitudinal Study. J. Clin. Med. 2020;9:2941. doi: 10.3390/jcm9092941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulak C.A.M., Borba V.Z.C., Junior J.K., Custodio M.R. Bone disease after transplantation: Osteoporosis and fractures risk. Arq. Bras. Endocrinol. Metabol. 2014;58:484–492. doi: 10.1590/0004-2730000003343. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook P., Henderson N.K., Keogh A., MacDonald P., Glanville A., Spratt P., Bergin P., Ebeling P., Eisman J. Effect of calcitriol on bone loss after cardiac or lung transplantation. J. Bone Miner Res. 2000;15:1818–1824. doi: 10.1359/jbmr.2000.15.9.1818. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Delgado I., Prieto S., Gil-Fraguas L., Robles E., Rufilanchas J.J., Hawkins F. Calcitonin, etidronate, and calcidiol treatment in bone loss after cardiac transplantation. Calcif. Tissue Int. 1997;60:155–159. doi: 10.1007/s002239900206. [DOI] [PubMed] [Google Scholar]
- 46.Chokshi A., Cheema F.H., Schaefle K.J., Jiang J., Collado E., Shahzad K., Khawaja T., Farr M., Takayama H., Naka Y., et al. Hepatic dysfunction and survival after orthotopic heart transplantation: Application of the MELD scoring system for outcome prediction. J. Heart Lung Transplant. 2012;31:591–600. doi: 10.1016/j.healun.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zittermann A., Ernst J.B., Prokop S., Fuchs U., Berthold H.K., Gouni-Berthold I., Gummert J.F., Pilz S. A 3 year post-intervention follow-up on mortality in advanced heart failure (EVITA vitamin D supplementation trial) ESC Heart Fail. 2020;7:3754–3761. doi: 10.1002/ehf2.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbarawi M., Kheiri B., Zayed Y., Barbarawi O., Dhillon H., Swaid B., Yelangi A., Sundus S., Bachuwa G., Alkotob M.L., et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83,000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol. 2019;4:765–776. doi: 10.1001/jamacardio.2019.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhandari R., Malvar J., Sacapano A., Aguayo-Hiraldo P., Jodele S., Orgel E. Association between Vitamin D and Risk for Early and Late Post-Transplant Complications. Biol. Blood Marrow Transplant. 2020;26:343–350. doi: 10.1016/j.bbmt.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Bienaimé F., Girard D., Anglicheau D., Canaud G., Claude Souberbielle J., Kreis H., Noël L., Friedlander G., Elie C., Legendre C., et al. Vitamin D Status and Outcomes After Renal Transplantation. JASN. 2013;24:831–841. doi: 10.1681/ASN.2012060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thorsen I.S., Bleskestad I.H., Åsberg A., Hartmann A., Skadberg Ø., Brede C., Ueland T., Pasch A., Reisaeter A.V., Gøransson L.G. Vitamin D as a risk factor for patient survival after kidney transplantation: A prospective observational cohort study. Clin. Transplant. 2019;33:e13517. doi: 10.1111/ctr.13517. [DOI] [PubMed] [Google Scholar]
- 52.Cubbon R.M., Lowry J.E., Drozd M., Hall M., Gierula J., Paton M.F., Byrom R., Kearney L.C., Barth J.H., Kearney M.T., et al. Vitamin D deficiency is an independent predictor of mortality in patients with chronic heart failure. Eur. J. Nutr. 2019;58:2535–2543. doi: 10.1007/s00394-018-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace G., Jodele S., Howell J., Myers K.C., Teusink A., Zhao X., Setchell K., Holtzapfel C., Lane A., Taggart C., et al. Vitamin D Deficiency and Survival in Children after Hematopoietic Stem Cell Transplant. Biol. Blood Marrow Transplant. 2015;21:1627–1631. doi: 10.1016/j.bbmt.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foroutan F., Doumouras B.S., Ross H., Alba A.C. Impact of pretransplant recipient body mass index on post heart transplant mortality: A systematic review and meta-analysis. Clin. Transplant. 2018;32:e13348. doi: 10.1111/ctr.13348. [DOI] [PubMed] [Google Scholar]
- 55.Doumouras B.S., Fan C.S., Dipchand A.I., Manlhiot C., Stehlik J., Ross H.J., Alba A.C. The effect of pre-heart transplant obesity on post-transplant mortality: Analysis of the ISHLT registry data. J. Heart Lung Transplant. 2017;36:S190. doi: 10.1016/j.healun.2017.01.499. [DOI] [PubMed] [Google Scholar]
- 56.Nagendran J., Moore M., Norris C., Khani-Hanjani A., Graham M.M., Freed D.H. The varying effects of obesity and morbid obesity on outcomes following cardiac transplantation. Int. J. Obes. 2016;40:721–724. doi: 10.1038/ijo.2016.20. [DOI] [PubMed] [Google Scholar]
- 57.Paschou S.A., Kosmopoulos M., Nikas I.P., Spartalis M., Kassi E., Goulis D.G., Lambrinoudaki I., Siasos G. The Impact of Obesity on the Association between Vitamin D Deficiency and Cardiovascular Disease. Nutrients. 2019;11:2458. doi: 10.3390/nu11102458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.