Abstract
Metabolic syndrome and its associated disorders such as obesity, insulin resistance, atherosclerosis and type 2 diabetes mellitus are globally prevalent. Different molecules showing therapeutic potential are currently available for the management of metabolic syndrome, although their efficacy has often been compromised by their poor bioavailability and side effects. Studies have been carried out on medicinal plant extracts for the treatment and prevention of metabolic syndrome. In this regard, isolated pure compounds have shown promising efficacy for the management of metabolic syndrome, both in preclinical and clinical settings. Apigenin, a natural bioactive flavonoid widely present in medicinal plants, functional foods, vegetables and fruits, exerts protective effects in models of neurological disorders and cardiovascular diseases and most of these effects are attributed to its antioxidant action. Various preclinical and clinical studies carried out so far show a protective effect of apigenin against metabolic syndrome. Herein, we provide a comprehensive review on both in vitro and in vivo evidence related to the promising antioxidant role of apigenin in cardioprotection, neuroprotection and renoprotection, and to its beneficial action in metabolic-syndrome-dependent organ dysfunction. We also provide evidence on the potential of apigenin in the prevention and/or treatment of metabolic syndrome, analysing the potential and limitation of its therapeutic use.
Keywords: apigenin, antioxidants, flavonoids, intracellular signalling, metabolic syndrome
1. Introduction
Public-health-related chronic diseases, such as diabetes, cancer, depression, hypertension, stroke, Alzheimer’s disease and metabolic syndrome are prevalent worldwide. Both healthy diet and physical activities can combat or delay these diseases [1,2,3]. A major contribution to the healthy diet is provided by fruits and vegetables, which are rich in natural bioactive chemical compounds. Phytochemicals are a vital part of a healthy diet and have been shown to play a predominant role in combating these chronic diseases. There are numerous phytochemical compounds, such as polyphenols, alkaloids, tannins and glycosides, which have been described as having no toxicity but high therapeutic potential in several disease settings. Polyphenols are the largest contributing group within natural product compounds consisting of subgroups such as flavonoids, flavanones, isoflavonoids, flavones and flavanols [1,4,5,6,7].
Flavonoids are the secondary metabolites which have widespread metabolic functions in plants. They are widely distributed in fruits, flowers, seeds, vegetables and in both legumes and non-legume plants. More than 6000 flavonoid compounds have been isolated and investigated for different biological purposes and the number is constantly increasing. They are known to have diverse pharmacological activities, acting as anticancer, antioxidant and anti-inflammatory agents, and also inhibiting platelet aggregation and viral replication [6,7,8].
Moreover, several studies have indicated that the consumption of dietary flavonoids can be associated with a reduced risk of metabolic syndrome. Metabolic syndrome is considered to be a worldwide epidemic complex disorder that is defined by a cluster of interconnected factors that are able to significantly augment the risk of several cardiovascular diseases (CVD), including coronary heart disease (CHD), atherosclerosis and diabetes mellitus type 2 [9]. Thanks to their antioxidative and anti-inflammatory effects, flavonoids have been widely studied for the prevention and/or amelioration of metabolic syndrome and its related disorders. However, the beneficial effects of flavonoids need to be corroborated, also contextualizing their effects with the different individual risk factors associated with metabolic syndrome, as well as the different effects of flavonoids and their metabolites on the body [10].
Among the flavonoids, apigenin is one of the most investigated and isolated compounds in human health. It is abundant in plants such as parsley, onion, oranges, chamomile, celery, spices and honey and some plant-origin beverages such as wine, tea and beer. Diverse biological effects of apigenin have been reported in both in vitro and in vivo studies and several studies revealed that apigenin could display anti-inflammatory, antioxidant and anti-obesity actions, as well as antiproliferative and anticancer activities [11,12,13]. On the other hand, emerging evidence established a potential interaction between apigenin and human gut microbiota, which contains enzymes that could degrade apigenin; this is of particular interest in the context of metabolic disorders, since, as revealed by several studies conducted on humans and animal models, gut microbiota may exert a strong influence in the pathogenesis of metabolic syndrome. Accordingly, an imbalance between gut microbes and the host’s immune system could induce “metabolic endotoxemia”, leading to systemic inflammation and insulin resistance [14]. The intense investigation carried out over the last twenty years regarding the possibility of manipulating gut microbiota through dietary polyphenols for preventing metabolic disorders suggests that apigenin can also play a beneficial role. To date, although the potential activity of dietary apigenin for the modulation of the colonic microbial population composition or activity is still limited, some studies demonstrated that apigenin can affect both the growth and gene expression of selective gut bacteria strains, paving the way for further investigation [15,16].
Although extensive basic and clinical research is warranted to assess the relevance of apigenin in metabolic syndrome, an increasing number of studies provided evidence regarding the direct metabolic involvement of apigenin and its potential in ameliorating metabolic diseases, which is mainly attributed to its capacity to inhibit oxidative stress, regulate glucose and lipid metabolism and attenuate mitochondrial dysfunction. However, the metabolic implication of apigenin in pathophysiological conditions has not yet been fully elucidated. Therefore, the present review aims to summarize the findings, in particular obtained over the last five years, regarding the growing metabolic implications of apigenin and its beneficial effect against metabolic syndrome.
2. Chemistry and Biosynthesis of Apigenin
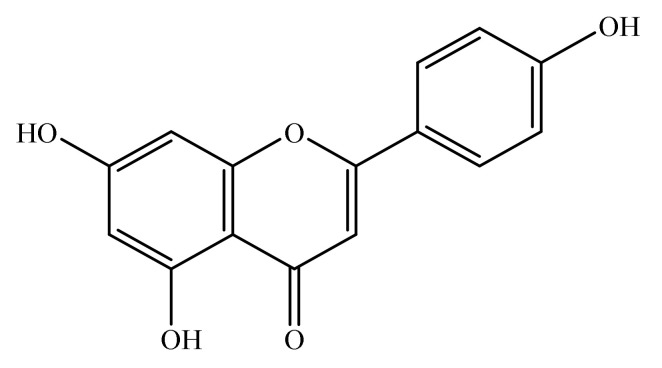
Apigenin is a natural bioactive flavonoid. Chemically, it is 4′,5,7-trihydroxyflavone (Figure 1) and is basically related to flavone, a subclass of flavonoids [17]. Apigenin is widely distributed in the plant kingdom, and is found in vegetables such as onions and parsley, fruits such as oranges and grape fruits and plant-derived beverages such as wine and tea. Apigenin is richly found in the plant species belonging to family Achillea, Sideritis, Fabaceae, Asteraceae, Teucrium, Genista, Artemisia, Matricaria and Lamiaceae [18,19,20,21,22].
Figure 1.
Chemical structure of apigenin (4′,5,7-trihydroxyflavone).
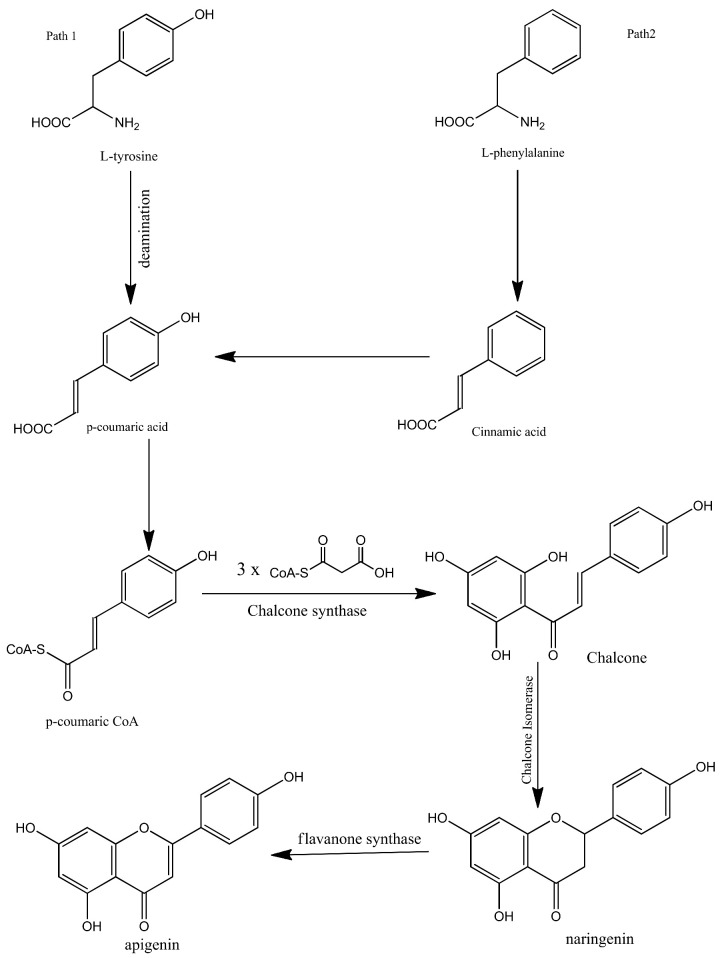
Apigenin is synthesized biogenetically by the phenylpropanoid pathway. It is synthesized from tyrosine and phenylalanine precursors. Tyrosine is directly deaminated to p-coumaric acid while phenylalanine is converted into cinnamic acid via non-oxidative deamination which is further oxidized at C-4 and then converted into p-coumaric acid. After the synthesis of p-coumaric acid by both pathways, the synthesized p-coumaric acid is further activated with Coenzyme-A. Three molecules of malonyl-CoA are then condensed with the p-coumarate. The compound is then aromatized to chalcone by chalcone synthase. Chalcone isomerase then isomerizes the chalcone and converts it into naringenin. In the final step, naringenin is oxidized into apigenin by flavanone synthase. The biosynthesis of apigenin is summarized in Figure 2 [1,23,24,25].
Figure 2.
Biosynthesis of apigenin.
3. Pharmacokinetics of Apigenin
Apigenin is characterized by poor systemic availability, due to its low lipid and water solubility; it is either excreted unabsorbed in the urine or faeces or rapidly metabolized after absorption [16].
3.1. Absorption
Absorption of apigenin takes place throughout the gastrointestinal (GI) tract from stomach (deglycosylated) to colon (non-deglycosylated). Apigenin is transported via active carrier transport and passive diffusion in the duodenum and jejunum, while in the ileum and colon it is transported only by passive diffusion [26,27]. It has been established by Chen et al. that systemically absorbed apigenin was in conjugated form [28]. Li et al. found that topically administered apigenin was absorbed into local skin tissues despite transdermal blood circulation [29].
3.2. Distribution
The presence of apigenin was not found in the kidneys and GI lumen after 12 h of administration of apigenin glycosides (flavonoid extract). In the liver it was detected in 1.5 and 12 h [27]. Orally administered single-dose radiolabelled apigenin was detected after 10 days with radioactivity of 0.4% in kidneys, 1.2% in liver, 9.4% in intestine, 12% in faeces and 51% in urine [30]. Apigenin was also detected in human red blood cells [31].
Zhang et al. [32] (2013) indicated that apigenin binds to human serum transferrin glycoprotein at the Fe3+-binding site. Because of the high plasma binding capacity, apigenin distributes to tissues well and has a large volume of distribution in vivo [33].
3.3. Metabolism
Due to the poor bioavailability of apigenin, complete metabolism of apigenin occurs. The metabolism of apigenin occurs through phase II conjugation (sulfation and glucuronidation) reaction. Phase II biotransformation of apigenin involves both enteric and enterohepatic cycling [34]. Gradollato et al. found, in the rat liver, that the metabolism of apigenin follows phase I process via cytochrome P450 and nicotinamide adenine dinucleotide phosphate (NADPH). This phase I was followed by phase II process (glucuronidation and sulfation). One metabolite from sulfation and 3 β-monoglucuronides were seen after glucuronidation reaction [30]. The metabolites of both reactions were detected in plasma. Some other studies reported that sulfation of apigenin was less as compared to glucuronidation in both in vitro and in vivo assays [35].
Apigenin is metabolized by conjugation reactions. Prior to absorption of apigenin into blood and liver, the extensive conjugation of apigenin takes place in the gastrointestinal tract [36]. Some studies have reported that apigenin is also metabolized by hydrolysis in the liver and intestine. Deglycosylation of apigenin glucosides occurs in the epithelial β-glucosidases. Liver microsomes also metabolize apigenin [37].
3.4. Excretion
Apigenin is mostly excreted in urine and faeces. In particular, after a single oral dose of apigenin, 51.0% and 12.0% of apigenin were detected in urine and faeces. Most of the administered apigenin is excreted in unabsorbed form [30].
3.5. Bioavailability
Apigenin has been categorized as a class II drug with high intestinal membrane permeability and poor solubility according to the Biopharmaceutics Classification System [38]. Apigenin is poorly soluble in non-polar solvents (0.001–1.63 mg/mL), while it is soluble as more than 100 mg/mL (freely) in dimethylsulfoxide. The solubility in the phosphate buffer was 2.16 μg/mL at pH 7.5. Innovative techniques are used to enhance the bioavailability of apigenin [26,39]. For instance, carbon nanopowders with apigenin system was used and it was found that dissolution of apigenin was improved about 275% as compared to pure apigenin in 60 min, resulting in an increase of apigenin bioavailability of 183% [40]. In vitro assays reported that apigenin nanocrystals have faster dissolution velocity than coarse powder. These are prepared by a supercritical antisolvent process [41].
4. Metabolic Syndrome: An Overview
Metabolic syndrome is a combination of interlinked different clinical, physiological, biochemical and metabolic disorders that enhances the chances of atherosclerosis, type 2 diabetes mellitus and cardiovascular disorders, eventually leading to death [11]. Different factors such as high blood pressure, atherogenic dyslipidaemia, visceral adiposity, insulin resistance, genetic susceptibility and endothelial dysfunctions are involved in the pathogenesis of metabolic syndrome. These factors are interconnected by various mechanisms and pathways [42,43]. Metabolic syndrome has adverse effects on different body systems as summarized in Table 1.
Table 1.
Adverse effects of metabolic syndrome on different body systems.
| Target Sites | Systemic Effects of Metabolic Syndrome | References |
|---|---|---|
| Skin | Psoriasis, systemic lupus erythematosus, alopecia, acne inversa, burn-induced insulin resistance, skin cancer | [44] |
| Eye | Open angle glaucoma, age-related cataract, oculomotor nerve palsy, central retinal artery occlusion, non-diabetic retinopathy | [42] |
| Liver | Hepatic fibrosis, non-alcoholic steatohepatitis, elevated serum transaminase, cirrhosis and non-alcoholic fatty liver | [45] |
| Kidney | Glomerulomegaly, microalbuminuria, focal segmental glomerulosclerosis, hyperfiltration, hypofiltration and chronic kidney disorder | [46] |
| Cardiovascular System | Myocardial infarction, stroke and coronary heart disease | [47] |
| Reproductive System | Erectile dysfunction, polycystic ovarian syndrome and hypogonadism | [48] |
According to the survey of International Diabetic Federation (IDF) and findings from the third National Health and Nutrition Examination Survey, 25% of the total adult population of the world succumbs from metabolic syndrome. According to the National Health and Nutrition Examination Survey, the prevalence of metabolic syndrome is found to be 60% in obese individuals, 22% in overweight and 5% among normal weight individuals [42,49,50,51]. However, it should be noted that the prevalence of metabolic syndrome varies across different racial/ethnic populations depending on the definitions used [9]. For instance, as systematically reviewed by Adjei et al., a higher prevalence of metabolic syndrome has been registered in Europe among migrants/ethnic minorities than host populations; in addition, a higher prevalence of metabolic syndrome has been reported in non-Hispanic Whites compared to Black populations. Furthermore, in the USA, some racial/ethnic differences have been found [52]. Therefore, a careful consideration of the influence of the race/ethnic group is needed in defining the prevalence and incidence of metabolic syndrome.
Metabolic syndrome is diagnosed by the definitions of World Health Organization (WHO), European Group for the Study of Insulin Resistance (EGIR), International Diabetes Federation (IDF) and American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) as shown in Table 2. In addition, criteria for the metabolic syndrome diagnosis as represented by the National Cholesterol Education Program’s Adult Treatment Panel III report (ATP III) were evaluated in comparison to IDF. The latter was found to be more accurate [53].
Table 2.
Definitions and criteria for metabolic syndromes according to World Health Organization (WHO), European Group for the Study of Insulin Resistance (EGIR), International Diabetes Federation (IDF) and American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI). According to IDF, 25% of the total adult population of the world succumbs from metabolic syndrome. According to the National Health and Nutrition Examination Survey, the prevalence of metabolic syndrome is found to be 60% in obese individuals, 22% in overweight and 5% among normal weight individuals. * According to Alberti et al. (2009) [54], it is recommended that the IDF cut-off points be used for non-Europeans and either the IDF or AHA/NHLBI cut-off points be used for people of European origin until more data are available. HDL: high density lipoprotein, Rx: pharmacologic treatment, TG: triglycerides, BMI: body mass index; waist circumference in EGIR is for European men and women.
| Criteria | WHO (1998) | IDF (2005) | EGIR (1999) | AHA/NHLBI (2009) |
|---|---|---|---|---|
| Diabetes or Insulin Resistance + Two of the Four Criteria Below | Obesity + Two of the Four Criteria Below | Hyperinsulinemia + Two of the Four Criteria Below | 3 of the Following 5 Risk Factors | |
| Hyperglycaemia | Insulin resistance already required | Fasting glucose ≥ 100 mg/dL | Insulin resistance already required | Fasting glucose ≥ 100 mg/dL |
| Hypertension | ≥140/90 mmHg | >130 mmHg systolic or >85 mmHg diastolic | ≥140/90 mmHg | ≥130 mmHg systolic or ≥85 mmHg diastolic |
| Obesity | Waist/hip ratio: >0.90 (M) > 0.85 (F) or BMI > 30 kg/m2 |
Central obesity already required | Waist circumference: ≥94 cm (M) ≥80 cm (F) |
* Waist circumference: ≥102 cm (M) ≥88 cm (F) TG ≥ 150 mg/dL |
| Dyslipidaemia | TG ≥ 150 mg/dL or HDL-C < 35 mg/dL (M) and <39 mg/dL (F) |
TG ≥ 150 mg/dL or Rx | TG ≥ 177 mg/dL or HDL-C < 39 mg/dL |
HDL-C < 40 mg/dL (M) and <50 mg/dL (F) |
In the context of the multiple derangements characterizing metabolic syndrome, insulin resistance, dyslipidaemia and hypertension represent important features that confer an increased risk for CVD and type 2 diabetes mellitus [55,56]. Individuals with insulin resistance show intolerance or impaired glucose metabolism characterized by excessive arterial blood pressure, increased fasting glucose level or a decrease in the actions of intravenous administered insulin, such as suppression of endogenous glucose production or insulin-mediated glucose clearance [57,58]. Dyslipidaemia is a spectrum of lipid abnormalities characterized by distortion in the structure, composition, biological activity and metabolism of atherogenic and anti-atherogenic lipoproteins and includes increased levels of triglycerides, elevated apolipoprotein B, decreased levels of high-density lipoprotein (HDL) cholesterol and elevated levels of small particles of low-density lipoproteins (LDL). Atherogenic dyslipidaemia is caused by insulin resistance via different mechanisms. Various metabolic abnormalities such as obesity, dyslipidaemia and glucose intolerance are commonly associated with essential hypertension [59]. Several studies have shown that the renin-angiotensin system is activated by hyperinsulinemia and hyperglycaemia via expressing angiotensin receptors, which lead to hypertension associated with insulin resistance [60].
It is noteworthy that human visceral obesity also represents a major risk factor for obesity-related diseases. Obesity is also responsible for the increased release of several biologically active substances, including adipokines, which act as classic hormones, thus exerting systemic metabolic effects; adipokines and cytokines may impair the insulin sensitivity of tissues and induce inflammation and chronic complications [61]. Therefore, a dysregulated signalling of adipokines may lead to metabolic disorders and chronic complications during obesity, representing a crucial mechanism by which dysfunctional adipose tissue promotes type 2 diabetes mellitus and CVD [61,62].
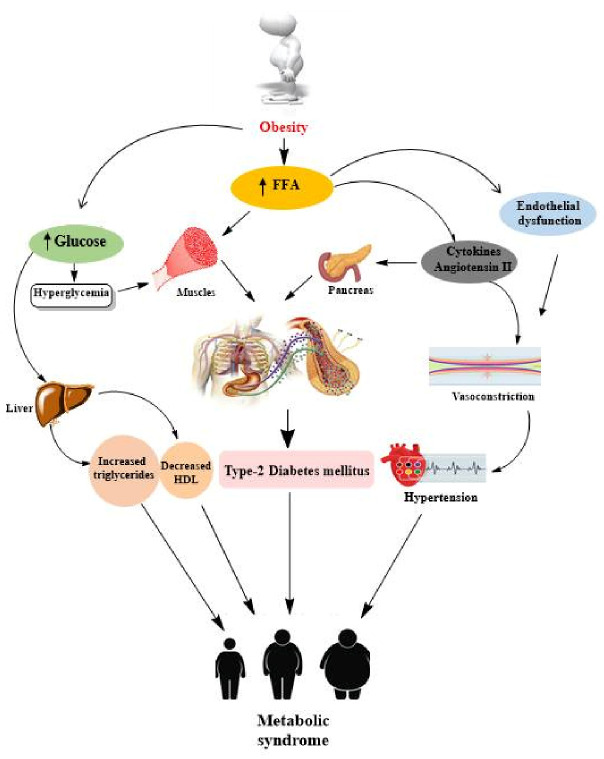
A schematic representation of the pathophysiology of metabolic syndrome is depicted in Figure 3.
Figure 3.
Pathophysiology of metabolic syndrome. Obesity, hypertension and insulin resistance are the major contributing factors in metabolic syndrome. FFA: free fatty acids; high-density lipoprotein (HDL) cholesterol.
5. Broad-Spectrum Antioxidant Activity of Apigenin with a Main Focus on Dysmetabolic Conditions
It is widely accepted that flavonoids possess free-radical scavenger activity acting as potent antioxidant agents due to their polyphenolic structure [63]. Accordingly, epidemiological studies indicate that a diet rich in flavonoids and flavones has potential anticancer effects, especially for the digestive tract, prostate, pancreas, breast, haematological and skin cancer, and is associated with reduced risk of other chronic diseases, including CVD and neurodegenerative disorders [64,65,66]. Although in flavanones and flavones the hydroxyl group at position 3 and the catechol structure in the B-ring are absent, in reference to apigenin, the double bond at 2,3 carbon makes its structure more reactive, resulting in an antioxidant compound [67]. Moreover, apigenin scavenges superoxide, singlet oxygen and hydroxyl radicals in vitro [68]. Several research studies have proven the potential capacity of apigenin to act as a health-promoting agent [1 and references therein]. This natural flavone has been shown to exert therapeutic effects in neurological disorders and CVD [17]. Other studies showed that apigenin has hepatoprotective, antidepressant, anticancer and antihypertensive effects; it is also able to prevent autoimmune myocarditis, atherogenesis and asthma and induces beneficial action against osteoporosis, type 2 diabetes mellitus and pancreatitis [69,70,71,72,73,74,75,76,77,78,79,80]. Importantly, in addition to the involvement of apigenin in inflammation modulation, mRNA splicing regulation, cell cycle arrest and regulation of apoptosis [81], most of these findings support the hypothesis that the beneficial effects of apigenin are also attributed to its capacity to counteract oxidative stress, which is known to be a key component of many diseases, including cardiovascular, pulmonary, neurodegenerative diseases, cancer and metabolic disorders [82]. In particular, many studies strongly support the concept of the crucial impact of oxidative stress in the complex pathogenesis of metabolic syndrome and its major clinical manifestations (atherosclerosis, hypertension and diabetes), responsible for mitochondrial dysfunction, ectopic lipid accumulation and alteration of gut microbiota [83]. Therefore, a therapeutic approach targeting oxidative stress may ameliorate the risk factors associated with metabolic syndrome, delaying or preventing the progression of metabolic disease complications.
In the following sections, we provide information about the in vitro and in vivo antioxidant potential of apigenin in protecting different organs (i.e., heart, brain and kidney) against several toxic stimuli in order to introduce its beneficial action against some alterations in the above organs, which often occur during metabolic syndrome, before addressing the direct involvement of apigenin in dysmetabolism and metabolic syndrome.
5.1. Cardioprotective Potential of Apigenin through Antioxidant Action
Over the last five years, a large amount of in vitro and in vivo evidence indicates that apigenin has a broad-spectrum efficacy against oxidative-stress-dependent tissue/organ (including the heart) damage, since it has been shown to exert both direct antioxidant effect, by counteracting ROS formation, and indirect action by regulating the endogenous antioxidant defence system. It is known that oxidative stress is dramatically implicated in the onset and in the progression of CVD, such as cardiac arrhythmia, atherosclerosis, myocardial ischaemia/reperfusion (I/R) injury and heart failure (HF) [84,85]. Therefore, apigenin was extensively studied for its potential in attenuating cardiac injury from different types of insult by preserving heart function and regulating the oxidative cell balance. For instance, apigenin induces cardioprotection against myocardial hypertrophy by employing isoproterenol (ISO), widely used to induce cardiac hypertrophy and dysfunction in vitro and in vivo [86,87]. Buwa et al. (2016) demonstrated that apigenin can mitigate the hemodynamic, biochemical and histopathological changes in an ISO-induced rat model of myocardial infarction mainly by regulating the antioxidant defence system and improving the action of proliferator-activated receptor gamma (PPARγ) receptor signalling, which regulates the inflammatory response and may represent a potential therapeutic target to prevent cardiac hypertrophy and HF during metabolic disorders [88]. On the other hand, Thangaiyan and colleagues (2018) showed the beneficial action of apigenin on the single cardiomyocyte component since it prevented ISO-induced lipid peroxidative levels, DNA damage and antioxidant depletion in H9c2 cardiomyocytes [89]. These finding were corroborated by very recent data that evaluated the involvement of apigenin in the onset and progression of hypertension and hypertension-induced cardiac hypertrophy. In spontaneously hypertensive rats (SHRs), Gao et al. (2021) have found that bilateral hypothalamic paraventricular nucleus (PVN) chronic infusion of apigenin attenuated the increase of mean arterial pressure, cardiac hypertrophy and fibrosis, positively affecting PVN oxidative stress due to its ability to modulate the NADPH oxidase-dependent ROS generation and to restore the activity of superoxide dismutase 1 (Cu/Zn-SOD) and the 67-kDa isoform of glutamate decarboxylase (GAD67) [90].
It is widely known that anthracyclines (ANTs), including doxorubicin (DOX), are among the most prescribed chemotherapeutic drugs because of their strong efficacy in both solid and haematological tumours; however, their clinical use is hampered by dose-dependent and cumulative cardiotoxicity [91]. In the context of the complexity of ANTs-induced multifactorial cardiotoxicity, redox cycling and ROS generation play an undoubted role, although they do not represent the single and/or the principal event [92]. Interestingly, in addition to the recognized ability of apigenin in increasing DOX efficiency in different cancer cell lines, promising experimental data have recently been obtained on its cardioprotective role following DOX treatment that is mediated, at least in part, through reduction of oxidative stress. This is the case of the study carried out by Zare et al. (2019) [93] and Sahu et al. (2019) [94] and as reviewed by Navarro-Hortal et al. (2020) [95].
Apigenin has also been shown to precondition the heart against susceptibility to infarction; in particular, this flavone reversed vascular endothelial dysfunction in a cell culture model of early atherosclerosis, suppressing arterial ROS production and oxidative stress and protecting the heart against I/R damage [96]. In this regard, nutritional preconditioning with apigenin improved cardiac function in rats exposed to I/R by decreasing lipid peroxidation and enhancing SOD, glutathione peroxidase (GPX) and catalase (CAT) myocardial activities in a mechanism that targets a mitochondrial pathway mediated by the Notch1/Hes1 signalling [97]. Besides, apigenin reduced the oxidative stress and ROS production in I/R-induced H9c2 cells by activating the PI3K/Akt pathway [98].
Role of Apigenin in Dysmetabolism-Dependent Heart Dysfunction
The endothelial protective effect of apigenin was further evaluated in selective in vitro and in vivo models of endothelial dysfunction (ED), raising the hypothesis that this flavone could also be beneficial during dysmetabolic conditions. Indeed, ED is a key event in the progression of atherosclerosis, representing a hallmark of diabetes mellitus and obesity, which contributes to CVD [99]. ED in CVD is the major cause of the initial lesion in the vessel, representing an early damage marker for the development of vascular complications in diabetes; thus, the preservation of endothelial integrity is crucial for preventing diabetic complications. Regarding this aspect, interesting data have been obtained by Qin et al. (2016), who provided direct evidence that apigenin protected endothelial cells against high glucose-induced ED; mechanistically, apigenin improved the endothelium activity via inhibiting phosphorylation of protein kinase C βII (PKCβII) expression and downstream ROS production, and restoring the NO depletion via increasing eNOS activity [100]. Xu and collaborators (2021) provided evidence regarding the capacity of intragastric administration of apigenin to significantly ameliorate lipid profile by reducing total cholesterol, triglyceride and LDL cholesterol, and prevent atherosclerosis in hyperlipidaemic rats. Interestingly, apigenin also suppressed LDL oxidation and regulated the expression levels of lectin-like oxidized low-density lipoprotein (LDL) receptor-1 (LOX-1), Bcl-2 and Bax in the aorta of hyperlipidaemic rats, suggesting a promising anti-atherosclerotic role of apigenin in vivo [101].
Other studies moved towards a possible clinical application of apigenin for the treatment of myocardial complications during diabetes mellitus by employing in vivo models. Streptozotocin-treated rats exposed to myocardial infarction chronically treated with apigenin exhibited an improved haemodynamic function and a recovered redox status; by a pharmacological approach, the authors also found that apigenin acts through PPARγ stimulation [102]. As above reported, PPARγ may be considered a novel therapeutic target to prevent the cardiac complications and HF in metabolic disorders, since PPARγ agonists exhibited beneficial action against CVD [88]. Therefore, the ability of apigenin to significantly modulate PPARγ activity should be regarded with interest. Accordingly, other flavonoids modulate PPARγ signalling, thereby regulating the glucose/lipid metabolism [103] and corroborating the metabolic involvement of apigenin during CVD. It has been recently reported that hypoxia inducible factor (HIF)-lα represents a key regulator of glucolipid metabolism and is involved in the conversion of hypoxic myocardial energy utilization [104]. Notably, HIF-lα, strongly expressed in hypertrophied hearts, can be inhibited by apigenin; this finding, together with the ability of apigenin to selectively modulate PPARγ, corroborates the important role of apigenin in affecting blood pressure and glucolipid metabolism. In this regard, a recent study [105] examined the chronic effects of apigenin in a rat model of cardiac hypertrophy induced by renovascular hypertension, in which selective metabolic changes in myocardial energy utilization, based on the switch from fatty acids to glucose, occur [106]. The authors highlighted the beneficial contribution of apigenin in improving hypertensive cardiac hypertrophy and abnormal myocardial glucolipid metabolism by reducing myocardial HIF-1α expression and upregulating the expressions of myocardial PPARγ and its target genes, glycerol-3-phosphate acyltransferase genes (GPAT) and glucose transporter (GLUT-4) [105].
Other evidence emerges from the study of Liu et al. (2017), who demonstrated that apigenin administration improved the cardiac functions and mitigated the cardiac hypertrophy and interstitial fibrosis in a mouse model of diabetic cardiomyopathy by attenuating myocardial oxidative stress (i.e., 4-hydroxynonenal and malondialdehyde) and improving the activity of SOD and GPX [107]. The beneficial effects of apigenin on glucose and lipid metabolism are quite established, since apigenin improves glucose intolerance in miRNA103-overexpressing transgenic mice [108] and increases NAD+ levels through inhibition of cluster of differentiation 38 (CD38) in obese mice, ameliorating glucose and lipid homeostasis [109]. It is noteworthy that it has been shown that targeting NAD+ pathway may represent a strategy to ameliorate metabolic dysfunction since an increase in tissue intracellular NAD+ levels can protect against obesity, metabolic syndrome and type 2 diabetes [110,111]. However, further studies are necessary to better decipher and corroborate the cardioprotective role of apigenin by affecting metabolism during diabetic cardiomyopathy.
5.2. Neuroprotective Role of Apigenin through Antioxidant Action
In very recent years, apigenin showed great potential in counteracting neurodegenerative diseases and other brain disorders, offering neuroprotection through multiple mechanisms, including antioxidant action [112]. Focusing on the involvement of apigenin in redox regulation, the study by Shao and collaborators (2020) identified, by screening analyses, apigenin as a possible candidate to act as an antiepileptic agent [113]. By developing a two-photon fluorescence probe for the real-time tracking of endogenous hypochlorite fluxes in vivo and ex vivo in the brain of kainic-acid-induced epileptic mice, the authors demonstrated that apigenin was effective in reducing the expression of intracellular myeloperoxidase, a crucial source of free radicals generating oxidative stress in epileptic brains, and in regulating the activity of glutathione peroxidase 4 (GPX-4), thioredoxin reductase (TXNRD), glutathione (GSH) and SIRT1, thereby conferring neuroprotection through regulation of kainic-acid-induced ferroptosis and oxidative stress [113].
Diverse studies have indicated that ROS contributes to the disruption of the blood–brain barrier by disturbing tight junction proteins [114]; moreover, oxidative stress alters the amyloid β-peptide kinetics and is a major factor responsible for its toxicity [115]. In view of the powerful antioxidant effect of apigenin, in the last few years, several studies have shown its promising effect during neurodegeneration. Extensive literature data on both experimental and clinical models have been elegantly reviewed by Nabavi et al. (2018) concerning these aspects, pointing out apigenin as a promising bioactive compound for which future clinical trials should be encouraged to target neurodegenerative disorders [112].
Very recent studies [116,117,118] also explored the antioxidant protective role of apigenin in experimental models of ischemic stroke and cerebral I/R damage and subarachnoid haemorrhage. In both in vivo (murine models of middle cerebral artery occlusion) and in vitro (an oxygen-glucose deprivation/reoxygenation model of human brain microvascular endothelial cells and PC12 cells exposed to cobalt chloride) models, apigenin attenuated brain damage and neurological deficiency and improved neurological function, supporting previous evidence and indicating its capacity to ameliorate in vivo post-stroke cognitive impairments through regulating histone deacetylase and brain-derived neurotrophic factor (BDNF) [119].
Apigenin in Dysmetabolism-Dependent Brain Alteration
Starting from the assumption that apigenin is able to bind and interact with dipeptidyl peptidase-IV (DPP-IV), inhibiting its activity in the hippocampus of high fat, high fructose diet (HFFD)-fed insulin-resistant rats [120], Kalivarathan et al. (2017) demonstrated that apigenin exerted antioxidant action, improving antioxidant enzymes (i.e., SOD, CAT and GPX) and reducing the oxidative stress-related kinases (i.e., kappa B kinase beta (IKKβ) and c-Jun NH2 terminal kinase (JNK), and the nuclear translocation and activation of nuclear factor kappa B (NF-κB)) activation in the hippocampus of HFFD-fed rats [120]. These finding are in accordance with another recent report that extended the knowledge of the molecular mechanism by which apigenin protects the brain during dysmetabolism; the authors found that similarly to sitagliptin (STG), a selective DPP-IV inhibitor, apigenin can improve cognitive function by activating cAMP response element-binding protein (CREB)-BDNF axis and by improving brain insulin signalling in HFFD-fed rats [120]. Notably, STG is approved for the treatment of type 2 diabetes mellitus due to its effect in improving glucose and lipid metabolism and insulin sensitivity; STG is also known to exert neuroprotective action in insulin-resistant rats due to its ability to ameliorate recognition memory, oxidative stress and hippocampal neurogenesis [121]. Considering the ability of apigenin to act as a DPP-IV inhibitor with an efficiency comparable to STG, it is conceivable to hypothesize that apigenin could be useful in counteracting neurodegenerative events associated with insulin resistance and metabolic syndrome. Of course, additional studies need to be performed in order to establish the involvement of apigenin in dysmetabolic-dependent neurological impairment.
5.3. Renoprotection of Apigenin against Oxidative Stress-Induced Injury
Thanks to its antioxidant action, apigenin can inhibit oxidative stress to attenuate the nephrotoxicity secondary to different toxic stimuli, including pharmacological agents such as cisplatin and DOX. Cisplatin is a highly effective antineoplastic drug that in combination chemotherapy regimens is currently used as front-line therapy in the treatment of several cancers; however, nephrotoxicity is a serious and dose-limiting toxicity of cisplatin [122]. Promising preventive effects of apigenin against cisplatin-induced nephrotoxicity have been obtained in experimental studies by different researches; in particular, Hassan et al. (2017) and Wu et al. (2021) showed that chronic administration of apigenin relieved the oxidative injury dependent on cisplatin due to its antioxidant effect and its ability to enhance SOD and GPX activities [123,124]. On the other hand, He et al. (2016) confirmed the protective effect of apigenin in a mouse model of DOX-induced nephrotoxicity [125]. Further studies are however necessary to evaluate whether apigenin could hamper the anticancer efficacy of these chemotherapeutics in vivo. The antioxidant capacity of apigenin in other kidney-related pathological contexts was confirmed by various recent studies. In this regard, Liu et al. (2017) showed that a 24 h pre-treatment with apigenin protected against 45 min of renal ischaemia, followed by 24 h of reperfusion in a rat model [126], while Wang and colleagues [127] have found that apigenin improved SOD, GSH and CAT enzyme levels to mitigate renal pathological changes in mice exposed to mesoporous silica nanoparticles, a promising drug delivery system whose toxicity has been documented [128]. Similar findings have been obtained by Ali et al. (2021), who showed the beneficial action of apigenin against kidney damage induced by nickel oxide nanoparticles (NiONPs) exposure in rats. NiONPs have important medical applications; unfortunately, they showed a significant toxic profile mainly due to pro-oxidative action [129]. Moreover, Zamani and collaborators indicated, in rats, that apigenin was effective in exerting renal mitochondrial protection and antioxidant action following the exposure of carbon nanotubes, which induce mitochondrial dysfunction and oxidative stress and accumulate in several organs and tissues, including kidneys, with consequent organ damage [130].
Apigenin in Renal Complication following Dysmetabolism
As above mentioned, and as will be more detailed in the next part of the present review, the antidiabetic activity of apigenin is extensively studied because of its capacity to regulate glucose metabolism, inhibit α-glucosidase activity and increase insulin secretion [1,131]. Conversely, the effectiveness of apigenin in ameliorating the renal complication following dysmetabolism was less addressed. One of the first studies that evaluated the action of apigenin in diabetic nephropathy was carried out by Malik and colleagues (2017) [132]. It is well known that diabetic nephropathy is one of the major microvascular complications of diabetes mellitus, where oxidative stress plays a predominant role in its progression [133]. In a rat model of streptozotocin-induced diabetic nephropathy, the authors indicate that apigenin significantly attenuated renal impairment by suppressing oxidative stress (as evinced by reduced MDA and increased SOD, CAT and GSH) and inhibiting MAPK pathway. The use of streptozotocin is widely reported for studying antioxidant potential of pharmacological and bioactive substances in hyperglycaemic contexts, since this drug selectively induces an excess of ROS formation via exertion of heavy electron pressure on the mitochondrial electron transport chain from oxidation of overproduced NADH [134]. Notably, the authors also found that the effects of apigenin were similar to those of ramipril, which induces renoprotection by blocking angiotensin type 1 receptor (AT1R)-mediated oxidative stress, indicating that apigenin is strictly involved in reducing free radicals’ formation and oxidative stress during diabetic nephropathy. Additional mechanistic knowledge regarding the renoprotection of apigenin in diabetic conditions was provided by a very recent study [135]. Here, the authors demonstrated that apigenin administration in Zucker diabetic fatty (fa/fa) rats effectively downregulated CD38 expression and mitochondrial oxidative stress, thus reducing tubulointerstitial fibrosis and tubular cell damage; this flavone also increased the kidney intracellular NAD+/NADH ratio and Sirt3-mediated mitochondrial antioxidant enzymes. Based on these findings, it is conceivable that apigenin plays a crucial role in the pathogenesis of diabetic kidney disease and that additional studies are warranted to elucidate its mechanism of action.
Table 3 recapitulates the main cardioprotective, neuroprotective and renoprotective effects of apigenin following different toxic stimuli and during dysmetabolic-induced organ dysfunction.
Table 3.
Cardioprotective, neuroprotective and renoprotective effects of apigenin following different toxic stimuli and during dysmetabolic-induced organ dysfunction.
| Organ | Effects of Apigenin through Antioxidant Action | Effects of Apigenin during Dysmetabolic-Dependent Organ Alteration |
|---|---|---|
|
||
|
||
| HEART | ||
|
||
|
||
|
||
| BRAIN |
|
|
| KIDNEY |
|
ISO = isoproterenol, DOX = doxorubicin, LDL = low density lipoproteins, I/R = ischaemia/reperfusion, NiONPs = nickel oxide nanoparticles.
6. Effects of Apigenin in Metabolic Syndrome
As above mentioned, metabolic syndrome is a progression of health-related abnormalities which is characterized by dyslipidaemia, hypertension, insulin resistance and fatty liver. Metabolic syndrome worsens the pathological conditions of CVD and diabetes. [136,137,138,139,140]. An increasing number of studies confirm that oxidative stress is one of the leading causes of metabolic syndrome and ensuing pathological conditions such as diabetes and hypertension [138,141]. Therefore, treating or preventing oxidative stress can represent a promising strategy to attenuate insulin resistance and metabolic syndrome and the associated CVD and type 2 diabetes mellitus [1,142,143,144].
Several studies report that apigenin can be effective in the management of metabolic syndrome due to its ability to improve glucose and lipid metabolism during dysmetabolic conditions [1,131,145]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is considered to be a key endogenous antioxidant transcription factor. Antioxidant proteins are expressed by the interaction of Nrf2 and antioxidant response element (ARE) in DNA [146,147]. Under normal conditions, Nrf2 forms a complex with the cytoplasmic Kelch-like ECH-associated protein 1 (Keap1) which results in proteolysis of Nrf2. When the Nrf2-Keap1 complex is exposed to an oxidant or electrophile, Nrf2 translocates into the nucleus and forms a complex with ARE. The Nrf2-ARE complex starts the transcription of numerous antioxidant genes. Dissociation of the Nrf2-Keap1 complex results in the activation of Nrf2, which further facilitates the nuclear transcription of antioxidant genes. Molecular docking coupled with expression studies have shown that apigenin interferes with the binding of Nrf2 and Kaep1 and thus dissociates Nrf2, increasing the latter’s nuclear translocation and antioxidant activity [138,148,149].
Emerging reports indicate that mammalian cells have key NAD+ ase in the form of CD38 and that the manipulation of NAD+ metabolism may represent a plausible strategy to ameliorate obesity, metabolic syndrome and type 2 diabetes. It has been reported that overexpression of CD38 leads to acetylation of proteins which results in the protein degeneration in obesity. Studies have shown that CD38 is abundantly found in mitochondria where the sirtuin-1 (SIRT1) activity and NAD+ ase level are maintained. In this regard, Escande et al. carried out in vivo analysis and confirmed the mechanistic pathways of apigenin as a remedy for the management of metabolic syndrome [109]. The inhibition of CD38 by apigenin can enhance the homeostasis of glucose and thus improve liver oxidation of fatty acids, with the observation that the apigenin-treated mice for 4 days had markedly decreased levels of blood glucose relative to the control mice [109]. The authors showed that CD38 is a crucial target site for controlling metabolic syndrome and found that apigenin acts as a CD38 inhibitor. Apigenin was administered to obese mice, raising the NAD+ levels in the liver by inhibiting CD38 and reducing protein acetylation. Lipid metabolism, glucose homeostasis and glucose tolerance were improved in obese mice by inhibiting CD38 [109,110,150,151]. Thus, apigenin appears to be a CD38 inhibitor that may be used as a promising strategy for the treatment of metabolic syndrome via activation of NAD+ and SIRT1.
Besides, Jung et al. evaluated the long-term supplementary effects of low-dose apigenin on obesity, establishing that this flavone is effective in treating metabolic syndrome in obese mice (induced by high fat diet) by ameliorating the insulin resistance, dyslipidaemia and hepatic steatosis [140]. They reported that apigenin significantly normalized the fasting blood glucose level by normalizing plasma insulin levels and the β-cell function. Apigenin also attenuated inflammation by significantly lowering the plasma concentrations of pro-inflammatory mediators like IFN-γ, IL-6, TNF-α and MCP-1. They also found that in apigenin-treated obese mice, the concentrations of plasma-free fatty acid were significantly reduced. Apigenin also markedly reduced the total plasma cholesterol levels, plasma apoB/A1 ratios and plasma apoB. Importantly, apigenin significantly decreased the activity of glucose-6 phosphatase and phosphoenolpyruvate carboxykinase (PEPCK), two key regulatory enzymes of gluconeogenesis [140,152]. It has been reported that an impairment of gluconeogenesis promotes hepatic glucose production, aggravating insulin resistance and further contributing to the pathogenesis of hyperglycaemia and glucose intolerance [152,153]. These data reinforce the hypothesis according to which apigenin improves insulin sensitivity and glucose tolerance, modulating hepatic gluconeogenesis. These findings are supported by the study of Cazarolli and collaborators who tested the effect of apigenin-6-C-(2″-O-α-L-rhamnopyranosyl)- β-L-fucopyranoside, obtained from Averrhoa carambola L. leaves, on 14C-glucose uptake. The authors showed that this compound was effective in lowering blood glucose and stimulated glucose-induced insulin secretion after oral treatment in hyperglycaemic rats acting as an insulin-mimetic agent [154].
Another important finding deriving from this and other studies regards the pancreatic beneficial action of apigenin during dysmetabolism. Pancreatic β-cells are very sensitive to oxidative stress, which is one of the major causes of cell damages in diabetes. Accordingly, in vivo and in vitro findings showed that pre-treatment with apigenin protected RINm5F pancreatic beta cells against streptozotocin-induced oxidative damages and decreased the intracellular ROS production, oxidative stress (i.e., lipid peroxidation and protein carbonylation) and cellular DNA damage and mitigated apoptosis of β-cells induced by streptozotocin [155].
6.1. Peroxisome Proliferator-Activated Receptor γ (PPARγ) as a Main Metabolic Target of Apigenin
Feng et al. performed two separate studies involving both in vivo and in vitro examinations which provided molecular insights into the apigenin-induced metabolic effect via targeting peroxisomes PPARγ [156,157]. In the first study, the authors showed that apigenin mitigated HFD-induced non-alcoholic fatty liver disease (NAFLD) progression, attenuating lipid accumulation and oxidative stress in mice; these effects were mainly attributed to the ability of apigenin to activate Nrf2 by promoting its translocation into the nucleus that, in turn, inhibited the function of PPARγ [156]. These findings are of significant interest since PPARγ, as a ligand-dependent transcription factor, is crucially involved in lipid metabolism and oxidative stress. Indeed, its results increased in fatty liver disease associated with obesity in both animal and human models and the inhibition/knockdown of PPARγ ameliorates fatty liver in obese and NAFLD conditions [158,159]. In the other study, the authors observed that apigenin ameliorated the insulin resistance, metabolic abnormalities and obesity-related inflammation by also acting on PPARγ [157]. It has been reported that PPARγ has a key role in the low-grade inflammation and adipogenesis in metabolic syndrome. PPARγ is a member of ligand inducible transcription factors (PPAR) and regulates the functional polarization of macrophages acting as anti-inflammatory regulators in atherosclerosis [160,161]. Macrophages can be found in a polarized inflammatory state (macrophages 1; M1) which generates inflammatory cytokines, such as interleukin-6 (IL-6), tumour necrosis factor (TNF) and IL-1R and effector molecules such as nitric oxide and ROS. Macrophages may also be found (macrophages 2; M2) in a polarized anti-inflammatory state (IL-10, IL-12 and IL-23). M1 macrophages have inherent activation of genes such as C-C chemokine receptor type 7 (CCR7) and CD38, while in M2, genes such as mannose receptors type C, arginase, chitinase-like Ym-1 and certain scavengers are activated [162,163]. Feng et al. established a relationship between macrophage polarization and apigenin and found that apigenin binds to PPARγ and alters the equilibrium between M1 and M2 polarization, thus inducing its biological actions in metabolic syndrome. In their in vitro cell model, they found that apigenin decreased the levels of CD80, CCR7, IL-6, TNF and nitric oxide in M1 cell model, while in M2 cell model, apigenin increased the levels of mRNA gene expression, IL-10 and arginase1 activity on the surface of macrophages. This in vitro analysis confirmed that apigenin favours the upregulation of M2 markers and downregulation of M1 markers. Thus, apigenin could inhibit the inflammatory macrophages (enhance polarization of M2), attenuating obesity-related inflammation [157]. Similarly, another in vitro study confirmed that apigenin binds to PPARγ, acting as allosteric effector. Salam et al. also confirmed that apigenin is a PPARγ agonist [164,165].
It should be noted that during adipocytes differentiation, PPARγ, in addition to CCAAT/enhancer binding protein-α (C/EBPα), represents a key transcription factor; PPARγ and C/EBPα act in concert to activate the gene expression of fatty-acid-binding protein and PEPCK and to promote lipid synthesis [166]. In this regard, the 3T3-L1 preadipocytes cell line is widely employed to reproduce an adipocyte differentiation model system for studying the metabolic mechanisms of obesity and the molecular mechanisms of adipogenesis since these cells differentiate into mature adipocytes in vitro [167]. It is noteworthy that in recent years, apigenin was also found to inhibit the differentiation of 3T3-L1 preadipocytes; in particular, apigenin exhibited marked synergistic effect together with emodin in both differentiation and pancreas lipase assays [168]. According to these reports, apigenin alleviated the glucose metabolic disorders in HFD mice [140] and abrogated weight gain potentiation after HFD re-exposure by improving gut microbes; interestingly, apigenin also increased energy expenditure with an increase in Ucp1 expression, suggestive of adipose tissue browning and increased non-shivering thermogenesis [169]. These data further corroborate the role of apigenin in obesity and support the conclusions of Escande et al. (2013), according to which apigenin regulates NAD+ levels and global protein acetylation through sirtuin-dependent metabolic disorders [109].
6.2. Additional Insight in the Mechanism Underlying Apigenin-Induced Metabolic Effects
Another research paper published in 2021 analysed the function and mechanism of apigenin during insulin resistance by reproducing in vitro and in vivo models (intracellular fat accumulation model cells using palmitate and HFD–fed mice); the authors observed that apigenin downregulated sterol regulatory element-binding protein 1c (SREBP-1c), sterol regulatory element-binding protein 2 (SREBP-2), fatty acid synthase, stearyl-CoA desaturase 1 and 3-hydroxy-3-methyl-glutaryl-CoA reductase and endoplasmic reticulum stress in vitro and in vivo; these effects were functionally associated with an improvement of lipid profile and insulin resistance [170]. The authors highlight a novel mechanism by which apigenin can mitigate hyperlipidaemia-related states.
Excessive accumulation of visceral fat inside the abdominal cavity (surrounding vital organs) is referred to as visceral obesity. Most evidence indicates that visceral obesity is directly related to metabolic syndrome. High mortality rate and greater risk of metabolic syndrome (insulin resistance, glucose intolerance, dyslipidaemia and hypertension) are associated with excessive visceral fat accumulation [171,172]. It has been observed that in the pathogenesis of visceral obesity, signal transducer and activator of transcription 3 (STAT3) is a key factor. STAT3 is constitutively active in obese individuals and stimulates the visceral adipose tissues [173]. Moreover, growing evidence indicates that increased levels of cluster determinant 36 (CD36) are directly linked to obesity. Deficiency of CD36 reduces the visceral fat accumulation and thus decreases the progression of obesity [174]. Interestingly, Tao et al. provide additional knowledge about the mechanism of action underlying the antivisceral obesity effect of apigenin; here, the authors found that apigenin can counteract metabolic syndrome by decreasing the visceral obesity via inhibiting CD36 and STAT3 signals and consequently reducing PPARγ expression in adipocytes [175]. These data indicate the STAT3/CD36/PPARγ-signalling axis as an important therapeutic target in the apigenin-mediated antivisceral and anti-obesity action.
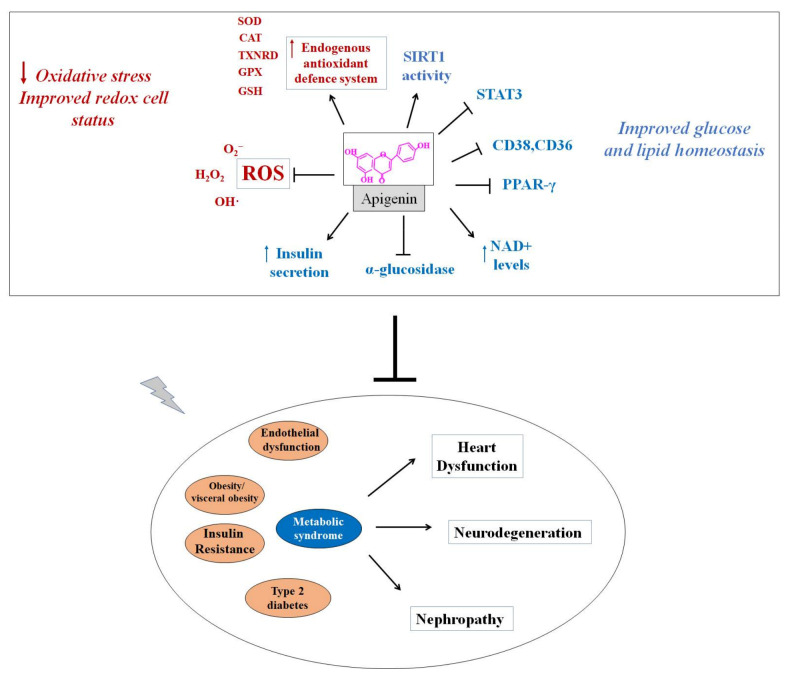
The schematization depicted in Figure 4 recapitulates the antioxidant action and metabolic effects by which apigenin could improve glucose and lipid metabolism, ameliorating endothelial dysfunction and insulin resistance and reducing visceral obesity and hyperglycaemia, thus resulting in cardio-, neuro- and renoprotection during metabolic syndrome.
Figure 4.
Proposed mechanism of action of apigenin in metabolic syndrome. CAT: catalase; CD36: cluster determinant 36; CD38: cluster of differentiation 38; GPX: glutathione peroxidase; GSH: glutathione; H2O2: hydrogen peroxide; O2−: superoxide anion; OH·: hydroxyl radical; SIRT1: sirtuin-1; SOD: superoxide dismutase; STAT3: signal transducer and activator of transcription 3; TXNRD: thioredoxin reductase.
7. Does Apigenin Have Potential Clinical Impact in Metabolic Syndrome?
The potential clinical uses of apigenin have been evaluated for different diseases such as depression, insomnia, Alzheimer’s disease, cancer and amnesia. Of course, additional experimental and clinical studies are warranted to establish the real therapeutic impact of apigenin in the near future [1,112] and references therein. However, to date, most of the findings regarding the ability of apigenin in preventing and/or treating metabolic syndrome and its related complications are limited to pre-clinical models. Indeed, there is a lack of research related to the potential of apigenin in dysmetabolic conditions for humans. On the other hand, it should be noted that important targets taking part in the mechanism of action by which apigenin induces beneficial effect in dysmetabolism have been studied in clinical practice for the management of metabolic syndrome. For instance, Feng et al. have found that the homeostasis of macrophages 1 (M1) and macrophages 2 (M2) phenotypes is necessary for the management of inflammatory disorders. PPARγ inhibits the inflammation via activation of M2 phenotypes. PPARγ ligands have been established as stimulating the polarization of macrophages in anti-inflammation and insulin resistance. In clinical practice, PPARγ agonists such as pioglitazone and rosiglitazone have been used for a long time for the management of insulin resistance, which decreases the activation of MCP1, IL-6 and TNF and regulates the synthesis of adiponectin (anti-inflammatory molecule) [176,177]. Recently, clinical trials have been performed on PPARγ agonists such as rosiglitazone, pioglitazone (thiazolidinedione) and sitagliptin and metformin combination with rosiglitazone for the management of insulin resistance in metabolic syndrome. Though PPARγ agonists (thiazolidinedione) are approved for clinical practice, their use has been limited due to severe liver toxicity, bone disorders and cardiovascular side effects [178]. Therefore, researchers are now trying to find out natural compounds for the management of abnormalities associated with metabolic syndrome with high efficacy and lessened side effects. As it has been established earlier that PPARγ ligands have a promising role in the treatment of metabolic syndrome, therefore much more attention has been paid towards newer and safer natural PPARγ agonist compounds.
Apigenin has been established as a PPARγ ligand; moreover, Escande et al. have shown that apigenin is a NAD+ ase CD38 inhibitor, and Nicholas et al. found apigenin to be a modulator of NF-kB in macrophages which improves metabolic syndrome [109,157,165,179]. Feng et al. have shown that apigenin is effective in the management of metabolic syndrome via PPARγ with lesser side effects than thiazolidinedione. Overall, these results on pre-clinical models indicate that the effects of apigenin in metabolic syndrome are worthy of emphasis and investigation in future clinical studies.
8. Future Perspectives and Limitations
Bioactive compounds deriving from food and plants are used as supplements or nutraceutical products [180,181], but the challenging aspect of isolation of bioactive compounds, their bioavailability and stability, should be resolved via different techniques by recovering them from some other natural sources like microalgae. This challenging process has been overcome for apigenin by recovering it from agrofood wastes and microalgae using different techniques, such as microwave-assisted extraction, supercritical CO2 and subcritical water technologies and enzymatic techniques [182,183,184]. Researchers have found apigenin to be the safest therapeutic moiety. However, it has been reported that high doses (30 mg/kg, 100 mg/kg, 200 mg/kg) of apigenin may induce hepatocyte degeneration and toxicity, mild sedative effect and muscle relaxation in murine models [185,186,187]. Apigenin, being a potential therapeutic agent, needs to be evaluated in novel therapeutic formulations through nanodelivery and microdelivery techniques so as to enhance its therapeutic efficacy and target specificity [188,189].
9. Conclusions
Extensive research has provided indication of the impact of diet and lifestyle in preventing the onset of diseases that are strictly linked to negative lifestyle and dietary habits, such as metabolic syndrome. Therefore, lifestyle modifications can represent the main therapeutic strategy for the treatment and management of metabolic syndrome. Randomized controlled trial studies clearly indicated that selective dietary patterns based on Mediterranean dietary patterns (MDP), dietary approaches to stop hypertension (DASH) diet and Nordic diet exhibit beneficial action against metabolic syndrome. Their effect may be attributed to the high presence of fruits, vegetables, whole grains, fish and nuts [190]. Additional evidence reported that plant-based diets, if conducted according to dietary guidelines and recommendations, may significantly reduce the risk of coronary heart disease and other cardio-cerebrovascular diseases, as well as of metabolic syndrome and type 2 diabetes, indicating that these regimens can be effective in preventing and treating cardio-metabolic and metabolic diseases.
Many of these chronic diseases require pharmacological intervention, while others can be prevented by including selective nutraceuticals in the diet. Apigenin possesses diverse organoleptic and nutritional properties in natural phenolic compounds. Due to its countless therapeutic uses and beneficial health-related properties, it is important to further delineate the mechanism of actions of apigenin for its inclusion in nutraceutical formulations [12,191,192]. Experimental evidence from in vivo and in vitro reports and clinical studies showed that apigenin can be considered a promising therapeutic agent for the prevention and/or management of metabolic syndrome and its related risk factors. Although randomized controlled clinical trials indicate that apigenin can exhibit interesting neuroprotective properties against different neurological disorders (i.e., depression, Alzheimer’s disease and Parkinson’s disease) [1,112], there is a lack of research related to the potential of apigenin in dysmetabolic conditions and metabolic syndrome for humans. Therefore, additional cellular, animal models and clinical studies are mandatory to characterize the safety and efficacy of apigenin in reducing and/or slowing the onset of metabolic syndrome, mainly as a preventive agent able to act “beyond the diet but before the need to use a drug”.
Acknowledgments
C.R. acknowledges Programma Operativo Regionale (POR) Calabria (Italy) FESR-FSE 2014/2020-Azione 10.5.12-Linea B (DR n. 683 del 21/05/2019) for financial support for RTDa position.
Abbreviations
| ARE | Antioxidant response element |
| AT II | Angiotensin II |
| ATP III | National Cholesterol Education Program’s Adult Treatment Panel III report |
| BMI | Body mass index |
| CAT | Catalase |
| CD36 | Cluster determinant 36 |
| CD38 | Cluster of differentiation 38 |
| CETP | Cholesterol ester transport protein |
| CHD | Coronary heart disease |
| Cmax | Maximum concentration |
| CVD | Cardiovascular diseases |
| EGIR | European Group for the Study of Insulin Resistance |
| FFA | Free fatty acids |
| GPX | Glutathione peroxidase |
| GSH | Glutathione |
| HDL | High-density lipoproteins |
| HF | Heart failure |
| I/R | Ischaemia/reperfusion |
| IDF | International Diabetes Federation |
| IL | Interleukin |
| IL-6 | Interleukin 6 |
| ISO | Isoproterenol |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LDL | Low-density lipoproteins |
| M1 | Macrophages 1 |
| M2 | Macrophages 2 |
| MRP | Multidrug resistance proteins |
| MW | Molecular weight |
| NAD | Nicotinamide adenine dinucleotide |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NHANES | National Health and Nutritional Examination Survey |
| NO | Nitric oxide |
| NAFLD | Non-alcoholic Fatty Liver Disease |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PPARγ | Peroxisomes proliferator activated receptor gamma |
| SIRT1 | Sirtuin-1 |
| SOD | Superoxide dismutase |
| STAT 3 | Signal transducer and activator of transcription 3 |
| TGs | Triglycerides |
| TNF | Tumour Necrosis factor |
| TXNRD | Thioredoxin reductase |
| VLDL | Very low-density lipoproteins |
| VO | Visceral obesity |
| WHO | World Health Organization |
Author Contributions
Conceptualization: W.A., C.R., H.K., Y.H., M.A., A.D.B., N.A., T.A. and W.S.C.; data curation: W.A., C.R., H.K. and A.D.B.; writing—original draft: W.A., C.R., H.K., Y.H., M.A. and W.S.C.; Writing—review and editing: W.A., C.R., H.K., N.A., T.A. and W.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
N.A. was supported by grants from Italian Association for Cancer Research (IG24449) and Italian Ministry of Health (GR-2016-02361523).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Salehi B., Venditti A., Sharifi-Rad M., Kręgiel D., Sharifi-Rad J., Durazzo A., Lucarini M., Santini A., Souto E.B., Novellino E., et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019;20:1305. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandola A., Ashdown-Franks G., Hendrikse J., Sabiston C.M., Stubbs B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019;107:525–539. doi: 10.1016/j.neubiorev.2019.09.040. [DOI] [PubMed] [Google Scholar]
- 3.Spence J.D. Nutrition and Risk of Stroke. Nutrients. 2019;11:647. doi: 10.3390/nu11030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabera J.N., Semana E., Mussa A.R., He X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014;2:377–392. [Google Scholar]
- 5.Amarowicz R., Carle R., Dongowski G., Durazzo A., Galensa R., Kammerer D., Maiani G., Piskula M.K. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009;53((Suppl. S2)):S151–S183. doi: 10.1002/mnfr.200700486. [DOI] [PubMed] [Google Scholar]
- 6.Falcone Ferreyra M.L., Rius S.P., Casati P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012;3:222. doi: 10.3389/fpls.2012.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madunić J., Madunić I.V., Gajski G., Popić J., Garaj-Vrhovac V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018;413:11–22. doi: 10.1016/j.canlet.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 8.Ferrer J.L., Austin M.B., Stewart C., Jr., Noel J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008;46:356–370. doi: 10.1016/j.plaphy.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kassi E., Pervanidou P., Kaltsas G., Chrousos G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galleano M., Calabro V., Prince P.D., Litterio M.C., Piotrkowski B., Vazquez-Prieto M.A., Miatello R.M., Oteiza P.I., Fraga C.G. Flavonoids and metabolic syndrome. Ann. N. Y. Acad. Sci. 2012;1259:87–94. doi: 10.1111/j.1749-6632.2012.06511.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang C.S., Landau J.M., Huang M.T., Newmark H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 12.Hostetler G.L., Ralston R.A., Schwartz S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017;8:423–435. doi: 10.3945/an.116.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberti K.G., Zimmet P., Shaw J., IDF Epidemiology Task Force Consensus Group The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 14.Festi D., Schiumerini R., Eusebi L.H., Marasco G., Taddia M., Colecchia A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014;20:16079–16094. doi: 10.3748/wjg.v20.i43.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M., Firrman J., Zhang L., Arango-Argoty G., Tomasula P., Liu L., Xiao W., Yam K. Apigenin Impacts the Growth of the Gut Microbiota and Alters the Gene Expression of Enterococcus. Molecules. 2017;22:1292. doi: 10.3390/molecules22081292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M., Firrman J., Liu L., Yam K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed Res. Int. 2019;2019:7010467. doi: 10.1155/2019/7010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shukla S., Gupta S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ornano L., Venditti A., Donno Y., Sanna C., Ballero M., Bianco A. Phytochemical analysis of non-volatile fraction of Artemisia caerulescens subsp. densiflora (Viv.)(Asteraceae), an endemic species of La Maddalena Archipelago (Sardinia–Italy) Nat. Prod. Res. 2016;30:920–925. doi: 10.1080/14786419.2015.1079189. [DOI] [PubMed] [Google Scholar]
- 19.Venditti A., Maggi F., Vittori S., Papa F., Serrilli A.M., Di Cecco M., Ciaschetti G., Mandrone M., Poli F., Bianco A. Volatile compounds from Achillea tenorii (Grande) growing in the Majella National Park (Italy) Nat. Prod. Res. 2014;28:1699–1704. doi: 10.1080/14786419.2014.940349. [DOI] [PubMed] [Google Scholar]
- 20.Venditti A., Guarcini L., Bianco A., Rosselli S., Bruno M., Senatore F. Phytochemical analysis of Achillea ligustica All. from Lipari Island (Aeolian Islands) Nat. Prod. Res. 2016;30:912–919. doi: 10.1080/14786419.2015.1079188. [DOI] [PubMed] [Google Scholar]
- 21.Sharifi-Rad M., Nazaruk J., Polito L., Morais-Braga M.F.B., Rocha J.E., Coutinho H.D.M., Salehi B., Tabanelli G., Montanari C., Del Mar Contreras M., et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018;215:76–88. doi: 10.1016/j.micres.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Venditti A., Frezza C., Guarcini L., Foddai S., Serafini M., Bianco A. Phytochemical Study of a Species with Ethnopharmacological Interest: Sideritis romana L. Eur. J. Med. Plants. 2016;12:1–9. doi: 10.9734/EJMP/2016/23809. [DOI] [Google Scholar]
- 23.Forkmann G. Flavonoids as flower pigments: The formation of the natural spectrum and its extension by genetic engineering. Plant Breed. 1991;106:1–26. doi: 10.1111/j.1439-0523.1991.tb00474.x. [DOI] [Google Scholar]
- 24.Herrmann K.M. The shikimate pathway as an entry to aromatic secondary metabolism. Plant Physiol. 1995;107:7–12. doi: 10.1104/pp.107.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martens S., Forkmann G., Matern U., Lukacin R. Cloning of parsley flavone synthase I. Phytochemistry. 2001;58:43–46. doi: 10.1016/S0031-9422(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J., Liu D., Huang Y., Gao Y., Qian S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012;436:311–317. doi: 10.1016/j.ijpharm.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Pforte H., Hempel J., Jacobasch G. Distribution pattern of a flavonoid extract in the gastrointestinal lumen and wall of rats. Nahrung. 1999;43:205–208. doi: 10.1002/(SICI)1521-3803(19990601)43:3<205::AID-FOOD205>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 28.Chen J., Lin H., Hu M. Metabolism of flavonoids via enteric recycling: Role of intestinal disposition. J. Pharmacol. Exp. Ther. 2003;304:1228–1235. doi: 10.1124/jpet.102.046409. [DOI] [PubMed] [Google Scholar]
- 29.Li B., Birt D.F. In vivo and in vitro percutaneous absorption of cancer preventive flavonoid apigenin in different vehicles in mouse skin. Pharm. Res. 1996;13:1710–1715. doi: 10.1023/A:1016453009818. [DOI] [PubMed] [Google Scholar]
- 30.Gradolatto A., Basly J.P., Berges R., Teyssier C., Chagnon M.C., Siess M.H., Canivenc-Lavier M.C. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab. Dispos. 2005;33:49–54. doi: 10.1124/dmd.104.000893. [DOI] [PubMed] [Google Scholar]
- 31.Bader N., Bosy-Westphal A., Koch A., Mueller M.J. Influence of Vitamin C and E Supplementation on Oxidative Stress Induced by Hyperbaric Oxygen in Healthy Men. Ann. Nutr. Metab. 2006;50:173–176. doi: 10.1159/000090737. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X., Han R., Sun X., Li G., Yang Q., Li Q., Gai W., Zhang M., Chen L., Yang G., et al. The Effect of the Skeleton Structure of Flavanone and Flavonoid on Interaction with Transferrin. Bioorg. Med. Chem. Lett. 2013;23:6677–6681. doi: 10.1016/j.bmcl.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 33.DeRango-Adem E.F., Blay J. Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers? Front. Pharmacol. 2021;12:681477. doi: 10.3389/fphar.2021.681477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang L., Zhou J., Yang C.H., Xia B.J., Hu M., Liu Z.Q. Systematic studies of sulfation and glucuronidation of 12 flavonoids in the mouse liver S9 fraction reveal both unique and shared positional preferences. J. Agric. Food Chem. 2012;60:3223–3233. doi: 10.1021/jf201987k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffiths L.A., Smith G.E. Metabolism of apigenin and related compounds in the rat. Metabolite formation in vivo and by the intestinal microflora in vitro. Biochem. J. 1972;128:901–911. doi: 10.1042/bj1280901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galijatovic A., Otake Y., Walle U.K., Walle T. Extensive metabolism of the flavonoid chrysin by human Caco-2 and Hep G2 cells. Xenobiotica. 1999;29:1241–1256. doi: 10.1080/004982599237912. [DOI] [PubMed] [Google Scholar]
- 37.Day A.J., DuPont M.S., Ridley S., Rhodes M., Rhodes M.J., Morgan M.R., Williamson G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998;436:71–75. doi: 10.1016/S0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- 38.Tang D., Chen K., Huang L., Li J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin. Drug Metab. Toxicol. 2017;13:323–330. doi: 10.1080/17425255.2017.1251903. [DOI] [PubMed] [Google Scholar]
- 39.Li B., Robinson D.H., Birt D.F. Evaluation of properties of apigenin and [G-3H]apigenin and analytic method development. J Pharm Sci. 1997;86:721–725. doi: 10.1021/js960383s. [DOI] [PubMed] [Google Scholar]
- 40.Ding S.M., Zhang Z.H., Song J., Cheng X.D., Jiang J., Jia X.B. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014;9:2327–2333. doi: 10.2147/IJN.S60938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J., Huang Y., Liu D., Gao Y., Qian S. Preparation of apigenin nanocrystals using supercritical antisolvent process for dissolution and bioavailability enhancement. Eur. J. Pharm. Sci. 2013;48:740–747. doi: 10.1016/j.ejps.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 42.Kaur J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014;2014:21. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A., Gordon D.J., Krauss R.M., Savage P.J., Smith S.C., Jr., et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 44.Padhi T.G. Metabolic syndrome and skin: Psoriasis and beyond. Indian J. Dermatol. 2013;58:299–305. doi: 10.4103/0019-5154.113950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruce K.D., Byrne C.D. The metabolic syndrome: Common origins of a multifactorial disorder. Postgrad. Med. J. 2009;85:614–621. doi: 10.1136/pgmj.2008.078014. [DOI] [PubMed] [Google Scholar]
- 46.Locatelli F., Pozzoni P., Del Vecchio L. Renal manifestations in the metabolic syndrome. J. Am. Soc. Nephrol. 2006;4((Suppl. S2)):S81–S85. doi: 10.1681/ASN.2005121332. [DOI] [PubMed] [Google Scholar]
- 47.Standl E. Aetiology and consequences of the metabolic syndrome. Eur. Heart J. Suppl. 2005;7((Suppl. D)):D10–D13. doi: 10.1093/eurheartj/sui023. [DOI] [Google Scholar]
- 48.Dokras A., Bochner M., Hollinrake E., Markham S., Vanvoorhis B., Jagasia D.H. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet. Gynecol. 2005;106:131–137. doi: 10.1097/01.AOG.0000167408.30893.6b. [DOI] [PubMed] [Google Scholar]
- 49.Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 50.Ford E.S., Giles W.H., Dietz W.H. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 51.Cornier M.A., Dabelea D., Hernandez T.L., Lindstrom R.C., Steig A.J., Stob N.R., Van Pelt R.E., Wang H., Eckel R.H. The Metabolic Syndrome. Endocr. Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adjei N.K., Samkange-Zeeb F., Kebede M. Racial/ethnic differences in the prevalence and incidence of metabolic syndrome in high-income countries: A protocol for a systematic review. Syst. Rev. 2020;9:134. doi: 10.1186/s13643-020-01400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park Y., Zhu S., Palaniappan L., Heshka S., Carnethon M.R., Heymsfield S.B. The Metabolic Syndrome: Prevalence and Associated Risk Factor Findings in the US Population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch. Intern. Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alberti K.G.M.M., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P.T., Loria C.M., Smith S.C., Jr. Harmonizing the Metabolic Syndrome. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 55.Gill H., Mugo M., Whaley-Connell A., Stump C., Sowers J.R. The key role of insulin resistance in the cardiometabolic syndrome. Am. J. Med. Sci. 2005;330:290–294. doi: 10.1097/00000441-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Morse S.A., Zhang R., Thakur V., Reisin E. Hypertension and the metabolic syndrome. Am. J. Med. Sci. 2005;330:303–310. doi: 10.1097/00000441-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Brinkman A.K. Management of Type 1 Diabetes. Nurs Clin. N. Am. 2017;52:499–511. doi: 10.1016/j.cnur.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Hermansen K., Bohl M., Schioldan A.G. Insulin Aspart in the Management of Diabetes Mellitus: 15 Years of Clinical Experience. Drugs. 2016;76:41–74. doi: 10.1007/s40265-015-0500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrannini E., Natali A. Essential hypertension, metabolic disorders, and insulin resistance. (Pt 2)Am. Heart J. 1991;121:1274–1282. doi: 10.1016/0002-8703(91)90433-I. [DOI] [PubMed] [Google Scholar]
- 60.Malhotra A., Kang B.P., Cheung S., Opawumi D., Meggs L.G. Angiotensin II promotes glucose-induced activation of cardiac protein kinase C isozymes and phosphorylation of troponin I. Diabetes. 2001;50:1918–1926. doi: 10.2337/diabetes.50.8.1918. [DOI] [PubMed] [Google Scholar]
- 61.Zorena K., Jachimowicz-Duda O., Ślęzak D., Robakowska M., Mrugacz M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020;21:3570. doi: 10.3390/ijms21103570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chait A., den Hartigh L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020;7:22. doi: 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Treml J., Šmejkal K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016;15:720–738. doi: 10.1111/1541-4337.12204. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y., Stevens V.L., Shah R., Peterson J.J., Dwyer J.T., Gapstur S.M., McCullough M.L. Dietary flavonoid and proanthocyanidin intakes and prostate cancer risk in a prospective cohort of US men. Am. J. Epidemiol. 2014;179:974–986. doi: 10.1093/aje/kwu006. [DOI] [PubMed] [Google Scholar]
- 65.Cassidy A., O’Reilly É.J., Kay C., Sampson L., Franz M., Forman J.P., Curhan G., Rimm E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011;93:338–347. doi: 10.3945/ajcn.110.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J., Lee H.J., Lee K.W. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J. Neurochem. 2010;112:1415–1430. doi: 10.1111/j.1471-4159.2009.06562.x. [DOI] [PubMed] [Google Scholar]
- 67.Sichel G., Corsaro C., Scalia M., Di Bilio A.J., Bonomo R.P. In vitro scavenger activity of some flavonoids and melanins against O2-(.) Free Radic. Biol. Med. 1991;11:1–8. doi: 10.1016/0891-5849(91)90181-2. [DOI] [PubMed] [Google Scholar]
- 68.Han J.Y., Ahn S.Y., Kim C.S., Yoo S.K., Kim S.K., Kim H.C., Hong J.T., Oh K.W. Protection of apigenin against kainate-induced excitotoxicity by anti-oxidative effects. Biol. Pharm. Bull. 2012;35:1440–1446. doi: 10.1248/bpb.b110686. [DOI] [PubMed] [Google Scholar]
- 69.Yue S., Xue N., Li H., Huang B., Chen Z., Wan X. Hepatoprotective Effect of Apigenin Against Liver Injury via the Non-canonical NF-κB Pathway In Vivo and In Vitro. Inflammation. 2020;43:1634–1648. doi: 10.1007/s10753-020-01238-5. [DOI] [PubMed] [Google Scholar]
- 70.Ji J., Yu Q., Dai W., Wu L., Feng J., Zheng Y., Li Y., Guo C. Apigenin Alleviates Liver Fibrosis by Inhibiting Hepatic Stellate Cell Activation and Autophagy via TGF-β1/Smad3 and p38/PPARα Pathways. PPAR Res. 2021;2021:6651839. doi: 10.1155/2021/6651839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X., Bu H., Jiang Y., Sun G., Jiang R., Huang X., Duan H., Huang Z., Wu Q. The antidepressant effects of apigenin are associated with the promotion of autophagy via the mTOR/AMPK/ULK1 pathway. Mol. Med. Rep. 2019;20:2867–2874. doi: 10.3892/mmr.2019.10491. [DOI] [PubMed] [Google Scholar]
- 72.Perrott K.M., Wiley C.D., Desprez P.-Y., Campisi J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. Geroscience. 2017;39:161–173. doi: 10.1007/s11357-017-9970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qin Y., Zhao D., Zhou H.-g., Wang X.-h., Zhong W.-l., Chen S., Gu W.G., Wang W., Zhang C.H., Liu Y.R., et al. Apigenin inhibits NF-κB and snail signaling, EMT and metastasis in human hepatocellular carcinoma. Oncotarget. 2016;7:41421. doi: 10.18632/oncotarget.9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang S., Liu X., Sun C., Yang J., Wang L., Liu J., Gong L., Jing Y. Apigenin Attenuates Experimental Autoimmune Myocarditis by Modulating Th1/Th2 Cytokine Balance in Mice. Inflammation. 2016;39:678–686. doi: 10.1007/s10753-015-0294-y. [DOI] [PubMed] [Google Scholar]
- 75.Ren K., Jiang T., Zhou H., Liang Y., Zhao G.-J. Apigenin Retards Atherogenesis by Promoting ABCA1-Mediated Cholesterol Efflux and Suppressing Inflammation. Cell Physiol. Biochem. 2018;47:2170–2184. doi: 10.1159/000491528. [DOI] [PubMed] [Google Scholar]
- 76.Li J.M., Zhang B.F. Apigenin protects ovalbumin-induced asthma through the regulation of Th17 cells. Fitoterapia. 2013;91:298–304. doi: 10.1016/j.fitote.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Goto T., Hagiwara K., Shirai N., Yoshida K., Hagiwara H. Apigenin inhibits osteoblastogenesis and osteoclastogenesis and prevents bone loss in ovariectomized mice. Cytotechnology. 2015;67:357–365. doi: 10.1007/s10616-014-9694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lampropoulos P., Lambropoulou M., Papalois A., Basios N., Manousi M., Simopoulos C., Tsaroucha A.K. The role of apigenin in an experimental model of acute pancreatitis. J. Surg. Res. 2013;183:129–137. doi: 10.1016/j.jss.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 79.Mrazek A.A., Porro L.J., Bhatia V., Falzon M., Spratt H., Zhou J., Chao C., Hellmich M.R. Apigenin inhibits pancreatic stellate cell activity in pancreatitis. J. Surg. Res. 2015;196:8–16. doi: 10.1016/j.jss.2015.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou X., Wang F., Zhou R., Song X., Xie M. Apigenin: A current review on its beneficial biological activities. J. Food Biochem. 2017;41:e12376. doi: 10.1111/jfbc.12376. [DOI] [Google Scholar]
- 81.Arango D., Morohashi K., Yilmaz A., Kuramochi K., Parihar A., Brahimaj B., Grotewold E., Doseff A.I. Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Proc. Natl. Acad. Sci. USA. 2013;110:E2153–E2162. doi: 10.1073/pnas.1303726110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forman H.J., Zhang H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021;20:689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spahis S., Borys J.M., Levy E. Metabolic syndrome as a multifaceted risk factor for oxidative stress. Antioxid. Redox Signal. 2017;26:445–461. doi: 10.1089/ars.2016.6756. [DOI] [PubMed] [Google Scholar]
- 84.Seddon M., Looi Y.H., Shah A.M. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rocca C., Pasqua T., Boukhzar L., Anouar Y., Angelone T. Progress in the emerging role of selenoproteins in cardiovascular disease: Focus on endoplasmic reticulum-resident selenoproteins. Cell. Mol. Life Sci. 2019;76:3969–3985. doi: 10.1007/s00018-019-03195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rocca C., Grande F., Granieri M.C., Colombo B., De Bartolo A., Giordano F., Rago V., Amodio N., Tota B., Cerra M.C., et al. The chromogranin A1-373 fragment reveals how a single change in the protein sequence exerts strong cardioregulatory effects by engaging neuropilin-1. Acta Physiol. 2021;231:e13570. doi: 10.1111/apha.13570. [DOI] [PubMed] [Google Scholar]
- 87.Grande F., De Bartolo A., Occhiuzzi M.A., Caruso A., Rocca C., Pasqua T., Carocci A., Rago V., Angelone T., Sinicropi M.S. Carbazole and Simplified Derivatives: Novel Tools toward β-Adrenergic Receptors Targeting. Appl. Sci. 2021;11:5486. doi: 10.3390/app11125486. [DOI] [Google Scholar]
- 88.Rangwala S.M., Lazar M.A. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol. Sci. 2004;25:331–336. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 89.Thangaiyan R., Robert B.M., Arjunan S., Govindasamy K., Nagarajan R.P. Preventive effect of apigenin against isoproterenol-induced apoptosis in cardiomyoblasts. J. Biochem. Mol. Toxicol. 2018;32:e22213. doi: 10.1002/jbt.22213. [DOI] [PubMed] [Google Scholar]
- 90.Gao H.L., Yu X.J., Hu H.B., Yang Q.W., Liu K.L., Chen Y.M., Zhang Y., Zhang D.D., Tian H., Zhu G.Q., et al. Apigenin Improves Hypertension and Cardiac Hypertrophy Through Modulating NADPH Oxidase-Dependent ROS Generation and Cytokines in Hypothalamic Paraventricular Nucleus. Cardiovasc. Toxicol. 2021;21:721–736. doi: 10.1007/s12012-021-09662-1. [DOI] [PubMed] [Google Scholar]
- 91.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D., Aboyans V., Asteggiano R., Galderisi M., Habib G., Lenihan D.J., Lip G.Y.H., Lyon A.R., et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur. Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 92.Rocca C., Pasqua T., Cerra M.C., Angelone T. Cardiac Damage in Anthracyclines Therapy: Focus on Oxidative Stress and Inflammation. Antioxid. Redox Signal. 2020;32:1081–1097. doi: 10.1089/ars.2020.8016. [DOI] [PubMed] [Google Scholar]
- 93.Zare M.F.R., Rakhshan K., Aboutaleb N., Nikbakht F., Naderi N., Bakhshesh M., Azizi Y. Apigenin attenuates doxorubicin induced cardiotoxicity via reducing oxidative stress and apoptosis in male rats. Life Sci. 2019;232:116623. doi: 10.1016/j.lfs.2019.116623. [DOI] [PubMed] [Google Scholar]
- 94.Sahu R., Dua T.K., Das S., De Feo V., Dewanjee S. Wheat phenolics suppress doxorubicin-induced cardiotoxicity via inhibition of oxidative stress, MAP kinase activation, NF-κB pathway, PI3K/Akt/mTOR impairment, and cardiac apoptosis. Food Chem. Toxicol. 2019;125:503–519. doi: 10.1016/j.fct.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 95.Navarro-Hortal M.D., Varela-López A., Romero-Márquez J.M., Rivas-García L., Speranza L., Battino M., Quiles J.L. Role of flavonoids against adriamycin toxicity. Food Chem. Toxicol. 2020;146:111820. doi: 10.1016/j.fct.2020.111820. [DOI] [PubMed] [Google Scholar]
- 96.Clayton Z.S., Hutton D.A., Brunt V.E., VanDongen N.S., Ziemba B.P., Casso A.G., Greenberg N.T., Mercer A.N., Rossman M.J., Campisi J., et al. Apigenin restores endothelial function by ameliorating oxidative stress, reverses aortic stiffening, and mitigates vascular inflammation with aging. Am. J. Physiol. Heart Circ. Physiol. 2021;321:H185–H196. doi: 10.1152/ajpheart.00118.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang H., Lai S., Luo Y., Wan Q., Wu Q., Wan L., Qi W., Liu J. Nutritional Preconditioning of Apigenin Alleviates Myocardial Ischemia/Reperfusion Injury via the Mitochondrial Pathway Mediated by Notch1/Hes1. Oxidative Med. Cell. Longev. 2019;2019:7973098. doi: 10.1155/2019/7973098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou Z., Zhang Y., Lin L., Zhou J. Apigenin suppresses the apoptosis of H9C2 rat cardiomyocytes subjected to myocardial ischemia reperfusion injury via upregulation of the PI3K/Akt pathway. Mol. Med. Rep. 2018;18:1560–1570. doi: 10.3892/mmr.2018.9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McVeigh G.E., Cohn J.N. Endothelial dysfunction and the metabolic syndrome. Curr. Diabetes Rep. 2003;3:87–92. doi: 10.1007/s11892-003-0059-0. [DOI] [PubMed] [Google Scholar]
- 100.Qin W., Ren B., Wang S., Liang S., He B., Shi X., Wang L., Liang J., Wu F. Apigenin and naringenin ameliorate PKCβII-associated endothelial dysfunction via regulating ROS/caspase-3 and NO pathway in endothelial cells exposed to high glucose. Vasc. Pharmacol. 2016;85:39–49. doi: 10.1016/j.vph.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 101.Xu Q., Li Y.C., Du C., Wang L.N., Xiao Y.H. Effects of Apigenin on the Expression of LOX-1, Bcl-2, and Bax in Hyperlipidemia Rats. Chem. Biodivers. 2021;18:e2100049. doi: 10.1002/cbdv.202100049. [DOI] [PubMed] [Google Scholar]
- 102.Mahajan U.B., Chandrayan G., Patil C.R., Arya D.S., Suchal K., Agrawal Y.O., Ojha S., Goyal S.N. The Protective Effect of Apigenin on Myocardial Injury in Diabetic Rats mediating Activation of the PPAR-γ Pathway. Int. J. Mol. Sci. 2017;18:756. doi: 10.3390/ijms18040756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maron D.J. Flavonoids for reduction of atherosclerotic risk. Curr. Atheroscler. Rep. 2004;6:73–78. doi: 10.1007/s11883-004-0119-1. [DOI] [PubMed] [Google Scholar]
- 104.Czibik G. Complex role of the HIF system in cardiovascular biology. J. Mol. Med. 2010;88:1101–1111. doi: 10.1007/s00109-010-0646-x. [DOI] [PubMed] [Google Scholar]
- 105.Zhu Z.Y., Gao T., Huang Y., Xue J., Xie M.L. Apigenin ameliorates hypertension-induced cardiac hypertrophy and down-regulates cardiac hypoxia inducible factor-lα in rats. Food Funct. 2016;7:1992–1998. doi: 10.1039/C5FO01464F. [DOI] [PubMed] [Google Scholar]
- 106.Pasqua T., Rocca C., Giglio A., Angelone T. Cardiometabolism as an Interlocking Puzzle between the Healthy and Diseased Heart: New Frontiers in Therapeutic Applications. J. Clin. Med. 2021;10:721. doi: 10.3390/jcm10040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu H.J., Fan Y.L., Liao H.H., Liu Y., Chen S., Ma Z.G., Zhang N., Yang Z., Deng W., Tang Q.Z. Apigenin alleviates STZ-induced diabetic cardiomyopathy. Mol. Cell. Biochem. 2017;428:9–21. doi: 10.1007/s11010-016-2913-9. [DOI] [PubMed] [Google Scholar]
- 108.Ohno M., Shibata C., Kishikawa T., Yoshikawa T., Takata A., Kojima K., Akanuma M., Kang Y.J., Yoshida H., Otsuka M., et al. The flavonoid apigenin improves glucose tolerance through inhibition of microRNA maturation in miRNA103 transgenic mice. Sci. Rep. 2013;3:2553. doi: 10.1038/srep02553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Escande C., Nin V., Price N.L., Capellini V., Gomes A.P., Barbosa M.T., O’Neil L., White T.A., Sinclair D.A., Chini E.N. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: Implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes. 2013;62:1084–1093. doi: 10.2337/db12-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barbosa M.T., Soares S.M., Novak C.M., Sinclair D., Levine J.A., Aksoy P., Chini E.N. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007;21:3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- 111.Yoshino J., Mills K.F., Yoon M.J., Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nabavi S.F., Khan H., D’onofrio G., Šamec D., Shirooie S., Dehpour A.R., Argüelles S., Habtemariam S., Sobarzo-Sanchez E. Apigenin as neuroprotective agent: Of mice and men. Pharmacol. Res. 2018;128:359–365. doi: 10.1016/j.phrs.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 113.Shao C., Yuan J., Liu Y., Qin Y., Wang X., Gu J., Chen G., Zhang B., Liu H.K., Zhao J., et al. Epileptic brain fluorescent imaging reveals apigenin can relieve the myeloperoxidase-mediated oxidative stress and inhibit ferroptosis. Proc. Natl. Acad. Sci. USA. 2020;117:10155–10164. doi: 10.1073/pnas.1917946117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Butterfield D.A., Drake J., Pocernich C., Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid beta-peptide. Trends Mol. Med. 2001;7:548–554. doi: 10.1016/S1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 116.Pang Q., Zhao Y., Chen X., Zhao K., Zhai Q., Tu F. Apigenin Protects the Brain against Ischemia/Reperfusion Injury via Caveolin-1/VEGF In Vitro and In Vivo. Oxidative Med. Cell. Longev. 2018;2018:7017204. doi: 10.1155/2018/7017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cai M., Ma Y., Zhang W., Wang S., Wang Y., Tian L., Peng Z., Wang H., Qingrong T. Apigenin-7-O-β-D-(-6″-p-coumaroyl)-Glucopyranoside Treatment Elicits Neuroprotective Effect against Experimental Ischemic Stroke. Int. J. Biol. Sci. 2016;12:42–52. doi: 10.7150/ijbs.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Han Y., Zhang T., Su J., Zhao Y., Chenchen Wang Li X. Apigenin attenuates oxidative stress and neuronal apoptosis in early brain injury following subarachnoid hemorrhage. J. Clin. Neurosci. 2017;40:157–162. doi: 10.1016/j.jocn.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 119.Tu F., Pang Q., Huang T., Zhao Y., Liu M., Chen X. Apigenin Ameliorates Post-Stroke Cognitive Deficits in Rats through Histone Acetylation-Mediated Neurochemical Alterations. Med. Sci. Monit. 2017;23:4004–4013. doi: 10.12659/MSM.902770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kalivarathan J., Chandrasekaran S.P., Kalaivanan K., Ramachandran V., Carani Venkatraman A. Apigenin attenuates hippocampal oxidative events, inflammation and pathological alterations in rats fed high fat, fructose diet. Biomed. Pharmacother. 2017;89:323–331. doi: 10.1016/j.biopha.2017.01.162. [DOI] [PubMed] [Google Scholar]
- 121.Araki E., Inagaki N., Tanizawa Y., Oura T., Takeuchi M., Imaoka T. Efficacy and safety of once-weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once-daily insulin glargine in Japanese patients with type 2 diabetes: A randomized, open-label, phase III, non-inferiority study. Diabetes Obes. Metab. 2015;17:994–1002. doi: 10.1111/dom.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pabla N., Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 123.Hassan S.M., Khalaf M.M., Sadek S.A., Abo-Youssef A.M. Protective effects of apigenin and myricetin against cisplatin-induced nephrotoxicity in mice. Pharm. Biol. 2017;55:766–774. doi: 10.1080/13880209.2016.1275704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu Q., Li W., Zhao J., Sun W., Yang Q., Chen C., Xia P., Zhu J., Zhou Y., Huang G., et al. Apigenin ameliorates doxorubicin-induced renal injury via inhibition of oxidative stress and inflammation. Biomed. Pharmacother. 2021;137:111308. doi: 10.1016/j.biopha.2021.111308. [DOI] [PubMed] [Google Scholar]
- 125.He X., Li C., Wei Z., Wang J., Kou J., Liu W., Shi M., Yang Z., Fu Y. Protective role of apigenin in cisplatin-induced renal injury. Eur. J. Pharmacol. 2016;789:215–221. doi: 10.1016/j.ejphar.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y., Wang L., Du Y., Chen Z., Guo J., Weng X., Wang X., Wang M., Chen D., Liu X. Effects of apigenin pretreatment against renal ischemia/reperfusion injury via activation of the JAK2/STAT3 pathway. Biomed. Pharmacother. 2017;95:1799–1808. doi: 10.1016/j.biopha.2017.09.091. [DOI] [PubMed] [Google Scholar]
- 127.Wang T., Zhang Z., Xie M., Li S., Zhang J., Zhou J. Apigenin Attenuates Mesoporous Silica Nanoparticles-Induced Nephrotoxicity by Activating FOXO3a. Biol. Trace Elem. Res. 2021 doi: 10.1007/s12011-021-02871-3. [DOI] [PubMed] [Google Scholar]
- 128.Hozayen W.G., Mahmoud A.M., Desouky E.M., El-Nahass E.S., Soliman H.A., Farghali A.A. Cardiac and pulmonary toxicity of mesoporous silica nanoparticles is associated with excessive ROS production and redox imbalance in Wistar rats. Biomed. Pharmacother. 2019;109:2527–2538. doi: 10.1016/j.biopha.2018.11.093. [DOI] [PubMed] [Google Scholar]
- 129.Ali A.A., Mansour A.B., Attia S.A. The potential protective role of apigenin against oxidative damage induced by nickel oxide nanoparticles in liver and kidney of male Wistar rat, Rattus norvegicus. Environ. Sci. Pollut. Res. Int. 2021;28:27577–27592. doi: 10.1007/s11356-021-12632-3. [DOI] [PubMed] [Google Scholar]
- 130.Zamani F., Samiei F., Mousavi Z., Azari M.R., Seydi E., Pourahmad J. Apigenin ameliorates oxidative stress and mitochondrial damage induced by multiwall carbon nanotubes in rat kidney mitochondria. J. Biochem. Mol. Toxicol. 2021;35:1–7. doi: 10.1002/jbt.22762. [DOI] [PubMed] [Google Scholar]
- 131.Pamunuwa G., Karunaratne D.N., Waisundara V.Y. Antidiabetic Properties, Bioactive Constituents, and Other Therapeutic Effects of Scoparia dulcis. Evid. -Based Complementary Altern. Med. 2016;2016:8243215. doi: 10.1155/2016/8243215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Malik S., Suchal K., Khan S.I., Bhatia J., Kishore K., Dinda A.K., Arya D.S. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-κB-TNF-α and TGF-β1-MAPK-fibronectin pathways. Am. J. Physiol. Renal Physiol. 2017;313:F414–F422. doi: 10.1152/ajprenal.00393.2016. [DOI] [PubMed] [Google Scholar]
- 133.Hu C., Sun L., Xiao L., Han Y., Fu X., Xiong X., Xu X., Liu Y., Yang S., Liu F., et al. Insights into the Mechanisms Involved in the Expression and Regulation of Extracellular Matrix Proteins in Diabetic Nephropathy. Curr. Med. Chem. 2015;22:2858–2870. doi: 10.2174/0929867322666150625095407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rains J.L., Jain S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ogura Y., Kitada M., Xu J., Monno I., Koya D. CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging. 2020;12:11325–11336. doi: 10.18632/aging.103410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Basciano H., Federico L., Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mellor K., Ritchie R.H., Meredith G., Woodman O.L., Morris M.J., Delbridge L.M. High-fructose diet elevates myocardial superoxide generation in mice in the absence of cardiac hypertrophy. Nutrition. 2010;26:842–848. doi: 10.1016/j.nut.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 138.Yang M., Jiang Z.H., Li C.G., Zhu Y.J., Li Z., Tang Y.Z., Ni C.L. Apigenin prevents metabolic syndrome in high-fructose diet-fed mice by Keap1-Nrf2 pathway. Biomed. Pharmacother. 2018;105:1283–1290. doi: 10.1016/j.biopha.2018.06.108. [DOI] [PubMed] [Google Scholar]
- 139.Herman M.A., Samuel V.T. The Sweet Path to Metabolic Demise: Fructose and Lipid Synthesis. Trends Endocrinol. Metab. 2016;27:719–730. doi: 10.1016/j.tem.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jung U.J., Cho Y.Y., Choi M.S. Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice. Nutrients. 2016;8:305. doi: 10.3390/nu8050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ceriello A., Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 142.Ford E.S., Mokdad A.H., Giles W.H., Brown D.W. The metabolic syndrome and antioxidant concentrations: Findings from the Third National Health and Nutrition Examination Survey. Diabetes. 2003;52:2346–2352. doi: 10.2337/diabetes.52.9.2346. [DOI] [PubMed] [Google Scholar]
- 143.Evans J.L., Maddux B.A., Goldfine I.D. The molecular basis for oxidative stress-induced insulin resistance. Antioxid. Redox Signal. 2005;7:1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 144.Houstis N., Rosen E.D., Lander E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 145.Zhang K., Song W., Li D., Jin X. Apigenin in the regulation of cholesterol metabolism and protection of blood vessels. Exp. Ther. Med. 2017;13:1719–1724. doi: 10.3892/etm.2017.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Peng S., Hou Y., Yao J., Fang J. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017;8:997–1007. doi: 10.1039/C7FO00054E. [DOI] [PubMed] [Google Scholar]
- 147.Peng S., Yao J., Liu Y., Duan D., Zhang X., Fang J. Activation of Nrf2 target enzymes conferring protection against oxidative stress in PC12 cells by ginger principal constituent 6-shogaol. Food Funct. 2015;6:2813–2823. doi: 10.1039/C5FO00214A. [DOI] [PubMed] [Google Scholar]
- 148.Bhakkiyalakshmi E., Dineshkumar K., Karthik S., Sireesh D., Hopper W., Paulmurugan R., Ramkumar K.M. Pterostilbene-mediated Nrf2 activation: Mechanistic insights on Keap1:Nrf2 interface. Bioorganic Med. Chem. 2016;24:3378–3386. doi: 10.1016/j.bmc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 149.Bello M., Morales-González J.A. Molecular recognition between potential natural inhibitors of the Keap1-Nrf2 complex. (Pt 1)Int. J. Biol. Macromol. 2017;105:981–992. doi: 10.1016/j.ijbiomac.2017.07.117. [DOI] [PubMed] [Google Scholar]
- 150.Bai P., Cantó C., Oudart H., Brunyánszki A., Cen Y., Thomas C., Yamamoto H., Huber A., Kiss B., Houtkooper R.H., et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aksoy P., White T.A., Thompson M., Chini E.N. Regulation of intracellular levels of NAD: A novel role for CD38. Biochem. Biophys. Res. Commun. 2006;345:1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 152.Barthel A., Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2003;285:E685–E692. doi: 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- 153.Casacchia T., Scavello F., Rocca C., Granieri M.C., Beretta G., Amelio D., Gelmini F., Spena A., Mazza R., Toma C.C., et al. Leopoldia comosa prevents metabolic disorders in rats with high-fat diet-induced obesity. Eur. J. Nutr. 2019;58:965–979. doi: 10.1007/s00394-018-1609-1. [DOI] [PubMed] [Google Scholar]
- 154.Cazarolli L.H., Folador P., Moresco H.H., Brighente I.M., Pizzolatti M.G., Silva F.R. Mechanism of action of the stimulatory effect of apigenin-6-C-(2″-O-alpha-l-rhamnopyranosyl)-beta-L-fucopyranoside on 14C-glucose uptake. Chem.-Biol. Interact. 2009;179:407–412. doi: 10.1016/j.cbi.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 155.Wang N., Yi W.J., Tan L., Zhang J.H., Xu J., Chen Y., Qin M., Yu S., Guan J., Zhang R. Apigenin attenuates streptozotocin-induced pancreatic β cell damage by its protective effects on cellular antioxidant defense. In Vitro Cell Dev. Biol. Anim. 2017;53:554–563. doi: 10.1007/s11626-017-0135-4. [DOI] [PubMed] [Google Scholar]
- 156.Feng X., Yu W., Li X., Zhou F., Zhang W., Shen Q., Li J., Zhang C., Shen P. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem. Pharmacol. 2017;136:136–149. doi: 10.1016/j.bcp.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 157.Feng X., Weng D., Zhou F., Owen Y.D., Qin H., Zhao J., Wen Y., Huang Y., Chen J., Fu H., et al. Activation of PPARγ by a Natural Flavonoid Modulator, Apigenin Ameliorates Obesity-Related Inflammation Via Regulation of Macrophage Polarization. EBioMedicine. 2016;9:61–76. doi: 10.1016/j.ebiom.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Machado M.V., Michelotti G.A., Xie G., Almeida Pereira T., Boursier J., Bohnic B., Guy C.D., Diehl A.M. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS ONE. 2015;10:e0127991. doi: 10.1371/journal.pone.0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tailleux A., Wouters K., Staels B. Roles of PPARs in NAFLD: Potential therapeutic targets. Biochim. Biophys. Acta. 2012;1821:809–818. doi: 10.1016/j.bbalip.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 160.Cipolletta D., Feuerer M., Li A., Kamei N., Lee J., Shoelson S.E., Benoist C., Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bouhlel M.A., Derudas B., Rigamonti E., Dièvart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 162.Chinetti-Gbaguidi G., Staels B. Macrophage polarization in metabolic disorders: Functions and regulation. Curr. Opin. Lipidol. 2011;22:365–372. doi: 10.1097/MOL.0b013e32834a77b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 164.Guevara I., Iwanejko J., Dembińska-Kieć A., Pankiewicz J., Wanat A., Anna P., Gołabek I., Bartuś S., Malczewska-Malec M., Szczudlik A. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin. Chim. Acta. 1998;274:177–188. doi: 10.1016/S0009-8981(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 165.Salam N.K., Huang T.H., Kota B.P., Kim M.S., Li Y., Hibbs D.E. Novel PPAR-gamma agonists identified from a natural product library: A virtual screening, induced-fit docking and biological assay study. Chem. Biol. Drug Des. 2008;71:57–70. doi: 10.1111/j.1747-0285.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- 166.Zhang T., Yamamoto N., Yamashita Y., Ashida H. The chalcones cardamonin and flavokawain B inhibit the differentiation of preadipocytes to adipocytes by activating ERK. Arch Biochem. Biophys. 2014;554:44–54. doi: 10.1016/j.abb.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 167.Boschi F., Rizzatti V., Zamboni M., Sbarbati A. Lipid droplets fusion in adipocyte differentiated 3T3-L1 cells: A Monte Carlo simulation. Exp. Cell Res. 2014;321:201–208. doi: 10.1016/j.yexcr.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 168.Guo X., Liu J., Cai S., Wang O., Ji B. Synergistic interactions of apigenin, naringin, quercetin and emodin on inhibition of 3T3-L1 preadipocyte differentiation and pancreas lipase activity. Obes. Res. Clin. Pract. 2016;10:327–339. doi: 10.1016/j.orcp.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 169.Chilloux J., Dumas M.E. Are Gut Microbes Responsible for Post-dieting Weight Rebound? Cell Metab. 2017;25:6–7. doi: 10.1016/j.cmet.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 170.Wu L., Guo T., Deng R., Liu L., Yu Y. Apigenin Ameliorates Insulin Resistance and Lipid Accumulation by Endoplasmic Reticulum Stress and SREBP-1c/SREBP-2 Pathway in Palmitate-Induced HepG2 Cells and High-Fat Diet-Fed Mice. J. Pharmacol. Exp. Ther. 2021;377:146–156. doi: 10.1124/jpet.120.000162. [DOI] [PubMed] [Google Scholar]
- 171.Shuster A., Patlas M., Pinthus J.H., Mourtzakis M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012;85:1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bouchi R., Takeuchi T., Akihisa M., Ohara N., Nakano Y., Nishitani R., Murakami M., Fukuda D., Fujita M., Minami I., et al. High visceral fat with low subcutaneous fat accumulation as a determinant of atherosclerosis in patients with type 2 diabetes. Cardiovasc. Diabetol. 2015;14:136. doi: 10.1186/s12933-015-0302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Priceman S.J., Kujawski M., Shen S., Cherryholmes G.A., Lee H., Zhang C., Kruper L., Mortimer J., Jove R., Riggs A.D., et al. Regulation of adipose tissue T cell subsets by Stat3 is crucial for diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA. 2013;110:13079–13084. doi: 10.1073/pnas.1311557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu M., Tso P., Woods S.C. Receptor CD36 links a risk-associated allele to obesity and metabolic disorders. J. Biol. Chem. 2018;293:13349–13350. doi: 10.1074/jbc.H118.004818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tao S., Chunhua H., Chunfang Y., Ting J., Junfang S., Minting C., Sarwat F., Ruihong G., Xianjing H., Zhaoxiang B., et al. Apigenin inhibits STAT3/CD36 signaling axis and reduces visceral obesity. Pharmacol. Res. 2020;152:104586. doi: 10.1016/j.phrs.2019.104586. [DOI] [PubMed] [Google Scholar]
- 176.Dasu M.R., Park S., Devaraj S., Jialal I. Pioglitazone inhibits Toll-like receptor expression and activity in human monocytes and db/db mice. Endocrinology. 2009;150:3457–3464. doi: 10.1210/en.2008-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang W.S., Jeng C.Y., Wu T.J., Tanaka S., Funahashi T., Matsuzawa Y., Wang J.P., Chen C.L., Tai T.Y., Chuang L.M. Synthetic peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care. 2002;25:376–380. doi: 10.2337/diacare.25.2.376. [DOI] [PubMed] [Google Scholar]
- 178.Lehrke M., Lazar M.A. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 179.Nicholas C., Batra S., Vargo M.A., Voss O.H., Gavrilin M.A., Wewers M.D., Guttridge D.C., Grotewold E., Doseff A.I. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J. Immunol. 2007;179:7121–7127. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- 180.Andrade L.M., Andrade C.J., Dias M., Nascimento C.A.O., Mendes M.A. Chlorella and spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements; an overview. MOJ Food Process Technol. 2018;6:45–58. doi: 10.15406/mojfpt.2018.06.00144. [DOI] [Google Scholar]
- 181.Zuin V.G., Ramin L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. Top. Curr. Chem. 2018;376:3. doi: 10.1007/s41061-017-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shoubaky G.A.E., Abdel-Daim M.M., Mansour M.H., Salem E.A. Isolation and Identification of a Flavone Apigenin from Marine Red Alga Acanthophora spicifera with Antinociceptive and Anti- Inflammatory Activities. J. Exp. Neurosci. 2016;10:21–29. doi: 10.4137/JEN.S25096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nguyen V.T. Recovering Bioactive Compounds from Agricultural Wastes. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2017. Potential, uses and future perspectives of agricultural wastes; pp. 1–32. [DOI] [Google Scholar]
- 184.Lucarini M., Durazzo A., Romani A., Campo M., Lombardi-Boccia G., Cecchini F. Bio-Based Compounds from Grape Seeds: A Biorefinery Approach. Molecules. 2018;23:1888. doi: 10.3390/molecules23081888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Singh P., Mishra S.K., Noel S., Sharma S., Rath S.K. Acute exposure of apigenin induces hepatotoxicity in Swiss mice. PLoS ONE. 2012;7:e31964. doi: 10.1371/journal.pone.0031964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Viola H., Wasowski C., Levi de Stein M., Wolfman C., Silveira R., Dajas F., Medina J.H., Paladini A.C. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995;61:213–216. doi: 10.1055/s-2006-958058. [DOI] [PubMed] [Google Scholar]
- 187.Venigalla M., Gyengesi E., Münch G. Curcumin and Apigenin—novel and promising therapeutics against chronic neuroinflammation in Alzheimer’s disease. Neural Regen. Res. 2015;10:1181–1185. doi: 10.4103/1673-5374.162686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ross J.A., Kasum C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 189.Huang Y., Zhao X., Zu Y., Wang L., Deng Y., Wu M., Wang H. Enhanced Solubility and Bioavailability of Apigenin via Preparation of Solid Dispersions of Mesoporous Silica Nanoparticles. Iran. J. Pharm. Res. 2019;18:168–182. [PMC free article] [PubMed] [Google Scholar]
- 190.Calton E.K., James A.P., Pannu P.K., Soares M.J. Certain dietary patterns are beneficial for the metabolic syndrome: Reviewing the evidence. Nutr. Res. 2014;34:559–568. doi: 10.1016/j.nutres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 191.De Felice S.L. The nutraceutical revolution: Its impact on food industry R& D. Trends Food Sci. Technol. 1995;6:59–61. [Google Scholar]
- 192.Santini A., Novellino E. Nutraceuticals: Beyond the diet before the drugs. Curr. Bioact. Compd. 2014;10:1–12. doi: 10.2174/157340721001140724145924. [DOI] [Google Scholar]