Abstract
Stroke is one of the main causes of neurological disability worldwide and the second cause of death in people over 65 years old, resulting in great economic and social burden. Ischemic stroke accounts for 85% of total cases, and the approved therapies are based on re-establishment of blood flow, and do not directly target brain parenchyma. Thus, novel therapies are urgently needed. In this review, limb remote ischemic conditioning (RIC) is revised and discussed as a potential therapy against ischemic stroke. The review targets both (i) fundamental research based on experimental models and (ii) clinical research based on clinical trials and human interventional studies with healthy volunteers. Moreover, it also presents two approaches concerning RIC mechanisms in stroke: (i) description of the underlying cerebral cellular and molecular mechanisms triggered by limb RIC that promote neuroprotection against stroke induced damage and (ii) the identification of signaling factors involved in inter-organ communication following RIC procedure. Limb to brain remote signaling can occur via circulating biochemical factors, immune cells, and/or stimulation of autonomic nervous system. In this review, these three hypotheses are explored in both humans and experimental models. Finally, the challenges involved in translating experimentally generated scientific knowledge to a clinical setting are also discussed.
Keywords: Ischemic stroke, Remote ischemic conditioning, Hormesis, Neuroprotection, Neuroinflammation, Oxidative stress
Introduction
Stroke is one of the main causes of death and disability worldwide, with high social and economic burden [1], being 85% ischemic and 15% hemorrhagic. Ischemic stroke results from a vessel blockage resulting in lack of cerebral blood supply, limiting oxygen and nutrient availability in the brain. The only approved therapies are based on the re-establishment of blood flow in the ischemic area, either by lysis of thrombi with recombinant tissue-activated plasminogen (thrombolysis) or by mechanical removal (endovascular treatment) [2]. Both treatments present limitations: risk of hemorrhage transformation, narrow selection criteria including short time window or need for advanced imaging, and no direct action on brain parenchyma [3–5]. Thus, innovative conceptual and methodological approaches targeting brain parenchyma are urgently needed.
The ischemic brain tissue can be divided in two regions: (i) the ischemic core, where severe ischemia rapidly results in cell necrosis and tissue loss; and (ii) the ischemic penumbra, a surrounding rim of hypoperfused tissue that may remain viable for several hours, or even days. Thus, the main aim of any acute stroke treatment is to salvage the penumbra area by re-establishment of blood flow and/or by limiting neural cell death and neuronal dysfunction. Over the last three decades, great efforts have been made to develop new therapeutic drugs targeting penumbra, namely minocycline, natalizumab, fingolimod, or uric acid, among others. Despite promising pre-clinical results, clinical trials were disappointing [4, 6].
An alternative strategy may be to take advantage of endogenous mechanisms to protect the brain from ischemia. Indeed, the brain can activate several different responses to stress and trigger mechanisms of defense against ischemia. Hormesis, for example, or conditioning (also known as preconditioning), is based on the activation and strengthening of endogenous defense mechanisms. In fact, hormesis or conditioning is a procedure by which a noxious stimulus (such as ischemia), below the threshold of damage, is applied to a tissue or system. Without causing any lesion, conditioning promotes a cellular protective state (tolerance or cytoprotection) against more severe noxious stimuli given beyond the threshold of damage [7].
In 1986, it was demonstrated for the first time the cardioprotective effect of ischemic preconditioning [8]. Five minutes of occlusion followed by 5-min reperfusion of the left anterior descending artery decreased cardiac tissue lesion from 40 min of ischemia [8]. Since then, much experimental and clinical data has been generated concerning cardioprotection and more recently neuroprotection has also been explored mainly in animal models [7, 9]. This protective effect of ischemic preconditioning can also be found when applied to a distant organ or tissue and is called remote ischemic conditioning. It was first described that 5-min period of circumflex branch artery occlusion decreased infarct size in a remote myocardium region [10]. The concept of limb remote ischemic preconditioning was first explored in 1997 by Oxman and colleagues for promoting cardioprotection against reperfusion tachyarrhythmia [11]. For the last two decades, the remote ischemic conditioning procedure has received much attention due to its non-invasive nature, safety, and feasibility in the clinical setting (Fig. 1).
Fig. 1.
Limb remote ischemic conditioning (RIC) and neuroprotection.Scheme for RIC applied in the arm and the potential signaling that confers neuroprotection
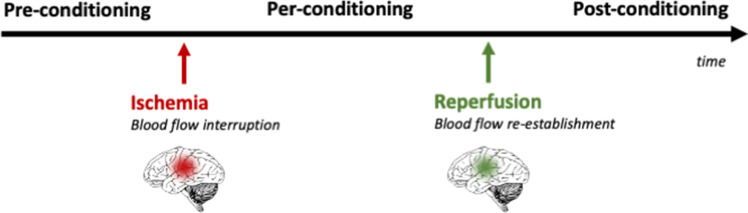
In the context of stroke, conditioning can be divided in three categories depending on timing: preconditioning, per-conditioning, and post-conditioning [7, 12] (Fig. 2). Limb RIC can be applied at several distinct time points, which in turn induce early- (hours) and late- (days) phase conditioning responses. Pre-RIC might be applied as a protective conditioning in the event of a future stroke in at-risk individuals. Per-RIC refers to RIC applied during the early acute stroke phase before reperfusion. Rapid post-RIC might provide neuroprotection when applied immediately after ischemic stroke and initial reperfusion, and delayed post-RIC could be equally applied days after a stroke. Herein, the used nomenclature is remote ischemic conditioning (RIC) in order to encompass pre-, per-, and post-conditioning.
Fig. 2.
Timing for ischemic conditioning.There are three different times for the application of ischemic conditioning in the context of ischemic stroke: pre-conditioning (before ischemia), per-conditioning (after ischemia before reperfusion), and post-conditioning (after the onset of reperfusion)
Major Molecular and Cellular Mechanisms Following Cerebral Ischemia and Reperfusion
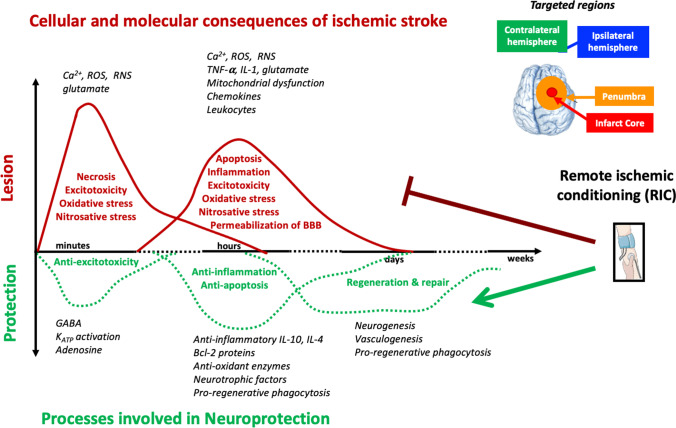
Remote ischemic conditioning can target different cellular and molecular processes occurring during ischemia and reperfusion. Cerebral ischemia causes excitotoxicity, oxidative and nitrosative stress, neuroinflammation, and bioenergy catastrophe, which are associated with neuronal dysfunction, neural cell death, blood–brain barrier permeabilization, and tissue loss (Fig. 3).
Fig. 3.
Chronological cellular and molecular consequences of ischemic stroke and the associated processes involved in neuroprotection.Following ischemic stroke, there is rapid generation of excitotoxicity, necrosis, oxidative, and nitrosative stress in the core of stroke. Later on (hours up to few days), there is apoptosis, neuroinflammation, bioenergy catastrophe, BBB permeabilization, and still oxidative and nitrosative stress, which are more associated with penumbra area of stroke
Excitotoxicity was the first identified molecular mechanism following brain ischemia and is due to excessive and rapid release of the excitatory amino acid glutamate, without the necessary reuptake by astrocytes. Glutamate leads to the activation of N-methyl-D-aspartate (NMDA), amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), and kainate receptors, which in turn promotes great Ca2+ influx [13]. Intracellular Ca2+ accumulation activates lytic enzymes (proteases) and promotes mitochondrial dysfunction. Mitochondria are key organelles for bioenergy production, control of cell death, and generation of reactive oxygen species (ROS); thus, their dysfunction promotes unbalanced bioenergy status, apoptosis and necrosis, and oxidative stress [13]. Likewise, activation of enzymes such as nitric oxide synthase, cyclooxygenase, or NADPH oxidase is coupled with excitotoxicity [4, 14]. Paradoxically, during reperfusion, when blood flow is reestablished, there is a great increase on ROS and reactive nitrogen species (RNS) formation, probably due to the unbalanced re-establishment of oxidative metabolism and by overwhelming the endogenous antioxidant defenses [8]. Exacerbated inflammation occurs in the microvasculature and in the brain parenchyma, including the release of pro-inflammatory and neurotoxic factors along with the accumulation of leukocytes [4]. In response to stress, microglia (the brain resident immune cells) become reactive releasing neurotoxic factors such as TNF-α, IL-1, ROS, or RNS [15]. Nevertheless, microglia are also key players in the clearance of apoptotic cells by phagocytosis, which in turn reduces inflammation and neurotoxicity, promoting re-generation of brain parenchyma [16, 17]. Likewise, oxidative stress, neural cell death, and/or neuroinflammation lead to increased permeability of blood–brain barrier, which in turn exacerbate inflammation by infiltration of more circulating immune cells and pro-inflammatory factors. The main events occurring during ischemia reperfusion are described in Fig. 3.
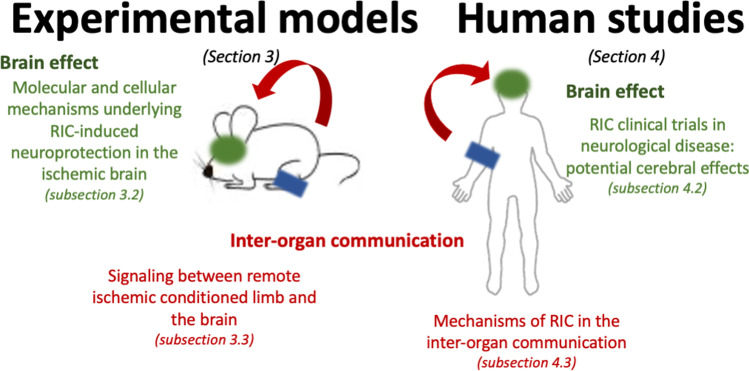
With our review, we aimed to cover experimental models (the “Experimental Models” section) and clinical trials and human interventional studies (the “Human Studies and Clinical Trials of Remote Ischemic Conditioning (RIC)” section). Two main aspects of RIC are approached: (i) RIC-induced protective effect in the brain following stroke and its potential underlying molecular and cellular mechanisms (“Molecular and Cellular Mechanisms Underlying RIC-Induced Neuroprotection in the Ischemic Brain” section for models and “RIC Clinical Trials in Neurological Disease: Potential Cerebral Effects” section for humans) and (ii) how inter-organ communication occurs between limb and brain (“Inter-organ Communication: Signaling Between Remote Ischemic Conditioned Limb and the Brain” section and “Mechanisms of RIC in the Inter-organ Communication” section for experimental models and humans, respectively). The rationale for the review is represented in Fig. 4.
Fig. 4.
Rationale of the review.Organization of data generated by experimental models or human-based studies (including clinical trials). Data were divided in two: description of the underlying cerebral cellular and molecular mechanisms triggered by limb RIC that promote neuroprotection against stroke-induced damage and the identification of signaling factors involved in inter-organ communication following RIC procedure
Experimental Models
In this part of the review, we summarize the cellular and molecular neuroprotective signals and mechanisms, derived from experimental evidence, which support limb RIC as neuroprotective in order to characterize the specific physiology of limb remote preconditioning that will support its translation to future clinical trials. It is organized in two main parts: (i) which are the cerebral mechanisms activated by RIC and (ii) which are the RIC-activated blood circulating factors or autonomic nervous system components that may in turn drive protection in the ischemic brain.
Introduction to Experimental Models
Although the first application in rats of limb RIC as a cardioprotective strategy was shown in 1997, it was only in 2004 that RIC was explored for neuroprotective purposes [18]. Neuroprotection by remote ischemic preconditioning performed on a limb was first demonstrated in a rat model of experimental brain ischemia to induce neuroprotection against ischemia–reperfusion injury, by application of 3 cycles of 10-min RIC and 10-min reperfusion [18]. Neural protection via RIC has since been observed in both young male and female rodent models [19–21] and aged male rats [22].
Molecular and Cellular Mechanisms Underlying RIC-Induced Neuroprotection in the Ischemic Brain
Herein, the RIC-induced cerebral pathways, gene expression, or neural cellular responses that can potentially play a role in neuroprotection are described. The main information is summarized in Table 1 and Fig. 5.
Table 1.
Experimental models of neuroprotection by limb RIC: molecular and cellular mechanisms underlying RIC-induced neuroprotection in the ischemic brain
| Model | Pathway/mechanism researched | Reference |
|---|---|---|
|
Rat: Sprague–Dawley (m/270-330 g) IS model: 30 min BCCAO post-RIC: directly after IS, 3 h or 6 h 3 cycles: 15 min RIC + 15 min RF |
Protein synthesis Afferent nerves |
[23] |
|
Rat: Sprague–Dawley (m/250–280 g) IS model: 1 h MCAO post-RIC: after RF 3 cycles: 10 min RIC + 10 min RF |
HIF-1α | [24] |
|
Rat: Sprague–Dawley (m/250–330 g) IS model: 1 h MCAO, 48 h RF pre-RIC: before MCAO 3 cycles: 15 min RIC + 15 min RF |
Aquaporin-4 | [25] |
|
Mouse: CD1 (adult) IS model: 1 h MCAO post-RIC: after MCAO 3 cycles: 5 min RIC + 5 min RF |
Nrf2, HO-1, NQO-1 Nrf2-ARE pathway oxidative stress |
[26] |
|
Rat: Sprague–Dawley (m/220–280 g) IS model: 2 h MCAO + 24 h RF per-RIC/post-RIC (at RF) 3 cycles: 10 min RIC + 10 min RF |
p38 MAPK-ATF2 pathway | [27] |
|
Rat: Wistar (m/220-250 g) IS model: 20 min BCCAO post-RIC: before MCAO 3 cycles: 10 min RIC + 10 min RF |
HO-1, BDNF, TNFα oxidative stress nitrite neuroinflammation |
[28] |
|
Rat: Wistar (m/220-250 g) IS model: 20 min BCCAO post-RIC: after BCCAO 3 cycles: 10 min RIC + 10 min RF |
nitrite, oxidative stress, lipid peroxidation AChE, BDNF, CREB, GSK-3β, TNFα |
[29] |
|
Rat: Sprague–Dawley (m/280-320 g) IS model: 1.5 h MCAO, 24 h RF pre-RIC: 1 h before MCAO 4 cycles: 5 min RIC + 5 min RF |
AMPK, HSP70, HIF1α, Bcl2, caspase 3 and 9, apoptosis, inflammation, IL-1β, TNFα, IL-6, oxidative damage | [30] |
|
Mouse: C57BL/6 J (m/22-24 g) IS model: 1 h MCAO, 24 h RF post RIC: (after MCAO) 3 cycles: 10 min RIC + 10 min RF |
pSTAT3/STAT3 GFAPα, GS |
[31] |
|
Rat: Sprague–Dawley (m/280-320 g) IS model: 1.5 h MCAO pre-RIC: 24 h before MCAO 3 cycles: 10 min RIC + 10 min RF |
Brain: IL-4, IL-10, IL-1β, IFN𝛾 Blood: HIF-1α Plasma:IL-4, IL-10, IL-1β,IFN𝛾 |
[32] |
|
Rat: Sprague–Dawley (m/250-280 g) IS model: 1 h MCAO, 24 h RF pre-RIC: daily for 3 days 4 cycles: 5 min RIC + 5 min RF Cell model: Primary hippocampal neurons, 30 min pre-OGD (precondition), 3 h OGD + 24 h recovery |
Notch1/NICD/Hes1 IKKβ/NF-kB_p65 |
[33] |
|
Rat: Sprague–Dawley (m/250-300 g) IS model: MCAO 90 min post-RIC: after MCAO 3 cycles: 5 min RIC + 5 min RF |
apoptosis (Bcl-2/Bax) TNF-α, NF-kB, pSTAT3 |
[34] |
|
Rat: Sprague–Dawley (m/20 mo) IS model: 90 min MCAO pre-RIC: 24 h before MCAO 3 cycles: 10 min RIC + 10 min RF |
HIF1A, HIF2A, IL-1β, IL-6, TNFα, IFN-γ, IL-4, IL-10, pAkt, pERK | [22] |
|
Rat: Wistar (m/250-300 g) IS model: global 4-VO + BCCAO 8 min pre-RIC: before IS 3 cycles: 10 min RIC + 10 min RF |
Neuroglobin (Ngb), mt membrane potential, mt Na + /K + -ATPase activity | [35] |
|
Rat: Sprague–Dawley (m/280–320 g) IS model: 1.5 h MCAO + 24 h RF per-RIC, post-RIC: 24 h after RF, daily 14d 3 cycles: 10 min RIC + 10 min RF |
Ngb, apoptosis, ROS | [36] |
|
Rat: Sprague–Dawley (m/280-320 g) IS model: MCAO 2 h pre-RIC: 1 h before MCAO 3 cycles: 5 min RIC + 5 min RF |
adenosine, adenosine A1 receptor (ADORA1), lipid peroxidation, mitochondrial stress, oxidative stress, TNFα | [37] |
|
Mouse: C57BL/6 J (m/12wo) Bmal1 KO IS model: 60 min MCAO pre-RIC: before MCAO 2 cycles: 10 min RIC + 10 min RF |
sleep circadian system |
[38] |
|
Rat: Wistar (m/250–300 g,11-12wo) IS model: 4-VO + BCCAO 8 min pre-RIC: before IS 3 cycles: 10 min RIC + 10 min RF |
adenosine adenosine A1 receptor p-p38 MAPK pERK |
[39] |
|
Rat: Sprague–Dawley (m/260-280 g) IS model: 1.5 h MCAO per-RIC 3 cycles: 10 min RIC + 10 min RF |
pial collateral flow | [40] |
|
Rat: Sprague–Dawley (m/16–18 mo) IS model: MCAO per-RIC: 1 h after MCAO 3 cycles: 15 min RIC + 15 min RF |
collateral flow | [41] |
|
Rat: Sprague–Dawley (m/age NA) IS model: 1 h MCAO, 24 h RF per-RIC: immediately after MCAO 3 cycles: 15 min RIC + 15 min RF |
Apoptosis, MMP-9, MMP-2, p-AMPK, eNOS | [42] |
|
Rat: Sprague–Dawley (m/280-320 g) IS model: 1.5 h MCAO per-RIC, post-RIC: 1d after, daily 7-14d 3 cycles: 10 min RIC + 10 min RF |
arteriogenesis, focal CBF, collateral circulation, Notch signaling | [43] |
|
Rat: Sprague–Dawley (m/220-260 g) IS model: 2-VO (3d hypoperfusion) chronic Post-RIC: after VO, daily 28d 3 cycles: 10 min RIC + 10 min RF |
p-eNOS/eNOS, CBF, angiogenesis | [44] |
|
Mouse: C57BL/6 J (m/10 wo) IS model: BCAS (30 min + 30 min) post RIC: 1w after BCAS, daily 3w, 1-4mo 4 cycles: 5 min RIC + 5 min RF |
Endothelium, VEC (NO), EPC, Macrophages, Angiogenesis, Arteriogenesis | [45] |
|
Rat: Sprague–Dawley (m/250-300 g) IS model: 1.5 h MCAO, 21d RF post-RIC: 2d after MCAO 3 cycles: 10 min RIC + 10 min RF |
Kallikrein 1 bradykinin B2 receptor |
[46] |
|
Mouse: C57BL/6 (m/9-10wo) IS model: 3-VO post-RIC: immediately after 3-VO 3 cycles: 10 min RIC + 10 min RF |
leptomeningeal collateral flow, granulocyte colony‐ stimulating factor (G‐CSF), monocytes/macrophages | [47] |
|
Rat: Sprague–Dawley (m/270-330 g) IS model: 30 min left CCAsO & dMCAO pre-RIC: before CCAsO & dMCAO 3 cycles: 15 min RIC + 15 min RF |
LCN2 Bim BOCT |
[48] |
|
Rat: Sprague–Dawley (m/260-280 g) IS model: 1.5 h MCAO post-RIC: after MCAO 3 cycles: 10 min RIC + 10 min RF |
synaptogenesis PSD95 GAP43 Synapsin1 |
[49] |
|
Rat: Wistar (m/adult) IS model: Modified 2-VO post-RIC: 1w after IS, 4x/day for 2 weeks 4 cycles: 5 min RIC + 5 min RF |
Autophagolysosomal pathway, TFEB, Apoptosis | [50] |
|
Mouse: C57BL/6 (sex NA/adult 8-9wo) IS model: 90 min MCAO, 48 h RF pre-RIC: 48 h before MCAO 3 cycles: 3 min RIC + 5 min RF |
COX-IV, HSP60, EndoG/AIF Apoptosis, MDV |
[51] |
|
Rat: Wistar (m/200-250 g) IS model: 4-VO + 8 min BCCAO pre-RIC: before IS 3 cycles: 10 min RIC + 10 min RF |
hippocampus CA1 apoptosis (DNA fragmentation, apoptotic bodies) |
[18] |
|
Rat: Wistar (m/280-320 g) IS model: global 4-VO + BCCAO 8 min pre-RIC: before IS 3 cycles: 10 min RIC + 10 min RF |
hippocampus CA1, CA3/DG MEK-1/pERK1/2 neural cell death |
[52] |
|
Rat: Wistar (m/250-300 g) IS model: global 4-VO + BCCAO 8 min pre-RIC: before IS 3 cycles: 10 min RIC + 10 min RF |
hippocampus CA1 p38 MAPK neural cell death |
[53] |
|
Rat: Wistar (m/250-300 g) IS model: global 4-VO + BCCAO 8 min pre-RIC: before IS 3 cycles: 10 min RIC + 10 min RF |
p38 MAPK/HSP 70 | [54] |
|
Mouse: C57BL/6 J (m/20wo) IS model: eMCAO (embolic) per-RIC (2 h after eMCAO) 5 cycles: 5 min RIC + 5 min RF Condition: tPA 4 h after eMCAO |
Infarct size, CBF, pAkt | [20] |
|
Rat: Sprague–Dawley (m/adult) IS model: 8 min 4-VO post-RIC: after IS 4 cycles: 5 min RIC + 4 min RF |
Apoptosis (Bcl-2/Bax), NO, eNOS, p-eNOS, PI3K-pAkt/p-eNOS | [55] |
|
Rat: Sprague–Dawley (m/300-320 g) IS model: MCAO 2 h + post-RIC: 0 min,10 min, 30 min after IS 3 cycles: 10 min RIC + 10 min RF |
Akt/GSK3b-dependent autophagy | [56] |
|
Rat: Sprague–Dawley (m/adult) IS model: focal MCAO 90 min + 72 h RF post-RIC: 3 h-6 h after RF 3 cycles: 5 min RIC + 4 min RF |
KATP channels | [57] |
|
Rat: Wistar (m/f, 250-350 g) IS model: global 4-VO 10 min delayed post-RIC: 20 min RIC, 2d after IS |
very delayed post RIC | [21] |
|
Rat: C57BL/6 J (ovariectomized f/20wo) IS model: eMCAO (embolic) per-RIC (2 h after eMCAO) 5 cycles: 5 min RIC + 5 min RF |
Infarct size, CBF | [19] |
|
Rat: Sprague–Dawley (m/280-320 g) IS model: 120 min MCAO, 3 h and 24 h RF per-RIC (10 min after MCAO) 4 cycles: 10 min RIC + 10 min RF |
Apoptosis, Autophagy-lysosome pathway (ALP) | [58] |
|
[66]Rat: Sprague–Dawley (m/300–320 g) IS model: 2 h MCAO + 22 h RF per-RIC/post-RIC (I-30 min, RF-0) 3 cycles: 10 min RIC + 10 min RF |
Akt/p-Bcl-2/Beclin activation of autophagy | [59] |
|
Rat: Sprague–Dawley (f/250-280 g) IS model: 1 h MCAO post-RIC: after MCAO 3 cycles: 10 min RIC + 10 min RF |
TLR4/NF-кB pathway (…inflammation/cytokine production…) |
[60] |
|
Rat: Sprague–Dawley (m/260-280 g) IS model: 1.5 h MCAO post-RIC: after MCAO 3 cycles: 10 min RIC + 10 min RF |
neurogenesis | [61] |
|
Rat: Sprague–Dawley (m/300–320 g) IS model: 2 h MCAO, 24 h RF post-RIC: after MCAO, before RF 3 cycles: 10 min RIC + 10 min RF |
pAkt, fibulin 5, claudin, occludin, BBB | [62] |
|
Rat: Sprague–Dawley (m/250–280 g) IS model: 2 h MCAO, 24 h RF post-RIC: after MCAO, before RF 3 cycles: 15 min RIC + 15 min RF |
mTOR p70S6K |
[63] |
|
Mouse: C57BL/6 J (m/20-25 g) IS model: 2 h MCAO post RIC: after MCAO 3 cycles: 10 min RIC + 10 min RF |
p-AMPKα, p-mTOR, p-ACC, p-ULK1, autophagy, apoptosis | [64] |
|
Rat: Sprague–Dawley (f/16wo) IS model: 1 h MCAO post-RIC: just after MCAO 3 cycles: 10 min RIC + 10 min RF |
BBB permeability, MMP-9, claudin-5, GFAP | [65] |
|
Mouse: C57BL/6 (8–10 wo) IS model: 45 min MCAO pre-, early pre-, per-, post- RIC: 4 cycles 5 min RIC + 5 min RF |
collateral circulation | [66] |
|
Rat: Sprague–Dawley (m/250-280 g)) IS model: 90 min MCAO post-RIC: just after MCAO 1–3 cycles: 5/10/15 min RIC + 5/10/15 min RF |
BBB permeability, apoptosis | [67] |
Fig. 5.
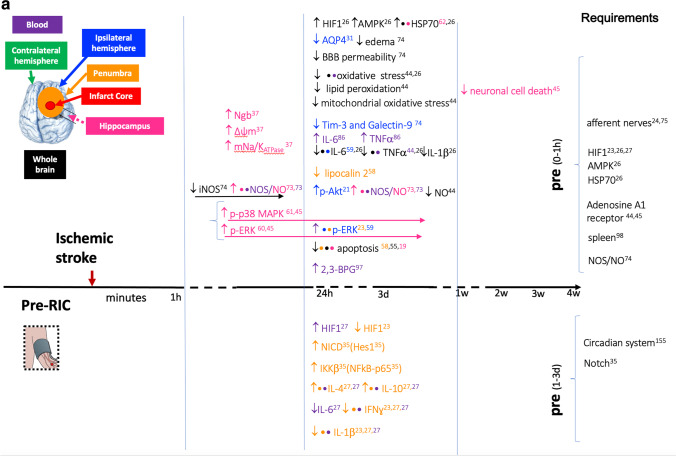
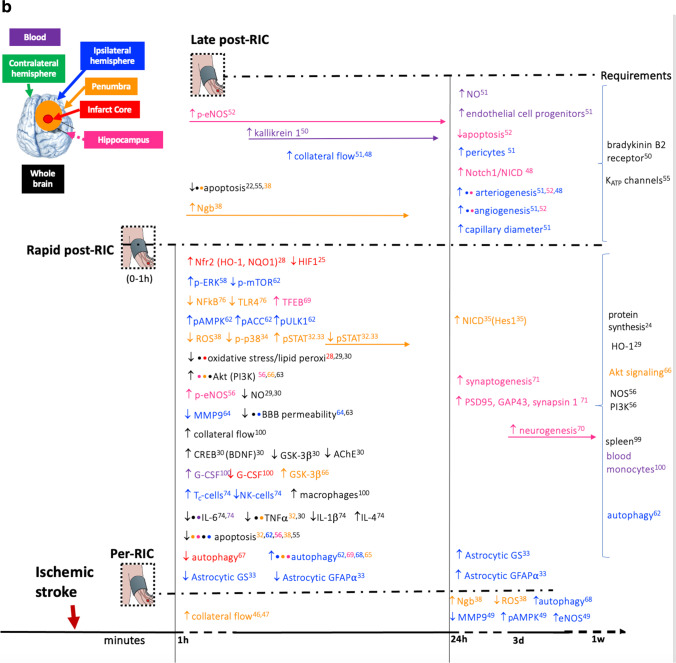
A Pathways and gene expression that are altered by limb pre-RIC following ischemic stroke and that are related to neuroprotection in experimental models.There is a timeline with the altered pathways and gene expression that occurs when limb RIC is applied before the ischemic stroke (pre-RIC) in comparison with ischemic stroke without RIC. When pre-RIC is induced up to 1h before ischemic stroke, the altered events are represented in the upper part of the figure. In the lower part of the figure, there are the events occurring when pre-RIC is induced between 1h and 3 days before ischemic stroke. In the right hand side, the required conditions for pre-RIC to protect the brain against ischemic stroke are described. The altered pathways and different gene expressions are described accordingly with brain region that is represented by different colors. The reference number is described in Tables 1 and 2. The used symbols are for upregulated/increased and for downregulated/reduced. B Pathways and gene expression that are altered by limb per- and post-RIC following ischemic stroke and that are related to neuroprotection in experimental models.There is a timeline with the altered pathways and gene expression that occurs when limb RIC is applied after the ischemic insult before reperfusion (per-RIC) and after ischemia and reperfusion up to 1h (rapid post-RIC) or at later stages (post-RIC). In all three cases, alterations in pathways and gene expression are compared with ischemic stroke without RIC treatment. In the right hand side, the required conditions for per- and post-RIC to protect the brain against ischemic stroke are described. The altered pathways and different gene expressions are described accordingly with brain region that is represented by different colors. The reference number is described in Tables 1 and 2. The used symbols are for upregulated/increased and for downregulated/reduced
Involvement of Protein Synthesis and Transcriptional Regulation
The protein synthesis inhibitor cycloheximide was shown to block the neuroprotective effects of limb post-RIC in rat [23] indicating that de novo protein synthesis is required for neuroprotection via limb RIC.
Activation of the transcription factor hypoxia-inducible factor 1α (HIF-1α) is triggered by lack of oxygen and coordinates adaptation to hypoxia via transcriptional regulation of more than 200 genes. HIF-1α mRNA expression is upregulated in ischemic cerebral cortex 24 h after IS but declines to control level within 3 days, but protein remains in both neurons and astrocytes for up to 7 days post-ischemia in a rat model of experimental ischemic stroke (IS) [24]. Post-RIC, applied immediately after IS, inhibited HIF-1α mRNA expression in ischemic cerebral cortex by around 50% within 24 h, and protein up to the third day after IS [24]. In another rat model using pre-RIC, 1 h before IS, overall, the protein expression of HIF-1α was increased in whole brain extracts after IS, and increased a further twofold in the pre-RIC model [30]. In aged rats, HIF1α and HIF2α are also upregulated in ischemic penumbra 48 h after IS but not when pre-RIC is applied 24 h before IS [22]. HIF1α was also increased in whole blood after post-RIC [32]. Exogenous systemic activation of HIF-1α, by IP injection 60 min after reperfusion (RF), in a rat model of IS, mimics the neuroprotective effects as well as the pro- and anti-inflammatory cytokine levels in ischemic brain seen in post-RIC, while inhibition of HIF-1α in post-RIC abolished these effect [32].
Intravenous administration of a HIF-1α inhibitor has also been shown to reverse the neuroprotective effects of pre-RIC within 24–48 h, described as decreased neurological deficit scores, lower brain water content, and increase HSP70 protein expression, and reversed inflammatory cytokine profiles in brain and peripheral blood, as seen in rat after pre-RIC [22, 30].
The full significance to neuroprotection of HIF1α inhibition by post-RIC specifically in the ischemic cerebral core, but overall increase in pre-RIC whole brain extract, remains to be fully clarified; it may reflect differential action of different RIC procedures or regional brain differences [30].
Another transcription factor that regulates endogenous antioxidant capacity is nuclear factor erythroid 2-related factor 2 (Nrf2/NFE2L2), which was upregulated in ischemic cortex, by post-RIC in the CD1 mouse model 24 h after IS [26]. In the nucleus, Nrf2 binds to the antioxidant response element (ARE) on target genes initiating expression of cytoprotective genes including NAD(P)H quinone dehydrogenase 1 (NQO1), a reductant, and heme oxygenase-1 (HO-1/HMOX1) that degrades heme into iron ions, biliverdin, and antiapoptotic CO. Expression of both NQO1 and HO-1 is upregulated by post-RIC 24 h after IS [26]. However, inhibition of HO-1 in post-RIC abolished the neuroprotective effects of post-RIC [28], including the upregulation of BDNF (involved in hippocampal neurogenesis and brain plasticity); hippocampal structural abnormalities; and the decrease in the pro-inflammatory cytokine TNFα which are triggered by post-RIC [28]. The protein expression of cAMP response element-binding protein (CREB), which was reduced in whole brain at 72 h in a rat model of IS, was rescued by post-RIC [29]; this may account for the increased expression of BDNF observed after post-RIC, as BDNF harbors CREB response elements on its promoter. Increased glycogen synthase kinase 3 beta (GSK3β) protein expression is observed after IS in rat brain model after 72 h, being partly rescued by post-RIC [29].
The glial water channel aquaporin-4 (AQP4), a downstream target of hypoxia-inducible factor 1 alpha (HIF-1α) and inflammatory cytokines, is upregulated in ischemic hemisphere of rat model of IS, at 48 h, and downregulated by pre-RIC [25]. This could be attributed to HIF1α inhibition and explain reduced edema by pre-RIC.
Several pathways that are activated by cytokines or growth factors and regulate gene expression have been shown to be involved in limb RIC. A twofold increase in phosphorylated STAT3 was observed in rat ischemic penumbra 24 h after middle cerebral artery occlusion (MCAO), rising to fourfold after 3 days and still at 2.5 fold after 14 days [31, 34], while 24 h after post-RIC pSTAT3 is initially higher than in IS, but by 3 days, the levels are down to half that in IS, decreasing even more after 14 days [31, 34]. This suggests that endogenous neuroprotection may act via activation of the pSTAT3 transcription factor at early stages (24 h after IS) but deactivation 3–14 days after IS.
The neuroprotective effects of post-RIC on penumbra region may also be linked to suppression of another transcription factor p-ATF2 via the p38 MAPK-ATF2 pathway [27]. Mechanistically, p-ATF2 would activate transcription of genes by binding cAMP-responsive elements (CRE) and might also be a histone acetyltransferase, thus directly affecting chromatin.
Also implicated in limb RIC neuroprotection against IS in rats are NF-κB pathways [33] that regulate the transcription of over 150 genes controlling inflammation, immune cell development, cell cycle, proliferation, and cell death, including the expression of cytokines, chemokines, immunoreceptors, and regulators of proliferation and apoptosis. NF-kB can be activated by a variety of intracellular and extracellular signals, including TNFα. In the context of limb RIC neuroprotection against IS in rat, NF-kB pathways activated by limb RIC were shown to be dependent on activation of upstream Notch1 signaling and downstream Notch regulated transcription of Hes1 [33]. Curiously, in the latter model, in the absence of limb pre-RIC, inhibition of Notch1 by itself reduced infarct volume, improved neurological deficit score, and attenuated apoptosis in hippocampus, evidence that neuroprotection by Notch1 activation or inhibition is not linear.
Neuroglobin
Neuroglobin (Ngb) is a major player in mediating neuroprotective effects of limb RIC. Ngb, an oxygen-binding heme protein found in the brain that can also be detected in serum [68], is increased in rodent brain neurons following hypoxia suggesting that it may play a role in the brain’s response to hypoxic-ischemic injury. In rat, pre-RIC, increased Ngb expression, was associated with neuroprotection, and improved mitochondrial Na+-K+-ATPase activity and mitochondrial membrane potential after global ischemia [35]. In rat, per-RIC, increased Ngb in peri-infarct region, was only observed 1 h after IS [36], while daily repeated post-RIC for 14 days resulted in sustaining increased levels of Ngb. The redox state of Ngb during acute hypoxia regulates the stability of the transcription factors HIF-1α and Nrf2 as well as release of cytochrome c from mitochondria that triggers apoptosis [69].
Purinergic Signaling
Adenosine plays an important role in the endogenous neuroprotective mechanisms induced by RIC. While extracellular ATP is in the nanomolar range, intracellular ATP is in the millimolar range, so that damage to cell membranes during trauma (like an ischemic event) leads to massive increase in extracellular ATP, and rapid formation of adenosine which can activate four distinct subtypes of G-protein-coupled receptors, named A1, A2A, A2B, and A3 [70, 71]. Adenosine A1 and A2A receptors in particular have inhibitory functions in most tissues including the brain, slowing metabolic activity and reducing synaptic vesicle release [72] placing brain cells in a neuroprotective quiescent state.
Circulating adenosine is also an endogenous distress signal that modulates tissue damage and repair [72, 73]. In rat Hu et al. 2012 showed that limb pre-RIC, performed 1 h before experimental IS, resulted in reduced systemic and cerebral inflammation and oxidative stress just 1 h after the IS, and that this was dependent on activation of adenosine A1 receptors throughout the organism [37]. Another study has also shown that an adenosine A1 receptor antagonist dose-dependently blocked pre-RIC-induced brain ischemic tolerance and delayed neuronal cell death up to 7 days post-IS [39], supporting the premise that adenosine signaling through adenosine A1 receptors is required for pre-RIC neuroprotection. The same study showed that femoral injection of adenosine, 10 min prior to IS, mimics the neuroprotective effects of pre-RIC, dose-dependently attenuating neuronal cell death and increasing expression of both p-p38 MAPK and pERK in CA1 hippocampus 12 h after IS [39].
Brain Blood Flow and Blood Vessel Development
Limb RIC increases brain blood flow and promotes blood vessel development. Transient localized ischemic stroke, by its very nature, causes reduced regional cerebral blood flow, which in turn leads to oxidative stress, while the reperfusion that follows aggravates the ischemic damage through increased production of reactive oxygen species (ROS). In a rat model of MCAO, both per-RIC and post-RIC were shown to prevent collateral vessel collapse and increase collateral flow into the middle cerebral artery which may help rescue the penumbral region, the marginal zone around the ischemic core, which can be in part rescued by RIC [40, 41]. Post-RIC applied daily for 7 days increased focal cerebral blood flow in the ischemic ipsilateral hemisphere [43]; after 14 days, arteriogenesis was observed, specifically in the form of growth of functional leptomeningeal collateral arteries [43] suggesting that RIC reinforces the development of supplemental networks of vessels to compensate defective blood flow.
Ischemic stroke and RIC also affect expression of matrix metalloproteinases (MMPs) in the brain, which may be linked to changes in tissue and/or vessel remodeling. Ischemic stroke modeled in rat by itself increases expression of MMP-2 and MMP-9 [42], while per-RIC, immediately after 1-h MCAO, significantly reduced MMP-9 but not MMP-2 expression and activity in ipsilateral ischemic hemisphere after 24 h [42].
Post-RIC performed 2 days after IS in rat resulted in upregulation of endogenous tissue kallikrein (TK), detected in plasma from day 3 for 21 days, an enzyme which produces Lys-bradykinin, a potent vasodilator that contributes to hypotension in systemic circulation [46]; the latter study also showed that the selective bradykinin B2 receptor antagonist HOE-140 reverses the neuroprotective effects of post-RIC.
Chronic post-RIC, applied daily for 3 weeks in rat, increased capillary density and diameter, angiogenesis, arteriogenesis and collaterals, as well as expression of pericytes colocalized with cerebral blood vessels, concomitant with increased endothelial cell progenitors in blood [45]. In this context, the highly conserved Notch signaling pathway, regulating cell proliferation, differentiation, and apoptosis, appears to be central for RIC neuroprotection via activation of blood vessel plasticity. Chronic limb post-RIC in rat for 21 days was shown to increase angiogenesis in CA1 hippocampus [44], and in 14 days, it increased arteriogenesis concomitant with increased Notch1 and NICD expression in the ischemic brain arteries in rat after IS [43]. Pre-activation of Notch1 and downstream NF-κB pathways in neurons also appears to be required for successful neuroprotective effect of pre-RIC against cerebral I/R injury [33].
Vascular cognitive impairment (VCI) is caused by brain hypoperfusion; it presents similar mechanisms to ischemic stroke and constitutes a major aging-related public health concern, with no available treatment. Bilateral common carotid artery stenosis is used as a model of VCI, since it induces hypoperfusion by decreasing cerebral blood flow, which in turn causes accumulation of amyloid and promotes white matter loss [74]. When post-RIC was applied daily for 2 weeks, there was an improvement in cerebral blood flow and a reduction of neurodegenerative features such as amyloid beta accumulation, inflammation and cell death [74].
Antioxidant
Neuroprotection by limb RIC acts by reducing oxidative stress. The brain’s high oxidative metabolic rate and high lipid content make it a vulnerable target for ischemia-derived ROS that causes general oxidative damage and lipid peroxidation. In rat, limb pre-RIC applied 1 h before MCAO was shown to reduce mitochondrial and overall oxidative stress, lipid peroxidation, oxidative DNA damage, and oxidative damage to proteins in whole brain 24 h later [30, 37]. Combination of per-RIC and chronic post-RIC for 3 days also reduced ROS in peri-infarct region but did not have an additive effect when used in combination [36]. In a mouse CD1 model of IS, post-RIC reduced oxidative stress, as determined by increased activity of super oxide dismutase, and reduced lipid peroxidation, as determined by lower levels of MDA (malondialdehyde) in ischemic cortex 24 h after IS [26]. In a rat post-RIC model, 72 h later, lower lipid peroxidation (MDA), and nitrite, and increased antioxidant GSH were observed, witness to post-RIC effects on reducing oxidative stress even after 3 days [29]. In a cellular model, 10 min of post-RIC human plasma (high molecular weight dialysate hydrophobic fraction) has also been shown to reduce apoptosis and oxidative stress of human neural stem cells in culture when subjected 24 h of oxygen glucose deprivation [75].
Neuronal Cell Death and Apoptosis
Marked increase in neuronal apoptosis is observed in the hippocampal CA1 region in animal models of transient global ischemia that mimic ischemia/reperfusion (IS/RF) injury and delayed neuronal death induced by a cerebral ischemic insult such as an ischemic stroke. Several studies have shown that limb remote ischemic conditioning (limb RIC) reduces neuronal apoptosis in the CA1 hippocampus region (72 h after IS/RF), when applied before experimental stroke as limb pre-RIC [18], and in whole brain at 48 h [51] concomitant with improved glucose metabolism, or at 24 h [30] witnessed by increased Bcl2 and reduced caspases 3 and 9. Likewise, immediately after stroke (MCAO 90 min) as limb rapid post-RIC (3 cycles: 5 min RIC + 4 min RF) [57]; or in delayed post-RIC, 3 h and 6 h after focal IS/RF [57], as evidenced by DNA fragmentation and or apoptotic body formation. Very significant neuroprotection in CA1 hippocampal region in rat was also observed by a single 20-min limb RIC, applied as very delayed post-RIC 2 days after a global 10-min ischemic insult [21]. Increased Bcl-2/Bax ratio has been consistently observed in rat 24–48 h after post-RIC [34, 55] indication that reduced neuronal cell death in limb RIC is at least in part regulated by reducing apoptosis. Combination of per-RIC and chronic post-RIC for 14 days does not have an added effect on reduced neural apoptosis in peri-infarct region [36]. Reduced neuronal cell death was also observed after 21 days of chronic post-RIC [44]. In rat, increased production over 72 h of the pro-apoptotic protein Bim was observed in neurons, which was reversed by pre-RIC in salvage area [48].
Mitochondrial function and integrity in CA1 hippocampus region are compromised by ischemia/reperfusion. This has been observed in rat by deterioration of mitochondrial membrane potential, a marker of neuronal cell death, and reduced mitochondrial Na + -K + -ATPase activity, both of which are improved by pre-RIC [35].
In a rat model of delayed post-RIC, up to 6 h, blockade of potassium channels was shown to reduce the neuroprotective and antiapoptotic effects of post-RIC on the brain, while potassium channel activation mimicked the antiapoptotic and neuroprotective effects of post-RIC [57]
MAPK, AMPK, and PI3K-Akt Pathways
Several mitogen-activated protein kinase (MAPK) cascades have been shown to play a role in neural ischemic conditioning. pERK was decreased after IS in mouse [76] and aged rats [22] while pre-RIC greatly increased pERK levels in ischemic ipsilateral hemisphere [22, 76], as did post-RIC though to a lesser extent [76]. Neuroprotection was also shown to be dependent on MEK-1 dependent increase in pERK1/2, as observed in CA1 hippocampus region 6 h after pre-RIC and 8-min MCAO, peaking at 12 h and returning to normal after 5 days [52]. Ischemic tolerance induced by pre-RIC has also been shown to be dependent on activation of the p38 MAPK cascade at 6 h, peaking at 12 h and normalizing 1 day after pre-RIC in CA1 hippocampus [53] at least partly by downstream upregulation of HSP 70 expression which is detected 1 day later and peaks 2 days after pre-RIC [54]. Limb pre-RIC-induced upregulation of both p-p38 MAPK and pERK in CA1 hippocampus 12 h after IS appears to be adenosine dependent [39]. These activations may also be triggered by cytokines or growth factors inducing activation of p38 MAPK or ERK1/2 that translocate to the nucleus to trans-activate transcription factors regulating expression of genes involved in several pathways involving differentiation and cell survival.
AMPK is activated when AMP/ATP or ADP/ATP ratios in cells rise due to physiological stresses, including ischemia. In rat, AMPK protein in whole brain [30] and p-AMPK in ischemic ipsilateral hemisphere [42] were increased 24 h after MCAO alone, and increased further in pre-RIC performed 1 h before MCAO [30] or after per-RIC [42]. Inhibition of AMPK reversed the neuroprotective effects of pre-RIC, namely, the better neurological deficit scores, lower brain water content, and increase HSP70 protein expression observed after pre-RIC [30].
In a post-RIC mouse model of IS, AMPK pathway was also activated in the cerebral cortex, 12 h after RF, as witnessed by increased p-AMPK, p-ACC, and p-ULK1 and decreased p-mTOR [64]. In per-RIC performed immediately after 1-h MCAO in rat, p-AMPK was increased more than twofold after 24-h reperfusion [42].
Limb RIC also interferes with PI3K-Akt pathway. Results relating to activation of Akt in IS and RIC are not always consistent. Reduction of pAkt was observed in ischemic hemisphere in mouse brain 48 h after experimental embolic stroke (2-h eMCAO) [20], 45-min MCAO [76], or 90-min MCAO in aged rats [22], which was partially rescued by pre-RIC 24 h before MCAO, per-RIC (2 h after eMCAO), or tPA (4 h after eMCAO), per-RIC and tPA together having an added effect on pAkt rescue [20] but not by pre-RIC or post-RIC in a diabetic mouse model of IS [76] or in presence of HIF inhibitor [22]. In a rat model using 8-min global cerebral ischemia, no change was observed in pAkt in hippocampal CA1 region 48 h after reperfusion, while rapid post-RIC-induced activation of pAkt [55]. However, in a rat model of post-RIC performed immediately after 2-h MCAO, both pAkt1(Thr308) and pAkt1(Ser473) were highly upregulated 24 h after post-RIC [62]. In aged rats, pAkt was increased 48 h after IS and pre-RIC had no effect [22]. PI3K/Akt enhanced signaling in limb RIC may be responsible for decreased blood–brain barrier permeability via fibulin-5 [62], as pAkt-dependent fibulin-5 overexpression was observed 24 h after post-RIC with concomitant fibulin-5-dependent claudin-5 and occludin post-RIC upregulation [62, 65]. Post-RIC in rat also reduced the increase in matrix metalloproteinase 9 (MMP-9) that is observed after IS which is implicated in BBB breakdown [65].
Autophagy
In a mouse model of ischemic stroke, limb post-RIC activates autophagy in cerebral cortex of right hemisphere, as witnessed by increased LC1-II/LC3-I, Beclin-1, and Atg7, and decreased SQSTM1/P62 at 12 h, and reduces apoptosis [64]. Inhibition of autophagy using intracerebroventricular (ICV) injection of 3-methyladenine (3-MA) reverses the neuroprotective effects of limb post-RIC observed after 12 h [64] as witnessed by increased neurological deficit score, brain water content, infarct volume, and apoptosis. Post-RIC-induced autophagy, in cerebral cortex of right hemisphere, was also dependent on AMPK pathway activation, as demonstrated by reversion of the post-RIC observed increase in p-AMPKα, p-ACC, and p-ULK1, and decrease in p-mTOR, using AMPK inhibitor compound C [64].
Experimental results measuring autophagy can vary according to the brain region observed. In a rat model, 2-h MCAO did not change amount of autophagosomes in penumbral tissue 24 h after RF, but immediate rapid post-RIC (3–4 cycles: 10 min RIC + 10 min RF) increased amount of autophagosomes and autophagy-lysosomal pathway in penumbral tissue 24 h after reperfusion [58] in a p(S473)Akt/p(S9)GSK3β-dependent manner [56]. In an identical model of rapid post-RIC (3 cycles: 15 min RIC + 15 min RF), MCAO increased amount of autophagosomes in ischemic cortex which was decreased 24 h after post-RIC [63]. Therefore, it appears that in penumbral tissue, autophagy is activated only by post-RIC, whereas in ischemic cortex, autophagy is activated during I/R and reduced by post-RIC. In rat ischemic hemisphere, both per-RIC and post-RIC promote Akt-dependent S70 phosphorylation of Bcl-2 triggering dissociation of Bcl-2/Beclin1 complex and Beclin-1-dependent autophagosome formation [59].
In rat ischemic cortex of the right middle cerebral artery region, autophagy was also shown to be increased during acute ischemia/reperfusion injury via reduced mTOR/p70S6K signaling [63]. Conversely, autophagy was reduced in rat, in response to limb post-RIC during reperfusion, via increased mTOR/p70S6K signaling [63]. The question remains as to how signals from the limb post-RIC trigger this reduced autophagy. Limb post-RIC, in a rat model of chronic cerebral ischemia, was shown to attenuate neural damage in cortex and hippocampus via activation of transcription factor EB (TFEB), a driver of autophagy via the autophagolysosome pathway [50].
Neurogenesis
In addition to reducing infarction size and improving functional outcome, post-RIC in rat has been shown to induced neurogenesis for up to 28 days, in rat after 90-min IS, in the hippocampus region where adult neurogenesis occurs, the subgranular zone (SGZ), and the subventricular zone (SVZ) [61]. Rapid post-RIC in rat was also shown to promote synaptogenesis in ischemic penumbra and to increase expression, in the infarct cortex, of Post-Synaptic Density Protein 95 (PSD95) that is critical for synaptogenesis and synaptic plasticity, Growth Associated Protein 43 (GAP43), a major component of growth cones of elongating axons, and synapsin involved in regulation of axonogenesis and synaptogenesis [49].
Nitric Oxide Synthase (NOS)/Nitric Oxide signaling
In rat, experimental ischemic stroke alone does not change endothelial nitric oxide synthase (eNOS) expression in ischemic ipsilateral hemisphere compared to contralateral hemisphere, but per-RIC performed immediately after 1-h MCAO, resulted in eNOS increase after 24-h reperfusion in ischemic hemisphere compared to MCAO alone [42]. In another rat model using 8-min global cerebral ischemia, limb pre-RIC increased NO and NOS activity following a double peak pattern in both serum (0 h and 48 h) and CA1 hippocampal region (6 h and 48 h) [77]. Limb rapid post-RIC increased eNOS expression and activation in hippocampal CA1 region 48 h after ischemic stroke (both p-eNOS(S1177) and eNOS), along with increased pAkt, in a PI3K-dependent manner, the neuroprotective effects (reduced apoptosis and increased neuronal density in CA1 and reduced behavioral deficits) of rapid post-RIC being abolished by both non-selective NOS or PI3K inhibitors [55], indicating that eNOS activation in this CA1 region occurs, at least in part, via PI3K dependent phosphorylation of pAkt. In chronic post-RIC, applied daily, plasma nitrite levels were increased at 3 weeks, but no longer at 1 month or 4 months [45]. In a model of cerebral hypoperfusion, p-eNOS rose in CA1 hippocampus at day 1 and maintained for 2 weeks declining at 3 weeks, but chronic post-RIC applied daily for 28 days sustained p-eNOS beyond 4 weeks [44].
In another rat model of focal ischemia, iNOS expression doubled, from 1 to 24 h after ischemia, in pooled brain tissue (pooled ischemic core, penumbra, and cortex), and pre-RIC completely inhibited iNOS expression below normal levels [78], indication that RIC blocks the pro-inflammatory expression of iNOS in IS. Twenty-four hours after stroke, a reduced nitrite/nitrate ratio was observed in rat whole brain with limb pre-RIC applied 1 h before 2-h MCAO, indicating reduced inflammation in the whole brain [37]. These results indicate that limb RIC globally reduces the pro-inflammatory iNOS/NO production, but locally (e.g., in hippocampal C1 region), PI3K-Akt/p-eNOS/NO is involved in signaling that triggers conditioning pathways of neuroprotection that reduce apoptosis and increase neuronal density in CA1.
Neuroinflammation
Limb RIC reduces brain neuroinflammation. Oxidative stress precipitated by ischemic stroke in the brain results in chronic expression of pro-inflammatory genes, which can further aggravate neuronal injury. In rat, limb pre-RIC applied immediately before ischemia was shown to inhibit brain edema and blood–brain barrier permeability measured 2 days after stroke [78]. Rat models of ischemic stroke have shown that pro-inflammatory markers and cytokines such as MPO (myeloperoxidase), TNFα, IL-1β, and IL-6, known to be releases by activated microglia, are all highly increased in whole brain tissue 24 h after IS [30, 34]; IL-1β and IFNγ are increased in ischemic penumbra 48 h after IS but not with pre-RIC 24 h before IS, while no changes were observed in IL-6, TNFα, IL-4, and IL-10 [22]; but 72 h after IS, TNFα [29] was increased in whole brain homogenate. In another study, 48 h after IS in rat cortical penumbra surrounding the ischemic core, IL-1β and IL-6 are not changed, but IL-1β is decreased by post-RIC; while IS-induced decrease of anti-inflammatory cytokines IL-4 and IL-10 in cortical penumbra is reversed by post-RIC [32]. An increase in IL-6 and TNFα in ischemic ipsilateral hemisphere was observed 48 h after reperfusion in a diabetic mouse model of IS, which for IL-6 was partially compensated for by pre-RIC but not post-RIC [76], while no changes were observed in IL-1β, IFN-γ, or IL-4 before or after IS or RIC.
Pre-RIC, applied 1 h before IS, reverses this increase for MPO and IL-6, and significantly reduced this increase for IL-1β and TNFα [30]. Reduction in TNFα was also observed in ischemic penumbra after 1 h using rapid post-RIC [34] and in whole brain 72 h after post-RIC [29]. In ischemic brain 48 h after post-RIC, anti-inflammatory cytokines IL-4 and IL-10 are increased, and pro-inflammatory cytokines IL-1β and IFNγ are decreased while no change is seen in IL-6 [32]. In whole brain, IL-6 and IL-1β mRNA are markedly increased by ischemic stroke after 48 h, while post-RIC attenuates this increase [79]. IL-4 mRNA appears to be decreased in whole brain after ischemia and markedly increased 48 h after post-RIC [79]. In ischemic ipsilateral hemisphere of a diabetic IS model, 48 h after reperfusion, helper T cells (CD4 +), cytotoxic T cells (CD8a +), and NK cells increase is compensated by pre-RIC, while post-RIC also markedly decreased cytotoxic T cells and NK cells, having no effect of helper T cells [76].
After ischemic stroke in rat, increased production over 72 h of lipocalin 2 (LCN2) from reactive astrocytes in salvage area is reversed by pre-RIC [48]. Lipocalin has been heralded as a therapeutic target to reduce neuroinflammation and neuronal cell death in brain injury [80].
In mouse model of ischemic stroke, post-RIC was shown to induce changes in astrocyte protoplasmic (glutamine synthetase, GS expressing) or fibrous (GFAP) types [31]. GS expression is upregulated by IS after 3 days but no longer after 14 days, while post-RIC upregulates GS expression only after 14 days. GFAP, specifically GFAPα, in ipsilateral side of brain, is increased almost twofold 3 days later after IS, with increase maintained at 50% 14 days later [31]. This is reversed by post-RIC but only at 3 days. This occurs with no concomitant change of the GFAPδ isoform, resulting in increased GFAPα/GFAPδ ratio in post-RIC brain that reflects altered astrocyte intermediate filament networks [31].
Inflammatory processes in non-infectious reactions can be triggered through Toll-like receptors (TLRs) through interaction with endogenous molecules released from damaged tissues or dead cells. These TLR inflammatory pathways can drive early- and late-phase cytokine or chemokine production via NF-kB. In rat experimental IS, both TLR4 and NF-kB mRNA levels are highly overexpressed in ischemic cortex, 1 day after IS, with levels returning to normal after 7 days, but protein levels were still maintained at 7 days in neurons but not astrocytes in peripheral ischemic tissue [60], evidence of a pro-inflammatory response to IS driven by neurons in the brain; when post-RIC was applied, both TLR4 and NF-kB expression were inhibited 1 or 7 days later, evidence of a long-lasting anti-inflammatory effect of post-RIC [60].
In rat, pre-RIC inhibited expression of Tim-3 and Galectin-9 measured 24-h post-stroke in the brain (pooled ischemic core, penumbra, cortex) which increase after IS and may regulate cell death in lymphocytes [78].
Final Remarks on Experimental Models and RIC Time-frames
The vast majority of experimental animal models demonstrating RIC-induced neural protection are of young male adult rodents, with only a few studies using female rodents [19–21] and aged male rats [22]. In relation to co-morbidities, although diabetes is a key co-morbidity present in stroke patients, in particular in aged ones, only one study [76] performed MCAO in a diabetic mouse model. In the latter, Liu and colleagues showed that neither pre-RIC nor post-RIC rescued IS-induced pAkt reduction in ischemic hemisphere, as seen in other studies in young adult models, which stresses the need to clarify how dysmetabolism may interfere with RIC-induced protection and signaling. More experimental model studies are needed to clarify the effects sex, age, and co-morbidities on the beneficial effects of RIC observed so far in young adult models.
The precise RIC protocol that might afford neuroprotection against stroke in humans is likely to require optimization. The dose (acute or chronic) and the time point of limb RIC application will only be effective in precise and limited frames. In experimental models of limb post-RIC, reduced infarction was observed after 2 days when RIC was applied up to 3 h after stroke, but not if RIC was applied after 6 h or 2 months later when only behavioral outcome was ameliorated [23]. In the gerbil brain, for example, induction of ischemic tolerance was shown to require at least 2 min of ischemic preconditioning, at least 1 day before a damaging cerebral ischemic event [81], with a 2-day interval providing even more neuroprotection. This early study also showed that two episodes of preconditioning on consecutive days provided complete tolerance to 5-min cerebral ischemia after 2 days. A later systematic analysis of post-RIC time courses revealed that the total cumulative time of repeated limb occlusion/reperfusions may be fundamental for effective remote post-conditioning, with the maximum protective effect attained in a rat model with 2 cycles of 15 min each, and 2 or 3 cycles of 10 min each and a total time of 40 to 60 min [67].
In a mouse model of 45-min MCAO, which compared delayed pre-RIC (24 h before MCAO), early pre-RIC (just before MCAO), per-RIC (during MCAO), and post-RIC (just after MCAO), only the per-RIC procedure showed a clear neuroprotection possibly resulting from enhanced collateral circulation [66].
In a rat model, with ischemic preconditioning via middle cerebral artery occlusion, neuroprotection against 100 min of ischemic insult did not last beyond 7 days, while repeated post-RIC (chronic RIC), for 14 consecutive days, was associated with stronger neuroprotection against cerebral ischemia/reperfusion injury [36]. However, there seems to be a limit in the effectiveness of chronic RIC. Application of 1 month or 4 months daily limb RIC, in a model of vascular cognitive impairment and dementia, was equally effective [45]. The effective therapeutic window, including the cycles and duration of reperfusion and occlusion, which have been recently reviewed [82], remains to be determined precisely and is certain to impact on the degree and time window of neuroprotection.
Inter-organ Communication: Signaling Between Remote Ischemic Conditioned Limb and the Brain
Experimental models have also been explored to disclose how the communication between the ischemic conditioned limb and the brain occurs. The main hypotheses are the activation of the autonomous nervous system and/or the presence of signaling circulating factors in blood, namely biochemical molecules, immune cells, and/or exosomes carrying microRNAs. Data are described in the present subsection and summarized in Table 2.
Table 2.
Experimental models of neuroprotection by limb RIC: limb RIC targets autonomous nervous system and affects circulating blood factors, exosomes and blood cells
| Model | Pathway/mechanism | Reference |
|---|---|---|
|
Rat: Sprague–Dawley (m/250–350 g) IS model: BCCAO 30 min, perm. MCAO pre-RIC: immediately before IS 3 cycles: 15 min RIC + 15 min RF |
galectin-9/Tim-3 inflammatory cell signaling pathway, NO, iNOS, BBB, afferent nerves | [78] |
|
Rat: Wistar (m/250-320 g) IS model: global 4-VO + BCCAO 8 min pre-RIC: before IS 3 cycles: 10 min RIC + 10 min RF |
hippocampus CA1, serum, NO/NOS | [77] |
|
Rat: Sprague–Dawley (m/240–250 g) IS model: MCAO 90 min per-RIC: 30 min before reperfusion 3 cycles: 5 min RIC + 5 min RF |
platelet-derived microparticles | [83] |
|
Rat: Sprague–Dawley (m/280–320 g) IS model: 1.5 h MCAO + 24 h RF per-RIC/post-RIC (I-30, RF-30) 3 cycles: 10 min RIC + 10 min RF |
No neural transmission in per-RIC | [84] |
|
Rat: Sprague–Dawley (m/280-320 g) IS model: 1.5 h MCAO, 3d RF pre-RIC: 1 h before MCAO 4 cycles: 5 min RIC + 4 min RF |
TNFα, IL-6, IL-10; Lymphocytes: Tc, Th, NKT, B, NK; monocytes | [85] |
|
Rat: Sprague–Dawley (m/age NA) IS model: MCAO (90 min w/isoflurane) pre-RIC: before IS 4 cycles: 5 min RIC + 4 min RF |
spleen, T lym, Tc, NKT, B-lym, RIC immune response is dependent on the spleen | [86] |
|
Mouse: type 2 diabetic BKS.Cg-Dock7m + / + Leprdb/Nju, db/db (m/8wo) IS model: 45 min MCAO + 48 h RF pre-/post-RIC: 1d before MCA/after RF 3 cycles: 10 min RIC + 10 min RF |
Lymphocytes: Th, Tc, NK, B, pERK, pAKT, TNF-α, IL-6, IL-1β, IFN-γ, IL-4 | [76] |
|
Mouse: C57BL/6 (m/8–10 wo) IS model: 70 min MCAO, 24 h RF per-RIC: during MCAO 3 cycles: 10 min RIC + 10 min RF |
erythrocytes oxygen delivery 2,3-biphosphoglycerate |
[87] |
|
Mouse: C57BL/6, CCR2 KO (m/f, 12wo) IS model: 30 min MCAO post-RIC: 2 h after MCAO, 2d RF 5 cycles: 5 min RIC + 5 min RF RIC only: single, 3 × daily, 7 × daily |
monocytes: splenic, anti-inflam., pro-inflam.; resident microglia | [88] |
|
Mouse: C57BL/6 (m/20-22 g) IS model: 45 min MCAO post-RIC: after MCAO, 2d RF 3 cycles: 10 min RIC + 10 min RF |
Lymphocytes: B, Th, Tc, NK, NKT cells, non-inflam. monocytes, IL-10, IL-6, TNF-α, IL-4, IL-1β | [79] |
|
Cell model: HUVEC cells, SH-SY5Y cells Rat: Sprague–Dawley (m/f, 220-250 g) IS model: 2 h MCAO, 24 h RF post-RIC: after MCAO, 3 × femoral artery |
CD63, HSP70 and TSG101 in plasma, exosomes (endothelial) | [89] |
|
Human & Mouse pre-RIC 4 cycles: 5 min RIC + 5 min RF |
miR-144 | [90] |
|
Rat: Wistar (m/8 wo) IS model: permanent MI post-RIC: (4w after MI), daily for 4w 5 cycles: 5 min RIC + 5 min RF |
msiR-29a | [91] |
|
Mouse: C57BL/6 (20-22 g) IS model: dMCAO electrocoagulation pre-RIC: 24 h before MCAO 3 cycles: 10 min RIC + 10 min RF |
Exosomes, HIF-1α, CD63, TSG101, CD81 | [92] |
|
Mouse: C57BL6: (sex NA/8–10 wo) IS model: MCAO (1 h) pre-RIC: (1 h before MCAO) 4 cycles: 10 min RIC + 10 min RF |
miR-144, p-PTEN, pAkt, apoptosis | [93] |
|
Human: (n = 4) male/young RIC: non-dominant arm cuff 200 mmHg 5 cycles: 5 min RIC + 5 min RF Cell model: SH-SY5Y cells |
plasma exosomes, miR-126, DNMT3B, DNA methylation, cell cycle, p21 (CDKN1A) | [94] |
Autonomous Nervous System
The neuroprotective effects of limb RIC require afferent nerves and the blood factors. Experimental models show that the neuroprotective mechanisms afforded by limb RIC require complementary and interdependent neural elements and factors circulating in the bloodstream [23, 78]. Afferent sensory nerve blocker capsaicin or ganglion blocker hexamethonium completely abrogated the neuroprotective effects of limb pre- and post-RIC in rat [23, 78], suggesting that limb pre- and post-RIC procedures activate an essential neural afferent pathway. However, RIC neural transmission pathways may not be significant in per-RIC, when the RIC procedure is performed during ischemia, before reperfusion [84], giving added importance to other factors.
Circulating Blood Factors
Blood factors, engendered by RIC, have also been shown to provide a degree of neuroprotection. Plasma proteomics in a rat model revealed that RIC results in significant changes in plasma protein profiles [95, 96]. Platelet-derived microparticles (MP) extracted from healthy rat RIC plasma, and injected before reperfusion into rats subjected to 90-min MCAO, had reduced infarction area 24 h later, albeit to a lesser degree than per-RIC itself [83]. Likewise, in a rat model using 8-min global cerebral ischemia, limb pre-RIC increased NO levels and NOS activity following an double peak pattern, both in serum (0 h and 48 h) and CA1 hippocampal region (6 h and 48 h) [77]. In chronic post-RIC, applied daily, plasma nitrite levels were increased at 3 weeks, but no longer at 1 month or 4 months [45].
Cytokines circulating in blood are also altered by ischemic stroke and limb RIC. In mouse model, limb pre-RIC applied 1 h before MCAO [37] reduced TNFα in plasma measured 24 h later, indicating that limb pre-RIC initially reduces systemic inflammation. In rat, TNFα mRNA was increased in whole blood 48 h after IS [79] with similar trends observed in whole brain. However, in serum, 3 days after IS, TNFα levels are normal, while pre-RIC TNFα levels in serum after 3 days were markedly increased [85]. In the same study, serum interleukin 6 (IL-6) was increased after IS in rat [85] and diabetic mouse [76], while pre-RIC further exacerbated this increase at 3 days after IS in rat [85], and pre-RIC and post-RIC mitigated the increase in diabetic mouse at 48 h [76] with no change in IL-1β, IFN-γ, TNF-α, or IL-4. However, post-RIC in another study of rat IS markedly reduced IL-6 mRNA in both whole blood and whole brain 48 h later [79]. Two days after brain ischemia in rat, protein levels of anti-inflammatory cytokines IL-4 and IL-10 are reduced in plasma and brain in one study [32] but IL-10 mRNA is increased in blood after IS. When RIC is applied in the absence of cerebral ischemia in rat, plasma shows a 50% increase in protein levels of anti-inflammatory cytokines IL-4 and IL-10 with no change in pro-inflammatory cytokines IL-1β, IL-6, and IFN-γ at 48 h [32]. However, when post-RIC is performed just after ischemic stroke, 48 h later, plasma protein levels of anti-inflammatory cytokines IL-4 and IL-10 are 2- to threefold increased [32], but mRNA of IL-10 is decreased in whole blood [79]. Pro-inflammatory cytokines IL-1β, IL-6, and IFN-γ were decreased in plasma after post-RIC [32]; while systemic inhibition of HIF1α reverses both the neuroprotective effects of post-RIC and protein cytokine profiles in plasma [32]. In aged rats, IL-1β, IL-6, and IFN-γ were increased 48 h after IS but not when pre-RIC was applied 24 h before, while no changes were observed in TNFα, IL-4, and IL-10 [22].
Exosomes and MicroRNAs
Exosomes and MicroRNAs may be involved in neuroprotective effects of limb RIC. Endothelial-derived exosomes have been shown to provide a degree of protection against ischemia/reperfusion injury in neuronal cells, including reducing apoptosis and increasing proliferation [89]. Pre-RIC plasma exosomes from mice are rich in HIF-1α, CD63, TSG101, and CD81, and attenuate infarct size and neurological function when infused into IS model [92]. Exosomes extracted from human RIC plasma reduced DNA methylation and provided a degree of tolerance to oxygen/glucose deprivation in vitro in SH-SY5Y neuroblastoma cells [94]. Fifty differentially expressed microRNAs have been identified in human plasma collected immediately after RIC, including miR-126, which was upregulated and is postulated to target DNA (cytosine-5)-methyltransferase 3B (DNMT3B) which may in part explain the RIC exosome induced reduction in DNA methylation [94].
MicroRNAs (miRNAs) are also candidate molecules that could be involved in induction of limb RIC neuroprotection. miRNAs can be found in blood linked to the carrier protein Argonaute-2 or inside exosomes that are produced by a variety of cells both of which can traverse the blood–brain barrier [97, 98]. In mouse plasma, 13 miRNAs were downregulated and 18 upregulated 24 h after limb RIC performed on abdominal aorta [99]. However, different RIC protocols can result in different miRNA expression profiles [100]. In rat, presumed muscle-derived miR-29a was upregulated in plasma after limb RIC was performed once a day for 4 weeks [91]. In both mouse and human, miR-144 was upregulated in plasma after limb RIC 4 cycles of 5-min ischemia/reperfusion [90]; and in an MCAO stroke mouse model, miR-144 introduced into circulation targeted PTEN resulting in increased Akt pathway activity and inhibiting of apoptosis in brain [93].
Circulating Blood Cells
Limb RIC causes changes in blood cell populations that may have direct neuroprotective effects. In a mouse model of IS, pre-RIC resulted in increased levels of 2,3-biphosphoglycerate (2,3-BPG) in circulating erythrocytes after 24 h [87]. In fact, 2,3-BPG facilitates the dissociation of oxygen-hemoglobin bound, shifting the oxygen dissociation curve toward increased oxygen delivery to ischemic brain tissue [87].
RIC also alters circulating immune cell composition which appears to be dependent on an intact spleen [86, 88]. In addition, whereas normally splenic lymphocytes positively correlate with circulating B and T lymphocytes, following RIC, a negative correlation with circulating T lymphocytes was observed. In rat experimental ischemic stroke, limb pre-RIC increased splenic volume, total lymphocytes and the percentages of cytotoxic and natural killer T cells, and B lymphocytes in the spleen after 3 days [86], whereas an increase in CD8 + cytotoxic T cells is seen after 1 h and is maintained after 3 days. In the blood, leukocyte populations are also altered by RIC. The percentage of CD4 + helper T cells is reduced by IS in rat and pre-RIC does not change this. In the same pre-RIC model, however, the downregulation in CD8 + cytotoxic T cells, NKT cells, B cells, and noninflammatory resident monocytes, triggered by IS, appears to be partially rescued by pre-RIC, 3 days after IS [85]. In a mouse model of IS, after 2 days, CD4 + helper T cells are decreased in the spleen and lymph nodes, and post-RIC completely reverses this, with increasing trend also observed in blood [79]. Cytotoxic CD8 + T cells and NKT cells appear reduced in lymph nodes, spleen, and blood after IS, with this trend being reversed by post-RIC [79]. B cells and NK cells on the contrary appear to be increased by IS in the lymph nodes, spleen, and blood after 24 h, with this trend being reversed by post-RIC [79]. In a diabetic IS stroke mouse model, the decrease in blood helper T cells (CD4 +) and cytotoxic T cells (CD8 +) was reversed by pre-RIC but unaltered by post-RIC after 48 h, while no changes were observed in B cells or NK cells [76]. In mouse, IS-induced reduction in splenic monocytes/macrophages is reversed by rapid post-RIC within 24 h [47].
Noninflammatory monocytes were also increased after IS in mouse blood but not in the spleen or lymph nodes, while post-RIC reversed this; no changes in inflammatory monocytes were observed in the blood, spleen, or lymph nodes after IS and/or post-RIC [79, 88]. Although post-RIC did not change relative values of pro-inflammatory or anti-inflammatory monocyte subsets in spleen, pro-inflammatory monocytes are increased in blood. In vitro cultured splenocytes treated with post-RIC serum converted from anti-inflammatory monocytes into pro-inflammatory monocytes, with a greater shift observed with serum from severe stroke with post-RIC [88]. This supports the notion that limb post-RIC produces circulating factors that induce the spleen to produce pro-inflammatory monocytes into circulation that can infiltrate the brain and have a protective role. In rat brain, contralateral to stroke lesion or in ipsilateral hemisphere, post-RIC did not affect resident microglia measured 3 days after stroke; however, in ipsilateral ischemic hemisphere, there was increased infiltration of pro-inflammatory monocytes, and reduced mRNA expression of MCP-1 and IL-1β [88].
In mouse, blood monocyte depletion reversed the neuroprotective effects of rapid post-RIC on infarct volume and brain monocytes/macrophages [47]. In the same model, rapid post-RIC attenuates the large increase of granulocyte colony‐stimulating factor (G-CSF) in ischemic cortex, and the reduction in plasma G-CSF and splenic tissue monocytes/macrophages induced by ischemic stroke [47], and this regulation of inflammatory stimuli increases leptomeningeal collateral circulation [47].
Human Studies and Clinical Trials of Remote Ischemic Conditioning (RIC)
Introduction
Similarly to experimental research settings, the first clinical studies about RIC were made in myocardial infarction patients. RIC procedure was applied in ST-segment elevation myocardial infarction, and has reported reductions in infarcted area, some improvement of left-ventricle ejection fractions, reduction of creatinine-kinase myocardial plasma release or decreased troponin I levels [101–105]. Likewise, in patients with stable ischemic heart failure chronically treated with RIC (2 procedures per day during 6 weeks) improved left-ventricle ejection fraction volume and decreased B type natriuretic peptide levels were registered [106]. In rheumatic heart disease patients undergoing valve replacement, remote ischemic conditioning decreased the release of serum cardiac troponin I and also improved liver and lung biomarkers, indicating a potential systemic protective effect [107]. Recently, it was also found that strength training in healthy volunteers along with 8 sessions of RIC procedure improved muscle strength in wrist extensor muscle [108].
Herein, the main focus is to review the potential protective effects of RIC targeting the brain in clinical settings. Because there is not as much data for ischemic stroke as in experimental models and also because the cellular mechanisms RIC-mediated neuroprotection are similar for several different disorders, our review also highlights other neurological diseases besides ischemic stroke.
RIC Clinical Trials in Neurological Disease: Potential Cerebral Effects
In neurological disease, RIC-based strategies have been tested for safety and efficacy in patients with acute ischemic stroke, hemorrhagic stroke, middle cerebral or carotid artery stenosis, and spinal cord lesion. RIC has also been evaluated for cerebral complications associated with coronary artery bypass grafting. Clinical trial RIC data relating to ischemic stroke and other cerebral diseases is summarized in Tables 3 and 4, respectively.
Table 3.
Clinical trials of remote ischemic conditioning (RIC) targeting ischemic stroke
| Name of study | Type of patients/pathology | size and phase of study | Primary outcomes | Secondary outcomes | RIC protocol* | Main results/observations | Status | Ref |
|---|---|---|---|---|---|---|---|---|
| RESIST | Acute ischemic stroke and intracerebral hemorrhage | intention of including 1000 patients | 3 months modified Rankin Scale score | 3 categories: clinical, neuroimaging and plasma biomarkers | 5 cycles pre-hospital and in hospital | Not available | ongoing | [109] |
| RECAST | Acute ischemic stroke | pilot study with 26 patients | feasibility and tolerability | blood biomarkers and 90 day NIHSS score | 4 cycles in the first 24 h after stroke onset | RIC decreased 90 day NIHSS score and increased plasma levels of HSP-27 | completed | [110] |
| RECAST-2 | Acute ischemic stroke | 60 stroke patients phase IIb | feasibility and tolerability | blood biomarkers and 90 day NIHSS score | 4 cycles in the first 24 h after stroke onset | no changes in NIHSS score but RIC decreased S100ß plasma concentration | completed | [111] |
| RECAST-3 | Acute ischemic stroke | 1300 stroke patients phase III | Death or dependency at day 90 (modified Rankin Scale) |
At day 90: Cerebrovascular events; major adverse cardiac and cerebral events; acute kidney injury; COVID-19 status; disability; cognition; mood; frailty; quality of life; safety |
4 doses of 4 cycles Dose 1 in the first 6 h after stroke onset; second dose 2 h after dose 1 and 2 doses at day 2 | Multicenter clinical trial | ongoing | [112] |
| no name | Acute ischemic stroke | proof-of-concept study, 443 patients | penumbral salvage (assessed on multimodal magnetic resonance imaging) | infarct growth at 24 h and 1 month follow-up | 4 cycles during the first 4 h (when transportation was too short less cycles were done) | mostly neutral, some indication that RIC reduces the risk of infarction after 1 month | completed | [113, 114] |
| RESCUE BRAIN | Carotid acute ischemic stroke | multicenter including 188 patients | Infarct volume at 24 h | NIHSS score at 24 h and at 90 days: activities of daily living, degree of disability, excellent outcome, successful recanalization | 4 cycles up to 6 h after stroke onset | at 90 days no significant difference in mortality or symptomatic intracerebral hemorrhage | completed | [115, 116] |
| REPOST | Acute ischemic stroke | intention of including 200 acute ischemic stroke patients | Infarct size (MRI diffusion-weighted image) at the end of hospitalization | Modified Rankin Scale, NIHSS, quality of life, and cardiovascular and cerebrovascular morbidity and mortality | 4 cycles twice daily during the hospitalization for a maximum of 4 days | Not available | ongoing | [117] |
| no name | Acute minor ischemic stroke or transient ischemic attack | 165 patients, phase IIa | prevention of secondary stroke | not applicable | 5 cycles on bilateral upper limbs twice a day for 90 days | RIC to be used and tested along with the antiplatelet strategy (aspirin and/or clopidogrel) | ongoing | [118] |
| no name | Acute ischemic stroke in patients treated with thrombectomy | pilot study with 20 patients treated with thrombectomy | safety, feasibility and assessment of any serious RIC-related adverse events | not applicable | 4 cycles applied before and immediately after recanalization, and once a day for 7 days | no RIC-induced effect on intracranial pressure, cranial perfusion, mean arterial pressure or middle artery systolic flow velocity | completed | [119] |
| no name | Acute ischemic stroke in patients treated intravenous thrombolysis | pilot study with 49 patients treated with intravenous thrombolysis | safety | not applicable | 4 cycles applied within 6-24 h of intravenous thrombolysis |
No difference was found in: hemorrhagic transformation, clinical score, adverse events, blood pressure But RIC group presented lower levels of C reactive protein |
completed | [120] |
Table 4.
Clinical trials of remote ischemic conditioning (RIC) targeting other cerebral diseases
| Name of study | Type of patients/pathology | size and phase of study | Primary outcomes | Secondary outcomes | RIC protocol* | Main results/observations | Status | Ref |
|---|---|---|---|---|---|---|---|---|
| no name | Symptomatic intracranial arterial stenosis | proof-of-concept study, 68 patients with symptomatic intracranial arterial stenosis | stroke recurrence | time to which modified Rankin scale recovers to 0–1 | 5 cycles applied bilaterally and daily for 300 days | RIC improvement of brain perfusion and reduction from 26.7% to 7.9% of stroke recurrence at day 300 | completed | [121] |
| no name | Symptomatic intracranial arterial stenosis | 58 octo and nonagenarian patients with symptomatic intracranial arterial stenosis | stroke recurrence | improvement of the NIHSS score (≥ 8 points or ≤ 1 point from the baseline) or modified Rankin scale of 0–2 | 5 cycles applied bilaterally and daily for 180 days | no clear effect: 2 infarctions and 7 transient ischemic attacks in RIC group while 8 infarctions and 11 transient ischemic attacks in sham group | completed | [122] |
| no name | Middle cerebral artery stenosis | pilot study, 10 patients with unilateral MCA stenosis and 24 healthy volunteers | safety and feasibility | heart rate, oxygenation index or mean flow velocity | 5 cycles (single application) | no effect on heart rate, oxygenation index or mean flow velocity in MCA stenosis patients, but reduction on blood pressure in healthy volunteers | completed | [123] |
| no name | Cervical spondylotic myelopathy patients prior to undergoing elective decompression surgery | pilot study, 40 patients | safety and feasibility for a larger clinical trial | Median nerve somatosensory- evoked potentials (SEPs) S100ß and neuron-specific enolase plasma levels | 3 cycles (single application before surgery—preconditioning) | decrease of S100ß plasma concentration | completed | [124] |
| no name | Aneurysmal subarachnoid hemorrhage (SAH) | pilot study 4 patients | Cerebral hemodynamic and metabolic features | not applicable | 4 cycles applied in non-consecutive days 2 to 12 days following SAH (leg) | Reduction of lactate/pyruvate ration and glycerol levels in the brain; increase on intracranial pressure and decrease the mean velocities of middle cerebral artery | completed | [125] |
| no name | Aneurysmal subarachnoid hemorrhage (SAH) | phase I, 20 patients | safety and feasibility for a larger clinical trial (development of deep venous thrombosis, bruising, or injury to the conditioned limb) | development of new neurological deficits or cerebral infarct and neurological features at follow-up | 4 cycles applied every other day for 12 days (leg) | RIC procedure is safe and feasible | completed | [126] |
| no name | Aneurysmal subarachnoid hemorrhage (SAH) | phase Ib, 33 patients | development of venous thrombosis or injury in the limb, safety | Transcranial Doppler spasm, delayed cerebral ischemia and 3 month modified Rankin scale | 3 cycles** applied every 24 or 48 h during 14 days (leg) | RIC procedure is safe and feasible, with no difference in the analyzed parameters | completed | [127] |
| RICH-1 | Intracranial hemorrhage | Randomized pilot study | safety | Hematoma and perihematomal edema volume, hematoma expansion | 4 cycles daily for one week | cranial tomography will be performed at days 1, 3, 7 and 14 after stroke onset | ongoing | [128] |
| no name | Small vessel disease—Mild cognitive impairment | pilot study, 30 patients | change in brain lesions | changes of cognitive function, plasma biomarkers, and cerebral hemodynamic parameters at 1-year follow-up | 5 cycles twice a day for 1 year | RIC decreased white matter hyperintensities, improved visuospatial and executive abilities and reduced plasma levels of tryglycerides, total cholesterol, LDL and homocysteine | completed | [129] |
| no name | Subcortical ischemic vascular dementia | pilot study, 37 patients | change in neuropsychological assessments | Changes in high-sensitive C reactive protein concentration, white matter lesion volume, diffusion tension imaging metrics of white matter | 5 cycles daily for 6 months | RIC improved cognitive function assessed by Hopkins Verbal Learning Test-Revised, Controlled Oral Word Association Test, Trail Making Test A and B and Judgment of Line Orientation | completed | [130] |
Ischemic Stroke
In acute ischemic stroke, a proof-of-concept clinical trial with 171 stroke patients showed that RIC is safe and can be applied in the pre-hospital setting [113, 114]. In this study, the primary outcome was penumbral salvage assessed on multimodal magnetic resonance imaging and secondary outcome was infarct growth at 24-h and 1-month follow-up. Despite most results were neutral, RIC should have potential benefit in reducing the risk of infarction growth after 1 month [113, 114].
RECAST (Remote Ischemic Conditioning After Stroke Trial) was a pilot randomized placebo controlled phase II trial in acute ischemic stroke. RECAST was first developed with 26 patients and had a primary outcome of feasibility and tolerability and a secondary outcome including blood biomarkers and 90-day NIHSS [110]. Despite RIC increased plasma concentration of heat shock protein-27 (HSP-27), no plasma level changes in S100ß, troponin-T, HSP-60, HSP-70, HSP-90, or matrix metalloproteinase-9 (MMP-9) were observed [110]. Finally, RIC improved neurological outcome assessed by a decrease on 90-day NIHSS [110]. RECAST was further developed as a phase IIb clinical trial with 60 acute ischemic stroke patients randomized 1:1 to receive RIC [111]. Again, feasibility and safety were warranted, but no changes in NIHSS or in plasma levels of neuron-specific enolase or MMP-9, except of S100ß plasma concentration that decreased in RIC group [111]. RECAST-3 is the phase III prospective randomized multicenter clinical trial that is in progress and will include 1300 participants [112]. In this phase III, four doses of RIC (4 cycles of 5 min of ischemia) will be applied: the first one within 6 h or less of stroke onset, second dose at 2 h after the first dose, and doses 3 and 4 will be applied at the second day after stroke onset.
Another pilot study was performed to assess feasibility and safety of RIC as adjuvant therapy along with thrombectomy for acute ischemic stroke [119]. Twenty patients were treated with RIC procedure at 3 time points: immediately before canalization, immediately after canalization, and 7 days after thrombectomy. No RIC-induced effect was found on intracranial pressure, cranial perfusion, mean arterial pressure, or middle artery systolic flow velocity [119].
Symptomatic intracranial arterial stenosis is a major cause of stroke. Daily and bilateral application of RIC (5 cycles of 5 min of ischemia and 5 min of reperfusion) for about 300 days reduced (from 26.7 to 7.9%) the stroke recurrence in patients with intracranial arterial stenosis [121]. Likewise, in octo- and nonagenarians, the same study was performed for 180 days with a reduction of stroke recurrence in RIC group patients [122]. Nevertheless, a single procedure of RIC (5 cycles of 5 min of ischemia and 5 min of reperfusion) did not alter heart rate, oxygenation index, or mean flow velocity in patients with middle cerebral artery stenosis, a reduction in blood pressure in healthy volunteers was the single RIC effect found [123]. Thus, these data suggest that chronic application of RIC might present more efficient results in neuroprotection than a single acute treatment.
The potential protection of RIC against ischemic injury in patients with severe carotid artery stenosis undergoing carotid artery stenting was tested [131]. Two RIC procedures applied daily during 2 weeks before carotid artery stenting significantly decreased the incidence of new ischemic lesions assessed by new diffusion-weighted imaging lesions 48 h after stenting. Nevertheless, RIC did not change plasma concentrations of neuron-specific enolase or S100ß nor clinical outcomes after 6 months [131].
RESCUE BRAIN was a French multicenter randomized clinical trial of remote ischemic per-conditioning in the treatment of acute ischemic stroke, which included 188 patients receiving either remote ischemic per-conditioning or sham procedure during or after reperfusion treatments, up to 6 h after the stroke onset. Infarct volume at 24 h was the primary outcome, but no significant changes were observed between intervention and control groups [115]. Likewise, at 3 months, no significant difference was found in mortality or symptomatic intracerebral hemorrhage [115].
RESIST is a clinical trial of remote ischemic per- and post-conditioning for treatment of acute stroke ongoing in Denmark whose protocol was described in [109]. Authors intend to include 1000 patients with diagnosis of acute ischemic stroke and intracerebral hemorrhage along with 1500 patients with a pre-hospital presumed stroke, with the 3-month follow-up modified ranking scale score as the primary outcome [109]. Since this clinical trial is larger than the previous ones, it holds expectations for potential beneficial outcomes of RIC.
REPOST is another randomized clinical trial for remote ischemic post-conditioning (RIPostC) in ischemic stroke that is ongoing in the Netherlands [117]. In this clinical study, RIPostC procedure is applied to stroke patients twice a day during hospitalization; primary outcome is infarct size assessed by MRI diffusion-weighted image at the end of hospitalization [117]. In fact, one may speculate that remote conditioning triggers mild responses by the organism, which would need chronic application for generating beneficial effects. Accordingly, Liu and colleagues have described a protocol, which is ongoing and only includes patients with acute minor ischemic stroke or transient ischemic attack [118]. In this clinical trial, RIC is used as adjuvant treatment for secondary stroke prevention. Again, it may be considered that RIC might present a mild and long-term effect, which can be more efficient for less severe ischemic events.
Other clinical trials are in progress, namely REMOTE-CAT (NCT03375762), REVISE 2 (NCT030445055), and RICE PAC (NCT03152799).
Another pilot study with 49 acute stroke patients assessed the effect of RIC in combination with treatment with intravenous thrombolysis [120]. RIC procedure was applied within 6 to 24 h of thrombolysis treatment. The two groups were followed up for 90 days and no differences between RIC and sham group were found regarding blood pressure, hemorrhage transformation, or adverse effects. Nevertheless, the levels of high sensitivity C reactive protein were lower for RIC group [120].
Finally, in stroke survivors, the application of RIC (5 cycles of 5 min of ischemia) at their paretic leg every other day for 2 weeks improved the self-selected walking speed and increased the time to fatigue, when compared the sham RIC applied patients [132]. Although not being a direct cerebral effect, RIC may improve stroke patient quality of life.
Subarachnoid Hemorrhage and Intracerebral Hemorrhage
RIC was first assessed in a small pilot study with 4 patients with aneurysmal subarachnoid hemorrhage (SAH). Three to four sessions of 4 cycles were applied during 2 to 12 non-consecutive days following SAH [125]. In the 4 tested patients, RIC appeared to increase the mean of intracranial pressure and decrease the mean velocities in the middle cerebral artery. Brain microdialysis revealed that RIC might reduce lactate/pyruvate ratio and glycerol levels [125]. One year later, same authors performed a similar study, considered phase I clinical trial with 20 aneurysmal SAH patients [126]. In this study, the primary outcome was the development of deep venous thrombosis, bruising, or injury to the conditioned limb. None of these outcomes was registered, meaning the clinical trial is safe and feasible [126]. The main conclusion is the need for subsequent larger clinical trials. A similar study was performed by Koch and colleagues with 34 aneurysmal SAH patients for safety and feasibility purposes [127]. The analyzed parameters were transcranial Doppler signs of vasospasm, delayed cerebral ischemia, and 3-month modified Rankin scale with no statistical difference between control and treated groups. Herein, the authors also tested different times of ischemia during RIC procedure, namely 5, 7.5, and 10 min of ischemia with no difference on outcomes or potential injury [127].
Finally, another pilot study will be performed for RIC in the treatment of intracerebral hemorrhage (RICH-1) [128]. Patients will be daily treated within 24–48 h of the onset for one week. Primary outcome is safety and secondary outcome will be the volume of hematoma and perihematomal edema, functional outcomes, and the incidence of adverse events. Moreover, cranial tomography will be performed at days 1, 3, 7, and 14 after stroke onset [128].
Other Potential Cerebral Beneficial Effects of RIC
Remote ischemic preconditioning (RIPC) was tested in patients with cervical spondylotic myelopathy prior to undergoing elective decompression surgery, including 20 patients in each group: intervention and control [124]. While RIPC has reduced serum levels of neuron-specific enolase and S100ß, no effect was found in the median nerve somatosensory-evoked potential [124]. This suggests some effects of RIPC on neurological complications following spinal cord surgery, but many more studies are needed.
Long-term administration of RIC procedure was tested in patients with mild cognitive impairment related to small vessel disease. In fact, 5 cycles of RIC were applied twice a day during 1 year in 14 patients, while 16 patients corresponded to control group without any treatment [129]. After 1 year, RIC reduced the volume of white matter hyperintensities and improved the visuospatial and executive abilities, while no difference was found in the number of lacunes between the two groups. The major effects were found in plasma characteristics, namely RIC promoted marked reduction in plasma triglycerides, total cholesterol, low-density lipoprotein, and homocysteine [129]. Despite the reduced number of patients, this is a promising study since RIC might act more efficiently when applied in a long-term (chronic) manner due to its mild immediate effect. Likewise, in patients with subcortical ischemic vascular dementia, chronic RIC has been tested as daily applications for 6 months [130]. This randomized study included 37 patients: 18 underwent 5 cycles of brief bilateral upper limb compression at 200 mmHg; 9 controls underwent the same procedure with compression at 60 mmHg; and 10 controls had no procedure. RIC-treated patients showed cognitive improvement assessed by neuropsychological tests [130].
Following coronary artery bypass grafting (CABG), silent episodes of brain ischemia can occur; thus, RIC procedure holds potential as a pre-operatory preventive treatment. RIC or sham procedure were tested in 70 patients undergoing CABG, with structural brain magnetic resonance imaging (MRI) for connectivity analysis and neurological evaluation [133]. No difference between groups was found in new ischemic brain lesions or functional connectivity profile; however, RIC reduced the pooled volume of ischemic brain lesions [133].
Finally, young healthy adults underwent a cognitive and motor training along with 7-day daily RIC procedure or sham procedure for the evaluation of cognitive and motor learning. In fact, RIC promoted cognitive and motor learning enhancement, which can potentially by used in rehabilitation of cerebral injured patients [134].
Mechanisms of RIC in the Inter-organ Communication
Most of these clinical trials are mainly focused on disclosing the potential beneficial cerebral and cardiovascular effect of RIC in humans. Nevertheless, some of them were also devoted to describe the underlying mechanism of remote protection and inter-organ communication, as described in the following section. Furthermore, we also review several publications concerning human interventional studies in healthy volunteers for the assessment of RIC-associated mechanisms, which are mainly based on modulation of autonomic nervous system or humoral factors. Likewise, other RIC-induced responses can be associated with microcirculation, endothelial function, or inflammation control. These responses can be considered beneficial consequences of RIC or as signaling events for inter-organ communication and neuroprotection.
Autonomic Nervous System
Data generated in experimental models pointed to the involvement of autonomic nervous system in RIC signaling and inter-organ communication. In fact, blockade of opioid receptors or muscarinic receptors, bilateral vagotomy, or spinal cord resection abolished RIC protection in animal models [135–137]. In healthy volunteers, ischemia–reperfusion (IR) procedure was applied in forearm for promoting lesion which was assessed by the activation of muscle sympathetic nerve activity, finger reactive hyperemia, and oxidative stress (reduced glutathione (GSH) in erythrocytes). Pre-treatment with 2 cycles of 5 min of RIC in one leg delayed the IR-induced increase on muscle sympathetic nerve activity measured in contralateral leg by microneurography [138]. Likewise, RIC limited the increase on erythrocyte-derived GSH levels during ischemia and promoted vasodilation [138]. Other studies used heart rate variability (HRV) to follow autonomic nervous system response to RIC in healthy volunteers. In a pilot study using young and senior (> 60 years old) sub-groups, HRV analysis demonstrated that the non-linear parameter SD2 (associated with long-term HRV) increased significantly in response to RIC procedure, suggesting the activation of both sympathetic and parasympathetic nervous system, in particular via the slow sympathetic response to the baroreceptors stimulation and via fast vagal parasympathetic response [139]. In addition, another study with 50 subjects analyzed HRV is several time points: 1 h before RIC procedure and 1, 3, 3, 6, 9, 12, and 24 h after. Similarly, the time domain SDNN and the non-linear SD2 parameters increased 1 h after RIC, while 12 h later only SD2 increase [140]. Khaliulin and colleagues found a reduction on porphyrin fluorescence in the blood, as well as an increase in power of very low frequency band in ECG recording; both facts indicate an increased stress resistance response [141].
Humoral Factors
In human healthy subjects, RIC procedure increased the levels of nitrite in plasma and platelet rich plasma [142]. Of note, nitrite is an oxidation product of NO, being an indirect measure of NO. Although NO is associated with vasomodulation, Dezfulian and colleagues have demonstrated that RIC-derived human plasma protects myocytes against cell death induced by hypoxia/reoxygenation in an in vitro cell model [142]. Moreover, RIC reduced platelet mitochondrial respiration and increased mitochondrial anion superoxide production, which can be associated to NO binding to mitochondrial respiratory chain reaction complexes [142]. Exosomes-mediated intercellular communication may be another factor involved in RIC signaling to distant organs due to the ability of transferring microRNAs, lipids, and proteins. In fact, exosomal miRNA 126 was found in plasma of healthy volunteers subjected to RIC procedure [94]. Similar to miRNA 126-induced neuroprotection against stroke in experimental models [143], exosomes isolated from RIC-derived human plasma prevented neuronal cell death using the in vitro model of SH-SY5Y cells [94].
A microarray for the analysis of blood gene expression was done 15 min and 24 h after RIC procedure in 4 healthy volunteers subject to RIC, with the identification of 169 genes differently expressed [144]. Konstantinov and colleagues highlighted the potential anti-inflammatory pattern of gene expression derived from RIC, pointing to the upregulation of anti-inflammatory heat shock protein (HSP) 70 and calpastatin genes, while downregulation of immunity response genes, namely TLR4 and TNF signaling pathway TNFR6 [144]. Proteomic studies based on 2 dimensional difference in gel electrophoresis (2DIGE) and mass spectrometry (MS) were performed to identify potential modifications on the composition of circulating plasma proteins following RIC [145]. Six proteins were found to change in response to RIC when analysis was done by 2DIGE, while 48 proteins were found in MS analyses, being only 3 of them common in both analysis. This sort of large proteomic approaches must follow further validations with different analytical techniques, as well as functional validation using experimental models. In another study, three cycles (5 min ischemia and 5 min of reperfusion) of RIC were applied in 60 healthy young volunteers followed by proteomic analysis at 1, 3, 8, 24, and 48 h [146]. Fourteen proteins were found differently present in the serum, most of them (11 proteins) at 1 h after RIC. These proteins are involved in mechanisms related to coagulation, apoptosis, lipid metabolism, and immune system [146]. Furthermore, mass spectrometry proteomic analysis was done in plasma derived from children with cyanotic heart disease undergoing cardiopulmonary bypass surgery treated or not with remote ischemic preconditioning (RIPreC). Protein pattern in the plasma was different only 6 h after RIPreC with the increased concentration of six proteins: inter-alpha globulin inhibitor, fibrinogen preproprotein, complement-C3 precursor, complement C4B, apolipoprotein B100, and urinary proteinase inhibitor [147]. In fact, the large heterogeneity of reported data and lack of reproducibility might be due to inter-donor variability, age, and different data analysis processes [148].
RIC procedure applied in traumatic brain injury or cervical spondylotic myelopathy patients reduced the levels of neuron-specific enolase (NSE) and S100ß, which are serum biomarkers for acute brain injury [124, 149]. Despite being a good indication of RIC-induced neuroprotection, NSE and S100ß may be not involved in neuroprotection signaling triggered by RIC.
Finally, recent metabolomic profiling of human cerebrospinal fluid after daily RIC, applied during 3 consecutive days, showed increased creatine, ethanolamine, and isobutyrate, and decreased phenylalanine, hypoxanthine, and tyrosine [150]; beyond being evidence of altered pathways of energy and amino-acids metabolism in the central nervous system, the significance of these results to RIC-induced neuroprotection remains to be determined.
Endothelial Function and Blood Circulation
Endothelial function and blood circulation (vasodilation), which are key events in response and protection against ischemia and reperfusion, can be assessed by blood flow-mediated dilation of the brachial artery or by skin microcirculation using cutaneous vascular conductance. Several studies in clinical settings were conducted to evaluate blood circulation and endothelial function in response to RIC. In the arm, 20 min of ischemia followed by reperfusion decreased endothelial function, which was assessed by blood flow-mediated dilation. The ischemia–reperfusion-induced decrease of flow-mediated dilation was partially reverted by RIC procedure in the contralateral arm or leg, applied before (pre) or after (post) ischemia [151, 152]. Moreover, RIC-triggered improvement of flow-mediated dilation was reverted by pharmacological treatment with glibenclamide, a sulfonylurea that inhibits ATP-sensitive potassium channels, indicating that endothelial protection depends on KATP channel activation in humans [151]. Likewise, administration of the autonomic ganglion blocker trimetaphan also reduced the RIC-induced vasoprotection [152]. In addition, RIC procedure in the arm of healthy subjects improved microcirculation assessed in the thigh, in particular at the third cycle tissue oxygen saturation and capillary blood flow increased [153]. Likewise, 7 days daily based RIC improved flow-mediated dilation and skin microcirculation (cutaneous vascular conductance assessment) in the RIC arm and the contralateral arm, suggesting a local and distant effect of RIC [154]. Finally, 1 week of twice a day, RIC application improved coronary microcirculation in healthy and heart failure patients [155]. Coronary microcirculation was measured by transthoracic Doppler echocardiography assessed by coronary flow reserve [155].
RIC Controversies and Remaining Questions
Translation of Knowledge from Experimental Models and Basic Research to Clinical Settings
RIC-based clinical trials have just begun and much data is still needed in order to reveal the real potential protective role of RIC in clinical settings. Nevertheless, one can partially predict less successful results in clinical trials than experimental models, with a complex translation of knowledge due to the features of the animals used. In fact, in the majority of models, the animals are young, in some cases adolescent, without any other co-morbidities that usually are associated with stroke patients, namely hypertension, diabetes, atherosclerosis, etc. Although the maintenance and use of aged animals are highly expensive, this should be taken into account when performing pre-clinical basic research about age-related disease.
In addition, basic research-associated technology (proteomic, metabolomics, transcriptomics, etc.) should be more explored to identify potential biochemical factors and circulating cells (leukocytes and erythrocytes) involved in the communication between the remote ischemic conditioned limb and the brain in human subjects. Taking advantage of the mild invasive procedure, such as blood collection, it is possible to investigate the RIC-associated molecular and cellular underlying mechanisms within human samples. Thus, a deep knowledge about the signaling mechanisms of RIC in humans could be reached.
Real Therapeutic Properties of RIC in Ischemic Stroke Clinical Settings
For acute ischemic stroke, several clinical trials have revealed the feasibility and safety of RIC. Although the first results regarding the beneficial outcomes of RIC are not yet clear, there are some clues indicating the potential beneficial effects of RIC. Thus, ongoing clinical trials are still promising. For example, RESIST, Danish clinical trial of remote ischemic per- and post-conditioning, will include 1000 stroke patients [109].
Patient selection may be a key factor to access the therapeutical potential of RIC, since age and co-morbidities may influence RIC efficacy. Since RIC triggers endogenous defense mechanism, one could argue that its effect are mild, and therefore only beneficial in non-severe strokes. On the other hand, RIC impact on cerebral blood flow and on brain parenchyma can be dependent on the presence of salvageable penumbra. Thus, it would be important to correlate RIC improvement in patient’s outcome with the existence of salvageable penumbra. Also, RIC can have reduced impact in patients with early recanalization, but useful in those with longer treatments delays or failed recanalization therapies.
Finally, how co-morbidities can influence the beneficial effect of RIC is still unclear. Most of the completed RIC clinical studies are pilot studies that only provide preliminary data. Thus, larger clinical trials, allowing subgroup analysis, are needed for understanding the potential influence of co-morbidities such as diabetes, atherosclerosis, hypertension, age, and others on the benefits RIC treatment.
RIC Application for Chronic Cerebral Diseases
RIC may also be more efficient in long-term applications in chronic diseases. In fact, Meng and colleagues have demonstrated that daily application of RIC during 300 or 180 days (the last one for octo- and nonagenarians) decreased the recurrence of stroke in patients with intracranial arterial stenosis [121, 122]. RIC was also tested for 1 year in patients with mild cognitive impairment caused by small vessel disease, improving some visuospatial and executive abilities and decreasing the volume of white matter hyperintensities, despite being a pilot study with only 29 patients [129]. A second study with 37 patients with vascular dementia, that applied RIC daily during 6 months, also reported an improvement in cognitive functions [130]. Likewise, chronic application of post-RIC in an experimental model of vascular cognitive impairment also improved cerebral blood flow and protected the brain parenchyma [74]. Thus, one can speculate that chronic RIC treatment holds great beneficial potential if applied chronically for patients with high risk of cerebral vascular diseases, including vascular dementia and white matter disease, among others. As a secondary stroke prevention treatment, RIC beneficial effects may also depend on ischemic stroke etiology, being potentially more effective in patients with large vessel disease when compared with cardioembolic stroke.
Concluding Remarks and Perspectives
Pre-clinical studies showed the potential neuroprotective effect of RIC and disclose its associated pathways and signaling. This therapy is based on the promotion of physiological protective mechanisms, and can inspire new targeted therapies built upon these same principals. In the translation to clinical practice, RIC results are modest so far. Type of RIC, clinical application, and patient selection criteria are of utmost importance in order to establish the RIC role in patient care. If efficient, RIC will most certainly be applied as an add-on therapy, together in other strategies for acute stroke treatment or stroke prevention.
Acknowledgements
The authors would like to acknowledge Dr Cláudia Queiroga for her work, ideas, and fruitful discussions in the beginning of this project, and the funding agency that supported the work “Fundação para a Ciência e Tecnologia” (FCT) with 4 projects: Applied Molecular Biosciences Unit-UCIBIO (UID/Multi/04378/2019); iNOVA4Health, Programme in Translational Medicine (UID/Multi/04462/2013); LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy; and PTDC/MEC-NEU/28750/2017.
Author contribution
All authors participate in the bibliographic research, writing, and revising of the manuscript.
Funding
This work was supported by funding from the Portuguese Fundação para a Ciência e Tecnologia (FCT) in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences, UCIBIO; the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy, i4HB; the project iNOVA4Health, Programme in Translational Medicine (UID/Multi/04462/2013); and the grant PTDC/MEC-NEU/28750/2017.
Data and Materials Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T et al (2018) 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 49. 10.1161/STR.0000000000000158 [DOI] [PubMed]
- 3.Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 5.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14:1363–1368. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 9.Hess DC, Blauenfeldt RA, Andersen G, et al. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat Rev Neurol. 2015;11:698–710. doi: 10.1038/nrneurol.2015.223. [DOI] [PubMed] [Google Scholar]
- 10.Przyklenk K, Bauer B, Ovize M, et al. Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.CIR.87.3.893. [DOI] [PubMed] [Google Scholar]
- 11.Oxman T, Arad M, Klein R, et al. Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol Circ Physiol. 1997;273:H1707–H1712. doi: 10.1152/ajpheart.1997.273.4.H1707. [DOI] [PubMed] [Google Scholar]
- 12.Stetler RA, Leak RK, Gan Y, et al. Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol. 2014;114:58–83. doi: 10.1016/j.pneurobio.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuyama N, Takizawa S, Ishida H, et al. Peroxynitrite Formation in Focal Cerebral Ischemia—Reperfusion in Rats Occurs Predominantly in the Peri-Infarct Region. J Cereb Blood Flow Metab. 1998;18:123–129. doi: 10.1097/00004647-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Jayaraj RL, Azimullah S, Beiram R, et al. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawabori M, Kacimi R, Kauppinen T, et al. Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) Deficiency Attenuates Phagocytic Activities of Microglia and Exacerbates Ischemic Damage in Experimental Stroke. J Neurosci. 2015;35:3384–3396. doi: 10.1523/JNEUROSCI.2620-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deczkowska A, Keren-Shaul H, Weiner A, et al. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell. 2018;173:1073–1081. doi: 10.1016/j.cell.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Zhao HG, Bin Li W, Li QJ, et al. Limb ischemic preconditioning attenuates apoptosis of pyramidal neurons in the CA1 hippocampus induced by cerebral ischemia-reperfusion in rats. Acta Physiol Sin. 2004;56:407–412. [PubMed] [Google Scholar]
- 19.Hoda MN, Bhatia K, Hafez SS, et al. Remote Ischemic Perconditioning is Effective After Embolic Stroke in Ovariectomized Female Mice. Transl Stroke Res. 2014 doi: 10.1007/s12975-013-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoda MN, Siddiqui S, Herberg S, et al. Remote ischemic perconditioning is effective alone and in combination with intravenous tissue-type plasminogen activator in murine model of embolic stroke. Stroke. 2012;43:2794–2799. doi: 10.1161/STROKEAHA.112.660373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burda R, Danielisova V, Gottlieb M, et al. Delayed remote ischemic postconditioning protects against transient cerebral ischemia/reperfusion as well as kainate-induced injury in rats. Acta Histochem. 2014 doi: 10.1016/j.acthis.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Du X, Yang J, Liu C, et al. Hypoxia-Inducible Factor 1α and 2α Have Beneficial Effects in Remote Ischemic Preconditioning Against Stroke by Modulating Inflammatory Responses in Aged Rats. Front Aging Neurosci. 2020 doi: 10.3389/fnagi.2020.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren C, Yan Z, Wei D, et al. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 2009 doi: 10.1016/j.brainres.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zong Y, Jiang L, Zhang M, et al. Limb remote ischemic postconditioning protects cerebral ischemia from injury associated with expression of HIF-1aα in rats. BMC Neurosci. 2015 doi: 10.1186/s12868-015-0235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q, Wu F, Peng X, et al. Limb remote ischemic preconditioning protects against cerebral ischemia through down-regulation of aquaporin-4. Int J Clin Exp Med. 2016;9:13878–13889. [Google Scholar]
- 26.Li P, Su L, Li X, et al. Remote limb ischemic postconditioning protects mouse brain against cerebral ischemia/reperfusion injury via upregulating expression of Nrf2, HO-1 and NQO-1 in mice. Int J Neurosci. 2016 doi: 10.3109/00207454.2015.1042973. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Zhou S, Wu L, et al. The role of p38MAPK signal pathway in the neuroprotective mechanism of limb postconditioning against rat cerebral ischemia/reperfusion injury. J Neurol Sci. 2015 doi: 10.1016/j.jns.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Ramagiri S, Taliyan R. Protective effect of remote limb post conditioning via upregulation of heme oxygenase-1/BDNF pathway in rat model of cerebral ischemic reperfusion injury. Brain Res. 2017 doi: 10.1016/j.brainres.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Ramagiri S, Taliyan R. Remote limb ischemic post conditioning during early reperfusion alleviates cerebral ischemic reperfusion injury via GSK-3β/CREB/ BDNF pathway. Eur J Pharmacol. 2017 doi: 10.1016/j.ejphar.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 30.Xia M, Ding Q, Zhang Z, Feng Q. Remote Limb Ischemic Preconditioning Protects Rats Against Cerebral Ischemia via HIF-1α/AMPK/HSP70 Pathway. Cell Mol Neurobiol. 2017 doi: 10.1007/s10571-016-0444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng X, Zhao H, Yan F, et al. Limb remote ischemic post-conditioning mitigates brain recovery in a mouse model of ischemic stroke by regulating reactive astrocytic plasticity. Brain Res. 2018 doi: 10.1016/j.brainres.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Liu C, Du X, et al. Hypoxia inducible factor 1α plays a key role in remote ischemic preconditioning against stroke by modulating inflammatory responses in rats. J Am Heart Assoc. 2018 doi: 10.1161/JAHA.117.007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang W, Lin C, Yuan L, et al. Preactivation of Notch1 in remote ischemic preconditioning reduces cerebral ischemia-reperfusion injury through crosstalk with the NF-κB pathway. J Neuroinflammation. 2019 doi: 10.1186/s12974-019-1570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Z, Li L, Mo X, et al. Non-invasive remote limb ischemic postconditioning protects rats against focal cerebral ischemia by upregulating STAT3 and reducing apoptosis. Int J Mol Med. 2014 doi: 10.3892/ijmm.2014.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li SQ, Bin LW, Zhang M, et al. The role of neuroglobin in the neuroprotection of limb ischemic preconditioning in rats. Mol Neurobiol. 2013;47:197–208. doi: 10.1007/s12035-012-8373-7. [DOI] [PubMed] [Google Scholar]
- 36.Ren C, Wang P, Wang B, et al. Limb remote ischemic per-conditioning in combination with post-conditioning reduces brain damage and promotes neuroglobin expression in the rat brain after ischemic stroke. Restor Neurol Neurosci. 2015;33:369–379. doi: 10.3233/RNN-140413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu S, Dong H, Zhang H, et al. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res. 2012 doi: 10.1016/j.brainres.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 38.Brager AJ, Yang T, Ehlen JC, et al. Sleep is critical for remote preconditioning-induced neuroprotection. Sleep. 2016 doi: 10.5665/sleep.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan Q, Jia HX, Li SQ, et al. The role of adenosine in up-regulation of p38 MAPK and ERK during limb ischemic preconditioning-induced brain ischemic tolerance. Brain Res. 2019 doi: 10.1016/j.brainres.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Ma J, Ma Y, Dong B, et al. Prevention of the collapse of pial collaterals by remote ischemic perconditioning during acute ischemic stroke. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X16680636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma J, Ma Y, Shuaib A, Winship IR. Improved collateral flow and reduced damage after remote ischemic perconditioning during distal middle cerebral artery occlusion in aged rats. Sci Rep. 2020 doi: 10.1038/s41598-020-69122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parray A, Ma Y, Alam M, et al. An increase in AMPK/e-NOS signaling and attenuation of MMP-9 may contribute to remote ischemic perconditioning associated neuroprotection in rat model of focal ischemia. Brain Res. 2020 doi: 10.1016/j.brainres.2020.146860. [DOI] [PubMed] [Google Scholar]
- 43.Ren C, Li S, Wang B, et al. Limb remote ischemic conditioning increases Notch signaling activity and promotes arteriogenesis in the ischemic rat brain. Behav Brain Res. 2018 doi: 10.1016/j.bbr.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 44.Ren C, Li N, Li S, et al. Limb ischemic conditioning improved cognitive deficits via eNOS-dependent augmentation of angiogenesis after chronic cerebral hypoperfusion in rats. Aging Dis. 2018 doi: 10.14336/AD.2017.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan MB, Hafez S, Hoda MN, et al. Chronic Remote Ischemic Conditioning Is Cerebroprotective and Induces Vascular Remodeling in a VCID Model. Transl Stroke Res. 2018 doi: 10.1007/s12975-017-0555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang D, He XB, Wang Z, et al. Remote limb ischemic postconditioning promotes motor function recovery in a rat model of ischemic stroke via the up-regulation of endogenous tissue kallikrein. CNS Neurosci Ther. 2018 doi: 10.1111/cns.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Ma L, Ren C, et al. Immediate remote ischemic postconditioning reduces cerebral damage in ischemic stroke mice by enhancing leptomeningeal collateral circulation. J Cell Physiol. 2019 doi: 10.1002/jcp.27858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M, Chen J, Zhang S, Ren C. Downregulation of lipocalin-2 and Bim expression after remote limb preconditioning in the ischemic rat brain. Brain Res. 2018 doi: 10.1016/j.brainres.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Zhang Z, Zhang L, et al. RLIPostC protects against cerebral ischemia through improved synaptogenesis in rats. Brain Inj. 2018 doi: 10.1080/02699052.2018.1483029. [DOI] [PubMed] [Google Scholar]
- 50.Li Z, Cui X, Lv H, et al. Remote ischemic postconditioning attenuates damage in rats with chronic cerebral ischemia by upregulating the autophagolysosome pathway via the activation of TFEB. Exp Mol Pathol. 2020 doi: 10.1016/j.yexmp.2020.104475. [DOI] [PubMed] [Google Scholar]
- 51.Lv J, Guan W, You Q, et al. RIPC provides neuroprotection against ischemic stroke by suppressing apoptosis via the mitochondrial pathway. Sci Rep. 2020 doi: 10.1038/s41598-020-62336-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin RL, Bin LW, Li QJ, et al. The role of extracellular signal-regulated kinases in the neuroprotection of limb ischemic preconditioning. Neurosci Res. 2006 doi: 10.1016/j.neures.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Sun XC, Bin LW, Li QJ, et al. Limb ischemic preconditioning induces brain ischemic tolerance via p38 MAPK. Brain Res. 2006 doi: 10.1016/j.brainres.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 54.Sun XC, Xian XH, Bin LW, et al. Activation of p38 MAPK participates in brain ischemic tolerance induced by limb ischemic preconditioning by up-regulating HSP 70. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Peng B, Guo QL, He ZJ, et al. Remote ischemic postconditioning protects the brain from global cerebral ischemia/reperfusion injury by up-regulating endothelial nitric oxide synthase through the PI3K/Akt pathway. Brain Res. 2012 doi: 10.1016/j.brainres.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 56.Qi ZF, Luo YM, Liu XR, et al. AKT/GSK3β-Dependent Autophagy Contributes to the Neuroprotection of Limb Remote Ischemic Postconditioning in the Transient Cerebral Ischemic Rat Model. CNS Neurosci Ther. 2012 doi: 10.1111/cns.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun J, Tong L, Luan Q, et al. Protective effect of delayed remote limb ischemic postconditioning: Role of mitochondrial K ATP channels in a rat model of focal cerebral ischemic reperfusion injury. J Cereb Blood Flow Metab. 2012 doi: 10.1038/jcbfm.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su J, Zhang T, Wang K, et al. Autophagy activation contributes to the neuroprotection of remote ischemic perconditioning against focal cerebral ischemia in rats. Neurochem Res. 2014 doi: 10.1007/s11064-014-1396-x. [DOI] [PubMed] [Google Scholar]
- 59.Qi Z, Dong W, Shi W, et al. Bcl-2 Phosphorylation Triggers Autophagy Switch and Reduces Mitochondrial Damage in Limb Remote Ischemic Conditioned Rats After Ischemic Stroke. Transl Stroke Res. 2015 doi: 10.1007/s12975-015-0393-y. [DOI] [PubMed] [Google Scholar]
- 60.Qi W, Zhou F, Li S, et al. Remote ischemic postconditioning protects ischemic brain from injury in rats with focal cerebral ischemia/reperfusion associated with suppression of TLR4 and NF-κB expression. NeuroReport. 2016 doi: 10.1097/WNR.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 61.Huang D, Liu H, Qu Y, Wang P. Non-invasive remote ischemic postconditioning stimulates neurogenesis during the recovery phase after cerebral ischemia. Metab Brain Dis. 2017 doi: 10.1007/s11011-017-0068-3. [DOI] [PubMed] [Google Scholar]
- 62.Zhang W, Wang Y, Bi G. Limb remote ischaemic postconditioning-induced elevation of fibulin-5 confers neuroprotection to rats with cerebral ischaemia/reperfusion injury: Activation of the AKT pathway. Clin Exp Pharmacol Physiol. 2017 doi: 10.1111/1440-1681.12742. [DOI] [PubMed] [Google Scholar]
- 63.Chen Gz, Shan Xy, Li Xs, Tao Hm. Remote ischemic postconditioning protects the brain from focal ischemia/reperfusion injury by inhibiting autophagy through the mTOR/p70S6K pathway. Neurol Res. 2018 doi: 10.1080/01616412.2018.1424696. [DOI] [PubMed] [Google Scholar]
- 64.Guo H, Zhao L, Wang B, et al. Remote limb ischemic postconditioning protects against cerebral ischemia-reperfusion injury by activating AMPK-dependent autophagy. Brain Res Bull. 2018 doi: 10.1016/j.brainresbull.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Hu XS, Zhou FF, et al. Limb remote ischemic postconditioning protects integrity of the blood-brain barrier after stroke. Neural Regen Res. 2018 doi: 10.4103/1673-5374.237122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kitagawa K, Saitoh M, Ishizuka K, Shimizu S. Remote Limb Ischemic Conditioning during Cerebral Ischemia Reduces Infarct Size through Enhanced Collateral Circulation in Murine Focal Cerebral Ischemia. J Stroke Cerebrovasc Dis. 2018 doi: 10.1016/j.jstrokecerebrovasdis.2017.09.068. [DOI] [PubMed] [Google Scholar]
- 67.Xu C, Yi C, Guo H, et al. Limb remote ischemic postconditioning is effective but also time-course-limited in protecting the brain from I/R injury. Turkish J Med Sci. 2012 doi: 10.3906/sag-1106-2. [DOI] [Google Scholar]
- 68.Xue L, Chen H, Lu K, et al. Clinical significance of changes in serum neuroglobin and HIF-1α concentrations during the early-phase of acute ischemic stroke. J Neurol Sci. 2017 doi: 10.1016/j.jns.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 69.Hota KB, Hota SK, Srivastava RB, Singh SB. Neuroglobin regulates hypoxic response of neuronal cells through Hif-1α- and Nrf2-mediated mechanism. J Cereb Blood Flow Metab. 2012 doi: 10.1038/jcbfm.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borea PA, Varani K, Vincenzi F, et al. The a3 adenosine receptor: History and perspectives. Pharmacol Rev. 2015 doi: 10.1124/pr.113.008540. [DOI] [PubMed] [Google Scholar]
- 71.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets-what are the challenges? Nat Rev Drug Discov. 2013 doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14(7):1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 73.Trautmann A (2009) Extracellular ATP in the immune system: More than just a “danger signal.” Sci Signal. 10.1126/scisignal.256pe6 [DOI] [PubMed]
- 74.Khan MB, Hoda MN, Vaibhav K, et al. Remote Ischemic Postconditioning: Harnessing Endogenous Protection in a Murine Model of Vascular Cognitive Impairment. Transl Stroke Res. 2015;6:69–77. doi: 10.1007/s12975-014-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Motomura A, Shimizu M, Kato A, et al. Remote ischemic preconditioning protects human neural stem cells from oxidative stress. Apoptosis. 2017 doi: 10.1007/s10495-017-1425-8. [DOI] [PubMed] [Google Scholar]
- 76.Liu C, Yang J, Zhang C, et al. Remote ischemic conditioning reduced cerebral ischemic injury by modulating inflammatory responses and ERK activity in type 2 diabetic mice. Neurochem Int. 2020 doi: 10.1016/j.neuint.2020.104690. [DOI] [PubMed] [Google Scholar]
- 77.Zhao HG, Sun XC, Xian XH, et al. The role of nitric oxide in the neuroprotection of limb ischemic preconditioning in rats. Neurochem Res. 2007 doi: 10.1007/s11064-007-9381-2. [DOI] [PubMed] [Google Scholar]
- 78.Wei D, Ren C, Chen X, Zhao H. The Chronic Protective Effects of Limb Remote Preconditioning and the Underlying Mechanisms Involved in Inflammatory Factors in Rat Stroke. PLoS ONE. 2012;7:e30892. doi: 10.1371/journal.pone.0030892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu C, Yang J, Zhang C, et al. The changes of systemic immune responses during the neuroprotection induced by remote ischemic postconditioning against focal cerebral ischemia in mice. Neurol Res. 2019 doi: 10.1080/01616412.2018.1523037. [DOI] [PubMed] [Google Scholar]
- 80.Suk K (2016) Lipocalin-2 as a therapeutic target for brain injury: An astrocentric perspective. Prog Neurobiol. 10.1016/j.pneurobio.2016.08.001 [DOI] [PubMed]
- 81.Kitagawa K, Matsumoto M, Tagaya M, et al. “Ischemic tolerance” phenomenon found in the brain. Brain Res. 1990 doi: 10.1016/0006-8993(90)90189-I. [DOI] [PubMed] [Google Scholar]
- 82.Yang J, Shakil F, Cho S. Peripheral Mechanisms of Remote Ischemic Conditioning. Cond Med. 2019;2:61–68. [PMC free article] [PubMed] [Google Scholar]
- 83.Shan LY, Li JZ, Zu LY, et al. Platelet-derived microparticles are implicated in remote ischemia conditioning in a rat model of cerebral infarction. CNS Neurosci Ther. 2013 doi: 10.1111/cns.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ren C, Liu K, Li N, et al. Neural transmission pathways are involved in the neuroprotection induced by post- but not perischemic limb remote conditioning. Brain Circ. 2015 doi: 10.4103/2394-8108.172897. [DOI] [Google Scholar]
- 85.Liu ZJ, Chen C, Li XR, et al. Remote Ischemic Preconditioning-Mediated Neuroprotection against Stroke is Associated with Significant Alterations in Peripheral Immune Responses. CNS Neurosci Ther. 2016 doi: 10.1111/cns.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen C, Jiang W, Liu Z, et al. Splenic responses play an important role in remote ischemic preconditioning-mediated neuroprotection against stroke. J Neuroinflammation. 2018 doi: 10.1186/s12974-018-1190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L, Ren C, Li Y, et al. Remote ischemic conditioning enhances oxygen supply to ischemic brain tissue in a mouse model of stroke: Role of elevated 2,3-biphosphoglycerate in erythrocytes. J Cereb Blood Flow Metab. 2020 doi: 10.1177/0271678X20952264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang J, Balkaya M, Beltran C, et al. Remote Postischemic Conditioning Promotes Stroke Recovery by Shifting Circulating Monocytes to CCR2+ Proinflammatory Subset. J Neurosci. 2019 doi: 10.1523/JNEUROSCI.2699-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiao B, Chai Y, Lv S, et al. Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int J Mol Med. 2017 doi: 10.3892/ijmm.2017.3106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Li J, Rohailla S, Gelber N, et al. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109:423. doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 91.Yamaguchi T, Izumi Y, Nakamura Y, et al. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol. 2015 doi: 10.1016/j.ijcard.2014.10.144. [DOI] [PubMed] [Google Scholar]
- 92.Li Y, Ren C, Li H, et al. Role of exosomes induced by remote ischemic preconditioning in neuroprotection against cerebral ischemia. NeuroReport. 2019 doi: 10.1097/WNR.0000000000001280. [DOI] [PubMed] [Google Scholar]
- 93.Zhong SJ, Cui MM, Gao YT, et al. MicroRNA-144 promotes remote limb ischemic preconditioning-mediated neuroprotection against ischemic stroke via PTEN/Akt pathway. Acta Neurol Belg. 2020 doi: 10.1007/s13760-020-01500-5. [DOI] [PubMed] [Google Scholar]
- 94.Cui J, Liu N, Chang Z, et al. Exosomal MicroRNA-126 from RIPC Serum Is Involved in Hypoxia Tolerance in SH-SY5Y Cells by Downregulating DNMT3B. Mol Ther - Nucleic Acids. 2020;20:649–660. doi: 10.1016/j.omtn.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hibert P, Prunier-Mirebeau D, Beseme O, et al. Modifications in rat plasma proteome after remote ischemic preconditioning (RIPC) stimulus: Identification by a SELDI-TOF-MS approach. PLoS ONE. 2014 doi: 10.1371/journal.pone.0085669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hibert P, Prunier-Mirebeau D, Beseme O, et al. Apolipoprotein A-I Is a Potential Mediator of Remote Ischemic Preconditioning. PLoS ONE. 2013 doi: 10.1371/journal.pone.0077211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011 doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferreira R, Santos T, Amar A, et al. Argonaute-2 promotes miR-18a entry in human brain endothelial cells. J Am Heart Assoc. 2014 doi: 10.1161/JAHA.114.000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ueno K, Samura M, Nakamura T, et al. Increased plasma VEGF levels following ischemic preconditioning are associated with downregulation of miRNA-762 and miR-3072-5p. Sci Rep. 2016 doi: 10.1038/srep36758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duan X, Ji B, Wang X, et al. Expression of MicroRNA-1 and MicroRNA-21 in different protocols of ischemic conditioning in an isolated rat heart model. Cardiol. 2012 doi: 10.1159/000338149. [DOI] [PubMed] [Google Scholar]
- 101.Crimi G, Pica S, Raineri C, et al. Remote Ischemic Post-Conditioning of the Lower Limb During Primary Percutaneous Coronary Intervention Safely Reduces Enzymatic Infarct Size in Anterior Myocardial Infarction. JACC Cardiovasc Interv. 2013;6:1055–1063. doi: 10.1016/j.jcin.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 102.Munk K, Andersen NH, Schmidt MR, et al. Remote Ischemic Conditioning in Patients With Myocardial Infarction Treated With Primary Angioplasty: Impact on Left Ventricular Function Assessed by Comprehensive Echocardiography and Gated Single-Photon Emission CT. Circ Cardiovasc Imaging. 2010;3:656–662. doi: 10.1161/CIRCIMAGING.110.957340. [DOI] [PubMed] [Google Scholar]
- 103.Prunier F, Angoulvant D, Saint Etienne C, et al. The RIPOST-MI study, assessing remote ischemic perconditioning alone or in combination with local ischemic postconditioning in ST-segment elevation myocardial infarction. Basic Res Cardiol. 2014;109:400. doi: 10.1007/s00395-013-0400-y. [DOI] [PubMed] [Google Scholar]
- 104.Rentoukas I, Giannopoulos G, Kaoukis A, et al. Cardioprotective Role of Remote Ischemic Periconditioning in Primary Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2010;3:49–55. doi: 10.1016/j.jcin.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 105.White SK, Frohlich GM, Sado DM, et al. Remote Ischemic Conditioning Reduces Myocardial Infarct Size and Edema in Patients With ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Interv. 2015;8:178–188. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 106.Chen L, Zhou Q, Jin H, et al. Effects of Remote Ischaemic Conditioning on Heart Rate Variability and Cardiac Function in Patients With Mild Ischaemic Heart Failure. Hear Lung Circ. 2018;27:477–483. doi: 10.1016/j.hlc.2017.03.164. [DOI] [PubMed] [Google Scholar]
- 107.Hu Q, Luo W, Huang L, et al. Multiorgan protection of remote ischemic perconditioning in valve replacement surgery. J Surg Res. 2016;200:13–20. doi: 10.1016/j.jss.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 108.Surkar SM, Bland MD, Mattlage AE, et al. Effects of remote limb ischemic conditioning on muscle strength in healthy young adults: A randomized controlled trial. PLoS ONE. 2020;15:e0227263. doi: 10.1371/journal.pone.0227263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blauenfeldt RA, Hjort N, Gude MF, et al. A multicentre, randomised, sham-controlled trial on REmote iSchemic conditioning In patients with acute STroke (RESIST) – Rationale and study design. Eur Stroke J. 2020;5:94–101. doi: 10.1177/2396987319884408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.England TJ, Hedstrom A, O’Sullivan S, et al. RECAST (Remote Ischemic Conditioning After Stroke Trial) Stroke. 2017;48:1412–1415. doi: 10.1161/STROKEAHA.116.016429. [DOI] [PubMed] [Google Scholar]
- 111.England TJ, Hedstrom A, O’Sullivan SE et al (2019) Remote Ischemic Conditioning After Stroke Trial 2: A Phase IIb Randomized Controlled Trial in Hyperacute Stroke. J Am Heart Assoc 8.10.1161/JAHA.119.013572 [DOI] [PMC free article] [PubMed]
- 112.England T (2021) Remote Ischaemic Conditioning After Stroke 3 (RECAST-3): A multicentre randomised controlled trial. https://fundingawards.nihr.ac.uk/award/NIHR128240
- 113.Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning in thrombolysed stroke patients: Randomized study of activating endogenous neuroprotection - design and MRI measurements. Int J Stroke. 2013;8:141–146. doi: 10.1111/j.1747-4949.2012.00786.x. [DOI] [PubMed] [Google Scholar]
- 114.Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: A randomized trial. Stroke. 2014;45:159–167. doi: 10.1161/STROKEAHA.113.001346. [DOI] [PubMed] [Google Scholar]
- 115.Pico F, Lapergue B, Ferrigno M, et al. Effect of In-Hospital Remote Ischemic Perconditioning on Brain Infarction Growth and Clinical Outcomes in Patients With Acute Ischemic Stroke. JAMA Neurol. 2020;77:725. doi: 10.1001/jamaneurol.2020.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pico F, Rosso C, Meseguer E, et al. A multicenter, randomized trial on neuroprotection with remote ischemic per-conditioning during acute ischemic stroke: the REmote iSchemic Conditioning in acUtE BRAin INfarction study protocol. Int J Stroke. 2016;11:938–943. doi: 10.1177/1747493016660098. [DOI] [PubMed] [Google Scholar]
- 117.Landman T, Schoon Y, Warlé M, et al. The effect of repeated remote ischemic postconditioning on infarct size in patients with an ischemic stroke (REPOST): study protocol for a randomized clinical trial. Trials. 2019;20:167. doi: 10.1186/s13063-019-3264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu S-M, Zhao W-L, Song H-Q, et al. Rationale and Study Design for a Single-Arm Phase IIa Study Investigating Feasibility of Preventing Ischemic Cerebrovascular Events in High-Risk Patients with Acute Non-disabling Ischemic Cerebrovascular Events Using Remote Ischemic Conditioning. Chin Med J (Engl) 2018;131:347–351. doi: 10.4103/0366-6999.223849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao W, Che R, Li S, et al. Remote ischemic conditioning for acute stroke patients treated with thrombectomy. Ann Clin Transl Neurol. 2018;5:850–856. doi: 10.1002/acn3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He Y, Guo Z, Qin C, et al. Remote ischemic conditioning combined with intravenous thrombolysis for acute ischemic stroke. Ann Clin Transl Neurol. 2020;7:972–979. doi: 10.1002/acn3.51063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–1861. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- 122.Meng R, Ding Y, Asmaro K, et al. Ischemic Conditioning Is Safe and Effective for Octo- and Nonagenarians in Stroke Prevention and Treatment. Neurotherapeutics. 2015;12:667–677. doi: 10.1007/s13311-015-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li S, Ma C, Shao G, et al. Safety and Feasibility of Remote Limb Ischemic Preconditioning in Patients with Unilateral Middle Cerebral Artery Stenosis and Healthy Volunteers. Cell Transplant. 2015;24:1901–1911. doi: 10.3727/096368914X683520. [DOI] [PubMed] [Google Scholar]
- 124.Hu S, Dong H, Li Y, et al. Effects of Remote Ischemic Preconditioning on Biochemical Markers and Neurologic Outcomes in Patients Undergoing Elective Cervical Decompression Surgery. J Neurosurg Anesthesiol. 2010;22:46–52. doi: 10.1097/ANA.0b013e3181c572bd. [DOI] [PubMed] [Google Scholar]
- 125.Gonzalez NR, Hamilton R, Bilgin-Freiert A, et al. Cerebral Vasospasm: Neurovascular Events After Subarachnoid Hemorrhage. Vienna: Springer Vienna; 2013. Cerebral Hemodynamic and Metabolic Effects of Remote Ischemic Preconditioning in Patients with Subarachnoid Hemorrhage; pp. 193–198. [DOI] [PubMed] [Google Scholar]
- 126.Gonzalez NR, Connolly M, Dusick JR, et al. Phase I Clinical Trial for the Feasibility and Safety of Remote Ischemic Conditioning for Aneurysmal Subarachnoid Hemorrhage. Neurosurgery. 2014;75:590–598. doi: 10.1227/NEU.0000000000000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote Ischemic Limb Preconditioning After Subarachnoid Hemorrhage. Stroke. 2011;42:1387–1391. doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao W, Jiang F, Li S et al (2020) Remote Ischemic Conditioning for Intracerebral Hemorrhage (RICH-1): Rationale and Study Protocol for a Pilot Open-Label Randomized Controlled Trial. Front Neurol 11.10.3389/fneur.2020.00313 [DOI] [PMC free article] [PubMed]
- 129.Wang Y, Meng R, Song H, et al. Remote Ischemic Conditioning May Improve Outcomes of Patients With Cerebral Small-Vessel Disease. Stroke. 2017;48:3064–3072. doi: 10.1161/STROKEAHA.117.017691. [DOI] [PubMed] [Google Scholar]
- 130.Liao Z, Bu Y, Li M, et al. Remote ischemic conditioning improves cognition in patients with subcortical ischemic vascular dementia. BMC Neurol. 2019;19:206. doi: 10.1186/s12883-019-1435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao W, Meng R, Ma C, et al. Safety and Efficacy of Remote Ischemic Preconditioning in Patients With Severe Carotid Artery Stenosis Before Carotid Artery Stenting. Circulation. 2017;135:1325–1335. doi: 10.1161/CIRCULATIONAHA.116.024807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Durand MJ, Boerger TF, Nguyen JN, et al. Two weeks of ischemic conditioning improves walking speed and reduces neuromuscular fatigability in chronic stroke survivors. J Appl Physiol. 2019;126:755–763. doi: 10.1152/japplphysiol.00772.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gasparovic H, Kopjar T, Rados M, et al. Impact of remote ischemic preconditioning preceding coronary artery bypass grafting on inducing neuroprotection. J Thorac Cardiovasc Surg. 2019;157:1466–1476.e3. doi: 10.1016/j.jtcvs.2018.08.116. [DOI] [PubMed] [Google Scholar]
- 134.Cherry-Allen KM, Gidday JM, Lee J-M, et al. Remote Limb Ischemic Conditioning at Two Cuff Inflation Pressures Yields Learning Enhancements in Healthy Adults. J Mot Behav. 2017;49:337–348. doi: 10.1080/00222895.2016.1204268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Donato M, Buchholz B, Rodríguez M, et al. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Exp Physiol. 2013;98:425–434. doi: 10.1113/expphysiol.2012.066217. [DOI] [PubMed] [Google Scholar]
- 136.Mei B, Li W, Cheng X, et al. Activating mu-opioid receptors in the spinal cord mediates the cardioprotective effect of remote preconditioning of trauma. Cardiol J. 2017;24:314–323. doi: 10.5603/CJ.a2016.0062. [DOI] [PubMed] [Google Scholar]
- 137.Basalay MV, Mastitskaya S, Mrochek A, et al. Glucagon-like peptide-1 (GLP-1) mediates cardioprotection by remote ischaemic conditioning. Cardiovasc Res. 2016;112:669–676. doi: 10.1093/cvr/cvw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lambert EA, Thomas CJ, Hemmes R, et al. Sympathetic nervous response to ischemia-reperfusion injury in humans is altered with remote ischemic preconditioning. Am J Physiol Circ Physiol. 2016;311:H364–H370. doi: 10.1152/ajpheart.00369.2016. [DOI] [PubMed] [Google Scholar]
- 139.Noronha Osório D, Viana-Soares R, Marto JP et al (2019) Autonomic nervous system response to remote ischemic conditioning: Heart rate variability assessment. BMC Cardiovasc Disord 19.10.1186/s12872-019-1181-5 [DOI] [PMC free article] [PubMed]
- 140.Qu Y, Liu J, Guo Z-N, et al. The Impact of Remote Ischaemic Conditioning on Beat-to-Beat Heart Rate Variability Circadian Rhythm in Healthy Adults. Hear Lung Circ. 2020 doi: 10.1016/j.hlc.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 141.Khaliulin I, Fleishman AN, Shumeiko NI, et al. Neuro-autonomic changes induced by remote ischemic preconditioning (RIPC) in healthy young adults: Implications for stress. Neurobiol Stress. 2019;11:100189. doi: 10.1016/j.ynstr.2019.100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dezfulian C, Taft M, Corey C, et al. Biochemical signaling by remote ischemic conditioning of the arm versus thigh: Is one raise of the cuff enough? Redox Biol. 2017;12:491–498. doi: 10.1016/j.redox.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang J, Zhang X, Chen X, et al. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol Ther - Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Konstantinov IE, Arab S, Kharbanda RK, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19:143–150. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- 145.Hepponstall M, Ignjatovic V, Binos S, et al. Remote Ischemic Preconditioning (RIPC) Modifies Plasma Proteome in Humans. PLoS ONE. 2012;7:e48284. doi: 10.1371/journal.pone.0048284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pang T, Zhao Y, Zhang N-R, et al. Transient Limb Ischemia Alters Serum Protein Expression in Healthy Volunteers: Complement C3 and Vitronectin May Be Involved in Organ Protection Induced by Remote Ischemic Preconditioning. Oxid Med Cell Longev. 2013;2013:1–9. doi: 10.1155/2013/859056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hepponstall M, Ignjatovic V, Binos S, et al. Remote Ischemic Preconditioning (RIPC) Modifies the Plasma Proteome in Children Undergoing Repair of Tetralogy of Fallot: A Randomized Controlled Trial. PLoS ONE. 2015;10:e0122778. doi: 10.1371/journal.pone.0122778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Helgeland E, Breivik LE, Vaudel M, et al. Exploring the Human Plasma Proteome for Humoral Mediators of Remote Ischemic Preconditioning - A Word of Caution. PLoS ONE. 2014;9:e109279. doi: 10.1371/journal.pone.0109279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Joseph B, Pandit V, Zangbar B, et al. Secondary brain injury in trauma patients. J Trauma Acute Care Surg. 2015;78:698–705. doi: 10.1097/TA.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 150.Wang H, He Z, Zhang Y, Zhang J. 1H NMR metabolic signature of cerebrospinal fluid following repetitive lower-limb remote ischemia preconditioning. Neurochem Int. 2018 doi: 10.1016/j.neuint.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 151.Loukogeorgakis SP, Williams R, Panagiotidou AT, et al. Transient Limb Ischemia Induces Remote Preconditioning and Remote Postconditioning in Humans by a K ATP Channel-Dependent Mechanism. Circulation. 2007;116:1386–1395. doi: 10.1161/CIRCULATIONAHA.106.653782. [DOI] [PubMed] [Google Scholar]
- 152.Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, et al. Remote Ischemic Preconditioning Provides Early and Late Protection Against Endothelial Ischemia-Reperfusion Injury in Humans. J Am Coll Cardiol. 2005;46:450–456. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 153.Kraemer R, Lorenzen J, Kabbani M, et al. Acute effects of remote ischemic preconditioning on cutaneous microcirculation - A controlled prospective cohort study. BMC Surg. 2011 doi: 10.1186/1471-2482-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jones H, Hopkins N, Bailey TG, et al. Seven-Day Remote Ischemic Preconditioning Improves Local and Systemic Endothelial Function and Microcirculation in Healthy Humans. Am J Hypertens. 2014;27:918–925. doi: 10.1093/ajh/hpu004. [DOI] [PubMed] [Google Scholar]
- 155.Fukuda S, Kono Y, Hanatani A et al (2014) Remote ischemic conditioning improves coronary microcirculation in healthy subjects and patients with heart failure. Drug Des Devel Ther 1175.10.2147/DDDT.S68715 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.