Abstract
A large body of research has demonstrated that human stearoyl-CoA desaturase 1 (SCD1), a universally expressed fatty acid Δ9-desaturase that converts saturated fatty acids (SFA) into monounsaturated fatty acids, is a central regulator of metabolic and signaling pathways involved in cell proliferation, differentiation, and survival. Unlike SCD1, stearoyl-CoA desaturase 5 (SCD5), a second SCD isoform found in a variety of vertebrates, including humans, has received considerably less attention but new information on its catalytic properties, regulation and biological functions of this enzyme has begun to emerge. This review will examine the new evidence that supports key metabolic and biological roles for SCD5, as well as the potential implication of this desaturase in the mechanisms of human diseases.
1. Introduction
Cells must produce a large variety of molecular species of lipids to meet the demand for structural, energetic, and signaling molecules required for proliferation, growth, differentiation, and survival [1]. Acyl-containing lipids, such as phospholipids, mono-, di- and triacylglycerols, cholesteryl esters, and other quantitatively minor lipids, contain a functionally appropriate combination of saturated (SFA), monounsaturated (MUFA), and polyunsaturated (PUFA) fatty acids of different carbon chain length and unsaturation level. SFA and MUFA are the most abundant fatty acids in mammalian organisms, accounting for over 80% of all fatty acid species present in cell lipids. Therefore, changes in the content and distribution of these fatty acids will modify a plethora of biological processes mediated by acyl-containing lipids, including cell membrane architecture and mobility, intracellular and circulating forms of energy storage, lipid-mediated signal transduction events, cellular trafficking, and transcriptional activation, among many other functions that promote and preserve major cellular activities [2, 3, 4].
2. Human SCD activity and fatty acid desaturation
Synthesis of MUFA is a universal metabolic feature that is essential in all stages in the life of a cell [1, 4]. Enzymes that produce MUFA, including fatty acyl desaturases such as SCDs, are ubiquitously present in all organisms, going from bacteria and yeast to humans [5]. A variety of Scd-like genes are expressed in Saccharomyces cerevisiae (ole1), Caenorhabditis elegans (fat-5, fat-6, and fat-7), and in the vertebrates (scd1, scd2, scd3, scd4, and scd5) [6]. Mammalian SCDs are endoplasmic reticulum-resident enzymes that introduce the first double bond in the cis-Δ9 position of saturated fatty acyl-CoAs, chiefly palmitoyl-CoA and stearoyl-CoA, to produce palmitoleyl-CoA and oleyl-CoA, respectively (Figure 1) [7, 8]. These desaturases have been extensively investigated in diverse mammalian organisms, particularly in rodent models, as well as in human tissues and cultured cells. Mice contain four SCD isoforms (SCD1–4) expressed in a tissue-specific manner, with the SCD1 isoform present in all tissues, particularly in the liver, whereas expression of SCD2, SCD3 and SCD4 is highly restricted to brain, skin, and heart, respectively [9, 10, 11]. Rats express two isoforms SCD1 and SCD2 [12]. Also, two SCD isozymes, SCD1 and SCD5, have been reported in humans [13, 14, 15].
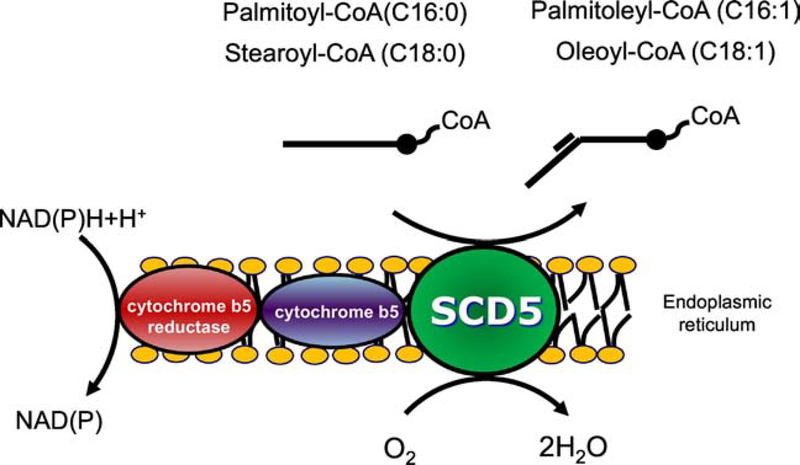
Figure 1. Stearoyl-CoA desaturase reaction.
Mammalian stearoyl-CoA desaturases (SCD), including human SCD1 and SCD5, catalyze the Δ9-desaturation of saturated long chain acyl-CoAs, mainly 16-carbon palmitoyl-CoA and 18-carbon stearoyl-CoA, yielding palmitoleoyl-CoA and oleoyl-CoA, respectively. For this reaction, the desaturase uses electrons are donated by NAD(P)H via cytochrome b5 reductase and cytochrome b5. The final acceptor of the electrons is molecular O2, with the reaction producing H2O as an additional catalytic product.
Structural, catalytic and metabolic features of murine and human SCD1, as well as its transcriptional and posttranscriptional regulation by dietary, hormonal and environmental factors, have been thoroughly discussed elsewhere [4, 11, 16, 17, 18]. A wealth of experimental data points to SCD1 activity as the central modulator of the SFA-to-MUFA ratio in cell lipids [16, 17, 19]. Furthermore, a perturbation in the balance of these two fatty acid types has been implicated in several prevalent diseases in humans, including obesity, diabetes, fatty liver, cardiovascular, and neoplastic diseases [4, 20, 21, 22]. In contrast, the catalytic properties, regulation, and biological roles of SCD5, the second Δ9-desaturase isoform expressed in humans and other vertebrates, as well as its relevance in the mechanisms of disease, have been poorly characterized. However, a growing number of studies have started to shed light on the mechanisms by which SCD5 participates in the control of cellular fatty acid distribution, lipid synthesis, cellular signaling, and, thus, the rate of cell growth, replication, and survival. Moreover, recent data suggest that SCD5 may be involved in the onset and progression of several human developmental and chronic diseases.
3. SCD5: structural, enzymatic, and biological features
SCD5, which was initially named ACOD4 due to its homology with other mammalian Δ9-desaturases, was first cloned in the chromosome inversion site of a family with cleft lip [14]. Interestingly, while SCD1 is found in chromosome 10, the scd5 gene is located in chromosome 4 [14], indicating that this SCD variant is a distinct Δ9-desaturating enzyme. SCD5 shares 54–58% identity and 72–74% similarity with SCD1–4 isoforms [14, 15].Two variants of SCD5 are described in the NCBI reference sequences and UniprotKB databases: SCD5 variant 1 (NM_ 001037582.3,) and SCD5 variant 2 (NM_024906.3 ), which encode for proteins containing Δ−9 fatty acyl desaturase-like domains. However, variant 2 presents multiple differences in the 3’ coding region resulting in a protein with a shorter and distinct C terminus region (NP_079182.2, Q86SK9–2, 256 aa ), compared with the protein encoded by variant 1 (NP_001032671.2, Q86SK9–1, 330 aa). The analysis of the protein structure of SCD5 variant 1 predicts the presence of four potential transmembrane domains and three histidine boxes (HRLWSH, HRVHH, HNYHH), which are a typical find in fatty acyl desaturases, including SCD1 [23, 24]. Another critical structural difference between SCD1 and SCD5 is the lack of PEST sequences, which are rich in proline, serine, threonine, glutamic acid and aspartic acid, in the latter enzyme [25]. Deletion of N-terminal amino acids containing PEST sequences in the SCD1 protein have been shown to reduce the half-life of the desaturase [26, 27]. Although the absence of a PEST sequence in SCD5 may give this desaturase shorter half-life than SCD1, no studies have been conducted, so far, to address this subject.
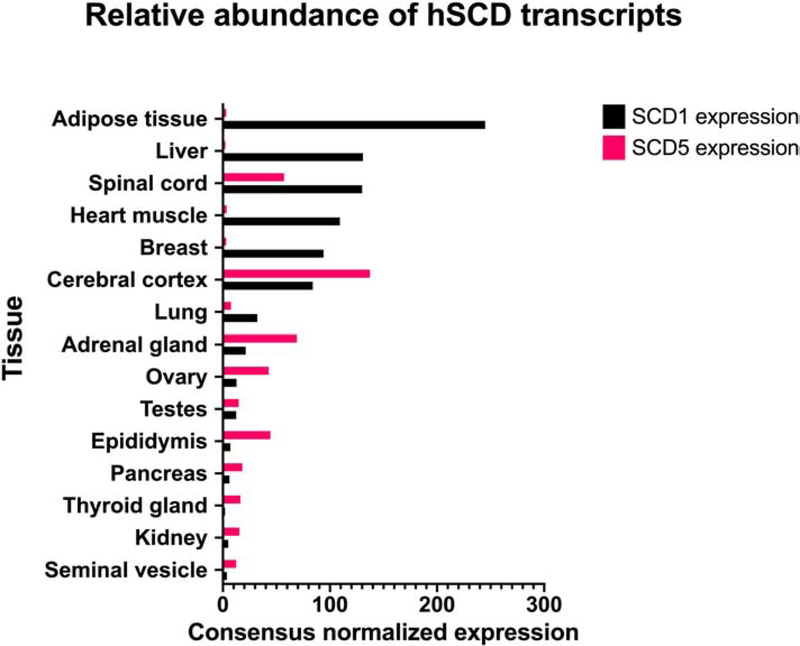
Although SCD5 was initially thought to be present exclusively in primates, SCD5 orthologues have also been found in a variety of vertebrates, including ruminants, pigs, dogs, and birds [25, 28, 29] (Figure 2). Several phylogenetic analyses of mammalian SCD variants hint at the possibility that a primordial Δ9-desaturase gene diverged into scd1 and scd5 as independently duplicated genes early in vertebrate evolution [5, 25, 28]. Further, work of Wu et al. [30] suggests that the pattern of gene expression after gene duplication may have resulted in functional specialization of SCD5, which may help explain its distinct expression profile in tissues and organs when compared to SCD1 (www.proteinatlas.com) [31] (Figure 3). The human scd1 gene is ubiquitously found in all tissues, but more predominantly present in adipose tissue, liver, brain, heart, breast, and lung, among other tissues [13]. SCD1 is also highly expressed in human oncogene-transformed and cancer cells [32]. In contrast to SCD1, human SCD5 is primarily found in fetal brain, and in adult brain and pancreas, adrenal gland, gonads, and to a lesser degree in kidney, lung, and normal and obese human adipose tissue (www.proteinatlas.com) [14, 15, 31, 33]. A similar expression pattern of SCD5 has been described in other vertebrates [25, 28]. As it will be discussed in more detail below, since optimally balanced levels of SFA and MUFA are required for brain development [34, 35], the particular high expression of SCD5 in fetal brain suggest that this desaturase could provide this and other embryonic tissues with a distinctive desaturase activity that supplies appropriate amounts of SFA and MUFA for the specific needs to the developing tissue. Additionally, SCD5 was also detected in human cumulus cells, which surrounds the oocyte and are crucial for its development [36, 37]. These studies showed that the desaturase transcript levels increase with nuclear maturity and are thought to predict the quality of oocytes and viability of embryos. Noteworthy, SCD5 expression has also been observed in several human oncogene-transformed and cancer cells [32, 38, 39].
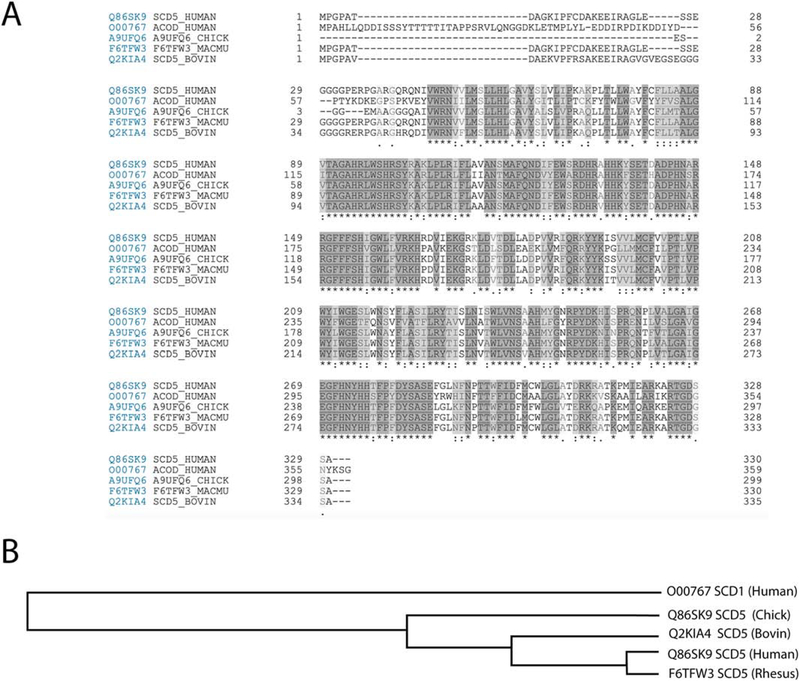
Figure 2: Protein sequences of SCD1 and SCD5.
A) Alignment of sequences of human SCD1 and paralog SCD5 from four different species using Clustal Omega program in UniProt. The asterisk denotes positions which have a single, fully conserved residue (dark highlight). While colon denotes conservation between groups of strongly similar properties (gray highlight), period indicates conservation between groups of weakly similar properties (light gray highlight). Overall, the degree of conservation among the five proteins is 47%. B) Tree depicting relationships among proteins aligned in section A.
Figure 3. Expression of human SCD1 and SCD5 in tissues.
Relative expression of SCD1 and SCD5 in several human tissues (adapted from Human Protein Atlas, www.proteinatlas.org). Data represented in graph is based on RNA-seq data from Human Protein Atlas and the Genotype-Tissue Expression project, as well as CAGE data from FANTOM5 project.
Despite the limited sequence homology of SCD5 with other vertebrate Δ9-desaturases, the subcellular localization and enzymatic activity of this desaturase are similar to other SCDs. Wang et al. [15] showed that human SCD5, like SCD1, resides in the endoplasmic reticulum compartment. In the same study, in vitro determinations demonstrated that SCD5 was able to desaturate stearic acid to oleic acid, but desaturation of palmitic acid was not determined. Sinner et al. [38] reported that palmitoleic acid increased in mouse neuronal cells that stably expressed human SCD5, whereas the relative content of oleic acid remained unchanged, suggesting that SCD5 not only can desaturate palmitic acid but that this fatty acid acts as preferred substrate for this enzyme. Moreover, in studies done in bovine tissues, single nucleotide polymorphisms in SCD5 were associated with higher C16:1/C16:0 index, suggesting, again, a greater Δ9-desaturating activity for palmitic acid substrate [40]. However, lentivirus-mediated expression of SCD5 in human melanoma and murine mammary carcinoma cells markedly increased the oleic acid/stearic acid ratio with minimal changes in the palmitoleic acid/palmitic acid ratio [41]. In this connection, a liver-specific overexpression of human SCD5 in mice with global SCD1 depletion increased the levels of oleic acid, but not palmitoleic acid, indicating a preference for stearate as substrate for SCD5 [42]. Finally, the expression of human SCD5 in mouse 3T3-L1 preadipocytes was shown to significantly elevate oleic acid without modifying the content of palmitoleic acid (R. Ariel Igal, unpublished observations). Whether these different desaturation rates of 16- and 18-carbon fatty acid substrates by SCD5 reported in the above studies are dictated by a cell-type specific abundance of substrates remains an open question.
4. Regulation of SCD5
The structural features of the scd5 gene offer some clues on the regulation and function of this desaturase. A number of transcription binding sites at the 5’-UTR region of human scd5 gene indicate that sequences corresponding to C/EBP-α, AP1, SP1, NF-1, NF-Y, T3R, PPAR-a, and SREBP1 are present in the promoter region of the gene [5, 25, 30]. Although the binding of transcription factors to the aforementioned transcription sites are known to control the expression of SCD1 [43], the actual functional activity of these promoter regions in the scd5 gene remains largely undetermined. Studies done by Lengi and Corl [44] showed that transcription factors SREBP-1a, a master regulator of lipogenesis, and EGR2 are able to bind to and activate bovine SCD5 promoter regions. Interestingly, EGR2 is a transcriptional regulator that plays a central role in peripheral nerve myelination and activate the expression of SCD2, a murine SCD that, like SCD5, is highly present in brain cells [45]. SREBP-1a has been shown to potentiate the EGR2-mediated activation of mouse SCD2 promoter [46], but this synergistic effect was not observed upon binding of these two transcription factors to bovine SCD5 promoter [44]. The lack of a substantial induction of SCD5 mRNA synthesis upon binding of either or both SREBP1a and EGR2 to the SCD5 promoter may suggest the presence of an antagonistic mechanism that limits transcriptional activity [44]. In addition, analysis of the 3’-UTR region of the scd5 gene predicts the presence of several conserved sites of microRNA families among vertebrates, including two conserved sites for mammals [30]. Importantly, these microRNA clusters are associated with several diseases including NAFLD, schizophrenia, autism, as well as brain and pancreatic cancers [30]. T-cells from patients with rheumatoid arthritis are characterized by increased levels of microRNA 34b and a decrease in SCD5, a target for this microRNA [47], suggesting a connection between the regulation of the desaturase levels and immune-inflammatory functions in humans.
Mammalian SCD1 is notably sensitive to changes in the levels of macronutrients in the diet, particularly unsaturated fatty acids [2, 17], but dietary factors appear to have a less significant influence on the levels of SCD5. In experiments done in cows fed rapeseed, soybean or linseed oils, which are rich in oleic, linoleic, and gamma-linoleic acids, respectively, SCD1 transcription levels in mammary glands were significantly reduced by the dietary oils whereas the expression of SCD5, which is low in basal conditions, remained unmodified [48]. However, in a human glioma cell line, SCD5 levels were markedly decreased by treatment with gamma-linolenic acid [49], which may insinuate a selective regulation of SCD5 by polyunsaturated fatty acids. Human SCD5 also appears to be unresponsive to lipidic factors in the serum. Roongta et al. [50] observed that, while SCD1 expression markedly increased when cancer cells were grown in 2% fetal bovine serum compared to 10% fetal bovine serum, the inhibitory effect of serum on the desaturase levels disappeared when lipids were removed. In the same study, the expression of SCD5 was unmodified by changing concentrations of fetal bovine serum or by delipidated serum. Furthermore, although retinoic acid, a retinoid that is known to suppress cell proliferation and stimulate differentiation [51], is known to induce the transcription of SCD1 in human cells [52], the levels of SCD5 in both normal human skin fibroblasts and SH-SY5Y human neuroblastoma cells remained unchanged upon retinoic acid treatment [38]. Taken together, the aforementioned studies suggest that, compared to SCD1, SCD5 seems less sensible to regulation by lipid factors. Future investigations employing a variety of experimental models and conditions are needed to the determine the degree by which SCD5 is modulated by other nutritional, hormonal, and environmental factors that affect SCD1 expression and function.
5. SCD5: role in metabolism, and in cell proliferation, differentiation and survival
A large body of evidence has conclusively determined that SCD1 not only controls the ratio SFA-to-MUFA and the metabolic fate of these fatty acids in mammalian cells, including humans, but also the rate of overall lipogenesis [4, 16, 17]. Studies published in recent years, albeit mostly performed in cell cultured models, have begun to answer the question of whether SCD5 plays a similar role in lipid metabolism. The first evidence of a potential implication of SCD5 in the control of the de novo synthesis of acyl-containing lipids was reported by Sinner et al. [38]. This work showed that the stable expression of human SCD5 in mouse neuroblastoma cells increased the incorporation of 14C-labeled glucose into phosphatidylcholine and cholesterol esters. Thus, SCD5, similar to SCD1, may not only determine the MUFA:SFA ratio but also the rate of polar and neutral lipid synthesis in fast-replicating cells. Further supporting a role for SCD5 in lipid regulation, it was observed that in mice with a global SCD1 knockout the liver-specific expression of human SCD5 fully reversed the decreased levels of hepatic triacylglycerol observed in SCD1-ablated animals [42]. In this study, however, the rate of synthesis of triacylglycerols, as well as other major lipids in liver, such as phospholipids and cholesterol esters, was not determined.
It has been firmly established that the synthesis of MUFA is a key component of the mechanisms of cell proliferation, growth, differentiation, and survival of mammalian cells, including human normal and cancer cells [3, 4, 19]. Since human fetal brain cells exhibit high expression levels of SCD5 [14, 15], this desaturase could be implicated in the highly synchronized sequence of cell division, exit of cell cycle, and entry in the program of differentiation of neurons. In this connection, initial studies performed in mouse neuronal cells showed that, compared to mock-transfected cells, the expression of human SCD5 increased the cell replication rate [38]. The faster cell proliferation induced by ectopic SCD5 could have been caused by an acceleration of the cell cycle promoted by increased levels of cyclin D1, a crucial regulator of the G1/S phase transition [53], observed in SCD5-overexpressing cells. In cancer cells, SCD1 activity propels cell cycle progression via a modification in the expression of cyclin D1 and CDK6 [54], suggesting that both human Δ9-desaturases are implicated in the mechanisms of cell division by targeting key checkpoint proteins in a similar regulatory fashion. Further reinforcing the notion that SCD5 activity is critically involved in cell proliferation, HEI-OC1 hair cells ectopically expressing human SCD5 exhibited an increase in cell replication whereas the expression of a mutant SCD harboring a c.626G > C mutation that affects a conserved tryptophan residue showed no effect on cell proliferation [55].
SCD5 activity also appears to be crucially linked to the mechanisms of cell differentiation. In neuronal cells, constitutive expression of human SCD5 drastically impaired neuritogenesis as shown by a diminished growth of neurite prolongations in length and number [38]. Further, the detection of low levels of βIII-tubulin, an early marker of neuronal differentiation [56], in SCD5-expressing cells in both basal and differentiating conditions suggests that the desaturase regulates the initial stages of differentiation [38]. Although data indicate that SCD enzymes, particularly SCD1, control both cell proliferation and cell differentiation [4, 38, 55], evidence at a mechanistic level is still lacking. The question of whether both SCD isoforms exhibit separate or complementary roles in both biological processes remains unanswered. However, the abovementioned studies in neuronal cells stably expressing SCD5 underscore the possibility that this enzyme is integral to the intertwined modulation of cell replication and differentiation, processes whose balance determines the fate of cells. Moreover, exit from cell cycle is a prerequisite for the differentiation of neurons [57], therefore, a more active cycle of cell replication could explain the delay, or even failure, in fully developing into differentiated neurons of those cells expressing ectopic SCD5. This view is supported by the observation that although retinoic acid in neuronal cells markedly halted cell proliferation in both SCD5-expressing and controls cells the anti-differentiation effect of the retinoid was less prominent in the SCD5 overexpressors [38].
The changes in the biological phenotype of neuronal cells promoted by the expression of SCD5, such as alterations in cell proliferation and neurite outgrowth, could be traced, at least in part, to a modification of signaling mechanisms, such as Akt, ERK1/2, and Wnt [38]. In this study, it was observed that in cells conditioned for differentiation SCD5 blunted the EGF-induced phosphorylation and activation of EGFR, as well as its downstream signals ERK and Akt, which are central players in neuronal replication and functional maturation [57, 58]. In light of the findings showing that SCD1 activity is required for the full induction of Akt and ERK pathways [32, 59, 60], the observation that SCD5 modulates these two signaling systems in an opposite manner suggests that human SCD isoforms may exhibit divergent functions in human cells, at least in certain cell types.
Wnt are secreted proteins that control cell replication, differentiation, and polarity, as well as organ development, in a large number of organisms [61]. By binding to their receptors, Wnt proteins trigger the activation of three signaling branches: the canonical β-catenin/TCF pathway, the β-catenin independent pathways JNK/PCP pathway, and the Wnt/calcium pathway [62, 63]. Posttranslational acylation of Wnt proteins with palmitic and palmitoleic acids of conserved cysteine and serine residues, respectively, is a major determinant of Wnt production and secretion, as well as in their activation of signaling targets in both the β-catenin-dependent and-independent pathways (Figure 4) [64, 65]. Interestingly, changing levels of SCD1 activity and, consequently, the ratio palmitoleic to palmitic acid, have been shown to modify the rate of Wnt synthesis, secretion, and biological activity by altering the acylation of Wnt proteins [61]. SCD5 also appears to be central for the regulation of the Wnt signaling system in target cells. It was observed that the expression of human SCD5 in mouse neuronal cells decreases the activity of canonical Wnt while the non-canonical Wnt pathway was stimulated [38]. In the same study, SCD5 expression led to a greater secretion of recombinant Wnt5a, a non-canonical Wnt, whereas it diminished the cellular and secreted levels of canonical Wnt7b, reinforcing the notion that this desaturase evokes regulatory effects, albeit divergent, on Wnt pathways. Further, it was observed that SCD activity produces a MUFA substrate for the Wnt-associated acyltransferase Porcupine, which incorporates a MUFA, but not SFA, into Wnt ligands, subsequently modifying their biological activity [66, 67]. Similarly, in hepatocytes, SCD1 catalyzed the synthesis of palmitoleate which serves as the substrate for the Porcupine-mediated acylation and consequent activation of Wnt ligands [68]. The acylated Wnt proteins were able to induce the Wnt/β-catenin signaling cascade that is necessary for liver functional zonation and hepatic metabolism [68]. The requirement of SCD activity seems to be unique to the acylation and biological activity of Wnt ligands, as other lipid-modified ligands, such as Sonic hedgehog, are not affected by the loss of SCD activity [66, 67]. Based on the aforementioned data, it is tempting to speculate that SCD1 and SCD5 may functionally interact with Porcupine in the process of Wnt acylation with MUFA, thereby controlling the activation of these proteins. Therefore, in this hypothetical mechanism, SCD1 and SCD5, separately or in coordination, could control the mechanisms of differentiation and morphogenesis during development, at least partly, by modulating the activation rate of Wnt signals. In addition, a feedforward loop involving SCD activity and Wnt signaling appear to take place since it was observed that Wnt signals target SCD1 for activation in adipocytes [69]. Future research will help determine whether SCD1 and SCD5 activities have similar or distinctive effects on the modulation of Wnt signaling pathways and, also, whether the regulation of Wnt by the desaturases exhibits a time-, stage- and Wnt-specific behavior.
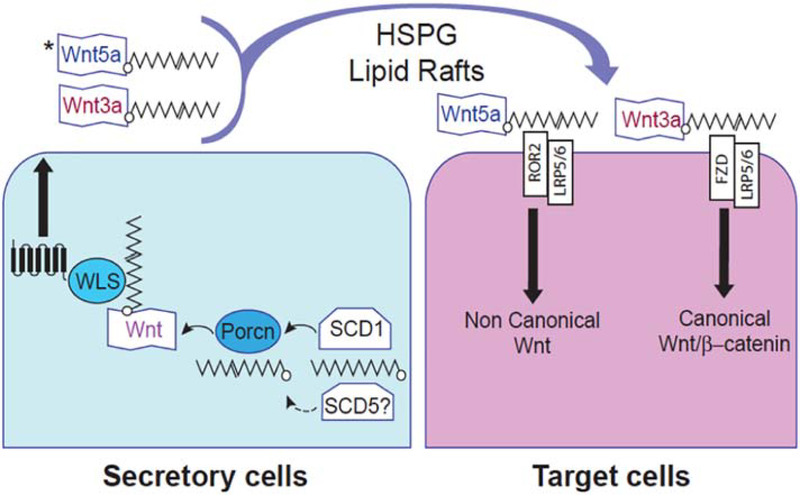
Figure 4. Potential mechanism by which SCD activity modulates Wnt signaling activity.
Wnt ligands are lipid-modified molecules that are acylated in the endoplasmic reticulum of secretory cells with monounsaturated palmitoleic acid (16:1). SCD1, and possibly SCD5, mediates the desaturation of palmitic to palmitoleic acid, providing the substrate for the O-acyl-transferase Porcupine (Porcn) that acylates Wnt ligands. The acylation is critical for Wnt release from secretory cells (left) by the cargo receptor Wls (Wntless), and for inducing signaling activity upon binding to receptors in the target cell (right). While a role of SCD1 in Wnt3a synthesis and signaling has been established, SCD5 could also participate in the mechanisms of synthesis and signaling of Wnt ligands, including Wnt5a. HSPG: Heparan sulfate proteoglycan; ROR2: Receptor tyrosine kinase like orphan receptor 2; FZD: frizzled class receptor; LRP5/LRP6 LDL receptor related protein 6/ protein 5.
The role of SCD1 as a key pro-survival factor that attenuates cell stress and prevents the dismissal of cells by necrosis or apoptosis has been well documented in mammalian cells, particularly in human cancer cells [3, 4]. The potential involvement of SCD5 in the mechanisms of cell death is, so far, less conclusive. An early study by Roongta et al. [50] reported that the inhibition of SCD5 expression in lung cancer cells did not significantly affect the cell apoptotic rate, suggesting that this desaturase may not be implicated in cancer cell survival. However, it can be hypothesized that SCD5 may attain a distinct role as a survival factor in stressful metabolic conditions that may lead to cellular damage, such in a context of excess levels of SFA promoted by either excessive de novo synthesis (i.e., cancer cells) or overload from plasma. It has been firmly demonstrated that SCD1, by processing SFA into MUFA, operates as a critical barrier against SFA-induced cellular damage in mammalian cells, including human cells [70, 71]. A Δ9-desaturase activity like SCD5, whose expression appears less sensitive to regulation than SCD1, may, therefore, be part of a highly conserved mechanism by which cells avoid the deleterious effects of an SFA buildup, even more critically so if SCD1 activity were reduced. This hypothesis finds support in the work of Liu et al. [72], in which it was observed that liver-targeted expression of human SCD5 counteracts endoplasmic reticulum stress and inflammatory effects of a highly lipogenic sucrose-rich diet in SCD1-ablated mice. Further reinforcing this notion, in human pancreatic β-cells, isoxazole, a small molecule that influences cell proliferation and differentiation, was found to stimulate the expression of SCD5 as part of a mechanism put in place by β–cells to prevent lipotoxicity [73]. Whether the anti-lipotoxic action of SCD5 is especially prominent in tissues that express high levels of this desaturase, such as brain and, especially, pancreas, a tissue that is highly sensitive to SFA-mediated cytotoxicity [74], is an issue that remains unresolved.
6. Potential implication of SCD5 in human diseases
a). Cancer.
A large body of research has demonstrated that the activation of SCD1 is a hallmark of oncogenic transformation and, also, that MUFA synthesis is an essential requirement for the maintenance of most metabolic and biological traits of cancer cells [4]. A growing number of studies hints at the possibility that SCD5 may also have a role in the modulation of mitogenic and tumorigenic events. Analysis of alternative splicing signatures, which are thought to be implicated in cancer development, performed in over ten thousand genes in kidney renal clear cell carcinomas showed that scd5 was among the seven genes that are considered potential diagnostic biomarkers of carcinoma prognosis [75]. High expression of SCD5 was observed in uveal melanoma, the most prevalent, and highly metastatic, form of intraocular cancer in adults [76]. Also, importantly, this study showed that elevated SCD5 was positively associated with poor prognosis of this cancer. Furthermore, Bellenghi et al. [41] reported that, compared to normal tissues, mRNA and protein levels of SCD5 were elevated in primary and low-invasive melanoma tissues. Intriguingly, these authors found that the expression of SCD5 in human melanoma cells lowered the rate of tumor formation suggesting that this enzyme may display a cancer cell type-specific function or, also, interfere with the pro-oncogenic function of SCD1[4].
SCD5 activity may also be required for providing cancer cells with the appropriate local environment to thrive. Cancer-associated fibroblasts in the tumor stroma were shown to induce SCD5 expression in mammary cancer cells at both transcriptional and translational levels [77]. In this work, ablation of SCD5 induced greater necrosis of cancer cells, an effect that was reversed by addition of oleic acid, but did not affect the rate of programmed cell death. Furthermore, in contrast to findings in SCD1-ablated cells, inhibition of SCD5 expression did not affect the motility of cancer cells [77]. The aforementioned observations, in conjunction with previous studies [4], suggest that SCD1 and SCD5 play specific roles in the mechanisms of cancer. However, future studies that more comprehensibly address the role of SCD5 in varied cell- and tissue-specific cancers will provide more in-depth information about the involvement of SCD5 in the processes of cell replication, survival, and invasiveness. In addition, since SCD1 has been identified as a main modulator of both the replication and differentiation of human pluripotent stem cells, as well as the proliferation of cancer stem cells [78, 79], future experiments should also address the question of whether SCD5 is also implicated in the biological fate of human normal and cancer stem cells.
b). Craniofacial development.
As mentioned above, the high expression levels of SCD5 in the embryo and in fetal tissues, particularly in brain and pancreas [14], indicate that this desaturase, among other biological roles, may be implicated in critical developmental functions. In this regard, the gene encoding for SCD5 was first identified in two patients, father and son, with cleft palate in which a pericentric inversion of chromosome 4 led to a deletion in the desaturase gene [13]. The association of SCD5 expression with a clefting phenotype was also observed patients with 4q21 microdeletion syndrome [80]. Also, a disruption in the scd5 gene due to gene microtriplication in chromosome 4 was found in a patient with macrocephaly, frontal bossing, and development delay [81], all clinical features that are also observed in 4q21 microdeletions [82]. In addition, we observed that Xenopus embryos microinjected with human SCD5 RNA exhibited brain malformation, absence of left eye, and an abnormally uncoiled intestine [Débora Sinner and R. Ariel Igal, unpublished observations]. Despite limitations associated with the gain of function assay, the aforementioned anomalies were not observed in embryos injected with a control plasmid.
While the above described reports, particularly human genetic studies, point to a role of SCD5 in craniofacial development and disease, mechanistic data for this association are lacking. Signaling pathways regulated by SCD5, such as Wnt [38], are known to regulate ciliogenesis, which is critical for craniofacial development [83], and are also associated with ciliopathies affecting hearing and sight [84]. Lipid modifications in cilia, especially protein acylation, are crucial for protein trafficking and signaling mechanisms [85], therefore, it is tempting to speculate that SCD5 may influence the activity of primary cilia and, consequently, craniofacial formation. In this regard, Willemarck et al. [86] observed that activation of fatty acid biosynthesis in Xenopus embryos by ectopic expression of SREBP1c, or the addition of palmitic acid, blocked the formation of primary cilia, which led to tissue malformation. This study also found that the alteration of embryo development was associated with SREBP1c-induced changes in Hedgehog and Wnt signaling activity. However, whether fatty acid desaturases, particularly SCD5, play a role in producing a SFA:MUFA ratio that is appropriate for the timely activation of fatty acid-sensitive signaling proteins that control ciliar functions is currently unknown.
Finally, normal craniofacial development also depends on cranial neural crest cells that give rise to the majority of the bone and cartilage of the head and face, as well as to nerve ganglia, smooth muscle, connective tissue, and pigment cells [87]. Disruptions in the generation, replication, migration, or differentiation of cranial neural crest cells are known to cause congenital malformations, such as cleft palate [87, 88]. Thus, determining whether SCD5 activity is a prerequisite for the normal biological processes in neural crest cells will help clarify a potential implication of the desaturase in craniofacial development. In addition, elucidating the role of SCD5 in craniofacial formation at a mechanistic level will lay the foundation for the development of novel approaches to prevent and/or treat developmental diseases, such as malformations in central nervous system, craniofacial developmental alterations, and related pathologies.
c). Neurodegenerative disease.
As discussed earlier, SCD5 activity appears to be crucial in the processes of neuronal proliferation and differentiation, suggesting that functions of the central nervous system, as well as pathological alterations, may be influenced by the rate of activity of this enzyme. An indication of the potential involvement of SCD5 in neurological disorders is described in a recent report in which the mRNA expression of this desaturase was found elevated in the brain of patients with Alzheimer’s disease [89]. Schizophrenia, bipolar disorder, and major depressive disorder, clinical entities that are thought to be genetically connected, display few susceptibility genes located on chromosome 4, including scd5 [90]. However, evidence for a potential mechanistic participation of the desaturase in the onset of these diseases is lacking. In addition, a novel missense mutation in the scd5 gene was identified in a large family whose members were diagnosed with hearing loss of autosomal dominant inheritance [91]. Although the molecular mechanisms by which SCD5 activity modulates the auditory pathways are unknown, the desaturase may be essential not only for the integrity and functionality of spiral ganglion cells in the cochlea [91] but also for the auditory processes in brain cells, which express high levels of SCD5 [89].
d). Pulmonary hypertension.
The expression of SCD5 was found elevated in lymphocytes from patients with vasodilator-responsive pulmonary artery hypertension when compared to the non-responsive phenotype [92], indicating the desaturase may be implicated in the mechanisms of hypertension. This study also suggests that the detection of SCD5 in peripheral blood, in conjunction with other genes identified in the study, could be utilized as a tool to more efficiently diagnose and treat pulmonary arterial hypertension. Interestingly, in the same patient group, increase in the expression of DSG2, a desmosomal cadherin involved in Wnt/β-catenin signaling, was also observed [92]. Since desaturase activity, by providing substrate for protein acylation, has been linked to mechanisms of Wnt activation, further investigations should provide more concrete evidence on a functional association of SCD5 and Wnt activity in pulmonary artery hypertension.
7. Concluding remarks
Despite significant advances in the research of SCD5 in different mammalian organisms in later years, many aspects of the structure, nutritional and hormonal regulation of this desaturase, as well as its biological functions, remain to be elucidated. Unlike SCD1, which is exquisitely sensitive to variations in nutritional composition and caloric levels of diets, as well as hormonal status, SCD5 has been shown, so far, to exhibit a lack of responsiveness to changing levels of dietary fatty acids and circulating factors. However, future studies will conclusively answer the question of whether SCD5 expression and activity are susceptible to fluctuations in nutritional, hormonal, and environmental factors or, as it has been hypothesized, this enzyme expresses a more constitutive Δ9-desaturating activity that is required to preserve essential metabolic and biological functions in cells.
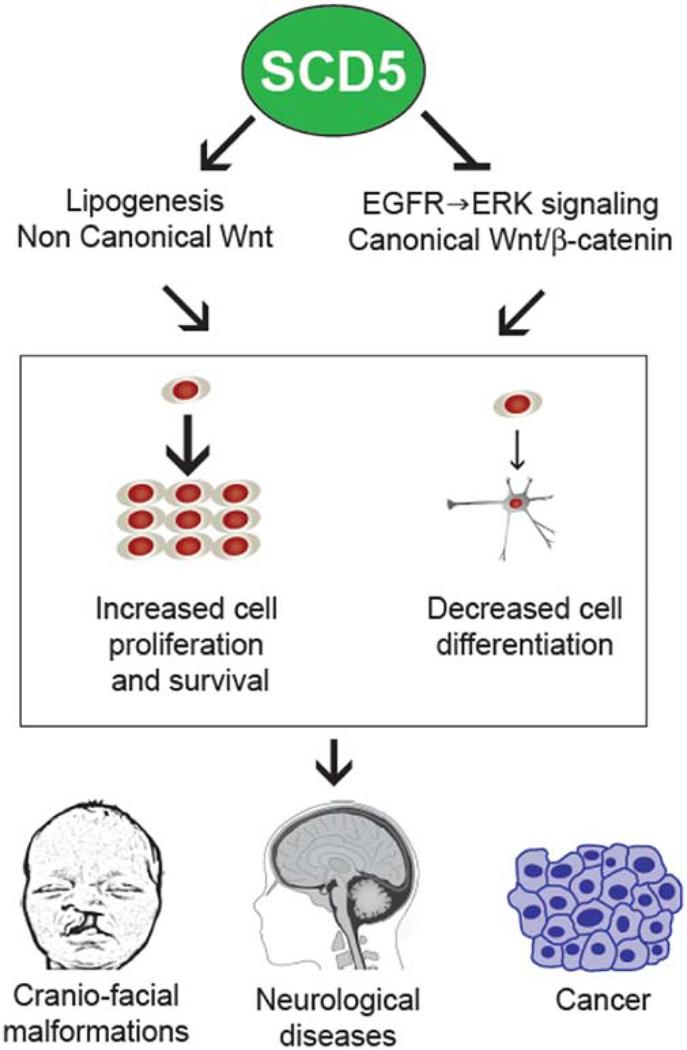
Published data suggest that, similarly to SCD1, SCD5 activity is mechanistically connected to metabolic and signaling pathways that promote proliferation and survival, and inhibit differentiation, at least in some cell types (Figure 5). However, an elevated MUFA-to-SFA ratio, caused by an increase in either SCD1 or SCD5 expression, was shown to both activate and deactivate signaling pathways, such as EGFR-mediated Akt and ERK kinases, and Wnt [32, 38, 66, 67, 93, 94]. Additional studies are required for establishing whether the desaturases exhibit unique cell-, tissue- and organism-specific roles in the regulation of different signaling pathways. The implication of SCDs in the modulation of signal transduction pathways that are essential for development suggests a potential role for these enzymes, in particular SCD5, in promoting morphogenetic events that are critical for the differentiation of the nervous system and craniofacial structures in the developing embryo. Redundancy in the activities of SCD isoforms, together with the lack of a murine SCD5, has prevented a more definitive conclusion regarding the participation of SCD5 during mammalian development. While redundancy may pose a limitation to mouse studies, other experimental models, such as the chick embryo, could provide valuable information on the role of the enzyme during development. The SCD5 variant expressed in Gallus gallus share similarities with the human SCD5, accounting for 85.3% of identity (Figure 2), and, similarly to human SCD5, expression of chicken SCD5 was the highest in brain and pancreas [25]. Since chick embryo is an established model for developmental studies, it could be a suitable model to explore potential mechanisms by which endogenous SCD5 participates in the process of morphogenesis.
Figure 5. Proposed model for the implication of SCD5 activity in pathobiological mechanisms of disease.
Excess SCD5 expression evokes a chronically greater ratio MUFA-to-SFA in cells, with consequent alterations in key metabolic and signaling pathways involved in cell proliferation, differentiation and survival. While the molecular mechanisms by which SCD5 is linked to developmental and neurological pathologies, as well as in and cancer, remain to be elucidated, human genetic data and experimental studies suggest that abnormal SCD5 may be implicated in the onset and progression of disease.
Finally, clinical and epidemiological studies have not, so far, addressed a possible relationship between abnormal levels of SCD5 and the onset and progression of diseases. Further studies should focus on establishing the specific roles of SCD5-derived metabolites in the regulation of biosynthetic, bioenergetic, and signaling pathways required for cell proliferation, growth, and differentiation, as well as in organ development, particularly in the central nervous system. SCD5 may, therefore, represent an attractive new target for preventive and therapeutic interventions in a number of diseases, including cancer, congenital malformations, as well as Alzheimer’s disease, and other neurodegenerative conditions for which effective treatments are lacking. While some potent small molecule inhibitors of SCD1 activity have been generated [95, 96], no specific pharmacological inhibitors of SCD5 activity are currently available, a situation that poses a significant challenge for the study of SCD5 as a target in disease treatment. Further research tackling the unknown molecular and biological aspects of SCD5, as well the development of SCD5-specific blockers, will provide the necessary validation for a potential use of SCD5 as a useful therapeutic target.
Highlights:
SCD5, a second fatty acid Δ9-desaturase isoform present in a number of vertebrates, including humans, is predominantly expressed in fetal brain, and in adult brain and pancreas.
SCD5 activity stimulates biological mechanisms that induce cancer cell proliferation and inhibit cell differentiation.
SCD5 is involved in the control of signal transduction mechanisms that modulate cell proliferation and differentiation, as well as organ development.
Alteration in the expression of SCD5 is associated with cancer, neurological and developmental diseases.
Acknowledgements.
The authors are indebted to Chuck Crimmel for his help with graphics. This work was partly supported by the Institute of Human Nutrition, Columbia University Irving Medical Center (R.A.I) and by the National Institute of Health Grant R01 HL144774 (D.I.S). The authors would like to apologize to those researchers whose work in the field could not be cited in this review due to space constrains.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Storck EM, Ozbalci C, Eggert US Lipid Cell Biology: A Focus on Lipids in Cell Division Annual Rev. Biochem, 87 (2018), pp.839–869 [DOI] [PubMed] [Google Scholar]
- [2].Ntambi JM Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol J. Lipid Res, 40 (1999), pp. 1549–1558 [PubMed] [Google Scholar]
- [3].Igal RA Stearoyl-CoA desaturase-1: a novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer Carcinogenesis, 31 (2010), pp. 1509–1515 [DOI] [PubMed] [Google Scholar]
- [4].Igal RA Stearoyl CoA desaturase-1: New insights into a central regulator of cancer metabolism. Biochim Biophys Acta, 1861 (2016) pp. 1865–1880 [DOI] [PubMed] [Google Scholar]
- [5].Castro LF, Wilson JM, Gonçalves O, Galante-Oliveira S, Rocha E, Cunha I The evolutionary history of the stearoyl-CoA desaturase gene family in vertebrates BMC Evol. Biol, 11 (2011), pp. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wu X, Zou X, Chang Q, Zhang Y, Li Y, Zhang L, Huang J, Liang B The evolutionary pattern and the regulation of stearoyl-CoA desaturase genes. Biomed. Res. Int. 2013 (2013), pp. 856521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bloch K Enzymic synthesis of monounsaturated fatty acids Acc. Chem. Res, 2 (1969), pp. 193–202 [Google Scholar]
- [8].Enoch HG, Catala A, Strittmatter P Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid J. Biol. Chem, 251 (1976), pp. 5095–5103 [PubMed] [Google Scholar]
- [9].Zheng Y, Prouty SM, Harmon A, Sundberg JP, Stenn KS, Parimoo S Scd3--a novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics, 71 ( 2001), pp. 182–191 [DOI] [PubMed] [Google Scholar]
- [10].Miyazaki M, Jacobson MJ, Man WC, Cohen P, Asilmaz E, Friedman JM, Ntambi JM Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors J. Biol. Chem, 278 (2003), pp. 33904–33911 [DOI] [PubMed] [Google Scholar]
- [11].Ntambi JM, Miyazaki M Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res, 43 (2004), pp. 91–104 [DOI] [PubMed] [Google Scholar]
- [12].Mihara K Structure and regulation of rat liver microsomal stearoyl-CoA desaturase gene. J. Biochem, 108 (1990), pp. 1022–9 [DOI] [PubMed] [Google Scholar]
- [13].Zhang L, Ge L, Parimoo S, Stenn K, Prouty SM Human stearoyl-CoA desaturase: alternative transcripts generated from a single gene by usage of tandem polyadenylation sites. Biochem. J, 340 (1999), pp. 255–264 [PMC free article] [PubMed] [Google Scholar]
- [14].Beiraghi S, Zhou M, Talmadge CB, Went-Sumegi N, Davis JR, Huang D, Saal H, Seemayer TA, Sumegi J Identification and characterization of a novel gene disrupted by a pericentric inversion inv(4)(p13.1q21.1) in a family with cleft lip Gene, 302 (2003), pp. 11–21 [DOI] [PubMed] [Google Scholar]
- [15].Wang J, Yu L, Schmidt RE, Su C, Huang X, Gould K, Cao Characterization of G HSCD5, a novel human stearoyl-CoA desaturase unique to primates Biochem. Biophys. Res. Commun, 332 (2005), pp. 735–742 [DOI] [PubMed] [Google Scholar]
- [16].Paton CM, Ntambi Biochemical JM and physiological function of stearoyl-CoA desaturase Am. J. Physiol. Endocrinol. Metab, 297 (2009), pp. 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hodson L, Fielding BA Stearoyl-CoA desaturase: rogue or innocent bystander? Prog. Lipid Res, 52 (2013), pp. 15–42 [DOI] [PubMed] [Google Scholar]
- [18].Nagao K, Murakami A, Umeda M Structure and Function of Δ9-Fatty Acid Desaturase. Chem Pharm Bull (Tokyo), 67 (2019), pp. 327–332. [DOI] [PubMed] [Google Scholar]
- [19].Igal RA Roles of StearoylCoA Desaturase-1 in the Regulation of Cancer Cell Growth, Survival and Tumorigenesis Cancers, 3 (2011), pp. 2462–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Popeijus HE, Saris WH, Mensink RP Role of stearoyl-CoA desaturases in obesity and the metabolic syndrome Int. J. Obes. (Lond), 32 (2008), pp. 1076–1082 [DOI] [PubMed] [Google Scholar]
- [21].Brown JM, Rudel LL Stearoyl-coenzyme A desaturase 1 inhibition and the metabolic syndrome: considerations for future drug discovery Curr. Opin. Lipidol, 21 (2010), pp. 192–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Narce M, Bellenger J, Rialland M, Bellenger S Recent advances on stearoyl-Coa desaturase regulation in fatty liver diseases Curr. Drug Metab, 13 (2012), pp. 1454–1463 [DOI] [PubMed] [Google Scholar]
- [23].Wang H, Klein MG, Zou H, Lane W, Snell G, Levin I, Li K, Sang BC Crystal structure of human stearoyl-coenzyme A desaturase in complex with substrate Nat. Struct. Mol., Biol. 7 (2015), pp. 581–585 [DOI] [PubMed] [Google Scholar]
- [24].Bai Y, McCoy JG, Levin EJ, Sobrado P, Rajashankar KR, Fox BG, Zhou M X-ray structure of a mammalian stearoyl-CoA desaturase Nature, 524 (2015), pp. 252–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lengi AJ, Corl BA Comparison of pig, sheep and chicken SCD5 homologs: evidence for an early gene duplication event Comp. Biochem. Physiol. B Biochem. Mol. Biol, 150 (2008), pp. 440–446 [DOI] [PubMed] [Google Scholar]
- [26].Mziaut H, Korza G, Ozols J The N terminus of microsomal delta 9 stearoyl-CoA desaturase contains the sequence determinant for its rapid degradation. Proc. Natl. Acad. Sci U S A, 97 (2000), pp. 8883–8888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kato H, Sakaki K, Mihara K Ubiquitin-proteasome-dependent degradation of mammalian ER stearoyl-CoA desaturase. J. Cell Sci, 119 (2006), pp. 2342–2353. [DOI] [PubMed] [Google Scholar]
- [28].Lengi AJ, Corl BA Identification and characterization of a novel bovine stearoyl-CoA desaturase isoform with homology to human SCD5 Lipids, 42 (2007), pp. 499–508 [DOI] [PubMed] [Google Scholar]
- [29].The UniProt Consortium UniProt: a worldwide hub of protein knowledge Nucleic Acids Res. 47 (2019), pp. 506–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wu X, Zou X, Chang Q, Zhang Y, Li Y, Zhang L, Huang J, Liang B The evolutionary pattern and the regulation of stearoyl-CoA desaturase genes. Biomed. Res. Int, 2013 (2013), pp. 85652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Uhlén M, Fagerberg L, Hallström BM et al. Proteomics. Tissue-based map of the human proteome. Science 347 (2015), pp. 1260419. [DOI] [PubMed] [Google Scholar]
- [32].Nashed M, Chisholm JW, Igal RA Stearoyl-CoA desaturase activity modulates the activation of epidermal growth factor receptor in human lung cancer cells Exp. Biol. Med, 237 (2012), pp. 1007–1017 [DOI] [PubMed] [Google Scholar]
- [33].Mika A, Kaska L, Korczynska J, Mirowska A, Stepnowski P, Proczko M, Ratnicki-Sklucki K, Goyke E, Sledzinski T; Visceral and subcutaneous adipose tissue stearoyl-CoA desaturase-1 mRNA levels and fatty acid desaturation index positively correlate with BMI in morbidly obese women Eur. J. Lipid Sci. Technol, 117 (2015), pp. 926–932 [Google Scholar]
- [34].Polo-Hernández E, Tello V, Arroyo AA, Domínguez-Prieto M, de Castro F, Tabernero A, Medina JM Oleic acid synthesized by stearoyl-CoA desaturase (SCD-1) in the lateral periventricular zone of the developing rat brain mediates neuronal growth, migration and the arrangement of prospective synapses. Brain Res, 1570 (2014), pp. 13–25 [DOI] [PubMed] [Google Scholar]
- [35].Hamilton KL, Fernandes KJL Neural stem cells and adult brain fatty acid metabolism: Lessons from the 3xTg model of Alzheimer’s disease. Biol. Cell, 110 (2018), pp. 6–25 [DOI] [PubMed] [Google Scholar]
- [36].Feuerstein P, Cadoret V, Dalbies-Tran R, Guerif F, Bidault R, Royere D Gene expression in human cumulus cells: one approach to oocyte competence. Hum. Reprod, 22 (2007), pp. 3069–3077. [DOI] [PubMed] [Google Scholar]
- [37].Liu Q, Zhang J, Wen H, Feng Y, Zhang X, Xiang H, Cao Y, Tong X, Ji Y, Xue Z Analyzing the Transcriptome Profile of Human Cumulus Cells Related to Embryo Quality via RNA Sequencing. Biomed. Res. Int, 2018 (2018), pp. 9846274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sinner DI, Kim GJ, Henderson GC, Igal StearoylCoA desaturase-R. A.5: a novel regulator of neuronal cell proliferation and differentiation PLoS One, 7 (2012), pp. e39787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].He M, Guo S, Li Z In situ characterizing membrane lipid phenotype of breast cancer cells using mass spectrometry profiling. Sci. Rep, 5 (2015), pp. 11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Baeza MC, Corva PM, Soria LA, Pavan E, Rincon G, Medrano JF Genetic variants in a lipid regulatory pathway as potential tools for improving the nutritional quality of grass-fed beef. Anim. Genet, 44 (2013), pp. 121–129 [DOI] [PubMed] [Google Scholar]
- [41].Bellenghi M, Puglisi R, Pedini F, De Feo A, Felicetti F, Bottero L, Sangaletti S, Errico MC, Petrini M, Gesumundo C, Denaro M, Felli N, Pasquini L, Tripodo C, Colombo MP, Carè A, Mattia G SCD5-induced Oleic Acid Production Reduces Melanoma Malignancy by Intracellular Retention of SPARC and Cathepsin B. J. Pathol, 236 (2015), pp. 315–325 [DOI] [PubMed] [Google Scholar]
- [42].Burhans MS, Flowers MT, Harrington KR, Bond LM, Guo CA, Anderson RM, Ntambi JM Hepatic oleate regulates adipose tissue lipogenesis and fatty acid oxidation. J. Lipid Res, 56 (2015), pp. 304–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mauvoisin D, Mounier C Hormonal and nutritional regulation of SCD1 gene expression Biochimie, 93 (2011), pp. 78–86 [DOI] [PubMed] [Google Scholar]
- [44].Lengi AJ, Corl BA Regulation of the bovine SCD5 promoter by EGR2 and SREBP1. Biochem. Biophys. Res. Commun, 421 (2012), pp. 375–379 [DOI] [PubMed] [Google Scholar]
- [45].Garbay B, Boiron-Sargueil F, Shy M, Chbihi T, Jiang H, Kamholz J, Cassagne C Regulation of oleoyl-CoA synthesis in the peripheral nervous system: demonstration of a link with myelin synthesis. J. Neurochem, 71 (1998), pp. 1719–1726 [DOI] [PubMed] [Google Scholar]
- [46].Leblanc SE, Srinivasan R, Ferri C, Mager GM, Gillian-Daniel AL, Wrabetz L, Svaren J Regulation of cholesterol/lipid biosynthetic genes by Egr2/Krox20 during peripheral nerve myelination J. Neurochem. 93 (2005) 737–748. [DOI] [PubMed] [Google Scholar]
- [47].Lu MC, Yu CL, Chen HC, Yu HC, Huang HB, Lai NS Increased miR-223 expression in T cells from patients with rheumatoid arthritis leads to decreased insulin-like growth factor-1-mediated interleukin-10 production. Clin. Exp. Immunol, 177 (2014), pp. 641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jacobs AA, van Baal J, Smits MA, Taweel HZ, Hendriks WH, van Vuuren AM, Dijkstra J Effects of feeding rapeseed oil, soybean oil, or linseed oil on stearoyl-CoA desaturase expression in the mammary gland of dairy cows. J. Dairy Sci, 94 (2011), pp. 874–887 [DOI] [PubMed] [Google Scholar]
- [49].Antal O, Péter M, Hackler L Jr, Mán I, Szebeni G, Ayaydin F, Hideghéty K, Vigh L, Kitajka K, Balogh G, Puskás LG Lipidomic analysis reveals a radiosensitizing role of gamma-linolenic acid in glioma cells. Biochim. Biophys. Acta, 1851 (2015), pp. 1271–1282 [DOI] [PubMed] [Google Scholar]
- [50].Roongta UV, Pabalan JG, Wang X, Ryseck RP, Fargnoli J, Henley BJ, Yang WP, Zhu J, Madireddi MT, Lawrence RM, Wong TW, Rupnow BA Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy Mol. Cancer Res, 9 (2011), pp. 1551–1561 [DOI] [PubMed] [Google Scholar]
- [51].Shea TB, Fischer I, Sapirstein VS Effect of retinoic acid on growth and morphological differentiation of mouse NB2a neuroblastoma cells in culture. Brain Res, 353 (1985), pp. 307–314 [DOI] [PubMed] [Google Scholar]
- [52].Samuel W, Kutty RK, Nagineni S, Gordon JS, Prouty SM, Chandraratna RA, Wiggert B Regulation of stearoyl coenzyme A desaturase expression in human retinal pigment epithelial cells by retinoic acid J. Biol. Chem, 276 (2001), pp. 28744–28750 [DOI] [PubMed] [Google Scholar]
- [53].Malumbres M, Barbacid Cell cycle M, CDKs and cancer: a changing paradigm Nat. Rev. Cancer, 9 (2009), pp. 153–166 [DOI] [PubMed] [Google Scholar]
- [54].Hess D, Chisholm JW, Igal RA Inhibition of stearoyl-CoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells PLoS ONE, 5 (2010), pp. e11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lu X, Zhang Y, Chen L, Wang Q, Zeng Z, Dong C, Qi Y, Liu Y Whole exome sequencing identifies SCD5 as a novel causative gene for autosomal dominant nonsyndromic deafness. Eur. J. Med. Genet. (2020), in press. [DOI] [PubMed] [Google Scholar]
- [56].Katsetos CD, Herman MM, Mörk SJ Class III beta-tubulin in human development and cancer. Cell Motil. Cytoskeleton, 55 (2003), pp. 77–96 [DOI] [PubMed] [Google Scholar]
- [57].Hirabayashi Y, Gotoh Y Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neurosci. Res, 51 (2005), pp. 331–336 [DOI] [PubMed] [Google Scholar]
- [58].Marcucci H, Paoletti L, Jackowski S, Banchio C Phosphatidylcholine biosynthesis during neuronal differentiation and its role in cell fate determination. J. Biol. Chem, 285 (2010), pp. 25382–25393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Scaglia N, Igal RA Inhibition of Stearoyl-CoA Desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis Int. J. Oncol, 33 (2008), pp. 839–850 [PubMed] [Google Scholar]
- [60].Fritz V, Benfodda Z, Rodier G, Henriquet C, Iborra F, Avancès C, Allory Y, de la Taille A, Culine S, Blancou H, Cristol JP, Michel F, Sardet C, Fajas L Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice Mol. Cancer Ther, 9 (2010), pp. 1740–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Torres VI, Godoy JA, Inestrosa NC Modulating Wnt signaling at the root: Porcupine and Wnt acylation. Pharmacol. Ther, 198 (2019), pp. 34–45. [DOI] [PubMed] [Google Scholar]
- [62].Seidensticker MJ, Behrens J Biochemical interactions in the wnt pathway. Biochim. Biophys. Acta, 1495 (2000), pp. 168–182. [DOI] [PubMed] [Google Scholar]
- [63].Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M M, Moon Dishevelled activates RT Ca2+ flux, PKC, and CamKII in vertebrate embryos. J. Cell Biol, 161 (2003), pp. 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL IL, Reya T, Yates III JR, Nusse R Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature, 423 (2003), pp. 448–452 [DOI] [PubMed] [Google Scholar]
- [65].Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev. Cell, 11 (2006), pp. 791–801 [DOI] [PubMed] [Google Scholar]
- [66].Rios-Esteves J, Resh MD Stearoyl CoA desaturase is required to produce active, lipid-modified Wnt proteins Cell Rep, 4 (2013), pp. 1072–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rios-Esteves J, Haugen B, Resh MD Identification of key residues and regions important for porcupine-mediated Wnt acylation. J. Biol. Chem, 289 (2014), pp. 17009–17019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cabrae R, Dubuquoy C, Caüzac M, Morzyglod L, Guilmeau S, Noblet B, Fève B, Postic C, Burnol AF, Moldes M Insulin activates hepatic Wnt/β-catenin signaling through stearoyl-CoA desaturase 1 and Porcupine. Sci. Rep, 10 (2020), pp. 5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bagchi DP, Li Z, Corsa CA, Hardij J, Mori H, Learman BS, Lewis KT, Schill RL, Romanelli SM, MacDougald OA Wntless regulates lipogenic gene expression in adipocytes and protects against diet-induced metabolic dysfunction. Mol. Metab. (2020) in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr., Ory DS, Schaffer JE Triglyceride accumulation protects against fatty acid-induced lipotoxicity Proc. Natl. Acad. Sci. U S A, 100 (2003), pp. 3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Scaglia N, Igal RA Stearoyl-CoA desaturase is involved in the control of proliferation, anchorage-independent growth, and survival in human transformed cells J. Biol. Chem, 280 (2005), pp. 25339–25349 [DOI] [PubMed] [Google Scholar]
- [72].Liu X, Burhans MS, Flowers MT, Ntambi JM Hepatic oleate regulates liver stress response partially through PGC-1α during high-carbohydrate feeding. J. Hepatol, 65 (2016), pp. 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kalwat MA, Huang Z, Wichaidit C, McGlynn K, Earnest S, Savoia C, Dioum EM, Schneider JW, Hutchison MR, Cobb MH Isoxazole Alters Metabolites and Gene Expression, Decreasing Proliferation and Promoting a Neuroendocrine Phenotype in β-Cells. ACS Chem. Biol, 11 (2016), pp. 1128–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Green CD, Olson LK Modulation of palmitate-induced endoplasmic reticulum stress and apoptosis in pancreatic β-cells by stearoyl-CoA desaturase and Elovl6. Am. J. Physiol. Endocrinol. Metab, 300 (2011), pp. E640–649. [DOI] [PubMed] [Google Scholar]
- [75].Song J, Liu YD, Su J, Yuan D, Sun F, Zhu J Systematic analysis of alternative splicing signature unveils prognostic predictor for kidney renal clear cell carcinoma. J. Cell Physiol, 234 (2019), pp. 22753–22764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Xu Y, Han W, Xu WH, Wang Y, Yang XL, Nie NL, Yao J, Shen GL, Zhang XF Identification of differentially expressed genes and functional annotations associated with metastases of the uveal melanoma. J. Cell Biochem, 120 (2019), pp. 19202–19214 [DOI] [PubMed] [Google Scholar]
- [77].Angelucci C, D’Alessio A, Iacopino F, Proietti G, Di Leone A, Masetti R, Sica G Pivotal role of human stearoyl-CoA desaturases (SCD1 and 5) in breast cancer progression: oleic acid-based effect of SCD1 on cell migration and a novel pro-cell survival role for SCD5. Oncotarget, 36 (2018), pp. 24364–24380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ben-David U, Gan QF, Golan-Lev T, Arora P, Yanuka O, Oren YS, Leikin-Frenkel A, Graf M, Garippa R, Boehringer M, Gromo G, Benvenisty N Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen Cell Stem Cell, 12 (2013), pp. 167–179 [DOI] [PubMed] [Google Scholar]
- [79].Noto A, Raffa S, De Vitis C, Roscilli G, Malpicci D, Coluccia P, Di Napoli A, Ricci A, Giovagnoli MR, Aurisicchio L, Torrisi MR, Ciliberto G, Mancini R Stearoyl-CoA desaturase-1 is a key factor for lung cancer-initiating cells Cell Death Dis, 4 (2013), pp. 4:e947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bhoj E, Halbach S, McDonald-McGinn D, Tan C, Lande R, Waggoner D, Zackai E Expanding the spectrum of microdeletion 4q21 syndrome: a partial phenotype with incomplete deletion of the minimal critical region and a new association with cleft palate and Pierre Robin sequence. Am. J. Med. Genet. A, 161 (2013), pp. 2327–2333 [DOI] [PubMed] [Google Scholar]
- [81].Lebedev IN, Nazarenko LP, Skryabin NA, Babushkina NP, Kashevarova AA A de novo microtriplication at 4q21.21-q21.22 in a patient with a vascular malignant hemangioma, elongated sigmoid colon, developmental delay, and absence of speech. Am. J. Med. Genet. A, 170 (2016), pp. 2089–2096 [DOI] [PubMed] [Google Scholar]
- [82].Bonnet C, Andrieux J, Béri-Dexheimer M, Leheup B, Boute O, Manouvrier S, Delobel B, Copin H, Receveur A, Mathieu M, Thiriez G, Le Caignec C, David A, de Blois MC, Malan V, Philippe A, Cormier-Daire V, Colleaux L, Flori E, Dollfus H, Pelletier V, Thauvin-Robinet C, Masurel-Paulet A, Faivre L, Tardieu M, Bahi-Buisson N, Callier P, Mugneret F, Edery P, Jonveaux P, Sanlaville D Microdeletion at chromosome 4q21 defines a new emerging syndrome with marked growth restriction, mental retardation and absent or severely delayed speech. J. Med. Genet, 47 (2010), pp. 377–384 [DOI] [PubMed] [Google Scholar]
- [83].Brugmann SA, Cordero DR, Helms Craniofacial ciliopathies JA: A new classification for craniofacial disorders. Am. J. Med. Genet. A, 152A (2010), pp. 2995–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wheway G, Nazlamova L, Hancock JT Signaling through the Primary Cilium. Front. Cell Dev. Biol, 6 (2018), pp. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Roy K, Marin EP Lipid Modifications in Cilia Biology. J. Clin. Med, 8 (2019), pp. E921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Willemarck N, Rysman E, Brusselmans K, Van Imschoot G, Vanderhoydonc F, Moerloose K, Lerut E, Verhoeven G, van Roy F, Vleminckx K, Swinnen JV Aberrant activation of fatty acid synthesis suppresses primary cilium formation and distorts tissue development. Cancer Res, 70 (2010), pp. 9453–62. [DOI] [PubMed] [Google Scholar]
- [87].Cordero DR, Brugmann S, Chu Y, Bajpai R, Jame M, Helms JA Cranial neural crest cells on the move: their roles in craniofacial development. Am. J. Med. Genet, 115A (2011), pp. 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Achilleos A, Trainor PA Neural crest stem cells: discovery, properties and potential for therapy. Cell Res, 22 (2012), pp. 288–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Astarita G, Jung KM, Vasilevko V, Dipatrizio NV, Martin SK, Cribbs DH, Head E, Cotman CW, Piomelli D Elevated stearoyl-CoA desaturase in brains of patients with Alzheimer’s disease. PLoS One, 6 (2011), pp. e24777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Tang J, Chen X, Cai B, Chen G A logical relationship for schizophrenia, bipolar, and major depressive disorder. Part 4: Evidence from chromosome 4 high-density association screen. J. Comp. Neurol, 527 (2019), pp. 392–405 [DOI] [PubMed] [Google Scholar]
- [91].Lu X, Zhang Y, Chen L, Wang Q, Zeng Z, Dong C, Qi Y, Liu Y Whole exome sequencing identifies SCD5 as a novel causative gene for autosomal dominant nonsyndromic deafness. Eur. J. Med. Genet. (2020) in press [DOI] [PubMed] [Google Scholar]
- [92].Hemnes AR, Trammell AW, Archer SL, Rich S, Yu C, Nian H, Penner N, Funke M, Wheeler L, Robbins IM, Austin ED, Newman JH, West J Peripheral blood signature of vasodilator-responsive pulmonary arterial hypertension. Circulation, 131 (2015), pp. 401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Binczek E, Jenke B, Holz B, Günter RH, Thevis M, Stoffel W Obesity resistance of the stearoyl-CoA desaturase-deficient (scd1−/−) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation Biol. Chem, 388 (2007), pp. 405–418 [DOI] [PubMed] [Google Scholar]
- [94].Scaglia N, Chisholm JW, Igal RA Inhibition of stearoylCoA desaturase-1 inactivates acetyl-CoA carboxylase and impairs proliferation in cancer cells: role of AMPK PLoS One, 4 (2009), pp. e6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Powell DA An overview of patented small molecule stearoyl coenzyme-A desaturase inhibitors (2009 – 2013) Expert Opin. Ther. Pat, 24 (2014), pp. 155–175. [DOI] [PubMed] [Google Scholar]
- [96].Theodoropoulos PC, Gonzales SS, Winterton SE, Rodriguez-Navas C, McKnight JS, Morlock LK, Hanson JM, Cross B, Owen AE, Duan Y, Moreno JR, Lemoff A, Mirzaei H, Posner BA, Williams NS, Ready JM, Nijhawan D Discovery of tumor-specific irreversible inhibitors of stearoyl CoA desaturase Nat. Chem. Biol, 12 (2016), pp. 218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]