Abstract
Simple Summary
Information about the relative length of patient stays, primary care, and prereferral intervals (from symptom onset to specialist referral) is very scarce, and how the presenting symptoms influence these intervals and referral routes remains unknown. This study assesses the impact of presenting symptoms on time intervals, number of visits at the primary care level, and referral pattern of patients with symptomatic oral cancer. This approach will allow targets to be identified for future interventions and the optimization of the treatment pathway for symptomatic oral cancer patients.
Abstract
This investigation was aimed at determining the time intervals from the presenting symptoms until the beginning of oral cancer treatment and their relative contribution to the total time, and to assess the impact of the presenting symptom on diagnostic timelines and patient referral routes. A cross-sectional, ambispective study was designed to investigate symptomatic incident cases. The Aarhus statement was used as a conceptual framework. Strategies for minimizing potential recall biases were implemented. A sample of 181 patients was recruited (power: 99.5%; α = 0.05). The patient interval reached 58.2 days (95% CI, 40.3–76.2), which accounted for 74% of the whole prereferral interval and for more than one third of the total time interval. The presenting symptom (trigger for consultation) influenced both the number of primary care consultations and the length of time to diagnosis. General dental practitioners generated longer intervals to diagnosis (p < 0.005) and needed more consultations before referring a patient (RR = 0.76; 95% CI, 0.61–0.93), than general medical practitioners. The current study identifies the patient as the main target for interventions to improve awareness and reinforces the need for increased alertness amongst healthcare professionals about presenting symptoms of oral cancer and to diminish the number of prereferral consultations in order to optimize the primary care interval.
Keywords: early diagnosis, time intervals, diagnostic delay, primary care interval, symptomatic oral cancer
1. Introduction
Oral and pharyngeal neoplasms combined are the seventh most frequent cancer and the ninth-leading cause of cancer deaths worldwide [1,2]. Growing incidences have been observed for these tumors in Eastern and Central Europe and the USA [2,3].
The wide variations observed in incidence (up to 20-fold) in different geographical areas, or even within the same country for minorities or subpopulations, can be explained by dissimilarities in socioeconomic status and regional differences in risk factors [3,4,5]. In addition to the 177,757 new cases reported worldwide in 2020, an increase in incident cases of over 40% is expected for the next 20 years (280,539), along with the subsequent associated mortality [1], which unveils a global public health problem.
Despite improvements in diagnostic and therapeutic procedures, about half of oral cancers have already reached an advanced state (III or IV) when diagnosed, which implies a poor five-year survival rate (20–50%), with only minor improvements (<5%) in the last 20 years [6,7]. This circumstance has been attributed to delayed diagnoses [6,7]. In this vein, it has been suggested that early diagnosis is the most important factor influencing survival to oral cancer and that early diagnoses and treatments would be linked to survival rates beyond 80% after five years for oral cancer [8]. On the other hand, long time intervals (“delays”) to the diagnosis and treatment of symptomatic oral cancers—as well as breast, melanoma, colorectal, and also head and neck cancers—seem to be associated with poorer outcomes [9,10]. In addition, several meta-analyses disclosed diagnostic delays (>1 month) as being associated with advanced disease stage at diagnosis [9,10,11].
In order to improve the quality of research on this topic, methodological protocols have been developed in the last decade to study the journeys of symptomatic cancer patients within the model of pathways to treatment (from symptom onset to the start of treatment) framework, which allows time intervals and their prognostic implications to be identified, together with allowing intervention strategies to be designed and to minimize bias [12,13,14,15]. One result of these strategies is the finding that the longer the diagnostic-to-treatment interval, the poorer the overall survival for patients with oral squamous cell carcinomas [16]. In addition, patient delay influences survival of head-and-neck carcinomas [17,18], and diagnostic delay is a risk factor for mortality from head and neck cancer [9,11]. In particular, patients experiencing referral delay have shown a strong association with poor survival [17,19]. However, the tumour growth rate acts as a confounder when studying the liaison between delayed diagnosis and survival and it may justify the inconsistencies identified when measuring this association [9,20].
Conversely, and despite the fact that the patient interval may represent the main part of the total time interval to diagnosis and treatment, available information about the relative length of this interval, as well as about the primary care interval and the prereferral interval (from symptom onset to specialist referral), is very limited [15,21,22,23,24,25].
Although symptoms can intuitively condition both patient and primary care intervals as well as referral routes, there is no information on this issue, which is crucial for early diagnosis research [26]. Therefore, the aims of this investigation were to determine the time intervals from the first symptom (presenting symptom) until the beginning of treatment of oral cancer patients and their relative importance and to assess the impact of the presenting symptom on diagnostic timelines and patient referral routes.
2. Materials and Methods
A cross-sectional, ambispective, hospital-based study was designed in which the prospective component began when patients contacted the treating specialist.
Participants were recruited from among the incident cases in the 2015–2019 period with pathological diagnosis of oral squamous cell carcinoma at the CHUAC and POVISA hospitals in Galicia (North-Western Spain). Both hospitals are reference centers for oral cancer treatment under a public, free and universal healthcare scheme (Galician Health Service). The inclusion criterion was symptomatic patients, those whose physical (oral) changes or symptoms prompted them to seek care from a primary care health professional. Exclusion criteria included prevalent or recurrent cases, multiple carcinomas, secondary primary tumors, metastatic cancer, patients who had been treated at some stage at private clinics, patients with records of hospital admissions from hospital accident and emergency services, patients referred because of casual findings during unrelated consultations or as a consequence of screening programs.
These criteria permitted the identification of 280 cases during the study period, and a sample of 181 patients were recruited (participation rate: 64.6%).
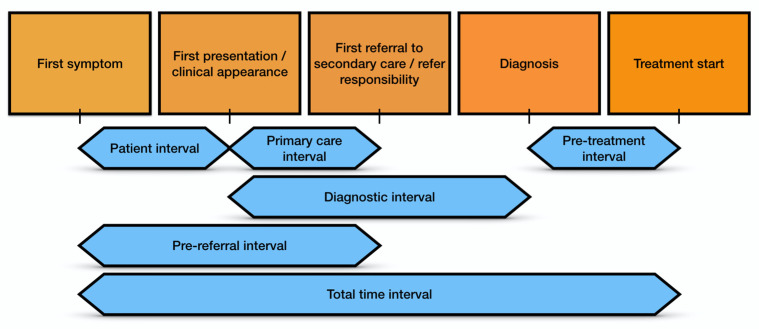
The model of pathways to treatment of symptomatic cancer patients and the Aarhus Statement were used as the conceptual framework for this investigation [12,13,14]. The intervals considered in this study were the patient interval (time from symptom onset to first consultation with a healthcare professional); the primary care interval (time from first consultation to referral for further investigation); and the overall prereferral interval (the time elapsed from symptom onset to referral and the number of prereferral consultations) [12,15,22]. The pretreatment interval (from diagnosis to start of treatment) and the overall time interval (from first symptom to the beginning of treatment) were also considered (see Figure 1) [12].
Figure 1.
The model of pathways to treatment of symptomatic cancer patients: Aarhus Statement.
The presenting symptom was defined as the first symptom reported at presentation at a primary care setting by a patient later diagnosed with an oral squamous cell carcinoma [15]. Symptoms were recorded at the time of diagnosis by the treating specialist using a structured questionnaire. All patients in the study answered the questionnaire. In order to minimize potential memory bias, the information reported by the patient was checked against clinical records at the primary care level and also with patients’ relatives. In case of inconsistencies, this information was discussed with patients letting them know the presenting symptoms recorded in their previous clinical records until a consensus was reached. For patients referred with more than one symptom, the oral and maxillofacial surgeon asked the patient to identify the first symptom, and this information was double-checked against the individual’s primary care clinical records. For those cases with multiple symptoms, these symptoms were added together, and the resulting number was considered a variable in the study. The number of consultations was quantified by disclosing the number of consultations related to the presenting symptom using the Galician Health Service electronic medical records (IanusTM) and its codification system (International Classification of Primary Care [ICPC-2 Plus]).
Finally, to compare dentists’ (GDPs) versus physicians’ (GPs) performance in the referral of oral cancer patients, the proportion of patients referred by each figure, the number of consultations at the primary care level, and the time intervals associated to each referral pattern were analyzed.
This investigation complies with the requirements of the Declaration of Helsinki and was approved by the Galician Research Ethics Committee (Ref. 2014/604).
Statistical Analysis
The estimated value for f2 according to the adjusted model, with a sample size of 181, is 0.988. The power for detecting significant effects in at least one of the variables is 99.5% with an alpha of 0.05.
For normal continuous variables, mean and standard deviation and/or 95% confidence intervals were estimated, while the median and interquartile range were obtained for noncontinuous variables. Raw frequencies and percentages were estimated for categorical variables. Differences in interval means regarding the referral pattern were tested with the Mann–Whitney–Wilcoxon test.
The odds ratios (ORs) and their 95% confidence intervals were estimated to analyze the association between presenting symptoms and referral pattern from primary care. The relationship between the number of prereferral consultations with presenting symptoms and referrals was evaluated through risk ratios at a 95% confidence interval. For variables with few events, ORs were estimated using small-sample adjustment: the most effective modification to the estimator of OR in small sample was the following: OS(ss) = ad/(b + 1) (c + 1).
Likelihood ratio tests (LRTs) for comparing models with and without confounders (null model) were estimated. Null hypothesis for the test is that the simplest model (model without confounder) is better, so a p-value greater than 0.05 favors the simplest model. Models without possible confounders were always selected by LRT (Supplementary File S1).
Generalized additive models (GAMs) were used to assess the effect of selected symptoms, number of prereferral consultations, number of symptoms, and type of primary care referral pattern. GAMs are extensions of generalized linear models that allow flexible effects of continuous covariates over the response using splines. Cubic splines were used for modelling the effects of number of consultations and symptoms. The response variable was log-transformed. All statistical analysis were performed with R statistical software [27].
3. Results
The study included a sample of 181 patients (63.9% males; mean age 65.8 ± 12.7 years-old), with carcinomas located on the tongue (C02: 45.2%) and less frequently on the palate (C05: 9.6%), floor of the mouth (C04: 8.3%), gums (C03: 7.6%), base of the tongue (C01: 3.1%), and other sites within the oral cavity (C06: 26.1%).
The majority of patients were diagnosed at advanced disease stages (TNM III-IV: 56.7%), and total time interval from initial symptom to start of treatment was = 159.8 days (95% CI, 136.6–182.9). The prereferral interval (time until the patient was sent for hospital care) was the largest contributor to the time spent on the patients’ pathway to treatment ( = 96.0 days; 95% CI, 70.8–121.1). In addition, the patient interval (signs/symptoms detection till consultation with a primary healthcare professional) averaged 58.2 days (95% CI, 40.3–76.2), which accounted for 74% of the prereferral interval and for more than one third of the total time interval in the Aarhus framework (Table 1).
Table 1.
Time intervals (days) in the journey of oral cancer patients from symptom to treatment.
| Variable | Mean (CI) | Standard Error | 1st Quartile |
Median | 3rd Quartile |
|---|---|---|---|---|---|
| Total interval | 159.80 (136.68, 182.95) | 139.34 | 75.25 | 109.00 | 189.75 |
| Patient interval | 58.29 (40.38, 76.20) | 96.10 | 7.00 | 31.00 | 61.00 |
| Primary care interval | 28.08 (14.36, 41.81) | 63.25 | 59.00 | 31.00 | 115.00 |
| Overall prereferral interval | 96.00 (70.81, 121.19) | 114.65 | 26.50 | 58.00 | 133.50 |
| Diagnostic interval | 70.12 (52.45, 87.80) | 91.33 | 15.00 | 35.00 | 82.00 |
| Treatment interval | 32.25 (25.51, 38.99) | 39.15 | 16.75 | 23.00 | 33.25 |
| Mean ratio of the patient interval over other time intervals | |||||
| Patient interval/primary care interval | 2.94 (2.57–3.31) | 0.22 | 0.04 | 1.98 | 7.98 |
| Patient interval/Prereferral interval | 0.74 (0.64,0.83) | 0.40 | 0.56 | 0.98 | 1.00 |
| Patient interval/Total interval | 0.36 (0.31,0.42) | 0.29 | 0.10 | 0.33 | 0.53 |
Together, physicians (GPs) and dentists (GDPs), took mean of 28 days (95% CI, 14.3–41.81) and two consultations (IQR: 2–3) to refer patients for specialized care, making the primary care interval the shortest interval in the study.
The presenting symptom (trigger for consultation) influenced both the number of consultations at the primary care level and the time until diagnosis (Table 2 and Table 3). Pain (27.6%), ulceration (24.8%), and lumps (22.1%) were the most frequent presenting symptoms. Presenting symptoms different from white patch (14.9%) showed frequencies below 3.5%. While pain was linked to longer primary care intervals and more consultations (RR: 1.39; p < 0.001), lumps (RR: 0.75; p < 0.001) were associated with shorter diagnostic intervals and fewer consultations. The number of consultations at the primary care level showed significant positive correlation with the primary care interval (0.54 [0.36–0.68]; p < 0.001) and with the diagnostic interval (0.38 [0.20–0.54]; p < 0.001) (Table 2).
Table 2.
Time intervals and number of consultations at primary care by presenting symptom.
| Presenting Symptom |
Patient Interval | Primary Care Interval | Prereferral Interval |
Diagnostic Interval |
Number of Consultations | ||
|---|---|---|---|---|---|---|---|
| Median | RR (95% CI) | p-Value | |||||
| Pain | |||||||
| No (n = 131) | 62.94 (108.34) | 15.60 (38.11) * | 99.80 (128.10) | 61.25 (74.74) | 2.51 | 1.39 (1.17–1.66) | p < 0.001 * |
| Yes (n = 50) | 50.72 (72.44) | 42.49 (81.59) | 91.38 (97.37) | 83.43 (111.39) | 3.51 | ||
| Oral lump | |||||||
| No (n = 141) | 51.24 (71.26) | 37.28 (72.25) * | 95.27 (90.22) | 83.70 (100.24) * | 3.27 | 0.75 (0.63–0.89) | p < 0.001 * |
| Yes (n = 40) | 71.67 (131.09) | 8.67 (30.81) | 97.48 (155.05) | 39.16 (56.77) | 2.46 | ||
| Oral ulceration | |||||||
| No (n = 136) | 61.66 (113.03) | 30.24 (59.91) | 102.96 (130.11) | 80.89 (99.56) | 3.20 | 0.84 (0.69–1.02) | p = 0.08 |
| Yes (n = 45) | 52.60 (57.98) | 24.20 (69.74) | 83.93 (81.89) | 47.65 (67.02) | 2.71 | ||
| White patch | |||||||
| No (n = 154) | 57.39 (95.22) | 23.94 (55.88) | 90.55 (113.66) | 60.85 (82.09) | 3.04 | 1.02 (0.83–1.26) | p = 0.82 |
| Yes (n = 27) | 62.50 (102.52) | 40.52 (81.87) | 116.82 (119.55) | 107.24 (116.61) | 2.00 | ||
| Red patch | |||||||
| No (n = 175) | 55.94 (94.15) | 26.14 (62.95) | 90.99 (111.68) | 69.43 (92.05) | 3.00 | 1.35 (0.87–2.09) | p = 0.17 |
| Yes (n = 6) | 100.17 (129.27) | 58.80 (66.86) | 173.20 (145.84) | 84.00 (83.05) | 3.00 | ||
| Bleeding | |||||||
| No (n = 178) | 58.21 (96.90) | 28.42 (63.55) | 96.54 (115.50) | 71.17 (91.91) | 2.98 | 0.65 (0.25–1.35) | p = 0.31 |
| Yes (n = 3) | 61.33 (73.53) | 0.00 (NA) | 74.50 (99.70) | 16.50 (6.36) | 3.06 | ||
| Burning sensation | |||||||
| No (n = 178) | 57.85 (96.97) | 29.07 (64.20) | 96.77 (116.25) | 70.52 (91.99) | 2.95 | 1 (0.72–1.38) | p = 1 |
| Yes (n = 3) | 74.33 (65.76) | 1.33 (2.31) | 75.67 (68.06) | 56.67 (78.31) | 1 | ||
| Ill-fitted dentures | |||||||
| No (n = 178) | 55.21 (90.76) | 28.42 (63.55) | 92.21 (110.07) | 70.67 (91.96) | 2.99 | 1.11 (0.58–2.13) | p = 0.74 |
| Yes (n = 3) | 403.00 (NA) | 0.00 (NA) | 403.00 (NA) | 42.00 (56.57) | 3.33 | ||
| Tooth mobility | |||||||
| No (n = 179) | 57.52 (96.18) | 28.42 (63.55) | 95.40 (115.23) | 70.57 (92.03) | 2.98 | 1.34 (0.66–2.70) | p = 0.40 |
| Yes (n = 2) | 145.00 (NA) | 0.00 (NA) | 145.00 (NA) | 47.00 (49.50) | 4.00 | ||
(RR: Relative Risk; (*): (p < 0.05).
Table 3.
Distribution of the presenting symptom and number of consultations by referral.
| Presenting Symptom | Dental Referral (GDP n = 35) |
Medical Referral (GP n = 66) |
Odds Ratio (95% CI) |
p Ratio |
p Overall |
|
|---|---|---|---|---|---|---|
| Pain | No | 21 (35.0%) | 39 (65.0%) | Ref. | ||
| Yes | 14 (34.1%) | 27 (65.9%) | 1.04.0.45;2.44] | 0.934 | 1 | |
| Oral lump | No | 27 (37.0%) | 46 (63.0%) | Ref. | ||
| Yes | 8 (28.6%) | 20 (71.4%) | 1.45.0.57;3.96] | 0.442 | 0.574 | |
| Oral ulceration | No | 27 (40.3%) | 40 (59.7%) | Ref. | ||
| Yes | 8 (23.5%) | 26 (76.5%) | 2.16.0.87;5.81] | 0.099 | 0.146 | |
| Other symptoms: | No | 26 (35.1%) | 48 (64.9%) | Ref. | ||
| Yes | 9 (33.3%) | 18 (66.7%) | 0.96.0.43;2.66] | 0.878 | 1 | |
| Number of consultations | Median (95% CI) | Median (95% CI) | RR (LCL-UCL) | p-value | ||
| 3.57 (2.93–4.21) | 2.71 (2.39–3.04) | 0.76 (0.61–0.93) | 0.008 * | |||
Percentages are estimated by row. GDP: General Dental Practitioner; GP: General Medical Practitioner; RR: Relative Risk; CI, Confidence interval; (*): p < 0.05).
The healthcare professional who first received the patient conditioned both the diagnostic interval and the number of consultations at the primary care level. Physicians (GPs) needed a significantly lower number (OR: 0.66; 95% CI, 0.47–0.92) of consultations (Median: 2; IQR: 2.0–3.0) than GDPs (dentists) (Median: 3; IQR: 2.72–4.0), resulting in a shorter primary care interval (p = 0.029) (Table 3 and Table 4).
Table 4.
Description of time intervals by referral.
| Variable | Dental Referral (GDP) n = 35 |
Medical Referral (GP) n = 66 |
Others Referring n = 80 |
p-Value |
|---|---|---|---|---|
| Total interval (days) | 157.10 (103.92, 210.28) | 166.16 (131.02, 201.30) | 154.67 (115.00,194.34) | 0.59 |
| Patient interval (days) | 42.45 (21.29, 63.60) | 83.40 (40.08, 120.72) | 34.88 (21.98, 47.78) | 0.23 |
| Primary care interval (days) | 50.87 (11.33.90.41) | 23.79 (8.35,39.22) | 5.07 (0.67, 9.48) | 0.02 * |
| Prereferral interval (days) | 94.00 (50.97, 137.03) | 109.42 (68.59, 150.25) | 60.88 (31.57, 90.18) | 0.05 * |
(GDP: General Dental Practitioner; GP: General Medical Practitioner; Others referring: Healthcare professional outside the National Health Service Primary Care network.) (*) p ≤ 0.05).
However, GDPs generated more efficient in-hospital routes by referring these patients to oral and maxillofacial surgery services, resulting in significantly shorter total time intervals (p = 0.05) (Table 5).
Table 5.
Influence of the pattern of referral on in-hospital routes.
| Variable | Oral & Maxillofacial Surgery Services n = 138 |
Other Hospital Services n = 43 | OR (95% CI) | p Ratio | p Overall |
|---|---|---|---|---|---|
| Dental Referral | 32 (23%) | 3 (6.98%) | Ref. | 0.05 * | |
| Medical Referral | 47 (34.1%) | 19 (44.2%) | 4.10 (1.25;19.4) | 0.01 * | |
| Others Referring | 59 (42.8%) | 21 (48.8%) | 3.62 (1.12;16.9) | 0.03 | |
| Total interval (days) | 152.2 (125.4, 179.1) | 183 (136.1, 229.8) | 0.05 |
Preliminary analysis did not show modifications of the effect of the presenting symptoms or number of consultations over primary care interval when possible confounders were included in the model (age, gender, TNM-Stage, co-morbidity). Variables that did not show significant effects were not modified. Anova tables for fixed and flexible effects of the models including confounders are detailed in Supplementary File S1.
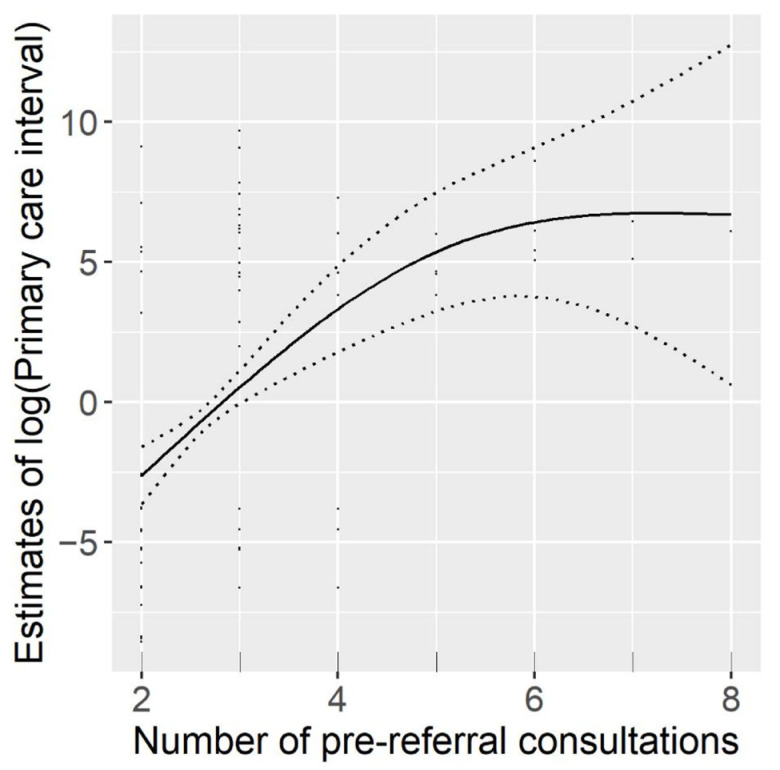
In the multivariate model for the primary care interval, gender and age were included in the initial model, but no effects on this interval were observed. Moreover, the LRT favored the model that excluded gender and age (LRT = 1.81; p = 0.403). Multivariate regression analysis identified the presenting symptom “lump” as being associated with a shorter primary care interval. Conversely, an increase in the number of prereferral consultations was linked to longer primary care intervals (Figure 2 and Table 6).
Figure 2.
Flexible effect of number of prereferral interval on logarithm of primary care interval (mean effect centered at 0). Dotted lines representing 95% credible interval.
Table 6.
Multivariate model for the primary care interval.
| Multivariate Regression Model. Fixed Effects | ||||||
|---|---|---|---|---|---|---|
| Variable | Estimate | Std. Error | Conf. Low | Conf. High | Statistics | p-Value |
| Referral pattern: GPs | −1.408 | 1.367 | −4.135 | 1.319 | −1.030 | 0.306 |
| Referral pattern: others | −0.004 | 1.858 | −3.712 | 3.703 | −0.002 | 0.998 |
| Presenting symptom: pain | 1.902 | 1.201 | −0.495 | 4.299 | 1.583 | 0.118 |
| Presenting symptom: oral lump | −2.785 | 1.277 | −5.332 | 0.238 | −2.182 | 0.033 * |
| Presenting symptom: oral ulceration | 0.140 | 1.157 | −2.169 | 2.449 | 0.121 | 0.904 |
| Multivariate regression model. Flexible effects | ||||||
| Variable | Edf. | Ref. Edf. | F | p-value | ||
| Number of symptoms | 1.737 | 1.929 | 1.381 | 0.222 | ||
| Number of consultations | 1.821 | 1.968 | 15.095 | 0.000 * | ||
(GPs: General Medical Practitioners; Edf: Effective degrees of freedom; (*): p < 0.05).
4. Discussion
The current study reports comprehensive quantitative data on the impact of the presenting symptom on each time interval in the pathways to treatment of symptomatic oral squamous cell carcinoma patients and on their referral routes. Our study provides relevant information for both clinicians and policymakers. The patient interval accounts for most of the prereferral and primary care intervals, and the most frequent presenting symptoms influence the number of consultations at the primary care level and thus the primary care interval. The referring units also condition the intervals and patients’ routes to treatment.
4.1. Strengths and Limitations
The main strengths of our study are the use of a conceptual framework for improving the design and reporting of studies on early cancer diagnosis (Aarhus Statement) [12], the designation of clearly defined events and time intervals and the use of an ambispective design, which increased the quality of the data collected. In addition, detailing information about the relative contribution of each interval to the overall time interval will allow for prioritization of interventions aimed at diminishing delays.
As these kind of studies gathers information about all time intervals in patients’ journeys from the detection of a bodily change, fully prospective designs are virtually impossible. Potential recall biases were prevented by double-checking the information provided by patients against details given by their relatives and the data recorded in primary care clinical charts. Comorbidity may cause both misattribution and a poor recording of the presenting symptom, although this phenomenon was not observed in our sample. Conversely, our sample may be affected by selection bias because it is hospital-based (participation rate: 64.6%), but this bias is highly unlikely because the features of the sample are very similar to those of the incident cases who declined the invitation to enter the study and to those of the general population with oral cancer [1]. In addition, and despite the fact that an early diagnosis and treatment of symptomatic cancer depends on many individual and health system-related factors, there is no evidence about differences in the relative frequency of the presenting symptoms of oral cancer across different countries. Our findings may be particularly relevant for regions with universal health coverage schemes with primary care gatekeepers. Patients were recruited before the onset of the COVID-19 pandemic, avoiding the impact of this new core contributing factor which conditions the self-management and help-seeking attitudes of patients and affects both referrals and appointments and shapes the planning and scheduling of treatment. Although data are scarce, several short communications have reported fewer oral cancer diagnoses during the pandemic, as well as a lack of control of potentially malignant oral disorders and an increase in the proportion of cancers diagnosed at advanced stages and longer therapeutic delays compared to the same period of the previous year [28].
4.2. Time Intervals and the Relative Length of “Patient Delay”
In order to improve both study design and comparability among studies on early cancer diagnosis, previous researchers in the field have recommended the use of the Aarhus guidelines [12]. Some reports that have applied this conceptual framework and used heterogeneous criteria suggested that “patient delay” is the most important contributor to delays in the diagnosis of oral cancer [25]. Reports from the Netherlands and Finland have described patient delays shorter than 1.5 months [17,19,29], while others undertaken in the UK, USA, Australia, India, and Iran have reported durations exceeding three months for this interval [25,30,31]. However, these studies show marked inconsistencies, even within the same country [19,32], probably due to the utilization of heterogeneous criteria and to the absence of a conceptual framework. In addition, symptom recognition—crucial in the patient interval—depends on the cultural and social characteristics of the patient, which hinders comparisons between populations [13,33].
The current study reports an average patient interval (80 days) that is shorter than the average reported by a quantitative systematic review [25], but its relative length compared to the primary care interval is markedly longer, which casts light on an issue for future interventions, as this also occurs with other neoplasms (breast, melanoma, testicular, vulval, cervix, or endometrial) [15]. The patient interval accounts for more than a third of the total time interval.
Little research has been conducted to investigate the primary care interval, and developed countries display the shortest intervals (<1 month) [25,34], as shown by our results, whereas the longest delays are reported from countries with weaker healthcare systems [35], although, wide, above-average intervals (187 days) have been identified in highly developed countries (Australia, USA) [25,30,36]. In addition, oral cancer treatment requires complex planning during the pretreatment interval. Surprisingly, this interval is not usually considered in studies about early diagnosis and treatment [37,38].
4.3. Presenting Symptoms and Time Intervals
Reports on the impact of symptoms on diagnostic timeliness have been restricted to a handful of carcinomas (breast, colon, lung, and pancreas) [26], and there is no information available about oral cancers. However, recognition of symptoms seems to be a particularly relevant factor for this neoplasm and paramount for the patient interval [13].
Oral ulcerations are one of the most frequent presenting symptoms of oral cancer (31–51%) [20,33] and were present in about one quarter (24.8%) of the patients in our study. It is worth mentioning that there are no pathognomonic signs or symptoms of oral cancer, and nonhealing ulcers, sores, or changes in symptoms may prompt patients to seek help [13,39]. The same applies to other early signs, which frequently include plain, changes in color and texture and/or precursor lesions (leukoplakia, erythroplakia) [39,40] (18.2% in our series). Misinterpretations of these bodily changes usually result in longer appraisal intervals, with a paramount influence in the total time to diagnosis [40,41].
4.4. Prereferral Interval (GP vs. GDP)
Oral cancer is the only neoplasm which can be referred for specialized care by both GDPs and primary care physician GPs [31]. Both types of healthcare professionals refer patients in similar proportions (GPs: 50% [13–86%]; GDPs: 40% [15–80%]) [19]. This referral pattern shows wide regional differences, even within the same country. The highest proportions of dentists referring for specialized care were reported in the UK [41], Australia [42], Denmark [43], the USA [44], Japan [45], and Argentina [37], without showing significant differences in terms of delay when compared to GPs [19]. However, our results indicate significant differences in the primary care interval and the prereferral interval depending on the referral pattern: patients referred by GDPs had shorter prereferral intervals. In addition, most dentists referred directly to hospital oral and maxillofacial surgery, ensuring a more efficient pathway through the healthcare system with shorter time intervals.
The reasons patients chose a GP or a GDP for consultation when experiencing possible symptoms of oral cancer is not known, although financial reasons or tumour sites may have played a role [46]. A recent community-based study showed that patients with a persistent oral ulceration—the most frequent presenting symptom—preferred consultation with a GP [47]. In addition, the number of consultations before referral has been proposed as a subrogated indicator of the primary care interval [48], especially for “harder to suspect” cancers (multiple myeloma, or pancreatic or stomach carcinomas), which generate more consultations and longer primary care intervals [22,48]. However, the association between the number of prereferral consultations and the primary care interval for oral cancer revealed by our results may make this indicator useful for “intermediate or easier to suspect” neoplasms.
On average, patients had two or three consultations before referral [23], and our results show GPs needed a significantly lower number of consultations and had shorter primary care intervals than did GDPs, probably because the latter undertook further investigation or “treatment trials” (removing irritating factors such as ill-fitted dentures, etc.) [23,36,49]. In addition, patients with oral ulcerations were mainly seen by GPs and seem to have experienced fewer consultations. Other less well-known patient-mediated factors and, though unlikely a factor, suboptimal clinical training of GDPs should also be considered as potential causes for the higher number of consultations by GDPs. In Spain, GPs and GDPs refer patients for hospital services independently. In contrast, referral within the primary care system may cause delayed referrals for hospital treatment.
4.5. Practical Implications for Research, Clinical Practice, and Health Policy
An overview of the existing research on the patient interval reveals a high risk for bias, which can be minimized by using an adequate theoretical framework, and the “Model of Pathways to Treatment” (Aarhus Statement) is highly recommended [12,13,14]. In this vein, the processes of symptom interpretation need further research and the use of validated questionnaires for collecting data from patients is strongly recommended [40].
Increasing patient awareness may have an important impact on the patient interval, and priority should be given to interventions focused on increasing public knowledge of cancer symptoms and risk factors [9] to decrease the burden of oral carcinomas. These interventions have proved more effective if based on theoretical models [50] and addressed to high-risk groups in a way that incorporate the sociocultural environment of the community.
These interventions should pay special attention to detailing the most frequent signs and symptoms of oral cancer (lumps or swelling and white or red patches) and to highlighting those with higher positive predictive value (e.g., nonhealing ulcerations) [36,37], as the main trigger for consultation is symptomatology [42]. The risk of misinterpreting symptoms as banal conditions should also be explained [41], and patients should be warned about the prognostic importance of seeking help quickly. In addition, reducing the number or prereferral consultations may be a useful early diagnosis initiative to reduce the primary care interval. However, a referral policy that is too broad may increase patients’ anxiety and hospital costs. Nevertheless, fast tracks have been useful in diminishing the time between referral and the beginning of cancer treatment [23]. Refining referral guidelines are necessary to clarify the roles of GDPs and GPs in the patient referral pathway, as is the implementation of new interventions aimed at reducing the prereferral interval of patients with oral cancer [27,51,52,53,54,55].
5. Conclusions
The patient interval is almost three times longer than the primary care interval and constitutes the major component of the prereferral interval. It accounts for about one third of the total time interval to treatment. This protagonist role has permitted its identification as a potential target for intervention to increase early diagnosis of oral cancer. The presenting symptom can influence the number of consultations needed by the healthcare professional and the length of the different time intervals to diagnosis. Moreover, time intervals are also conditioned by the referral pattern: while GDPs generate longer primary care intervals and a higher number of consultations, GPs use less efficient in-hospital routes causing longer total intervals. Therefore, a better understanding of the presenting symptoms, a reduction in the number of consultations, and the optimization of referral pathways with specific fast tracks tailored to the different healthcare environments would contribute to diminishing the time intervals until the start of treatment.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13205163/s1, Supplementary File S1. Anova tables of the models.
Author Contributions
Conception: P.I.V.-C., J.S. and J.L.L.-C.; Validation: D.P.L., A.R.-M. and Á.G.-R.; Formal analysis: P.I.V.-C., J.S., J.L.L.-C. and P.C.B.; Investigation: P.I.V.-C., P.C.B. and J.S.; Resources: D.P.L., A.R.-M. and Á.G.-R.; Data synthesis: D.P.L., A.R.-M., P.C.B. and Á.G.-R.; Writing—original draft preparation: P.I.V.-C. and J.S.; Writing—review and editing: P.I.V.-C., J.S., J.L.L.-C. and P.C.B.; Supervision: D.P.L., A.R.-M. and Á.G.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding: Research project PI 14/01446 (COF), Spanish National R&D&I Programme 2013–2016, co-founded by the ISCIII-Subdirección General de Evaluación y Fomento de la Investigación and the European Regional Development Fund (ERDF).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Santiago-Lugo Galician Ethics Committee (protocol code 2014/604. Date of approval 17 December 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to legal constraints.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Agency of Research on Cancer Global Cancer Observatory. Cancer Tomorrow. [(accessed on 25 June 2021)]. Available online: http://gco.iarc.fr.
- 2.Bosetti C., Carioli G., Santucci C., Bertuccio P., Gallus S., Garavello W., Negri E., La Vecchia C. Global trends in oral and pharyngeal cancer incidence and mortality. Int. J. Cancer. 2020;147:1040–1049. doi: 10.1002/ijc.32871. [DOI] [PubMed] [Google Scholar]
- 3.Shrestha A.D., Vedsted P., Kallestrup P., Neupane D. Prevalence and incidence of oral cancer in low- and middle-income countries: A scoping review. Eur. J. Cancer Care Engl. 2020;29:e13207. doi: 10.1111/ecc.13207. [DOI] [PubMed] [Google Scholar]
- 4.Dhanuthai K., Rojanawatsirivej S., Thosaporn W., Kintarak S., Subarnbhesaj A., Darling M., Kryshtalskyj E., Chiang C.P., Shin H.I., Choi S., et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Cir. Bucal. 2018;23:e23–e29. doi: 10.4317/medoral.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonhson N.W., Warnakulasuriya S., Gupta P.C., Dimba E., Chindia M., Otoh E.C., Sankaranarayanan S., Califano J., Kowalski L. Global oral health inequalities in incidence and outcomes for oral cancer: Causes and solutions. Adv. Dent. Res. 2011;23:237–246. doi: 10.1177/0022034511402082. [DOI] [PubMed] [Google Scholar]
- 6.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Warnakulasuriya S. Living with oral cancer: Epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol. 2010;46:407–410. doi: 10.1016/j.oraloncology.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Silverman S., Kerr A.R., Epstein J.B. Oral and pharyngeal cancer control and early detection. Oral and pharyngeal cancer control and early detection. J. Cancer Educ. 2010;25:279–281. doi: 10.1007/s13187-010-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neal R.D., Tharmanathan P., France B., Din N.U., Cotton S., Fallon-Ferguson J., Hamilton W., Hendry A., Hendry M., Lewis R., et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer. 2015;112:S92–S107. doi: 10.1038/bjc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gómez I., Seoane J., Varela-Centelles P., Diz P., Takkouche B. Is diagnostic delay related to advanced-stage oral cancer? A meta-analysis. Eur. J. Oral Sci. 2009;117:541–546. doi: 10.1111/j.1600-0722.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 11.Seoane J., Takkouche B., Varela-Centelles P., Tomás I., Seoane-Romero J.M. Impact of delay in diagnosis on survival to head and neck carcinomas: A systematic review with meta-analysis. Clin. Otolaryngol. 2012;37:99–106. doi: 10.1111/j.1749-4486.2012.02464.x. [DOI] [PubMed] [Google Scholar]
- 12.Weller D., Vedsted P., Rubin G., Walter F.M., Emery J., Scott S., Campbell C., Andersen R.S., Hamilton W., Olesen F., et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer. 2012;106:1262–1267. doi: 10.1038/bjc.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varela-Centelles P., López-Cedrún J.L., Fernández-Sanromán J., Seoane-Romero J.M., Santos de Melo N., Álvarez-Nóvoa P., Gómez I., Seoane J. Key points and time intervals for early diagnosis in symptomatic oral cancer: A systematic review. Int. J. Oral Maxillofac. Surg. 2017;46:1–10. doi: 10.1016/j.ijom.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Seoane J., Alvarez-Novoa P., Gomez I., Takkouche B., Diz P., Warnakulasiruya S., Seoane-Romero J.M., Varela-Centelles P. Early oral cancer diagnosis: The Aarhus statement perspective. A systematic review and meta-analysis. Head Neck. 2016;38:E2182–E2189. doi: 10.1002/hed.24050. [DOI] [PubMed] [Google Scholar]
- 15.Lyratzopoulos G., Saunders C., Abel G.A., McPhail S., Neal R.D., Wardle J., Rubin G.P. The relative length of the patient and the primary care interval in patients with 28 common and rarer cancers. Br. J. Cancer. 2015;112((Suppl. 1)):S35–S40. doi: 10.1038/bjc.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graboyes E.M., Kompelli A.R., Neskey D.M., Brennan E., Nguyen S., Sterba K.R., Warren G.W., Hughes-Halbert C., Nussenbaum B., Day T.A. Association of Treatment Delays With Survival for Patients With Head and Neck Cancer: A Systematic Review. JAMA Otolaryngol. Head Neck Surg. 2019;145:166–177. doi: 10.1001/jamaoto.2018.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teppo H., Alho O.P. Relative importance of diagnostic delays in different head and neck cancers. Clin. Otolaryngol. 2008;33:325–330. doi: 10.1111/j.1749-4486.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 18.Koivunen P., Rantala N., Hyrynkangas K., Jokinen K., Alho O.P. The impact of patient and professional delay on survival in pharyngeal cancer. Cancer. 2001;92:2885–2891. doi: 10.1002/1097-0142(20011201)92:11<2885::AID-CNCR10119>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Kantola S., Jokinen K., Hyrynkangas K., Mäntyselkä P., Alho O.P. Detection of tongue cancer in primary care. Br. J. Gen. Pract. 2001;51:106–111. [PMC free article] [PubMed] [Google Scholar]
- 20.Seoane J., Pita-Fernández S., Gómez I., Vazquez I., López-Cedrún J.L., De Agustin D., Varela-Centelles P. Proliferative activity and diagnostic delay in oral cancer. Head Neck. 2010;32:1377–1384. doi: 10.1002/hed.21338. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Cedrún J.L., Otero-Rico A., Vázquez-Mahía I., Seoane J., García-Caballero L., Seoane-Romero J.M., Varela-Centelles P. Association between hospital interval and survival in patients with oral cancer: A waiting time paradox. PLoS ONE. 2019;14:e0224067. doi: 10.1371/journal.pone.0224067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyratzopoulos G., Abel G.A., McPhail S., Neal R.D., Rubin G. Measures of promptness of cancer diagnosis in primary care: Secondary analysis of national audit data on patients with 18 common and rarer cancers. Br. J. Cancer. 2013;108:686–690. doi: 10.1038/bjc.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grafton-Clarke C., Chen K.W., Wilcock J. Diagnosis and referral delays in primary care for oral squamous cell cancer: A systematic review. Br. J. Gen. Pract. 2019;69:e112–e126. doi: 10.3399/bjgp18X700205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutkowska M., Hnitecka S., Nahajowski M., Dominiak M., Gerber H. Oral cancer: The first symptoms and reasons for delaying correct diagnosis and appropriate treatment. Adv. Clin. Exp. Med. 2020;29:735–743. doi: 10.17219/acem/116753. [DOI] [PubMed] [Google Scholar]
- 25.Varela-Centelles P., Seoane J., Lopez-Cedrun J.L., Fernandez-Sanroman J., García-Martin J.M., Takkouche B., Alvarez-Novoa P., Seoane-Romero J.M. The length of patient and primary care time interval in the pathways to treatment in symptomatic oral cancer. A quantitative systematic review. Clin. Otolaryngol. 2018;43:164–171. doi: 10.1111/coa.12919. [DOI] [PubMed] [Google Scholar]
- 26.Koo M.M., Hamilton W., Walter F.M., Rubin G.P., Lyratzopoulos G. Symptom Signatures and Diagnostic Timeliness in Cancer Patients: A Review of Current Evidence. Neoplasia. 2018;20:165–174. doi: 10.1016/j.neo.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team . R: A Language and Environment for Statistical Computing. R. Foundation for Statistical Computing; Vienna, Austria: 2020. [(accessed on 6 July 2021)]. Available online: https://www.R-project.org/ [Google Scholar]
- 28.Arduino P.G., Conrotto D., Broccoletti R. The outbreak of Novel Coronavirus disease (COVID-19) caused a worrying delay in the diagnosis of oral cancer in north-west Italy: The Turin Metropolitan Area experience. Oral Dis. 2021;27((Suppl. 3)):742–743. doi: 10.1111/odi.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brouha X.D., Tromp D.M., Hordijk G.J., Winnubst J.A., de Leeuw J.R. Oral and pharyngeal cancer: Analysis of patient delay at different tumor stages. Head Neck. 2005;27:939–945. doi: 10.1002/hed.20270. [DOI] [PubMed] [Google Scholar]
- 30.Peacock Z.S., Pogrel M.A., Schmidt B.L. Exploring the reasons for delay in treatment of oral cancer. J. Am. Dent. Assoc. 2008;139:1346–1352. doi: 10.14219/jada.archive.2008.0046. [DOI] [PubMed] [Google Scholar]
- 31.Sargeran K., Murtomaa H., Safavi S.M., Teronen O. Delayed diagnosis of oral cancer in Iran: Challenge for prevention. Oral Health Prev. Dent. 2009;7:69–76. [PubMed] [Google Scholar]
- 32.Jovanovic A., Kostense P.J., Schulten E.A., Snow G.B., van der Waal I. Delay in diagnosis of oral squamous cell carcinoma; a report from the Netherlands. Oral Oncol. 1992;28:37–38. doi: 10.1016/0964-1955(92)90009-P. [DOI] [PubMed] [Google Scholar]
- 33.Andersen R.S., Vedsted P., Olesen F., Bro F., Søndergaard J. Patient delay in cancer studies: A discussion of methods and measures. BMC Health Serv. Res. 2009;9:189. doi: 10.1186/1472-6963-9-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers S.N., Pabla R., McSorley A., Lowe D., Brown J.S., Vaughan E.D. An assessment of deprivation as a factor in the delays in presentation, diagnosis and treatment in patients with oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2007;43:648–655. doi: 10.1016/j.oraloncology.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Joshi P., Nair S., Chaturvedi P., Nair D., Agarwal J.P., D′Cruz A.K. Delay in seeking specialized care for oral cancers: Experience from a tertiary cancer center. Indian J. Cancer. 2014;51:95–97. doi: 10.4103/0019-509X.137934. [DOI] [PubMed] [Google Scholar]
- 36.Kaing L., Manchella S., Love C., Nastri A., Wiesenfeld D. Referral patterns for oral squamous cell carcinoma in Australia: 20 years progress. Aust. Dent. J. 2016;61:29–34. doi: 10.1111/adj.12314. [DOI] [PubMed] [Google Scholar]
- 37.Morelatto R.A., Herrera M.C., Fernández E.N., Corball A.G., López de Blanc S.A. Diagnostic delay of oral squamous cell carcinoma in two diagnosis centers in Córdoba Argentina. J. Oral Pathol. Med. 2007;36:405–408. doi: 10.1111/j.1600-0714.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 38.Kowalski L.P., Carvalho A.L. Influence of time delay and clinical upstaging in the prognosis of head and neck cancer. Oral Oncol. 2001;37:94–98. doi: 10.1016/S1368-8375(00)00066-X. [DOI] [PubMed] [Google Scholar]
- 39.Suspected Cancer: Recognition and Referral. National Institute for Health and Care Excellence (NICE) 2015. [(accessed on 25 June 2021)]. Available online: https://www.nice.org.uk/guidance/ng12/chapter/1-recommendations. [PubMed]
- 40.van der Waal I., de Bree R., Brakenhoff R., Coebergh J.W. Early diagnosis in primary oral cancer: Is it possible? Med. Oral Patol. Oral Cir. Bucal. 2011;16:e300–e305. doi: 10.4317/medoral.16.e300. [DOI] [PubMed] [Google Scholar]
- 41.Scott S.E., Walter F.M., Webster A., Sutton S., Emery J. The model of pathways to treatment: Conceptualization and integration with existing theory. Br. J. Health Psychol. 2013;18:45–65. doi: 10.1111/j.2044-8287.2012.02077.x. [DOI] [PubMed] [Google Scholar]
- 42.Dimitroulis G., Reade P., Wiesenfeld D. Referral patterns of patients with oral squamous cell carcinoma, Australia. Eur. J. Cancer Part B Oral Oncol. 1992;28:23–27. doi: 10.1016/0964-1955(92)90007-N. [DOI] [PubMed] [Google Scholar]
- 43.Pinholt E.M., Rindum J., Pindborg J. Oral cancer: A retrospective study of 100 Danish cases. Br. J. Oral Maxillofac. Surg. 1997;35:77–80. doi: 10.1016/S0266-4356(97)90679-3. [DOI] [PubMed] [Google Scholar]
- 44.Holmes J.D., Dierks E.J., Homer L.D., Potter B.E. Is detection of oral and oropharyngeal squamous cancer by a dental health care provider associated with a lower stage at diagnosis? J. Oral Maxillofac. Surg. 2003;61:285–291. doi: 10.1053/joms.2003.50056. [DOI] [PubMed] [Google Scholar]
- 45.Onizawa K., Nishihara K., Yamagata K., Yusa H., Yanagawa T., Yoshida H. Factors associated with diagnostic delay of oral squamous cell carcinoma. Oral Oncol. 2003;39:781–788. doi: 10.1016/S1368-8375(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 46.Kerdpon D., Sriplung H. Factors related to advanced stage oral squamous cell carcinoma in southern Thailand. Oral Oncol. 2001;37:216–221. doi: 10.1016/S1368-8375(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 47.Varela-Centelles P., Seoane J., Ulloa-Morales Y., Estany-Gestal A., Blanco-Hortas A., García-Pola M.J., Seoane-Romero J.M. People would rather see a physician than a dentist when experiencing a long-standing oral ulceration. A population-based study in Spain. Med. Oral Patol. Y Oral Cir. Bucal. 2020;25:e455–e460. doi: 10.4317/medoral.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyratzopoulos G., Wardle J., Rubin G. Rethinking diagnostic delay in cancer: How difficult is the diagnosis? BMJ. 2014;349:g7400. doi: 10.1136/bmj.g7400. [DOI] [PubMed] [Google Scholar]
- 49.Langton S., Cousin G.C.S., Plüddemann A., Bankhead C.R. Comparison of primary care doctors and dentists in the referral of oral cancer: A systematic review. Br. J. Oral Maxillofac. Surg. 2020;58:898–917. doi: 10.1016/j.bjoms.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Luryi A.L., Yarbrough W.G., Niccolai L.M., Roser S., Reed S.G., Nathan C.h.O., Moore M.G., Day T., Judson B.L. Public awareness of head and neck cancers: A cross-sectional survey. JAMA Otolaryngol. Head Neck Surg. 2014;140:639–646. doi: 10.1001/jamaoto.2014.867. [DOI] [PubMed] [Google Scholar]
- 51.Mansell G., Shapley M., Jordan J.L., Jordan K. Interventions to reduce primary care delay in cancer referral: A systematic review. Br. J. Gen. Pract. 2011;61:e821–e835. doi: 10.3399/bjgp11X613160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koo M.M., Unger-Saldaña K., Mwaka A.D., Corbex M., Ginsburg O., Walter F.M., Calanzani N., Moodley J., Rubin G.P., Lyratzopoulos G. Conceptual Framework to Guide Early Diagnosis Programs for Symptomatic Cancer as Part of Global Cancer Control. JCO Glob. Oncol. 2021;7:35–45. doi: 10.1200/GO.20.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Virgilsen L.F., Pedersen A.F., Vedsted P., Petersen G.S., Jensen H. Alignment between the patient’s cancer worry and the GP’s cancer suspicion and the association with the interval between first symptom presentation and referral: A cross-sectional study in Denmark. BMC Fam. Pract. 2021;22:129. doi: 10.1186/s12875-021-01480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeung C.A. Referrals to dentists by GPs could delay diagnosis of oral cancer. BMJ. 2017;356:i6784. doi: 10.1136/bmj.i6784. [DOI] [PubMed] [Google Scholar]
- 55.Lyratzopoulos G., Neal R.D., Barbiere J.M., Rubin G.P., Abel G.A. Variation in number of general practitioner consultations before hospital referral for cancer: Findings from the 2010 National Cancer Patient Experience survey in England. Lancet Oncol. 2012;13:353–365. doi: 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to legal constraints.