Abstract
The apolipoprotein E (APOE) ε4 allele is a risk factor for Alzheimer’s disease (AD) that has been linked to changes in brain structure and function as well as to different biological subtypes of the disease. The present study aimed to investigate the association of APOE ε4 genotypes with brain functional impairment, as assessed by quantitative EEG (qEEG) in patients on the AD continuum. The study population included 101 amyloid positive patients diagnosed with mild cognitive impairment (MCI) (n = 50) and AD (n = 51) that underwent resting-state EEG recording and CSF Aβ42 analysis. In total, 31 patients were APOE ε4 non-carriers, 42 were carriers of one, and 28 were carriers of two APOE ε4 alleles. Quantitative EEG analysis included computation of the global field power (GFP) and global field synchronization (GFS) in conventional frequency bands. Amyloid positive patients who were carriers of APOE ε4 allele(s) had significantly higher GFP beta and significantly lower GFS in theta and beta bands compared to APOE ε4 non-carriers. Increased global EEG power in beta band in APOE ε4 carriers may represent a brain functional compensatory mechanism that offsets global EEG slowing in AD patients. Our findings suggest that decreased EEG measures of global synchronization in theta and beta bands reflect brain functional deficits related to the APOE ε4 genotype in patients that are on a biomarker-verified AD continuum.
Keywords: quantitative electroencephalography, apolipoprotein E, Alzheimer’s disease, mild cognitive impairment, amyloid pathology
1. Introduction
Alzheimer’s disease (AD) is the most common form of dementia with distinct neuropathological changes and a heterogeneous clinical presentation. The disease is neuropathologically characterized by the gradual accumulation of amyloid-β 42 (Aβ42) and hyperphosphorylated tau proteins in the brain tissue in the form of senile plaques and neurofibrillary tangles, respectively [1,2]. However, AD can present with different clinical symptomatology in its typical and atypical forms, depending on whether memory or other specific cognitive domains are affected [3]. These individual differences in the vulnerability of the human brain to the AD-associated neuropathology depend on several elements, including genetic predisposition, environmental effect, and lifestyle factors [4,5,6].
Even though an increasing number of genetic variants have been associated with AD [6], the apolipoprotein ε4 (APOE) genotype remains the main known genetic risk factor for the more common sporadic form of AD [7,8]. The APOE gene encodes three alleles—ε2, ε3, and ε4—with an allele-frequency ranging from 4–8%, 72–87%, and 9–19%, respectively, among the healthy population worldwide [7]. An extensive meta-analysis demonstrated that heterozygous and homozygous APOE ε4 carriers have a 1.1–5.6- and 2.2–33.1-fold increased risk, respectively, for developing AD compared to non-carriers [7]. The mechanism by which APOE ε4 exerts these effects remains to be fully elucidated; however, its physiological role in generating and maintaining synapses suggests impaired synaptic connections as the contributing factor, especially in the event of brain injury [8,9]. APOE ε4 has been additionally related to increased Aβ accumulation [10], altered Aβ clearance [11], interaction with tau protein [12], and neuroinflammation in brain tissue [13].
Previous studies have shown that APOE ε4 affects brain functional activity and connectivity, even before the presence of Aβ pathology in the brain tissue [14,15]. These findings highlight the relationship between genetic factors and brain functional changes in subjects that are at increased risk for dementia. The heritability and genetic origin of brain functioning have been particularly emphasized by studies employing electroencephalography (EEG) [16,17,18].
EEG is a non-invasive diagnostic procedure that registers brain activity in real time [19] and that can be further used to quantify and localize changes in the temporal and spatial organization of brain neuronal networks [20]. Quantitative EEG (qEEG), therefore, provides objective and comprehensive assessment of brain functional activity commonly analyzed across its slow (delta and theta) and fast (alpha and beta) frequency bands that are thought to reflect distinct physiological processes [19]. Individual differences in these EEG signals were shown to have strong genetic origin [18], including a high concordance of EEG signals between identical twins [16,21,22].
A number of studies have assessed qEEG changes in patients with cognitive impairment and AD, coming from a standpoint that AD is a disease of synaptic failure [23,24] and that EEG is the only available method that can directly mirror synaptic activity with a millisecond time resolution [25]. The most consistent qEEG findings in patients on the clinical AD continuum include generalized EEG-slowing and reduced EEG synchronization in fast frequency bands [26,27,28,29,30,31]. These qEEG changes have been additionally related to the severity of cognitive impairment [32,33,34,35], future cognitive decline and progression to dementia [27,36,37], as well as with a cerebrospinal fluid (CSF) profile of AD biomarker changes [38,39].
Several studies have further addressed a potential relationship between qEEG changes and the APOE status in patients with AD; however, the results have been conflicting thus far, reporting accentuated EEG slowing in APOE ε4 carriers [40,41], more severe EEG slowing in APOE ε4 non-carriers [42], and no differences in relation to the APOE status [43]. Notably, these studies involved patients whose diagnoses were based on clinical criteria and without the biomarker evidence of AD pathology, which, considering the pathophysiological heterogeneity of cognitive disorders, may have affected the interpretation of results.
The aim of the present study was to investigate relationship between APOE genotype and brain functional impairment in memory clinic patients on a biomarker-verified AD continuum. We employed two qEEG measures of global power and synchronization that have been utilized previously in studies on AD and cognitive disorders [28,29,34,38]. Our hypothesis was that the APOE genotype has an intrinsic effect on brain oscillatory activity in MCI and dementia patients that have positive biomarkers of AD pathology. Furthermore, we investigated whether comprehensive qEEG analyses, including measures of both power and synchronization of EEG oscillations, may provide complementary information on these brain functional changes.
2. Materials and Methods
2.1. Study Population
The study population included in total 101 patients clinically diagnosed with MCI (n = 50) according to the Winblad et al., 2004 criteria [44] or with AD (n = 51) according to the ICD-10 criteria [45]. All patients were recruited at the Clinic for Cognitive Disorders, Karolinska University Hospital Huddinge, Stockholm, Sweden and underwent comprehensive clinical assessment, computed tomography (CT) and/or magnetic resonance brain imaging (MRI), resting-state EEG recording, CSF sampling, analysis of AD biomarkers (Aβ42, phospho tau (p-tau), and total tau (t-tau)), and APOE genotyping of peripheral blood-DNA.
All diagnostic tests were part of the baseline cognitive assessment and patients were, therefore, drug naïve with respect to AD medication. The severity of cognitive impairment was, among other tests, assessed using the Mini-Mental State Examination (MMSE) [46]. The exclusion criteria included the presence of any significant psychiatric or neurological comorbidity, history of brain trauma, use of antiepileptic or neuroleptic medications, and any other dementia diagnosis.
Patients were stratified into three groups based on their APOE status and numbers of ε4 alleles including APOE ε4 non-carriers (n = 31), APOE ε4 heterozygous carriers—one allele (n = 42) and APOE ε4 homozygous carriers—two alleles (n = 28). All MCI and AD patients included in this study were amyloid positive according to their CSF Aβ42 levels and, therefore, meet the research and biomarker criteria for Alzheimer’s disease continuum [3,47]. MCI and AD patients were pooled in the following analyses due to a limited number of patients that prevented separate analyses of diagnostic groups. The number of MCI and AD patients within each APOE genotype group are presented in Table 1.
Table 1.
Demographic and clinical characteristics in APOE ε4 non-carriers and carriers.
|
APOE ε4 Non-Carriers |
APOEε4 Heterozygous Carriers (One Allele) |
APOEε4 Homozygous Carriers (Two Alleles) |
p-Value | |
|---|---|---|---|---|
| N (total) | 31 | 42 | 28 | |
| MCI | 13 | 22 | 15 | |
| AD | 18 | 20 | 13 | |
| Age (years) | 65.03 ± 9.17 | 65.79 ± 8.54 | 64.04 ± 5.31 | 0.766 |
| Sex (M/F) | 15/16 | 17/25 | 9/19 | 0.447 |
| Education (years) | 11.97 ± 3.80 | 12.39 ± 3.80 | 12.68 ± 3.42 | 0.631 |
| MMSE a | 24.73 ± 4.32 | 26.71 ± 2.76 | 25.11 ± 4.14 | 0.092 |
Data presented as the means ± standard deviation. a Missing values for MMSE variables: one patient per each APOE ε4 group. Independent-Samples Kruskal–Wallis Test and Chi-Square test over the three APOE genotype groups as appropriate. AD = Alzheimer’s disease; APOE = Apolipoprotein E; CSF = cerebrospinal fluid; MCI = mild cognitive impairment; and MMSE = Mini Mental State Examination.
2.2. CSF Analysis
CSF samples were obtained by a standard lumbar puncture procedure between the L3/L4 or L4/L5 intervertebral space. All CSF samples were sampled using a 25-gauge needle, collected in 12 mL polypropylene tube, centrifuged at 1000 rpm (10 min), and frozen at −70 °C. Conventional CSF markers of AD pathology, including Aβ42, p-tau, and t-tau protein concentrations, were analyzed at the clinical chemistry laboratory Karolinska University Hospital, Huddinge, using xMAP technology and the INNO-BIA AlzBio3 kit (Innogenetics) [48]. Patients on the AD continuum present with decreased CSF Aβ42 levels, which is thought to reflect increased deposition and reduced clearance of Aβ42 into the CSF [49]. The clinical cut-off for pathological CSF levels, defined by Karolinska University Hospital laboratory, was CSFAβ42 levels <550 ng/L. MCI and AD patients with pathological CSF Aβ42 levels were defined as amyloid positive in the present study.
2.3. Resting-State EEG Recordings and Analyses
All patients underwent resting-state EEG recordings at the Department of Clinical Neurophysiology within 6 months of the baseline clinical assessment. EEGs were recorded on the Nervus system (NicoletOne EEG Reader v5.93.0.424, Natus NicoletOne, Pleasanton, CA, USA) using 21 electrodes and standard electrode placement according to the 10/20 system. The electrode impedances were kept below 5 kU. The EEGs were recorded with a sampling rate of 256 Hz and band-pass filter between 0.5 and 70 Hz.
All digital EEG files were exported for research purposes. The EEG recordings were first assessed for any physiological and non-physiological artifacts as well as periods of drowsiness by visual inspection. Artifacts and periods of drowsiness were then removed by manual selection and the rejection of referred EEG segments. Eye movements and electrocardiographic artifacts were additionally removed using independent component analysis algorithm. The EEGs were first segmented in the 2-s artifact-free epochs. Next, Fast Fourier Transform (FFT) was performed on all available 2-s EEG epochs in order to translate EEG data into the frequency domain.
The present study employed two global frequency domain qEEG measures that have been validated previously in the context of cognitive impairment and AD [28,36,38] named GFP and GFS. GFP is a single and generalized measure of the strength of scalp potential fields [25,50]. It can be further employed in the frequency domain where its formula corresponds to the root mean of squared spectral amplitudes across all EEG channels and, therefore, summarizes global EEG power across pre-defined frequency bands [25,28,38]. GFS is a global measure of brain functional connectivity that reflects the phase synchrony of EEG oscillations across all electrode sites [51]. In more detail, FFT analysis of EEG epochs yields, for a given frequency, sine and cosine coefficients of all electrodes that can be entered into a sine–cosine diagram. Multichannel-EEG recording, at a given time point or time epoch, can, therefore, be presented as a cloud spread of electrode points in the sine–cosine diagram. For the computation of GFS at a given frequency, these points were submitted to a principal component analysis (PCA): GFS is then defined as the ratio of the two resulting PCA eigenvalues. The more of the variance is explained by the first principal component, the more of the electrode entry points approximate the straight line and the closer GFS is to 1, which would imply that all EEG sources observable at the given frequency oscillated either in phase or in antiphase. GFS is, therefore, a measure of a common phase of EEG oscillations at all electrode sites at a certain frequency point and can obtain a value between 0 and 1. Both GFP and GFS measures were averaged over all EEG epochs and conventional frequency bands were defined as delta (1–3.5 Hz), theta (4–7.5 Hz), alpha (8–11.5 Hz), and beta (12–19.5 Hz). EEG preprocessing and quantitative analysis were performed in Brain Vision Analyzer, version 2.0, software (Gilching, Germany).
2.4. Statistical Analysis
Statistical analyses were performed in SPSS (version 26, IBM, New York, NY, USA) and STATA (version 16.1, StataCorp LLC, College Station, TX, USA). Demographics (age, gender, and education) and MMSE levels were compared between APOE ε4 non-carriers, heterozygous, and homozygous carriers using the Independent-samples Kruskal–Wallis Test for continuous variables and Chi-Square test for categorical variables.
qEEG measure GFP in all frequency bands was transformed with zero-skewness natural logarithmic transformation in order to obtain non-skewed data distributions. In the figures, the differences in GFP and GFS across APOE groups were presented using the original untransformed data. P-values are based on one-way analysis of variance (ANOVA) on the transformed variables. Since the assumption of homogeneity of variance was not met for all GFP/GFS variables (Levene’s test < 0.05), the p-values from Welch’s ANOVA were reported. Welch’s ANOVA was run separately for GFP/GFS measures in each frequency band. The level of statistical significance was p < 0.05.
3. Results
3.1. Demographics and Clinical Characteristics
Demographics and clinical characteristics of our study population and across the three APOE ε4 genotype groups (non-carriers, carriers with one allele, and carriers with two alleles) are presented in Table 1. There were no statistically significant differences in age (p = 0.766), education (p = 0.631), or distribution of females versus males (p = 0.447) between the three groups. The APOE ε4 non-carriers, heterozygous, and homozygous carriers did not differ significantly in the global severity of cognitive impairment as assessed by MMSE (p = 0.092).
3.2. Relationship between Global EEG Power and APOE Genotype in Amyloid Positive MCI and AD Patients
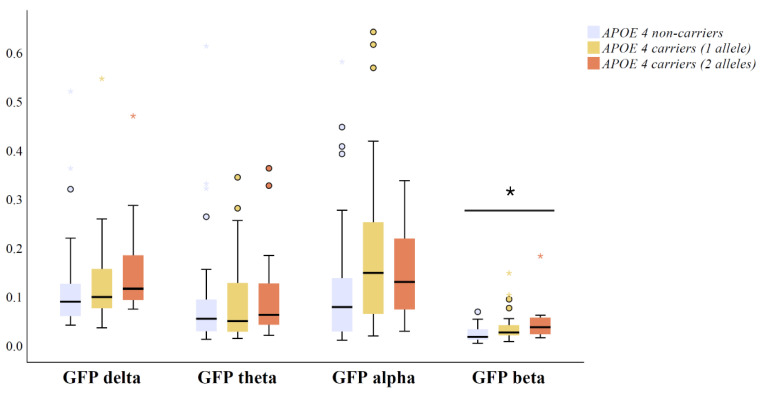
GFP medians and interquartile ranges in four conventional frequency bands across APOE ε4 genotype groups are presented in Figure 1. One outlying GFP alpha data point from an APOE ε4 heterozygous carrier was excluded from the analysis (GFP alpha > 2 µV). There was a statistically significant gradient-like increase in GFP beta (p = 0.001) in amyloid positive APOE ε4 heterozygous and homozygous carriers compared to non-carriers (Figure 1). Interestingly, there were no significant differences in GFP in delta (p = 0.065), theta (p = 0.491), or alpha bands (p = 0.084) between amyloid positive APOE genotype groups (Figure 1).
Figure 1.
Differences in qEEG measures of global field power (GFP) between APOE ε4 carriers with two alleles (n = 28), carriers with one allele (n = 42) and non-carriers (n = 31) including CSF Aβ42 positive (<550 ng/L) MCI and AD patients. The original data on GFP are presented as the median (solid line), interquartile range (box), and minimum and maximum values (whiskers) across four conventional frequency bands. p-Values are based on ANOVA over the three genotype groups using GFP measures transformed with zero skewness natural log-transformation; * p < 0.05. Outlier and extreme values are denoted as circles and stars, respectively. Abbreviations: ANOVA = analysis of variance; and APOE = apolipoprotein E.
3.3. Relationship between Global EEG Synchronization and APOE Genotype in Amyloid Positive MCI and AD Patients
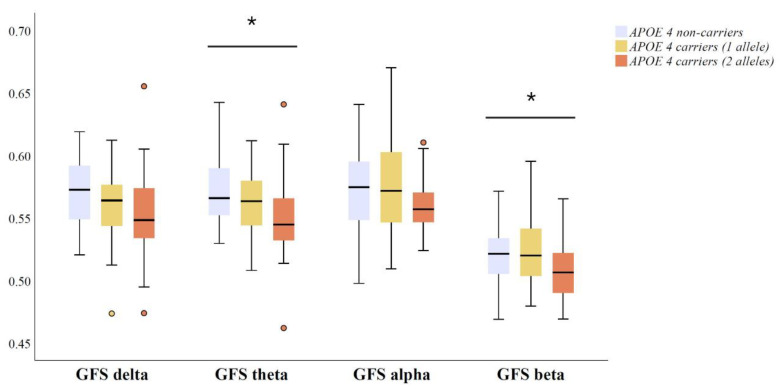
GFS medians and interquartile ranges in four conventional frequency bands across APOE genotype groups are presented in Figure 2. Amyloid positive APOE ε4 non-carriers, heterozygous and homozygous carriers differed in GFS measure in theta (p = 0.041) and beta bands (p = 0.036). That is, APOE ε4 carriers exhibited lower EEG synchronization in theta and beta bands compared to non-carriers (Figure 2). Even though a trend was observed, the differences in GFS in delta (p = 0.090) and alpha (p = 0.079) band did not reach statistical significance (Figure 2).
Figure 2.
Differences in qEEG measures of global field synchronization (GFS) between APOE ε4 carriers with two alleles (n = 28), carriers with one allele (n = 42) and non-carriers (n = 31), including CSF Aβ42 positive (<550 ng/L) MCI and AD patients. The original data on GFS are presented as the median (solid line), interquartile range (box), and minimum and maximum values (whiskers) across four conventional frequency bands. p-Values are based on ANOVA over the three genotype groups; * p < 0.05. Outlier and extreme values are denoted as circles and stars, respectively. Abbreviations: ANOVA = analysis of variance; and APOE = apolipoprotein E.
4. Discussion
The main finding of the present study was that a decrease in qEEG measures of global brain synchronization in theta and beta bands is associated with the presence of an APOE ε4 genotype in MCI and AD patients with amyloid biomarker changes indicative of AD pathology. Additionally, amyloid positive APOE ε4 carriers exhibited an increase in global EEG power in beta band compared to non-carriers. Our study, therefore, demonstrated an association between APOE ε4 genotypes and intrinsic brain activity and connectivity, as assessed by qEEG analyses, in patients with cognitive dysfunction that are on a biomarker-verified AD continuum.
Decreased global EEG synchronization in theta and beta bands may reflect more severe brain functional impairment in patients that are on the AD continuum and carry the APOE ε4 allele compared to non-carriers. Reduced EEG synchronization in fast frequencies, including alpha and beta bands, has been supported by numerous studies involving MCI and AD patients and is consistent across various qEEG measures of brain functional connectivity [29,43,52,53,54]. The level of decrease in alpha and beta synchronization has been additionally associated with the disease severity [29,34,35], performance on neuropsychological tests [31], and AD biomarker changes in CSF, including a decrease in Aβ42 and increase in p-tau and t-tau levels [38]. Importantly, beta activity has been related to a number of cognitive processes including working and episodic memory [55,56,57], language processing [58], visual perception [59], decision making [60], and attentional processes [61,62]. Therefore, alterations in the idle, “resting-state” beta activity and synchronization may be associated with inadequate engagement of beta rhythms during various cognitive tasks and, consequently, with impairment of multiple cognitive domains that is characteristic of AD dementia.
We further report that Aβ positive APOE ε4 carriers exhibit lower GFS in the theta band compared to APOE ε4 non-carriers. There is an overlap between the brain areas that are typically affected in amnestic syndromes and brain regions that are thought to generate theta rhythm including hippocampus and entorhinal cortex [19]. In this context, decreased global theta synchronization in amyloid positive APOE ε4 carriers may reflect a limbic predominant pathology associated with clinical presentation of typical AD. A recent meta-analysis that addressed heterogeneity of biologically subtypes of AD reported several characteristics of limbic-predominant AD, including amnestic syndrome, late-onset sporadic presentation, and the presence of the APOE ε4 genotype [63].
Several previous studies have assessed the relationship between APOE status and changes in EEG power across conventional frequency bands. Most of them reported a pattern of qEEG changes that are characteristic for “EEG slowing”, including increase in delta and theta and decrease in alpha and beta power and/or amplitude in MCI and AD patients that were APOE ε4 carriers compared to non-carriers [40,41,64,65]. One of the studies reported no changes in EEG power with respect to the APOE ε4 status; however, it included a single measure of EEG power ratio and a modest number of study participants [43].
In contrast, a more recent large-scale study by de Waal and colleagues included 320 AD patients and 246 healthy controls and demonstrated higher EEG delta and theta and lower alpha power in AD patients that were APOE ε4 non-carriers compared to carriers, indicating more pronounced EEG slowing in APOE ε4 non-carriers [42]. Our results may provide some insights on these contradictory reports. We reported an increase in beta power (and a non-significant trend towards an increase in alpha and delta power) as the only significant change in global EEG power measures in amyloid positive APOE ε4 heterozygous and homozygous carriers compared to non-carriers, partly supporting recent reports from de Waal and colleagues that demonstrated less severe EEG slowing in APOE ε4 carriers. Increases in EEG beta power may appear counterintuitive in the context of qEEG changes in AD; however, these results stem from MCI and AD patient groups that have not been contrasted to healthy controls, i.e., the comparison was made to APOE ε4 negative and amyloid positive MCI and AD patients. In that regard, increased EEG beta power may reflect a mechanism of brain functional compensation in amyloid positive APOE ε4 carriers that are at increased risk for future cognitive deterioration [8,66]. Overall, our results suggest that the main drawback of previous studies is lack of biological and pathological characterization of the study population.
The increases in delta and theta power in previous studies may reflect a non-specific qEEG alterations in cognitively impaired APOE ε4 carriers, driven by a number of subjects without underlying AD pathology. As it is known from the literature and clinical practice, a number of MCI subjects will progress to other types of dementias, including dementia with Lewy bodies (DLB), Parkinson disease dementia (PDD), and vascular dementia or remain cognitively stable over time [67,68], while a substantial proportion of clinically diagnosed AD patients may have underlying vascular, mixed, or tau-related pathologies [69,70,71]. Interestingly, APOE ε4 has also been associated with increased risk for Lewy Body disease, including DLB and PDD [72], as well as with vascular dementia [73].
It would be of interest to further investigate these associations in cognitively healthy APOE ε4 carriers with and without evidence of AD pathology. These studies would elucidate whether APOE ε4 carriers exhibit disturbances in brain functional connectivity even before the clinical appearance of symptoms and amyloid pathology as reported by some of the functional MRI studies [14,15]. A limitation of the present study is that a rather conservative and binary cut-off for CSF amyloid positivity was used for the stratification of both MCI and AD patients. These biomarker cut-offs were initially derived from AD patient cohorts in order to clinically support a diagnosis of AD dementia, and a less stringent cut-off may be required for mild cognitive disorders [47,74].
Modification of brain activity and functional connectivity by the APOE genotype could further aid selection of qEEG parameters that may contribute to the prediction of AD or even different clinico-biological AD subtypes. In the quest for such sensitive biomarkers, qEEG analysis could be extended to sleep EEG and different provocation methods during standard EEG recordings, such as hyperventilation and photostimulation. Interestingly, Ponomareva and colleagues reported that cognitively healthy relatives of AD patients that were APOE ε4 carriers had higher occurrence of synchronous delta and theta as well as sharp-waves during hyperventilation condition compared to relatives that were APOE ε4 non-carriers [65]. This study indicated that an EEG activation paradigm may be required to accentuate early brain functional impairment in cognitively healthy patients who are at a genetically increased risk for dementia.
In conclusion, our study demonstrated that decreased EEG global synchronization in theta and beta bands reflect brain functional deficits related to the APOE ε4 genotype in patients with a cognitive dysfunction and biomarker-verified AD pathology. Future studies on novel qEEG approaches and both established and new AD risk genes are required to demonstrate whether qEEG parameters could serve as potential endophenotypes for cognitive disorders due to AD.
Acknowledgments
We thank Lars Hyllienmark and Atif Sepic from Department of Clinical Neurophysiology at Karolinska University Hospital for their kind help and support during data collection and Bengt Winblad for all the scientific discussions and kind support of our research studies.
Author Contributions
Conceptualization, U.S. and V.J.; methodology, U.S., I.K., T.K. and V.J.; software, U.S., T.K. and I.K.; formal analysis, U.S. and V.J.; investigation, U.S., V.J., C.J. and C.G.; resources, V.J. and U.S.; writing—original draft preparation, U.S.; writing—review and editing, V.J. and I.K., T.K., C.J. and C.G.; visualization, U.S.; supervision, V.J. and C.G.; funding acquisition, U.S. and V.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Gun and Bertil Stohne’s Research Scholarship (US), Gun and Bertil Stohne’s Research Grant (US), Gamla Tjänarinnor grant (US, VJ), and Swedish State Support for Clinical Research (#ALF-591660) (VJ).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Regional Ethical Review Board in Stockholm, Sweden (Dnr: 2020-00678, 2011/1978-31/4).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during this study are available from the study’s senior and corresponding authors on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 3.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K., DeKosky S.T., Gauthier S., Selkoe D., Bateman R., et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 4.Lourida I., Hannon E., Littlejohns T.J., Langa K.M., Hyppönen E., Kuzma E., Llewellyn D.J. Association of Lifestyle and Genetic Risk With Incidence of Dementia. JAMA. 2019;322:430–437. doi: 10.1001/jama.2019.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant W.B., Campbell A., Itzhaki R.F., Savory J. The significance of environmental factors in the etiology of Alzheimer’s disease. J. Alzheimers Dis. 2002;4:179–189. doi: 10.3233/JAD-2002-4308. [DOI] [PubMed] [Google Scholar]
- 6.Karch C.M., Goate A.M. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R., Myers R.H., Pericak-Vance M.A., Risch N., van Duijn C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. doi: 10.1001/jama.1997.03550160069041. [DOI] [PubMed] [Google Scholar]
- 8.Liu C.C., Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J., Basak J.M., Holtzman D.M. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiman E.M., Chen K., Liu X., Bandy D., Yu M., Lee W., Ayutyanont N., Keppler J., Reeder S.A., Langbaum J.B., et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellano J.M., Kim J., Stewart F.R., Jiang H., DeMattos R.B., Patterson B.W., Fagan A.M., Morris J.C., Mawuenyega K.G., Cruchaga C., et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci. Transl. Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brecht W.J., Harris F.M., Chang S., Tesseur I., Yu G.Q., Xu Q., Dee Fish J., Wyss-Coray T., Buttini M., Mucke L., et al. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J. Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch J.R., Morgan D., Mance J., Matthew W.D., Laskowitz D.T. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J. Neuroimmunol. 2001;114:107–113. doi: 10.1016/S0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- 14.Sheline Y.I., Morris J.C., Snyder A.Z., Price J.L., Yan Z., D’Angelo G., Liu C., Dixit S., Benzinger T., Fagan A., et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J. Neurosci. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., Smith S.M., Matthews P.M., Beckmann C.F., Mackay C.E. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl. Acad. Sci. USA. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stassen H.H., Lykken D.T., Propping P., Bomben G. Genetic determination of the human EEG. Hum. Genet. 1988;80:165–176. doi: 10.1007/BF00702862. [DOI] [PubMed] [Google Scholar]
- 17.Lykken D.T., Tellegen A., Thorkelson K. Genetic determination of EEG frequency spectra. Biol. Psychol. 1974;1:245–259. doi: 10.1016/0301-0511(74)90001-5. [DOI] [PubMed] [Google Scholar]
- 18.Smit D.J., Posthuma D., Boomsma D.I., Geus E.J. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42:691–697. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 19.Schomer D.L., Lopes da Silva F. Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Wolters Kluwer Health; Philadelphia, PA, USA: 2015. [Google Scholar]
- 20.Smailovic U., Jelic V. Neurophysiological Markers of Alzheimer’s Disease: Quantitative EEG Approach. Neurol. Ther. 2019;8:37–55. doi: 10.1007/s40120-019-00169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Beijsterveldt C.E., Molenaar P.C., de Geus E.J., Boomsma D.I. Heritability of human brain functioning as assessed by electroencephalography. Am. J. Hum. Genet. 1996;58:562–573. [PMC free article] [PubMed] [Google Scholar]
- 22.van Beijsterveldt C.E., van Baal G.C. Twin and family studies of the human electroencephalogram: A review and a meta-analysis. Biol. Psychol. 2002;61:111–138. doi: 10.1016/S0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 23.Selkoe D.J. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 24.Koffie R.M., Hyman B.T., Spires-Jones T.L. Alzheimer’s disease: Synapses gone cold. Mol. Neurodegener. 2011;6:63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michel C.M. Electrical Neuroimaging. Cambridge University Press; Cambridge, UK: 2009. 238p [Google Scholar]
- 26.Dierks T., Ihl R., Frolich L., Maurer K. Dementia of the Alzheimer type: Effects on the spontaneous EEG described by dipole sources. Psychiatry Res. 1993;50:151–162. doi: 10.1016/0925-4927(93)90027-F. [DOI] [PubMed] [Google Scholar]
- 27.Jelic V., Johansson S.E., Almkvist O., Shigeta M., Julin P., Nordberg A., Winblad B., Wahlund L.O. Quantitative electroencephalography in mild cognitive impairment: Longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol. Aging. 2000;21:533–540. doi: 10.1016/S0197-4580(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 28.Huang C., Wahlund L., Dierks T., Julin P., Winblad B., Jelic V. Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: A cross-sectional and longitudinal study. Clin. Neurophysiol. 2000;111:1961–1967. doi: 10.1016/S1388-2457(00)00454-5. [DOI] [PubMed] [Google Scholar]
- 29.Koenig T., Prichep L., Dierks T., Hubl D., Wahlund L.O., John E.R., Jelic V. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging. 2005;26:165–171. doi: 10.1016/j.neurobiolaging.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Jelic V., Shigeta M., Julin P., Almkvist O., Winblad B., Wahlund L.O. Quantitative electroencephalography power and coherence in Alzheimer’s disease and mild cognitive impairment. Dementia. 1996;7:314–323. doi: 10.1159/000106897. [DOI] [PubMed] [Google Scholar]
- 31.Adler G., Brassen S., Jajcevic A. EEG coherence in Alzheimer’s dementia. J. Neural Transm. 2003;110:1051–1058. doi: 10.1007/s00702-003-0024-8. [DOI] [PubMed] [Google Scholar]
- 32.Pozzi D., Petracchi M., Sabe L., Golimstock A., Garcia H., Starkstein S. Quantified electroencephalographic correlates of neuropsychological deficits in Alzheimer’s disease. J. Neuropsychiatry Clin. Neurosci. 1995;7:61–67. doi: 10.1176/jnp.7.1.61. [DOI] [PubMed] [Google Scholar]
- 33.Kim J.S., Lee S.H., Park G., Kim S., Bae S.M., Kim D.W., Im C.H. Clinical implications of quantitative electroencephalography and current source density in patients with Alzheimer’s disease. Brain Topogr. 2012;25:461–474. doi: 10.1007/s10548-012-0234-1. [DOI] [PubMed] [Google Scholar]
- 34.Park Y.-M., Che H.-J., Im C.-H., Jung H.-T., Bae S.-M., Lee S.-H. Decreased EEG synchronization and its correlation with symptom severity in Alzheimer’s disease. Neurosci. Res. 2008;62:112–117. doi: 10.1016/j.neures.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Ma C.C., Liu A.J., Liu A.H., Zhou X.Y., Zhou S.N. Electroencephalogram global field synchronization analysis: A new method for assessing the progress of cognitive decline in Alzheimer’s disease. Clin. EEG Neurosci. 2014;45:98–103. doi: 10.1177/1550059413489669. [DOI] [PubMed] [Google Scholar]
- 36.Smailovic U., Kåreholt I., Koenig T., Ashton N.J., Winblad B., Höglund K., Nilsson P., Zetterberg H., Blennow K., Jelic V. Synaptic molecular and neurophysiological markers are independent predictors of progression in Alzheimer’s disease. J. Alzheimers Dis. 2021;83:355–366. doi: 10.3233/JAD-201234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luckhaus C., Grass-Kapanke B., Blaeser I., Ihl R., Supprian T., Winterer G., Zielasek J., Brinkmeyer J. Quantitative EEG in progressing vs stable mild cognitive impairment (MCI): Results of a 1-year follow-up study. Int. J. Geriatr. Psychiatry. 2008;23:1148–1155. doi: 10.1002/gps.2042. [DOI] [PubMed] [Google Scholar]
- 38.Smailovic U., Koenig T., Kareholt I., Andersson T., Kramberger M.G., Winblad B., Jelic V. Quantitative EEG power and synchronization correlate with Alzheimer’s disease CSF biomarkers. Neurobiol. Aging. 2018;63:88–95. doi: 10.1016/j.neurobiolaging.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Stomrud E., Hansson O., Minthon L., Blennow K., Rosen I., Londos E. Slowing of EEG correlates with CSF biomarkers and reduced cognitive speed in elderly with normal cognition over 4 years. Neurobiol. Aging. 2010;31:215–223. doi: 10.1016/j.neurobiolaging.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 40.Lehtovirta M., Partanen J., Könönen M., Hiltunen J., Helisalmi S., Hartikainen P., Riekkinen Sr P., Soininen H. A Longitudinal Quantitative EEG Study of Alzheimer’s Disease: Relation to Apolipoprotein E Polymorphism. Dement. Geriatr. Cogn. Disord. 2000;11:29–35. doi: 10.1159/000017210. [DOI] [PubMed] [Google Scholar]
- 41.Babiloni C., Benussi L., Binetti G., Cassetta E., Dal Forno G., Del Percio C., Ferreri F., Ferri R., Frisoni G., Ghidoni R., et al. Apolipoprotein E and alpha brain rhythms in mild cognitive impairment: A multicentric Electroencephalogram study. Ann. Neurol. 2006;59:323–334. doi: 10.1002/ana.20724. [DOI] [PubMed] [Google Scholar]
- 42.de Waal H., Stam C.J., de Haan W., van Straaten E.C., Blankenstein M.A., Scheltens P., van der Flier W.M. Alzheimer’s disease patients not carrying the apolipoprotein E epsilon4 allele show more severe slowing of oscillatory brain activity. Neurobiol. Aging. 2013;34:2158–2163. doi: 10.1016/j.neurobiolaging.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Jelic V., Julin P., Shigeta M., Nordberg A., Lannfelt L., Winblad B., Wahlund L.O. Apolipoprotein E epsilon4 allele decreases functional connectivity in Alzheimer’s disease as measured by EEG coherence. J. Neurol. Neurosurg. Psychiatry. 1997;63:59–65. doi: 10.1136/jnnp.63.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.O., Nordberg A., Bäckman L., Albert M., Almkvist O., et al. Mild cognitive impairment—beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization . The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. WHO; Geneva, Switzerland: 1992. [Google Scholar]
- 46.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 47.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsson A., Vanderstichele H., Andreasen N., De Meyer G., Wallin A., Holmberg B., Rosengren L., Vanmechelen E., Blennow K. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin. Chem. 2005;51:336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- 49.Motter R., Vigo-Pelfrey C., Kholodenko D., Barbour R., Johnson-Wood K., Galasko D., Chang L., Miller B., Clark C., Green R., et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann. Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- 50.Lehmann D., Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr. Clin. Neurophysiol. 1980;48:609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- 51.Koenig T., Lehmann D., Saito N., Kuginuki T., Kinoshita T., Koukkou M. Decreased functional connectivity of EEG theta-frequency activity in first-episode, neuroleptic-naive patients with schizophrenia: Preliminary results. Schizophr. Res. 2001;50:55–60. doi: 10.1016/S0920-9964(00)00154-7. [DOI] [PubMed] [Google Scholar]
- 52.Fonseca L.C., Tedrus G.M., Prandi L.R., Andrade A.C. Quantitative electroencephalography power and coherence measurements in the diagnosis of mild and moderate Alzheimer’s disease. Arquivos de Neuro-Psiquiatria. 2011;69:297–303. doi: 10.1590/S0004-282X2011000300006. [DOI] [PubMed] [Google Scholar]
- 53.Stam C.J., Van Der Made Y., Pijnenburg Y.A.L., Scheltens P. EEG synchronization in mild cognitive impairment and Alzheimer’s disease. Acta Neurologica Scandinavica. 2003;108:90–96. doi: 10.1034/j.1600-0404.2003.02067.x. [DOI] [PubMed] [Google Scholar]
- 54.Stam C.J., Nolte G., Daffertshofer A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007;28:1178–1193. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Axmacher N., Schmitz D.P., Wagner T., Elger C.E., Fell J. Interactions between medial temporal lobe, prefrontal cortex, and inferior temporal regions during visual working memory: A combined intracranial EEG and functional magnetic resonance imaging study. J. Neurosci. 2008;28:7304–7312. doi: 10.1523/JNEUROSCI.1778-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanslmayr S., Spitzer B., Bäuml K.H. Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb. Cortex. 2009;19:1631–1640. doi: 10.1093/cercor/bhn197. [DOI] [PubMed] [Google Scholar]
- 57.Hanslmayr S., Staresina B.P., Bowman H. Oscillations and Episodic Memory: Addressing the Synchronization/Desynchronization Conundrum. Trends Neurosci. 2016;39:16–25. doi: 10.1016/j.tins.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss S., Mueller H.M. “Too Many betas do not Spoil the Broth”: The Role of Beta Brain Oscillations in Language Processing. Front. Psychol. 2012;3:201. doi: 10.3389/fpsyg.2012.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piantoni G., Kline K.A., Eagleman D.M. Beta oscillations correlate with the probability of perceiving rivalrous visual stimuli. J. Vis. 2010;10:18. doi: 10.1167/10.13.18. [DOI] [PubMed] [Google Scholar]
- 60.Wimmer K., Ramon M., Pasternak T., Compte A. Transitions between Multiband Oscillatory Patterns Characterize Memory-Guided Perceptual Decisions in Prefrontal Circuits. J. Neurosci. 2016;36:489–505. doi: 10.1523/JNEUROSCI.3678-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamiński J., Brzezicka A., Gola M., Wróbel A. β band oscillations engagement in human alertness process. Int. J. Psychophysiol. 2012;85:125–128. doi: 10.1016/j.ijpsycho.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Gola M., Kamiński J., Brzezicka A., Wróbel A. β band oscillations as a correlate of alertness--changes in aging. Int. J. Psychophysiol. 2012;85:62–67. doi: 10.1016/j.ijpsycho.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Ferreira D., Nordberg A., Westman E. Biological subtypes of Alzheimer disease: A systematic review and meta-analysis. Neurology. 2020;94:436–448. doi: 10.1212/WNL.0000000000009058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lehtovirta M., Partanen J., Könönen M., Soininen H., Helisalmi S., Mannermaa A., Ryynänen M., Hartikainen P., Riekkinen P. Spectral analysis of EEG in Alzheimer’s disease: Relation to apolipoprotein E polymorphism. Neurobiol. Aging. 1996;17:523–526. doi: 10.1016/0197-4580(96)00024-3. [DOI] [PubMed] [Google Scholar]
- 65.Ponomareva N.V., Korovaitseva G.I., Rogaev E.I. EEG alterations in non-demented individuals related to apolipoprotein E genotype and to risk of Alzheimer disease. Neurobiol. Aging. 2008;29:819–827. doi: 10.1016/j.neurobiolaging.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 66.Olsson B., Lautner R., Andreasson U., Öhrfelt A., Portelius E., Bjerke M., Hölttä M., Rosén C., Olsson C., Strobel G., et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 67.Mitchell A.J., Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—Meta-analysis of 41 robust inception cohort studies. Acta Psychiatr. Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 68.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 69.DeTure M.A., Dickson D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019;14:32. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crary J.F. Primary age-related tauopathy and the amyloid cascade hypothesis: The exception that proves the rule? J. Neurol. Neuromedicine. 2016;1:53–57. doi: 10.29245/2572.942X/2016/6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jack C.R., Jr. PART and SNAP. Acta Neuropathol. 2014;128:773–776. doi: 10.1007/s00401-014-1362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsuang D., Leverenz J.B., Lopez O.L., Hamilton R.L., Bennett D.A., Schneider J.A., Buchman A.S., Larson E.B., Crane P.K., Kaye J.A., et al. APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70:223–228. doi: 10.1001/jamaneurol.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chuang Y.F., Hayden K.M., Norton M.C., Tschanz J., Breitner J.C., Welsh-Bohmer K.A., Zandi P.P. Association between APOE epsilon4 allele and vascular dementia: The Cache County study. Dement. Geriatr. Cogn. Disord. 2010;29:248–253. doi: 10.1159/000285166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Müller E.G., Edwin T.H., Stokke C., Navelsaker S.S., Babovic A., Bogdanovic N., Knapskog A.B., Revheim M.E. Amyloid-β PET-Correlation with cerebrospinal fluid biomarkers and prediction of Alzheimer´s disease diagnosis in a memory clinic. PLoS ONE. 2019;14:e0221365. doi: 10.1371/journal.pone.0221365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during this study are available from the study’s senior and corresponding authors on reasonable request.