Abstract
Simple Summary
Colorectal MANECs are highly aggressive carcinomas defined by a distinct neuroendocrine morphology and positivity for synaptophysin in the neuroendocrine component. It is unclear whether a neuroendocrine differentiation in conventional adenocarcinomas without a suggestive morphology is of clinical relevance. We tested 1002 conventional colorectal carcinomas with a non-neuroendocrine morphology for synaptophysin expression and correlated the results with clinicopathological characteristics as well as patient survival and compared the survival characteristics of synaptophysin expression groups to those of true MANECs. We found no survival differences between synaptophysin expression groups within conventional colorectal adenocarcinomas. MANECs, on the other hand, showed significantly worse survival characteristics. Our data suggest that synaptophysin expression in conventional colorectal adenocarcinomas is of minor prognostic relevance and that conventional adenocarcinomas with a diffuse synaptophysin expression should not be classified as MANECs.
Abstract
Background: Colorectal mixed adenoneuroendocrine carcinomas (MANECs) are clinically highly aggressive neoplasms. MANECs are composed of variable adenocarcinoma components combined with morphologically distinct neuroendocrine carcinoma components, which are confirmed by synaptophysin immunohistochemistry, the gold standard marker of a neuroendocrine differentiation. However, the biological behavior of adenocarcinomas that express synaptophysin but do not show a typical neuroendocrine morphology remains unclear. Methods: We investigated synaptophysin expression in 1002 conventional colorectal adenocarcinomas and correlated the results with clinicopathological characteristics and patient survival and compared the survival characteristics of synaptophysin expression groups to MANECs. Results: Synaptophysin expression in conventional colorectal adenocarcinomas was associated with a shortened disease-free survival (p = 0.037), but not with overall survival or disease-specific survival (DSS) in univariate analyses and without any survival impact in multivariate analyses. Patients with “true” MANECs, on the other hand, showed a significantly shorter survival than all conventional adenocarcinomas with or without synaptophysin expression in uni- and multivariate analyses (e.g., multivariate DSS: p < 0.001, HR: 5.20). Conclusions: Our study demonstrates that synaptophysin expression in conventional colorectal adenocarcinomas, in contrast to MANECs, is not associated with a significantly poorer clinical outcome when compared to adenocarcinomas without synaptophysin expression. Furthermore, our data suggest that conventional adenocarcinomas with a diffuse synaptophysin expression should not be classified as MANECs, also strongly arguing that synaptophysin testing should be reserved for carcinomas with an H&E morphology suggestive of a neuroendocrine differentiation.
Keywords: neuroendocrine differentiation, colorectal adenocarcinomas, MANEC
1. Introduction
Epithelial tumors composed of a neuroendocrine and a non-neuroendocrine component are called mixed neuroendocrine–non-neuroendocrine neoplasms (MiNENs) [1]. In the colon, they usually present as mixed adenoneuroendocrine carcinomas (MANEC), in which the adenocarcinoma component is combined with either a large cell or, rarely, a small cell neuroendocrine carcinoma (NEC). Genetically, colorectal MANECs as well as NECs have been found to be closely related to conventional colorectal adenocarcinomas, as they share crucial driver mutations [2,3]. However, MANECs differ from conventional colorectal carcinomas in their prognosis. In a recent study of a large cohort of more than 1000 colorectal carcinomas, we found that MANEC patients have a significantly worse clinical outcome than all patients with the other colorectal adenocarcinoma subtypes that are listed in the 2019 WHO Classification of Tumors of the Digestive System (WHO) [4]. They behave more like NECs and are therefore often treated like NECs [5,6]. Therefore, MANECs have to be correctly identified to be reliably distinguished from conventional colorectal adenocarcinomas. This is usually easy, since the histological component suggestive of a neuroendocrine differentiation is recognizable in many cases on H&E-stained sections [1]. However, it can be difficult in conventional colorectal adenocarcinomas that only reveal their neuroendocrine differentiation when immunohistochemically stained for synaptophysin, the immunohistochemical gold standard for the detection of neuroendocrine differentiation [7,8], which represents an integral membrane glycoprotein that is found in presynaptic vesicles of neurons as well as normal neuroendocrine epithelial cells (e.g., pancreatic islets) [9]. The neuroendocrine cells are usually found scattered in the mucin producing epithelium of the conventional adenocarcinomas and their numbers do not exceed the 30% threshold level that arbitrarily separates colorectal MANEC from colorectal adenocarcinoma with a neuroendocrine component [10]. However, there are occasional cases in which the number of synaptophysin-expressing cells in the epithelium of the neoplastic glands is so high that it is close to or even exceeds the 30% threshold level. Since such observations raise the question of the prognostic and clinical significance of neuroendocrine differentiation in these otherwise histologically inconspicuous conventional adenocarcinomas, a number of studies have dealt with this problem, but so far produced controversial results. While the extent of neuroendocrine differentiation was prognostically relevant in some studies, other studies were unable to confirm this statement [11,12,13,14,15,16,17,18,19]. Therefore, the prognostic assessment of colorectal adenocarcinomas with a neuroendocrine differentiation that is only demonstrable by immunohistochemistry remains a problem for diagnostic pathologists, especially if the neuroendocrine cell number appears to exceed the 30% cut-off level.
In this study, we investigated the frequency and extent of neuroendocrine differentiation, identified by synaptophysin expression, in a large cohort of 1013 colorectal carcinomas (1002 “conventional” adenocarcinomas with a non-neuroendocrine morphology on H&E sections and 11 colorectal MANECs) and correlated it with clinicopathological features and survival. Specifically, the following questions were addressed: (1) What is the frequency and extent of a neuroendocrine differentiation demonstrated by synaptophysin immunohistochemistry in conventional colorectal adenocarcinomas with a non-neuroendocrine morphology? (2) Are these colorectal carcinomas associated with certain clinicopathological parameters? (3) Are there significant differences in patient survival compared to, on the one hand, conventional adenocarcinomas without an immunohistochemically detectable neuroendocrine differentiation, and on the other hand, to typical colorectal MANECs?
2. Materials and Methods
2.1. Study Population
A total of 1002 colorectal adenocarcinomas and 11 colorectal MANECs from patients who underwent surgical resection between 1997 and 2019 at the University Hospital rechts der Isar of the Technical University of Munich were analyzed. All patients with colorectal carcinomas from this time span with fully available clinicopathological/survival data and with available tumor tissue on the Tissue Micro Array were included in this study. Formalin-fixed paraffin-embedded (FFPE) tumor samples from the tumor center and the invasive margin were assembled into the used tissue microarray (TMA) using a fully automated Tissue Microarrayer (TMA Grandmaster, sysmex, Budapest, Hungary) with a core size of 2 mm. All samples of a respective tumor region were extracted from areas harboring a high burden of invasive carcinoma, which were marked by an experienced pathologist (M.J.). Other tumors of the colorectal system (e.g., neuroendocrine tumors, non-epithelial tumors, etc.) were excluded. One case of an undifferentiated carcinoma from the original cohort was also excluded to avoid statistical bias. The clinicopathological characteristics as well as survival data for all patients were extracted from the Munich Cancer Registry and from hospital records. For overall survival (OS), all recorded patient deaths were noted. For disease-specific survival (DSS), only tumor-associated deaths were recorded as events. For disease-free survival (DFS), loco-regional or distant recurrence was noted as an event. Endpoints of all survival comparisons were either events or a loss of follow-up before 120 months, in which case the patients were censored at the time of the last available entry regarding the specific patient. All patients alive after 120 months were also censored. OS/DSS/DFS times were calculated using the date of the primary surgery as a starting point. The treatment concepts of included patients followed internal policies, which were based on the given German guidelines at the time of diagnosis, generally meaning that all patients were intended to receive stage-adapted treatment. Most of these tumors (1997–2018) were also examined in a recent study on incidence and critical relevance of morphological parameters in colorectal carcinoma subtypes as defined by the 2019 WHO classification of tumors of the digestive system [4].
The microsatellite status (MSI) was determined in the previous study [4], where all carcinomas were classified and subtyped according to the criteria of the 2019 WHO classification of tumors the digestive system, and pathological staging was reassessed using the current TNM classification of malignant tumors [1,20]. The detailed characteristics of the cohort, including age, sex, TNM, UICC-stage, resection-status, MSI-status, WHO grade, localization and tumor type, are depicted in Supplementary Table S1. This study was approved by the local ethics committee of the Technical University of Munich (reference number: 252/16 s).
2.1.1. Histomorphological Characterization
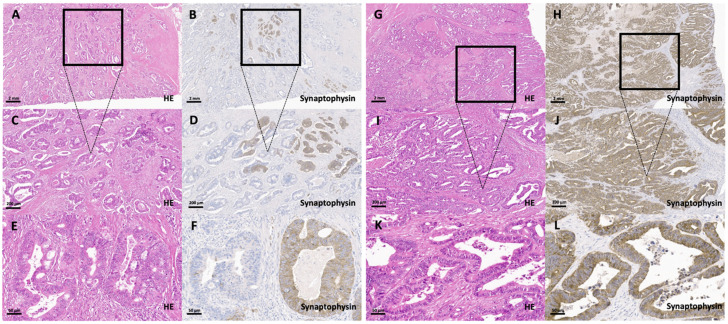
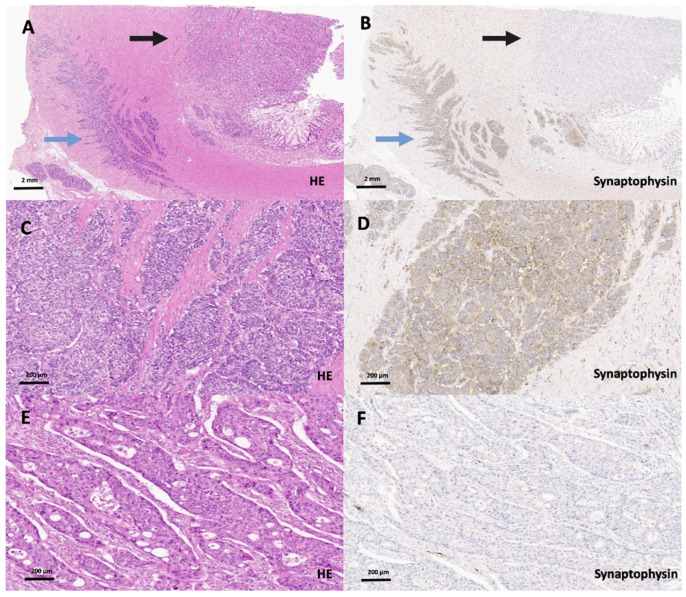
Full block H&E slides from 1013 colorectal carcinomas that were (mostly) part of a previously published collective were rescreened on full block slides at the beginning of this study [4], where the carcinomas were re-classified in accordance with the subtypes listed in the 2019 WHO classification of tumors of the digestive system. Tumors that were not part of the previous cohort but added to the collective were classified as described previously [4]. The final investigated cohort comprised 1002 colorectal adenocarcinomas of various subtypes that showed no morphologic features suggestive of a neuroendocrine carcinoma (Figure 1). Eleven colorectal cancers were diagnosed as MANECs on full block slides as they showed adenocarcinomas that were mixed with a tumor component >30% that was morphologically suggestive of a neuroendocrine carcinoma and that expressed synaptophysin (and Chromogranin A), according to current WHO guidelines (Figure 2). These 11 colorectal MANECs were used as a statistical control group for further analyses.
Figure 1.
Synaptophysin-expressing groups in conventional colorectal adenocarcinomas with a non-neuroendocrine morphology. (A–F) Conventional colorectal adenocarcinoma with a non-neuroendocrine morphology (partial synaptophysin expression group; 10–29%) on H&E (A (2×), C (20×), E (40×)) and synaptophysin staining (B (2×), D (20×), F (40×)) with a group of synaptophysin-positive cells accounting for 15% of the whole tumor. (E–H) Conventional colorectal adenocarcinoma with a non-neuroendocrine morphology with a diffuse synaptophysin expression in all tumor cells on H&E (G (2×), I (20×), K (40×)) and synaptophysin staining (H (2×), J (20×), L (40×)).
Figure 2.
Scanning magnification (A, HE, 2×; B, synaptophysin, 2×) of a true colorectal MANEC (blue arrow: NEC, black arrow: adenocarcinoma component). Higher magnification of the NEC component on H&E (C, 20×) and synaptophysin staining (D, 20×) showing the typical NEC morphology. Higher magnification of the poorly differentiated, synaptophysin-negative adenocarcinoma component (E, HE, 20×; F, synaptophysin, 20×) of this colorectal MANEC that does not show a neuroendocrine histomorphology.
2.1.2. Immunohistochemistry
The TMA was stained with synaptophysin (polyclonal, Ventana medical systems, Tucson, AZ, USA, prediluted). A cytoplasmic synaptophysin expression was considered specific. A scattered expression pattern was defined by us as a discontinuous expression of synaptophysin while a staining of more than 50 continuous cells was stated as block-like (Supplementary Figure S1). Finally, for each core, the synaptophysin-positive cells were counted and the mean percentage of positive cells for both cores was assigned for each tumor.
2.2. Statistics
Statistical analyses were performed using SPSS version 26 (SPSS Institute, Chicago, IL, USA). Associations were calculated with an χ2 test as well as an χ2 test for trends and Fisher’s exact test. The Bonferroni method was used to correct for multiple testing. Survival probabilities were plotted with the Kaplan–Meier method, and a log-rank test was used to probe for the significance of differences in survival. Multivariate survival analyses (including age, gender, UICC stage, synaptophysin expression groups in conventional adenocarcinomas (+typical MANECs), WHO grade) were performed with the Cox proportional hazard model. p-values ≤ 0.05 were considered significant. All statistical tests were performed two-sided.
3. Results
3.1. Clinicopathological Features and Survival
The cohort of 1013 colorectal carcinomas included 1002 colorectal adenocarcinomas without histological features suggestive of neuroendocrine differentiation (“conventional colorectal adenocarcinomas”). Eleven carcinomas had a neuroendocrine carcinoma component that showed a typical neuroendocrine histology (MANECs), characterized by a solid and diffuse growth pattern with necrotic foci and cells containing large vesicular nuclei with nucleoli and easy recognizable mitoses. The median age of patients at diagnosis was 69 years (range: 9–87). There were 581 male patients and 432 female patients. As expected, pTNM/UICC stage, WHO grade and resection status were significantly associated with OS, DSS and DFS (Supplementary Table S1) [4].
3.2. Synaptophysin Expression in Conventional Colorectal Adenocarcinomas without Histological Features Suggestive of a Neuroendocrine Differentiation
The 1002 conventional adenocarcinomas included 763 synaptophysin-negative (76%) and 239 synaptophysin-positive tumors. The expression of synaptophysin (Table 1) ranged from single scattered synaptophysin-positive tumor cells to a strong and diffuse expression in almost all tumor cells. The tumors of the first and largest group (126/239; 53%) had only single positive cells which accounted for less than 1% of the tumor cell population. The second largest group contained adenocarcinomas with a range from >1–9% of scattered synaptophysin-positive tumor cells (72/239; 30%). The third group encompassed 15 carcinomas with synaptophysin-positive cells ranging from 10–29% (15/239; 6%) and the fourth group had 14 carcinomas with a diffuse synaptophysin staining of 30–99% of all tumor cells (14/239; 6%). The fifth and last group summarized 12/239 (5%) adenocarcinomas with diffuse and intense synaptophysin staining of almost all cells of the neoplastic glands. After initial statistical analysis (and under consideration of the used WHO threshold) [10], four subgroups of adenocarcinomas were compiled: synaptophysin-negative adenocarcinomas (763/1002), adenocarcinomas with scattered (<1–9% of the tumor cells; 198/1002), partial (synaptophysin expression in 10–29% of the tumor cells; 15/1002, Figure 1) and diffuse (synaptophysin expression in 30–100% of the tumor cells; 26/1002, Figure 1) synaptophysin expression.
Table 1.
Distribution and statistical associations to clinicopathological characteristics of synaptophysin expression groups in conventional adenocarcinomas with a non-neuroendocrine morphology.
| Overall | Adenocarcinoma Synaptophysin-Negative Cells | Adenocarcinoma 1–9% Synaptophysin-Positive Cells | Adenocarcinoma 10–29% Synaptophysin-Positive Cells | Adenocarcinoma 30–100% Synaptophysin-Positive Cells | p-Value | ||
|---|---|---|---|---|---|---|---|
| 1002 (100%) | 763 (76%) | 198 (20%) | 15 (1%) | 26 (3%) | |||
| Age | 0.46 | ||||||
| below median | 485 (48%) | 367 (48%) | 102 (51%) | 5 (33%) | 11 (42%) | ||
| above median | 517 (52%) | 396 (52%) | 96 (49%) | 10 (67%) | 15 (58%) | ||
| Sex | 0.52 | ||||||
| male | 575 (57%) | 431 (56%) | 118 (60%) | 8 (53%) | 18 (69%) | ||
| female | 427 (43%) | 332 (44%) | 80 (40%) | 7 (47%) | 8 (31%) | ||
| pT | 0.49 | ||||||
| 1 | 73 (7%) | 51(7%) | 21 (11%) | 0 | 1 (7%) | ||
| 2 | 180 (18%) | 136 (18%) | 36 (18%) | 4 (27%) | 4 (18%) | ||
| 3 | 558 (56%) | 429 (56%) | 104 (52%) | 7 (46%) | 18 (69%) | ||
| 4 | 191 (19%) | 147 (19%) | 37 (19%) | 4 (47%) | 3 (19%) | ||
| pN | <0.001 | ||||||
| 0 | 560 (56%) | 426 (56%) | 120 (61%) | 5 (33%) | 9 (35%) | ||
| 1 | 284(28%) | 225 (29%) | 46 (23%) | 7 (47%) | 6 (23%) | ||
| 2 | 158 (16%) | 112 (15%) | 32 (16%) | 3 (20%) | 11 (42%) | ||
| pM | 0.09 | ||||||
| 0 | 859 (86%) | 663 (87%) | 168 (85%) | 11 (73%) | 17 (64%) | ||
| 1 | 143 (14%) | 100 (13%) | 30 (15%) | 4 (27%) | 9 (36%) | ||
| UICC Stage | 0.01 | ||||||
| 1 | 201 (20%) | 144 (19%) | 51 (26%) | 2 (13%) | 4 (15%) | ||
| 2 | 342 (34%) | 269 (35%) | 66 (33%) | 2(13%) | 5 (19%) | ||
| 3 | 310 (31%) | 245 (32%) | 50 (25%) | 7 (47%) | 8 (31%) | ||
| 4 | 149 (15%) | 105 (14%) | 31 (16%) | 4 (27%) | 9 (35%) | ||
| Lymphatic | 0.02 | ||||||
| Invasion | not present | 498 (49%) | 381 (50%) | 106 (54%) | 4 (27%) | 7 (27%) | |
| present | 504 (51%) | 382 (50%) | 92 (46%) | 11 (73%) | 19 (73%) | ||
| Vascular | 0.19 | ||||||
| Invasion | not present | 867 (86.5%) | 663 (87%) | 169 (86%) | 15 (100%) | 20 (77%) | |
| present | 135 (13.5%) | 100 (13%) | 29 (14%) | 0 | 6 (23%) | ||
| Resection | 0.19 | ||||||
| Margin | R0 | 933 (93%) | 709 (93%) | 188 (85%) | 13 (87%) | 23 (88%) | |
| R1 | 41 (4%) | 34 (4%) | 3 (2%) | 2 (13%) | 2 (8%) | ||
| R2 | 28 (3%) | 20 (3%) | 7 (3%) | 0 | 1 (4%) | ||
| Localization | 0.51 | ||||||
| right colon | 488 (49%) | 368 (48%) | 96 (48%) | 10 (67%) | 14 (54%) | ||
| left colon | 514 (51%) | 395 (52%) | 102 (52%) | 5 (33%) | 12 (46%) | ||
| WHO Tumor Type | 0.01 | ||||||
| Adenocarcinoma NOS | 629 (63%) | 480 (61%) | 125 (62%) | 8 (53%) | 16 (61%) | ||
| Mucinous adenocarcinoma | 86 (8%) | 71 (9%) | 13 (6%) | 1 (7%) | 1 (4%) | ||
| Signet-ring cell carcinoma | 9 (1%) | 6 (1%) | 1 (1%) | 0 | 2 (8%) | ||
| Medullary carcinoma | 31 (3%) | 24 (4%) | 6 (3%) | 1 (7%) | 0 | ||
| Micropapillary adenocarcinoma | 128 (13%) | 102 (13%) | 17 (9%) | 3 (20%) | 6 (23%) | ||
| Serrated adenocarcinoma | 88 (9%) | 60 (8%) | 25 (13%) | 2 (13%) | 1 (4%) | ||
| Adenoma-like adenocarcinoma | 31 (3%) | 20 (4%) | 11 (6%) | 0 | 0 | ||
| Microsatellite | 0.19 | ||||||
| Status | microsatellite stable | 846 (84%) | 634 (83%) | 175 (88%) | 13 (87%) | 24 (92%) | |
| microsatellite instable | 156 (16%) | 129 (17%) | 23 (12%) | 2 (13%) | 2 (8%) | ||
| WHO Grade | 0.19 | ||||||
| low-grade | 687 (68%) | 520 (68%) | 144 (73%) | 8 (53%) | 15 (69%) | ||
| high-grade | 315 (32%) | 243 (32%) | 54 (27%) | 7 (47%) | 11 (31%) | ||
3.3. Correlation of Synaptophysin Expression in Conventional Colorectal Adenocarcinomas with Clinicopathological Data
Scattered synaptophysin-expressing cells were seen in all subtypes. The diffuse expression pattern was weakly associated with adenocarcinoma subtypes (p = 0.05). Synaptophysin positivity was significantly associated with lymph node metastases (p < 0.001), UICC stage (p = 0.01) and lymphatic invasion (p = 0.02) but not with age, sex, T stage, vascular invasion, perineural invasion, resection margin, microsatellite status, localization or WHO grade (Table 1).
3.4. Correlation of Synaptophysin Expression in Conventional Colorectal Adenocarcinomas with Survival Parameters
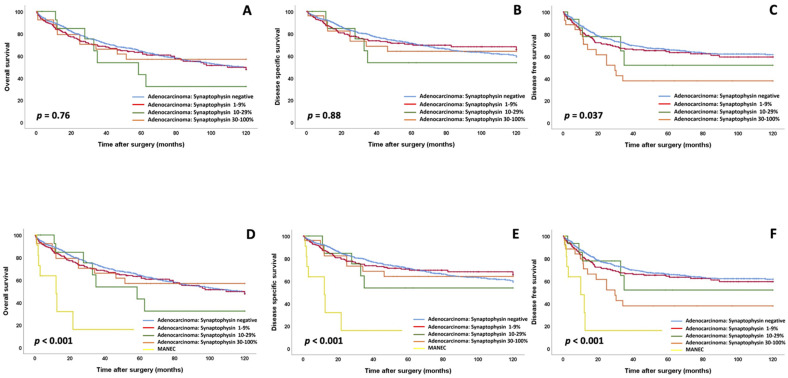
In univariate analysis of the 1002 conventional colorectal adenocarcinomas, synaptophysin-expressing groups showed an association with DFS (p = 0.037), but not with OS or DSS (Figure 3, Supplementary Table S2). There was no impact on all survival parameters in specific stage groups (UICC stage I and II versus UICC stage III and IV) or clinicopathological subcohorts (low vs. high WHO grade, T stage groups, N stage groups, data not shown). In multivariate analysis (including age, sex, UICC stage and WHO grade), only UICC stage and WHO grade, but not synaptophysin expression, impacted OS, DSS and DFS (DFS: p = 0.49, Table 2; DSS: p = 0.23, OS: p = 0.16).
Figure 3.
(A–C) Overall survival, disease-specific survival and disease-free survival for synaptophysin expression groups in conventional adenocarcinomas with a non-neuroendocrine morphology (A–C); note that there are no survival differences between synaptophysin expression groups in conventional adenocarcinomas and only a slightly reduced disease-free survival for conventional adenocarcinomas with a synaptophysin expression of 30% or more. (D–F): Overall survival, disease-specific survival and disease-free survival for synaptophysin expression groups in conventional adenocarcinomas compared to typical MANECs (D–F); please note that while the conventional adenocarcinomas show similar survival characteristics, the MANECs show significantly unfavorable survival characteristics.
Table 2.
Multivariate analysis for disease-free survival including synaptophysin expression groups in conventional adenocarcinomas with a non-neuroendocrine morphology, UICC stage, WHO grade, age and sex.
| HR (DFS) | Lower CI (95%) | Upper CI (95%) | p-Value | ||
|---|---|---|---|---|---|
| Conventional Adenocarcinoma Synaptophysin Subgroups | 0.49 | ||||
| Conventional adenocarcinoma synaptophysin negative | 1.0 | ||||
| Conventional adenocarcinoma 1–9% synaptophysin positive | 1.2 | 0.92 | 1.60 | ||
| Conventional adenocarcinoma 10–29% synaptophysin positive | 0.8 | 0.34 | 1.97 | ||
| Conventional adenocarcinoma 30–100% synaptophysin positive | 1.1 | 0.64 | 1.94 | ||
| WHO grade | 0.01 | ||||
| Low grade | 1.00 | ||||
| High grade | 1.34 | 1.07 | 1.69 | ||
| UICC Stage | I | 1.00 | <0.001 | ||
| II | 2.16 | 1.30 | 3.50 | ||
| III | 3.94 | 2.48 | 6.25 | ||
| IV | 11.87 | 7.40 | 19.05 | ||
| Age group | 0.72 | ||||
| Below median | 1.00 | ||||
| Median and above | 1.05 | 0.84 | 1.32 | ||
| Sex | male | 1.0 | 0.48 | ||
| female | 1.08 | 0.86 | 1.35 |
3.5. Survival of True Colorectal MANECs Compared to Colorectal Adenocarcinomas without Histological Features Suggestive of a Neuroendocrine Differentiation
The true MANECs showed a significantly worse clinical outcome (OS: p < 0.001; DSS: p < 0.001; DFS: p < 0.001; Table 3, Supplementary Table S3) compared to the group of diffuse synaptophysin-expressing conventional adenocarcinomas (e.g., mean DFS: 16.98 months vs. 77.81 months) in univariate analyses. These results were confirmed for all parameters by multivariate analyses including WHO grade, UICC stage, age and sex (e.g., DFS: p = 0.001, HR: 3.87, Table 3; DSS: p < 0.001, HR: 5.20, Supplementary Table S4; OS: p < 0.001, HR: 4.16).
Table 3.
Multivariate analysis for disease-free survival including synaptophysin expression groups in conventional adenocarcinomas with a non-neuroendocrine morphology compared to MANECs and UICC stage, WHO grade, age and sex.
| HR (DFS) | Lower CI (95%) | Upper CI (95%) | p-Value | ||
|---|---|---|---|---|---|
| Conventional Adenocarcinoma Synaptophysin Subgroups | 0.001 | ||||
| versus MANEC/NEC | |||||
| Conventional Adenocarcinoma synaptophysin negative | 1.0 | ||||
| Conventional Adenocarcinoma 1–9% synaptophysin positive | 1.20 | 0.91 | 1.59 | ||
| Conventional Adenocarcinoma 10–29% synaptophysin positive | 0.83 | 0.34 | 2.01 | ||
| Conventional Adenocarcinoma 30–100% synaptophysin positive | 1.12 | 0.65 | 1.96 | ||
| MANEC/NEC | 3.87 | 1.79 | 8.37 | ||
| WHO grade | 0.011 | ||||
| Low grade | 1.00 | ||||
| High grade | 1.34 | 1.07 | 1.68 | ||
| UICC Stage | I | 1.00 | <0.001 | ||
| II | 2.10 | 1.31 | 3.44 | ||
| III | 4.17 | 2.52 | 6.33 | ||
| IV | 12.16 | 7.39 | 19.02 | ||
| Age group | 0.61 | ||||
| Below median | 1.00 | ||||
| Median and above | 1.05 | 0.86 | 1.36 | ||
| Sex | male | 1.0 | 0.37 | ||
| female | 1.14 | 0.83 | 1.31 |
4. Discussion
In a previous study [4], we demonstrated that colorectal MANECs with a histologically recognizable neuroendocrine carcinoma component confirmed by synaptophysin positivity are associated with a significantly poorer prognosis when compared to conventional adenocarcinomas NOS and other adenocarcinoma subtypes [4]. However, this study did not examine the clinical relevance of the expression of synaptophysin, the gold standard marker for the immunohistochemical confirmation of a neuroendocrine differentiation [21], in conventional colorectal adenocarcinomas without a histological pattern suggestive of neuroendocrine differentiation. In the current study, we screened 1002 conventional adenocarcinomas for synaptophysin expression and found that approximately a quarter of these tumors harbored synaptophysin-positive cells, albeit mostly as scattered tumor cells embedded in the epithelium of the neoplastic glands. A significant synaptophysin expression in at least 10% of the tumor cell population was only found in 4% of all cases, with more than half of them with an expression of at least 30% of the tumor cells, thereby reaching the immunohistochemical WHO threshold level qualifying a colorectal carcinoma for a MANEC [10].
The most important result of this study was that none of the synaptophysin-expressing groups of conventional colorectal adenocarcinomas (adenocarcinoma NOS and specific WHO subtypes) showed significantly different overall survival or disease-specific survival parameters compared to non-synaptophysin-expressing conventional colorectal carcinomas. In conventional adenocarcinomas with a synaptophysin expression of more than 30% of the tumor cell population, a slightly poorer disease-free survival was noted in univariate analysis, but this result was not confirmed by multivariate analysis including UICC stage, WHO grade, age and gender. Our data thus suggest that synaptophysin expression in conventional colorectal adenocarcinomas without any component suggestive of a neuroendocrine differentiation in H&E-stained sections is of minor prognostic relevance, at best.
In the next step, we compared the survival data of synaptophysin-expressing conventional adenocarcinomas with those of true colorectal MANECs. In uni- and multivariate analyses (including age, sex, UICC stage, WHO grade), we observed that the MANECs had a significantly shorter overall survival, disease-specific survival and disease-free survival than all synaptophysin-expressing adenocarcinomas, including conventional adenocarcinomas with diffuse synaptophysin expression in more than 30% of the cells of the neoplastic glands. These data suggest that the clinical relevance of synaptophysin expression in colorectal adenocarcinomas is strongly related to a histologically recognizable neuroendocrine component, usually with the features of a large cell neuroendocrine carcinoma. The composition of the exocrine and the neuroendocrine component to each other may differ from case to case but can morphologically be traced back to a collision, combined or amphicrine type in most cases [2,3].
Many studies investigated the prognostic impact of neuroendocrine differentiation in gastrointestinal carcinomas [12,14,17,18,19,22,23,24], and all studies showed that the expression of neuroendocrine markers such as synaptophysin is linked to a poor prognosis when the tumor has a histological pattern suggestive of neuroendocrine differentiation in H&E-stained sections. However, conflicting results were produced by studies that defined a neuroendocrine differentiation solely by immunohistochemistry regardless of the carcinoma morphology, either reporting poor prognosis [13], association with distant metastasis [14] or not showing any prognostic impact at all [17,18].
The correct recognition of MANECs is not only important for the assessment of the clinical course, but also for the therapeutic strategy that derives from this assessment, as the presence of a poorly differentiated neuroendocrine component usually qualifies these patients for specific chemotherapy regimens (often a combination of platinum derivatives and topoisomerase inhibitors such as Cisplatin and Etoposid) [5,6,25]. Nevertheless, our study has some limitations: this is a retrospective analysis, and the results of this paper should be validated in a prospective fashion. Furthermore, based on our data, we are not able to make further conclusions about the molecular underpinnings and possible differences in treatment response of synaptophysin-expressing conventional colorectal adenocarcinomas without a morphologically recognizable neuroendocrine component. Further studies including clinical trials are needed to address this issue.
5. Conclusions
In conclusion, we demonstrated that synaptophysin expression in conventional colorectal adenocarcinomas, in contrast to colorectal MANECs, is not associated with a significantly poorer clinical outcome when compared to conventional adenocarcinomas without synaptophysin expression. Therefore, our data strongly suggest that synaptophysin testing should be restricted to carcinomas whose morphology on H&E-stained sections indicates a neuroendocrine differentiation. It also means that conventional adenocarcinomas, in which the cells of the neoplastic glands diffusely express synaptophysin and exceed in number the 30% threshold level, should not be classified as MANECs.
Acknowledgments
We thank the Comparative Experimental Pathology Unit of the Institute of Pathology and especially Marion Mielke and Olga Seelbach for excellent technical assistance. Furthermore, we thank Simone Köppel and Christina Schott from the Biobank of the Klinikum rechts der Isar (MTBio) for their excellent support.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13205111/s1. Supplementary Table S1: Clinicopathological characteristics of the overall cohort including conventional adenocarcinomas and MANECs with respect to survival parameters (overall, disease-specific, disease-free survival). Supplementary Table S2: Impact of synaptophysin expression groups on survival parameters in conventional adenocarcinomas with a non-neuroendocrine morphology (log-rank test). Supplementary Table S3: Impact of synaptophysin expression groups on survival parameters in adenocarcinomas with a non-neuroendocrine morphology compared to MANECs (log-rank test). Supplementary Table S4: Multivariate analysis for disease-specific survival including synaptophysin expression groups in conventional adenocarcinomas with a non-neuroendocrine morphology compared to MANECs including UICC stage, WHO grade, age and sex. Supplementary Figure S1: Overview of synaptophysin staining patterns.
Author Contributions
M.J. designed the study; M.J. and B.K. wrote the manuscript with assistance from G.K., A.K., S.F., S.L., A.v.W., C.D. (Carsten Denkert), W.W., M.J., B.K., A.K., F.S. and M.S. performed histopathological analyses. M.J., C.D. (Carsten Denkert), S.F., W.W. and P.J. performed statistical analyses. M.J., D.W., S.L., G.K., T.G., L.M.S., K.S. (Kristina Schwamborn), C.L., C.D. (Claire Delbridge), A.K., F.S. and K.S. (Katja Steiger). collected clinicopathological data. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants of the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) (project ID 360372040—SFB 1335 to W.W. and project ID 39535707—SFB 1371 to K.S.). The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. The local ethics committee of the Technical University of Munich approved this study (2016-12-14; reference number: 252/16 s).
Informed Consent Statement
Patients signed a general informed consent protocol during admission to the hospital.
Data Availability Statement
All data relevant for this study are given with the main paper including figures, tables and the supplemental files. The tissue investigated for this study is archived in the Institute of Pathology of the Technical University of Munich.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Classification of Tumours Editorial Board . Digestive System Tumours. 5th ed. IARC; Lyon, France: 2019. [Google Scholar]
- 2.Woischke C., Schaaf C.W., Yang H.M., Vieth M., Veits L., Geddert H., Markl B., Stommer P., Schaeffer D.F., Frolich M., et al. In-depth mutational analyses of colorectal neuroendocrine carcinomas with adenoma or adenocarcinoma components. Mod. Pathol. 2017;30:95–103. doi: 10.1038/modpathol.2016.150. [DOI] [PubMed] [Google Scholar]
- 3.Jesinghaus M., Konukiewitz B., Keller G., Kloor M., Steiger K., Reiche M., Penzel R., Endris V., Arsenic R., Hermann G., et al. Colorectal mixed adenoneuroendocrine carcinomas and neuroendocrine carcinomas are genetically closely related to colorectal adenocarcinomas. Mod. Pathol. 2017;30:610–619. doi: 10.1038/modpathol.2016.220. [DOI] [PubMed] [Google Scholar]
- 4.Jesinghaus M., Schmitt M., Lang C., Reiser M., Scheiter A., Konukiewitz B., Steiger K., Silva M., Tschurtschenthaler M., Lange S., et al. Morphology Matters: A Critical Reappraisal of the Clinical Relevance of Morphologic Criteria From the 2019 WHO Classification in a Large Colorectal Cancer Cohort Comprising 1004 Cases. Am. J. Surg. Pathol. 2021;45:969–978. doi: 10.1097/PAS.0000000000001692. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi T., Machida N., Morizane C., Kasuga A., Takahashi H., Sudo K., Nishina T., Tobimatsu K., Ishido K., Furuse J., et al. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014;105:1176–1181. doi: 10.1111/cas.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka T., Kaneko M., Nozawa H., Emoto S., Murono K., Otani K., Sasaki K., Nishikawa T., Kiyomatsu T., Hata K., et al. Diagnosis, Assessment, and Therapeutic Strategy for Colorectal Mixed Adenoneuroendocrine Carcinoma. Neuroendocrinology. 2017;105:426–434. doi: 10.1159/000478743. [DOI] [PubMed] [Google Scholar]
- 7.La Rosa S., Marando A., Sessa F., Capella C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers. 2012;4:11–30. doi: 10.3390/cancers4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volante M., Rindi G., Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: A comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006;449:499–506. doi: 10.1007/s00428-006-0306-2. [DOI] [PubMed] [Google Scholar]
- 9.Wiedenmann B., Franke W.W., Kuhn C., Moll R., Gould V.E. Synaptophysin: A marker protein for neuroendocrine cells and neoplasms. Proc. Natl. Acad. Sci. USA. 1986;83:3500–3504. doi: 10.1073/pnas.83.10.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagtegaal I.D., Arends M., Odze R.D., Lam A.K. Digestive System Tumours: Tumours of the Colon and Rectum. 5th ed. International Agency for Research on Cancer (IARC); Lyon, France: 2019. [Google Scholar]
- 11.Grabowski P., Schindler I., Anagnostopoulos I., Foss H.D., Riecken E.O., Mansmann U., Stein H., Berger G., Buhr H.J., Scherubl H. Neuroendocrine differentiation is a relevant prognostic factor in stage III-IV colorectal cancer. Eur. J. Gastroenterol. Hepatol. 2001;13:405–411. doi: 10.1097/00042737-200104000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Liu F., Meng Q., Ma S. Is neuroendocrine differentiation a prognostic factor in poorly differentiated colorectal cancer? World J. Surg. Oncol. 2017;15:71. doi: 10.1186/s12957-017-1139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bruine A.P., Wiggers T., Beek C., Volovics A., von Meyenfeldt M., Arends J.W., Bosman F.T. Endocrine cells in colorectal adenocarcinomas: Incidence, hormone profile and prognostic relevance. Int. J. Cancer. 1993;54:765–771. doi: 10.1002/ijc.2910540510. [DOI] [PubMed] [Google Scholar]
- 14.Shinji S., Naito Z., Ishiwata T., Tanaka N., Furukawa K., Suzuki H., Seya T., Kan H., Tsuruta H., Matsumoto S., et al. Neuroendocrine cell differentiation of poorly differentiated colorectal adenocarcinoma correlates with liver metastasis. Int. J. Oncol. 2006;29:357–364. doi: 10.3892/ijo.29.2.357. [DOI] [PubMed] [Google Scholar]
- 15.Hamada Y., Oishi A., Shoji T., Takada H., Yamamura M., Hioki K., Yamamoto M. Endocrine cells and prognosis in patients with colorectal carcinoma. Cancer. 1992;69:2641–2646. doi: 10.1002/1097-0142(19920601)69:11<2641::AID-CNCR2820691104>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 16.Zeng Y.J., Lai W., Liu L., Wu H., Luo X.X., Wang J., Chu Z.H. Prognostic significance of neuroendocrine differentiation in colorectal adenocarcinoma after radical operation: A meta-analysis. J. Gastrointest. Surg. 2014;18:968–976. doi: 10.1007/s11605-014-2480-x. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd R.V., Schroeder G., Bauman M.D., Krook J.E., Jin L., Goldberg R.M., Farr G.H., Jr. Prevalence and Prognostic Significance of Neuroendocrine Differentiation in Colorectal Carcinomas. Endocr. Pathol. 1998;9:35–42. doi: 10.1007/BF02739950. [DOI] [PubMed] [Google Scholar]
- 18.Foley E.F., Gaffey M.J., Frierson H.F., Jr. The frequency and clinical significance of neuroendocrine cells within stage III adenocarcinomas of the colon. Arch. Pathol. Lab. Med. 1998;122:912–914. [PubMed] [Google Scholar]
- 19.Suresh P.K., Sahu K.K., Pai R.R., Sridevi H.B., Ballal K., Khandelia B., Minal J., Annappa R. The Prognostic Significance of Neuroendocrine Differentiation in Colorectal Carcinomas: Our Experience. J. Clin. Diagn. Res. 2015;9:EC01–EC04. doi: 10.7860/JCDR/2015/12800.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gospodarowicz M.K., Brierley J.D., Wittekind C. TNM Classification of Malignant Tumours. John Wiley & Sons; Hoboken, NJ, USA: 2017. [Google Scholar]
- 21.Yu S., Hornick J.L., Gonzalez R.S. An algorithmic approach utilizing CK7, TTF1, beta-catenin, CDX2, and SSTR2A can help differentiate between gastrointestinal and pulmonary neuroendocrine carcinomas. Virchows Arch. 2021 doi: 10.1007/s00428-021-03085-7. [DOI] [PubMed] [Google Scholar]
- 22.Chavez-Blanco A., Taja-Chayeb L., Cetina L., Chanona-Vilchis G., Trejo-Becerril C., Perez-Cardenas E., Segura-Pacheco B., Acuna-Gonzalez C., Duenas-Gonzalez A. Neuroendocrine marker expression in cervical carcinomas of non-small cell type. Int. J. Gynecol. Pathol. 2002;21:368–374. doi: 10.1097/00004347-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Yao G.Y., Zhou J.L., Lai M.D., Chen X.Q., Chen P.H. Neuroendocrine markers in adenocarcinomas: An investigation of 356 cases. World J. Gastroenterol. 2003;9:858–861. doi: 10.3748/wjg.v9.i4.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogimi T., Sadahiro S., Kamei Y., Chan L.F., Miyakita H., Saito G., Okada K., Suzuki T., Kajiwara H. Distribution of Neuroendocrine Marker-Positive Cells in Colorectal Cancer Tissue and Normal Mucosal Tissue: Consideration of Histogenesis of Neuroendocrine Cancer. Oncology. 2019;97:294–300. doi: 10.1159/000501521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitry E., Baudin E., Ducreux M., Sabourin J.C., Rufie P., Aparicio T., Aparicio T., Lasser P., Elias D., Duvillard P., et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br. J. Cancer. 1999;81:1351–1355. doi: 10.1038/sj.bjc.6690325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant for this study are given with the main paper including figures, tables and the supplemental files. The tissue investigated for this study is archived in the Institute of Pathology of the Technical University of Munich.