Abstract
Simple Summary
Intrahepatic cholangiocarcinoma is the second most common primary liver malignancy. Among patients with operable disease, surgical resection is the cornerstone of therapy. Among the majority of patients who present with advanced disease treatment, systemic or targeted therapy is indicated. Recent advancements have provided more novel therapeutic approaches to a subset of patients with intrahepatic cholangiocarcinoma.
Abstract
Although rare, intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy and the incidence of ICC has increased 14% per year in recent decades. Treatment of ICC remains difficult as most people present with advanced disease not amenable to curative-intent surgical resection. Even among patients with operable disease, margin-negative surgical resection can be difficult to achieve and the incidence of recurrence remains high. As such, there has been considerable interest in systemic chemotherapy and targeted therapy for ICC. Over the last decade, the understanding of the molecular and genetic foundations of ICC has reshaped treatment approaches and strategies. Next-generation sequencing has revealed that most ICC tumors have at least one targetable mutation. These advancements have led to multiple clinical trials to examine the safety and efficacy of novel therapeutics that target tumor-specific molecular and genetic aberrations. While these advancements have demonstrated survival benefit in early phase clinical trials, continued investigation in randomized larger-scale trials is needed to further define the potential clinical impact of such therapy.
Keywords: intrahepatic, cholangiocarcinoma, biomarkers, outcomes, trials
1. Introduction
Cholangiocarcinoma is defined by anatomic location and can arise throughout the biliary tree: distally, peri-hilar region, or intrahepatic. The different anatomic locations of cholangiocarcinoma correspond to varied etiologies of the disease. Specifically, extrahepatic cholangiocarcinoma likely derives from stem cells in peribiliary glands while intrahepatic cholangiocarcinoma (ICC) arises from hepatocyte stem cells [1,2]. In turn, the varied anatomic locations of cholangiocarcinoma can have implications for diagnosis, surgical planning, and resectability. In particular, ICC is the second most common primary hepatic malignancy. ICC is a rare cancer, with approximately 8000 cases diagnosed each year in the United States, comprising only 3% of gastrointestinal malignancies diagnosed globally per year [3,4]. The incidence of ICC has increased in recent decades, with some studies demonstrating a 14% increase in incidence per year since the early 1990s [5]. The increase in ICC incidence is likely due to the rising global prevalence of hepatitis C infection, as well as obesity associated non-alcoholic fatty liver disease and non-alcoholic steatohepatitis, which are known risk factors for ICC [5,6,7]. There are also regional differences in the risk factors associated with ICC. For example, the relative incidence of hepatolithiasis, liver fluke infection, as well as viral hepatitis is markedly higher among patients with ICC in Eastern countries. In contrast, among patients in Western countries, ICC is more often associated with primary sclerosing cholangitis and other diseases associated with chronic liver inflammation (non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, and cirrhosis) [8].
Unfortunately, approximately one-half of patients who present with a new diagnosis of ICC will have advanced disease that is not amenable to curative-intent resection (R0 resection) [5]. While the best chance for cure, an R0 resection can only be achieved in 32–88% of patients who are candidates for curative-intent resection [5,9]. Unfortunately, even with R0 resection, 22% of patients will recur within 6 months of hepatectomy [10]. The majority (50%) of patients who recur have intrahepatic recurrence, while 20% have peritoneal recurrence and 20–30% recur with portal lymph node metastasis [11,12,13,14]. As such, long-term prognosis generally remains poor with a 5 year survival of 25% for patients with local disease, 8% for patients with regional disease, and 2% for patients with distant disease [4]. After controlling for patient and disease factors, patients who present with multiple tumors, tumor size (>5 cm), and lymph node metastases have a reduced 5 year overall survival [9,10,15]. Other machine-based learning platforms have also highlighted the importance of the cumulative impact of tumor size, number of tumors, clinical nodal status, and albumin–bilirubin grade on outcomes following curative-intent treatment of ICC [16].
Overall tumor response to systemic chemotherapy ranges from 10% to 30% [9,17,18]. While there are several randomized clinical trials examining the efficacy of adjuvant systemic therapies in ICC, a survival benefit has been most consistently demonstrated with gemcitabine plus cisplatin [19]. This was demonstrated in a randomized trial of gemcitabine plus cisplatin versus gemcitabine alone in patients with locally advanced or metastatic cholangiocarcinoma [20]. Median overall survival of patients with advanced or metastatic disease who received gemcitabine-cisplatin was 11.7 months versus 8.1 months for patients who received just gemcitabine (hazard ratio 0.64, 95% CI 0.52–0.8) [20]. Despite these results, 5 year survival with either therapy is poor [20]. As such, there has been increased interest in novel therapeutic targets to treat cholangiocarcinoma.
Given the high incidence of advanced stage at presentation, poor prognosis associated with ICC, and the limited efficacy of current treatment modalities, there has been considerable interest in the molecular and genetic underpinnings of ICC [21]. Improved understanding of the molecular pathways that promote biliary oncogenesis and the genetic mutational foundations of ICC may elucidate potential targets for molecular and genetic-based treatment therapies, with the ultimate goal of improving overall survival for all-stage ICC. We herein review the pathogenesis of ICC and the associated molecular processes and genetic mutations that may promote biliary oncogenesis. We also describe novel and evolving targeted molecular and genetic-based therapies for the treatment of ICC.
2. Pathogenesis of ICC
ICC most often occurs in the setting of chronic biliary inflammation and stasis. Patients with disease processes that promote chronic biliary stasis and inflammation (primary sclerosing cholangitis, hepatolithiasis, liver fluke infection, chronic hepatitis and cirrhosis) are therefore at risk of developing ICC [22,23]. Biliary stasis and inflammation promote excessive generation of nitric oxide, which potentiates oxidative damage to DNA and inhibits DNA repair and cellular apoptosis. Additionally, cyclo-oxygenase-2 (COX-2) is upregulated by induced nitric oxide species and acts to promote cholangiocyte growth and survival. Bile acids have also been demonstrated to facilitate cholangiocarcinogenesis through interference with cell signaling pathways and normal cellular apoptosis [22,23].
Common genetic mutations associated with ICC include KRAS, BRAF, TP53, and epidermal growth factor receptor [24,25,26,27,28,29,30]. KRAS mutations are the most commonly recognized mutation associated with ICC; however, their incidence varies widely from 8 to 53% [30]. BRAF mutations are also common and have a reported incidence of 0–22% in ICC tumors [30]. Interpretation of these data are complicated, however, as studies examining genetic mutations associated with cholangiocarcinoma typically categorize ICC with peri-hilar cholangiocarcinoma and distal cholangiocarcinoma, which may have different genetic mutational patterns [25,31,32]. In a study of isolated ICC, 7% of ICC tumors were associated with KRAS mutations and 7% had BRAF mutations [30]. In this study, tumors associated with KRAS and BRAF mutations also had a more advanced TNM stage mostly due to lymph node metastasis. Tumor size, number, grade, evidence of vascular invasion or perineural invasion, or satellite lesions did not correlate with mutational status. Patients with tumors exhibiting either KRAS or BRAF mutations had worse median survival versus wild-type tumors (23 months versus 34 months, respectively, p = 0.05) [30]. Patients with KRAS mutations also had a worse 5 year overall survival compared with patients who had ICC tumors with BRAF mutations (13.5 months vs. 23.2 months, respectively, p = 0.05) [30].
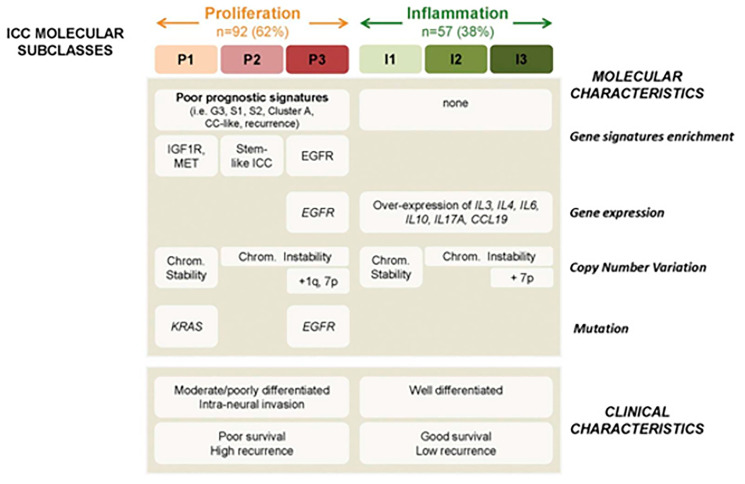
More recently, studies employing gene expression profiling, high-density single-nucleotide array, and mutation analyses of formalin-fixed ICC samples have identified two predominant biological types of ICC: inflammatory and proliferative (Figure 1) [32]. These analyses integrate components of previously distinct hypotheses on the epigenetic, molecular, and genetic origins of ICC. Inflammatory type ICC accounts for 38% of ICC and is defined by activation of pro-inflammatory signaling pathways via interleukin-10 (IL-10), interleukin-4 (IL-4), interleukin-6 (IL-6), overexpression of other cytokines, and activation of signal transducer and activator of transcription 3 protein (STAT3) (Figure 1) [32]. STAT3 has been noted to play a role in many types of cancer and has been associated with a worse prognosis in specific cancers. STAT3 is activated by upstream cytokines and growth factors, including IL-6 and human epidermal growth factor (EGFR) [33]. STAT3 relays signals from activated cytokine and growth factor-receptors at the cellular plasma membranes to the nucleus to promote gene transcription that promotes cellular differentiation, proliferation, angiogenesis, and immune response and inhibits apoptosis [33]. Gene expression that is induced by STAT3 produces cytokines that can re-activate STAT3 (i.e., IL-6), leading to an intrinsic and cyclic propagation of this oncogenic pathway [34]. Therefore, in conditions of chronic biliary inflammation and stasis that activate STAT3, STAT3 also acts to propagate local inflammation and oncogenesis.
Figure 1.
ICC Molecular Subclasses, reproduced from Sia et al. [32]. Summary of characteristics of ICC classes. Specific molecular and clinical characteristics differ between ICC classes. Molecular characteristics such as signatures of poor prognosis (i.e., cluster A, CC-like, G3, S1, S2, and stem-cell like ICC), oncogenic pathways (i.e., IGF1R, MET, EGFR), gene expression (i.e., EGFR, ILs), copy number variations, and oncogenes mutations (KRAS and EGFR) are differentially enriched in the proliferation and inflammation classes. Clinical characteristics such as moderate/poorly differentiated tumors and intraneural invasion are more frequent in the proliferation class. Differences in survival and recurrence were observed.
The proliferative type of ICC accounts for 62% of ICC and is defined by activation of oncogenic signaling pathways, most notably through receptor tyrosine kinases (RTK) [32]. RTKs are cellular plasma membrane receptor proteins (consisting of an extracellular ligand binding domain, transmembrane helix, and intracellular domain) that mediate cellular communication and signaling [35]. There are 58 RTKs that are encoded within the human genome, which are grouped into 20 different classes, differentiated by signaling pathway and function [35]. The extracellular domain of RTKs binds growth factor ligands to signal downstream intracellular processes, including cellular proliferation, differentiation, motility, and metabolism [36]. Aberrant expression and mutations within genes encoding the component parts of RTKs (i.e., the extracellular domain, transmembrane helix, or the intracellular domain) promote various mechanisms of abnormal RTK function. The main mutations types that drive RTK dysfunction are gain of function mutations (EGFR), overexpression and genomic amplification of RTKs (EGFR, MET), chromosomal rearrangements (fusion of genes encoding BCR and ABL tyrosine kinases, or PDGFR tyrosine kinases), and constitutive activation by kinase domain duplication [36]. Furthermore, in subgroup analyses, Sia et al. demonstrated that proliferative-type ICC could be further divided into subclasses (named P1–P3), with distinct prognostic implications reflective of their gene expression predominance [32]. Proliferative-type ICC also demonstrated enrichment of several oncogenic pathways, that are described in hepatocellular cancer (cluster A, G3 proliferation, S1 signature), which may help explain its relatively worse prognosis when compared with inflammatory type ICC [32].
When comparing clinical phenotypes of inflammatory versus proliferative ICC, several important differences have been described. For example, proliferative-type ICC tumors are more likely to be moderately to poorly differentiated, while inflammatory type tumors are more likely to be well differentiated. Survival analyses of proliferative versus inflammatory type ICC reveal that patients with proliferative-type ICC have shorter time to recurrence (15 vs. 37 months, p = 0.03) and a reduced median survival (24.3 vs. 47.2, p = 0.048) [32]. In addition, the incidence of KRAS mutations was 8% in proliferative-type ICC tumors and 7% in inflammatory type ICC tumors; the incidence of BRAF mutations was 5% among proliferative-type ICC tumors and 2% among inflammatory type ICC tumors [32]. The proliferative class of ICC may share common progenitor cells with HCC, which may explain the similar aggressive phenotypes. On sub-analyses that utilized DNA copy number variation and gene expression-based classes to predict recurrence and survival, certain subclasses of the proliferative-type ICC had poor prognostic signatures similar to signatures associated with HCC (Figure 1) [32]. This hypothesis may also be supported by the observation that progenitor cells, which give rise to both hepatocytes and cholangiocytes, are activated in adult human livers by hepatocyte replicative senescence and microenvironment inflammation and damage. Therefore, in the right epigenetic context, progenitor cells may give rise to both HCC and ICC or even cases of combined HCC-ICC [37,38].
The tumor microenvironment has also been used to define genomic and molecular subgroupings related to cholangiocarcinoma. To this point, Job et al. described four subtypes of ICC that were distinguished by cell type and functional transcriptomic markers within tumor specific microenvironments [39]. These subtypes correlated with prognosis and overall survival, highlighting that immunogenic ICC subtypes may be more amenable to treatment with immune checkpoint inhibitors than others ICC lesions [39]. Similarly, Andersen et al. profiled transcriptomes from resected cholangiocarcinoma within the context of their tumor microenvironment and identified certain microenvironment characteristics associated with worse prognosis [40]. These investigators also reported better treatment response to lapatinib, a dual inhibitor of EGFR and HER2, as well as trastuzumab, a selective inhibitor of HER2, among specific ICC subtypes [40].
Despite the considerable progress made in understanding the molecular and genetic underpinnings of ICC in recent decades, many of these studies have been only hypothesis generating. The data do, however, suggest a complicated network of microenvironment, molecular, and genetic factors that strongly influence cholangiocarcinogenesis. Importantly, there may be several different ICC phenotypes that reflect differences in etiology and pathogenesis. In turn, better characterization of different phenotypic-genomic ICC tumors has facilitated the investigation of novel and targeted treatment approaches for ICC.
3. Identified Targetable Mutations
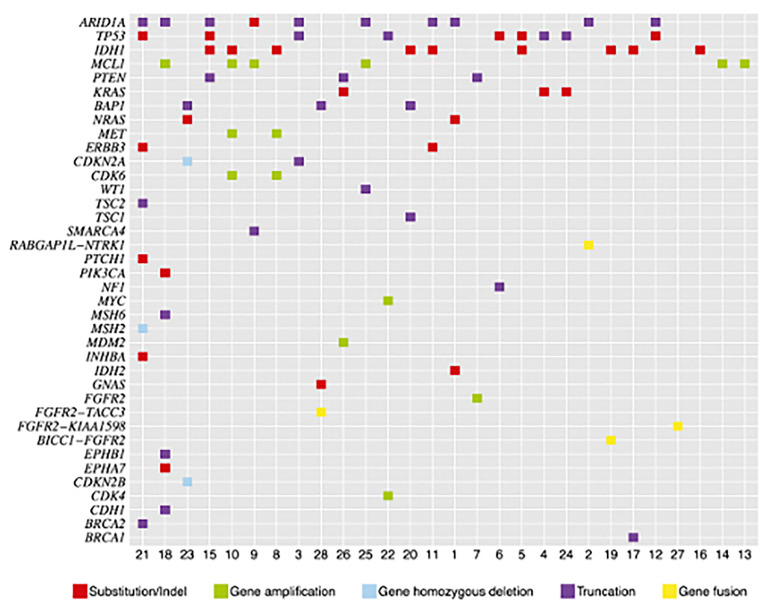
The development of next-generation DNA sequencing has shifted the treatment landscape for many different types of cancer including ICC. In fact, up to 70% of ICC tumors may have at least one targetable gene aberration and on average have anywhere from 1 to 2 targetable mutations per tumor examined [41,42]. Several early small studies observed the most common mutations in ICC within AT-rich interactive domain-containing protein 1A (ARID1A, 36%), isocitrate dehydrogenase 1/2 (IDH1/2, 36%), TP53 (36%), and Myeloid Leukemia and Chlamydia (MCL1, 21%) genes [41]. Common actionable mutations (those with FDA-approved drugs available for treatment) included fibroblast growth factor receptor 2 (FGFR2, 14%), KRAS (11%), phosphatase and tensin homolog (PTEN, 11%), cyclin-dependent kinase inhibitor 2A/B (CDKN2B, 7%), Erb-B2 Receptor Tyrosine Kinase 3 (ERBB3, 7%), MET (7%), NRAS (7%), CDK6 (7%), BRCA1 (4%), BRCA2 (4%), NF1 (4%), PIK3CA (4%), PTCH1 (4%), and TSC1 (4%) genes (Figure 2) [41]. More recently, other larger studies have demonstrated that the most frequent genetic alterations in ICC occur to be in TP53 (TP53; 27%), cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B; 27%), KRAS (22%), AT-rich interactive domain-containing protein 1A (ARID1A; 18%), and isocitrate dehydrogenase 1/2 (IDH1; 20%), BRCA1 associated protein (BAP1, 15%), FGFR2 (11%), and MET (2%) genes [42]. After controlling for patient and disease factors, multivariable analyses demonstrate that TP53 aberrations were associated with worse survival (HR 1.68, p = 0.015), while FGFR aberrations were associated with improved overall survival (HR 0.478, p = 0.03) [42].
Figure 2.
Tile plot of genomic alterations in 28 cases of ICC, reproduced from Ross et al. [41].
Programmed Death-Ligand 1 (PD-L1) expression has also been reported to be present in 10–70% of ICC tumor specimens [43,44,45]. PD-L1 positivity has been associated with more aggressive ICC characteristics and worse survival [43]. While uncommon, microsatellite-instability (MSI) has been explored as a biomarker and target for personalized ICC therapy. Due to the rarity of MSI in ICC, definitive conclusions regarding its incidence and prognostic implications have been difficult to decipher. The available data suggest that microsatellite unstable tumors (as defined by loss of DNA mismatch repair proteins MLH1, PMS2, MSH2, MSH6) are uncommon and occur only in a minority of patients with ICC (ranges from 1 to 10%) [43,46].
4. Results of Targeted Therapies for ICC
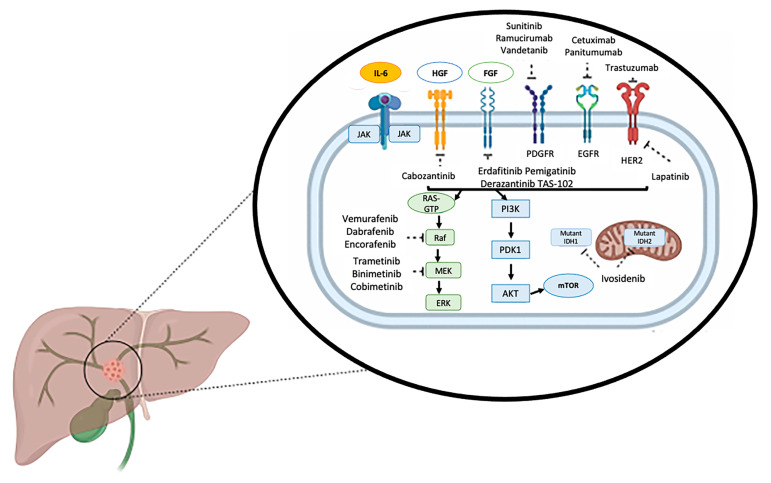
The evolution in the understanding of molecular and genetic pathways associated with ICC has facilitated several early phase clinical trials. These studies have generally investigated novel targeted therapies used either as mono-therapy or in combination with other systemic therapies. Novel therapies and their genetic or molecular targets has been well summarized in schematic form by Rizzo et al. [43] (Figure 3). Although various genetic aberrations have been identified in ICC, the incidence of many mutations remain low, which has been made the use of targeted therapy challenging. Early phase clinical trials have been initiated, however, for some of the more commonly occurring genetic aberrations including those involving IDH1/2, FGFR, EGFR, and VEGF [21,43].
Figure 3.
Schematic representation of therapeutically relevant signaling pathways and selected targeted therapies currently under evaluation in biliary tract cancer, reproduced from Rizzo et al. [47]. AKT: protein kinase B; EGFR: epidermal growth factor receptor; FGF: fibroblast growth factor; HER2: epidermal growth factor receptor 2; HGF: hepatocyte growth factor; IL-6: interleukin 6; IDH: isocitrate dehydrogenase; JAK: Janus kinase; mTOR: mammalian target of rapamycin; PDGFR: platelet derived growth factor receptor; PDK1: phosphoinositide-dependent kinase-1; PI3K: phosphoinositide 3-kinase.
4.1. Targeted Therapy: Isocitrate Dehydrogenase
Since IDH1/2 mutations are present in approximately 10–20% of ICC lesions, this genetic aberration has been the target of therapeutic intervention [41]. The mechanism by which IDH aberrations promote cholangiocarcinogenesis are still being fully elucidated. Animal models suggest that mutation of IDH induces an abnormal response to hepatocyte injury and inflammation, as well as silencing of HNF4-alpha, a transcription factor crucial to hepatocyte differentiation that is a potent anti-proliferative and tumor suppressor. In mouse models, mutant IDH-associated silencing of HNF4-alpha has resulted in a pro-neoplastic state with a biliary predominance [48].
The safety and efficacy of Ivosidenib, an inhibitor of mutated IDH1, have been investigated in a multicenter randomized double-blinded placebo-controlled phase 3 trial among patients with advanced cholangiocarcinoma who had progressed on either fluorouracil or gemcitabine-based chemotherapy [49]. The trial included patients with all types cholangiocarcinoma (i.e., intrahepatic, hilar, distal); however, the majority of accrued patients had ICC (89% in treatment arm and 77% in placebo arm) [49]. Among the 185 patients (124 in treatment arm and 61 in placebo arm), Ivosidenib was associated with an improved progression-free survival (median 2.7 months vs. 1.4 months, HR 0.37, 95% CI 0.25–0.54, p < 0.0001) and comparable safety profiles (30% of patients in the treatment arm patients had serious adverse events vs. 22% in the control arm) [49]. While other phase 1 and 2 studies have examined the safety profiles of inhibitors of mutated IDH1/2, a definitive benefit in the treatment of ICC has yet to be established [50].
4.2. Targeted Therapy: Fibroblast Growth Factor Receptor
FGFR aberrations have been identified in 10–15% of ICC tumors [41,42,51]. FGFR is expressed on multiple cell types and consists of four transmembrane receptors (FGFR1–4) with intracellular tyrosine kinase domains. The binding of these FGFR receptors leads to unregulated activation of several cellular proliferation pathways, including RAS-Raf-MAPK, JAK-STAT, and PI3-AKT-mTOR, leading to angiogenesis and unregulated cellular proliferation [52].
Pemigatinib is an oral kinase inhibitor, which inhibits FGFR 1, FGFR2, and FGFR3. Several Phase 2 trials have examined the safety and antitumor activity of Pemigatinib among patients with advanced cholangiocarcinoma with FGFR rearrangement. In one trial, among 146 enrolled patients, 35% had an objective treatment response, 42% of patients died from disease progression, and 45% of patients had serious adverse events [53]. Pemigatinib is currently being investigated in a phase 3, open-label, randomized, active-controlled multicenter trial that compares efficacy and safety of Pemigatinib versus standard of care (gemcitabine-cisplatin) in patients with advanced or metastatic cholangiocarcinoma with FGFR2 aberration (ClinicalTrials.gov, accessed on 21 September 2021, Identifier: NCT03656536). Futibatinib is a different highly selective irreversible oral inhibitor of FGFR 1–4. Its safety has been studied in a multicenter, phase 2 trial of patients with advanced or metastatic ICC with FGFR2 gene rearrangements who had disease progression after first line therapy (gemcitabine-cisplatin). Among 103 patients who enrolled, 34% had an objective response and the disease control rate was 76% at ≥6 months follow-up. Serious adverse events were experienced by 73% of patients (≥grade 3) [52]. The safety and efficacy of Futibatinib are now being compared with standard of care (gemcitabine-cisplatin) in a multicenter phase 3 study of patients with advanced cholangiocarcinoma with FGFR2 gene rearrangements (NCT04093362). In addition to these more well-known drugs, there are a number of other early phase studies examining drug safety and efficacy of inhibitors of mutant FGFR, as well as several upstream or downstream processes related to FGFR-based pathways [42,50]. Javle et al. published results from a single-arm phase II study that examined efficacy and safety of infigratinib, a FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma [49,51,54]. In a cohort of 108 patients with advanced or metastatic cholangiocarcinoma, the objective response rate was 23%, the median duration of response was 5.0 months, and the median progression-free survival was 7.3 months (95% CI 5.6–7.6 months) [51,54,55]. The majority of patients experienced hyperphosphatemia despite taking a prophylactic phosphate binder (77%) and non-severe eye disorders (68%), while a minority of patients experienced serious eye disorders (16%, central serous retinopathy and retinal pigment epithelium detachment) [54,55]. Based on these results, a phase III clinical trial of infigratinib versus standard of care gemcitabine/cisplatin is currently being conducted (NCT 03773302). Additional ongoing trials examining treatment efficacy and safety of FGFR targeted therapies are listed in Table 1.
Table 1.
Ongoing trials evaluating FGFR and IDH1/2 inhibitors among patients with advanced solid tumors or ICC with or without known FGFR or IDH1/2 mutations (clinicaltrials.gov, accessed on 21 September 2021).
| NCT No. | Phase | Setting | Arm A | Arm B | Agent Description | Primary Outcomes |
|---|---|---|---|---|---|---|
| 01752920 | I/II | Advanced solid tumor with FGFR alteration | Derazantinib | FGFR inhibitor | NAE | |
| 02150967 | II | Advanced cholangiocarcinoma, FGFR2 gene mutation | BGJ398 | BGJ398: FGFR inhibitor | ORR | |
| 02465060 | Advanced solid tumors, lymphomas or multiple myeloma |
Multiple genetic-alteration-specific drugs | Diverse mechanisms | ORR | ||
| 02924396 | II | Advanced cholangiocarcinoma | Pemigatinib | FGFR2 inhibitor | ORR | |
| 03230318 | II | ICC or HCC with FGFR gene fusions | Derazantinib | FGFR inhibitor | ORR, PFS at 3 months | |
| 03656536 | II | Advanced bile duct cancer | Pemigatinib | Gemcitabine + cisplatin | Pemigatinib: FGFR inhibitor |
PFS |
| 03773302 | III | Advanced cholangiocarcinoma with FGFR2 gene mutation | BGJ398 | Gemcitabine + cisplatin | BGJ398: FGFR inhibitor | PFS |
| 04093362 | III | Advanced cholangiocarcinoma with FGFR2 rearrangements | TAS-120/Futibatinib | Gemcitabine + cisplatin | TAS-120: FGFR inhibitor | PFS |
| 0421168 | II | Advanced biliary tract cancer |
Toripalimab + Lenvatinib |
Toripalimab: Recombinant anti-human PDI IgG4Lenvatinib: angiogenesis inhibitor that targets multiple tyrosine kinases including FGFR |
ORR | |
| 04238715 | II | Advanced cholangiocarcinoma with FGFR2 gene fusion | E7090 | FGFR2 inhibitor | ORR | |
| 04256980 | II | Advanced cholangiocarcinoma including those with FGFR2 rearrangements |
Pemigatinib | FGFR2 inhibitor | ORR | |
| 04353375 | II | Advanced ICC with FGFR2 fusion |
HMPL-453 | FGFR inhibitor | ORR at 6 months | |
| 04526106 | I | Advanced solid tumor with FGFR2 amplification, mutation, rearrangement, translocation, activation | RLY-4008 | FGFR2 inhibitor | MTD, NAE | |
| 04919642 | II | Advanced cholangiocarcinoma with FGFR mutation | TT-00420 | Multi-kinase inhibitor including FGFR |
ORR | |
| 05039892 | II | Advanced bile duct cancer with FGFR2 mutation | 3D185 | FGFR inhibitor | ORR | |
| 03212274 | II/III | Advanced solid neoplasms with IDH1/2 mutation | Olaparib | PARP inhibitor | ORR | |
| 04521686 | II/III | Advanced solid neoplasms with IDH1/2 mutation | LY3410738 | mIDH1 inhibitor | ORR | |
| 03878095 | II/III | Advanced solid neoplasm with IDH1/2 mutation | Olaparib + Ceralasertib | ATR inhibitor | ORR |
Ongoing trials evaluating FGFR and IDH1/2 inhibitors in patients with advanced solid tumors or ICC with or without known FGFR or IDH1/2 mutations, as listed on clinicaltrials.gov, accessed on 21 September 2021. HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; FGFR, fibroblast growth factor receptor; MTD, maximum tolerated dose; NAE number adverse events; ORR objective response rate; PFS, progression-free survival; PD1, programmed cell death protein.
4.3. Targeted Therapy: Epidermal Growth Factor Receptor
The HER tyrosine kinase family includes EGFR and HER1–4 aberrations. EGFR aberrations have been identified in 25% of ICC tumors [56]. EGFR is a subclass of the tyrosine kinase transmembrane receptor that binds to epidermal growth factor and activates signaling pathways involved in cell motility, cell adhesion, angiogenesis and invasion [57]. HER/EGFR aberration causes unregulated activation of these pathways.
Several early phase trials examining safety and efficacy of inhibitors of aberrant EGFR and HER (lapatinib, erlotinib, pertuzumab, trastuzumab) in patients with cholangiocarcinoma and other solid tumors are either pending or have failed to show significant objective treatment response. Analyses, have been limited, however, by patient selection and heterogeneity of diagnosis [50,58].
4.4. Targeted Therapy: Immune Checkpoint Inhibitors
Programmed Death-Ligand 1 (PD-L1) has been noted in 10–70% of ICC tumor specimens and its expression has been associated with tumor aggressiveness and diminished survival [43]. PD-L1 is expressed on tumor cells and binds PD-1 receptors on activated T cells to inhibit cytotoxic action, thereby resisting the immune mediated defense. The safety and efficacy of a number of PD-L1 inhibitors have been examined in relation to advanced or metastatic PD-L1-positive cholangiocarcinoma (Table 2) [43]. These early phase trials failed to demonstrate definitive drug related benefits, yet initial results do suggest potential for both treatment efficacy and safety [43]. As such, while MSI-ICC is rare and small study numbers preclude robust analyses, some studies have reported relatively prolonged survival of patients with MSI-ICC treated with targeted immune checkpoint inhibitors. There are a number of ongoing early phase trials evaluating immune checkpoint inhibitor treatment efficacy and safety in patients with advanced MS- ICC (Table 3) [43].
Table 2.
Ongoing Phase I-III clinical trials evaluating immune checkpoint inhibitors in biliary tract cancer patients with advanced disease [43].
| NCT No. | Phase | Setting | Arm A | Arm B | Agent Description | Primary Outcomes |
|---|---|---|---|---|---|---|
| 04066491 | II/III | First line | Bintrafusp alfa (M7824) plus CisGem | Placebo + CisGem |
Bintrafusp alfa: bifunctional fusion protein composed by PD-L1 antibody fused with 2 extracellular domains of TGF-B receptor | DLTs, OS |
| 03875235 | III | First line | Durvalumab + CisGem | Placebo + CisGem |
Durvalumab: PD-L1 inhibitor | OS |
| 03260712 | II | First line | Pembrolizumab + CisGem |
Pembrolizumab: PD-L1 antibody | PFS at 6 months | |
| 04300959 | II | First line | Anlotinib + tremelimumab + CisGem |
CisGem | Anlotinib: TKI inhibiting PDGFR, FGFR, VEGFR, c-KIT kinase Sintilimab: PD-L1 inhibitor |
OS at 12 months |
| 03046862 | II | First line | Durvalumab + tremelimumab + CisGem |
Durvalumab: PD-L1 inhibitor Tremelimumab: anti-CTLA-4 agent |
||
| 03796429 | II | First line | Toripalimab + S-1 + Gem | Toripalimab: PD-1 antibody | PFS, OS | |
| 04172402 | II | First line | Nivolumab + S-1 + Gem | Nivolumab: PD-1 antibody | ORR | |
| 04027764 | II | First line | Toripalimab + S-1 + albumin + paclitaxel | Toripalimab: PD-1 antibody | ORR | |
| 03478488 | III | First line | KN035 + GEMOX | GEMOX | KN035: PD-L1 inhibitor | OS |
| 04191343 | II | First line | Toripalimab + GEMOX | Toripalimab: PD-1 antibody | ORR | |
| 04003636 | III | First line | Pembrolizumab + CisGem |
Placebo + CisGem |
Pembrolizumab: PD-L1 antibody | PFS, OS |
| 03937895 | I/II | First or later line | Pembrolizumab + allogenic NK cell (SMT-NK) |
Pembrolizumab: PD-L1 antibody SMT-NK: allogenic natural killer cell |
DLTs, ORR | |
| 03639935 | II | Maintenance after platinum-based first line | Nivolumab + Rucaparib | Nivolumab: PD-1 antibody Rucaparib: PARP inhibitor |
PFS at 4 months | |
| 03785873 | I/II | Second line | Nivolumab + 5-FU + Nallri | Nivolumab: PD-1 antibody | DLTs, PFS | |
| 04298021 | II | Second line | AZD6738 + Durvalumab |
AZD6738 + Olaparib | AZD6738: ATR and ATM inhibitor Durvalumab: PD-L1 inhibitor Olaparib: PARP inhibitor |
DCR |
| 03110328 | II | Second line | Pembrolizumab | Pembrolizumab: PD-L1 antibody | PFS, OS | |
| 04211168 | II | Second line | Toripalimab + Lenvatinib |
Toripalimab: PD-L antibody Lenvatinib: TKI |
ORR, AEs | |
| 03797326 | II | Second line | Pembrolizumab + Lenvatinib |
Pembrolizumab: PD-L1 antibody Lenvatinib: TKI |
||
| 04010071 | II | Second line | Toripalimab + axitinib | Toripalimab: PD-1 antibody Axitinib: TKI |
ORR, PFS | |
| 03704480 | II | Second line | Durvalumab + tremelimumab | Durvalumab + tremelimumab + paclitaxel | Durvalumab: PD-L1 inhibitor Tremelimumab: anti-CTLA-4 agent |
PFS |
| 04003636 | III | First line | Pembrolizumab + CisGem |
Placebo + CisGem |
Pembrolizumab: PD-1 antibody | PFS, OS |
| 03937895 | I/II | First or later line | Pembrolizumab + allogenic NK cell (SMT-NK) |
Pembrolizumab: PD-1 antibody SMT-NK: allogenic natural killer |
DLTs, ORR | |
| 03999658 | II | Second or later line | STI-3031 | St3031: PD-L1 inhibitor | ORR | |
| 03475953 | I/II | Second or later line | Avelumab + regorafenib |
Avelumab: PD-L1 inhibitor Regorafenib: TKI |
RP2D | |
| 03801083 | II | Second or later line | TILs | TILs: tumor-infiltrating Lymphocytes | ORR | |
| 04057365 | II | Second or later line | Nivolumab + DKN-01 | Nivolumab: PD-1 antibody DKN-01: humanized monoclonal antibody against DKK1 protein |
ORR | |
| 04298008 | II | Third line | AZD6738 + Durvalumab |
AZD6738: ATR and ATM inhibitor Durvalumab: PD-L1 inhibitor |
DCR |
Ongoing phase I to III clinical trials evaluating immune checkpoint inhibitors in biliary tract cancer patients with advanced disease, reproduced from Rizzo et al. [43]. This table includes ongoing clinical trials assessing immunotherapy as first-, second-, or later-line treatment. 5-FU: 5-fluorouracil; AEs, adverse events; ATM, ataxia-telangiectasia mutation; BTC, biliary tract cancer; CisGem, cisplatin plus gemcitabine combination; CTLA-4, cytotoxic T-lymphocyte antigen 4; DCR: disease control rate; DLTs, dose-limiting toxicities; FGFR, fibroblast growth factor receptor; GBC, gallbladder cancer; GEMOX, gemcitabine plus oxaliplatin; ORR, overall response rate; OS, overall survival; PARP, poly ADP ribose polymerase; PDGFR, platelet-derived growth factor receptor; PD-1, programmed death 1, PFS, progression-free survival; RP2D, recommended phase II dose; S-1: tegafur/gimeracil/oteracil; TILs: tumor-infiltrating lymphocytes; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor.
Table 3.
Reported outcomes of single-agent immune checkpoint inhibitors in advanced biliary tract cancer (BTC) [43].
| Phase | Setting | Immune Check Point Inhibitor | Agent Description | Outcomes |
|---|---|---|---|---|
| Ib [58] | Second line or later | Pembrolizumab | Pembrolizumab: PD-1 inhibitor | mPFS 1.8 months mOS 5.7 months ORR 13% SD rate 17% |
| II [58] | Second line or later | Pembrolizumab | Pembrolizumab: PD-1 inhibitor | mPFS 2.0 months mOS 7.4 months ORR 5.8% |
| II [59] | Second line or later | Nivolumab | Nivolumab: PD-1 inhibitor | mPFS 1.4 months mOS 5.2 months PR rate 3% |
| II [60] | Second line or later | Nivolumab | Nivolumab: PD-1 inhibitor | mPFS 3.7 months mOS 14.2 months ORR 20% DCR 50% |
| II [61] | Second line or later | Durvalumab | Durvalumab: PD-L1 inhibitor | mPFS 1.5 months mOS 8.1 months PR rate 4.2% |
| I [62] | Second line or later | M7824 | M7824: PD-L1 inhibitor | mOS 12.7 months ORR 02% |
Reported outcomes of single-agent immune checkpoint inhibitors in advanced biliary tract cancer (BTC), reproduced from Rizzo et al. [43].
4.5. Targeted Therapies: BRAF Mutations
BRAF mutations occur in 5–7% of ICC [63]. BRAF is a tyrosine kinase member of the RAS-RAF-MEK-ERK pathway (mitogen-activated protein kinase, MAPK), which is an integral pathway that mitigates cell proliferation, differentiation, transformation, and apoptosis [64]. BRAF mutations have been associated with higher TNM stage, resistance to systemic chemotherapies, and worse survival [26,30]. Targeted therapies pertaining to BRAF-positive ICC have demonstrated mixed results [65]. Many of studies to date have been “bucket” studies that included many different kinds of solid tumors harboring BRAF-mutations [65]. As such, interpretation of these data and the identification of disease-specific efficacy has been challenging. While several ongoing trials pertaining to patients with metastatic biliary tract cancer are ongoing, results from these trials are still pending (Table 4) [65].
Table 4.
Ongoing trials evaluating BRAF targeted therapies in advanced biliary tract cancer registered on ClinicalTrials.gov, accessed on 21 September 2021 [65].
| NCT No. | Phase | Setting | Arm A | Arm B | Agent Description | Primary Outcomes |
|---|---|---|---|---|---|---|
| 04190328 | I | Second or later line; BRAF-mutant solid tumors, including BTC | ABM-1310 | ABM-1310: BRAF inhibitor | MTD/RP2D | |
| 04249843 | I | Second or later line; BRAF-mutant solid tumors, including BTC | BGB-3245 | BGB-3245: BRAF inhibitor | DLT MTD/RP2D |
|
| 03839342 | II | Second or later line; BRAF-mutant solid tumors, including BTC | Binimetinib + encorafenib | Binimetinib: BRAF inhibitor Encorafenib: BRAF inhibitor |
ORR | |
| 01989585 | I/II | Second or later line; BRAF-mutant solid tumors, including BTC | Dabrafenib + trametinib | Dabrafenib + trametinib + navitoclax | Dabrafenib: BRAF inhibitor Trametinib: BRAF inhibitor Navitoclax: Bcl-2 inhibitor |
MTD CR rate |
| 04418167 | I | Second or later line; BRAF-mutant solid tumors, including BTC with MAPK pathway mutations | JSI-I 187 | JSI-I 187 + dabrafenib | JSI-I 187: ERK inhibitor Dabrafenib: BRAF inhibitor |
AEs |
| 03272464 | I | Second or later line; BRAF-mutant solid tumors, including BTC | Dabrefenib + trametinib + itacitinib | Dabrafenib: BRAF inhibitor Trametinib: BRAF inhibitor Icatinib: JAK I inhibitor |
MTD |
Ongoing trials evaluating BRAF-targeted therapies in advanced biliary tract cancer, reproduced from Rizzo et al. [65]. Abbreviations: AEs: adverse events; BTC: biliary tract cancer; CR: complete response; DLTs: dose-limiting toxicities; MTD: maximum tolerated dose; JAK1: Janus-associated kinase 1; MEK: mitogen-activated protein kinase; ORR: overall response rate; RP2D: recommended phase 2 dose.
5. Conclusions
Considerable progress has been made in understanding the molecular and genetic pathogenesis of ICC in recent decades. This evolution in knowledge has facilitated development and study of novel targeted therapies for patients with advanced ICC. While some targeted therapies have demonstrated significant potential relative to disease progression and even survival, the data are still emerging. Notwithstanding these limitations, patients with advanced ICC should have genetic profiling performed to identify potential targeted therapies. As with many rare disease, it is critical that patients be appropriately enrolled in clinical trials to help better define the role, efficacy and safety of targeted therapies for ICC.
Author Contributions
Conceptualization, A.W.A., A.P., A.E., D.T. and T.M.P.; methodology, A.W.A., A.P., A.E., D.T. and T.M.P.; software, A.W.A.; validation, A.W.A., A.P., A.E., D.T. and T.M.P.; formal analysis, A.W.A.; investigation, A.W.A., A.P., A.E., D.T. and T.M.P.; resources, A.W.A., A.P., A.E., D.T. and T.M.P.; data curation, A.W.A.; writing—original draft preparation, A.W.A., A.P., A.E., D.T. and T.M.P.; writing—review and editing, A.W.A., A.P., A.E., D.T. and T.M.P.; visualization, A.W.A., A.P., A.E., D.T. and T.M.P.; supervision, T.M.P.; project administration, A.W.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cardinale V. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World J. Gastrointest. Oncol. 2010;2:407–416. doi: 10.4251/wjgo.v2.i11.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oishi N., Kumar M.R., Roessler S., Ji J., Forgues M., Budhu A., Zhao X., Andersen J.B., Ye Q.-H., Jia H.-L., et al. Transcriptomic profiling reveals hepatic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology. 2012;56:1792–1803. doi: 10.1002/hep.25890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan S., Toledano M., Taylor-Robinson S. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB. 2008;10:77–82. doi: 10.1080/13651820801992641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Cancer Society Key Statistics for Bile Duct Cancer. 2021. [(accessed on 23 August 2021)]. Available online: https://www.cancer.org/cancer/bile-duct-cancer/about/key-statistics.html.
- 5.Endo I., Gonen M., Yopp A.C., Dalal K.M., Zhou Q., Klimstra D., D’Angelica M., DeMatteo R.P., Fong Y., Schwartz L., et al. Intrahepatic Cholangiocarcinoma. Ann. Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 6.Khan S.A., Thomas H.C., Davidson B.R., Taylor-Robinson S.D. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 7.Nathan H., Pawlik T.M., Wolfgang C.L., Choti M.A., Cameron J.L., Schulick R.D. Trends in Survival after Surgery for Cholangiocarcinoma: A 30-Year Population-Based SEER Database Analysis. J. Gastrointest. Surg. 2007;11:1488–1497. doi: 10.1007/s11605-007-0282-0. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A., Dixon E. Epidemiology and risk factors: Intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2017;6:101–104. doi: 10.21037/hbsn.2017.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maithel S.K., Gamblin T.C., Kamel I., Corona-Villalobos C.P., Thomas M., Pawlik T.M. Multidisciplinary approaches to intrahepatic cholangiocarcinoma. Cancer. 2013;119:3929–3942. doi: 10.1002/cncr.28312. [DOI] [PubMed] [Google Scholar]
- 10.Tsilimigras D.I., Sahara K., Wu L., Moris D., Bagante F., Guglielmi A., Aldrighetti L., Weiss M., Bauer T.W., Alexandrescu S., et al. Very Early Recurrence After Liver Resection for Intrahepatic Cholangiocarcinoma. JAMA Surg. 2020;155:823–831. doi: 10.1001/jamasurg.2020.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyder O., Hatzaras I., Sotiropoulos G.C., Paul A., Alexandrescu S., Marques H.P., Pulitano C., Barroso E., Clary B.M., Aldrighetti L., et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153:811–818. doi: 10.1016/j.surg.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi S.-B., Kim K.-S., Choi J.-Y., Park S.W., Lee W.J., Chung J.-B. The Prognosis and Survival Outcome of Intrahepatic Cholangiocarcinoma Following Surgical Resection: Association of Lymph Node Metastasis and Lymph Node Dissection with Survival. Ann. Surg. Oncol. 2009;16:3048–3056. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M., Takasaki K., Otsubo T., Katsuragawa H., Katagiri S. Recurrence after surgical resection of intrahepatic cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2001;8:154–157. doi: 10.1007/s005340170039. [DOI] [PubMed] [Google Scholar]
- 14.Weber S.M., Ribero D., O’Reilly E.M., Kokudo N., Miyazaki M., Pawlik T.M. Intrahepatic Cholangiocarcinoma: Expert consensus statement. HPB. 2015;17:669–680. doi: 10.1111/hpb.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagante F., Spolverato G., Merath K., Weiss M., Alexandrescu S., Marques H.P., Aldrighetti L., Maithel S.K., Pulitano C., Bauer T.W., et al. Intrahepatic cholangiocarcinoma tumor burden: A classification and regression tree model to define prognostic groups after resection. Surgery. 2019;166:983–990. doi: 10.1016/j.surg.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Tsilimigras D.I., Mehta R., Moris D., Sahara K., Bagante F., Paredes A.Z., Moro A., Guglielmi A., Aldrighetti L., Weiss M., et al. A Machine-Based Approach to Preoperatively Identify Patients with the Most and Least Benefit Associated with Resection for Intrahepatic Cholangiocarcinoma: An International Multi-Institutional Analysis of 1146 Patients. Ann. Surg. Oncol. 2019;27:1110–1119. doi: 10.1245/s10434-019-08067-3. [DOI] [PubMed] [Google Scholar]
- 17.Blechacz B., Komuta M., Roskams T., Gores G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011;8:512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aishima S., Fujita N., Mano Y., Kubo Y., Tanaka Y., Taketomi A., Shirabe K., Maehara Y., Oda Y. Different roles of S100P Overexpression in Intrahepatic Cholangiocarcinoma. Am. J. Surg. Pathol. 2011;35:590–598. doi: 10.1097/PAS.0b013e31820ffdf1. [DOI] [PubMed] [Google Scholar]
- 19.Cloyd J.M., Ejaz A., Pawlik T.M. The Landmark Series: Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2020;27:2859–2865. doi: 10.1245/s10434-020-08621-4. [DOI] [PubMed] [Google Scholar]
- 20.Valle J. ABC-02. CDDP + GEM vs. GEM for biliary tract cancer: OS better with CDDP + GEM. N. Engl. J. Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 21.Ntanasis-Stathopoulos I., Tsilimigras D.I., Gavriatopoulou M., Schizas D., Pawlik T.M. Cholangiocarcinoma: Investigations into pathway-targeted therapies. Expert Rev. Anticancer Ther. 2020;20:765–773. doi: 10.1080/14737140.2020.1807333. [DOI] [PubMed] [Google Scholar]
- 22.Sirica A.E. Cholangiocarcinoma: Molecular targeting strategies for chemoprevention and therapy. Hepatology. 2004;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 23.Maemura K., Natsugoe S., Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J. Hepatobiliary Pancreat. Sci. 2014;21:754–760. doi: 10.1002/jhbp.126. [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg D., Rosenbaum E., Argani P., Wistuba I.I., Sidransky D., Thuluvath P.J., Hidalgo M., Califano J., Maitra A. The V599E BRAF mutation is uncommon in biliary tract cancers. Mod. Pathol. 2004;17:1386–1391. doi: 10.1038/modpathol.3800204. [DOI] [PubMed] [Google Scholar]
- 25.Tannapfel A., Benicke M., Katalinic A., Uhlmann D., Köckerling F., Hauss J., Wittekind C. Frequency of p16INK4A alterations and k-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721–727. doi: 10.1136/gut.47.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tannapfel A., Sommerer F., Benicke M., Katalinic A., Uhlmann D., Witzigmann H., Hauss J., Wittekind C. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706–712. doi: 10.1136/gut.52.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahrendt S., Rashid A., Chow J.T., Eisenberger C.F., Pitt H.A., Sidransky D. p53 overexpression and K- ras gene mutations in primary sclerosing cholangitis-associated biliary tract cancer. J. Hepatobiliary Pancreat. Surg. 2000;7:426–431. doi: 10.1007/s005340070039. [DOI] [PubMed] [Google Scholar]
- 28.Furubo S., Harada K., Shimonishi T., Katayanagi K., Tsui W., Nakanuma Y. Protein expression and genetic alterations of p53 and ras in intrahepatic cholangiocarcinoma. Histopathology. 1999;35:230–240. doi: 10.1046/j.1365-2559.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 29.Gwak G.-Y., Yoon J.-H., Shin C.M., Ahn Y.J., Chung J.K., Kim Y.A., Kim T.-Y., Lee H.-S. Detection of response-predicting mutations in the kinase domain of the epidermal growth factor receptor gene in cholangiocarcinomas. J. Cancer Res. Clin. Oncol. 2005;131:649–652. doi: 10.1007/s00432-005-0016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson S., Hyder O., Dodson R., Nayar S.K., Poling J., Beierl K., Eshleman J.R., Lin M.-T., Pawlik T.M., Anders R.A. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Human Pathol. 2013;44:2768–2773. doi: 10.1016/j.humpath.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sia D., Tovar V., Moeini A., Llovet J.M. Intrahepatic cholangiocarcinoma: Pathogenesis and rationale for molecular therapies. Oncogene. 2013;32:4861–4870. doi: 10.1038/onc.2012.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sia D., Hoshida Y., Villanueva A., Roayaie S., Ferrer-Fabrega J., Tabak B., Peix J., Sole M., Tovar V., Alsinet C., et al. Integrative Molecular Analysis of Intrahepatic Cholangiocarcinoma Reveals 2 Classes That Have Different Outcomes. Gastroenterology. 2013;144:829–840. doi: 10.1053/j.gastro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston P., Grandis J.R. STAT3 SIGNALING: Anticancer Strategies and Challenges. Mol. Interv. 2011;11:18–26. doi: 10.1124/mi.11.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rébé C., Végran F., Berger H., Ghiringhelli F. STAT3 activation. JAK-STAT. 2013;2:e23010. doi: 10.4161/jkst.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner J.P., Wolf-Yadlin A., Sevecka M., Grenier J.K., Root D.E., Lauffenburger D.A., MacBeath G. Receptor Tyrosine Kinases Fall into Distinct Classes Based on Their Inferred Signaling Networks. Sci. Signal. 2013;6:ra58. doi: 10.1126/scisignal.2003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du Z., Lovly C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer. 2018;17:1–13. doi: 10.1186/s12943-018-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818–3822. doi: 10.1038/sj.onc.1209558. [DOI] [PubMed] [Google Scholar]
- 38.Wakasa T., Wakasa K., Shutou T., Hai S., Kubo S., Hirohashi K., Umeshita K., Monden M. A histopathological study on combined hepatocellular and cholangiocarcinoma: Cholangiocarcinoma component is originated from hepatocellular carcinoma. Hepatogastroenterology. 2007;54:508–513. [PubMed] [Google Scholar]
- 39.Job S., Rapoud D., dos Santos A., Gonzalez P., Desterke C., Pascal G., Elarouci N., Ayadi M., Adam R., Azoulay D., et al. Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Hepatology. 2019;72:965–981. doi: 10.1002/hep.31092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersen J.B., Spee B., Blechacz B.R., Avital I., Komuta M., Barbour A., Conner E.A., Gillen M.C., Roskams T., Roberts L., et al. Genomic and Genetic Characterization of Cholangiocarcinoma Identifies Therapeutic Targets for Tyrosine Kinase Inhibitors. Gastroenterology. 2012;142:1021–1031.e15. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross J.S., Wang K., Gay L., Al-Rohil R., Rand J.V., Jones D.M., Lee H.J., Sheehan C.E., Otto G.A., Palmer G., et al. New Routes to Targeted Therapy of Intrahepatic Cholangiocarcinomas Revealed by Next-Generation Sequencing. Oncologist. 2014;19:235–242. doi: 10.1634/theoncologist.2013-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Javle M., Bekaii-Saab T., Jain A., Wang Y., Kelley R.K., Wang K., Kang H.C., Catenacci D., Ali S., Krishnan S., et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer. 2016;122:3838–3847. doi: 10.1002/cncr.30254. [DOI] [PubMed] [Google Scholar]
- 43.Rizzo A., Ricci A.D., Brandi G. PD-L1, TMB, MSI, and Other Predictors of Response to Immune Checkpoint Inhibitors in Biliary Tract Cancer. Cancers. 2021;13:558. doi: 10.3390/cancers13030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontugne J., Augustin J., Pujals A., Compagnon P., Rousseau B., Luciani A., Tournigand C., Cherqui D., Azoulay D., Pawlotsky J.-M., et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget. 2017;8:24644–24651. doi: 10.18632/oncotarget.15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walter D., Herrmann E., Schnitzbauer A.A., Zeuzem S., Hansmann M.L., Peveling-Oberhag J., Hartmann S. PD-L1 expression in extrahepatic cholangiocarcinoma. Histopathology. 2017;71:383–392. doi: 10.1111/his.13238. [DOI] [PubMed] [Google Scholar]
- 46.Goeppert B., Roessler S., Renner M., Singer S., Mehrabi A., Vogel M.N., Pathil A., Czink E., Köhler B., Springfeld C., et al. Mismatch repair deficiency is a rare but putative therapeutically relevant finding in non-liver fluke associated cholangiocarcinoma. Br. J. Cancer. 2018;120:109–114. doi: 10.1038/s41416-018-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizzo A. Targeted Therapies in Advanced Cholangiocarcinoma: A Focus on FGFR Inhibitors. Medicina. 2021;57:458. doi: 10.3390/medicina57050458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saha S.K., Parachoniak C.A., Ghanta K., Fitamant J., Ross K.N., Najem M.S., Gurumurthy S., Akbay E., Sia D., Cornella H., et al. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513:110–114. doi: 10.1038/nature13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abou-Alfa G.K., Macarulla T., Javle M.M., Kelley R.K., Lubner S.J., Adeva J., Cleary J.M., Catenacci D.V., Borad M.J., Bridgewater J., et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamarca A., Barriuso J., McNamara M.G., Valle J.W. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J. Hepatol. 2020;73:170–185. doi: 10.1016/j.jhep.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Javle M., Lowery M., Shroff R.T., Weiss K.H., Springfeld C., Borad M.J., Ramanathan R.K., Goyal L., Sadeghi S., Macarulla T., et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J. Clin. Oncol. 2018;36:276–282. doi: 10.1200/JCO.2017.75.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goyal L., Kongpetch S., Crolley V.E., Bridgewater J. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat. Rev. 2021;95:102170. doi: 10.1016/j.ctrv.2021.102170. [DOI] [PubMed] [Google Scholar]
- 53.Abou-Alfa G.K., Sahai V., Hollebecque A., Vaccaro G., Melisi D., Al-Rajabi R., Paulson A.S., Borad M.J., Gallinson D., Murphy A.G., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Javle M.M., Roychowdhury S., Kelley R.K., Sadeghi S., Macarulla T., Waldschmidt D.T., Goyal L., Borbath I., El-Khoueiry A.B., Yong W.-P., et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J. Clin. Oncol. 2021;39:265. doi: 10.1200/JCO.2021.39.3_suppl.265. [DOI] [Google Scholar]
- 55.Javle M., Roychowdhury S., Kelley R.K., Sadeghi S., Macarulla T., Weiss K.H., Waldschmidt D.-T., Goyal L., Borbath I., El-Khoueiry A., et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021;6:803–815. doi: 10.1016/S2468-1253(21)00196-5. [DOI] [PubMed] [Google Scholar]
- 56.Yoshikawa D., Ojima H., Iwasaki M., Hiraoka N., Kosuge T., Kasai S., Hirohashi S., Shibata T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br. J. Cancer. 2007;98:418–425. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raymond E., Faivre S., Armand J.P. Epidermal Growth Factor Receptor Tyrosine Kinase as a Target for Anticancer Therapy. Drugs. 2000;60:15–23. doi: 10.2165/00003495-200060001-00002. [DOI] [PubMed] [Google Scholar]
- 58.Harding J., Cleary J., Shapiro G., Braña I., Moreno V., Quinn D., Borad M., Loi S., Spanggaard I., Stemmer S., et al. Treating HER2-mutant advanced biliary tract cancer with neratinib: Benefits of HER2-directed targeted therapy in the phase 2 SUMMIT ‘basket’ trial. Ann. Oncol. 2019;30:iv127. doi: 10.1093/annonc/mdz154.004. [DOI] [Google Scholar]
- 59.Ueno M., Ikeda M., Morizane C., Kobayashi S., Ohno I., Kondo S., Okano N., Kimura K., Asada S., Namba Y., et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol. Hepatol. 2019;4:611–621. doi: 10.1016/S2468-1253(19)30086-X. [DOI] [PubMed] [Google Scholar]
- 60.Kim R.D., Chung V., Alese O.B., El-Rayes B.F., Li D., Al-Toubah T.E., Schell M.J., Zhou J.-M., Mahipal A., Kim B.H., et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020;6:888–894. doi: 10.1001/jamaoncol.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ioka T., Ueno M., Oh D.-Y., Fujiwara Y., Chen J.-S., Doki Y., Mizuno N., Park K., Asagi A., Hayama M., et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC) J. Clin. Oncol. 2019;37:387. doi: 10.1200/JCO.2019.37.4_suppl.387. [DOI] [Google Scholar]
- 62.Yoo C., Oh D.-Y., Choi H.J., Kudo M., Ueno M., Kondo S., Chen L.-T., Osada M., Helwig C., Dussault I., et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J. Immunother. Cancer. 2019;8:e000564. doi: 10.1136/jitc-2020-000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chakrabarti S., Kamgar M., Mahipal A. Targeted Therapies in Advanced Biliary Tract Cancer: An Evolving Paradigm. Cancers. 2020;12:2039. doi: 10.3390/cancers12082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang W., Liu H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 65.Rizzo A., Di Federico A., Ricci A.D., Frega G., Palloni A., Pagani R., Tavolari S., Di Marco M., Brandi G. Targeting BRAF-Mutant Biliary Tract Cancer: Recent Advances and Future Challenges. Cancer Control. 2020;27:1073274820983013. doi: 10.1177/1073274820983013. [DOI] [PMC free article] [PubMed] [Google Scholar]