Abstract
Yield is one of the most important agronomic traits for the breeding of rapeseed (Brassica napus L), but its genetic dissection for the formation of high yield remains enigmatic, given the rapid population growth. In the present review, we review the discovery of major loci underlying important agronomic traits and the recent advancement in the selection of complex traits. Further, we discuss the benchmark summary of high-throughput techniques for the high-resolution genetic breeding of rapeseed. Biparental linkage analysis and association mapping have become powerful strategies to comprehend the genetic architecture of complex agronomic traits in crops. The generation of improved crop varieties, especially rapeseed, is greatly urged to enhance yield productivity. In this sense, the whole-genome sequencing of rapeseed has become achievable to clone and identify quantitative trait loci (QTLs). Moreover, the generation of high-throughput sequencing and genotyping techniques has significantly enhanced the precision of QTL mapping and genome-wide association study (GWAS) methodologies. Furthermore, this study demonstrates the first attempt to identify novel QTLs of yield-related traits, specifically focusing on ovule number per pod (ON). We also highlight the recent breakthrough concerning single-locus-GWAS (SL-GWAS) and multi-locus GWAS (ML-GWAS), which aim to enhance the potential and robust control of GWAS for improved complex traits.
Keywords: quantitative trait loci, multi-locus GWAS, yield-related traits, high-throughput genotyping
1. Introduction
Rapeseed (Brassica napus L., genome AACC, 2n = 38) is one of the second top-most oilseed crops predominantly grown for protein meal and vegetable oil across the world [1,2,3,4]. Feeding the ever-expanding population is a major challenge due to the ever-expanding demand from humans and the production of biofuels increases global food security [5,6]. To fulfill the global food demand, grain production is expected to increase up to 50% by 2025 [7]. Therefore, to accomplish this exploiting demand, different plant varieties with improved agronomic traits will steadily need to be generated. Various agronomic traits include stress-inducive response, yield and yield-related traits, which are controlled by many genes that are being significantly influenced by the environment [8]. Therefore, to elaborate the actual mechanism of agronomic traits, the dissection and isolation of complex traits into the single chromosome locus and their characterization for each quantitative trait locus (QTL) is imperatively noticed.
With the rapid progress in sequencing technology and bioinformatics tools, QTL analysis, offering the ever-increasing opportunity, has been a highly significant, precise and efficient genotyping approach that utilizes molecular markers (e.g., single nucleotide polymorphism, SNPs) by engineering a powerful marker-based selection system that stringently controls the complex genomic traits [9]. For gene mapping, the population of 60 K illumines Infinium SNP arrays for B. napus can be sustainably transferred to a gene-based, low-cost and high-throughput genotype-based screening method [10]. To control the desired trait, this system is tremendously effective in mapping the QTLs within a narrow-range genomic level; it can also be the source of supply markers within the desired traits [11]. A previous study has incredibly triggered the rapid outcomes between Arabidopsis and Brassica species. These outcomes showed that 12 genes have been identified with 8 quantitative trait nucleotides (QTNs) underlying seed weight. Moreover, a single gene-specific marker (BnAP2) was also identified [12].
Linkage disequilibrium (LD) mapping, also known as association mapping, demonstrates the statistical relationship between the genetic markers and phenotypes within the natural populations, which constitutes an efficient approach for the association mapping of QTL traits. Genome-wide association studies (GWAS) are an effective and promising approach for partitioning complex traits [13,14]. More recently, GWAS has been promisingly involved for many crop varieties [15], including Avena sativa [16], Sorghum bicolor [17], Hordeum vulgare [18], Triticum aestivum [19], Glycine max [20], Oryza sativa [21], Zea mays [22], Arachis hypogaea [23] and Brassica napus [24].
The analysis of these crops by QTL/GWAS methods will be rapidly expanding and applied to cereals crops. Therefore, the present study mainly focuses on the rapeseed QTL characteristics that are an important model for future research. The current review also provides a benchmark summary of the recently studied literature with a major concern on rapeseed QTLs that demonstrate a significant role in future breeding strategies. Furthermore, this study also highlights the comprehensive information about both the single-locus GWAS (SL-GWAS) and multi-locus GWAS (ML-GWAS) approaches, which can expand the robustness of GWAS for complex genetic traits.
2. Breeding Objectives in Rapeseed Crop
To focus on breeding strategies in almost all cultivated plants to improve their yield, more specifically, seed yield is our major target. In rapeseed, seed yield remains an important breeding goal. In particular, oil quality, low erucic acid and glucosinolates contents have been important aspects for breeding in rapeseed cultivars [25,26]. These efforts have been utilized to reduce linolenic acid and to improve oleic acid contents, shelf-life and palatability of the oil for human consumption [27,28] and have led to the development of double-low cultivars [25,29], known as “HOLLi”. In addition to oil quality, seed oil contents are also becoming a key target. Researchers have concentrated their attention on exploring the genetic effects and mechanisms controlling oil production [30] to satisfy edible oil and biofuels production requirements. In this regard, several QTLs have been identified maintaining oil contents [31,32] and improving the quality of rapeseed breeding. The targets are not only focused on seed oil content but also on protein content to enhance the energy value of meals for livestock as a feed resource. To improve the feed meal, researchers have focused on reducing the level of glucosinolates, tannins and sinapate esters [26]. Genetic engineering strategies have successfully improved the seed protein content by increasing the utility of essential amino acids.
Further objectives have been focused on quality improvement, including reducing tannin contents in the seed protein and decreased fiber content [25,26] to boost meal quality and palatability; as mentioned, seed yield is the priority effort of breeders. According to Nesi et al. [26], seed yield has been increased by 50% during the last fifty years. However, the increasing demands on the plant’s edible oil and biofuels attracted the breeders to breed cultivars with increased production [29]. Besides yield improvement, researchers have also been focused on environmental stress and tolerance through engineered cultivars that resist environmental stresses, such as salinity and alkalinity stress [33,34], water stress [35] and nutritional deficiency [36,37,38]. These efforts will improve the final seed yield. Yield is the most important and complex feature in crop plants that reflects the environmental interaction and governs the developmental processes and growth events prevailing the entire lifecycle of the plant [39].
There are three direct factors for seed yield, including seed weight, seed number and silique number per plant. Other factors that indirectly affect the component traits include biomass, harvest index, plant architecture and adaptation, resistance to the biotic and abiotic constraints [40]. Hence, seed yield can be improved by keeping into consideration the direct component features and the other indirect contributing traits. Previous reports described some morphological and agronomic traits, such as siliques (pods) per plant, seed per silique, silique length, seed weight, plant height and oil contents [40,41,42], as yield accelerating traits. These yield accelerating traits can be used based upon selection criteria for yield improvement in rapeseed. Among the contributing traits, siliques per plant (SP) and seeds per silique contribute to the total number of seeds produced by the plant; thus, they directly control and determine the seed yield [43]. Moreover, these traits are further controlled by other plant traits such as plant height, branches per plant, silique density on the plant and silique traits (silique length, number of siliques, etc.). Analyzing the fundamental genetic mechanism and control of these traits separately will help to understand the dependent traits, hence improving seed yield in B. napus [44].
3. The Role of Genome-Wide Association Studies (GWAS) in Molecular Plant Breeding
The GWAS technique has been efficiently utilized to integrate novel traits in crops, which ameliorate the statistical correlation between genetic markers and phenotypic traits in the various crop varieties within the natural populations [45,46,47]. GWAS is a well-known technique in the framework of human genetics and possesses many useful aspects to cover and straighten out a variety of positive correlations between complicated diseases, as well as common/useful variants, but, due to the missing heritability, which still comes across as a problematic challenge, millions of molecular markers and the majority of individuals would be prerequisite to identify a wide range of QTLs. Nonetheless, in plant breeding, the missing heritability seems to be less serious due to some genetic variants, which explicitly demonstrates the phenotypic variation [48]. More recently, GWAS has been promisingly involved for many crop varieties [15], such as Oryza sativa [21], Zea mays [22], Triticum aestivum [19], Hordeum vulgare [18], Avena sativa [16], Brassica napus [24], Glycine max [20], Arachis hypogaea [23] and Sorghum bicolor [17], cataloged in Table 1. In plant breeding, GWAS methodologies seem to be more successful, because the previous findings coherently demonstrated the greater extent of phenotypic variations over the human GWAS outcomes [48]. The drawback of GWAS is the “fanciful” fabrication between the desired trait of interest and molecular markers. The previous outcomes demonstrated that the cryptic population is one of the significant determinants of fictitious relations [49,50]. Pritchard et al. [51] implicitly visualized the complete population structure that is based on the Bayesian clustering approach (STRUCTURE). They standardized a K population’s model where the individuals were nominated according to their genotype ratio among different population varieties. They also predicted the allele frequency of the population. Price et al. [52] developed a new strategy through the routine practice of principal component analysis in order to interpret the population structure in a given genetic dataset [50] that significantly governs the statistics of “axes of variation”. Moreover, various methods have been proved, although they have shown limited success [51,52]. Apart from these conventional methodologies adopted for phenotypic records and pedigree analysis, modification at the DNA level seems to contribute sufficient information about the principal population structure [53]. In crop breeding, the information about population structure can perform a vital role in establishing the manipulation of competent germplasm [54]. Thus, due to its wide range of applications, GWAS has the efficacy to be utilized directly in plant breeding programs [55].
Table 1.
The enigmatic role of genome-wide association studies in various crop species.
| Crop | Population Size | Markers | Traits | References |
|---|---|---|---|---|
| Aegilops tauschii | 322 | 7185 SNPs | Morphological traits | [56] |
| B. napus | 523 | 41 SNPs | Flowering time | [57] |
| B. napus | 248 | 60K SNPs | Seed germination and vigor | [58] |
| B. napus | 472 | 60,000 SNPs | Seed weight and quality | [47] |
| B. napus | 155 | 35,791 SNPs | Harvest index | [59] |
| B. napus | 521 | 60K SNPs | Seed oil content | [60] |
| B. napus | 523 | 60K SNPs | Flowering time | [61] |
| B. napus | 348 | 60K SNPs | Silique related traits | [62] |
| B. napus | 472 | 19,945 SNPs | Plant height and primary branch number | [63] |
| B. napus | 143 | 60K SNPs | Branch angle | [64] |
| B. napus | 520 | 60K SNPs | Branch angle trait | [65] |
| B. napus | 158 | 60K SNPs | Flowering time and yield responses | [66] |
| B. napus | 192 | 369 SSR, 740 AFLP | Yield-related traits | [44] |
| B. napus | 370 | 60K SNPs | Quantity of fatty acids | [67] |
| B. napus | 422 | 60K SNPs | Root development | [68] |
| B. napus | 333 | 60K SNPs | PH, BIH, and BN | [69] |
| B. napus | 588 | 385,692 SNPs | Oil content | [70] |
| B. napus | 300 | 201,187 SNP | Fatty acid composition | [71] |
| B. napus | 216 | 30,262 SNPs | Root architectural traits | [72] |
| B. napus | 157 | 742 SNPs | Seed weight and silique length | [73] |
| B. napus | 327 | 33,186 SNPs | Branching morphogenesis. | [74] |
| B. napus | 435 | 60K SNPs | Fatty acid composition and content | [75] |
| B. napus | 331 | 60K SNPs | Silique number | [76] |
| B. napus | 307 | 60K SNPs | Seed glucosinolates contents | [77] |
| B. napus | 419 | 60K SNPs | Seedling stage | [78] |
| B. napus | 300 | 201,817 SNPs | Earliness traits | [79] |
| B. napus | 368 | 60K SNPs | Salt tolerance-related traits | [34] |
| B. napus | 520 | 60K SNPs | Fatty acid composition | [80] |
| B. napus | 210 | 23,435 SNPs | Hypocotyl elongation | [81] |
| B. napus | 520 | Brassica 60K | Seven yield-determining traits | [82] |
| Barley | 175 | 107 SSRs | Seed aging and longevity | [83] |
| Barley | ~500 | 1536 SNPs | Morphological trait | [84] |
| Barley | 122 DH lines | 9680 SNPs | 14 main agronomic traits | [85] |
| Barley | 275 | 9K SNP, 3072 SNP | Six-rowed spring barley | [86] |
| Barley | 233 | 7864 SNPs | Root and shoot architecture traits | [87] |
| Barley | 25 | 9 K iSelect SNPs | Plant growth under drought stress | [88] |
| Barley | 222-2-303 6-rowed | 7864 SNPs. | Adherence of hulls to the caryopsis | [89] |
| Barley | 109 | 15,828 DArTseqs and 7829 SNPs | Chlorophyll fluorescence induction (OJIP) parameters | [90] |
| Barley | 166 | 777 DArT markers | Drought stress | [91] |
| Cotton | 169 | 53,848 SNPs | Fiber quality traits | [15] |
| Cotton | 319 | 55,060 SNPs | Drought stress | [92] |
| Cotton | 231 | 122 SSR and 4729 SNP markers | Fiber quality traits and yield components |
[93] |
| Cotton | 196 | CottonSNP80K | Salt tolerance | [94] |
| Cotton | 316 | 81,675 SNP | Drought tolerance | [95] |
| Cotton | 83 | 15,369 SNPs | Oil content | [96] |
| Cotton | 215 | ~1.57 million SNPs | Salt tolerance | [97] |
| Flax | 370 | 258,873 SNPs | Pasmo resistance | [98] |
| Foxtail millet | 916 | 0.8 m SNPs | Agronomic traits | [99] |
| Maize | 384 | 681,257 SNPs | Seedling root architecture traits | [100] |
| Maize | 999 | 56,110 SNPs | Northern corn leaf blight resistance | [101] |
| Maize | 368 | 1.03 m SNPs | Kernel oil concentration fatty acid composition | [102] |
| Maize | 25000 | SNPs | Southern leaf blight resistance | [103] |
| Maize | - | SNPs | Leaf architecture | [104] |
| Maize | 144 | 43,427 SNPs | The regenerative capacity of the embryonic callus | [13] |
| Maize | 257 | 48,193 SNPs | Stalk lodging resistance | [14] |
| Maize | 230 | 145,232 SNPs | Starch pasting properties | [61] |
| Rice | 413 | 44,100 SNPs | Agronomic traits | [105] |
| Rice | 9 | 71,710 SNPs | Agronomic traits | [106] |
| Rice | 220 | 6000 SNPs | Salinity tolerance | [107] |
| Rice | 517 | ∼3.6 m SNPs | Agronomic traits | [108] |
| Rice | 950 | SNPs | Flowering time grain-related traits | [109] |
| Rice | 20 | 32,655 SNPs | Agronomic traits | [110] |
| Rice | 236 | 147,692 SNPs | Cooked rice texture | [111] |
| Rice | 478 | 162,529 SNPs | Salt-tolerance | [112] |
| Sorghum | 971 | ~26,500 SNPs | Plant height and architecture | [17] |
| Sorghum | 245 | 85,885 | Forage quality-related traits | [113] |
| Soybean | 96 | SSRs | Plant height pods/plant 100-seed weight plant growth habit seeds/pod days to 50% flowering and maturity |
[114] |
| Soybean | 368 | 62,423 SNPs | Plant height and the number of nodes | [115] |
| Soybean | 219 | 292,035 SNPs | Photosynthetic response to low P stress | [116] |
| Soybean | 144 | SoySNP660k | Protein content | [117] |
| Sugarcane | 20 | 20 SSRs | Cane weight tillers/plant | [118] |
| Tomato | 174 | 182 SSRs | Flavor traits | [119] |
| Wheat | 382 | SNPs | Agronomic traits and carbon isotope | [120] |
| Wheat | 182 | 14,646 SNPs | 20 free amino acid | [121] |
| Wheat | 339 | 13,098 SNPs | Karnal bunt resistance | [122] |
| Wheat | 160 | 10,172 SNPs | Wheat quality and yield-related traits | [123] |
| Wheat | 635 | 10,802 SNPs | Flour yield and alveograph quality traits | [124] |
The Role of QTLs and GWAS in Three Yield-Related Traits in Rapeseed
Yield is the most important but one of the complex traits in crops. Because of the ever-growing population, the increase in the food demand has been deemed a global concern and is now becoming the major challenge for the speedy breeding of plant cultivars to generate high yield oilseed rape cultivars with increasing agricultural sustainability and productivity to fulfill the burgeoning demand globally [125,126]. Besides pod number, seeds per silique (SS) and thousand-seed weight (TSW) are the other two important yield-determining components of a single plant, both of which are directly associated with ovule development. According to a recent assessment of rapeseed cultivars, the approximated number of seeds per pod is about ~20, which is thought to be far lower than the germplasm ratio, which exhibited a higher range of ~30 [127,128]; this holds the scientists’ interest in the genetic modification and improvement of rapeseed cultivars by means of increasing the number of seed per silique. The most important factors that control the number of seeds per silique include the number of ovary/ovules and the number of unfertile/fertile ovule, as well as the number of fertile ovules that convert into seeds. In B. napus, the number of ovules per ovary is measured through different phases occurring during the ovule development [129]. In contrast, the number of unfertile/fertile ovules depends on various fertilization events, i.e., pollen/pollen tubes interaction, pollen sterility and ovule fertility [130,131]. The most desirable outcomes of the fertilized ovule that develops into a seed are principally governed by the biological process of seed development characterized by physiological and nutritional requirements, as well as many other environmental factors, such as abiotic and biotic stimulus [130,132]. QTL mapping for ovule numbers had been studied in other plants, such as Glycine max [133], Vicia faba [133] and Raphanus sativa [134]. More recently, Yuan and Kessler [135] found a locus associated with NERDI in A. thaliana, which plays a vital role in the regulation of ovule number in both female and male gametophytes. The above discussion suggests that limited literature is available in regard to the genetic basis for ovule number in crops, especially in oilseed rape. To the best of our knowledge, there is no study conducted for ON in B. napus yet.
Additionally, the development of high yielding varieties is a major goal in rapeseed breeding, which is determined by three yield components, i.e., siliques per plant (SP), SS and SW [43]. Previous studies articulately observed a negative interaction between silique-related traits. Furthermore, it was also determined that these traits had a derogatory impact on the breeding event by scrutinizing QTNs and genes, which is accomplished for each desirable trait [136,137]. Over the last ten years, TSW has shown rapid development in the field of molecular marker technology [40]. Recently, ~65 and ~168 QTLs, for SS and TSW, respectively, have been examined in 19 linkage groups [37,40,41,43,136,137,138,139,140,141,142,143,144,145,146,147,148,149].
Li et al. [150] detected 133 QTLs for 12 yield-related traits (including SS and TSW, etc.) in rapeseed, containing 14 QTLs consistently identified across two locations. Most of the QTLs were found on the N2 and N7 linkage positions. Radoev et al. [142] also identified 33 QTLs for seed yield and yield component traits at four locations. These results revealed that 10 QTLs had a significant effect on the target traits. On the other hand, Shi et al. [40] employed an extensive study on two populations, i.e., RC-F2 and TNDH, in ten environments. They found 85 QTLs for seed yield and 785 QTLs for 8 yield-related traits (seed number per silique, thousand seed weight, etc.). Bagheri et al. [151] identified 47 QTLs for 17 traits associated with seed yield and plant architecture, which explained from 6% to 56% of the total variance for the targeted traits. Shi et al. [137] found one major QTL (qSN. A06) for SS, which explained 32.1% of the phenotypic variation. Similarly, Yang et al. [129] also found a major QTL for SS on qSN. A06. Raman et al. [152] identified 2 QTLs on Chr A03 and Chr A07, explaining from 5% to 19% of the phenotypic variation for FT and SY. Zhao et al. [153] detected 18 QTLs for SYs and 208 QTLs for YDTs. Recently, Luo et al. [149] studied 22 traits and found 1904 QTLs; among them, 80 QTLs were associated with yield, while 535 QTLs contributed to SY.
Seed size/weight is also an essential factor in Sys [43], having greater heritability than the other yield component traits (YCTs) [40]. In different populations, 6, 4 and 7 QTLs for seed weight were identified located on N7, N17 and N19 linkage groups, respectively [141]. Shi and his group discovered a major QTL qSW.A07-2 [40], while Fan and his colleagues also identified 2 major QTLs TSWA07a and TSWA7b on the same chromosome, which explained from 27.6% to 37.9% of the variation in said trait [41]. Further, Yang and his group detected 1 major QTL (cqSWA09), which also explained high variation (28.2%) for silique length and seed weight [146]. Li et al. [47] carried out a comprehensive study about seed weight and silique length (SL) and 13 and 9 QTLs were identified, which showed the highest variations of 67% and 54%, respectively. Some QTLs were consistently detected through experiments and the authors suggested that these QTLs were more stable and reliable for future study. Using two different populations, 21 and 20 QTLs were identified for seed weight and silique length. The ranges of phenotypic variations for seed weight (SW) and seed length (SL) were observed to be from 24.4% to 62.9% and from 55.1% to 74.3%, respectively [154].
Based on a 60K SNP array, some studies were conducted on various traits of B. napus (flowering time and harvest index) [61,155]. Based on a 60K SNP array, Li et al. [47] identified significant SNPs for erucic acid content (A08 and C03), glucosinolates content (A09, C02, C07 and C09) and seed weight (A07 and A09). These results revealed that the identified significant SNPs were suitable for fine mapping complex traits of B. napus [47]. Cai et al. [44] employed GWAS for six yield-related traits using 192 inbred lines of rapeseed. They identified seven and nine associated markers for seed per silique and thousand seed weight. These lines were genotyped using 451 SLM markers and 740 AFLP markers. Schiessl et al. [66] studied seed yield and yield-determining traits using a 60K SNP array and identified 36 loci associated with target traits in B. napus. GWA studies have not been extensively adopted in the genetic dissection of ovule number, seed per silique and thousand seed weight in rapeseed [66].
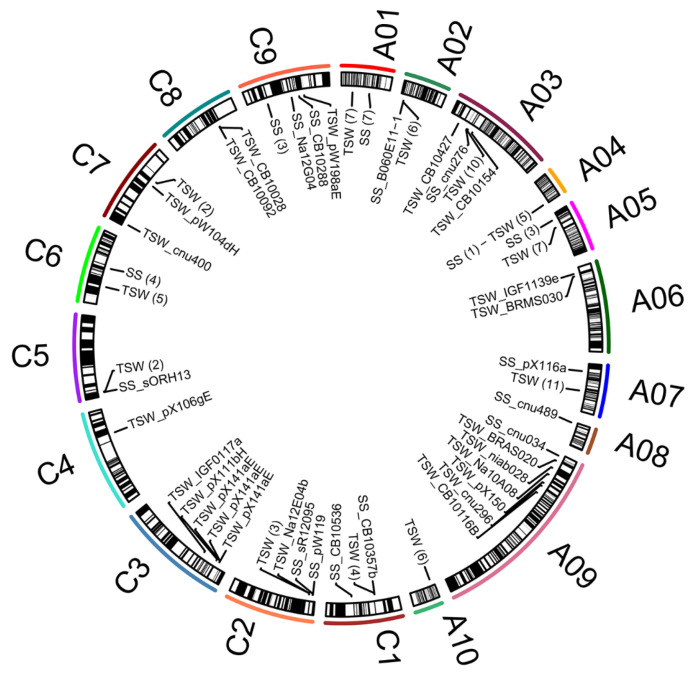
More recently, Khan et al. [4] carried out a GWAS analysis on 521 accessions of oilseed rape, genotyped with a Brassica 60 K SNP array, by using single-locus GWAS (SL-GWAS) and multi-locus GWAS (ML-GWAS) methods. The outcomes of this study presented the significant numbers of 31 and 280 QTLs/QTNs, that were detected by analyzing SL-GWAS and ML-GWAS methodologies, respectively. Among these sequences, 74 common significant QTNs (which include 8 for ovule number (ON), 32 for SS and 34 for TSW) were repeatedly detected by more than three ML-GWAS models and in multiple environments [4]. Figure 1 shows the distribution of important loci associated with seed per silique and thousand-seed weight across the chromosomes of rapeseed by QTL and GWAS studies.
Figure 1.
Distribution of QTLs for seeds per silique (SS) and seed weight (TSW) across the chromosomes (A01–A10; C1–C9) of rapeseed. Values between parentheses indicate the number of QTLs for a given trait (for details see Supplementary Table S1).
We greatly emphasize that the genetic modification of these traits may also improve rapeseed molecular breeding to develop eco-friendly and improved yield-related cultivars. The outcomes analyzed by this approach may principally favor a strong revolution for the improvement of rapeseed.
4. Candidate Genes and Superior Alleles of Seed Yield Related Loci Identified in Rapeseed
The candidate gene approach implies the identification of significant genes that are important for quantitative traits and agriculture. The candidate gene approach was first used for maize (flowering time) [156,157] and then also used for many important traits [158,159]. Recently, in B. napus three independent pieces of literature were available on candidate genes related to YDTs [126]. Zhao and his group identified candidate genes for seed yield (4), TSW (2) and plant height (1) [33]. Zheng and his colleagues also detected candidate genes, 31 for plant height, 15 for branch initiation height and 17 for branch number using diverse oilseed rape accessions [69]. Lu et al. [82] used 520 accessions of rapeseed and discovered 6, 7, 7 and 3 candidate genes for seed per silique, pod number, branch pod number and TSW, respectively. Khan et al. [4] further prophesied the genes associated with SS, TSW and ON, respectively. They found a total of 42 candidate genes, which were homologous to A. thaliana yield-determining traits, which lie in the range of QTLs. The candidate genes for ON, SS and TSW were identified in the numbers of 3, 17 and 20, respectively, whereas 2 candidate genes were linked with SS/TSW (Bn-A09-p30391674/Bn-A09-p30404228) or TSW/SS (Bn-A08-p16523108/Bn-scaff_16665-p54637).
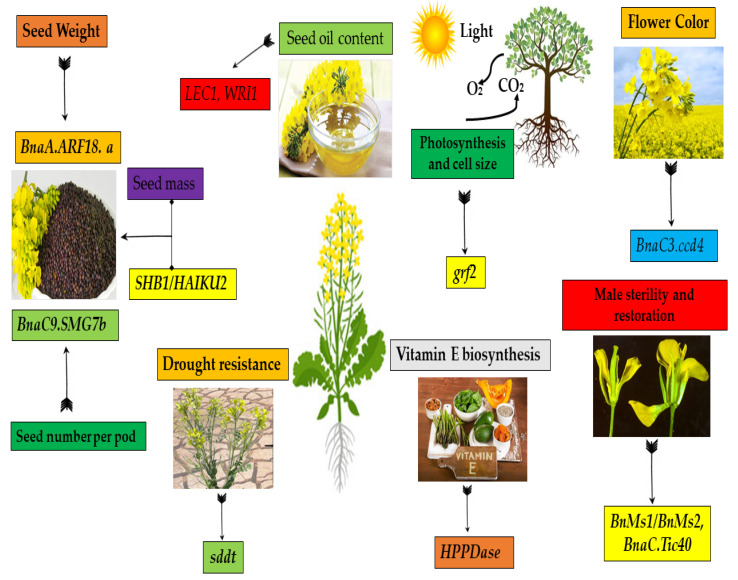
In rapeseed, only two findings were reported that demonstrated the gene cloning for TSW and SS, respectively. For SS, qSS.C9, the QTL is thought to be one of the important QTLs [160] that play a significant role in developing female gametophytes [131]. Additionally, the ARF18 gene controls both TSW and SL QTL formation [161]. Moreover, the ARF18 gene slowed down the function of the auxin gene and had an inhibitory effect on its biological activity, which was assumed to modulate the activity of silique wall development and TSW by regulating maternal genes [161]. Furthermore, many other genes also play a tremendous role in cloning many other important traits, viz. yield, abiotic stress, seed oil, disease resistance, etc., as presented in Figure 2.
Figure 2.
This figure presents the major role of cloned genes for key traits in rapeseed, viz: yield, drought stress resistance, seed oil, flower color, disease resistance, photosynthetic rate, etc.
More recently, Ma et al. [13] identified, in maize crop, 63 common QTNs within the 31 elite inbred lines. Of them, 36 QTNs were showed in <50% superior alleles, which anticipated that these identified alleles were not appropriate for the artificial selection method, whereas 27 further QTNs were identified to be >50%; therefore, these findings confirmed that these alleles were the best match for artificial selection [13]. Subsequently, the recent outcomes of Khan et al.’s study [4] identified 74 significant QTNs by utilizing multiple methods in a different environment. The identified QTNs were determined to be significant QTNs that were strongly associated with yield-determining traits. Of these, 8, 13 and 34 QTNs were observed to have superior alleles for ON, SS and TSW, respectively. In fact, the allele percentage ranged from 9.4% to 85.41%, from 7.67% to 82.34% and from 4.79% to 68.13%, for SS, TSW and ON, respectively. Interestingly, one of the identified QTN for ON (Bn-A09-p10297982) had the highest percentage proportion (67%), whereas the remaining seven showed less than 50%. For SS, 31 QTNs were identified, in which 13 had superior alleles >50%, whereas 18 of the identified QTNs had <50% allele ratio. Lastly, for TSW, among 34 identified QTNs, 14 QTNs showed superior alleles for more than 50%, whereas 20 QTNs showed fewer superior alleles, about 50%. Remarkably, the single QTNs for both TSW (Bn-A03-p24823015) and SS (Bn-A03-p403559) had the highest proportion of superior alleles, 82.34% and 85.41%, respectively. Therefore, the authors argued that these identified superior alleles showed an effective role in the above-aforementioned yield-determining traits. We strongly believe that the obtained high percentage of superior alleles will be convenient for high yield production in the revolution of rapeseed breeding. Until now, there is no significant study organized to find the superior alleles in rapeseed. In the marker-assisted selection (MAS) method, the highest percentage of superior alleles is very accessible. This strategy also showed significant insights into seed yield and other useful economic traits in many plant species.
5. Comparative Overview of Single-Locus and Multi-Locus GWAS Methodologies
To improve yield-determining traits (YDTs), contributing to a better understanding of their genetic basis and diversity, recently, genome-wide association studies (GWAS) approaches have been extensively used to dissect the complex traits in crops. Before this, most of the findings have been reported to have utilized single-locus GWAS, such as the mixed linear model (MLM) ([162,163]), while, recently, various new MLM-based models have been introduced [164]. More comprehensively, these novel strategies have various applications in the genetic integration of novel and omics-related traits, facilitating the recent breakthrough in the generation of bioinformatics and sequencing strategies. Additionally, single-locus models have some pitfalls, including the GLM principally showing high false-positive rates (FPR). In contrast, MLM used Bonferroni corrections for the identification of loci to mitigate the chances of FPR, though this process is very rigorous and the outcomes for some important loci data are still missing. To overcome these limitations, Zhang et al. [165] utilized different multi-locus GWAS approaches, including multi-locus random-SNP-effect mixed linear model (mrMLM) [166], iterative Modified-Sure Independence Screening EM-Bayesian LASSO (ISIS EM-BLASSO) [167], fast multi-locus random-SNP-effect EMMA (FASTmrEMMA) [168] and pLARmEB [169]. In a previous study, Li et al. [170] demonstrated that the comprehensive approach of GWAS is very effective in identifying the number of QTNs, more specifically in B. napus, by utilizing the ML-GWAS and SL-GWAS methods.
The mrMLM method enhances the identification of loci by more than 55% throughout the covered region. For instance, Misra et al. [171] determined the significant rice variants in determining rice grain by employing ML-GWS and SL-GWAS methods. Therefore, the combined utilization of the ML-GWAS and SL-GWAS methodologies was useful to detect the genetic locus of GWi7.1, GWi7.2 and to identify novel genes. Moreover, Xu et al. [172] utilized multi-locus and single-locus GWAS methods to quantify the significance of novel QTNs in the pasting traits of maize starch. In contrast to the ML and SL-GWAS methods, it was confirmed that ML-GWAS FASTmrEMMA had novel QTNs (29), whereas SL-GWAS (GEMMA) showed a much lower number of novel QTNs (7) [172]. More recently, Peng et al. [121] utilized six ML-GWAS methods to determine the genetic dissection of 20 amino acid levels in Triticum aestivum L. As a result, they achieved that ML-GWAS models are very authentic and dynamic [121]. Correspondingly, Cui et al. [112] confirmed that, via this multi-locus GWAS approach, most of the QTNs were discovered by following ISIS EM-BLASSO [112]. Additionally, Su et al. [173] showed research findings in the genetic dissection of upland cotton, in which 70 QTNs were identified. They concluded that the ML-GWAS methods are much more powerful and authentic than the single-locus GWAS (MLM) methods in TASSEL v5.0 [173]. Finally, the results mentioned above confirm the strengths of ML-GWAS strategies as compared to SL-GWAS methods, although, more recently, the outcomes of some studies recommend that the co-interaction of single-locus and multi-locus GWAS methods significantly enhances the identification of rationality and robustness of GWAS [97,115,165,172].
6. Conclusions, Future Trends, and Perspectives
This review presented the robustness and power of QTL/GWAS analyses, candidate gene association studies and superior allele identification in Brassica napus. Follow-up work can be carried out on the following aspects:
The genetic analysis of phenotypic traits has found novel and significant QTNs/QTLs/candidate genes, giving research a chance to progress, more specifically, in the identification of the activity of these genes and further recognize the mechanism of the genetic constitution of the traits of thousand seed weight, ovule number per ovary and seed number per silique through crosswise adverse geographical and climatic conditions.
The information collected from previous studies enriches the knowledge of variations in populations in the three above-mentioned yield-related traits; therefore, this warrants the need for supplementary authentication that will deliver markers through the integration into the host plant improvement.
The association analysis of the candidate gene method and the development of near-isogenic, transgenic, or mutant plants may be efficiently utilized to identify novel and significant alleles and how they interact with the above-mentioned yield-related traits.
Genomic regions reviewed in the present study merit attention for their further utilization in breeding programs that use marker-assisted selection (or genomics-assisted breeding) on Brassica napus.
Acknowledgments
Sunny Ahmar thanks to Agencia Nacional de Investigación y Desarrollo de Chile (ANID) Doctorado Nacional/2021-21210254.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biom11101516/s1, Table S1: Summary of the identified loci associated with seed per silique (SS) and thousand-seed weight (TSW) in rapeseed by QTL and GWAS studies.
Author Contributions
Conceptualization, S.U.K. and C.F.; writing—original draft preparation, S.S., S.U.K.; writing—review and editing, S.S., S.U.K., O.A., S.A., F.B. and F.M.-P.; resources, F.M.-P.; validation, R.S., S.U.K., M.H.U.K. and S.S.; visualization, R.S., M.H.U.K., S.S., O.A., S.A. and F.M.-P.; supervision, C.F. and F.M.-P. All authors have read and agreed to the published version of the manuscript.
Funding
F.M.-P. and S.A. thank the Chilean National Fund for Scientific and Technological Development (FONDECYT) Grant No. 1201973.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang Y., Zhu K., Li H., Han S., Meng Q., Khan S.U., Fan C., Xie K., Zhou Y. Precise editing of CLAVATA genes in Brassica napus L. regulates multilocular silique development. Plant Biotechnol. J. 2018;16:1322–1335. doi: 10.1111/pbi.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y., Shen Y., Li S., Ge X., Li Z. High density linkage map construction and QTL detection for three silique-related traits in Orychophragmus violaceus derived Brassica napus population. Front. Plant Sci. 2017;8:1512. doi: 10.3389/fpls.2017.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahid M., Cai G., Zu F., Zhao Q., Qasim M.U., Hong Y., Fan C., Zhou Y. Comparative Transcriptome Analysis of Developing Seeds and Silique Wall Reveals Dynamic Transcription Networks for Effective Oil Production in Brassica napus L. . Int. J. Mol. Sci. 2019;20:1982. doi: 10.3390/ijms20081982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan S.U., Yangmiao J., Liu S., Zhang K., Khan M.H.U., Zhai Y., Olalekan A., Fan C., Zhou Y. Genome-wide association studies in the genetic dissection of ovule number, seed number, and seed weight in Brassica napus L. Ind. Crop. Prod. 2019;142:111877. doi: 10.1016/j.indcrop.2019.111877. [DOI] [Google Scholar]
- 5.Takeda S., Matsuoka M. Genetic approaches to crop improvement: Responding to environmental and population changes. Nat. Rev. Genet. 2008;9:444–457. doi: 10.1038/nrg2342. [DOI] [PubMed] [Google Scholar]
- 6.Brown M.E., Funk C.C. Food Security under Climate Change. University of Nebraska; Lincoln, NE, USA: 2008. [Google Scholar]
- 7.Khush G.S. Challenges for meeting the global food and nutrient needs in the new millennium. Proc. Nutr. Soc. 2001;60:15–26. doi: 10.1079/PNS200075. [DOI] [PubMed] [Google Scholar]
- 8.Song X.-J., Ashikari M.J.R. Toward an optimum return from crop plants. Rice. 2008;1:135–143. doi: 10.1007/s12284-008-9018-3. [DOI] [Google Scholar]
- 9.Thomson M.J. High-throughput SNP genotyping to accelerate crop improvement. Plant Breed. Biotechnol. 2014;2:195–212. doi: 10.9787/PBB.2014.2.3.195. [DOI] [Google Scholar]
- 10.Clarke W.E., Higgins E.E., Plieske J., Wieseke R., Sidebottom C., Khedikar Y., Batley J., Edwards D., Meng J., Li R. A high-density SNP genotyping array for Brassica napus and its ancestral diploid species based on optimised selection of single-locus markers in the allotetraploid genome. Theor. Appl. Genet. 2016;129:1887–1899. doi: 10.1007/s00122-016-2746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar J., Gupta D.S., Gupta S., Dubey S., Gupta P., Kumar S. Quantitative trait loci from identification to exploitation for crop improvement. Plant Cell Rep. 2017;36:1187–1213. doi: 10.1007/s00299-017-2127-y. [DOI] [PubMed] [Google Scholar]
- 12.Cai G., Yang Q., Yang Q., Zhao Z., Chen H., Wu J., Fan C., Zhou Y. Identification of candidate genes of QTLs for seed weight in Brassica napus through comparative mapping among Arabidopsis and Brassica species. BMC Genet. 2012;13:105. doi: 10.1186/1471-2156-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma L., Liu M., Yan Y., Qing C., Zhang X., Zhang Y., Long Y., Wang L., Pan L., Zou C. Genetic dissection of maize embryonic callus regenerative capacity using multi-locus genome-wide association studies. Front. Plant Sci. 2018;9:561. doi: 10.3389/fpls.2018.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y., Liu P., Zhang X., Zheng Q., Chen M., Ge F., Li Z., Sun W., Guan Z., Liang T. Multi-locus genome-wide association study reveals the genetic architecture of stalk lodging resistance-related traits in maize. Front. Plant Sci. 2018;9:611. doi: 10.3389/fpls.2018.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C., Fu Y., Sun R., Wang Y., Wang Q. Single-locus and multi-locus genome-wide association studies in the genetic dissection of fiber quality traits in upland cotton (Gossypium hirsutum L.) Front. Plant Sci. 2018;9:1083. doi: 10.3389/fpls.2018.01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newell M., Cook D., Tinker N., Jannink J.-L. Population structure and linkage disequilibrium in oat (Avena sativa L.): Implications for genome-wide association studies. Theor. Appl. Genet. 2011;122:623–632. doi: 10.1007/s00122-010-1474-7. [DOI] [PubMed] [Google Scholar]
- 17.Morris G.P., Ramu P., Deshpande S.P., Hash C.T., Shah T., Upadhyaya H.D., Riera-Lizarazu O., Brown P.J., Acharya C.B., Mitchell S.E., et al. Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proc. Natl. Acad. Sci. USA. 2013;110:453–458. doi: 10.1073/pnas.1215985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visioni A., Tondelli A., Francia E., Pswarayi A., Malosetti M., Russell J., Thomas W., Waugh R., Pecchioni N., Romagosa I., et al. Genome-wide association mapping of frost tolerance in barley (Hordeum vulgare L.) BMC Genom. 2013;14:424. doi: 10.1186/1471-2164-14-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zegeye H., Rasheed A., Makdis F., Badebo A., Ogbonnaya F.C. Genome-wide association mapping for seedling and adult plant resistance to stripe rust in synthetic hexaploid wheat. PLoS ONE. 2014;9:e105593. doi: 10.1371/journal.pone.0105593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Song Q., Cregan P.B., Nelson R.L., Wang X., Wu J., Jiang G.-L. Genome-wide association study for flowering time, maturity dates and plant height in early maturing soybean (Glycine max) germplasm. BMC Genom. 2015;16:217. doi: 10.1186/s12864-015-1441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spindel J., Begum H., Akdemir D., Collard B., Redoña E., Jannink J., McCouch S. Genome-wide prediction models that incorporate de novo GWAS are a powerful new tool for tropical rice improvement. Heredity. 2016;116:395–408. doi: 10.1038/hdy.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y., Xu C., Xu S. Prediction and association mapping of agronomic traits in maize using multiple omic data. Heredity. 2017;119:174–184. doi: 10.1038/hdy.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X., Zhang J., He X., Wang Y., Ma X., Yin D. Genome-wide association study of major agronomic traits related to domestication in peanut. Front. Plant Sci. 2017;8:1611. doi: 10.3389/fpls.2017.01611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q., Zhou C., Zheng W., Mason A.S., Fan S., Wu C., Fu D., Huang Y. Genome-wide SNP markers based on SLAF-seq uncover breeding traces in rapeseed (Brassica napus L.) Front. Plant Sci. 2017;8:648. doi: 10.3389/fpls.2017.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittkop B., Snowdon R., Friedt W. Status and perspectives of breeding for enhanced yield and quality of oilseed crops for Europe. Euphytica. 2009;170:131. doi: 10.1007/s10681-009-9940-5. [DOI] [Google Scholar]
- 26.Nesi N., Delourme R., Brégeon M., Falentin C., Renard M. Genetic and molecular approaches to improve nutritional value of Brassica napus L. seed. Comptes Rendus Biol. 2008;331:763–771. doi: 10.1016/j.crvi.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Scarth R., Tang J. Modification of Brassica oil using conventional and transgenic approaches. Crop Sci. 2006;46:1225–1236. doi: 10.2135/cropsci2005.08-0245. [DOI] [Google Scholar]
- 28.Coonrod D.K. Inheritance of Oleic, Eicosenoic, and Erucic Acid Content in Canola. Colorado State University; Fort Collins, CO, USA: 2005. [Google Scholar]
- 29.Abbadi A., Leckband G. Rapeseed breeding for oil content, quality, and sustainability. Eur. J. Lipid Sci. Technol. 2011;113:1198–1206. doi: 10.1002/ejlt.201100063. [DOI] [Google Scholar]
- 30.Wang X., Wang H., Long Y., Li D., Yin Y., Tian J., Chen L., Liu L., Zhao W., Zhao Y. Identification of QTLs associated with oil content in a high-oil Brassica napus cultivar and construction of a high-density consensus map for QTLs comparison in B. napus. PLoS ONE. 2013;8:e80569. doi: 10.1371/journal.pone.0080569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmood T., Rahman M., Stringam G., Yeh F., Good A. Identification of quantitative trait loci (QTL) for oil and protein contents and their relationships with other seed quality traits in Brassica juncea. Theor. Appl. Genet. 2006;113:1211–1220. doi: 10.1007/s00122-006-0376-1. [DOI] [PubMed] [Google Scholar]
- 32.Zou J., Jiang C., Cao Z., Li R., Long Y., Chen S., Meng J. Association mapping of seed oil content in Brassica napus and comparison with quantitative trait loci identified from linkage mapping. Genome. 2010;53:908–916. doi: 10.1139/G10-075. [DOI] [PubMed] [Google Scholar]
- 33.Zhao W., Wang X., Wang H., Tian J., Li B., Chen L., Chao H., Long Y., Xiang J., Gan J. Genome-wide identification of QTL for seed yield and yield-related traits and construction of a high-density consensus map for QTL comparison in Brassica napus. Front. Plant Sci. 2016;7:17. doi: 10.3389/fpls.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan H., Chen L., Guo J., Li Q., Wen J., Yi B., Ma C., Tu J., Fu T., Shen J. Genome-wide association study reveals the genetic architecture underlying salt tolerance-related traits in rapeseed (Brassica napus L.) Front. Plant Sci. 2017;8:593. doi: 10.3389/fpls.2017.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., Lu G., Long W., Zou X., Li F., Nishio T. Recent progress in drought and salt tolerance studies in Brassica crops. Breed. Sci. 2014;64:60–73. doi: 10.1270/jsbbs.64.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding G., Yang M., Hu Y., Liao Y., Shi L., Xu F., Meng J. Quantitative trait loci affecting seed mineral concentrations in Brassica napus grown with contrasting phosphorus supplies. Ann. Bot. 2010;105:1221–1234. doi: 10.1093/aob/mcq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding G., Zhao Z., Liao Y., Hu Y., Shi L., Long Y., Xu F. Quantitative trait loci for seed yield and yield-related traits, and their responses to reduced phosphorus supply in Brassica napus. Ann. Bot. 2012;109:747–759. doi: 10.1093/aob/mcr323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang M., Ding G., Shi L., Xu F., Meng J. Detection of QTL for phosphorus efficiency at vegetative stage in Brassica napus. Plant Soil. 2011;339:97–111. doi: 10.1007/s11104-010-0516-x. [DOI] [Google Scholar]
- 39.Quarrie S., Pekic Quarrie S., Radosevic R., Rancic D., Kaminska A., Barnes J., Leverington M., Ceoloni C., Dodig D. Dissecting a wheat QTL for yield present in a range of environments: From the QTL to candidate genes. J. Exp. Bot. 2006;57:2627–2637. doi: 10.1093/jxb/erl026. [DOI] [PubMed] [Google Scholar]
- 40.Shi J., Li R., Qiu D., Jiang C., Long Y., Morgan C., Bancroft I., Zhao J., Meng J. Unraveling the complex trait of crop yield with quantitative trait loci mapping in Brassica napus. Genetics. 2009;182:851–861. doi: 10.1534/genetics.109.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan C., Cai G., Qin J., Li Q., Yang M., Wu J., Fu T., Liu K., Zhou Y. Mapping of quantitative trait loci and development of allele-specific markers for seed weight in Brassica napus. Theor. Appl. Genet. 2010;121:1289–1301. doi: 10.1007/s00122-010-1388-4. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L., Yang G., Liu P., Hong D., Li S., He Q. Genetic and correlation analysis of silique-traits in Brassica napus L. by quantitative trait locus mapping. Theor. Appl. Genet. 2011;122:21–31. doi: 10.1007/s00122-010-1419-1. [DOI] [PubMed] [Google Scholar]
- 43.Chen W., Zhang Y., Liu X., Chen B., Tu J., Tingdong F. Detection of QTL for six yield-related traits in oilseed rape (Brassica napus) using DH and immortalized F 2 populations. Theor. Appl. Genet. 2007;115:849–858. doi: 10.1007/s00122-007-0613-2. [DOI] [PubMed] [Google Scholar]
- 44.Cai D., Xiao Y., Yang W., Ye W., Wang B., Younas M., Wu J., Liu K. Association mapping of six yield-related traits in rapeseed (Brassica napus L.) Theor. Appl. Genet. 2014;127:85–96. doi: 10.1007/s00122-013-2203-9. [DOI] [PubMed] [Google Scholar]
- 45.Nordborg M., Weigel D. Next-generation genetics in plants. Nature. 2008;456:720. doi: 10.1038/nature07629. [DOI] [PubMed] [Google Scholar]
- 46.Mandel J.R., Nambeesan S., Bowers J.E., Marek L.F., Ebert D., Rieseberg L.H., Knapp S.J., Burke J.M. Association mapping and the genomic consequences of selection in sunflower. PLoS Genet. 2013;9:e1003378. doi: 10.1371/journal.pgen.1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li F., Chen B., Xu K., Wu J., Song W., Bancroft I., Harper A.L., Trick M., Liu S., Gao G. Genome-wide association study dissects the genetic architecture of seed weight and seed quality in rapeseed (Brassica napus L.) DNA Res. 2014;21:355–367. doi: 10.1093/dnares/dsu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brachi B., Morris G.P., Borevitz J.O. Genome-wide association studies in plants: The missing heritability is in the field. Genome Biol. 2011;12:232. doi: 10.1186/gb-2011-12-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C. Population subdivision with respect to multiple alleles. Ann. Hum. Genet. 1969;33:23–29. doi: 10.1111/j.1469-1809.1969.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 50.Lander E.S., Schork N.J. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- 51.Pritchard J.K., Stephens M., Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price A.L., Patterson N.J., Plenge R.M., Weinblatt M.E., Shadick N.A., Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006;38:904. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 53.Varshney R.K., Graner A., Sorrells M.E. Genic microsatellite markers in plants: Features and applications. TRENDS Biotechnol. 2005;23:48–55. doi: 10.1016/j.tibtech.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Bus A., Körber N., Snowdon R.J., Stich B. Patterns of molecular variation in a species-wide germplasm set of Brassica napus. Theor. Appl. Genet. 2011;123:1413–1423. doi: 10.1007/s00122-011-1676-7. [DOI] [PubMed] [Google Scholar]
- 55.Jannink J.-L., Lorenz A.J., Iwata H. Genomic selection in plant breeding: From theory to practice. Brief. Funct. Genom. 2010;9:166–177. doi: 10.1093/bfgp/elq001. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y., Wang L., Deng M., Li Z., Lu Y., Wang J., Wei Y., Zheng Y. Genome-wide association study of phosphorus-deficiency-tolerance traits in Aegilops tauschii. Theor. Appl. Genet. 2015;128:2203–2212. doi: 10.1007/s00122-015-2578-x. [DOI] [PubMed] [Google Scholar]
- 57.Xu Y., Li P., Yang Z., Xu C. Genetic mapping of quantitative trait loci in crops. Crop J. 2017;5:175–184. doi: 10.1016/j.cj.2016.06.003. [DOI] [Google Scholar]
- 58.Hatzig S.V., Frisch M., Breuer F., Nesi N., Ducournau S., Wagner M.-H., Leckband G., Abbadi A., Snowdon R.J. Genome-wide association mapping unravels the genetic control of seed germination and vigor in Brassica napus. Front. Plant Sci. 2015;6:221. doi: 10.3389/fpls.2015.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo X., Ma C., Yue Y., Hu K., Li Y., Duan Z., Wu M., Tu J., Shen J., Yi B. Unravelling the complex trait of harvest index in rapeseed (Brassica napus L.) with association mapping. BMC Genom. 2015;16:379. doi: 10.1186/s12864-015-1607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu S., Fan C., Li J., Cai G., Yang Q., Wu J., Yi X., Zhang C., Zhou Y. A genome-wide association study reveals novel elite allelic variations in seed oil content of Brassica napus. Theor. Appl. Genet. 2016;129:1203–1215. doi: 10.1007/s00122-016-2697-z. [DOI] [PubMed] [Google Scholar]
- 61.Xu L., Hu K., Zhang Z., Guan C., Chen S., Hua W., Li J., Wen J., Yi B., Shen J. Genome-wide association study reveals the genetic architecture of flowering time in rapeseed (Brassica napus L.) DNA Res. 2015;23:43–52. doi: 10.1093/dnares/dsv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X., Chen L., Wang A., Wang H., Tian J., Zhao X., Chao H., Zhao Y., Zhao W., Xiang J. Quantitative trait loci analysis and genome-wide comparison for silique related traits in Brassica napus. BMC Plant Biol. 2016;16:71. doi: 10.1186/s12870-016-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li F., Chen B., Xu K., Gao G., Yan G., Qiao J., Li J., Li H., Li L., Xiao X. A genome-wide association study of plant height and primary branch number in rapeseed (Brassica napus) Plant Sci. 2016;242:169–177. doi: 10.1016/j.plantsci.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 64.Liu J., Wang W., Mei D., Wang H., Fu L., Liu D., Li Y., Hu Q. Characterizing variation of branch angle and genome-wide association mapping in rapeseed (Brassica napus L.) Front. Plant Sci. 2016;7:21. doi: 10.3389/fpls.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun C., Wang B., Wang X., Hu K., Li K., Li Z., Li S., Yan L., Guan C., Zhang J. Genome-wide association study dissecting the genetic architecture underlying the branch angle trait in rapeseed (Brassica napus L.) Sci. Rep. 2016;6:33673. doi: 10.1038/srep33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schiessl S., Iniguez-Luy F., Qian W., Snowdon R.J. Diverse regulatory factors associate with flowering time and yield responses in winter-type Brassica napus. BMC Genom. 2015;16:737. doi: 10.1186/s12864-015-1950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang M., Zhang Y., Liu Y., Tong C., Cheng X., Zhu W., Li Z., Huang J., Liu S. Mapping loci controlling fatty acid profiles, oil and protein content by genome-wide association study in Brassica napus. Crop J. 2019;7:217–226. doi: 10.1016/j.cj.2018.10.007. [DOI] [Google Scholar]
- 68.He Y., Hu D., You J., Wu D., Cui Y., Dong H., Li J., Qian W. Genome-wide association study and protein network analysis for understanding candidate genes involved in root development at the rapeseed seedling stage. Plant Physiol. Biochem. 2019;137:42–52. doi: 10.1016/j.plaphy.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 69.Zheng M., Peng C., Liu H., Tang M., Yang H., Li X., Liu J., Sun X., Wang X., Xu J. Genome-wide association study reveals candidate genes for control of plant height, branch initiation height and branch number in rapeseed (Brassica napus L.) Front. Plant Sci. 2017;8:1246. doi: 10.3389/fpls.2017.01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao Z., Zhang C., Tang F., Yang B., Zhang L., Liu J., Huo Q., Wang S., Li S., Wei L. Identification of candidate genes controlling oil content by combination of genome-wide association and transcriptome analysis in the oilseed crop Brassica napus. Biotechnol. Biofuels. 2019;12:1–16. doi: 10.1186/s13068-019-1557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu Q., King G.J., Liu X., Shan N., Borpatragohain P., Baten A., Wang P., Luo S., Zhou Q. Identification of SNP loci and candidate genes related to four important fatty acid composition in Brassica napus using genome wide association study. PLoS ONE. 2019;14:e0221578. doi: 10.1371/journal.pone.0221578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arifuzzaman M., Oladzadabbasabadi A., McClean P., Rahman M. Shovelomics for phenotyping root architectural traits of rapeseed/canola (Brassica napus L.) and genome-wide association mapping. Mol. Genet. Genom. 2019;294:985–1000. doi: 10.1007/s00438-019-01563-x. [DOI] [PubMed] [Google Scholar]
- 73.Dong H., Tan C., Li Y., He Y., Wei S., Cui Y., Chen Y., Wei D., Fu Y., He Y. Genome-wide association study reveals both overlapping and independent genetic loci to control seed weight and silique length in Brassica napus. Front. Plant Sci. 2018;9:921. doi: 10.3389/fpls.2018.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He Y., Wu D., Wei D., Fu Y., Cui Y., Dong H., Tan C., Qian W. GWAS, QTL mapping and gene expression analyses in Brassica napus reveal genetic control of branching morphogenesis. Sci. Rep. 2017;7:15971. doi: 10.1038/s41598-017-15976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guan M., Huang X., Jia L., Wang S., Zhu M., Qiao C., Wei L., Xu X., Liang Y., Wang R. Association mapping analysis of fatty acid content in different ecotypic rapeseed using mrMLM. Front. Plant Sci. 2018;9:1872. doi: 10.3389/fpls.2018.01872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li S., Zhu Y., Varshney R., Zhan J., Zheng X., Shi J., Wang X., Liu G., Wang H. A systematic dissection of the mechanisms underlying the natural variation of silique number in rapeseed germplasm. Plant Biotechnol. J. 2019;18:568–580. doi: 10.1111/pbi.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei D., Cui Y., Mei J., Qian L., Lu K., Wang Z.M., Li J., Tang Q., Qian W. Genome-wide identification of loci affecting seed glucosinolate contents in Brassica napus L. J. Integr. Plant Biol. 2019;61:611–623. doi: 10.1111/jipb.12717. [DOI] [PubMed] [Google Scholar]
- 78.Chen L., Wan H., Qian J., Guo J., Sun C., Wen J., Yi B., Ma C., Tu J., Song L. Genome-wide association study of cadmium accumulation at the seedling stage in rapeseed (Brassica napus L.) Front. Plant Sci. 2018;9:375. doi: 10.3389/fpls.2018.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou Q., Han D., Mason A.S., Zhou C., Zheng W., Li Y., Wu C., Fu D., Huang Y. Earliness traits in rapeseed (Brassica napus): SNP loci and candidate genes identified by genome-wide association analysis. DNA Res. 2017;25:229–244. doi: 10.1093/dnares/dsx052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qu C., Jia L., Fu F., Zhao H., Lu K., Wei L., Xu X., Liang Y., Li S., Wang R. Genome-wide association mapping and Identification of candidate genes for fatty acid composition in Brassica napus L. using SNP markers. BMC Genom. 2017;18:232. doi: 10.1186/s12864-017-3607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo X., Xue Z., Ma C., Hu K., Zeng Z., Dou S., Tu J., Shen J., Yi B., Fu T. Joint genome-wide association and transcriptome sequencing reveals a complex polygenic network underlying hypocotyl elongation in rapeseed (Brassica napus L.) Sci. Rep. 2017;7:41561. doi: 10.1038/srep41561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu K., Peng L., Zhang C., Lu J., Yang B., Xiao Z., Liang Y., Xu X., Qu C., Zhang K. Genome-wide association and transcriptome analyses reveal candidate genes underlying yield-determining traits in Brassica napus. Front. Plant Sci. 2017;8:206. doi: 10.3389/fpls.2017.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagel M., Kranner I., Neumann K., Rolletschek H., Seal C.E., Colville L., Fernández-Marín B., Börner A. Genome-wide association mapping and biochemical markers reveal that seed ageing and longevity are intricately affected by genetic background and developmental and environmental conditions in barley. Plant Cell Environ. 2015;38:1011–1022. doi: 10.1111/pce.12474. [DOI] [PubMed] [Google Scholar]
- 84.Cockram J., White J., Zuluaga D.L., Smith D., Comadran J., Macaulay M., Luo Z., Kearsey M.J., Werner P., Harrap D. Genome-wide association mapping to candidate polymorphism resolution in the unsequenced barley genome. Proc. Natl. Acad. Sci. USA. 2010;107:21611–21616. doi: 10.1073/pnas.1010179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu X., Zuo J., Wang J., Liu L., Sun G., Li C., Ren X., Sun D. Multi-locus genome-wide association studies for 14 Main agronomic traits in barley. Front. Plant Sci. 2018;9:1683. doi: 10.3389/fpls.2018.01683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Almerekova S., Sariev B., Abugalieva A., Chudinov V., Sereda G., Tokhetova L., Ortaev A., Tsygankov V., Blake T., Chao S. Association mapping for agronomic traits in six-rowed spring barley from the USA harvested in Kazakhstan. PLoS ONE. 2019;14:e0221064. doi: 10.1371/journal.pone.0221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abdel-Ghani A.H., Sharma R., Wabila C., Dhanagond S., Owais S.J., Duwayri M.A., Al-Dalain S.A., Klukas C., Chen D., Lübberstedt T. Genome-wide association mapping in a diverse spring barley collection reveals the presence of QTL hotspots and candidate genes for root and shoot architecture traits at seedling stage. BMC Plant Biol. 2019;19:216. doi: 10.1186/s12870-019-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pham A.-T., Maurer A., Pillen K., Brien C., Dowling K., Berger B., Eglinton J.K., March T.J. Genome-wide association of barley plant growth under drought stress using a nested association mapping population. BMC Plant Biol. 2019;19:134. doi: 10.1186/s12870-019-1723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wabila C., Neumann K., Kilian B., Radchuk V., Graner A. A tiered approach to genome-wide association analysis for the adherence of hulls to the caryopsis of barley seeds reveals footprints of selection. BMC Plant Biol. 2019;19:95. doi: 10.1186/s12870-019-1694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rapacz M., Wójcik-Jagła M., Fiust A., Kalaji H.M., Koscielniak J. Genome-wide associations of chlorophyll fluorescence OJIP transient parameters connected with soil drought response in barley. Front. Plant Sci. 2019;10:78. doi: 10.3389/fpls.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang M., Fu M.-M., Qiu C.-W., Cao F., Chen Z.-H., Zhang G., Wu F. Response of Tibetan Wild Barley Genotypes to Drought Stress and Identification of Quantitative Trait Loci by Genome-Wide Association Analysis. Int. J. Mol. Sci. 2019;20:791. doi: 10.3390/ijms20030791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hou S., Zhu G., Li Y., Li W., Fu J., Niu E., Li L., Zhang D., Guo W. Genome-wide association studies reveal genetic variation and candidate genes of drought stress related traits in cotton (Gossypium hirsutum L.) Front. Plant Sci. 2018;9:1276. doi: 10.3389/fpls.2018.01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu R., Gong J., Xiao X., Zhang Z., Li J., Liu A., Lu Q., Shang H., Shi Y., Ge Q. GWAS analysis and QTL identification of fiber quality traits and yield components in upland cotton using enriched high-density SNP markers. Front. Plant Sci. 2018;9:1067. doi: 10.3389/fpls.2018.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan Y., Xing H., Zeng W., Xu J., Mao L., Wang L., Feng W., Tao J., Wang H., Zhang H. Genome-wide association and differential expression analysis of salt tolerance in Gossypium hirsutum L. at the germination stage. BMC Plant Biol. 2019;19:394. doi: 10.1186/s12870-019-1989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li H.-M., Liu S.-D., Ge C.-W., Zhang X.-M., Zhang S.-P., Chen J., Shen Q., Ju F.-Y., Yang Y.-F., Li Y. Analysis of Drought Tolerance and Associated Traits in Upland Cotton at the Seedling Stage. Int. J. Mol. Sci. 2019;20:3888. doi: 10.3390/ijms20163888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma J., Liu J., Pei W., Ma Q., Wang N., Zhang X., Cui Y., Li D., Liu G., Wu M. Genome-wide association study of the oil content in upland cotton (Gossypium hirsutum L.) and identification of GhPRXR1, a candidate gene for a stable QTLqOC-Dt5-1. Plant Sci. 2019;286:89–97. doi: 10.1016/j.plantsci.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 97.Dilnur T., Peng Z., Pan Z., Palanga K.K., Jia Y., Gong W., Du X. Association analysis of salt tolerance in Asiatic cotton (Gossypium arboretum) with SNP markers. Int. J. Mol. Sci. 2019;20:2168. doi: 10.3390/ijms20092168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He L., Xiao J., Rashid K.Y., Yao Z., Li P., Jia G., Wang X., Cloutier S., You F.M. Genome-wide association studies for pasmo resistance in flax (Linum usitatissimum L.) Front. Plant Sci. 2018;9:1982. doi: 10.3389/fpls.2018.01982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jia J., Zhao S., Kong X., Li Y., Zhao G., He W., Appels R., Pfeifer M., Tao Y., Zhang X. Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature. 2013;496:91. doi: 10.1038/nature12028. [DOI] [PubMed] [Google Scholar]
- 100.Pace J., Gardner C., Romay C., Ganapathysubramanian B., Lübberstedt T. Genome-wide association analysis of seedling root development in maize (Zea mays L.) BMC Genom. 2015;16:47. doi: 10.1186/s12864-015-1226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ding J., Zhang L., Chen J., Li X., Li Y., Cheng H., Huang R., Zhou B., Li Z., Wang J. Genomic dissection of leaf angle in maize (Zea mays L.) using a four-way cross mapping population. PLoS ONE. 2015;10:e0141619. doi: 10.1371/journal.pone.0141619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li X., Qian Q., Fu Z., Wang Y., Xiong G., Zeng D., Wang X., Liu X., Teng S., Hiroshi F. Control of tillering in rice. Nature. 2003;422:618. doi: 10.1038/nature01518. [DOI] [PubMed] [Google Scholar]
- 103.Kump K.L., Bradbury P.J., Wisser R.J., Buckler E.S., Belcher A.R., Oropeza-Rosas M.A., Zwonitzer J.C., Kresovich S., McMullen M.D., Ware D. Genome-wide association study of quantitative resistance to southern leaf blight in the maize nested association mapping population. Nat. Genet. 2011;43:163. doi: 10.1038/ng.747. [DOI] [PubMed] [Google Scholar]
- 104.Tian F., Bradbury P.J., Brown P.J., Hung H., Sun Q., Flint-Garcia S., Rocheford T.R., McMullen M.D., Holland J.B., Buckler E.S. Genome-wide association study of leaf architecture in the maize nested association mapping population. Nat. Genet. 2011;43:159. doi: 10.1038/ng.746. [DOI] [PubMed] [Google Scholar]
- 105.Zhao K., Tung C.-W., Eizenga G.C., Wright M.H., Ali M.L., Price A.H., Norton G.J., Islam M.R., Reynolds A., Mezey J. Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat. Commun. 2011;2:467. doi: 10.1038/ncomms1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Begum H., Spindel J.E., Lalusin A., Borromeo T., Gregorio G., Hernandez J., Virk P., Collard B., McCouch S.R. Genome-wide association mapping for yield and other agronomic traits in an elite breeding population of tropical rice (Oryza sativa) PLoS ONE. 2015;10:e0119873. doi: 10.1371/journal.pone.0119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kumar V., Singh A., Mithra S.A., Krishnamurthy S., Parida S.K., Jain S., Tiwari K.K., Kumar P., Rao A.R., Sharma S. Genome-wide association mapping of salinity tolerance in rice (Oryza sativa) DNA Res. 2015;22:133–145. doi: 10.1093/dnares/dsu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang X., Sang T., Zhao Q., Feng Q., Zhao Y., Li C., Zhu C., Lu T., Zhang Z., Li M. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 2010;42:961. doi: 10.1038/ng.695. [DOI] [PubMed] [Google Scholar]
- 109.Huang B.E., George A.W., Forrest K.L., Kilian A., Hayden M.J., Morell M.K., Cavanagh C.R. A multiparent advanced generation inter-cross population for genetic analysis in wheat. Plant Biotechnol. J. 2012;10:826–839. doi: 10.1111/j.1467-7652.2012.00702.x. [DOI] [PubMed] [Google Scholar]
- 110.Xu F.-F., Tang F.-F., Shao Y.-F., Chen Y.-L., Chuan T., Bao J.-S. Genotype× environment interactions for agronomic traits of rice revealed by association mapping. Rice Sci. 2014;21:133–141. doi: 10.1016/S1672-6308(13)60179-1. [DOI] [Google Scholar]
- 111.Misra G., Badoni S., Domingo C.J., Cuevas R.P., Llorente C., Mbanjo E.G.N., Sreenivasulu N. Deciphering the Genetic Architecture of Cooked Rice Texture. Front. Plant Sci. 2018;9:1405. doi: 10.3389/fpls.2018.01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cui Y., Zhang F., Zhou Y. The application of multi-locus GWAS for the detection of salt-tolerance loci in rice. Front. Plant Sci. 2018;9:1464. doi: 10.3389/fpls.2018.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li J., Tang W., Zhang Y.-W., Chen K.-N., Wang C., Liu Y., Zhan Q., Wang C., Wang S.-B., Xie S.-Q.X. Genome-wide association studies for five forage quality-related traits in sorghum (Sorghum bicolor L.) Front. Plant Sci. 2018;9:1146. doi: 10.3389/fpls.2018.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kumar B., Talukdar A., Bala I., Verma K., Lal S.K., Sapra R.L., Namita B., Chander S., Tiwari R. Population structure and association mapping studies for important agronomic traits in soybean. J. Genet. 2014;93:775–784. doi: 10.1007/s12041-014-0454-0. [DOI] [PubMed] [Google Scholar]
- 115.Chang F., Guo C., Sun F., Zhang J., Wang Z., Kong J., He Q., Sharmin R.A., Zhao T. Genome-wide association studies for dynamic plant height and number of nodes on the Main stem in summer sowing soybeans. Front. Plant Sci. 2018;9:1184. doi: 10.3389/fpls.2018.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lü H., Yang Y., Li H., Liu Q., Zhang J., Yin J., Chu S., Zhang X., Yu K., Lv L. Genome-wide association studies of photosynthetic traits related to phosphorus efficiency in soybean. Front. Plant Sci. 2018;9:1226. doi: 10.3389/fpls.2018.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ning H., Zhang K.X., Xue H., Li W.-X. Identification of QTNs Controlling Seed Protein Content in Soybean Using Multi-Locus Genome-Wide Association Studies. Front. Plant Sci. 2018;9:1690. doi: 10.3389/fpls.2018.01690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bilal M., Saeed M., Nasir I.A., Tabassum B., Zameer M., Khan A., Tariq M., Javed M.A., Husnain T. Association mapping of cane weight and tillers per plant in sugarcane. Biotechnol. Biotechnol. Equip. 2015;29:617–623. doi: 10.1080/13102818.2015.1008203. [DOI] [Google Scholar]
- 119.Zhang N., Gibon Y., Wallace J.G., Lepak N., Li P., Dedow L., Chen C., So Y.-S., Kremling K., Bradbury P.J. Genome-wide association of carbon and nitrogen metabolism in the maize nested association mapping population. Plant Physiol. 2015;168:575–583. doi: 10.1104/pp.15.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mora F., Castillo D., Lado B., Matus I., Poland J., Belzile F., von Zitzewitz J., del Pozo A. Genome-wide association mapping of agronomic traits and carbon isotope discrimination in a worldwide germplasm collection of spring wheat using SNP markers. Mol. Breed. 2015;35:69. doi: 10.1007/s11032-015-0264-y. [DOI] [Google Scholar]
- 121.Peng Y., Liu H., Chen J., Shi T., Zhang C., Sun D., He Z., Hao Y., Chen W. Genome-wide association studies of free amino acid levels by six multi-locus models in bread wheat. Front. Plant Sci. 2018;9:1196. doi: 10.3389/fpls.2018.01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gupta V., He X., Kumar N., Fuentes-Davila G., Sharma R.K., Dreisigacker S., Juliana P., Ataei N., Singh P.K. Genome Wide Association Study of Karnal Bunt Resistance in a Wheat Germplasm Collection from Afghanistan. Int. J. Mol. Sci. 2019;20:3124. doi: 10.3390/ijms20133124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Echeverry-Solarte M., Kumar A., Kianian S., Mantovani E.E., Simsek S., Alamri M.S., Mergoum M. Genome-wide genetic dissection of supernumerary spikelet and related traits in common wheat. Plant Genome. 2014;7:3. doi: 10.3835/plantgenome2014.03.0013. [DOI] [PubMed] [Google Scholar]
- 124.Kristensen P.S., Jensen J., Andersen J.R., Guzmán C., Orabi J., Jahoor A. Genomic Prediction and Genome-Wide Association Studies of Flour Yield and Alveograph Quality Traits Using Advanced Winter Wheat Breeding Material. Genes. 2019;10:669. doi: 10.3390/genes10090669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chalhoub B., Denoeud F., Liu S., Parkin I.A., Tang H., Wang X., Chiquet J., Belcram H., Tong C., Samans B. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science. 2014;345:950–953. doi: 10.1126/science.1253435. [DOI] [PubMed] [Google Scholar]
- 126.Raboanatahiry N., Chao H., Dalin H., Pu S., Yan W., Yu L., Wang B., Li M. QTL alignment for seed yield and yield related traits in Brassica napus. Front. Plant Sci. 2018;9:1127. doi: 10.3389/fpls.2018.01127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen B., Xu K., Li J., Li F., Qiao J., Li H., Gao G., Yan G., Wu X. Evaluation of yield and agronomic traits and their genetic variation in 488 global collections of Brassica napus L. Genet. Resour. Crop Evol. 2014;61:979–999. doi: 10.1007/s10722-014-0091-8. [DOI] [Google Scholar]
- 128.Yang Y., Wang Y., Zhan J., Shi J., Wang X., Liu G., Wang H. Genetic and Cytological Analyses of the Natural Variation of Seed Number per Pod in Rapeseed (Brassica napus L.) Front. Plant Sci. 2017;8:1890. doi: 10.3389/fpls.2017.01890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang Y., Shi J., Wang X., Liu G., Wang H. Genetic architecture and mechanism of seed number per pod in rapeseed: Elucidated through linkage and near-isogenic line analysis. Sci. Rep. 2016;6:24124. doi: 10.1038/srep24124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Y., Cheng Y., Cai G., Fan C., Zhou Y. Cytological basis and molecular mechanism of variation in number of seeds per pod in Brassica napus. Sci. Sin. Vitae. 2014;44:822–831. [Google Scholar]
- 131.Li S., Chen L., Zhang L., Li X., Liu Y., Wu Z., Dong F., Wan L., Liu K., Hong D. BnaC9. SMG7b functions as a positive regulator of the number of seeds per silique in Brassica napus by regulating the formation of functional female gametophytes. Plant Physiol. 2015;169:2744–2760. doi: 10.1104/pp.15.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu W., Fu Y., Dong H., Chen Z., Zhang Q., Mao S., He Y.-j., Qian W. Morphologic and physiologic characterization of an ovule abortion mutant in Brassica napus. Sci. Agric. Sin. 2014;47:2062–2068. [Google Scholar]
- 133.Tischner T., Allphin L., Chase K., Orf J., Lark K. Genetics of seed abortion and reproductive traits in soybean. Crop Sci. 2003;43:464–473. doi: 10.2135/cropsci2003.0464. [DOI] [Google Scholar]
- 134.Hashida T., Nakatsuji R., Budahn H., Schrader O., Peterka H., Fujimura T., Kubo N., Hirai M. Construction of a chromosome-assigned, sequence-tagged linkage map for the radish, Raphanus sativus L. and QTL analysis of morphological traits. Breed. Sci. 2013;63:218–226. doi: 10.1270/jsbbs.63.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yuan J., Kessler S.A. A genome-wide association study reveals a novel regulator of ovule number and fertility in Arabidopsis thaliana. PLoS Genet. 2019;15:e1007934. doi: 10.1371/journal.pgen.1007934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hall M.C., Basten C.J., Willis J.H. Pleiotropic quantitative trait loci contribute to population divergence in traits associated with life-history variation in Mimulus guttatus. Genetics. 2006;172:1829–1844. doi: 10.1534/genetics.105.051227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi J., Zhan J., Yang Y., Ye J., Huang S., Li R., Wang X., Liu G., Wang H. Linkage and regional association analysis reveal two new tightly-linked major-QTLs for pod number and seed number per pod in rapeseed (Brassica napus L.) Sci. Rep. 2015;5:14481. doi: 10.1038/srep14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Clarke J., Simpson G. Influence of irrigation and seeding rates on yield and yield components of Brassica napus cv. Tower. Can. J. Plant Sci. 1978;58:731–737. doi: 10.4141/cjps78-108. [DOI] [Google Scholar]
- 139.Butruille D.V., Guries R.P., Osborn T.C. Linkage analysis of molecular markers and quantitative trait loci in populations of inbred backcross lines of Brassica napus L. Genetics. 1999;153:949–964. doi: 10.1093/genetics/153.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qiu D., Morgan C., Shi J., Long Y., Liu J., Li R., Zhuang X., Wang Y., Tan X., Dietrich E. A comparative linkage map of oilseed rape and its use for QTL analysis of seed oil and erucic acid content. Theor. Appl. Genet. 2006;114:67–80. doi: 10.1007/s00122-006-0411-2. [DOI] [PubMed] [Google Scholar]
- 141.Quijada P.A., Udall J.A., Lambert B., Osborn T.C. Quantitative trait analysis of seed yield and other complex traits in hybrid spring rapeseed (Brassica napus L.): 1. Identification of genomic regions from winter germplasm. Theor. Appl. Genet. 2006;113:549–561. doi: 10.1007/s00122-006-0323-1. [DOI] [PubMed] [Google Scholar]
- 142.Radoev M., Becker H.C., Ecke W. Genetic analysis of heterosis for yield and yield components in rapeseed (Brassica napus L.) by quantitative trait locus mapping. Genetics. 2008;179:1547–1558. doi: 10.1534/genetics.108.089680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shi Y., Zhang X., Xu Z.Y., Li L., Zhang C., Schläppi M., Xu Z.Q. Influence of EARLI1-like genes on flowering time and lignin synthesis of Arabidopsis thaliana. Plant Biol. 2011;13:731–739. doi: 10.1111/j.1438-8677.2010.00428.x. [DOI] [PubMed] [Google Scholar]
- 144.Basunanda P., Radoev M., Ecke W., Friedt W., Becker H., Snowdon R. Comparative mapping of quantitative trait loci involved in heterosis for seedling and yield traits in oilseed rape (Brassica napus L.) Theor. Appl. Genet. 2010;120:271. doi: 10.1007/s00122-009-1133-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang L., Liu P., Hong D., Huang A., Li S., He Q., Yang G. Inheritance of seeds per silique in Brassica napus L. using joint segregation analysis. Field Crop. Res. 2010;116:58–67. doi: 10.1016/j.fcr.2009.11.010. [DOI] [Google Scholar]
- 146.Yang P., Shu C., Chen L., Xu J., Wu J., Liu K. Identification of a major QTL for silique length and seed weight in oilseed rape (Brassica napus L.) Theor. Appl. Genet. 2012;125:285–296. doi: 10.1007/s00122-012-1833-7. [DOI] [PubMed] [Google Scholar]
- 147.Li N., Shi J., Wang X., Liu G., Wang H. A combined linkage and regional association mapping validation and fine mapping of two major pleiotropic QTLs for seed weight and silique length in rapeseed (Brassica napus L.) BMC Plant Biol. 2014;14:114. doi: 10.1186/1471-2229-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Qi L., Mao L., Sun C., Pu Y., Fu T., Ma C., Shen J., Tu J., Yi B., Wen J. Interpreting the genetic basis of silique traits in B rassica napus using a joint QTL network. Plant Breed. 2014;133:52–60. doi: 10.1111/pbr.12131. [DOI] [Google Scholar]
- 149.Luo Z., Wang M., Long Y., Huang Y., Shi L., Zhang C., Liu X., Fitt B.D., Xiang J., Mason A.S., et al. Incorporating pleiotropic quantitative trait loci in dissection of complex traits: Seed yield in rapeseed as an example. Theor. Appl. Genet. 2017;130:1569–1585. doi: 10.1007/s00122-017-2911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li Y., Shen J., Wang T., Chen Q., Zhang X., Fu T., Meng J., Tu J., Ma C. QTL analysis of yield-related traits and their association with functional markers in Brassica napus L. Aust. J. Agric. Res. 2007;58:759–766. doi: 10.1071/AR06350. [DOI] [Google Scholar]
- 151.Bagheri H., El-Soda M., van Oorschot I., Hanhart C., Bonnema G., Jansen-van den Bosch T., Mank R., Keurentjes J., Meng L., Wu J. Genetic analysis of morphological traits in a new, versatile, rapid-cycling Brassica rapa recombinant inbred line population. Front. Plant Sci. 2012;3:183. doi: 10.3389/fpls.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Raman R., Diffey S., Carling J., Cowley R.B., Kilian A., Luckett D.J., Raman H. Quantitative genetic analysis of grain yield in an Australian Brassica napus doubled-haploid population. Crop Pasture Sci. 2016;67:298–307. doi: 10.1071/CP15283. [DOI] [Google Scholar]
- 153.Zhao B.-Y., Hu Y.-F., Li J.-j., Yao X. BnaABF2, a bZIP transcription factor from rapeseed (Brassica napus L.), enhances drought and salt tolerance in transgenic Arabidopsis. Bot. Stud. 2016;57:12. doi: 10.1186/s40529-016-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fu Y., Wei D., Dong H., He Y., Cui Y., Mei J., Wan H., Li J., Snowdon R., Friedt W. Comparative quantitative trait loci for silique length and seed weight in Brassica napus. Sci. Rep. 2015;5:14407. doi: 10.1038/srep14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang N., Chen B., Xu K., Gao G., Li F., Qiao J., Yan G., Li J., Li H., Wu X. Association mapping of flowering time QTLs and insight into their contributions to rapeseed growth habits. Front. Plant Sci. 2016;7:338. doi: 10.3389/fpls.2016.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Remington D.L., Thornsberry J.M., Matsuoka Y., Wilson L.M., Whitt S.R., Doebley J., Kresovich S., Goodman M.M., Buckler E.S. Structure of linkage disequilibrium and phenotypic associations in the maize genome. Proc. Natl. Acad. Sci. USA. 2001;98:11479–11484. doi: 10.1073/pnas.201394398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thornsberry J.M., Goodman M.M., Doebley J., Kresovich S., Nielsen D., Buckler E.S., IV Dwarf8 polymorphisms associate with variation in flowering time. Nat. Genet. 2001;28:286. doi: 10.1038/90135. [DOI] [PubMed] [Google Scholar]
- 158.Ehrenreich I.M., Hanzawa Y., Chou L., Roe J.L., Kover P.X., Purugganan M.D. Candidate gene association mapping of Arabidopsis flowering time. Genetics. 2009;183:325–335. doi: 10.1534/genetics.109.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li Y., Böck A., Haseneyer G., Korzun V., Wilde P., Schön C.-C., Ankerst D.P., Bauer E. Association analysis of frost tolerance in rye using candidate genes and phenotypic data from controlled, semi-controlled, and field phenotyping platforms. BMC Plant Biol. 2011;11:146. doi: 10.1186/1471-2229-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang L., Li S., Chen L., Yang G. Identification and mapping of a major dominant quantitative trait locus controlling seeds per silique as a single Mendelian factor in Brassica napus L. Theor. Appl. Genet. 2012;125:695–705. doi: 10.1007/s00122-012-1861-3. [DOI] [PubMed] [Google Scholar]
- 161.Liu J., Hua W., Hu Z., Yang H., Zhang L., Li R., Deng L., Sun X., Wang X., Wang H. Natural variation in ARF18 gene simultaneously affects seed weight and silique length in polyploid rapeseed. Proc. Natl. Acad. Sci. USA. 2015;112:E5123–E5132. doi: 10.1073/pnas.1502160112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang Y.-M., Mao Y., Xie C., Smith H., Luo L., Xu S. Mapping quantitative trait loci using naturally occurring genetic variance among commercial inbred lines of maize (Zea mays L.) Genetics. 2005;169:2267–2275. doi: 10.1534/genetics.104.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yu J., Pressoir G., Briggs W.H., Bi I.V., Yamasaki M., Doebley J.F., McMullen M.D., Gaut B.S., Nielsen D.M., Holland J.B. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006;38:203. doi: 10.1038/ng1702. [DOI] [PubMed] [Google Scholar]
- 164.Feng J., Wen Y., Zhang J., Zhang Y. Advances on methodologies for genome-wide association studies in plants. Acta Agron. Sin. 2016;42:945–956. doi: 10.3724/SP.J.1006.2016.00945. [DOI] [Google Scholar]
- 165.Zhang Y.-M., Jia Z., Dunwell J.M. The Applications of New Multi-Locus GWAS Methodologies in the Genetic Dissection of Complex Traits. Front. Plant Sci. 2019;10:100. doi: 10.3389/fpls.2019.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang S.-B., Feng J.-Y., Ren W.-L., Huang B., Zhou L., Wen Y.-J., Zhang J., Dunwell J.M., Xu S., Zhang Y.-M. Improving power and accuracy of genome-wide association studies via a multi-locus mixed linear model methodology. Sci. Rep. 2016;6:19444. doi: 10.1038/srep19444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tamba C.L., Ni Y.-L., Zhang Y.-M. Iterative sure independence screening EM-Bayesian LASSO algorithm for multi-locus genome-wide association studies. PLoS Comput. Biol. 2017;13:e1005357. doi: 10.1371/journal.pcbi.1005357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wen Y.-J., Zhang H., Ni Y.-L., Huang B., Zhang J., Feng J.-Y., Wang S.-B., Dunwell J.M., Zhang Y.-M., Wu R. Methodological implementation of mixed linear models in multi-locus genome-wide association studies. Brief. Bioinform. 2017;19:700–712. doi: 10.1093/bib/bbw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang J., Feng J., Ni Y., Wen Y., Niu Y., Tamba C., Yue C., Song Q., Zhang Y. pLARmEB: Integration of least angle regression with empirical Bayes for multilocus genome-wide association studies. Heredity. 2017;118:517. doi: 10.1038/hdy.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li H., Zhang L., Hu J., Zhang F., Chen B., Xu K., Gao G., Li H., Zhang T., Li Z. Genome-wide association mapping reveals the genetic control underlying branch angle in rapeseed (Brassica napus L.) Front. Plant Sci. 2017;8:1054. doi: 10.3389/fpls.2017.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Misra G., Badoni S., Anacleto R., Graner A., Alexandrov N., Sreenivasulu N. Whole genome sequencing-based association study to unravel genetic architecture of cooked grain width and length traits in rice. Sci. Rep. 2017;7:12478. doi: 10.1038/s41598-017-12778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu Y., Yang T., Zhou Y., Yin S., Li P., Liu J., Xu S., Yang Z., Xu C. Genome-wide association mapping of starch pasting properties in maize using single-locus and multi-locus models. Front. Plant Sci. 2018;9:1311. doi: 10.3389/fpls.2018.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Su J., Ma Q., Li M., Hao F., Wang C. Multi-Locus genome-wide association studies of fiber-quality related traits in Chinese early-maturity upland cotton. Front. Plant Sci. 2018;9:1169. doi: 10.3389/fpls.2018.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article.