Abstract
Context
Hypercalcemia of malignancy (HCM) has not been studied in a fashion to determine all possible mechanisms of hypercalcemia in any given patient.
Objective
The 2 objectives were to assess the completeness of evaluation and to determine the distribution of etiologies of HCM in a contemporary cohort of patients.
Methods
A retrospective analysis was performed of patients with cancer who developed hypercalcemia over 20 years at a single health system. Laboratory data were electronically captured from medical records to identify cases of parathyroid hormone (PTH)-independent hypercalcemia. The records were then manually reviewed to confirm the diagnosis of HCM, document the extent of evaluation, and determine underlying etiology(ies) of HCM in each patient.
Results
The initial data set included 167 551 adult patients with malignancy, of which 11 589 developed hypercalcemia. Of these, only a quarter (25.4%) had assessment of PTH with a third of the latter (30.9%) indicating PTH-independent hypercalcemia. Of those with PTH-independent hypercalcemia, a third (31.6%) had assessment of PTH-related peptide (PTHrP) and/or 1,25-dihydroxy (1,25-OH) vitamin D and constituted the 153 cases of HCM examined in this study. Eighty-three of these patients had an incomplete evaluation of their HCM. The distribution of etiologies of HCM was therefore determined from the remaining 70 patients who had assessment of all 3 possible etiologies (PTHrP, 1,25-OH vitamin D, and skeletal imaging) and was as follows: PTHrP, 27%; osteolytic metastases, 50%; and 1,25-OH vitamin D, 39%, with combinations of etiologies being common (approximately 20%).
Conclusion
HCM is incompletely evaluated in many patients. The distribution of etiologies of HCM in this report differs significantly from the previous literature, warranting further study to determine whether its causes have indeed changed over time.
Keywords: hypercalcemia of malignancy, rural, parathyroid hormone, parathyroid hormone-related peptide, 1, 25-dihydroxy vitamin D
Hypercalcemia of malignancy (HCM) occurs in 10% to 30% of patients with malignancy [1-4]. The underlying pathophysiology of this condition is almost always parathyroid hormone (PTH)-independent because PTH-dependent HCM occurs only in parathyroid carcinoma and in very rare cases of ectopic PTH secretion [1-4]. HCM can, therefore, be divided into 3 main categories: humoral hypercalcemia of malignancy [induced by PTH-related peptide ([PTHrP)], which the literature suggests makes up the majority of cases (80%); osteolytic metastases (OM), estimated to make up another 20% of cases; and excess 1,25-dihydroxy (1,25-OH) vitamin D, estimated to contribute to <1% of cases (most of these reported in lymphomas) [1-4]. The previously mentioned prevalence estimates have been commonly cited in the literature since the seminal review article by Stewart in the New England Journal of Medicine published in 2005 [1]. However, the classical distribution of etiologies listed in this article is mainly based on several studies between 1980 and 1990 at a time when PTHrP was just being identified and characterized [5-7]. Notably, 2 of the leading publications in this regard contained findings from small studies involving 50 [5] and 38 patients [7], respectively. While several other publications in the 1990s confirmed this 80% contribution of PTHrP [8-10], other publications around the same time showed a much lower prevalence of PTHrP-mediated HCM between 44% and 69% [11-13]. In a more recent study published in 2016, PTHrP contributed to only 38% of 242 cases of HCM, with OM contributing to approximately 27% (this number included both PTHrP and OM contributing in combination in 10% of cases), and the cause was undetermined in 40% [14]. Although these studies reported PTHrP levels in all subjects, many, but not all, patients also underwent skeletal imaging to assess for OM. However, virtually no assessment of 1,25-OH vitamin D levels was undertaken in most of these studies [6-14].
More recent literature has continued to challenge conventional thinking about the etiology of HCM, especially regarding the contribution of 1,25-OH vitamin D. In a review of 101 cases of 1,25-OH vitamin D–mediated hypercalcemia, 22% were related to malignancy [15]. In a 2020 publication by Chukir et al, 45% of 101 patients with HCM and solid tumors in whom 1,25-OH vitamin D was assessed had an elevated or inappropriately high-normal level of that hormone [16]. Thus, the long-standing belief as to the etiology of HCM is based largely on decades-old data obtained from incompletely evaluated patients.
Furthermore, HCM now appears to be less common in clinical practice than the often-quoted 10% to 30% in many reviews on the subject and is currently on the order of just 0.67% to 2.0% of patients with cancer [17,18]. To further characterize this apparent trend in HCM diagnosis, we undertook a retrospective chart review to identify the etiology, or combinations of etiologies, in patients with HCM at Marshfield Clinic Health System (Weston, WI, USA) over a contemporary 20-year time span (2000-2019). We had 2 goals: (1) to assess the degree of completeness of the diagnostic workup for HCM and (2) to review a cohort of patients that had undergone a conclusive evaluation with regards to the etiology of their HCM according to assessments of PTHrP levels, skeletal imaging, and 1,25-OH vitamin D levels.
Materials and Methods
Study Design
We queried the electronic medical records for adult (≥18 years of age) patients with a history of malignancy who developed hypercalcemia between January 1, 2000 and December 31, 2019. Records were initially screened for a diagnosis of malignancy using codes from the International Classification of Disease, versions 9 and 10. An electronic algorithm was then used to both identify cases of hypercalcemia from laboratory data and query its subsequent evaluation. Patients with a diagnosis of skin cancer or leukemia as well as patients with a record of normal, low, or no reports of serum calcium concentrations were excluded from analysis (Fig. 1). Although HCM can occur in skin cancers and leukemia, that is uncommon, and we therefore focused our analysis on solid malignancies and the 2 more common hematologic malignancies associated with HCM, lymphomas, and multiple myeloma. Also, while HIV-related cancers were not excluded, no patient in our cohort had HIV. PTH-independent hypercalcemia was defined as an albumin-corrected serum calcium (cCa2+) above the upper limit of the normal range with a PTH < 20 pg/mL, as described in the recent literature [3,19,20]. Medications possibly affecting serum calcium levels included thiazide diuretics, lithium, and supplemental doses of vitamin D > 1000 international units daily. No patients determined to have HCM were taking calcitriol. The algorithm identified 287 patients with cancer who developed PTH-independent hypercalcemia and had assessment of PTHrP, 1,25-OH vitamin D, or both. These medical records were manually reviewed to confirm cases of HCM by the method outlined in the following discussion. The 153 cases of HCM identified with 1 or more additional tests (Fig. 1, A) were used to further evaluate the degree of completeness/incompleteness of the evaluation. These patients had documented PTH-independent hypercalcemia with an assessment of PTHrP, 1,25-OH vitamin D, and/or skeletal imaging but not necessarily all 3 modalities. In the determination of the etiology of HCM, only the 70 patients with sufficient data to triangulate all components of the etiology of HCM were included (Fig. 1C).
Figure 1.
Flow diagram of the patient cohort at each stage of data extraction. Abbreviations: PTH, parathyroid hormone; PTHrP, parathyroid hormone-related peptide; 1,25-OH vitamin D, 1,25-dihydroxy vitamin D; HCM, hypercalcemia of malignancy.
Manual Data Extraction Process and Hypercalcemia of Malignancy Diagnosis
All charts were reviewed by a single reviewer (M.T.S.). For HCM to be diagnosed, at least 2 elevated calcium levels were required. While the algorithm was designed to capture PTH within 1 month of the first instance of hypercalcemia, it was always associated with hypercalcemia documented simultaneously on manual record review in those with HCM. The calcium and estimated glomerular filtration rate (eGFR) levels captured were, respectively, the highest and lowest levels documented within several days of the assessment of other biochemical parameters (PTH, PTHrP, 1,25-OH vitamin D, etc). In those patients with some degree of biochemical and/or skeletal imaging evaluation, persistent non-PTH-mediated hypercalcemia with elevated PTHrP, elevated 1,25-OH vitamin D, and/or OM were deemed to have HCM. In addition, the diagnosis of HCM was also based on the presence of an active malignancy and extensive review of both inpatient and outpatient medical records. This included oncology notes both before and after the onset of hypercalcemia, treatments for hypercalcemia instituted (intravenous bisphosphonate, subcutaneous denosumab etc) with such review oftentimes revealing an ominous clinical course consistent with HCM. This investigation allowed exclusion of other causes of hypercalcemia such as transient hypercalcemia without recurrence, chronic kidney disease or hypoparathyroidism with excess calcitriol dosage, severe dehydration, vitamin D intoxication, milk-alkali syndrome, and sarcoidosis, to name a few.
Assays Utilized
Total calcium was performed by modified calcium O-cresolphthalein complexone bichromatic endpoint technique. eGFR was calculated by the isotope dilution mass spectrometry traceable modification of diet in renal disease study equation.
25-OH vitamin D was performed by liquid chromatography-tandem mass spectrometry. PTH was performed by 2-site sandwich immunoassay using direct chemiluminometric technology by Siemens Centaur. PTHrP was performed by immunochemiluminometric assay. 1,25-OH vitamin D was performed by extraction/liquid chromatography-tandem mass spectrometry.
Normal Ranges
The normal range for total calcium varied from a lower limit of normal of 8.2 to 8.5 mg/dL to an upper limit of normal of 10.2 to 10.5 mg/dL. Our algorithm was designed to capture any calcium value greater than the upper limit of normal for whichever normal range was listed at the time. The other normal ranges were as follows: eGFR, ≥60 mL/min/1.73m2; 25-OH vitamin D, 30 to 80 ng/mL; PTH, 11 to 67 or 18 to 85 pg/mL (with a value < 20 pg/mL used to define PTH-independence in the setting of hypercalcemia); PTHrP, ≤4.2 pmol/L; and 1,25-OH vitamin D, 18 to 78 pg/mL (female) or 18 to 64 pg/mL (male) with a value in the upper third of the normal range being defined as inappropriately normal in the setting of PTH-independent hypercalcemia.
Statistical Analysis
Statistical comparisons of demographic characteristics were performed using the t-test, chi-squared test, and the Wilcoxon rank sum test as appropriate. SAS software (SAS Institute, Cary, NC, USA) was used for all analyses, and a P-value of 0.05 was set as the threshold for statistical significance.
Results
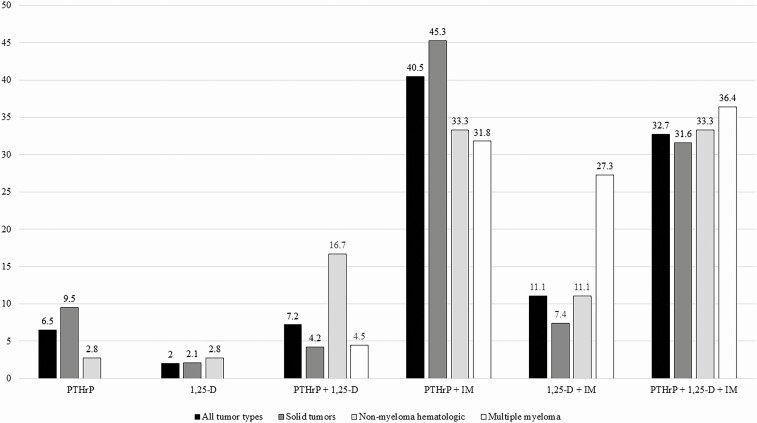
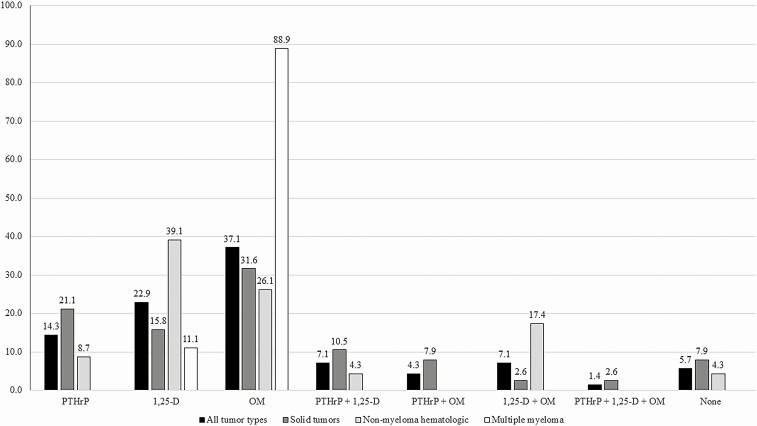
In our entire cohort of 167 551 adult patients with malignancy, 6.9% (11 589/167 521) developed hypercalcemia, of which only 25.4% had an assessment of PTH. Of those, 30.9% had a PTH < 20 pg/mL, and only 31.6% of that group had further biochemical assessment of PTHrP and/or 1,25-OH vitamin D (Fig. 1). One hundred fifty-three had some degree of evaluation to determine components of their HCM etiology (Fig. 1A). Of these, 70 patients had sufficient data to triangulate all components of the etiology of HCM (Fig. 1C), while in the remaining 83 cases the data were insufficient to do so (Fig. 1B). Table 1 depicts the demographics, malignancy distribution, degree of hypercalcemia, use of medications possibly affecting serum calcium levels, 25-OH vitamin D levels, and eGFR in each group. Aside from significantly more nonmyeloma hematologic malignancies in the conclusive evaluation group (P = 0.0440), the groups were not statistically different. Figure 2 depicts the degree of completeness of evaluation for HCM in all 153 patients with subgrouping of solid tumors, nonmyeloma hematologic malignancies, and multiple myeloma. Although multiple testing modalities were used in most patients, only about one third of cases had a complete evaluation. The least commonly assessed modality was measurement of 1,25-OH vitamin D levels, with an especially low frequency (45.3%) of testing in solid tumors compared to 90.6% of patients in that group having a PTHrP level tested. As a whole, the most frequent combination of tests performed was PTHrP and skeletal imaging (40.5%). Figure 3 depicts the frequency of assessment of each modality and the percentage of those tested having an abnormal result in the cohort of 153 patients for all tumors and subgroups. As mentioned previously, 1,25-OH vitamin D level measurement was the least commonly assessed modality at about 53% for all tumors, compared to approximately 85% each for PTHrP and skeletal imaging. The most frequently positive test (ie, abnormal result) in relation to the number of those tested in the overall tumor cohort was skeletal imaging, with about 61% of those being tested showing OM. In contrast, less than 40% of those patients tested for PTHrP, regardless of tumor type, showed a positive result. For patients with all tumor types in whom 1,25-OH vitamin D levels were measured, the results were abnormal in approximately 28%. A similar proportion of patients with solid tumors and measured 1,25-OH vitamin D levels had a positive result (over 22%).
Table 1.
Demographic and laboratory characteristics of the patient cohort grouped by comprehensiveness of evaluation for hypercalcemia of malignancy
| Conclusive evaluation | Nonconclusive evaluation | P-value | |
|---|---|---|---|
| Patients, n | 70 | 83 | |
| Gender, n (%) | 0.3055 | ||
| Female | 31 (44.3) | 30 (36.1) | |
| Male | 39 (55.7) | 53 (63.9) | |
| Age, years, median (range) | 70.0 (37.0-89.0) | 71.0 (37.0-90.0) | 0.7532 |
| Malignancy type, n (%) | 0.0440 | ||
| Solid | 38 (54.3) | 57 (68.7) | |
| Nonmyeloma hematologic | 23 (32.9) | 13 (15.7) | |
| Multiple myeloma | 9 (12.9) | 13 (15.7) | |
| cCa2 + mg/dL, mean ± SD | 13.7 ± 1.7 | 13.2 ± 1.7 | 0.1104 |
| Hypercalcemia severity, n (%) | 0.1928 | ||
| Mild (<12.0 mg/dL) | 12 (17.1) | 22 (26.5) | |
| Moderate (12.0-14.0 mg/dL) | 30 (42.9) | 38 (45.8) | |
| Severe (>14.0 mg/dL) | 28 (40.0) | 23 (27.7) | |
| Medication possibly affecting cCa2+, yes, n (%) | 8 (11.4) | 4 (4.8) | |
| 25-OH vitamin D ng/mL, mean ± SD | 27.5 ± 11.9 | 29.9 ± 14.6 | 0.4186 |
| eGFR mL/min mean ± SD | 46.6 ± 2 4.9 | 47.7 ± 22.9 | 0.7753 |
Abbreviations: cCa2+: albumin-corrected serum calcium; 25-OH vitamin D: 25-hydroxy vitamin D; eGFR: estimated glomerular filtration rate.
Figure 2.
Extent of evaluation of hypercalcemia of malignancy in patients from Figure 1A. All tumor types (n = 153). Solid tumors (n = 95). Nonmyeloma hematologic malignancies (n = 36). Multiple myeloma (n = 22). All values are percentages. Abbreviations: 1,25 vit D, 1,25-dihydroxy vitamin D; IM, skeletal imaging; PTHrP, parathyroid hormone-related peptide.
Figure 3.
Frequency of assessment of parathyroid hormone-related peptide, 25-hydroxy vitamin D, and skeletal imaging and percentage of those tested with an abnormal result in patients from Figure 1A. All tumor types (n = 153). Solid tumors (n = 95). Nonmyeloma hematologic malignancies (n = 36). Multiple myeloma (n = 22). All values are percentages. Abbreviations: abn., abnormal; 1,25 vit D, 1,25-dihydroxy vitamin D; IM, skeletal imaging; PTHrP, parathyroid hormone-related peptide.
The etiology(ies) of HCM in the 70 patients with sufficient data is depicted in Figure 4. Combinations of modalities were common, involving approximately 20% of patients overall. As expected, HCM in the multiple myeloma group was predominantly related to OM. When reviewing all tumor types, PTHrP was involved to some degree in approximately 27% of cases, with OM being seen in 50%, and 1,25-OH vitamin D being involved in approximately 39% of cases. As expected, 1,25-OH vitamin D was represented in the greatest proportion of patients in the nonmyeloma hematologic malignancy group (approximately 61%). However, even in solid tumors, 1,25-OH vitamin D contributed to HCM in some fashion in over 31% of cases. In the entire cohort, < 6% had all 3 modalities tested without any etiology being discerned for their non-PTH mediated hypercalcemia.
Figure 4.
Etiology(ies) of hypercalcemia of malignancy in patients with conclusive data for selecting one or more of the 3 modalities in patients from Figure 1C. All tumor types (n = 70). Solid tumors (n = 38). Nonmyeloma hematologic malignancies (n = 23). Multiple myeloma (n = 9). All values are percentages. Abbreviations: 1,25, 1,25-dihydroxy vitamin D; OM, osteolytic metastases; PTHrP, parathyroid hormone-related peptide.
Discussion
The long-standing orthodoxy in regards to the distribution of underlying pathophysiologies of HCM has remained unchallenged for decades. The classic review article published in 2005 [1] was based on several very small studies between 1980 and the early 1990s [5-7]. Those studies, along with multiple subsequent studies [8-13], were generally not designed to determine the exact distribution of etiologies of HCM, but rather to elucidate the humoral mediator of HCM in patients without OM, which to that point was unknown. However, even at that time, not all studies were in agreement with the finding of an 80% rate of PTHrP causing HCM [11-13], yet that number became the standard. Subsequent reviews of HCM have promulgated this assertion [2-4]. More recently, several studies have begun to challenge this notion [14], and the significance of 1,25-OH vitamin D as a contributor to HCM in solid malignancies has begun to be realized to not only be of academic interest but also, through findings from clinical practice, of importance for the management of these patients [15,16].
To our knowledge, ours is the first study to attempt to comprehensively document the completeness of evaluation for HCM and describe the distribution of etiologies of HCM in a contemporary cohort of patients. We speculate that an incomplete understanding of the etiology of HCM in most patients is likely related to the lack of proper assessment of their hypercalcemia. Indeed, only 25.4% of those patients with cancer in our cohort who developed hypercalcemia even had a PTH assessment. In those who did have documentation of PTH-independent hypercalcemia, less than one third had any further biochemical evaluation, with <8% having what would be defined as a conclusive evaluation. The previous studies cited, including those published in the 1980s and 1990s [5-13] and more recent publications [14], also show this same problem of limited evaluation. It should be noted that this incompleteness of evaluation is not necessarily a reflection of poor clinical care, but rather a feature of the urgent need to treat these patients with severe hypercalcemia. Management of acute, severe hypercalcemia is standardized, nonspecific, and readily available and does not require a specific underlying etiology to be known prior to initiation [1-4,6]. Lastly, a significant proportion of our patients presented with severe hypercalcemia as the first manifestation of their malignancy, which would also limit completeness of evaluation of HCM.
Our results contrast the long-standing belief as to the etiology of HCM. Several important points can be made from our data in this regard. First, combinations of etiologies are fairly common as a whole and in subgroups of malignancies, except for multiple myeloma where, as expected, OM predominate. Outside of multiple myeloma, approximately 20% of all cases of HCM had more than one contributor to the hypercalcemia, similar to the findings of Szymanski et al [14]. Second, our data show the importance of PTHrP to be much lower than classically thought, with a significant increase in importance of OM and 1,25-OH vitamin D. Lastly, 1,25-OH vitamin D plays a very significant role in the pathophysiology of HCM of solid tumors, which is contrary to the popular belief that elevated 1,25-OH vitamin D levels are rare in solid tumors [1-4,6,15]. The mechanism of increased 1,25-OH vitamin D in solid tumors appears to be similar to that which occurs in lymphomas, namely via ectopic activity of 1-α hydroxylase in tumor cells and/or peritumor macrophages [21-25]. Of note, PTHrP does not appear to stimulate 1-α hydroxylase [2-4]. It is of interest to note that in 1 of the classic publications, which has contributed significantly to the long-standing belief that 80% of cases of HCM are related to PTHrP, approximately 8% of the patients had an elevated or inappropriately high-normal level of 1,25-OH vitamin D, and 54% had bone scans consistent with OM, with 20% having had no skeletal imaging [5]. Therefore, even in the older data, a significant portion of patients had 1,25-OH vitamin D as a contribution to their HCM. As mentioned, this was likely overlooked at the time, since the main purpose of those older studies was to further characterize the as yet undefined humoral mediator eventually described to be PTHrP. Lastly, our results highlight the increased importance of 1,25-OH vitamin D in the etiology of HCM in solid tumors, which is consistent with another recent publication showing an abnormal result of this test in approximately 45% of patients with solid tumors, in whom 1,25-OH vitamin D levels were checked [16].
We might theorize as to why our findings are significantly different from the classic dogma. As already mentioned, none of the classic studies were designed to elucidate the distribution of etiologies of HCM. Beyond that, however, it is possible that HCM has evolved into a different entity than it was decades ago due to advancements in the management of various malignancies. That is, one can imagine that if cancer therapy itself improves outcomes, it will likely decrease the prevalence of HCM, with those cases still arising perhaps being of a different frequency and/or pathophysiology than they would have been years ago. Particular advancements in management likely to contribute along these lines are tyrosine kinase inhibitors, immune checkpoint inhibitors, and the standard, widespread prophylactic use of intravenous bisphosphonates and denosumab in patients known to have OM [26-30]. Furthermore, an increase in the apparent prevalence of OM in our data set could also be contributed in part to the more recent and widespread availability of positron emission tomography-computed tomography scans allowing a greater sensitivity for the diagnosis of OM [31,32]. If HCM has truly evolved into a different entity than it was decades ago, then a better understanding of the etiology is certainly of more than academic interest, as further alterations in management would be of utmost importance. Toward this end, the recent publication showing recalcitrant HCM in solid tumors with an elevated 1,25-OH vitamin D with or without concomitant PTHrP elevation is but 1 example [16]. Also recent case reports have shown the potential utility of oral cinacalcet in the management of HCM caused by both PTHrP [33] and 1,25-OH vitamin D [34]. Although the exact mechanism of this effect is unknown, it may involve actions of cinacalet at the nonparathyroid calcium-sensing receptor in the bone, kidney, and intestine [34].
There are several limitations of our study. First, the retrospective nature of this study is a major limitation, especially with regard to the incomplete evaluation we have documented in our own cohort while simultaneously attempting to expand our knowledge of the modalities contributing to HCM. Related to this is the lack of documentation of increased 1-α hydroxylase in any tumor material in our cohort. However, there has been demonstration of 1-α hydroxylase activity in tumor cells in several of the same malignancies to whom increased 1,25-OH vitamin D was deemed, in our cohort, to be a contributor to their HCM including soft tissue myofibrosarcoma [21], methothelioma [22], and clear cell renal cell carcinoma [23]. Second, this incomplete evaluation of most of the cases of HCM makes our current dataset relatively small, including only 70 patients (38 with solid tumors, 23 with nonmyeloma hematologic malignancies, and 9 with multiple myeloma) with a conclusive evaluation. Prospective studies with larger numbers of patients undergoing a conclusive evaluation are needed to either confirm or refute our findings. The fact that ~6% of our completely evaluated patients had no discernable cause of hypercalcemia may be a reflection of the limitations of the available testing. That is, interleukins 1 and 6 as well as tumor necrosis factor α have been shown to be rare causes of HCM. Furthermore, currently available imaging modalities have a sensitivity of less than 100% for OM, allowing some cases to be missed [31,32]. Another potential limitation is that this group of patients might be a biased subset, perhaps representing a group with more severe disease and/or hypercalcemia. However, as shown in Table 1, there was no significant difference in mean cCa2+ or the distribution of severity of cCa2+ between the groups with a conclusive vs a less than conclusive evaluation, although the number of patients in each group was small. Unfortunately, with such a large proportion of our cohort not even having a PTH assessed (8651 patients) or no evaluation of PTHrP or 1,25-OH vitamin D in the setting of PTH-independent hypercalcemia (620 patients), we cannot assure our sample was not a biased subset. Lastly, we abstracted data from the medical records of a predominantly rural white/Caucasian population, so our findings may not be generalizable to more diverse, urban populations. However, as many of our patients primarily receive care at Marshfield Clinic Health System facilities, the strength of our data set lies in the long-term monitoring of these patients, which not only decreases the number of patients lost to follow-up but also permits a comprehensive analysis of changes in clinical practice over time.
Conclusions
Our results challenge classical thinking in regard to the underlying etiology of HCM. Contrary to standard dogma, it appears that PTHrP may only contribute to HCM in approximately 27% of cases, with OM contributing in almost 50% and 1,25-OH vitamin D making up a substantially higher proportion than previously believed at almost 40% of cases. This discrepancy may be due in part to incomplete patient evaluation in prior studies. However, HCM may very well have evolved into a different entity than it was in previous decades. A key implication for practice from this study is that the pretest probability of OM and 1,25-OH vitamin D as etiologies of HCM seem much higher than previously thought and should be considered in tandem with exclusion of PTH- or PTHrP-related hypercalcemia. Prospective studies of patients with hypercalcemia in malignancy that include a comprehensive biochemical and skeletal imaging evaluation are needed to verify these findings. A better understanding of the exact etiology(ies) of HCM in any given patient may improve management of this condition.
Acknowledgments
The authors acknowledge Emily Andreae and Marie Fleisner from the Marshfield Clinic Research Institute for assistance in editing and preparing this manuscript for submission.
Funding Source: None.
Author Contributions: M.T.S. and A.A.O. designed the study and wrote the manuscript. M.T.S., Y.H.L., and A.A.O. contributed to the data collection. M.T.S., Y.H.L., S.A.D., and A.A.O. analyzed the data and contributed to writing the manuscript. M.T.S. and A.A.O. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373-379. [DOI] [PubMed] [Google Scholar]
- 2. Sternlicht H, Glezerman IG. Hypercalcemia of malignancy and new treatment options. Ther Clin Risk Manag. 2015;11:1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zagzag J, Hu MI, Fisher SB, Perrier ND. Hypercalcemia and cancer: differential diagnosis and treatment. CA Cancer J Clin. 2018;68(5):377-386. [DOI] [PubMed] [Google Scholar]
- 4. Asonitis N, Angelousi A, Zafeiris C, Lambrou GI, Dontas I, Kassi E. Diagnosis, pathophysiology and management of hypercalcemia in malignancy: a review of the literature. Horm Metab Res. 2019;51(12):770-778. [DOI] [PubMed] [Google Scholar]
- 5. Stewart AF, Horst R, Deftos LJ, Cadman EC, Lang R, Broadus AE. Biochemical evaluation of patients with cancer-associated hypercalcemia: evidence for humoral and nonhumoral groups. N Engl J Med. 1980;303(24):1377-1383. [DOI] [PubMed] [Google Scholar]
- 6. Insogna KL, Broadus AE. Hypercalcemia of malignancy. Annu Rev Med. 1987;38:241-256. [DOI] [PubMed] [Google Scholar]
- 7. Burtis WJ, Brady TG, Orloff JJ, et al. . Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N Engl J Med. 1990;322(16):1106-1112. [DOI] [PubMed] [Google Scholar]
- 8. Blind E, Raue F, Götzmann J, Schmidt-Gayk H, Kohl B, Ziegler R. Circulating levels of midregional parathyroid hormone-related protein in hypercalcaemia of malignancy. Clin Endocrinol (Oxf). 1992;37(3):290-297. [DOI] [PubMed] [Google Scholar]
- 9. Walls J, Ratcliffe WA, Howell A, Bundred NJ. Parathyroid hormone and parathyroid hormone-related protein in the investigation of hypercalcaemia in two hospital populations. Clin Endocrinol (Oxf). 1994;41(4):407-413. [DOI] [PubMed] [Google Scholar]
- 10. Ratcliffe WA, Hutchesson AC, Bundred NJ, Ratcliffe JG. Role of assays for parathyroid-hormone-related protein in investigation of hypercalcaemia. Lancet. 1992;339(8786):164-167. [DOI] [PubMed] [Google Scholar]
- 11. Blind E, Raue F, Meinel T, et al. . [Diagnostic significance of parathyroid hormone-related protein in tumor patients with hypercalcemia]. Dtsch Med Wochenschr. 1993;118(10):330-335. [DOI] [PubMed] [Google Scholar]
- 12. Blind E, Raue F, Meinel T, et al. . Levels of parathyroid hormone-related protein in hypercalcemia of malignancy: comparison of midregional radioimmunoassay and two-site immunoradiometric assay. Clin Investig. 1993;71(1):31-36. [DOI] [PubMed] [Google Scholar]
- 13. Kao PC, Klee GG, Taylor RL, Heath H 3rd. Parathyroid hormone-related peptide in plasma of patients with hypercalcemia and malignant lesions. Mayo Clin Proc. 1990;65(11):1399-1407. [DOI] [PubMed] [Google Scholar]
- 14. Szymanski JJ, Otrock ZK, Patel KK, Scott MG. Incidence of humoral hypercalcemia of malignancy among hypercalcemic patients with cancer. Clin Chim Acta. 2016;453:190-193. [DOI] [PubMed] [Google Scholar]
- 15. Donovan PJ, Sundac L, Pretorius CJ, d’Emden MC, McLeod DS. Calcitriol-mediated hypercalcemia: causes and course in 101 patients. J Clin Endocrinol Metab. 2013;98(10):4023-4029. [DOI] [PubMed] [Google Scholar]
- 16. Chukir T, Liu Y, Hoffman K, Bilezikian JP, Farooki A. Calcitriol elevation is associated with a higher risk of refractory hypercalcemia of malignancy in solid tumors. J Clin Endocrinol Metab. 2020;105:(4):e1115-e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jick S, Li L, Gastanaga VM, Liede A. Prevalence of hypercalcemia of malignancy among cancer patients in the UK: analysis of the clinical practice research datalink database. Cancer Epidemiol. 2015;39(6):901-907. [DOI] [PubMed] [Google Scholar]
- 18. Gastanaga VM, Schwartzberg LS, Jain RK, et al. . Prevalence of hypercalcemia among cancer patients in the United States. Cancer Med. 2016;5(8):2091-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bilezikian JP. Primary hyperparathyroidism. J Clin Endocrinol Metab. 2018;103(11):3993-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bilezikian JP, Bandeira L, Khan A, Cusano NE. Hyperparathyroidism. Lancet. 2018;391(10116):168-178. [DOI] [PubMed] [Google Scholar]
- 21. Ogose A, Kawashima H, Morita O, Hotta T, Umezu H, Endo N. Increase in serum 1,25-dihydroxyvitamin D and hypercalcaemia in a patient with inflammatory myofibroblastic tumour. J Clin Pathol. 2003;56(4):310-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JM, Pou K, Sadow PM, et al. . Vitamin D-mediated hypercalcemia and cushing syndrome as manifestations of malignant pleural mesothelioma. Endocr Pract. 2008;14(8):1011-1016. [DOI] [PubMed] [Google Scholar]
- 23. Shivnani SB, Shelton JM, Richardson JA, Maalouf NM. Hypercalcemia of malignancy with simultaneous elevation in serum parathyroid hormone–related peptide and 1,25-dihydroxyvitamin D in a patient with metastatic renal cell carcinoma. Endocr Pract. 2009;15(3):234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Evans KN, Taylor H, Zehnder D, et al. . Increased expression of 25-hydroxyvitamin D-1alpha-hydroxylase in dysgerminomas: a novel form of humoral hypercalcemia of malignancy. Am J Pathol. 2004;165(3):807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akai PS, Wong T, Chang-Poon V, Green F, Whitelaw WA, Hanley DA. Resectable bronchogenic carcinoma presenting with hypercalcemia: tumor-associated granulomatous reaction and probable production of 1,25-dihydroxyvitamin D. Clin Invest Med. 1989;12(3):212-216. [PubMed] [Google Scholar]
- 26. Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015;36(7):422-439. [DOI] [PubMed] [Google Scholar]
- 27. Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. 2018;29(1):84-91. [DOI] [PubMed] [Google Scholar]
- 28. Zheng GZ, Chang B, Lin FX, et al. . Meta-analysis comparing denosumab and zoledronic acid for treatment of bone metastases in patients with advanced solid tumours. Eur J Cancer Care (Engl). 2017;26(6). doi: 10.1111/ecc.12541 [DOI] [PubMed] [Google Scholar]
- 29. Diel IJ, Body JJ, Stopeck AT, et al. . The role of denosumab in the prevention of hypercalcaemia of malignancy in cancer patients with metastatic bone disease. Eur J Cancer. 2015;51(11):1467-1475. [DOI] [PubMed] [Google Scholar]
- 30. Ibrahim T, Farolfi A, Mercatali L, Ricci M, Amadori D. Metastatic bone disease in the era of bone-targeted therapy: clinical impact. Tumori. 2013;99(1):1-9. [DOI] [PubMed] [Google Scholar]
- 31. Yang HL, Liu T, Wang XM, Xu Y, Deng SM. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21(12):2604-2617. [DOI] [PubMed] [Google Scholar]
- 32. Wu F, Jiang Y, Ma L, Yu Q, Xue W, Wang Z. Detection of bone metastases in patients with cancer: 99m Tc-MDP bone scan and 18F-FDG PET/CT. Biomed Res. 2017;28:1299-1304. [Google Scholar]
- 33. Sheehan M, Tanimu S, Tanimu Y, Engel J, Onitilo A. Cinacalcet for the treatment of humoral hypercalcemia of malignancy: an introductory case report with a pathophysiologic and therapeutic review. Case Rep Oncol. 2020;13(1):321-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheehan MT, Wermers RA, Jatoi A, Loprinzi CL, Onitilo AA. Oral cinacalcet responsiveness in non-parathyroid hormone mediated hypercalcemia of malignancy. Med Hypotheses. 2020;143:110149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.