Abstract
Parkin plays an important role in ensuring efficient mitochondrial function and calcium homeostasis. Parkin-mutant human fibroblasts, with defective oxidative phosphorylation activity, showed high basal cAMP level likely ascribed to increased activity/expression of soluble adenylyl cyclase and/or low expression/activity of the phosphodiesterase isoform 4 and to a higher Ca2+ level. Overall, these findings support the existence, in parkin-mutant fibroblasts, of an abnormal Ca2+ and cAMP homeostasis in mitochondria. In our previous studies resveratrol treatment of parkin-mutant fibroblasts induced a partial rescue of mitochondrial functions associated with stimulation of the AMPK/SIRT1/PGC-1α pathway. In this study we provide additional evidence of the potential beneficial effects of resveratrol inducing an increase in the pre-existing high Ca2+ level and remodulation of the cAMP homeostasis in parkin-mutant fibroblasts. Consistently, we report in these fibroblasts higher expression of proteins implicated in the tethering of ER and mitochondrial contact sites along with their renormalization after resveratrol treatment. On this basis we hypothesize that resveratrol-mediated enhancement of the Ca2+ level, fine-tuned by the ER–mitochondria Ca2+ crosstalk, might modulate the pAMPK/AMPK pathway in parkin-mutant fibroblasts.
Keywords: resveratrol, parkin, mitochondria, cAMP, calcium (Ca2+), endoplasmic reticulum (ER)
1. Introduction
PARK2 and PARK6, which encode for parkin and PINK1 respectively, are genes responsible of the onset of familial Parkinson’s disease (PD) a progressive degenerative disorder of the central nervous system characterized by dopaminergic neurodegeneration in the substantia nigra pars compacta. Several studies in PD models proved hallmarked dysfunctions of mitochondria, in particular, defect of the respiratory chain complex I, which plays a central role in mitochondrial oxidative phosphorylation (OXPHOS) efficiency and capacity [1], depletion of ATP production, increased reactive oxygen species (ROS) and oxidative stress, anomalies in mitochondrial dynamics, trafficking and turnover, dysregulation in calcium homeostasis, and protein misfolding and aggregation [2,3,4,5,6,7,8,9,10].
Parkin, together with PINK1, is involved in one of the mitochondrial quality control pathways in the cells that identifies impaired mitochondria and selectively primes their elimination by mitophagy [11,12]. In particular, loss of mitochondrial membrane potential in damaged mitochondria induces the accumulation of PINK1, a serine/threonine kinase, on the outer mitochondrial membrane surface. Subsequently, PINK1 phosphorylates parkin, which translocates to damaged mitochondria mediating the selective removal of the damaged organelle, after ubiquitination of mitochondrial proteins [13,14,15]. Studies, in vitro and in vivo, on parkin-null models clearly indicate a role of parkin in the preservation of a functional mitochondrial compartment. Indeed, an altered mitochondrial respiration and morphology, decrease of mitochondrial ATP production and a higher susceptibility to neurotoxin 1-methyl-4-phenylpyridinium ion (MPP+) have been observed in parkin KO models [16,17,18,19,20]. Accordingly, mitochondrial impairment has been repeatedly observed in parkin-mutant non-neuronal cell-type-like fibroblasts [4,5,21,22,23].
Along with this line of evidence, we showed in earlier studies that primary parkin-mutant fibroblasts, carrying different mutations in the PARK2 gene, displayed severe ultrastructural abnormalities, mainly in mitochondria [4,24], altered expression of proteins involved in structural dynamics of cytoskeleton, oxidative stress response, Ca2+ homeostasis, and RNA processing [25,26]. Furthermore, parkin-mutant fibroblasts showed an altered lipid profile [27], dysfunctions of lysosomal function [28] and of clock gene-dependent energy metabolism [29], and higher Ca2+ and cAMP basal levels [4,5,30].
The higher cAMP level, observed in parkin-mutant fibroblasts, appeared linked to an increased expression of soluble adenylate cyclase (sAC), which produces cAMP, and to a lower expression of the phosphodiesterase isoform 4 (PDE4), which hydrolyzes cAMP and inactivates cAMP-mediated signaling [30]. PDE4 is the major isoform of the phosphodiesterase family, prominently expressed in fibroblasts and brain, and often associated with various pathophysiological conditions [31].
The higher basal intracellular Ca2+ level in the cytosol and, in particular, in the mitochondria could be responsible for the increased sAC-dependent cAMP level in parkin-mutant fibroblasts [30]. cAMP is one of the main second messengers proved to modulate mitochondrial metabolism [32,33,34,35,36,37,38], and it is strictly linked to Ca2+ homeostasis [39,40] that, in turn, is also involved in the regulation of mitochondria functions [41].
Resveratrol is a well-known nonflavonoid polyphenol endowed with powerful antioxidant properties, primarily found in red grapes/wine [42] and reported to have protective effects in several neurodegenerative diseases [43,44]. Evidences have been provided, in parkin-mutant fibroblasts, on the resveratrol ability to induce a partial rescue of mitochondrial dysfunctions [5]. Briefly, the resveratrol treatment induced an energy homeostasis improvement through activation of the AMP-mediated protein kinase (AMPK) pathway, resulting in increased expression of several PGC1α target genes involved in mitochondrial biogenesis and radical oxygen homeostasis [5]. It is reported that resveratrol modulates cellular cAMP level and Ca2+ homeostasis by inhibiting PDE4 and plasma membrane Ca2+-ATPase (PMCA) in C2C12 myotube and fibroblast cell cultures, respectively. Moreover, resveratrol modulates the expression of proteins present at the contact sites between mitochondria and endoplasmic reticulum (ER) in different cellular systems [45,46,47,48,49,50,51].
Organelle functions are strictly associated with the capacity to bind other organelles via membrane contact sites [52]. Specific contact sites are present between mitochondria and endoplasmic reticulum (ERMCSs) where several Ca2+-transport systems are localized [53,54,55,56,57,58]. The Ca2+ signaling plays a central role in the cellular function regulating autophagy, mitochondrial metabolism, and cell death [41]. Recently, PINK1 and parkin have been found to be mainly localized at the ERMCSs and, together with other several proteins, control the crosstalk between the two organelles [59,60]. ERMCSs represent essential structures, linked to multiple pathways, among which Ca2+ homeostasis, lipid transfer, autophagy, and mitochondrial dynamics [61,62,63] whose perturbations are associated with several diseases, including neurological disorders [64]. In mammals, several tethering molecules are involved in the formation of ERMCSs [65], among which are glucose-regulated protein 75 (GRP75), mitochondrial Rho GTPase 2 (Miro2), and mitofusin 2 (Mfn2). GRP75 physically bridges VDAC1, an outer mitochondrial membrane (OMM) protein, to the inositol 1,4,5-trisphosphate receptor (IP3R), an ER Ca2+-release channel [63,66]. Miro2 is an OMM protein that anchors mitochondria to microtubules and is required for normal mitochondrial cristae architecture, mitochondrial transport, and mitochondria-associated membranes (MAMs) function [67]. Mfn2 is a large GTPase, located on both the OMM and ER surface, which forms heterocomplexes with Mfn1. Indeed, Mfn2 has been found enriched at ERMCSs, where it regulates organelles tethering in different tissues [68]. Moreover, Miro2 and Mfn2 have been found to take part in the regulation of the Ca2+ flux from the ER to mitochondria [69].
In this study we evaluated the effect of resveratrol treatment on the basal cellular cAMP and Ca2+ levels and on the expression of specific proteins localized at ERMCSs in control and parkin-mutant fibroblasts, lacking the 50 kDa full-length parkin protein. The results attained suggest that resveratrol treatment of parkin-mutant fibroblasts induced a remodulation of the cAMP homeostasis by decreasing transmembrane adenylyl cyclases (tmAC) and increasing sAC contribution to cAMP level. In addition, resveratrol affects Ca2+ homeostasis, inducing a significant increase of cytosolic and mitochondrial Ca2+ levels. These effects might be additionally associated with a restoration of normal levels of Miro2 and Mfn2, upregulated in parkin-mutant fibroblasts.
2. Materials and Methods
2.1. Cell Cultures
Parkin-mutant fibroblasts from a patient affected by an early-onset PD, with compound heterozygous mutations (del exon7–9/Glu409X), lacking the 50 kDa full-length parkin protein, and control fibroblasts from one healthy subject, were obtained by explants from skin punch biopsy, after informed consent, and cultured as previously described [5,30]. Control and parkin-mutant primary fibroblasts were grown in a T25 Flask and used for experiments at 80% confluence. For resveratrol treatment the cells were incubated with dimethyl sulfoxide (0.02% DMSO), used as control vehicle, or 25 μM resveratrol (RSV) (Sigma Aldrich, St. Louis, MO, USA, Catalog number: R5010) for 30 min at 37 °C. Following extensive dose-dependence assays, a resveratrol concentration of 25 μM was chosen for the absence of cytotoxicity up to 48 h of treatment.
2.2. Cyclic Adenosine Monophosphate (cAMP) Assay
Control and RSV-treated cells were incubated in the absence or in the presence of 10 µM Rolipram, 100 µM 3-Isobutyl-1-methylxanthine (IBMX), 10 µM forskolin, and 100 µM SQ22536 for 30 min at 37 °C. For cAMP assays, the culture medium was removed and 1 mL of 0.1 M HCl was added to the cell layer, followed by 10 min incubation at 37 °C. The lysed cells were scraped, transferred into tubes, and centrifuged at 1300× g for 10 min at 4 °C. The supernatant was used for cAMP measurements using the direct cAMP ELISA Kit (Enzo Life Sciences, New York, NY, USA) according to the manufacturer’s instruction. Measurements were performed on a Victor 2030 multilabel reader (PerkinElmer, Waltham, MA, USA). The cAMP values were normalized to the protein concentration and expressed as pmol/mg protein.
2.3. Quantitative Fluorimetric Measurement of Cytosolic and Mitochondrial Ca2+ Levels
Cytosolic and mitochondrial Ca2+ levels were measured in control and parkin-mutant fibroblasts exposed to either vehicle (0.02% DMSO) or 25 μM resveratrol (RSV) for 30 min, by using the cell-permeable fluorescent Ca2+ indicator Fluo-4 AM or X-Rhod-1AM (Invitrogen, Carlsbad, CA, USA), respectively [70,71]. Once inside the cell, the lipophilic blocking groups of Fluo-4 AM are cleaved by nonspecific cell esterase, resulting in a negatively charged dye that stays inside cells, and whose fluorescence is greatly enhanced upon binding to Ca2+. X-Rhod-1AM is a cell permeable cationic fluorescent dye which can result in a membrane potential-driven uptake into mitochondria. For the Ca2+ level measurements, the cells at 80% confluence were incubated with 2.5 μM of the fluorescent probes for 30 min at 37 °C. Cell monolayers collected by trypsinization and centrifugation were resuspended in a buffer containing 10 mM Hepes and 6 mM D-Glucose (pH 7.4) at an approximate concentration of 1 × 105 cells in 1 mL. Fluorescence intensity was measured at 25 °C in a spectrofluorometer (Jasco FP6200 Mary’s Court Easton, MD, USA), equipped with a stirrer and temperature control, by the subsequent addition of 5 mM CaCl2, 0.1% Triton X-100 (for cytosolic Ca2+ level detection), 0.1% Na-Colate (for mitochondrial Ca2+ level detection), and 40 mM EGTA. The excitation/emission wavelengths were 495 nm/506 nm for Fluo-4 AM and 580 nm/602 nm for X-Rhod-1 AM. The cytosolic and mitochondrial Ca2+ levels were evaluated by using an apparent Kd (443 nM for Fluo-4AM and 700 nM for X-Rhod-1AM) according to the equation described by Grynkiewicz [72]. Where indicated, incubations with DMSO or RSV, in the presence or in the absence of 1 µM thapsigargin (TG), 10 µM dantrolene (Dan), 5 µM ruthenium red (RR), were performed for 30 min at 37 °C.
2.4. Western Blot Analysis
Total cell proteins (20 μg) from control and parkin-mutant fibroblasts exposed to either vehicle (0.02% DMSO) or 25 μM resveratrol (RSV) for 24 h were separated on a 8% Tris-Glycine SDS–PAGE, transferred to nitrocellulose membranes with 0.2 µm pore size (Bio-Rad, Hercules, CA, USA), and immunoblotted with specified primary antibodies against GRP75 (1:200; Santa Cruz Bio Technology, Dallas, TX, USA, Catalog number: sc-13967), Miro2 (1:1000; Cell Signaling Technology, Danvers, MA, USA, Catalog number: #14016), and Mfn2 (1:200; Millipore, Burlington, MA, USA, Catalog number: #ABC42). Sample loading was assessed with actin (1:3000; Sigma Aldrich, St. Louis, MO, USA, Catalog number: A1978). After incubation with the horseradish peroxidase-conjugated secondary antibody (1:3000; Bio-Rad, Hercules, CA, USA, Anti-mouse catalog number: #1706516; Anti-rabbit catalog number: #1707515), signals were settled by the chemiluminescence kit (ClarityTM Western ECL Substrate, Bio-Rad, Hercules, CA, USA). Immuno-revealed bands were acquired by ChemiDoc Imaging System XRS (Bio-Rad, Hercules, CA, USA) and analyzed with the Image J Lab software 1.8.0_112 (https://imagej.nih.gov/ij/index.html accessed on 21 July 2021).
2.5. Protein Measurement
The protein concentration was measured by the Quick Start™ Bradford Protein Assay (Bio-Rad, Hercules, CA, USA) and bovine serum albumin was used as the standard.
2.6. Statistical Analysis
Data are shown as mean ± SEM. The significance of any differences throughout the data sets presented was determined by one-way or two-way analysis of variance (ANOVA) with the Tukey post hoc test. The threshold for statistical significance (p-value) was set to 0.05.
3. Results
3.1. Resveratrol Decreases cAMP Level in Parkin-Mutant Fibroblasts
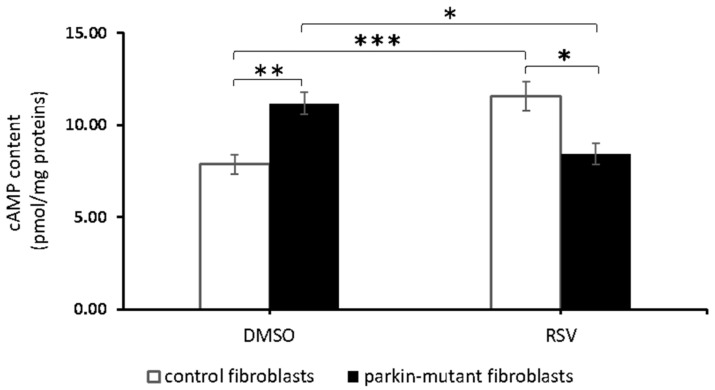
We previously showed a higher basal level of cAMP in parkin-mutant fibroblasts compared to control cells [30]. To examine the effect of the resveratrol (RSV) on the basal level of cAMP, we treated control and parkin-mutant fibroblasts with 25 μM RSV or vehicle for 30 min, as described in Materials and Methods. RSV-treatment induced a significant increase of cAMP level in control fibroblasts and, on the contrary, a significant decrease of the higher basal cAMP level present in parkin-mutant fibroblasts (Figure 1). To note, the cAMP level in RSV-treated parkin-mutant fibroblasts reached a value comparable to that of control fibroblasts under basal conditions.
Figure 1.
Effect of resveratrol on the basal cAMP cellular level in control and parkin-mutant fibroblasts. Basal cyclic adenosine monophosphate (cAMP) cellular level, expressed as pmol/mg protein, in control (open bar) and parkin-mutant (black bar) fibroblasts exposed to either vehicle (DMSO) or 25 μM resveratrol (RSV) for 30 min. The values represent the means ± SEM from 3 independent experiments under each condition. The significance was determined by two-way ANOVA with Tukey post hoc; *, p ˂ 0.01; **, p ˂ 0.001; ***, p ˂ 0.0005. For more details, see Materials and Methods.
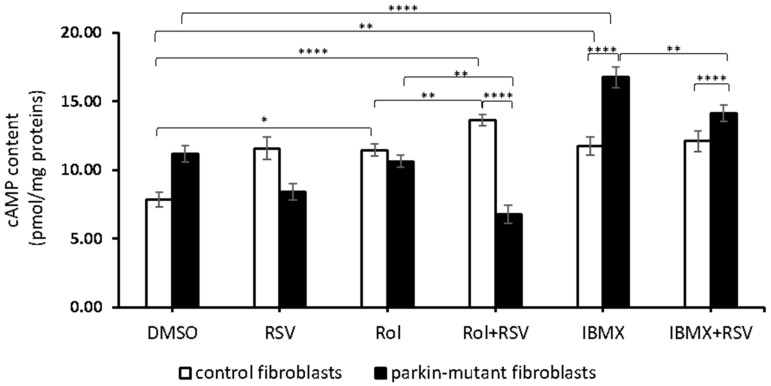
Since RSV is reported to modulate cAMP level by inhibiting PDE4 [45], we carried out experiments in the presence of rolipram (Rol), a selective inhibitor of PDE4. Rol-treatment led to a significant increase of the cAMP level in control fibroblasts without any significant effect in parkin-mutant fibroblasts, thus suggesting a lower expression of PDE4 [30]. The co-treatment with resveratrol (Rol+RSV) induced a further increase of cAMP level in control cells and a decrease in parkin-mutant fibroblasts as compared with Rol-treatment (Figure 2). In control cells, the treatment with IBMX, a pan-inhibitor of other phosphodiesterases (PDEs), resulted in a significant increase of the cAMP level [30], comparable with that attained by Rol-treatment, and no further changes were observed by co-treatment with resveratrol (Figure 2). In parkin-mutant fibroblasts, IBMX-treatment caused a significant increase of the cAMP level, much higher than in control cells [30], which was, however, significantly reduced in the presence of RSV (Figure 2). These results suggested that RSV, while in control fibroblasts, increased cAMP level, likely inhibiting PDE4 [45] in PDE4-defective parkin-mutant cells, and could act on different targets.
Figure 2.
Effect of resveratrol on the cAMP cellular level in the presence of rolipram and IBMX. cAMP content in control (open bar) and parkin-mutant (black bar) fibroblasts exposed for 30 min to vehicle (DMSO) or 25 μM resveratrol (RSV). Where indicated, the cells were treated for 30 min with 10 µM rolipram (Rol) or 100 µM IBMX (IBMX) alone or co-incubated with resveratrol, Rol+RSV, or IBMX+RSV. The values represent the means ± SEM from 3 independent experiments under each condition. The significance was determined by two-way ANOVA with Tukey post hoc; *, p ˂ 0.01; **, p ˂ 0.001; ****, p ˂ 0.0001. The statistical significance of cAMP level among DMSO and RSV treatments is presented in Figure 1 and omitted herein to streamline the figure. For more details, see Materials and Methods.
As the steady-state level of cAMP results from the balance between its synthesis and degradation, we considered the possibility of an inhibitory effect of RSV on the adenylate cyclases in parkin-mutant fibroblasts.
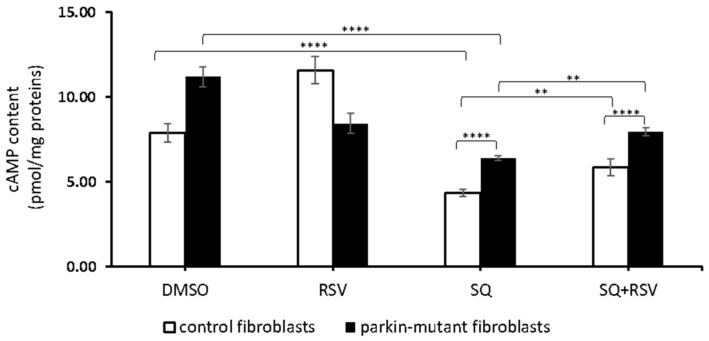
cAMP can be produced by transmembrane adenylyl cyclases (tmACs) and by soluble adenylyl cyclase (sAC). Therefore, we analyzed the effect of RSV on the cAMP level in the presence of SQ22536 (SQ), a specific inhibitor of tmACs [73]. As already reported [30], the SQ treatment resulted in a significant decrease of the cAMP level, both in control and parkin-mutant cells, though its level in parkin-mutant cells remained much higher than in control cells. The co-treatment with RSV (SQ+RSV) induced a significant increase of cAMP level with respect to SQ-treatment in both control and parkin-mutant fibroblasts (Figure 3). It is noteworthy that, even in the case of co-treatment (SQ+RSV), the level of cAMP in parkin-mutant fibroblasts was higher than that observed in control cells in the same experimental condition.
Figure 3.
Effect of resveratrol on cAMP cellular level in the presence of SQ22386. cAMP content in control (open bar) and parkin-mutant (black bar) fibroblasts exposed for 30 min to vehicle (DMSO) or 25 μM resveratrol (RSV). Where indicated, the cells were incubated for 30 min with 100 µM SQ22386 (SQ) or co-incubated with resveratrol (SQ+RSV). The values represent the means ± SEM from 3 independent experiments under each condition. The significance was determined by two-way ANOVA with Tukey post hoc; **, p ˂ 0.001; ****, p ˂ 0.0001. The statistical significance of cAMP level among DMSO and RSV treatments is presented in Figure 1 and omitted herein to streamline the figure. For more details, see Materials and Methods.
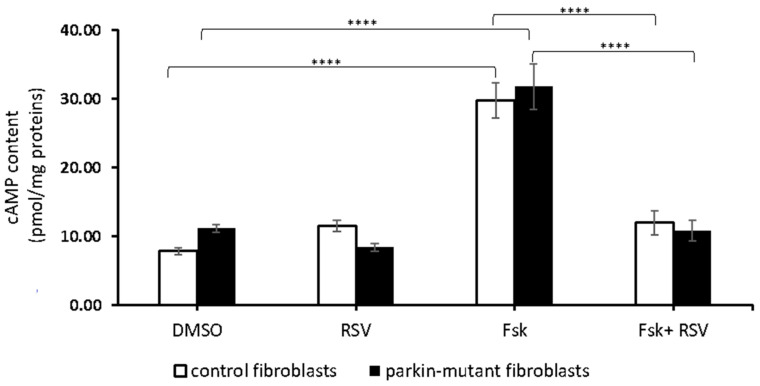
Next, we investigated the effect of resveratrol in the presence of forskolin (Fsk), an activator of tmAC. Fsk-treatment resulted in a strong enzymatic response in both control and parkin-mutant fibroblasts with a significant increase of cAMP level [28] that was completely prevented by co-treatment with resveratrol (Fsk+RSV) (Figure 4). This result suggested a possible inhibition of tmAC by resveratrol as already described [74].
Figure 4.
Effect of resveratrol on cAMP cellular level in the presence of forskolin. cAMP content in control (open bar) and parkin-mutant (black bar) fibroblasts exposed for 30 min to vehicle (DMSO) or 25 μM resveratrol (RSV). Where indicated, the cells were incubated 30 min with 10µM forskolin (Fsk) or co-incubated with resveratrol (Fsk+RSV). The values represent the means ± SEM from 3 independent experiments under each condition. The significance was determined by two-way ANOVA with Tukey post hoc; ****, p ˂ 0.0001. The statistical significance of cAMP level among DMSO and RSV treatments is presented in Figure 1 and omitted herein to streamline the figure. For more details, see Materials and Methods.
The reported results need to be reconciled with the opposite effect of resveratrol on the cAMP level observed in control and parkin-mutant fibroblasts. A reliable explanation could be that, in RSV-treated control cells, the likely inhibition of tmAC might be compensated by the inhibition of PDE4, thus resulting in an increased cAMP level. The same hypothesis does not apply to the case of RSV-dependent decrease of cAMP level observed in parkin-mutant fibroblasts as these cells lack efficient PDE4 activity.
3.2. Resveratrol Further Increases Cytosolic and Mitochondrial Ca2+ Levels in Parkin-Mutant Fibroblasts
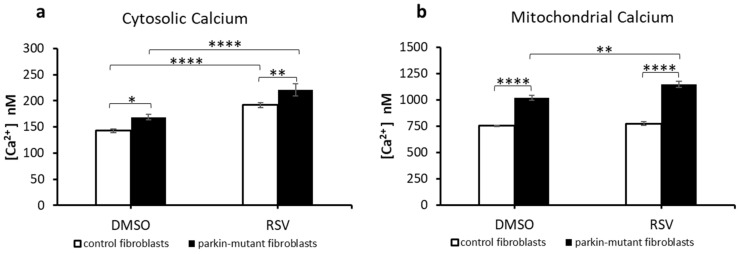
As previously reported, parkin-mutant fibroblasts showed deregulation of basal level of cAMP associated with higher steady state Ca2+ basal level in both cytosolic and mitochondrial compartment [28]. Since cAMP is strictly connected to calcium level [75] and, as we previously showed, a calcium overload in fibroblast cell cultures leads to an increase of cAMP [30], we measured the Ca2+ level in RSV-treated cells. In agreement with previous studies [47,48,76], RSV-treatment caused a significant increase of cytosolic Ca2+ level in both control and parkin-mutant cells (Figure 5a). Instead, RSV-treatment caused an increase of mitochondrial Ca2+ level only in parkin-mutant cells (Figure 5b). Of note, in the RSV-treated parkin-mutant fibroblasts, the Ca2+ level was significantly higher in both cytosolic and mitochondrial compartments than in control fibroblasts.
Figure 5.
Effect of resveratrol on the basal cytosolic and mitochondrial Ca2+ levels in control and parkin-mutant fibroblasts. Spectrofluorometric measurements of cytosolic (a) and mitochondrial Ca2+ (b) levels in control (open bar) and parkin-mutant (black bar) fibroblasts loaded, respectively, with Fluo-4AM and X-Rhod-1AM, exposed to either vehicle (DMSO) or 25 μM resveratrol for 30 min. The values, expressed as nM, represent the means ± SEM from 3 independent experiments under each condition. The significance was determined by two-way ANOVA with Tukey post hoc; *, p ˂ 0.01; **, p ˂ 0.001; ****, p ˂ 0.0001. For more details, see Materials and Methods.
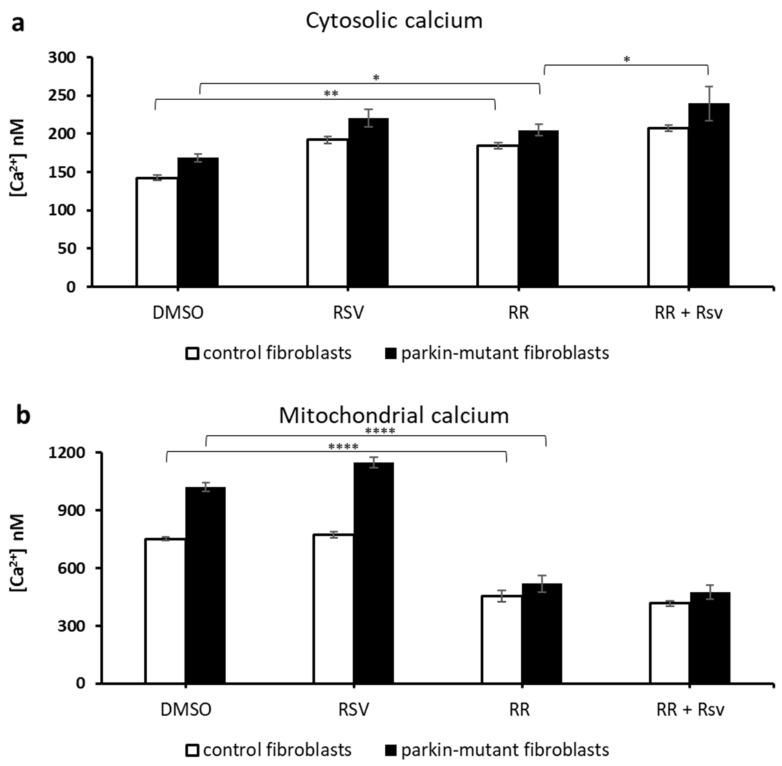
Treatment with ruthenium red (RR), a specific inhibitor of the mitochondrial Ca2+ uniporter (MCU) [77,78,79,80], induced a significant increase of cytosolic and a decrease of mitochondrial steady-state Ca2+ levels, both in control and parkin-mutant fibroblasts [30]. Co-treatment with resveratrol (RR+RSV) caused a further increase of cytosolic Ca2+ in parkin-mutant fibroblasts, as compared with RR-treated cells (Figure 6a), but no additional effect on the mitochondrial Ca2+ level (Figure 6b) was observed.
Figure 6.
Effect of resveratrol on the basal cellular Ca2+ level in control and parkin-mutant fibroblasts in the presence of ruthenium red (RR). Spectrofluorometric measurements of cytosolic (a) and mitochondrial (b) Ca2+ levels in control (open bar), and parkin-mutant (black bar) fibroblasts loaded, respectively, with Fluo-4AM and X-Rhod-1AM. The cells were exposed for 30 min to vehicle (DMSO) or 25 μM resveratrol (RSV). Where indicated, the cells were incubated for 30 min with 5 µM ruthenium red (RR) alone or co-incubated with resveratrol (RSV+RR). The values, expressed as nM, represent the means ± SEM from 3 independent experiments under each condition. The significance was determined by two-way ANOVA with Tukey post hoc; *, p ˂ 0.01; **, p ˂ 0.001; ****, p ˂ 0.0001. The statistical significance of Ca2+ level among DMSO and RSV treatments is presented in Figure 5 and omitted herein to streamline the figure. For more details, see Materials and Methods.
The cytosolic and the mitochondrial levels of Ca2+ are linked to ion release/uptake fluxes mainly controlled by intracellular stores. Taking in the account that parkin, localized at the ERMCSs, modulates at least in vitro ER mitochondria Ca2+ crosstalk [81,82,83,84], we tested the effect of thapsigargin (TG), a specific irreversible inhibitor of the ER Ca2+-ATPase (SERCA) [85] and of dantrolene (Dan), an inhibitor of the ryanodine receptor (RyR), an ER-resident Ca2+ -release channel [86].
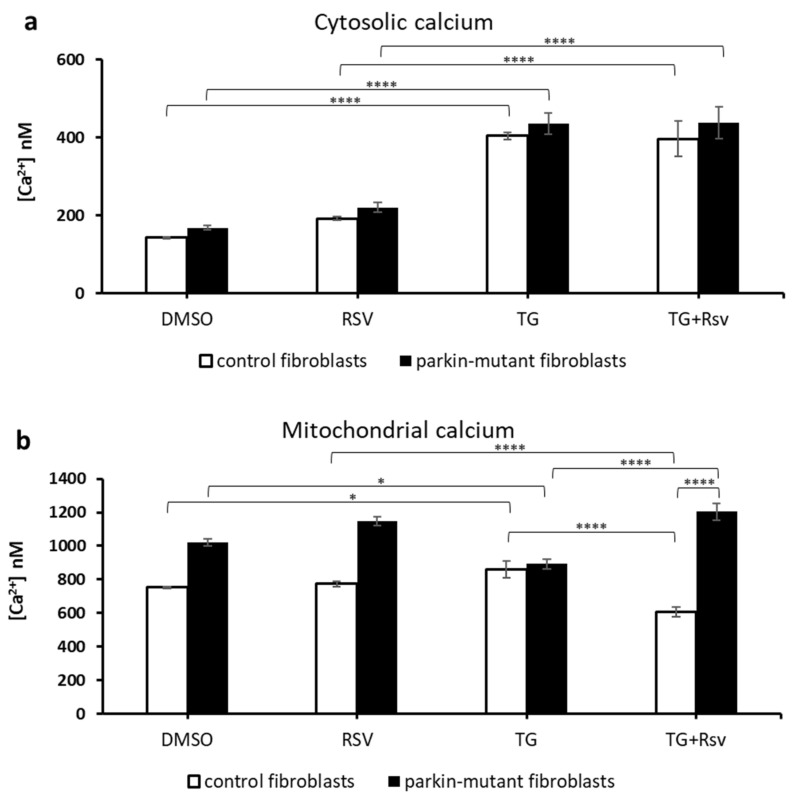
TG-treatment resulted in a significant increase of the cytosolic Ca2+ level in both control and parkin-mutant fibroblasts [30]. A small but significant increase of mitochondrial Ca2+ was observed in control fibroblasts, while a decrease of it was observed in parkin-mutant fibroblasts [30]. Co-treatment of TG with RSV (TG+RSV) did not cause appreciable changes in the cytosolic Ca2+ level in both cell samples as compared with that elicited by TG alone (Figure 7a), remaining significantly higher with respect to RSV-treatments. Conversely, the co-treatment (TG+RSV) caused a significant decrease of the mitochondrial Ca2+ level in control cells with respect to both TG-treatment and RSV-treatment and an increase in parkin-mutant fibroblasts compared to TG-treated cells (Figure 7b). Thus, although in control and parkin-mutant fibroblasts the TG treatment induced the same effect on Ca2+ level in both mitochondrial and cytosolic compartment, the co-treatment with RSV showed an opposite effect in mitochondria, inducing a decrease of Ca2+ level in control cells and an increase in parkin-mutant fibroblasts.
Figure 7.
Effect of resveratrol on the basal cellular Ca2+ level in control and parkin-mutant fibroblasts in the presence of thapsigargin (TG). Spectrofluorometric measurements of cytosolic (a) and mitochondrial (b) Ca2+ levels in control (open bar), and parkin-mutant (black bar) fibroblasts loaded, respectively, with Fluo-4AM and X-Rhod-1AM. The cells were exposed for 30 min to vehicle (DMSO) or 25 μM resveratrol (RSV). Where indicated, the cells were incubated for 30 min with 1 µM thapsigargin (TG) alone or co-incubated with resveratrol (RSV+TG). The values, expressed as nM, represent the means ± SEM from 3 independent experiments under each condition. The significance was determined by two-way ANOVA with Tukey post hoc; *, p ˂ 0.01; ****, p ˂ 0.0001. The statistical significance of Ca2+ level among DMSO and RSV treatments is presented in Figure 5 and omitted herein to streamline the figure. For more details, see Materials and Methods.
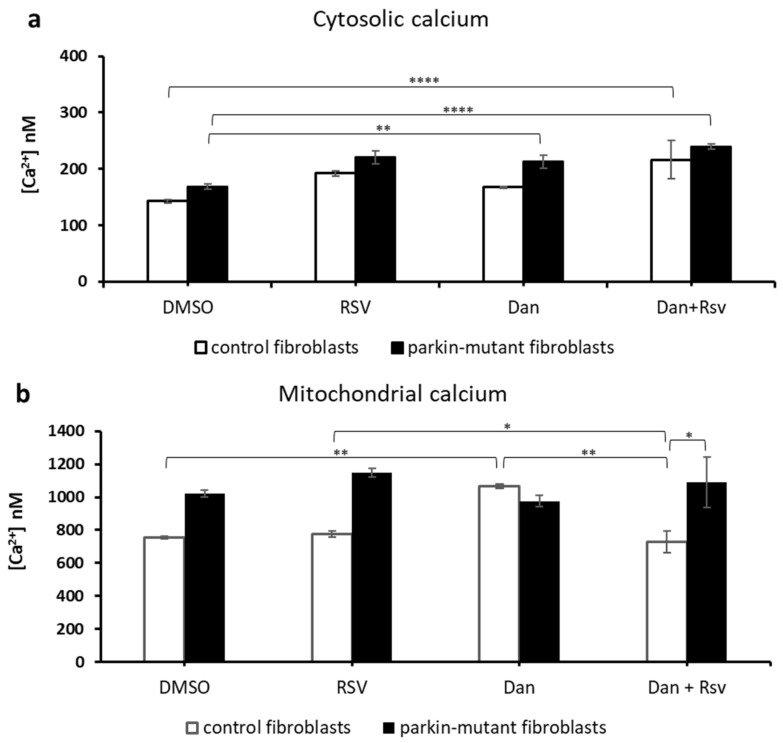
Regarding the effect elicited by Dan, we observed a slight but significant increase of cytosolic Ca2+ in parkin-mutant fibroblasts [30]. To note, Dan-treatment caused a significant increase of mitochondrial Ca2+ level in control cells and the co-treatment (Dan+RSV) induced a decrease of calcium level, which was lower than that observed with RSV alone (Figure 8a). No significant effect on mitochondrial Ca2+ level was observed in parkin-mutant fibroblasts irrespective of whether dantrolene was tested alone or in combination with RSV (Figure 8b). It is noteworthy that, even in the case of co-treatment (Dan+RSV), the level of mitochondrial Ca2+ in parkin-mutant fibroblasts was significantly higher than that observed in control cells (Figure 8b). On this basis we hypothesize that RSV-mediated enhancement of the Ca2+ level could be due to an altered Ca2+ exchange between ER and mitochondria.
Figure 8.
Effect of resveratrol on the basal cellular Ca2+ level in control and parkin-mutant fibroblasts in the presence of dantrolene (Dan). Spectrofluorometric measurements of cytosolic (a) and mitochondrial (b) Ca2+ levels in control (open bar), and parkin-mutant (black bar) fibroblasts loaded, respectively, with Fluo-4AM and X-Rhod-1AM. The cells were exposed for 30 min to vehicle (DMSO) or 25 μM resveratrol (RSV). Where indicated, the cells were incubated for 30 min with 10 µM dantrolene (Dan) alone or co-incubated with resveratrol (RSV+Dan). The values, expressed as nM, represent the means ± SEM from 3 independent experiments under each condition. The significance was determined by two-way ANOVA with Tukey post hoc; *, p ˂ 0.01; **, p ˂ 0.001; ****, p ˂ 0.0001. The statistical significance of Ca2+ level among DMSO and RSV treatments is presented in Figure 5 and omitted herein to streamline the figure. For more details, see Materials and Methods.
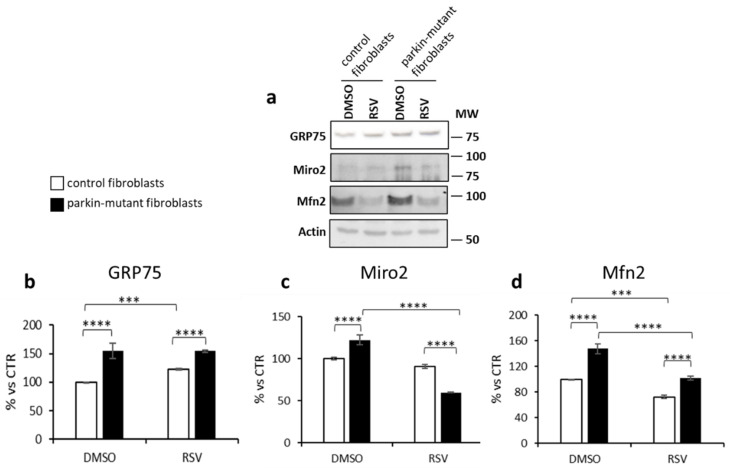
3.3. Parkin-Mutant Fibroblasts Show Higher Levels of GRP75, Miro2, and Mfn2 Proteins; Resveratrol Treatment Decreases Miro2 and Mfn2 Protein Levels
Several soluble and integral membrane proteins provide both structural and functional features in keeping the distance between mitochondria and ER in a proper range and in controlling inter-organelle Ca2+ homeostasis [62,87,88,89]. Therefore, we investigated the expression of some proteins known to be involved in the tethering/modulation of mitochondria-ER interface: GRP75, Miro2, and Mfn2.
Western blotting and densitometric analysis showed higher basal level of GRP75, Miro2, and Mfn2 in parkin-mutant fibroblasts, as compared to control cells (Figure 9). Interestingly, 24 h of RSV-treatment appeared to affect the level of these ER–mitochondria tethering proteins. In particular, GRP75 was significantly upregulated in control cells whereas Miro2 and Mfn2 were downregulated to a larger extent in parkin-mutant cells than in control cells.
Figure 9.
Effect of resveratrol on protein levels of GRP75, Miro2, and Mfn2 in control and parkin-mutant fibroblasts. (a) Representative image of Western blot of GRP75, Miro2, and Mfn2 performed on whole cell lysates from control and parkin-mutant fibroblasts exposed to either vehicle (DMSO) or 25 μM resveratrol for 24 h (MW, molecular weight). The graphs (panel (b), GRP75; panel (c), Miro2; panel (d), Mfn2) display the statistical densitometric analysis of band intensity of proteins normalized to the corresponding actin level, used as loading control. Data means ± SEM from 3 independent experiments under each condition are expressed as percentage of vehicle-treated control cells. The significance was determined by one-way ANOVA with Tukey post hoc; ***, p ˂ 0.0005; ****, p ˂ 0.0001. For more details, see Materials and Methods.
4. Discussion
Resveratrol is a natural polyphenolic compound with antioxidant and anti-inflammatory properties, able to modulate many cellular processes, including mitochondrial activity and ion homeostasis. These properties are not simply linked to the direct ROS scavenging activity of resveratrol but also to its ability to bind and modulate several intracellular targets [90,91,92]. Resveratrol can increase cytosolic Ca2+ in many cell types [46,47,48,76] by modulation of specific pathways involved in Ca2+ homeostasis. In our previous studies, we examined the effect of resveratrol treatment on parkin-mutant fibroblasts [5]. We showed that resveratrol induced an increase of mitochondrial complex I activity with a consequent significant increase of mitochondrial ATP content and a decrease in lactate level, suggesting a switch from glycolytic to oxidative metabolism. The resveratrol-dependent improvement of the mitochondrial oxidative function has been associated with a reduced oxidative stress and an increased expression of several PGC1α target genes involved in mitochondrial biogenesis. These effects have been linked to the AMPK-dependent SIRT1 activation [5] (see also [93,94,95,96,97]). In addition, in the same cellular model of parkin-mutant fibroblasts, we observed an altered mitochondrial cAMP and Ca2+ homeostasis [30]. It has been described that resveratrol can activate the CaMKKβ-AMPK pathway controlling both Ca2+ and cAMP homeostasis [45,98,99].
In this study, we assessed the effect of resveratrol-treatment on the altered mitochondrial Ca2+ and cAMP homeostasis in a cellular model of parkin-mutant fibroblasts. We first observed that resveratrol-treatment induced an increase of cAMP level in control cells, likely due to inhibition of PDE4 [45], and a decrease in parkin-mutant fibroblasts where the PDE4 is less expressed [30]. Taking into account that the cellular cAMP basal level in the parkin-mutant fibroblasts is higher than in control cells, we want to highlight that in resveratrol-treated parkin-mutant cells, the cAMP level decreased to a value comparable to the basal level observed in control cells. In addition, resveratrol-treatment induced a large decrease of the forskolin-stimulated adenylate cyclase activity in both control and parkin-mutant fibroblasts, thereby indicating a likely inhibitory effect of resveratrol on the tmACs. Thus, in parkin-mutant cells the inhibition of tmAC by resveratrol, not sufficiently compensated by an efficient PDE4 activity, could be responsible for the observed decrease in the cAMP level. These results are in agreement with the significant inhibition of forskolin-stimulated tmAC activity by low concentrations of resveratrol mediated by binding to AdoRs, observed in a glial cell model [74]. Further observations on the role of resveratrol in the modulation of cAMP level stemmed from experiments in the presence of SQ, a tmAC inhibitor. Taking into account that resveratrol inhibits PDE4 [45], it is conceivable that, in resveratrol-treated control cells, the observed increase of cAMP level, in spite of tmAC inhibition (in the presence of SQ), should be due to PDE4 inhibition. Conversely, in parkin-mutant cells, lacking the PDE4, the increase in cAMP level, observed in these conditions, should be linked to the resveratrol-dependent increase of mitochondrial Ca2+ which, in turn, primed sAC activity.
Since resveratrol activates AMPK/SIRT1/PGC1α signaling in control and parkin-mutant fibroblasts [5], and considering that Ca2+ modulates the sAC-dependent cAMP level and the Ca2+/CaMKKβ pathway activating the AMPK [98,99], we pointed the attention to the modulation of Ca2+ level by resveratrol. Higher basal Ca2+ level both in the cytosolic and, mainly, in the mitochondrial compartment has been already shown in parkin-mutant fibroblasts than in control cells [30]. Of note, proteomics studies in parkin-mutant fibroblasts showed downregulation of several Ca2+-binding proteins [26] among which calreticulin, a chaperone protein engaged in ER Ca2+ storage capacity [100], and three proteins of the S100 family, S100-A4, S100-A6, and S100-A10, involved in Ca2+-dependent regulation of a variety of intracellular activities such as intracellular Ca2+ homeostasis [101]. Resveratrol-treatment induced a further increase of cytosolic Ca2+ level in both control and parkin-mutant fibroblasts and an increase of the mitochondrial Ca2+ in parkin-mutant cells (see also [102]). The release of Ca2+ from extracellular and intracellular compartment, induced by resveratrol-treatment, could be responsible for the AMPK-dependent restore of mitochondrial respiration and ATP production previously described in parkin-mutant fibroblasts [5].
As the cellular Ca2+ homeostasis depends on various Ca2+ channels and active pumps, including MCU, SERCA, and RyR, which control Ca2+ release and uptake from intracellular stores, we evaluated on these the effect of resveratrol using specific inhibitors. Mitochondrial Ca2+ uptake is largely mediated by the MCU and driven by the mitochondrial membrane potential [103]. Parkin selectively regulates the turnover of MICU1, a subunit of MCU [104], by promoting its proteasome-mediated degradation. The loss of function in the parkin-mutant fibroblasts should enhance the MCU-mediated entry of Ca2+ into the mitochondria [see [30]]. The results obtained by the co-treatment with resveratrol and ruthenium red (RR), in parkin-mutant fibroblasts, showed a further increase in cytosolic Ca2+ level as compared with RR-treated cells and the absence of any effect on the mitochondrial Ca2+ level, therefore suggesting that resveratrol is not acting on MCU.
The endoplasmic reticular Ca2+ ATPase (SERCA) is involved in maintaining the low resting Ca2+ concentration in cytosolic compartment. In both control and parkin-mutant fibroblasts, the co-treatment with resveratrol and thapsigargin (TG+RSV) did not further increase the high cytosolic level of Ca2+ elicited by TG alone. This leads to assume a limited inhibition of SERCA by resveratrol as already described [105]. Furthermore, in the TG+RSV-treated control cells, we observed a larger decrease in mitochondrial Ca2+ level as compared to the TG-treated cells. This result is in agreement with the role of mitochondria in providing a local source of Ca2+ for ER refilling in Ca2+-depleted ER [106]. On the contrary, in parkin-mutant fibroblasts, the co-treatment TG+RSV induced an increase of mitochondrial Ca2+ level, as compared with TG-treated cells, showing a level of Ca2+ comparable to that measured in the presence of resveratrol alone. In parkin-mutant cells the TG+RSV co-treatment seems to prevent or, in any case, not to allow the mitochondrial Ca2+ ER refilling in Ca2+-depleted ER cells.
Previous studies clearly established in MAMs a functional and structural communication between mitochondria and ER [107,108,109,110], characterized by the presence of ryanodine receptors (RyRs) and inositol 1,4,5-triphosphate receptors (Ins (1,4,5) P3Rs). ER and the nearby mitochondria create microdomains through the VDACs and the MCU complex where intracellular Ca2+ transfer from ER to mitochondria takes place [62]. Co-incubation of resveratrol with dantrolene (Dan+RSV) induced a larger increase of Ca2+ level in the cytosolic compartment, in both control and parkin-mutant fibroblasts but a marked decrease of the mitochondrial Ca2+ only in control cells, as compared with Dan-treated cells. Under these conditions, in control cells, resveratrol could induce a partial inhibition of SERCA, causing an increase in cytosolic Ca2+ level and, as described for the TG-treatment, this could induce the ER refilling by the mitochondria. In parkin-mutant fibroblasts this process, even in this case, does not appear to occur and upon co-treatment (Dan+RSV) no significant changes of mitochondrial Ca2+ level were observed, as compared with Dan-treated cells.
In parkin-mutant fibroblasts, the increased resveratrol-dependent Ca2+ level could be responsible for the enhanced activity of intramitochondrial Ca2+-sensitive dehydrogenases. This leads to an increased supply of reducing equivalents for the respiratory chain activity and consequent increase of ATP synthesis [5,111]. We assumed that, in parkin-mutant fibroblasts, the basal high level of mitochondrial Ca2+ is related to dysfunctional mitochondria, mainly derived by the failed auto(mito)phagic process. In this context, resveratrol-induced Ca2+ increase could lead to an improvement of the oxidative phosphorylation system and oxidative stress condition in new functional mitochondria derived from a rebalanced mitochondrial biogenesis vs. mitophagy, resulting from the Ca2+-dependent AMPK/SIRT1/PGC1α activation.
The deregulation of Ca2+ homeostasis, in parkin-mutant cells, is object of debate. It is reported that parkin-null cells and fibroblasts expressing mutant parkin showed reduced ER–mitochondria contact sites associated with a decrease in mitochondrial Ca2+ [82]. Conversely, it has been found that the number of ER–mitochondria contact sites is augmented in fibroblasts from PARK2 knockout mice and in human fibroblasts harboring PARK2 mutations [84]. In addition, PINK1 deficiency results in mitochondrial Ca2+ overload associated with a lower threshold of mPTP-opening, making neurons vulnerable to apoptosis [112]. Although it is known that Ca2+ stimulates the mitochondrial respiratory chain, an excessive Ca2+ load is dangerous for mitochondria by opening mPTP, which results in mitochondrial membrane potential dissipation and respiratory chain uncoupling, associated with a decrease of mitochondrial ATP synthesis [113], culminating in cell death. Conversely, a decrease of mitochondrial Ca2+ uptake causes a reduction of mPTP opening making the cells resistant to apoptosis (for review see [41]). However, although in parkin-mutant fibroblasts we observed a further increase of mitochondrial Ca2+ after resveratrol treatment along with an increase of mitochondrial respiration and mitochondrial ATP production [5], we are inclined to rule out any involvement of the mPTP. In addition, it has been shown that resveratrol inhibited the mPTP opening [114,115]. Thus, in parkin-mutant fibroblasts, characterized by a deregulation of the crosstalk between cAMP and Ca2+, together with ER stress [25], resveratrol treatment can normalize the cAMP content and modulate Ca2+ level. This elucidates the mechanism by which resveratrol, by modulating both cAMP and Ca2+ levels, restored OXPHOS efficiency through AMPK/SIRT1/PGC1 α activation [5].
In keeping the importance of preserving a proper Ca2+ transfer between ER and mitochondria, we studied the effect of 24 h resveratrol-treatment on the expression level of GRP75, Mfn2, and Miro2, three proteins involved in MAMs. Noteworthy, we observed a higher expression level of GRP75 in parkin-mutant fibroblasts, as compared with control cells. GRP75 is part of a multiprotein complex gathering IP3R and VDAC1, functionally coupling ER and mitochondria and promoting Ca2+ exchanges [63]. In mouse primary neurons, it has been reported that the GRP75 overexpression induces an increase of ER–mitochondria tethering and of mitochondrial Ca2+ level [116,117]. Therefore, the high GRP75 protein level observed in parkin-mutant fibroblasts could contribute to the higher basal Ca2+ level measured in the mitochondrial compartment.
Moreover, parkin-mutant fibroblasts showed a higher expression of Mfn2 as compared with control cells, which was decreased by resveratrol-treatment. Mfn2 is mainly localized at the MAM-related contact sites [118,119,120,121], though its specific function is still matter of debate [68,122]. It has been shown that, in several cell lines, parkin selectively ubiquitinates mammalian Mfn1 and Mfn2 [123,124] for degradation. This is consistent with the higher basal protein level observed in parkin-mutant fibroblasts. In primary fibroblasts from parkin knockout mice and from parkin-mutant fibroblasts, a recent study showed an augmented ER–mitochondria tethering and ER-to-mitochondria Ca2+ transfer, likely due to increased Mfn2 level in MAMs [84]. Furthermore, it is also reported that Mfn2 suppression is associated with an increased number of ER–mitochondria contact sites and an increased Ca2+ transfer between the two organelles [119]. In the present study, in parkin-mutant fibroblasts, the high expression level of Mfn2, likely due to the lack of its ubiquitination, could be involved in the increased steady-state Ca2+ level.
Rho GTPases Miro1/2, localized in the mitochondrial outer membrane, are components of a complex that anchors mitochondria to motor proteins. Their ubiquitination by parkin leads to mitochondrial arrest that further facilitates the elimination of impaired mitochondria by mitophagy [125,126]. Recent studies revealed the role of Miro, containing two Ca2+-sensing EF hand domains, in the ER–mitochondria contact sites regulation [67,69]. Furthermore, the PINK1–parkin pathway should negatively regulate Miro level, through ubiquitination, resulting in an increased Miro protein level in PINK1 mutant mammalian cells [125]. Consistently, knock-down of Miro by RNAi decreased mitochondrial Ca2+ level in PINK1 mutant dopaminergic neurons [127]. As previously reported for GRP75 and Mfn2, we observed a higher expression level of Miro2 in parkin-mutant fibroblasts, which could be responsible of the high Ca2+ level therein. Furthermore, it is worth mentioning that the ER stress upregulates GRP75 [128] and Mfn2 [129] expressions, leading to increased MAM formation and mitochondrial Ca2+ overload, exactly as observed by our group in parkin-mutant cells.
Parkin plays a central role in the mitochondrial quality-control processes [13] in which a fine balance of mitochondrial autophagy and biogenesis is established [130]. We previously showed that resveratrol treatment caused an enhanced macroautophagic flux through an LC3-independent pathway activation [5]. This effect could be linked to the resveratrol-induced decrease of Miro2 and Mfn2 levels observed in parkin-mutant fibroblasts. Thus, the resveratrol treatment, by modulating specific signaling pathways such as AMPK//SIRT1/PGC1α [131], might lead to an increase of autophagic flux and mitochondrial biogenesis (see [5,131]), driving to the formation of new healthy mitochondria and to the proper cAMP and MAM proteins levels. This pathway could also be linked to Ca2+ homeostasis; indeed, in colon cancer cells it has been shown that resveratrol induces a metabolic reprogramming, increasing oxidative capacities, pyruvate dehydrogenase activity, and ATP production. These effects were abrogated by Ca2+ chelation or the blockade of the mitochondrial Ca2+ uniporter as well as by the inhibition of AMPK pathway [132].
5. Conclusions
The aim of the present study was to investigate the effect of resveratrol on deregulated cAMP and Ca2+ homeostasis in human skin parkin-mutant fibroblasts, a parkin-null cellular model. The OXPHOS efficiency improvement by resveratrol, via the AMPK/SIRT1/PGC1α pathway, which we reported in parkin-mutant fibroblasts in our previous study [5], can be mechanistically linked to three major causes, altered cAMP and Ca2+ levels and modulation of protein expression at the ER–mitochondria contact sites. In this study we showed that resveratrol induces a significant increase of cytosolic and mitochondrial Ca2+ level in parkin-mutant fibroblasts, resulting in a remodulation of the cAMP level. Moreover, resveratrol induces a significant downregulation of the expression level of Miro2 and Mfn2, proteins involved in the ERMCSs, highly expressed in parkin-mutant fibroblasts, likely regulating the Ca2+ traffic between ER and mitochondria. These findings might shed new light in identifying novel molecular targets for PD treatment.
Author Contributions
Conceptualization, T.C.; methodology A.S., A.F., P.T., and D.D.R.; formal analysis, C.P. and T.C.; investigation, A.S., A.F., P.T., M.L.M., A.V., and D.D.R.; data curation, A.S., A.F., C.P., D.D.R., and T.C.; writing—original draft preparation, T.C.; writing—review and editing, A.S., C.P., N.C., D.D.R., and T.C.; funding acquisition, A.S., C.P., D.D.R., and T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by local funds from University of Foggia to C.P. and from University of Bari to T.C., A.S., and D.D.R.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local Ethics Committee at the University of Bari Medical School (Deliberation n. 847, 30 June 2011).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Authors declare that data shared are in accordance with consent provided by participants.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cocco T., Pacelli C., Sgobbo P., Villani G. Control of OXPHOS Efficiency by Complex I in Brain Mitochondria. Neurobiol. Aging. 2009;30:622–629. doi: 10.1016/j.neurobiolaging.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Bose A., Beal M.F. Mitochondrial Dysfunction in Parkinson’s Disease. J. Neurochem. 2016;139((Suppl. S1)):216–231. doi: 10.1111/jnc.13731. [DOI] [PubMed] [Google Scholar]
- 3.Zanellati M.C., Monti V., Barzaghi C., Reale C., Nardocci N., Albanese A., Valente E.M., Ghezzi D., Garavaglia B. Mitochondrial Dysfunction in Parkinson Disease: Evidence in Mutant PARK2 Fibroblasts. Front. Genet. 2015;6:78. doi: 10.3389/fgene.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacelli C., De Rasmo D., Signorile A., Grattagliano I., di Tullio G., D’Orazio A., Nico B., Comi G.P., Ronchi D., Ferranini E., et al. Mitochondrial Defect and PGC-1α Dysfunction in Parkin-Associated Familial Parkinson’s Disease. Biochim. Biophys. Acta. 2011;1812:1041–1053. doi: 10.1016/j.bbadis.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Ferretta A., Gaballo A., Tanzarella P., Piccoli C., Capitanio N., Nico B., Annese T., Di Paola M., Dell’aquila C., De Mari M., et al. Effect of Resveratrol on Mitochondrial Function: Implications in Parkin-Associated Familiar Parkinson’s Disease. Biochim. Biophys. Acta. 2014;1842:902–915. doi: 10.1016/j.bbadis.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Surmeier D.J., Guzman J.N., Sanchez-Padilla J., Schumacker P.T. The Role of Calcium and Mitochondrial Oxidant Stress in the Loss of Substantia Nigra Pars Compacta Dopaminergic Neurons in Parkinson’s Disease. Neuroscience. 2011;198:221–231. doi: 10.1016/j.neuroscience.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calì T., Ottolini D., Brini M. Mitochondria, Calcium, and Endoplasmic Reticulum Stress in Parkinson’s Disease. Biofactors. 2011;37:228–240. doi: 10.1002/biof.159. [DOI] [PubMed] [Google Scholar]
- 8.Tan J.M.M., Wong E.S.P., Lim K.-L. Protein Misfolding and Aggregation in Parkinson’s Disease. Antioxid. Redox Signal. 2009;11:2119–2134. doi: 10.1089/ars.2009.2490. [DOI] [PubMed] [Google Scholar]
- 9.Prasuhn J., Davis R.L., Kumar K.R. Targeting Mitochondrial Impairment in Parkinson’s Disease: Challenges and Opportunities. Front. Cell Dev. Biol. 2020;8:615461. doi: 10.3389/fcell.2020.615461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grünewald A., Kumar K.R., Sue C.M. New Insights into the Complex Role of Mitochondria in Parkinson’s Disease. Prog. Neurobiol. 2019;177:73–93. doi: 10.1016/j.pneurobio.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Pickrell A.M., Youle R.J. The Roles of PINK1, Parkin, and Mitochondrial Fidelity in Parkinson’s Disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McWilliams T.G., Muqit M.M. PINK1 and Parkin: Emerging Themes in Mitochondrial Homeostasis. Curr. Opin. Cell Biol. 2017;45:83–91. doi: 10.1016/j.ceb.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Narendra D.P., Youle R.J. Targeting Mitochondrial Dysfunction: Role for PINK1 and Parkin in Mitochondrial Quality Control. Antioxid. Redox Signal. 2011;14:1929–1938. doi: 10.1089/ars.2010.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashrafi G., Schwarz T.L. The Pathways of Mitophagy for Quality Control and Clearance of Mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickles S., Vigié P., Youle R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018;28:R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palacino J.J., Sagi D., Goldberg M.S., Krauss S., Motz C., Wacker M., Klose J., Shen J. Mitochondrial Dysfunction and Oxidative Damage in Parkin-Deficient Mice. J. Biol. Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 17.Giguère N., Pacelli C., Saumure C., Bourque M.-J., Matheoud D., Levesque D., Slack R.S., Park D.S., Trudeau L.-É. Comparative Analysis of Parkinson’s Disease-Associated Genes in Mice Reveals Altered Survival and Bioenergetics of Parkin-Deficient Dopamine Neurons. J. Biol. Chem. 2018;293:9580–9593. doi: 10.1074/jbc.RA117.000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botella J.A., Bayersdorfer F., Gmeiner F., Schneuwly S. Modelling Parkinson’s Disease in Drosophila. Neuromol. Med. 2009;11:268–280. doi: 10.1007/s12017-009-8098-6. [DOI] [PubMed] [Google Scholar]
- 19.Greene J.C., Whitworth A.J., Kuo I., Andrews L.A., Feany M.B., Pallanck L.J. Mitochondrial Pathology and Apoptotic Muscle Degeneration in Drosophila Parkin Mutants. Proc. Natl. Acad. Sci. USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müftüoglu M., Elibol B., Dalmizrak O., Ercan A., Kulaksiz G., Ogüs H., Dalkara T., Ozer N. Mitochondrial Complex I and IV Activities in Leukocytes from Patients with Parkin Mutations. Mov. Disord. 2004;19:544–548. doi: 10.1002/mds.10695. [DOI] [PubMed] [Google Scholar]
- 21.Mortiboys H., Thomas K.J., Koopman W.J.H., Klaffke S., Abou-Sleiman P., Olpin S., Wood N.W., Willems P.H.G.M., Smeitink J.A.M., Cookson M.R., et al. Mitochondrial Function and Morphology Are Impaired in Parkin-Mutant Fibroblasts. Ann. Neurol. 2008;64:555–565. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auburger G., Klinkenberg M., Drost J., Marcus K., Morales-Gordo B., Kunz W.S., Brandt U., Broccoli V., Reichmann H., Gispert S., et al. Primary Skin Fibroblasts as a Model of Parkinson’s Disease. Mol. Neurobiol. 2012;46:20–27. doi: 10.1007/s12035-012-8245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González-Casacuberta I., Morén C., Juárez-Flores D.-L., Esteve-Codina A., Sierra C., Catalán-García M., Guitart-Mampel M., Tobías E., Milisenda J.C., Pont-Sunyer C., et al. Transcriptional Alterations in Skin Fibroblasts from Parkinson’s Disease Patients with Parkin Mutations. Neurobiol. Aging. 2018;65:206–216. doi: 10.1016/j.neurobiolaging.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Vergara D., Ferraro M.M., Cascione M., del Mercato L.L., Leporatti S., Ferretta A., Tanzarella P., Pacelli C., Santino A., Maffia M., et al. Cytoskeletal Alterations and Biomechanical Properties of Parkin-Mutant Human Primary Fibroblasts. Cell Biochem. Biophys. 2015;71:1395–1404. doi: 10.1007/s12013-014-0362-1. [DOI] [PubMed] [Google Scholar]
- 25.Vergara D., Gaballo A., Signorile A., Ferretta A., Tanzarella P., Pacelli C., Di Paola M., Cocco T., Maffia M. Resveratrol Modulation of Protein Expression in Parkin-Mutant Human Skin Fibroblasts: A Proteomic Approach. Oxid. Med. Cell. Longev. 2017;2017:2198243. doi: 10.1155/2017/2198243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippolis R., Siciliano R.A., Pacelli C., Ferretta A., Mazzeo M.F., Scacco S., Papa F., Gaballo A., Dell’Aquila C., De Mari M., et al. Altered Protein Expression Pattern in Skin Fibroblasts from Parkin-Mutant Early-Onset Parkinson’s Disease Patients. Biochim. Biophys. Acta. 2015;1852:1960–1970. doi: 10.1016/j.bbadis.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Lobasso S., Tanzarella P., Vergara D., Maffia M., Cocco T., Corcelli A. Lipid Profiling of Parkin-Mutant Human Skin Fibroblasts. J. Cell Physiol. 2017;232:3540–3551. doi: 10.1002/jcp.25815. [DOI] [PubMed] [Google Scholar]
- 28.Guerra F., Girolimetti G., Beli R., Mitruccio M., Pacelli C., Ferretta A., Gasparre G., Cocco T., Bucci C. Synergistic Effect of Mitochondrial and Lysosomal Dysfunction in Parkinson’s Disease. Cells. 2019;8:452. doi: 10.3390/cells8050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacelli C., Rotundo G., Lecce L., Menga M., Bidollari E., Scrima R., Cela O., Piccoli C., Cocco T., Vescovi A.L., et al. Parkin Mutation Affects Clock Gene-Dependent Energy Metabolism. Int. J. Mol. Sci. 2019;20:2772. doi: 10.3390/ijms20112772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanzarella P., Ferretta A., Barile S.N., Ancona M., De Rasmo D., Signorile A., Papa S., Capitanio N., Pacelli C., Cocco T. Increased Levels of CAMP by the Calcium-Dependent Activation of Soluble Adenylyl Cyclase in Parkin-Mutant Fibroblasts. Cells. 2019;8:250. doi: 10.3390/cells8030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhat A., Ray B., Mahalakshmi A.M., Tuladhar S., Nandakumar D.N., Srinivasan M., Essa M.M., Chidambaram S.B., Guillemin G.J., Sakharkar M.K. Phosphodiesterase-4 Enzyme as a Therapeutic Target in Neurological Disorders. Pharm. Res. 2020;160:105078. doi: 10.1016/j.phrs.2020.105078. [DOI] [PubMed] [Google Scholar]
- 32.Papa S., Sardanelli A.M., Scacco S., Petruzzella V., Technikova-Dobrova Z., Vergari R., Signorile A. The NADH: Ubiquinone Oxidoreductase (Complex I) of the Mammalian Respiratory Chain and the CAMP Cascade. J. Bioenerg. Biomembr. 2002;34:1–10. doi: 10.1023/A:1013863018115. [DOI] [PubMed] [Google Scholar]
- 33.Piccoli C., Scacco S., Bellomo F., Signorile A., Iuso A., Boffoli D., Scrima R., Capitanio N., Papa S. CAMP Controls Oxygen Metabolism in Mammalian Cells. FEBS Lett. 2006;580:4539–4543. doi: 10.1016/j.febslet.2006.06.085. [DOI] [PubMed] [Google Scholar]
- 34.Papa S., Scacco S., De Rasmo D., Signorile A., Papa F., Panelli D., Nicastro A., Scaringi R., Santeramo A., Roca E., et al. CAMP-Dependent Protein Kinase Regulates Post-Translational Processing and Expression of Complex I Subunits in Mammalian Cells. Biochim. Biophys. Acta. 2010;1797:649–658. doi: 10.1016/j.bbabio.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Valsecchi F., Ramos-Espiritu L.S., Buck J., Levin L.R., Manfredi G. CAMP and Mitochondria. Physiology. 2013;28:199–209. doi: 10.1152/physiol.00004.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang F., Zhang L., Qi Y., Xu H. Mitochondrial CAMP Signaling. Cell. Mol. Life Sci. 2016;73:4577–4590. doi: 10.1007/s00018-016-2282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Rasmo D., Micelli L., Santeramo A., Signorile A., Lattanzio P., Papa S. CAMP Regulates the Functional Activity, Coupling Efficiency and Structural Organization of Mammalian FOF1 ATP Synthase. Biochim. Biophys. Acta. 2016;1857:350–358. doi: 10.1016/j.bbabio.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Signorile A., Santeramo A., Tamma G., Pellegrino T., D’Oria S., Lattanzio P., De Rasmo D. Mitochondrial CAMP Prevents Apoptosis Modulating Sirt3 Protein Level and OPA1 Processing in Cardiac Myoblast Cells. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:355–366. doi: 10.1016/j.bbamcr.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 39.Valsecchi F., Konrad C., D’Aurelio M., Ramos-Espiritu L.S., Stepanova A., Burstein S.R., Galkin A., Magranè J., Starkov A., Buck J., et al. Distinct Intracellular SAC-CAMP Domains Regulate ER Ca2+ Signaling and OXPHOS Function. J. Cell Sci. 2017;130:3713–3727. doi: 10.1242/jcs.206318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Benedetto G., Gerbino A., Lefkimmiatis K. Shaping Mitochondrial Dynamics: The Role of CAMP Signalling. Biochem. Biophys. Res. Commun. 2018;500:65–74. doi: 10.1016/j.bbrc.2017.05.041. [DOI] [PubMed] [Google Scholar]
- 41.Modesti L., Danese A., Angela Maria Vitto V., Ramaccini D., Aguiari G., Gafà R., Lanza G., Giorgi C., Pinton P. Mitochondrial Ca2+ Signaling in Health, Disease and Therapy. Cells. 2021;10:1317. doi: 10.3390/cells10061317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Del Rio D., Rodriguez-Mateos A., Spencer J.P.E., Tognolini M., Borges G., Crozier A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun A.Y., Wang Q., Simonyi A., Sun G.Y. Resveratrol as a Therapeutic Agent for Neurodegenerative Diseases. Mol. Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magalingam K.B., Radhakrishnan A.K., Haleagrahara N. Protective Mechanisms of Flavonoids in Parkinson’s Disease. Oxid. Med. Cell. Longev. 2015;2015:314560. doi: 10.1155/2015/314560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park S.-J., Ahmad F., Philp A., Baar K., Williams T., Luo H., Ke H., Rehmann H., Taussig R., Brown A.L., et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting CAMP Phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sareen D., Darjatmoko S.R., Albert D.M., Polans A.S. Mitochondria, Calcium, and Calpain Are Key Mediators of Resveratrol-Induced Apoptosis in Breast Cancer. Mol. Pharm. 2007;72:1466–1475. doi: 10.1124/mol.107.039040. [DOI] [PubMed] [Google Scholar]
- 47.Campos-Toimil M., Elíes J., Orallo F. Trans- and Cis-Resveratrol Increase Cytoplasmic Calcium Levels in A7r5 Vascular Smooth Muscle Cells. Mol. Nutr. Food Res. 2005;49:396–404. doi: 10.1002/mnfr.200400108. [DOI] [PubMed] [Google Scholar]
- 48.Elíes J., Cuíñas A., García-Morales V., Orallo F., Campos-Toimil M. Trans-Resveratrol Simultaneously Increases Cytoplasmic Ca2+ Levels and Nitric Oxide Release in Human Endothelial Cells. Mol. Nutr. Food Res. 2011;55:1237–1248. doi: 10.1002/mnfr.201100240. [DOI] [PubMed] [Google Scholar]
- 49.Peterson J.A., Oblad R.V., Mecham J.C., Kenealey J.D. Resveratrol Inhibits Plasma Membrane Ca2+-ATPase Inducing an Increase in Cytoplasmic Calcium. Biochem. Biophys. Rep. 2016;7:253–258. doi: 10.1016/j.bbrep.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dekkers D.H.W., Bezstarosti K., Gurusamy N., Luijk K., Verhoeven A.J.M., Rijkers E.-J., Demmers J.A., Lamers J.M.J., Maulik N., Das D.K. Identification by a Differential Proteomic Approach of the Induced Stress and Redox Proteins by Resveratrol in the Normal and Diabetic Rat Heart. J. Cell. Mol. Med. 2008;12:1677–1689. doi: 10.1111/j.1582-4934.2008.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song C., Luo B., Gong L. Resveratrol Reduces the Apoptosis Induced by Cigarette Smoke Extract by Upregulating MFN2. PLoS ONE. 2017;12:e0175009. doi: 10.1371/journal.pone.0175009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolgin E. How Secret Conversations inside Cells Are Transforming Biology. Nature. 2019;567:162–164. doi: 10.1038/d41586-019-00792-9. [DOI] [PubMed] [Google Scholar]
- 53.Vance J.E. MAM (Mitochondria-Associated Membranes) in Mammalian Cells: Lipids and Beyond. Biochim. Biophys. Acta. 2014;1841:595–609. doi: 10.1016/j.bbalip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 54.Marchi S., Patergnani S., Pinton P. The Endoplasmic Reticulum-Mitochondria Connection: One Touch, Multiple Functions. Biochim. Biophys. Acta. 2014;1837:461–469. doi: 10.1016/j.bbabio.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 55.Krols M., Bultynck G., Janssens S. ER-Mitochondria Contact Sites: A New Regulator of Cellular Calcium Flux Comes into Play. J. Cell Biol. 2016;214:367–370. doi: 10.1083/jcb.201607124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Csordás G., Renken C., Várnai P., Walter L., Weaver D., Buttle K.F., Balla T., Mannella C.A., Hajnóczky G. Structural and Functional Features and Significance of the Physical Linkage between ER and Mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Csordás G., Várnai P., Golenár T., Roy S., Purkins G., Schneider T.G., Balla T., Hajnóczky G. Imaging Interorganelle Contacts and Local Calcium Dynamics at the ER-Mitochondrial Interface. Mol. Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Filadi R., Theurey P., Pizzo P. The Endoplasmic Reticulum-Mitochondria Coupling in Health and Disease: Molecules, Functions and Significance. Cell Calcium. 2017;62:1–15. doi: 10.1016/j.ceca.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Guardia-Laguarta C., Liu Y., Lauritzen K.H., Erdjument-Bromage H., Martin B., Swayne T.C., Jiang X., Przedborski S. PINK1 Content in Mitochondria Is Regulated by ER-Associated Degradation. J. NeuroSci. 2019;39:7074–7085. doi: 10.1523/JNEUROSCI.1691-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gelmetti V., De Rosa P., Torosantucci L., Marini E.S., Romagnoli A., Di Rienzo M., Arena G., Vignone D., Fimia G.M., Valente E.M. PINK1 and BECN1 Relocalize at Mitochondria-Associated Membranes during Mitophagy and Promote ER-Mitochondria Tethering and Autophagosome Formation. Autophagy. 2017;13:654–669. doi: 10.1080/15548627.2016.1277309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vance J.E. Phospholipid Synthesis in a Membrane Fraction Associated with Mitochondria. J. Biol. Chem. 1990;265:7248–7256. doi: 10.1016/S0021-9258(19)39106-9. [DOI] [PubMed] [Google Scholar]
- 62.Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. Close Contacts with the Endoplasmic Reticulum as Determinants of Mitochondrial Ca2+ Responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 63.Szabadkai G., Bianchi K., Várnai P., De Stefani D., Wieckowski M.R., Cavagna D., Nagy A.I., Balla T., Rizzuto R. Chaperone-Mediated Coupling of Endoplasmic Reticulum and Mitochondrial Ca2+ Channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erpapazoglou Z., Mouton-Liger F., Corti O. From Dysfunctional Endoplasmic Reticulum-Mitochondria Coupling to Neurodegeneration. Neurochem. Int. 2017;109:171–183. doi: 10.1016/j.neuint.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 65.Kornmann B., Currie E., Collins S.R., Schuldiner M., Nunnari J., Weissman J.S., Walter P. An ER-Mitochondria Tethering Complex Revealed by a Synthetic Biology Screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Stefani D., Bononi A., Romagnoli A., Messina A., De Pinto V., Pinton P., Rizzuto R. VDAC1 Selectively Transfers Apoptotic Ca2+ Signals to Mitochondria. Cell Death Differ. 2012;19:267–273. doi: 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Modi S., López-Doménech G., Halff E.F., Covill-Cooke C., Ivankovic D., Melandri D., Arancibia-Cárcamo I.L., Burden J.J., Lowe A.R., Kittler J.T. Miro Clusters Regulate ER-Mitochondria Contact Sites and Link Cristae Organization to the Mitochondrial Transport Machinery. Nat. Commun. 2019;10:4399. doi: 10.1038/s41467-019-12382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Brito O.M., Scorrano L. Mitofusin 2 Tethers Endoplasmic Reticulum to Mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 69.Lee S., Lee K.-S., Huh S., Liu S., Lee D.-Y., Hong S.H., Yu K., Lu B. Polo Kinase Phosphorylates Miro to Control ER-Mitochondria Contact Sites and Mitochondrial Ca2+ Homeostasis in Neural Stem Cell Development. Dev. Cell. 2016;37:174–189. doi: 10.1016/j.devcel.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hajnóczky G., Robb-Gaspers L.D., Seitz M.B., Thomas A.P. Decoding of Cytosolic Calcium Oscillations in the Mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 71.Mirabet M., Mallol J., Lluis C., Franco R. Calcium Mobilization in Jurkat Cells via A2b Adenosine Receptors. Br. J. Pharm. 1997;122:1075–1082. doi: 10.1038/sj.bjp.0701495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grynkiewicz G., Poenie M., Tsien R.Y. A New Generation of Ca2+ Indicators with Greatly Improved Fluorescence Properties. J. Biol. Chem. 1985;260:3440–3450. doi: 10.1016/S0021-9258(19)83641-4. [DOI] [PubMed] [Google Scholar]
- 73.Fabbri E., Brighenti L., Ottolenghi C. Inhibition of Adenylate Cyclase of Catfish and Rat Hepatocyte Membranes by 9-(Tetrahydro-2-Furyl)Adenine (SQ 22536) J. Enzyme Inhib. 1991;5:87–98. doi: 10.3109/14756369109069062. [DOI] [PubMed] [Google Scholar]
- 74.Sánchez-Melgar A., Albasanz J.L., Guixà-González R., Saleh N., Selent J., Martín M. The Antioxidant Resveratrol Acts as a Non-Selective Adenosine Receptor Agonist. Free Radic. Biol. Med. 2019;135:261–273. doi: 10.1016/j.freeradbiomed.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 75.Halls M.L., Cooper D.M.F. Regulation by Ca2+-Signaling Pathways of Adenylyl Cyclases. Cold Spring Harb. Perspect. Biol. 2011;3:a004143. doi: 10.1101/cshperspect.a004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J.-Q., Wu P.-F., Long L.-H., Chen Y., Hu Z.-L., Ni L., Wang F., Chen J.-G. Resveratrol Promotes Cellular Glucose Utilization in Primary Cultured Cortical Neurons via Calcium-Dependent Signaling Pathway. J. Nutr. Biochem. 2013;24:629–637. doi: 10.1016/j.jnutbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 77.Rizzuto R., Simpson A.W., Brini M., Pozzan T. Rapid Changes of Mitochondrial Ca2+ Revealed by Specifically Targeted Recombinant Aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 78.Giorgi C., Marchi S., Pinton P. The Machineries, Regulation and Cellular Functions of Mitochondrial Calcium. Nat. Rev. Mol. Cell Biol. 2018;19:713–730. doi: 10.1038/s41580-018-0052-8. [DOI] [PubMed] [Google Scholar]
- 79.Kirichok Y., Krapivinsky G., Clapham D.E. The Mitochondrial Calcium Uniporter Is a Highly Selective Ion Channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 80.Liu J.C., Liu J., Holmström K.M., Menazza S., Parks R.J., Fergusson M.M., Yu Z.-X., Springer D.A., Halsey C., Liu C., et al. MICU1 Serves as a Molecular Gatekeeper to Prevent In Vivo Mitochondrial Calcium Overload. Cell Rep. 2016;16:1561–1573. doi: 10.1016/j.celrep.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calì T., Ottolini D., Negro A., Brini M. Enhanced Parkin Levels Favor ER-Mitochondria Crosstalk and Guarantee Ca2+ Transfer to Sustain Cell Bioenergetics. Biochim. Biophys. Acta. 2013;1832:495–508. doi: 10.1016/j.bbadis.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 82.Basso V., Marchesan E., Peggion C., Chakraborty J., von Stockum S., Giacomello M., Ottolini D., Debattisti V., Caicci F., Tasca E., et al. Regulation of ER-Mitochondria Contacts by Parkin via Mfn2. Pharm. Res. 2018;138:43–56. doi: 10.1016/j.phrs.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 83.Zheng L., Bernard-Marissal N., Moullan N., D’Amico D., Auwerx J., Moore D.J., Knott G., Aebischer P., Schneider B.L. Parkin Functionally Interacts with PGC-1α to Preserve Mitochondria and Protect Dopaminergic Neurons. Hum. Mol. Genet. 2017;26:582–598. doi: 10.1093/hmg/ddw418. [DOI] [PubMed] [Google Scholar]
- 84.Gautier C.A., Erpapazoglou Z., Mouton-Liger F., Muriel M.P., Cormier F., Bigou S., Duffaure S., Girard M., Foret B., Iannielli A., et al. The Endoplasmic Reticulum-Mitochondria Interface Is Perturbed in PARK2 Knockout Mice and Patients with PARK2 Mutations. Hum. Mol. Genet. 2016;25:2972–2984. doi: 10.1093/hmg/ddw148. [DOI] [PubMed] [Google Scholar]
- 85.Treiman M., Caspersen C., Christensen S.B. A Tool Coming of Age: Thapsigargin as an Inhibitor of Sarco-Endoplasmic Reticulum Ca2+-ATPases. Trends Pharmacol. Sci. 1998;19:131–135. doi: 10.1016/S0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- 86.Fruen B.R., Mickelson J.R., Louis C.F. Dantrolene Inhibition of Sarcoplasmic Reticulum Ca2+ Release by Direct and Specific Action at Skeletal Muscle Ryanodine Receptors. J. Biol. Chem. 1997;272:26965–26971. doi: 10.1074/jbc.272.43.26965. [DOI] [PubMed] [Google Scholar]
- 87.Friedman J.R., Lackner L.L., West M., DiBenedetto J.R., Nunnari J., Voeltz G.K. ER Tubules Mark Sites of Mitochondrial Division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Csordás G., Hajnóczky G. Sorting of Calcium Signals at the Junctions of Endoplasmic Reticulum and Mitochondria. Cell Calcium. 2001;29:249–262. doi: 10.1054/ceca.2000.0191. [DOI] [PubMed] [Google Scholar]
- 89.Wu H., Carvalho P., Voeltz G.K. Here, there, and everywhere: The importance of ER membrane contact sites. Science. 2018;361:eaan5835. doi: 10.1126/science.aan5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tellone E., Galtieri A., Russo A., Giardina B., Ficarra S. Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2015;2015:392169. doi: 10.1155/2015/392169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xia N., Daiber A., Förstermann U., Li H. Antioxidant Effects of Resveratrol in the Cardiovascular System. Br. J. Pharm. 2017;174:1633–1646. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Oliveira M.R., Nabavi S.F., Manayi A., Daglia M., Hajheydari Z., Nabavi S.M. Resveratrol and the Mitochondria: From Triggering the Intrinsic Apoptotic Pathway to Inducing Mitochondrial Biogenesis, a Mechanistic View. Biochim. Biophys. Acta. 2016;1860:727–745. doi: 10.1016/j.bbagen.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 93.Dasgupta B., Milbrandt J. Resveratrol Stimulates AMP Kinase Activity in Neurons. Proc. Natl. Acad. Sci. USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chiang M.-C., Nicol C.J., Cheng Y.-C. Resveratrol Activation of AMPK-Dependent Pathways Is Neuroprotective in Human Neural Stem Cells against Amyloid-Beta-Induced Inflammation and Oxidative Stress. Neurochem. Int. 2018;115:1–10. doi: 10.1016/j.neuint.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 95.Price N.L., Gomes A.P., Ling A.J.Y., Duarte F.V., Martin-Montalvo A., North B.J., Agarwal B., Ye L., Ramadori G., Teodoro J.S., et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vingtdeux V., Giliberto L., Zhao H., Chandakkar P., Wu Q., Simon J.E., Janle E.M., Lobo J., Ferruzzi M.G., Davies P., et al. AMP-Activated Protein Kinase Signaling Activation by Resveratrol Modulates Amyloid-Beta Peptide Metabolism. J. Biol. Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 98.Hawley S.A., Pan D.A., Mustard K.J., Ross L., Bain J., Edelman A.M., Frenguelli B.G., Hardie D.G. Calmodulin-Dependent Protein Kinase Kinase-Beta Is an Alternative Upstream Kinase for AMP-Activated Protein Kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 99.Hurley R.L., Anderson K.A., Franzone J.M., Kemp B.E., Means A.R., Witters L.A. The Ca2+/Calmodulin-Dependent Protein Kinase Kinases Are AMP-Activated Protein Kinase Kinases. J. Biol. Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 100.Nakamura K., Zuppini A., Arnaudeau S., Lynch J., Ahsan I., Krause R., Papp S., De Smedt H., Parys J.B., Muller-Esterl W., et al. Functional Specialization of Calreticulin Domains. J. Cell Biol. 2001;154:961–972. doi: 10.1083/jcb.200102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gonzalez L.L., Garrie K., Turner M.D. Role of S100 Proteins in Health and Disease. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867:118677. doi: 10.1016/j.bbamcr.2020.118677. [DOI] [PubMed] [Google Scholar]
- 102.Sandebring A., Dehvari N., Perez-Manso M., Thomas K.J., Karpilovski E., Cookson M.R., Cowburn R.F., Cedazo-Mínguez A. Parkin Deficiency Disrupts Calcium Homeostasis by Modulating Phospholipase C Signalling. FEBS J. 2009;276:5041–5052. doi: 10.1111/j.1742-4658.2009.07201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pozzan T., Rizzuto R., Volpe P., Meldolesi J. Molecular and Cellular Physiology of Intracellular Calcium Stores. Physiol. Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 104.Matteucci A., Patron M., Vecellio Reane D., Gastaldello S., Amoroso S., Rizzuto R., Brini M., Raffaello A., Calì T. Parkin-Dependent Regulation of the MCU Complex Component MICU1. Sci. Rep. 2018;8:14199. doi: 10.1038/s41598-018-32551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.García-Casas P., Arias-Del-Val J., Alvarez-Illera P., Fonteriz R.I., Montero M., Alvarez J. Inhibition of Sarco-Endoplasmic Reticulum Ca2+ ATPase Extends the Lifespan in C. Elegans Worms. Front. Pharm. 2018;9:669. doi: 10.3389/fphar.2018.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arnaudeau S., Kelley W.L., Walsh J.V., Demaurex N. Mitochondria Recycle Ca2+ to the Endoplasmic Reticulum and Prevent the Depletion of Neighboring Endoplasmic Reticulum Regions. J. Biol. Chem. 2001;276:29430–29439. doi: 10.1074/jbc.M103274200. [DOI] [PubMed] [Google Scholar]
- 107.Kopach O., Kruglikov I., Pivneva T., Voitenko N., Fedirko N. Functional Coupling between Ryanodine Receptors, Mitochondria and Ca2+ ATPases in Rat Submandibular Acinar Cells. Cell Calcium. 2008;43:469–481. doi: 10.1016/j.ceca.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 108.Rizzuto R., Pozzan T. Microdomains of Intracellular Ca2+: Molecular Determinants and Functional Consequences. Physiol. Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 109.Bravo-Sagua R., Rodriguez A.E., Kuzmicic J., Gutierrez T., Lopez-Crisosto C., Quiroga C., Díaz-Elizondo J., Chiong M., Gillette T.G., Rothermel B.A., et al. Cell Death and Survival through the Endoplasmic Reticulum-Mitochondrial Axis. Curr. Mol. Med. 2013;13:317–329. doi: 10.2174/156652413804810781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jakob R., Beutner G., Sharma V.K., Duan Y., Gross R.A., Hurst S., Jhun B.S., O-Uchi J., Sheu S.-S. Molecular and Functional Identification of a Mitochondrial Ryanodine Receptor in Neurons. NeuroSci. Lett. 2014;575:7–12. doi: 10.1016/j.neulet.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Green D.R., Wang R. Calcium and Energy: Making the Cake and Eating It Too? Cell. 2010;142:200–202. doi: 10.1016/j.cell.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 112.Gandhi S., Wood-Kaczmar A., Yao Z., Plun-Favreau H., Deas E., Klupsch K., Downward J., Latchman D.S., Tabrizi S.J., Wood N.W., et al. PINK1-Associated Parkinson’s Disease Is Caused by Neuronal Vulnerability to Calcium-Induced Cell Death. Mol. Cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kwong J.Q., Molkentin J.D. Physiological and Pathological Roles of the Mitochondrial Permeability Transition Pore in the Heart. Cell Metab. 2015;21:206–214. doi: 10.1016/j.cmet.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guo Y.-J., Dong S.-Y., Cui X.-X., Feng Y., Liu T., Yin M., Kuo S.-H., Tan E.-K., Zhao W.-J., Wu Y.-C. Resveratrol Alleviates MPTP-Induced Motor Impairments and Pathological Changes by Autophagic Degradation of α-Synuclein via SIRT1-Deacetylated LC3. Mol. Nutr. Food Res. 2016;60:2161–2175. doi: 10.1002/mnfr.201600111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liao Z., Liu D., Tang L., Yin D., Yin S., Lai S., Yao J., He M. Long-Term Oral Resveratrol Intake Provides Nutritional Preconditioning against Myocardial Ischemia/Reperfusion Injury: Involvement of VDAC1 Downregulation. Mol. Nutr. Food Res. 2015;59:454–464. doi: 10.1002/mnfr.201400730. [DOI] [PubMed] [Google Scholar]
- 116.Honrath B., Metz I., Bendridi N., Rieusset J., Culmsee C., Dolga A.M. Glucose-Regulated Protein 75 Determines ER-Mitochondrial Coupling and Sensitivity to Oxidative Stress in Neuronal Cells. Cell Death Discov. 2017;3:17076. doi: 10.1038/cddiscovery.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee S., Wang W., Hwang J., Namgung U., Min K.-T. Increased ER-Mitochondria Tethering Promotes Axon Regeneration. Proc. Natl. Acad. Sci. USA. 2019;116:16074–16079. doi: 10.1073/pnas.1818830116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cosson P., Marchetti A., Ravazzola M., Orci L. Mitofusin-2 Independent Juxtaposition of Endoplasmic Reticulum and Mitochondria: An Ultrastructural Study. PLoS ONE. 2012;7:e46293. doi: 10.1371/journal.pone.0046293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Filadi R., Greotti E., Turacchio G., Luini A., Pozzan T., Pizzo P. Mitofusin 2 Ablation Increases Endoplasmic Reticulum–Mitochondria Coupling. Proc. Natl. Acad. Sci. USA. 2015;112:E2174–E2181. doi: 10.1073/pnas.1504880112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Detmer S.A., Chan D.C. Functions and Dysfunctions of Mitochondrial Dynamics. Nat. Rev. Mol. Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 121.Filadi R., Greotti E., Turacchio G., Luini A., Pozzan T., Pizzo P. On the Role of Mitofusin 2 in Endoplasmic Reticulum-Mitochondria Tethering. Proc. Natl. Acad. Sci. USA. 2017;114:E2266–E2267. doi: 10.1073/pnas.1616040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Naon D., Zaninello M., Giacomello M., Varanita T., Grespi F., Lakshminaranayan S., Serafini A., Semenzato M., Herkenne S., Hernández-Alvarez M.I., et al. Critical Reappraisal Confirms That Mitofusin 2 Is an Endoplasmic Reticulum-Mitochondria Tether. Proc. Natl. Acad. Sci. USA. 2016;113:11249–11254. doi: 10.1073/pnas.1606786113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tanaka A., Cleland M.M., Xu S., Narendra D.P., Suen D.-F., Karbowski M., Youle R.J. Proteasome and P97 Mediate Mitophagy and Degradation of Mitofusins Induced by Parkin. J. Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gegg M.E., Cooper J.M., Chau K.-Y., Rojo M., Schapira A.H.V., Taanman J.-W. Mitofusin 1 and Mitofusin 2 Are Ubiquitinated in a PINK1/Parkin-Dependent Manner upon Induction of Mitophagy. Hum. Mol. Genet. 2010;19:4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang X., Winter D., Ashrafi G., Schlehe J., Wong Y.L., Selkoe D., Rice S., Steen J., LaVoie M.J., Schwarz T.L. PINK1 and Parkin Target Miro for Phosphorylation and Degradation to Arrest Mitochondrial Motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu S., Sawada T., Lee S., Yu W., Silverio G., Alapatt P., Millan I., Shen A., Saxton W., Kanao T., et al. Parkinson’s Disease-Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria. PLoS Genet. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee K.-S., Huh S., Lee S., Wu Z., Kim A.-K., Kang H.-Y., Lu B. Altered ER-Mitochondria Contact Impacts Mitochondria Calcium Homeostasis and Contributes to Neurodegeneration in Vivo in Disease Models. Proc. Natl. Acad. Sci. USA. 2018;115:E8844–E8853. doi: 10.1073/pnas.1721136115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liang T., Hang W., Chen J., Wu Y., Wen B., Xu K., Ding B., Chen J. ApoE4 (Δ272–299) Induces Mitochondrial-Associated Membrane Formation and Mitochondrial Impairment by Enhancing GRP75-Modulated Mitochondrial Calcium Overload in Neuron. Cell Biosci. 2021;11:50. doi: 10.1186/s13578-021-00563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yuan M., Gong M., Zhang Z., Meng L., Tse G., Zhao Y., Bao Q., Zhang Y., Yuan M., Liu X., et al. Hyperglycemia Induces Endoplasmic Reticulum Stress in Atrial Cardiomyocytes, and Mitofusin-2 Downregulation Prevents Mitochondrial Dysfunction and Subsequent Cell Death. Oxid. Med. Cell. Longev. 2020;2020:6569728. doi: 10.1155/2020/6569728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gottlieb R.A., Carreira R.S. Autophagy in Health and Disease. 5. Mitophagy as a Way of Life. Am. J. Physiol. Cell Physiol. 2010;299:C203–C210. doi: 10.1152/ajpcell.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen K.-G., Kang R.-R., Sun Q., Liu C., Ma Z., Liu K., Deng Y., Liu W., Xu B. Resveratrol Ameliorates Disorders of Mitochondrial Biogenesis and Mitophagy in Rats Continuously Exposed to Benzo(a)Pyrene from Embryonic Development through Adolescence. Toxicology. 2020;442:152532. doi: 10.1016/j.tox.2020.152532. [DOI] [PubMed] [Google Scholar]
- 132.Saunier E., Antonio S., Regazzetti A., Auzeil N., Laprévote O., Shay J.W., Coumoul X., Barouki R., Benelli C., Huc L., et al. Resveratrol Reverses the Warburg Effect by Targeting the Pyruvate Dehydrogenase Complex in Colon Cancer Cells. Sci. Rep. 2017;7:6945. doi: 10.1038/s41598-017-07006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Authors declare that data shared are in accordance with consent provided by participants.