Abstract
Simple Summary
The Ras-Raf-MEK-ERK signaling pathway is responsible for regulating cell proliferation, differentiation, and survival. Overexpression and overactivation of members within the signaling cascade have been observed in many solid and blood cancers. Research often focuses on targeting the pathway to disrupt cancer initiation and progression. We aimed to provide an overview of the pathway’s physiologic role and regulation, interactions with other pathways involved in cancer development, and mutations that lead to malignancy. Several blood and solid cancers are analyzed to illustrate the impact of the pathway’s dysregulation, stemming from mutation or viral induction. Finally, we summarized different approaches to targeting the pathway and the associated novel treatments being researched or having recently achieved approval.
Abstract
The mitogen-activated protein kinase (MAPK) pathway, consisting of the Ras-Raf-MEK-ERK signaling cascade, regulates genes that control cellular development, differentiation, proliferation, and apoptosis. Within the cascade, multiple isoforms of Ras and Raf each display differences in functionality, efficiency, and, critically, oncogenic potential. According to the NCI, over 30% of all human cancers are driven by Ras genes. This dysfunctional signaling is implicated in a wide variety of leukemias and solid tumors, both with and without viral etiology. Due to the strong evidence of Ras-Raf involvement in tumorigenesis, many have attempted to target the cascade to treat these malignancies. Decades of unsuccessful experimentation had deemed Ras undruggable, but recently, the approval of Sotorasib as the first ever KRas inhibitor represents a monumental breakthrough. This advancement is not without novel challenges. As a G12C mutant-specific drug, it also represents the issue of drug target specificity within Ras pathway; not only do many drugs only affect single mutational profiles, with few pan-inhibitor exceptions, tumor genetic heterogeneity may give rise to drug-resistant profiles. Furthermore, significant challenges in targeting downstream Raf, especially the BRaf isoform, lie in the paradoxical activation of wild-type BRaf by BRaf mutant inhibitors. This literature review will delineate the mechanisms of Ras signaling in the MAPK pathway and its possible oncogenic mutations, illustrate how specific mutations affect the pathogenesis of specific cancers, and compare available and in-development treatments targeting the Ras pathway.
Keywords: Ras signaling, leukemia, ATLL, MEK, ERK, viral oncogenesis, solid tumors
1. Introduction
The MAPK cascade is a vital cellular network, which regulates apoptosis, development, differentiation, and proliferation (Reviewed in [1,2]). The network causes cellular changes through modulating gene expression. It achieves these alterations by integrating signaling induced receptor-ligand interactions, phosphorylation cascades, and modulation of transcription factor activities. Much progress has been made in understanding the role of MAPK signaling in cancer, which has resulted in the development of targeted therapies aimed at curbing aberrant MAPK signaling [3]. There are three main cascades in the mammalian MAPK family: classical MAPK (ERK), C-Jun N-terminal kinase (JNK), and p38 kinase [4]. The classical MAPK family involves the Ras-Raf-MEK-ERK cascade of proteins [5]. Many cancers have mutations in the classical MAPK cascade that contribute to unregulated cellular division. Studying the pathway’s normal physiology has allowed therapies to be developed that are able to treat malignancies by differential targeting of proteins implicated in the MAPK/ERK cascade (Figure 1). Notably, Ras, the master regulator of the MAPK pathway, is inherently associated with the development of cancer. This has recently (2020) been described in detail [6]. Once thought to be untargetable, recent breakthroughs in allele-specific covalent inhibitors have opened new doors for cancer therapy [7]. Downstream from Ras, all Raf isoforms enhance the catalytic activity of Mitogen activated protein kinase/ERK kinase (MEK1) but have differing efficacy. BRaf is generally shown to be the most effective at inducing MEK activation, and ARaf is shown to be the least effective [8,9]. However, in targeting BRaf, scientists have found the challenge of paradoxical activation and aberrant signaling independent of Ras activation [10]. Additionally, p21-activated kinases 1 (PAK1) phosphorylate MEK1 to increase association with Raf proteins, allowing c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) cross-pathway enhancement of the MAPK cascade [11]. MEK1 activates its sole downstream target, extracellular-signal-regulated kinases 1,2 (ERK1/2) by phosphorylation of threonine and tyrosine residues [12]. ERK has over 200 targets within the cell that contribute to proliferation, differentiation, cell survival, and other diverse cellular processes. The length and degree of ERK signaling plays an important role within the cell. Sustained moderate ERK signaling over many hours downregulates anti-proliferative genes which prevent cell-cycle progression from G0/G1 into S phase. This allows the expression of cellular signals to promote progression including cyclin D1 [13,14,15]. In contrast, transient higher levels of ERK signaling induce CDK-inhibitor protein expression, including p21 and p27, halting the cell-cycle progression [15,16,17].
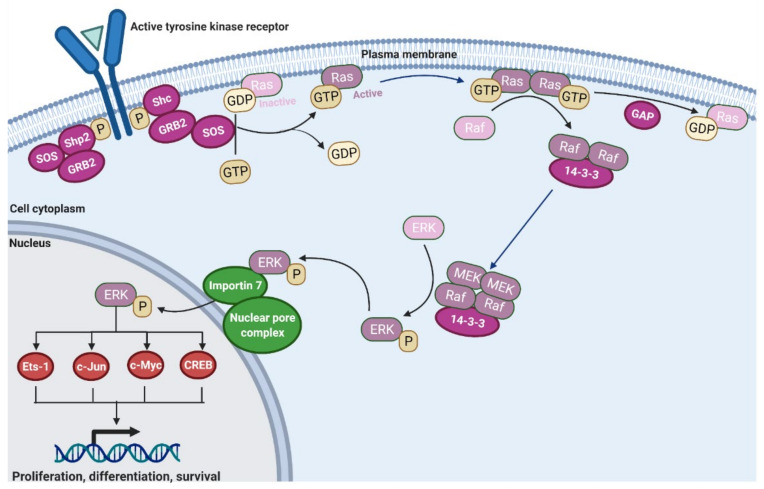
Figure 1.
The MAPK cascade. Once a ligand binds the tyrosine kinase receptor, it self-phosphorylates [18]. This creates binding sites for Shc and Shp2. GRB2 can associate with either and then recruit SOS [19,20]. SOS is a guanine exchange factor for Ras and induces the exchange of GDP for GTP [21]. Now active Ras will dimerize and bind Raf [21]. After activating Raf, GTPase activating proteins (GAP) will hydrolyze the GTP to GDP to return Ras to its resting inactive state [22]. The active Raf dimers will recruit MEK [23], which then activates ERK [3]. ERK interacts with Importin 7 at the nuclear envelope to facilitate its entry through the nuclear pore complex into the nucleus [24,25]. Once inside, it phosphorylates multiple transcription factors to alter gene expression in the cell and induce proliferation and survival [26].
Another important process that contributes to malignancy is viral oncogenesis, which is believed to comprise 12% of all clinically observed cancers and involves similar dysregulation of these conserved growth and signaling pathways [27]. There are several viruses known to be oncogenic in humans, including Epstein–Barr virus (EBV), hepatitis B and C viruses (HBV/HCV), human T-cell leukemia virus type 1 (HTLV-1), and human papillomaviruses (HPV). Although distinct, these viruses share the similar characteristic of modulating host cellular processes to proliferate and propagate themselves or to avoid immune detection and clearance from the body [28,29,30]. We have been investigating HTLV-1 infection, pathology, and associated adult T-cell leukemia/lymphoma (ATLL) [31,32,33,34,35,36,37].
Targeting the Ras signaling pathway has been a longstanding challenge, frustrating the efforts of scientists for decades with the Ras protein itself even being considered undruggable [7]. However, recent breakthroughs in MAPK/ERK pathway inhibitors have led to the approval of a novel, mutation-specific drug that directly inhibits Ras. Sotorasib is a first of its kind KRas G12C mutant targeting anti-cancer therapy and is currently approved for the treatment of non-small cell lung cancer (NSCLC) (Clinical Trial NCT03600883). Meanwhile, Sorafenib, the first FDA approved drug to treat HCC, is one of the only approved targeted drug therapies for advanced HCC, targeting Raf. However, challenges remain in the specificity of drugs to mutational profiles. Resistance easily arises due to tumor genetic heterogeneity, exacerbated by a lack of pan-Ras noncovalent inhibitors, and other factors [38,39,40,41]. Downstream, the challenge of paradoxical activation of the pathway by Ras-Raf inhibitors must also be addressed [10]. In this review, we have examined the role Ras-Raf signaling plays in the pathology of leukemias/lymphomas and solid tumors by compiling the current understanding of this signaling network in various cancers. The role of Ras-Raf signaling will also be discussed in the context of currently available therapies and ongoing clinical trials.
2. Activation of Ras and Raf Proteins
The master regulator of the classical MAPK cascade is the Ras protein, which is encoded by three genes, NRas, HRas, and KRas. These genes produce four active isoforms sharing a highly conserved structure and a unique C-terminal hypervariable region. These distinct C-terminal variations result in different post translational modifications that create different Ras isoforms with distinct efficacy, cellular distribution and functionality [42]. To localize to the plasma membrane where it can recruit Raf, all four Ras isoforms require post-translational modification after synthesis. A tetrapeptide signal at the carboxyl terminus, the CaaX box, serves as the motif recognized by farnesyl transferase (FTase) to initiate these changes (Figure 2) [43,44]. Once anchored onto the plasma membrane, signaling through Ras can be induced by via cytokine receptors, tyrosine kinase receptors, and G-protein-coupled receptors (GPCRs).
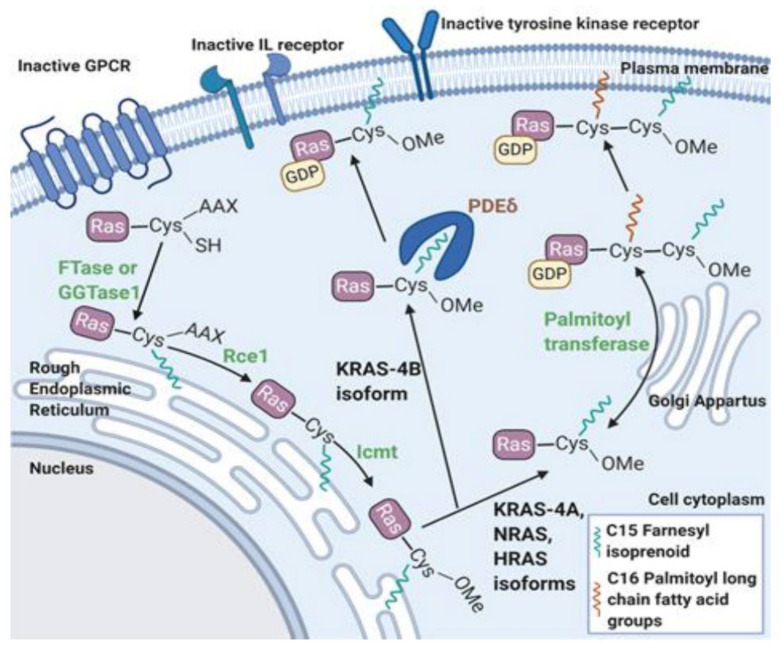
Figure 2.
Post translational farnesylation of Ras protein. The first modification is prenylation, preferentially performed by farnesyl transferase (FTase) [44]. This is initiated after recognition of the CaaX box on Ras’s C-terminus by FTase. Alternatively, the KRas-4B and NRas isoforms can be acted on by geranylgeranyl transferase 1 (GGTase1) if FTase is inhibited [43,45]. The farnesyl and geranylgeranyl moieties add enough hydrophobicity to enable Ras insertion into the endoplasmic reticulum membrane. Ras converting enzyme (Rce1) performs a final cleavage of the CaaX residues before isoprenylcysteine carboxyl methyltransferase (Icmt) adds a carboxymethyl group [44]. The final processing and transfer to the plasma membrane is isoform specific. Due to the farnesyl tail and a five amino acid sequence motif (Lys- Ser- Lys-Thr-Lys) in the C-terminus region, KRas-4B is directly chaperoned to the membrane by phosphodiesterase delta (PDEδ) [43,46,47]. Lacking the necessary motif, all other isoforms enter the Golgi apparatus for reversible palmitoylation by palmitoyl transferase. From the Golgi apparatus, HRas and NRas are trafficked to the plasma membrane on motile vesicles [48]. KRas-4A is trafficked by a poorly understood Golgi- independent route depending on mitochondrial function and class C vacuolar protein sorting (vps) proteins [49]. Afterwards, all isoforms associate with the membrane through their respective two-point anchors: the farnesyl modification and polybasic region of six lysines for KRas4b and the palmitoyl and farnesyl modifications for the other isoforms [50].
2.1. Ras Activation by the Tyrosine Kinase, Interleukin (IL) Receptors, and G-Protein Coupled Receptors
Tyrosine kinase and interleukin receptors utilize the same mechanism to interact with the Ras cascade. After cytokine binding, subsequent receptor dimerization allows transphosphorylation by the clustering Janus kinase 2 (JAK2) proteins bound to both β chains [51]. At high cytokine concentrations, adaptor protein Shc’s src homology 2 domain (SH2) binds to these phosphorylated tyrosine residues. While it lacks inherent catalytic function, Shc serves as a phosphorylated anchor for growth factor receptor bound protein 2 (Grb2) to bind. Grb2 then associates with son of sevenless (SOS), a guanine nucleotide exchange factor (GEF) for Ras [51,52,53,54].
Grb2 can also associate with src homology region 2 domain-containing phosphatase 2′s (Shp2) [55] (Figure 3). This protein acts as a scaffolding protein, serving as a link to tyrosine kinase receptors via its two SH2 domains and Grb2 at its C terminus tail. It also has protein tyrosine phosphatase domain, which inactivates several negative regulators of the MAPK pathway [56]. Shp2′s dephosphorylation releases additional Grb2 molecules from sequestration by Sprouty family 1–2 proteins [57,58]. It also dephosphorylates Ras’ docking sites of RASA, a Ras GTPase activating protein (Ras-GAP) that accelerates the hydrolysis of Ras’ bound GTP. By preventing RASA’s binding, Ras-GTP accumulates and propagates its effects longer [59,60]. Shp2 also indirectly affects Ras’s signaling through the Src family kinases (SFK), cytosolic protein tyrosine kinases that play roles in cell proliferation and survival. Src proteins have two tyrosine sites that play a role in regulation. Auto dephosphorylation of Tyr416 contributes to Src activation, while Tyr527 phosphorylation by C-terminal Src kinase (Csk) is inhibitory [61]. Before it can inhibit SFKs, Csk activity is determined by the docking protein Paxillin and the Csk binding protein (Cbp). CBP is also known as the phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG). When Shp2 is recruited by Gab1, it prevents the docking of Csk by dephosphorylating paxillin and Cbp/PAG. Csk molecules then dissociate from SFKs and allow them to propagate ERK signaling [62,63,64].
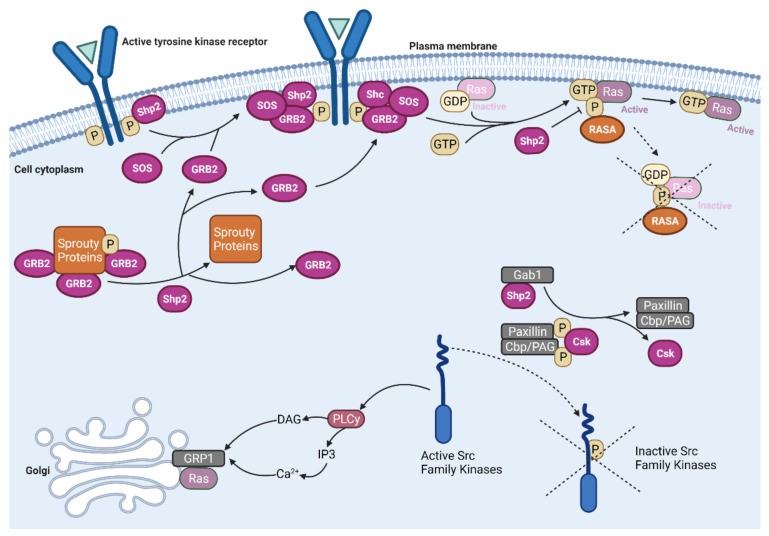
Figure 3.
The roles of src homology region 2 domain-containing phosphatase 2 (Shp2) in the Ras-Raf-MEK-ERK pathway. Shp2 is a GRB2 scaffolding protein that anchors it to tyrosine kinase receptors [56]. Additionally, Shp2 dephosphorylates Sprouty family proteins to release sequestered GRB2 molecules [57,58]. It also dephosphorylates Ras docking sites of RASA, a Ras GTPase activating protein (Ras-GAP) that accelerates the hydrolysis of Ras bound GTP [59,60]. This allows more active Ras-GTP molecules to accumulate instead of being converted to inactive Ras-GDP. After being recruited by Gab1, Shp2 can also dephosphorylate the binding sites of Paxillin and the Csk binding protein/phosphoprotein associated with glycosphingolipid-enriched microdomains (Cbp/PAG). This prevents the docking of C-terminal Src kinase (Csk) on Src family kinases (SFKs) and inhibits its activity by phosphorylating Tyr527 [61,62,63,64]. When active, SFKs initiate a signaling pathway through phospholipase C γ (PLCγ), diacylglycerol (DAG) and calcium. This results in the recruitment of the Ras guanine nucleotide exchange factor RasGRP1, directing it to the Golgi to activate intracellular Ras molecules [65,66].
At basal signaling levels, Ras proteins are bound to GDP and impeded from interacting with its effectors. When SOS is activated, it facilitates Ras’ exchange of GDP for GTP, resulting in an activated Ras confirmation. [43,46,67]. While SOS is ubiquitously expressed throughout the body to act downstream as part of receptor coupling, there are two additional guanine exchange factors (GEFs) that are tissue specific and facilitate the activation process. Both additional GEFs are distributed in the central nervous system [68,69]. Ras protein-specific guanine nucleotide releasing factor 1 (Ras-GRF1) is also expressed in the pancreas [70], while Ras-GRF 2 is additionally expressed in T-Cells [71,72].
While Ras has some slow intrinsic GTPase ability, its signal is usually terminated in conjunction with GTPase activating proteins [22,73]. Ras GAPs catalyze the hydrolysis of the bound GTP, accelerating the process by a factor of 105 [74,75]. By ending the signaling driving the MAPK pathway, these tumor suppressors prevent unlimited and unregulated cell proliferation and other cellular outcomes downstream of Ras [76,77]. Some of the most studied members of this family include neurofibromin and DAP2IP [76]. Whether GAP’s loss of function occurs through germline or somatic mutations [78], proteasomal degradation [79] or epigenetic silencing [80,81], the resulting proliferation of cells by prolonged Ras signaling are recognized in having a role in many cancers including lung [82], prostate [83,84], and hepatocellular cancers [85,86].
Ras molecules have also been shown to cluster together on different microdomains of the plasma membrane. Inactivated HRas-GDP isoforms have an affinity for lipid rafts on the plasma membrane near its triggering receptors and GEFs [67,87]. Lipid rafts are transient nanoscale clusters of protein and cholesterol within the plasma membranes. These liquid-ordered regions form around the HRas isoforms, partly due to the deep insertion of the palmitoyl moieties into the bilayer resulting from the GDP-induced confirmation [88]. Once GDP is exchanged for GTP, activated HRas’ conformational change induces changes in the N terminal catalytic domain and the hypervariable linker domain [89]. These changes decrease the extension of the palmitoyl moiety and release HRas-GTP from the liquid-ordered region. HRas-GTP enters a new liquid-disordered microdomain which allows for preferential interaction with the scaffolding protein Galectin-1 (Gal-1) as well as interaction with Raf and other subsequent signaling proteins [88,90]. Gal-1 is a known regulator of Ras nanoclustering specific to HRas. When Ras nanoclusters begin to recruit effector proteins, it induces Raf dimerization. Gal-1 binds to the Ras-binding domain on two Raf molecules, stabilizing the Raf dimer and conveying stability of Ras dimers and the whole nanocluster. While Gal-1 is specific to H-Ras, other scaffolds for the other isoforms include galectin-3, nucleophosmin and caveolae [21]. Due to the lack of palmitoylation motifs, KRas proteins have their own nonoverlapping, cholesterol-independent liquid-disordered microdomains. These clusters, like KRas signaling, are actin dependent [87]. NRas localizes to the borders of liquid ordered/liquid disordered microdomains as the GTP bound state preferentially localizes to cholesterol sensitive clusters [88,91]. These patterns of clustering and microdomain association have been a recent interest of research, as these patterns are suggested to play a role in determining how each isoform has a distinct function and signaling throughout the body. Where they cluster facilitates interactions with their activators, scaffolds and substrates, as well as with other Ras molecules to promote the dimerization that supports coupling with Raf. It can also determine the susceptibility of each isoform to different modifications. Differences in the microenvironments has been shown to determine that HRas and NRas can be targeted for ubiquitination signaling, allowing them to be transported to endosomes’ signaling network [92]. As the microdomains of Ras are better mapped and understood, they will provide more understanding on the regulation of Ras and how mutations in distinct isoforms may affect tissues differently.
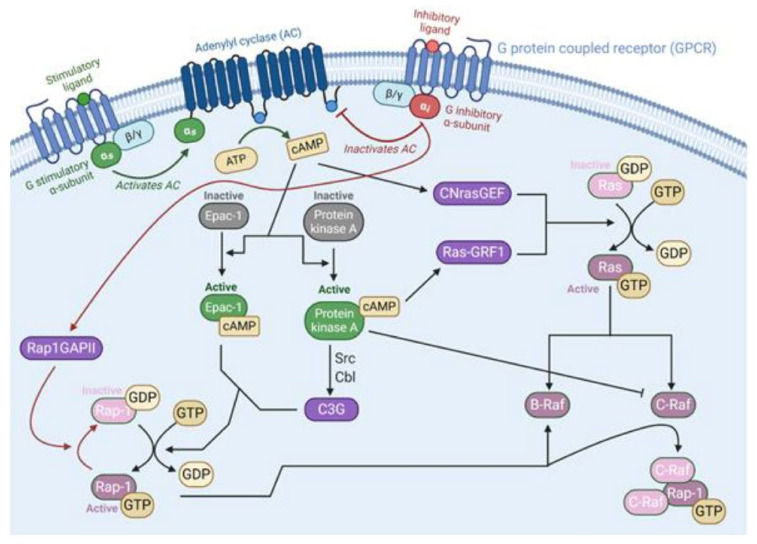
Recently, there has been a renewed interest in mapping the pathways of G-protein coupled receptors (GPCRs) and their roles in tumorigenesis. Many neuropeptides, including galanin, neurotensin, and gastrin-releasing peptide, have been found to stimulate proliferation and survival of small cell lung carcinoma cells through GPCR activation of ERK activity [93]. Each peptide signals through their specific GPCR, and several GPCR groups interact directly with the Ras-Raf-ERK-MAPK pathway as illustrated in Figure 4 and Figure 5. In various cell types, the effects differ depending which α-subunit isoform is utilized. Gαs induces adenylyl cyclase to produce the second messenger cAMP, triggering both protein kinase A (PKA) and exchange protein directly activated by cAMP (Epac-1). Downstream, this can modulate the activity of Raf proteins: increased BRaf activity or inhibition of c-Raf action [94,95,96]. In adrenal medullary cells, Gαs signals through cAMP, PKA and Rap-1 potentiates the effects of growth factors and supports differentiation into sympathetic neurons [97]. In comparison, Gαi subunits have the opposite effect by deactivating adenylyl cyclase and decreasing the amount of cAMP present [94]. Winitz et al. showcased Gi activation of CRaf-MEK-ERK in fibroblasts with the acetylcholine muscarinic m2 receptor [98] (Figure 5). Additionally, the βγi subunits of these receptors, as well as the Gq GPCR family, can have additional effects in some cells through the activation of phospholipase C-β (PLCβ), which will lead to the activation of the Raf-MEK-ERK pathway to induce chemotaxis and proliferation. Della Rocca et al. found that stimulation of α1B or α2A adrenergic receptors triggered these pathways to cause a rapid 5–10-fold increase in ERK phosphorylation. This activation relies on the phospholipase C, calmodulin, Pyk2 and Src pathways and suggested that fibroblasts, ovary cells and neuroblastoma cells used this mechanism for cellular proliferation [99]. Βγ subunits have also been shown to cause the accumulation of GTP-bound Ras proteins, prolonging their signals [100]. Furthermore, constitutively active Gq receptor’s Gα14 subunits have been shown to increase the formation of GTP-bound Ras and the downstream phosphorylation of ERK in hepatocellular carcinoma cells [101]. G protein-coupled receptors are also part of chemokine and environment-sensing axes that are being researched as possible drug targets in B-cell lymphomas as well as prostate and ovarian cancers [95,102,103,104,105]. In addition to direct activation of the Ras-Raf pathway, some GPCRs have also been shown to mediate and increase the interaction between the scaffolding protein 14-3-3 and CRaf to increase CRaf signaling [106].
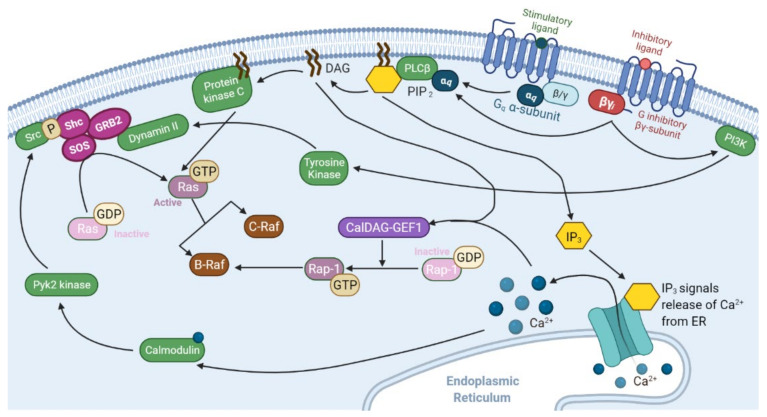
Figure 4.
G protein-coupled receptor subunits Gαq and Gβγi interaction with the Ras-Raf pathway. G-proteins are heterotrimeric guanine nucleotide binding proteins with α-, β-, and γ-subunits. When a ligand binds to the extracellular portion of the receptor, it confers a guanine nucleotide exchange factor confirmation that induces the α-subunit to exchange its bound GDP to GTP. This causes the α-subunit to disassociate from the receptor and βγ- subunit. Both the α and βγ subunits effect changes in the cell, before the α subunit hydrolyzes the GTP and returns the receptor complex to its inactive state [94,95,107,108]. Both αq and βγi activate phospholipase C-β (PLCβ) to create the second messengers of diacylglycerol (DAG) and inositol-1,4,5-triphosphate (IP3) through the hydrolyzation of phosphatidylinositol 4,5-bisphosphate (PIP2) [109]. DAG activates PKC, which directly phosphorylates and activates Ras proteins [110,111]. IP3 stimulates the calmodulin pathway and Pyk2 kinase by way of inducing calcium release from the endoplasmic reticulum [112]. The resulting phosphorylation provides the base for Shc anchoring and recruitment of Ras’ guanine exchange factor complex [113]. Both DAG and IP3 play a role in allosterically controlling CalDAG-GEF1, a guanine exchange factor for Rap1 [94,114]. Once Rap1 has exchanged its bound GDP for GTP, it can activate BRaf in the place of Ras [115]. Furthermore, βγi activates phosphoinositide 3-kinase (PI3K), which augments cell signaling from tyrosine kinase receptors to increase Dynamin II [116], an additional anchor for Shc and Ras’ GEF complex [117,118,119].
Figure 5.
G-protein-coupled receptor subunits Gαs and Gαi interaction with the Ras-Raf pathway. When activated by a ligand, the GPCR is induced to exchange its bound GDP for GTP, freeing the α subunit to act on the cell. αs acts on adenylyl cyclase (AC) to increase the production of the second messenger cAMP from ATP [120]. In turn, cAMP activates Epac-1 [121] and protein kinase A [120]. Epac-1 and C3G, a downstream molecule from PKA, are guanine-nucleotide exchange factors for Rap-1 and induce the exchange of GDP to GTP to activate it [121,122]. Both activate Rap-1, which can modulate the activity of BRaf [115]. Protein Kinase A has other mechanisms of action as well. It can directly prevent the activation of CRaf through phosphorylation. Both activate Rap-1, which can modulate the activity of BRaf [115]. Protein kinase A has other mechanisms of action as well. It can directly prevent the activation of CRaf through phosphorylation [123]. It also activates a Ras GEF, RasGRF1, to start the MAPK cascade in certain cells [124]. In contrast, αi inhibits adenylyl cyclase and produces opposite effects. It also activates a Ras GEF, RasGRF1, to start the MAPK cascade in certain cells [124]. In contrast, αi inhibits adenylyl cyclase and produces opposite effects.
2.2. Activation and Regulation of Raf Protein
The classical MAPK cascade continues with the activation of the serine/threonine kinase Raf. There are three Raf isoforms that can be activated: ARaf, BRaf, and CRaf (also called Raf-1). All three isoforms share a similar conserved two-lobe structure connected by an oscillating hinge. These structures can be further broken down into the Ras-binding domain (RBD), the cysteine-rich domain (CRD) and an acidic N-terminus (NTA) on the N-terminal side as well as a serine/threonine-kinase domain and 14-3-3 binding motif on the C-terminal side. The intervening hinge region has a second conserved region (CR2) with another 14-3-3 recognition site [125,126]. The CRD domain is a conserved C1 domain, or a small domain found in many proteins that are activated at the membrane [126]. Despite this similarity, each isoform has varying activity levels and roles throughout the cell.
Before activation, or in non-dividing cells, Raf’s N-terminus docks onto and auto-inhibits the kinase region to prevent any activity [127]. This autoinhibition is stabilized by the regulatory 14-3-3 proteins [126]. These are phosphoserine/phosphothreonine binding proteins with two Raf binding sites. In the inactive state, 14-3-3 binds to both the C-terminus and the CR2 site in the intervening hinge motifs of a single Raf molecule. [128,129,130,131]. To facilitate 14-3-3 binding, the CR2 site is phosphorylation by several different enzymes, including protein kinase A (PKA) [23,132], AKT [133,134], AMPK [135,136,137] serum/glucocorticoid regulated kinase (SGK) [128] and LATS1 [138]. All parts of this inactivating bundle are oriented by the CRD domain at the center of the complex. This positions active site of the C-terminal kinase domain to point outwards from the bundle and coordinate with Raf’s substrate, MEK. Both Raf and MEK remain inactive as their alpha helical domains are still displaced, but have their active sites aligned around an ADP molecule [126]. Additionally, the CRD domain is prevented from interacting with the cellular membrane [139].
To switch confirmations, the Raf protein must first be recruited to the plasma membrane. An anchored and activated Ras will bind to the RBD of Raf with nanomolar affinity due to hydrogen bonding and electrostatic interactions to form an extended β sheet structure. There is an inherent flexibility around this binding, allowing the Raf molecules to rotate to enter better positions. After this binding, the CRD domain is released from the autoinhibition complex. A zinc finger motif forms between the RBD, the CRD and the short five amino acid linker region between them, allowing the two domains to interact directly with each other and as one extended structure [139,140]. The CRD domain will then also creates a large hydrophobic interface with Ras to stabilize its interaction with Raf. The hydrophobic portions of CRD will also contact the bilayer’s phospholipids to anchor the complex with the membrane [139]. Furthermore, the release of CRD exposes the phosphorylated serine regulatory site in the CR2 region of Raf. Protein phosphatase 1 (PP1) and the leucine rich repeat scaffold protein SHOC2 removes the phosphorylation, disrupting the inhibitory interaction with 14-3-3 [141,142,143,144,145]. This dichotomy between the phosphorylation of the CR2 site acts as a valuable regulation point for Raf activity and allows many other pathways throughout the cell to feedback and influence Raf regulation. Further stabilization is due to the Ras clustering. Clustering close together allows nearby Raf molecules to dimerize [146]. The dimerization allows the autophosphorylation of the pair’s NTA motifs and activation loop segment on the C-lobe [146,147]. This is further supported when 14-3-3 binds to the C-terminal phosphoserine sites (Ser621 on Raf-1 and Ser729 on B-Raf) of both Raf proteins to strengthen the dimerization and promote Raf activity.
2.3. Ras-Raf-MEK-ERK Pathway Interaction with p53
In addition to the transcription factors that ERK targets, the Ras-Raf-MEK-ERK pathway interacts with several other key cellular pathways to mediate mitogenic signaling. To keep cells dividing, the activation of the classical MAPK cascade prevents the acetylation of p53′s DNA binding domain. Without this acetylation, p53 has decreased transcriptional activation of cyclin-dependent kinase inhibitor p21, effectively blocking the p53/p21 axis that would induce cell cycle arrest [148,149]. Additionally, inactivation of the p53/p21 cascade can induce cell division through the Raf-MEK-ERK cascade independent of Ras activation [148,149]. When Drosten et al. knocked down expression of p53 or p21 in Rasless cells, they observed proliferation and an activation of the Raf-MEK-ERK signaling pathway, as seen by increased phosphorylation of MEK and ERK. However, proliferation was not seen in cells lacking Raf, MEK or ERK. They therefore hypothesized that there was a p53-dependent feedback loop that decreased the activity of the Raf-MEK-ERK pathway [148]. In tumorigenesis, this inactivation could be due to lost p53 expression, a common mutagenic step in many cancers. Drosten’s hypothesis suggests that these common mutations remove negative feedback on cellular proliferation signals induced by the Raf-ERK-MEK pathway. This could explain some tumors’ continued proliferation despite DNA damage [150]. Furthermore, activating mutations of ERK can lead to cisplatin resistance in cancer when paired with inactivating p53 mutations [151]. This pair of mutations also often coexists in pancreatic cancer [152] and colon cancer [153].
Other research suggests that the two cascades interact through human double minute-2 protein (Hdm2), which inhibits p53 by sequestration to repress its transcriptional activity. Hdm2 also acts as an E3 ubiquitin ligase to promote nuclear export and degradation of p53 [154]. Once activated by ERK, Ets transcription factors bind to the promoter region of Hdm2, increasing its expression and ultimately causing the degradation of p53. In many cancers, p53 function is lost due to inactivating mutations, an overexpression of Hdm2 [153] or a decrease in p14ARF antagonization of Hdm2′s ubiquitin ligase activity [155]. In some tumor development, Ras mutations may increase Hdm2 levels enough to block p53 from inducing apoptosis or arresting growth in the face of DNA damage. This could induce the radiation resistance found in some tumors [153]. Physiologically, the classical MAPK pathway can be activated by hormones, growth factors, and differentiation factors. In malignant cells, additional activation can be due to aberrant function resulting from chromosomal abnormalities, genetic mutations, overexpression of upstream receptors, or innate mutations of the Ras-Raf-MEK-ERK pathway proteins themselves [156].
3. Ras-Raf Pathway Mutations
A major source of dysregulation for the MAPK-ERK pathway is the mutations affecting the proteins of the pathway. These mutations generally affect Ras and Raf and result in regulatory dysfunction that contributes to oncogenesis [157,158]. Therefore, it is important to discuss the frequency of these mutations and how they contribute to abnormal signaling.
3.1. Ras Mutations
Ras mutations are present in between 15 and 30% of cancers [159,160] and often result in pathway hyperactivation [161]. The frequency of these mutations and the location of each mutation are specific to each type of cancer [157,161]. Blood cancers commonly have NRas and KRas mutations (Table 1), whereas the majority of solid organ tumors with Ras mutations (Table 2), including colorectal and pancreatic cancers, generally only have KRas mutations. These KRas mutations are found at much higher rates than Ras mutations in blood cancer overall [160]. Depending on the Ras isoform subtype, there are hotspots for mutation that are unique: KRas is most commonly G12 mutated, and NRas and HRas are most commonly Q61 mutated, although HRas has an abundance of G12 and G13 mutations as well [160]. Mutations in these regions are oncogenic because they disrupt interactions between the Ras protein and GTP-ase activating proteins (GAPs) [22]. GAPs promote GTP hydrolysis and therefore functionally inactivate Ras. Without GAP-catalyzed GTP hydrolysis, Ras can remain active in the absence of an upstream signal and can contribute to oncogenesis. Loss of GAP function has been associated with neurofibromatosis and a series of cancers, including lung, hepatocellular, and prostate cancers [22,76,162].
Table 1.
Various blood cancers with Ras-Raf mutations.
| Blood Cancer | Ras-Raf Pathology | General Treatment Protocol | 5-Year Survival | References |
|---|---|---|---|---|
| Myelodysplastic Syndromes | NRas, KRas mutation in 7–48% of patients. | Use of erythropoietin stimulating agents to mitigate symptoms. Allogeneic stem cell transplant for higher risk patients. | 29% | [163,164,165,166,167] |
| Acute Myeloid Leukemia | NRas, KRas mutations in 10–27% of de novo patients. | Induction via cytarabine and the addition of an anthracycline for patients followed by consolidation. | 24% | [167,168,169,170] |
| Acute Lymphoblastic Leukemia | NRas, KRas mutations in 5–22% of patients. BRaf mutations have been found in infants and children with acute B and T-cell lymphoblastic leukemia. Small sample found ~21% of ALL patients with BRaf mutations. |
Induction using vincristine, corticosteroids, L asparaginase, and an anthracycline for patients followed by consolidation. | Between 30 and 45% | [171,172,173,174] |
| Chronic Myelomonocytic Leukemia | NRas, KRas mutations in 30–50% with CMML. Subset of patients with CMML-1 presented BRaf mutations (~7%). |
Lack of consensus about a treatment that markedly expands overall survival rate. Hypomethylating agents have shown some promise and are approved by the FDA for CMML. Allogeneic stem cell transplantation regarded as only curative treatment. | 18.5% | [175,176,177,178,179] |
| Chronic Myeloid Leukemia | NRas mutations in up to 1/3 of atypical CML. Chronic CML patients present up to 17% of Ras mutations, up to 58% of acute CML patients have Ras mutations. | Standard treatment involves the use of tyrosine kinase inhibitors in sequence. | 61% | [166,169,180,181] |
Table 2.
Various solid cancers associated with Ras-Raf mutations.
| Solid Cancer | Ras-Raf Pathology | General Treatment Protocol | 5-Year Survival Rate | References |
|---|---|---|---|---|
| Pancreatic Adenocarcinoma | KRas mutations in up to 90% of patients. BRaf mutation in 14% of patients. | Surgical resection and/or chemotherapy. | less than 5%. | [166,182,183,184,185] |
| Melanoma | Ras mutations in up to 36% of patients. BRaf mutations in 27–70% of patients. | Surgical resection. Chemotherapy and novel targeted therapies, including BRaf inhibitors, may be used when surgical resection is not possible. | 91%. | [169,186,187,188,189] |
| Non-Small Cell Lung Cancer | KRas mutations in 22–36% of patients. BRaf in ~2 to 5% of patients. | Surgical resection and/or chemotherapy. | 25% | [190,191,192,193,194,195] |
| Colorectal Cancer | KRas mutations in 40–60% of patients. BRaf mutation in 18% of patients. | Surgical resection and/or chemotherapy. | 65% | [169,187,196,197] |
| Seminoma | KRas, NRas mutations in 7–40% of patients. | Radical orchiectomy with subsequent chemotherapy. | 86.4% | [166,198,199,200] |
| Bladder Cancer | HRas, NRas mutations shown in up to 80% of patients, although Ras mutations generally considered present in 10% of patients. | Immunotherapy and/or chemotherapy, with radical cystectomy after invasion of muscle. | 80.8% | [201,202,203,204] |
| Hepatocellular Carcinoma | NRas mutations in 30%, KRas mutations in 1.6% of patients. BRaf mutations in 14% of patients. | Surgical resection, liver transplantation, and/or chemotherapy. | 15% | [156,166,187,205,206] |
| Ovarian Cancer | KRas mutation in 13.7% of patients. BRaf mutations in 4–30% of patients | Cytoreductive surgery followed by chemotherapy. | 40% | [187,188,207,208] |
| Renal Cell Carcinoma | Ras mutations in up to 13% of patients. | Tyrosine kinase, m-TOR, and VEGF inhibitors. Other targeting therapies including immunotherapy are used as well. | Between 92.5 and 12% depending on localization. | [166,209,210,211] |
The type of oncogenic cell expressing the Ras mutant can also drive mutations on the MAPK pathway. Some Ras mutants transform healthy cells more aggressively into malignant cells or to appear at earlier stages of malignancy [212]. Complicating the situation further, the missense mutations in the Ras codon hotspots have also been shown previously to result in different interactions with other proteins [157,213]. With many variables affecting how a specific Ras mutant behaves, researchers have found that response to pharmacological intervention can depend on the mutation subtype and currently the data suggest that new forms of treating cancer via Ras targeting may require distinct treatment methods [212]. As for how these mutations can be induced, Ras oncogenes have been known for decades to occur in rats treated with carcinogens [214]. The type of mutagen introduced has been shown to induce varying Ras mutant subtypes [157]. The type of mutagen introduced has been shown to induce varying Ras mutant subtypes [157]. Tissue exposure to mutagens drives some of the differences in mutational frequency seen in Ras mutant subtypes. This exposure does not explain why a tumor of a tissue may have Ras isoforms with different mutant subtypes (e.g., a tumor with HRas favoring 61 codon mutations but KRas 12 codon mutations). Work has been done showing that local tissue signaling networks may be responsible for a particular kind of Ras mutation and that certain co-mutation events involving KRas play a role in the specific location of the KRas mutation [215]. Ras isoforms have been shown to differ in DNA damage but not repair rates, with codon 12 binding better to carcinogens on KRas compared with HRas and NRas [157,216]. In addition to increased binding of carcinogens, KRas was shown to be more at risk for damage from UV radiation than NRas. This is despite NRas having a high frequency of mutation in malignant melanoma, a cancer associated with UV light exposure, and is due to the higher prevalence of thymine–thymine sites in the KRas gene [216]. KRas’s higher susceptibility to chemical and UV mutagenesis may explain why it is more frequently seen in cancers, although more work is needed to understand why certain codons may be preferentially mutated in Ras isoforms.
3.2. Raf Mutations
Raf mutations affecting the serine/threonine kinase contribute to a series of development disorders and are thought to contribute to approximately 8% of cancers [158,217]. Hundreds of missense mutations have been found affecting the Raf gene that result in various degrees of hyperactivation and downstream activation of ERK, resulting in oncogenesis [188]. Of the three Raf isoforms, ARaf, BRaf, and CRaf, BRaf is the isoform more commonly mutated in cancer [217]. The location of the mutation on BRaf has a large effect on its ability to increase activity several fold over wild type, as mutations in the activation loop and phosphate binding loops impair their inhibitory interactions leading to an active conformation of the Raf protein [217,218]. The V600 location on BRaf is the most important location for mutations on this protein, with mutations occurring here in 92% of oncogenic forms of BRaf [218]. Basal kinase activities as high as 480-fold occur depending on the mutant form with V600D, V600E, V600K, and other similar mutants having some of the highest basal kinase and phosphorylated ERK levels [187,218]. Alternate mutations other than V600E at the same location were shown to have higher or similar kinase activity, however the glutamate replacement requires a single base substitution and is therefore seen at higher rates in cancers. Various mutants with locations outside of the 600 amino residues have been found that lower rates of kinase activity. It is thought that there may need to be a varied activation of the BRaf protein due to the fact that overactivation of ERK leads to senescence [188].
3.3. MEK and ERK Mutations
Mutations in MEK and ERK are less studied but have been noted in developmental disorders and in both naturally occurring neoplasms and in response to BRaf inhibitors as a mechanism for resistance when treating cancers such as melanoma [219,220,221,222]. ERK, although often overactive due to abnormal upstream activity, is rarely mutated in cancer [223]. MEK and ERK mutations are not nearly as common as Ras and Raf mutations, however they are vital to understanding mechanisms of resistance. This importance can be seen with the use of Raf inhibitors for cancers such as melanoma, which results in brief clinical improvement, but often ends with patients developing resistance through genetic and nongenetic processes [221]. Depending on the location of the mutation, MEK1 and MEK2 mutations have been shown to reduce BRaf and/or MEK inhibition by dabrafenib and trametinib, respectively [220,221,224]. ERK mutations that are clinically relevant have been seen in response to ERK and Raf inhibitors and are thought to mediate drug resistance [225,226]. Mutations were found that obstruct proper ERK inhibitor binding, resulting in ERK catalytic activity despite inhibitor treatment. ERK mutation mediated resistance was circumvented using MEK inhibition, suggesting that resistance due to mutations of ERK in response to various MAPK/ERK inhibitors may be superseded through combination therapy targeting multiple proteins in the pathway [225,226]. Combined inhibition using various MAPK/ERK inhibitors also superseded MEK and NRas mutations and therefore represents an avenue for cancer therapy [220]. Experimentally derived and naturally observed ERK mutations are reviewed in [223].
4. Ras-Raf Signaling in Various Types of Cancers
Aberrant signaling in the MAPK cascade holds significant weight in the development of lymphomas and solid tumors. Disruptions in signaling may be from a variety of causes not limited to genetic predispositions, as demonstrated in cases of virally associated cancers such as adult T-cell leukemia/lymphoma, Burkitt’s lymphoma, and hepatocellular carcinoma.
4.1. Leukemia/Lymphoma
Adult T-cell leukemia/lymphoma (ATLL) is an aggressive T-cell neoplasm characterized by the clonal expansion of lymphocytes, which displays monoclonal integration of the human T-cell leukemia virus type 1 (HTLV-1) provirus [227,228]. HTLV-1 was initially found in a patient cell line diagnosed with cutaneous T-cell lymphoma in 1979 and is known as the first human retrovirus to be discovered [229,230]. HTLV-1 proviral integration into the host chromosome is believed to drive many of the observed consequences of genomic instability and disruption of genome integrity [231,232]. The transformation of T-cells present in patients with ATLL is almost certainly dependent on the activities of Tax1 protein in infection/disease progression. However, it is suggested that Tax dependence is only required early during infection as Tax1 is rarely detected in the leukemic cells of ATL patients [228,231]. Tax1 is the HTLV-1 viral, trans-activator protein that has pleiotropic effects in activating and dysregulating cellular processes involved in growth and immunosurveillance and has been extensively studied regarding its activities as a tumor initiator in ATL [233,234]. Tax1 overexpression performed in cancer cell lines (breast, colon, and hepatoma) resulted in increased proliferation as quantified by MTT assay [235]. Additionally, protein analysis by Western blot demonstrated an increase in phosphorylation of all the members in the Ras-Raf pathway, suggesting Tax1-mediated augmentation of signaling in driving proliferation [235]. A crucial effect of Tax-mediated dysregulation is increased cellular proliferation through activation of MAPK. A whole-genome integration study conducted on a cohort of 370 ATL cases revealed numerous genomic alterations, such as activating mutations, gene fusions, and insertion/deletions [236]. These alterations overlap with genes that are targeted by and known to interact with Tax1 during HTLV-1 infection. A different study analyzing Ras signaling found that Tax1 expression resulted in increased Ras-GTP levels and increased phosphorylation of ERK, and that this correlated with an anti-apoptotic state. Apoptotic resistance was overcome when a Ras farnesylcystein mimetic (FTS, S-farnesylthiosalicyclic acid) was used in Tax 1-expressing cells, which also resulted in decreased levels of Ras-GTP and phosphorylated ERK, suggesting that the Ras-Raf-MEK-ERK signaling pathway partially contributes to apoptosis resistance in ATL [237].
Burkitt’s leukemia/lymphoma (BL) is a subtype of non-Hodgkin’s lymphoma (NHL) that is diagnosed as an aggressive neoplasm of B lymphocytes [238]. BL can be categorized into three types, endemic, sporadic, or immunodeficiency-related, and is characterized by a high rate of proliferation and apoptosis. For the endemic BL, infection or association with the Epstein–Barr Virus (EBV) is always observed, whereas it is only observed in 25–40% of cases of sporadic and immunodeficiency-related BL [239,240]. The defining feature of BL across all subtypes is the translocation of the oncogene MYC into proximity with either the immunoglobulin heavy or light chain, which results in continuous MYC expression that is believed to drive the high proliferation rate [241,242]. As such, the development of methods to target MYC has seen preclinical and clinical attention and represents an important future avenue to pursue in the treatment of BL [243,244]. The use of chemotherapy is effective in treating pediatric BL, however, caution is taken when treating adults due to the risk of tumor lysis syndrome [239]. Although the role of MYC in driving BL oncogenesis has been well characterized, the importance of other oncogenes such as those in the MAPK cascade is not as well researched. A study that analyzed the genomes of BL patients at primary diagnosis and at relapse detected mutations within the MAPK pathway, specifically in NRas [245]. While MYC mutations were also found in patients at primary diagnosis, the detection of NRas mutations at relapse suggests that dysregulation of MAPK can provide therapeutic resistance in patients undergoing chemotherapy.
4.2. Solid Tumors
Liver cancer was the fourth most common cause of cancer related death worldwide in 2020, with the vast majority being hepatocellular carcinoma (HCC) [246]. HCC is usually observed in patients with preexisting liver conditions, such as cirrhosis or chronic hepatitis B or C viral infections [247]. Common treatment modalities include surgical resection, liver transplantation, trans-arterial chemoembolization, radiation, ablation, and systemic therapies, including sorafenib [246,247]. This is an aggressive and lethal cancer often diagnosed at an advanced stage, and there is a need to develop additional therapeutics. HBV infection, the leading driver of HCC worldwide, induces mutations in tumor protein p53 and activation of oncogenic signaling [248]. The MAPK-ERK signaling pathway is highly active (50–100%) in most observed HCC cases, however, mutations of Ras or Raf genes are rarely observed in humans [206,249]. The lack of Ras-Raf mutations in HCC suggests that there is either improper upstream signaling or that there is a lapse in MAPK/ERK inhibition and regulation which results in overactive signaling.
The Ras-Raf pathway is known to play a critical role in HCC. Several members in the MAPK-ERK pathway are overexpressed in HCC. CRaf and MEK were found to be heavily upregulated in both cirrhosis and carcinoma, and CRaf was found to be heavily phosphorylated in nearly all cirrhosis and carcinoma samples tested by Hwang et al. [250]. Overexpression of Ras, MEK, and particularly CRaf were associated with worse prognostic outcomes [251]. The upregulation of Raf in HCC is consistent with previous work showing that it is key in tumor growth and angiogenesis in many different solid cancers and with data showing that Raf inhibition disrupts these two processes in HCC [251]. Additionally, a study comparing MEK1/2 levels between tumor and healthy HCC cells, found that only tumor cells showed high phosphorylated MEK1/2 levels and that an increased expression of MEK1 in HCC tumor cells lead to more growth in vivo and resistance to apoptosis in response to MEK inhibitor U0126 [252]. This study and others have also found increased ERK activation, which mediates upregulation of important factors in cell growth and proliferation and correlated with tumor size [252,253,254].
Pancreatic adenocarcinoma (PAC) is classified as a malignant neoplasm of the ductal or acinar cells in the pancreas and is generally diagnosed through endoscopic ultrasound and/or other imaging modalities [255]. With surgical and adjuvant therapy resulting in very low survival rates, alternative forms of treatment are required to produce better prognoses (Table 1). Mutations of the KRas gene are very common in PAC with a frequency of over 90% reported in cancer cells and are associated with a poor prognosis [256]. KRas has been shown to be required for sustained tumorigenic growth in advanced PAC, with loss of KRas expression leading to tumor regression [257]. KRas-induced activation of ERK, the main effector of the MAPK-ERK pathway, is thought to be responsible for a host of tumorigenic properties including tumor cell chemoresistance, invasion of pancreatic tumors, and the proliferation of pancreatic tumor cells [256]. KRas has been shown to be required for sustained tumorigenic growth in advanced pancreatic carcinoma, with loss of KRas expression leading to tumor regression [257]. In addition, KRas works with a series of other pathways to induce transformation, evade cell death or suppression of growth, and other malignant processes as reviewed in [256]. Although KRas mutations have been shown to be sufficient in transforming pancreatic cells into premalignant cell lines that can transition into PAC, there are patients who harbor KRas mutations displaying premalignant cell lines that never develop PAC. This indicates that although KRas is important in driving initial stages in PAC, other mutations may be needed in concert to develop PAC [258]. CRaf is important for both the initiation of KRas-driven PAC and progression, while BRaf is seemingly only required for late-stage PAC progression [259].
Non-small cell lung cancer (NSCLC) makes up 85% of lung cancer cases and comprises three main types of lung cancer: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Treatment generally involves surgical resection with or without adjuvant therapy, however most diagnoses of NSCLC are made after metastasis where 5-year survival rates are approximately 6%, indicating a need for an alternative form of treatment [260]. KRas mutations and elevated KRas expression, as mentioned previously, are associated with driving forward metabolic activity associated with tumors (such as increased reliance on glucose, increased energy needs, increased TCA cycle activity, etc.) and previous research has shown similarities in metabolic activities between lung adenocarcinoma mouse models and human NSCLC tumor cells [261,262]. KRas mutation frequency and type can vary with lifestyle habits such as smoking [263]. The G12C mutation is associated with smoking tobacco use in patients with NSCLC [263]. Additionally, non-smokers and light smokers show fewer KRas mutations than heavy smokers and show a higher rate of the G12D mutation than the G12C mutation commonly seen in smokers and NSCLC generally [264]. There are conflicting reports concerning whether KRas mutations, including subtype (G12C, G12D, etc.) influence the survival outcome of NSCLC patients. Some reports have KRas mutations associated with poorer outcomes in NSCLC patients, especially those in early stages, going as far as finding that NSCLC patients with G12C and G12V KRas mutants had significantly reduced progression-free survival compared with those with G12D mutations or WT KRas [265,266]. Other reports, however, find that mutations, including those different in subtype, have no significant survival difference including during adjuvant chemotherapy [266,267,268].
In NSCLC and colorectal cancers (CRC), the KRas mutation has been linked to an increased expression in PD-L1, an immune checkpoint protein expressed in tumor cells that interacts with the PL1 receptor on T-cells as a method of avoiding immune-mediated cell death [269,270]. The MAPK-ERK pathway is known to both directly and indirectly result in the phosphorylation and inhibition of tristetraprolin, a protein that binds PD-L1 mRNA leading to its degradation [269]. An additional method by which the MAPK-ERK pathway mediates immune subversion is through the ability of KRas mutant tumor cells to generate suppressive T regulatory cells by secreting IL10 and TGFβ1 [271]. Modulation of antitumor immune responses occurs in other cancers with mutations in the MAPK-ERK pathway. Most notably, in BRaf mutant cancers like melanoma or CRC, immunosuppressive cytokines are upregulated inhibiting the release of inflammatory cytokines by dendritic cells and recruiting cells that downregulate antitumor responses [272]. Cancer-associated fibroblasts are among the cells mediating immunosuppression, and in BRaf mutant melanoma they have been found to upregulate PD-L1 aiding in immune evasion [273]. BRaf mutations have also been shown to reduce CD8+ T-cell tumor recognition and induce internalization of major-histocompatibility complex molecules in melanoma tumor cells [274,275]. Therefore, in patients with KRas and BRaf mutant cancers, immune checkpoint blockade has presented as an additional avenue of treatment to consider and has even produced positive results in patients with these mutations [276,277].
BRaf mutations are found in a smaller subset of NSCLC patients with the majority harboring V600E mutations and in adenocarcinomas [278]. In NSCLC mouse models, it has been found by various studies that CRaf was required for proper tumor initiation but not BRaf, as total ablation of BRaf but not CRaf allowed oncogenesis in KRas G12D and G12V mutant mice [279,280]. Additionally, depletion of CRaf but not BRaf in KRas mutant mice resulted in an inhibition of downstream ERK phosphorylation, an additional finding emphasizing the role of CRaf in KRas-driven cancer [281].
5. Targeting of Ras-Raf Signaling in Cancers
Exploring the role of MAPK signaling in the development of various cancers is vital in identifying potential therapeutic targets. Several MAPK signaling inhibitors relevant to cancer therapy have been compiled in Table 3. Upstream of Ras, SOS is a novel target for inhibiting the Ras protein. Several inhibitors have been manufactured including BI-3406 and BI 1701963 which bind to SOS and disrupt its interaction with Ras [282]. Preclinical research has shown that co-delivering SOS and MEK inhibitors counteracts the MEK resistance that is commonly seen after prolonged delivery of MEK inhibitors. There are multiple clinical trials testing BI 1701936 in solid cancer patients with and without KRas mutations. These trials involve the use of BI 1701963 in combination with different MAP/ERK protein inhibitors including KRas and MEK inhibitors (NCT04111458; NCT04975256; NCT04835714). Another novel target upstream of Ras is SHP2. Multiple phase I clinical trials using SHP2 inhibitors RMC-4630 and TNO155 are underway targeting KRas mutant NSCLC and other forms of cancer such as head and neck carcinoma (NCT04000529; NCT04330664; NCT03634982) [282]. Phase I clinical trials show that SHP2 inhibitors are tolerated well and reduced tumor volume in a subset of KRas G12C mutant NSCLC when combined with KRas inhibition [283]. Additional trials are being undertaken combining SHP2 inhibitors with other MAPK protein inhibitors, including the MEK inhibitor cobimetinib (NCT03989115; NCT03634982) [283]. SHP2 inhibitor BBP-398 is also being studied as a monotherapy in patients with advanced solid cancer without the BRaf V600E mutation (NCT04528836).
Table 3.
Inhibitors of MAPK cascade in active clinical trials.
| Inhibitor | Notes | Clinical Trials | Targeted Cancers |
|---|---|---|---|
| BI 1701963 | Binds SOS1 protein, inhibiting its activation of KRas |
NCT04111458, NCT04975256, NCT04835714 |
Lung, colon, and lung cancer |
| RMC-4630 | Selective inhibitor of Shp2, indirectly inhibiting KRas |
NCT03634982, NCT03989115, NCT04916236 |
Pancreatic, colorectal, and non-small cell lung cancer |
| TNO155 | Selective inhibitor of Shp2, indirectly inhibiting KRas |
NCT04330664, NCT04292119, NCT04000529 |
Lung, head and neck, esophageal, gastrointestinal and colorectal cancer |
| BBP-398 | Selective inhibitor of Shp2, indirectly inhibiting KRas | NCT04528836 | Advanced solid tumors |
| Sorafenib | Inhibitor of multiple intracellular and cell surface kinases such as CRaf and BRaf that are involved in tumor cell signaling, angiogenesis, and apoptosis | NCT01730937, NCT03518502, NCT01371981 | Liver and thyroid cancer, leukemias |
| Sotorasib (AMG 510) |
KRas inhibition specific to G12C mutation | NCT03600883, NCT04303780, NCT04933695 | Non-small cell lung cancer |
| MRTX849 | KRas inhibition selective to the G12C mutation | NCT04793958, NCT04685135 | Non-small cell lung and colorectal cancer |
| JAB-21822 | KRas inhibition selective to the G12C mutation | NCT05009329, NCT05002270 | Non-small cell lung and colorectal cancer |
| GFH925 | KRas inhibition selective to the G12C mutation | NCT05005234 | Advanced solid tumors |
| LY3537982 | KRas inhibition selective to the G12C mutation | NCT04956640 | Non-small cell lung, colorectal, endometrial, ovarian, and pancreatic cancer |
| Tipifarnib (R115777) |
Farnesyltransferase inhibitor that prevents post-translational processing of Ras proteins. Prevents Ras from membrane binding |
NCT03496766, NCT04997902, NCT03155620 |
Non-small cell lung and head and neck cancer, non-Hodgkin lymphoma |
| Rigosertib | Targets mutated Ras pathway in leukemia by interacting with effectors proteins containing Ras binding domains | NCT04263090, NCT04263090, NCT03786237 | Myelodysplastic syndrome, non-small cell lung cancer, and squamous cell carcinoma |
| Trametinib (GSK1120212) |
Non-ATP-competitive inhibitor of MEK1/2. FDA approved, suggested to use in combination with BRaf inhibitors due to resistance. | NCT03340506, NCT04940052, NCT02101788 | Non-small cell lung, thyroid, ovarian and peritoneal cancer, melanoma |
| Mirdametinib (PD0325901) |
MEK1/2 inhibitor (derivative of CI-1040) |
NCT02022982, NCT03905148, NCT04923126 |
Ovarian, endometrial, pancreatic, thyroid, and non-small cell lung cancer, melanoma, glioma |
| Selumetinib (AZD6244) |
Highly selective, non-ATP-competitive MEK1 inhibitor. | NCT04576117, NCT03705507, NCT03705507 | Non-small cell lung cancer, glioma, leukemia |
| Binimetinib (MEKTOVI; MEK162) |
Highly selective, non-ATP-competitive MEK1/2 inhibitor. | NCT04657991, NCT02928224, NCT03843775 | Colorectal cancer, melanoma, and other BRaf mutant malignancies |
| RO5126766 (CH5127566) |
Selective Raf/MEK1/2 inhibitor | NCT02407509, NCT03875820, NCT04720417 | Non-small cell lung, ovarian, and colorectal cancer, multiple myeloma, melanoma |
| HL-085 | Selective MEK1 inhibitor | NCT03973151, NCT03990077, NCT03781219 | Non-small cell lung cancer, melanoma, and BRaf mutant solid cancers |
| Ulixertinib (BVD-523) |
ATP-competitive, reversible inhibitor of ERK1/2 | NCT04145297, NCT03417739, NCT04488003 | Gastrointestinal cancers, melanoma, and BRaf mutant solid cancer |
Ras itself has been considered as a target for cancer therapy. However, due to the difficulty in targeting Ras, contemporary efforts focused on targeting other proteins in the MAPK-ERK pathway despite Ras, particularly KRas, having high rates of oncogenic mutations in multiple cancers, especially PAC [259]. Indirect methods of targeting Ras include the use of farnesyltransferase inhibitors, which inhibit proteins that result in Ras’s localization to the cell membrane. Tipifarnib has shown efficacy in multiple phase II trials for acute myelogenous leukemia and myelodysplastic disorders but has not shown success in targeting advanced PAC [284,285]. Tipifarnib has also recently been designated as a breakthrough therapy by the FDA for HRas mutant head and neck squamous cell carcinoma after positive results from a phase II clinical trial and has shown preclinical activity in HRas mutant thyroid cancer cell lines [286,287]. Additional clinical trials are underway using tipifarnib for HRas mutant NSCLC and head and neck squamous cell carcinoma (NCT03496766; NCT04997902). While significant challenges arise in targeting specific KRas oncoproteins, breakthroughs in targeting the KRas G12C mutant have yielded the approval of Sotorasib as treatment for NSCLC [40]. These drugs represent the newest attempt at targeting the MAPK-ERK pathway, and approval of Sotorasib marks the first successful case of covalent Ras inhibition [38]. Although this new avenue of therapeutics presents with much optimism, KRas G12C inhibitor resistance has already been seen in preclinical models [38,39,40,41]. Genetically heterogenous tumors with mutations untargetable by G12C-specific drugs will inevitably develop resistance once the selective pressure of a drug treatment is exerted. To exacerbate the issue of resistance, tumors which lack dependency on KRas signaling may have intrinsic resistance, demonstrated by continued viability of tumor cells despite complete ablation of KRas signaling [288]. Researchers have explored additional explanations and have found that the mesenchymal cancer cell phenotype, which has been previously associated with a lower reliance on KRas for tumorigenic processes, instead relies on PI3K signaling, mediating resistance to G12C inhibitors [41]. These inhibitors are also specific for the G12C mutation which limits their use as this mutation is not common in several cancers including PAC (~1%), where the G12D and G12V mutations are markedly more common [289]. In accordance with discovery of the G12C inhibitor, there have been recent successful attempts at developing a rudimentary KRas G12D inhibitor that showed efficacy in cell-based assays and may point toward an additional method of treatment for KRas-driven cancers in the future [290].
Looking beyond targeting Ras directly, BRaf inhibitors developed to target the popular V600E mutant (vemurafenib, dabrafenib, etc.) have been pursued for their disruption of the MAPK-ERK pathway in cancers with BRaf-driven cancers, including BRaf mutant melanoma and NSCLC [193]. These inhibitors, however, have been ineffective in KRas- driven cancer [259,291,292]. An issue that can arise with use of Raf inhibitors alone is paradoxical activation. This was seen clinically when a patient with KRas mutant NSCLC was treated with vemurafenib and showed signs of a tumor flare indicative of paradoxical activation that may occur when weakly inhibiting Raf kinases [293]. Raf inhibitors were developed to avoid paradoxical activation by also targeting Raf dimers (e.g., LXH254, LY3009120, PLX8394, etc.) [259]. These have resulted either in high toxicity or were unable to inhibit CRaf, the particular Raf isoform important in the initiation of PAC [259]. Pan-Raf inhibitors are currently undergoing investigation for various solid tumors, aiming to avoid paradoxical activation. Sorafenib, a potent oral inhibitor of CRaf and BRaf, as well as several tyrosine kinases have been approved for advanced HCC [247,250,252,294,295,296]. There are several issues with the use of sorafenib as a monotherapy for advanced HCC. HCC commonly does not respond to sorafenib due to the heterogeneity of HCC cells [297,298,299]. When HCC does respond, the clinical benefit of the drug is limited to stabilizing HCC and resistance is also commonly seen after 6 months of treatment [297,298,299]. Synergistic regimen featuring sorafenib codelivery with many therapeutic drugs that are not MAPK/ERK inhibitors have been investigated for advanced HCC and include Artesunate, an antimalarial drug that suppresses angiogenesis and cell proliferation in HCC cell lines [300]. Artesunate is responsible for the creation of reactive oxygen species that contribute to increased apoptosis and, in the process, create phosphorylated ERK and STAT3 that concomitant sorafenib delivery reduces greatly, ultimately leading to reduced tumor growth [300]. Alternatively, sorafenib has been used alongside various compounds that inhibit the PI3K-AKT-mTOR pathway [299,301,302].
Downstream of Raf, MEK inhibitors have been developed to impede the MAPK-ERK pathway. Although effective for melanoma alongside codelivery of BRaf inhibitors, MEK inhibitors have generally also failed to treat PAC despite preclinical successes [259]. MEK inhibition has been hampered by two main issues, toxicity, specifically ocular toxicity, and resistance [258]. Drug resistance to MEK inhibitors that develops after prolonged use is thought to limit its effectiveness [303]. Mutations increasing MEK1 activation and bolstering resistance to MEK inhibitors have been shown to develop in colon cancer cell lines and even in a patient with resistant melanoma that developed after MEK inhibitor use [303]. Intratumor genetic heterogeneity has also been shown to develop in response to MEK inhibitor delivery in pancreatic cancer cell lines and may contribute to the lackluster results of MEK inhibitor use in clinical trials for patients with advanced PAC [304]. In addition, efforts to inhibit MEK have notably led to the removal of negative feedback against upstream receptor tyrosine kinases, resulting in continued Ras signaling despite MEK inhibition that may also contribute to resistance [305]. This issue is not isolated to MEK inhibitors as similar treatment evasion has been reported for G12C inhibitors, with tyrosine kinase blockade overcoming newly developed resistance, hinting that a potentially similar mechanism of evasion may exist for G12C inhibitors [288]. Raf inhibitors developed to avoid paradoxical activation by also targeting Raf dimers (e.g., LXH254, LY3009120, PLX8394, etc.) have resulted either in high toxicity or a lack of inhibition of CRaf, the particular Raf isoform important in the initiation of PAC [259].
Following MEK, ERK1/2 is thought to contribute to resistance to Raf and MEK inhibitors through loss of ERK feedback inhibition and subsequent ERK reactivation [306]. ERK1/2 feedback inhibition occurs via phosphorylation of proline rich regions on MEK that reduce MEK1 activity and interrupt activating phosphorylation and protein binding for MEK1/2 [307]. In addition, phosphorylated ERK levels have been associated with worse outcomes in patients with PAC [308]. Looking at preclinical models where resistance was developed in response to BRaf or MEK inhibitors, ERK inhibition was shown to be effective against resistant tumor cells [309,310]. Targeting PI3K and/or mTOR effector proteins downstream of KRas, has been an alternative route apart from targeting the MAPK-ERK protein pathway that is synergistic with combined MAPK/ERK inhibition and similar to ERK inhibition and has overcome resistant cancer cell lines [311,312]. Additionally, immune checkpoint blockade via the inhibition of PD1, a protein expressed on T-cells that can be manipulated by tumor cells in order to avoid immune activity, has also led to reduced resistance to MEK inhibitor trametinib and there are currently multiple Phase I and II trials combining MAPK/ERK inhibitors with PD1/PD1L inhibitors in patients with KRas G12C NSCLC (NCT03600883; NCT03600701; NCT02902029) [270]. The use of autophagy inhibitors alongside ERK inhibitors, as in the case of combining MEK and PDEδ inhibitors, has also shown enhanced antitumorigenic activity [308,312,313,314,315]. ERK inhibition, shown to upregulate PAC tumor cell reliance on autophagy and downregulate dependence on other metabolic processes, worked synergistically with hydroxychloroquine to inhibit growth in preclinical models of PAC [313]. ERK reactivation is often seen in EGFR inhibitor resistant NSCLC tumors, and therefore ERK1/2 inhibitors can be used as a part of a multidrug regimen to improve the effectiveness of these inhibitors representing another innovative use for ERK inhibitors [316,317]. There are concerns about the efficiency of delivering ERK inhibitors due to possible off-target toxicities and solubility problems, similar roadblocks shared by MEK inhibitors [318]. To solve these issues, there are efforts to deliver SCH779284 and other ERK inhibitors alongside standard chemotherapeutics more efficiently using nanoparticles [318].
6. Conclusions and Future Perspectives
The Ras-Raf-MEK-ERK pathway is a critical component of cell cycle control, exerting strict regulation over proliferation and differentiation. Mutations within this highly conserved signaling pathway have proven to be key drivers of numerous human blood and solid cancers. As key regulatory points within the MAPK pathway, Ras and Raf exist as multiple isoforms with different characteristics regarding activity and involvement in oncogenesis. Thus, targeting Ras signaling has been a topic of discussion and research. Most notably, a recent breakthrough in the approval of Sotorasib as a KRas G12C inhibitor has ignited hope in what was previously considered an undruggable target. In addition, there are a plethora other KRas G12C inhibitors in clinical trials for NSCLC and CRC such as JAB-21822, GFH925, LY3537982, and most notably MRTX849 which is leading with phase III trials (Table 3). Other novel targeting involving the MAPK-ERK pathway include SOS inhibitors, particularly BI 1701963 which is undergoing phase I/II clinical trials, and Shp2 inhibitors (Table 3). However, challenges remain in mutational subgroup specificity leading to drug resistance by tumor genetic heterogeneity, and a need for pan-inhibitors remains. Furthermore, downstream challenges may arise in paradoxical activation by MAPK/ERK inhibitors, highlighting the necessity of continued research to circumvent these challenges.
Alternatively, nutraceuticals are under-researched and interact with MAPK pathway. Silibinin and Curcumin have been used with sorafenib and have interacted synergistically to inhibit tumor growth in HCC preclinical models [319,320]. Silibinin as a monotherapy has inhibited tumor cell proliferation, metastatic potential, ERK1/2 phosphorylation, and levels of downstream cyclin proteins [319,321,322,323]. Meanwhile, Curcumin, one of many plant-derived polyphenols, has been investigated for use in multiple cancers and pathologies, including HBV-induced HCC, due to its antioxidant, antiviral, and anticancer functions [324,325]. Further research is needed to study the use of plant-derived compounds for use with and without other MAPK/ERK inhibitors. Other novel methods of targeting the Ras-Raf pathway include chimeric antigen receptor T-cell therapy, which involves the use of modified T-cells that recognize antigens specific to KRas mutant cancer cells and induce T-cell response independent of the usual processing and presentation by antigen presenting cells [326]. There is currently an active phase I/II clinical trial using G12V-specific T-cell therapy for pancreatic cancer (NCT04146298). Additional methods using the body’s immune system include peptide vaccines that work by inducing an immune response to a synthetic peptide associated with tumor cells that are processed and presented by class I and class II major histocompatibility complex molecules. There are two phase I clinical trials using KRas-targeted peptide vaccines: one prophylactically for patients at risk of developing pancreatic cancer and the other in patients with resected microsatellite stable pancreatic or colorectal cancer (NCT05013216; NCT04117087). Lastly, vaccines using mRNA instead of peptides are being adopted for use against cancer and have a series of improvements over peptide vaccines including the ability to encode entire tumor antigens, resulting in greater epitope presentation to T-cells and stimulating a larger T-cell response [327]. The mRNA vaccine mRNA-5671 targets mutant KRas tumor cells and is in a phase I clinical trial for patients with advanced or metastatic NSCLC, PAC, and CRC (NCT03948763).
Furthermore, there are certain types of cancers that have not been thoroughly investigated in regards to the role played by the MAPK/ERK pathway such as acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), hematologic malignancies that are differentiated by clonal proliferation of either myeloid or lymphocytic cells, respectively. More work is needed to elucidate the involvement of Ras signaling in AML and ALL. The same is true for HPV infection and its associated cervical, oral, anal, and other cancers. Similarly, while the involvement of the Ras-Raf pathway is increasingly understood in ATLL and BL, no treatments targeting it exist for these cancers. While much progress has been made in targeting MAPK/ERK signaling and the growing body of knowledge surrounding Ras-Raf involvement in oncogenesis yields great potential, substantial efforts must be made to translate these targets into safe, efficacious treatment for a wide variety of cancers.
Acknowledgments
All figures were created on Biorender.
Abbreviations
| MAPK | Mitogen-activated protein kinase |
| ATLL | Adult T-cell leukemia/lymphoma |
| HTLV-1 | Human T-cell lymphotropic virus type 1 |
| ERK | Extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| EBV | Epstein–Barr virus |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HPV | Human papillomaviruses |
| GDP | Guanosine diphosphate |
| GTP | Guanosine triphosphate |
| Ras-GRF | Ras protein-specific guanine nucleotide releasing factor 1 |
| FTase | Farnesyl transferase |
| GGTase1 | Geranylgeranyl transferase 1 |
| Rce1 | Ras converting enzyme |
| Icmt | Isoprenylcysteine carboxyl methyltransferase |
| PDEδ | Phosphodiesterase delta |
| GPCR | G-protein-coupled receptors |
| IL | Interleukin |
| JAK2 | Janus kinase 2 |
| SH2 | Src homology 2 domain |
| Grb2 | Growth factor receptor bound protein 2 |
| SOS | Son of sevenless |
| GEF | Guanine nucleotide exchange factor |
| Gal-1 | Galectin-1 |
| EGFR | Epidermal growth factor receptor |
| PKA | Protein kinase A |
| PLCβ | Phospholipase C-β |
| RBD | Ras-binding domain |
| PAK | p21-activated protein kinase |
| PKC | Protein kinase C |
| BL | Burkitt’s lymphoma |
| NHL | non-Hodgkin’s lymphoma |
| HCC | Hepatocellular carcinoma |
| NSCLC | Non-small cell lung cancer |
| PAC | Pancreatic adenocarcinoma |
| CRC | Colorectal cancer |
| Shrp2 | Src homology region 2 domain-containing phosphatase 2 |
| Csk | C-terminal Src kinase |
| SFKs | Src family kinases (SFKs) |
| Epac-1 | Exchange protein directly activated by cAMP |
| PLCγ | Phospholipase C-γ |
| PAG | Phosphoprotein associated with glycosphingolipid-enriched microdomains |
| PP1 | Protein phosphatase 1 |
Author Contributions
M.D., A.L., E.L., D.S., and R.P. put together the manuscript draft under the direct supervision and guidance of P.J. who conceptualized the idea, edited drafts, and formulated the final format for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIH/NINDS grant number R01 NS097147.
Conflicts of Interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marshall C.J. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 1994;4:82–89. doi: 10.1016/0959-437X(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 2.Sun Y., Liu W.-Z., Liu T., Feng X., Yang N., Zhou H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 3.Roberts P.J., Der C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W., Liu H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 5.Mor A., Philips M.R. Compartmentalized ras/mapk signaling. Annu. Rev. Immunol. 2006;24:771–800. doi: 10.1146/annurev.immunol.24.021605.090723. [DOI] [PubMed] [Google Scholar]
- 6.Weiss R.A. A perspective on the early days of RAS research. Cancer Metastasis Rev. 2020;39:1023–1028. doi: 10.1007/s10555-020-09919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore A.R., Rosenberg S.C., McCormick F., Malek S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020;19:533–552. doi: 10.1038/s41573-020-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X., Noh S.J., Zhou G., Dixon J.E., Guan K.-L. Selective Activation of MEK1 but Not MEK2 by A-Raf from Epidermal Growth Factor-stimulated Hela Cells. J. Biol. Chem. 1996;271:3265–3271. doi: 10.1074/jbc.271.6.3265. [DOI] [PubMed] [Google Scholar]
- 9.Marais R., Light Y., Paterson H.F., Mason C.S., Marshall C.J. Differential Regulation of Raf-1, A-Raf, and B-Raf by Oncogenic Ras and Tyrosine Kinases. J. Biol. Chem. 1997;272:4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- 10.Gibney G.T., Messina J.L., Fedorenko I.V., Sondak V.K., Smalley K.S.M. Paradoxical oncogenesis—The long-term effects of BRAF inhibition in melanoma. Nat. Rev. Clin. Oncol. 2013;10:390–399. doi: 10.1038/nrclinonc.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coles L.C., Shaw P.E. PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene. 2002;21:2236–2244. doi: 10.1038/sj.onc.1205302. [DOI] [PubMed] [Google Scholar]
- 12.Payne D.M., Rossomando A.J., Martino P., Erickson A.K., Her J.H., Shabanowitz J., Hunt D.F., Weber M.J., Sturgill T.W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase) EMBO J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto T., Ebisuya M., Ashida F., Okamoto K., Yonehara S., Nishida E. Continuous ERK Activation Downregulates Antiproliferative Genes throughout G1 Phase to Allow Cell-Cycle Progression. Curr. Biol. 2006;16:1171–1182. doi: 10.1016/j.cub.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 14.Alao J.P. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer. 2007;6:24–30. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sewing A., Wiseman B., Lloyd A.C., Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip. Mol. Cell. Biol. 1997;17:5588–5597. doi: 10.1128/MCB.17.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods D., Parry D., Cherwinski H., Bosch E., Lees E., McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip. Mol. Cell. Biol. 1997;17:5598–5611. doi: 10.1128/MCB.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirza A.M., Gysin S., Malek N., Nakayama K.-I., Roberts J.M., McMahon M. Cooperative Regulation of the Cell Division Cycle by the Protein Kinases RAF and AKT. Mol. Cell. Biol. 2004;24:10868–10881. doi: 10.1128/MCB.24.24.10868-10881.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertics P.J., Gill G.N. Self-phosphorylation enhances the protein-tyrosine kinase activity of the epidermal growth factor receptor. J. Biol. Chem. 1985;260:14642–14647. doi: 10.1016/S0021-9258(17)38618-0. [DOI] [PubMed] [Google Scholar]
- 19.Batzer A.G., Rotin D., Ureña J.M., Skolnik E.Y., Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol. Cell. Biol. 1994;14:5192–5201. doi: 10.1128/MCB.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margolis B., Skolnik E.Y. Activation of Ras by receptor tyrosine kinases. J. Am. Soc. Nephrol. 1994;5:1288–1299. doi: 10.1681/ASN.V561288. [DOI] [PubMed] [Google Scholar]
- 21.Blaževitš O., Mideksa Y.G., Šolman M., Ligabue A., Ariotti N., Nakhaeizadeh H., Fansa E.K., Papageorgiou A., Wittinghofer A., Ahmadian M.R., et al. Galectin-1 dimers can scaffold Raf-effectors to increase H-ras nanoclustering. Sci. Rep. 2016;6:24165. doi: 10.1038/srep24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vigil D., Cherfils J., Rossman K.L., Der C.J. Ras superfamily GEFs and GAPs: Validated and tractable targets for cancer therapy? Nat. Rev. Cancer. 2010;10:842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavoie H., Therrien M. Regulation of RAF protein kinases in ERK signalling. Nat. Rev. Mol. Cell Biol. 2015;16:281–298. doi: 10.1038/nrm3979. [DOI] [PubMed] [Google Scholar]
- 24.James B.P., Bunch T.A., Krishnamoorthy S., Perkins L.A., Brower D.L. Nuclear localization of the ERK MAP kinase mediated by Drosophila αPS2βPS integrin and importin-7. Mol. Biol. Cell. 2007;18:4190–4199. doi: 10.1091/mbc.e06-07-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorenzen J., Baker S., Denhez F., Melnick M., Brower D., Perkins L. Nuclear import of activated D-ERK by DIM-7, an importin family member encoded by the gene moleskin. Development. 2001;128:1403–1414. doi: 10.1242/dev.128.8.1403. [DOI] [PubMed] [Google Scholar]
- 26.Chang F., Steelman L.S., Lee J.T., Shelton J.G., Navolanic P.M., Blalock W.L., Franklin R.A., McCubrey J. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: Potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 27.Mesri E.A., Feitelson M.A., Munger K. Human Viral Oncogenesis: A Cancer Hallmarks Analysis. Cell Host Microbe. 2014;15:266–282. doi: 10.1016/j.chom.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olagnier D., Sze A., Hadj S.B., Chiang C., Steel C., Han X., Routy J.-P., Lin R., Hiscott J., Van Grevenynghe J. HTLV-1 Tax-Mediated Inhibition of FOXO3a Activity Is Critical for the Persistence of Terminally Differentiated CD4+ T Cells. PLOS Pathog. 2014;10:e1004575. doi: 10.1371/journal.ppat.1004575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuda M., Longnecker R. Latent membrane protein 2A inhibits transforming growth factor-beta 1-induced apoptosis through the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 2004;78:1697–1705. doi: 10.1128/JVI.78.4.1697-1705.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L., Wu J., Ling M.T., Zhao L., Zhao K.-N. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol. Cancer. 2015;14:1–13. doi: 10.1186/s12943-015-0361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito M., Jain P., Tsukasaki K., Bangham C. HTLV-1 Infection and Its Associated Diseases. Leuk. Res. Treat. 2012;2012:1. doi: 10.1155/2012/123637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman S., Khan Z.K., Wigdahl B., Jennings S.R., Tangy F., Jain P. Murine FLT3 Ligand-Derived Dendritic Cell-Mediated Early Immune Responses Are Critical to Controlling Cell-Free Human T Cell Leukemia Virus Type 1 Infection. J. Immunol. 2010;186:390–402. doi: 10.4049/jimmunol.1002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mostoller K., Norbury C.C., Jain P., Wigdahl B. Human T-cell leukemia virus type I Tax induces the expression of dendritic cell markers associated with maturation and activation. J. Neurovirol. 2004;10:358–371. doi: 10.1080/13550280490521104. [DOI] [PubMed] [Google Scholar]
- 34.Jain P., Lavorgna A., Sehgal M., Gao L., Ginwala R., Sagar D., Harhaj E.W., Khan Z.K. Myocyte enhancer factor (MEF)-2 plays essential roles in T-cell transformation associated with HTLV-1 infection by stabilizing complex between Tax and CREB. Retrovirology. 2015;12:1–24. doi: 10.1186/s12977-015-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain P., Manuel S.L., Khan Z.K., Ahuja J., Quann K., Wigdahl B. DC-SIGN Mediates Cell-Free Infection and Transmission of Human T-Cell Lymphotropic Virus Type 1 by Dendritic Cells. J. Virol. 2009;83:10908–10921. doi: 10.1128/JVI.01054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain P., Ahuja J., Khan Z.K., Shimizu S., Meucci O., Jennings S.R., Wigdahl B. Modulation of dendritic cell maturation and function by the Tax protein of human T cell leukemia virus type. J. Leukoc. Biol. 2007;82:44–56. doi: 10.1189/jlb.1006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ginwala R., Caruso B., Khan Z.K., Pattekar A., Chew G.M., Corley M.J., Loonawat R., Jacobson S., Sreedhar S., Ndhlovu L.C., et al. HTLV-1 Infection and Neuropathogenesis in the Context of Rag1(-/-)gammac(-/-) (RAG1-Hu) and BLT Mice. J. Neuroimmune Pharmacol. 2017;12:504–520. doi: 10.1007/s11481-017-9740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janes M.R., Zhang J., Li L.S., Hansen R., Peters U., Guo X., Chen Y., Babbar A., Firdaus S.J., Darjania L., et al. Targeting KRAS Mutant Cancers with a Covalent G12C-Specific Inhibitor. Cell. 2018;172:578–589. doi: 10.1016/j.cell.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Hallin J., Engstrom L.D., Hargis L., Calinisan A., Aranda R., Briere D.M., Sudhakar N., Bowcut V., Baer B.R., Ballard J.A., et al. The KRAS(G12C) Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020;10:54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canon J., Rex K., Saiki A.Y., Mohr C., Cooke K., Bagal D., Gaida K., Holt T., Knutson C.G., Koppada N., et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 41.Adachi Y., Ito K., Hayashi Y., Kimura R., Tan T.Z., Yamaguchi R., Ebi H. Epithelial-to-Mesenchymal Transition is a Cause of Both Intrinsic and Acquired Resistance to KRAS G12C Inhibitor in KRAS G12C–Mutant Non–Small Cell Lung Cancer. Clin. Cancer Res. 2020;26:5962–5973. doi: 10.1158/1078-0432.CCR-20-2077. [DOI] [PubMed] [Google Scholar]
- 42.McCubrey J.A., Steelman L.S., Chappell W.H., Abrams S.L., Wong E., Chang F., Lehmann B., Terrian D.M., Milella M., Tafuri A., et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007;1773:1263–1268. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brock E.J., Ji K., Reiners J.J., Mattingly R.R. How to Target Activated Ras Proteins: Direct Inhibition vs. Induced Mislocalization. Mini-Rev. Med. Chem. 2016;16:358–369. doi: 10.2174/1389557515666151001154002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J., Yao X., Huang J. New tricks for human farnesyltransferase inhibitor: Cancer and beyond. MedChemComm. 2017;8:841–854. doi: 10.1039/C7MD00030H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox A.D., Fesik S.W., Kimmelman A.C., Luo J., Der C.J. Drugging the undruggable RAS: Mission Possible? Nat. Rev. Drug Discov. 2014;13:828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porru M., Pompili L., Caruso C., Biroccio A., Leonetti C. Targeting KRAS in metastatic colorectal cancer: Current strategies and emerging opportunities. J. Exp. Clin. Cancer Res. 2018;37:1–10. doi: 10.1186/s13046-018-0719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dharmaiah S., Bindu L., Tran T.H., Gillette W.K., Frank P.H., Ghirlando R., Nissley D.V., Esposito D., McCormick F., Stephen A.G., et al. Structural basis of recognition of farnesylated and methylated KRAS4b by PDEdelta. Proc. Natl. Acad. Sci. USA. 2016;113:E6766–E6775. doi: 10.1073/pnas.1615316113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choy E., Chiu V.K., Silletti J., Feoktistov M., Morimoto T., Michaelson D., Ivanov I.E., Philips M.R. Endomembrane Trafficking of Ras: The CAAX Motif Targets Proteins to the ER and Golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 49.Wang G., Deschenes R.J. Plasma Membrane Localization of Ras Requires Class C Vps Proteins and Functional Mitochondria in Saccharomyces cerevisiae. Mol. Cell. Biol. 2006;26:3243–3255. doi: 10.1128/MCB.26.8.3243-3255.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cox A.D., Der C.J., Philips M.R. Targeting RAS Membrane Association: Back to the Future for Anti-RAS Drug Discovery? Clin. Cancer Res. 2015;21:1819–1827. doi: 10.1158/1078-0432.CCR-14-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dougan M., Dranoff G., Dougan S.K. GM-CSF, IL-3, and IL-5 Family of Cytokines: Regulators of Inflammation. Immunity. 2019;50:796–811. doi: 10.1016/j.immuni.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 52.Broughton S., Dhagat U., Hercus T., Nero T., Grimbaldeston M.A., Bonder C.S., Lopez A.F., Parker M.W. The GM-CSF/IL-3/IL-5 cytokine receptor family: From ligand recognition to initiation of signaling. Immunol. Rev. 2012;250:277–302. doi: 10.1111/j.1600-065X.2012.01164.x. [DOI] [PubMed] [Google Scholar]
- 53.Reddy E.P., Korapati A., Chaturvedi P., Rane S. IL-3 signaling and the role of Src kinases, JAKs and STATs: A covert liaison unveiled. Oncogene. 2000;19:2532–2547. doi: 10.1038/sj.onc.1203594. [DOI] [PubMed] [Google Scholar]
- 54.Ahmed S., Prignent S. Insights into the Shc Family of Adaptor Proteins. J. Mol. Signal. 2017;12:1–17. doi: 10.5334/1750-2187-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward A.C., Monkhouse J.L., Hamilton J.A., Csar X.F. Direct binding of Shc, Grb2, SHP-2 and p40 to the murine granulocyte colony-stimulating factor receptor. Biochim. Biophys. Acta Bioenerg. 1998;1448:70–76. doi: 10.1016/S0167-4889(98)00120-7. [DOI] [PubMed] [Google Scholar]
- 56.Kerr D.L., Haderk F., Bivona T.G. Allosteric SHP2 inhibitors in cancer: Targeting the intersection of RAS, resistance, and the immune microenvironment. Curr. Opin. Chem. Biol. 2021;62:1–12. doi: 10.1016/j.cbpa.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Hanafusa H., Torii S., Yasunaga T., Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat. Cell Biol. 2002;4:850–858. doi: 10.1038/ncb867. [DOI] [PubMed] [Google Scholar]
- 58.Jarvis L.A., Toering S.J., Simon M.A., Krasnow M.A., Smith-Bolton R. Sprouty proteins are in vivo targets of Corkscrew/SHP-2 tyrosine phosphatases. Development. 2006;133:1133–1142. doi: 10.1242/dev.02255. [DOI] [PubMed] [Google Scholar]
- 59.Montagner A., Yart A., Dance M., Perret B., Salles J.-P., Raynal P. A Novel Role for Gab1 and SHP2 in Epidermal Growth Factor-induced Ras Activation. J. Biol. Chem. 2005;280:5350–5360. doi: 10.1074/jbc.M410012200. [DOI] [PubMed] [Google Scholar]
- 60.Agazie Y.M., Hayman M.J. Molecular Mechanism for a Role of SHP2 in Epidermal Growth Factor Receptor Signaling. Mol. Cell. Biol. 2003;23:7875–7886. doi: 10.1128/MCB.23.21.7875-7886.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roskoski R., Jr. Src protein–tyrosine kinase structure and regulation. Biochem. Biophys. Res. Commun. 2004;324:1155–1164. doi: 10.1016/j.bbrc.2004.09.171. [DOI] [PubMed] [Google Scholar]
- 62.Song Y., Zhao M., Zhang H., Yu B. Double-edged roles of protein tyrosine phosphatase SHP2 in cancer and its inhibitors in clinical trials. Pharmacol. Ther. 2021:107966. doi: 10.1016/j.pharmthera.2021.107966. [DOI] [PubMed] [Google Scholar]
- 63.Jiang L.Q., Feng X., Zhou W., Knyazev P.G., Ullrich A., Chen Z. Csk-binding protein (Cbp) negatively regulates epidermal growth factor-induced cell transformation by controlling Src activation. Oncogene. 2006;25:5495–5506. doi: 10.1038/sj.onc.1209554. [DOI] [PubMed] [Google Scholar]
- 64.Hrdinka M., Horejsi V. PAG—A multipurpose transmembrane adaptor protein. Oncogene. 2013;33:4881–4892. doi: 10.1038/onc.2013.485. [DOI] [PubMed] [Google Scholar]
- 65.Bivona T.G., De Castro I.P., Ahearn I.M., Grana T.M., Chiu V.K., Lockyer P.J., Cullen P.J., Pellicer A., Cox A.D., Philips M.R. Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature. 2003;424:694–698. doi: 10.1038/nature01806. [DOI] [PubMed] [Google Scholar]
- 66.McKay M.M., Morrison D.K. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 67.Degirmenci U., Wang M., Hu J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells. 2020;9:198. doi: 10.3390/cells9010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernández-Medarde A., Santos E. The RasGrf family of mammalian guanine nucleotide exchange factors. Biochim. Biophys. Acta Bioenerg. 2011;1815:170–188. doi: 10.1016/j.bbcan.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Le D.T., Shannon K.M. Ras processing as a therapeutic target in hematologic malignancies. Curr. Opin. Hematol. 2002;9:308–315. doi: 10.1097/00062752-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 70.De Mora J.F., Esteban L.M., Burks D.J., Núñez A., Garcés C., García-Barrado M.J., Iglesias-Osma M.C., Moratinos J., Ward J.M., Santos E. Ras-GRF1 signaling is required for normal beta-cell development and glucose homeostasis. EMBO J. 2003;22:3039–3049. doi: 10.1093/emboj/cdg280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruiz S., Santos E., Bustelo X.R. RasGRF2, a Guanosine Nucleotide Exchange Factor for Ras GTPases, Participates in T-Cell Signaling Responses. Mol. Cell. Biol. 2007;27:8127–8142. doi: 10.1128/MCB.00912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stone J.C. Regulation and Function of the RasGRP Family of Ras Activators in Blood Cells. Genes Cancer. 2011;2:320–334. doi: 10.1177/1947601911408082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qu L., Pan C., He S.-M., Lang B., Gao G.-D., Wang X.-L., Wang Y. The Ras Superfamily of Small GTPases in Non-neoplastic Cerebral Diseases. Front. Mol. Neurosci. 2019;12:121. doi: 10.3389/fnmol.2019.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gideon P., John J., Frech M., Lautwein A., Clark R., Scheffler J.E., Wittinghofer A. Mutational and kinetic analyses of the GTPase-activating protein (GAP)-p21 interaction: The C-terminal domain of GAP is not sufficient for full activity. Mol. Cell. Biol. 1992;12:2050–2056. doi: 10.1128/MCB.12.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rudack T., Xia F., Schlitter J., Kötting C., Gerwert K. Ras and GTPase-activating protein (GAP) drive GTP into a precatalytic state as revealed by combining FTIR and biomolecular simulations. Proc. Natl. Acad. Sci. USA. 2012;109:15295–15300. doi: 10.1073/pnas.1204333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maertens O., Cichowski K. An expanding role for RAS GTPase activating proteins (RAS GAPs) in cancer. Adv. Biol. Regul. 2014;55:1–14. doi: 10.1016/j.jbior.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 77.Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987;238:542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- 78.Patil S., Chamberlain R.S. Neoplasms associated with germline and somatic NF1 gene mutations. Oncologist. 2012;17:101–116. doi: 10.1634/theoncologist.2010-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGillicuddy L.T., Fromm J.A., Hollstein P.E., Kubek S., Beroukhim R., De Raedt T., Johnson B.W., Williams S.M., Nghiemphu P., Liau L.M., et al. Proteasomal and Genetic Inactivation of the NF1 Tumor Suppressor in Gliomagenesis. Cancer Cell. 2009;16:44–54. doi: 10.1016/j.ccr.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dote H., Toyooka S., Tsukuda K., Yano M., Ota T., Murakami M., Naito M., Toyota M., Gazdar A.F., Shimizu N. Aberrant promoter methylation in human DAB2 interactive protein (hDAB2IP) gene in gastrointestinal tumour. Br. J. Cancer. 2005;92:1117–1125. doi: 10.1038/sj.bjc.6602458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen H., Tu S.-W., Hsieh J.-T. Down-regulation of Human DAB2IP Gene Expression Mediated by Polycomb Ezh2 Complex and Histone Deacetylase in Prostate Cancer. J. Biol. Chem. 2005;280:22437–22444. doi: 10.1074/jbc.M501379200. [DOI] [PubMed] [Google Scholar]
- 82.Yano M., Toyooka S., Tsukuda K., Dote H., Ouchida M., Hanabata T., Aoe M., Date H., Gazdar A.F., Shimizu N. Aberrant promoter methylation of human DAB2 interactive protein (hDAB2IP) gene in lung cancers. Int. J. Cancer. 2005;113:59–66. doi: 10.1002/ijc.20531. [DOI] [PubMed] [Google Scholar]
- 83.Xie D., Gore C., Liu J., Pong R.-C., Mason R., Hao G., Long M., Kabbani W., Yu L., Zhang H., et al. Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc. Natl. Acad. Sci. USA. 2010;107:2485–2490. doi: 10.1073/pnas.0908133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Min J., Zaslavsky A., Fedele G., McLaughlin S.K., Reczek E.E., De Raedt T., Guney I., Strochlic D.E., MacConaill L.E., Beroukhim R., et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat. Med. 2010;16:286–294. doi: 10.1038/nm.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang X., Li N., Li X., Zhao W., Qiao Y., Liang L., Ding Y. Low expression of DAB2IP contributes to malignant development and poor prognosis in hepatocellular car-cinoma. J. Gastroenterol. Hepatol. 2012;27:1117–1125. doi: 10.1111/j.1440-1746.2011.07049.x. [DOI] [PubMed] [Google Scholar]
- 86.Chen S., Wang L., Yao B., Liu Q., Guo C. miR-1307-3p promotes tumor growth and metastasis of hepatocellular carcinoma by repressing DAB2 inter-acting protein. Biomed. Pharmacother. 2019;117:109055. doi: 10.1016/j.biopha.2019.109055. [DOI] [PubMed] [Google Scholar]
- 87.Prior I., Muncke C., Parton R.G., Hancock J.F. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J. Cell Biol. 2003;160:165–170. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Henis Y., Hancock J., Prior I.A. Ras acylation, compartmentalization and signaling nanoclusters (Review) Mol. Membr. Biol. 2009;26:80–92. doi: 10.1080/09687680802649582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rotblat B., Prior I.A., Muncke C., Parton R.G., Kloog Y., Henis Y., Hancock J.F. Three Separable Domains Regulate GTP-Dependent Association of H-ras with the Plasma Membrane. Mol. Cell. Biol. 2004;24:6799–6810. doi: 10.1128/MCB.24.15.6799-6810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prior I.A., Harding A., Yan J., Sluimer J., Parton R.G., Hancock J.F. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat. Cell Biol. 2001;3:368–375. doi: 10.1038/35070050. [DOI] [PubMed] [Google Scholar]
- 91.Weise K., Triola G., Brunsveld L., Waldmann H., Winter R. Influence of the Lipidation Motif on the Partitioning and Association of N-Ras in Model Membrane Subdomains. J. Am. Chem. Soc. 2009;131:1557–1564. doi: 10.1021/ja808691r. [DOI] [PubMed] [Google Scholar]
- 92.Tebar F., Enrich C., Rentero C., Grewal T. GTPases Rac1 and Ras Signaling from Endosomes. Mol. Evol. Evid. Monophyly Metazoa. 2018;57:65–105. doi: 10.1007/978-3-319-96704-2_3. [DOI] [PubMed] [Google Scholar]
- 93.Cristea S., Sage J. Is the Canonical RAF/MEK/ERK Signaling Pathway a Therapeutic Target in SCLC? J. Thorac. Oncol. 2016;11:1233–1241. doi: 10.1016/j.jtho.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goldsmith Z.G., Dhanasekaran D.N. G Protein regulation of MAPK networks. Oncogene. 2007;26:3122–3142. doi: 10.1038/sj.onc.1210407. [DOI] [PubMed] [Google Scholar]
- 95.Dubrovska A., Cojoc M., Peitzsch C., Trautmann F., Polishchuk L., Telegeev G.D. Emerging targets in cancer management: Role of the CXCL12/CXCR4 axis. OncoTargets Ther. 2013;6:1347–1361. doi: 10.2147/OTT.S36109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gesty-Palmer D., Chen M., Reiter E., Ahn S., Nelson C.D., Wang S., Eckhardt A.E., Cowan C.L., Spurney R.F., Luttrell L.M., et al. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone recep-tor-stimulated ERK1/2 activation. J. Biol. Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 97.Vossler M.R., Yao H., York R.D., Pan M.-G., Rim C.S., Stork P.J. cAMP Activates MAP Kinase and Elk-1 through a B-Raf- and Rap1-Dependent Pathway. Cell. 1997;89:73–82. doi: 10.1016/S0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 98.Winitz S., Russell M., Qian N.X., Gardner A., Dwyer L., Johnson G.L. Involvement of Ras and Raf in the Gi-coupled acetylcholine muscarinic m2 receptor activation of mito-gen-activated protein (MAP) kinase kinase and MAP kinase. J. Biol. Chem. 1993;268:19196–19199. doi: 10.1016/S0021-9258(19)36498-1. [DOI] [PubMed] [Google Scholar]
- 99.Della Rocca G.J., van Biesen T., Daaka Y., Luttrell D.K., Luttrell L., Lefkowitz R.J. Ras-dependent Mitogen-activated Protein Kinase Activation by G Protein-coupled Receptors. J. Biol. Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 100.Gutkind J.S. The Pathways Connecting G Protein-coupled Receptors to the Nucleus through Divergent Mitogen-activated Protein Kinase Cascades. J. Biol. Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 101.Kwan D.H., Yung L.Y., Ye R.D., Wong Y.H. Activation of ras-dependent signaling pathways by G 14 -coupled receptors requires the adaptor protein TPR. J. Cell. Biochem. 2012;113:3486–3497. doi: 10.1002/jcb.24225. [DOI] [PubMed] [Google Scholar]
- 102.Nugent A., Proia R.L. The role of G protein-coupled receptors in lymphoid malignancies. Cell. Signal. 2017;39:95–107. doi: 10.1016/j.cellsig.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mashiko M., Kurosawa A., Tani Y., Tsuji T., Takeda S. GPR31 and GPR151 are activated under acidic conditions. J. Biochem. 2019;166:317–322. doi: 10.1093/jb/mvz042. [DOI] [PubMed] [Google Scholar]
- 104.Fehrenbacher N., Philips M.R. Targeting RAS—Will GPR31 deliver us a new path forward? Mol. Cell. Oncol. 2017;4:e1359228. doi: 10.1080/23723556.2017.1359228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fehrenbacher N., Da Silva I.T., Ramirez C., Zhou Y., Cho K.-J., Kuchay S., Shi J., Thomas S., Pagano M., Hancock J., et al. The G protein–coupled receptor GPR31 promotes membrane association of KRAS. J. Cell Biol. 2017;216:2329–2338. doi: 10.1083/jcb.201609096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li H., Eishingdrelo A., Kongsamut S., Eishingdrelo H. G-protein-coupled receptors mediate 14-3-3 signal transduction. Signal. Transduct. Target. Ther. 2016;1:16018. doi: 10.1038/sigtrans.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McCudden C.R., Hains M.D., Kimple R.J., Siderovski D., Willard F.S. G-protein signaling: Back to the future. Cell. Mol. Life Sci. 2005;62:551–577. doi: 10.1007/s00018-004-4462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ito A., Satoh T., Kaziro Y., Itoh H. G protein βγ subunit activates Ras, Raf, and MAP kinase in HEK 293 cells. FEBS Lett. 1995;368:183–187. doi: 10.1016/0014-5793(95)00643-N. [DOI] [PubMed] [Google Scholar]
- 109.Harden T.K. G Protein-dependent Regulation of Phospholipase C by Cell Surface Receptors. Am. Rev. Respir. Dis. 1990;141:119–122. doi: 10.1164/ajrccm/141.3_Pt_2.S119. [DOI] [PubMed] [Google Scholar]
- 110.Bivona T.G., Quatela S.E., Bodemann B.O., Ahearn I.M., Soskis M.J., Mor A., Miura J., Wiener H.H., Wright L., Saba S.G., et al. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mito-chondria and induces apoptosis. Mol. Cell. 2006;21:481–493. doi: 10.1016/j.molcel.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 111.Alvarez-Moya B., López-Alcalá C., Drosten M., Bachs O., Agell N. K-Ras4B phosphorylation at Ser181 is inhibited by calmodulin and modulates K-Ras activity and function. Oncogene. 2010;29:5911–5922. doi: 10.1038/onc.2010.298. [DOI] [PubMed] [Google Scholar]
- 112.Kohno T., Matsuda E., Sasaki H., Sasaki T. Protein-tyrosine kinase CAKbeta/PYK2 is activated by binding Ca2+/calmodulin to FERM F2 alpha2 helix and thus forming its dimer. Biochem. J. 2008;410:513–523. doi: 10.1042/BJ20070665. [DOI] [PubMed] [Google Scholar]
- 113.Blaukat A., Ivankovic-Dikic I., Gronroos E., Dolfi F., Tokiwa G., Vuori K., Dikic I. Adaptor Proteins Grb2 and Crk Couple Pyk2 with Activation of Specific Mitogen-activated Protein Kinase Cascades. J. Biol. Chem. 1999;274:14893–14901. doi: 10.1074/jbc.274.21.14893. [DOI] [PubMed] [Google Scholar]
- 114.Guo F.F., Kumahara E., Saffen D. A CalDAG-GEFI/Rap1/B-Raf cassette couples M(1) muscarinic acetylcholine receptors to the activation of ERK1/2. J. Biol. Chem. 2001;276:25568–25581. doi: 10.1074/jbc.M101277200. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Y.L., Wang R.C., Cheng K., Ring B.Z., Su L. Roles of Rap1 signaling in tumor cell migration and invasion. Cancer. Biol. Med. 2017;14:90–99. doi: 10.20892/j.issn.2095-3941.2016.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feng H., Liu K.W., Guo P., Zhang P., Cheng T., McNiven M.A., Johnson G.R., Hu B., Cheng S.Y. Dynamin 2 mediates PDGFRalpha-SHP-2-promoted glioblastoma growth and invasion. Oncogene. 2012;31:2691–2702. doi: 10.1038/onc.2011.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wunderlich L., Faragó A., Buday L. Characterization of Interactions of Nck with Sos and Dynamin. Cell. Signal. 1999;11:25–29. doi: 10.1016/S0898-6568(98)00027-8. [DOI] [PubMed] [Google Scholar]
- 118.Kranenburg O., Verlaan I., Hordijk P.L., Moolenaar W.H. Gi-mediated activation of the Ras/MAP kinase pathway involves a 100 kDa tyrosine-phosphorylated Grb2 SH3 binding protein, but not Src nor Shc. EMBO J. 1997;16:3097–3105. doi: 10.1093/emboj/16.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kranenburg O., Verlaan I., Moolenaar W.H. Gi-mediated tyrosine phosphorylation of Grb2 (growth-factor-receptor-bound protein 2)-bound dynamin-II by lysophosphatidic acid. Pt 1Biochem. J. 1999;339:11–14. doi: 10.1042/bj3390011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sassone-Corsi P. The cyclic AMP pathway. Cold Spring Harb. Perspect. Biol. 2012;4:a011148. doi: 10.1101/cshperspect.a011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ster J., De Bock F., Guerineau N., Janossy A., Barrere-Lemaire S., Bos J.L., Bockaert J., Fagni L. Exchange protein activated by cAMP (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2+-dependent K+ channels in cerebellar neurons. Proc. Natl. Acad. Sci. USA. 2007;104:2519–2524. doi: 10.1073/pnas.0611031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schmitt J.M., Stork P.J. PKA phosphorylation of Src mediates cAMP’s inhibition of cell growth via Rap1. Mol. Cell. 2002;9:85–94. doi: 10.1016/S1097-2765(01)00432-4. [DOI] [PubMed] [Google Scholar]
- 123.Schramm K., Niehof M., Radziwill G., Rommel C., Moelling K. Phosphorylation of c-Raf-1 by Protein Kinase A Interferes with Activation. Biochem. Biophys. Res. Commun. 1994;201:740–747. doi: 10.1006/bbrc.1994.1763. [DOI] [PubMed] [Google Scholar]
- 124.Umeda K., Negishi M., Katoh H. RasGRF1 mediates brain-derived neurotrophic factor-induced axonal growth in primary cultured cortical neurons. Biochem. Biophys. Rep. 2018;17:56–64. doi: 10.1016/j.bbrep.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu H., Nazmun N., Hassan S., Liu X., Yang J. BRAF mutation and its inhibitors in sarcoma treatment. Cancer Med. 2020;9:4881–4896. doi: 10.1002/cam4.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park E., Rawson S., Li K., Kim B.W., Ficarro S.B., Gonzalez-Del Pino G., Sharif H., Marto J.A., Jeon H., Eck M.J. Architecture of autoinhibited and active BRAF-MEK1-14-3-3 complexes. Nature. 2019;575:545–550. doi: 10.1038/s41586-019-1660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cutler R.E., Stephens R.M., Saracino M.R., Morrison D.K. Autoregulation of the Raf-1 serine/threonine kinase. Proc. Natl. Acad. Sci. USA. 1998;95:9214–9219. doi: 10.1073/pnas.95.16.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Obsilova V., Obsil T. The 14-3-3 Proteins as Important Allosteric Regulators of Protein Kinases. Int. J. Mol. Sci. 2020;21:8824. doi: 10.3390/ijms21228824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liau N.P.D., Venkatanarayan A., Quinn J.G., Phung W., Malek S., Hymowitz S.G., Sudhamsu J. Dimerization Induced by C-Terminal 14–3–3 Binding Is Sufficient for BRAF Kinase Activation. Biochemistry. 2020;59:3982–3992. doi: 10.1021/acs.biochem.0c00517. [DOI] [PubMed] [Google Scholar]
- 130.Pennington K.L., Chan T.Y., Torres M., Andersen J.L. The dynamic and stress-adaptive signaling hub of 14-3-3: Emerging mechanisms of regulation and context-dependent protein–protein interactions. Oncogene. 2018;37:5587–5604. doi: 10.1038/s41388-018-0348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yaffe M.B. How do 14-3-3 proteins work?—Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 2002;513:53–57. doi: 10.1016/S0014-5793(01)03288-4. [DOI] [PubMed] [Google Scholar]
- 132.Dhillon A.S., Pollock C., Steen H., Shaw P.E., Mischak H., Kolch W. Cyclic AMP-Dependent Kinase Regulates Raf-1 Kinase Mainly by Phosphorylation of Serine. Mol. Cell. Biol. 2002;22:3237–3246. doi: 10.1128/mcb.22.10.3237-3246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rommel C., Clarke B.A., Zimmermann S., Nuñez L., Rossman R., Reid K., Moelling K., Yancopoulos G.D., Glass D.J. Differentiation Stage-Specific Inhibition of the Raf-MEK-ERK Pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 134.Zimmermann S., Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 135.Yuan J., Ng W.H., Yap J., Chia B., Huang X., Wang M., Hu J. The AMPK inhibitor overcomes the paradoxical effect of RAF inhibitors through blocking phospho–Ser-621 in the C terminus of CRAF. J. Biol. Chem. 2018;293:14276–14284. doi: 10.1074/jbc.RA118.004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yuan J., Dong X., Yap J., Hu J. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020;13:113. doi: 10.1186/s13045-020-00949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shen C.-H., Yuan P., Perez-Lorenzo R., Zhang Y., Lee S.X., Ou Y., Asara J.M., Cantley L., Zheng B. Phosphorylation of BRAF by AMPK Impairs BRAF-KSR1 Association and Cell Proliferation. Mol. Cell. 2013;52:161–172. doi: 10.1016/j.molcel.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Romano D., Nguyen L., Matallanas D., Halasz M., Doherty C., Kholodenko B., Kolch W. Protein interaction switches coordinate Raf-1 and MST2/Hippo signalling. Nat. Cell Biol. 2014;16:673–684. doi: 10.1038/ncb2986. [DOI] [PubMed] [Google Scholar]
- 139.Tran T.H., Chan A.H., Young L.C., Bindu L., Neale C., Messing S., Dharmaiah S., Taylor T., Denson J.-P., Esposito D., et al. KRAS interaction with RAF1 RAS-binding domain and cysteine-rich domain provides insights into RAS-mediated RAF activation. Nat. Commun. 2021;12:1–16. doi: 10.1038/s41467-021-21422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Luo Z., Diaz B., Marshall M.S., Avruch J. An intact Raf zinc finger is required for optimal binding to processed Ras and for ras-dependent Raf activation in situ. Mol. Cell. Biol. 1997;17:46–53. doi: 10.1128/MCB.17.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abraham D., Podar K., Pacher M., Kubicek M., Welzel N., Hemmings B.A., Dilworth S.M., Mischak H., Kolch W., Baccarini M. Raf-1-associated Protein Phosphatase 2A as a Positive Regulator of Kinase Activation. J. Biol. Chem. 2000;275:22300–22304. doi: 10.1074/jbc.M003259200. [DOI] [PubMed] [Google Scholar]
- 142.Jaumot M., Hancock J.F. Protein phosphatases 1 and 2A promote Raf-1 activation by regulating 14-3-3 interactions. Oncogene. 2001;20:3949–3958. doi: 10.1038/sj.onc.1204526. [DOI] [PubMed] [Google Scholar]
- 143.Ory S., Zhou M., Conrads T.P., Veenstra T.D., Morrison D.K. Protein Phosphatase 2A Positively Regulates Ras Signaling by Dephosphorylating KSR1 and Raf-1 on Critical 14-3-3 Binding Sites. Curr. Biol. 2003;13:1356–1364. doi: 10.1016/S0960-9822(03)00535-9. [DOI] [PubMed] [Google Scholar]
- 144.Young L.C., Hartig N., del Río I.B., Sari S., Ringham-Terry B., Wainwright J.R., Jones G.G., McCormick F., Rodriguez-Viciana P. SHOC2–MRAS–PP1 complex positively regulates RAF activity and contributes to Noonan syndrome pathogenesis. Proc. Natl. Acad. Sci. USA. 2018;115:E10576–E10585. doi: 10.1073/pnas.1720352115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yin Q., Han T., Fang B., Zhang G., Zhang C., Roberts E.R., Izumi V., Zheng M., Jiang S., Yin X., et al. K27-linked ubiquitination of BRAF by ITCH engages cytokine response to maintain MEK-ERK signaling. Nat. Commun. 2019;10:1–15. doi: 10.1038/s41467-019-09844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Abankwa D., Gorfe A. Mechanisms of Ras Membrane Organization and Signaling: Ras Rocks Again. Biomolecules. 2020;10:1522. doi: 10.3390/biom10111522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hu J., Stites E.C., Yu H., Germino E.A., Meharena H.S., Stork P.J., Kornev A., Taylor S.S., Shaw A.S. Allosteric Activation of Functionally Asymmetric RAF Kinase Dimers. Cell. 2013;154:1036–1046. doi: 10.1016/j.cell.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Drosten M., Sum E.Y.M., Lechuga C.G., Simón-Carrasco L., Jacob H.K.C., García-Medina R., Huang S., Beijersbergen R., Bernards R., Barbacid M. Loss of p53 induces cell proliferation via Ras-independent activation of the Raf/Mek/Erk signaling pathway. Proc. Natl. Acad. Sci. USA. 2014;111:15155–15160. doi: 10.1073/pnas.1417549111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Drosten M., Barbacid M. Ras and p53: An unsuspected liaison. Mol. Cell. Oncol. 2015;3:e996001. doi: 10.1080/23723556.2014.996001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Serrano M., Lin A.W., McCurrach M.E., Beach D., Lowe S.W. Oncogenic ras Provokes Premature Cell Senescence Associated with Accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 151.Zhang X., Qi Z., Yin H., Yang G. Interaction between p53 and Ras signaling controls cisplatin resistance via HDAC4- and HIF-1alpha-mediated reg-ulation of apoptosis and autophagy. Theranostics. 2019;9:1096–1114. doi: 10.7150/thno.29673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lu L., Zeng J. Evaluation of K-ras and p53 expression in pancreatic adenocarcinoma using the cancer genome atlas. PLoS ONE. 2017;12:e0181532. doi: 10.1371/journal.pone.0181532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ries S., Biederer C., Woods D., Shifman O., Shirasawa S., Sasazuki T., McMahon M., Oren M., McCormick F. Opposing Effects of Ras on p53: Transcriptional Activation of mdm2 and Induction of p19ARF. Cell. 2000;103:321–330. doi: 10.1016/S0092-8674(00)00123-9. [DOI] [PubMed] [Google Scholar]
- 154.Sui X., Shin S., Zhang R., Firozi P.F., Yang L., Abbruzzese J.L., Reddy S.A.G. Hdm2 is regulated by K-Ras and mediates p53-independent functions in pancreatic cancer cells. Oncogene. 2008;28:709–720. doi: 10.1038/onc.2008.423. [DOI] [PubMed] [Google Scholar]
- 155.Esteller M., Tortola S., Toyota M., Capella G., Peinado M.A., Baylin S.B., Herman J.G. Hypermethylation-associated inactivation of p14(ARF) is independent of p16(INK4a) methylation and p53 mutational status. Cancer Res. 2000;60:129–133. [PubMed] [Google Scholar]
- 156.Li L., Zhao G.-D., Shi Z., Qi L.-L., Zhou L.-Y., Fu Z.-X. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol. Lett. 2016;12:3045–3050. doi: 10.3892/ol.2016.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Prior I.A., Lewis P.D., Mattos C. A Comprehensive Survey of Ras Mutations in Cancer. Cancer Res. 2012;72:2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hussain M.R.M., Baig M., Mohamoud H.S.A., Ul-Haq Z., Hoessli D.C., Khogeer G.S., Al-Sayed R.R., Al-Aama J.Y. BRAF gene: From human cancers to developmental syndromes. Saudi J. Biol. Sci. 2015;22:359–373. doi: 10.1016/j.sjbs.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aoki Y., Niihori T., Inoue S.-I., Matsubara Y. Recent advances in RASopathies. J. Hum. Genet. 2016;61:33–39. doi: 10.1038/jhg.2015.114. [DOI] [PubMed] [Google Scholar]
- 160.Prior I.A., Hood F.E., Hartley J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020;80:2969–2974. doi: 10.1158/0008-5472.CAN-19-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hobbs A., Der C.J., Rossman K.L. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 2016;129:1287–1292. doi: 10.1242/jcs.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Khrenova M., Mironov V., Grigorenko B., Nemukhin A.V. Modeling the Role of G12V and G13V Ras Mutations in the Ras-GAP-Catalyzed Hydrolysis Reaction of Guanosine Triphosphate. Biochemistry. 2014;53:7093–7099. doi: 10.1021/bi5011333. [DOI] [PubMed] [Google Scholar]
- 163.Greenberg P.L., Stone R.M., Al-Kali A., Barta S.K., Bejar R., Bennett J.M., Carraway H., De Castro C.M., Deeg H.J., DeZern A.E., et al. Myelodysplastic Syndromes, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016;15:60–87. doi: 10.6004/jnccn.2017.0007. [DOI] [PubMed] [Google Scholar]
- 164.Steensma D.P. Myelodysplastic syndromes current treatment algorithm. Blood Cancer J. 2018;8:1–7. doi: 10.1038/s41408-018-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ma X. Epidemiology of Myelodysplastic Syndromes. Am. J. Med. 2012;125:S2–S5. doi: 10.1016/j.amjmed.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bos J.L. ras oncogenes in human cancer: A review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 167.Kadia T.M., Kantarjian H., Kornblau S., Borthakur G., Faderl S., Freireich E.J., Luthra R., Garcia-Manero G., Pierce S., Cortes J., et al. Clinical and proteomic characterization of acute myeloid leukemia with mutated RAS. Cancer. 2012;118:5550–5559. doi: 10.1002/cncr.27596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tallman M.S., Wang E.S., Altman J.K., Appelbaum F.R., Bhatt V.R., Bixby D., Coutre S.E., De Lima M., Fathi A.T., Fiorella M., et al. Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019;17:721–749. doi: 10.6004/jnccn.2019.0028. [DOI] [PubMed] [Google Scholar]
- 169.DeSantis C.E., Lin C.C., Mariotto A.B., Siegel R.L., Stein K.D., Kramer J.L., Alteri R., Robbins A.S., Jemal A. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 170.Neubauer A., Maharry K., Mrózek K., Thiede C., Marcucci G., Paschka P., Mayer R.J., Larson R.A., Liu E.T., Bloomfield C.D. Patients with Acute Myeloid Leukemia and RAS Mutations Benefit Most From Postremission High-Dose Cytarabine: A Cancer and Leukemia Group B Study. J. Clin. Oncol. 2008;26:4603–4609. doi: 10.1200/JCO.2007.14.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brown P., Inaba H., Annesley C., Beck J., Colace S., Dallas M., DeSantes K., Kelly K., Kitko C., Lacayo N., et al. Pediatric Acute Lymphoblastic Leukemia, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020;18:81–112. doi: 10.6004/jnccn.2020.0001. [DOI] [PubMed] [Google Scholar]
- 172.Jaime-Pérez J.C., Jiménez-Castillo R.A., Herrera-Garza J.L., Gutiérrez-Aguirre H., Marfil-Rivera L.J., Gómez-Almaguer D. Survival Rates of Adults with Acute Lymphoblastic Leukemia in a Low-Income Population: A Decade of Experience at a Single Institution in Mexico. Clin. Lymphoma Myeloma Leuk. 2017;17:60–68. doi: 10.1016/j.clml.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 173.Knight T., Irving J.A. Ras/Raf/MEK/ERK Pathway Activation in Childhood Acute Lymphoblastic Leukemia and Its Therapeutic Targeting. Front. Oncol. 2014;4:160. doi: 10.3389/fonc.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gustafsson B., Angelini S., Sander B., Christensson B., Hemminki K., Kumar R. Mutations in the BRAF and N-ras genes in childhood acute lymphoblastic leukaemia. Leukemia. 2004;19:310–312. doi: 10.1038/sj.leu.2403589. [DOI] [PubMed] [Google Scholar]
- 175.Pleyer L., Germing U., Sperr W.R., Linkesch W., Burgstaller S., Stauder R., Girschikofsky M., Schreder M., Pfeilstocker M., Lang A.H., et al. Azacitidine in CMML: Matched-pair analyses of daily-life patients reveal modest effects on clinical course and survival. Leuk. Res. 2014;38:475–483. doi: 10.1016/j.leukres.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 176.Gelsi-Boyer V., Trouplin V., Adélaïde J., Aceto N., Remy V., Pinson S., Houdayer C., Arnoulet C., Sainty D., Bentires-Alj M., et al. Genome profiling of chronic myelomonocytic leukemia: Frequent alterations of RAS and RUNX1genes. BMC Cancer. 2008;8:299. doi: 10.1186/1471-2407-8-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Onida F., Kantarjian H.M., Smith T.L., Ball G., Keating M.J., Estey E.H., Glassman A.B., Albitar M., Kwari M.I., Beran M. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: A retrospective analysis of 213 patients. Blood. 2002;99:840–849. doi: 10.1182/blood.V99.3.840. [DOI] [PubMed] [Google Scholar]
- 178.Patnaik M.M., Tefferi A. Cytogenetic and molecular abnormalities in chronic myelomonocytic leukemia. Blood Cancer J. 2016;6:e393. doi: 10.1038/bcj.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang L., Singh R.R., Patel K.P., Stingo F., Routbort M., You M.J., Miranda R.N., Garcia-Manero G., Kantarjian H.M., Medeiros L.J., et al. BRAF kinase domain mutations are present in a subset of chronic myelomonocytic leukemia with wild-type RAS. Am. J. Hematol. 2014;89:499–504. doi: 10.1002/ajh.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Radich J.P., Deininger M., Abboud C.N., Altman J.K., Berman E., Bhatia R., Bhatnagar B., Curtin P., DeAngelo D.J., Gotlib J., et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018;16:1108–1135. doi: 10.6004/jnccn.2018.0071. [DOI] [PubMed] [Google Scholar]
- 181.Liu E., Hjelle B., Bishop J.M. Transforming genes in chronic myelogenous leukemia. Proc. Natl. Acad. Sci. USA. 1988;85:1952–1956. doi: 10.1073/pnas.85.6.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tempero M.A., Malafa M.P., Chiorean E.G., Czito B., Scaife C., Narang A.K., Fountzilas C., Wolpin B.M., Al-Hawary M., Asbun H., et al. Pancreatic Adenocarcinoma, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019;17:202–210. doi: 10.6004/jnccn.2019.0014. [DOI] [PubMed] [Google Scholar]
- 183.Le N., Sund M., Vinci A., Beyer G., Javed M.A., Krug S., Neessee A., Schober M. Prognostic and predictive markers in pancreatic adenocarcinoma. Dig. Liver Dis. 2016;48:223–230. doi: 10.1016/j.dld.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 184.Caldas C., Kern S.E. K-ras mutation and pancreatic adenocarcinoma. Int. J. Pancreatol. 1995;18:1–6. doi: 10.1007/BF02825415. [DOI] [PubMed] [Google Scholar]
- 185.Schultz N.A., Roslind A., Christensen I.J., Horn T., Høgdall E., Pedersen L.N., Kruhøffer M., Burcharth F., Wøjdemann M., Johansen J.S. Frequencies and Prognostic Role of KRAS and BRAF Mutations in Patients with Localized Pancreatic and Ampullary Adenocarcinomas. Pancreas. 2012;41:759–766. doi: 10.1097/MPA.0b013e31823cd9df. [DOI] [PubMed] [Google Scholar]
- 186.Davis L.E., Shalin S.C., Tackett A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019;20:1366–1379. doi: 10.1080/15384047.2019.1640032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 188.Garnett M.J., Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6:313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 189.Ball N.J., Yohn J.J., Morelli J.G., Norris D.A., Golitz L.E., Hoeffler J.P. RAS Mutations in Human Melanoma: A Marker of Malignant Progression. J. Investig. Dermatol. 1994;102:285–290. doi: 10.1111/1523-1747.ep12371783. [DOI] [PubMed] [Google Scholar]
- 190.Duma N., Santana-Davila R., Molina J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 191.Ettinger D.S., Wood D.E., Aggarwal C., Aisner D.L., Akerley W., Bauman J.R., Bharat A., Bruno D.S., Chang J.Y., Chirieac L.R., et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J. Natl. Compr. Cancer Netw. 2019;17:1464–1472. doi: 10.6004/jnccn.2019.0059. [DOI] [PubMed] [Google Scholar]
- 192.Riely G.J., Marks J., Pao W. KRAS Mutations in Non-Small Cell Lung Cancer. Proc. Am. Thorac. Soc. 2009;6:201–205. doi: 10.1513/pats.200809-107LC. [DOI] [PubMed] [Google Scholar]
- 193.Sánchez-Torres J.M., Viteri S., Molina M.A., Rosell R. BRAF mutant non-small cell lung cancer and treatment with BRAF inhibitors. Transl. Lung Cancer Res. 2013;2:244–250. doi: 10.3978/j.issn.2218-6751.2013.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mitsudomi T., Viallet J., Mulshine J., Linnoila R.I., Minna J.D., Gazdar A.F. Mutations of ras genes distinguish a subset of non-small-cell lung cancer cell lines from small-cell lung cancer cell lines. Oncogene. 1991;6:1353–1362. [PubMed] [Google Scholar]
- 195.Molina J.R., Yang P., Cassivi S.D., Schild S.E., Adjei A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008;83:584–594. doi: 10.1016/S0025-6196(11)60735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gustavsson B., Carlsson G., Machover D., Petrelli N., Roth A., Schmoll H.-J., Tveit K.-M., Gibson F. A Review of the Evolution of Systemic Chemotherapy in the Management of Colorectal Cancer. Clin. Color. Cancer. 2015;14:1–10. doi: 10.1016/j.clcc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 197.Breivik J., Meling G.I., Spurkland A., Rognum T.O., Gaudernack G. K-ras mutation in colorectal cancer: Relations to patient age, sex and tumour location. Br. J. Cancer. 1994;69:367–371. doi: 10.1038/bjc.1994.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Oldenburg J., Fosså S.D., Nuver J., Heidenreich A., Schmoll H.-J., Bokemeyer C., Horwich A., Beyer J., Kataja V. Testicular seminoma and non-seminoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013;24:vi125–vi132. doi: 10.1093/annonc/mdt304. [DOI] [PubMed] [Google Scholar]
- 199.Dong W., Gang W., Liu M., Zhang H. Analysis of the prognosis of patients with testicular seminoma. Oncol. Lett. 2016;11:1361–1366. doi: 10.3892/ol.2015.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mulder M.P., Keijzer W., Verkerk A., Boot A.J., Prins M.E., Splinter T.A., Bos J.L. Activated ras genes in human seminoma: Evidence for tumor heterogeneity. Oncogene. 1989;4:1345–1351. [PubMed] [Google Scholar]
- 201.DeGeorge K.C., Holt H.R., Hodges S.C. Bladder Cancer: Diagnosis and Treatment. Am. Fam. Physician. 2017;96:507–514. [PubMed] [Google Scholar]
- 202.Abdollah F., Gandaglia G., Thuret R., Schmitges J., Tian Z., Jeldres C., Passoni N.M., Briganti A., Shariat S.F., Perrotte P., et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: A trend analysis. Cancer Epidemiol. 2013;37:219–225. doi: 10.1016/j.canep.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 203.Oxford G., Theodorescu D. Review Article: The Role of Ras Superfamily Proteins in Bladder Cancer Progression. J. Urol. 2003;170:1987–1993. doi: 10.1097/01.ju.0000088670.02905.78. [DOI] [PubMed] [Google Scholar]
- 204.Inamura K. Bladder Cancer: New Insights into Its Molecular Pathology. Cancers. 2018;10:100. doi: 10.3390/cancers10040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bosetti C., Turati F., La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pr. Res. Clin. Gastroenterol. 2014;28:753–770. doi: 10.1016/j.bpg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 206.Taketomi A., Shirabe K., Muto J., Yoshiya S., Motomura T., Mano Y., Ikegami T., Yoshizumi T., Sugio K., Maehara Y. A rare point mutation in the Ras oncogene in hepatocellular carcinoma. Surg. Today. 2013;43:289–292. doi: 10.1007/s00595-012-0462-8. [DOI] [PubMed] [Google Scholar]
- 207.Orr B., Edwards R.P. Diagnosis and Treatment of Ovarian Cancer. Hematol. Clin. North. Am. 2018;32:943–964. doi: 10.1016/j.hoc.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 208.Protani M.M., Nagle C.M., Webb P. Obesity and Ovarian Cancer Survival: A Systematic Review and Meta-analysis. Cancer Prev. Res. 2012;5:901–910. doi: 10.1158/1940-6207.CAPR-12-0048. [DOI] [PubMed] [Google Scholar]
- 209.Alsharedi M., Katz H. Check point inhibitors a new era in renal cell carcinoma treatment. Med. Oncol. 2018;35:85. doi: 10.1007/s12032-018-1147-y. [DOI] [PubMed] [Google Scholar]
- 210.Motzer R.J., Jonasch E., Michaelson M.D., Nandagopal L., Gore J.L., George S., Alva A., Haas N., Harrison M.R., Plimack E.R., et al. NCCN Guidelines Insights: Kidney Cancer, Version 2.2020. J. Natl. Compr. Cancer Netw. 2019;17:1278–1285. doi: 10.6004/jnccn.2019.0054. [DOI] [PubMed] [Google Scholar]
- 211.Bayrak O., Sen H., Bulut E., Cengiz B., Karakok M., Erturhan S., Seckiner I. Evaluation of EGFR, KRAS and BRAF gene mutations in renal cell carcinoma. J. Kidney Cancer VHL. 2014;1:40–45. doi: 10.15586/jkcvhl.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wiesweg M., Kasper S., Worm K., Herold T., Reis H., Sara L., Metzenmacher M., Abendroth A., Darwiche K., Aigner C., et al. Impact of RAS mutation subtype on clinical outcome—A cross-entity comparison of patients with advanced non-small cell lung cancer and colorectal cancer. Oncogene. 2019;38:2953–2966. doi: 10.1038/s41388-018-0634-0. [DOI] [PubMed] [Google Scholar]
- 213.Terrell E.M., Durrant D.E., Ritt D.A., Sealover N.E., Sheffels E., Spencer-Smith R., Esposito D., Zhou Y., Hancock J.F., Kortum R., et al. Distinct Binding Preferences between Ras and Raf Family Members and the Impact on Oncogenic Ras Signaling. Mol. Cell. 2019;76:872–884.e5. doi: 10.1016/j.molcel.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Barbacid M. ras oncogenes: Their role in neoplasia. Eur. J. Clin. Investig. 2008;20:225–235. doi: 10.1111/j.1365-2362.1990.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 215.Cook J.H., Melloni G.E.M., Gulhan D.C., Park P.J., Haigis K.M. The origins and genetic interactions of KRAS mutations are allele- and tissue-specific. Nat. Commun. 2021;12:1–14. doi: 10.1038/s41467-021-22125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nair S.G., Loppnow G.R. Comparison of K-Ras and N-Ras Mutagenic Hot Spots for UVC Damage. ACS Omega. 2019;4:3469–3475. doi: 10.1021/acsomega.8b03017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Holderfield M., Deuker M.M., McCormick F., McMahon M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat. Rev. Cancer. 2014;14:455–467. doi: 10.1038/nrc3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wan P.T., Garnett M.J., Roe S.M., Lee S., Niculescu-Duvaz D., Good V.M., Cancer Genome Project. Jones C.M., Marshall C.J., Springer C.J., et al. Mechanical acts RAF-Erk signal pathway by oncological mutations B-Raf. Cell. 2004;116:855–867. doi: 10.1016/S0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 219.Hanrahan A.J., Sylvester B.E., Chang M.T., ElZein A., Gao J., Han W., Liu Y., Xu D., Gao S.P., Gorelick A.N., et al. Leveraging Systematic Functional Analysis to Benchmark an In Silico Framework Distinguishes Driver from Passenger MEK Mutants in Cancer. Cancer Res. 2020;80:4233–4243. doi: 10.1158/0008-5472.CAN-20-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Greger J.G., Eastman S.D., Zhang V., Bleam M.R., Hughes A.M., Smitheman K.N., Dickerson S.H., Laquerre S.G., Liu L., Gilmer T.M. Combinations of BRAF, MEK, and PI3K/mTOR Inhibitors Overcome Acquired Resistance to the BRAF Inhibitor GSK2118436 Dabrafenib, Mediated by NRAS or MEK Mutations. Mol. Cancer Ther. 2012;11:909–920. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
- 221.Van Allen E.M., Wagle N., Sucker A., Treacy D.J., Johannessen C.M., Goetz E.M., Place C.S., Taylor-Weiner A., Whittaker S., Kryukov G.V., et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4:94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rodriguez-Viciana P., Rauen K.A. Biochemical characterization of novel germline BRAF and MEK mutations in car-dio-facio-cutaneous syndrome. Methods Enzymol. 2008;438:277–289. doi: 10.1016/S0076-6879(07)38019-1. [DOI] [PubMed] [Google Scholar]
- 223.Smorodinsky-Atias K., Soudah N., Engelberg D. Mutations That Confer Drug-Resistance, Oncogenicity and Intrinsic Activity on the ERK MAP Kinases—Current State of the Art. Cells. 2020;9:129. doi: 10.3390/cells9010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Emery C.M., Vijayendran K.G., Zipser M.C., Sawyer A.M., Niu L., Kim J.J., Hatton C., Chopra R., Oberholzer P.A., Karpova M.B., et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc. Natl. Acad. Sci. USA. 2009;106:20411–20416. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Goetz E.M., Ghandi M., Treacy D.J., Wagle N., Garraway L.A. ERK Mutations Confer Resistance to Mitogen-Activated Protein Kinase Pathway Inhibitors. Cancer Res. 2014;74:7079–7089. doi: 10.1158/0008-5472.CAN-14-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jaiswal B.S., Durinck S., Stawiski E.W., Yin J., Wang W., Lin E., Moffat J.G., Martin S.E., Modrusan Z., Seshagiri S. ERK Mutations and Amplification Confer Resistance to ERK-Inhibitor Therapy. Clin. Cancer Res. 2018;24:4044–4055. doi: 10.1158/1078-0432.CCR-17-3674. [DOI] [PubMed] [Google Scholar]
- 227.Phillips A.A., Harewood J.C.K. Adult T Cell Leukemia-Lymphoma (ATL): State of the Art. Curr. Hematol. Malign. Rep. 2018;13:300–307. doi: 10.1007/s11899-018-0458-6. [DOI] [PubMed] [Google Scholar]
- 228.Mehta-Shah N., Ratner L., Horwitz S.M. Adult T-Cell Leukemia/Lymphoma. J. Oncol. Pract. 2017;13:487–492. doi: 10.1200/JOP.2017.021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Poiesz B.J., Ruscetti F.W., Gazdar A.F., Bunn P.A., Minna J.D., Gallo R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gallo R.C. History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. Oncogene. 2005;24:5926–5930. doi: 10.1038/sj.onc.1208980. [DOI] [PubMed] [Google Scholar]
- 231.Yamagishi M., Watanabe T. Molecular Hallmarks of Adult T Cell Leukemia. Front. Microbiol. 2012;3:334. doi: 10.3389/fmicb.2012.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Krump N.A., You J. Molecular mechanisms of viral oncogenesis in humans. Nat. Rev. Genet. 2018;16:684–698. doi: 10.1038/s41579-018-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bangham C.R., Ratner L. How does HTLV-1 cause adult T-cell leukaemia/lymphoma (ATL)? Curr. Opin. Virol. 2015;14:93–100. doi: 10.1016/j.coviro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yasunaga J.-I. Strategies of Human T-Cell Leukemia Virus Type 1 for Persistent Infection: Implications for Leukemogenesis of Adult T-Cell Leukemia-Lymphoma. Front. Microbiol. 2020;11:979. doi: 10.3389/fmicb.2020.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Song C., Wang W., Li M., Liu Y., Zheng D. Tax1 enhances cancer cell proliferation via Ras-Raf-MEK-ERK signaling pathway. IUBMB Life. 2009;61:685–692. doi: 10.1002/iub.221. [DOI] [PubMed] [Google Scholar]
- 236.Kataoka K., Nagata Y., Kitanaka A., Shiraishi Y., Shimamura T., Yasunaga J.-I., Totoki Y., Chiba K., Sato-Otsubo A., Nagae G., et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015;47:1304–1315. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 237.Stoppa G., Rumiato E., Saggioro D. Ras signaling contributes to survival of human T-cell leukemia/lymphoma virus type 1 (HTLV-1) Tax-positive T-cells. Apoptosis. 2011;17:219–228. doi: 10.1007/s10495-011-0676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ziegler J.L. Burkitt’s Lymphoma. N. Engl. J. Med. 1981;305:735–745. doi: 10.1056/NEJM198109243051305. [DOI] [PubMed] [Google Scholar]
- 239.Dunleavy K., Little R.F., Wilson W.H. Update on Burkitt Lymphoma. Hematol. Clin. North. Am. 2016;30:1333–1343. doi: 10.1016/j.hoc.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 240.De-Thé G., Geser A., Day N.E., Tukei P.M., Williams E.H., Beri D.P., Smith P.G., Dean A.G., Bornkamm G.W., Feorino P., et al. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt’s lymphoma from Ugandan prospective study. Nature. 1978;274:756–761. doi: 10.1038/274756a0. [DOI] [PubMed] [Google Scholar]
- 241.Casulo C., Friedberg J. Treating Burkitt Lymphoma in Adults. Curr. Hematol. Malign. Rep. 2015;10:266–271. doi: 10.1007/s11899-015-0263-4. [DOI] [PubMed] [Google Scholar]
- 242.Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R.C., Croce C.M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc. Natl. Acad. Sci. USA. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang J., Meng L., Jiang W., Zhang H., Zhou A., Zeng N. Identification of clinical molecular targets for childhood Burkitt lymphoma. Transl. Oncol. 2020;13:100855. doi: 10.1016/j.tranon.2020.100855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Strati P., Nastoupil L.J., Davis R.E., Fayad L.E., Fowler N., Hagemeister F.B., Kwak L., Oki Y., Wang M., Westin J., et al. A phase 1 trial of alisertib and romidepsin for relapsed/refractory aggressive B-cell and T-cell lymphomas. Haematologica. 2019;105:e26–e28. doi: 10.3324/haematol.2019.220012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wever C.M., Geoffrion D., Grande B.M., Yu S., Alcaide M., Lemaire M., Riazalhosseini Y., Hébert J., Gavino C., Vinh D., et al. The genomic landscape of two Burkitt lymphoma cases and derived cell lines: Comparison between primary and relapse samples. Leuk. Lymphoma. 2018;59:2159–2174. doi: 10.1080/10428194.2017.1413186. [DOI] [PubMed] [Google Scholar]
- 246.Kim E., Viatour P. Hepatocellular carcinoma: Old friends and new tricks. Exp. Mol. Med. 2020;52:1898–1907. doi: 10.1038/s12276-020-00527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Balogh J., Victor D., III, Asham E.H., Burroughs S.G., Boktour M., Saharia A., Li X., Ghobrial R.M., Monsour H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Qi L.-N., Bai T., Chen Z.-S., Wu F.-X., Chen Y.-Y., De Xiang B., Peng T., Han Z.-G., Li L.-Q. The p53 mutation spectrum in hepatocellular carcinoma from Guangxi, China: Role of chronic hepatitis B virus infection and aflatoxin B1 exposure. Liver Int. 2014;35:999–1009. doi: 10.1111/liv.12460. [DOI] [PubMed] [Google Scholar]
- 249.Hou W., Liu J., Chen P., Wang H., Ye B.C., Qiang F. Mutation analysis of key genes in RAS/RAF and PI3K/PTEN pathways in Chinese patients with hepatocellular carcinoma. Oncol. Lett. 2014;8:1249–1254. doi: 10.3892/ol.2014.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hwang Y.H., Choi J.Y., Kim S., Chung E.S., Kim T., Koh S.S., Lee B., Bae S.H., Kim J., Park Y.M. Over-expression of c-raf-1 proto-oncogene in liver cirrhosis and hepatocellular carcinoma. Hepatol. Res. 2004;29:113–121. doi: 10.1016/j.hepres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 251.Chen L., Shi Y., Jiang C.Y., Wei L.X., Wang Y.L., Dai G.H. Expression and prognostic role of pan-Ras, Raf-1, pMEK1 and pERK1/2 in patients with hepatocellular carcinoma. Eur. J. Surg. Oncol. 2011;37:513–520. doi: 10.1016/j.ejso.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 252.Huynh H., Nguyen T.T.T., Chow K.-H.K.-P., Tan P.H., Soo K.C., Tran E. Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: Its role in tumor progression and apoptosis. BMC Gastroenterol. 2003;3:19. doi: 10.1186/1471-230X-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ito Y., Sasaki Y., Horimoto M., Wada S., Tanaka Y., Kasahara A., Ueki T., Hirano T., Yamamoto H., Fujimoto J., et al. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27:951–958. doi: 10.1002/hep.510270409. [DOI] [PubMed] [Google Scholar]
- 254.Yoshida T., Hisamoto T., Akiba J., Koga H., Nakamura K., Tokunaga Y., Hanada S., Kumemura H., Maeyama M., Harada M., et al. Spreds, inhibitors of the Ras/ERK signal transduction, are dysregulated in human hepatocellular carcinoma and linked to the malignant phenotype of tumors. Oncogene. 2006;25:6056–6066. doi: 10.1038/sj.onc.1209635. [DOI] [PubMed] [Google Scholar]
- 255.Vareedayah A.A., Alkaade S., Taylor J.R. Pancreatic Adenocarcinoma. MO. Med. 2018;115:230–235. [PMC free article] [PubMed] [Google Scholar]
- 256.Jonckheere N., Vasseur R., Van Seuningen I. The cornerstone K-RAS mutation in pancreatic adenocarcinoma: From cell sig-naling network, target genes, biological processes to therapeutic targeting. Crit. Rev. Oncol. Hematol. 2017;111:7–19. doi: 10.1016/j.critrevonc.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 257.Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014;371:2140–2141. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 258.Neuzillet C., Hammel P., Tijeras-Raballand A., Couvelard A., Raymond E. Targeting the Ras–ERK pathway in pancreatic adenocarcinoma. Cancer Metastasis Rev. 2012;32:147–162. doi: 10.1007/s10555-012-9396-2. [DOI] [PubMed] [Google Scholar]
- 259.Drosten M., Barbacid M. Targeting the MAPK Pathway in KRAS-Driven Tumors. Cancer Cell. 2020;37:543–550. doi: 10.1016/j.ccell.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 260.Goebel C., Louden C.L., McKenna R., Onugha O., Wachtel A., Long T. Diagnosis of Non-small Cell Lung Cancer for Early Stage Asymptomatic Patients. Cancer Genom. Proteom. 2019;16:229–244. doi: 10.21873/cgp.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kerr E.M., Gaude E., Turrell F.K., Frezza C., Martins C.P. Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities. Nat. Cell Biol. 2016;531:110–113. doi: 10.1038/nature16967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hensley C.T., Faubert B., Yuan Q., Lev-Cohain N., Jin E., Kim J., Jiang L., Ko B., Skelton R., Loudat L., et al. Metabolic Heterogeneity in Human Lung Tumors. Cell. 2016;164:681–694. doi: 10.1016/j.cell.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang X., Ricciuti B., Nguyen T., Li X., Rabin M.S., Awad M.M., Lin X., Johnson B.E., Christiani D.C. Association between Smoking History and Tumor Mutation Burden in Advanced Non–Small Cell Lung Cancer. Cancer Res. 2021;81:2566–2573. doi: 10.1158/0008-5472.CAN-20-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Dogan S., Shen R., Ang D.C., Johnson M.L., D’Angelo S.P., Paik P.K., Brzostowski E.B., Riely G.J., Kris M., Zakowski M.F., et al. Molecular Epidemiology of EGFR and KRAS Mutations in 3,026 Lung Adenocarcinomas: Higher Susceptibility of Women to Smoking-Related KRAS-Mutant Cancers. Clin. Cancer Res. 2012;18:6169–6177. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ihle N.T., Byers L.A., Kim E.S., Saintigny P., Lee J.J., Blumenschein G.R., Tsao A., Liu S., Larsen J., Wang J., et al. Effect of KRAS Oncogene Substitutions on Protein Behavior: Implications for Signaling and Clinical Outcome. J. Natl. Cancer Inst. 2012;104:228–239. doi: 10.1093/jnci/djr523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Meng D., Yuan M., Li X., Chen L., Yang J., Zhao X., Ma W., Xin J. Prognostic value of K-RAS mutations in patients with non-small cell lung cancer: A systematic review with meta-analysis. Lung Cancer. 2013;81:1–10. doi: 10.1016/j.lungcan.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 267.Yu H.A., Sima C.S., Shen R., Kass S., Gainor J., Shaw A., Hames M., Iams W., Aston J., Lovly C., et al. Prognostic Impact of KRAS Mutation Subtypes in 677 Patients with Metastatic Lung Adenocarcinomas. J. Thorac. Oncol. 2015;10:431–437. doi: 10.1097/JTO.0000000000000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shepherd F.A., Domerg C., Hainaut P., Jänne P.A., Pignon J.-P., Graziano S., Douillard J.-Y., Brambilla E., Le Chevalier T., Seymour L., et al. Pooled Analysis of the Prognostic and Predictive Effects of KRAS Mutation Status and KRAS Mutation Subtype in Early-Stage Resected Non–Small-Cell Lung Cancer in Four Trials of Adjuvant Chemotherapy. J. Clin. Oncol. 2013;31:2173–2181. doi: 10.1200/JCO.2012.48.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Glodde N., Hölzel M. RAS and PD-L1: A Masters’ Liaison in Cancer Immune Evasion. Immunity. 2017;47:1007–1009. doi: 10.1016/j.immuni.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 270.Coelho M.A., Trécesson S.D.C., Rana S., Zecchin D., Moore C., Molina-Arcas M., East P., Spencer-Dene B., Nye E., Barnouin K., et al. Oncogenic RAS Signaling Promotes Tumor Immunoresistance by Stabilizing PD-L1 mRNA. Immunity. 2017;47:1083–1099.e6. doi: 10.1016/j.immuni.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zdanov S., Mandapathil M., Abu Eid R., Adamson-Fadeyi S., Wilson W., Qian J., Carnie A., Tarasova N., Mkrtichyan M., Berzofsky J.A., et al. Mutant KRAS Conversion of Conventional T Cells into Regulatory T Cells. Cancer Immunol. Res. 2016;4:354–365. doi: 10.1158/2326-6066.CIR-15-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sumimoto H., Imabayashi F., Iwata T., Kawakami Y. The BRAF–MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Khalili J.S., Liu S., Rodríguez-Cruz T.G., Whittington M., Wardell S., Liu C., Zhang M., Cooper Z., Frederick D.T., Li Y., et al. Oncogenic BRAF(V600E) Promotes Stromal Cell-Mediated Immunosuppression Via Induction of Interleukin-1 in Melanoma. Clin. Cancer Res. 2012;18:5329–5340. doi: 10.1158/1078-0432.CCR-12-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Boni A., Cogdill A., Dang P., Udayakumar D., Njauw C.-N.J., Sloss C.M., Ferrone C.R., Flaherty K.T., Lawrence D.P., Fisher D.E., et al. Selective BRAFV600E Inhibition Enhances T-Cell Recognition of Melanoma without Affecting Lymphocyte Function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 275.Bradley S., Melendez B., Talukder A., Lizée G. Trouble at the core: BRAF(V600E) drives multiple modes of T-cell suppression in melanoma. Oncoimmunology. 2015;5:e1078966. doi: 10.1080/2162402X.2015.1078966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E., et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ribas A., Lawrence D., Atkinson V., Agarwal S., Miller W.H., Carlino M.S., Fisher R., Long G.V., Hodi F.S., Tsoi J., et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat. Med. 2019;25:936–940. doi: 10.1038/s41591-019-0476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Pratilas C.A., Hanrahan A.J., Halilovic E., Persaud Y., Soh J., Chitale D., Shigematsu H., Yamamoto H., Sawai A., Janakiraman M., et al. Genetic Predictors of MEK Dependence in Non–Small Cell Lung Cancer. Cancer Res. 2008;68:9375–9383. doi: 10.1158/0008-5472.CAN-08-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Karreth F.A., Frese K.K., DeNicola G., Baccarini M., Tuveson D.A. C-Raf Is Required for the Initiation of Lung Cancer by K-RasG12D. Cancer Discov. 2011;1:128–136. doi: 10.1158/2159-8290.CD-10-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Blasco R.B., Francoz S., Santamaría D., Cañamero M., Dubus P., Charron J., Baccarini M., Barbacid M. c-Raf, but Not B-Raf, Is Essential for Development of K-Ras Oncogene-Driven Non-Small Cell Lung Carcinoma. Cancer Cell. 2011;19:652–663. doi: 10.1016/j.ccr.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Takezawa K., Okamoto I., Yonesaka K., Hatashita E., Yamada Y., Fukuoka M., Nakagawa K. Sorafenib Inhibits Non–Small Cell Lung Cancer Cell Growth by Targeting B-RAF in KRAS Wild-Type Cells and C-RAF in KRAS Mutant Cells. Cancer Res. 2009;69:6515–6521. doi: 10.1158/0008-5472.CAN-09-1076. [DOI] [PubMed] [Google Scholar]
- 282.Hofmann M.H., Gmachl M., Ramharter J., Savarese F., Gerlach D., Marszalek J.R., Sanderson M.P., Kessler D., Trapani F., Arnhof H., et al. BI-3406, a Potent and Selective SOS1–KRAS Interaction Inhibitor, Is Effective in KRAS-Driven Cancers through Combined MEK Inhibition. Cancer Discov. 2021;11:142–157. doi: 10.1158/2159-8290.CD-20-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Koczywas M., Haura E., Janne P.A., Pacheco J.M., Ulahannan S., Wang J.S., Burris H.A., Riess J.W., McCoach C., Gordon M.S., et al. Abstract LB001: Anti-tumor activity and tolerability of the SHP2 inhibitor RMC-4630 as a single agent in patients with RAS-addicted solid cancers. Cancer Res. 2021;81 doi: 10.1158/1538-7445.AM2021-LB001. [DOI] [Google Scholar]
- 284.Karp J.E., Lancet J.E. Development of the farnesyltransferase inhibitor tipifarnib for therapy of hematologic malignancies. Futur. Oncol. 2005;1:719–731. doi: 10.2217/14796694.1.6.719. [DOI] [PubMed] [Google Scholar]
- 285.Van Cutsem E., Hidalgo M., Canon J., Macarulla T., Bazin I., Poddubskaya E., Manojlovic N., Radenkovic D., Verslype C., Raymond E., et al. Phase I/II trial of pimasertib plus gemcitabine in patients with metastatic pancreatic cancer. Int. J. Cancer. 2018;143:2053–2064. doi: 10.1002/ijc.31603. [DOI] [PubMed] [Google Scholar]
- 286.Ho A.L., Brana I., Haddad R., Bauman J., Bible K., Oosting S., Wong D.J., Ahn M.-J., Boni V., Even C., et al. Tipifarnib in Head and Neck Squamous Cell Carcinoma with HRAS Mutations. J. Clin. Oncol. 2021;39:1856–1864. doi: 10.1200/JCO.20.02903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Untch B.R., Dos Anjos V., Garcia-Rendueles M.E.R., Knauf J.A., Krishnamoorthy G.P., Saqcena M., Bhanot U.K., Socci N.D., Ho A.L., Ghossein R., et al. Tipifarnib Inhibits HRAS-Driven Dedifferentiated Thyroid Cancers. Cancer Res. 2018;78:4642–4657. doi: 10.1158/0008-5472.CAN-17-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Dunnett-Kane V., Nicola P., Blackhall F., Lindsay C. Mechanisms of Resistance to KRAS(G12C) Inhibitors. Cancers. 2021;13:151. doi: 10.3390/cancers13010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Witkiewicz A.K., McMillan E.A., Balaji U., Baek G., Lin W.-C., Mansour J.C., Mollaee M., Wagner K.-U., Koduru P., Yopp A.C., et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 2015;6:6744. doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Sakamoto K., Kamada Y., Sameshima T., Yaguchi M., Niida A., Sasaki S., Miwa M., Ohkubo S., Sakamoto J.-I., Kamaura M., et al. K-Ras(G12D)-selective inhibitory peptides generated by random peptide T7 phage display technology. Biochem. Biophys. Res. Commun. 2017;484:605–611. doi: 10.1016/j.bbrc.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 291.Carlino M.S., Kwan V., Miller D.K., Saunders C.A., Yip D., Nagrial A.M., Tomlinson J., Grimmond S.M., Scolyer R.A., Kefford R.F., et al. New RAS-Mutant Pancreatic Adenocarcinoma with Combined BRAF and MEK Inhibition for Metastatic Melanoma. J. Clin. Oncol. 2015;33:e52–e56. doi: 10.1200/JCO.2013.51.5783. [DOI] [PubMed] [Google Scholar]
- 292.Boussemart L., Girault I., Malka-Mahieu H., Mateus C., Routier E., Rubington M., Kamsu-Kom N., Thomas M., Tomasic G., Agoussi S., et al. Secondary Tumors Arising in Patients Undergoing BRAF Inhibitor Therapy Exhibit Increased BRAF–CRAF Heterodimerization. Cancer Res. 2016;76:1476–1484. doi: 10.1158/0008-5472.CAN-15-2900-T. [DOI] [PubMed] [Google Scholar]
- 293.Mellema W.W., Burgers S.A., Smit E.F. Tumor flare after start of RAF inhibition in KRAS mutated NSCLC: A case report. Lung Cancer. 2015;87:201–203. doi: 10.1016/j.lungcan.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 294.Miyauchi S., Shien K., Takeda T., Araki K., Nakata K., Miura A., Takahashi Y., Kurihara E., Ogoshi Y., Namba K., et al. Antitumor Effects of Pan-RAF Inhibitor LY3009120 Against Lung Cancer Cells Harboring Oncogenic BRAF Mutation. Anticancer Res. 2020;40:2667–2673. doi: 10.21873/anticanres.14237. [DOI] [PubMed] [Google Scholar]
- 295.Semrad T.J., Gandara D.R., Lara P.N. Enhancing the clinical activity of sorafenib through dose escalation: Rationale and current experience. Ther. Adv. Med. Oncol. 2011;3:95–100. doi: 10.1177/1758834010396117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Villanueva A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 297.Sun T., Liu H., Ming L. Multiple Roles of Autophagy in the Sorafenib Resistance of Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2017;44:716–727. doi: 10.1159/000485285. [DOI] [PubMed] [Google Scholar]
- 298.Kim J.S., Choi G.H., Jung Y., Kim K.M., Jang S.-J., Yu E.S., Lee H.C. Downregulation of Raf-1 kinase inhibitory protein as a sorafenib resistance mechanism in hepatocellular carcinoma cell lines. J. Cancer Res. Clin. Oncol. 2018;144:1487–1501. doi: 10.1007/s00432-018-2672-y. [DOI] [PubMed] [Google Scholar]
- 299.Ye L., Mayerle J., Ziesch A., Reiter F.P., Gerbes A.L., De Toni E.N. The PI3K inhibitor copanlisib synergizes with sorafenib to induce cell death in hepatocellular carcinoma. Cell Death Discov. 2019;5:86. doi: 10.1038/s41420-019-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Yao X., Zhao C.-R., Yin H., Wang K., Gao J.-J. Synergistic antitumor activity of sorafenib and artesunate in hepatocellular carcinoma cells. Acta Pharmacol. Sin. 2020;41:1609–1620. doi: 10.1038/s41401-020-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Gedaly R., Angulo P., Hundley J., Daily M., Chen C., Koch A., Evers B.M. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30:4951–4958. [PMC free article] [PubMed] [Google Scholar]
- 302.Gedaly R., Angulo P., Hundley J., Daily M., Chen C., Evers B.M. PKI-587 and Sorafenib Targeting PI3K/AKT/mTOR and Ras/Raf/MAPK Pathways Synergistically Inhibit HCC Cell Proliferation. J. Surg. Res. 2012;176:542–548. doi: 10.1016/j.jss.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 303.Wang H., Daouti S., Li W.H., Wen Y., Rizzo C., Higgins B., Packman K., Rosen N., Boylan J.F., Heimbrook D., et al. Identification of the MEK1(F129L) activating mutation as a potential mechanism of acquired resistance to MEK inhi-bition in human cancers carrying the B-RafV600E mutation. Cancer Res. 2011;71:5535–5545. doi: 10.1158/0008-5472.CAN-10-4351. [DOI] [PubMed] [Google Scholar]
- 304.Pedersen K., Bilal F., Morales C.B., Salcedo M.T., Macarulla T., Massó-Vallés D., Mohan V., Vivancos A., Carreras M.-J., Serres-Créixams X., et al. Pancreatic cancer heterogeneity and response to Mek inhibition. Oncogene. 2017;36:5639–5647. doi: 10.1038/onc.2017.174. [DOI] [PubMed] [Google Scholar]
- 305.Kun E., Tsang Y., Ng C., Gershenson D., Wong K. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat. Rev. 2021;92:102137. doi: 10.1016/j.ctrv.2020.102137. [DOI] [PubMed] [Google Scholar]
- 306.Hayes T.K., Neel N.F., Hu C., Gautam P., Chenard M., Long B., Aziz M., Kassner M., Bryant K.L., Pierobon M., et al. Long-Term ERK Inhibition in KRAS-Mutant Pancreatic Cancer Is Associated with MYC Degradation and Senes-cence-like Growth Suppression. Cancer Cell. 2016;29:75–89. doi: 10.1016/j.ccell.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lake D., Correa S.A., Muller J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell. Mol. Life Sci. 2016;73:4397–4413. doi: 10.1007/s00018-016-2297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Yan Z., Ohuchida K., Fei S., Zheng B., Guan W., Feng H., Kibe S., Ando Y., Koikawa K., Abe T., et al. Inhibition of ERK1/2 in cancer-associated pancreatic stellate cells suppresses cancer-stromal interaction and metastasis. J. Exp. Clin. Cancer Res. 2019;38:221. doi: 10.1186/s13046-019-1226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Bhagwat S.V., McMillen W.T., Cai S., Zhao B., Whitesell M., Shen W., Kindler L., Flack R.S., Wu W., Anderson B., et al. ERK Inhibitor LY3214996 Targets ERK Pathway–Driven Cancers: A Therapeutic Approach Toward Precision Medicine. Mol. Cancer Ther. 2019;19:325–336. doi: 10.1158/1535-7163.MCT-19-0183. [DOI] [PubMed] [Google Scholar]
- 310.Hatzivassiliou G., Liu B., O’Brien C., Spoerke J.M., Hoeflich K.P., Haverty P.M., Soriano R., Forrest W.F., Heldens S., Chen H., et al. ERK Inhibition Overcomes Acquired Resistance to MEK Inhibitors. Mol. Cancer Ther. 2012;11:1143–1154. doi: 10.1158/1535-7163.MCT-11-1010. [DOI] [PubMed] [Google Scholar]
- 311.Ning C., Liang M., Liu S., Wang G., Edwards H., Xia Y., Polin L., Dyson G., Taub J.W., Mohammad R.M., et al. Targeting ERK enhances the cytotoxic effect of the novel PI3K and mTOR dual inhibitor VS-5584 in preclinical models of pancreatic cancer. Oncotarget. 2017;8:44295–44311. doi: 10.18632/oncotarget.17869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Shi H., Kong X., Ribas A., Lo R.S. Combinatorial treatments that overcome PDGFRbeta-driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res. 2011;71:5067–5074. doi: 10.1158/0008-5472.CAN-11-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Bryant K.L., Stalnecker C.A., Zeitouni D., Klomp J.E., Peng S., Tikunov A.P., Gunda V., Pierobon M., Waters A.M., George S.D., et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med. 2019;25:628–640. doi: 10.1038/s41591-019-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Martin S., Dudek-Perić A.M., Maes H., Garg A.D., Gabrysiak M., Demirsoy S., Swinnen J.V., Agostinis P. Concurrent MEK and autophagy inhibition is required to restore cell death associated danger-signalling in Vemuraf-enib-resistant melanoma cells. Biochem. Pharmacol. 2015;93:290–304. doi: 10.1016/j.bcp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 315.Xu H., Zhou S., Xia H., Yu H., Tang Q., Bi F. MEK nuclear localization promotes YAP stability via sequestering beta-TrCP in KRAS mutant cancer cells. Cell Death Differ. 2019;26:2400–2415. doi: 10.1038/s41418-019-0309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Becker J.H., Gao Y., Soucheray M., Pulido I., Kikuchi E., Rodríguez M.L., Gandhi R., Lafuente-Sanchis A., Aupí M., Fernández-Coronado J.A., et al. CXCR7 Reactivates ERK Signaling to Promote Resistance to EGFR Kinase Inhibitors in NSCLC. Cancer Res. 2019;79:4439–4452. doi: 10.1158/0008-5472.CAN-19-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Neto J.M.F., Nadal E., Bosdriesz E., Ooft S.N., Farre L., McLean C., Klarenbeek S., Jurgens A., Hagen H., Wang L., et al. Multiple low dose therapy as an effective strategy to treat EGFR inhibitor-resistant NSCLC tumours. Nat. Commun. 2020;11:1–9. doi: 10.1038/s41467-020-16952-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Ray P., Dutta D., Haque I., Nair G., Mohammed J., Parmer M., Kale N., Orr M., Jain P., Banerjee S., et al. pH-Sensitive Nanodrug Carriers for Codelivery of ERK Inhibitor and Gemcitabine Enhance the Inhibition of Tumor Growth in Pancreatic Cancer. Mol. Pharm. 2021;18:87–100. doi: 10.1021/acs.molpharmaceut.0c00499. [DOI] [PubMed] [Google Scholar]
- 319.Mao J., Yang H., Cui T., Pan P., Kabir N., Chen D., Ma J., Chen X., Chen Y., Yang Y. Combined treatment with sorafenib and silibinin synergistically targets both HCC cells and cancer stem cells by enhanced inhibition of the phosphorylation of STAT3/ERK/AKT. Eur. J. Pharmacol. 2018;832:39–49. doi: 10.1016/j.ejphar.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 320.Bian Y., Guo D. Targeted Therapy for Hepatocellular Carcinoma: Co-Delivery of Sorafenib and Curcumin Using Lactosylated pH-Responsive Nanoparticles. Drug Des. Dev. Ther. 2020;14:647–659. doi: 10.2147/DDDT.S238955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kawazoe T., Taniguchi K. The Sprouty/Spred family as tumor suppressors: Coming of age. Cancer Sci. 2019;110:1525–1535. doi: 10.1111/cas.13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Momeny M., Khorramizadeh M.R., Ghaffari S.H., Yousefi M., Yekaninejad M.S., Esmaeili R., Jahanshiri Z., Nooridaloii M.R. Effects of silibinin on cell growth and invasive properties of a human hepatocellular carcinoma cell line, HepG-2, through inhibition of extracellular signal-regulated kinase 1/2 phosphorylation. Eur. J. Pharmacol. 2008;591:13–20. doi: 10.1016/j.ejphar.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 323.Varghese L., Agarwal C., Tyagi A., Singh R.P., Agarwal R. Silibinin Efficacy against Human Hepatocellular Carcinoma. Clin. Cancer Res. 2005;11:8441–8448. doi: 10.1158/1078-0432.CCR-05-1646. [DOI] [PubMed] [Google Scholar]
- 324.Ailioaie L.M., Litscher G. Curcumin and Photobiomodulation in Chronic Viral Hepatitis and Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020;21:7150. doi: 10.3390/ijms21197150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Abdelmoaty A.A.A., Zhang P., Lin W., Fan Y.J., Ye S.N., Xu J.H. C0818, a novel curcumin derivative, induces ROS-dependent cytotoxicity in human hepatocellular car-cinoma cells in vitro via disruption of Hsp90 function. Acta Pharmacol. Sin. 2021 doi: 10.1038/s41401-021-00642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sadelain M., Brentjens R., Rivière I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Miao L., Zhang Y., Huang L. mRNA vaccine for cancer immunotherapy. Mol. Cancer. 2021;20:1–23. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]