Abstract
Simple Summary
Cancer metabolism plays a fundamental role in cancer biology in the tumor microenvironment. Under the reaction of one-carbon metabolism-dependent S-adenosylmethionine, methylation defines a biologically malignant phenotype of cancer. As methylation is closely associated with malignant phenotypes in various cell types, including epithelial cancer cells, immune cells, and cancer-associated fibroblasts, in tumor tissues, a cancer sieging strategy targeting the “methylosystem” may be an effective therapeutic approach against refractory cancers.
Abstract
As cancer is a genetic disease, methylation defines a biologically malignant phenotype of cancer in the association of one-carbon metabolism-dependent S-adenosylmethionine (SAM) as a methyl donor in each cell. Methylated substances are involved in intracellular metabolism, but via intercellular communication, some of these can also be secreted to affect other substances. Although metabolic analysis at the single-cell level remains challenging, studying the “methylosystem” (i.e., the intercellular and intracellular communications of upstream regulatory factors and/or downstream effectors that affect the epigenetic mechanism involving the transfer of a methyl group from SAM onto the specific positions of nucleotides or other metabolites in the tumor microenvironment) and tracking these metabolic products are important research tasks for understanding spatial heterogeneity. Here, we discuss and highlight the involvement of RNA and nicotinamide, recently emerged targets, in SAM-producing one-carbon metabolism in cancer cells, cancer-associated fibroblasts, and immune cells. Their significance and implications will contribute to the discovery of efficient methods for the diagnosis of and therapeutic approaches to human cancer.
Keywords: methylation, one-carbon metabolism, RNA, nicotinamide, cancer-associated fibroblasts
1. Introduction
Cancer consists of highly heterogeneous tissues and comprises mutation-driven biologically malignant cancer cells, immune cells, cancer-associated fibroblasts (CAFs), and blood vessel endothelial cells [1]. The tumor microenvironment (TME) contains components that adapt to the originally occurring primary tumor sites because of their anatomical location, immunosuppressive environment, and unique metabolism. Moreover, TME refers to metastatic sites in distant organs [2]. Although the tumor mutation burden is defined by the total number of somatic mutations in cancer cell DNA, research has elucidated that metabolism plays a fundamental role in cancer biology in TME [3]. Research has demonstrated that interactions among cancer cells, immune cells, and CAFs emerge via metabolites based on biomass and the energy production of one-carbon metabolism (Figure 1) [4]. This mechanism is an attractive target for efficient cancer drug discovery. Further, as an aspect of cancer prevention during the early stages of cancer, an association between food and nutrient intake has emerged. A recent study from a broad review of meta-analyses of observational studies evaluating the strength and validity of the evidence for factors associated with the risk of developing or dying from 11 primary cancers indicated that the consumption of dairy products, milk, calcium, and whole grains is inversely associated with cancer risk, suggesting an effective outcome of nutrient intake [5]. Given that folates are important nutrients obtained through the diet, recent studies indicated that the dietary intake of one-carbon metabolism-related nutrients is associated, inversely or positively, with pancreatic cancer [6] and melanoma risk [7], suggesting that metabolites are involved in intra- or intercellular metabolism under the influence of essential nutrients. This finding might be beneficial for cancer medicine and aging research, although it warrants further investigation [8].
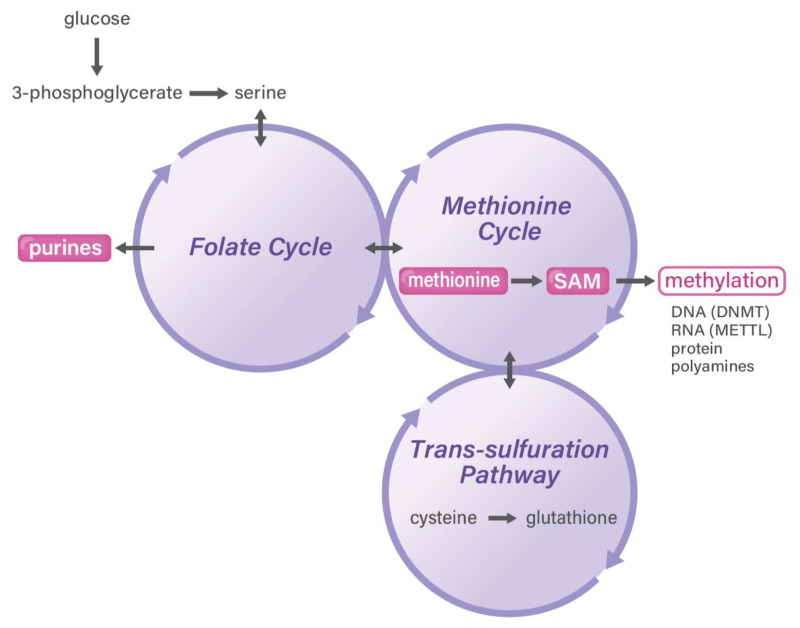
Figure 1.
One-carbon metabolism in cancer. One-carbon metabolism is a mechanism that is coupled with three reactions of the folate cycle, methionine cycle, and trans-sulfuration pathway. The circled and highlighted areas indicate those playing a role in cancer and stem cells, as highlighted in the text. DNMT, DNA methyltransferase; METTL, adenosine-methyltransferase.
2. Genome and Metabolome
Recent developments in next-generation sequencing have enabled studies at the single-cell level. Spatial transcriptome analysis, a new method for obtaining positional information on cells in cancer via single-cell analysis, was applied to various clinical specimens to investigate the transcriptome at the intracellular and intercellular levels [9]; however, measuring metabolites in single cells remains challenging [10]. As a metabolite of one-carbon metabolism, SAM plays a critical role in the methylation of various targets, including DNA, RNA, and proteins, and of several other metabolites [11]. Methylated substances are involved in intracellular metabolism in TME, but some can also be secreted to affect other substances via intercellular communication [12]. In this review, we focused on the elucidation of intercellular and intracellular communications between upstream regulatory factors and/or downstream effectors that affect the epigenetic mechanism involving the transfer of a methyl group from SAM onto the specific positions of nucleotides or other metabolites in TME as a whole tissue; in TME, various cells, including epithelial cancer cells, CAFs, endothelial cells, immune cells, and others, are involved and track important products. To understand the spatial heterogeneity, we refer to this as the “methylosystem.” The significance and implication of this review will contribute to the discovery of efficient methods for diagnosing and devising therapeutic approaches to human cancer. Understanding the cellular and molecular mechanisms underlying the methylosystem could be a novel strategy for disrupting cancer cell interactions and contribute to the development of efficient and safe therapeutic strategies to treat cancer. Furthermore, research findings can be used as cancer diagnostic tools for developing precision medicine by precisely predicting and monitoring cancer therapy outcomes.
3. Oncogenes and TME-Driven Metabolism Alterations
Otto Heinrich Warburg was awarded the 1931 Nobel Prize in physiology for his “discovery of the nature and mode of action of the respiratory enzyme” [13]. Warburg was rewarded as his discovery, termed the Warburg effect, explained that cancer cells largely depend on aerobic glycolysis, whereas, in sharp contrast, normal differentiated cells primarily rely on oxidative phosphorylation in mitochondria to generate energy and biomaterials needed for cellular processes [14].
Recent studies have indicated that cancers indeed exhibit the Warburg effect, an increased uptake and conversion of glucose to lactate, but cancer cells are also associated with alterations in glutamine and fatty acid metabolism [15]. In this malignant mechanism, the MYC oncogene contributes to alterations in cellular metabolism to facilitate tumorigenesis by altering nucleotide metabolism and DNA replication induced by the attenuated expression of E2F1; hypoxia-inducible transcription factor 1; lactate dehydrogenase A; and several microRNAs, such as miR-23a/b, to increase the protein expression of glutaminase [15]. In addition to the regulation of gene expression at the transcriptional level, research has revealed the significance of the ubiquitin-proteasome system (UPS), which controls various signaling factors in the glycolysis pathway via ubiquitination or deubiquitination [16]. As a result of UPS, deubiquitination acts as either a tumor-promoting oncoprotein or as a tumor-restricting suppressor protein [16].
Cellular metabolism in cancer cells is altered in terms of the mechanism of oxidative phosphorylation within the mitochondria. First, in TME, hypoxia promotes the isocitrate dehydrogenase (IDH)-dependent carboxylation of α-ketoglutarate to citrate, which further contributes to the malignant phenotype of cancer cells; these phenotypes include rapid cell growth, extended survival under hypoxia, malnutrition, and therapeutic resistance [17]. Second, a study on malignant cells found that the reduced glutamine metabolism by IDH1 mediates lipogenesis under a hypoxic TME; this indicates a critical role for the oxidative process in regulating carbon use for producing acetyl coenzyme A, the central biosynthetic precursor that supports fatty acid synthesis and protein acetylation in mammalian cells [18]. Another study on colorectal cancer indicated that the metabolism of the IDH-dependent carboxylation of α-ketoglutarate to citrate was altered by the oncometabolite D-2-hydroxyglurate (HG), which directly induced the epithelial-mesenchymal transition and was associated with the distant metastasis of cancer cells [19]. A mathematical analysis of cancer patient data predicted that imbalanced IDH1/2 expression is associated with the 2-HG-inactivating β-oxygenation pathway in colorectal cancer [20]. Third, given that the KRAS oncogene is among the most frequently mutated genes in pancreatic cancer, a study on the metabolism of pancreatic cancer cells indicated that they rely on a distinctive pathway. Via this pathway, glutamine supports pancreatic cancer growth via a KRAS-regulated metabolic pathway; in this pathway, it can be converted into oxaloacetate by aspartate transaminase and this oxaloacetate is further converted into malate and then pyruvate, which contributes to an increase in the NADPH/NADP+ ratio and leads to the maintenance of the cellular redox state [21]. It has been suggested that the essentiality of this pathway in pancreatic ductal adenocarcinoma and the fact that it is critical in normal cells may provide novel therapeutic approaches for treating these refractory tumors [21]. By contrast, in a study on colorectal cancer, cases with KRAS mutations demonstrated a different role of this oncogene in the malignant phenotype, suggesting a role of KRAS in the metabolic adaptation mechanism to nutritional stress in colorectal cancer [22]. Further, in colorectal cancer, the V600E mutation in the BRAF oncogene was found to be involved in AMP-activated protein kinase-mediated autophagy and therapeutic resistance in cancer cells [23].
4. Methionine and One-Carbon Metabolism Pathway in Cancer
As somatic stem cells, hematopoietic stem cells play a functional role at the center of hematopoiesis, and specific metabolic changes in hematopoietic stem and progenitor cells (HSPCs) have been linked with the induction of alterations in myelopoiesis in the bone marrow as well as with HSPC dysfunction in aging and clonal hematopoiesis [8]. HSPC function is regulated by metabolic processes during various stimuli, such as immunologic and inflammatory responses [24]. Folate metabolism is among the most functionally important metabolic processes of hematopoiesis and the immune response, which is consistent with the fact that hematopoiesis and the immune response are the most proliferative processes in the body [25]. For example, long-term dietary folate deficiency can induce macrocytic anemia. Antifolate metabolism antagonists against hematopoietic malignancies and other solid cancers are the most important methods as chemotherapy to reduce cancer-specific metabolism and inhibit the proliferation of cancer cells [26]. Although recent research has shown that immune cells are sensitive to exposure to conventional antifolate therapies, which can limit the effective doses required to eradicate cancer cells, the antifolate reagent methotrexate is an anchoring drug in chronic arthritis and systemic lupus erythematosus [27]. It has emerged that more sensitive antifolate metabolism antagonists in immune cells are necessary for clinical use.
Diet is a major source of one-carbon units and includes three groups: (1) glucose and its glycolysis product, serine; (2) methionine cycle products (such as methionine and choline); and (3) glycine, which can be derived from threonine via the reaction of L-threonine dehydrogenase (TDH) in rodents, but not in humans, as the human TDH encodes a pseudogene without functional catalytic activity [25]. Moreover, histidine can be incorporated into one-carbon units in an alternative pathway.
The one-carbon pathway differs among various cell types (Figure 2). First, in cancer cells, which proliferate quickly, one-carbon metabolism is activated and plays a role in the production of purine, which is a precursor of nucleotides (such as DNA and RNA). In cancer cells, glucose can be predominantly used and gives rise to serine and purines. In a study on clinical samples, the enzyme status of one-carbon folate metabolism was shown to predict the survival rate of patients with gastrointestinal cancer; this finding provides a rationale for this pathway as certain anticancer drug targets [28], such as methylenetetrahydrofolate dehydrogenase (MTHFD) 2 [29], suggesting the druggability of one-carbon metabolism in cancer diagnostic and therapeutic approaches [30]. Previous reports have indicated that >80% of cancer cells eventually depend on the uptake of extracellular methionine and that cancer cells can rapidly synthesize methionine from homocysteine, which is consistent with the general requirements of cancer cells for methionine for altered metabolic flux via a pathway linked to SAM usage [31]. The high demand or “addiction” of cancer cells to exogenously provided methionine is not caused by the cancer cells’ inability to synthesize methionine but rather by their high demands of methionine-derived metabolites [32], including processes involving Cdc6 and prereplication complexes [33], nucleoside metabolism and polyamine synthesis [34], and cell cycle arrest in G1 involving p38 mitogen-activated protein kinase [35]. The dependence of cancer cells on methionine is referred to as the methionine stress sensitivity of cancer cells or the Hoffman effect [31]. Of note, Sugimura et al. provided the in vivo evidence of tumor dependency on dietary methionine, demonstrating that tumor growth in rats is significantly affected by the restriction of individual amino acids such as methionine [36]. However, the magnitude of the dependence of SAM synthesis on folate metabolism or betaine on cancer cells remains unclear.
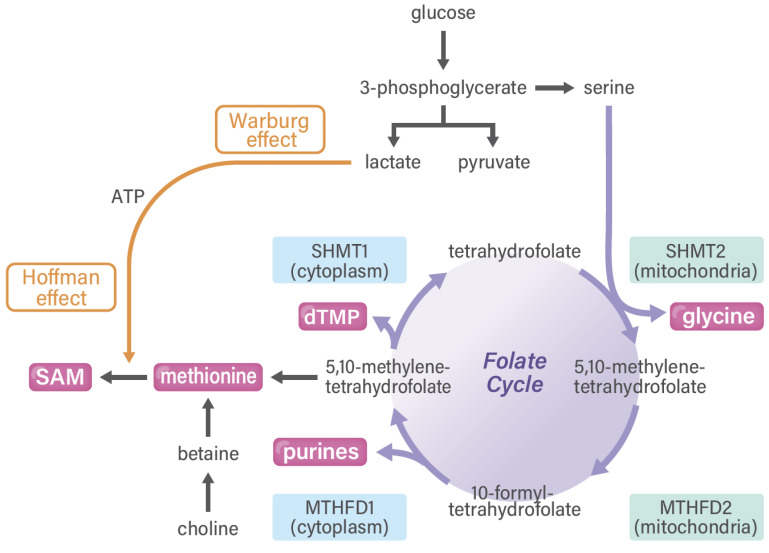
Figure 2.
Folate cycle and SAM in cancer. The Warburg and Hoffman effects collaborate with each other via one-carbon metabolism. MTHFD and SHMT are druggable targets for cancer treatment. MTHFD, methylenetetrahydrofolate dehydrogenase; SAM, S-adenosylmethionine; SHMT, serine hydroxymethyltransferase.
Second, glucose-dependent serine synthesis and folate metabolism are vital for the production of purine in somatic stem cells, which is required for cancer and embryonic development [25]. A previous study on an animal model of MTHFD2−/− embryos indicated that MTHFD2 has functional importance in hematopoietic lineages, including immune cells [37]. In TME, T cells are required for rapid proliferation and prompt transcriptional responses to various stimuli, which necessitate rapid one-carbon metabolism. In addition, a biochemical study on T cell function [38] and a clinical investigation on gastrointestinal cancer [28] noted large changes in the gene expression of genes in the serine hydroxymethyltransferase (SHMT)2 to MTHFD2 metabolic pathway. A study on immune cells with the antioxidant N-acethylcycteine indicated that the activation of T cells requires both the generation of a one-carbon unit and redox defense (Figure 3) [39], suggesting that T cell activation is linked to the trans-sulfuration pathway in one-carbon metabolism [4] and that there is a great need for developing MTHFD2-specific reagents [25]. Nonetheless, one-carbon metabolism and drug discovery in cancer stem cells (i.e., a fraction of stemness-possessing cancer cells in whole-tumor tissues) remains to be completely understood. Of note, the authors of a rat study reported the indispensable role of several amino acids in maintaining somatic stem cells, which is indicative of the finding that depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation [40]. In previous studies, some dietary factors, including casein [41], folate, and amino acids [42], were found to play important roles in the recovery after granulocytopenia in rats. Research has further extended the use of compounds in long-term ex vivo hematopoietic stem cell expansion, which will enable nonconditioned transplantation [43].
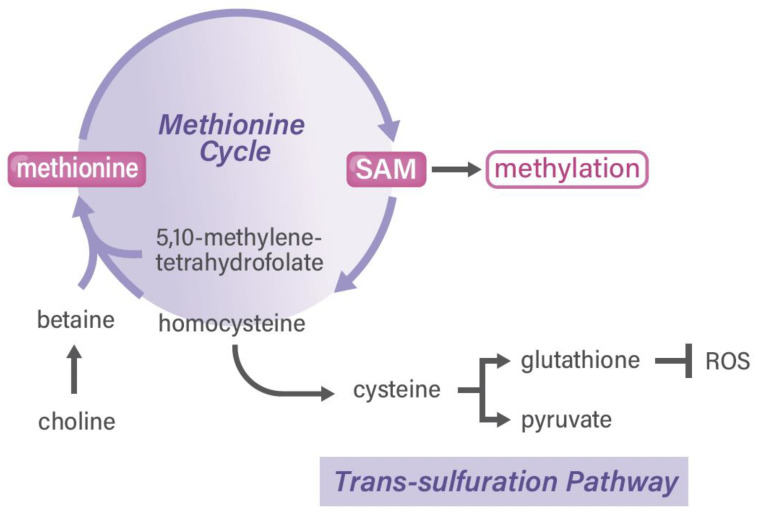
Figure 3.
Methionine cycle and SAM in cancer. ROS, reactive oxygen species; SAM, S-adenosylmethionine.
Third, SAM can be synthesized not only from folate in differentiated somatic cells but also from betaine, which is derived from choline. As mentioned above, in sharp contrast to cancer cells, noncancerous somatic cells can use betaine to produce sufficient SAM to maintain the homeostasis of the methylation of nucleotides and protein in cells [25]. Cancer cells’ requirement for methionine is purported to be caused by the high demand of SAM and one-carbon metabolism-related metabolites in cancer cells. It has been reported that SAM is involved in ornithine decarboxylase in the polyamine pathway of cancer stem cells in osteosarcoma [44] and cervical cancer [45], which is useful for drug discovery in targeting esophageal cancer [46]. Furthermore, polyamine flux suppresses histone lysine demethylases and enhances ID1 expression in cancer stem cells [47]. Taken together, the one-carbon metabolism pathway plays a role in different cell types, although understanding the dietary factors warrants further studies.
5. RNA Methylation Pathways in Cancer
One-carbon metabolism is known to be associated with nucleotide methylation pathways, including for both DNA and RNA. Because DNA methylation is an important characteristic of cancer, it has been studied extensively and has broadened the scope of epigenetics as a functional assay to elucidate disease pathophysiology [48]. DNA methylation in liquid biopsies in blood has emerged in the initial diagnosis of cancer but also in the early detection of relapse after therapy [49]. In contrast to DNA modification being predominant in 5-methyl cytosine, RNA modifications occur in 6-methyl adenine (m6A) and in various positions of nucleotides, which characterize heterogeneous cancer types (Figure 4A) [50]. Measuring m6A in microRNAs, which are small noncoding RNAs, is useful for detecting the early stages and relapse phases of cancer [51].
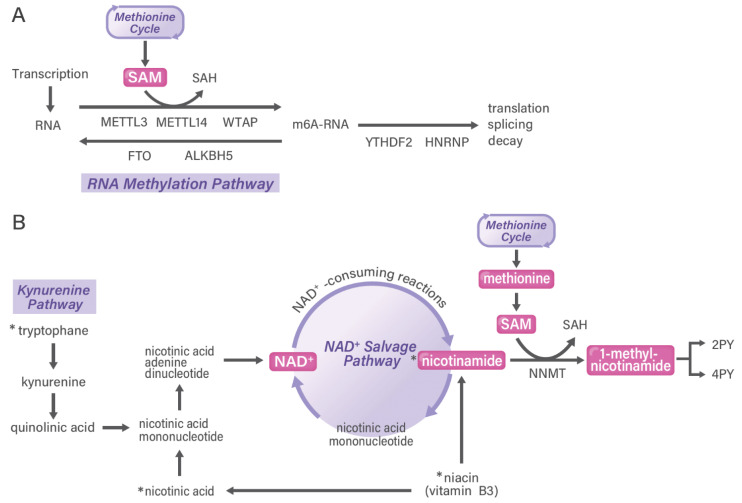
Figure 4.
RNA methylation pathway and NAD+ salvage pathway in cancer. In tumor tissues, heterogeneous cell populations exert differential functions of the RNA methylation pathway (A) and the NAD+ salvage pathway (B), which are examples of the methylosystem. Nicotinamide metabolism is shown as the NAD+ salvage pathway in the correlation of the kynurenine pathway and the one-carbon pathway. Although NAD+ can be synthesized from tryptophan in the kynurenine pathway, this metabolic pathway is less effective in humans than in mice. 2PY, N-methyl-2-pyridone-5-carboxamide; 4PY, N-methyl-4-pyridone-3-carboxamide; NAD+, nicotinamide adenine dinucleotide; SAM, S-adenosylmethionine; SAH, S-adenosyl-homocysteine. Asterisks indicate dietary intake. Asterisks (*) indicate ones from diet.
Human N6-adenosine-methyltransferase complex catalytic subunit (METTL3) and METTL14 were shown to form a stable heterodimer core complex that functions in catalyzing m6A RNA methylation [52]. WT1-associated protein (WTAP) interacts with this complex and affects methylation [52]. The core complex of METTL3-14 with WTAP plays a role in writing epitranscriptome (RNA modification) codes [53]. By contrast, demethylases that reverse this methylation, termed erasers, have been identified: fat mass and obesity-associated protein [54] and α-ketoglutarate-dependent dioxygenase homolog 5 [55]. These modifications can be recognized to execute function by readers: heterogeneous nuclear ribonucleoproteins and YT521-B homology (YTH) N6-methyladenosine RNA binding protein 1 (YTHDF1). A study on colorectal cancer found that the expression of the m6A reader YTHDF1 is controlled by the oncogene c-myc [56]. Taken together, one-carbon metabolism and its metabolites are involved in dynamic m6A modifications, which can be recognized by different binding proteins to exert the biologically malignant phenotypes of cancer.
6. Nicotinamide Adenine Dinucleotide (NAD+) Salvage Pathway in Cancer
In 1951, Cantoni first partially purified nicotinamide N-methyltransferase (NNMT) from rat liver [57] and subsequently discovered the structure of the cofactor SAM, which is an active methyl donor [58]. The enzymatic activity of NNMT is important for preventing the nicotinamide (NAM)-mediated inhibition of NAD+-consuming enzymes (such as poly-adenosine-diphosphate, ribose polymerases, and sirtuins) [59]. NNMT activity alteration has been reported in oral, stomach, colon, rectum, liver, pancreas, breast, bladder, prostate, ovary, and lung tumors as well as in glioma, lymphoma, and insulinoma [60,61,62]. The clinical significance of the involvement of NNMT was examined in a systematic review and meta-analysis [63], which indicated the prognostic value of NNMT expression in patients with solid tumors. Table 1 highlights the subsequent original reports and other basic research reports.
The involvement of gene expression via epigenetic regulation has been reported via the histone H3 lysine 9 demethylation mechanism [64]. In addition, NNMT has been shown to be involved in the epithelial-mesenchymal transition under the condition of glucose deprivation [65]. A study on gastric cancer showed that NNMT promotes the epithelial-mesenchymal transition [66]. NNMT downregulation also inhibits migration and the epithelial-mesenchymal transition in esophageal squamous cell carcinoma [67]. Moreover, another study on esophageal squamous carcinoma showed that NNMT is involved in metabolic reprogramming and promotes the Warburg effect [68]. NNMT and 1-methylnicotinamide (MNAM) are reportedly involved in the mechanism of inhibition of the apoptosis signal-regulating kinase 1-p38 MAPK pathway, resulting in increased colorectal cancer cell resistance to 5-FU [69]. In nasopharyngeal carcinoma, NNMT is associated with the phosphorylation of Akt and worse patient prognosis [70].
A recent study indicated that the overexpression of NNMT suppressed the m6A methylation of CD44 mRNA, thereby enhancing CD44v3 formation; this process contributes to vascular invasion and distant metastasis in hepatocellular carcinoma [71]. By contrast, NNMT knockdown increased the m6A methylation of the RRACH motif (R demotes G or A; H is A, C, or U) on exon 12 and exon 19 of CD44 mRNA [71]. The researchers suggested that NNMT-modulated CD44 m6A demethylation improves RNA stability [71]. Taken together, as NNMT, the enzyme that converts nicotinamide to MNAM, is overexpressed in a variety of human cancers, NNMT and metabolite productions are suggested to play a role in the malignant phenotype of cancer, including the involvement of the cancer stem cell phenotype.
The activity of NNMT is tightly linked to the maintenance of the NAD+ level in cells [59]. A recent study on TME via a combination of metabolomics and single-cell RNA sequencing analysis indicated that cells within ascites and ovarian cancer showed a notable enrichment in MNAM in tumor-infiltrating T cells. Of note, although MNAM levels were elevated in T cells, NNMT expression was restricted to fibroblasts and tumor cells. The study also indicated that MNAM induces T cells to secrete the tumor-promoting cytokine tumor necrosis factor-alpha. Furthermore, the study found that TME-derived MNAM can modulate T cell function and suggested that this could be a potential immunotherapy target against ovarian cancer (Figure 4B) [72].
7. Targeting Metabolism in TME
Although studies have examined the significance and implication of CAFs, the drug discovery of CAF-targeting molecules or reagents for cancer diagnosis and therapeutic purposes has recently emerged (Table 1 and Table 2). A recent study indicated that fibroblast activation protein (FAP), which promotes tumor growth and progression, is overexpressed in the CAFs of many human epithelial cancers, including pancreatic cancer, and is an attractive target for marking by 64Cu- and 225Ac-labeled FAP inhibitor FAPI-04, which have been used as theranostics for treating FAP-expressing pancreatic cancer, as shown in a proof-of-concept study [73]. In a study on hepatocellular carcinoma, the usefulness of 68Ga-FAPI-04 positron emission tomography/computed tomography (PET/CT) was demonstrated, and 68Ga-FAPI-04 PET/CT was found to be more sensitive than 18F-FDG PET/CT in detecting hepatocellular carcinoma lesions, as 68Ga-FAPI-04 uptake is primarily correlated with tumor size, suggesting clinical benefits [74]. Another study reported that FAP-specific PET/CT imaging in fibrotic interstitial lung diseases and lung cancer provided potential clinical value for the monitoring and therapeutic evaluation of fibrotic interstitial lung diseases and suggested that these areas be investigated in future studies [75]. NNMT stabilizes sirtuin 1 in prostate cancer cells [76], whereas NNMT increases complex I activity in SH-SY5Y human neuroblastoma cells via sirtuin 3, suggesting a central role of NNMT in regulating energy homeostasis [77]. Taken together, therapy targeting FAP in the cancer stroma is effective and will contribute to the development of new treatment strategies (Figure 5).
Table 1.
NNMT and cancer.
| Cancer Type | Function | References |
|---|---|---|
| Melanoma | Gene silencing enhances chemosensitivity | [78] |
| Colorectal cancer | Vanillin downregulates NNMT | [79] |
| HeLa cells | Inhibitor of NNMT shows antiproliferative activity | [80] |
| Gastric cancer | Exosomal NNMT promotes metastasis | [81] |
| Ovarian cancer | Low NNMT benefits from bevacizumab treatment | [82] |
| Gastric carcinoma | NNMT in cancer-associated fibroblasts | [83] |
| Hepatoblastoma | NNMT downregulation by DNA hypermethylation | [84] |
| Gastric cancer | Prognostic biomarker correlated with immune | [85] |
| Breast cancer | NNMT inhibits autophagy through AMPK pathway | [86] |
| Esophageal squamous carcinoma | Metabolic reprogramming and promoting the Warburg effect | [68] |
| Ovarian cancer | Overexpression is associated with poor prognosis | [87] |
| Bladder, lung, colorectal, and osteosarcoma | Cancer stem cell enrichment is associated with NNMT expression | [88] |
| Colorectal cancer | High stromal NNMT expression | [89] |
| Endometrial cancer | NNMT associates with patient survival | [90] |
| Skin cancer | NNMT associates with nonmelanoma skin cancers | [91] |
| Renal cell carcinoma | NNMT controls metabolism during progression | [92] |
| Cutaneous squamous cell carcinoma | NNMT induces the proliferation and invasion | [93] |
| Hepatocellular carcinoma | Hepatic stellate cells induce NNMT and metastasis via regulation of CD44v3 | [71] |
| Esophageal squamous cell carcinoma | Downregulation of NNMT inhibits migration and epithelial-mesenchymal transition | [67] |
| Breast cancer | NNMT enhances chemoresistance through SIRT1 | [94] |
| Ovarian cancer | NNMT is a master metabolic regulator of cancer-associated fibroblasts | [95] |
| Cervical carcinoma | Clinical significance of NNMT was evaluated | [96] |
| Oral melanoma | Potential prognostic significance | [97] |
| Non-Small-Cell Lung Cancer | Targeting NNMT and miR-449a in EGFR-TKI resistance | [98] |
| Prostate cancer | NNMT stabilizes sirtuin 1 | [76] |
| Gastric cancer | NNMT promotes epithelial-mesenchymal transition | [66] |
| Melanoma | Potential involvement in tumor | [99] |
| Adenoid cystic carcinoma | Deregulation of NNMT and gap junction protein Alpha-1 causes metastasis | [100] |
| Neuroblastoma | NNMT in involved in sirtuin 3 | [77] |
| Gastric carcinoma | A potential biomarker for worse prognosis | [101] |
| Renal cell carcinoma | Stage-specific changes | [102] |
| Pancreatic cancer | Prognostic value of NNMT in patients | [103] |
| Breast cancer | Downregulation of NNMT induces apoptosis via mitochondria pathway | [104] |
| Oral carcinoma | Silencing of NNMT inhibits tumorigenicity | [105] |
| Nasopharyngeal carcinoma | NNMT is associated with Akt phosphorylation and worse prognosis | [70] |
| Bladder cancer | Potential for a urine-based diagnostic test | [106] |
| Oral squamous cell carcinoma | Basis for developing a noninvasive diagnostic test | [107] |
| Mesenchymal cancer stem cell | Cancer stem cell NNMT enhances cellular radiation resistance | [108] |
| Renal cell carcinoma | NNMT activates matrix metalloproteinase-2 | [109] |
| Glioma | Interferon-gamma elevates NNMT | [110] |
| Oral squamous cell carcinoma | NNMT correlates with tumor differentiation | [111] |
| Lung cancer | Serum levels of NNMT in patients | [112] |
| Hepatocellular carcinoma | NNMT is associated with poor prognosis | [113] |
| Bladder cancer | Metallothionein 1E and NNMT as novel regulators of cell migration | [114] |
| Hepatocellular carcinoma | Stat3 upregulates NNMT | [115] |
| Oral squamous cell carcinoma | NNMT inversely correlates with lymph node metastasis | [116] |
| Renal carcinoma | NNMT as a tumor marker | [117] |
| Colorectal cancer | Serum tumor marker | [118] |
| Papillary thyroid cancer | Activation of NNMT gene promoter by hepatocyte nuclear factor-1beta | [119] |
| Bladder cancer | Heat shock proteins and NNMT in predicting response to radiation | [120] |
| Colon cancer | NNMT as a marker of cancer cachexia in mice | [121] |
| Ehrlich ascites tumor | Preferential increase of activity of NNMT | [122] |
| Ehrlich ascites tumor | NNMT for malignant tumor burden | [123] |
NNMT, nicotinamide N-methyltransferase; AMPK, AMP-activated protein kinase; SIRT1, Sirtuin 1 gene; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; Stat3, signal transducer and activator of transcription 3.
Table 2.
Recently emerged CAF-targeting FAP medicine.
| Cancer Type | Drug Discovery and Application | References |
|---|---|---|
| Glioblastoma | Mesenchymal cells promote angiogenesis | [124] |
| Lung cancer | Specific PET/CT imaging | [75] |
| Advanced cancers | 177Lu-FAPI-46 | [125] |
| Prostate cancer | FAPI | [126] |
| Pancreatic cancer | 68Ga-FAPI-04 PET/MR | [127] |
| Breast cancer | (68)Ga-FAPI-04 | [127] |
| Diverse adenocarcinomas | (177)Lu-FAP-2286 | [128] |
| Various cancers | Al(18)F-NOTA-FAPI | [129] |
| Gynecological malignancies | 68Ga-FAPI-PET/CT | [130] |
| Lymphoma | (68)Ga-FAPI-PET/CT | [131] |
| Colorectal cancer | FAP binds to enolase1 and activates NF-kappaB pathway to promote metastasis | [132] |
| Adenoid cystic carcinomas | 68Ga-FAPI-PET/CT | [133] |
| Sarcoma | Ga-68-FAPI | [134] |
| Murine HPV-positive head and neck tumors | FAP-targeted CD40 agonist (FAP-CD40) | [134] |
| Murine tumor models | FAP-targeted CD40 agonist induces effective antitumor immunity | [135] |
| Hepatocellular carcinoma | Use of nanoparticle formulation | [136] |
| Cancer xenografts | (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine-based small molecules | [137] |
| Cancers | H-ferritin nanocages loaded with navitoclax | [138] |
| Esophageal cancer | FAP-targeted near-infrared photoimmunotherapy (NIR PIT) | [139] |
| Pancreatic cancer | 68Ga-FAPI-PET/CT imaging | [140] |
| Hepatic nodules | (68)Ga-FAPI-04 PET/CT | [141] |
| Cancers | Liposomes bearing HER2 and FAP single-chain antibody fragments | [142] |
| Esophageal cancer | FAPI-PET/CT | [143] |
| Cancer | Bifunctional DOTA and DATA(5m) chelators | [144] |
| Non-small-cell lung cancer and epithelial ovarian cancer | FAP-targeted 4-1BB agonist (FAP-4-1BBL) | [145] |
| Head and neck cancers | FAP inhibitor PET | [146] |
| Cancer, heart diseases, and pulmonary fibrosis | (18)F-Labeled FAPI | [147] |
| Cancers | 99mTc-Labeled FAPI tracers | [148] |
| Cancers | (68)Ga-FAPI-46 PET imaging | [149] |
| Pancreatic cancer xenograft mouse models | 64 Cu- and 225 Ac-labeled FAPI-04 | [73] |
| Cancers | (68)Ga-FAPI-PET/CT | [150] |
| Cancers | (68)Ga-FAPI-PET/CT | [151] |
| Cancers | Tetravalent FAP-(death receptor) DR5 antibody | [152] |
| Cancers | FAPI with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine scaffold | [153] |
| Metastatic colorectal cancer (phase II trial) | Val-boroPro (talabostat) inhibiting FAP | [154] |
| Non-small-cell lung cancer | Sibrotuzumab directed against human FAP | [155] |
PET, positron emission tomography; CT, computed tomography; FAP, fibroblast activation protein; FAPI, FAP inhibitor; HER2, human epidermal growth factor type 2.
Figure 5.
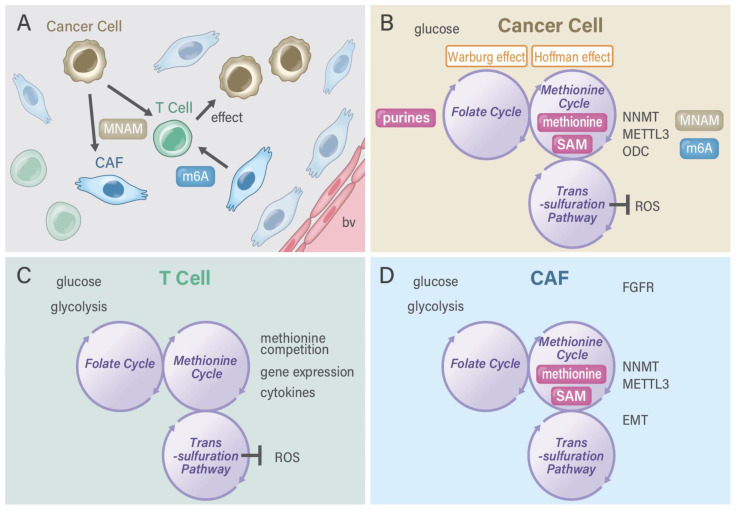
Methylosystem in the tumor microenvironment. (A) Various cells exist in the tumor microenvironment, including cancer cells, immune cells, and cancer-associated fibroblasts. (B) Cancer cells possess activated one-carbon metabolism, which comprises the folate cycle, methionine cycle, and trans-sulfuration pathway. One-carbon metabolism collaborates with the Warburg and Hoffman effects. Cancer cells can secrete microRNAs with m6A from the RNA methylation pathway and MNAM from the NAD+ salvage pathway. METTL3 function is connected with NNMT in CD44v3 cancer cells [71]. (C) T cells have unique redox regulation as the trans-sulfuration pathway of one-carbon metabolism plays a critical role in the maintenance and execution of T cell function [25]. (D) Pathway of one-carbon metabolism plays a role in maintenance of CAFs, which communicate with surrounding cells including T cells and cancer cells. CAF, cancer-associated fibroblast; ODC, ornithine decarboxylase; FGFR, fibroblast growth factor receptor; EMT, epithelial-mesenchymal transition; bv, blood vessels; MNAM, 1-methylnicotinamide.
8. Conclusions
Although transcription can be examined using high-speed next-generation RNA sequencing, single-cell-level analysis remains challenging for studying cancer metabolism. There is a need to clarify and understand the communication between cells across different cancers; thus, an analysis technology that is similar to single-cell analysis is required and future technological development is needed. The analysis of individual cell types is currently progressing, and as mentioned in this review, the exchange of metabolites among cells can be used to obtain an essential understanding of TME based on the methylosystem. The one-carbon metabolism-dependent modulation of nicotinamide and RNA are examples that have emerged recently as possible applications for early diagnosis and therapeutic approaches in precision medicine. Recent studies have presented increasing evidence regarding the unique metabolism of CAF-surrounding cancer cells, and CAF-targeting technology has been developed. By manipulating CAFs, it is possible to block the mechanism activating cancer cells; as a result, research expects that the approach can target CAF and modulate their function, as an efficient therapeutic strategy. Understanding such a methylosystem is expected to be an important tool in future precision medicine, such as in the development of preventive intervention methods as well as in the development of methods for early cancer diagnosis and breakthrough treatments.
Acknowledgments
The authors are thankful to all lab members.
Abbreviations
| AMPK | AMP-activated protein kinase |
| CAF | Cancer-associated fibroblast |
| CT | computed tomography |
| DNMT | DNA methyltransferase |
| EMT | epithelial-mesenchymal transition |
| FAP | fibroblast activation protein |
| FAPI | FAP inhibitor |
| FGFR | fibroblast growth factor receptor |
| EGFR-TKI | epidermal growth factor receptor |
| TKI | tyrosine kinase inhibitor |
| HER2 | human epidermal growth factor type 2 |
| HG | D-2-hydroxyglurate |
| HSPC | hematopoietic stem and progenitor cell |
| IDH | isocitrate dehydrogenase |
| METTL | adenosine-methyltransferase |
| MNAM | 1-methylnicotinamide |
| MTHFD | methylenetetrahydrofolate dehydrogenase |
| NAD | Nicotinamide adenine dinucleotide |
| NAM | nicotinamide |
| NNMT | nicotinamide N-methyltransferase |
| ODC | ornithine decarboxylase |
| 2PY | N-methyl-2-pyridone-5-carboxamide |
| 4PY | N-methyl-4-pyridone-3-carboxamide |
| ROS | reactive oxygen species |
| SAH | S-adenosylhomocysteine |
| SAM | S-adenosylmethionine |
| SHMT | serine hydroxymethyltransferase |
| Stat | signal transducer and activator of transcription |
| TDH | L-threonine dehydrogenase |
| TME | tumor microenvironment |
| UPS | ubiquitin-proteasome system |
| PET | Positron emission tomography |
| PTRT | Peptide-targeted radionuclide therapy |
| WTAP | WT1-associated protein |
| YTHDF1 | YT521-B homology (YTH) N6-methyladenosine RNA binding protein 1 |
Author Contributions
H.I. and K.O. (Kazuhiko Ogawa) conceptualized the study objectives and obtained funding. S.T., K.O. (Ken Ofusa), R.C., K.T., K.O. (Kazuhiko Ogawa) and H.I. wrote the manuscript. S.T., R.C., A.V. and H.I. designed the review and outlined the content. S.T., K.O. (Ken Ofusa), A.V., K.T. and H.I. suggested which disease information should be addressed in the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (19K22658; 20H00541; 21K19526; AMED, Japan; 17cm0106414h0002) to H.I. Partial support was received from Princess Takamatsu Cancer Research Fund, Senshin Medical Research Foundation, and Mitsubishi Foundation to H.I.
Data Availability Statement
Not applicable.
Conflicts of Interest
Partial institutional endowments were received from Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan), Hirotsu Bio Science Inc. (Tokyo, Japan), Kinshu-kai Medical Corporation (Osaka, Japan), Kyowa-kai Medical Corporation (Osaka, Japan), IDEA Consultants Inc. (Tokyo, Japan), and Unitech Co. Ltd. (Chiba, Japan). K.O. is an employee of IDEA Consultants Inc. (Tokyo, Japan).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gorchs L., Kaipe H. Interactions between Cancer-Associated Fibroblasts and T Cells in the Pancreatic Tumor Microenvironment and the Role of Chemokines. Cancers. 2021;13:2995. doi: 10.3390/cancers13122995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X., Ramadori P., Pfister D., Seehawer M., Zender L., Heikenwalder M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer. 2021;21:541–557. doi: 10.1038/s41568-021-00383-9. [DOI] [PubMed] [Google Scholar]
- 3.Moindjie H., Rodrigues-Ferreira S., Nahmias C. Mitochondrial Metabolism in Carcinogenesis and Cancer Therapy. Cancers. 2021;13:3311. doi: 10.3390/cancers13133311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeda Y., Chijimatsu R., Vecchione A., Arai T., Kitagawa T., Ofusa K., Yabumoto M., Hirotsu T., Eguchi H., Doki Y., et al. Impact of One-Carbon Metabolism-Driving Epitranscriptome as a Therapeutic Target for Gastrointestinal Cancer. Int. J. Mol. Sci. 2021;22:7278. doi: 10.3390/ijms22147278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papadimitriou N., Markozannes G., Kanellopoulou A., Critselis E., Alhardan S., Karafousia V., Kasimis J.C., Katsaraki C., Papadopoulou A., Zografou M., et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat. Commun. 2021;12:4579. doi: 10.1038/s41467-021-24861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J.Y., Butler L.M., Wang R., Jin A., Koh W.P., Yuan J.M. Dietary Intake of One-Carbon Metabolism-Related Nutrients and Pancreatic Cancer Risk: The Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 2016;25:417–424. doi: 10.1158/1055-9965.EPI-15-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pieroth R., Paver S., Day S., Lammersfeld C. Folate and its impact on cancer risk. Curr. Nutr. Rep. 2018;7:70–84. doi: 10.1007/s13668-018-0237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitakaze M., Chijimatsu R., Vecchione A., Kitagawa T., Doki Y., Eguchi H., Ishii H. Epithelial Cell Transformation and Senescence as Indicators of Genome Aging: Current Advances and Unanswered Questions. Int. J. Mol. Sci. 2021;22:7544. doi: 10.3390/ijms22147544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagasawa S., Kashima Y., Suzuki A., Suzuki Y. Single-cell and spatial analyses of cancer cells: Toward elucidating the molecular mechanisms of clonal evolution and drug resistance acquisition. Inflamm. Regen. 2021;41:22. doi: 10.1186/s41232-021-00170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L., Xu T., Zhang J., Wong S.C.C., Ritchie M., Hou H.W., Wang Y. Single Cell Metabolite Detection Using Inertial Microfluidics-Assisted Ion Mobility Mass Spectrometry. Anal. Chem. 2021;93:10462–10468. doi: 10.1021/acs.analchem.1c00106. [DOI] [PubMed] [Google Scholar]
- 11.Sun Q., Huang M., Wei Y. Diversity of the reaction mechanisms of SAM-dependent enzymes. Acta Pharm. Sin. B. 2021;11:632–650. doi: 10.1016/j.apsb.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauinger L., Kaiser P. Sensing and Signaling of Methionine Metabolism. Metabolites. 2021;11:83. doi: 10.3390/metabo11020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 14.Vander Heiden M.G.C.L., Thompson C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang C.V., Le A., Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin. Cancer Res. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S.-H., Baek K.-H. Regulation of Cancer Metabolism by Deubiquitinating Enzymes: The Warburg Effect. Int. J. Mol. Sci. 2021;22:6173. doi: 10.3390/ijms22126173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise D.R., Ward P.S., Shay J.E.S., Cross J.R., Gruber J.J., Sachdeva U.M., Platt J.M., DeMatteo R.G., Simon M.C., Thompson C.B. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. USA. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metallo C.M., Gameiro P.A., Bell E.L., Mattaini K.R., Yang J., Hiller K., Jewell C.M., Johnson Z.R., Irvine D.J., Guarente L., et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colvin H., Nishida N., Konno M., Haraguchi N., Takahashi H., Nishimura J., Hata T., Kawamoto K., Asai A., Tsunekuni K., et al. Oncometabolite D-2-Hydroxyglurate Directly Induces Epithelial-Mesenchymal Transition and is Associated with Distant Metastasis in Colorectal Cancer. Sci. Rep. 2016;6:36289. doi: 10.1038/srep36289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koseki J., Colvin H., Fukusumi T., Nishida N., Konno M., Kawamoto K., Tsunekuni K., Matsui H., Doki Y., Mori M., et al. Mathematical analysis predicts imbalanced IDH1/2 expression associates with 2-HG-inactivating β-oxygenation pathway in colorectal cancer. Int. J. Oncol. 2015;46:1181–1191. doi: 10.3892/ijo.2015.2833. [DOI] [PubMed] [Google Scholar]
- 21.Son J., Lyssiotis C.A., Ying H., Wang X., Hua S., Ligorio M., Perera R.M., Ferrone C.R., Mullarky E., Shyh-Chang N., et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyo M., Konno M., Nishida N., Sueda T., Noguchi K., Matsui H., Colvin H., Kawamoto K., Koseki J., Haraguchi N., et al. Metabolic Adaptation to Nutritional Stress in Human Colorectal Cancer. Sci. Rep. 2016;6:38415. doi: 10.1038/srep38415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sueda T., Sakai D., Kawamoto K., Konno M., Nishida N., Koseki J., Colvin H., Takahashi H., Haraguchi N., Nishimura J., et al. BRAFV600E inhibition stimulates AMP-activated protein kinase-mediated autophagy in colorectal cancer cells. Sci. Rep. 2016;6:18949. doi: 10.1038/srep18949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajishengallis G., Li X., Chavakis T. Immunometabolic control of hematopoiesis. Mol. Aspects Med. 2021;77:100923. doi: 10.1016/j.mam.2020.100923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ducker G.S., Rabinowitz J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017;25:27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller D.R. A tribute to Sidney Farber—The father of modern chemotherapy. Br. J. Haematol. 2006;134:20–26. doi: 10.1111/j.1365-2141.2006.06119.x. [DOI] [PubMed] [Google Scholar]
- 27.Bedoui Y., Guillot X., Sélambarom J., Guiraud P., Giry C., Jaffar-Bandjee M.C., Ralandison S., Gasque P. Methotrexate an Old Drug with New Tricks. Int. J. Mol. Sci. 2019;20:5023. doi: 10.3390/ijms20205023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koseki J., Konno M., Asai A., Colvin H., Kawamoto K., Nishida N., Sakai D., Kudo T., Satoh T., Doki Y., et al. Enzymes of the one-carbon folate metabolism as anticancer targets predicted by survival rate analysis. Sci. Rep. 2018;8:303. doi: 10.1038/s41598-017-18456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asai A., Koseki J., Konno M., Nishimura T., Gotoh N., Satoh T., Doki Y., Mori M., Ishii H. Drug discovery of anticancer drugs targeting methylenetetrahydrofolate dehydrogenase 2. Heliyon. 2018;4:e01021. doi: 10.1016/j.heliyon.2018.e01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asai A., Konno M., Koseki J., Taniguchi M., Vecchione A., Ishii H. One-carbon metabolism for cancer diagnostic and therapeutic approaches. Cancer Lett. 2020;470:141–148. doi: 10.1016/j.canlet.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser P. Methionine Dependence of Cancer. Biomolecules. 2020;10:568. doi: 10.3390/biom10040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stern P.H., Wallace C.D., Hoffman R.M. Altered methionine metabolism occurs in all members of a set of diverse human tumor cell lines. J. Cell Physiol. 1984;119:29–34. doi: 10.1002/jcp.1041190106. [DOI] [PubMed] [Google Scholar]
- 33.Booher K., Lin D.W., Borrego S.L., Kaiser P. Downregulation of Cdc6 and pre-replication complexes in response to methionine stress in breast cancer cells. Cell Cycle. 2012;11:4414–4423. doi: 10.4161/cc.22767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borrego S.L., Fahrmann J., Datta R., Stringari C., Grapov D., Zeller M., Chen Y., Wang P., Baldi P., Gratton E., et al. Metabolic changes associated with methionine stress sensitivity in MDA-MB-468 breast cancer cells. Cancer Metab. 2016;4:9. doi: 10.1186/s40170-016-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin D.W., Chung B.P., Kaiser P. S-adenosylmethionine limitation induces p38 mitogen-activated protein kinase and triggers cell cycle arrest in G1. J. Cell Sci. 2014;127:50–59. doi: 10.1242/jcs.127811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugimura T., Birnbaum S.M., Winitz M., Greenstein J.P. Quantitative nutritional studies with water-soluble, chemically defined diets. VIII. The forced feeding of diets each lacking in one essential amino acid. Arch. Biochem. Biophys. 1959;81:448–455. doi: 10.1016/0003-9861(59)90225-5. [DOI] [PubMed] [Google Scholar]
- 37.Pietro E.D., Sirois J., Tremblay M.L., MacKenzie R.E. Mitochondrial NAD-dependent methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase is essential for embryonic development. Mol. Cell Biol. 2002;22:4158–4166. doi: 10.1128/MCB.22.12.4158-4166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ron-Harel N., Santos D., Ghergurovich J.M., Sage P.T., Reddy A., Lovitch S.B., Dephoure N., Satterstrom F.K., Sheffer M., Spinelli J.B., et al. Mitochondrial biogenesis and proteome remodeling promote one-carbon metabolism for T cell activation. Cell Metab. 2016;24:104–117. doi: 10.1016/j.cmet.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ducker G.S., Chen L., Morscher R.J., Ghergurovich J.M., Esposito M., Teng X., Kang Y., Rabinowitz J.D. Reversal of Cytosolic One-Carbon Flux Compensates for Loss of the Mitochondrial Folate Pathway. Cell Metab. 2016;23:1140–1153. doi: 10.1016/j.cmet.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taya Y., Ota Y., Wilkinson A.C., Kanazawa A., Watarai H., Kasai M., Nakauchi H., Yamazaki S. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science. 2016;354:1152–1155. doi: 10.1126/science.aag3145. [DOI] [PubMed] [Google Scholar]
- 41.Kornberg A., Daft F.S., Sebrell W.H. Granulocytopenia and Anemia in Rats Fed Diets of Low Casein Content. Science. 1946;103:646–648. doi: 10.1126/science.103.2682.646. [DOI] [PubMed] [Google Scholar]
- 42.Kornberg A. Amino acids in the production of granulocytes in rats. J. Biol. Chem. 1946;64:203–212. doi: 10.1016/S0021-9258(18)43060-8. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson A.C., Ishida R., Kikuchi M., Sudo K., Morita M., Crisostomo R.V., Yamamoto R., Loh K.M., Nakamura Y., Watanabe M., et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature. 2019;571:117–121. doi: 10.1038/s41586-019-1244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamari K., Hayashi K., Ishii H., Kano Y., Konno M., Kawamoto K., Nishida N., Koseki J., Fukusumi T., Hasegawa S., et al. Identification of chemoradiation-resistant osteosarcoma stem cells using an imaging system for proteasome activity. Int. J. Oncol. 2014;45:2349–2354. doi: 10.3892/ijo.2014.2671. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi K., Tamari K., Ishii H., Konno M., Nishida N., Kawamoto K., Koseki J., Fukusumi T., Kano Y., Nishikawa S., et al. Visualization and characterization of cancer stem-like cells in cervical cancer. Int. J. Oncol. 2014;45:2468–2474. doi: 10.3892/ijo.2014.2670. [DOI] [PubMed] [Google Scholar]
- 46.Kano Y., Konno M., Kawamoto K., Tamari K., Hayashi K., Fukusumi T., Satoh T., Tanaka S., Ogawa K., Mori M., et al. Novel drug discovery system for cancer stem cells in human squamous cell carcinoma of the esophagus. Oncol. Rep. 2014;31:1133–1138. doi: 10.3892/or.2013.2952. [DOI] [PubMed] [Google Scholar]
- 47.Tamari K., Konno M., Asai A., Koseki J., Hayashi K., Kawamoto K., Murai N., Matsufuji S., Isohashi F., Satoh T., et al. Polyamine flux suppresses histone lysine demethylases and enhances ID1 expression in cancer stem cells. Cell Death Discov. 2018;4:104. doi: 10.1038/s41420-018-0117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chater-Diehl E., Goodman S.J., Cytrynbaum C., Turinsky A.L., Choufani S., Weksberg R. Anatomy of DNA methylation signatures: Emerging insights and applications. Am. J. Hum. Genet. 2021;108:1359–1366. doi: 10.1016/j.ajhg.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adashek J.J., Janku F., Kurzrock R. Signed in Blood: Circulating Tumor DNA in Cancer Diagnosis, Treatment and Screening. Cancers. 2021;13:3600. doi: 10.3390/cancers13143600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konno M., Taniguchi M., Ishii H. Significant epitranscriptomes in heterogeneous cancer. Cancer Sci. 2019;110:2318–2327. doi: 10.1111/cas.14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konno M., Koseki J., Asai A., Yamagata A., Shimamura T., Motooka D., Okuzaki D., Kawamoto K., Mizushima T., Eguchi H., et al. Distinct methylation levels of mature microRNAs in gastrointestinal cancers. Nat. Commun. 2019;10:3888. doi: 10.1038/s41467-019-11826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J., Yue Y., Han D., Wang X., Fu Y., Zhang L., Jia G., Yu M., Lu Z., Deng X., et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer K.D., Jaffrey S.R. Rethinking m6A Readers, Writers, and Erasers. Annu Rev. Cell Dev. Biol. 2017;33:319–342. doi: 10.1146/annurev-cellbio-100616-060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y., Yi C., Lindahl T., Pan T., Yang Y.G. He CN6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated, FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C.M., Li C.J., Vågbø C.B., Shi Y., Wang W.L., Song S.H., et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishizawa Y., Konno M., Asai A., Koseki J., Kawamoto K., Miyoshim N., Takahashi H., Nishida N., Haraguchi N., Sakai D., et al. Oncogene c-Myc Promotes Epitranscriptome m6A Reader YTHDF1 Expression in Colorectal Cancer. Oncotarget. 2017;9:7476–7486. doi: 10.18632/oncotarget.23554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cantoni G.L. Methylation of nicotinamide with soluble enzyme system from rat liver. J. Biol. Chem. 1951;189:203–216. doi: 10.1016/S0021-9258(18)56110-X. [DOI] [PubMed] [Google Scholar]
- 58.Cantoni G.L. The nature of the active methyl donor formed enzymatically from L-methionine and adenosinetriphosphate. J. Am. Chem. Soc. 1952;74:2942–2943. doi: 10.1021/ja01131a519. [DOI] [Google Scholar]
- 59.Kujundžić R.N., Prpićm Đaković N., Dabelić N., Tomljanović M., Mojzeš A., Fröbe A., Trošelj K.G. Nicotinamide N-Methyltransferase in Acquisition of Stem Cell Properties and Therapy Resistance in Cancer. Int. J. Mol. Sci. 2021;22:5681. doi: 10.3390/ijms22115681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pissios P. Nicotinamide methyltransferase: More than a vitamin B3 clearance enzyme. Trends Endocrinol. Metab. 2017;28:340–353. doi: 10.1016/j.tem.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramsden D.B., Waring R.H., Barlow D.J., Parsons R.B. Nicotinamide N-methyltransferase in health and cancer. Int. J. Tryptophan. Res. 2017;10:1177. doi: 10.1177/1178646917691739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu X.M., Long H. Nicotinamide N-methyltransferase as a potential marker for cancer. Neoplasma. 2018;65:656–663. doi: 10.4149/neo_2018_171024N680. [DOI] [PubMed] [Google Scholar]
- 63.Li S., Qiao L., Yang Z., He C. Prognostic Value of Nicotinamide N-Methyltransferase Expression in Patients With Solid Tumors: A Systematic Review and Meta-Analysis. Front. Physiol. 2018;9:1407. doi: 10.3389/fphys.2018.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Q., He M.D., Mao L., Wang X., Jiang Y.L., Li M., Lu Y.H., Yu Z.P., Zhou Z. Nicotinamide N-Methyltransferase Suppression Participates in Nickel-Induced Histone H3 Lysine9 Dimethylation in BEAS-2B Cells. Cell Physiol. Biochem. 2017;41:2016–2026. doi: 10.1159/000475432. [DOI] [PubMed] [Google Scholar]
- 65.Kanska J., Aspuria P.P., Taylor-Harding B., Spurka L., Funar I.V., Orsulic S., Karlan B.Y., Wiedemeyer W.R. Glucose deprivation elicits phenotypic plasticity via ZEB1-mediated expression of NNMT. Oncotarget. 2017;8:26200–26220. doi: 10.18632/oncotarget.15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang L., Zeng M., Pan H., Liu H., He Y. Nicotinamide N-methyltransferase promotes epithelial-mesenchymal transition in gastric cancer cells by activating transforming growth factor-beta1 expression. Oncol. Lett. 2018;15:4592–4598. doi: 10.3892/ol.2018.7885. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Cui Y., Zhang L., Wang W., Ma S., Liu H., Zang X., Zhang Y., Guan F. Downregulation of nicotinamide N-methyltransferase inhibits migration and epithelial-mesenchymal transition of esophageal squamous cell carcinoma via Wnt/beta-catenin pathway. Mol. Cell Biochem. 2019;460:93–103. doi: 10.1007/s11010-019-03573-0. [DOI] [PubMed] [Google Scholar]
- 68.Cui Y., Yang D., Wang W., Zhang L., Liu H., Ma S., Guo W., Yao M., Zhang K., Li W., et al. Nicotinamide N-methyltransferase decreases 5-fluorouracil sensitivity in human esophageal squamous cell carcinoma through metabolic reprogramming and promoting the Warburg effect. Mol. Carcinog. 2020;59:940–954. doi: 10.1002/mc.23209. [DOI] [PubMed] [Google Scholar]
- 69.Xie X., Liu H., Wang Y., Zhou Y., Yu H., Li G., Ruan Z., Li F., Wang X., Zhang J. Nicotinamide N-methyltransferase enhances resistance to 5-fluorouracil in colorectal cancer cells through inhibition of the ASK1-p38 MAPK pathway. Oncotarget. 2016;7:45837–45848. doi: 10.18632/oncotarget.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Win K.T., Lee S.W., Huang H.Y., Lin L.C., Lin C.Y., Hsing C.H., Chen L.T., Li C.F. Nicotinamide N-methyltransferase overexpression is associated with Akt phosphorylation and indicates worse prognosis in patients with nasopharyngeal carcinoma. Tumour Biol. 2013;34:3923–3931. doi: 10.1007/s13277-013-0980-z. [DOI] [PubMed] [Google Scholar]
- 71.Li J., You S., Zhang S., Hu Q., Wang F., Chi X., Zhao W., Xie C., Zhang C., Yu Y., et al. Elevated N-methyltransferase expression induced by hepatic stellate cells contributes to the metastasis of hepatocellular carcinoma via regulation of the CD44v3 isoform. Mol. Oncol. 2019;13:1993–2009. doi: 10.1002/1878-0261.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kilgour M.K., MacPherson S., Zacharias L.G., Ellis A.E., Sheldon R.D., Liu E.Y., Keyes S., Pauly B., Carleton G., Allard B., et al. 1-Methylnicotinamide is an immune regulatory metabolite in human ovarian cancer. Sci. Adv. 2021;7:eabe1174. doi: 10.1126/sciadv.abe1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watabe T., Liu Y., Kaneda-Nakashima K., Shirakami Y., Lindner T., Ooe K., Toyoshima A., Nagata K., Shimosegawa E., Haberkorn U., et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: 64 Cu- and 225 Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2020;61:563–569. doi: 10.2967/jnumed.119.233122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang H., Zhu W., Ren S., Kong Y., Huang Q., Zhao J., Guan Y., Jia H., Chen J., Lu L., et al. 68Ga-FAPI-04 Versus 18F-FDG PET/CT in the Detection of Hepatocellular Carcinoma. Front. Oncol. 2021;11:693640. doi: 10.3389/fonc.2021.693640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Röhrich M., Leitz D., Glatting F.M., Wefers A.K., Weinheimer O., Flechsig P., Kahn N., Mall M.A., Giesel F.L., Kratochwil C., et al. Fibroblast Activation Protein specific PET/CT imaging in fibrotic interstitial lung diseases and lung cancer: A translational exploratory study. J. Nucl. Med. 2021;62 doi: 10.2967/jnumed.121.261925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.You Z., Liu Y., Liu X. Nicotinamide N-methyltransferase enhances the progression of prostate cancer by stabilizing sirtuin 1. Oncol. Lett. 2018;15:9195–9201. doi: 10.3892/ol.2018.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu K.Y., Mistry R.J., Aguirre C.A., Fasouli E.S., Thomas M.G., Klamt F., Ramsden D.B., Parsons R.B. Nicotinamide N-methyltransferase increases complex I activity in SH-SY5Y cells via sirtuin 3. Biochem. Biophys. Res. Commun. 2015;467:491–496. doi: 10.1016/j.bbrc.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 78.Campagna R., Salvolini E., Pompei V., Pozzi V., Salvucci A., Molinelli E., Brisigotti V., Sartini D., Campanati A., Offidani A., et al. Nicotinamide N-methyltransferase gene silencing enhances chemosensitivity of melanoma cell lines. Pigment. Cell Melanoma Res. 2021 doi: 10.1111/pcmr.12993. [DOI] [PubMed] [Google Scholar]
- 79.Li G., Kong B., Tong Q., Li Y., Chen L., Zeng J., Yu H., Xie X., Zhang J. Vanillin downregulates NNMT and attenuates NNMT-related resistance to 5-fluorouracil via ROS-induced cell apoptosis in colorectal cancer cells. Oncol Rep. 2021;45:110. doi: 10.3892/or.2021.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akar S., Duran T., Azzawri A.A., Koçak N., Çelik Ç., Yıldırım H.İ. Small molecule inhibitor of nicotinamide N-methyltransferase shows anti-proliferative activity in HeLa cells. J. Obestet. Gynaecol. 2021:1–9. doi: 10.1080/01443615.2020.1854696. [DOI] [PubMed] [Google Scholar]
- 81.Zhu A.K., Shan Y.Q., Zhang J., Liu X.C., Ying R.C., Kong W.C. Exosomal NNMT from peritoneum lavage fluid promotes peritoneal metastasis in gastric cancer. Kaohsiung J. Med. Sci. 2021;37:305–313. doi: 10.1002/kjm2.12334. [DOI] [PubMed] [Google Scholar]
- 82.Li J., Yue H., Yu H., Lu X., Xue X. Patients with low nicotinamide N-methyltransferase expression benefit significantly from bevacizumab treatment in ovarian cancer. BMC Cancer. 2021;21:67. doi: 10.1186/s12885-021-07785-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang L., Song M., Zhang F., Yuan H., Chang W., Yu G., Niu Y. Accumulation of Nicotinamide N-Methyltransferase (NNMT) in Cancer-associated Fibroblasts: A Potential Prognostic and Predictive Biomarker for Gastric Carcinoma. J. Histochem Cytochem. 2021;69:165–176. doi: 10.1369/0022155420976590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rivas M.P., Aguiar T.F.M., Maschietto M., Lemes R.B., Caires-Júnior L.C., Goulart E., Telles-Silva K.A., Novak E., Cristofani L.M., Odone V., et al. Hepatoblastomas exhibit marked NNMT downregulation driven by promoter DNA hypermethylation. Tumour Biol. 2020;42:1010428320977124. doi: 10.1177/1010428320977124. [DOI] [PubMed] [Google Scholar]
- 85.Wu M., Hu W., Wang G., Yao Y., Yu X.F. Nicotinamide N-Methyltransferase Is a Prognostic Biomarker and Correlated With Immune Infiltrates in Gastric Cancer. Front. Genet. 2020;11:580299. doi: 10.3389/fgene.2020.580299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu H., Zhou X., Wang Y., Huang X., Yang J., Zeng J., Li G., Xie X., Zhang J. Nicotinamide N-methyltransferase inhibits autophagy induced by oxidative stress through suppressing the AMPK pathway in breast cancer cells. Cancer Cell Int. 2020;20:191. doi: 10.1186/s12935-020-01279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harmankaya İ., Akar S., Uğraş S., Güler A.H., Ezveci H., Aydoğdu M., Çelik Ç. Nicotinamide N-methyltransferase overexpression may be associated with poor prognosis in ovarian cancer. J. Obs. Gynaecol. 2021;41:248–253. doi: 10.1080/01443615.2020.1732891. [DOI] [PubMed] [Google Scholar]
- 88.Pozzi V., Salvolini E., Lucarini G., Salvucci A., Campagna R., Rubini C., Sartini D., Emanuelli M. Cancer stem cell enrichment is associated with enhancement of nicotinamide N-methyltransferase expression. IUBMB Life. 2020;72:1415–1425. doi: 10.1002/iub.2265. [DOI] [PubMed] [Google Scholar]
- 89.Song M., Li Y., Miao M., Zhang F., Yuan H., Cao F., Chang W., Shi H., Song C. High stromal nicotinamide N-methyltransferase (NNMT) indicates poor prognosis in colorectal cancer. Cancer Med. 2020;9:2030–2038. doi: 10.1002/cam4.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akar S., Harmankaya İ., Uğraş S., Çelik Ç. Nicotinamide N-methyltransferase expression and its association with phospho-Akt, p53 expression, and survival in high-grade endometrial cancer. Turk. J. Med. Sci. 2019;49:1547–1554. doi: 10.3906/sag-1907-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pompei V., Salvolini E., Rubini C., Lucarini G., Molinelli E., Brisigotti V., Pozzi V., Sartini D., Campanati A., Offidani A., et al. Nicotinamide N-methyltransferase in nonmelanoma skin cancers. Eur. J. Clin. Invest. 2019;49:e13175. doi: 10.1111/eci.13175. [DOI] [PubMed] [Google Scholar]
- 92.Holstein S., Venz S., Junker H., Walther R., Stope M.B., Zimmermann U. Nicotinamide N-Methyltransferase and Its Precursor Substrate Methionine Directly and Indirectly Control Malignant Metabolism During Progression of Renal Cell Carcinoma. Anticancer Res. 2019;39:5427–5436. doi: 10.21873/anticanres.13736. [DOI] [PubMed] [Google Scholar]
- 93.Hah Y.S., Cho H.Y., Jo S.Y., Park Y.S., Heo E.P., Yoon T.J. Nicotinamide N-methyltransferase induces the proliferation and invasion of squamous cell carcinoma cells. Oncol. Rep. 2019;42:1805–1814. doi: 10.3892/or.2019.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y., Zeng J., Wu W., Xie S., Yu H., Li G., Zhu T., Li F., Lu J., Wang G.Y., et al. Nicotinamide N-methyltransferase enhances chemoresistance in breast cancer through SIRT1 protein stabilization. Breast Cancer Res. 2019;21:64. doi: 10.1186/s13058-019-1150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eckert M.A., Coscia F., Chryplewicz A., Chang J.W., Hernandez K.M., Pan S., Tienda S.M., Nahotko D.A., Li G., Blaženović I., et al. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature. 2019;569:723–728. doi: 10.1038/s41586-019-1173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akar S., Harmankaya İ., Uğraş S., Çelik Ç. Expression and Clinical Significance of Nicotinamide N-Methyltransferase in Cervical Squamous Cell Carcinoma. Int. J. Gynecol. Pathol. 2020;39:289–295. doi: 10.1097/PGP.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 97.Mascitti M., Santarelli A., Sartini D., Rubini C., Colella G., Salvolini E., Ganzetti G., Offidani A., Emanuelli M. Analysis of nicotinamide N-methyltransferase in oral malignant melanoma and potential prognostic significance. Melanoma Res. 2019;29:151–156. doi: 10.1097/CMR.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 98.Bach D.H., Kim D., Bae S.Y., Kim W.K., Hong J.Y., Lee H.J., Rajasekaran N., Kwon S., Fan Y., Luu T.T., et al. Targeting Nicotinamide N-Methyltransferase and miR-449a in EGFR-TKI-Resistant Non-Small-Cell Lung Cancer Cells. Mol. Nucleic Acids. 2018;11:455–467. doi: 10.1016/j.omtn.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ganzetti G., Sartini D., Campanati A., Rubini C., Molinelli E., Brisigotti V., Cecati M., Pozzi V., Campagna R., Offidani A., et al. Nicotinamide N-methyltransferase: Potential involvement in cutaneous malignant melanoma. Melanoma Res. 2018 28:82–88. doi: 10.1097/CMR.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 100.Ishibashi K., Ishii K., Sugiyama G., Sumida T., Sugiura T., Kamata Y.U., Seki K., Fujinaga T., Kumamaru W., Kobayashi Y., et al. Deregulation of Nicotinamide N-Methyltransferase and Gap Junction Protein Alpha-1 Causes Metastasis in Adenoid Cystic Carcinoma. Anticancer Res. 2018;38:187–197. doi: 10.21873/anticanres.12207. [DOI] [PubMed] [Google Scholar]
- 101.Chen C., Wang X., Huang X., Yong H., Shen J., Tang Q., Zhu J., Ni J., Feng Z. Nicotinamide N-methyltransferase: A potential biomarker for worse prognosis in gastric carcinoma. Am. J. Cancer Res. 2016;6:649–663. [PMC free article] [PubMed] [Google Scholar]
- 102.Neely B.A., Wilkins C.E., Marlow L.A., Malyarenko D., Kim Y., Ignatchenko A., Sasinowska H., Sasinowski M., Nyalwidhe J.O., Kislinger T., et al. Proteotranscriptomic Analysis Reveals Stage Specific Changes in the Molecular Landscape of Clear-Cell Renal Cell Carcinoma. PLoS ONE. 2016;11:e0154074. doi: 10.1371/journal.pone.0154074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu Y., Liu P., Zheng D.H., Wu N., Zhu L., Xing C., Zhu J. Expression profile and prognostic value of NNMT in patients with pancreatic cancer. Oncotarget. 2016;7:19975–19981. doi: 10.18632/oncotarget.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J., Wang Y., Li G., Yu H., Xie X. Down-regulation of nicotinamide N-methyltransferase induces apoptosis in human breast cancer cells via the mitochondria-mediated pathway. PLoS ONE. 2014;9:e89202. doi: 10.1371/journal.pone.0089202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pozzi V., Sartini D., Morganti S., Giuliante R., Di Ruscio G., Santarelli A., Rocchetti R., Rubini C., Tomasetti M., Giannatempo G., et al. RNA-mediated gene silencing of nicotinamide N-methyltransferase is associated with decreased tumorigenicity in human oral carcinoma cells. PLoS ONE. 2013;8:e71272. doi: 10.1371/journal.pone.0071272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sartini D., Muzzonigro G., Milanese G., Pozzi V., Vici A., Morganti S., Rossi V., Mazzucchelli R., Montironi R., Emanuelli M. Upregulation of tissue and urinary nicotinamide N-methyltransferase in bladder cancer: Potential for the development of a urine-based diagnostic test. Cell Biochem. Biophys. 2013;65:473–483. doi: 10.1007/s12013-012-9451-1. [DOI] [PubMed] [Google Scholar]
- 107.Sartini D., Pozzi V., Renzi E., Morganti S., Rocchetti R., Rubini C., Santarelli A., Lo Muzio L., Emanuelli M. Analysis of tissue and salivary nicotinamide N-methyltransferase in oral squamous cell carcinoma: Basis for the development of a noninvasive diagnostic test for early-stage disease. Biol Chem. 2012;393:505–511. doi: 10.1515/hsz-2012-0112. [DOI] [PubMed] [Google Scholar]
- 108.D’Andrea F.P., Safwat A., Kassem M., Gautier L., Overgaard J., Horsman M.R. Cancer stem cell overexpression of nicotinamide N-methyltransferase enhances cellular radiation resistance. Radiother. Oncol. 2011;99:373–378. doi: 10.1016/j.radonc.2011.05.086. [DOI] [PubMed] [Google Scholar]
- 109.Tang S.W., Yang T.C., Lin W.C., Chang W.H., Wang C.C., Lai M.K., Lin J.Y. Nicotinamide N-methyltransferase induces cellular invasion through activating matrix metalloproteinase-2 expression in clear cell renal cell carcinoma cells. Carcinogenesis. 2011;32:138–145. doi: 10.1093/carcin/bgq225. [DOI] [PubMed] [Google Scholar]
- 110.Yamada K., Miyazaki T., Hara N., Tsuchiya M. Interferon-gamma elevates nicotinamide N-methyltransferase activity and nicotinamide level in human glioma cells. J. Nutr. Sci. Vitam. 2010;56:83–86. doi: 10.3177/jnsv.56.83. [DOI] [PubMed] [Google Scholar]
- 111.Emanuelli M., Santarelli A., Sartini D., Ciavarella D., Rossi V., Pozzi V., Rubini C., Lo Muzio L. Nicotinamide N-Methyltransferase upregulation correlates with tumour differentiation in oral squamous cell carcinoma. Histol. Histopathol. 2010;25:15–20. doi: 10.14670/HH-25.15. [DOI] [PubMed] [Google Scholar]
- 112.Tomida M., Mikami I., Takeuchi S., Nishimura H., Akiyama H. Serum levels of nicotinamide N-methyltransferase in patients with lung cancer. J. Cancer Res. Clin. Oncol. 2009;135:1223–1229. doi: 10.1007/s00432-009-0563-y. [DOI] [PubMed] [Google Scholar]
- 113.Kim J., Hong S.J., Lim E.K., Yu Y.S., Kim S.W., Roh J.H., Do I.G., Joh J.W., Kim D.S. Expression of nicotinamide N-methyltransferase in hepatocellular carcinoma is associated with poor prognosis. J. Exp. Clin. Cancer Res. 2009;28:20. doi: 10.1186/1756-9966-28-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu Y., Siadaty M.S., Berens M.E., Hampton G.M., Theodorescu D. Overlapping gene expression profiles of cell migration and tumor invasion in human bladder cancer identify metallothionein 1E and nicotinamide N-methyltransferase as novel regulators of cell migration. Oncogene. 2008;27:6679–6689. doi: 10.1038/onc.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tomida M., Ohtake H., Yokota T., Kobayashi Y., Kurosumi M. Stat3 up-regulates expression of nicotinamide N-methyltransferase in human cancer cells. J. Cancer Res. Clin. Oncol. 2008;134:551–559. doi: 10.1007/s00432-007-0318-6. [DOI] [PubMed] [Google Scholar]
- 116.Sartini D., Santarelli A., Rossi V., Goteri G., Rubini C., Ciavarella D., Lo Muzio L., Emanuelli M. Nicotinamide N-methyltransferase upregulation inversely correlates with lymph node metastasis in oral squamous cell carcinoma. Mol. Med. 2007;13:415–421. doi: 10.2119/2007-00035.Sartini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sartini D., Muzzonigro G., Milanese G., Pierella F., Rossi V., Emanuelli M. Identification of nicotinamide N-methyltransferase as a novel tumor marker for renal clear cell carcinoma. J. Urol. 2006;176:2248–2254. doi: 10.1016/j.juro.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 118.Roessler M., Rollinger W., Palme S., Hagmann M.L., Berndt P., Engel A.M., Schneidinger B., Pfeffer M., Andres H., Karl J., et al. Identification of nicotinamide N-methyltransferase as a novel serum tumor marker for colorectal cancer. Clin. Cancer Res. 2005;11:6550–6557. doi: 10.1158/1078-0432.CCR-05-0983. [DOI] [PubMed] [Google Scholar]
- 119.Xu J., Capezzone M., Xu X., Hershman J.M. Activation of nicotinamide N-methyltransferase gene promoter by hepatocyte nuclear factor-1beta in human papillary thyroid cancer cells. Mol. Endocrinol. 2005;19:527–539. doi: 10.1210/me.2004-0215. [DOI] [PubMed] [Google Scholar]
- 120.Kassem H.S., Sangar V., Cowan R., Clarke N., Margison G.P. A potential role of heat shock proteins and nicotinamide N-methyl transferase in predicting response to radiation in bladder cancer. Int. J. Cancer. 2002;101:454–460. doi: 10.1002/ijc.10631. [DOI] [PubMed] [Google Scholar]
- 121.Okamura A., Ohmura Y., Islam M.M., Tagawa M., Horitsu K., Moriyama Y., Fujimura S. Increased hepatic nicotinamide N-methyltransferase activity as a marker of cancer cachexia in mice bearing colon 26 adenocarcinoma. Jpn. J. Cancer Res. 1998;89:649–656. doi: 10.1111/j.1349-7006.1998.tb03267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hanazawa Y., Sato K., Kuroiwa N., Ogawa M., Kuriyama A., Asanagi M., Kato N., Moriyama Y., Horitsu K., Fujimura S. Characterization of nicotinamide methyltransferase in livers of mice bearing Ehrlich ascites tumors: Preferential increase of activity. Tumour Biol. 1994;15:7–16. doi: 10.1159/000217868. [DOI] [PubMed] [Google Scholar]
- 123.Nakagawa K., Miyazaki M., Okui K., Kato N., Moriyama Y., Fujimura S. N1-methylnicotinamide level in the blood after nicotinamide loading as further evidence for malignant tumor burden. Jpn. J. Cancer Res. 1991;82:1277–1283. doi: 10.1111/j.1349-7006.1991.tb01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Balaziova E., Vymola P., Hrabal P., Mateu R., Zubal M., Tomas R., Netuka D., Kramar F., Zemanova Z., Svobodova K., et al. Fibroblast Activation Protein Expressing Mesenchymal Cells Promote Glioblastoma Angiogenesis. Cancers. 2021;13:3304. doi: 10.3390/cancers13133304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Assadi M., Rekabpour S.J., Jafari E., Divband G., Nikkholgh B., Amini H., Kamali H., Ebrahimi S., Shakibazad N., Jokar N., et al. Feasibility and Therapeutic Potential of 177Lu-Fibroblast Activation Protein Inhibitor-46 for Patients With Relapsed or Refractory Cancers: A Preliminary Study. Clin. Nucl. Med. 2021;46:e523–e530. doi: 10.1097/RLU.0000000000003810. [DOI] [PubMed] [Google Scholar]
- 126.Kesch C., Yirga L., Dendl K., Handke A., Darr C., Krafft U., Radtke J.P., Tschirdewahn S., Szarvas T., Fazli L., et al. High fibroblast-activation-protein expression in castration-resistant prostate cancer supports the use of FAPI-molecular theranostics. Eur. J. Nucl. Med. Mol. Imaging. 2021 doi: 10.1007/s00259-021-05423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shou Y., Xue Q., Yuan J., Zhao J. 68Ga-FAPI-04 PET/MR is helpful in differential diagnosis of pancreatitis from pancreatic malignancy compared to 18F-FDG PET/CT: A case report. Eur. J. Hybrid. Imaging. 2021;5:12. doi: 10.1186/s41824-021-00106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baum R.P., Schuchardt C., Singh A., Chantadisai M., Robiller F.C., Zhang J., Mueller D., Eismant A., Almaguel F., Zboralski D., et al. Feasibility, Biodistribution and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy (PTRT) of Diverse Adenocarcinomas using (177)Lu-FAP-2286: First-in-Human Results. J. Nucl. Med. 2021;62 doi: 10.2967/jnumed.120.259192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang S., Zhou X., Xu X., Ding J., Liu S., Hou X., Li N., Zhu H., Yang Z. Clinical translational evaluation of Al(18)F-NOTA-FAPI for fibroblast activation protein-targeted tumour imaging. Eur. J. Nucl. Med. Mol. Imaging. 2021 doi: 10.1007/s00259-021-05470-5. [DOI] [PubMed] [Google Scholar]
- 130.Dendl K., Koerber S.A., Finck R., Mokoala K.M.G., Staudinger F., Schillings L., Heger U., Röhrich M., Kratochwil C., Sathekge M., et al. 68Ga-FAPI-PET/CT in patients with various gynecological malignancies. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:4089–4100. doi: 10.1007/s00259-021-05378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jin X., Wei M., Wang S., Wang G., Lai Y., Shi Y., Zhang Y., Yang Z., Wang X. Detecting fibroblast activation proteins in lymphoma using (68)Ga-FAPI PET/CT. J. Nucl. Med. 2021;62 doi: 10.2967/jnumed.121.262134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yuan Z., Hu H., Zhu Y., Zhang W., Fang Q., Qiao T., Ma T., Wang M., Huang R., Tang Q., et al. Colorectal cancer cell intrinsic fibroblast activation protein alpha binds to Enolase1 and activates NF-kappaB pathway to promote metastasis. Cell Death Dis. 2021;12:543. doi: 10.1038/s41419-021-03823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Röhrich M., Syed M., Liew D.P., Giesel F.L., Liermann J., Choyke P.L., Wefers A.K., Ritz T., Szymbara M., Schillings L., et al. 68Ga-FAPI-PET/CT improves diagnostic staging and radiotherapy planning of adenoid cystic carcinomas - Imaging analysis and histological validation. Radiother. Oncol. 2021;160:192–201. doi: 10.1016/j.radonc.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kessler L., Ferdinandus J., Hirmas N., Bauer S., Dirksen U., Zarrad F., Nader M., Chodyla M.K., Milosevic A., Umutlu L., et al. Ga-68-FAPI as diagnostic tool in sarcoma: Data from the FAPI-PET prospective observational trial. J. Nucl. Med. 2021;62 doi: 10.2967/jnumed.121.262096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sum E., Rapp M., Fröbel P., Le Clech M., Dürr H., Giusti A.M., Perro M., Speziale D., Kunz L., Menietti E., et al. Fibroblast Activation Protein alpha-Targeted CD40 Agonism Abrogates Systemic Toxicity and Enables Administration of High Doses to Induce Effective Antitumor Immunity. Clin. Cancer Res. 2021;27:4036–4053. doi: 10.1158/1078-0432.CCR-20-4001. [DOI] [PubMed] [Google Scholar]
- 136.Yang Z., Zhang L., Zhu H., Zhou K., Wang H., Wang Y., Su R., Guo D., Zhou L., Xu X., et al. Nanoparticle formulation of mycophenolate mofetil achieves enhanced efficacy against hepatocellular carcinoma by targeting tumour-associated fibroblast. J. Cell Mol. Med. 2021;25:3511–3523. doi: 10.1111/jcmm.16434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Slania S.L., Das D., Lisok A., Du Y., Jiang Z., Mease R.C., Rowe S.P., Nimmagadda S., Yang X., Pomper M.G. Imaging of Fibroblast Activation Protein in Cancer Xenografts Using Novel (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine-Based Small Molecules. J. Med. Chem. 2021;64:4059–4070. doi: 10.1021/acs.jmedchem.0c02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sitia L., Bonizzi A., Mazzucchelli S., Negri S., Sottani C., Grignani E., Rizzuto M.A., Prosperi D., Sorrentino L., Morasso C., et al. Selective Targeting of Cancer-Associated Fibroblasts by Engineered H-Ferritin Nanocages Loaded with Navitoclax. Cells. 2021;10:328. doi: 10.3390/cells10020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Katsube R., Noma K., Ohara T., Nishiwaki N., Kobayashi T., Komoto S., Sato H., Kashima H., Kato T., Kikuchi S., et al. Fibroblast activation protein targeted near infrared photoimmunotherapy (NIR PIT) overcomes therapeutic resistance in human esophageal cancer. Sci. Rep. 2021;11:1693. doi: 10.1038/s41598-021-81465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Röhrich M., Naumann P., Giesel F.L., Choyke P.L., Staudinger F., Wefers A., Liew D.P., Kratochwil C., Rathke H., Liermann J., et al. Impact of 68Ga-FAPI PET/CT Imaging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas. J. Nucl. Med. 2021;62:779–786. doi: 10.2967/jnumed.120.253062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shi X., Xing H., Yang X., Li F., Yao S., Zhang H., Zhao H., Hacker M., Huo L., Li X. Fibroblast imaging of hepatic carcinoma with (68)Ga-FAPI-04 PET/CT: A pilot study in patients with suspected hepatic nodules. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:196–203. doi: 10.1007/s00259-020-04882-z. [DOI] [PubMed] [Google Scholar]
- 142.Tansi F.L., Rüger R., Böhm C., Steiniger F., Raasch M., Mosig A.S., Kontermann R.E., Teichgräber U.K., Fahr A., Hilger I. Rapid Target Binding and Cargo Release of Activatable Liposomes Bearing HER2 and FAP Single-Chain Antibody Fragments Reveal Potentials for Image-Guided Delivery to Tumors. Pharmaceutics. 2020;12:972. doi: 10.3390/pharmaceutics12100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ristau J., Giesel F.L., Haefner M.F., Staudinger F., Lindner T., Merkel A., Schlittenhardt J., Kratochwil C., Choyke P.L., Herfarth K., et al. Impact of Primary Staging with Fibroblast Activation Protein Specific Enzyme Inhibitor (FAPI)-PET/CT on Radio-Oncologic Treatment Planning of Patients with Esophageal Cancer. Mol. Imaging Biol. 2020;22:1495–1500. doi: 10.1007/s11307-020-01548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moon E.S., Elvas F., Vliegen G., De Lombaerde S., Vangestel C., De Bruycker S., Bracke A., Eppard E., Greifenstein L., Klasen B., et al. Targeting fibroblast activation protein (FAP): Next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA(5m) chelators. EJNMMI Radiopharm. Chem. 2020;5:19. doi: 10.1186/s41181-020-00102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Trüb M., Uhlenbrock F., Claus C., Herzig P., Thelen M., Karanikas V., Bacac M., Amann M., Albrecht R., Ferrara-Koller C., et al. Fibroblast activation protein-targeted-4-1BB ligand agonist amplifies effector functions of intratumoral T cells in human cancer. J. Immunother. Cancer. 2020;8:e000238. doi: 10.1136/jitc-2019-000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Syed M., Flechsig P., Liermann J., Windisch P., Staudinger F., Akbaba S., Koerber S.A., Freudlsperger C., Plinkert P.K., Debus J., et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:2836–2845. doi: 10.1007/s00259-020-04859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Toms J., Kogler J., Maschauer S., Daniel C., Schmidkonz C., Kuwert T., Prante O. Targeting Fibroblast Activation Protein: Radiosynthesis and Preclinical Evaluation of an (18)F-Labeled FAP Inhibitor. J. Nucl. Med. 2020;61:1806–1813. doi: 10.2967/jnumed.120.242958. [DOI] [PubMed] [Google Scholar]
- 148.Lindner T., Altmann A., Krämer S., Kleist C., Loktev A., Kratochwil C., Giesel F., Mier W., Marme F., Debus J., et al. Design and Development of 99mTc-Labeled FAPI Tracers for SPECT Imaging and 188Re Therapy. J. Nucl. Med. 2020;61:1507–1513. doi: 10.2967/jnumed.119.239731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meyer C., Dahlbom M., Lindner T., Vauclin S., Mona C., Slavik R., Czernin J., Haberkorn U., Calais J. Radiation Dosimetry and Biodistribution of (68)Ga-FAPI-46 PET Imaging in Cancer Patients. J. Nucl. Med. 2020;61:1171–1177. doi: 10.2967/jnumed.119.236786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kratochwil C., Flechsig P., Lindner T., Abderrahim L., Altmann A., Mier W., Adeberg S., Rathke H., Röhrich M., Winter H., et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019;60:801–805. doi: 10.2967/jnumed.119.227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Giesel F.L., Kratochwil C., Lindner T., Marschalek M.M., Loktev A., Lehnert W., Debus J., Jäger D., Flechsig P., Altmann A., et al. (68)Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019;60:386–392. doi: 10.2967/jnumed.118.215913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brünker P., Wartha K., Friess T., Grau-Richards S., Waldhauer I., Koller C.F., Weiser B., Majety M., Runza V., Niu H., et al. RG7386, a Novel Tetravalent FAP-DR5 Antibody, Effectively Triggers FAP-Dependent, Avidity-Driven DR5 Hyperclustering and Tumor Cell Apoptosis. Mol. Cancer Ther. 2016;15:946–957. doi: 10.1158/1535-7163.MCT-15-0647. [DOI] [PubMed] [Google Scholar]
- 153.Jansen K., Heirbaut L., Cheng J.D., Joossens J., Ryabtsova O., Cos P., Maes L., Lambeir A.M., De Meester I., Augustyns K., et al. Selective Inhibitors of Fibroblast Activation Protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine Scaffold. ACS Med. Chem. Lett. 2013;4:491–496. doi: 10.1021/ml300410d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Narra K., Mullins S.R., Lee H.O., Strzemkowski-Brun B., Magalong K., Christiansen V.J., McKee P.A., Egleston B., Cohen S.J., Weiner L.M., et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting Fibroblast Activation Protein in patients with metastatic colorectal cancer. Cancer Biol. Ther. 2007;6:1691–1699. doi: 10.4161/cbt.6.11.4874. [DOI] [PubMed] [Google Scholar]
- 155.Scott A.M., Wiseman G., Welt S., Adje I.A., Lee F.T., Hopkins W., Divgi C.R., Hanson L.H., Mitchell P., Gansen D.N., et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res. 2003;9:1639–1647. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.