Abstract
Scientific research on neuro-cognitive mechanisms of autism often focuses on circuits that support social functioning. However, autism is a heterogeneous developmental variation in multiple domains, including social communication, but also language, cognition, and sensory-motor control. This suggests that the underlying mechanisms of autism share a domain-general foundation that impacts all of these processes. In this Perspective Review, we propose that autism is not a social deficit that results from an atypical “social brain”. Instead, typical social development relies on learning. In social animals, infants depend on their caregivers for survival, which makes social information vitally salient. The infant must learn to socially interact in order to survive and develop, and the most prominent learning in early life is crafted by social interactions. Therefore, the most prominent outcome of a learning variation is atypical social development. To support the hypothesis that autism results from a variation in learning, we first review evidence from neuroscience and developmental science, demonstrating that typical social development depends on two domain-general processes that determine learning: (a) motivation, guided by allostatic regulation of the internal milieu; and (b) multi-modal associations, determined by the statistical regularities of the external milieu. These two processes are basic ingredients of typical development because they determine allostasis-driven learning of the social environment. We then review evidence showing that allostasis and learning are affected among individuals with autism, both neurally and behaviorally. We conclude by proposing a novel domain-general framework that emphasizes allostasis-driven learning as a key process underlying autism. Guided by allostasis, humans learn to become social, therefore, the atypical social profile seen in autism can reflect a domain-general variation in allostasis-driven learning. This domain-general view raises novel research questions in both basic and clinical research and points to targets for clinical intervention that can lower the age of diagnosis and improve the well-being of individuals with autism.
Keywords: autism, learning, allostasis, social development, domain-general neural circuits, multi-modal integration, parent-infant synchrony
1. Introduction
Autism is a heterogeneous developmental variation, defined by the American Psychiatric Association as a persistent deficit in social communication and interaction [1]. As such, scientific investigations on autism often focus on social behaviors and their underlying neural mechanisms. While this approach provides insight into autism, it has two major challenges. First, social variation is not an inclusive description of autism, as individuals with autism also exhibit atypical verbal, cognitive, and sensory-motor profiles [2,3,4,5]. Second, the field of social neuroscience still debates the idea that social processing in the brain is produced by a dedicated tissue, or a “social brain” [6,7]. An alternative approach considers social processing and social cognition as a complex neural computation, supported by multiple domain-general processes, including motivation, perception, and motor control [8]. Considering the complex autism phenotype, along with the challenges of neurally defining social processing as a discrete module, we propose that the developmental variation seen in autism stems from variations in domain-general processes, which are not necessarily “social” per se.
In this Perspective Review, we propose a hypothesis, by which autism is not a “social” deficit, but rather a variation in learning. Specifically, we propose that in social animals, newborns depend on their caregiver for allostasis regulation, which is defined as the ongoing process of regulating the internal milieu [9,10]. This makes the social environment vitally salient and reinforces allostasis-driven learning of social knowledge and skills. Given that social knowledge and skills are acquired through learning, domain-general neural processes that underlie learning can impact social development, including perception, motor control, allostasis regulation, and multi-modal integration. Variation in such domain-general processes can affect allostasis-driven learning of social knowledge and skills and manifest as autism.
To support this view, we review lines of evidence. The first shows that social processing in the brain does not depend on a dedicated neural tissue, but rather involves domain-general neural circuits controlling allostasis-regulation and multi-modal integration. The second line of evidence shows that typical social development depends on allostasis-driven learning. We then review evidence demonstrating that the same domain-general processes of allostasis and learning vary in autism, both behaviorally and neurally. Finally, we integrate the evidence to propose that autism is not the result of a “broken social brain” but the manifestation of a variation in allostasis-driven learning.
2. Current Theories on Cognitive Mechanisms of Autism Focus on Social Deficits
Several theoretical accounts in the literature portray the cognitive and psychological mechanisms that underlie autism [11]. Autism is often explained by impairments in social cognition, including the ability for the theory of mind and empathy. The theory of mind account focuses on the ability to mentalize, which is the ability to internally represent the mind of others, including abstract and hidden information about others’ emotions, intentions, knowledge, and beliefs [12,13]. Individuals with autism have difficulties in mentalizing the mental states of others [14,15], have difficulties inferring others’ emotions and intentions by looking at their eyes [16], distinguishing their own knowledge from knowledge attributed to others [17,18], and attributing mental states spontaneously [19]. Moreover, the individual ability for the theory of mind is associated with the severity of further autistic symptoms, such as impeded social communication and repetitive restrictive behaviors [20]. According to the Double Empathy Problem account [21], individuals with autism are not only impaired in understanding others but are also less understood by neurotypical individuals. At the level of the brain, regions that are implicated to support the theory of mind and empathy display atypical activation and connectivity patterns among individuals with autism. Specifically, the medial prefrontal cortex (mPFC), and the posterior and anterior cingulate cortices (PCC and ACC, respectively), which are often referred to as the default mode network and are associated with theory of mind performance [22,23], have decreased connectivity in individuals with autism [24,25,26], as well as atypical structure and activations pattern [27,28,29,30]. Moreover, individuals with autism have an atypical developmental trajectory [31] and impaired functional connectivity [25,32] between the temporo-parietal junction and the inferior frontal gyrus, which were also associated with the theory of mind [33,34].
In addition to social cognition, other accounts explain autism by decreased internal motivation for social interactions [11]. For example, the Social Motivation theory posits that individuals with autism experience less internal rewards from social interactions compared to neurotypical individuals, and seek less social contact [35,36]. This is often explained in terms of reduced “salience” or the importance of social information for individuals with autism [35]. In line with this, the Social Orientating hypothesis [37] predicts the earliest indicator of autism is the lack of attention to social stimuli. Neuroimaging research shows that individuals with autism have atypical function in the orbitofrontal–striatum–amygdala circuit and expression of oxytocin receptors within it, which are implicated in reward and motivation [35,38,39,40]. This finding is highly consistent across the neuroimaging literature in the field of autism research [41].
Both the social cognition and social motivation accounts capture important variations seen in autism, each focusing on a distorted function in a specific social feature, and its underlying neural circuitry. Yet, can social motivation and social cognition be considered dedicated modules that are categorically separated from non-social motivational and cognitive processes? Other accounts explain autism using more general cognitive processes. For example, the Executive Function theory [42,43], suggests that autism is caused by impairments in the ability to organize actions and thoughts, and control impulses. Other theories propose that the core deficit in autism is differentiating important information from the context, which makes it difficult for individuals with autism to process complex stimuli and shift attention. These accounts include the Weak Central Coherence theory [44], the Context Blindness theory [45], and the Monotropism theory [46]. According to the Extreme Male Brain theory [47], autism reflects an extreme form of the typical male profile, favoring systemizing over empathizing [47,48]. Individuals with autism are superior in tasks that favor systemizing [49,50], have increased attention to detail [50], and are impaired in tasks of empathy and theory of mind [16,19,51]. This account is supported by the significantly higher prevalence of autism in males compared to females [52]. Moreover, males and females with autism are more similar to each other than to neurotypical males or females, respectively, in neural connectivity [53] and grey matter volume in the left inferior parietal lobe and operculum [54]. It is suggested that elevated exposure to testosterone during pregnancy could impact the neural development of the fetus, favoring extreme masculinization of the brain [55].
We propose that not only autism, but sociality at large is not a dedicated module and that its mechanisms can be explained with a domain-general account. Specifically, social processing does not depend on a dedicated neural system. Instead, social processing involves domain-general neural mechanisms that control allostasis-regulation and multi-modal integration. These circuits support the processing of both social and non-social information, with no categorical distinction between “types” of information [8]. The idea that social processing is not a dedicated module but rather, is supported by domain-general neural circuits, has implications for the conceptualization of autism and its underlying neural mechanisms.
3. Neural Basis of Social Processing and How It Varies in Autism
A large body of evidence from the field of Social Neuroscience portrays the neural circuits that are consistently involved in social processing. Specifically, neural activity and connectivity within a group of cortico-limbic brain regions are consistently involved in the neural processing of social information. These regions include the subcortical regions in the amygdala, nucleus accumbens (NAcc), and hypothalamus [56,57,58,59], and cortical regions in perception and action cortices [60], limbic cortices in the ventral anterior insula and cingulate cortex [61,62], and other association cortices in the ventromedial prefrontal cortex (vmPFC), ACC, and PCC [63,64]. Moreover, coordinated function and dopamine secretion within these regions is associated with improved social behavior in humans [65,66,67] and non-human mammals [68,69,70].
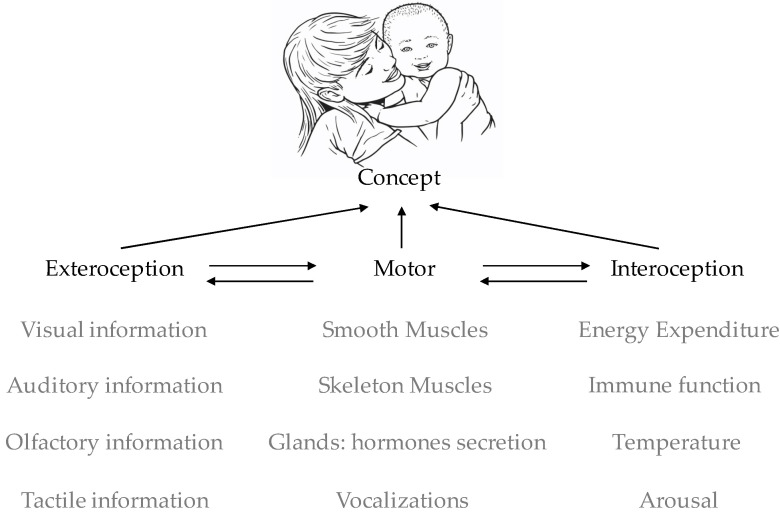
While these regions are repeatedly reported to underlie social processing across the literature, they are not dedicated or specific to social processing. The amygdala, NAcc insula, and ACC have a visceromotor role and via the hypothalamus, they regulate the internal milieu of the body through the autonomic nervous system [71], and the endocrine system [71,72,73]. The amygdala, dorsal ACC, and ventral anterior insula are also considered part of the salience network, which engages when facing important information in the world [74,75,76], and together with sensory and motor regions patriciate in perception and action in salient situations, whether social or non-social [77,78,79,80]. Association cortices in the vmPFC and PCC are involved in the representation of information about the world, including rudimentary and abstract concepts, and are considered to be part of the default mode network, which has a role in mentalization and theory of mind [22,23]. Altogether, the evidence suggests that social processing in the brain relies on domain-general processes of visceromotor control on the internal milieu of the body (allostasis), perception-action in face of salient stimuli, and representation of abstract knowledge (Figure 1).
Figure 1.
Domain-general neural circuits support social processing. Social processing in the brain does not rely on dedicated tissue, but on domain-general circuits that control allostasis, and multi-modal integration, including: (A) Visceromotor circuits that control allostasis, including, the amygdala, nucleus accumbens (NAcc), hypothalamus, and pituitary (in yellow) [71]; (B) Cortices of perception and action that perceive the environment and control behavior (in green); (C) Association cortices including limbic cortices in the anterior cingulate cortex (ACC) and insula, as well as the medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC) [81]. With experience, association cortices integrate multi-modal information and represent it as concepts, including emotion concepts and concepts about the mind of others [30,64] (in blue). Altered function in these circuits, which characterizes individuals with autism [82,83,84], supports the domain-general view of autism.
Individuals with autism show atypical connectivity within the salience and the default-mode networks [82,83,84]. Children with autism show hyper-connectivity within regions of the default-mode network, compared to neurotypical children, which is associated with the degree of their social impairment [24,26]. Adults and adolescents with autism show reduced connectivity in the default mode network compared to neurotypical controls [85], and children with autism display hypo-connectivity in the salience network, compared to neurotypical children, which is associated with sensory and social symptoms [86]. In addition to the default mode and salience networks, altered connectivity patterns are also found in other domain-general neural circuits among individuals with autism. This includes weaker connectivity between the amygdala and the medial prefrontal cortex [38,87], between both the amygdala and the PCC and temporal lobes [87,88], and between the amygdala and the striatum [87]. Atypical function in this circuit is associated with decreased social motivation and social saliency [35]. Altogether, when considering the neural phenotype of autism in humans, the domain-general nature of the neural circuits that are affected in autism supports the idea that the neural variation that underlies autism is not exclusive to social processing.
4. Social Development Depends on Allostasis-Driven Learning
4.1. Allostasis Regulation Shapes Social Learning
Optimal social development and well-being in children depends on the extent to which infants and parents are attentive to one another, and reciprocate affective states and behavioral communication cues, a term coined as parent-infant bio-behavioral synchrony [89,90]. The idea that synchronous parental care is adaptive for children is intuitive. However, the mechanisms in the child via which synchrony promotes learning and development remain unknown. In order to understand the mechanisms of social development in children, a key scientific challenge is to recognize key processes in the child via which parent–child synchrony in early life optimizes learning and development.
Recent literature points out that parent–child synchrony is adaptive because it helps to regulate the biological and psychological demands of the child, or their allostasis [8]. Since human infants are helpless in maintaining their ongoing regulatory needs, they depend on a dedicated caregiver for allostasis regulation. Parents feed their infants to regulate their diet and immune system, sing and touch their infants to regulate their arousal and temperature, and control many aspects of the infants’ autonomous nervous system [8,91,92]. Parent–child synchrony is a useful strategy for the social regulation of allostasis, which is highly rewarding to both parents and children, and effectively reinforces physical well-being, as well as social bonding and social learning. By being cared for, infants learn to socially interact in order to meet their allostatic demands, as biological primal needs for survival motivate infants toward social learning. Therefore, it is not synchrony per se that supports development and learning but rather its regulatory consequences on children’s allostasis [8,93].
As allostasis regulation is key for social development and learning [8,94,95], variation in children’s ability for allostasis regulation can adversely affect the rewarding experience of social interactions. This can result in reduced social orienting, social seeking, social liking, and as a result, deficient social learning, which are central characteristics of autism [35]. Indeed, children with autism show impaired patterns of allostasis regulation (see a review of empirical evidence in the next section), and it is suggested here that this can shift the trajectory of learning and by that determine the developmental phenotype of autism.
4.2. The Social Environment Shapes the Multi-Modal Representations of Perception and Action Patterns
The human brain is relatively immature at birth. One of the prominent neural features that are missing in newborns is the ability for multi-sensory integration, whereby information from different modalities is synthesized and used in concert [96]. This integrative capability gradually develops during postnatal life as the brain gains experience perceiving consistent multi-modal patterns in the environment, and gradually representing them as rudimentary concepts [8,97,98]. Since humans are social animals and infants completely rely on the caregiver for survival, the infant’s environment is essentially social, and the most prominent sensory patterns in the infant’s environment are of other humans. As a result, the first rudimentary concepts that infants acquire are social concepts [8,92]. For example, by repeated interaction with the caregiver, infants gradually recognize the spatial organization of the caregiver’s visual, auditory, olfactory, and tactile features and form a concept of mommy (or any other caregiver) [8].
Mommy is not only the most consistent pattern of information in the environment, it is also the most salient and potentially rewarding because it is essential for the infant’s allostasis. By integrating exteroceptive social information about the caregiver with interoceptive rewarding information about allostasis, the caregiver concept is quickly learned and repeatedly reinforced. High statistical regularity in the integration between the caregiver and allostasis regulation promotes social motivation and reinforces learning of social concepts and behaviors. As caregivers regulate infants’ allostasis, infants gain experience not only in rudimentary perceptual concepts, but also in more abstract concepts such as emotion concepts, and representations of others’ minds [8,95], and perception-action concepts, such as breastfeeding, synchrony, or vocalizations [95]. Thereafter, infants learn to share their attention with the caregiver [99] and to coordinate explicit knowledge (social and non-social) by acquiring language [100]. Synchronous parenting fosters the child’s ability to learn and use concepts [101] and can determine the content of concept learning by infants. For example, parental use of mental state language during parent–infant interactions promotes children to label their own emotions [102] and later, to infer and represent other people’s mental states [95,102,103,104].
Learning concepts and skills is a key domain-general process that underlies typical development. Social regulation of allostasis during development promotes social motivation and provides a strong driving force for social learning. However, social learning is not a special type of learning but rather more pronounced in early life. This is because of the high relevance of social information to the infant’s allostasis and the high prevalence of social information in the infant’s environment (see Box 1 and Figure 2).
Box 1. Learning in Typical Social Development and Autism.
1. Concepts
Concepts () are multi-modal representations of statistically regular patterns of information in the external world and the internal milieu. A central aspect of development is constructing concepts. During development, infants detect statistical regularities in the environment and organize them into concepts [105,106,107]. Concepts can be thought of as spatial and temporal patterns of information that enable the brain to perceive the world in a multi-modal meaningful way and to prepare for upcoming allostatic and environmental demands [71,108]. Since humans are social animals and depend on their caregiver, the first concepts infants acquire are social [8]. These include rudimentary sensory concepts such as a “face” to more abstract concepts like “mommy”, and more complex sensory-motor concepts like “breastfeeding” and “synchrony” or “crying” [95]. Consistent patterns of non-social exteroceptive-interoceptive-motor information are similarly constructed as concepts, and there is no categorical difference between social and non-social learning.
is a function of mental representations of consistent multi-modal patterns of information from interoceptive, exteroceptive, and motor sources (Figure 2).
Learning and updating of concepts depends on the rate of learning (η) novel information (ΔI) from interoceptive, exteroceptive, and motor sources.
2. Learning Concepts Depends on Allostasis
The elementary ongoing process of optimizing the body’s internal milieu shapes learning through motivation and reward. Patterns in the world that are relevant for allostasis (learning them reinforces allostasis regulation) are efficiently learned as concepts.
The rate of learning (η) of a concept depends on its impact on allostatic regulation
Here, the sensitivity of allostasis to a certain concept is represented as (∂log(A))/(∂log(C)). For example, concepts (C) with a large impact on allostasis (A), including social concepts such as mommy or any other caregiver, are learned faster than concepts with minor impact on allostasis, such as many non-social concepts.
3. Autism
According to this model, autism results from an atypical rate of learning, due to variation in domain-general processes of perception (interoception, exteroception), motor control, allostasis regulation, or their multi-modal integration into concepts. Variation in any of these processes can interfere with the rate and proposition of concepts and manifest as atypical social development. These domain-general processes are not orthogonal to one another, but rather represent different levels of observation, which can be prognostic of autism. For example, an atypical phenotype in infants in physiological measurements of allostasis, behavioral measurements such as synchrony with the caregiver, or neural measurements such as the formation of multi-modal neural tracts can serve as bio-behavioral indicators for future diagnosis of autism.
Figure 2.
Basic ingredients of learning favor social development. During development, infants organize sensory-motor information into concepts. Information includes the exteroceptive perception of the environment, interoceptive perception of the body, execution of motor control over behavior (skeleton muscles), and visceromotor control of the internal milieu via the autonomic and endocrine systems (contraction of smooth muscles, hormonal secretion from glands). Two domain-general processes that impact learning are statistical learning of the environment [109,110,111] and motivated learning guided by allostatic demands [93]. In social animals, the environment is primarily social, and allostasis is regulated primarily via social interactions. Therefore, the first and most prominent learning humans do is social. Accordingly, a deficiency in multi-modal integration of the basic ingredients that determine the ability to learn concepts, including perception, motor control, and allostasis regulation, results in atypical social development.
5. Individuals with Autism Show Variations in Domain-General Processes of Learning
The hypothesis that autism results from a variation in allostasis-driven learning raises specific predictions by which individuals with autism show an atypical phenotype in the domain-general processes of perception (interoception, exteroception), motor control, allostasis regulation, or their multi-modal integration. In the next section, we review evidence that the basic ingredients of allostasis-driven learning vary in autism.
5.1. Individuals with Autism Show Atypical Patterns of Allostasis Regulation
Empirical studies demonstrate that individuals with autism have atypical patterns of allostasis regulation [112,113]. Specifically, children and adults with autism exhibit atypical eating patterns [114], excessive water drinking [115], and disturbed sleeping patterns [116]. Such disturbed control over allostatic processes potentially stems from disturbances in the accurate perception of the internal milieu or interoception. Individuals with autism have reduced awareness of interoceptive signals of their body, such as reduced thirst awareness [117], reduced performance in heartbeat tracking tasks [118], and hyposensitivity to pain and proprioception [119], compared to neurotypical individuals. An infant’s ability for interoception is necessary to ensure attuned parental care, which is adapted to the infant’s allostatic needs. For example, to be fed, the infant’s brain must sense a decrease in plasma glucose levels through interoception, and socially communicate it to the caregiver via motor control over cry. This signals the allostatic need to the caregivers and elicits parental response aimed to regulate the infant allostasis. By attending to the infant’s allostatic needs, the parent reinforces further social behavior in the infant [8]. Disrupted interoception in infancy can interfere in three central processes that impact social development. First, it impairs the infant’s signals for regulation and therefore the caregiver’s ability to accurately regulate the infant, and later-on the infant’s ability for self-regulation [120,121,122]. Second, it impairs social motivation of the infant by reducing the rewarding value of social care. Third, it impairs social learning by disrupting the statistical regularity of allostatic-based reinforcement derived from social care (see Box 1).
5.2. Individuals with Autism Show Atypical Perception and Motor Function
In addition to altered interoception in autism, several studies report perceptual variation in autism across different exteroceptive modalities [123,124,125,126,127]. For example, individuals with autism have altered visual perception, including superior performance in detail or pattern recognition tasks [128,129], and in visual search tasks [130], along with significant deficits in motion processing tasks [131,132]. Auditory perception variation in autism is also reported [133,134], showing that individuals with autism tend to have improved pitch memory [135] but lower recognition of speech in noise [136] compared to neurotypical controls. Individuals with autism also show a variation in motor function [137] and often show stereotypical repetitive motor movements [138] and stereotypical vocalizations [139]. A growing body of evidence highlights the importance of such sensory-motor variations in the prognosis of individuals with autism [140]. Importantly, the sensory phenotype of children with and without autism significantly correlates with their social skills [141,142]. Thus, sensory-motor variations can impair learning (both social and non-social), as it can impair the formation of multi-modal associations and constructing concepts. Given the social environment human infants develop in and the importance of social interactions to the infant’s allostasis, many perception–action patterns that infants learn are social. For example, forming action-dependent concepts and their quick updating is crucial for synchronization and communication with the caregiver during social interaction. Evidence shows that motor control over vocalizations is crucial for synchronous social behavior [143,144], and mother–infant attunement [67]. As such, variation in perception and in motor functions during development can result in atypical trajectory of social development.
5.3. Individuals with Autism Show Atypical Learning
Impairments in learning among individuals with autism are reported at the neural and behavioral levels. At the neural level, evidence in multiple animal models of autism shows that genetic mutations associated with autism cause altered expression and translation of dendritic proteins that mediate synaptic plasticity [145,146,147,148,149,150]. In humans, individuals with autism display impairments in long-term potentiation evoked by transcranial magnetic stimulation [151,152]. At the behavioral level, individuals with autism show impairments in reinforcement learning [153,154,155]. Reinforcement learning is guided by the motivational state of the organism approaching a reward or avoiding a punishment [156,157]. Allostatic demands impact the motivational state of an individual and are therefore a central organizing principle of the nervous system, which guides learning and behavior [158]. Dopamine transmission in cortico-striatal circuits is central in learning about rewards and punishments and disturbances of the dopaminergic system in cortico-striatal circuits have a key role in psychopathologies that involve reinforcement learning [158,159,160]. Individuals with autism do not conform to standard patterns of reinforcement learning and are less affected by rewards and punishments [153,155,161]. Accordingly, autism is associated with aberrant patterns of dopamine signaling in the ventral tegmental area and substantia nigra, affecting reward processing and goal-directed behavior [162]. The distorted patterns of reinforcement-learning support the significance of allostasis-driven learning in autism. In addition to reinforcement learning, individuals with autism show impaired motor learning, specifically in tasks involving the acquisition of novel movement patterns acquisition [163], and implicit procedural learning tasks [164]. Moreover, individuals with autism show impaired language learning, and up to a third of children with autism are minimally verbal [165]. Among verbal children with autism, language is acquired in a substantial developmental delay. While typically developing children produce their first words at the ages of 8 to 14 months, the mean age of first word-use in children with autism is three years [166]. Importantly, the age of first word-use is correlated with the prognosis of children with autism [167]. Individuals with autism also display an atypical use of language, for example, children with autism over-imitate phrases they hear (“echolalia”, [168]), and exhibit syntactic impairments [169]. Such impairments in language acquisition can reflect impairments in concept use and acquisition. Children and adults with autism show differences in conceptualizations and have difficulties categorizing new information by forming prototypes and generalizing previously learned concepts to new situations [170]. Leider and colleagues recently demonstrated that individuals with autism rely more on longer-term statistics rather than recent events and show a slower motor response to changing sensory patterns of non-social stimuli. This suggests that individuals with autism are slower to form and represent statistically regular patterns of information [171] (i.e., concepts), possibly because of aberrant updating of probabilistic representations of the environment [172]. This evidence emphasizes that the different aspects of the autism phenotype, from low-level perception-action through complex social behaviors, are associated with a general variation in learning. This suggests that a domain-general view to development, by which variation in basic processes of learning can manifest as atypical social development, should be considered as a mechanistic approach to studying autism.
6. Implications
Considering the role of allostasis regulation, and multi-modal perception and action in social development can impact the way autism is studied. Our framework portrays a line of new hypotheses and research questions on the neurobehavioral mechanisms of autism. Specifically, this framework calls for testing the mechanistic role of learning in autism, along with the domain-general ingredients of learning including allostasis regulation, perception (both interoception and exteroception), motor control, and multi-modal association.
This framework can also impact how autism is diagnosed and treated. As of today, the average age of diagnosis in children is three years, when developmental delays are observed in communication and social behavior [173]. The age of diagnosis is important for useful interventions that improve the child outcomes, including self-reliance and well-being [174]. Decreasing the age of diagnosis is one of the key priorities in the field of autism research [175,176]. According to our model, distinct neural and behavioral features that are potentially relevant for identifying autism can be reliably measured in very young infants. For example, current research methods enable to measure in very young infants allostasis regulation [177], parent-infant synchrony [89], the rate of learning new concepts [178,179], and the formation of multi-sensory neural tracts [180,181]. We hypothesize that variation in these measurements in early infancy can be indicative for a later prognosis for atypical social development, and can potentially serve as bio-behavioral markers for autism early on. Moreover, there is a long-standing debate regarding the clinical distinction between autism and other spectrum disorders, such as Asperger’s syndrome or pervasive developmental delay not otherwise specified (PDD-NOS) [182,183]. Future empirical research is called for in order to test whether the distinct neural and behavioral features described here, including perception, motor control, and learning can differentiate autism from typical development and other spectrum disorders in young children [182,183].
The model proposed here points to potential measures that can be taken to improve the behavioral outcome in children. For example, clinically targeting the dyad, which is considered here as the developmental medium of infants, can be operative in optimizing the regulation and development of every child. Numerous studies show the importance of family involvement in therapy programs [184,185]. Multiple intervention programs target parental care to improve the infant’s behavior, symptoms, and well-being [186,187]. For example, Naturalistic Developmental Behavioral Interventions (NDBI) [188] and Applied Behavioral Analysis (ABA) [189] are evidence-based approaches for early intervention in infants with autism. Both NDBI and ABA target parental behavior and were shown to induce significant improvements in the social development of infants [189,190,191,192]. This supports the proposed model, which emphasizes the importance of the social environment, and proposes a potential mechanism for how social regulation of allostasis determines concept learning, and therefore social learning [8]. Future research that clinically aims to improve the dyadic synchronization and allostatic regulation of children can potentially improve children’s well-being, their skill of synchrony, and potentially optimize social learning and development in both typically and atypically developing children. Moreover, our approach also points to basic processes of physiological regulation, as well as interoception as basic ingredients of optimal social development, and targets of clinical intervention that can improve children’s well-being and development.
7. Conclusions
We propose an alternative framework for autism, by which it is not a social disorder, but a general variation in allostasis-driven learning. This calls for both basic and clinical research to provide novel in-depth knowledge on mechanisms of autism as well as novel clinical paths for intervention that can improve the prognosis of individuals with autism and lower the age of diagnosis. This framework also invites investigating other psychopathologies with a domain-general view to better understand their mechanisms and develop new treatments.
Acknowledgments
We thank Yuval Hart, Lior Zeevi and Lior Abramson for their valued feedback on earlier versions of the manuscript.
Author Contributions
Conceptualization, M.D., S.O. and S.A.; investigation, M.D., S.O. and S.A.; resources, S.A..; writing—original draft preparation, M.D., S.A.; writing—review and editing, M.D., S.O. and S.A.; visualization, S.A.; supervision, S.A.; funding acquisition, S.A.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; Washington, DC, USA: 2013. Neurodevelopmental Disorders. [Google Scholar]
- 2.Gernsbacher M., Geye H., Ellis Weismer S. The Role of Language and Communication Impairments within Autism. John Benjamins Publishing; Amsterdam, The Netherlands: 2005. [Google Scholar]
- 3.Pellicano E. The development of core cognitive skills in autism: A 3-year prospective study. Child Dev. 2010;81:1400–1416. doi: 10.1111/j.1467-8624.2010.01481.x. [DOI] [PubMed] [Google Scholar]
- 4.Coll S.M., Foster N.E.V., Meilleur A., Brambati S.M., Hyde K.L. Sensorimotor skills in autism spectrum disorder: A meta-analysis. Res. Autism Spectr. Disord. 2020;76:101570. doi: 10.1016/j.rasd.2020.101570. [DOI] [Google Scholar]
- 5.Canu D., Van der Paelt S., Canal-Bedia R., Posada M., Vanvuchelen M., Roeyers H. Early non-social behavioural indicators of autism spectrum disorder (ASD) in siblings at elevated likelihood for ASD: A systematic review. Eur. Child Adolesc. Psychiatry. 2021;30:497–538. doi: 10.1007/s00787-020-01487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lockwood P.L., Apps M.A.J., Chang S.W.C. Is There a ‘Social’ Brain? Implementations and Algorithms. Trends Cogn. Sci. 2020;24:802–813. doi: 10.1016/j.tics.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett L.F., Satpute A.B. Large-scale brain networks in affective and social neuroscience: Towards an integrative functional architecture of the brain. Curr. Opin. Neurobiol. 2013;23:361–372. doi: 10.1016/j.conb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atzil S., Gao W., Fradkin I., Barrett L.F. Growing a social brain. Nat. Hum. Behav. 2018;2:624–636. doi: 10.1038/s41562-018-0384-6. [DOI] [PubMed] [Google Scholar]
- 9.Sterling P. Allostasis: A model of predictive regulation. Physiol. Behav. 2012;106:5–15. doi: 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 10.McEwen B.S., Wingfield J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/S0018-506X(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher-Watson S., Happé F. Autism. 1st ed. Routledge; London, UK: 2019. p. 208. [Google Scholar]
- 12.Premack D., Woodruff G. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 2010;1:515–526. doi: 10.1017/S0140525X00076512. [DOI] [Google Scholar]
- 13.Byom L.J., Mutlu B. Theory of mind: Mechanisms, methods, and new directions. Front. Hum. Neurosci. 2013;7:413. doi: 10.3389/fnhum.2013.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frith U., Morton J., Leslie A.M. The cognitive basis of a biological disorder: Autism. Trends Neurosci. 1991;14:433–438. doi: 10.1016/0166-2236(91)90041-R. [DOI] [PubMed] [Google Scholar]
- 15.Tager-Flusberg H. Autistic children’s talk about psychological states: Deficits in the early acquisition of a theory of mind. Child Dev. 1992;63:161–172. doi: 10.2307/1130910. [DOI] [PubMed] [Google Scholar]
- 16.Baron-Cohen S., Jolliffe T., Mortimore C., Robertson M. Another advanced test of theory of mind: Evidence from very high functioning adults with autism or asperger syndrome. J. Child Psychol. Psychiatry. 1997;38:813–822. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 17.Baron-Cohen S., Leslie A.M., Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 18.Wimmer H. Beliefs about beliefs: Representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983;13:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- 19.Senju A., Southgate V., White S., Frith U. Mindblind eyes: An absence of spontaneous theory of mind in Asperger syndrome. Science. 2009;325:883–885. doi: 10.1126/science.1176170. [DOI] [PubMed] [Google Scholar]
- 20.Jones C.R.G., Simonoff E., Baird G., Pickles A., Marsden A.J.S., Tregay J., Happe F., Charman T. The association between theory of mind, executive function, and the symptoms of autism spectrum disorder. Autism Res. 2018;11:95–109. doi: 10.1002/aur.1873. [DOI] [PubMed] [Google Scholar]
- 21.Milton D.E.M. On the ontological status of autism: The ‘double empathy problem’. Disabil. Soc. 2012;27:883–887. doi: 10.1080/09687599.2012.710008. [DOI] [Google Scholar]
- 22.Raichle M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 23.Mars R., Neubert F.-X., Noonan M., Sallet J., Toni I., Rushworth M. On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch C.J., Uddin L.Q., Supekar K., Khouzam A., Phillips J., Menon V. Default mode network in childhood autism: Posteromedial cortex heterogeneity and relationship with social deficits. Biol. Psychiatry. 2013;74:212–219. doi: 10.1016/j.biopsych.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kana R.K., Maximo J.O., Williams D.L., Keller T.A., Schipul S.E., Cherkassky V.L., Minshew N.J., Just M.A. Aberrant functioning of the theory-of-mind network in children and adolescents with autism. Mol. Autism. 2015;6:59. doi: 10.1186/s13229-015-0052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uddin L.Q., Supekar K., Lynch C.J., Khouzam A., Phillips J., Feinstein C., Ryali S., Menon V. Salience network–based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70:869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert S.J., Bird G., Brindley R., Frith C.D., Burgess P.W. Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: An fMRI study of two executive function tasks. Neuropsychologia. 2008;46:2281–2291. doi: 10.1016/j.neuropsychologia.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agam Y., Joseph R.M., Barton J.J., Manoach D.S. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage. 2010;52:336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oblak A.L., Rosene D.L., Kemper T.L., Bauman M.L., Blatt G.J. Altered posterior cingulate cortical cyctoarchitecture, but normal density of neurons and interneurons in the posterior cingulate cortex and fusiform gyrus in autism. Autism Res. 2011;4:200–211. doi: 10.1002/aur.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee P.S., Yerys B.E., Della Rosa A., Foss-Feig J., Barnes K.A., James J.D., VanMeter J., Vaidya C.J., Gaillard W.D., Kenworthy L.E. Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: A fcMRI study of response inhibition. Cereb. Cortex. 2009;19:1787–1794. doi: 10.1093/cercor/bhn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igelstrom K.M., Webb T.W., Graziano M.S.A. Functional Connectivity Between the Temporoparietal Cortex and Cerebellum in Autism Spectrum Disorder. Cereb. Cortex. 2017;27:2617–2627. doi: 10.1093/cercor/bhw079. [DOI] [PubMed] [Google Scholar]
- 33.Schurz M., Tholen M.G. What brain imaging did (not) tell us about the Inferior Frontal Gyrus in theory of mind—A commentary on Samson et al., (2015) Cortex. 2016;74:329–333. doi: 10.1016/j.cortex.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Lombardo M.V., Chakrabarti B., Bullmore E.T., Consortium M.A., Baron-Cohen S. Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. Neuroimage. 2011;56:1832–1838. doi: 10.1016/j.neuroimage.2011.02.067. [DOI] [PubMed] [Google Scholar]
- 35.Chevallier C., Kohls G., Troiani V., Brodkin E.S., Schultz R.T. The social motivation theory of autism. Trends Cogn. Sci. 2012;16:231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clements C.C., Zoltowski A.R., Yankowitz L.D., Yerys B.E., Schultz R.T., Herrington J.D. Evaluation of the Social Motivation Hypothesis of Autism: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2018;75:797–808. doi: 10.1001/jamapsychiatry.2018.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawson G., Meltzoff A.N., Osterling J., Rinaldi J., Brown E. Children with autism fail to orient to naturally occurring social stimuli. J. Autism Dev. Disord. 1998;28:479–485. doi: 10.1023/A:1026043926488. [DOI] [PubMed] [Google Scholar]
- 38.Li L., He C., Jian T., Guo X., Xiao J., Li Y., Chen H., Kang X., Chen H., Duan X. Attenuated link between the medial prefrontal cortex and the amygdala in children with autism spectrum disorder: Evidence from effective connectivity within the “social brain”. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2020;111:110147. doi: 10.1016/j.pnpbp.2020.110147. [DOI] [PubMed] [Google Scholar]
- 39.Delmonte S., Gallagher L., O’Hanlon E., Mc Grath J., Balsters J. Functional and structural connectivity of frontostriatal circuitry in Autism Spectrum Disorder. Front. Hum. Neurosci. 2013;7:430. doi: 10.3389/fnhum.2013.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohls G., Chevallier C., Troiani V., Schultz R.T. Social ‘wanting’ dysfunction in autism: Neurobiological underpinnings and treatment implications. J. Neurodev. Disord. 2012;4:10. doi: 10.1186/1866-1955-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bachevalier J., Loveland K.A. The orbitofrontal–amygdala circuit and self-regulation of social–emotional behavior in autism. Neurosci. Biobehav. Rev. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Hill E.L. Evaluating the theory of executive dysfunction in autism. Dev. Rev. 2004;24:189–233. doi: 10.1016/j.dr.2004.01.001. [DOI] [Google Scholar]
- 43.Craig F., Margari F., Legrottaglie A.R., Palumbi R., de Giambattista C., Margari L. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr. Dis. Treat. 2016;12:1191–1202. doi: 10.2147/NDT.S104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Happe F., Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. J. Autism Dev. Disord. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- 45.Vermeulen P. Context Blindness in Autism Spectrum Disorder. Focus Autism Other Dev. Disabil. 2014;30:182–192. doi: 10.1177/1088357614528799. [DOI] [Google Scholar]
- 46.Murray D., Lesser M., Lawson W. Attention, monotropism and the diagnostic criteria for autism. Autism. 2005;9:139–156. doi: 10.1177/1362361305051398. [DOI] [PubMed] [Google Scholar]
- 47.Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn. Sci. 2002;6:248–254. doi: 10.1016/S1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- 48.Baron-Cohen S., Cassidy S., Auyeung B., Allison C., Achoukhi M., Robertson S., Pohl A., Lai M.C. Attenuation of typical sex differences in 800 adults with autism vs. 3900 controls. PLoS ONE. 2014;9:e102251. doi: 10.1371/journal.pone.0102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baron-Cohen S., Richler J., Bisarya D., Gurunathan N., Wheelwright S. The systemizing quotient: An investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358:361–374. doi: 10.1098/rstb.2002.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baron-Cohen S., Ashwin E., Ashwin C., Tavassoli T., Chakrabarti B. Talent in autism: Hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baron-Cohen S., Wheelwright S. The empathy quotient: An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J. Autism Dev. Disord. 2004;34:163–175. doi: 10.1023/B:JADD.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- 52.Loomes R., Hull L., Mandy W.P.L. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56:466–474. doi: 10.1016/j.jaac.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Alaerts K., Swinnen S.P., Wenderoth N. Sex differences in autism: A resting-state fMRI investigation of functional brain connectivity in males and females. Soc. Cogn. Affect. Neurosci. 2016;11:1002–1016. doi: 10.1093/scan/nsw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beacher F.D., Minati L., Baron-Cohen S., Lombardo M.V., Lai M.C., Gray M.A., Harrison N.A., Critchley H.D. Autism attenuates sex differences in brain structure: A combined voxel-based morphometry and diffusion tensor imaging study. AJNR Am. J. Neuroradiol. 2012;33:83–89. doi: 10.3174/ajnr.A2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baron-Cohen S., Auyeung B., Norgaard-Pedersen B., Hougaard D.M., Abdallah M.W., Melgaard L., Cohen A.S., Chakrabarti B., Ruta L., Lombardo M.V. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry. 2015;20:369–376. doi: 10.1038/mp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gingrich B., Liu Y., Cascio C., Wang Z., Insel T.R. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behav. Neurosci. 2000;114:173–183. doi: 10.1037/0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- 57.Baez-Mendoza R., Schultz W. The role of the striatum in social behavior. Front. Neurosci. 2013;7:233. doi: 10.3389/fnins.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emery N.J., Amaral D.G. Cognitive Neuroscience of Emotion. Oxford University Press; New York, NY, USA: 2000. The role of the amygdala in primate social cognition; pp. 156–191. [Google Scholar]
- 59.Ross H.E., Young L.J. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocr. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Preston S.D., de Waal F.B. Empathy: Its ultimate and proximate bases. Behav. Brain Sci. 2002;25:1–20. doi: 10.1017/S0140525X02000018. discussion 20–71. [DOI] [PubMed] [Google Scholar]
- 61.Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menon V., Uddin L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Bos W., Guroglu B. The role of the ventral medial prefrontal cortex in social decision making. J. Neurosci. 2009;29:7631–7632. doi: 10.1523/JNEUROSCI.1821-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith D.V., Clithero J.A., Boltuck S.E., Huettel S.A. Functional connectivity with ventromedial prefrontal cortex reflects subjective value for social rewards. Soc. Cogn. Affect. Neurosci. 2014;9:2017–2025. doi: 10.1093/scan/nsu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Atzil S., Hendler T., Feldman R. Specifying the neurobiological basis of human attachment: Brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36:2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yaniv A.U., Salomon R., Waidergoren S., Shimon-Raz O., Djalovski A., Feldman R. Synchronous caregiving from birth to adulthood tunes humans’ social brain. Proc. Natl. Acad. Sci. USA. 2021;118:e2012900118. doi: 10.1073/pnas.2012900118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atzil S., Touroutoglou A., Rudy T., Salcedo S., Feldman R., Hooker J.M., Dickerson B.C., Catana C., Barrett L.F. Dopamine in the medial amygdala network mediates human bonding. Proc. Natl. Acad. Sci. USA. 2017;114:2361–2366. doi: 10.1073/pnas.1612233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Champagne F.A., Chretien P., Stevenson C.W., Zhang T.Y., Gratton A., Meaney M.J. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J. Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silva M.R., Bernardi M.M., Felicio L.F. Effects of dopamine receptor antagonists on ongoing maternal behavior in rats. Pharm. Biochem. Behav. 2001;68:461–468. doi: 10.1016/S0091-3057(01)00471-3. [DOI] [PubMed] [Google Scholar]
- 70.Johns J.M., Joyner P.W., McMurray M.S., Elliott D.L., Hofler V.E., Middleton C.L., Knupp K., Greenhill K.W., Lomas L.M., Walker C.H. The effects of dopaminergic/serotonergic reuptake inhibition on maternal behavior, maternal aggression, and oxytocin in the rat. Pharm. Biochem. Behav. 2005;81:769–785. doi: 10.1016/j.pbb.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barrett L.F., Simmons W.K. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015;16:419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ulrich-Lai Y.M., Herman J.P. Neural Regulation of Endocrine and Autonomic Stress Responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luiten P.G., Ter Horst G.J., Steffens A.B. The hypothalamus, intrinsic connections and outflow pathways to the endocrine system in relation to the control of feeding and metabolism. Prog. Neurobiol. 1987;28:1–54. doi: 10.1016/0301-0082(87)90004-9. [DOI] [PubMed] [Google Scholar]
- 74.Chen T., Cai W., Ryali S., Supekar K., Menon V. Distinct global brain dynamics and spatiotemporal organization of the salience network. PLoS Biol. 2016;14:e1002469. doi: 10.1371/journal.pbio.1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seeley W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019;39:9878–9882. doi: 10.1523/JNEUROSCI.1138-17.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jilka S.R., Scott G., Ham T., Pickering A., Bonnelle V., Braga R.M., Leech R., Sharp D.J. Damage to the Salience Network and interactions with the Default Mode Network. J. Neurosci. 2014;34:10798–10807. doi: 10.1523/JNEUROSCI.0518-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bickart K.C., Wright C.I., Dautoff R.J., Dickerson B.C., Barrett L.F. Amygdala volume and social network size in humans. Nat. Neurosci. 2011;14:163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barrett L.F., Bliss-Moreau E., Duncan S.L., Rauch S.L., Wright C.I. The amygdala and the experience of affect. Soc. Cogn. Affect. Neurosci. 2007;2:73–83. doi: 10.1093/scan/nsl042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bickart K.C., Dickerson B.C., Barrett L.F. The amygdala as a hub in brain networks that support social life. Neuropsychologia. 2014;63:235–248. doi: 10.1016/j.neuropsychologia.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cooper J.C., Knutson B. Valence and salience contribute to nucleus accumbens activation. Neuroimage. 2008;39:538–547. doi: 10.1016/j.neuroimage.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baldassano C., Hasson U., Norman K.A. Representation of Real-World Event Schemas during Narrative Perception. J. Neurosci. 2018;38:9689–9699. doi: 10.1523/JNEUROSCI.0251-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen H., Uddin L.Q., Duan X.J., Zheng J.J., Long Z.L., Zhang Y.X., Guo X.N., Zhang Y., Zhao J.P., Chen H.F. Shared atypical default mode and salience network functional connectivity between autism and schizophrenia. Autism Res. 2017;10:1776–1786. doi: 10.1002/aur.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kernbach J.M., Satterthwaite T.D., Bassett D.S., Smallwood J., Margulies D., Krall S., Shaw P., Varoquaux G., Thirion B., Konrad K., et al. Shared endo-phenotypes of default mode dysfunction in attention deficit/hyperactivity disorder and autism spectrum disorder. Transl. Psychiatry. 2018;8:1–11. doi: 10.1038/s41398-018-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oldehinkel M., Mennes M., Marquand A., Charman T., Tillmann J., Ecker C., Dell’Acqua F., Brandeis D., Banaschewski T., Baumeister S., et al. Altered Connectivity Between Cerebellum, Visual, and Sensory-Motor Networks in Autism Spectrum Disorder: Results from the EU-AIMS Longitudinal European Autism Project. Biol. Psychiatry-Cogn. Neurosci. Neuroimaging. 2019;4:260–270. doi: 10.1016/j.bpsc.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 85.von dem Hagen E.A., Stoyanova R.S., Baron-Cohen S., Calder A.J. Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Soc. Cogn. Affect. Neurosci. 2013;8:694–701. doi: 10.1093/scan/nss053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abbott A.E., Nair A., Keown C.L., Datko M., Jahedi A., Fishman I., Muller R.A. Patterns of Atypical Functional Connectivity and Behavioral Links in Autism Differ Between Default, Salience, and Executive Networks. Cereb. Cortex. 2016;26:4034–4045. doi: 10.1093/cercor/bhv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shen M.D., Li D.D., Keown C.L., Lee A., Johnson R.T., Angkustsiri K., Rogers S.J., Muller R.A., Amaral D.G., Nordahl C.W. Functional Connectivity of the Amygdala Is Disrupted in Preschool-Aged Children with Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55:817–824. doi: 10.1016/j.jaac.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Monk C.S., Peltier S.J., Wiggins J.L., Weng S.-J., Carrasco M., Risi S., Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feldman R. Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J. Child Psychol. Psychiatry. 2007;48:329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- 90.Feldman R. Parent–infant synchrony: A biobehavioral model of mutual influences in the formation of affiliative bonds. Monogr. Soc. Res. Child. Dev. 2012;77:42–51. doi: 10.1111/j.1540-5834.2011.00660.x. [DOI] [Google Scholar]
- 91.Winberg J. Mother and newborn baby: Mutual regulation of physiology and behavior—A selective review. Dev. Psychobiol. 2005;47:217–229. doi: 10.1002/dev.20094. [DOI] [PubMed] [Google Scholar]
- 92.Hofer M.A. Hidden regulators in attachment, separation, and loss. Monogr. Soc. Res. Child Dev. 1994;59:192–207. doi: 10.1111/j.1540-5834.1994.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 93.Keramati M., Gutkin B. Homeostatic reinforcement learning for integrating reward collection and physiological stability. eLife. 2014;3:e04811. doi: 10.7554/eLife.04811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Atzil S., Barrett L.F. Social Regulation of Allostasis: Commentary on “Mentalizing Homeostasis: The Social Origins of Interoceptive Inference” by Fotopoulou & Tsakiris. Neuropsychoanalysis. 2017;19:29–33. doi: 10.1080/15294145.2017.1295214. [DOI] [Google Scholar]
- 95.Atzil S., Gendron M. Bio-behavioral synchrony promotes the development of conceptualized emotions. Curr. Opin. Psychol. 2017;17:162–169. doi: 10.1016/j.copsyc.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stein B.E., Stanford T.R., Rowland B.A. Development of multisensory integration from the perspective of the individual neuron. Nat. Rev. Neurosci. 2014;15:520–535. doi: 10.1038/nrn3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aydede M., Guzeldere G. Cognitive architecture, concepts, and introspection: An information-theoretic solution to the problem of phenomenal consciousness. Nous. 2005;39:197–255. doi: 10.1111/j.0029-4624.2005.00500.x. [DOI] [Google Scholar]
- 98.Arcaro M.J., Livingstone M.S. On the relationship between maps and domains in inferotemporal cortex. Nat. Rev. Neurosci. 2021;22:573–583. doi: 10.1038/s41583-021-00490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Charman T., Swettenham J., Baron-Cohen S., Cox A., Baird G., Drew A. Infants with autism: An investigation of empathy, pretend play, joint attention, and imitation. Dev. Psychol. 1997;33:781–789. doi: 10.1037/0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- 100.Fisher A.V., Godwin K.E., Matlen B.J., Unger L. Development of Category-Based Induction and Semantic Knowledge. Child Dev. 2015;86:48–62. doi: 10.1111/cdev.12277. [DOI] [PubMed] [Google Scholar]
- 101.Feldman R., Greenbaum C.W. Affect regulation and synchrony in mother—Infant play as precursors to the development of symbolic competence. Infant Ment. Health J. 1997;18:4–23. doi: 10.1002/(SICI)1097-0355(199721)18:1<4::AID-IMHJ2>3.0.CO;2-R. [DOI] [Google Scholar]
- 102.Taumoepeau M., Ruffman T. Stepping stones to others’ minds: Maternal talk relates to child mental state language and emotion understanding at 15, 24, and 33 months. Child Dev. 2008;79:284–302. doi: 10.1111/j.1467-8624.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 103.Brown J.R., Dunn J. ‘You can cry, mum’: The social and developmental implications of talk about internal states. Br. J. Dev. Psychol. 1991;9:237–256. doi: 10.1111/j.2044-835X.1991.tb00874.x. [DOI] [Google Scholar]
- 104.Rollo D., Sulla F. Maternal Talk in Cognitive Development: Relations between Psychological Lexicon, Semantic Development, Empathy, and Temperament. Front. Psychol. 2016;7:394. doi: 10.3389/fpsyg.2016.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murphy G. The Big Book of Concepts. MIT Press; Cambridge, MA, USA: 2004. [Google Scholar]
- 106.Barsalou L.W. Ad hoc categories. Mem. Cogn. 1983;11:211–227. doi: 10.3758/BF03196968. [DOI] [PubMed] [Google Scholar]
- 107.Gweon H. Inferential social learning: Cognitive foundations of human social learning and teaching. Trends Cogn. Sci. 2021 doi: 10.1016/j.tics.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 108.Chanes L., Barrett L.F. Redefining the Role of Limbic Areas in Cortical Processing. Trends Cogn. Sci. 2016;20:96–106. doi: 10.1016/j.tics.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krogh L., Vlach H.A., Johnson S.P. Statistical learning across development: Flexible yet constrained. Front. Psychol. 2012;3:598. doi: 10.3389/fpsyg.2012.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siegelman N., Frost R. Statistical learning as an individual ability: Theoretical perspectives and empirical evidence. J. Mem. Lang. 2015;81:105–120. doi: 10.1016/j.jml.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saffran J.R., Aslin R.N., Newport E.L. Statistical learning by 8-month-old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- 112.Schaaf R.C., Benevides T.W., Leiby B.E., Sendecki J.A. Autonomic dysregulation during sensory stimulation in children with autism spectrum disorder. J. Autism Dev. Disord. 2015;45:461–472. doi: 10.1007/s10803-013-1924-6. [DOI] [PubMed] [Google Scholar]
- 113.McCormick C.E.B., Sheinkopf S.J., Levine T.P., LaGasse L.L., Tronick E., Lester B.L. Diminished respiratory sinus arrhythmia response in infants later diagnosed with autism spectrum disorder. Autism Res. 2018;11:726–731. doi: 10.1002/aur.1929. [DOI] [PubMed] [Google Scholar]
- 114.Nadeau M.V., Richard E., Wallace G.L. The Combination of Food Approach and Food Avoidant Behaviors in Children with Autism Spectrum Disorder: “Selective Overeating”. J. Autism Dev. Disord. 2021 doi: 10.1007/s10803-021-04945-6. [DOI] [PubMed] [Google Scholar]
- 115.Terai K., Munesue T., Hiratani M. Excessive water drinking behavior in autism. Brain Dev. 1999;21:103–106. doi: 10.1016/S0387-7604(98)00079-5. [DOI] [PubMed] [Google Scholar]
- 116.Richdale A.L. Sleep problems in autism: Prevalence, cause, and intervention. Dev. Med. Child. Neurol. 1999;41:60–66. doi: 10.1017/S0012162299000122. [DOI] [PubMed] [Google Scholar]
- 117.Fiene L., Brownlow C. Investigating interoception and body awareness in adults with and without autism spectrum disorder. Autism Res. 2015;8:709–716. doi: 10.1002/aur.1486. [DOI] [PubMed] [Google Scholar]
- 118.Garfinkel S.N., Tiley C., O’Keeffe S., Harrison N.A., Seth A.K., Critchley H.D. Discrepancies between dimensions of interoception in autism: Implications for emotion and anxiety. Biol. Psychol. 2016;114:117–126. doi: 10.1016/j.biopsycho.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 119.Elwin M., Ek L., Schroder A., Kjellin L. Autobiographical accounts of sensing in Asperger syndrome and high-functioning autism. Arch. Psychiatr. Nurs. 2012;26:420–429. doi: 10.1016/j.apnu.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 120.Singletary W.M. An integrative model of autism spectrum disorder: ASD as a neurobiological disorder of experienced environmental deprivation, early life stress and allostatic overload. Neuropsychoanalysis. 2015;17:81–119. doi: 10.1080/15294145.2015.1092334. [DOI] [Google Scholar]
- 121.McEwen B.S. A key role for allostatic overload in ASD and other disorders. Commentary on “An integrative model of autism spectrum disorder: ASD as a neurobiological disorder of experienced environmental deprivation, early life stress, and allostatic overload” by William M. Singletary, MD. Neuropsychoanalysis. 2016;18:9–14. doi: 10.1080/15294145.2016.1149686. [DOI] [Google Scholar]
- 122.Wass S. Allostasis and metastasis: The yin and yang of childhood self-regulation. Dev. Psychopathol. 2021:1–12. doi: 10.1017/S0954579421000833. [DOI] [PubMed] [Google Scholar]
- 123.Bogdashina O. Sensory Perceptual Issues in Autism and Asperger Syndrome: Different Sensory Experiences—Different Perceptual Worlds. Jessica Kingsley Publishers; London, UK: 2003. p. 217. [Google Scholar]
- 124.Jones R.S.P., Quigney C., Huws J.C. First-hand accounts of sensory perceptual experiences in autism: A qualitative analysis. J. Intellect. Dev. Disabil. 2003;28:112–121. doi: 10.1080/1366825031000147058. [DOI] [Google Scholar]
- 125.Chung S., Son J.W. Visual Perception in Autism Spectrum Disorder: A Review of Neuroimaging Studies. J. Korean Acad. Child Adolesc. Psychiatry. 2020;31:105–120. doi: 10.5765/jkacap.200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Behrmann M., Thomas C., Humphreys K. Seeing it differently: Visual processing in autism. Trends Cogn. Sci. 2006;10:258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 127.O’Connor K. Auditory processing in autism spectrum disorder: A review. Neurosci. Biobehav. Rev. 2012;36:836–854. doi: 10.1016/j.neubiorev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 128.Shah A., Frith U. An islet of ability in autistic children: A research note. J. Child Psychol. Psychiatry. 1983;24:613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 129.Plaisted K., O’Riordan M., Baron-Cohen S. Enhanced discrimination of novel, highly similar stimuli by adults with autism during a perceptual learning task. J. Child Psychol. Psychiatry. 1998;39:765–775. doi: 10.1111/1469-7610.00375. [DOI] [PubMed] [Google Scholar]
- 130.O’Riordan M.A., Plaisted K.C., Driver J., Baron-Cohen S. Superior visual search in autism. J. Exp. Psychol. Hum. Percept. Perform. 2001;27:719–730. doi: 10.1037/0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- 131.Milne E., Swettenham J., Hansen P., Campbell R., Jeffries H., Plaisted K. High motion coherence thresholds in children with autism. J. Child Psychol. Psychiatry. 2002;43:255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- 132.Spencer J., O’Brien J., Riggs K., Braddick O., Atkinson J., Wattam-Bell J. Motion processing in autism: Evidence for a dorsal stream deficiency. Neuroreport. 2000;11:2765–2767. doi: 10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- 133.Kellerman G.R., Fan J., Gorman J.M. Auditory abnormalities in autism: Toward functional distinctions among findings. CNS Spectr. 2005;10:748–756. doi: 10.1017/S1092852900019738. [DOI] [PubMed] [Google Scholar]
- 134.Del Nieto Rincon P.L. Autism: Alterations in auditory perception. Rev. Neurosci. 2008;19:61–78. doi: 10.1515/REVNEURO.2008.19.1.61. [DOI] [PubMed] [Google Scholar]
- 135.Heaton P. Pitch memory, labelling and disembedding in autism. J. Child Psychol. Psychiatry. 2003;44:543–551. doi: 10.1111/1469-7610.00143. [DOI] [PubMed] [Google Scholar]
- 136.Alcantara J.I., Weisblatt E.J., Moore B.C., Bolton P.F. Speech-in-noise perception in high-functioning individuals with autism or Asperger’s syndrome. J. Child Psychol. Psychiatry. 2004;45:1107–1114. doi: 10.1111/j.1469-7610.2004.t01-1-00303.x. [DOI] [PubMed] [Google Scholar]
- 137.Ming X., Brimacombe M., Wagner G.C. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007;29:565–570. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 138.Lewis M.H., Bodfish J.W. Repetitive behavior disorders in autism. Ment. Retard. Dev. Disabil. Res. Rev. 1998;4:80–89. doi: 10.1002/(SICI)1098-2779(1998)4:2<80::AID-MRDD4>3.0.CO;2-0. [DOI] [Google Scholar]
- 139.Aiken J.M., Salzberg C.L. The effects of a sensory extinction procedure on stereotypic sounds of two autistic children. J. Autism Dev. Disord. 1984;14:291–299. doi: 10.1007/BF02409580. [DOI] [PubMed] [Google Scholar]
- 140.Pastor-Cerezuela G., Fernandez-Andres M.I., Sanz-Cervera P., Marin-Suelves D. The impact of sensory processing on executive and cognitive functions in children with autism spectrum disorder in the school context. Res. Dev. Disabil. 2020;96:103540. doi: 10.1016/j.ridd.2019.103540. [DOI] [PubMed] [Google Scholar]
- 141.Miller Kuhaneck H., Britner P.A. A preliminary investigation of the relationship between sensory processing and social play in autism spectrum disorder. OTJR Occup. Particip. Health. 2013;33:159–167. doi: 10.3928/15394492-20130614-04. [DOI] [PubMed] [Google Scholar]
- 142.Humes L.E., Busey T.A., Craig J., Kewley-Port D. Are age-related changes in cognitive function driven by age-related changes in sensory processing? Atten. Percept. Psychophys. 2013;75:508–524. doi: 10.3758/s13414-012-0406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kessler S.E., Radespiel U., Hasiniaina A.I., Leliveld L.M., Nash L.T., Zimmermann E. Modeling the origins of mammalian sociality: Moderate evidence for matrilineal signatures in mouse lemur vocalizations. Front. Zool. 2014;11:14. doi: 10.1186/1742-9994-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seltzer L.J., Ziegler T.E., Pollak S.D. Social vocalizations can release oxytocin in humans. Proc. R. Soc. Lond. B Biol. Sci. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kelleher R.J., 3rd, Bear M.F. The autistic neuron: Troubled translation? Cell. 2008;135:401–406. doi: 10.1016/j.cell.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 146.Bourgeron T. A synaptic trek to autism. Curr. Opin. Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 147.Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat. Rev. Neurosci. 2015;16:551–563. doi: 10.1038/nrn3992. [DOI] [PubMed] [Google Scholar]
- 148.Gkogkas C.G., Khoutorsky A., Ran I., Rampakakis E., Nevarko T., Weatherill D.B., Vasuta C., Yee S., Truitt M., Dallaire P., et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493:371–377. doi: 10.1038/nature11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Santini E., Huynh T.N., MacAskill A.F., Carter A.G., Pierre P., Ruggero D., Kaphzan H., Klann E. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature. 2013;493:411–415. doi: 10.1038/nature11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Santini E., Klann E. Reciprocal signaling between translational control pathways and synaptic proteins in autism spectrum disorders. Sci. Signal. 2014;7:re10. doi: 10.1126/scisignal.2005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oberman L.M., Ifert-Miller F., Najib U., Bashir S., Heydrich J.G., Picker J., Rotenberg A., Pascual-Leone A. Abnormal Mechanisms of Plasticity and Metaplasticity in Autism Spectrum Disorders and Fragile X Syndrome. J. Child Adolesc. Psychopharmacol. 2016;26:617–624. doi: 10.1089/cap.2015.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pedapati E.V., Gilbert D.L., Erickson C.A., Horn P.S., Shaffer R.C., Wink L.K., Laue C.S., Wu S.W. Abnormal Cortical Plasticity in Youth with Autism Spectrum Disorder: A Transcranial Magnetic Stimulation Case-Control Pilot Study. J. Child Adolesc. Psychopharmacol. 2016;26:625–631. doi: 10.1089/cap.2015.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schuetze M., Rohr C.S., Dewey D., McCrimmon A., Bray S. Reinforcement Learning in Autism Spectrum Disorder. Front. Psychol. 2017;8 doi: 10.3389/fpsyg.2017.02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Solomon M., Smith A.C., Frank M.J., Ly S., Carter C.S. Probabilistic reinforcement learning in adults with autism spectrum disorders. Autism Res. Off. J. Int. Soc. Autism Res. 2011;4:109–120. doi: 10.1002/aur.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yechiam E., Arshavsky O., Shamay-Tsoory S.G., Yaniv S., Aharon J. Adapted to explore: Reinforcement learning in Autistic Spectrum Conditions. Brain Cogn. 2010;72:317–324. doi: 10.1016/j.bandc.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 156.Maia T.V. Reinforcement learning, conditioning, and the brain: Successes and challenges. Cogn. Affect. Behav. Neurosci. 2009;9:343–364. doi: 10.3758/CABN.9.4.343. [DOI] [PubMed] [Google Scholar]
- 157.Botvinick M.M., Niv Y., Barto A.G. Hierarchically organized behavior and its neural foundations: A reinforcement learning perspective. Cognition. 2009;113:262–280. doi: 10.1016/j.cognition.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maia T.V., Frank M.J. From reinforcement learning models to psychiatric and neurological disorders. Nat. Neurosci. 2011;14:154–162. doi: 10.1038/nn.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shiner T., Seymour B., Wunderlich K., Hill C., Bhatia K.P., Dayan P., Dolan R.J. Dopamine and performance in a reinforcement learning task: Evidence from Parkinson’s disease. Brain. 2012;135:1871–1883. doi: 10.1093/brain/aws083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Silvetti M., Wiersema J.R., Sonuga-Barke E., Verguts T. Deficient reinforcement learning in medial frontal cortex as a model of dopamine-related motivational deficits in ADHD. Neural. Netw. 2013;46:199–209. doi: 10.1016/j.neunet.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 161.Minassian A., Paulus M., Lincoln A., Perry W. Adults with autism show increased sensitivity to outcomes at low error rates during decision-making. J. Autism Dev. Disord. 2007;37:1279–1288. doi: 10.1007/s10803-006-0278-8. [DOI] [PubMed] [Google Scholar]
- 162.Pavăl D. A Dopamine Hypothesis of Autism Spectrum Disorder. Dev. Neurosci. 2017;39:355–360. doi: 10.1159/000478725. [DOI] [PubMed] [Google Scholar]
- 163.Gidley Larson J.C., Mostofsky S.H. Evidence that the pattern of visuomotor sequence learning is altered in children with autism. Autism Res. 2008;1:341–353. doi: 10.1002/aur.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mostofsky S.H., Goldberg M.C., Landa R.J., Denckla M.B. Evidence for a deficit in procedural learning in children and adolescents with autism: Implications for cerebellar contribution. J. Int. Neuropsychol. Soc. 2000;6:752–759. doi: 10.1017/S1355617700677020. [DOI] [PubMed] [Google Scholar]
- 165.Norrelgen F., Fernell E., Eriksson M., Hedvall A., Persson C., Sjolin M., Gillberg C., Kjellmer L. Children with autism spectrum disorders who do not develop phrase speech in the preschool years. Autism. 2015;19:934–943. doi: 10.1177/1362361314556782. [DOI] [PubMed] [Google Scholar]
- 166.Howlin P. Outcome in high-functioning adults with autism with and without early language delays: Implications for the differentiation between autism and Asperger syndrome. J. Autism Dev. Disord. 2003;33:3–13. doi: 10.1023/A:1022270118899. [DOI] [PubMed] [Google Scholar]
- 167.Mayo J., Chlebowski C., Fein D.A., Eigsti I.M. Age of first words predicts cognitive ability and adaptive skills in children with ASD. J. Autism Dev. Disord. 2013;43:253–264. doi: 10.1007/s10803-012-1558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tager-Flusberg H., Calkins S. Does imitation facilitate the acquisition of grammar? Evidence from a study of autistic, Down’s syndrome and normal children. J. Child Lang. 1990;17:591–606. doi: 10.1017/S0305000900010898. [DOI] [PubMed] [Google Scholar]
- 169.Eigsti I.M., Bennetto L., Dadlani M.B. Beyond pragmatics: Morphosyntactic development in autism. J. Autism Dev. Disord. 2007;37:1007–1023. doi: 10.1007/s10803-006-0239-2. [DOI] [PubMed] [Google Scholar]
- 170.Klinger L.G., Dawson G. Prototype formation in autism. Dev. Psychopathol. 2001;13:111–124. doi: 10.1017/S0954579401001080. [DOI] [PubMed] [Google Scholar]
- 171.Lieder I., Adam V., Frenkel O., Jaffe-Dax S., Sahani M., Ahissar M. Perceptual bias reveals slow-updating in autism and fast-forgetting in dyslexia. Nat. Neurosci. 2019;22:256–264. doi: 10.1038/s41593-018-0308-9. [DOI] [PubMed] [Google Scholar]
- 172.Palmer C.J., Lawson R.P., Hohwy J. Bayesian approaches to autism: Towards volatility, action, and behavior. Psychol. Bull. 2017;143:521–542. doi: 10.1037/bul0000097. [DOI] [PubMed] [Google Scholar]
- 173.van’t Hof M., Tisseur C., van Berckelear-Onnes I., van Nieuwenhuyzen A., Daniels A.M., Deen M., Hoek H.W., Ester W.A. Age at autism spectrum disorder diagnosis: A systematic review and meta-analysis from 2012 to 2019. Autism. 2021;25:862–873. doi: 10.1177/1362361320971107. [DOI] [PubMed] [Google Scholar]
- 174.Elder J.H., Kreider C.M., Brasher S.N., Ansell M. Clinical impact of early diagnosis of autism on the prognosis and parent-child relationships. Psychol. Res. Behav. Manag. 2017;10:283–292. doi: 10.2147/PRBM.S117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Interagency Autism Coordinating Committee . 2016–2017 Interagency Autism Coordinating Committee Strategic Plan for Autism Spectrum Disorder. The U.S. Department of Health and Human Services Interagency Autism Coordinating Committee; Washington, DC, USA: 2017. [Google Scholar]
- 176.Allison C., Matthews F.E., Ruta L., Pasco G., Soufer R., Brayne C., Charman T., Baron-Cohen S. Quantitative Checklist for Autism in Toddlers (Q-CHAT). A population screening study with follow-up: The case for multiple time-point screening for autism. BMJ Paediatr. Open. 2021;5:e000700. doi: 10.1136/bmjpo-2020-000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Irani M., Klein Selle N., Zeevi L., Bdarne S., Atzil S. A novel approach to study social regulation in parents and infants. in preparation.
- 178.Kording K.P., Wolpert D.M. Bayesian integration in sensorimotor learning. Nature. 2004;427:244–247. doi: 10.1038/nature02169. [DOI] [PubMed] [Google Scholar]
- 179.Ashourian P., Loewenstein Y. Bayesian inference underlies the contraction bias in delayed comparison tasks. PLoS ONE. 2011;6:e19551. doi: 10.1371/journal.pone.0019551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Narayan A., Rowe M.A., Palacios E.M., Wren-Jarvis J., Bourla I., Gerdes M., Brandes-Aitken A., Desai S.S., Marco E.J., Mukherjee P. Altered Cerebellar White Matter in Sensory Processing Dysfunction Is Associated with Impaired Multisensory Integration and Attention. Front. Psychol. 2021;11 doi: 10.3389/fpsyg.2020.618436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Owen J.P., Marco E.J., Desai S., Fourie E., Harris J., Hill S.S., Arnett A.B., Mukherjee P. Abnormal white matter microstructure in children with sensory processing disorders. Neuroimage Clin. 2013;2:844–853. doi: 10.1016/j.nicl.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.de Giambattista C., Ventura P., Trerotoli P., Margari M., Palumbi R., Margari L. Subtyping the Autism Spectrum Disorder: Comparison of Children with High Functioning Autism and Asperger Syndrome. J. Autism Dev. Disord. 2019;49:138–150. doi: 10.1007/s10803-018-3689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Walker D.R., Thompson A.N.N., Zwaigenbaum L., Goldberg J., Bryson S.E., Mahoney W.J., Strawbridge C.P., Szatmari P. Specifying PDD-NOS: A Comparison of PDD-NOS, Asperger Syndrome, and Autism. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43:172–180. doi: 10.1097/00004583-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 184.Rankin J.A., Paisley C.A., Tomeny T.S., Eldred S.W. Fathers of Youth with Autism Spectrum Disorder: A Systematic Review of the Impact of Fathers’ Involvement on Youth, Families, and Intervention. Clin. Child Fam. Psychol. Rev. 2019;22:458–477. doi: 10.1007/s10567-019-00294-0. [DOI] [PubMed] [Google Scholar]
- 185.Sharabi A., Marom-Golan D. Social Support, Education Levels, and Parents’ Involvement: A Comparison Between Mothers and Fathers of Young Children with Autism Spectrum Disorder. Top. Early Child. Spec. Educ. 2018;38:54–64. doi: 10.1177/0271121418762511. [DOI] [Google Scholar]
- 186.Koller J., David T., Bar N., Lebowitz E.R. The Role of Family Accommodation of RRBs in Disruptive Behavior Among Children with Autism. J. Autism Dev. Disord. 2021 doi: 10.1007/s10803-021-05163-w. [DOI] [PubMed] [Google Scholar]
- 187.Feldman I., Koller J., Lebowitz E.R., Shulman C., Ben Itzchak E., Zachor D.A. Family Accommodation in Autism Spectrum Disorder. J. Autism Dev. Disord. 2019;49:3602–3610. doi: 10.1007/s10803-019-04078-x. [DOI] [PubMed] [Google Scholar]
- 188.Schreibman L., Dawson G., Stahmer A.C., Landa R., Rogers S.J., McGee G.G., Kasari C., Ingersoll B., Kaiser A.P., Bruinsma Y., et al. Naturalistic Developmental Behavioral Interventions: Empirically Validated Treatments for Autism Spectrum Disorder. J. Autism Dev. Disord. 2015;45:2411–2428. doi: 10.1007/s10803-015-2407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Peters-Scheffer N., Didden R., Korzilius H., Sturmey P. A meta-analytic study on the effectiveness of comprehensive ABA-based early intervention programs for children with Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2011;5:60–69. doi: 10.1016/j.rasd.2010.03.011. [DOI] [Google Scholar]
- 190.Minjarez M.B., Karp E.A., Stahmer A.C., Brookman-Frazee L. Empowering parents through parent training and coaching. In: Paul H., editor. Naturalistic Developmental Behavioral Interventions for Autism Spectrum Disorder. Brookes Publishing Co.; Baltimore, MD, USA: 2020. pp. 77–98. [Google Scholar]
- 191.Landa R.J. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int. Rev. Psychiatry. 2018;30:25–39. doi: 10.1080/09540261.2018.1432574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fami Tafreshi F., Mohammadi M.R., Sharifi Saki S., Ahmadi H., Karimi R., Aakhte M. Effectiveness of Training Applied Behavior Analysis to Parents on Increasing Self-help of Children with Autism. Q. J. Child. Ment. Health. 2016;3:9–18. [Google Scholar]