Summary
Since 2016 there has been a 20-fold increase in known burns injury from personal mobility device (PMD) related fires. The root cause is the failure of high-density lithium ion (Li-ion) battery packs powering the PMDs. This failure process, known as thermal runaway, is well documented in applied science journals. Importantly, the liberation of hydrogen fluoride from failing Li-ion batteries may contribute to unrecognized chemical burns. A clinical gap in knowledge exists in the understanding of the explosive nature of Li-ion batteries. We reviewed the electrochemical pathophysiology of a failing Li-ion cell as it impacts clinical management of burn injuries. This retrospective study was carried out in two major institutions in Singapore. All admitted PMD-related burns and follow up appointments were captured and reviewed from 2016 - 2020. Thirty patients were admitted to tertiary hospitals, 43% of patients were in the pediatric population and 57% were adult patients, aged from 0.3 to 77 years. TBSA of burns ranged from 0 to 80% with a mean 14.5%. 73% of cases presented with inhalation injury, 8 of whom did not suffer any cutaneous burns. 50% of patients sustained both cutaneous and inhalation burn injuries. 27% of patients sustained major burns of >20% TBSA, with 2 in the pediatric group. Mortali ty rate was 10% from PMD-related fires. This cause of burn injury has proven to be fa tal. Prevention of PMD-related fires by ensuring proper battery utilization, adherence to PMD sanctions for battery standards and public education is vital to reducing the morbidity and mortality of this unique type of thermal injury.
Keywords: burns, personal mobility devices, electric scooter, lithium ion battery, thermal runaway, burns critical care
Abstract
Depuis 2016, les rapports de brûlures après incendie de véhicules électriques personnels (VEP) ont été multipliés par 20. La cause essentielle en est le dysfonctionnement de la batterie lithium/ion (Li/ion) les motorisant. Ce dysfonctionnement est connu sous le terme d’emballement thermique, bien décrit dans les revues technologiques. La libération de fluorure d’hydrogène lors de cette réaction peut entraîner des brûlures chimiques ignorées et la physiopathologie exacte de ces brûlures reste largement méconnue des cliniciens. Nous avons revu les mécanismes physico- chimiques de l’emballement thermique des batteries Li/ion et leur conséquences sur la prise en charge des brûlures occasionnées. Cette étude rétrospective a été réalisée par 2 grosses structures singapouriennes. Tous les dossiers d’accidents de VEP survenus entre 2016 et 2020, comprenant le suivi à distance, ont été revus. Ils regroupaient 30 patients âgés de 3 mois à 77 ans, dont 43% d’enfants. La surface brûlée représentait 0 à 80% de SCT (moyenne 14,5%) et 27% des patients (dont 2 enfants) étaient brûlés sur plus de 20% SCT. Une inhalation était retrouvée dans 73% des cas (dont 8 sans brûlure cutanée). La moitié des patients avaient une brûlure et une inhalation. La mortalité s’élevait à 10%. La prévention de ces accidents par le contrôle- qualité des batteries (sanctions à l’appui) et l’éducation à l’utilisation correcte des VEP et de leur batterie est nécessaire pour éviter ces dysfonctionnements potentiellement létaux.
Introduction
The use of personal mobility devices (PMD) in the form of electric scooters/bicycles (e-scooters/bikes) has been on the increase worldwide with the rising demand of alternative energy to curb fossil fuel dependency. Arguably, electric mobility devices are “green” as they reduce carbon emission and provide an alternative and sustainable commuting tool that is clean, easy and renewable.
Singapore has one of the highest numbers of PMD riders per capita. The economics of this is due to the astronomical cost of vehicle ownership. The average cost of an automobile is an estimated $120,000 SGD according to the Land Transport Authority Singapore.
Since 2016 there have been 9 reported incidences of e-scooter-related fires. This jumped up to 49 in 2017, a 444% increase in PMD-related fires, as reported by Singapore Civil Defence Force (SCDF) Annual Fire, Ambulance and Enforcement Statistics 2017.1,2 These figures are still climbing, with a reported 74 cases of PMD-related fires in 2018 alone.3
The root cause of these fires has been distilled to the detonation of high-density lithium ion (Li-ion) polymer battery packs powering these PMDs. Explosions were postulated to occur during the overcharged state.1,2 The only case in burns literature to date was reported by Khor et al. from the Singapore General Hospital (SGH) Regional Burns Unit in 2018, quoting a 30% total body surface area (TBSA) thermal burn with inhalational injury citing the same mechanism.4 In this study, we review the first series of thermal injury in adults and children from this novel mechanism of burns.
Purpose
Due to the soaring numbers of thermal burns admitted concurrently to the SGH Burns Unit and the Kendang Kerbau Women’s and Children’s Hospital (KKH) Burns Unit for PMD related burn injuries, a retrospective study captured over 2016-2020 across these two institutions was performed.
Primary aims
To understand the electrochemical physics behind the thermal runaway phenomenon of failing Liion battery.
To examine the demographics, outcomes and patterns of burns injuries sustained by PMD-related fires as it relates to management.
Secondary aims
To explain the process and toxicity of hydrogen fluoride (HF) gas venting prior to explosion during Li-ion cell failure which is unrecognized.
Prevention and safety strategies for PMD-related fires
Methodology
All admitted PMD-related burn injuries and follow up appointments were captured and reviewed. The electronic medical records via Allscripts Sunrise Clinical Manager System 5.5TM (Eclipsys) were reviewed for all our patients across SGH Burns Unit and KKH Burns Unit from 2016 to 2020.
Data were collected and analysed for patient demographics (race, age, gender, date of injury, preexisting co-morbidities, smoking status), burn severity index (TBSA, burn depth, inhalational injury, associated injuries), treatment (burns ICU admission, high dependency admission, intubation, surgical treatment), length of stay and mortality.
All statistical analysis was performed using SPSS Version 23 software (IBM, USA). All continuous data were expressed as mean ± standard deviation.
All categorical data were expressed as frequency ratio or percentages. Student’s t-test was used to test for significance for continuous data and Chi-square or Fisher’s exact test was used to test for significance for categorical data. A p-value of <0.05 is considered to be statistically significant.
Results
Admissions
From 2016 to 2020 there were 30 admissions for PMD-related burn injuries. Seventeen of these patients were admitted to the SGH regional burns unit, and 13 were admitted to the KKH pediatric burns unit for treatment. Combined admissions to both tertiary institutions show a 20-fold increase in admissions for PMD-related fires to the present date (Fig. 1).
Fig. 1. Hospital conveyance to admissions by year and admission to percent total body surface area burns.
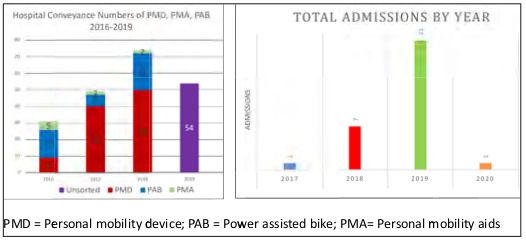
Table I. Demographic details of patients admitted for PMD-related burn injuries 2017- 2020.
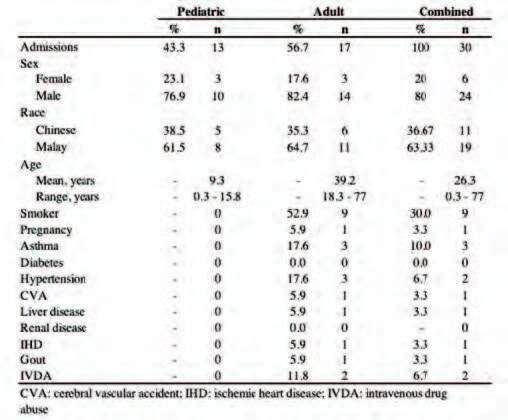
Fig. 2. Admission to percent total body surface area burns.
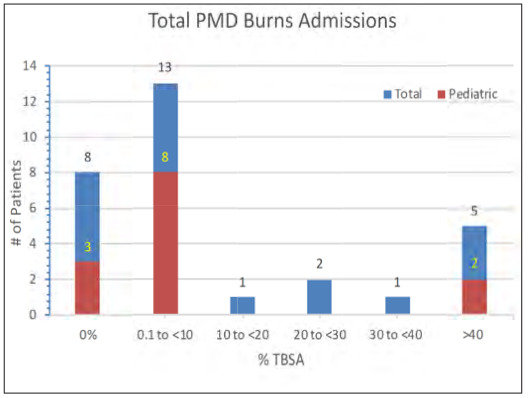
Table II. Clinical details of patients admitted for PMD-related burn injuries 2017-2020.
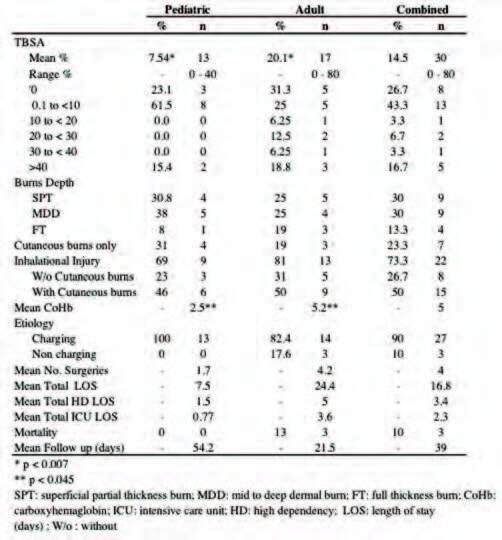
Fig. 3. 40% TBSA partial to deep dermal burns over chest, bilateral upper limbs and lower limbs. This patient had extensive inhalation injury and sustained cardiovascular collapse after re-entering his residence to rescue the fire. He went into multi organ failure secondary to burn shock and demised on post admission day 2 from myocardial collapse.
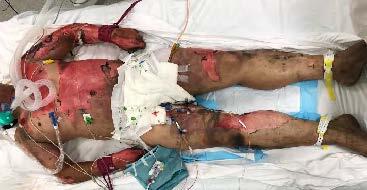
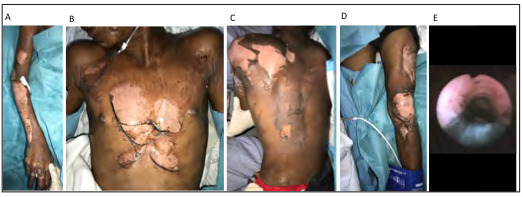
Fig. 4. A) 72-year-old male who sustained 80% TBSA partial to deep dermal burns to anterior chest extending to posterior thorax and (B) abdomen; C) Deep to full thickness burns of bilateral anterior and posterior lower limbs, sparing the perineum and genitalia, as well as full thickness burns of bilateral upper limbs requiring escharotomy; D) Left lower leg shrapnel injury with exposed tibial bone from battery casing which was removed.
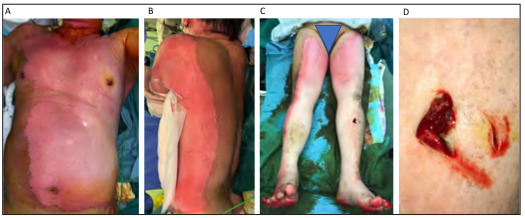
Fig. 5. - Li Ion Cell Structure. A) Four functional components: a positive cathode consisting of a lithium metal oxide, a negative anode, typically a lithium graphite, a separator segregating the oppositely charged electrodes but permitting Li ionic flow, and an electrolyte, which insulates the passage of electrons within the battery. The solid electrolyte interphase (SEI) is a protective layer coated at the anode during the cell formation process; B) In discharge, the de-intercalated Li ion flow travels toward the anode. The anode is oxidized as electron flow travels toward the cathode, which is reduced. In charging, the process is the opposite; C). A Li ion polymer pack typically used to power personal mobility devices; D) An X-ray image shows the multilaminated structure of a single battery cell where each layer represents an anode and cathode layer as a functional unit of a Li ion battery. Image reproduced with permission from Wang et al.
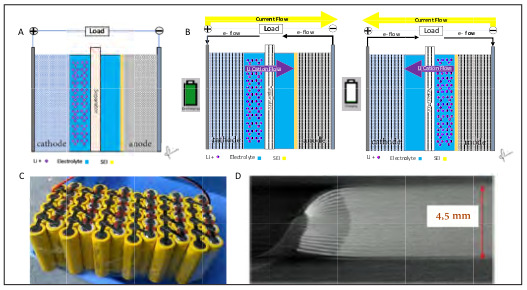
Fig. 6. Fire Triangle and Tetrahedron. Classical fire triangle model describes fuel, oxygen and heat as the three pillars of combustion. Paradigm shift to the fire tetrahedron model which adds an uninhibited chain reaction propagating the combustion process.
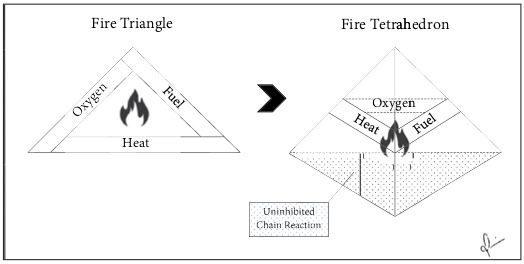
Fig. 7. Thermal Runaway and Gas Release. A) Illustrates the ejection phase of thermal runaway where high pressure gases including hydrogen fluoride gases are vented; B) Time rendered photo-capture of the thermal runaway process. Notice that combustion may or may not occur during the ejection phase as compared to A. Image reproduced with permission from Wang et al.

Fig. 8. Thirteen-year-old pediatric burns with 40% TBSA cutaneous partial to deep dermal burns with inhalation injury after Li ion battery explosion. A) He sustained mixed cutaneous burns to left upper arm, anterior and positioner thorax (B,C), and D) left lower limb; E) He had significant inhalation injury with evidence of soot in the lower airway requiring lavage.
Of those patients conveyed to the hospital, 21% were admitted under patient acuity category 1 in 2018. This figure doubled to 40% in 2019 based on local conveyance to admission data.1-3
Cause of burns and mechanism of combustion
All burns were a result of detonation of the Liion battery pack. In 90% (n=27) of patients, burns were associated with the combustion of electric scooters left unattended during recharging. 10% (n=3) of burns were a result of combustion of non-recharging or stand-alone Li-ion battery packs.
Burn patient demographics
In our combined burns patient cohort, the mean age corresponded to 26.3 (SD ± 19.4), a median age of 18.5 with a range of 0.3 to 77. This population is younger than the calculated mean age of 42.6 (SD ± 17.5) years from our center’s burns epidemiology from 2011 to 2013.4
A total 55% (n=17) of our patients were adults above the age of 18, whereas the remaining 45% (n=13) of patients were pediatric. Our patients were predominantly male with a male to female ratio of 4:1. One third of the patients were Chinese (n=11) with the remaining two-thirds identified as Malay (n=19) (Table I).
Subgroup analysis of the pediatric burns cohort (<18 years of age) yielded a mean age of 9.3 (SD ± 5), a median age of 9.2, with a range of 0.3 to 15.8 years at presentation. Conversely, the subgroup analysis of the adult population (>18 years of age) yielded a mean age of 39.2 (SD ± 17.9), a median of 32.8, with a range of 18.3 to 77 years at presentation.
Casualties per incident and a community patternof burns injury
In our cohort there were 4 social or family clusters comprising of 14 patients (5 adults and 9 children) who suffered burn injuries from 4 independent PMD-related fires. Two of the four family clusters consisted of 2 pairs of brothers trapped in a confined room when the PMD detonated. One cluster consisted of 5 schoolboys at a sleepover when the PMD detonated in the living room. The last cluster consisted of four family members who sustained mixed burn injuries as a result of PMD combustion. As a result of the fire, a neighbour in the neighbouring residential unit sustained inhalation injury from the smoke generated and required hospital admission. This subgroup comprises 48% (n=14) of our burns cohort and yields a casualty to incidence ratio of 1.5:1 per PMD explosion event.
Trauma activation
Forty-three percent (n=13) of all patients were trauma-activated based on high suspicion of blast injury, respiratory compromise and/or hemodynamic instability at presentation to the emergency department. Of this cohort, 4 were children under the age of 14 and 9 were adults. All trauma-activated patients required either burns intensive care unit or high dependency unit admission for stabilization and management.
Extent of burns
The mean total body surface area (TBSA) for our admissions was 14.5% (SD ± 22.3) with a range of 0 to 80% (Fig. 2). This is higher than the reported mean TBSA of 9.5% (SD ± 14.2) from our burn center’s 2011-2013 epidemiology studies. Patients with <10% TBSA burns made up the majority of admissions at 43.3% (n=13), one third of which underwent surgical intervention for superficial partial to deep dermal burns requiring biobrane application and/or secondary split thickness skin grafting. 3.3% of patients (n=1) sustained 10-20% TBSA burns, 6.7% of patients (n=2) sustained 20-30% TBSA burn injuries, 3.3% of patients (n=1) sustained 30-40% TBSA burn injury and 16.7% of patients sustained greater than 40% TBSA burn injuries (Table II).
In terms of burn depth, 30% (n=9) of patients presented with superficial partial thickness burns; 30% (n=9) presented with mid to deep dermal thickness burns and 13% (n=4) presented with full thickness burns. There was no statistically significant correlation between mechanism of combustion and burn depth. There were two statistically significant factors that correlated with a higher TBSA burn injury: age and mechanism of burn. Adult patients (n=17) presented with a higher mean TBSA of 20.1% compared to pediatric (n=13) mean TBSA of 7.54% (p ± 0.007). Detonation of a non-charging battery was associated with a higher TBSA compared to detonation of a charging battery (p ± 0.05).
Inhalation injury
Seventy-three percent (n=22) of our patients presented with airway injury evidenced by soot and edema in the upper airway. Twenty-seven percent (n=8) presented with inhalation injury alone and 50% (n=15) presented with cutaneous burns with inhalation injury. Subgroup analysis showed that the incidence of inhalation injury in the pediatric subgroup was 69% (n=9) and 81% (n=13) in the adult subgroup. While adults presented with increased incidences of inhalational injury, this difference was not statistically significant (Table II).
Additionally, there was no statistically significant correlation with the mechanism of battery combustion and inhalation injury.
There was, however, statistical significance with the presenting carboxy-hemoglobin (CoHb) level between the pediatric versus adult sub populations. Adults presented with a higher initial CoHb level, with a mean of 5.2% (R 0-29%) versus 2.5% (R 0-5.1%) in the pediatric sub group (p ± 0.045). CoHb levels correlated statistically significantly with total length of stay and mortality (Table II).
Length of hospitalization
The mean length of hospitalization was 39 days for when surgery was indicated. This is a 25% increase compared to the reported mean from our 2011 to 2013 burns epidemiology study (Table II).4 However, in those patients in whom surgery was not required, there was a reduction in the average length of stay from 4.6 days to 3.5.
Hospitalization duration was statistically longer in patients who required surgical intervention (p = 0.0001). Additionally, full thickness burns were positively correlated with increased length of stay (p ± 0.003)
Similarly, in those patients requiring operative management, there was a statistically significant correlation with a longer stay in the intensive care unit, on average 6.09 days (p ± 0.02), and high dependency unit on average 6.55 days (p ± 0.001)
Surgical intervention
Forty-one percent (n=12) of our burn patients required surgery for management of their cutaneous burns. The mean number of operations was a reported 7.4 (SD ± 7.8). There was a correlation with increased number of surgeries in those patients with larger extent of burns and those with deeper burns. In 28% (n=8) of patients with <10% TBSA burns, surgery was not required. These patients underwent cleansing and recovered with interval dressing changes.
Mortality
The overall mortality rate was high at 10% (n=3).
All three patients were adults. 27% (n=8) of our patients were admitted to the ICU. Mortality was associated with two statistically significant factors. First, a TBSA ± 40% was associated with increased mortality (p ± 0.036). The mean TBSA of those who did not survive was 55% compared to the average TBSA of patients who survived, which was 14.5%. This is consistent with our burn center’s 2011 to 2013 data, which showed that a TBSA ± 35% is a predictor of mortality.4 Secondly, a high CoHb level at presentation was significantly correlated with increased mortality. The mean CoHb in the non-survival group was 28.6% compared to 2.2% in those who survived. This difference was found to be statistically significant with a p-value ± 0.036. Inhalation injury was not discovered to be a significant factor for mortality.
Patient one was a healthy 40-year-old male with no previous pre morbid conditions. He sustained 40% TBSA superficial partial thickness to mid dermal burns in addition to inhalation injury. He sustained a cardiovascular collapse on site with a down time of 22 minutes (Fig. 3). On admission to our Burns Intensive Care Unit, he was intubated and maintained on triple ionotropic support. He was severely acidotic with an arterial pH of 7.16 with type II respiratory failure and hypocalcemic with a corrected calcium level of 1.37 mmol/L and hypomagnesemia. Significantly he had a high anion gap of 23. He also had a significant carbon monoxide load with a carboxyhemaglobin level of 28.4%. This patient developed worsening acute kidney injury with oliguria requiring continuous renal replacement therapy in addition to multi organ failure secondary to burns shock by post admission day 2. Despite supportive efforts, this patient sustained a second myocardial infarction that led to his demise.
The second mortality was in a healthy 77-yearold patient who had a history of well controlled hypertension and hyperlipidemia. This patient was trapped in his living room after an e-scooter battery pack exploded during charging. He was found a distance away from the site of explosion by the paramedics in a smoke-filled flat with a sustained cardiac arrest of unknown duration. Return of spontaneous circulation was achieved after 40 minutes of cardiopulmonary resuscitation. He sustained a 2% TBSA deep dermal burn to the scapula region in addition to inhalation injury. Intubated in ICU, he was
in severe metabolic acidosis with a pH of 6.97 with a high anion gap of 27.5. The patient, again, presented with hypocalcemia with a corrected calcium of 1.6 mmol/L and hypomagnesemia. Interestingly, his CoHb index was normal at 0.9%. He was supported on single inotropic support; however, he suffered a second myocardial infarction as the cause of death.
The third patient was a 72-year-old with a past history of diabetes, hypertension and previous left hemiparetic stroke. This presented with 80% TBSA mixed partial to full thickness burns and inhalational injury after an e-scooter on charge exploded in the same room (Fig. 4). Arterial blood gas at presentation showed metabolic acidosis with a pH of 7.1 and anion gap of 22. His CoHb index was elevated at 3.4%. He was hypocalcemic with a corrected calcium of 1.8 mmol/L and hypomagnesemia. He was found to have ST depressions and a raised troponin T level of 206 ng/L. He underwent burns scrub down, escharotomy of bilateral upper limbs and application of Biobrane (UDL Laboratories, Rockford, IL, USA) to the trunk, abdomen, back and bilateral lower limbs. He developed multiorgan failure, sustained a myocardial infarction secondary to burns shock and multi-source sepsis with resultant demise.
Discussion
Since the commercialization of Li-ion batteries in 1991 by Sony Inc., the energy density and charge capacity have skyrocketed. The ubiquity of this technology has also seen its equivalent share of fires and explosions worldwide, some of which have caused grave harm to human life and galvanized international manufacturer recalls.5-6
The Tesla electric car battery combustion in 2012, the Li-ion battery fire while inflight on a Boeing 787 aircraft in 2013, the Samsung Note 7 pocket explosions in 2016, and the recall of Audi’s first all-electric vehicle due to its lithium ion battery fire risk in 2019 are landmarks feeding global media attention and apprehension.6-7
The potential incendiary nature of Li-ion batteries is well known. The in-depth documentation of the dangers and potential instabilities of Li-ion batteries exist mainly in a plethora of electrochemical, engineering, and applied science journals.8-13 In the medical literature, however, representation of thermal injury from Li-ion batteries is sparse and limited to case reports.14-18
The disequilibrium between the two bodies of literature signifies a substantial gap in knowledge in the understanding of the explosive nature of Li-ion batteries.
An understanding of the electrochemical “pathophysiology” of a failing Li-ion cell is important as it may dictate patterns of injury and affect acute stabilization of the patient and further inpatient management.
How lithium ion batteries work
Li-ion cells are known as ‘secondary’ batteries because their oxidation reduction (redox) reaction is reversible. This allows them to be recharged, which is dependent on the movement of the Li-ions between electrodes. Like all batteries, the Li-ion cell unit contains four functional components: 1) a positive electrode (cathode) consisting of a lithium metal oxide; 2) a negative electrode (anode), typically a lithium graphite; 3) a separator segregating the oppositely charged electrodes but permitting Li-ionic flow in between; 4) an electrolyte, which insulates the passage of electrons within the battery.5,6,12 This functional unit is arranged in concentric circles or cells within a battery (Fig. 5).
During charging, the battery is plugged in providing an electric current to the positive terminal, which drives the Li-ions from cathode through the electrolyte and intercalating onto the anode. The battery is ‘fully charged’ (maximum electrochemical potential) when no more Li-ion can be driven into the anode. This is the terminal voltage we see on battery labels.
During discharging, the process is the opposite. The natural state of a battery is to discharge. When the plug is removed, the Li-ions, seeking electroneutrality, will drive back to the cathode. The accompanying electron flow or electric current, insulated by the electrolyte solution within the battery, flows through an external circuit, powering the attached electronic device, as it travels back to the cathode to complete the electric circuit (Fig. 5).5,6,8,18
The boon and bane of lithium
Lithium is almost perfect. It is the lightest solid metallic element and possesses the highest electrochemical potential.19Weight-for-weight, lithium packs more power than the more toxic and heavier alternatives, i.e. lead, cadmium, mercury. Its light weight-to-highenergy-density ratio creates batteries which are portable, compact, enduring and marketable.
Furthermore, Li-ion batteries have long battery lives due to Li’s high energy density to low discharge rate (<5% per month) tempered by its lack of “memory effect” - loss of capacitance with cycles of partial charge and discharge.5,6,8,19
Volatility is the lithium ion trade off. Belonging to the group 1 alkali earth metals, with the same valency and thus reactivity characteristics of elemental sodium and potassium, Li is highly volatile as it easily gives up its single valence electron. Thus, elemental Li is highly combustible and never found in its pure form in nature.
Li combusts with oxygen exposure to form lithium oxide in an exothermic reaction:
4Li(solid) + O2(gas)→ 2Li2O (solid)
With exposure to water, Li is rapidly oxidized into lithium hydroxide (a strong alkaline base) and the highly flammable hydrogen gas as a by-product:
2Li(solid) + 2H2O(liquid) → 2LiOH(aqueous)+ H2(gas)
Given its inherent instability, Li-ion batteries are classified precisely by their battery chemistry which aims to balance the stabilization of Li to achieved energy capacitance as a trade-off. For example, Li manganese oxide at the cathode provides for a greater thermal stability at higher temperatures than lithium cobalt oxide but at a disadvantage of a lower energy density.12,19
Li-ion battery fires
Li-ion batteries contain all combustible components of the fire tetrahedron model. Fire is the quintessential redox reaction, where hydrocarbon fuel is rapidly oxidized and oxygen is equivalently reduced in the presence of an ignition source. In fire research, the two dimensional fire triangle model has historically canonized the vital reactants needed for combustion: 1. Fuel 2. Heat 3. Oxygen. The American National Fire Protection Agency has since evolved this model by adding a 4th component; the uninhibited chemical chain reaction, as the third dimension of the fire triangle, creating the fire tetrahedron model (Fig. 6).19 Removal of any one element of this tetrahedron halts the exothermic process.
In parallel, the same redox reaction occurs in a Li-ion cell at the cathode and anode; albeit in a closed system. Implicitly, all vital reactants of fire generation are contained and compacted within a Li-ion battery system: 1. The fuel source is the intrinsically flammable organic alky carbonate electrolyte solvent in addition to volatile Li itself. 2. The provision of heat may be external or internal. Overcharging, where a battery is charged beyond the designed voltage or capacitance, is the commonly observed external source of heat which then creates internal heat sources.1-3,5-6. Increased temperatures cause decomposition of the Li metal oxide material at the cathode which releases oxygen.4 Lastly, high temperatures further drive the chemical reaction rates inside the cell in a positive feedback cascade known as thermal runaway – the terminal failure event of the Li-ion battery system.5,6,20
Three stages to thermal runaway – overheating, ejection, detonation
Thermal runaway is an unrestrained, self-sustaining positive feedback loop where heat not only auto-catalyses but also increases the reaction rates of parallel exothermic chemical reactions during cell failure, akin to a simultaneous para and autocrine effect in cell biology. This “domino effect” leads to a failure cascade where heat generated from a singular failing cell drives adjacent cells into thermal runaway (Fig. 7).19-22
The first stage is overheating. Generally, Li-ion battery chemistries operate at an optimal temperature window, -20 to 55°C.19-21 In the context of this study, 90% of the batteries in our series exploded during unwitnessed charging. Though impossible to retrospectively determine the state of charge (SOC) of these batteries during detonation, Mendoza-Hernandez et al. have identified through isolated Li-ion cell experiments that under-charged batteries may be subject to conditions conducive of thermal runaway. They have found that 50% to 120% SOC is the range where an onset of exponential spikes in internal battery temperatures occurs, reaching a zenith of 176.4°C and 189.8°C in Li cobalt oxide and Li manganese oxide cells, respectively.21 Comparatively, at 80°C is the point of thermal escape where a multitude of non-consecutive events begins to occur. This includes free oxygen generation at the cathode from lithium metal oxide decomposition; separator failure leading to internal short circuits (rapid electrical discharge) and heat generation, driving the second stage of cell failure.20-22
In the second stage or ejection phase, occurring at temperatures >80°C, there are highly exothermic decompositions at both electrodes. At the anode, decomposition of the solid electrolyte interphase (SEI) layer releases combustible hydrocarbon gases. The SEI is an electrically insulating “protective layer” commonly consisting of meta-stable lithium ethylene dicarbonate (CH2OCO2Li)2, Li hexafluorophosphate (LiPF6), and carbonate electrolytes (ethylene carbonate (EC) + dimethyl carbonate (DMC) + propylene carbonate (PC)) that are plated at the anode during the cell’s formative process in Li-ion battery chemistry.5-6,8,19-22
An example of SEI decomposition at the anode - an exothermic reaction forming free oxygen species:6,20-21
(CH2OCO2Li)2 → Li2CO3 + C2H4 + CO2 + O2
Intercalated Li-ion at the anode further reacts exothermically with carbonate electrolytes forming highly combustible hydrocarbon gases – ethylene (C2H4), propene (C3H6), and ethane (C2H6):6,19-22
2Li + C3H4O3 (EC) → Li2CO3 + C2H4
2Li + C4H6O3 (PC) → Li2CO3 + C3H6
2Li + C3H6O3 (DMC) → Li2CO3 + C2H6
At the cathode, lithium metal oxide cathode decomposition may simultaneously release more free oxygen gas. An example of LiCoO2 is as follows:5,6,20-21
LixCoO2→ xLiCoO2 + (1 − x) Co3O4 + (1 − x)O2
Co3O4→ 3CoO + O2
CoO → Co + O2
These key reactions occur non-consecutively and expediently, driven by the heat generated off each other. This leads to rapid heat and pressure build up within the pack. Built-in safety vents within the battery allows for release and decompression of the system at the cost of releasing these flammable, noxious gases into the air. At this point, the gases may not be ignited immediately.
The third stage, detonation, is heralded by pack rupture when an overwhelmed venting system and excessive heat sparks gaseous explosion of the hydrocarbon gases and/or of the electrolytes upon exposure to air and moisture (Fig. 7). These alkyl carbonate electrolytes (EC, PC, DMC) are themselves combustible, even without reacting with lithium, as they exhibit high vapor pressures (degree to which a fuel vaporizes) of 4.8 kPa at room temperature and extremely low flash points (lowest temperature at which a solvent forms an ignitable mixture with air) of 25° ± 1°C.23 These gas explosions contribute to flash burns, primary or secondary blast, and inhalation injury, as seen in our case series (Video 1 - Reproduced with permission from Salgrom Technologies and Wetrax GmbH www.wetraxgmbh. de).
It is very important to understand that exothermic peaks and corresponding detonations of each cell can occur independently and unpredictably of one another. Simply speaking, each cell may be at different phases of thermal runaway. However, the heat release is the shared factor driving the overall process. This understanding is critical as it often dictates the patterns of thermal injury.
Major burns from Li-ion battery explosions: patterns of burn injuries to mechanism of combustion.
An explosion is a violent shattering or blowing apart. In the context of the thermal runaway process, once cell failure reaches stage 3, there is rapid expansion, gas release and ignition of fuel with extreme heat generation, fulfilling the criteria of an explosive device. Additionally, rupture and fragmentation of the shell of a cell pack may contribute to additional projectile shrapnel injury and has been reported. 24-30 In our series, we had one patient who had an associated injury from this mechanism where he sustained a full thickness laceration down to the left tibial bone from a shrapnel fragment, likely from the battery shell, which was removed (Fig. 4).
In terms of thermal injury, there is a clear demarcation in the pattern of burns between adults and children. The incidence of major burns >20% TBSA in adults was 2.3 fold higher at 35% compared to 15.4% in the pediatric sub group. This is consistent with the mean %TBSA of burns in the adult at 20.1% compared to 7.54% in children, which was statistically significant (Table II). Adults also presented with deeper burns compared to those of children. Nineteen percent of adults presented with full thickness burns compared to 8% children (Fig. 8).
The primary postulate based on patient history as to why adults sustained higher surface area and depth of burns is primarily because of the response to the explosion. It is frequently reported that the adult patients would re-enter the building or residence to rescue belongings in an attempt to extinguish the fire. This can be deadly given the context of how Li-ion batteries fail: each cell may fail nonsynchronously but positively driven by heat released from an adjacent cell. As one cell detonates, its fuel is consumed, and fire abates; this does not equate that the adjacent cell is in the same state. In fact, the adjacent cell may be at the cusp of detonation. This may translate to secondary thermal re-injury and noxious gas exposure.
Another critical observation is that burns from PMD-related fires exemplify a communal pattern of injury. Forty-eight percent of our patients (n=14) were from 4 family or social clusters injured by 4 independent PMD explosions. With a casualty to PMD explosion incidence ratio of 1.5:1, the clinician needs to be cognizant that PMD fires may result in a multiple casualties incident. This is compounded by the high risk of multiple patients with inhalation injury with or without cutaneous burns, which may come through the emergency department concurrently. A potential scenario of a sudden high volume of mixed cutaneous burns with inhalation burn injuries may overwhelm an admitting institution not prepped to handle such a multitude of burn casualties.
Hydrogen fluoride emission, toxicity and chemical burns
In the second stage or ejection phase, there is an unrecognized emission of hydrogen fluoride (HF) and phosphoryl fluoride (POF3) gas through the decomposition of lithium fluoride compounds in the cell pack (Fig 7). The electrolyte, anode/cathode components, separator composition, the cell binder often containing polyvinylidene fluoride (PVdF), are all potential fluorine sources.12 The reaction of Li hexafluorophosphate (LiPF6), a common electrolyte salt in Li-ion cells, is well described:5-6,12
LiPF6 → LiF + PF5
PF5 + H2O → POF3 + 2HF
LiPF6 + H2O → LiF + POF3 + 2HF
The primary step is the pyrolysis of LiPF6 to form phosphorous pentafluoride (PF5), a short- lived intermediary, which then readily reacts with water (either moisture contaminate inside the cell or external moisture) to generate HF gas. At the same time, POF3 reacts with water to form HF gas.31
Larson et al. presented his pivotal study published in Nature, which concluded that gas emission is greater than heat production and that there is a positive correlation of HF gas release with increased SOC.12 Furthermore, through two independent highfidelity measurement techniques in conjunction with fire tests on 7 commercial Li-ion batteries, he identified that the average HF release ranged from 20-200 mg/watt hour (Wh).12 Watt hour is a unit of measurement for battery capacity as a product of Ampere hours (Ah) x Voltage (V).
Extrapolating that the electronic scooter has battery capacities ranging from 250 to 3000 Wh, the lowest range of gas release to battery capacity corresponds to 5 grams (g) of HF released (250 Wh x 20mg/Wh) from a standard battery.25 Now, the toxicity of HF is well known.26,27 The immediately dangerous to life or health value of HF is 25mg/m3 versus the lethal 10-minute value of 139mg/m3 according to the Acute Exposure Guideline Levels (AEGL).12,24,26 Simple math dictates that a 5g HF release in a small confined space of 35 m3 or 115 square feet would easily reach the lethal threshold.
Since HF gas is not combustible, it presides even in the presence of fire, and it is not consumed by the burning process. Compounding to its detriment, gaseous HF, being completely water-soluble, forms hydrofluoric acid when in contact with mucosal surfaces, raising the utmost and unbeknownst concern of not only cutaneous hydrofluoric acid burns but also acidic burns to the aerodigestive tract as it is corrosive to the eyes, mucous membrane and respiratory tract.26,27 This is extensively documented in human and animal studies.26,27,31
Hydrogen fluoride gas release and inhalation injury: special considerations in the pediatric population
Infants and toddlers possess unfavourable anatomical differences compared to adults that predispose them to higher risk of upper airway obstruction: smaller airway cross sectional area, limited upper airway space due to shorter mandibles, prominent adenoids and larger tongues, as well as infra-thyroid cartilage tracheal narrowing can all contribute to air passage resistance in the context of acute inhalation injury.32
Furthermore, children possess a higher lung surface area to body weight ratio as well as increased minute volume to body weight. They are therefore at higher risk of increased HF toxicity compared to adults exposed to the same concentration of HF gas.
Additionally, given the smaller calibre of pediatric airways, children are more vulnerable to corrosive effects in the aerodigestive tract (Fig. 8).27 While the pediatric children with inhalational injury in our cohort sustained mild inhalation injury compared to the adult cohort, there should, nevertheless, be a low threshold for suspicion of inhalation injury in pediatric patients from PMD fires. Additionally, vigilance for chemical burn from HF gas solubilized into mucosal membranes in the oral and upper airway is paramount and may be overlooked during acute stabilization.
Little is known or written about HF acid inhalation injury. Cutaneous HF exposure has been well described with documented lethal exposure as miniscule as 2.5% TBSA; however, historically, the outcomes of inhaled industrial concentrations of HF have been rapidly lethal with few case reports on primary systemic and nebulized calcium gluconate therapy outcomes33-38 (Supplementary Fig.1). The authors believe that this undetected mechanism of chemical airway injury may have contributed significantly to mortalities in our cohort.
Mortality
The mortality rate in our cohort is 10% (n=3), which is four-times higher than our reported mortality rate from our burn injury epidemiological study from 2011 to 2013.29 All three male patients who died were from the adult cohort and sustained mixed cutaneous burns with inhalation injury. Two patients were found to be in cardiopulmonary arrest on site.
The third patient, while not in cardiac arrest on site, was found to have NSTEMI based on cardiac enzymes and ECG findings. Initial blood gases revealed high anion gap metabolic acidosis with concomitant hypocalcemia and hypomagnesemia in all three patients suspicious for systemic fluorosis.
It is our hypothesis that this HF toxicity could be a critically overlooked component contributing to high mortality in the context of cardiovascular insult and collapse from Li-ion battery explosions. Experimental data in animal studies have shown that HF in the upper airway is rapidly absorbed into the circulation, leading to systemic fluoride toxicity.37-39
Mechanistically, the permeating fluoride anion chelates and leeches cellular calcium and magnesium into insoluble calcium fluoride and magnesium fluoride. The depletion of calcium produces an inhibition of the sodium potassium ATPase leading to local hyperkalemia which can be a plausible cause of cardiac arrest in addition to reduced myocardial contractility, coronary vasospasm and dysrhythmias provoked by deranged serum calcium and magnesium levels.
Key management and prevention strategies
The best treatment for trauma is prevention. In PMD-related fires, secondary burn and inhalation injury after initial e-scooter battery explosion is a pattern seen in injury clusters.
Ten percent (n=3) of Li-ion battery packs that detonated in our series were not actively charging. Parallel to this, Mendoza et al., studying the thermal runaway behaviour of Li-ion batteries at different states of charge, established that the runaway process can initiate as low as 50% charge capacity.21 Thus, Li-ion batteries do not need to be overcharged or even charging in order to detonate. The keystone is for vigilance of both charging and non-charging devices, as both have been shown to undergo cell failure and detonation.
Heat is the catalyst of the thermal runaway process. Internal temperatures of 80°C is the cusp of where thermal escape occurs.20-22 To halt overheating is most practical in the prevention of Li-ion batteries. This can be as simple as tactile monitoring the battery temperature periodically and removing it from charge if the casing is hot to touch, or adequate storage in a cool and dry environment.
In cell failure, it is important to understand that the chain reaction of thermal runaway is a capricious process. In a failing cell, it is impossible to predict the timing of exothermic peaks of adjacent cells which can detonate and cause secondary burns and/or inhalation injury. It is, therefore, imperative that one does not re-enter the site of explosion with the expectation that the combustion process is complete, as thermal runaway is non sequential and varies from cell to cell.
The most efficient way to halt the thermal runaway process is to disable one or more limbs of the fire tetrahedron. In the context of a battery pack which contains all the constituents of the fire pyramid model, the best method to extinguish a Li-ion battery fire is to utilize a category D dry powder fire extinguisher.18 For combustible metal fires in the context of batteries, using water to douse the flames may cause further exothermic reactions with unconsumed lithium ions and/or result in further internal short circuits, leading to rapid electrical discharge and heat generation. Dry-powder extinguishers smother the process by removing oxygen and act as a heat sink, removing the catalyst of the thermal runaway process.
If faced with patients retrieved from Li-ion battery fires, it is crucial to be highly suspicious of a blast pattern type of traumatic injury. Advanced Trauma Life Support is vital in all cases.
Specifically, it is also necessary to examine for any shrapnel injury as failing batteries, once they reach stage 3, can detonate, fragmenting the casing violently. This was not only observed in our patient but also reported in the medical literature.30
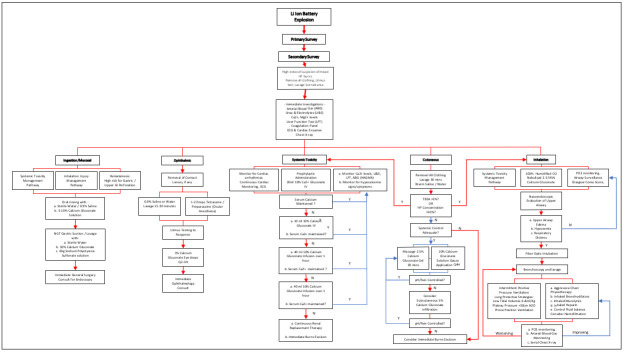
In a standardized approach, there should be high suspicion of mixed thermal and chemical burns inclusive of inhalation injury40 (Supplementary Fig. 1).
This should be at an extremely low threshold of suspicion if the history corroborates re-entry or collapse at the site of explosion. If systemic fluoride toxicity is suspected from initial blood investigations, chelation of fluoride ions to halt the consumption of calcium and magnesium through all routes is lifesaving, in addition to intravenous repletion of consumed electrolytes.
Supplementary Fig. 1. Algorithm for the management of concomitant hydrofluoric acid burns in PMD Li ion battery explosions.
Conclusion
Li-ion battery is not only an incendiary but also an explosive device. Detonation due to a Li-ion battery failure may not be singular but is often times plural, producing secondary upon primary cutaneous and inhalation burn injuries. Significantly, given the electrochemistry of the failing process, there should be high suspicion of mixed hydrofluoric acid burns, as failing Li-ion cells forcibly vent non-combustible hydrogen fluoride gas. As this gas is highly miscible with water, it rapidly forms hydrofluoric acid on contact with mucosal and moist tissue surfaces, exacerbating concomitant thermal cutaneous and inhalation burn injuries.
Most dangerously so, it is crucial to understand that Li-ion batteries need not be actively charging or overcharged to exhibit thermal runaway.
References
- 1. https://www.todayonline.com/singapore/e-scooter-fires-soar-300-2017-even-fire-cases-hit-40-year-low-scdf . [Google Scholar]
- 2. https://www.scdf.gov.sg/docs/default-source/scdf-library/publications/amb-fire-inspection-statistics/fire-ambulance-enforcement-statistics-2017.pdf . [Google Scholar]
- 3. https://www.scdf.gov.sg/docs/default-source/scdf-library/ambfire-inspection-statistics/scdf-annual-statistics-2018.pdf . [Google Scholar]
- 4.Hwee E, Song C, Tan KC, Tan BK, Chong SK. The trends of burns epidemiology in a tropical regional burns centre. Burns. 2016;42(3):682–686. doi: 10.1016/j.burns.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Khor SN, Chong SJ, Tan KC. Electric scooter burns and the danger of personal mobility device battery. ANZ J Surg. 2018;88(3):250–250. doi: 10.1111/ans.14391. [DOI] [PubMed] [Google Scholar]
- 6.Liu K, Liu Y, Lin DC, Allen P, Cui Y. Materials for lithium-ion battery safety. Sci Adv. 2018;4:eaas9820–eaas9820. doi: 10.1126/sciadv.aas9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang QS, Sun JH, Chu Lithium ion battery fire and explosion. Fire Safety Science. 2005:375–382. [Google Scholar]
- 8. https://www.bloomberg.com/news/articles/2019-06-10/audi-recalls-first-electric-vehicle-in-u-s-on-battery-fire-risk . [Google Scholar]
- 9.Galushkin NE, Yazvinskayay NN, Galushkin DN. Mechanism of thermal runaway in lithium ion cells. J Electrochem Soc. 2018;165(7):A1303–A1308. [Google Scholar]
- 10.Ouyang DX, Liu JH, Chen MY, Wang J. Investigation into fire hazards of lithium ion batteries under overcharging. Appl Sic. 2017;7:13–14. [Google Scholar]
- 11.Jhu CY, Wang YW, Shu CM, Chang CM, Wu HC. Thermal explosion hazards on 18650 lithium ion batteries with a VSP2 adiabatic calorimeter. J Hazard Mater. 2017;192:99–107. doi: 10.1016/j.jhazmat.2011.04.097. [DOI] [PubMed] [Google Scholar]
- 12.Huang P, Wang QS, Li K, Sun PP, Sun JH. The combustion behaviour of large-scale lithium titanate battery. Nature: Scientific Reports. 2005;5:7788–7788. doi: 10.1038/srep07788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsson F, Andersson P, Blomqvist P, Mellander BE. Toxic fluoride gas emissions from lithium ion battery fires. Nature: Scientific Reports. 2017;7:10018–10018. doi: 10.1038/s41598-017-09784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shastry S, Langdorf M. Electronic vapor cigarette battery explosions causing shotgun like superficial wounds and contusions. Western J Em Med. 2016;17(2):177–180. doi: 10.5811/westjem.2016.1.29410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marqa Q, Mohamed AT, Salib M, Morris S. Too hot for your pocket! Burns from e-cigarette lithium battery explosions: a case series. J Burn Care Res. 2018;XX:1–6. doi: 10.1093/jbcr/irx015. [DOI] [PubMed] [Google Scholar]
- 16.Dalal AK, Kumar B, Sunaina Case report on abdominal injury due to mobile battery blast. J Dent Med Sci. 2018;17(4):28–30. [Google Scholar]
- 17.Cherubino M, Pellegatta I, Sallam D, Pulera E, Valdatta L. Enzymatic debridment after mobile phone explosion: a case report. Ann Burns Fire Disasters. 2016;29(4):273–275. [PMC free article] [PubMed] [Google Scholar]
- 18.Fadeyibi IO, Izegnu MC, Benebo AS. Fatal cometic accident from a 1.5 volt dry cell battery explosion as seen in Lagos State University Teaching Hospital, Ikeja, Lagos, Nigeria. Ann Burns Fire Disasters. 2008;21(4):219–220. [PMC free article] [PubMed] [Google Scholar]
- 19. https://www.nfpa.org/News-and-Research/Publications-andmedia/Press-Room/Reporters-Guide-to-Fire-and-NFPA/Allabout-fire . [Google Scholar]
- 20.Murashko L. Thermal Modelling of Commercial Lithium Ion Batteries. Dissertation, Lappeenranta University of Technology. 2016 [Google Scholar]
- 21.Wang OS, Ping P, Zhao XJ, Chu GQ. Thermal runaway caused fire and explosion of lithium ion battery. J Power Sources. 2012;208:210–224. [Google Scholar]
- 22.Mendoza-Hernandez OS, Ishikawa H, Nishikawa Y, Maruyama Y, Umeda M. Cathode material comparison of thermal runaway behaviour of Li-ion cells at different states of charges including over charge. J Power Sources. 2015;280:499–504. [Google Scholar]
- 23.Ma S, Jiang M, Tao P, Song C. Temperature effect and thermal impact in lithium-ion batteries: a review. Progress in Natural Science: Materials International. 2018;26(6):653–666. [Google Scholar]
- 24.Durkee J. Health and safety hazards associated with cleaning agents. Management of Industrial Cleaning Technology and Processes. 2006:99–189. [Google Scholar]
- 25.Sturk D, Rosell L, Blomqvist P, Tidblad AA. Analysis of li-ion battery gases vented in an inert atmosphere thermal test chamber. Batteries. 2019;5(3):61–61. [Google Scholar]
- 26. https://electric-scooter.guide/guides/definitive-guide-electricscooters/ [Google Scholar]
- 27.Documentation for immediately dangerous to life or health concentrations (IDLHs) for hydrogen fluoride (as F). The National Institute for Occupational Safety and Health (NOISH) 1994 [Google Scholar]
- 28.Hydrogen Fluoride (HF) CAS 7664-39-3; UN 1052 (anhydrous), UN 1790 (solution). Agency for Toxic Substances & Disease Registry (ATSDR) Center for Disease Control (CDC) 2014 [Google Scholar]
- 29.Lim A, Kwek J, Ang L, Arulanadam S, Shali S. The impact of fires related to personal mobility device and power-assisted bicycle in Singapore. Burns Trauma. 2019;7(Suppl 1):27–27. [Google Scholar]
- 30.Shastry S, Landorf MI. Electronic vapor cigarette battery explosion causing shotgun like superficial wounds and contusion. Western J Emerg Med. 2016;27(2):177–180. doi: 10.5811/westjem.2016.1.29410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JS. Hydrogen fluoride and other soluble inorganic fluorides. Texas Commission on Environmental Quality. 2009 Cas Registry Number 7664-39-3. [Google Scholar]
- 32.Sen S. Pediatric inhalation injury. Burns Trauma. 2017;5:31–31. doi: 10.1186/s41038-017-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKee D, Thoma A, Bailey K, Fish J. A review of hydrofluoric acid burn management. Plast Surg (Oakv) 2014;22(2):95–98. [PMC free article] [PubMed] [Google Scholar]
- 34.Tsonis L, Hantsch-Bardsley C, Gamelli RL. Hydrofluoric acid inhalation Injury. J Burn Care Res. 2008;29(5):852–855. doi: 10.1097/BCR.0b013e3181848b7a. [DOI] [PubMed] [Google Scholar]
- 35.Tepperman PB. Fatality due to acute systemic fluoride poisoning following a hydrofluoric acid skin burn. J Occup Med. 1980;22:691–692. doi: 10.1097/00043764-198010000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Chela A, Reig R, Sanz P, Huguet P, Corbella J. Death due to hydrofluoric acid. Am J Forensic Med Pathol. 1989;10:47–48. doi: 10.1097/00000433-198903000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Dote T, Kono K, Usuda K, Shimizu H. Lethal inhalation exposure during maintenance operation of a hydrogen fluoride liquefying tank. Toxicol Ind Health. 2003;19:51–54. doi: 10.1191/0748233703th174oa. [DOI] [PubMed] [Google Scholar]
- 38.Kono K, Watanabe T, Dote T. Successful treatments of lung injury and skin burn due to hydrofluoric acid exposure. Arch Occup Environ Health. 2000;73:93–97. doi: 10.1007/pl00014634. [DOI] [PubMed] [Google Scholar]
- 39.Mayer TG, Gross PL. Fatal systemic fluorosis due to hydrofluoric acid burns. Ann Emerg Med. 1985;14(2):149–153. doi: 10.1016/s0196-0644(85)81078-7. [DOI] [PubMed] [Google Scholar]
- 40.Kirkpatrick JJR, Burd DAR. An algorithmic approach to the treatment of hydrofluoric acid burns. Burns. 1995;21(7):495–499. doi: 10.1016/0305-4179(95)00025-7. [DOI] [PubMed] [Google Scholar]