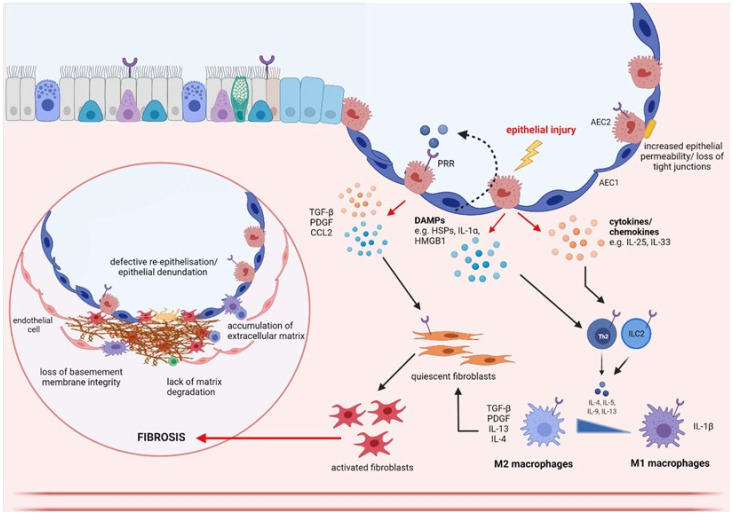
Figure 3.
Schematic diagram of downstream effects of epithelial damage in IPF. Upon injury, epithelial cells release chemokine/cytokines and DAMPs (e.g., high-mobility group box-1 (HMGB1), heat shock proteins (HSPs) and interleukin (IL)-1α) into the extracellular space. DAMPs can activate pattern recognition receptors (PRRs) on neighbouring epithelial cells and immune cells, directly stimulating the release of profibrotic cytokines including tumour growth factor (TGF)-β, PDGF and CCL2, which are involved in the activation of fibroblasts. Epithelial cells also secrete proinflammatory cytokines which recruit and activate innate immune cells (e.g., neutrophils, macrophages and dendritic cells), as well as adaptive immune cells (e.g., T lymphocytes and B lymphocytes), which further secrete pro fibrotic factors including IL-33, IL-4, IL-5, IL-13. For example, IL-33 promotes the differentiation of macrophages towards to a pro-fibrotic M2 phenotype, causing upregulation of pro-fibrotic cytokines including monocyte chemoattractant protein (MCP-1), IL-6 and TGF-β. Once activated, fibroblasts begin secretion of extracellular matrix (ECM) and pro-fibrotic factors to promote edge contractility and facilitate wound closure. Fibrosis is thought to occur in response to persistent epithelial damage leading to continued proliferation and migration of myofibroblasts, deposition of extracellular matrix (ECM) and recruitment of pro-inflammatory and pro-fibrotic markers with detrimental effects. Created using Biorender.com.